Abstract
Appropriate mechanical function of the uterine cervix is critical for maintaining a pregnancy to term so that the fetus can develop fully. At the end of pregnancy, however, the cervix must allow delivery, which requires it to markedly soften, shorten and dilate. There are multiple pathways to spontaneous preterm birth, the leading global cause of death in children less than 5 years old, but all culminate in premature cervical change, because that is the last step in the final common pathway to delivery. The mechanisms underlying premature cervical change in pregnancy are poorly understood, and therefore current clinical protocols to assess preterm birth risk are limited to surrogate markers of mechanical function, such as sonographically measured cervical length. This is what motivates us to study the cervix, for which we propose investigating clinical cervical function in parallel with a quantitative engineering evaluation of its structural function. We aspire to develop a common translational language, as well as generate a rigorous integrated clinical-engineering framework for assessing cervical mechanical function at the cellular to organ level. In this review, we embark on that challenge by describing the current landscape of clinical, biochemical, and engineering concepts associated with the mechanical function of the cervix during pregnancy. Our goal is to use this common platform to inspire novel approaches to delineation of normal and abnormal cervical function in pregnancy.
Keywords: Cervix, Pregnancy, Preterm birth
1. Introduction
The cervix is contiguous with the lower part of the uterus. Its proximal portion is located in the abdomen and its distal portion in the vagina. It has a narrow central canal which runs along its entire length, connecting the uterine cavity and the lumen of the vagina. The opening of this canal into the uterus is called the internal os and the opening into the vagina the external os (Fig. 1A). During pregnancy, the primary biomechanical function of the cervix is to maintain the fetus within the uterus. This requires withstanding multiple forces from the uterus, including the weight of the growing fetus and amniotic sac, as well as passive pressure from the uterine wall. Then, in a dramatic reversal of roles, the cervix markedly softens, shortens and dilates to allow delivery of the fetus. Shortly after delivery, the cervix reforms into its previous shape and consistency. How the cervix manages these complex dynamic changes is an interesting and understudied biomechanics problem.
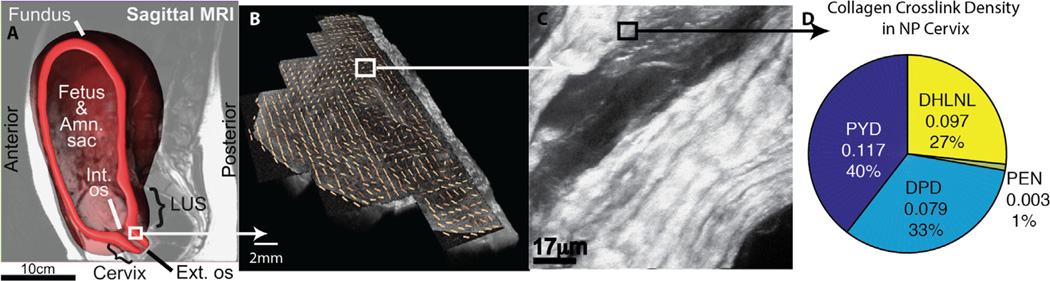
Fig. 1.
Biological length scales in the human cervix. (A) The location of the uterine cervix based on segmentation of magnetic resonance imaging (MRI) data of a 20 week pregnant patient (Fernandez et al., 2015). (B) Collagen fiber directionality of an axial slice of a nonpregnant (NP) cervix imaged via optical coherence tomography (Gan et al., 2014). (C) NP cervical collagen fiber imaged via second harmonic generation (Myers et al., 2009). (D) NP cervical crosslink density (mole-per-mole basis with collagen) content, pyridinoline (PYD), deoxypyridinoline (DPD), dihydroxylysinonorleucine (DHLNL), pentosidine [PEN] (Zork et al., 2015).
Critical problems can occur when the timing and extent of the biomechanical changes are altered. Specifically, premature softening, shortening and dilation, which may be considered early mechanical failure, occurs in cases of spontaneous preterm birth (sPTB). The underlying pathophysiology of these changes is poorly understood despite that preterm birth affects 15 million babies annually, is the leading cause of childhood (< 5 years old) death, and in 2013 was responsible for 1 million deaths (World Health Organization, 2014). The rate of preterm birth has significantly decreased by 2 decades of intense research effort into its pathophysiologies and associated molecular mechanisms. We believe that this lack of progress is partly due to lack of crosstalk between clinicians, engineers and basic scientists, and that progress will require multidisciplinary collaboration between previously distinct areas of expertise such as clinical obstetrics and engineering.
To begin that dialogue, here we provide an engineering framework for studying the mechanical function of the cervix during pregnancy. The main steps include modeling the material behavior of the cervix, characterizing the pelvic anatomy, capturing the appropriate contact conditions between the pelvic soft tissues, and understanding the relevant loading and boundary conditions. Accomplishing this is a challenge because, in addition to understanding the basic material and anatomical parameters, one must consider the changes that occur throughout pregnancy to accommodate the growth and ultimate delivery of the fetus. However, overcoming these engineering challenges could lead to a better understanding of normal and pathological cervical deformation.
2. The clinical problem of preterm birth
2.1. Scope of the problem
Preterm birth is defined as delivery between 20 weeks and 36 weeks+6 days gestation. Medically indicated preterm birth may result from maternal factors (e.g. preeclampsia or placenta previa) or fetal factors (e.g. oligohydramnios or growth restriction). Spontaneous preterm birth (sPTB) was formerly divided into two general categories, namely cervical dysfunction (cervical insufficiency or cervical incompetence) or preterm labor (typically thought to be the result of intrauterine infection or bleeding). A more current understanding is that sPTB from all causes can be seen as part of an extremely complex continuum involving multiple phenotypes (Barros et al., 2015; Solomon and Iams, 2014). The etiology of sPTB is multifactorial, involving diverse precipitating factors such as infection and inflammation, bleeding, poor nutrition, demographics, stress, ethnicity and race, genetic predispositions and many others, all presumably with individual, and overlapping, molecular mechanisms (Gravett et al., 2010). A recent attempt to categorize phenotypes of preterm birth showed that approximately 25% of these births are neither medically indicated nor associated with any known phenotype (Barros et al., 2015).
Organizations such as the March of Dimes have recently celebrated a decline in the preterm birth rate (from 12.8% in 2006 to 11.4% by 2013), but data from the Centers for Disease Control shows that the sPTB rate in 2012 was nearly identical to that in 1997 (Martin et al., 2013; Schoen et al., 2014; Solomon and Iams, 2014). Strategies to address known risk factors (e.g. genitourinary infection and poor nutrition) have been ineffective, as have drug therapies targeted against uterine contractions, infection, or inflammation (Gravett et al., 2010; Solomon and Iams, 2014). The American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine promote intramuscular progesterone treatment in patients with a history of sPTB, and vaginal progesterone supplementation or cerclage (a suture tied around the cervix) in patients with a short cervix in the current pregnancy (American College of Obstetricians and Gynecologists, 2012; Society for Maternal-Fetal Medicine Publications Committee, 2012). Importantly however, the decline in preterm birth has been attributed primarily to provider education, which has resulted in fewer nonmedically indicated deliveries < 39 weeks, fewer teenage pregnancies, less smoking in pregnancy, and fewer twin and triplet pregnancies (Schoen et al., 2014; Solomon and Iams, 2014). Progesterone supplementation and cerclage in selected patients are “probably contributing” according to a 2015 review (Schoen et al., 2014), but this is obviously unclear, as are the mechanisms by which these treatments work, which must contribute to the reason current interventions that are ineffective in the vast majority of patients (American College of Obstetricians and Gynecologists, 2012; Conde-Agudelo et al., 2013; Grobman et al., 2012).
As stated recently by Norman and Shennan, the fact that 95% of sPTB is intractable to current therapies suggests that substantial further research is needed (Norman and Shennan, 2013). An understanding of the molecular mechanisms of the multiple pathways to sPTB is essential to the development of etiologic- and patient-specific interventions for patients at high risk and avoidance of unnecessary and potentially detrimental treatment in those at low risk (Feltovich et al., 2012; Iams and Berghella, 2010).We think the cervix is the logical place to start this investigation because cervical ripening is the last step before labor and delivery in the sPTB pathway, the final common denominator of a multitude of overlapping etiologies (Gracie et al., 2011; Gravett et al., 2010).
2.2. The role of the cervix in PTB and the importance of cervical remodeling
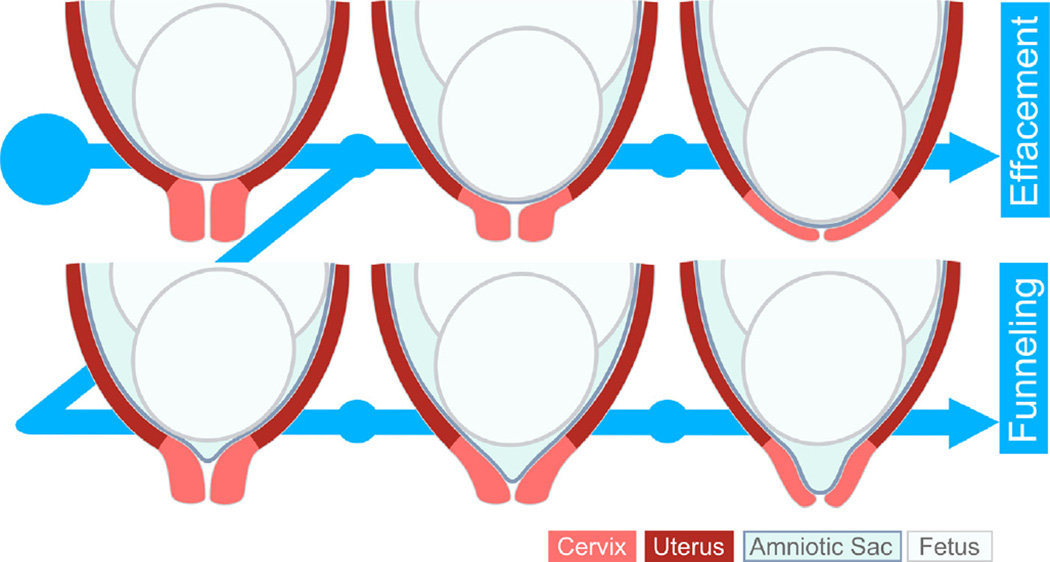
Clinicians use terms such as softening, shortening, funneling, effacing, and dilating to describe the cervical deformation that occurs during pregnancy (Fig. 2). Collectively, these changes are called cervical remodeling and refer to both the tissue's intrinsic material property changes and its resultant anatomical changes. Mouse models demonstrate a distinct separation between an early phase of remodeling that starts soon after conception and continues through delivery (cervical softening) and a later phase, near delivery, that involves rapid, marked softening and shortening (cervical ripening) (Akgul et al., 2012; Holt et al., 2011; Mahendroo, 2012; Read et al., 2007; Timmons et al., 2010a; Word et al., 2007). This process is not yet well characterized in human pregnancy. As in the mouse, cervical softening in human pregnancy begins early, a fact that was exploited to detect pregnancy as early as 6 weeks of gestation in the 19th century before diagnostic blood and urine tests were developed. Recent in vivo mechanical interrogation of the cervix (Badir et al., 2013a; Parra-Saavedra et al., 2011) supports the clinical finding of early, progressive cervical softening (see Section 4.2.2). Starting in mid-pregnancy, the normal human cervix begins to shorten until delivery, confirmed by longitudinal study of ultrasound cervical length (Iams et al., 1996). In addition, there is a clear relationship between cervical softening and the organization and composition of its extracellular matrix (ECM); specifically, cervical softening and shortening relate to dysfunctional ECM remodeling (House et al., 2009b).
Fig. 2.
Cervical deformation patterns and clinical definitions: Cervical length is clinically measured as the portion of the cervix that is closed. Effacement progresses in normal pregnancy when the fetal head descends and shortens the cervix. Funneling is a pathologic condition related to an abnormal cervical deformation pattern when the membranes slip into the inner canal and the cervix prematurely shortens.
Despite this, clinical assessment of softening remains entirely subjective; the clinician describes the cervix as soft, medium, or firm (Bishop, 1964). Compounding the matter, they often use the terms softening and ripening and remodeling interchangeably. This imprecision obscures the clinician's ability to effectively describe a patient's clinical findings to another clinician, let alone someone outside of the obstetric profession. To the engineer, cervical softness is a material property that must be defined with appropriate constitutive equations and cervical shortening is a tissue deformation that results from an evolving three-dimensional (3D) stress state and the intrinsic remodeling of the materials constituents (Fernandez et al., 2015; House et al., 2012, 2013; House and Socrate, 2006; Paskaleva, 2007). However, the engineer currently has no effective means to apply these definitions to the clinical situation because of the language mismatch between clinicians and basic scientists. This obscures the search for specific markers of possible pathogenic processes, such as abnormal tissue material property changes or abnormal anatomical considerations. In other words, a common language seems fundamental to progress toward understanding the problem of spontaneous preterm birth.
3. The multi-scale mechanical environment of pregnancy
Studying and characterizing reproductive organs in real-time throughout gestation is understandably challenging. Pregnancy is a protected environment and accessing organs to measure either geometry or material properties during this time is difficult. There is a wide range of biologic length scales that determine the structural response of the reproductive organs during pregnancy (Fig. 1A). These factors include features of the pelvic anatomy and the hierarchal material characteristics of the cervix and its surrounding tissues, from the collagen fibrils (~ 10–500 nm) bundling together to form collagen fibers (~ 1–500 µm, Fig. 1C) and the collagen fibers bundling together in a preferred anatomic direction to form an overall tissue ultrastructure (~ 1–10 mm, Fig. 1B).
Insight into the physiologic loads experienced during pregnancy and the load-carrying capability of the cervix have been derived from finite element models (Fernandez et al., 2015; House et al., 2012, 2013; Mahmoud et al., 2013; Paskaleva, 2007), mechanical and biochemical studies of ex vivo tissue specimens (Conrad et al., 1980; Conrad and Ueland, 1976, 1979; Fernandez et al., 2013; Gan et al., 2014; Myers et al., 2008, 2010; Oxlund et al., 2010a,b; Petersen et al., 1991; Rechberger et al., 1988; Yao et al., 2014), in vivo mechanical and biochemical interrogations of the cervix (Badir et al., 2013a; Bauer et al., 2007; Feltovich et al., 2010, 2012; Feltovich and Hall, 2013; Hee et al., 2014; House et al., 2005, 2009; Hricak et al., 1990; Maldjian et al., 1999; Mazza et al., 2006, 2013; Parra-Saavedra et al., 2011), and theoretical mechanics (Liao et al., 2014; Myers and Ateshian, 2014; Paskaleva, 2007). At the present time, there is no single set of correlating geometric and material property data from a single pregnant patient throughout gestation.
3.1. Anatomy of the pregnant pelvis organ and physiologic loading
Knowledge of the 3D anatomy of the cervix and surrounding structures is essential for a comprehensive understanding of the biomechanical mechanisms leading to clinically-observed cervical shortening (House et al., 2013). A short cervix, associated with an increased risk of sPTB, is clinically defined as less than 25 mm (To et al., 2001). An MRI study of the 3D geometry of the pelvis illustrates the diverse range of the volumetric dimensions of the uterus and the cervix (House et al., 2009). The cervix is generally 3 cm long and 2.5 cm in diameter, although these dimensions vary considerably between patients depending on age, number of previous births, and the menstrual cycle (Bauer et al., 2007). The cervix and upper vagina are supported in the pelvis by the cardinal and uterosacral ligaments, which maintain the position of the cervix during uterine growth (Ramanah et al., 2012). Within the uterus, the fetal membranes are in direct contact with the superior surface of the cervix at the internal os. (Fig. 1A)
Both magnetic resonance imaging (MRI) and ultrasound (US) imaging studies suggest that the anatomical structure most essential to normal cervical function is the internal os. Sonographic images of the cervix reveal that cervical shortening invariably begins at the internal os (Zilianti et al., 1995), where the cervix starts to dilate, leading to a funneled cervical canal (Fig. 2). Early finite element analysis (FEA) demonstrate this clinically-observed deformation pattern showing that the internal os opposes the lateral pull of the uterine wall and the hydrostatic forces of the uterine cavity (Paskaleva, 2007; House et al., 2012, 2013; Fernandez et al., 2015). It is viewed that the internal os dilates first because tissue stresses are higher there compared with the external os (House et al., 2013), and the external os is not significantly loaded until the cervix has significantly shortened (Fernandez et al., 2015). Building FEA models is difficult because of the complexity of pregnancy-related anatomy, incomplete data on tissue properties and the difficulty in defining appropriate boundary conditions and contact. A full validation of these FEA models are still needed, but hypotheses can still be made about the key mechanical factors that influence the load-carrying capability of the cervix during pregnancy. These factors include: cervical material properties, cervical geometry, fetal membrane properties and adhesion, static loading, and uterine contractions.
Cervical material properties
The load-bearing region of the cervix is its dense collagen-rich core, called the cervical stroma (see Section 3.2). Its mechanical load bearing properties arise from the hierarchal organization of its collagen network. The material response of the cervical tissue to loading is nonlinear, anisotropic, and time-dependent (see Section 4). During pregnancy, the stroma undergoes a complex growth and remodeling process that is incompletely understood (Myers and Ateshian, 2014). The outcome of cervical remodeling is tissue that is orders of magnitude softer during pregnancy compared with nonpregnant tissue (Table 1) (Conrad and Ueland, 1976; Myers et al., 2008, 2010; Rechberger et al., 1988; Yao et al., 2014). Premature softening is associated with preterm shortening and sPTB, but it is not clear how these material characteristics of the cervix influence its mechanical function under in vivo loading conditions.
Table1.
Cervical tissue tensile tangent moduli from uni-axial ex vivo mechanical tests. The strain level reported is location of the tangent moduli. PG=pregnant, NP=nonpregnant, ex.=external, int.=internal, circ=circumferential, long.=longitudinal, PGE2=Prostaglandin E2, CI=cervical insufficiency, instan=instantaneous, eq=equilibrium.
| Reference | Specimen description | Strain rate strain level | Tangent modulus (MPa) |
|---|---|---|---|
| Conrad and Ueland, 1976 | NP and PG Term | Rate: 0.22 %/s | NP: 0.055 |
| ex. os biopsy (5 × 10 mm) | Level: 0.8 | PG: 0.021 | |
| Conrad and Ueland (1979) | PG Term Non-treated | Rate: 0.22 %/s | PG: 0.027 |
| PG Term Oxytocin- & PGE2-treated | PG Oxytocin: 0.029 | ||
| ex. os biopsy | PG PGE2 0.015 | ||
| Conrad et al. (1980) | NP int. os | Not given | NP inner: 0.0643 |
| rectangular circ. strips | NP middle: 0.0413 | ||
| in 3 radial zones | NP outer: 0.0242 | ||
| Rechberger et al. (1988) | NP and PG at post partum | Rate: 1.11 %/s | NP inner: 40.3 |
| ex. os biopsy (2 × 2 × 12 mm) | Level NP: 0.61 | PG: 1.84 | |
| Level PG: 0.93 | |||
| Petersen et al. (1991) | NP int. and ex. os | Rate: 5.55 %/s | NP int os circum: 4 |
| biopsy taken in | Strain: level: 0.7 | NP int os long: 3.9 | |
| circ. and long. directions | NP ex. os circum: 3.6 | ||
| (1–2 × 10 mm) | NP ex. os long: 3.2 | ||
| Oxlund et al. (2010a,b) | NP with and w/out history of CI | Rate: 4.17 %/s | NP no history: 5.33 |
| ex. os biopsy | Level: 0.49 | NP w/ history of CI: 5.41 | |
| taken in long. direction (2 × 15 mm) | |||
| Myers et al. (2010) | NP and PG Term | Rate: 0.1 %/s | NP instan: 0.837 eq. 0.554 |
| rectangular circ. strips (2 × 4 × 10 mm) | Level NP: 0.25 | PG instan: 0.003 eq. 0.003 | |
| Level PG: 0.35 |
Cervical geometry
The 3D geometry of the cervix and the uterocervical angle has an important effect on the load distribution and stretch pattern within the internal os (Fernandez et al., 2015; Paskaleva, 2007). Large anatomical changes occur with fetal growth and these changes are associated with changes in cervical length, cervical volume, and uterocervical angle (House et al., 2009). A better understanding of relationships between anatomical geometry and cervical loading has direct clinical relevance. For example, modification of the uterocervical angle was postulated to be the mechanism of PTB prevention for the Arabin pessary in recent clinical trials (Goya et al., 2012).
Fetal membrane material properties and adhesion to the lower uterine segment
An under-appreciated factor that influences the amount of stress in the cervical tissue is the fetal membrane. The degree of adhesion between the fetal membrane and lower uterine segment and the material properties of the fetal membrane influence the amount of load that is placed on the cervix (Fernandez et al., 2015; Paskaleva, 2007). Simulation reveals that load sharing occurs between the membrane and cervix when the interface is intact (Fernandez et al., 2015; Paskaleva, 2007) and when the stiffness of the fetal membrane is high (Fernandez et al., 2015). When the adhesion is disrupted or if the fetal membrane stiffness decreases, cervical stresses are increased. We expect better understanding of the mechanical role of the fetal membrane will help clarify (1) how the cervix shortens in response to fundal pressure (the dynamic cervix) and (2) the mechanics of membrane prolapse, which is observed in cases of premature cervical shortening.
Static loading
In the absence of the uterine contractions, the cervix is loaded by intrauterine pressure and gravity. In addition, growth of the amniotic sac results in tensile stresses from the uterine wall. These forces also depend on the support action pelvic floor structures and abdominal wall. We hypothesize that static loading through a combination of uterine growth, hydrostatic pressure and gravity are the dominant loads that cause cervical shortening. Most patients with cervical shortening show no clinical signs of preterm uterine contractions. A better understanding of these static loads is critically needed for a better understanding of cervical deformation.
Uterine contractions
Although uterine contractility is clearly important for preterm cervical changes, many patients with preterm uterine contractions do not develop cervical shortening. In contrast, cervical shortening is often seen when preterm contractions are not prominent. An explanation for these contrasting observations is lacking and highlights a significant knowledge gap. An important goal of future efforts will be study the relative contributions of static loading and uterine contractility and their relative contribution to cervical shortening.
3.2. Cervical stroma ECM components and remodeling
Along with anatomical and loading considerations, the biological composition of the cervix and its surrounding tissues play key roles in how the weight of the fetus will be supported. The load-bearing qualities of these hydrated soft tissues are due to the ECM of the stroma. The cervix is a layered structure with a large core of dense connective tissue called the stroma. The cervical ECM components are maintained by interspersed cervical fibroblasts and smooth muscle cells, where the ECM remodels during pregnancy. (A review of human cervical ECM is presented by House et al. (2009b), with a summary and update of key ECM features and open research questions given here.)
The stroma is primarily hydrated, approximately 75–80% for nonpregnant and 85% for term pregnant tissue (House et al., 2009b). Its ECM is composed of a preferentially-aligned (Aspden, 1988; Gan et al., 2014) and crosslinked (Zork et al., 2015) collagen network that is embedded in a viscous groundsubstance of glycosaminoglycans (GAGs) (Myers et al., 2009; Osmers et al., 1993; Rechberger et al., 1988; Shimizu et al., 1980; Uldbjerg et al., 1983). The type and amount of collagen crosslinking and GAGs, the directionality and dispersion of collagen fibers, the relative amount of cells versus connective tissue, and the content of other matricellular proteins are still being elucidated for nonpregnant and pregnant tissue. The majority of studies have been conducted on ex vivo hysterectomy samples, which are limited to nonpregnant tissue and rare cases of cesarean hysterectomy. ECM remodeling characteristics for human tissue during pregnancy are largely unknown because getting access to the entire cervix, from internal to external os, is rare. Most ECM remodeling studies have been conducted on rodent models of pregnancy, which are hypothesized to have similar ECM changes as humans (Akgul et al., 2012; Akins et al., 2010, 2011; Holt et al., 2011; Mahendroo, 2012; Timmons et al., 2010b), although comparison between animal models and humans is lacking.
3.2.1. Cervical collagen
The cervical collagen fiber network is a collection of entangled and crosslinked collagen fibers, where deformation mechanisms most likely occur at length-scales ranging from the molecule to the tissue (~ 10 nm–10 mm). According to standard colorimetric biochemical assays of biopsy and hysterectomy tissue samples, the collagen content (Types I and III) can range from 54 to 77% of the tissue's dry weight (House et al., 2009b). Reports on the collagen content on term pregnant tissue vary greatly, with reports stating that the collagen content does not decrease during pregnancy (Myers et al., 2009) and reports from others stating it can decrease up to 40% (Uldbjerg et al., 1983). Interestingly, collagen content per dry weight for nonpregnant tissue taken at the internal and external os measured via ultra performance liquid chromatography-electrospray ionization tandem mass spectrometry (UPLC-ESI-MS/MS) is measured to be 34% (Zork et al., 2015). Again, it is unclear why there is such a discrepancy in the reported data for collagen content. Sample size, location, and preparation can answer some of these questions. A large scale mapping study of the collagen network is currently underway (Gan et al., 2014; Zork et al., 2015).
Collagen fiber bundles arrange themselves within the cervix according to anatomical directions (Fig. 1B). The main feature of this fiber ultrastructure is the circumferential band of fibers circling around the inner canal. Early reports suggest that there are zones of preferential collagen fibers, with a middle zone of circumferential collagen and an inner and outer zone of fibers parallel to the inner canal (Aspden, 1988; Weiss et al., 2006). The existence and size of these zones are still being elucidated for the human cervix in both the nonpregnant and pregnant state. Early reports of the collagen organization of the pregnant tissue show that the collagen fibers still maintain their preferential direction, but they are more disorganized and dispersed about the main fiber direction (Gan et al., 2014).
The collagen fibers themselves have a characteristic wave and crimp (Fig. 1C) that influence the nonlinearity of the material response. The fibers are composed of collagen fibrils, which are composed of crosslinked triple-helix collagen molecules. The amount and type of collagen crosslinks are still being elucidated. A recent UPLC-ESI-MS/MS study reveals that nonpregnant tissue contains a mix of mature trivalent crosslinks [pyridinoline (PYD) and deoxypyridinoline (DPD)], immature divalent crosslinks [dihydroxylysinonorleucine (DHLNL)], and the nonenzymatic advanced glycation end product pentosidine [PEN] (Fig. 1D) (Zork et al., 2015). On a mole-per-mole basis the collagen crosslink density for nonpregnant tissue is 0.123, 0.045, 0.094, and 0.005 for PYD, DPD, DHLNL, and PEN, respectively (Zork et al., 2015). The mature crosslinks are hypothesized to confer collagen fiber stiffness (Marturano et al., 2014), where an increase in collagen crosslinks may cause an alteration to the magnitude and nonlinearity of the collagen fiber material response. These crosslinks have yet to be measured for pregnant human tissue and correlated to mechanical properties for human tissue.
Cervical remodeling during pregnancy has been measured mainly in rodent tissue and in some human tissue studies (Akins et al., 2011; Danforth, 1983, 1947; Danforth et al., 1960, 1974; Holt et al., 2011; Leppert, 1992, 1995, 1998; Timmons et al., 2010b; Word et al., 2007; Yoshida et al., 2014a,b), where cervical softening during pregnancy is shown to depend on alterations in the collagen fiber organization, collagen fiber crosslinking, and the GAG and water content. In our preliminary collagen studies, we find evidence of collagen remodeling during pregnancy at multiple hierarchal length-scales of the collagen fiber network. Interestingly, the total collagen content per dry weight is thought to not change throughout remodeling in both humans (Myers et al., 2009) and mice (Akins et al., 2011; Yoshida et al., 2014a). Instead, pregnant human tissue is more soluble in weak acids indicating a reduction of collagen crosslinks (Myers et al., 2009). In mouse cervical tissue, there is a significant decrease in mature crosslinks (pyridinoline [PYD] and deoxypyridinoline [DPD]) during the cervical softening phase in early pregnancy (Yoshida et al., 2014a). This collagen crosslink decrease is accompanied by a decrease in the structural stiffness of whole tissue specimens. After this early softening stage, there is a continued decrease in mechanical properties as gestation progresses but no further decrease in cervical collagen crosslinking density. Hence, this finding indicates that different mechanisms take over in the later stages of cervical remodeling to further soften the tissue. These collagen crosslinking changes remain to be determined for human tissue.
3.2.2. Glycosaminoglycan ground substance
The contents of the ground substance are still being elucidated, and the role of glycosaminoglycans (GAGs) remains to be determined. Cervical GAGs are present either in the form of proteoglycans (e.g. dermatan sulfate in decorin), or they are embedded in the matrix without a core protein (e.g. hyaluronic acid (HA)) (Myers et al., 2009; Osmers et al., 1993; Shimizu et al., 1980; Uldbjerg et al., 1983). The decorin acts as a collagen organizer, and in other load-bearing tissues it is responsible for maintaining a uniform collagen fibril diameter and consistent collagen fibril distance (Danielson et al., 1997). In the cervical stroma, the role of HA is to imbibe fluid to maintain the osmotic swelling pressure, counteracting the volume restoring forces of the crosslinked collagen. In pregnancy it is hypothesized that the HA is responsible for increasing the swelling to break up the vulnerable collagen network during the last phase of cervical remodeling just before dilation (Akgul et al., 2012). However, recent evidence shows that there is no change in cervical hydration in HA knock-out mice (Akgul et al., 2014).
4. Cervical tissue mechanical properties
Characterizing the material behavior of human cervical tissue using either in vivo or ex vivo methodologies remains a challenge. Each methodology has advantages and drawbacks in terms of its ability to capture tissue remodeling characteristics of pregnant tissue or to obtain enough data to derive fully predictive 3D constitutive material equations. Animal models of pregnancy offer opportunities to test gestation-timed, genetically-altered, or chemically-treated samples. However, it is unclear whether the factors driving tissue remodeling in these animal models are similar enough to humans, e.g. the hormonal landscape and/or the in vivo loading state of the cervix (Elovitz and Mrinalini, 2004). Given such challenges, constitutive model development and understanding the mechanical environment of pregnancy must rely on several complementary approaches. Here we describe the conclusions gained from recent reports on the mechanical properties of the human cervix, discuss the validation of multiple results from separate studies, and describe how these results can be used together to draw conclusions about the role of the cervix in pregnancy.
4.1. Ex vivo tissue analysis
Ex vivo mechanical tests allow for control over definitions of boundary and loading conditions such that careful analysis of the tissue's stress response to various loading scenarios can be assessed. Age is known to affect the mechanical properties of nonpregnant cervical tissue. Spherical indentation tests reveal that older samples have a lower force response to compressive indentation (Yao et al., 2014), while in another study the uni-axial tensile response to loading for older tissue is higher (Oxlund et al., 2010b). Age is an important factor because most ex vivo studies have been conducted on hysterectomy specimens, and the age of the hysterectomy patient is often older than that of a typical pregnant patient. Mechanical testing data on pregnant tissue samples are rare because collecting tissue samples is, of course, difficult. Additionally, pregnant tissue is fragile and tends to damage under specimen preparation and loading. Whole tissue samples have been obtained and tested from cesarean hysterectomy cases of patients with placenta accreta, which may not be representative of normal cervical tissue because accreta is abnormal growth of the placenta into the uterine wall, and it is not known whether similar abnormalities exist within the cervix in these cases.
Table 1 summarizes uni-axial tension results reported in the literature for human cervical tissue. The tangent moduli are calculated as the linear slope of the engineering stress versus strain curve. Each study measured this tangent at different strain rates and levels of strain. Given that material response of cervical tissue is nonlinear and time-dependent, pulling the tissue at various rates and measuring the tangent modulus at different strains will influence the modulus reported. Additionally, because the tissue is known to be anisotropic, the direction of testing also influences results. To compare, all results are converted to MPa and the strain rates and levels are listed. The tangent moduli in uni-axial tension for nonpregnant tissue are reported to be in the range 0.0242–40.3 MPa, and the percent change in the moduli from nonpregnant to term ranges from −62% (Conrad and Ueland, 1976) to −95% (Rechberger et al., 1988) and −99.5% (Myers et al., 2010). It is unclear why such a large range in reported moduli exist for human cervical tissue. The variation in testing protocols, tissue age, and parity could explain the orders in magnitude in difference in the tangent tensile modulus between the studies. Hence, comparisons can only be made between samples of the same study and patients with similar obstetric backgrounds.
Taking these limitations into account, important key features of the material behavior have been discovered. Human tissue tests reveal that the dense collagenous core of the cervix is similar to other load-bearing soft tissues in that its material response to loading is anisotropic, nonlinear, and time-dependent. The main features of cervical tissue behavior are: (1) term pregnant tissue is orders of magnitude softer than nonpregnant tissue (Table 1), (2) pregnant tissue has a higher hydraulic permeability compared to nonpregnant tissue (Fernandez et al., 2013), (3) the tensile stress response to deformation is larger than the compression response (Myers et al., 2008, 2010, 2015; Yao et al., 2014), (4) tissue displays a large amount of stress relaxation after a ramp-hold in deformation (Myers et al., 2008, 2010; Petersen et al., 1991; Yao et al., 2014), and (5) there is a large range in tissue properties between patients due to diverse obstetric backgrounds (as seen in Table 1) Myers et al., 2008, 2010; Oxlund et al., 2010a,b; Yao et al., 2014. Continued mechanical testing is needed to determine the extent of anisotropy, heterogeneity, and time-dependent visco- or poroelastic deformation mechanisms on the material behavior of the tissue at different length and time scales. Once these challenges are overcome and an appropriate constitutive model is derived, datasets in the literature can be better interpreted and compared.
4.2. In vivo tissue analysis
In vivo interrogation of the cervix during pregnancy allows characterization of longitudinal changes over the course of the gestation. The driving motivation for the development of in vivo tools is both investigational and diagnostic in nature, where the ultimate clinical goal is to uncover cervical biomarkers that are predictive of sPTB. Before this clinical goal is met, engineering challenges must be overcome. These challenges include noninvasive design while sufficiently probing the tissue and interpretation of results given complex material behavior and boundary conditions. Here we review the latest in vivo mechanical testing data on human cervical tissue, and discuss the advances of mechanical aspiration and ultrasound on the study of cervical material characterization.
4.2.1. Balloon inflation – EndoFlip
The most recent mechanical testing data for pregnant cervical tissue reports the inflation-displacement response of 5 early pregnant and 6 term pregnant patients, where the inner canal of the cervix was pressurized using a balloon inflation device called the EndoFlip (Hee et al., 2014). Because of the positioning of the device in the inner canal, it was able to capture the deformation behavior from the internal to the external os. The pressure-displacement results are interpreted based on a fiber composite material model that assumes three preferentially aligned fiber families homogeneously distributed throughout a thick-walled cylinder (Liao et al., 2014). Fiber directionality is informed by a separate set of x-ray diffraction data, which elucidates the fiber directionality of the collagen in nonpregnant cervix (Aspden, 1988). The balloon is deployed sufficiently slowly such that it is assumed that the transient deformation mechanisms have died away.
Comparing pressure-displacement profiles between the early and late pregnancy groups, results indicate cervical softening from early to late pregnancy (Hee et al., 2014). Further, inner canal displacement is even along the cervical length in the late pregnant patients, suggesting that the external os remodels to the same extent as the internal os, which conflicts with other studies. This may be because balloon inflation response is influenced by the complex boundary conditions and shape of the cervical anatomy. For example, pressure-displacement results are influenced by potential bending of the cervix and the complex interplay between the cervix, lower uterine segment, and fetal membrane. This example of boundary condition interaction highlights the challenges in interpreting in vivo mechanical testing data. An approach that employs accurate geometric factors and additional information in regard to the tissue's ultrastructure is needed to determine comparative tissue material properties. Regardless, these results are invaluable because obtaining such data is difficult to accomplish. Combining these data, along with other evidence, should aid in the determination of cervical remodeling characteristics.
4.2.2. Mechanical aspiration
The aspiration technique is among the few existing quantitative, in situ and in vivo methods to determine biomechanical properties of soft human tissue. It uses a handheld device applied on the tissue of interest during noninvasive procedures, as well as in minimally invasive and invasive surgery. It employs a measuring principle called pipette aspiration, originally developed to handle cells under the microscope and later to investigate their mechanical properties. This approach enables local, non-destructive and thus in vivo-compatible measurement of soft tissue biomechanical properties. Applications on the liver and intra-abdominal organs (Hollenstein et al., 2013; Mazza et al., 2007) provide a basis for benchmarking corresponding constitutive model formulations and information about changes related to various pathologies. First aspiration measurements on the cervix were reported in 2009 (Bauer et al., 2009; Mazza et al., 2006).
Measurement procedure
After speculum insertion, visual monitoring via a mini-camera allows gentle placement of the tip of the aspiration tube on the anterior lip of the distal cervix that is protruding into the vagina. The pressure in the tube is reduced by extraction of air through a thin pipe via a peristaltic pump and the cervical tissue is pulled into the aspiration cylinder through the circular opening (8 mm diameter) until the tissue vault reaches and closes the thin pipe at four millimeters peak extension. The obtained value of the closing (suction) pressure pcl is a measure of the stiffness of the tissue. During a typical experiment, a (negative) suction pressure of up to 500 mbar is applied. The on-line version of Badir et al. (2013a) includes a video showing a representative aspiration measurement on the cervix.
Results of measurements on the pregnant cervix
Fig. 3 summarizes the results of aspiration measurements at different gestational ages (Badir et al., 2013b, 2013a). Lower values of pcl correspond to lower stiffness. In a series of 448 measurements in patients throughout pregnancy (n=50) and on nonpregnant subjects (n=50), stiffness in early pregnancy (first trimester) is significantly lower than in the nonpregnant cervix. Further, the negative pressure needed to deform the cervix (pcl) decreases during gestation, indicating a decrease in stiffness. After delivery (average 6 weeks postpartum) consistency recovers to the level of early pregnancy.
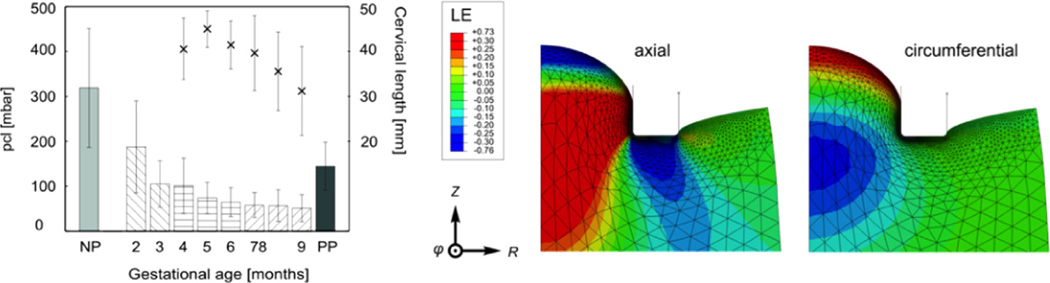
Fig. 3.
Closure pressure pcl of nonpregnant (NP), pregnant (months 2–9, n=50) and post-partum (PP) patients are shown as vertical bars, crosses indicate cervical length (CL, second vertical axis). Standard deviations are indicated as vertical lines. On the right: maximum logarithmic strains (LE) in the aspiration experiment calculated by finite element analysis (axisymmetric model with a neo-Hookean material, from Badir et al., 2013a, with the aspirated tissue in the middle and the aspirator edge as horizontal line next to it). Reproduced with permission from Badir et al. (2013a,b). (For interpretation of the references to color in this figure caption, the reader is referred to the web version of this article.)
The stiffness in late pregnancy drops by a factor of 5 when compared to the nonpregnant cervix (Fig. 3). In Maurer et al. (2015) and Mazza et al. (2013) it is shown that these results are in line with the findings reported by Parra-Saavedra et al. (2011) using the cervical consistency index (CCI), a semi-quantitative measurement of cervical softness obtained by applying pressure to the cervix with a transvaginal probe and quantifying the degree of cervical compression via ultrasound imaging. The time course of biomechanical changes, as indicated by aspiration and CCI measurements, is different from that of cervical length, suggesting that the two approaches might provide different or complementary information. The results of aspiration measurements can also be used to verify the predictive capabilities of constitutive models of cervical tissue. However, this technique suffers from important limitations which might affect its relevance for both diagnosis and biomechanical characterization, as explained in the following paragraphs.
The influence of measurement uncertainties is analyzed in Badir et al. (2013a) based on corresponding finite element based parametric studies using a neo-Hookean material (Fig. 3). The two factors with influence on the measurement of pcl are: (i) the friction coefficient between aspirator and cervix, and (ii) the contact force applied when placing the aspirator on the cervix. The values of pcl are shown to be affected by up to ± 15% due to this uncertainty, which is in line with evaluations of repeatability of aspiration measurements reported in Badir et al. (2013a). Thus, part of the scatter associated with pcl values of each gestational age (Fig. 3) is due to the difficulties of controlling contact conditions between aspirator and cervix. That said, cervical length assessment and ultrasound based methods (see next section) are subject to similar variability.
Another potential limitation in the context of biomechanical characterization of the cervix is that the aspiration test interrogates a relatively small amount of tissue, and only on the distal cervix. This measurement depends on the properties of cervical tissue up to only 10 mm below the epithelium (Badir et al., 2013a), shown by the extension of the region with large deformations shown in Fig. 3 (axial and circumferential strain). Thus, no conclusions can be drawn about the mechanical properties of the middle or the proximal cervix based on the aspiration measurement, and a constitutive model developed to represent the response of the distal cervix as observed in aspiration experiments fails to represent the mechanical behavior of the bulk organ (Maurer et al., 2015). Consequently, more information is needed in order to predict cervical deformation when subjected to physiological loading.
Further, as shown in Fig. 3, the state of deformation is multiaxial, with regions subjected to large elongation and others to large compression, in radial, circumferential and axial directions of the organ. This state of loading is representative of physiological conditions in terms of duration (quasi-static loading) and magnitude (large strains), but not in terms of direction. In fact, the most prominent values of elongation, extending over a large region below the epithelium, are those related to the axial direction (LE-axial in Fig. 3), while physiological loading is expected to elongate the cervix mainly in circumferential direction. Positive strains in this direction are present in the aspiration experiment (red area in circumferential strain, Fig. 3) but they are confined to the surface, close to the epithelium, where the ECM, does not contain the same high density of collagen fibers as the rest of the organ. For small strains, good agreement was found (Badir et al., 2013a) between the mechanical model parameters obtained from an inverse analysis of the aspiration experiment and the results from ex vivo mechanical testing of cervical tissue in Myers et al. (2010). This might indicate that the findings from aspiration measurements can be used to characterize the non-collagenous part of the ECM, having significant influence on the small strain mechanical response. In other words, characterization of the evolution of this component of the ECM during pregnancy might be relevant clinically, and studies to investigate this are currently underway.
4.2.3. Ultrasound
Ultrasound techniques have a distinct advantage in in vivo soft tissue assessment because they are noninvasive and the equipment is relatively inexpensive, safe, portable, and provides real-time results. The ECM has been shown to be a major contributing source of backscatter (echo signals) in soft tissue (Hall et al., 2000). Standard ultrasound methods provide grayscale images that are proportional to the echo signal amplitude, but the amplitude, and therefore those images, depends on soft tissue characteristics as well as ultrasound system settings. Quantitative ultrasound (QUS) methods can overcome some of these limitations because they directly address soft tissue characteristics are less reliant on system settings. QUS methods applicable to the cervix can be generally categorized into techniques that are primarily sensitive to changes in the time-dependent material response of the cervix tissue, tissue hydration status, or ECM (specifically collagen) structure, although these properties are interrelated (Feltovich et al., 2012).
Palpation-type elastography
Palpation-type elastography (quasistatic elastography) typically produces images of mechanical strain, the relative (local) deformation of the tissue. These methods were initially developed for breast imaging but soon after were extended to the prostate, thyroid and beyond. The basic concept is that an ultrasound image (usually the underlying radiofrequency (RF) echo signal or the analytic signal version of the same information) is acquired under an initial loading condition (such as the transducer barely in contact with the tissue). After a small deformation, another ultrasound image is acquired. The source and magnitude of the deformation varies among the systems and tissues of interest. In some cases the deformation is on the order of a few hundred microns at the transducer surface and is typically obtained by lightly pressing on the tissue with the ultrasound transducer. In other cases the deformation is on the order of a few microns and can be applied in a number of ways (such as quiver of the hand holding the transducer, or physiological motion of the subject or organ). Regardless, the two ultrasound images are compared to track motion and calculate strain resulting from the incremental load.
The results of these studies are most easily interpreted when the loading conditions result in uniform stress fields. One caution is that, because most tissues have nonlinear material behavior, the results of these studies can be highly dependent on loading conditions. In the most successful applications of strain elastography, the clinical task is to detect and characterize a local variation in tissue stiffness (such as a tumor) surrounded by normal tissue. Without the use of numerically-intensive modulus reconstructions (Barbone and Bamber, 2002), these methods are not sensitive to overall changes in tissue stiffness. The technique has been attempted by several groups on the cervix, all of which found it challenging to standardize the transducer force for meaningful data interpretation and comparisons between patients (Hernandez-Andrade et al., 2014; Molina et al., 2012; Swiatkowska-Freund and Preis, 2011). Molina et al. noted no statistically significant differences between cervices except in the precise area that received the force from the transducer. Various approaches are currently being employed to address this problem of transducer force standardization (Fruscalzo et al., 2015; Hee et al., 2013; Hernandez-Andrade et al., 2014).
Shear wave elastography
The fact that acoustic wave energy is lost as it propagates through tissue means that there is a net acoustic (radiation) force on the tissue. If the intensity of the wave is high enough, and the duration of the acoustic pulse long enough, the net force on the tissue will be large enough to cause a local (in the location of the acoustic beam) displacement of the tissue on the order of a few microns. This is sufficient motion to induce a transverse wave in the tissue (generally referred to as a shear wave). Since the shear wave speed in soft tissue is on the order of 1–10 m/s, and the ultrasound wave travels a thousand times faster, ultrasound imaging techniques can be used to monitor the propagation of the shear wave and measure its speed. The speed of the shear wave is, under idealized conditions, proportional to the shear modulus of the tissue (Palmeri et al., 2013). Although the results are dependent on initial loading conditions due to the nonlinear elasticity of most tissues, the results are far less dependent on the skills of the user, unlike palpation-type elastography techniques. Further, the methods provide a measure proportional to absolute, as opposed to relative, stiffness and therefore are well suited to estimating changes in overall tissue stiffness (not just local variations in stiffness).
To measure shear wave speeds (SWS) in the cervix, we used a prototype transducer (128 element, 3 mm diameter, 14 mm aperture) because the small aperture array was essential for these studies given that tissue assessment occurs very close to the transducer surface. Further, the pressure distribution near the transducer surface is very complicated and the location of the peak pressure can be relatively far away from the beam axis, which can induce a depth-dependent bias in shear wave speed estimates. These problems were avoided with this transducer. We demonstrated that shear wave speeds (SWS) are sensitive to the stiffness differences in ripened (softened with a prostaglandin agent used clinically for preparing the cervix for invasive procedures or induction of labor) versus unripened cervical tissue both ex vivo and in vivo (Carlson et al., 2014a,b). Additionally we demonstrated that in the former, SWS estimates vary within the cervix (Carlson et al., 2014a), consistent with reported variation in the ECM based on early studies (Aspden, 1988; Danforth, 1983). An in vivo feasibility study of this technique before and after cervical ripening for induction of labor at term demonstrated a highly statistically significant decrease in stiffness (Carlson et al., 2014b). This technique shows promise for assessing cervical softness, although a significant limitation is that precise acquisition and prototype equipment seems necessary to make appropriate conclusions. Another is that this technique provides information on the response to small strain and high rate of deformation, where additional mechanical tests are needed to validate a hyperelastic form of a material constitutive model.
Attenuation
Acoustic attenuation is a measure of the rate of loss in acoustic pressure as a wave propagates. It primarily addresses hydration status, but also collagen structure. The mechanisms for loss of acoustic wave energy are unknown in the cervix, but it has been proposed that changes in tissue hydration and the amount of unbound interstitial material affects attenuation in the cervix, thus attenuation would change throughout pregnancy (McFarlin et al., 2010). Controlled studies in phantoms and animal models have demonstrated that attenuation parameters can be accurately measured and the results are independent of the imaging system used (Nam et al., 2011; Wirtzfeld et al., 2010, 2013). Cross-sectional data during human pregnancy showed a trend toward decreasing attenuation with gestational age, although there was a very large variance in the attenuation estimates (Labyed et al., 2011).
Our recent studies in nonpregnant ripened versus unripened hysterectomy specimens demonstrated that controlling for expected sources of anatomical variation in collagen structure significantly reduces variance in attenuation estimates (Guerrero et al., 2014). Studies in structurally aligned tissues have shown that attenuation is dependent on the angle between the acoustic beam and structural orientation (Insana et al., 1992; Mottley and Miller, 1990; Nassiri et al., 1979; Topp and O'Brien, 2000) and attenuation versus steering angle provides evidence for aligned structures in the cervical ECM (presumably collagen). As well, attenuation estimates in nonpregnant ripened versus unripened hysterectomy specimens correlate with SWS findings.
Backscatter
Acoustic backscatter is the formal name for the echo signals (ultrasound waves) received back by an ultrasound transducer after they have been sent into a tissue or phantom. This QUS technique most directly addresses collagen structure. When ultrasound beams are electronically steered away from normal (01), system-dependent losses occur as some of the echo signal is lost because of changes in transducer sensitivity. This property can be exploited to evaluate tissue microstructure because there will be an increase in backscattered power loss if the beam encounters an anisotropic scatterer (e.g. an aligned, rod-like structure such as collagen), as opposed to if the beam encounters an isotropic scatterer (e.g. sphere, which looks the same from any angle). The excess backscattered power loss (eBSPL; the difference in the normalized backscattered power from tissue versus spherical scatterers) is a simple method to determine the presence of anisotropic scattering structures in the tissue of interest.
As with attenuation estimation, controlled studies in phantoms and in animal models have demonstrated that backscatter parameters can be accurately measured and the results are independent of the imaging system used (Anderson et al., 2010; Nam et al., 2011, 2012a,b). Backscatter parameters have proven useful for monitoring changes in tissues and diagnosing disease (Feleppa et al., 1996; Garra et al., 1989; Insana et al., 1992, 1995). In nonpregnant hysterectomy specimens, significant eBSPL suggests evidence for aligned rod-like scattering sources in the tissue (presumably collagen) (Feltovich et al., 2010). Our recent studies have demonstrated that the eBSPL varies along the length of the cervix, consistent with heterogeneity noted with other QUS parameters (SWS and attenuation estimates).
4.3. Comparing material properties – in vivo versus ex vivo
Different equilibrium constitutive material models have been used to describe cervical ex vivo tension and compression uni-axial material behavior (Myers et al., 2008, 2010, 2015), the in vivo pressure-displacement inner canal response recorded with the EndoFlip balloon inflation device (Liao et al., 2014; Hee et al., 2014), and the in vivo aspiration response of the external os of the cervix (Badir et al., 2013a,b). The two proposed fiber composite models, a continuously distributed fiber model (Myers et al., 2015) and a 3-fiber family model (Liao et al., 2014), are similar in that they attempt to account for the cervical collagen ultrastructure using early reports of fiber directionality (Aspden, 1988; Gan et al., 2014). They differ such that the material model based on the optical coherence tomography data and ex vivo mechanical tests uses a continuously distributed elliptical fiber distribution (EFD) (Gan et al., 2014; Myers et al., 2015), where fibers can only sustain tensile stresses, and the 3-fiber family model based on nonpregnant x-ray diffraction and EndoFlip data uses a fiber network with no fiber dispersion and the fiber strain energy density can sustain both tension and compression (Aspden, 1988; Hee et al., 2014; Liao et al., 2014). The third model, fit to the aspiration data, is a neo-Hookean material, where the tissue is assumed to be incompressible (Badir et al., 2013a). This modeling strategy is suitable because one set of mechanical testing data is considered and the collagen ultrastructure is mostly unknown in the pregnancy.
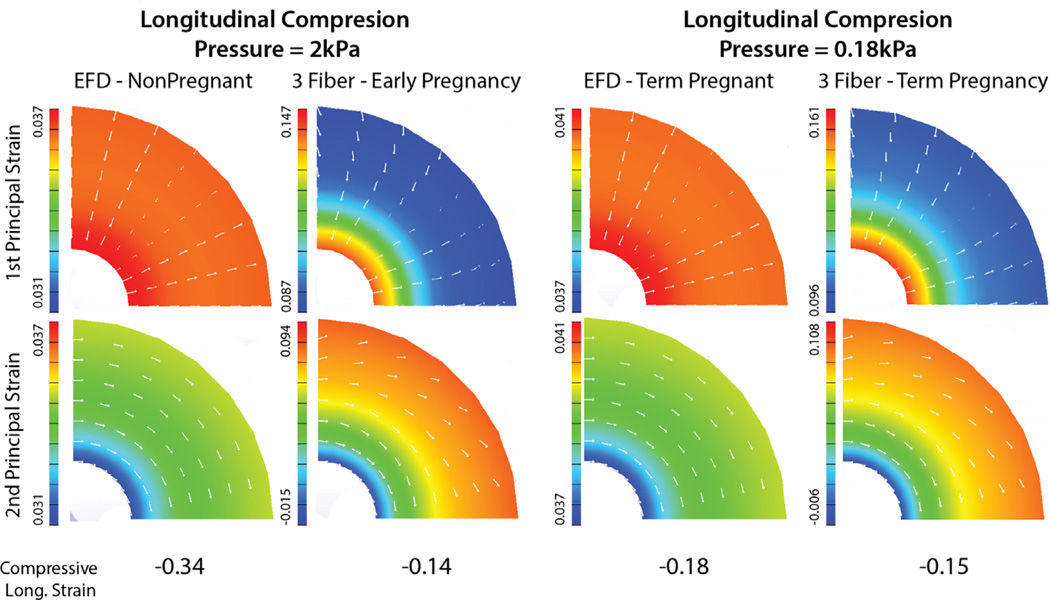
To compare material properties reported in these studies, the principal strain response of a thick-walled cylinder under longitudinal compression (Fig. 4) was calculated using FEA (methodology reported in Myers et al., 2015). Cervical material models and corresponding parameters for the EFD fiber, 3-fiber family, and the neo-Hookean models are reported in Myers et al. (2015), Liao et al. (2014) and Badir et al. (2013a), respectively. Under a uniform compression of the cylinder, the 3-fiber family model produces a twist about the inner canal because of the helical fibers circumferentially ringing around the inner canal (Aspden, 1988; Liao et al., 2014), whereas the EFD and neo-Hookean materials do not produce this twist. Both fiber composite models, with preferential circumferential fibers, reduce the amount of circumferential strain. However, because the 3-fiber family material model does not have fiber reinforcement in the radial direction, strains in this direction are larger than an EFD prediction with similar fiber moduli. Additionally, there is a large gradient in the radial and circumferential strain for the 3-fiber family model because the 3-fiber families are fully aligned with no dispersion. The EFD produces a small strain gradient from the inner to the outer radius, and as expected the isotropic neo-Hookean model produces the same amount of radial and circumferential strains (0.083, 0.17, and 0.32 for NP, 1st trimester, 2nd trimester tissue, respectively, under 2 kPa of compressive load and 0.05 for 3rd trimester tissue under 0.18 kPa).
Fig. 4.
Comparing fiber composite material models proposed in Myers et al. (2015) and Liao et al. (2014) for the human cervix. Principal strain magnitude and directionality are calculated within a thick-walled cylinder (inner radius=8 mm, outer radius=28 mm, length=38 mm) under compressive longitudinal pressure for an elliptically-shaped continuous fiber distribution (EFD) material model (Myers et al., 2015) fit to NP and PG ex vivo mechanical data and a 3-fiber family model fit to PG in vivo pressure-dilation data (Liao et al., 2014). The EFD material produces a more uniform strain pattern compared to the 3-fiber family model, where the 3-fiber family model produces a twist of the cylinder under uniform compression. The two models predict similar levels of longitudinal compression for the term pregnant condition. However, the two models predict different patterns of strain, where the 3-fiber family model produces a larger gradient of strain through the thickness of the cylinder.
These predicted deformation patterns need to be validated with bulk tissue mechanical tests. However, overlapping findings from these studies confirm that the term pregnant cervix is orders of magnitude softer than the nonpregnant cervix. Additionally, comparing the nonpregnant ex vivo EFD results with the in vivo EndoFlip and aspirator data, early softening is present in the 1st trimester of pregnancy. In terms of longitudinal strain, the results from all three models predicted similar values, with 0.18, 0.15, and 0.08 strain throughout the cylinder for the EFD, 3-fiber family, and neo-Hookean models, respectively.
5. In summary: integrating strategies
Complementary methods to characterize cervical remodeling during pregnancy can help derive a fully descriptive material constitutive model for tissue behavior under mechanical loading. Mechanical characterization methodologies described here have limitations and advantages. In terms of capturing overall bulk tissue properties during cervical remodeling, in vivo QUS methods and mechanical aspiration offer noninvasive techniques to characterize cervical properties during pregnancy. These tools are critical to understand cervical tissue changes in early pregnancy and to develop mechanical biomarkers for diagnosis. The major technical advantage of in vivo methods is the ability to conduct longitudinal studies in pregnancy to relate cervical remodeling to clinical outcomes. Small studies of biomechanical and QUS parameters in human ex vivo and in vivo tissue support a model of cervical remodeling that is consistent with animal studies, specifically, that early softening is primarily due to loss of mature collagen crosslinking with preservation of general collagen alignment (microstructure) while late ripening is associated with marked increase in tissue hydration within a vulnerable ECM (Mahendroo, 2012; Yoshida et al., 2014a).
That said, this is a challenging problem and much more information about both ex vivo and in vivo human tissue is necessary for comprehensive understanding of the complex process of cervical remodeling. In terms of constitutive model development, QUS and mechanical aspiration data are limited by complex boundary conditions, contact, testing locations, directionality, strain levels, and strain rates. In vivo mechanical aspiration is currently limited to interrogation of the external os. Conversely, while QUS methods can interrogate the entire structure, they involve small strain and high rate of deformation, which are difficult to interpret on soft anisotropic materials, which in turns makes fitting a 3-D material constitutive model to intrinsic tissue material properties a challenge. Ex vivo mechanical testing offers insight into the large deformation tissue material behavior and allows for predictive material modeling, but ex vivo mechanical tests are limited by possible tissue damage, possible tissue degradation, tissue swelling, ill definitions of the tissue micro and ultrastructure, and the inability to access pregnant tissue. These drawbacks are apparent in the wide range of tensile moduli reported in the literature (Table 1).
Extensive cross-validation of ex vivo and in vivo methodologies is needed to identify meaningful and comparative cervical tissue material properties. This requires overcoming the limitations outlined here, which can certainly be facilitated through a multidisciplinary approach, encompassing medical imaging, quantitative ultrasound, fresh tissue imaging, in vivo mechanical interrogation, advanced material modeling, biochemical analysis, and correlation with animal models of normal and abnormal cervical remodeling. We hope this approach will allow reframing of clinical definitions of cervical remodeling and deformation based on intrinsic material property changes, and rigorous definitions of mechanical loading conditions and strain with respect to the reference configuration of the cervix. Through combining these individual approaches in a collaborative engineering-clinical framework, we hope to begin to develop a biomechanical rubric that will aid in understanding the mechanical function of the cervix, and its role in spontaneous preterm birth.
Supplementary Material
Acknowledgments
K.M., J.V., and R.W. acknowledge the support of the National Science Foundation BRIGE1125670 award, the Office of the Provost at Columbia University, and the Columbia University Medical Center Irving Institute for Clinical and Translational Research, which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health through Grant no. UL1 TR000040. M.B. and E.M. gratefully acknowledge financial support by Swiss National Science Foundation, Grant no. 32003B_156450/1. H.F. and T.J.H. gratefully acknowledge the support of NIH R21HD063031, NIH R21 HD061896, NIH R01HD072077 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and Intermountain Research & Medical Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation, National Institutes of Health, nor the Swiss National Science Foundation.
Footnotes
Conflict of interest statement
None declared.
Uncited reference
Appendix A. Supplementary data
Supplementary data associated with this paper can be found in the online version at http://dx.doi.org/10.1016/j.jbiomech.2015.02.065.
References
- Akgul Y, Holt R, Mummert M, Word A, Mahendroo M. Dynamic changes in cervical glycosaminoglycan composition during normal pregnancy and preterm birth. Endocrinology. 2012;153:3493–3503. doi: 10.1210/en.2011-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgul Y, Word RA, Ensign LM, Yamaguchi Y, Lydon J, Hanes J, Mahendroo M. Hyaluronan in cervical epithelia protects against infection-mediated preterm birth. J. Clin. Invest. 2014;124:5481–5489. doi: 10.1172/JCI78765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins M, Luby-Phelps K, Mahendroo M. Second harmonic generation imaging as a potential tool for staging pregnancy and predicting preterm birth. J. Biomed. Opt. 2010;15:026020. doi: 10.1117/1.3381184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins ML, Luby-Phelps K, Bank R, Mahendroo M. Cervical softening during pregnancy: regulated changes in collagen cross-linking and composition of matricellular proteins in the mouse. Biol. Reprod. 2011;84:1053–1062. doi: 10.1095/biolreprod.110.089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG (2012a) practice bulletin no. 130: Prediction and prevention of preterm birth. American College of Obstetricians and Gynecologists. Obstet. Gynecol. 2012;120:964–973. doi: 10.1097/AOG.0b013e3182723b1b. [DOI] [PubMed] [Google Scholar]
- Anderson JJ, Herd MT, King MR, Haak A, Hafez ZT, Song J, Oelze ML, Madsen EL, Zagzebski JA, O'Brien WD, Hall TJ. Interlaboratory comparison of backscatter coefficient estimates for tissue-mimicking phantoms. Ultrason. Imaging. 2010;32:48–64. doi: 10.1177/016173461003200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspden R. Collagen organization in the cervix and its relation to mechanical function. Coll. Relat. Res. 1988;8:103–112. doi: 10.1016/s0174-173x(88)80022-0. [DOI] [PubMed] [Google Scholar]
- Badir S, Bajka M, Mazza E. A novel procedure for the mechanical characterization of the uterine cervix during pregnancy. J. Mech. Behav. Biomed. Mater. 2013a;27:143–153. doi: 10.1016/j.jmbbm.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Badir S, Mazza E, Zimmermann R, Bajka M. Cervical softening occurs early in pregnancy: characterization of cervical stiffness in 100 healthy women using the aspiration technique. Prenat. Diagn. 2013b;33:737–741. doi: 10.1002/pd.4116. [DOI] [PubMed] [Google Scholar]
- Barbone PE, Bamber JC. Quantitative elasticity imaging: what can and cannot be inferred from strain images. Phys. Med. Biol. 2002;47:2147–2164. doi: 10.1088/0031-9155/47/12/310. [DOI] [PubMed] [Google Scholar]
- Barros FC, Papageorghiou AT, Victora CG, Noble JA, Pang R, Iams J, Ismail LC, Goldenberg RL, Lambert A, Kramer MS, et al. The distribution of clinical phenotypes of preterm birth syndrome: implications for prevention. JAMA Pediatr. 2015 doi: 10.1001/jamapediatrics.2014.3040. [DOI] [PubMed] [Google Scholar]
- Bauer M, Mazza E, Jabareen M, Sultan L, Bajka M, Lang U, Zimmermann R, Holzapfel GA. Assessment of the in vivo biomechanical properties of the human uterine cervix in pregnancy using the aspiration test. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;144:S77–S81. doi: 10.1016/j.ejogrb.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Bauer M, Mazza E, Nava A, Zeck W, Eder M, Bajka M, Cacho F, Lang U, Holzapfel GA. In vivo characterization of the mechanics of human uterine cervices. Ann. N. Y. Acad. Sci. 2007;1101:186–202. doi: 10.1196/annals.1389.004. [DOI] [PubMed] [Google Scholar]
- Bishop E. Pelvic scoring for elective induction. Obstet. Gynecol. 1964;24:266. [PubMed] [Google Scholar]
- Carlson LC, Feltovich H, Palmeri ML. Estimation of shear wave speed in the human uterine cervix. Ultrasound Obstet. Gynecol. 2014a;43:452–458. doi: 10.1002/uog.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LC, Romero ST, Palmeri ML, Muñoz del Rio A, Esplin SM, Rotemberg VM, Hall TJ, Feltovich H. Changes in shear wave speed pre and post induction of labor: a feasibility study. Ultrasound Obstet. Gynecol. 2014 doi: 10.1002/uog.14663. http://dx.doi.org/10.1002/uog.14663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Agudelo A, Romero R, Nicolaides K, Chaiworapongsa T, O’Brien JM, Cetingoz E, Da Fonseca E, Creasy G, Soma-Pillay P, Fusey S, Cam C, Alfirevic Z, Hassan SS. Reports of major impact. Am. J. Obstet. Gynecol. 2013;208:42.e1–42.e18. doi: 10.1016/j.ajog.2012.10.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad JT, Tokarz RD, Williford JF. Dilatation of the Uterine Cervix: Connective Tissue Biology and Clinical Management. New York: Raven Press; 1980. [Google Scholar]
- Conrad JT, Ueland K. Reduction of the stretch modulus of human cervical tissue by prostaglandin E2. Am. J. Obstet. Gynecol. 1976;126:218–223. doi: 10.1016/0002-9378(76)90278-7. [DOI] [PubMed] [Google Scholar]
- Conrad JT, Ueland K. The stretch modulus of human cervical tissue in spontaneous, oxytocin-induced, and prostaglandin E2-induced labor. Am. J. Obstet. Gynecol. 1979;133:11–14. doi: 10.1016/0002-9378(79)90403-4. [DOI] [PubMed] [Google Scholar]
- Danforth D. The morphology of the human cervix. Clin. Obstet. Gynecol. 1983;26:7–13. doi: 10.1097/00003081-198303000-00005. [DOI] [PubMed] [Google Scholar]
- Danforth DN. The fibrous nature of the human cervix, and its relation to the isthmic segment in gravid and nongravid uteri. Am. J. Obstet. Gynecol. 1947;53:541–560. doi: 10.1016/0002-9378(47)90273-1. [DOI] [PubMed] [Google Scholar]
- Danforth DN, Buckingham JC, Roddick JW. Connective tissue changes incident to cervical effacement. Am. J. Obstet. Gynecol. 1960;80:939–945. doi: 10.1016/0002-9378(60)90472-5. [DOI] [PubMed] [Google Scholar]
- Danforth DN, Veis A, Breen M, Weinstein HG, Buckingham JC, Manalo P. The effect of pregnancy and labor on the human cervix: changes in collagen glycoproteins and glycosaminoglycans. Am. J. Obstet. Gynecol. 1974;120:641–651. doi: 10.1016/0002-9378(74)90608-5. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovitz M, Mrinalini C. Animal models of preterm birth. Trends Endocrinol. Metab. 2004;15:479–487. doi: 10.1016/j.tem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Feleppa E, Kalisz A, Sokil-Melgar J, Lizzi F, Liu T, Rosado A, Shao M, Fair W, Wang Y, Cookson M, et al. Typing of prostate tissue by ultrasonic spectrum analysis. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1996;43:609–619. [Google Scholar]
- Feltovich H, Hall T, Berghella V. Beyond cervical length: emerging technologies for assessing the pregnant cervix. Am. J. Obstet. Gynecol. 2012:1–43. doi: 10.1016/j.ajog.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltovich H, Hall TJ. Quantitative imaging of the cervix: setting the bar. Ultrasound Obstet. Gynecol. 2013;41:121–128. doi: 10.1002/uog.12383. [DOI] [PubMed] [Google Scholar]
- Feltovich H, Nam K, Hall TJ. Quantitative ultrasound assessment of cervical microstructure. Ultrason. Imaging. 2010;32:131–142. doi: 10.1177/016173461003200302. [DOI] [PubMed] [Google Scholar]
- Fernandez M, House M, Jambawalikar S, Vink J, Wapner R, Zweben N, Myers K. Investigating the mechanical function of the cervix during pregnancy using finite element models derived from high resolution 3D MRI. Comput. Methods Biomech. Biomed. Eng. 2015 doi: 10.1080/10255842.2015.1033163. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M, Vink J, Yoshida K, Wapner R, Myers K. Direct measurement of the permeability of human cervical tissue. J. Biomech. Eng. 2013;135:021024. doi: 10.1115/1.4023380. [DOI] [PubMed] [Google Scholar]
- Fruscalzo A, Londero AP, Fröhlich C, Meyer-Wittkopf M, Schmitz R. Quantitative elastography of the cervix for predicting labor induction success. Ultraschall Med. 2015 doi: 10.1055/s-0033-1355572. [DOI] [PubMed] [Google Scholar]
- Gan Y, Yao W, Myers KM, Hendon CP. 36th Annual International Conference of the IEEE on Engineering in Medicine and Biology Society (EMBC) IEEE; 2014. An automated 3d registration method for optical coherence tomography volumes; pp. 3873–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garra BS, Insana MF, Shawker TH, Wagner RF, Bradford M, Russell M. Quantitative ultrasonic detection and classification of diffuse liver disease. Comparison with human observer performance. Invest. Radiol. 1989;24:196–203. doi: 10.1097/00004424-198903000-00004. [DOI] [PubMed] [Google Scholar]
- Goya M, Pratcorona L, Merced C, Rodó C, Valle L, Romero A, Juan M, Rodríguez A, Muñoz B, Santacruz B, Bello-Muñoz JC, Llurba E, Higueras T, Cabero L, Carreras E Pesario Cervical para Evitar Prematuridad PECEP Trial Group. Cervical pessary in pregnant women with a short cervix PECEP: an open-label randomised controlled trial. The Lancet. 2012;379:1800–1806. doi: 10.1016/S0140-6736(12)60030-0. [DOI] [PubMed] [Google Scholar]
- Gracie S, Pennell C, Ekman-Ordeberg G, Lye S, McManaman J, Williams S, Palmer L, Kelley M, Menon R, Gravett M, Group tPOR. An integrated systems biology approach to the study of preterm birth using “-omic” technology – a guideline for research. BMC Pregnancy Childbirth. 2011;11:71. doi: 10.1186/1471-2393-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravett MG, Rubens CE, Nunes TM GAPPS Review Group. Global report on preterm birth and stillbirth (2 of 7): discovery science. BMC Pregnancy Childbirth. 2010;10(Suppl. 1):S2. doi: 10.1186/1471-2393-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobman WA, Thom EA, Spong CY, Iams JD, Saade GR, Mercer BM, Tita ATN, Rouse DJ, Sorokin Y, Wapner RJ, Leveno KJ, Blackwell S, Esplin MS, Tolosa JE, Thorp JM, Caritis SN, Van Dorsten JP Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network. 17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. Am. J. Obstet. Gynecol. 2012;207:390.e1–390.e8. doi: 10.1016/j.ajog.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero QW, Carlson LC, Feltovich H, Hall T. 2014 IEEE International Ultrasonics Symposium (IUS) IEEE; 2014. Quantitative ultrasound backscatter parameters in the human cervix; pp. 224–227. [Google Scholar]
- Hall CS, Scott MJ, Lanza GM, Miller JG, Wickline SA. The extracellular matrix is an important source of ultrasound backscatter from myocardium. J. Acoust. Soc. Am. 2000;107:612–619. doi: 10.1121/1.428327. [DOI] [PubMed] [Google Scholar]
- Hee L, Liao D, Sandager P, Gregersen H, Uldbjerg N. Cervical stiffness evaluated in vivo by endoflip in pregnant women. PLoS ONE. 2014;9:e91121. doi: 10.1371/journal.pone.0091121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hee L, Sandager P, Petersen O, Uldbjerg N. Quantitative sonoelastography of the uterine cervix by interposition of a synthetic reference material. Acta Obstet. Gynecol. Scand. 2013;92:1244–1249. doi: 10.1111/aogs.12246. [DOI] [PubMed] [Google Scholar]
- Hernandez-Andrade E, Romero R, Korzeniewski SJ, Ahn H, Aurioles-Garibay A, Garcia M, Schwartz AG, Yeo L, Chaiworapongsa T, Hassan SS. Cervical strain determined by ultrasound elastography and its association with spontaneous preterm delivery. J. .Perinat. Med. 2014;42:159–169. doi: 10.1515/jpm-2013-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenstein M, Bugnard G, Joos R, Kropf S, Villiger P, Mazza E. Towards laparoscopic tissue aspiration. Med. Image Anal. 2013;17:1037–1045. doi: 10.1016/j.media.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Holt R, Timmons B, Akgul Y, Akins M, Mahendroo M. The molecular mechanisms of cervical ripening differ between term and preterm birth. Endocrinology. 2011;152:1036–1046. doi: 10.1210/en.2010-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House M, Bhadelia R, Myers K, Socrate S. Magnetic resonance imaging of three-dimensional cervical anatomy in the second and third trimester. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;144(Suppl. 1):S65–S69. doi: 10.1016/j.ejogrb.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House M, Feltovich H, Hall T, Stack T, Patels A, Socrate S. Threedimensional, extended field-of-view ultrasound method for estimating large strain mechanical properties of the cervix during pregnancy. Ultrason. Imaging. 2012;34:1–14. doi: 10.1177/016173461203400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House M, Kaplan D, Socrate S. Relationships between mechanical properties and extracellular matrix constituents of the cervical stroma during pregnancy. Semin. Perinatol. 2009b;33:300–307. doi: 10.1053/j.semperi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House M, McCabe R, Socrate S. Using imaging-based, three-dimensional models of the cervix and uterus for studies of cervical changes during pregnancy. Clin. Anat. (New York, N.Y.) 2013;26:97–104. doi: 10.1002/ca.22183. [DOI] [PubMed] [Google Scholar]
- House M, OCallaghan M, Bahrami S, Chelmow D, Kini J, Wu D, Patz S, Bhadelia R. Magnetic resonance imaging of the cervix during pregnancy: effect of gestational age and prior vaginal birth. Am. J. Obstet. Gynecol. 2005;193:1554–1560. doi: 10.1016/j.ajog.2005.03.042. [DOI] [PubMed] [Google Scholar]
- House M, Socrate S. The cervix as a biomechanical structure. Ultrasound Obstet. Gynecol. 2006;28:745–749. doi: 10.1002/uog.3850. [DOI] [PubMed] [Google Scholar]
- Hricak H, Chang YC, Cann CE, Parer JT. Cervical incompetence: preliminary evaluation with MR imaging. Radiology. 1990;174:821–826. doi: 10.1148/radiology.174.3.2305065. [DOI] [PubMed] [Google Scholar]
- Iams JD, Berghella V. Care for women with prior preterm birth. Am. J. Obstet. Gynecol. 2010;203:89–100. doi: 10.1016/j.ajog.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N. Engl. J. Med. 1996;334:567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- Insana MF, Wood JG, Hall TJ. Identifying acoustic scattering sources in normal renal parenchyma in vivo by varying arterial and ureteral pressures. Ultrasound Med. Biol. 1992;18:587–599. doi: 10.1016/0301-5629(92)90073-j. [DOI] [PubMed] [Google Scholar]
- Insana MF, Wood JG, Hall TJ, Cox GG, Harrison LA. Effects of endothelin-1 on renal microvasculature measured using quantitative ultrasound. Ultrasound Med. Biol. 1995;21:1143–1151. doi: 10.1016/0301-5629(95)02008-x. [DOI] [PubMed] [Google Scholar]
- Labyed Y, Bigelow TA, Mcfarlin BL. Estimate of the attenuation coefficient using a clinical array transducer for the detection of cervical ripening in human pregnancy. Ultrasonics. 2011;51:34–39. doi: 10.1016/j.ultras.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert P. Cervical softening, effacement, and dilatation: a complex biochemical cascade. J. Matern. Fetal Neonatal. Med. 1992;1:213–223. [Google Scholar]
- Leppert P. Anatomy and physiology of cervical ripening. Clin. Obstet. Gynecol. 1995;38:267–279. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- Leppert P. The biochemistry and physiology of the uterine cervix during gestation and parturition. Prenat. Neonat. Med. 1998;3:103–105. [Google Scholar]
- Liao D, Hee L, Sandager P, Uldbjerg N, Gregersen H. Identification of biomechanical properties in vivo in human uterine cervix. J. Mech. Behav. Biomed. Mater. 2014;39:27–37. doi: 10.1016/j.jmbbm.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Mahendroo M. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction. 2012;143:429–438. doi: 10.1530/REP-11-0466. [DOI] [PubMed] [Google Scholar]
- Mahmoud H, Wagoner Johnson A, Chien EK, Poellmann MJ, McFarlin B. System-level biomechanical approach for the evaluation of term and preterm pregnancy maintenance. J. Biomech. Eng. 2013;135:021009. doi: 10.1115/1.4023486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian C, Adam R, Pelosi M. MRI appearance of cervical incompetence in a pregnant patient. Magn. Reson. Imaging. 1999;17:1399–1402. doi: 10.1016/s0730-725x(99)00073-9. [DOI] [PubMed] [Google Scholar]
- Martin J, Hamilton B, Ventura S, Osterman M, Mathews T. Births: final data for 2011. Natl. Vital Stat. Rep. 2013;62:1–90. [PubMed] [Google Scholar]
- Marturano J, Xylas J, Sridharan G, Georgakoudi I, Kuo C. Acta biomaterialia. Acta Biomater. 2014;10:1370–1379. doi: 10.1016/j.actbio.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, Badir S, Bajka M, Abitabile P, Zimmermann R, Mazza E. In vivo assessment of the biomechanical properties of the cervix: an analysis of available procedures. J. Biomech. 2015 doi: 10.1016/j.jbiomech.2015.02.038. submitted for publication. [DOI] [PubMed] [Google Scholar]
- Mazza E, Nava A, Bauer M, Winter R, Bajka M, Holzapfel GA. Mechanical properties of the human uterine cervix: an in vivo study. Med. Image Anal. 2006;10:125–136. doi: 10.1016/j.media.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Mazza E, Nava A, Hahnloser D, Jochum W, Bajka M. The mechanical response of human liver and its relation to histology: an in vivo study. Med. Image Anal. 2007;11:663–672. doi: 10.1016/j.media.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Mazza E, Parra-Saavedra M, Bajka M, Gratacos E, Nicolaides K, Deprest J. In vivo assessment of the biomechanical properties of the uterine cervix in pregnancy. Prenat. Diagn. 2013:33–41. doi: 10.1002/pd.4260. [DOI] [PubMed] [Google Scholar]
- McFarlin BL, Bigelow TA, Laybed Y, O'Brien WD, Oelze ML, Abramowicz JS. Ultrasonic attenuation estimation of the pregnant cervix: a preliminary report. Ultrasound Obstet. Gynecol. 2010;36:218–225. doi: 10.1002/uog.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina FS, Gómez LF, Florido J, Padilla MC, Nicolaides KH. Quantification of cervical elastography: a reproducibility study. Ultrasound Obst. Gynecol. 2012;39:685–689. doi: 10.1002/uog.11067. [DOI] [PubMed] [Google Scholar]
- Mottley JG, Miller JG. Anisotropy of the ultrasonic attenuation in soft tissues: measurements in vitro. J. Acoust. Soc. Am. 1990;88:1203–1210. doi: 10.1121/1.399751. [DOI] [PubMed] [Google Scholar]
- Myers K, Ateshian G. Interstitial growth and remodeling of biological tissues: tissue composition as state variables. J. Mech. Behav. Biomed. Mater. 2014;29:544–556. doi: 10.1016/j.jmbbm.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers K, Hendon C, Gan Y, Yao W, Vink J, Wapner R. A continuous fiber distribution material model for human cervical tissue. J. Biomech. 2015 doi: 10.1016/j.jbiomech.2015.02.060. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers K, Paskaleva A, House M, Socrate S. Mechanical and biochemical properties of human cervical tissue. Acta Biomater. 2008;4:104–116. doi: 10.1016/j.actbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Myers K, Socrate S, Paskaleva A, House M. A study of the anisotropy and tension/compression behavior of human cervical tissue. J. Biomech. Eng. 2010;132:021003. doi: 10.1115/1.3197847. [DOI] [PubMed] [Google Scholar]
- Myers K, Socrate S, Tzeranis D, House M. Changes in the biochemical constituents and morphologic appearance of the human cervical stroma during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;144(Suppl. 1):S82–S89. doi: 10.1016/j.ejogrb.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Nam K, Rosado-Mendez IM, Wirtzfeld LA, Ghoshal G, Pawlicki AD, Madsen EL, Lavarello RJ, Oelze ML, Zagzebski JA, O'Brien WD, Hall TJ. Comparison of ultrasound attenuation and backscatter estimates in layered tissue-mimicking phantoms among three clinical scanners. Ultrason. Imag. 2012a;34:209–221. doi: 10.1177/0161734612464451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam K, Rosado-Mendez IM, Wirtzfeld LA, Kumar V, Madsen EL, Ghoshal G, Pawlicki AD, Oelze ML, Lavarello RJ, Bigelow TA, Zagzebski JA, O'Brien WD, Hall TJ. Cross-imaging system comparison of backscatter coefficient estimates from a tissue-mimicking material. J. Acoust. Soc. Am. 2012b;132:1319–1324. doi: 10.1121/1.4742725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam K, Rosado-Mendez IM, Wirtzfeld LA, Pawlicki AD, Kumar V, Madsen EL, Ghoshal G, Lavarello RJ, Oelze ML, Bigelow TA, Zagzebski JA, O'Brien WD, Hall TJ. Ultrasonic attenuation and backscatter coefficient estimates of rodent-tumor-mimicking structures: comparison of results among clinical scanners. Ultrason. Imaging. 2011;33:233–250. doi: 10.1177/016173461103300403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassiri DK, Nicholas D, Hill CR. Attenuation of ultrasound in skeletal muscle. Ultrasonics. 1979;17:230–232. doi: 10.1016/0041-624x(79)90054-4. [DOI] [PubMed] [Google Scholar]
- Norman JE, Shennan AH. Prevention of preterm birth—why can't we do any better? The Lancet. 2013;381:184–185. doi: 10.1016/S0140-6736(12)61956-4. [DOI] [PubMed] [Google Scholar]
- Osmers R, Rath W, Pflanz MA, Kuhn W, Stuhlsatz HW, Szeverényi M. Glycosaminoglycans in cervical connective tissue during pregnancy and parturition. Obstet. Gynecol. 1993;81:88–92. [PubMed] [Google Scholar]
- Oxlund BS, Ørtoft G, Brüel A, Danielsen C, Oxlund H, Uldbjerg N. Cervical collagen and biomechanical strength in non-pregnant women with a history of cervical insufficiency. Reprod. Biol. Endocrinol. 2010a;8:92. doi: 10.1186/1477-7827-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxlund BS, Ørtoft G, Brüel A, Danielsen CC, Bor P, Oxlund H, Uldbjerg N. Collagen concentration and biomechanical properties of samples from the lower uterine cervix in relation to age and parity in non-pregnant women. Reprod. Biol. Endocrinol. 2010b;8:82. doi: 10.1186/1477-7827-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeri M, Feltovich H, Homyk A, Carlson L, Hall T. Evaluating the feasibility of acoustic radiation force impulse shear wave elasticity imaging of the uterine cervix with an intracavity array: a simulation study; IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control; 2013. pp. 2053–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Saavedra M, Gómez L, Barrero A, Parra G, Vergara F, Navarro E. Prediction of preterm birth using the cervical consistency index. Ultrasound Obstet. Gynecol. 2011;38:44–51. doi: 10.1002/uog.9010. [DOI] [PubMed] [Google Scholar]
- Paskaleva A. Ph.D. thesis. MIT; 2007. Biomechanics of Cervical Function in Pregnancy – Case of Cervical Insufficiency. [Google Scholar]
- Petersen LK, Oxlund H, Uldbjerg N, Forman A. In vitro analysis of muscular contractile ability and passive biomechanical properties of uterine cervical samples from nonpregnant women. Obstet. Gynecol. 1991;77:772–776. [PubMed] [Google Scholar]
- Ramanah R, Berger MB, Chen L, Riethmuller D, DeLancey JOL. See it in 3D! Researchers examined structural links between the cardinal and uterosacral ligaments. Am. J. Obstet. Gynecol. 2012;207:437.e1–437.e7. doi: 10.1016/j.ajog.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction. 2007;134:327–340. doi: 10.1530/REP-07-0032. [DOI] [PubMed] [Google Scholar]
- Rechberger T, Uldbjerg N, Oxlund H. Connective tissue changes in the cervix during normal pregnancy and pregnancy complicated by cervical incompetence. Obstet. Gynecol. 1988;71:563–567. [PubMed] [Google Scholar]
- Schoen CN, Tabbah S, Iams JD, Caughey AB, Berghella V. Why the united states preterm birth rate is declining. Am. J. Obstet. Gynecol. 2014 doi: 10.1016/j.ajog.2014.12.011. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Endo M, Yosizawa Z. Glycoconjugates (glycosaminoglycans and glycoproteins) and glycogen in the human cervix uteri. Tohoku. J. Exp. Med. 1980;131:289–299. doi: 10.1620/tjem.131.289. [DOI] [PubMed] [Google Scholar]
- Society for Maternal-Fetal Medicine Publications Committee. Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am. J. Obstet. Gynecol. 2012;206:376–386. doi: 10.1016/j.ajog.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Solomon C, Iams J. Prevention of preterm parturition. N. Engl. J. Med. 2014;370:254–261. doi: 10.1056/NEJMcp1103640. [DOI] [PubMed] [Google Scholar]
- Swiatkowska-Freund M, Preis K. Elastography of the uterine cervix: implications for success of induction of labor. Ultrasound Obstet. Gynecol. 2011;38:52–56. doi: 10.1002/uog.9021. [DOI] [PubMed] [Google Scholar]
- Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol. Metab. 2010a;21:353–361. doi: 10.1016/j.tem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol. Metab. 2010b;21:353–361. doi: 10.1016/j.tem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To MS, Skentou C, Liao AW, Cacho A, Nicolaides KH. Cervical length and funneling at 23 weeks of gestation in the prediction of spontaneous early preterm delivery. Ultrasound Obstet. Gynecol. 2001;18:200–203. doi: 10.1046/j.1469-0705.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- Topp KA, O'Brien WD. Anisotropy of ultrasonic propagation and scattering properties in fresh rat skeletal muscle in vitro. J. Acoust. Soc. Am. 2000;107:1027–1033. doi: 10.1121/1.428282. [DOI] [PubMed] [Google Scholar]
- Uldbjerg N, Ekman G, Malmstrom A, Olsson K, Ulmsten U. Ripening of the human uterine cervix related to changes in collagen glycosaminoglycans and collagenolytic activity. Am. J. Obstet. Gynecol. 1983;147:662–666. doi: 10.1016/0002-9378(83)90446-5. [DOI] [PubMed] [Google Scholar]
- Weiss S, Jaermann T, Schmid P, Staempfli P, Boesiger P, Niederer P, Caduff R, Bajka M. Three-dimensional fiber architecture of the nonpregnant human uterus determined ex vivo using magnetic resonance diffusion tensor imaging. Anat. Rec. 2006;288:84–90. doi: 10.1002/ar.a.20274. Part A. [DOI] [PubMed] [Google Scholar]
- Wirtzfeld LA, Ghoshal G, Hafez ZT, Nam K, Labyed Y, Anderson JJ, Herd MT, Haak A, He Z, Miller RJ, Sarwate S, Simpson DG, Zagzebski JA, Bigelow TA, Oelze ML, Hall TJ, O'Brien WD. Cross-imaging platform comparison of ultrasonic backscatter coefficient measurements of live rat tumors. J. Ultrasound Med. 2010;29:1117–1123. doi: 10.7863/jum.2010.29.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtzfeld LA, Ghoshal G, Rosado-Mendez IM, Nam K, Park Y, Pawlicki AD, Miller R, Simpson DG, Zagzebski JA, Oelze ML, Hall TJ, OBrien W. Quantitative ultrasound comparison of MAT and 4T1 mammary 1 tumors in mice and rats across multiple imaging systems. J. Ultrasound Med. 2015 doi: 10.7863/ultra.34.8.1373. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtzfeld LA, Nam K, Labyed Y, Ghoshal G, Haak A, Sen-Gupta E, He Z, Hirtz NR, Miller RJ, Sarwate S, Simpson DG, Zagzebski JA, Bigelow TA, Oelze ML, Hall TJ, O'Brien WD. Techniques and evaluation from a crossplatform imaging comparison of quantitative ultrasound parameters in an in vivo rodent fibroadenoma model. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2013;60:1386–1400. doi: 10.1109/TUFFC.2013.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Word RA, Li X, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin. Reprod. Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- World Health Organization. [accessed 12.03.14];World Health Organization Fact Sheet No. 363. 2014 Nov 17; 2014. http://www.who.int/mediacentre/factsheets/fs363/en/
- Yao W, Yoshida K, Fernandez M, Vink J, Wapner R, Ananth C, Oyen M, Myers K. Measuring the compressive viscoelastic mechanical properties of human cervical tissue using indentation. J. Mech. Behav. Biomed. Mater. 2014;34:18–26. doi: 10.1016/j.jmbbm.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Jiang H, Kim M, Vink J, Cremers S, Paik D, Wapner R, Mahendroo M, Myers K. Quantitative evaluation of collagen crosslinks and corresponding tensile mechanical properties in mouse cervical tissue during normal pregnancy. PLoS ONE. 2014a;9:e112391. doi: 10.1371/journal.pone.0112391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Reeves C, Vink J, Kitajewski J, Wapner R, Jiang H, Cremers S, Myers K. Cervical collagen network remodeling in normal pregnancy and disrupted parturition in Antxr2 deficient mice. J. Biomech. Eng. 2014b;136:021017. doi: 10.1115/1.4026423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilianti M, Azuaga A, Calderon F, Pagés G, Mendoza G. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J. Ultrasound Med. 1995;14:719–724. doi: 10.7863/jum.1995.14.10.719. [DOI] [PubMed] [Google Scholar]
- Zork NM, Myers KM, Yoshida K, Cremers S, Jiang H, Ananth CV, Wapner RJ, Kitajewski J, Vink J. A systematic evaluation of collagen cross-links in the human cervix. Am. J. Obstet. Gynecol. 2015 doi: 10.1016/j.ajog.2014.09.036. URL: 〈 http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=25281365&retmode=ref&cmd=prlinks〉, http://dx.doi.org/10.1016/j.ajog.2014.09.036. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.