Diabetes is a complex, chronic disease that is characterized by poor glycemic control and results from impaired insulin secretion, impaired insulin action, or both.1 Type 2 diabetes is the most common form of the disease and accounts for more than 90% of the estimated 29.1 million cases in the United States.2
Diabetes is a major public health issue around the globe. An estimated 382 million individuals have diabetes, and the worldwide prevalence is projected to increase to nearly 600 million in less than 25 years.3
In alignment with global trends, data indicate that the diabetes epidemic is worsening in the United States. A recent study that was based on surveillance data from the Centers for Disease Control and Prevention estimated that the prevalence of diabetes among US adults grew by 75% during the past 20 years, with the greatest increase among patients aged ≥65 years.4 This study used National Health and Nutrition Examination Survey (NHANES) data that were gathered during 3 periods: 1988–1994, 1999–2004, and 2005–2010. Based on the total population of the United States during these periods, the researchers estimated that the prevalence of diabetes increased from 14.9 million cases to 26.1 million cases within approximately 20 years.4
Public health experts project that the number of Americans with diabetes will increase from 1 in 10 adults today to as many as 1 in 3 adults by 2050, unless significant steps are taken to slow the growing incidence of diabetes.5 This trend is attributed to a confluence of factors, including an aging population that is at an increased risk for type 2 diabetes, increases in minority groups that are at high risk for type 2 diabetes, and increases in life expectancy for individuals with type 2 diabetes.5
The concurrent obesity epidemic in the United States is strongly associated with the increased prevalence of diabetes. During 1994–1998, 1999–2004, and 2005–2010, the incidence of individuals with a body mass index (BMI) of 30 kg/m2 or higher increased from 22% to 30% to 34.6%, respectively, and the prevalence of individuals with a BMI of 40 kg/m2 or higher increased dramatically from 2.7% to 4.9% to 6.4%, respectively.4
The situation is particularly concerning for younger Americans; childhood obesity has more than doubled in children and quadrupled in adolescents in the past 30 years.6–8 Obese adolescents are more likely to have prediabetes—a condition that is characterized by elevated glycemic levels but not high enough for a diagnosis of diabetes9—compared with adolescents who are not overweight.10 Furthermore, obese children and adolescents are at an increased risk for type 2 diabetes, as well as other health conditions.9
One epidemiologic study estimated that during 1980–1989 and 2000–2004, the proportion of individuals aged 18 years who would develop diabetes in their lifetimes increased by nearly 50% among women and approximately doubled among men.11 This development, which is projected to negatively affect diabetes-free life expectancy, was primarily attributed to increases in the prevalence of diabetes among obese men and women.11
The impact of increased diabetes-related morbidity and mortality in younger populations is particularly disturbing. For patients with young-onset type 2 diabetes (ie, individuals diagnosed at age <45 years), poor glycemic control is predictive of a high risk for complications over time.12 As a result, the mortality rate for patients with early-onset type 2 diabetes is projected to be approximately double the rate as for patients of a similar age and duration with type 1 diabetes; this increase in premature mortality is expected to be primarily driven by cardiovascular events.13
Economic Burden of Type 2 Diabetes
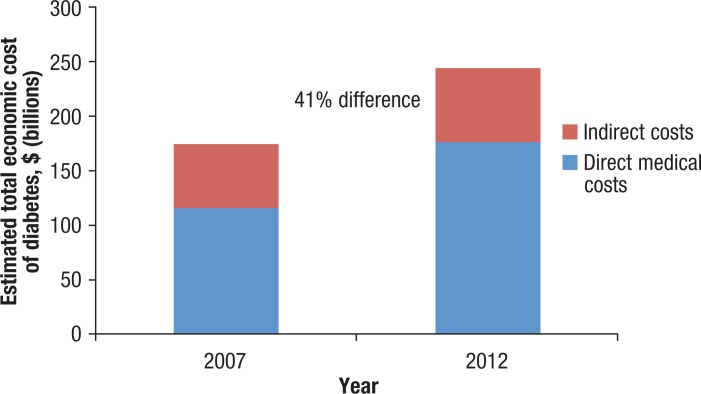
In addition to its impact on the rising morbidity and mortality rates, the diabetes epidemic is substantially affecting healthcare utilization and cost.14 Patients with type 2 diabetes use a disproportionate amount of healthcare services compared with that of individuals without type 2 diabetes. According to the American Diabetes Association (ADA), the total estimated cost for patients with diagnosed diabetes in 2012 was $245 billion, representing a 41% increase from the total estimated cost in 2007 (Figure 1).14,15 Overall, patients with diagnosed diabetes incur medical expenses that are on average 2.3 times higher than that of individuals without diabetes.14
Figure 1. Economic Cost of Diabetes Between 2007 and 2012.
Sources: American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596–615; American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046.
In 2012, inpatient hospital care accounted for 43% of the total diabetes-related medical expenses, based on estimates from the ADA.14 A total of 630,000 adults with diabetes were discharged from hospitals in 2010,16 and an estimated 15.7% of the 168 million hospital inpatient days incurred in 2012 were attributed to patients with diabetes; of these inpatient days, more than 60% were attributed directly to diabetes and diabetes-related complications.14
Disease Progression
Individuals with type 2 diabetes are often asymptomatic and may therefore remain undiagnosed for an extended period.17 During that time, however, poor glycemic control is silently damaging the body. Studies have found that many individuals with undiagnosed diabetes begin to develop microvascular and macrovascular complications, including chronic kidney disease (CKD), heart failure, and retinopathy. These complications may substantially progress by the time the patient is diagnosed with diabetes.18–20
Disease progression is a product of type 2 diabetes pathophysiology, which is believed to involve a broad and interrelated spectrum of organs and tissues.21 Beta-cell impairment, muscle tissue, and the brain and liver all contribute to insulin resistance. In addition, incretin resistance and/or deficiency in the gut, accelerated lipolysis and ketogenesis that are stimulated by fat cells, increased glucose reabsorption in the kidney, and alpha-cell activity that results in hyperglucagonemia are all thought to contribute to glucose intolerance in patients with type 2 diabetes.21
Despite treatment with antidiabetes agents, the majority of patients with type 2 diabetes experience loss of glycemic control over time.22,23 The United Kingdom Prospective Diabetes Study (UKPDS) showed that therapy with metformin, sulfonylurea, or insulin substantially lowered hemoglobin (Hb) A1c levels and fasting plasma glucose levels compared with conventional therapy; however, during 10 years, glycemic control gradually eroded.22,23 A similar pattern was observed with sulfonylureas after a median of 4 years in the ADOPT (A Diabetes Outcome Progression Trial) study.24
The decline in beta-cell function is strongly associated with progressively poorer glycemic control over time. Using the homeostasis model assessment to quantify beta-cell function, researchers in the UKPDS clinical trial demonstrated that beta-cell function continued to deteriorate in association with progressively increasing hyperglycemia, despite treatment.25
Complications of Type 2 Diabetes
The burden of type 2 diabetes–related vascular complications is substantial. Patients with microvascular complications use nearly twice the amount of healthcare resources compared with patients without microvascular complications.26
Diabetic retinopathy, an ocular disorder associated with damage to the retina, is the leading cause of new cases of blindness among US adults aged 20 to 74 years.27 In the United States, diabetic retinopathy affects 28.5% of adults aged ≥40 years, whereas vision-threatening diabetic retinopathy affects an estimated 4.4% of patients in this age-group.28 In addition to its devastating effect on the patient, diabetes-related blindness accounts for nearly $500 million annually in total costs.29
Diabetic neuropathy, a nerve disorder that is often characterized by impaired sensation in the extremities, affects 60% to 70% of patients with diabetes.30 Diminished pain sensation results in an increased risk for skin breakdown and infection, in addition to injuries. Without prompt treatment, patients may have foot ulcers; in severe cases, amputation may become necessary.31 One study showed that the costs for patients with diabetic neuropathy were 5 times higher than for diabetic patients without neuropathy.32 Fortunately, the early recognition and management of risk factors can prevent or delay the adverse outcomes of neuropathy.31
CKD, characterized by proteinurea and progressive kidney failure, occurs in 20% to 40% of patients with diabetes and is the leading cause of end-stage renal disease.31,33 The majority of patients with CKD are asymptomatic until their CKD has significantly progressed; therefore, they remain unaware of their condition.34 CKD is common in patients with diabetes, and in one analysis, nearly 42% of individuals with undiagnosed diabetes and 18% of individuals with prediabetes had CKD.18 It should be noted that although CKD frequently occurs in patients with type 2 diabetes, it is not limited to the diabetic population.
Macrovascular complications are also prevalent in patients with type 2 diabetes. In 2010, researchers reported that the hospitalization rates for myocardial infarction and stroke were 80% and 50% higher, respectively, among adults aged ≥20 years with diagnosed diabetes than among adults without diagnosed diabetes.2
The mortality rates are also higher among patients with diabetes compared with individuals without diabetes. Between 2003 and 2006, cardiovascular disease mortality rates were approximately 70% higher among adults aged ≥18 years with diagnosed diabetes compared with individuals without diagnosed diabetes.2 Furthermore, according to the ADA, of the 687,000 deaths where cardiovascular disease is listed as the primary cause, an estimated 110,000 (16%) are attributable to diabetes.14
The Paramount Importance of Glycemic Control
Epidemiologic studies have shown a strong association between glycemic control and microvascular complications; lowering HbA1c levels to ≤7% has been shown to reduce microvascular complications of diabetes.22 In fact, every 1% reduction in HbA1c levels is correlated with a 40% reduction in diabetes-related microvascular complications.30,35
In addition, evidence suggests that maintaining glycemic control early in the disease process is associated with long-term reduction in macrovascular disease.31 Although cardiovascular disease is a more common cause of mortality than microvascular complications, several population studies indicate that cardiovascular disease is less affected by hyperglycemia levels or by intensity of glycemic control in patients with type 2 diabetes than microvascular complications.31
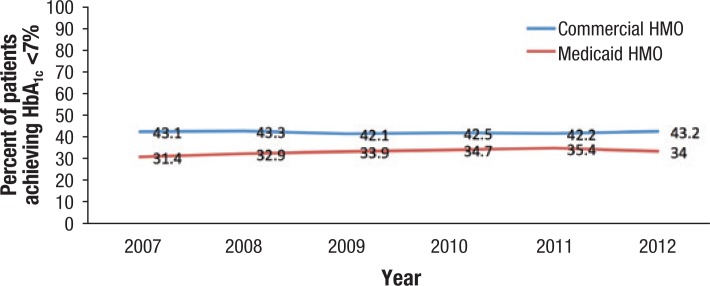
Evidence suggests that glycemic control remains suboptimal and is challenging for patients to manage. According to the National Committee for Quality Assurance, only 43.2% of patients with commercial HMO plans achieved HbA1c levels of <7% in 2012, which was approximately the same proportion as in 2007 (Figure 2).36 Another analysis that used the NHANES data (between 1999 and 2008) showed a significant improvement in reaching HbA1c targets in adults with diabetes aged ≥20 years.37 Despite the positive trend, however, an estimated 45% of patients with diabetes had not reached target HbA1c levels by 2008.37 Among minority populations, the rate was substantially lower than among whites, with less than 50% of African Americans and Mexican Americans reaching glycemic control (ie, HbA1c <7%) between 2005 and 2008.37
Figure 2. Percentage of Patients with Type 2 Diabetes Achieving HbA1c <7%.
Hb indicates hemoglobin.
Source: National Committee for Quality Assurance. Improving quality and patient experience. www.ncqa.org/Portals/0/Newsroom/SOHC/2013/SOHC-web_version_report.pdf.
These data clearly demonstrate that there remains a major gap in the quality of diabetes management, underscoring the need for ongoing strategies to improve glycemic control, as well as other relevant measures, including blood pressure, low-density lipoprotein cholesterol, and weight management.
Medication Nonadherence Remains a Major Issue for Patients with Chronic Diseases
Clinical trials have demonstrated that antidiabetes medications can substantially improve glycemic control in accordance with a key measure, namely HbA1c levels.22,23,25 Patients with diabetes are often asymptomatic or minimally symptomatic, particularly during the early stages of the disease.38 Along with other factors, lack of symptoms may contribute to patients not being adherent to their prescribed medications.39
Medication adherence is defined as the degree to which patients follow prescriber recommendations in starting, implementing, and discontinuing pharmacologic treatment.40 Medication nonadherence can take on many forms, including missing doses, taking incorrect doses, not having a prescription filled, taking a medication at the wrong time, or discontinuing therapy before all of the prescribed medication has been taken.41 Depending on the class of medication, it is estimated that 13% to 26% of patients do not fill their first new diabetes prescriptions within 30 days of the prescription fill date.42 The causes of medication nonadherence are often complex and multifactorial, and may be related to the patient, the healthcare provider, and/or a specific treatment. In addition, the patient's decision to deviate from the agreed course of action may be intentional or unintentional.41
This seemingly intractable problem represents a major public health concern, with many adverse consequences at the individual and societal levels. The rates of medication nonadherence vary with different disease states,43 but are especially high among patients with chronic diseases, averaging approximately 50% in developed countries.44 Patients with chronic diseases, such as diabetes, typically require long-term medications to control symptoms and to prevent complications, and they must often make significant behavioral changes to remain adherent to pharmacotherapy and lifestyle modifications.45 However, only approximately 15% to 25% of patients improve their health practices after their disease diagnosis, suggesting that it is difficult for the majority of patients to successfully integrate these changes into their lives.45,46
Nonadherence to pharmacotherapy has been shown to reduce productivity and to increase disease morbidity, mortality, physician office visits, hospitalizations, and admissions to nursing homes.47 Elderly patients may be particularly susceptible to the adverse effects of nonadherence, and data indicate that 10% of hospital admissions and up to 23% of nursing home admissions may result from medication nonadherence.48 Furthermore, it is estimated that nonadherence to prescribed medications results in treatment failure in 30% to 50% of the cases and is responsible for nearly 125,000 deaths annually.49,50
The Burden of Nonadherence to Antidiabetes Therapy
The rates of nonadherence to type 2 antidiabetes medications are relatively similar to the corresponding rates for other chronic diseases,51 and despite the introduction of several new classes of antidiabetes therapies in the past decade, suboptimal glycemic control and medication nonadherence remain major concerns. Nonadherence is prevalent among all antidiabetes medications, and approximately 50% of new diabetes medication users fail to consume at least 80% of prescribed doses during their first year of therapy.52,53
The individual and health system consequences of medication nonadherence are substantial. At the patient level, medication nonadherence is associated with poor HbA1c control and with diabetes disease progression.54 Furthermore, nonadherence to antidiabetes medication is associated with a significant increase in mortality.54
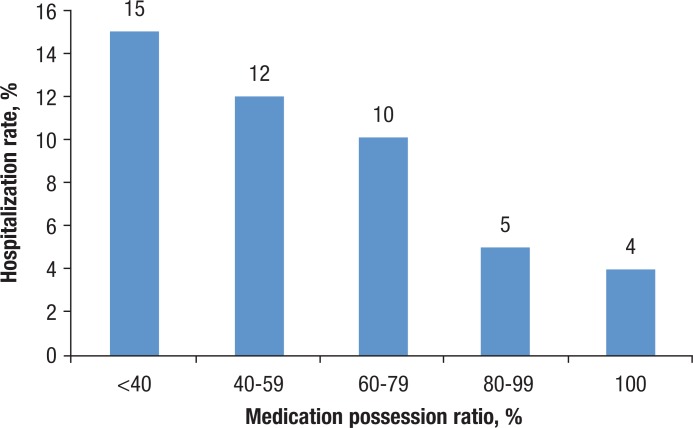
At the health system level, medication nonadherence increases the resource utilization and healthcare costs. In one analysis, nonadherence to oral antidiabetes medication was associated with a risk of hospitalization of up to 2.5 times higher than that of adherent patients, after adjusting for demographics, disease severity, and comorbid conditions (Figure 3).55
Figure 3. Impact of Adherence to Oral Antiglycemic Drugs on Hospitalization Risk, 2001.
Source: Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care. 2004;27:2149–2153.
In terms of healthcare costs, Sokol and colleagues sought to quantify the impact of medication adherence on the total healthcare costs among patients with diabetes and ischemic heart disease. The authors found that nonadherent patients were responsible for nearly double the yearly mean patient healthcare costs of adherent patients ($16,499 vs $8886).56 In addition, Balkrishnan and colleagues estimated that each 10% increase in medication adherence results in a mean decrease of 28.9% in healthcare costs.57
Furthermore, an analysis by Egede and colleagues supported the hypothesis that improved adherence to antidiabetes medications could result in substantial cost-savings, showing that the inpatient costs for nonadherent patients with type 2 diabetes were 41% higher compared with that of adherent patients.58 Nationally, it was estimated that improved adherence to diabetes medications would result in the estimated annual cost-savings that ranged from approximately $661 million to $1.16 billion.58
Addressing Barriers to Antidiabetes Therapy Adherence: Unmet Needs
That approximately half of patients with type 2 diabetes do not achieve glycemic control clearly indicates that an ongoing unmet need exists for therapeutic and interventional strategies that help to achieve and maintain glycemic control, reduce body weight, and decrease cardiovascular risk, while ensuring patient adherence to therapy.44
A broad constellation of factors contribute to medication nonadherence in type 2 diabetes. For example, communication gaps between patients and providers, including insufficient or incomprehensible information or instructions, are frequently cited as reasons for medication nonadherence.59 In addition, social, cognitive, and personal factors may also limit adherence.59,60 Furthermore, side effects of certain antidiabetes medications may outweigh the perceived benefits, causing some patients to become nonadherent.59 In addition, patients may have difficulty affording their medications, which can lead to cost-related nonadherence.59,61
In type 2 diabetes, treatment regimens are often complex—comprising different drugs with different routes of administration and different dosing schedules.59,60 Because type 2 diabetes is a chronic, progressive disease, these therapies must be taken long-term, and sometimes lifelong, to benefit the patient. However, medication adherence to all chronic disease medications, including diabetes, tends to decrease over time.62
Odegard and Gray conducted a study to characterize the barriers to adherence and medication management for adults with poorly controlled type 2 diabetes—defined as HbA1c levels of 9% or higher—and to identify specific adherence characteristics that are associated with poor diabetes control.60 The most common adherence challenges included paying for medications (34%), remembering doses (31%), reading prescription labels (21%), and obtaining refills (21%). The researchers found that the dosing frequency (P = .02), defined as taking more than 2 doses of diabetes medication daily, and difficulty reading the antidiabetes medication prescription label (P = .04) were significantly associated with higher HbA1c levels compared with other baseline characteristics, such as the number of prescribed medications or side effects.60
Efforts to Promote Medication Adherence in Type 2 Diabetes
Because healthcare decision makers recognize that medication nonadherence is a critical barrier to achieving and maintaining glycemic control, they are investing in a variety of initiatives to improve adherence to therapy among patients with diabetes. Interventions include pharmacist coaching programs,59,63 telephonic support,64 hospital-based adherence education,65 integrated care management programs,66 and financial incentives.61,63 Several studies have attempted to assess the effectiveness of antidiabetes medication adherence programs (Table).59,61,63–66
Table.
Summary of Interventions Designed to Promote Medication Adherence in Patients with Type 2 Diabetes
| Study [Sponsor] | Nature of intervention | Impact on nonadherence | Findings | Limitations |
|---|---|---|---|---|
| Antoine (2014)a | Coaching by pharmacist | No impact | US studies identified by the systematic literature review found no impact of pharmacist intervention on adherence • Grant (2003): Very high rates of self-reported adherence with and without pharmacist intervention • Odegard (2005): Also no impact on HbA1c control |
• Limited number of RCT studies available for the review |
| O'Connor (2014) [Geisinger] | Diabetes mellitus educator or pharmacist-scripted call | No impact | No improvement in first fill with intervention observed during RCT (10%-20% of patients do not fill first new diabetes mellitus medications) | • No assessment of adherence response beyond first fill • Small sample/limited representativeness (2378 patients, single payer) |
| Magee (2014) [Sanofi] | 3-month education program on adherence | Improved likelihood of high adherence | Odds of “high adherence” multiplied by 3.75 at month 3 (patient scale assessment, not based on actual prescription claims) | • No control arm • Nonrandomized study • Self-reported adherence measure (using MMAS-4 screening tool) • Representativeness may be limited (intervention in hospitalized patients with severely uncontrolled diabetes, a majority of African American women) |
| Elliot (2013) [CCHS] | Copayment waiver | From 41% to 17.5% after 12 months (cost-related only) | Intervention helped reduce cost-related nonadherence, but patients experienced no change in HbA1c, HCRU, or healthcare costs | • No control arm • Study did not provide estimates of impact on nonadherence due to reasons other than cost • By its nature, copayment waiver will remove perception of non-adherence being cost-related, but overall nonadherence may not have been impacted • Self-reported adherence measure |
| Wertz (2012) [Kroger, BCBS OH, Novartis] | Copayment support (>$500) and pharmacist coaching | From 22% to 14% after 12 months (estimated as 1-PDC) | • Intervention helped improve adherence to noninsulin antidiabetic agents, reducing HbA1c from 7.9% to 7.1% • However, medical costs increased more for the intervention versus control cohort (+33.2% vs +20.8%—even without considering cost of intervention) |
• Nonrandomized study (control based on retrospective matching) • Kroger employees may be particularly sensitive to pharmacy intervention |
| Schmittdiel (2009) [Kaiser] | Nurse-led integrated CM program | No impact | After 12 months: • % of adherent patients was not significantly different for the CM vs matched control patients • However, HbA1c levels of CM patients were 0.3% lower than that of their controls after multivariate adjustment (P <.01) |
• Nonrandomized study (control based on retrospective propensity score matching) |
Literature review of systematic randomized controlled clinical trials.
CM indicates care management; Hb, hemoglobin; HCRU, healthcare resource utilization; MMAS, Morisky Medication Adherence Scale; PDC, proportion of days covered; RCT, randomized controlled trial.
As the studies in the Table highlight, heterogeneous approaches have been used and tested to improve adherence to antidiabetes medications by researchers and by payers.59,61,63–66 Some studies suggest that these intervention programs are effective at improving adherence, but other studies find no such evidence.59,61,63–66 However, the benefits of these intervention programs are modest compared with the level of nonadherence observed in real-world clinical practice. In addition, the quality of the studies that assess these intervention programs are predominantly low. For example, few studies used randomized intervention design, and several studies did not have a control arm. In addition, many studies relied on self-reported adherence measures as opposed to actual medication use, which is likely to bias responses toward higher adherence in patients who received the intervention compared with patients who did not receive the intervention.59,61,63–66
The limited degree of success associated with different interventions indicates that unmet needs remain in conceptualizing, developing, and implementing effective strategies to improve medication adherence in patients with type 2 diabetes. Complex and coordinated strategies that involve physicians, nurses, and case managers may require substantial cost and resource commitment.67
Simplifying Treatment Regimens
Complex treatment regimens can lead to patient confusion about dosing instructions and to a lack of certainty about drug effectiveness, ultimately increasing the risk for medication nonadherence.41,59,68
There may be opportunities to simplify treatment regimens for patients with diabetes and with associated cardiometabolic diseases, especially for elderly patients. Farrell and colleagues reported on the implementation of a systematic approach to streamline a complex treatment regimen in an elderly patient with multiple chronic comorbidities.68 Medications that did not deliver a clear therapeutic benefit were discontinued, along with drugs that increased the risk for adverse drug reactions. In addition, fixed combination formulations and agents with once-daily dosing were used to further reduce the pill burden.68 The authors concluded that these strategies were effective in managing polypharmacy, reducing the pill burden, and improving medication management, and recommended adopting these interventions in the broader patient population.68
Although the medical literature is not equivocal, evidence suggests that reducing the pill burden can improve adherence. In a retrospective study of oral antihyperglycemic medications, Donnan and colleagues reported that the administration of 1 tablet daily was associated with greater adherence than multiple tablets.69 Patient feedback echoes this finding, and, in a recent study, patients reported that they would be more likely to remain adherent to type 2 diabetes medications if their dosing was simplified and their pill burden was reduced.70
Reducing Side Effects of Antidiabetes Drugs
Some antidiabetes therapies are associated with side effects that may cause patients to take lower-than-prescribed doses or skip doses, resulting in poor glycemic control. For example, because of their mechanisms of action, sulfonylureas and meglitinides are associated with hypoglycemia, and thiazolidinediones and sulfonylureas frequently cause weight gain.71
Evidence indicates that weight loss may play a role in promoting medication adherence in type 2 diabetes. McAdam-Marx and colleagues conducted a retrospective cohort study of patients with type 2 diabetes who were treated in a managed, integrated-care setting; the authors reported that a weight loss of ≥3% was associated with reaching HbA1c target of <7.0% after starting a newly prescribed class of antidiabetes medications72; this finding corroborated data from a previous longitudinal study that found an association between weight change and glycemic control.73 In addition, the researchers found that self-reported adherence to diabetes medication was also associated with a weight loss of ≥3%.72 Although the initial findings have been positive, additional research is needed to establish a definitive link between weight loss, medication adherence, and glycemic control.
The Future of Type 2 Diabetes Care
Nonadherence to medications that are prescribed for chronic illnesses, including diabetes, remains a major public health issue across the globe.3 In type 2 diabetes, nonadherence is strongly associated with poorer glycemic control, increased hospitalizations, and increased mortality.54,55,58
Despite the myriad efforts to address medication nonadherence, the majority of interventions that promote adherence have had limited sustained success and have not been cost-effective.59,61,63–66 Therefore, an unmet need clearly remains for innovative solutions to improve adherence to antidiabetes medication.
A number of issues should be taken into account when considering potential approaches for improving adherence to antidiabetes medications. With any self-administered medication, it is ultimately the patients' responsibility to take the drug as prescribed by their healthcare provider. Patients may diverge from the prescribed regimen for a host of cognitive, cultural, psychosocial, and financial reasons. Perhaps an ideal solution would be to relieve patients of their adherence burden and allow them to concentrate on other issues that may be affecting their lives. This could be accomplished through a long-term drug delivery device that would eliminate the need for day-to-day decision-making by the patient.
A long-term drug delivery device could be coupled with a therapeutic agent that has demonstrated proved efficacy, safety, and improved outcomes. Optimally, this agent should deliver sustained reduction in HbA1c levels, result in minimal weight change, and have a favorable side effect profile to minimize the chance of discontinuation. In addition, the agent must have the ability to be delivered through a long-term delivery device.
Glucagon-like peptide (GLP)-1 receptor agonists represent an important therapeutic option for many patients; these agents have demonstrated efficacy in achieving glycemic control and weight reduction in patients with type 2 diabetes.74,75 In long-term studies with GLP-1 receptor agonists, glycemic control and weight loss were maintained, and the incidence of new-onset side effects remained low after up to 3 years of therapy.76–79
However, the injectable administration and the frequent dosing that is required of GLP-1 receptor agonists may negatively impact patient tolerability, adherence, and persistence during long-term treatment, which, in turn, may result in suboptimal outcomes and increased healthcare costs.51,54,58
The use of external insulin pumps to continuously administer insulin, a relatively recent practice in type 2 diabetes, has been touted as an alternative to multiple daily injections.80 Although data from randomized clinical trials remain limited, longitudinal data suggest that continuous insulin administration may be advantageous in patients with severe insulin resistance and poor glycemic control.80 However, because of complicated handling requirements, current pumps require comprehensive and detailed education programs, which may limit the populations that could benefit from their use.80
The development of a novel, noninsulin long-term drug delivery system that delivers a GLP-1 receptor agonist could represent a reasonable approach to administering therapeutic agents that require frequent oral dosing or injections. Optimally, such a device should maintain long-term drug stability and ensure consistent and timely delivery of therapeutic agents for an extended time, while providing built-in adherence advantages over current injectable formulations.
The combination of an antidiabetes agent, such as a GLP-1 receptor agonist that is approved by the US Food and Drug Administration and with well-established efficacy and safety profiles, and a long-term drug delivery device could serve as an effective component of a broad intervention strategy to improve medication adherence in patients with type 2 diabetes and help to achieve glycemic control (in accordance with patients' treatment goals), delay disease progression, and improve favorable outcomes.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37(suppl 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 13, 2015.
- 3.International Diabetes Federation. IDF Diabetes Atlas, 6th edition. 2013. www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf. Accessed January 13, 2015.
- 4.Cheng YJ, Imperatore G, Geiss LS, et al. Secular changes in the age-specific prevalence of diabetes among U.S. adults: 1988–2010. Diabetes Care. 2013; 36: 2690–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Number of Americans with diabetes projected to double or triple by 2050. Press release. October 22, 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 13, 2015.
- 6.Ogden CL, Caroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014; 311: 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Childhood obesity facts. Updated December 11, 2014. www.cdc.gov/healthyyouth/obesity/facts.htm. Accessed January 14, 2015.
- 8.Centers for Disease Control and Prevention. Health, United States, 2011 with special feature on socioeconomic status and health. Hyattsville, MD: National Center for Health Statistics; 2012. www.cdc.gov/nchs/data/hus/hus11.pdf. Accessed May 21, 2012. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Prediabetes. Updated October 21, 2014. www.cdc.gov/diabetes/basics/prediabetes.html. Accessed January 13, 2015.
- 10.Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care. 2009; 32: 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham SA, Riosmena F, Wang J, et al. Decreases in diabetes-free life expectancy in the U.S. and the role of obesity. Diabetes Care. 2011; 34: 2225–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillier TA, Pedula KL. Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care. 2003; 26: 2999–3005. [DOI] [PubMed] [Google Scholar]
- 13.Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013; 36: 3863–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013; 36: 1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008; 31: 596–615. [DOI] [PubMed] [Google Scholar]
- 16.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014; 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Screening for diabetes. Diabetes Care. 2002; 25(suppl 1):S21–S24. [DOI] [PubMed] [Google Scholar]
- 18.Plantinga LC, Crews DC, Coresh J, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010; 5: 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores-Le Roux JA, Comin J, Pedro-Botet J, et al. Seven-year mortality in heart failure patients with undiagnosed diabetes: an observational study. Cardiovasc Diabetol. 2011; 10: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spijkerman AM, Dekker JM, Nijpels G, et al. Microvascular complications at time of diagnosis of type 2 diabetes are similar among diabetic patients detected by targeted screening and patients newly diagnosed in general practice: the hoorn screening study. Diabetes Care. 2003; 26: 2604–2608. [DOI] [PubMed] [Google Scholar]
- 21.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009; 58: 773–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352: 837–853. [PubMed] [Google Scholar]
- 23.UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352: 854–865. Erratum in: Lancet. 1998; 352: 1558. [PubMed] [Google Scholar]
- 24.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006; 355: 2427–2443. Erratum in: N Engl J Med. 2007; 356: 1387–1388. [DOI] [PubMed] [Google Scholar]
- 25.UK Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995; 44: 1249–1258. Erratum in: Diabetes. 1996; 45: 1655. [PubMed] [Google Scholar]
- 26.Pelletier EM, Shim B, Ben-Joseph R, Caro JJ. Economic outcomes associated with microvascular complications of type 2 diabetes mellitus: results from a US claims data analysis. Pharmacoeconomics. 2009; 27: 479–490. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Common eye disorders. Updated April 23, 2013. www.cdc.gov/visionhealth/basic_information/eye_disorders.htm. Accessed January 14, 2015.
- 28.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. 2010; 304: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javitt JC, Aiello LP, Chiang Y, et al. Preventive eye care in people with diabetes is cost-saving to the federal government. Implications for health-care reform. Diabetes Care. 1994; 17: 909–917. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. National diabetes fact sheet, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed January 15, 2015.
- 31.American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014; 37(suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 32.Le TK, Able SL, Lage MJ. Resource use among patients with diabetes, diabetic neuropathy, or diabetes with depression. Cost Eff Resour Alloc. 2006; 4: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loon NR. Diabetic kidney disease: preventing dialysis and transplantation. Clin Diabetes. 2003; 21: 55–62. [Google Scholar]
- 34.Cleveland Clinic. Chronic kidney disease. www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/nephrology/chronic-kidney-disease/. Accessed January 14, 2015.
- 35.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Committee for Quality Assurance. Improving quality and patient experience: the state of healthcare quality 2013. October 2013. www.ncqa.org/Portals/0/Newsroom/SOHC/2013/SOHC-web_version_report.pdf. Accessed Januray 5, 2014.
- 37.Ford ES. Trends in the control of risk factors for cardiovascular disease among adults with diagnosed diabetes: findings from the National Health and Nutrition Examination Survey 1999–2008∗. J Diabetes. 2011; 3: 337–347. [DOI] [PubMed] [Google Scholar]
- 38.American Heart Association. Symptoms, diagnosis & monitoring of diabetes. www.heart.org/HEARTORG/Conditions/Diabetes/SymptomsDiagnosisMonitoringofDiabetes/Symptoms-Diagnosis-Monitoring-of-Diabetes_UCM_002035_Article.jsp?appName=MobileApp. Accessed January 14, 2015.
- 39.Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: a review from the patient's perspective. Ther Clin Risk Manag. 2008; 4: 269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012; 73: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hugtenburg JG, Timmers L, Elders PJ, et al. Definitions, variants, and causes of nonadherence with medication: a challenge for tailored interventions. Patient Prefer Adherence. 2013; 7: 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah NR, Hirsch AG, Zacker C, et al. Factors associated with first-fill adherence rates for diabetic medications: a cohort study. J Gen Intern Med. 2009; 24: 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blandford L, Dans PE, Ober JD, Wheelock C. Analyzing variations in medication compliance related to individual drug, drug class, and prescribing physician. J Managed Care Pharm. 1999; 5: 47–51. [Google Scholar]
- 44.World Health Organization. Adherence to long-term therapies: evidence for action. 2003. http://whqlibdoc.who.int/publications/2003/9241545992.pdf. Accessed January 14, 2015.
- 45.de Ridder D, Geenen R, Kuijer R, van Middendorp H. Psychological adjustment to chronic disease. Lancet. 2008; 372: 246–255. [DOI] [PubMed] [Google Scholar]
- 46.Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. J Clin Epidemiol. 2001; 54(suppl 1):S57–S60. [DOI] [PubMed] [Google Scholar]
- 47.MacLaughlin EJ, Raehl CL, Treadway AK, et al. Assessing medication adherence in the elderly: which tools to use in clinical practice? Drugs Aging. 2005; 22: 231–255. [DOI] [PubMed] [Google Scholar]
- 48.Center for Technology and Aging. Technologies for optimizing medication use in older adults. 2009. www.techandaging.org/MedOpPositionPaper.pdf. Accessed January 14, 2015.
- 49.Stuart B, Briesacher B. Medication decisions—right and wrong. Med Care Res Rev. 2002; 59: 123–145. [DOI] [PubMed] [Google Scholar]
- 50.Smith DL. Compliance packaging: a patient education tool. Am Pharm. 1989; NS29:42–45, 49–53. [DOI] [PubMed] [Google Scholar]
- 51.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008; 28: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005; 353: 487–497. [DOI] [PubMed] [Google Scholar]
- 53.Blackburn DF, Swidrovich J, Lemstra M. Non-adherence in type 2 diabetes: practical considerations for interpreting the literature. Patient Prefer Adherence. 2013; 7: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006; 166: 1836–1841. [DOI] [PubMed] [Google Scholar]
- 55.Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care. 2004; 27: 2149–2153. [DOI] [PubMed] [Google Scholar]
- 56.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005; 43: 521–530. [DOI] [PubMed] [Google Scholar]
- 57.Balkrishnan R, Rajagopalan R, Camacho FT, et al. Predictors of medication adherence and associated health care costs in an older population with type 2 diabetes mellitus: a longitudinal cohort study. Clin Ther. 2003; 25: 2958–2971. [DOI] [PubMed] [Google Scholar]
- 58.Egede LE, Gebregziabher M, Dismuke CE, et al. Medication nonadherence in diabetes: longitudinal effects on costs and potential cost savings from improvement. Diabetes Care. 2012; 35: 2533–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Antoine SL, Pieper D, Mathes T, Eikermann M. Improving the adherence of type 2 diabetes mellitus patients with pharmacy care: a systematic review of randomized controlled trials. BMC Endocr Disord. 2014; 14: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Odegard PS, Gray SL. Barriers to medication adherence in poorly controlled diabetes mellitus. Diabetes Educ. 2008; 34: 692–697. [DOI] [PubMed] [Google Scholar]
- 61.Elliott DJ, Robinson EJ, Anthony KB, Stillman PL. Patient-centered outcomes of a value-based insurance design program for patients with diabetes. Popul Health Manag. 2013; 16: 99–106. [DOI] [PubMed] [Google Scholar]
- 62.Agency for Healthcare Research and Quality. Medication adherence interventions: comparative effectiveness. September 2012. http://effectivehealthcare.ahrq.gov/ehc/products/296/1249/EvidenceReport208_CQGMedAdherence_ExecutiveSummary_20120904.pdf. Accessed January 14, 2015.
- 63.Wertz D, Hou L, DeVries A, et al. Clinical and economic outcomes of the Cincinnati Pharmacy Coaching Program for diabetes and hypertension. Manag Care. 2012; 21: 44–54. [PubMed] [Google Scholar]
- 64.O'Connor PJ, Schmittdiel JA, Pathak RD, et al. Randomized trial of telephone outreach to improve medication adherence and metabolic control in adults with diabetes. Diabetes Care. 2014; 37: 3317–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Magee MF, Khan NH, Desale S, Nassar CM. Diabetes to go: knowledge- and competency-based hospital survival skills diabetes education program improves postdischarge medication adherence. Diabetes Educ. 2014; 40: 344–350. [DOI] [PubMed] [Google Scholar]
- 66.Schmittdiel JA, Uratsu CS, Fireman BH, Selby JV. The effectiveness of diabetes care management in managed care. Am J Manag Care. 2009; 15: 295–301. [PubMed] [Google Scholar]
- 67.Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff (Millwood). 2011; 30: 91–99. [DOI] [PubMed] [Google Scholar]
- 68.Farrell B, French Merkley V, Ingar N. Reducing pill burden and helping with medication awareness to improve adherence. Can Pharm J (Ott). 2013; 146: 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donnan PT, MacDonald TM, Morris AD. Adherence to prescribed oral hypoglycaemic medication in a population of patients with type 2 diabetes: a retrospective cohort study. Diabet Med. 2002; 19: 279–284. [DOI] [PubMed] [Google Scholar]
- 70.Hauber AB, Han S, Yang JC, et al. Effect of pill burden on dosing preferences, willingness to pay, and likely adherence among patients with type 2 diabetes. Patient Prefer Adherence. 2013; 7: 937–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 72.McAdam-Marx C, Bellows BK, Unni S, et al. Impact of adherence and weight loss on glycemic control in patients with type 2 diabetes: cohort analyses of integrated medical record, pharmacy claims, and patient-reported data. J Manag Care Pharm. 2014; 20: 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McAdam-Marx C, Mukherjee J, Bellows BK, et al. Evaluation of the relationship between weight change and glycemic control after initiation of antidiabetic therapy in patients with type 2 diabetes using electronic medical record data. Diabetes Res Clin Pract. 2014; 103: 402–411. [DOI] [PubMed] [Google Scholar]
- 74.Fakhoury WK, Lereun C, Wright D. A meta-analysis of placebo-controlled clinical trials assessing the efficacy and safety of incretin-based medications in patients with type 2 diabetes. Pharmacology. 2010; 86: 44–57. [DOI] [PubMed] [Google Scholar]
- 75.Shyangdan DS, Royle P, Clar C, et al. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011:CD006423. [DOI] [PMC free article] [PubMed]
- 76.Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012; 36: 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diamant M, Van Gaal L, Stranks S, et al. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care. 2012; 35: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gallwitz B, Guzman J, Dotta F, et al. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet. 2012; 379: 2270–2278. [DOI] [PubMed] [Google Scholar]
- 79.Macconell L, Pencek R, Li Y, et al. Exenatide once weekly: sustained improvement in glycemic control and cardiometabolic measures through 3 years. Diabetes Metab Syndr Obes. 2013; 6: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reznik Y, Cohen O. Insulin pump for type 2 diabetes: use and misuse of continuous subcutaneous insulin infusion in type 2 diabetes. Diabetes Care. 2013; 36(suppl 2):S219–S225. [DOI] [PMC free article] [PubMed] [Google Scholar]