Abstract
As essential mediators of red cell production, erythropoietin (EPO) and its cell surface receptor (EPO receptor [EPOR]) have been intensely studied. Early investigations defined basic mechanisms for hypoxia-inducible factor induction of EPO expression, and within erythroid progenitors EPOR engagement of canonical Janus kinase 2/signal transducer and activator of transcription 5 (JAK2/STAT5), rat sarcoma/mitogen-activated protein kinase/extracellular signal-regulated kinase (RAS/MEK/ERK), and phosphatidylinositol 3-kinase (PI3K) pathways. Contemporary genetic, bioinformatic, and proteomic approaches continue to uncover new clinically relevant modulators of EPO and EPOR expression, and EPO’s biological effects. This Spotlight review highlights such factors and their emerging roles during erythropoiesis and anemia.
Introduction
Early parabiotic experiments with anemic and nephrectomized rats predicted the existence of erythropoietin (EPO) as a blood-borne kidney-derived activator of erythropoiesis.1 Evidence that EPO occurs as a unique glycoprotein hormone was further advanced via arduous fractionations and bioassays of urinary proteins from anemia patients.2 The purification, partial sequencing, and cloning of erythropoietin3 have led to the generation of recombinant human EPO (rhEPO) (and derivatives) for the treatment of anemia associated with chronic kidney disease, chemotherapy, and low-risk myelodysplastic syndrome.4 The subsequent discovery of the EPO receptor (EPOR) as a plasma membrane single-pass homodimer5,6 elevated the EPO/EPOR system as a paradigm for hematopoietic cytokine receptor action. The EPOR, for example, was among the first discovered to associate with a Janus kinase (JAK),7 to transduce signals via transmembrane conformational mechanisms,8 and to be causally associated with polycythemia.9 EPO’s clinical and scientific successes have prompted in-depth investigations into EPO/EPOR biology. This review focuses on intriguing advances in understanding the regulation of EPO and EPOR expression, and the nature of novel EPO/EPOR signals that regulate erythroid progenitor cell (EPC) development. EPO also has been reported to exert survival, proliferative, and/or developmental effects in a wide range of nonhematopoietic tissues.10-16 In such cell types, however, EPOR protein expression (including cell surface levels) can be nominal,17 thereby complicating interpretations for direct vs indirect effects. Nonetheless, incisive EPOR loss-of-function approaches have revealed interesting EPO effects in cardiomyocyte mitochondrial biogenesis,18 retinal cell cytoprotection,19 melanoma cell survival,20 and adipogenesis.21 This broad area of investigation, however, lies beyond the scope of the present report.
EPO expression
The nature of rare Epo-producing cells is first becoming more clearly defined. During primitive erythropoiesis, studies using an Epo gene green fluorescent protein knock-in mouse indicate predominant Epo expression by neural crest and neuroepithelial cells.22 Tracking studies of myelin protein-zero23 marked peripheral neural cells demonstrate that Epopos embryonic neural crest fibroblasts migrate to the kidney,24 and perinatally reside within peritubular interstitia.25 Renal fibrosis due to ureteral obstruction can promote transdifferentiation of Epohigh fibroblasts to Epolow myofibroblasts.24 Epo levels in myofibroblasts can be increased, however, via neurotropin or dexamethasone dosing. During stress erythropoiesis, Epo expression can also be induced in the liver,26 as well as bone marrow (BM) osteoblasts as demonstrated upon von Hippel–Lindau factor (VHL) inactivation.27
New insight has also been gained into EPO gene regulation. Early investigations of hypoxia-induced EPO expression established important roles for a downstream EPO enhancer (E-3′) as a binding site for hypoxia-inducible factor (HIF) and hepatocyte nuclear factor 4 transcriptional regulators.28 In vivo studies in mice with a green fluorescent protein-marked Epo allele demonstrate that E-3′ deletion results in embryonic and neonatal anemia.25,26 In juvenile and adult kidney, however, Epo production is unexpectedly regained in the absence of E-3′,26 whereas hepatic Epo production continues to depend upon E-3′ effects.26 For renal EPO production, this raises new questions concerning activation mechanisms.
Among HIF1α, -2α, and -3α, HIF2α has been defined as a prime component of an EPO gene activating complex.29 New insight into HIF2α regulation (beyond requisite heterodimerization with HIFβ/aryl hydrocarbon receptor nuclear translocator)30 has also been gained. Hif2α’s translation first has been shown to be suppressed via iron response element binding protein 1 (Irp1) (in a knockout [KO] mouse model), thus connecting iron levels to Hif2α/HIFβ-regulated Epo gene expression.31 HIF2α’s activity is also modulated by lysine acetylation and deacetylation via CREB-binding protein (CBP) and sirtuin, respectively.32 For acetylation, acetyl-CoA levels during stress erythropoiesis can become physiologically limiting. Specifically, Hif2α’s acetylation, CBP association, and enhanced activity have been shown in an Acss2-KO model to depend upon acetyl-CoA synthetase-2.33 Moreover, acetate supplementation in vivo elevates Epo levels as well as hematocrits in hemolytic, partial nephrectomy chronic kidney disease, and Kit mutant models. The turnover of HIFs is promoted via hydroxylation by prolyl 4-hydroxylases (PHDs), and ubiquitination by VHL.30 Notably, several PHD inhibitors have been developed as HIF stabilizers to enhance EPO and erythrocyte production (eg, roxadustat [FibroGen/Astellas], AKB-6548 [Akebia Therapeutics], and GSK1278863 [GlaxoSmithKline]).34
EPO receptor expression and EPOR signal modifiers
The expression of EPO’s receptor is stringently regulated and is at a low level (∼1100 EPORs per primary human EPC and ∼300 per late-stage erythroblast) as determined via 125I-EPO binding studies.35 At the EpoR locus, Gata1,36 Sp1,37 and Scl/Tal138 stimulate transcription, but additional regulators are not well defined. For EPOR trafficking, certain new insights have been gained. Over-expression studies in murine myeloid 32D cells suggest ligand-independent EpoR turnover, with replenishment from a predicted large intracellular pool.39 For the endogenous EPOR, however, studies in human UT7epo and/or primary EPCs demonstrate substantial up-modulation of cell surface EPORs when EPO is limited, marked down-modulation upon EPO exposure, and only modest intracellular EPOR pools.40 During EPOR endocytosis, coordinated roles for p85-α (PI3 kinase regulatory subunit), ubiquitinated casitas B-lineage lymphoma and Epsin1 have been described.41,42 In UT7epo cells, β-transducin repeat containing E3 ubiquitin ligase (β-TRCP) subsequently promotes EPOR degradation.43 Dynamic down-modulation of low-level EPOR cell surface expression emphasizes a need for cautious interpretation of apparent EPOR levels, and the use of high-specificity reagents.17,40
Several new EPOR interacting factors have been described. Proteomic analyses of biotin-EPO/EPOR complexes have identified transferrin receptor 2 (TFR2) as an EPOR partner.44 In UT7epo cells, TFR2 facilitates EPOR processing and transport to the cell surface.44 In primary human EPCs, TFR2 knockdown decreased hemoglobinized cell formation, and increased numbers of early stage EPCs.44 EPCs from Tfr2−/− mice exhibit decreased EPO sensitivity and erythroid colony-forming unit formation.44 During iron deficiency, Tfr2 also acts to balance erythrocyte production with available iron.45 Beyond its established roles in hepatocyte iron transport,46 Tfr2 also therefore modulates EPO-dependent erythropoiesis. In addition, phospho-proteomic analyses have identified the integral plasma membrane protein regulator of human erythroid cell expansion (RHEX) as a new EPOR-associated factor. In UT7epo cells, RHEX co-IP’s with EPOR/JAK2 complexes, and its tyrosine phosphorylation is strongly induced by EPO. In primary EPCs, RHEX exhibits stage-specific expression,47 and its knockdown attenuates extracellular signal-regulated kinase 1/2 (ERK1/2) activation as well as late-stage human erythroblast development. Interestingly, RHEX is not represented among rat, mouse, or lower vertebrates.
Transferrin receptor 1 (TFR1) can also modulate EPOR signaling. Specifically, Tfr1 ligation by polymeric-IgA1 (p-IgA1) in murine erythroblasts increases EPO/EPOR-dependent mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling.48 This occurs in the absence of transferrin binding, but depends upon a Tfr1 endocytic motif. In a knock-in model, human p-IgA1 enhances recovery from anemia due to 5-fluorouracil, hypoxia, and hemolytic anemia. p-IgA1 also binds CD89, an Fc-α receptor and suppressor of inflammatory cytokine production.49 By speculation, p-IgA1 might also aid stress erythropoiesis by lessening inflammation, a mechanism implicated for an activin receptor-IIA ligand trap as a new anti-anemia agent.50
EPOR signal transduction via rat sarcoma/mek kinase/mitogen-activated protein kinase (RAS/RAF/MEK)/ERK circuits
As demonstrated in primary human EPCs, balanced activation of RAS pathways is required for effective EPO-dependent erythroblast formation.51 In mouse models, the deletion of K-Ras (but not H-Ras or N-Ras) generates severe anemia,52 and compromises EPO-dependent fetal liver EPC development. Activated K-RasG12D likewise induces severe anemia due to ineffective fetal erythropoiesis,53 and persistently stimulates Erk1/2, Akt, and signal transducer and activator of transcription 5 (Stat5).54 Of clinical interest, the inhibition of RAS farnesylation by tipifarnib decreases polycythemia vera erythroid burst-forming unit hyperproliferation.55 During erythropoiesis, RAS is also regulated by newly emerging guanosine triphosphate (GTP)/guanosine diphosphate exchange factors. One is the Ras-GTPase activating protein Rasa3, which when mutated in Scat mice leads to anemia and thrombocytopenia.56 A second is neurofibromin (Nf1), for which mutations have been associated with juvenile myelomonocytic leukemia, including anemia due to limited EPC differentiation.57
Regulation of RAS-modulated targets is also important for EPO-dependent erythropoiesis. C-Raf deletion results in embryonic anemia.58 And in β-thalassemia proerythroblasts, phospho-C-Raf levels correlate with increased ERK activation.59 Mek2 is dispensable for mouse development,60 implicating prime roles for Mek1 in Erk1/2 signaling. In mice expressing a truncated EpoR-HM allele, pharmacologic inhibition of Mek reverses stage-specific EPC differentiation defects,61 whereas in mice with somatic inactivation of Nf1, Mek1/2 inhibition decreases splenomegaly and enhances erythropoiesis.57 For ERKs, Erk1−/− mice exhibit heightened splenic erythropoiesis and hematocrits.62
RAS-like GTPases can also regulate EPO-dependent EPC formation. As a new EPO/EPOR target gene and Roco family GTPase, malignant fibrous histiocytoma-amplified sequences with leucine-rich tandem repeats 1 (MASL1) supports C-RAF/MEK/ERK activation and primary human erythroblast development.63 As Ras homolog family GTPases, RACs also regulate EPC development and erythroblast enucleation.64 In 32D-EpoR and UT7epo cells, EPO rapidly activates RAC1, implicating possible EPO/EPOR regulation of RACs.65 In oncology contexts, as new inhibitors of RAS and RAS-like factors are developed,66 their potential negative effects on erythropoiesis should therefore be evaluated.
EPO/EPOR cytoprotective circuits
EPO’s best-known effects are cytoprotective.67 Koulnis et al have described EPO’s slow yet persistent down-modulation of proapoptotic Bcl2-like 11 (Bim) in murine splenic EPCs.68 Prior studies in HCD57 cells and primary murine EPCs also demonstrated EPO-induced Bim phosphorylation and proteasomal degradation.69 The inhibition of Bim therefore represents one EPO-induced EPC survival mechanism. Post-EPO dosing, Bcl-xL levels in splenic EPCs transiently increase,68 and in 32D-EPOR cells Bcl-xL is an EPO/EPOR/STAT5 target gene.70 These latter EPO effects, however, are not observed in erythroid colony-forming unit-like murine BM EPCs,71 and EPO can efficiently cytoprotect Bclx-KO EPCs.72 Important questions therefore arise concerning possible additional mediators of EPO/EPOR cytoprotection.
Via gene profiling of murine BM EPCs, an intracellular Spi2A serpin has been identified as a new EPO/EPOR/JAK2/Stat5 target, and cytoprotective factor.71 Spi2A inhibits B- and L-cathepsins, which when leached from damaged lysosomes can trigger apoptosis.73 Spi2A-KO mice exhibit compromised EPO-induced EPC formation, and worsened anemia due to hemolysis or irradiation. Spi2A further cytoprotects erythroblasts against ROS, an effect that is phenocopied by a cathepsin-B inhibitor. Pharmacologically, selective cathepsin inhibitors therefore might act to limit cell loss due to oxidant damage in thalassemia and/or sickle cell EPCs.74,75 During stress erythropoiesis, Fas ligand (FasL)/Fas levels can also substantially modulate murine splenic EPC survival.76 In human EPCs, tumor necrosis factor (TNF)-related apoptosis inducing ligand or TRAIL may be a more potent pro-apoptotic TNF, and FASL may support caspase-dependent late differentiation events.77 As further illustrated by recent gene profiling studies of human and murine EPCs,78 complexities can exist in erythroid regulator utilization among species (and erythroid tissues) that require reconciliation.
Additional emerging EPO response circuits
In the context of erythroid neoplasia, JAK2 hyperactivation due to a V617F mutation (within an inhibitory pseudokinase domain) is a frequently contributing factor.79 Polycythemia vera V617F EPCs can develop in the absence of EPO, although at lower efficiencies.80 In a Ba/F3 cell model, JAK2V617F’s transforming potential is also promoted by EPOR (or thrombopoietin receptor [MPL]) expression.81 For JAK2 R867Q or S755R/R938Q mutations (as associated primarily with thrombocytosis), however, transformation is supported by MPL and not the EPOR. These findings implicate selective EPOR (and MPL) interactions with mutated JAK2 alleles in a context of myeloproliferative disease. JAK2’s degradation interestingly has been demonstrated to involve VHL-mediated ubiquitination. This is illustrated in Chuvash polycythemia in which the VHL mutation R200W alters properties of VHL-SOCS1 E3 ligase complexes, and limits activated phospho-JAK2 turnover.82
Although EPO/EPOR effects at large require JAK2, supporting roles for Src family kinases are also emerging. Murine EPCs deficient in Lyn exhibit diminished EPO-dependent erythroblast formation.83 And Src, but not Jak2, may mediate posttranslational modification of Cbl, a ubiquitin ligase which promotes EPOR down-modulation (as studied in F-36P cells).84 Mouse KO models additionally have revealed nonredundant Stat5-independent roles for phospholipase-cγ1 (Plc-γ1) in promoting EPOR/Jak2 signals for EPC development.85 In oncology contexts, promising inhibitors of SRC are being developed that additionally can affect Plc-γ1 and/or RAS circuits.86,87 For these agents, potential compromising effects on erythropoiesis should also be considered.
Via EPOR/Jak2/Stat5 signaling, EPO can also induce cytokine expression by EPCs,88 with the C1q/TNF cytokine family member erythroferrone (ERFE/Ctrp-15) as a new example.89 Specifically, ERFE has been discovered to act on hepatocytes to suppress hepcidin production, thereby lessening hepcidin’s inhibitory effects on iron efflux from enterocytes, hepatocytes, and macrophages.90 Following phlebotomy, ERFE expression is heightened, with ERFE-KO mice exhibiting delayed recovery from blood-loss–induced anemia. By comparison, the disruption of ERFE in β-thalassemia mice diminishes iron overload.89 ERFE modulation therefore has therapeutic potential for balancing systemic iron levels. Unexpectedly, EPO has also been shown to exert effects on pluripotent hematopoietic progenitor cells. Specifically, EPO at elevated levels can alter the transcriptomes of multi- and bi-potent progenitors.91 This generates lineage bias, and increases erythroid output while decreasing myelopoiesis.91 EPO therefore may guide EPC differentiation, as previously implicated in studies of EPO/EPOR-stimulated Akt phosphorylation of Gata1.92 Such EPO actions might contribute to rhEPO’s enhancement of erythroid recovery following allogeneic transplant.93
Summary
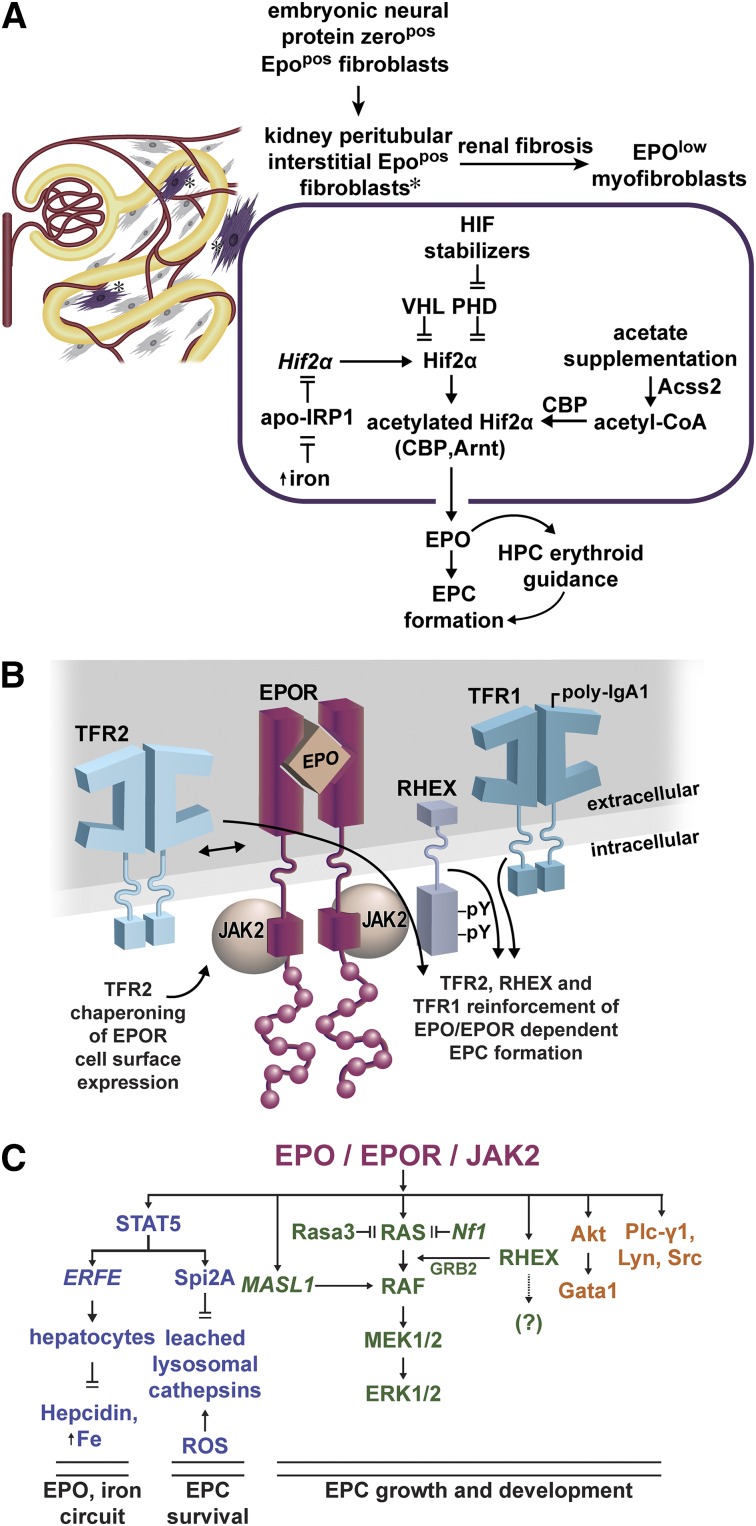
Within EPO and EPOR circuits, important new components are being revealed. Several regulate endogenous EPO expression (Figure 1A). Apo-IRP1 inhibits Hif2α transcript translation,31 whereas iron reverses this effect, heightening HIF2α and EPO levels. Pharmacologically, PHD inhibitors that stabilize HIF2α can likewise increase EPO expression,34 as does acetate supplementation via enhancement of Hif2α acetylation during stress erythropoiesis.33
Figure 1.
Emerging EPO and EPOR regulators, and action circuits. (A) Regulators of EPO expression in renal peritubular interstitial fibroblasts: during embryogenesis, neural tissue derived EPOpos protein-zeropos fibroblasts occupy interstitial peritubular sites within the neonatal kidney. Renal damage and fibrosis can convert these cells to EPOlow myofibroblasts.24 Within EPOpos interstitial fibroblasts, the EPO production is modulated in part by HIF2α, which itself is regulated at multiple levels. Iron reverses apo-IRP inhibition of HIF2α translation.31 HIF2α turnover is promoted by VHL and PHDs,30 and pharmacologic inhibitors of PHDs stabilize HIF2α.34 During stress erythropoiesis, acetate supplementation can further enhance HIF2α complex acetylation, activity, and EPO production via an Acss2-CBP circuit.33 (B) Modulation of EPOR signaling by interacting plasma membrane proteins: TFR2 associates with the EPOR and can modulate its trafficking.44 Upon p-IgA1 ligation, Tfr1 can also enhance EPOR signaling.48 RHEX also associates with the hEPOR, and promotes EPO-dependent human erythroblast formation.47 (C) Recently defined EPO/EPOR signal transduction circuits: newly discovered EPO/EPOR response genes include ERFE, Spi2A, and MASL1. As a secreted TNF-related cytokine, ERFE completes a circuit between EPO action, and regulation of systemic iron levels.89 By inhibiting leached lysosomal cathepsins, Spi2A cytoprotects erythroblasts against consequences of oxidative damage.71 MASL1 acts within a central RAS/MEK/ERK circuit,63 together with RHEX, to reinforce ERK1/2 activation.47 Further dynamic balancing of essential RAS/MEK/ERK signals (and of EPC formation) occurs via RAS down-modulation by Rasa356 and Nf1.57 Pro-erythropoietic effects also are being established for Akt, Plc-γ1, Lyn, and Src kinases. Akt can affect erythroid development via serine phosphorylation of Gata1,92 whereas Lyn and Src can act to enhance EPO/EPOR activated growth/development signals,83,84 and to modulate Cbl’s E3 ligase effects on EPOR turnover.74,75 For each of these EPO/EPOR signal transducers, their engagement and actions appear to become especially important during anemia and/or stress erythropoiesis.
Within EPCs, EPOR activity can be unexpectedly augmented by interactions with several plasma membrane proteins (Figure 1B). TRF2 acts via association with EPOR complexes, whereas Tfr1 is engaged upon p-IgA1 ligation, with each bolstering EPC formation.44,48 This ties two iron importers to EPOR’s effects. In addition, the novel hEPC protein RHEX associates with the hEPOR, enhances ERK1/2 activation, and supports erythroblast development.47
Important downstream EPOR signal transducers are also being discovered (Figure 1C). Within a central RAS/MEK module, these include MASL1,63 Rasa3,56 and neurofibrin,57 that act to balance ERK1/2 signaling and EPC production. For EPC cytoprotection, an EPO-induced Spi2A serpin and small molecule inhibitors of leached lysosomal cathepsins have emerged that lessen ROS-associated damage. Pro-erythropoietic actions of Akt, Plc-γ1, and Src family kinases are also being more clearly defined. Finally, EPO is proving to exert guiding effects on early hematopoietic progenitors,91 pointing to new EPO target populations (and indicating an ability of EPO to affect cells harboring few EPORs). High merit therefore persists for continued investigations of novel EPO/EPOR action mechanisms.
Acknowledgments
This study was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute grant R01 HL044491 and National Insitute of Diabetes and Digestive and Kidney Diseases grant R01 DK089439 (D.M.W.) and National Heart, Lung, and Blood Institute grant F32 HL120596 (D.K.).
Authorship
Contribution: D.K. and D.M.W. contributed in substantial ways to review concepts and construction.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Don M. Wojchowski, Center of Excellence in Stem and Progenitor Cell Biology and Regenerative Medicine, Maine Medical Center Research Institute, 81 Research Drive, Scarborough, ME 04074; e-mail: wojchd@mmc.org.
References
- 1.Reissmann KR. Studies on the mechanism of erythropoietic stimulation in parabiotic rats during hypoxia. Blood. 1950;5(4):372–380. [PubMed] [Google Scholar]
- 2.Miyake T, Kung CK, Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977;252(15):5558–5564. [PubMed] [Google Scholar]
- 3.Jacobs K, Shoemaker C, Rudersdorf R, et al. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature. 1985;313(6005):806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- 4.Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology and the American Society of Clinical Oncology Practice Guideline Update Committee. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood. 2010;116(20):4045–4059. doi: 10.1182/blood-2010-08-300541. [DOI] [PubMed] [Google Scholar]
- 5.D’Andrea AD, Lodish HF, Wong GG. Expression cloning of the murine erythropoietin receptor. Cell. 1989;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- 6.Constantinescu SN, Wu H, Liu X, Beyer W, Fallon A, Lodish HF. The anemic Friend virus gp55 envelope protein induces erythroid differentiation in fetal liver colony-forming units-erythroid. Blood. 1998;91(4):1163–1172. [PubMed] [Google Scholar]
- 7.Witthuhn BA, Quelle FW, Silvennoinen O, et al. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 8.Livnah O, Johnson DL, Stura EA, et al. An antagonist peptide-EPO receptor complex suggests that receptor dimerization is not sufficient for activation. Nat Struct Biol. 1998;5(11):993–1004. doi: 10.1038/2965. [DOI] [PubMed] [Google Scholar]
- 9.Sokol L, Luhovy M, Guan Y, Prchal JF, Semenza GL, Prchal JT. Primary familial polycythemia: a frameshift mutation in the erythropoietin receptor gene and increased sensitivity of erythroid progenitors to erythropoietin. Blood. 1995;86(1):15–22. [PubMed] [Google Scholar]
- 10.Bartnicki P, Kowalczyk M, Rysz J. The influence of the pleiotropic action of erythropoietin and its derivatives on nephroprotection. Med Sci Monit. 2013;19:599–605. doi: 10.12659/MSM.889023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debeljak N, Solár P, Sytkowski AJ. Erythropoietin and cancer: the unintended consequences of anemia correction. Front Immunol. 2014;5:563. doi: 10.3389/fimmu.2014.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mastromarino V, Musumeci MB, Conti E, Tocci G, Volpe M. Erythropoietin in cardiac disease: effective or harmful? J Cardiovasc Med (Hagerstown) 2013;14(12):870–878. doi: 10.2459/JCM.0b013e328362c6ae. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen AQ, Cherry BH, Scott GF, Ryou MG, Mallet RT. Erythropoietin: powerful protection of ischemic and post-ischemic brain. Exp Biol Med (Maywood) 2014;239(11):1461–1475. doi: 10.1177/1535370214523703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchis-Gomar F, Garcia-Gimenez JL, Pareja-Galeano H, Romagnoli M, Perez-Quilis C, Lippi G. Erythropoietin and the heart: physiological effects and the therapeutic perspective. Int J Cardiol. 2014;171(2):116–125. doi: 10.1016/j.ijcard.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Di L, Noguchi CT. Erythropoietin, a novel versatile player regulating energy metabolism beyond the erythroid system. Int J Biol Sci. 2014;10(8):921–939. doi: 10.7150/ijbs.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Wang L, Dey S, et al. Erythropoietin action in stress response, tissue maintenance and metabolism. Int J Mol Sci. 2014;15(6):10296–10333. doi: 10.3390/ijms150610296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott S, Sinclair A, Collins H, Rice L, Jelkmann W. Progress in detecting cell-surface protein receptors: the erythropoietin receptor example. Ann Hematol. 2014;93(2):181–192. doi: 10.1007/s00277-013-1947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carraway MS, Suliman HB, Jones WS, Chen CW, Babiker A, Piantadosi CA. Erythropoietin activates mitochondrial biogenesis and couples red cell mass to mitochondrial mass in the heart. Circ Res. 2010;106(11):1722–1730. doi: 10.1161/CIRCRESAHA.109.214353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mowat FM, Gonzalez F, Luhmann UF, et al. Endogenous erythropoietin protects neuroretinal function in ischemic retinopathy. Am J Pathol. 2012;180(4):1726–1739. doi: 10.1016/j.ajpath.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar SM, Zhang G, Bastian BC, et al. Erythropoietin receptor contributes to melanoma cell survival in vivo. Oncogene. 2012;31(13):1649–1660. doi: 10.1038/onc.2011.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teng R, Gavrilova O, Suzuki N, et al. Disrupted erythropoietin signalling promotes obesity and alters hypothalamus proopiomelanocortin production. Nat Commun. 2011;2:520. doi: 10.1038/ncomms1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki N, Hirano I, Pan X, Minegishi N, Yamamoto M. Erythropoietin production in neuroepithelial and neural crest cells during primitive erythropoiesis. Nat Commun. 2013;4:2902. doi: 10.1038/ncomms3902. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi Y, Hayashi A, Campagnoni CW, Kimura A, Inuzuka T, Baba H. L-MPZ, a novel isoform of myelin P0, is produced by stop codon readthrough. J Biol Chem. 2012;287(21):17765–17776. doi: 10.1074/jbc.M111.314468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asada N, Takase M, Nakamura J, et al. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest. 2011;121(10):3981–3990. doi: 10.1172/JCI57301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan X, Suzuki N, Hirano I, Yamazaki S, Minegishi N, Yamamoto M. Isolation and characterization of renal erythropoietin-producing cells from genetically produced anemia mice. PLoS ONE. 2011;6(10):e25839. doi: 10.1371/journal.pone.0025839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki N, Obara N, Pan X, et al. Specific contribution of the erythropoietin gene 3′ enhancer to hepatic erythropoiesis after late embryonic stages. Mol Cell Biol. 2011;31(18):3896–3905. doi: 10.1128/MCB.05463-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rankin EB, Wu C, Khatri R, et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell. 2012;149(1):63–74. doi: 10.1016/j.cell.2012.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bunn HF. Erythropoietin. Cold Spring Harb Perspect Med. 2013;3(3):a011619. doi: 10.1101/cshperspect.a011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scortegagna M, Ding K, Zhang Q, et al. HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood. 2005;105(8):3133–3140. doi: 10.1182/blood-2004-05-1695. [DOI] [PubMed] [Google Scholar]
- 30.Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27(1):41–53. doi: 10.1016/j.blre.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson SA, Nizzi CP, Chang YI, et al. The IRP1-HIF-2α axis coordinates iron and oxygen sensing with erythropoiesis and iron absorption. Cell Metab. 2013;17(2):282–290. doi: 10.1016/j.cmet.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen R, Xu M, Hogg RT, et al. The acetylase/deacetylase couple CREB-binding protein/Sirtuin 1 controls hypoxia-inducible factor 2 signaling. J Biol Chem. 2012;287(36):30800–30811. doi: 10.1074/jbc.M111.244780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu M, Nagati JS, Xie J, et al. An acetate switch regulates stress erythropoiesis. Nat Med. 2014;20(9):1018–1026. doi: 10.1038/nm.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnan M, Nissenson AR. New alternatives in anemia treatment: biosimilars and HIF stabilizers. Nephrol News Issues. 2014;28(6):24–28. [PubMed] [Google Scholar]
- 35.Broudy VC, Lin N, Brice M, Nakamoto B, Papayannopoulou T. Erythropoietin receptor characteristics on primary human erythroid cells. Blood. 1991;77(12):2583–2590. [PubMed] [Google Scholar]
- 36.Zon LI, Youssoufian H, Mather C, Lodish HF, Orkin SH. Activation of the erythropoietin receptor promoter by transcription factor GATA-1. Proc Natl Acad Sci USA. 1991;88(23):10638–10641. doi: 10.1073/pnas.88.23.10638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng D, Kan YW. The binding of the ubiquitous transcription factor Sp1 at the locus control region represses the expression of beta-like globin genes. Proc Natl Acad Sci USA. 2005;102(28):9896–9900. doi: 10.1073/pnas.0502041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lacombe J, Krosl G, Tremblay M, et al. Genetic interaction between Kit and Scl. Blood. 2013;122(7):1150–1161. doi: 10.1182/blood-2011-01-331819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker V, Schilling M, Bachmann J, et al. Covering a broad dynamic range: information processing at the erythropoietin receptor. Science. 2010;328(5984):1404–1408. doi: 10.1126/science.1184913. [DOI] [PubMed] [Google Scholar]
- 40.Singh S, Verma R, Pradeep A, et al. Dynamic ligand modulation of EPO receptor pools, and dysregulation by polycythemia-associated EPOR alleles. PLoS ONE. 2012;7(1):e29064. doi: 10.1371/journal.pone.0029064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sulahian R, Cleaver O, Huang LJ. Ligand-induced EpoR internalization is mediated by JAK2 and p85 and is impaired by mutations responsible for primary familial and congenital polycythemia. Blood. 2009;113(21):5287–5297. doi: 10.1182/blood-2008-09-179572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bulut GB, Sulahian R, Yao H, Huang LJ. Cbl ubiquitination of p85 is essential for Epo-induced EpoR endocytosis. Blood. 2013;122(24):3964–3972. doi: 10.1182/blood-2013-05-506212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer L, Deau B, Forejtníková H, et al. beta-Trcp mediates ubiquitination and degradation of the erythropoietin receptor and controls cell proliferation. Blood. 2007;109(12):5215–5222. doi: 10.1182/blood-2006-10-055350. [DOI] [PubMed] [Google Scholar]
- 44.Forejtnikovà H, Vieillevoye M, Zermati Y, et al. Transferrin receptor 2 is a component of the erythropoietin receptor complex and is required for efficient erythropoiesis. Blood. 2010;116(24):5357–5367. doi: 10.1182/blood-2010-04-281360. [DOI] [PubMed] [Google Scholar]
- 45.Nai A, Lidonnici MR, Rausa M, et al. The second transferrin receptor regulates red blood cell production in mice. Blood. 2015;125(7):1170–1179. doi: 10.1182/blood-2014-08-596254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Chloupková M. Abnormal iron uptake and liver cancer. Cancer Biol Ther. 2009;8(18):1699–1708. doi: 10.4161/cbt.8.18.9146. [DOI] [PubMed] [Google Scholar]
- 47.Verma R, Su S, McCrann DJ, et al. RHEX, a novel regulator of human erythroid progenitor cell expansion and erythroblast development. J Exp Med. 2014;211(9):1715–1722. doi: 10.1084/jem.20130624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coulon S, Dussiot M, Grapton D, et al. Polymeric IgA1 controls erythroblast proliferation and accelerates erythropoiesis recovery in anemia. Nat Med. 2011;17(11):1456–1465. doi: 10.1038/nm.2462. [DOI] [PubMed] [Google Scholar]
- 49.Wu J, Ji C, Xie F, et al. FcalphaRI (CD89) alleles determine the proinflammatory potential of serum IgA. J Immunol. 2007;178(6):3973–3982. doi: 10.4049/jimmunol.178.6.3973. [DOI] [PubMed] [Google Scholar]
- 50.Dussiot M, Maciel TT, Fricot A, et al. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in β-thalassemia. Nat Med. 2014;20(4):398–407. doi: 10.1038/nm.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arcasoy MO, Jiang X. Co-operative signalling mechanisms required for erythroid precursor expansion in response to erythropoietin and stem cell factor. Br J Haematol. 2005;130(1):121–129. doi: 10.1111/j.1365-2141.2005.05580.x. [DOI] [PubMed] [Google Scholar]
- 52.Khalaf WF, White H, Wenning MJ, Orazi A, Kapur R, Ingram DA. K-Ras is essential for normal fetal liver erythropoiesis. Blood. 2005;105(9):3538–3541. doi: 10.1182/blood-2004-05-2021. [DOI] [PubMed] [Google Scholar]
- 53.Braun BS, Archard JA, Van Ziffle JA, Tuveson DA, Jacks TE, Shannon K. Somatic activation of a conditional KrasG12D allele causes ineffective erythropoiesis in vivo. Blood. 2006;108(6):2041–2044. doi: 10.1182/blood-2006-01-013490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, Liu Y, Beard C, et al. Expression of oncogenic K-ras from its endogenous promoter leads to a partial block of erythroid differentiation and hyperactivation of cytokine-dependent signaling pathways. Blood. 2007;109(12):5238–5241. doi: 10.1182/blood-2006-09-047050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Larghero J, Gervais N, Cassinat B, et al. Farnesyltransferase inhibitor tipifarnib (R115777) preferentially inhibits in vitro autonomous erythropoiesis of polycythemia vera patient cells. Blood. 2005;105(9):3743–3745. doi: 10.1182/blood-2004-07-2949. [DOI] [PubMed] [Google Scholar]
- 56.Blanc L, Ciciotte SL, Gwynn B, et al. Critical function for the Ras-GTPase activating protein RASA3 in vertebrate erythropoiesis and megakaryopoiesis. Proc Natl Acad Sci USA. 2012;109(30):12099–12104. doi: 10.1073/pnas.1204948109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang T, Krisman K, Theobald EH, et al. Sustained MEK inhibition abrogates myeloproliferative disease in Nf1 mutant mice. J Clin Invest. 2013;123(1):335–339. doi: 10.1172/JCI63193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubiolo C, Piazzolla D, Meissl K, et al. A balance between Raf-1 and Fas expression sets the pace of erythroid differentiation. Blood. 2006;108(1):152–159. doi: 10.1182/blood-2005-09-3866. [DOI] [PubMed] [Google Scholar]
- 59.Wannatung T, Lithanatudom P, Leecharoenkiat A, Svasti S, Fucharoen S, Smith DR. Increased erythropoiesis of beta-thalassaemia/Hb E proerythroblasts is mediated by high basal levels of ERK1/2 activation. Br J Haematol. 2009;146(5):557–568. doi: 10.1111/j.1365-2141.2009.07794.x. [DOI] [PubMed] [Google Scholar]
- 60.Bélanger LF, Roy S, Tremblay M, et al. Mek2 is dispensable for mouse growth and development. Mol Cell Biol. 2003;23(14):4778–4787. doi: 10.1128/MCB.23.14.4778-4787.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menon MP, Fang J, Wojchowski DM. Core erythropoietin receptor signals for late erythroblast development. Blood. 2006;107(7):2662–2672. doi: 10.1182/blood-2005-02-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guihard S, Clay D, Cocault L, et al. The MAPK ERK1 is a negative regulator of the adult steady-state splenic erythropoiesis. Blood. 2010;115(18):3686–3694. doi: 10.1182/blood-2009-09-242487. [DOI] [PubMed] [Google Scholar]
- 63.Kumkhaek C, Aerbajinai W, Liu W, et al. MASL1 induces erythroid differentiation in human erythropoietin-dependent CD34+ cells through the Raf/MEK/ERK pathway. Blood. 2013;121(16):3216–3227. doi: 10.1182/blood-2011-10-385252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalfa TA, Zheng Y. Rho GTPases in erythroid maturation. Curr Opin Hematol. 2014;21(3):165–171. doi: 10.1097/MOH.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arai A, Kanda E, Miura O. Rac is activated by erythropoietin or interleukin-3 and is involved in activation of the Erk signaling pathway. Oncogene. 2002;21(17):2641–2651. doi: 10.1038/sj.onc.1205346. [DOI] [PubMed] [Google Scholar]
- 66.Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: mission possible? Nat Rev Drug Discov. 2014;13(11):828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koury MJ, Bondurant MC. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248(4953):378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- 68.Koulnis M, Porpiglia E, Porpiglia PA, et al. Contrasting dynamic responses in vivo of the Bcl-xL and Bim erythropoietic survival pathways. Blood. 2012;119(5):1228–1239. doi: 10.1182/blood-2011-07-365346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abutin RM, Chen J, Lung TK, Lloyd JA, Sawyer ST, Harada H. Erythropoietin-induced phosphorylation/degradation of BIM contributes to survival of erythroid cells. Exp Hematol. 2009;37(2):151–158. doi: 10.1016/j.exphem.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98(2):181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 71.Dev A, Byrne SM, Verma R, Ashton-Rickardt PG, Wojchowski DM. Erythropoietin-directed erythropoiesis depends on serpin inhibition of erythroblast lysosomal cathepsins. J Exp Med. 2013;210(2):225–232. doi: 10.1084/jem.20121762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rhodes MM, Kopsombut P, Bondurant MC, Price JO, Koury MJ. Bcl-x(L) prevents apoptosis of late-stage erythroblasts but does not mediate the antiapoptotic effect of erythropoietin. Blood. 2005;106(5):1857–1863. doi: 10.1182/blood-2004-11-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Repnik U, Hafner Cesen M, Turk B. Lysosomal membrane permeabilization in cell death: concepts and challenges. Mitochondrion. 2014;19(pt A):49–57. doi: 10.1016/j.mito.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 74.De Franceschi L, Bertoldi M, Matte A, et al. Oxidative stress and β-thalassemic erythroid cells behind the molecular defect. Oxid Med Cell Longev. 2013;2013:985210. doi: 10.1155/2013/985210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sangokoya C, Telen MJ, hi JT. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood. 2010;116(20):4338–4348. doi: 10.1182/blood-2009-04-214817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koulnis M, Liu Y, Hallstrom K, Socolovsky M. Negative autoregulation by Fas stabilizes adult erythropoiesis and accelerates its stress response. PLoS ONE. 2011;6(7):e21192. doi: 10.1371/journal.pone.0021192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carlile GW, Smith DH, Wiedmann M. A non-apoptotic role for Fas/FasL in erythropoiesis. FEBS Lett. 2009;583(4):848–854. doi: 10.1016/j.febslet.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 78.An X, Schulz VP, Li J, et al. Global transcriptome analyses of human and murine terminal erythroid differentiation. Blood. 2014;123(22):3466–3477. doi: 10.1182/blood-2014-01-548305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 80.Jedidi A, Marty C, Oligo C, et al. Selective reduction of JAK2V617F-dependent cell growth by siRNA/shRNA and its reversal by cytokines. Blood. 2009;114(9):1842–1851. doi: 10.1182/blood-2008-09-176875. [DOI] [PubMed] [Google Scholar]
- 81.Marty C, Saint-Martin C, Pecquet C, et al. Germ-line JAK2 mutations in the kinase domain are responsible for hereditary thrombocytosis and are resistant to JAK2 and HSP90 inhibitors. Blood. 2014;123(9):1372–1383. doi: 10.1182/blood-2013-05-504555. [DOI] [PubMed] [Google Scholar]
- 82.Russell RC, Sufan RI, Zhou B, et al. Loss of JAK2 regulation via a heterodimeric VHL-SOCS1 E3 ubiquitin ligase underlies Chuvash polycythemia. Nat Med. 2011;17(7):845–853. doi: 10.1038/nm.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karur VG, Lowell CA, Besmer P, Agosti V, Wojchowski DM. Lyn kinase promotes erythroblast expansion and late-stage development. Blood. 2006;108(5):1524–1532. doi: 10.1182/blood-2005-09-008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shintani T, Ohara-Waki F, Kitanaka A, Tanaka T, Kubota Y. Cbl negatively regulates erythropoietin-induced growth and survival signaling through the proteasomal degradation of Src kinase. Blood Cells Mol Dis. 2014;53(4):211–218. doi: 10.1016/j.bcmd.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 85.Schnöder TM, Arreba-Tutusaus P, Griehl I, et al. Epo-induced erythroid maturation is dependent on Plcγ1 signaling [published online ahead of print November 14, 1014]. Cell Death Differ. doi: 10.1038/cdd.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Girotti MR, Lopes F, Preece N, et al. Paradox-breaking RAF inhibitors that also target SRC are effective in drug-resistant BRAF mutant melanoma. Cancer Cell. 2015;27(1):85–96. doi: 10.1016/j.ccell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Lavallade H, Khoder A, Hart M, et al. Tyrosine kinase inhibitors impair B-cell immune responses in CML through off-target inhibition of kinases important for cell signaling. Blood. 2013;122(2):227–238. doi: 10.1182/blood-2012-11-465039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Menon MP, Karur V, Bogacheva O, Bogachev O, Cuetara B, Wojchowski DM. Signals for stress erythropoiesis are integrated via an erythropoietin receptor-phosphotyrosine-343-Stat5 axis. J Clin Invest. 2006;116(3):683–694. doi: 10.1172/JCI25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rochette L, Gudjoncik A, Guenancia C, Zeller M, Cottin Y, Vergely C. The iron-regulatory hormone hepcidin: a possible therapeutic target? Pharmacol Ther. 2015;146:35–52. doi: 10.1016/j.pharmthera.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 91.Grover A, Mancini E, Moore S, et al. Erythropoietin guides multipotent hematopoietic progenitor cells toward an erythroid fate. J Exp Med. 2014;211(2):181–188. doi: 10.1084/jem.20131189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao W, Kitidis C, Fleming MD, Lodish HF, Ghaffari S. Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway. Blood. 2006;107(3):907–915. doi: 10.1182/blood-2005-06-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hellström-Lindberg E, van de Loosdrecht A. Erythropoiesis stimulating agents and other growth factors in low-risk MDS. Best Pract Res Clin Haematol. 2013;26(4):401–410. doi: 10.1016/j.beha.2013.09.007. [DOI] [PubMed] [Google Scholar]