Abstract
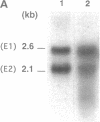
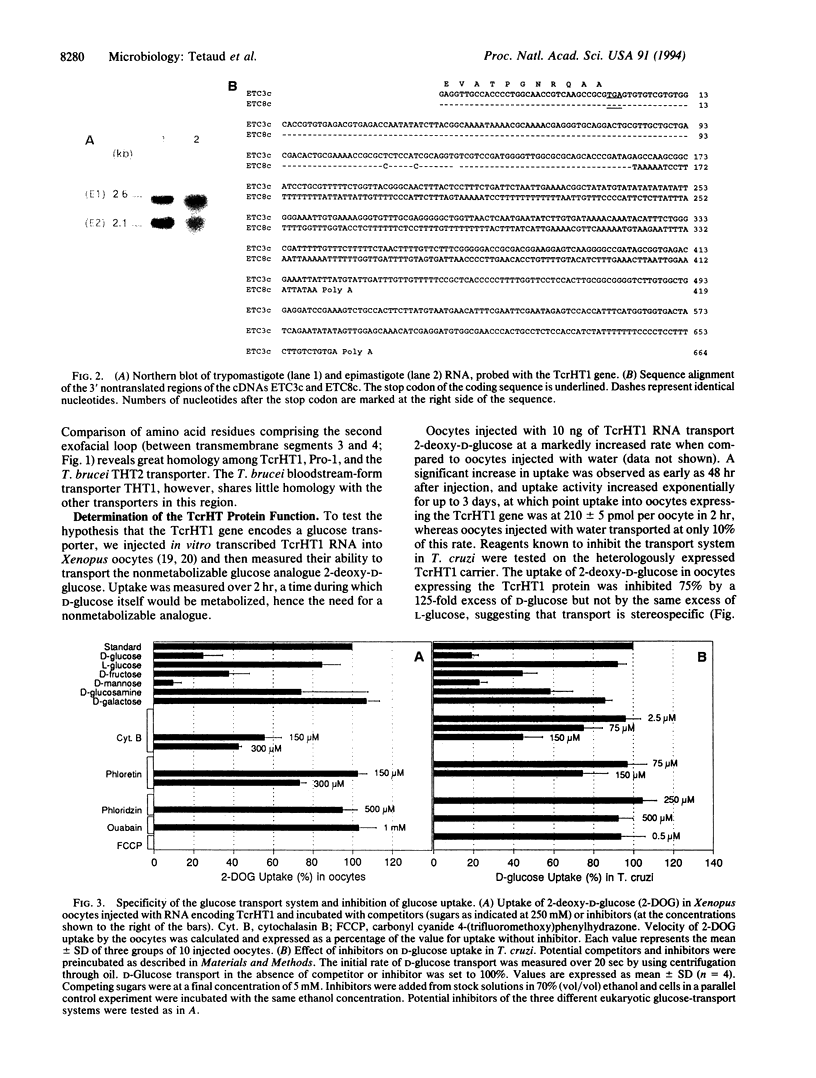
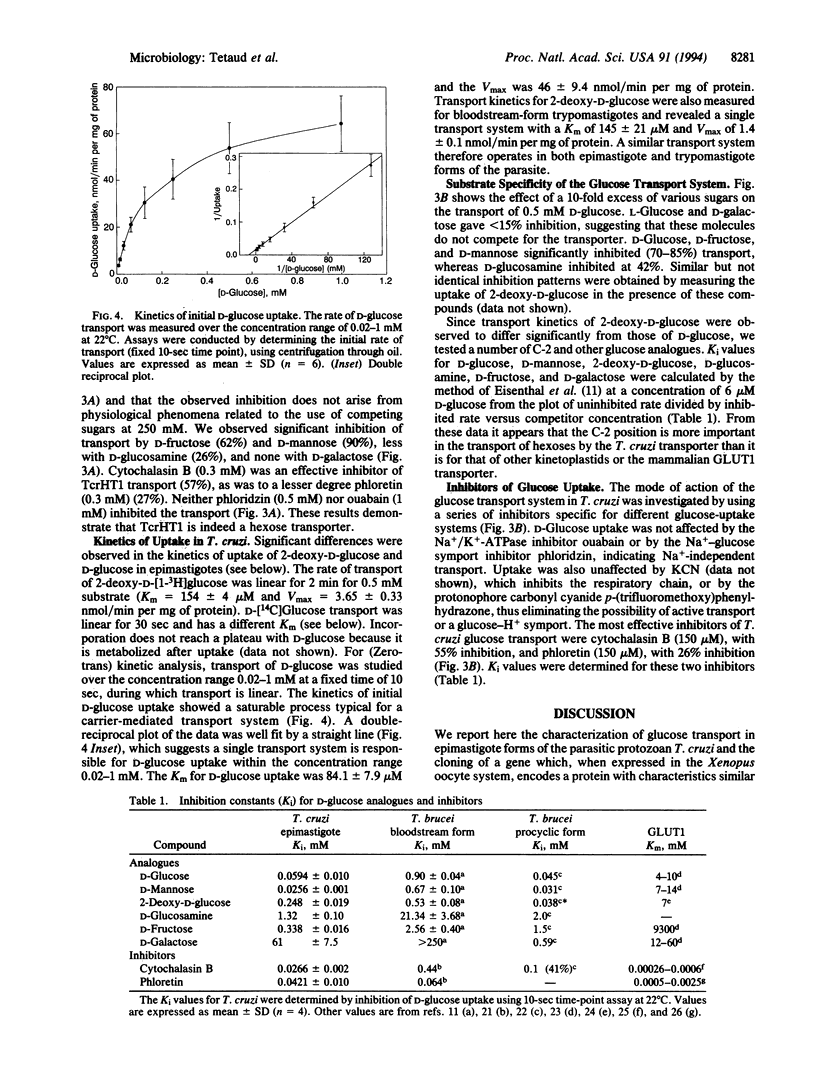
A gene from Trypanosoma cruzi, TcrHT1, which encodes a member of the glucose transporter superfamily has been cloned. The gene is similar in sequence to the T. brucei hexose transporter THT1 and the Leishmania transporter Pro-1 and is present in the T. cruzi genome as a cluster of at least eight tandemly reiterated copies. Northern blot analysis revealed two mRNA transcripts which differ in size with respect to their 3' untranslated regions. When injected with in vitro transcribed TcrHT1 mRNA, Xenopus oocytes express a hexose transporter with properties similar to those of T. cruzi. Glucose transport in T. cruzi is mediated via a carrier with unique properties when compared with the other glucose transporters already characterized among the Kinetoplastida. It is a facilitated transporter with a high affinity for D-glucose (Km = 84.1 +/- 7.9 microM and Vmax = 46 +/- 9.4 nmol/min per mg of protein) that shares with other kinetoplastid hexose transporters the ability to recognize D-fructose, which distinguishes these carriers from the human erythrocyte glucose transporter GLUT1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin S. A. Mammalian passive glucose transporters: members of an ubiquitous family of active and passive transport proteins. Biochim Biophys Acta. 1993 Jun 8;1154(1):17–49. doi: 10.1016/0304-4157(93)90015-g. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Bringaud F., Baltz T. A potential hexose transporter gene expressed predominantly in the bloodstream form of Trypanosoma brucei. Mol Biochem Parasitol. 1992 May;52(1):111–121. doi: 10.1016/0166-6851(92)90040-q. [DOI] [PubMed] [Google Scholar]
- Bringaud F., Baltz T. African trypanosome glucose transporter genes: organization and evolution of a multigene family. Mol Biol Evol. 1994 Mar;11(2):220–230. doi: 10.1093/oxfordjournals.molbev.a040104. [DOI] [PubMed] [Google Scholar]
- Bringaud F., Baltz T. Differential regulation of two distinct families of glucose transporter genes in Trypanosoma brucei. Mol Cell Biol. 1993 Feb;13(2):1146–1154. doi: 10.1128/mcb.13.2.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns B. R., Collard M. W., Landfear S. M. Developmentally regulated gene from Leishmania encodes a putative membrane transport protein. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7682–7686. doi: 10.1073/pnas.86.20.7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville C. A., Seatter M. J., Jess T. J., Gould G. W., Thomas H. M. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochem J. 1993 Mar 15;290(Pt 3):701–706. doi: 10.1042/bj2900701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Game S., Holman G. D. Specificity and kinetics of hexose transport in Trypanosoma brucei. Biochim Biophys Acta. 1989 Oct 2;985(1):81–89. doi: 10.1016/0005-2736(89)90107-7. [DOI] [PubMed] [Google Scholar]
- Fry A. J., Towner P., Holman G. D., Eisenthal R. Transport of D-fructose and its analogues by Trypanosoma brucei. Mol Biochem Parasitol. 1993 Jul;60(1):9–18. doi: 10.1016/0166-6851(93)90023-q. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Bell G. I. Facilitative glucose transporters: an expanding family. Trends Biochem Sci. 1990 Jan;15(1):18–23. doi: 10.1016/0968-0004(90)90125-u. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Holman G. D. The glucose transporter family: structure, function and tissue-specific expression. Biochem J. 1993 Oct 15;295(Pt 2):329–341. doi: 10.1042/bj2950329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Solomon A. K. Interaction between phloretin and the red blood cell membrane. J Gen Physiol. 1976 Apr;67(4):381–397. doi: 10.1085/jgp.67.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C. Y., Rampal A. L. Cytochalasin B binding sites and glucose transport carrier in human erythrocyte ghosts. J Biol Chem. 1977 Aug 10;252(15):5456–5463. [PubMed] [Google Scholar]
- Knodler L. A., Schofield P. J., Edwards M. R. Glucose transport in Crithidia luciliae. Mol Biochem Parasitol. 1992 Nov;56(1):1–13. doi: 10.1016/0166-6851(92)90149-e. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Langford C. K., Ewbank S. A., Hanson S. S., Ullman B., Landfear S. M. Molecular characterization of two genes encoding members of the glucose transporter superfamily in the parasitic protozoan Leishmania donovani. Mol Biochem Parasitol. 1992 Oct;55(1-2):51–64. doi: 10.1016/0166-6851(92)90126-5. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R., Borst P. Localization of nine glycolytic enzymes in a microbody-like organelle in Trypanosoma brucei: the glycosome. FEBS Lett. 1977 Aug 15;80(2):360–364. doi: 10.1016/0014-5793(77)80476-6. [DOI] [PubMed] [Google Scholar]
- Parsons M., Nielsen B. Active transport of 2-deoxy-D-glucose in Trypanosoma brucei procyclic forms. Mol Biochem Parasitol. 1990 Sep-Oct;42(2):197–203. doi: 10.1016/0166-6851(90)90162-f. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., Thomas J. A., Twomey C. E. The growth of Trypanosoma cruzi in human diploid cells for the production of trypomastigotes. Parasitology. 1980 Feb;80(1):153–162. doi: 10.1017/s0031182000000615. [DOI] [PubMed] [Google Scholar]
- Ter Kuile B. H., Opperdoes F. R. Glucose uptake by Trypanosoma brucei. Rate-limiting steps in glycolysis and regulation of the glycolytic flux. J Biol Chem. 1991 Jan 15;266(2):857–862. [PubMed] [Google Scholar]
- Ter Kuile B. H., Opperdoes F. R. Uptake and turnover of glucose in Leishmania donovani. Mol Biochem Parasitol. 1993 Aug;60(2):313–321. doi: 10.1016/0166-6851(93)90142-k. [DOI] [PubMed] [Google Scholar]
- Vera J. C., Rosen O. M. Functional expression of mammalian glucose transporters in Xenopus laevis oocytes: evidence for cell-dependent insulin sensitivity. Mol Cell Biol. 1989 Oct;9(10):4187–4195. doi: 10.1128/mcb.9.10.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J. C., Rosen O. M. Reconstitution of an insulin signaling pathway in Xenopus laevis oocytes: coexpression of a mammalian insulin receptor and three different mammalian hexose transporters. Mol Cell Biol. 1990 Feb;10(2):743–751. doi: 10.1128/mcb.10.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Dwyer D. M. Protonmotive force-driven active transport of D-glucose and L-proline in the protozoan parasite Leishmania donovani. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1716–1720. doi: 10.1073/pnas.82.6.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D. Transport of nutrients and ions across membranes of trypanosomatid parasites. Adv Parasitol. 1993;32:261–291. doi: 10.1016/s0065-308x(08)60209-2. [DOI] [PubMed] [Google Scholar]
- ter Kuile B. H., Opperdoes F. R. Mutual adjustment of glucose uptake and metabolism in Trypanosoma brucei grown in a chemostat. J Bacteriol. 1992 Feb;174(4):1273–1279. doi: 10.1128/jb.174.4.1273-1279.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]