Abstract
JC virus (JCV) causes progressive multifocal leukoencephalopathy (PML), the fatal demyelinating infection of oligodendrocytes, in up to 5% of AIDS patients. An intron-differential RNA PCR was developed to study the expression of alternately spliced JCV early mRNAs in brain tissues from PML patients with and without AIDS and in JCV-induced hamster brain tumors. The method utilizes primers that span the large tumor (T) and small tumor (t) antigen introns allowing amplification of specific cDNAs in the presence of contaminating viral genomic DNA. Hybridization with specific junctional probes and DNA sequence analysis confirmed the identity of the PCR products. Sequencing showed that JCV early mRNA is alternatively spliced as previously predicted by analogy to simian virus 40. Large T antigen mRNA was detected in all the brain tissues from PML patients with and without AIDS. The expression of small t antigen mRNA varied depending upon the association of PML with AIDS and upon other unknown factors. Of the 12 PML/AIDS brain tissue samples, 11 (92%) expressed small t antigen mRNA, whereas only 8 of 13 (62%) brain samples from patients with PML alone showed detectable levels of small t antigen mRNA. Human immunodeficiency virus 1 proviral DNA was detected in 10 of 12 PML/AIDS brain samples. The results indicate that alternative splicing of JCV early mRNA is regulated in the human brain and that the production of small t antigen may not be essential for the pathogenesis of PML.
Full text
PDF

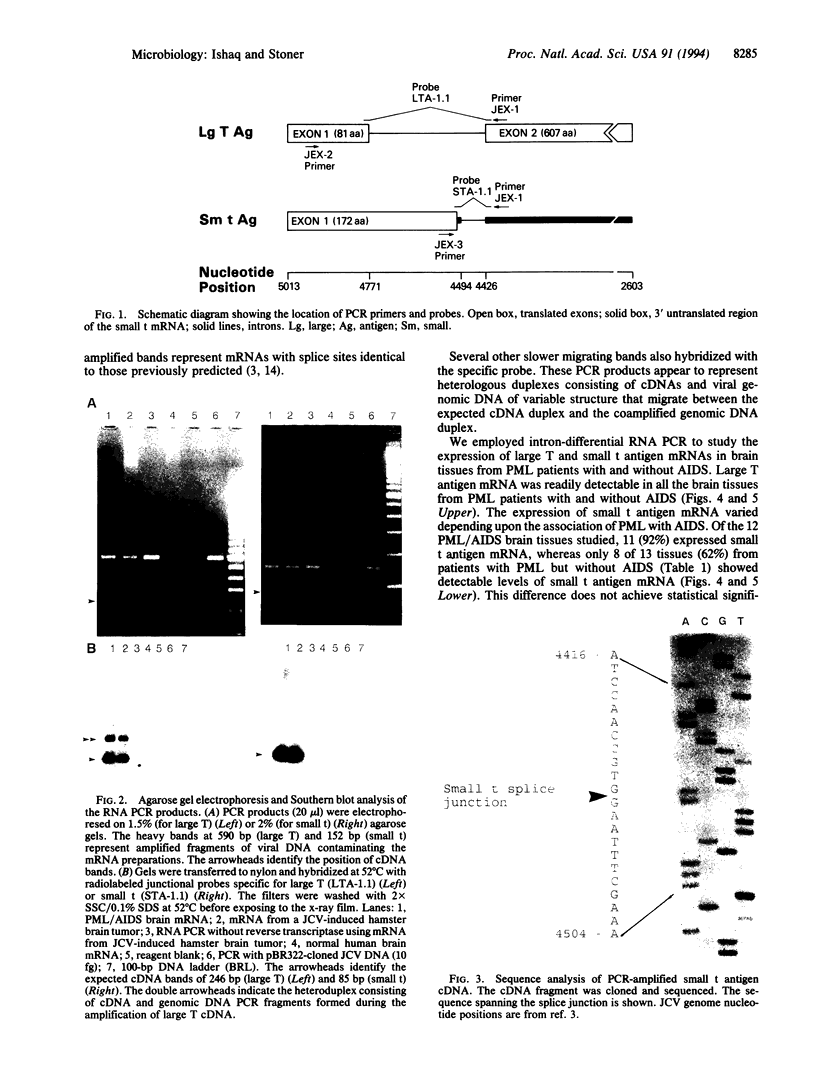


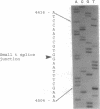
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ault G. S., Stoner G. L. Human polyomavirus JC promoter/enhancer rearrangement patterns from progressive multifocal leukoencephalopathy brain are unique derivatives of a single archetypal structure. J Gen Virol. 1993 Aug;74(Pt 8):1499–1507. doi: 10.1099/0022-1317-74-8-1499. [DOI] [PubMed] [Google Scholar]
- Ault G. S., Stoner G. L. Two major types of JC virus defined in progressive multifocal leukoencephalopathy brain by early and late coding region DNA sequences. J Gen Virol. 1992 Oct;73(Pt 10):2669–2678. doi: 10.1099/0022-1317-73-10-2669. [DOI] [PubMed] [Google Scholar]
- Berger J. R., Levy R. M. The neurologic complications of human immunodeficiency virus infection. Med Clin North Am. 1993 Jan;77(1):1–23. doi: 10.1016/s0025-7125(16)30269-3. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikel I., Loeken M. R. Involvement of simian virus 40 (SV40) small t antigen in trans activation of SV40 early and late promoters. J Virol. 1992 Mar;66(3):1489–1494. doi: 10.1128/jvi.66.3.1489-1494.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikel I., Montano X., Agha M. E., Brown M., McCormack M., Boltax J., Livingston D. M. SV40 small t antigen enhances the transformation activity of limiting concentrations of SV40 large T antigen. Cell. 1987 Jan 30;48(2):321–330. doi: 10.1016/0092-8674(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Chowdhury M., Taylor J. P., Chang C. F., Rappaport J., Khalili K. Evidence that a sequence similar to TAR is important for induction of the JC virus late promoter by human immunodeficiency virus type 1 Tat. J Virol. 1992 Dec;66(12):7355–7361. doi: 10.1128/jvi.66.12.7355-7361.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury M., Taylor J. P., Tada H., Rappaport J., Wong-Staal F., Amini S., Khalili K. Regulation of the human neurotropic virus promoter by JCV-T antigen and HIV-1 tat protein. Oncogene. 1990 Dec;5(12):1737–1742. [PubMed] [Google Scholar]
- Elsner C., Dörries K. Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology. 1992 Nov;191(1):72–80. doi: 10.1016/0042-6822(92)90167-n. [DOI] [PubMed] [Google Scholar]
- Epstein L. G., Gendelman H. E. Human immunodeficiency virus type 1 infection of the nervous system: pathogenetic mechanisms. Ann Neurol. 1993 May;33(5):429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]
- Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J. Regulatory sequences and virus-cell interactions of JC virus. Prog Clin Biol Res. 1983;105:41–59. [PubMed] [Google Scholar]
- Fu X. Y., Manley J. L. Factors influencing alternative splice site utilization in vivo. Mol Cell Biol. 1987 Feb;7(2):738–748. doi: 10.1128/mcb.7.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H., Manley J. L. A protein factor, ASF, controls cell-specific alternative splicing of SV40 early pre-mRNA in vitro. Cell. 1990 Jul 13;62(1):25–34. doi: 10.1016/0092-8674(90)90236-8. [DOI] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Conway G. C., Kozak D. The essential pre-mRNA splicing factor SF2 influences 5' splice site selection by activating proximal sites. Cell. 1990 Jul 13;62(1):35–42. doi: 10.1016/0092-8674(90)90237-9. [DOI] [PubMed] [Google Scholar]
- Loeken M., Bikel I., Livingston D. M., Brady J. trans-activation of RNA polymerase II and III promoters by SV40 small t antigen. Cell. 1988 Dec 23;55(6):1171–1177. doi: 10.1016/0092-8674(88)90261-9. [DOI] [PubMed] [Google Scholar]
- Major E. O., Amemiya K., Tornatore C. S., Houff S. A., Berger J. R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992 Jan;5(1):49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura T., Jikuya H., Soeda E., Yoshiike K. Genomic structure of human polyoma virus JC: nucleotide sequence of the region containing replication origin and small-T-antigen gene. J Virol. 1983 Jan;45(1):73–79. doi: 10.1128/jvi.45.1.73-79.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Pallas D. C., Shahrik L. K., Martin B. L., Jaspers S., Miller T. B., Brautigan D. L., Roberts T. M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990 Jan 12;60(1):167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- Pipas J. M. Common and unique features of T antigens encoded by the polyomavirus group. J Virol. 1992 Jul;66(7):3979–3985. doi: 10.1128/jvi.66.7.3979-3985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Mumby M. C., Rundell K., Walter G. Dephosphorylation of simian virus 40 large-T antigen and p53 protein by protein phosphatase 2A: inhibition by small-t antigen. Mol Cell Biol. 1991 Apr;11(4):1996–2003. doi: 10.1128/mcb.11.4.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E., Fedorov S., Kamibayashi C., Robbins D., Cobb M., Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993 Dec 3;75(5):887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- Stoner G. L. Polyomavirus models of brain infection and the pathogenesis of multiple sclerosis. Brain Pathol. 1993 Jul;3(3):213–227. doi: 10.1111/j.1750-3639.1993.tb00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H., Rappaport J., Lashgari M., Amini S., Wong-Staal F., Khalili K. Trans-activation of the JC virus late promoter by the tat protein of type 1 human immunodeficiency virus in glial cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3479–3483. doi: 10.1073/pnas.87.9.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takechi H., Hosokawa N., Hirayoshi K., Nagata K. Alternative 5' splice site selection induced by heat shock. Mol Cell Biol. 1994 Jan;14(1):567–575. doi: 10.1128/mcb.14.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp W. C. Variable defectiveness for lytic growth of the dl 54/59 mutants of simian virus 40. J Virol. 1980 Mar;33(3):1208–1210. doi: 10.1128/jvi.33.3.1208-1210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnav Y. N., Wong-Staal F. The biochemistry of AIDS. Annu Rev Biochem. 1991;60:577–630. doi: 10.1146/annurev.bi.60.070191.003045. [DOI] [PubMed] [Google Scholar]
- White F. A., 3rd, Ishaq M., Stoner G. L., Frisque R. J. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J Virol. 1992 Oct;66(10):5726–5734. doi: 10.1128/jvi.66.10.5726-5734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. I., Lickteig R. L., Estes R., Rundell K., Walter G., Mumby M. C. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol Cell Biol. 1991 Apr;11(4):1988–1995. doi: 10.1128/mcb.11.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerrahn J., Knippschild U., Winkler T., Deppert W. Independent expression of the transforming amino-terminal domain of SV40 large I antigen from an alternatively spliced third SV40 early mRNA. EMBO J. 1993 Dec;12(12):4739–4746. doi: 10.1002/j.1460-2075.1993.tb06162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]