Abstract
Background and Purpose
Kaempferol, a plant flavonoid present in normal human diet, can modulate vasomotor tone. The present study aimed to elucidate the signalling pathway through which this flavonoid enhanced relaxation of vascular smooth muscle.
Experimental Approach
The effect of kaempferol on the relaxation of porcine coronary arteries to endothelium-dependent (bradykinin) and -independent (sodium nitroprusside) relaxing agents was studied in an in vitro organ chamber setup. The whole-cell patch-clamp technique was used to determine the effect of kaempferol on potassium channels in porcine coronary artery smooth muscle cells (PCASMCs).
Key Results
At a concentration without direct effect on vascular tone, kaempferol (3 × 10−6 M) enhanced relaxations produced by bradykinin and sodium nitroprusside. The potentiation by kaempferol of the bradykinin-induced relaxation was not affected by Nω-nitro-L-arginine methyl ester, an inhibitor of NO synthase (10−4 M) or TRAM-34 plus UCL 1684, inhibitors of intermediate- and small-conductance calcium-activated potassium channels, respectively (10−6 M each), but was abolished by tetraethylammonium chloride, a non-selective inhibitor of calcium-activated potassium channels (10−3 M), and iberiotoxin, a selective inhibitor of large-conductance calcium-activated potassium channel (KCa1.1; 10−7 M). Iberiotoxin also inhibited the potentiation by kaempferol of sodium nitroprusside-induced relaxations. Kaempferol stimulated an outward-rectifying current in PCASMCs, which was abolished by iberiotoxin.
Conclusions and Implications
The present results suggest that, in smooth muscle cells of the porcine coronary artery, kaempferol enhanced relaxations caused by endothelium-derived and exogenous NO as well as those due to endothelium-dependent hyperpolarization. This vascular effect of kaempferol involved the activation of KCa1.1 channels.
Tables of Links
| TARGETS |
|---|
| Ion channelsa |
| KCa1.1, large-conductance calcium-activated potassium channel |
| KCa2.3, small-conductance calcium-activated potassium channel |
| KCa3.1, intermediate-conductance calcium-activated potassium channel |
| Enzymesb |
| PKA |
| Nuclear hormone receptorsc |
| Oestrogen receptor (NR3A2) |
| LIGANDS | |
|---|---|
| Apamin | TEA, tetraethylammonium chloride |
| Apigenin | TRAM-34 |
| Bradykinin | U46619 |
| Charybdotoxin | UCL 1684 |
| Iberiotoxin | |
| Indomethacin | |
| L-NAME | |
| Quercetin |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a b cAlexander et al., 2013a, b, c).
Introduction
Kaempferol is a flavonoid present in many plants (including tea, grapes and mulberry) (Olszewska, 2008; Chao et al., 2013; Nile et al., 2013). It is also one of the major bioactive components of the Chinese herbal medicine Carthamus tinctorius (Fan et al., 2009) used for the management of various cardiovascular disorders including thrombosis, hypertension and coronary artery disease. In vitro, kaempferol causes endothelium-independent relaxations in both conductance and resistance arteries of different animals (Duarte et al., 1993; Ho et al., 2002; Pérez-Vizcaíno et al., 2002; Xu et al., 2006). Although this is tempting to assume that this direct dilator effect of kaempferol may contribute to its therapeutic value, the concentration (more than 10−5 M) of the flavonoid required to elicit relaxation may be too high to be clinically relevant.
An average Western diet contains about 0.5 g of flavonoids per day (Manach et al., 1996), of which kaempferol represents about 0.3–0.7% (Hertog et al., 1993; Sampson et al., 2002). The plasma concentration of kaempferol reaches 10−7 M in humans following an oral intake of 9 mg, which is considered to be a relatively low dose (DuPont et al., 2004). The achievable plasma concentration of kaempferol in humans is approximately less than, or at the most, 10−5 M (Xu et al., 2006). Previous work from this laboratory demonstrated that at this concentration, kaempferol, although unable to directly alter vascular tone, enhanced the responses of porcine coronary arteries to various relaxing agents (Xu et al., 2006). This modulatory effect of kaempferol appears to be independent of the presence of the endothelium (Xu et al., 2006), suggesting the added therapeutic value of efficacy under pathological conditions in which the endothelium is dysfunctional. Little is known regarding the cellular targets and signalling pathways involved in the vascular effects of kaempferol. Previous work suggested that kaempferol does not act as an antioxidant to enhance vascular relaxation (Xu et al., 2006). The present study aimed to characterize the signalling pathway(s) responsible for the modulatory effect of lower concentrations (≤10−5 M) of kaempferol in porcine coronary arteries.
Methods
Tissue preparation
Hearts of pigs (of either sex; 50–70 kg) were collected from the local slaughter house. The animals were killed according to the regulations laid down by the Food and Environmental Hygiene Department of the Government of Hong Kong. The hearts were immersed in ice-cold modified Krebs–Henseleit solution (control solution), with the following composition (in 10-3 M): NaCl (120), KCl (4.76), MgSO4 (1.18), CaCl2 (1.25), NaHCO3 (25), NaH2PO4 (1.18) and glucose (5.5); and the right coronary arteries were isolated. Excess fat and connective tissues were removed and the arteries were cut into rings (3 mm wide). The rings were placed in organ chambers filled with 5 mL of the control solution and placed under a resting tension, measured by isometric force transducer (Model FT03, Grass Instrument Co., Quincy, MA, USA), of 2 g. The preparations were allowed to equilibrate for 120 min under oxygenated conditions (95% O2: 5% CO2) at 37°C. A basal tension of 2 g was maintained continuously throughout the equilibration period, during which the control solution was changed every 25 min.
Functional studies
After the equilibration period, a viability test was carried out. Rings were incubated with indomethacin (COX inhibitor; 10−5 M) for 20 min (to prevent prostanoid production). Afterwards, the rings were contracted with U46619; a thromboxane A2 mimetic, (3 × 10−8 M) and then relaxed with bradykinin (10−6 M). Rings contracting to U46619 with more than 4 g and relaxing to bradykinin by more than 80% were included in the study. They were allowed to return to the basal tension while changing the control solution every 15 min until the actual experiment.
All experiments were carried out in the presence of indomethacin (10−5 M), which was added to the rings 30 min before other pharmacological treatments. The effect of several flavonoids with similar chemical structures (Figure 1) on relaxations was investigated by incubating rings with kaempferol (3 × 10−6 and 10−5 M), quercetin (10−5 M), myricetin (10−5 M), rutin (10−5 M) or apigenin (10−5 M) for 30 min, before contracting them with U46619 (3 × 10−8 M). Rings incubated with the corresponding amount of vehicle (ethanol, 0.1%) were used as controls. After a stable and sustained contraction was achieved with U46619, the relaxing agent, bradykinin (10−11 to 10−6 M) or sodium nitroprusside (10−9 to 10−4 M), was added to the rings in cumulative manner to obtain concentration–relaxation curves. In order to determine the mechanism of actions of kaempferol, some porcine coronary arterial rings were incubated with Nω-nitro-L-arginine methyl ester (L-NAME, inhibitor of NOSs, 10−4 M; Ng et al., 2008), 1H-[1,2,4]oxadiazolo[4,2-a] quinoxalin-1-one (ODQ, inhibitor of soluble GC, 10−5 M; Chan et al., 2011), iberiotoxin [inhibitor of large-conductance calcium-activated potassium channel (KCa1.1), 10−7 M; Liang et al., 2010 ], charybdotoxin [inhibitor of KCa1.1 and intermediate-conductance calcium-activated potassium channel (KCa3.1), 10−7 M; Ng et al., 2008 ], 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34, inhibitor of KCa3.1, 10−6 M; Yeung et al., 2013), 6,12,19,20,25,26-hexahydro-5,27:13,18:21,24-trietheno-11,7-metheno-7H-dibenzo[b,n][1,5,12,16]tetraazacyclotricosine-5,13-diium dibromide (UCL 1684), inhibitor of small-conductance calcium-activated potassium channel (KCa2.3), 10−6 M; Yeung et al., 2013 ], apamin (inhibitor of KCa2.3, 10−6 M; Ng et al., 2008) and/or tetraethylammonium chloride [TEA, non-selective inhibitor of calcium-activated potassium channel (KCa), 10−3 M; Shimizu et al., 2000], in the absence and presence of kaempferol (3 × 10−6 M) for 30 min, before obtaining a sustained contraction with U46619 followed by relaxation to cumulative addition of bradykinin or sodium nitroprusside.
Figure 1.
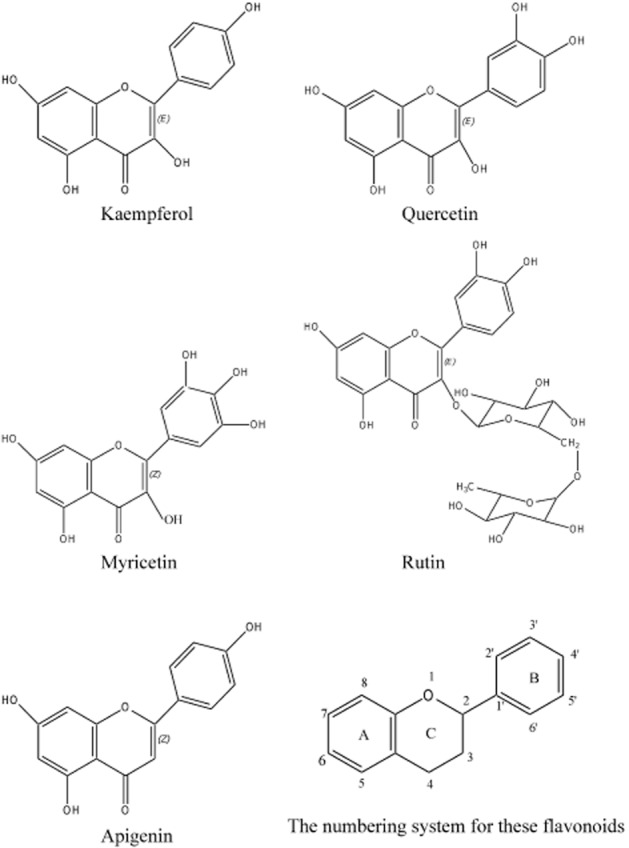
Molecular structure of the flavonoids studied.
Whole-cell patch-clamp study
Porcine coronary artery smooth muscle cells (PCASMCs) were obtained from the American Type culture collection (Manassas, VA, USA) and cultured in Eagle's minimum essential medium supplemented with FBS (10%, v/v), penicillin (100 U·mL−1), streptomycin (10−4 g·mL−1) and insulin growth factor (10−6 g·mL−1) at 37°C in 95% air/5% CO2. During the experiments, the PCASMCs (passages 2–5) were continuously superfused with the control solution at a pH adjusted to 7.4. Patch pipettes (tip resistance 3–5 MΩ) were pulled from glass capillary tubes, fire polished, and filled with an internal solution containing (in 10−3 M) KCl (35), K-gluconate (90), NaCl (10), HEPES (10), and 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA; 10), with pH adjusted to 7.2 with KOH (5 M). GTP (5 × 10−4 M) was added to provide a substrate for the signal transduction pathways. Because the intracellular concentration of calcium ions ([Ca2+]i) may affect potassium channel activity, [Ca2+]i was adjusted to a physiological level (∼7.5 × 10−8 M) by adding CaCl2 (3 × 10−3 M) to the intracellular solution. The [Ca2+]i was estimated using WEBMAXC v2.22 (Stanford University, Stanford, CA, USA; http://www.stanford.edu/~cpatton/maxc.html). MgATP (5 × 10−3 M) was included to inhibit ATP-sensitive potassium currents and to provide a substrate for energy-dependent processes. The composition of the pipette solution is appropriate for the study of Kv channels and KCa channels (Shimoda et al., 1998; 1999,). Seal resistances ranged from 1 to 10 GΩ after seal formation. Whole-cell currents were recorded with an Axopatch-1D amplifier (Axon Instruments, Sunnyvale, CA, USA) in voltage-clamp mode. Voltage-clamp protocols were applied with pClamp 9.0 software (Axon Instruments). Data were filtered at 5 kHz, and collected at a frequency of 50 kHz, digitized with a Digidata 1200 analogue-to-digital converter (Axon Instruments), and analysed with pClamp 9.0 software. The cell capacitance was calculated from the area under the capacitive current elicited by a 10 mV hyperpolarizing pulse from a holding potential of −70 mV. The whole-cell current was normalized to cell capacitance and is expressed as pA/pF. The series resistance was always less than 15MΩ. Capacitance subtraction and series resistance compensation (80%) were used in all recordings. The external solution was changed using a rapid-exchange system with a multibarrel pipette connected to a common orifice positioned 100–200 μM from the cells studied. Complete solution exchange was achieved in less than 1 s. All experiments were conducted at room temperature (22–25°C).
Data analysis
Results are expressed as the mean ± SEM and n represents the number of rings from different pigs used in the experiments. Relaxation was expressed as percentage of decreases in tension from the contracting level induced by U46619 (3 × 10−8 M). The concentrations at which bradykinin and sodium nitroprusside induced 50% of maximal relaxation (EC50) and their maximal relaxation (Emax) were determined with the computer software, GraphPad Prism version 5.00 (La Jolla, CA, USA) using the four-parameter logistic equation (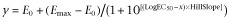 ). Two- and one-way anovas (with post hoc Bonferroni's test), using GraphPad Prism version 5.00, determined significant differences, if any, between concentration–relaxation curves and EC50, respectively, in different treatment groups. P-values less than 0.05 were considered to indicate statistically significant differences.
). Two- and one-way anovas (with post hoc Bonferroni's test), using GraphPad Prism version 5.00, determined significant differences, if any, between concentration–relaxation curves and EC50, respectively, in different treatment groups. P-values less than 0.05 were considered to indicate statistically significant differences.
Materials
U46619 was obtained from Biomol (Plymouth Meeting, PA, USA). All other drugs and chemicals were purchased from Sigma (St. Louis, MO, USA). Stock solutions of bradykinin, sodium nitroprusside L-NAME, iberiotoxin, charybdotoxin, TRAM-34, UCL 1684, apamin and TEA were dissolved in distilled water. For U46619, kaempferol, quercetin, myricetin, rutin and apigenin, ethanol was used as the solvent, and the final concentration of ethanol in the organ chamber solution was always ≤0.1%. Indomethacin was dissolved in sodium carbonate solution (10−3 M). ODQ was dissolved in DMSO. All stock solutions were kept refrigerated and used within 1 week of preparation except bradykinin and indomethacin, which were more stable and were used within 1 month. The control solution was used to make up the final working solutions. The control solution had the following composition (in 10−3 M): NaCl (120), KCl (4.76), MgSO4 (1.18), CaCl2 (1.25), NaHCO3 (25), NaH2PO4 (1.18) and glucose (5.5).
Results
Effects of flavonoids on bradykinin-induced relaxation
Bradykinin caused relaxation in a concentration-dependent manner in porcine coronary arterial rings contracted with U46619 (3 × 10−8 M). Kaempferol [3 × 10−6 and 10−5 M (concentrations at which it did not directly affect vascular tone on its own; Xu et al., 2006)], significantly potentiated relaxations to bradykinin (Figure 2A). There was no significant difference between the potentiating effects of the two concentrations of kaempferol on bradykinin-induced relaxations, as the EC50 of the kinin was similar in the presence of the two concentrations of kaempferol (Table 1). The vehicle, ethanol (0.1%), had no significant effect on bradykinin-induced relaxations when compared with time controls (without any treatment; Table 1).
Figure 2.
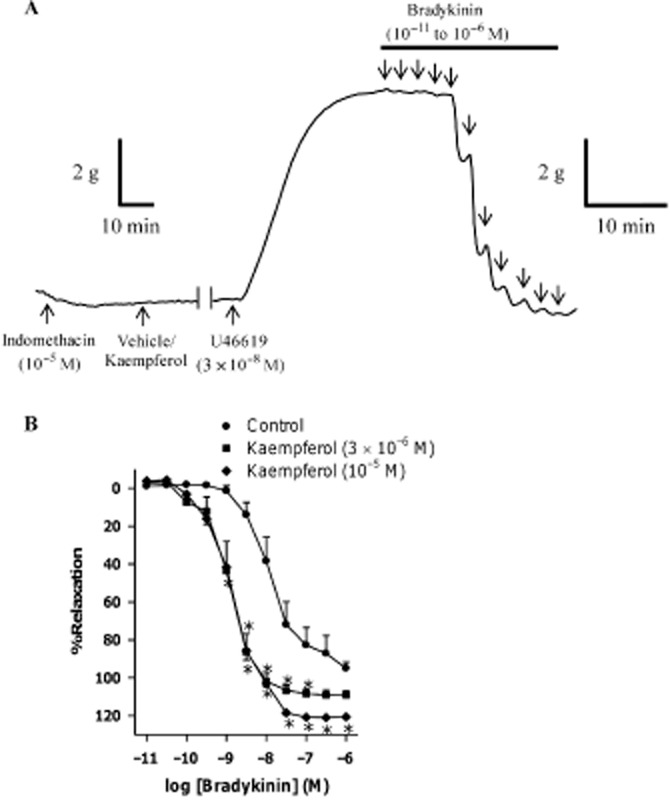
(A) A representative tracing showing responses of porcine coronary arterial rings to U46619 (3 × 10−8 M) followed by cumulative additions, in half-log increments, of bradykinin (10−11 to 10−6 M). (B) Concentration–response curves for bradykinin in the absence or presence of kaempferol. Rings from porcine coronary arteries were incubated with either kaempferol (3 × 10−6 or 10−5 M) or its vehicle control (ethanol, 0.1%) for 30 min. Rings were then contracted with U46619 (3 × 10−8 M) before bradykinin was added cumulatively. n = 8–10. *P < 0.05, significantly different from the control group.
Table 1.
Effects of acute treatment with different flavonoids on the EC50 and Emax values of concentration–relaxation curves of bradykinin (10−11 to 10−6 M) in porcine coronary arteries contracted by U46619 (3 × 10−8 M)
| Treatment | EC50 (log M) | Emax (%) |
|---|---|---|
| Control | −8.3 ± 0.1 | 102 ± 2.2 |
| Ethanol (0.1%) | −8.4 ± 0.1 | 109 ± 2.1 |
| Kaempferol (3 × 10−6 M) | −8.8 ± 0.1* | 112 ± 3.9 |
| Kaempferol (10−5 M) | −8.8 ± 0.1* | 114 ± 2.0* |
| Apigenin (10−5 M) | −8.1 ± 0.1 | 110 ± 1.2 |
| Myricetin (10−5 M) | −7.9 ± 0.2 | 96 ± 3.0 |
| Quercetin (10−5 M) | −8.4 ± 0.1 | 108 ± 1.8 |
| Rutin (10−5 M) | −8.3 ± 0.2 | 97 ± 2.2 |
n = 4–8 in each group.
P < 0.05, significantly different from the control group.
Flavonoids quercetin, myricetin, rutin and apigenin have similar chemical structures to kaempferol (Figure 1). Among those, only quercetin (10−5 M), significantly potentiated bradykinin-induced relaxations (Figure 3); this potentiating effect was smaller than that of kaempferol at the same concentration, as indicated by the absence of effect of quercetin on the EC50 and Emax of bradykinin (Table 1). As kaempferol produced the greatest potentiating effect compared with the other flavonoids tested, it was selected for further investigations of the signalling pathways involved.
Figure 3.
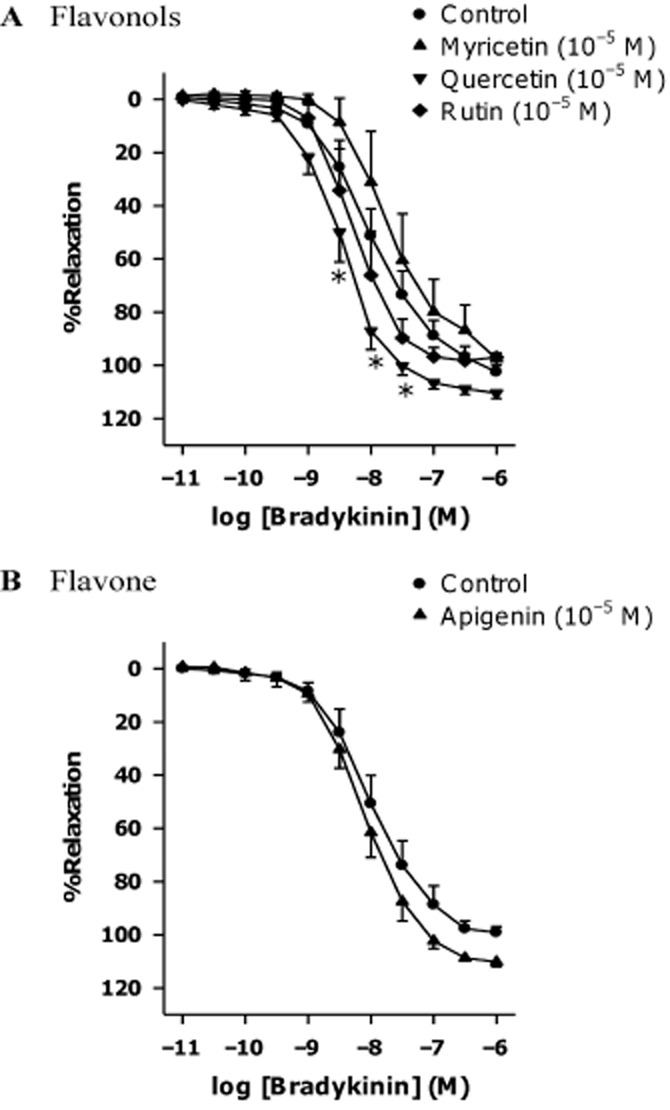
Effects of different flavonoids on bradykinin-induced relaxation. Rings from porcine coronary arteries were incubated with (A) flavonols (quercetin, rutin or myricetin), (B) flavone (apigenin) at the same concentration (10−5 M) or the vehicle control (ethanol, 0.1%) for 30 min. Rings were then contracted with U46619 (3 × 10−8 M) before bradykinin was added cumulatively. n = 4–8. *P < 0.05, significantly different from the control group.
Effects of kaempferol on non-prostanoid, non-NO-mediated bradykinin-induced relaxation
In porcine coronary arterial rings contracted by U46619, incubation with L-NAME (inhibitor of NOS, 10−4 M) or ODQ (inhibitor of soluble GC, 10−5 M) shifted the concentration–relaxation curves of bradykinin to the right (Table 2 and Supporting Information Fig. S1). Under these conditions, kaempferol (3 × 10−6 M) significantly enhanced relaxations to bradykinin (Figures 4A and 4B).
Table 2.
Effects of different pharmacological treatments, in the absence or presence of kaempferol (3 × 10−6 M), on the EC50 and Emax values of concentration–relaxation curves of bradykinin (10−11 to 10−6 M) in porcine coronary arteries contracted by U46619 (3 × 10−8 M)
| Treatment | EC50 (log M) | Emax (%) | ||
|---|---|---|---|---|
| Vehicle (ethanol, 0.1%) | Kaempferol (3 × 10−6 M) | Vehicle (ethanol, 0.1%) | Kaempferol (3 × 10−6 M) | |
| Control | −8.3 ± 0.2 | −8.9 ± 0.1# | 105 ± 3.0 | 106 ± 2.2 |
| L-NAME (10−4 M) | −7.2 ± 0.2* | −7.6 ± 0.2 | 68 ± 9.0* | 79 ± 3.1* |
| ODQ (10−5 M) | −7.3 ± 0.1* | −7.8 ± 0.4 | 73 ± 12* | 81 ± 7.5* |
| TRAM-34 (10−6 M) + UCL 1684 (10−6 M) | −8.2 ± 0.1 | −8.7 ± 0.1# | 94 ± 4.0 | 107 ± 4.2 |
| Iberiotoxin (3 × 10−7 M) | −8.1 ± 0.2 | −8.3 ± 0.2 | 100 ± 1.3 | 100 ± 1.7 |
n = 6–8 in each group.
P < 0.05 versus control group
P < 0.05 versus respective group without kaempferol. Iberiotoxin, inhibitor of large-conductance calcium-activated potassium channels; L-NAME, inhibitor of NOS; ODQ, inhibitor of soluble GC; TRAM-34, inhibitor of intermediate-conductance calcium-activated potassium channels; UCL 1684, inhibitor of small-conductance calcium-activated potassium channels.
Figure 4.
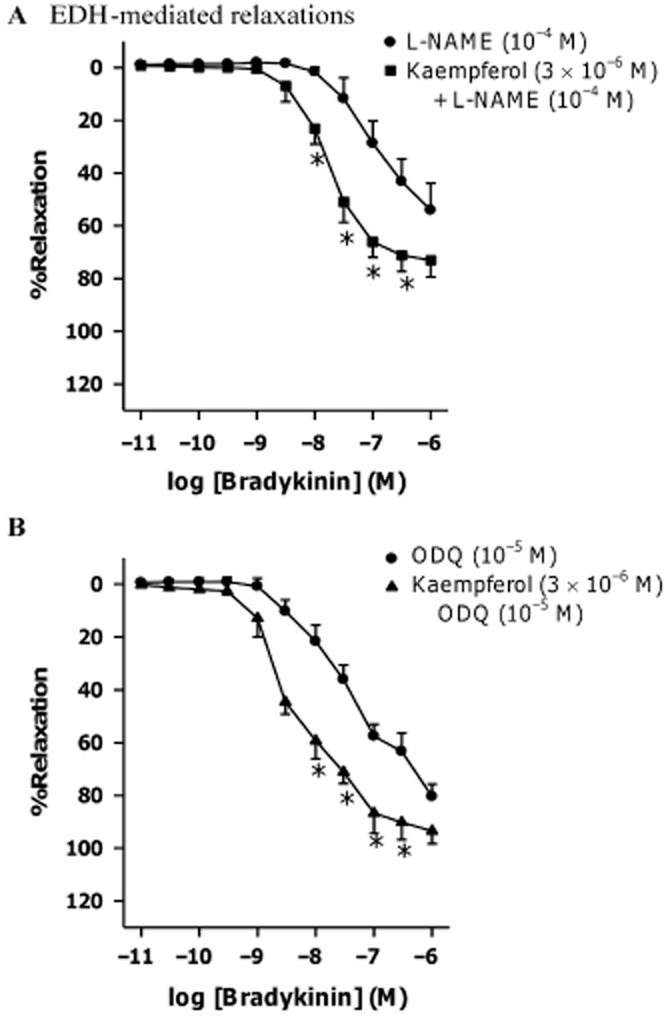
Effect of kaempferol on bradykinin-induced relaxations in the presence of (A) NOS inhibitor or (B) GC inhibitor. Rings from porcine coronary arteries were incubated with L-NAME (10−4 M) or ODQ (10−5 M), in the presence of kaempferol (3 × 10−6 M) or the vehicle control (ethanol, 0.1%) for 30 min. Rings were then contracted with U46619 (3 × 10−8 M) before bradykinin was added cumulatively. n = 6–7. *P < 0.05, significantly different from the group without kaempferol.
Effects of potassium channel blockers on the kaempferol-induced potentiations of bradykinin-induced relaxation
The combination of TRAM-34 (KCa3.1 inhibitor, 10−6 M) plus UCL 1684 (KCa2.3 inhibitor, 10−6 M) or charybdotoxin (non-selective KCa1.1 and KCa3.1 inhibitor, 10−7 M) plus apamin (KCa2.3 inhibitor, 10−6 M) reduced bradykinin-induced relaxations (Supporting Information Figs S2A and S3A respectively). While the combination of TRAM-34 plus UCL 1684 had no effect on the potentiation of bradykinin-induced relaxation by kaempferol (3 × 10−6 M; Figure 5A), this potentiation was no longer observed in the presence of charybdotoxin plus apamin (Supporting Information Fig. S3A). Both iberiotoxin (KCa1.1 inhibitor, 10−7 M) and TEA (non-selective KCa inhibitor, 10−3 M) did not affect bradykinin-induced relaxations (Supporting Information Figs S2B and S3B, respectively), but instead abolished the potentiating effect of kaempferol (Figure 5B and Supporting Information Fig. S3B respectively). The EC50s and Emax of bradykinin in inducing relaxation in the presence of different potassium channel blockers are shown in Table 2. In the combined presence of L-NAME, TRAM-34 plus UCL 1684, the bradykinin-induced relaxation was abolished (Supporting Information Fig. S4).
Figure 5.
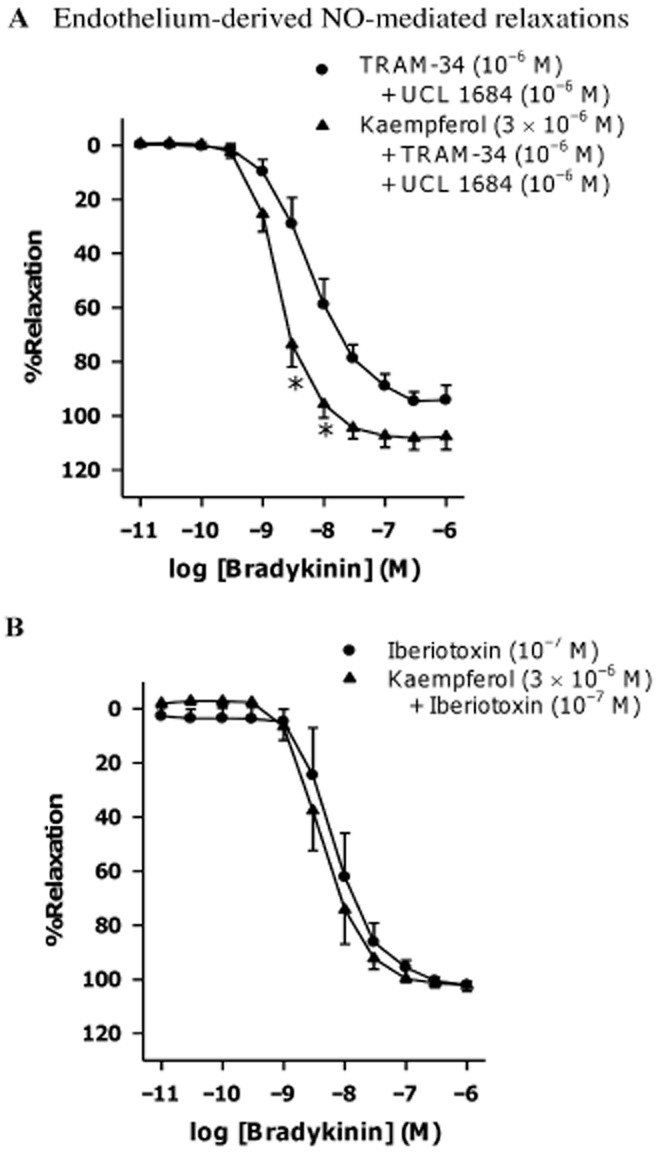
Effect of kaempferol on bradykinin-induced relaxations in the absence or presence of (A) intermediate- plus small-conductance calcium-activated potassium channel inhibitors or (B) large-conductance calcium-activated potassium channel inhibitor. Rings from porcine coronary arteries were incubated with TRAM-34 (10−6 M) plus UCL 1684 (10−6 M) or iberiotoxin (10−7 M), in the presence of kaempferol (3 × 10−6 M) or the vehicle control (ethanol, 0.1%) for 30 min. Rings were then contracted with U46619 (3 × 10−8 M) before bradykinin was added cumulatively. n = 7–8. *P < 0.05, significantly different from the group without kaempferol.
Effects of potassium channel blockers on kaempferol-induced potentiations of sodium nitroprusside-induced relaxation
Sodium nitroprusside (an NO donor) caused concentration-dependent relaxation in porcine coronary arterial rings contracted with U46619 (3 × 10−8 M). Kaempferol (3 × 10−6 M) potentiated this relaxation (Figure 6A and Table 3) and this potentiating effect was inhibited by iberiotoxin (10−7 M; Figure 6B and Table 3), which did not significantly affect sodium nitroprusside-induced relaxations (Table 3 and Supporting Information Fig. S5).
Figure 6.
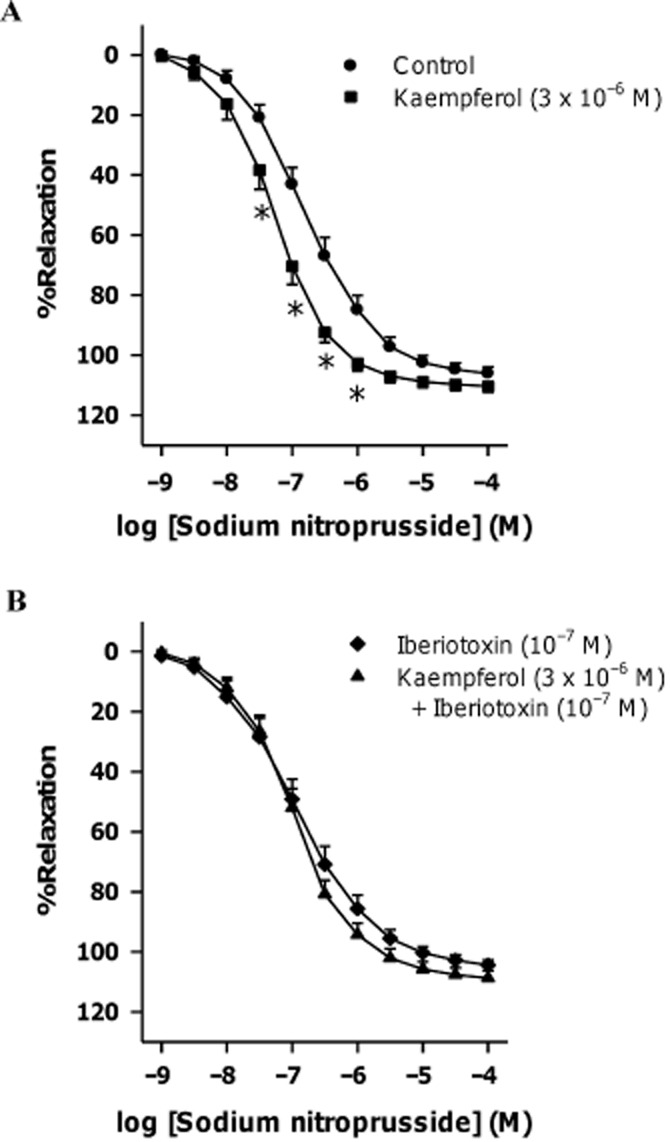
Concentration–response curves for sodium nitroprusside in the absence or presence of (A) kaempferol or (B) large-conductance calcium-activated potassium channel inhibitor with or without kaempferol. Rings from porcine coronary arteries were incubated with kaempferol (3 × 10−6 M), iberiotoxin (10−7 M) together with kaempferol (3 × 10−6 M) or the vehicle control (ethanol, 0.1%) for 30 min. Rings were then contracted with U46619 (3 × 10−8 M) before sodium nitroprusside was added cumulatively. n = 5–6. *P < 0.05, significantly different from the control group.
Table 3.
Effects of iberiotoxin, an inhibitor of large-conductance calcium-activated potassium channels, in the absence or presence of kaempferol (3 × 10−6 M), on the EC50 and Emax values of concentration–relaxation curves of sodium nitroprusside (10−9 to 10−4 M) in porcine coronary arteries contracted by U46619 (3 × 10−8 M)
| Treatment | EC50 (log M) | Emax (%) | ||
|---|---|---|---|---|
| Vehicle (ethanol, 0.1%) | Kaempferol (3 × 10−6 M) | Vehicle (ethanol, 0.1%) | Kaempferol (3 × 10−6 M) | |
| Control | −6.8 ± 0.1 | −7.3 ± 0.1* | 106 ± 2.3 | 110 ± 2.1 |
| Iberiotoxin (10−7 M) | −6.9 ± 0.1 | −7.0 ± 0.1 | 105 ± 1.8 | 109 ± 2.9 |
n = 5–6 in each group.
P < 0.05 versus control group.
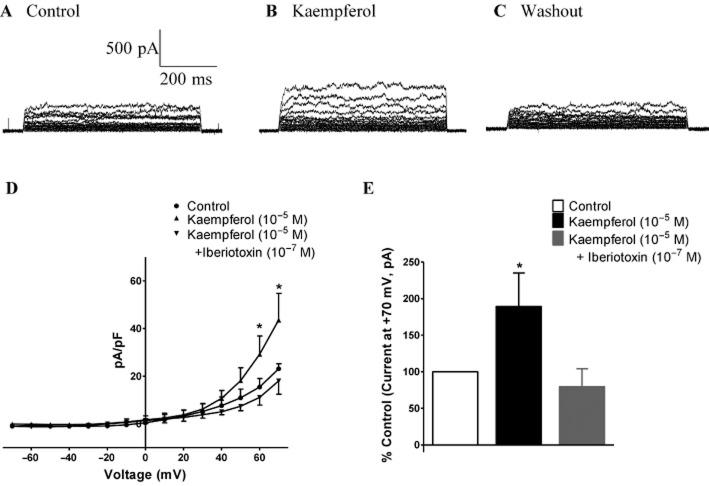
Effects of kaempferol on potassium currents in PCASMCs
The average cell capacitance of PCASMCs was 35.23 ± 4.22 pF (n = 3). Step depolarizations from a holding potential of −70 mV to test potentials between −70 and +70 mV elicited a voltage-dependent outward current (Figures 7A and 7D). Kaempferol (10−5 M) increased this outward current only at positive potentials (Figures 7B and 7D). This effect of kaempferol occurred within 10 s and was readily reversed upon washout of the flavonoid (Figure 7C). The current at the end of the pulse (recorded at the holding potential of +70 mV) was significantly greater in the presence than in the absence of kaempferol (10−5 M; Figure 7E). The outward current induced by kaempferol (10−5 M) in PCASMCs was abolished by iberiotoxin (10−7 M; Figures 7D and 7E).
Figure 7.
Effect of kaempferol on whole-cell current in PCASMC. Whole-cell currents were studied at different voltages generated by stepwise depolarization, in steps of 10 mV, from −70 to +70 mV with a holding potential of −70 mV. Currents were recorded under (A) control conditions, (B) in the presence of kaempferol (10−5 M) and (C) after washout of kaempferol. (D) Average current–voltage relationships, with the current normalized to cell capacitance and expressed as pA/pF, under control conditions and in the presence of kaempferol (10−5 M) with or without iberiotoxin (large-conductance calcium-activated potassium channel inhibitor, 10−7 M), and (E) percentage increase from the control condition of the current recorded at the end of the pulse at +70 mV. n = 3–6. *P < 0.05, significantly different from the control group.
Discussion and conclusions
The present data confirm previous findings that kaempferol, at clinically relevant concentrations, enhances endothelium-dependent relaxations to bradykinin in the porcine coronary artery (Xu et al., 2006). This potentiation is not selective for kaempferol, but is also observed, albeit to a lesser extent, with quercetin, but not with other flavonoids of similar molecular structure. The potentiating effect of kaempferol was observed for both NO-dependent and -independent relaxations, and was mediated by activation of KCa1.1 channels in the coronary vascular smooth muscle cells.
Kaempferol belongs to the superfamily of flavonoids, which can be subdivided into different families according to their molecular structures. A previous study demonstrated that some of these flavonoids caused relaxation in porcine coronary artery at high concentrations (>10−5 M) and the efficacy of this relaxation correlated with the molecular structure (Xu et al., 2007). In the present study, lower concentrations of kaempferol and quercetin, but not myricetin, rutin and apigenin, potentiated bradykinin-induced relaxations. Kaempferol, quercetin, myricetin and rutin belong to the flavonol class of flavonoids. The major differences among them is the number of hydroxyl groups on the B-ring [kaempferol has a single B-ring hydroxyl group (4′ position); quercetin and rutin have two and myricetin has three (Figure 1)] and the presence of glycosylation in rutin (Figure 1). As such, increased hydroxylation in the B-ring or presence of a bulky oligosaccharide apparently reduces the efficacy of the flavonoids in potentiating the response to bradykinin. This structure–activity relationship was also observed for the direct relaxing effect of these flavonols at high concentrations (>10−5 M; Xu et al., 2007). By contrast, apigenin, which, unlike kaempferol, does not possess a hydroxyl group at the 3 position of the C-ring, does not potentiate bradykinin-induced relaxation at 10−5 M, but is as effective as kaempferol in inducing relaxation at higher concentrations.
Among the flavonoids tested, kaempferol was the most effective in potentiating relaxation to bradykinin in isolated porcine coronary arteries. Thus, kaempferol may have the greatest therapeutic potential in the prevention or treatment of cardiovascular disorders. Cardiovascular diseases are associated with endothelial dysfunction, in which the synthesis or bioavailability of endothelium-derived NO is greatly reduced (Cardillo and Panza, 1998; Félétou and Vanhoutte, 2006; Vanhoutte et al., 2009). The present data indicate that the beneficial vascular action of kaempferol persists when NO biosynthesis was inhibited by L-NAME. Furthermore, inhibition with ODQ of soluble GC, the downstream molecular target of NO, did not affect the enhancement of relaxation by kaempferol. These findings suggest that the NO signalling pathway does not play a role in the modulatory effect of the flavonoid.
In the presence of indomethacin and L-NAME, bradykinin causes relaxation mediated by endothelium-dependent hyperpolarization (EDH) in porcine coronary arteries (Cowan and Cohen, 1991; Leung et al., 1997; Ng et al., 2008). As kaempferol potentiated bradykinin-induced relaxations under these conditions, it must also facilitate EDH-mediated relaxations. On the other hand, inhibition of EDH component by the combination of TRAM-34 and UCL 1684 in the presence of indomethacin did not affect the potentiation by kaempferol of bradykinin-induced relaxations, in the absence of L-NAME. The concentrations of TRAM-34 and UCL 1684 used in the present study are sufficient to abolish the EDH component, as they can abolish the bradykinin-induced relaxation that is resistant to indomethacin and L-NAME (Supporting Information Fig. S4). Therefore, kaempferol enhances endothelium-dependent relaxation whether mediated by NO or EDH. Moreover, kaempferol also enhances endothelium-independent relaxations to the NO donor, sodium nitroprusside. These findings suggest that, to enhance relaxation, kaempferol does not interfere with the signalling cascades that result in the release of endothelium-derived NO or the initiation of EDH.
The potentiating effect of kaempferol depends on the activation of KCa1.1 channels, which do not play a significant role in the relaxations to bradykinin and sodium nitroprusside (Supporting Information Figs. S2 and S3). Earlier work demonstrated that kaempferol activates KCa1.1 channels in HUVECs in a concentration-dependent manner (Xu et al., 2008). In the present study using PCASMCs, kaempferol also induced an outward current that has the characteristics of that carried by KCa1.1 channels, that is, non-inactivation with substantial noise (Nelson and Ouayle, 1995) and sensitivity to inhibition by iberiotoxin. As the increase in KCa1.1 current by kaempferol is prominent only at very positive holding potentials, activation of the channel by the flavonoid is likely to occur more effectively during depolarization, that is when the smooth muscle contracts. KCa1.1 channels are activated by increases in intracellular calcium concentration, and its activity is coupled to the stimulation of the inositol trisphosphate pathway (Nelson et al., 1995; Zhao et al., 2010; Yang et al., 2013a), a downstream effect of U46619-induced activation of thromboxane receptors (Huang et al., 2004). The induction of an outward potassium current in vascular smooth muscle results in hyperpolarization, which inhibits calcium influx through L-type calcium channels and thus leads to relaxation. However, kaempferol, at the concentration (10−5 M) that activates PCASMC KCa1.1 channels, did not cause significant relaxation per se (Xu et al., 2006), which suggests that the degree of outward KCa1.1 current induced by this lower concentration of kaempferol may only be sufficient to potentiate relaxations.
The KCa1.1 channel is a tetramer consisting of α- and β-subunits (mainly β1 in smooth muscle); the latter regulates the calcium and voltage sensitivity of the channel (Tanaka et al., 1997; Bao and Cox, 2005). The calcium sensitivity and opening probability of the channel increase when the ratio of β1- to α-subunit increases (Yang et al., 2013b). It is unlikely that kaempferol increases KCa1.1 current by modulating the α- to β1-subunits ratio, as its effect is readily reversible. While stimulation of either the inositol trisphosphate pathway (Zhao et al., 2010; Yang et al., 2013a) or transient receptor potential canonical 1 receptors (Kwan et al., 2009; Kochukov et al., 2013) is also coupled to KCa1.1 channel activation, these two mechanisms lead to increases in intracellular calcium concentration. The absence of contraction, therefore, argues against their possible role in kaempferol-induced activation of KCa1.1 channels. Being a flavonoid, kaempferol may possess antioxidative properties, but this effect appears not involved in the direct relaxation that it causes (Xu et al., 2006). In view of the similarity in chemical structure with 17β-estradiol, it is possible that kaempferol may act through a similar signalling pathway as the female hormone. The latter enhances vascular relaxation through activation of PKA (Teoh and Man, 2000). It is also a stimulator of KCa1.1 channels and this action depends on the presence of β1-subunits (Yang et al., 2013b). Previous studies demonstrate that inhibition of PKA inhibits kaempferol-induced increases in the KCa1.1 current in HUVECs (Xu et al., 2008). Taken in conjunction, these observations may imply that kaempferol may bind to cell membrane oestrogen receptor causing activation of PKA, which in turn phosphorylates the β1-subunit of KCa1.1 channels, hence increasing its opening. Further experiments are warranted to verify this interpretation.
In conclusion, kaempferol enhances relaxation of isolated porcine coronary arteries to bradykinin, irrespective of their mediation by either endothelium-derived NO or EDH signalling. This beneficial effect of kaempferol is likely to involve opening of KCa1.1 channels. As a similar potentiating effect on relaxations is observed with the structurally similar quercetin, KCa1.1 channels may be the cellular target for the beneficial effects of flavonols in the vasculature.
Acknowledgments
The authors thank Mr Godfrey Man for his excellent technical assistance. This study was supported in part by the Small Project Funding of the University of Hong Kong Research Council.
Glossary
- EDH
endothelium-dependent hyperpolarization
- KCa
calcium-activated potassium channel
- KCa1.1
large-conductance calcium-activated potassium channel
- KCa2.3
small-conductance calcium-activated potassium channel
- KCa3.1
intermediate-conductance calcium-activated potassium channel
- KHS
Krebs–Henseleit solution
- L-NAME
Nω-nitro-L-arginine methyl ester
- ODQ
1H-[1,2,4]oxadiazolo[4,2-a] quinoxalin-1-one
- PCASMCs
porcine coronary artery smooth muscle cells
- TEA
tetraethylammonium chloride
- TRAM-34
1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole
- U46619
9,11-dideoxy-9α,11α-methanoepoxy PGF2α
- UCL 1684
6,12,19,20,25,26-hexahydro-5,27:13,18:21,24-trietheno-11,7-metheno-7H-dibenzo[b,n][1,5,12,16]tetraazacyclotricosine-5,13-diium dibromide
Author contributions
Y. C. X. conducted the experiments. Y. C. X., S. W. S. L., G. P. H. L. and R. Y. K. M. participated in research design, performed data analysis, and wrote or contributed to the writing of the paper.
Conflict of interests
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supporting Information
Figure S1 Effect of (A) NOS inhibitor or (B) GC inhibitor on bradykinin-induced relaxations. Rings from porcine coronary arteries were incubated with either L-NAME (10−4 M) or ODQ (10−5 M) for 30 min. They were then contracted with U46619 (3 × 10−8 M) before bradykinin was cumulatively added. n = 6–7. *P < 0.05, significantly different from the control group.
Figure S2 Effect of (A) intermediate- plus small-conductance calcium-activated potassium channels inhibitors or (B) large-conductance calcium-activated potassium channel inhibitor on bradykinin-induced relaxations. Rings from porcine coronary arteries were incubated with TRAM-34 (10−6 M) plus UCL 1684 (10−6 M) or iberiotoxin (10−7 M) for 30 min. They were then contracted with U46619 (3 × 10−8 M) before bradykinin was cumulatively added. n = 7–8. *P < 0.05, significantly different from the control group.
Figure S3 Effect of (A) large and intermediate plus small-conductance calcium-activated potassium channels inhibitors and (B) non-selective calcium-activated potassium channel inhibitor on bradykinin-induced relaxations in the absence or presence of kaempferol. Rings from porcine coronary arteries were incubated with charybdotoxin (10−7 M) plus apamin (10−6 M), TEA (10−3 M), with or without kaempferol (3 × 10−6 M), for 30 min. They were then contracted with U46619 (3 × 10−8 M) before bradykinin was cumulatively added. n = 7–8. *P < 0.05, significantly different from the control group.
Figure S4 Effect of the combination of NOS and intermediate plus small-conductance calcium-activated potassium channels inhibitors on bradykinin-induced relaxations in the absence or presence of kaempferol. Rings from porcine coronary arteries were incubated with L-NAME (10−4 M), TRAM-34 (10−6 M) plus UCL 1684 (10−6 M) and/or kaempferol (3 × 10−6 M), for 30 min. They were then contracted with U46619 (3 × 10−8 M) before bradykinin was cumulatively added. n = 7–8. *P < 0.05, significantly different from the control group.
Figure S5 Effect of large-conductance calcium-activated potassium channel inhibitor on sodium nitroprusside-induced relaxations. They from porcine coronary arteries were incubated with or without iberiotoxin (10−7 M) for 30 min. They were then contracted with U46619 (3 × 10−8 M) before sodium nitroprusside was cumulatively added. n = 6–7.
Table S1 Effects of acute treatment with different flavonoids on the contraction to U46619 (3 × 10−8 M) before relaxations to bradykinin (10−11 to 10−6 M) in porcine coronary arteries.
Table S2 Effects of different pharmacological treatments, in the absence or presence of kaempferol (3 × 10−6 M), on the contraction to U46619 (3 × 10−8 M) before relaxations to bradykinin (10−11 to 10−6 M) in porcine coronary arteries.
Table S3 Effects of different potassium channel inhibitor, in the absence or presence of kaempferol (3 × 10−6 M), on the EC50 and Emax values of concentration–relaxation curves of bradykinin (10−11 to 10−6 M) in porcine coronary arteries contracted by U46619 (3 × 10−8 M).
Table S4 Effects of large-conductance calcium-activated potassium channel inhibitor, in the absence or presence of kaempferol (3 × 10−6 M), on the contraction to U46619 (3 × 10−8 M) before relaxations to sodium nitroprusside (10−9 to 10−4 M) in porcine coronary arteries.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013a;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Nuclear Hormone Receptors. Br J Pharmacol. 2013c;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Cox DH. Gating and ionic currents reveal how the BKCa channel's Ca2+ sensitivity is enhanced by its beta1 subunit. J Gen Physiol. 2005;126:393–412. doi: 10.1085/jgp.200509346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo C, Panza JA. Impaired endothelial regulation of vascular tone in patients with systemic arterial hypertension. Vasc Med. 1998;3:138–144. doi: 10.1177/1358836X9800300208. [DOI] [PubMed] [Google Scholar]
- Chan CK, Mak J, Gao Y, Man RY, Vanhoutte PM. Endothelium-derived NO, but not cyclic GMP, is required for hypoxic augmentation in isolated porcine coronary arteries. Am J Physiol Heart Circ Physiol. 2011;301:H2313–H2321. doi: 10.1152/ajpheart.00258.2011. [DOI] [PubMed] [Google Scholar]
- Chao PY, Lin KH, Chiu CC, Yang YY, Huang MY, Yang CM. Inhibitive effects of mulberry leaf-related extracts on cell adhesion and inflammatory response in human aortic endothelial cells. Evid Based Complement Alternat Med. 2013;2013:267217. doi: 10.1155/2013/267217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CL, Cohen RA. Two mechanisms mediate relaxation by bradykinin of pig coronary artery: NO-dependent and -independent responses. Am J Physiol. 1991;260:H830–H835. doi: 10.1152/ajpheart.1991.261.3.H830. [DOI] [PubMed] [Google Scholar]
- Duarte J, Pérez Vizcaíno F, Utrilla P, Jiménez J, Tamargo J, Zarzuelo A. Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen Pharmacol. 1993;24:857–862. doi: 10.1016/0306-3623(93)90159-u. [DOI] [PubMed] [Google Scholar]
- DuPont MS, Day AJ, Bennett RN, Mellon FA, Kroon PA. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur J Clin Nutr. 2004;58:947–954. doi: 10.1038/sj.ejcn.1601916. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao HY, Xu M, Zhou L, Guo H, Han J, et al. Qualitative evaluation and quantitative determination of 10 major active components in Carthamus tinctorius L. by high-performance liquid chromatography coupled with diode array detector. J Chromatogr A. 2009;1216:2063–2070. doi: 10.1016/j.chroma.2008.03.046. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr Cancer. 1993;20:21–29. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- Ho HM, Chen R, Huang Y, Chen ZY. Vascular effects of a soy leaves (Glycine max) extract and kaempferol glycosides in isolated rat carotid arteries. Planta Med. 2002;68:487–491. doi: 10.1055/s-2002-32545. [DOI] [PubMed] [Google Scholar]
- Huang JS, Ramamurthy SK, Lin X, Le Breton GC. Cell signalling through thromboxane A2 receptors. Cell Signal. 2004;16:521–533. doi: 10.1016/j.cellsig.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Kochukov MY, Balasubramanian A, Noel RC, Marrelli SP. Role of TRPC1 and TRPC3 channels in contraction and relaxation of mouse thoracic aorta. J Vasc Res. 2013;50:11–20. doi: 10.1159/000342461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan HY, Shen B, Ma X, Kwok YC, Huang Y, Man YB, et al. RPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res. 2009;104:670–678. doi: 10.1161/CIRCRESAHA.108.188748. [DOI] [PubMed] [Google Scholar]
- Leung SW, Teoh H, Quan A, Man RY. Endothelial dysfuncation exacerbates the impairment of relaxation by lysophosphatidylcholine in porcine coronary artery. Clin Exp Pharmacol Physiol. 1997;24:984–986. doi: 10.1111/j.1440-1681.1997.tb02735.x. [DOI] [PubMed] [Google Scholar]
- Liang CF, Au AL, Leung SW, Ng KF, Félétou M, Kwan YW, et al. Endothelium-derived nitric oxide inhibits the relaxation of the porcine coronary artery to natriuretic peptides by desensitizing big conductance calcium-activated potassium channels of vascular smooth muscle. J Pharmacol Exp Ther. 2010;334:223–231. doi: 10.1124/jpet.110.166652. [DOI] [PubMed] [Google Scholar]
- Manach C, Regerat F, Texier O, Agullo G, Demigne C, Remesy C. Bioavailability, metabolism and physiological impact of 4-oxo-flavonoids. Nutr Res. 1996;16:517–544. [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, et al. Relaxation of arterial muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Ng KF, Leung SW, Man RY, Vanhoutte PM. Endothelium-derived hyperpolarizing factor mediated relaxations in pig coronary arteries do not involve Gi/o proteins. Acta Pharmacol Sin. 2008;29:419–424. doi: 10.1111/j.1745-7254.2008.00905.x. [DOI] [PubMed] [Google Scholar]
- Nile SH, Kim SH, Ko EY, Park SW. Polyphenolic contents and antioxidant properties of different grape (V. viniferaV. labrusca, and V. hybrid) cultivars. Biomed Res Int. 2013;2013:718065. doi: 10.1155/2013/718065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewska M. Separation of quercetin, sexangularetin, kaempferol and isorhamnetin for simultaneous HPLC determination of flavonoid aglycones in inflorescences, leaves and fruits of three Sorbus species. J Pharm Biomed Anal. 2008;48:629–635. doi: 10.1016/j.jpba.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Vizcaíno F, Ibarra M, Cogolludo AL, Duarte J, Zaragozá-Arnáez F, Moreno L, et al. Endothelium-independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J Pharmacol Exp Ther. 2002;302:66–72. doi: 10.1124/jpet.302.1.66. [DOI] [PubMed] [Google Scholar]
- Sampson L, Rimm E, Hollman PC, de Vries JH, Katan MB. Flavonol and flavone intakes in US health professionals. J Am Diet Assoc. 2002;102:1414–1420. doi: 10.1016/s0002-8223(02)90314-7. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Yokoshiki H, Sperelakis N, Paul RJ. Role of voltage-dependent and Ca(2+)-activated K(+) channels on the regulation of isometric force in porcine coronary artery. J Vasc Res. 2000;37:16–25. doi: 10.1159/000025709. [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Sylvester JT, Sham JS. Inhibition of voltage-gated K+ current in rat intrapulmonary arterial myocytes by endothelin-1. Am J Physiol. 1998;274:L842–L853. doi: 10.1152/ajplung.1998.274.5.L842. [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Sylvester JT, Sham JS. Chronic hypoxia alters effects of endothelin and angiotensin on K+ currents in pulmonary arterial myocytes. Am J Physiol. 1999;277:L431–L439. doi: 10.1152/ajplung.1999.277.3.L431. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha + beta submit complexes. J Physiol. 1997;502:545–557. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh H, Man RY. Enhanced relaxation of porcine coronary arteries after acute exposure to a physiological level of 17β-estradiol involves non-genomic mechanisms and the cyclic AMP cascade. Br J Pharmacol. 2000;129:1739–1747. doi: 10.1038/sj.bjp.0703252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EHC, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Xu YC, Yeung DK, Man RY, Leung SW. Kaempferol enhances endothelium-independent and dependent relaxation in the porcine coronary artery. Mol Cell Biochem. 2006;287:61–67. doi: 10.1007/s11010-005-9061-y. [DOI] [PubMed] [Google Scholar]
- Xu YC, Leung SW, Yeung DK, Hu LH, Chen GH, Che CM, et al. Structure–activity relationships of flavonoids for vascular relaxation in porcine coronary artery. Phytochemistry. 2007;68:1179–1188. doi: 10.1016/j.phytochem.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Xu YC, Leung GP, Wong PY, Vanhoutte PM, Man RY. Kaempferol stimulates large conductance Ca2+-activatied K+ (BKCa) channels in human umbilical vein endothelial cells via a cAMP/PKA-dependent pathway. Br J Pharmacol. 2008;154:1247–1253. doi: 10.1038/bjp.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li PY, Cheng J, Cai F, Lei M, Tan XQ, et al. IP3 decreases coronary artery tone via activating the BKCa channel of coronary artery smooth muscle cells in pigs. Biochem Biophys Res Commun. 2013a;439:363–368. doi: 10.1016/j.bbrc.2013.08.079. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sohma Y, Nourian Z, Ella SR, Li M, Stupica A, et al. Mechanisms underlying regional differences in the sensitivity of BKCa current in arterial smooth muscle. J Physiol. 2013b;591:1277–1293. doi: 10.1113/jphysiol.2012.241562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung YY, Lee SS, Vanhoutte PM, Leung SW. Prolonged exposure to lopinavir impairs endothelium-dependent hyperpolarization-mediated relaxation in rat mesenteric arteries. J Cardiovasc Pharmacol. 2013;62:397–404. doi: 10.1097/FJC.0b013e31829fdd01. [DOI] [PubMed] [Google Scholar]
- Zhao G, Neeb ZP, Leo MD, Pachuau J, Adebiyi A, Ouyang K, et al. Type 1 IP3 receptors activate BKCa channels via local molecular coupling in arterial smooth muscle cells. J Gen Physiol. 2010;136:283–291. doi: 10.1085/jgp.201010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Effect of (A) NOS inhibitor or (B) GC inhibitor on bradykinin-induced relaxations. Rings from porcine coronary arteries were incubated with either L-NAME (10−4 M) or ODQ (10−5 M) for 30 min. They were then contracted with U46619 (3 × 10−8 M) before bradykinin was cumulatively added. n = 6–7. *P < 0.05, significantly different from the control group.
Figure S2 Effect of (A) intermediate- plus small-conductance calcium-activated potassium channels inhibitors or (B) large-conductance calcium-activated potassium channel inhibitor on bradykinin-induced relaxations. Rings from porcine coronary arteries were incubated with TRAM-34 (10−6 M) plus UCL 1684 (10−6 M) or iberiotoxin (10−7 M) for 30 min. They were then contracted with U46619 (3 × 10−8 M) before bradykinin was cumulatively added. n = 7–8. *P < 0.05, significantly different from the control group.
Figure S3 Effect of (A) large and intermediate plus small-conductance calcium-activated potassium channels inhibitors and (B) non-selective calcium-activated potassium channel inhibitor on bradykinin-induced relaxations in the absence or presence of kaempferol. Rings from porcine coronary arteries were incubated with charybdotoxin (10−7 M) plus apamin (10−6 M), TEA (10−3 M), with or without kaempferol (3 × 10−6 M), for 30 min. They were then contracted with U46619 (3 × 10−8 M) before bradykinin was cumulatively added. n = 7–8. *P < 0.05, significantly different from the control group.
Figure S4 Effect of the combination of NOS and intermediate plus small-conductance calcium-activated potassium channels inhibitors on bradykinin-induced relaxations in the absence or presence of kaempferol. Rings from porcine coronary arteries were incubated with L-NAME (10−4 M), TRAM-34 (10−6 M) plus UCL 1684 (10−6 M) and/or kaempferol (3 × 10−6 M), for 30 min. They were then contracted with U46619 (3 × 10−8 M) before bradykinin was cumulatively added. n = 7–8. *P < 0.05, significantly different from the control group.
Figure S5 Effect of large-conductance calcium-activated potassium channel inhibitor on sodium nitroprusside-induced relaxations. They from porcine coronary arteries were incubated with or without iberiotoxin (10−7 M) for 30 min. They were then contracted with U46619 (3 × 10−8 M) before sodium nitroprusside was cumulatively added. n = 6–7.
Table S1 Effects of acute treatment with different flavonoids on the contraction to U46619 (3 × 10−8 M) before relaxations to bradykinin (10−11 to 10−6 M) in porcine coronary arteries.
Table S2 Effects of different pharmacological treatments, in the absence or presence of kaempferol (3 × 10−6 M), on the contraction to U46619 (3 × 10−8 M) before relaxations to bradykinin (10−11 to 10−6 M) in porcine coronary arteries.
Table S3 Effects of different potassium channel inhibitor, in the absence or presence of kaempferol (3 × 10−6 M), on the EC50 and Emax values of concentration–relaxation curves of bradykinin (10−11 to 10−6 M) in porcine coronary arteries contracted by U46619 (3 × 10−8 M).
Table S4 Effects of large-conductance calcium-activated potassium channel inhibitor, in the absence or presence of kaempferol (3 × 10−6 M), on the contraction to U46619 (3 × 10−8 M) before relaxations to sodium nitroprusside (10−9 to 10−4 M) in porcine coronary arteries.