Abstract
Mycobacterium tuberculosis is an example of a bacterial pathogen with a specialized SecA2-dependent protein export system that contributes to its virulence. Our understanding of the mechanistic basis of SecA2-dependent export and the role(s) of the SecA2 pathway in M. tuberculosis pathogenesis has been hindered by our limited knowledge of the proteins exported by the pathway. Here, we set out to identify M. tuberculosis proteins that use the SecA2 pathway for their export from the bacterial cytoplasm to the cell wall. Using label-free quantitative proteomics involving spectral counting, we compared the cell wall and cytoplasmic proteomes of wild type M. tuberculosis to that of a ΔsecA2 mutant. This work revealed a role for the M. tuberculosis SecA2 pathway in the cell wall localization of solute binding proteins that work with ABC transporters to import solutes. Another discovery was a profound effect of SecA2 on the cell wall localization of the Mce1 and Mce4 lipid transporters, which contribute to M. tuberculosis virulence. In addition to the effects on solute binding proteins and Mce transporter export, our label-free quantitative analysis revealed an unexpected relationship between SecA2 and the hypoxia-induced DosR regulon, which is associated with M. tuberculosis latency. Nearly half of the transcriptionally controlled DosR regulon of cytoplasmic proteins were detected at higher levels in the ΔsecA2 mutant versus wild type M. tuberculosis. By increasing the list of M. tuberculosis proteins known to be affected by the SecA2 pathway, this study expands our appreciation of the types of proteins exported by this pathway and guides our understanding of the mechanism of SecA2-dependent protein export in mycobacteria. At the same time, the newly identified SecA2-dependent proteins are helpful for understanding the significance of this pathway to M. tuberculosis virulence and physiology.
Mycobacterium tuberculosis, the etiological agent of tuberculosis, remains a severe health concern, infecting an estimated one-third of the global population and causing an approximated 1.3 million deaths annually (1). Following inhalation into the lung, M. tuberculosis bacilli are engulfed by macrophages, which fail to destroy the pathogen and instead provide a niche for M. tuberculosis replication (2). M. tuberculosis proteins that are exported from the cytoplasm to the bacterial cell wall or into the host environment are ideally positioned for host-pathogen interactions or physiologic processes important to infection, such as nutrient uptake and cell wall biogenesis (3). M. tuberculosis has several systems for exporting proteins to extracytoplasmic locations, one of which is the SecA2-dependent protein export pathway (4). In M. tuberculosis, SecA2 is required for virulence in both mice and macrophage models of infection, making the identification of SecA2-dependent exported proteins important for understanding M. tuberculosis pathogenesis (5–7).
Mycobacteria, including M. tuberculosis, are among a select group of bacteria that have two nonredundant SecA ATPases, known as SecA1 and SecA2 (8). SecA1 is the housekeeping SecA, conserved throughout bacteria, and a central component of the essential general secretion (Sec) pathway. SecA1 powers translocation of unfolded proteins across a cytoplasmic membrane channel comprised of SecYEG proteins. Proteins exported by SecA1/SecYEG possess N-terminal Sec signal peptides that are cleaved following export to liberate the mature protein. In bacteria with two SecA proteins, SecA2 is generally nonessential but required for export of a limited set of proteins and, in the case of bacterial pathogens, SecA2 often contributes to virulence (8, 9). Bacterial SecA2 systems fall into two main groups based on the membrane channel they work with and the types of substrates they transport. One type of system, called a SecA2-SecY2 system, uses an accessory SecY2 channel protein and appears to be dedicated to exporting a single glycosylated protein (10–13). The other type of system, which is the case in mycobacteria, does not include an accessory SecY and the repertoire of exported proteins is more diverse (5, 14–17). In these SecA2-only or multisubstrate SecA2 systems, SecA2 appears to work with the SecYEG channel for protein translocation (9, 17–20). Studies conducted in mycobacteria suggest that the proteins exported by its SecA2-only pathway have a tendency to fold in the cytoplasm prior to export, which distinguishes them from proteins that remain unfolded and are exclusively exported by SecA1. This folding feature of SecA2-exported proteins may dictate the need for SecA2 in their export (21).
In mycobacteria, and more specifically M. tuberculosis, the number of known SecA2-dependent proteins remains small. In the nonpathogenic Mycobacterium smegmatis, there are two cell wall proteins that are well-established as being exported via the SecA2 pathway: Ms1704 and Ms1712 (15, 20, 21). Both of these proteins are lipoproteins with Sec signal peptides and members of the solute-binding protein (SBP) family (15). SBPs are cell wall proteins that work with membrane localized ABC transporters to import solutes into the bacterial cytoplasm. Like all mycobacteria, Mycobacterium marinum, a pathogen of fish and frogs, also has a SecA2-only system (16, 22). As many as 40 M. marinum proteins are predicted by proteomics to be SecA2-dependent (16). The most striking finding of this M. marinum study is that PknG, a protein associated with virulence and lacking a Sec signal peptide, is reduced in abundance in a cell envelope fraction of a M. marinum secA2 mutant compared with wild type (16, 23, 24). There are no direct orthologs of Ms1704 and Ms1712 in M. tuberculosis and the mode of PknG export by M. tuberculosis has not been established. Past efforts to identify SecA2-dependent proteins in M. tuberculosis are limited to comparative two-dimensional gel electrophoresis (2D-GE) of fully secreted proteins. With this approach, superoxide dismutase SodA (5) was identified as a protein requiring SecA2 for its secretion. Like PknG, SodA lacks a predicted Sec signal peptide. However, because inadequate SodA secretion is insufficient to explain the virulence defect of a M. tuberculosis ΔsecA2 mutant (7), there must exist additional M. tuberculosis SecA2-dependent proteins. Here, we set out to identify M. tuberculosis proteins dependent on SecA2 for their export by comparing the cell wall and cytoplasmic proteomes of M. tuberculosis wild type and a ΔsecA2 mutant using label-free quantitative (LFQ)1 shotgun proteomics.
Our LFQ analysis revealed reduced cell wall levels of almost all of the predicted M. tuberculosis SBPs in the ΔsecA2 mutant versus wild type, suggesting a broad role for SecA2 in the export of this family of proteins. Further, multiple protein components of Mce1 and Mce4 transporters were reduced in the ΔsecA2 mutant cell wall, suggesting a dependence on SecA2 for cell wall localization of these transport systems. Our proteomics approach also revealed an unexpected role for SecA2 in the DosR-regulated stress response of M. tuberculosis, with higher abundance of many cytoplasmic DosR-dependent proteins detected in the ΔsecA2 mutant compared with wild type. Finally, we obtained data consistent with M. tuberculosis PknG being exported by the SecA2 pathway. By expanding our knowledge of the types of proteins exported by the mycobacterial SecA2 system, this study helps our effort to understand the mechanism of this specialized protein export pathway. At the same time, the SecA2-dependent proteins identified in this work provide valuable insight into potential role(s) of the SecA2 pathway in M. tuberculosis virulence and physiology.
EXPERIMENTAL PROCEDURES
M. tuberculosis Strains and Plasmids Used in this Study
The following M. tuberculosis strains were used in this study: H37Rv (wild type), mc23112 (ΔsecA2), and a complemented strain MBTB476 (mc23112, pSM15) (5). Plasmid pSM15 is a derivative of pMV306.kan that carries the M. tuberculosis secA2 gene under the control of the hsp60 promoter. In experiments involving the complemented strain, H37Rv and ΔsecA2 strains carried the empty pMV261.kan plasmid to allow all strains to be grown in the presence of kanamycin.
Growth Conditions
M. tuberculosis strains were first grown at 37 °C in Middlebrook 7H9 medium (Difco, Sparks, MO) supplemented with 0.5% glycerol, 1× ADS [0.5% bovine serum albumin, 0.2% glucose, 0.85% NaCl], 0.05% Tyloxapol (Sigma St. Louis, MO), and 20 μg/ml kanamycin, if necessary. After reaching an OD600 of 2, cells were centrifuged and twice washed in modified 7H9 media: Middlebrook 7H9 supplemented with 0.1% glycerol, 1 mm propionic acid (Sigma), 0.5% bovine serum albumin, 0.1% Tyloxapol, pH adjusted to 6.5 and buffered with 100 mm 2-(4-morpholino)-ethane sulfonic acid. This modified 7H9 media was used in all subsequent steps of preparing samples and was designed to reflect features of the host environment encountered by M. tuberculosis. During infection, M. tuberculosis is exposed to a mildly acidic pH of 6.5 in a macrophage (25) and utilizes fatty acids as a carbon source (26–28). Washed cells were used to inoculate modified 7H9 medium at an OD600 of 0.08. Upon reaching an OD600 of 1.0, the cells were pelleted, washed with 1× phosphate-buffered saline (PBS) and sterilized by gamma-irradiation in a JL Shephard Mark I 137Cs irradiator (Department of Radiobiology, University of North Carolina at Chapel Hill) prior to removal from BSL-3 containment. In some experiments, a 40 ml volume of cells in modified 7H9 medium (OD600 of 0.8–1.0) was transferred to a 50 ml conical tube and let stand at 37 °C for 2 or 24 h as a strategy to induce the DosR-dependent regulon (29).
Preparation of Subcellular Fractions
Subcellular fractions were isolated as previously described (30). Briefly, irradiated cells suspended in 1× PBS containing protease inhibitors were lysed by passage through a French pressure cell. Unlysed cells were removed by centrifugation at 3000 × g to generate clarified whole cell lysates (WCLs), which were then spun at 27,000 × g for 30 min to pellet the cell wall fraction. The supernatant was then spun at 100,000 × g for 2 h to separate the cytoplasmic membrane fraction and collect the soluble cytoplasm-containing fraction. Protein concentrations were determined by bicinchoninic acid assay (Pierce, Rockford, IL).
SDS-PAGE Separation and In-gel Trypsin Digestion of Proteins
Cell wall proteins (34 μg) and cytoplasmic proteins (91 μg) from three biological replicates each of H37Rv and the ΔsecA2 mutant were separated on individual lanes of a precast 12% SDS-PAGE gel. Protein bands were visualized by Coomassie Blue R-250 staining (Bio-Rad, Hercules, CA) and each lane was cut into 32 gel slices for cell wall samples and 10 gel slices for cytoplasmic samples. In-gel trypsin digestion was performed, as previously described (31), with each gel slice being processed individually in a single well of a 96-well polypropylene plate. Peptides were stored at −80 °C until lyophilized.
Liquid Chromatography and Tandem Mass Spectrometry Analysis
Peptides were desalted using PepClean C18 spin columns (Pierce, Rockford, IL) and resuspended in an aqueous solution of 0.1% formic acid. Samples were analyzed by reversed phase LC-MS/MS using a 2D-nanoLC ultra system (Eksigent Inc, Dublin, CA) coupled to an LTQ-Orbitrap XL system with ETD (Thermo Scientific, San Jose, CA). The Eksigent system was configured to trap and elute peptides in 1D mode of operation via a sandwiched injection of ∼250 fmol of sample. The trapping was performed on a 3 cm long 100 μm i.d. C18 column, whereas elution was performed on a 15 cm long 75 μm i.d., 5 μm, 300Å particle; ProteoPep II integraFrit C18 column (New Objective Inc, Woburn, MA). Analytical separation of tryptic peptides was achieved with a linear gradient of 2–40% over 120 min at a 200 nL/min flow rate, where buffer A is aqueous solution of 0.1% formic acid and buffer B is a solution of acetonitrile in 0.1% formic acid. Mass spectrometric data acquisition was performed in a data dependent manner. A full scan mass analysis on an LTQ-Orbitrap (externally calibrated to a mass accuracy of < 1 ppm, and resolution of 60,000 at 400 Th) was followed by intensity dependent MS/MS of the 10 most abundant peptide ions. The dynamic exclusion time window was set to 60 s with monoisotopic precursor ion selection (MIPS) and charge state screening enabled for charges ≥ +2 for triggering data dependent MS/MS scans.
Peptide and Protein Identification
Mass spectra were processed, and peptide identification was performed using Mascot ver. 2.3 (Matrix Science Inc., Boston, MA) implemented on Proteome Discoverer ver. 1.3 (Thermo-Fisher Scientific, San Jose, CA). All searches were performed against the National Center for Biotechnology (NCBI) M. tuberculosis H37Rv protein sequence database (RefSeq NC_000962 uid 57777, 3906 protein entries). Peptides were identified using a target-decoy approach with a peptide false discovery rate (FDR) of 1% (32, 33). A precursor ion mass tolerance of 200 p.p.m. and a product ion mass tolerance of 0.5 Da (34) were used during the search to increase search space and reduce false-positive identifications with a maximum of two missed trypsin cleavage sites and oxidation of methionine residues as dynamic modification.
Peptide Validation and Spectral Count-based Label-free Quantitation (LFQ)
Peptide and protein validation and label-free spectral count-based quantitation was performed using ProteoIQ: ver 2.3.02 (PREMIER Biosoft international, Palo Alto, CA). Mascot search engine results against forward and decoy M. tuberculosis databases were obtained for all RAW data. Both forward and decoy search results were imported as DAT files into ProteoIQ to asses FDR. A peptide FDR of 1% and protein FDR of 5% were used to filter valid spectra. Peptide assignment to proteins was achieved by considering Occam's Razor principle that takes into account the presence of protein groups and penalizes proteins containing peptides identified in multiple proteins. The PROVALT algorithm in ProteoIQ was used to determine ion score thresholds and protein FDR (33). Mascot protein identifications were also subjected to probability-based confidence measurements using an independent implementation of the statistical models commonly known as peptide and protein Prophet deployed in ProteoIQ (35, 36). All protein hits were filtered with peptide Prophet using a minimum probability threshold of 0.5. Evaluation of sensitivity and error rates in this filtered data set for the cell wall proteome showed a sensitivity of 95% with a 4.8% error rate whereas the filtered data for the cytoplasm had 90% sensitivity with a 6.2% error rate. From a total of 2194 proteins detected in the cell wall and 2226 detected in cytoplasmic samples the data was filtered for proteins identified by a minimum of two peptides resulting in 1729 cell wall and 1810 cytoplasmic proteins identified, reported, and used in all further analysis in this study.
Relative protein quantitation was performed using spectral count-based LFQ. For each biological sample, data from the individual gel slices were combined. Statistical analysis was performed on all proteins identified with average spectral counts of ≥4 among the three replicates of at least one strain. The spectral count data was normalized by total spectral counts in each sample, using ProteoIQ, to adjust for differences in overall protein levels between samples. The normalized spectral count data was then used to calculate a ratio of the average spectral counts obtained for each strain ΔsecA2/H37Rv. Proteins were considered to have a significant difference in abundance if there was a difference of twofold or greater in average spectral counts between strains and a p value ≤ 0.01 using an unpaired two-tailed Student's t test. Multiple hypothesis testing was also applied to the data by computing q values using QVALUE software (http://genomics.princeton.edu/storeylab/qvalue/). The q values for quantitated proteins are reported on the data tables. The q value is an estimate of the minimum FDR for proteins judged to be significantly changed at a given p value (in our case p ≤ 0.01) (37). Spectral counts for all proteins and peptides identified in each replicate sample is provided in supplemental Tables S2 and S8 for cell wall identifications and supplemental Tables S9 and S15 for cytoplasmic identifications. For the proteins selected for relative quantitation (≥4 average spectral counts) spectral count data and statistics across replicates are shown in supplemental Tables S3–S7 for cell wall fractions and supplemental Tables S10–S14 for cytoplasmic fractions.
Immunoblotting
Equal protein content for whole cell lysates, cell wall, or cytoplasmic fractions from H37Rv, ΔsecA2 mutant, and the complemented strain were separated by SDS-PAGE and then transferred to nitrocellulose membranes. After blocking for 1 h at room temperature, proteins were detected using the following antibodies: antiMce1A at 1:10,000, antiMce1C at 1:5000, antiMce1F at 1:10,000, antiLprK at 1:5000, anti19kD at 1:20,000 (provided by Douglas Young, Imperial College, United Kingdom), antiPhoS1 at 1:20,000 (IT23, NIH Biodefense and Emerging Infections Research Resources Repository, NIAID), antiPknG at 1:5000 (provided by Yossef Av-Gay, University of British Columbia, Canada), and antiHspX at 1:1000 (IT20, NIH Biodefense and Emerging Infections Research Resources Repository, NIAID). Anti-mouse and anti-rabbit IgG conjugated horseradish peroxidase (Bio-Rad, Hercules, CA) secondary antibodies were used and signal was detected using Western Lightning Chemiluminescent detection reagent (Perkin-Elmer, Waltham, MA).
AntiMce Antibody Production
Antipeptide antibodies for Mce1A, 1C, 1F, and LprK were generated in rabbits using peptides conjugated to keyhole limpet hemocyanin by Open Biosystems (Huntsville, AL). The peptides used were the following: GRVDTISEVTRDGESA (Mce1A), DLLVDRKEDLAETLTILGR (Mce1C), SAVYSPASGELVGPDGVKY (Mce1F), and DPSPVPLKDGDTIPLKRS (LprK). Immunoaffinity chromatography was used for antibody purification.
Bioinformatic Analysis
Sec signal peptides were predicted using Signal P 4.01 and default options for Gram-positive bacteria (38). Tat signal peptide predictions were compiled using TatP 1.03, TATFIND v1.44, and TigrFAM5 (39–42). Transmembrane predictions were made using TMHMM 2.06 (43). Mycobacterial lipoproteins were predicted using the list compiled by Sutcliffe and Harrington (44). Protein associations were predicted using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) (version 9.1) set for a medium confidence level (0.4) (45).
Quantitative Real-Time PCR
Triplicate cultures were grown in modified 7H9 medium to an OD600 of 1.0 and pelleted by centrifugation at 3000 rpm for 10 min. Bacteria were lysed in 1 ml 3:1 chloroform-methanol, then vortexed with 5 ml TRIzol (Invitrogen) and incubated for 10 min at room temperature. Phases were separated by centrifugation at 3000 rpm for 15 min at 4 °C, and RNA was precipitated from the upper phase using 1× volume of isopropanol. RNA was pelleted by centrifugation at 12,000 rpm for 30 min at 4 °C, washed twice with cold 70% ethanol, and resuspended in RNase-free water. RNA samples were treated with DNase (Promega, Madison, WI) and then column purified (Zymo RNA clean and concentrator kit). Following RNA isolation, cDNA was synthesized with random primers using the iScript cDNA Synthesis Kit (Bio-Rad). Real-time PCR was completed using 25 ng of cDNA template in triplicate technical replicates using the SensiMix SYBR and fluorescein kit (Bioline, Tauton, MA). Transcripts were normalized to the housekeeping gene sigA (46). Primer sequences are provided (supplemental Table S1).
RESULTS
Proteomic Analysis of Differentially Abundant Proteins in the Cell Wall of H37Rv (wild type) versus ΔsecA2 Mutant M. tuberculosis
Triplicate cultures of M. tuberculosis H37Rv (wild type) and ΔsecA2 mutant were grown to mid-log phase, at which time cells were sterilized by gamma irradiation and lysed with a French pressure cell to generate whole cell lysates (WCL). WCLs were then subjected to differential ultracentrifugation to generate cell wall fractions. Immmunoblot analysis of the cell wall fractions showed the presence of the 19 kDa lipoprotein but not the cytoplasmic SigA and MtrA proteins, as expected (data not shown).
Because cell wall proteins often exhibit solubility problems, we combined SDS-solubilization and SDS-PAGE separation of cell wall fractions with LC-MS/MS to analyze the cell wall proteomes, as previously described (30). For each biological replicate, cell wall proteins were collected from an entire lane of an SDS-PAGE gel in 32 slices of equal size. Tryptic peptides from each slice were analyzed separately by LC-MS/MS. In total, we identified 1729 proteins with a minimum of two unique peptides among the cell wall fractions of H37Rv (wild type) and the ΔsecA2 mutant (supplemental Table S2). These cell wall-associated proteins fall into diverse functional categories (Fig. 1). The largest functional groups of proteins identified are predicted to be involved in cell wall processes, intermediary metabolism/respiration, or are conserved hypothetical proteins (47). This distribution of cell wall-associated proteins among functional groups is similar to what is seen in other M. tuberculosis cell wall proteome studies (48, 49).
Fig. 1.
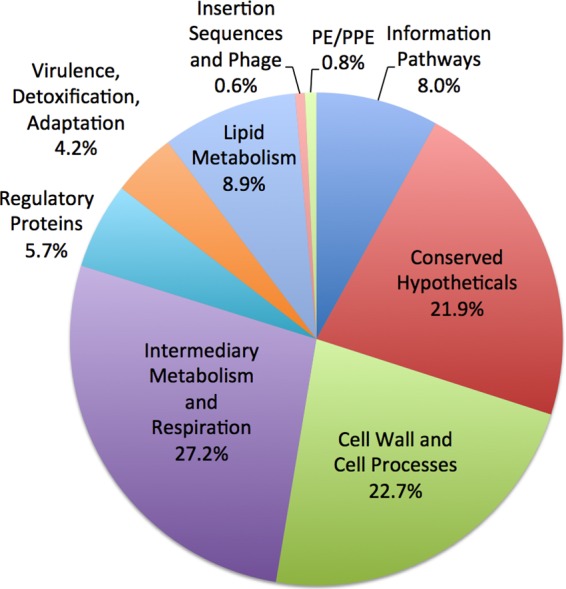
Functional categories of cell wall proteins identified by LC-MS/MS. The 1729 cell wall-associated proteins identified by LC-MS/MS by a minimum of two peptides were assigned to functional categories according to Tuberculist (47).
Of these 1729 proteins identified, 501 are predicted to be fully exported across or inserted into the cytoplasmic membrane because of the presence of a predicted Sec or Tat signal peptide (183 proteins) and/or a transmembrane domain (405 proteins). Additionally, 67 proteins were predicted to be lipoproteins, representing 70% of the lipoproteins predicted in the H37Rv genome (44), which is consistent with the role of lipidation in anchoring proteins to the bacterial cell envelope (50). The identification of proteins lacking a predicted signal peptide or transmembrane domain in the cell wall fraction was not surprising as past proteomic studies of mycobacterial membrane and cell wall fractions identify a similar percentage of proteins lacking export signals (31, 48, 49, 51, 52). Some of these proteins may be unconventional exported proteins lacking recognizable signals for export. However, because of the high sensitivity of mass spectrometry, cytoplasmic and cytoplasmic membrane contaminants can also be identified in the cell wall fraction.
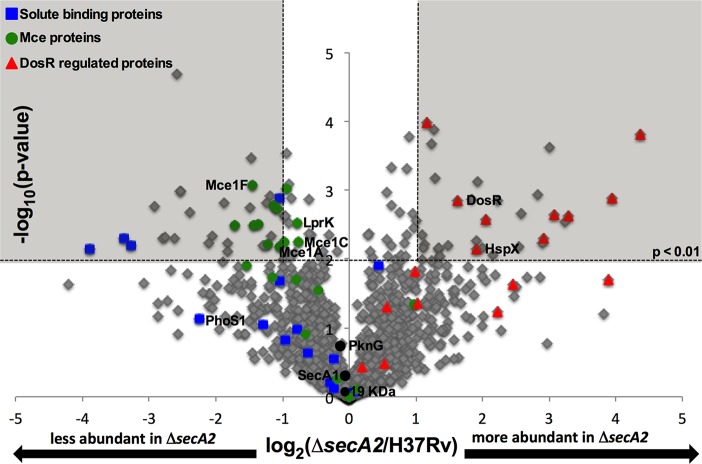
Using spectral counting, we compared the composition and relative levels of proteins in the ΔsecA2 mutant to H37Rv cell wall fractions. Spectral counting is based on the observation that the total number of MS/MS spectra assigned to a protein correlates with the abundance of that protein (53). In order to avoid low abundance proteins that fall outside of the linear dynamic range of spectral counting, we limited our analysis to the 1318 proteins identified by a minimum of two peptides with an average of ≥four spectral counts (53) among the replicates of at least one of the strains (H37Rv or ΔsecA2 mutant) (supplemental Table S3). When the spectral count data for each replicate was compared, a good correlation was revealed between biological replicates (Pearson's correlation coefficient of r>0.92) (supplemental Fig. S1). Following normalization by total spectral counts for each protein, spectral count ratios (log2(ΔsecA2/H37Rv)) were calculated for 1300 proteins that were detected in both strains (supplemental Table S3). For 18 proteins with average of ≥four spectral counts, spectral count ratios could not be calculated because these proteins were only identified in one of the two strains: Four proteins were exclusively identified in H37Rv (unidentified in the mutant) and 14 proteins were only identified in the ΔsecA2 mutant (supplemental Tables S4 and S5). Proteins exclusively identified in one of the strains are most likely present in the other strain, but at levels that fall below our limit of detection. For the 1300 proteins with spectral count ratios calculated, significant differences in protein levels between the two strains were determined by a Student's t test (p < 0.01) and by having a difference of twofold or greater between strains (log2 spectral count ratio of ±1.0). To visualize the distribution of spectral count ratios for the cell wall proteins, a volcano plot of the log2 ratio of ΔsecA2/H37Rv versus −log10 p value was generated (Fig. 2). The majority of proteins were present at similar levels in the two strains. Of note, the SecA1 ATPase was detected at equivalent levels in the ΔsecA2 mutant and H37Rv (Fig. 2), which reinforces past conclusions that the absence of SecA2 does not alter the level of SecA1 (54). Among the proteins exhibiting significant differences (>twofold difference, p < 0.01), there were 33 proteins with significantly decreased levels in the cell wall fraction of the ΔsecA2 mutant versus H37Rv and 33 proteins with significantly increased levels in the ΔsecA2 mutant cell wall fraction relative to H37Rv (supplemental Tables S6 and S7). Proteins chosen for independent validation in this study are labeled on the volcano plot.
Fig. 2.
Relative quantitation of proteins in H37Rv and ΔsecA2 mutant cell wall-fractions. Ratios for the 1300 proteins having average spectral counts of ≥4 in H37Rv and/or ΔsecA2 are shown plotted by log2 (ΔsecA2/H37Rv) and −log10(p value). The shaded area of the graph indicates proteins showing twofold differences, p < 0.01. Solute binding proteins (blue squares), Mce transporter proteins (green circles), and DosR-regulated proteins (red triangles) are marked on the plot, and proteins later validated are identified. In addition, SecA1, is identified on the plot as a protein expected to be present in similar amounts between strains (54).
From the spectral counting analysis, we expected to detect protein abundance differences resulting from SecA2-dependent export defects as well as regulatory consequences of the absence of SecA2. Altogether, there were 37 proteins whose level was either reduced or unidentified in the ΔsecA2 cell wall when compared with H37Rv. Of these proteins, which represent candidates for being exported by the SecA2 pathway, 46% possess a predicted Sec or Tat signal peptide or putative transmembrane domain, with four of these proteins being predicted lipoproteins. Conversely, there were 47 proteins either increased or uniquely identified in the ΔsecA2 mutant compared with H37Rv. In this latter collection of proteins, there were a smaller percentage of predicted exported proteins (28%) and only one predicted lipoprotein. This latter group of proteins seems likely to include more examples of contaminating cytoplasmic proteins.
Protein Families and Networks Exhibiting Differences in the ΔsecA2 Mutant versus H37Rv
To identify functional categories and protein families that are SecA2-dependent we plotted the percentage of proteins in a given functional group, as defined by Tuberculist (47), that exhibited differential levels between the ΔsecA2 mutant and H37Rv (Fig. 3). However, none of these broad functional categories revealed a strong association with SecA2-dependent proteins (Fig. 3).
Fig. 3.
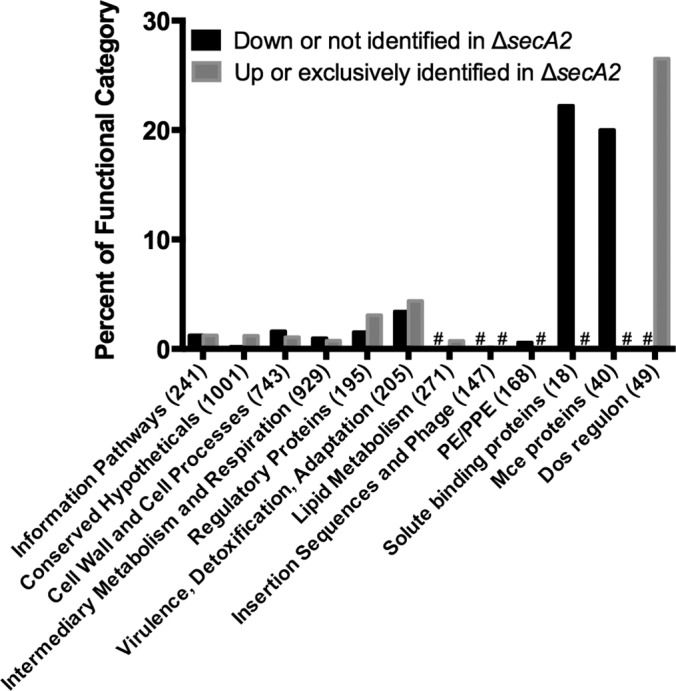
Percent of functional protein categories showing differences in the cell wall between H37Rv and the ΔsecA2 mutant. Shown is the percent of proteins in a given functional category showing SecA2-dependence, defined as ≥twofold difference, p < 0.01 between H37Rv and ΔsecA2 or exclusively identified in one of the two strains (supplemental Tables S4–S7). With the exception of SBPs (44, 55), Mce transporters (representing Mce1–4 systems) (68), and DosR-regulated proteins (58), functional groups were predicted by Tuberculist (47). The total number of proteins in each functional category is denoted in parentheses. # indicates no proteins in that category.
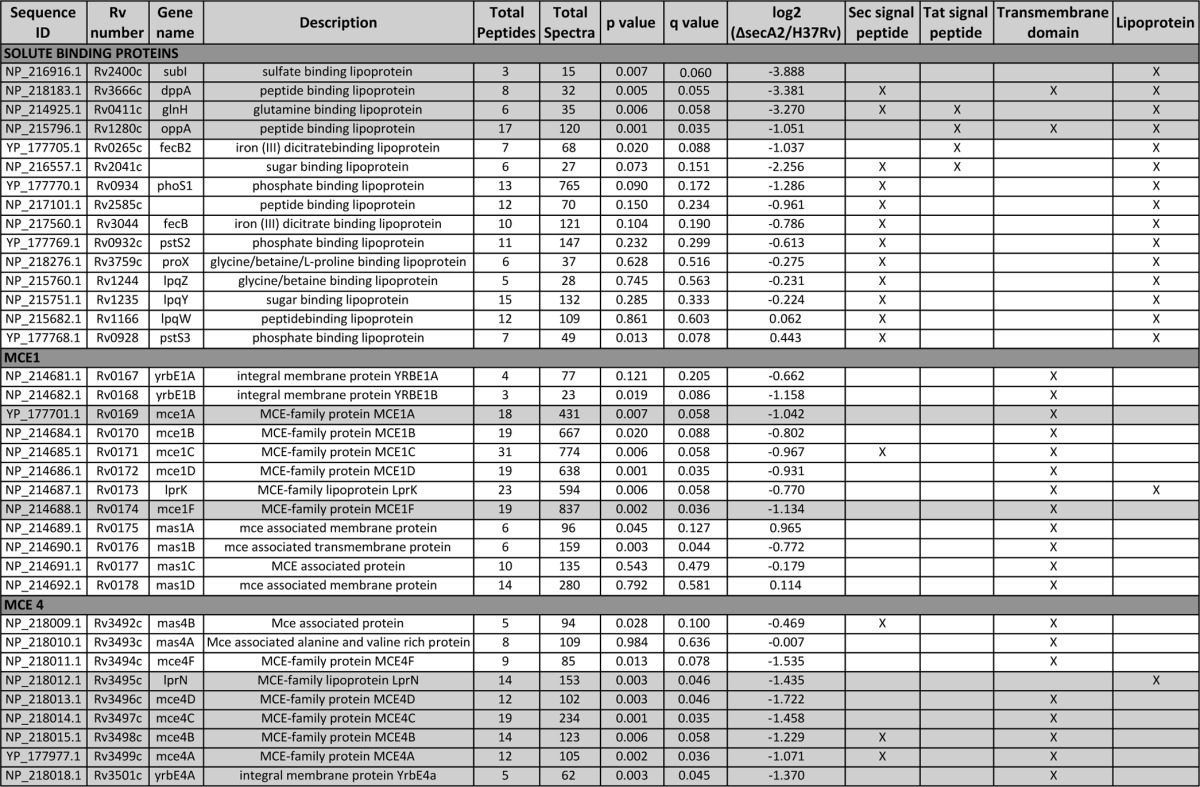
Solute Binding Proteins (SBPs)
Because of the precedent of two M. smegmatis SBPs being SecA2-dependent exported proteins (15), we inspected the spectral counting data for the 15 SBPs we identified in the cell wall fraction (18 SBPs are predicted to exist in M. tuberculosis) (44, 55). This analysis revealed a trend in SBPs being reduced in the cell wall of the ΔsecA2 mutant when compared with H37Rv (Fig. 3; Table I). There were four SBPs significantly reduced (≥twofold decreased, p < 0.01) in the ΔsecA2 mutant cell wall compared with H37Rv. Furthermore, there were an additional nine SBPs reduced in the ΔsecA2 mutant cell wall, although the reduction relative to H37Rv did not always reach twofold or achieve statistical significance. All of these SBPs are predicted lipoproteins, which is consistent with their localization to the cell wall. Altogether, 13 of the 15 SBPs we identified were reduced to some degree in the ΔsecA2 mutant cell wall.
Table I. Functional categories of proteins showing reduced abundance in the cell wall fraction of the M. tuberculosis ΔsecA2 mutant versus H37Rv. Shaded areas of the table indicate proteins that satisfied our criterion of having >twofold difference in abundance between the ΔsecA2 mutant and H37Rv and p < 0.01 or were exclusively identified in one of the strains.
Mce Proteins
As an alternate approach to find functional categories or networks influenced by SecA2, we entered the SecA2-dependent proteins showing significant differences between strains (≥twofold difference, p < 0.01, or unidentified) (supplemental Tables S4–S7) into the Search Tool for the Retrieval of Interacting Genes (STRING) database of known and predicted protein associations (supplemental Figs. S2 and S3) (45). For clarity, Fig. 4 only shows portions of the STRING analysis. Among the proteins reduced/unidentified in the cell wall of the ΔsecA2 mutant versus H37Rv, there was a cluster of members of two of the four Mce transporter systems (Mce1 and Mce4) (Fig. 4A). When the percentage of all Mce transporter components showing differential abundance in the ΔsecA2 mutant versus H37Rv was plotted, the reduction in Mce protein family members in the ΔsecA2 cell wall was evident (Fig. 3).
Fig. 4.
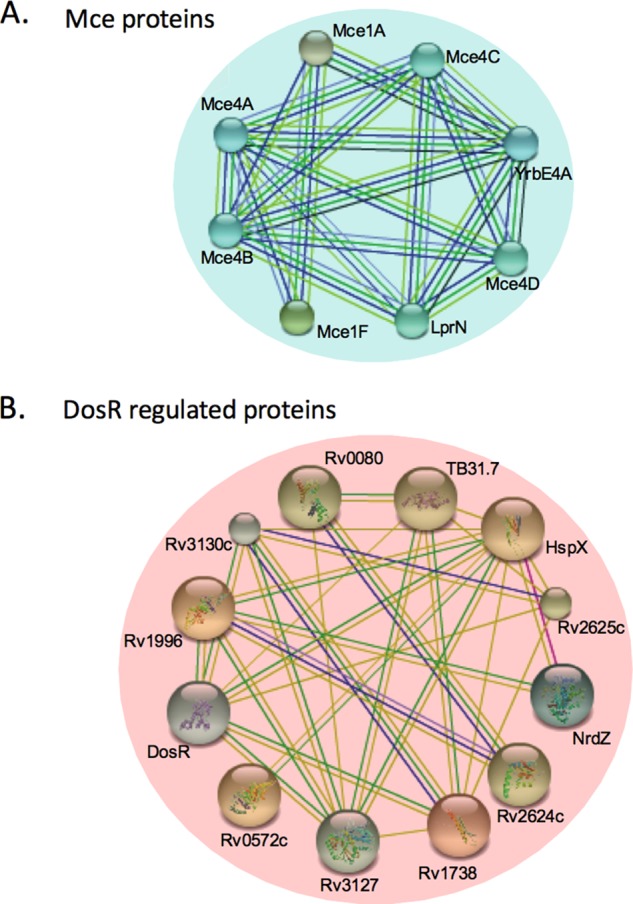
Protein associations among proteins identified as having differential abundance in the cell wall fractions of H37Rv versus the ΔsecA2 mutant. A, Proteins that were less abundant (≤twofold, p < 0.01) or not identified in the ΔsecA2 mutant and B proteins that were more abundant (≥twofold, p < 0.01) or exclusively identified in the ΔsecA2 mutant were examined for protein associations. A portion of the protein associations predicted using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) v9.1 using medium confidence are shown (45). Each line represents a different association between proteins.
Mce transporters are multiprotein complexes localized to the cell envelope (56, 57) that are proposed to function in lipid uptake by mycobacteria. Mce transporters are encoded by eight to 12 gene operons. Each system contains yrbE genes encoding putative membrane-spanning proteins, five mce genes encoding proteins with potential signal peptides or N-terminal transmembrane domains and one lpr gene encoding a lipoprotein (Fig. 5). Most Mce systems also have genes that encode Mce-associated proteins (mas) possessing transmembrane domains. Of the nine proteins encoded by the mce4 locus, six proteins were significantly reduced in the ΔsecA2 mutant cell wall (≥twofold, p < 0.01) (Fig. 5, Table I). Mce4F and Mas4B were also reduced in the ΔsecA2 mutant, although the differences did not meet one or both of our cutoffs. Among the 12 components of the Mce1 system, six proteins were significantly reduced in the ΔsecA2 mutant cell wall (p < 0.01); however, only two of them (Mce1A and Mce1F) were reduced by ≥twofold. The Mce-associated ATPase MceG (Mkl) was also significantly reduced in the ΔsecA2 mutant by 1.8-fold.
Fig. 5.
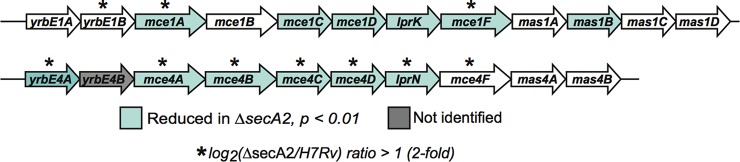
Multiple components of Mce1 and Mce4 transporters are reduced in the cell wall of the ΔsecA2 mutant. The M. tuberculosis H37Rv genome contains four mce loci encoding putative lipid transporters. The genomic regions encoding Mce1 and Mce4 transporter components are shown with Open Reading Frames (ORF) colored for significant differences between strains of p < 0.01, as observed by spectral counting. An asterisk above the ORF indicates a difference of at least twofold.
DosR-regulated Proteins
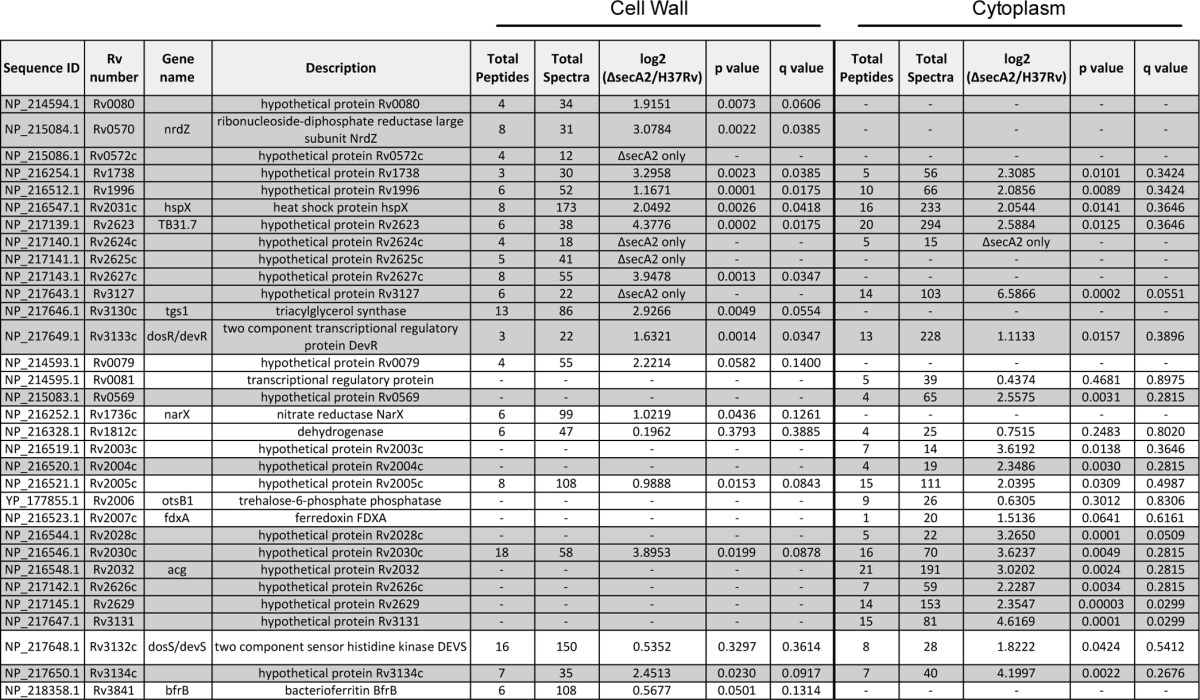
Among the proteins that were increased in the ΔsecA2 mutant relative to H37Rv, the STRING database identified a cluster of proteins that are members of the DosR regulon (58) (Figs. 3 and 4B). Of the 21 DosR-regulated proteins we identified, 13 were increased (≥twofold, p < 0.01) or exclusively identified in the ΔsecA2 cell wall. The mostly cytoplasmic proteins within the DosR regulon are under the transcriptional control of the DosR/S/T two component regulatory system. One of the proteins significantly increased in the ΔsecA2 mutant was the DosR response regulator, which is known to autoregulate itself at the transcriptional level (59). The remaining eight DosR-regulated proteins we identified in the cell wall followed the same trend of being increased in the ΔsecA2 mutant, although their differences failed to meet our significance and/or twofold cut-offs (Fig. 3, Table II).
Table II. DosR regulated proteins have altered abundance in the cell wall and cytoplasmic fractions of the M. tuberculosis ΔsecA2 mutant. Shaded areas of the table indicate proteins that satisfied our criterion of having >twofold difference in abundance between the ΔsecA2 mutant and H37Rv and p < 0.01 or were exclusively identified in one of the strains.
Validation of Cell Wall Protein Differences
To validate the spectral counting identifications of the above families and networks of differentially abundant proteins, we performed immunoblot analysis on sets of newly prepared cell wall samples that included a complemented strain, which represents the ΔsecA2 mutant carrying a plasmid expressing wild type secA2. Using antibodies to PhoS1, we monitored the abundance of this representative SBP in the cell wall fractions of H37Rv, ΔsecA2, and the complemented strain (Fig. 6A). As predicted by the spectral counting analysis, PhoS1 was partially reduced in the ΔsecA2 mutant cell wall. Importantly, the level of PhoS1 in the cell wall was restored in the complemented strain. PhoS1 levels in whole cell lysates were the same between strains (Fig. 6B) indicating that the reduced amount of PhoS1 in the ΔsecA2 cell wall is not caused by an effect on total PhoS1 levels. Rather, these results support PhoS1 being dependent on SecA2 for export to the cell wall. Using a series of antibodies to Mce1 components (Mce1A, Mce1C, Mce1F, and LprK) we similarly assessed the levels of Mce1 proteins in the cell wall. The levels of all four of these proteins were reduced in the cell wall of the ΔsecA2 mutant when compared with H37Rv and the complemented strain (Fig. 6A). In this case, lower levels of the Mce proteins were also observed in whole cell lysates of the ΔsecA2 mutant (Fig. 6B). Immunoblot analysis of cell wall fractions was also performed with anti19kDa lipoprotein antibodies. As predicted by the spectral counting data of cell wall fractions, the amount of the 19kDa lipoprotein was the same in the cell wall of all three strains.
Fig. 6.
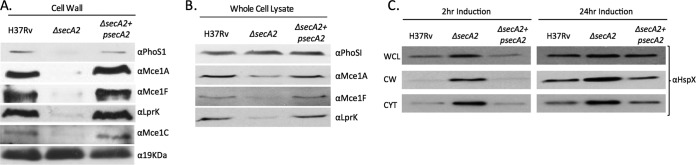
Immunoblot validation of protein abundance differences between H37Rv and the ΔsecA2 mutant. A, Equalized cell wall fractions of H37Rv, ΔsecA2, and complemented (ΔsecA2+psecA2) strains were analyzed by immunoblot using antiPhoS1, antiMce1A, antiMce1C, antiMce1F, antiLprK, and anti19KDa antibodies to monitor differences in protein levels. The 19 kDa lipoprotein was used as a control for a protein present in equal amounts across the strains. B, Equalized whole cell lysates of H37Rv, ΔsecA2, and complemented (ΔsecA2+psecA2) strains were analyzed by immunoblot using antiPhoS1, antiMce1A, antiMce1F, and antiLprK antibodies. C, H37Rv, ΔsecA2, and ΔsecA2+psecA2 samples in which the DosR regulon was induced for 2 or 24 h were analyzed. Whole cell lysate (WCL), cell wall (CW), and cytoplasmic (CYT) fractions were equalized for protein content and analyzed by immunoblot using antiHspX antibodies. For all data shown, a representative experiment is presented of a minimum of three that were performed.
We attempted immunoblot analysis with antibodies to HspX, as a representative DosR-regulated protein; however, the amount of HspX in the cell wall was below the level of detection. Therefore, in order to test the effect of SecA2 on HspX, we prepared samples from cultures that were left standing in 50 ml conical tubes for 2 or 24 h to induce DosR-dependent hspX expression (29). In these induced samples, HspX was detected in the cell wall and was present at higher levels in the ΔsecA2 mutant (Fig. 6C). Higher levels of HspX were also observed in the whole cell lysate as well as in the cytoplasm of the ΔsecA2 mutant indicating an overall increase in HspX levels in the ΔsecA2 mutant.
Transcriptional Effects do not Account for the Reduced Cell Wall Localization of Mce Proteins but can Account for DosR-regulated Effects
Genes in the mce1 and mce4 loci (Fig. 5) are organized in operons (61), which raises the possibility of a transcriptional effect accounting for the reduced levels of multiple Mce components that we observed in whole cell lysates and cell wall fractions of the ΔsecA2 mutant. To address the possibility of their being a transcriptional effect, we performed quantitative real time PCR (qRT-PCR) on RNA prepared from H37Rv, ΔsecA2, and complemented strains using primers for mce1A, mce1F, mce4A, and mce4F. Transcript levels were normalized to the housekeeping control sigA (46). For all four genes, there was no significant difference in transcript levels between H37Rv and the ΔsecA2 mutant (Fig. 7A). This result argues against a transcriptional effect on mce loci. Rather, it supports the alternate explanation for the immunoblot data, which is that there is a Mce export defect in the ΔsecA2 mutant and subsequent degradation of unexported Mce transporter components. In multiple experiments, the complemented strain showed a significantly lower level of mce1A, mce1F, mce4F transcript than either H37Rv or ΔsecA2. The explanation for this effect is not clear, although it could reflect feedback regulation of mce expression in the presence of higher than normal SecA2 levels resulting from the complementation construct.
Fig. 7.
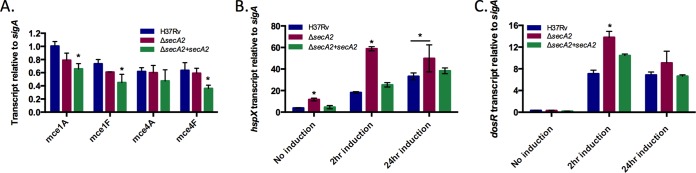
Transcript levels for mce genes are unchanged but transcript levels for DosR-regulated genes are higher in the ΔsecA2 mutant. A, RNA was isolated from H37Rv, ΔsecA2, and complemented (ΔsecA2+psecA2) strains. mce transcript levels were measured by quantitative RT-PCR and normalized to the level of sigA transcript. Data shown is for the mean of three biological replicates. (*p < 0.05 by ANOVA) B and C, RNA was isolated from H37Rv, ΔsecA2, and complemented (ΔsecA2+psecA2) stains in which the DosR regulon was not intentionally induced or was induced for 2 or 24 h. B, hspX transcript levels and C, dosR transcript levels were measured by quantitative RT-PCR and normalized to sigA transcript. Data represents the means of three biological replicates. (*p < 0.05 by ANOVA).
The DosR-regulated proteins identified as more abundant in the ΔsecA2 mutant are all under transcriptional control of the DosR/S/T system. To test if SecA2 affects transcription of DosR-regulated genes we measured hspX transcript levels in H37Rv, ΔsecA2, and complemented strains. RNA was prepared from uninduced samples and standing cultures that sat for 2 or 24 h to induce the DosR-regulon. In all conditions (± induction), the level of hspX transcript was higher in ΔsecA2 versus H37Rv or the complemented strain (Fig. 7B). We also found that the level of dosR transcript was higher in the ΔsecA2 mutant, although the difference was only statistically significant after 2 h induction. Interestingly, in both the hspX and dosR transcript analyses the difference between H37Rv and ΔsecA2 samples was most pronounced at 2 h and waned at 24 h. Overall, these results indicate that the higher abundance of DosR-regulated proteins in the ΔsecA2 mutant can be attributed to increased transcription.
Proteomic Analysis of Differentially Abundant Proteins in the Cytoplasm of H37Rv versus the ΔsecA2 Mutant
Our detection of up-regulated cytoplasmic members of the DosR regulon in the ΔsecA2 mutant was fortuitous: A likely consequence of these proteins being highly expressed contaminants of cell wall fractions. To more directly test for cytoplasmic effects in the ΔsecA2 mutant, we performed LFQ analysis on cytoplasmic fractions prepared from triplicate H37Rv and ΔsecA2 mutant cultures. A total of 1810 proteins were identified by a minimum of two peptides among the cytoplasmic fractions of H37Rv and the ΔsecA2 mutant. As expected, proteins identified in the cytoplasmic fraction fall into diverse functional categories (Fig. 8A). As with the cell wall proteome, intermediary metabolism/respiration and conserved hypothetical proteins were among the largest functional groups of proteins identified in the cytoplasmic proteome. However, a much smaller percentage of proteins with predicted functions in cell wall processes were identified in the cytoplasm versus the cell wall (9.7% versus 22.7%), as expected.
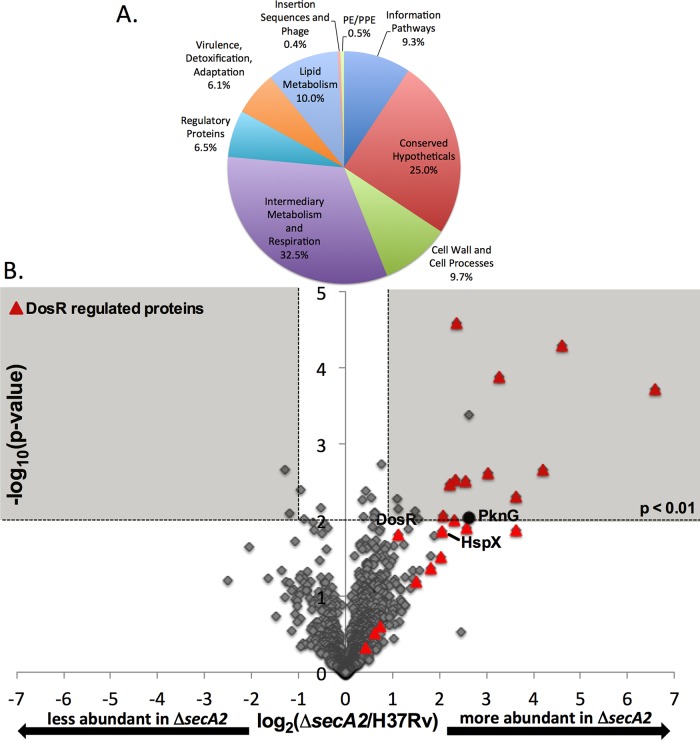
Fig. 8.
Relative quantitation of proteins in H37Rv and ΔsecA2 mutant cytoplasmic fractions. A, The 1810 cytoplasmic proteins identified by LC-MS/MS by a minimum of two peptides were assigned to functional categories according to Tuberculist (47). B, Ratios for the 1266 proteins identified by an average ≥4 spectral counts in H37Rv and/or ΔsecA2 are shown plotted by log2 (ΔsecA2/H37Rv) and −log10(p value). The shaded area of the graph indicates proteins showing twofold differences, p < 0.01. DosR-regulated proteins (red triangles) and PknG (black circle) are marked on the plot.
Spectral count ratios were calculated for the 1266 proteins in the cytoplasmic fraction having average spectral counts ≥4 across the biological replicates of at least one of the strains. Once again, there was strong correlation between the spectral count data of replicates (Pearson's correlation coefficient of r>0.99 between replicate cytoplasmic fractions, as shown in supplemental Fig. S4). A volcano plot of the log2 ratio of ΔsecA2/H37Rv shows the distribution of spectral count ratios (Fig. 8B). Among the proteins exhibiting significant differences (≥twofold, p < 0.01) between strains, there were 17 proteins with higher levels in the ΔsecA2 mutant and one protein with reduced levels of protein in the mutant. There were an additional three proteins with ≥4 average spectral counts that could not be quantitated because they were undetected in one of the two strains: two proteins were exclusively identified in ΔsecA2 mutant samples and SecA2 was the only protein exclusively identified in H37Rv. Of the proteins that were significantly increased or only identified in the ΔsecA2 mutant, 68% of them were DosR-regulated proteins (13 of 19 proteins) (Table II). As seen in the cell wall fractions, the other DosR-regulated proteins identified followed the same trend of being up-regulated in the ΔsecA2 mutant although the differences failed to meet our significance cut-offs. STRING analysis performed on proteins showing significant differences between strains (≥twofold difference, p < 0.01, or unidentified) identified no pathways, besides the DosR regulon, as being altered in the ΔsecA2 mutant.
PknG levels Are Increased in the Cytoplasm of the M. tuberculosis ΔsecA2 Mutant
Among the small number of nonDosR-regulated proteins detected at significantly higher levels in the cytoplasm of the ΔsecA2 mutant was the virulence factor PknG (6.1-fold increased, p = 0.009), which is reported to be SecA2-dependent in M. marinum (16). In some cases, when a protein is not being properly exported it will accumulate in the cytoplasm (15). Therefore, to follow up on this result, we performed immunoblot analysis with antiPknG antibodies on fractions prepared from H37Rv, ΔsecA2 mutant, and the complemented strain. Although PknG levels in the whole cell lysate were the same between the strains, the immunoblot confirmed a higher amount of PknG was present in the cytoplasmic fraction of the ΔsecA2 mutant (Fig. 9). Further, immunoblotting revealed less PknG in the cell wall fraction of the ΔsecA2 mutant when compared with H37Rv and the complemented strain. Therefore, although PknG did not meet our criteria of a significant difference in the LFQ analysis of the cell wall, our combined results from LFQ analysis of the cytoplasmic samples and immunoblot analysis are consistent with PknG being exported by the SecA2 pathway of M. tuberculosis, as reported to be the case for M. marinum (16).
Fig. 9.

The level of PknG is increased in the cytoplasm and reduced in the cell wall of the ΔsecA2 mutant. Equalized fractions of H37Rv, ΔsecA2, and complemented (ΔsecA2+psecA2) stains were analyzed by immunoblot using antiPknG antibodies. PknG levels in whole cell lysate (WCL), cell wall (CW), and cytoplasmic (CYT) fractions are shown. Shown is a representative of three experiments.
DISCUSSION
Despite progress in understanding the mycobacterial SecA2 system, the identity of the proteins exported by the SecA2 pathway, most specifically of M. tuberculosis, has remained a critical unknown. Here, we took an unbiased proteomic approach using LFQ analysis with spectral counting to discover new proteins exported by the SecA2 pathway of M. tuberculosis. Using a cutoff of a ≥twofold difference and p < 0.01 we substantially increased the list of mycobacterial proteins observed to be affected by deletion of secA2 (5, 15, 16). We mined this dataset to identify probable SecA2-dependent exported substrates and differences reflective of physiological effects of the SecA2 export pathway (Fig. 10). As with all proteomic studies of this sort, generating a comprehensive list of biologically significant differences is a challenge. Some differences and their significance will not be detected, as appears to have occurred for PknG in the cell wall analysis. Possible reasons for this occurring include quantitative measurement bias from missing data, incomplete sampling because of dynamic range issues, or matrix interference. At the same time, it is critical that differences detected in discovery experiments be validated with independently prepared samples, as performed for several proteins in this study.
Fig. 10.
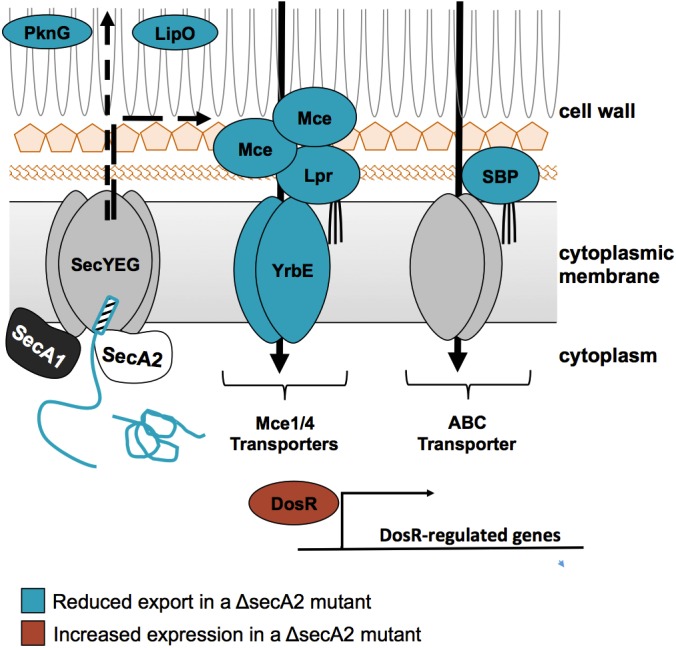
A model for SecA2-dependent effects in M. tuberculosis. In the current model for SecA2-dependent protein export, the SecA2 ATPase promotes the export of select proteins across the cytoplasmic membrane through the SecYEG channel, possibly with the help of SecA1 (8, 19, 20). Proteins identified as being exported by the SecA2 pathway include examples with signal peptides (hatched rectangle) (i.e. SBPs) and examples lacking signal peptides (i.e. PknG). The SecA2-dependent proteins identified in this study are shown in the model with proteins identified as reduced in the ΔsecA2 mutant colored blue (i.e. PknG, SBPs, LipO, Mce, and Lpr) and proteins identified as increased in the ΔsecA2 mutant colored red (i.e. DosR-regulated proteins).
Differences in Protein Families and Networks in the ΔsecA2 Mutant
A striking observation from this study was the lower level of nearly all signal peptide-containing SBP lipoproteins identified (13 of 15) in the ΔsecA2 mutant versus H37Rv cell wall. Although the best-studied mycobacterial SecA2 substrates are M. smegmatis SBPs (15, 20, 21), this is the first demonstration of SecA2-dependent SBP export in M. tuberculosis. The reduction in SBP localization to the M. tuberculosis ΔsecA2 cell wall was not complete (i.e. residual export occurred in the absence of SecA2). However, partial export defects were also seen with other proteins identified in this study and in past studies of SecA2-dependent proteins of M. tuberculosis, M.smegmatis, and M. marinum (5, 15, 16). Our data argues for a general property of SBPs that imparts a requirement for SecA2 in their export. In support of this idea, three SBPs are among the proteins reduced in a proteomic study of the M. marinum cell envelope of a secA2 mutant (16). An association of SBPs with SecA2-dependent export may also exist outside of mycobacteria. The Listeria monocytogenes SecA2-only system is reported to export three SBPs to the cell surface (14), although there are conflicting reports about the SecA2-dependent nature of some of these substrates (62, 63).
An interesting observation is that four of the 13 SBPs reduced in the M. tuberculosis ΔsecA2 mutant have predicted or proven Tat signal peptides (Table I) (39). The twin-arginine translocation (Tat) pathway operates independently and quite differently from the Sec pathway. The most notable feature of the Tat pathway is that it exports folded proteins across the cytoplasmic membrane (64). There are also examples of SBPs with Tat signal peptides in other bacteria, which suggests that cytoplasmic folding may be a common property of the SBP protein family (65, 66). A propensity for SecA2 exported proteins to fold in the cytoplasm is consistent with current models proposing a role for SecA2 in facilitating the export of such problematic proteins through the SecYEG channel that is built to accommodate proteins in an unfolded state (Fig. 10) (19, 21). These findings raise the possibility of at least some SBPs being compatible with Tat or SecA2 pathways, and for the Tat pathway being responsible for the residual export of SBPs that occurs in the absence of SecA2. It is important to note that only a subset of Tat substrates were SecA2-dependent in our study, ruling out a more general role for SecA2 in Tat export.
A new finding of this study was the effect of SecA2 on Mce1 and Mce4 transport systems. Because Mce2 or Mce3 proteins were not identified by an average of ≥four spectral counts, we do not know if other M. tuberculosis Mce systems depend on SecA2. However, reduced levels of LprM (Mce3E) as well as Mce4D were observed in the proteomic study of the M. marinum secA2 mutant cell envelope suggesting export of other Mce systems may also depend on SecA2 (16). With qRT-PCR, we ruled out transcriptional effects being the cause of reduced Mce levels. The large number of exported Mce components reduced in the ΔsecA2 mutant could reflect a role for SecA2 in exporting numerous Mce transporter components. However it is also possible that SecA2 exports one or a small number of Mce components and that a defect in localizing a single Mce protein in the ΔsecA2 mutant destabilizes the entire complex. Along these lines, we identified reduced MceG levels in the ΔsecA2 mutant versus H37Rv. MceG is a predicted Mce-associated cytoplasmic ATPase and previous studies show MceG stability is influenced by the presence of Mce transporters (67). Mce systems are proposed to import lipids in a manner analogous to solute import by ABC transporters (68, 69). Furthermore, Mce and Lpr proteins have been considered functionally similar to the SBPs of ABC transporters (68, 69), making our identification of both SBPs and Mce proteins as SecA2-dependent an interesting similarity.
Our studies of cell wall and cytoplasmic fractions revealed increased induction of the DosR regulon in the ΔsecA2 mutant (58, 70). Although first identified as a coordinated response to hypoxia, a condition associated with M. tuberculosis latency, the DosR regulon is now appreciated as being induced by a number of stresses (29, 70–75). Standing cultures of M. tuberculosis are reportedly able to induce expression of DosR-regulated proteins, presumably because of hypoxic conditions for the settled bacteria (29). Our storage of cell pellets prior to irradiation and fractionation likely provided a DosR-inducing signal, revealing an unanticipated SecA2-dependent effect on this regulatory response. Using qRT-PCR, we showed increased transcription of DosR-regulated genes occurs in the ΔsecA2 mutant. Interestingly, with both hspX and dosR transcripts the fold difference observed in the ΔsecA2 mutant was greater at earlier times points and less dramatic at 24 h postinduction. Together, our results show increased transcription and possibly accelerated induction of the DosR regulon in the ΔsecA2 mutant. The ΔsecA2 mutant may be altered in a way that allows it to recognize or respond more quickly to DosR inducing stimuli. Alternatively, the absence of SecA2 could produce an underlying level of stress that primes the mutant for DosR induction, although our LFQ analysis of cytoplasmic fractions did not reveal other stress pathways being up-regulated in the ΔsecA2 mutant. Our discovery of enhanced DosR responses could be useful for efforts to develop the M. tuberculosis ΔsecA2 mutant into a live attenuated vaccine (76, 77). Because of the desire for a tuberculosis vaccine to work with latent M. tuberculosis infections, a vaccine with improved ability to elicit immune responses to latency associated antigens, such as DosR-regulated proteins, would be advantageous (78).
SecA2-dependent Proteins with Functions in Virulence
SecA2 is required for growth and survival of M. tuberculosis in macrophages and mice (5–7). Although many differences detected by LFQ analysis remain to be validated, the results presented serve to expand the list of candidates to consider as SecA2-dependent effectors and help to develop new hypotheses for how SecA2 contributes to the pathogenesis of M. tuberculosis.
One way the SecA2 pathway functions in M. tuberculosis virulence is by enabling the bacterium to arrest phagosome maturation and avoid acidified phagolysosomes in macrophages (7). We identified two proteins that may help explain the role of M. tuberculosis SecA2 in arresting phagosome maturation. A predicted esterase LipO was significantly reduced in the ΔsecA2 mutant cell wall in comparison to H37Rv (6.9-fold reduced, p = 0.005). A lipO (rv1426c) mutant is defective in phagosome maturation arrest, indicating a potential role for LipO as an effector of this process (79). LipO has a predicted Sec signal peptide to account for its export to the cell wall. Although further studies are needed, our results raise the possibility of SecA2 localizing LipO to the cell wall and, thereby, enabling LipO to carry out a role in phagosome maturation arrest. An additional candidate for a being a SecA2 exported effector of phagosome maturation arrest is the protein kinase PknG, which is also implicated in phagosome maturation arrest (23). SecA2-dependent export of PknG likely contributes to phagosome maturation arrest, but there are probably additional SecA2-dependent effectors involved. This likelihood is supported by an experiment performed with the M. marinum secA2 mutant that shows restoration of PknG export in a secA2 mutant rescues some, but not all, phagosome maturation arrest in macrophages (16). Like the SecA2-dependent secreted SodA protein, PknG lacks an export signal. Thus, our results in support of PknG being exported by the SecA2 pathway of M. tuberculosis are also important in reinforcing earlier observations that SecA2-dependent substrates of mycobacteria include examples with and without signal peptides (5, 15, 16).
A role of SecA2 in exporting SBPs and Mce transporters may additionally contribute to M. tuberculosis virulence. Although most SBPs have yet to be investigated in terms of function, a wide range of solutes are predicted to be imported by these proteins. Collectively, the role of SecA2 in proper localization of SBPs could have a significant impact on nutrient acquisition or signaling during M. tuberculosis infection. Mce1 and Mce4 transporters are more firmly established as having roles in M. tuberculosis virulence. Although there is one conflicting report, multiple studies show Mce1 (like SecA2) is important for growth in macrophages and during the early phase of murine infection (5–7, 67, 80–83). The specific function of Mce1 during infection is not clear, but a potential role of Mce1 in importing free mycolic acids was recently proposed (57, 84). Mce4 is the best studied Mce transporter, with a shown role in cholesterol import (85, 86) and a role in virulence (85, 87). Thus, another way that the SecA2 pathway may contribute to M. tuberculosis infection could be through its role in SBP and Mce transporter localization.
CONCLUSION
Using a large-scale LFQ proteomic approach, we generated a list of candidates for cell wall proteins exported by the SecA2 pathway of M. tuberculosis. Our results are significant in highlighting SBPs as a family of SecA2-exported proteins and reinforcing prior observations that mycobacterial proteins exported in a SecA2-dependent fashion include examples with and without Sec signal peptides. At the same time, our results revealed unexpected contributions of SecA2 to Mce transport and DosR regulation. The proteins identified in this report represent a valuable resource for understanding the mechanism of SecA2-dependent export and contributions of this pathway to M. tuberculosis virulence and biology.
Supplementary Material
Acknowledgments
We thank Douglas Young, Murty Madiraju, and Yossef Av-Gay for providing antibodies and acknowledge members of the Braunstein laboratory and Dr. Henry S. Gibbons for critical reading the manuscript. We appreciate the assistance of Ellen Perkowski in assembling the bioinformatic predictions of export signals. The mass spectrometry proteomics data has been deposited to the ProteomeXchange Consortium with the dataset identifier PXD002144.
Footnotes
Author contributions: M.E.F., H.P.G., K.E.Z., X.C., and M.B. designed research; M.E.F., H.P.G., K.E.Z., and S.M. performed research; J.E.G. and C.M.S. contributed new reagents or analytic tools; M.E.F., H.P.G., K.E.Z., and M.B. analyzed data; M.E.F., K.E.Z., S.M., H.P.G., X.C., and M.B. wrote the paper.
* Support for this study was provided to M.B. by NIAID AI054540.
 This article contains supplemental Figs. S1 to S4 and Tables S1 to S15.
This article contains supplemental Figs. S1 to S4 and Tables S1 to S15.
1 The abbreviations used are:
- the following: LFQ
- label-free quantitation
- 2D-GE
- 2-dimensional gel electrophoresis
- ADS
- albumin-dextrose-saline
- WCL
- whole cell lysate
- CW
- cell wall
- SOL
- soluble
- FDR
- false discovery rate
- ETD
- electron transfer dissociation
- HCD
- higher energy collision dissociation
- MIPS
- monoisotopic precursor ion selection.
REFERENCES
- 1. WorldHealthOrganization (2012) Global tuberculosis report 2012 [Google Scholar]
- 2. Russell D. G. (2008) Mycobacterium tuberculosis: life and death in the phagosome. In: Kaufmann S. H., Rubin E. J., eds. Handbook of Tuberculosis: Molecular Biology and Biochemistry, pp. 307–322, Wiley-VCH [Google Scholar]
- 3. Hilbi H., Haas A. (2012) Secretive bacterial pathogens and the secretory pathway. Traffic 13, 1187–1197 [DOI] [PubMed] [Google Scholar]
- 4. Ligon L. S., Hayden J. D., Braunstein M. (2012) The ins and outs of Mycobacterium tuberculosis protein export. Tuberculosis 92, 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braunstein M., Espinosa B., Chan J., Belisle J. T., Jacobs W. R. J. (2003) SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 48, 453–464 [DOI] [PubMed] [Google Scholar]
- 6. Kurtz S., McKinnon K. P., Runge M. S., Ting J. P., Braunstein M. (2006) The SecA2 secretion factor of Mycobacterium tuberculosis promotes growth in macrophages and inhibits the host immune response. Infect. Immun. 74, 6855–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sullivan J. T., Young E. F., McCann J. R., Braunstein M. (2012) The Mycobacterium tuberculosis SecA2 system subverts phagosome maturation to promote growth in macrophages. Infect. Immun. 80, 996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feltcher M. E., Braunstein M. (2012) Emerging themes in SecA2-mediated protein export. Nat. Rev. Microbiol. 10, 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bensing B. A., Seepersaud R., Yen Y. T., Sullam P. M. (2014) Selective transport by SecA2: an expanding family of customized motor proteins. Biochim. Biophys. Acta 1843, 1674–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bensing B. A., Sullam P. M. (2002) An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44, 1081–1094 [DOI] [PubMed] [Google Scholar]
- 11. Siboo I. R., Chaffin D. O., Rubens C. E., Sullam P. M. (2008) Characterization of the accessory Sec system of Staphylococcus aureus. J. Bacteriol. 190, 6188–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Q., Wu H., Fives-Taylor P. M. (2004) Investigating the role of secA2 in secretion and glycosylation of a fimbrial adhesin in Streptococcus parasanguis FW213. Mol. Microbiol. 53, 843–856 [DOI] [PubMed] [Google Scholar]
- 13. Mistou M. Y., Dramsi S., Brega S., Poyart C., Trieu-Cuot P. (2009) Molecular dissection of the secA2 locus of group B Streptococcus reveals that glycosylation of the Srr1 LPXTG protein is required for full virulence. J. Bacteriol. 191, 4195–4206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lenz L. L., Mohammadi S., Geissler A., Portnoy D. A. (2003) SecA2-dependent secretion of autolytic enzymes promotes Listeria monocytogenes pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 100, 12432–12437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gibbons H. S., Wolschendorf F., Abshire M., Niederweis M., Braunstein M. (2007) Identification of two Mycobacterium smegmatis lipoproteins exported by a SecA2-dependent pathway. J. Bacteriol. 189, 5090–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Woude A. D., Stoop E. J., Stiess M., Wang S., Ummels R., van Stempvoort G., Piersma S. R., Cascioferro A., Jimenez C. R., Houben E. N., Luirink J., Pieters J., van der Sar A. M., Bitter W. (2014) Analysis of SecA2-dependent substrates in Mycobacterium marinum identifies protein kinase G (PknG) as a virulence effector. Cell Microbiol. 16, 280–295 [DOI] [PubMed] [Google Scholar]
- 17. Fagan R. P., Fairweather N. F. (2011) Clostridium difficile has two parallel and essential Sec secretion systems. J. Biol. Chem. 286, 27483–27493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rigel N. W., Braunstein M. (2008) A new twist on an old pathway–accessory Sec systems. Mol. Microbiol. 69, 291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ligon L. S., Rigel N. W., Romanchuk A., Jones C. D., Braunstein M. (2013) Suppressor analysis reveals a role for SecY in the SecA2-dependent protein export pathway of mycobacteria. J. Bacteriol. 195, 4456–4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rigel N. W., Gibbons H. S., McCann J. R., McDonough J. A., Kurtz S., Braunstein M. (2009) The accessory SecA2 system of mycobacteria requires ATP binding and the canonical SecA1. J. Biol. Chem. 284, 9927–9936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feltcher M. E., Gibbons H. S., Ligon L. S., Braunstein M. (2013) Protein export by the mycobacterial SecA2 system is determined by the preprotein mature domain. J. Bacteriol. 195, 672–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watkins B. Y., Joshi S. A., Ball D. A., Leggett H., Park S., Kim J., Austin C. D., Paler-Martinez A., Xu M., Downing K. H., Brown E. J. (2012) Mycobacterium marinum SecA2 promotes stable granulomas and induces tumor necrosis factor alpha in vivo. Infect. Immun. 80, 3512–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walburger A., Koul A., Ferrari G., Nguyen L., Prescianotto-Baschong C., Huygen K., Klebl B., Thompson C., Bacher G., Pieters J. (2004) Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science 304, 1800–1804 [DOI] [PubMed] [Google Scholar]
- 24. Cowley S., Ko M., Pick N., Chow R., Downing K. J., Gordhan B. G., Betts J. C., Mizrahi V., Smith D. A., Stokes R. W., Av-Gay Y. (2004) The Mycobacterium tuberculosis protein serine/threonine kinase PknG is linked to cellular glutamate/glutamine levels and is important for growth in vivo. Mol. Microbiol. 52, 1691–1702 [DOI] [PubMed] [Google Scholar]
- 25. Rohde K. H., Abramovitch R. B., Russell D. G. (2007) Mycobacterium tuberculosis invasion of macrophages: linking bacterial gene expression to environmental cues. Cell Host Microbe 2, 352–364 [DOI] [PubMed] [Google Scholar]
- 26. Schnappinger D., Ehrt S., Voskuil M. I., Liu Y., Mangan J. A., Monahan I. M., Dolganov G., Efron B., Butcher P. D., Nathan C., Schoolnik G. K. (2003) Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198, 693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKinney J. D., Honer zu Bentrup K., Munoz-Elias E. J., Miczak A., Chen B., Chan W. T., Swenson D., Sacchettini J. C., Jacobs W. R., Jr., Russell D. G. (2000) Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406, 735–738 [DOI] [PubMed] [Google Scholar]
- 28. Marrero J., Rhee K. Y., Schnappinger D., Pethe K., Ehrt S. (2010) Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc. Natl. Acad. Sci. U.S.A. 107, 9819–9824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kendall S. L., Movahedzadeh F., Rison S. C., Wernisch L., Parish T., Duncan K., Betts J. C., Stoker N. G. (2004) The Mycobacterium tuberculosis dosRS two-component system is induced by multiple stresses. Tuberculosis 84, 247–255 [DOI] [PubMed] [Google Scholar]
- 30. Gu S., Chen J., Dobos K. M., Bradbury E. M., Belisle J. T., Chen X. (2003) Comprehensive proteomic profiling of the membrane constituents of a Mycobacterium tuberculosis strain. Mol. Cell. Proteomics 2, 1284–1296 [DOI] [PubMed] [Google Scholar]
- 31. Gunawardena H. P., Feltcher M. E., Wrobel J. A., Gu S., Braunstein M., Chen X. (2013) Comparison of the membrane proteome of virulent Mycobacterium tuberculosis and the attenuated Mycobacterium bovis BCG vaccine strain by label-free quantitative proteomics. J. Proteome Res. 12, 5463–5474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elias J. E., Gygi S. P. (2007) Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 [DOI] [PubMed] [Google Scholar]
- 33. Weatherly D. B., Atwood J. A., 3rd, Minning T. A., Cavola C., Tarleton R. L., Orlando R. (2005) A heuristic method for assigning a false-discovery rate for protein identifications from Mascot database search results. Mol. Cell. Proteomics 4, 762–772 [DOI] [PubMed] [Google Scholar]
- 34. Hsieh E. J., Hoopmann M. R., MacLean B., MacCoss M. J. (2010) Comparison of database search strategies for high precursor mass accuracy MS/MS data. J. Proteome Res. 9, 1138–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keller A., Nesvizhskii A. I., Kolker E., Aebersold R. (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 [DOI] [PubMed] [Google Scholar]
- 36. Nesvizhskii A. I., Keller A., Kolker E., Aebersold R. (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646–4658 [DOI] [PubMed] [Google Scholar]
- 37. Storey J. D., Tibshirani R. (2003) Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. U.S.A. 100, 9440–9445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Petersen T. N., Brunak S., von Heijne G., Nielsen H. (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786 [DOI] [PubMed] [Google Scholar]
- 39. McDonough J. A., McCann J. R., Tekippe E. M., Silverman J. S., Rigel N. W., Braunstein M. (2008) Identification of functional Tat signal sequences in Mycobacterium tuberculosis proteins. J. Bacteriol. 190, 6428–6438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bendtsen J. D., Nielsen H., Widdick D., Palmer T., Brunak S. (2005) Prediction of twin-arginine signal peptides. BMC Bioinformatics 6, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rose R. W., Bruser T., Kissinger J. C., Pohlschroder M. (2002) Adaptation of protein secretion to extremely high-salt conditions by extensive use of the twin-arginine translocation pathway. Mol. Microbiol. 45, 943–950 [DOI] [PubMed] [Google Scholar]
- 42. Selengut J. D., Haft D. H., Davidsen T., Ganapathy A., Gwinn-Giglio M., Nelson W. C., Richter A. R., White O. (2007) TIGRFAMs and Genome Properties: tools for the assignment of molecular function and biological process in prokaryotic genomes. Nucleic Acids Res. 35, D260–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580 [DOI] [PubMed] [Google Scholar]
- 44. Sutcliffe I. C., Harrington D. J. (2004) Lipoproteins of Mycobacterium tuberculosis: an abundant and functionally diverse class of cell envelope components. FEMS Microbiol. Rev. 28, 645–659 [DOI] [PubMed] [Google Scholar]
- 45. Franceschini A., Szklarczyk D., Frankild S., Kuhn M., Simonovic M., Roth A., Lin J., Minguez P., Bork P., von Mering C., Jensen L. J. (2013) STRING v9.1: protein–protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 41, D808–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Manganelli R., Dubnau E., Tyagi S., Kramer F. R., Smith I. (1999) Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Mol. Microbiol. 31, 715–724 [DOI] [PubMed] [Google Scholar]
- 47. Lew J. M., Kapopoulou A., Jones L. M., Cole S. T. (2011) TubercuList–10 years after. Tuberculosis 91, 1–7 [DOI] [PubMed] [Google Scholar]
- 48. Mawuenyega K. G., Forst C. V., Dobos K. M., Belisle J. T., Chen J., Bradbury E. M., Bradbury A. R., Chen X. (2005) Mycobacterium tuberculosis functional network analysis by global subcellular protein profiling. Mol. Biol. Cell 16, 396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wolfe L. M., Mahaffey S. B., Kruh N. A., Dobos K. M. (2010) Proteomic definition of the cell wall of Mycobacterium tuberculosis. J. Proteome Res. 9, 5816–5826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hutchings M. I., Palmer T., Harrington D. J., Sutcliffe I. C. (2009) Lipoprotein biogenesis in Gram-positive bacteria: knowing when to hold 'em, knowing when to fold 'em. Trends Microbiol. 17, 13–21 [DOI] [PubMed] [Google Scholar]
- 51. Malen H., De Souza G. A., Pathak S., Softeland T., Wiker H. G. (2011) Comparison of membrane proteins of Mycobacterium tuberculosis H37Rv and H37Ra strains. BMC Microbiol. 11, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. He Z., De Buck J. (2010) Cell wall proteome analysis of Mycobacterium smegmatis strain MC2 155. BMC Microbiol. 10, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu H., Sadygov R. G., Yates J. R., 3rd (2004) A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 76, 4193–4201 [DOI] [PubMed] [Google Scholar]
- 54. Hou J. M., D'Lima N. G., Rigel N. W., Gibbons H. S., McCann J. R., Braunstein M., Teschke C. M. (2008) ATPase activity of Mycobacterium tuberculosis SecA1 and SecA2 proteins and its importance for SecA2 function in macrophages. J. Bacteriol. 190, 4880–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Braibant M., Gilot P., Content J. (2000) The ATP binding cassette (ABC) transport systems of Mycobacterium tuberculosis. FEMS Microbiol. Rev. 24, 449–467 [DOI] [PubMed] [Google Scholar]
- 56. Chitale S., Ehrt S., Kawamura I., Fujimura T., Shimono N., Anand N., Lu S., Cohen-Gould L., Riley L. W. (2001) Recombinant Mycobacterium tuberculosis protein associated with mammalian cell entry. Cell Microbiol. 3, 247–254 [DOI] [PubMed] [Google Scholar]
- 57. Forrellad M. A., McNeil M., Santangelo Mde L., Blanco F. C., Garcia E., Klepp L. I., Huff J., Niederweis M., Jackson M., Bigi F. (2014) Role of the Mce1 transporter in the lipid homeostasis of Mycobacterium tuberculosis. Tuberculosis 94, 170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Park H. D., Guinn K. M., Harrell M. I., Liao R., Voskuil M. I., Tompa M., Schoolnik G. K., Sherman D. R. (2003) Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 48, 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bagchi G., Chauhan S., Sharma D., Tyagi J. S. (2005) Transcription and autoregulation of the Rv3134c-devR-devS operon of Mycobacterium tuberculosis. Microbiology 151, 4045–4053 [DOI] [PubMed] [Google Scholar]
- 60. Boon C., Li R., Qi R., Dick T. (2001) Proteins of Mycobacterium bovis BCG induced in the Wayne dormancy model. J. Bacteriol. 183, 2672–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kumar A., Bose M., Brahmachari V. (2003) Analysis of expression profile of mammalian cell entry (mce) operons of Mycobacterium tuberculosis. Infect. Immun. 71, 6083–6087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Halbedel S., Hahn B., Daniel R. A., Flieger A. (2012) DivIVA affects secretion of virulence-related autolysins in Listeria monocytogenes. Mol. Microbiol. 83, 821–839 [DOI] [PubMed] [Google Scholar]
- 63. Renier S., Chambon C., Viala D., Chagnot C., Hebraud M., Desvaux M. (2013) Exoproteomic analysis of the SecA2-dependent secretion in Listeria monocytogenes EGD-e. J. Proteomics 80C, 183–195 [DOI] [PubMed] [Google Scholar]
- 64. Palmer T., Berks B. C. (2012) The twin-arginine translocation (Tat) protein export pathway. Nat. Rev. Microbiol. 10, 483–496 [DOI] [PubMed] [Google Scholar]
- 65. Shruthi H., Babu M. M., Sankaran K. (2010) TAT-pathway-dependent lipoproteins as a niche-based adaptation in prokaryotes. J. Mol. Evol. 70, 359–370 [DOI] [PubMed] [Google Scholar]
- 66. Chater K. F., Biro S., Lee K. J., Palmer T., Schrempf H. (2010) The complex extracellular biology of Streptomyces. FEMS Microbiol. Rev. 34, 171–198 [DOI] [PubMed] [Google Scholar]
- 67. Joshi S. M., Pandey A. K., Capite N., Fortune S. M., Rubin E. J., Sassetti C. M. (2006) Characterization of mycobacterial virulence genes through genetic interaction mapping. Proc. Natl. Acad. Sci. U.S.A. 103, 11760–11765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Casali N., Riley L. W. (2007) A phylogenomic analysis of the Actinomycetales mce operons. BMC Genomics 8, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kumar A., Chandolia A., Chaudhry U., Brahmachari V., Bose M. (2005) Comparison of mammalian cell entry operons of mycobacteria: in silico analysis and expression profiling. FEMS Immunol. Med. Microbiol. 43, 185–195 [DOI] [PubMed] [Google Scholar]
- 70. Sherman D. R., Voskuil M., Schnappinger D., Liao R., Harrell M. I., Schoolnik G. K. (2001) Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc. Natl. Acad. Sci. U.S.A. 98, 7534–7539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Boon C., Dick T. (2012) How Mycobacterium tuberculosis goes to sleep: the dormancy survival regulator DosR a decade later. Future Microbiol. 7, 513–518 [DOI] [PubMed] [Google Scholar]
- 72. Shiloh M. U., Manzanillo P., Cox J. S. (2008) Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe 3, 323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kumar A., Deshane J. S., Crossman D. K., Bolisetty S., Yan B. S., Kramnik I., Agarwal A., Steyn A. J. (2008) Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J. Biol. Chem. 283, 18032–18039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Voskuil M. I., Schnappinger D., Visconti K. C., Harrell M. I., Dolganov G. M., Sherman D. R., Schoolnik G. K. (2003) Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198, 705–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kumar A., Toledo J. C., Patel R. P., Lancaster J. R., Jr., Steyn A. J. (2007) Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor. Proc. Natl. Acad. Sci. U.S.A. 104, 11568–11573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hinchey J., Lee S., Jeon B. Y., Basaraba R. J., Venkataswamy M. M., Chen B., Chan J., Braunstein M., Orme I. M., Derrick S. C., Morris S. L., Jacobs W. R., Jr., Porcelli S. A. (2007) Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J. Clin. Invest. 117, 2279–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hinchey J., Jeon B. Y., Alley H., Chen B., Goldberg M., Derrick S., Morris S., Jacobs W. R., Jr., Porcelli S. A., Lee S. (2011) Lysine auxotrophy combined with deletion of the SecA2 gene results in a safe and highly immunogenic candidate live attenuated vaccine for tuberculosis. PLoS One 6, e15857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Geluk A., van Meijgaarden K. E., Joosten S. A., Commandeur S., Ottenhoff T. H. (2014) Innovative strategies to identify M. tuberculosis antigens and epitopes using genome-wide analyses. Front. Immunol. 5, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pethe K., Swenson D. L., Alonso S., Anderson J., Wang C., Russell D. G. (2004) Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc. Natl. Acad. Sci. U.S.A. 101, 13642–13647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McCann J. R., McDonough J. A., Sullivan J. T., Feltcher M. E., Braunstein M. (2011) Genome-wide identification of Mycobacterium tuberculosis exported proteins with roles in intracellular growth. J. Bacteriol. 193, 854–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rengarajan J., Bloom B. R., Rubin E. J. (2005) Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc. Natl. Acad. Sci. U.S.A. 102, 8327–8332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gioffre A., Infante E., Aguilar D., Santangelo M. P., Klepp L., Amadio A., Meikle V., Etchechoury I., Romano M. I., Cataldi A., Hernandez R. P., Bigi F. (2005) Mutation in mce operons attenuates Mycobacterium tuberculosis virulence. Microbes Infect. 7, 325–334 [DOI] [PubMed] [Google Scholar]
- 83. Shimono N., Morici L., Casali N., Cantrell S., Sidders B., Ehrt S., Riley L. W. (2003) Hypervirulent mutant of Mycobacterium tuberculosis resulting from disruption of the mce1 operon. Proc. Natl. Acad. Sci. U.S.A. 100, 15918–15923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cantrell S. A., Leavell M. D., Marjanovic O., Iavarone A. T., Leary J. A., Riley L. W. (2013) Free mycolic acid accumulation in the cell wall of the mce1 operon mutant strain of Mycobacterium tuberculosis. J. Microbiol. 51, 619–626 [DOI] [PubMed] [Google Scholar]
- 85. Pandey A. K., Sassetti C. M. (2008) Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. U.S.A. 105, 4376–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Klepp L. I., Forrellad M. A., Osella A. V., Blanco F. C., Stella E. J., Bianco M. V., Santangelo Mde L., Sassetti C., Jackson M., Cataldi A. A., Bigi F., Morbidoni H. R. (2012) Impact of the deletion of the six mce operons in Mycobacterium smegmatis. Microbes Infect. 14, 590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Senaratne R. H., Sidders B., Sequeira P., Saunders G., Dunphy K., Marjanovic O., Reader J. R., Lima P., Chan S., Kendall S., McFadden J., Riley L. W. (2008) Mycobacterium tuberculosis strains disrupted in mce3 and mce4 operons are attenuated in mice. J. Med. Microbiol. 57, 164–170 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.