Abstract
OBJECTIVE
To review the assessment and treatment of treatment-refractory pediatric obsessive-compulsive disorder (OCD).
METHOD
A PubMed search was conducted to identify controlled trials in pediatric OCD. Additionally, practice guidelines for the treatment of adults and children were further reviewed for references in treatment-refractory OCD across the lifespan.
RESULTS
Pharmacotherapy with selective serotonin reuptake inhibitors (SSRIs) and cognitive-behavioral therapy are effective treatments for pediatric OCD. Evidence suggests that CBT is additionally effective even in pediatric patients with refractory OCD symptoms. Antipsychotic augmentation, raising SSRI dosage, and several glutamate-modulating agents have some evidence of efficacy in adults with treatment-refractory OCD but have not been studied in pediatric populations.
CONCLUSION
Several pharmacological treatment options exist for children with refractory OCD symptoms. However, little evidence-based data exist to guide treatment for our most challenging pediatric OCD patients. Further research is needed to evaluate the efficacy/side-effect profile of commonly used interventions in treatment-refractory pediatric OCD.
Keywords: obsessive-compulsive disorder, cognitive-behavioral therapy, serotonin uptake inhibitors, antipsychotic agents, systematic review
INTRODUCTION
Despite the existence of highly effective, safe treatments in pediatric obsessive-compulsive disorder (OCD), a substantial fraction of children with OCD do not achieve adequate symptom relief. Roughly one-quarter to one-third of children do not experience a treatment response with first-line treatments for OCD.1 A large number of children with OCD judged to be “clinical responders” in treatment studies still have significant residual symptoms. Even with the best evidence-based care provided by the most experienced OCD clinicians (combined treatment in the POTS trial), over 46% of children do not achieve a remission of their OCD symptoms.1 Perhaps an even greater issue in the treatment of pediatric OCD than the efficacy of evidence-based treatments is the lack of availability of these treatments to many children with OCD.
Children with OCD symptoms that do not respond to evidence-based treatments are among the most challenging and difficult patients with OCD to treat. Perhaps the most frustrating aspect in the care of these children is the lack of pediatric research devoted to the problem. This clinical review will focus on the assessment and treatment of children with refractory OCD symptoms after briefly reviewing first-line treatments. Treatment-refractory pediatric OCD is defined as failing to achieve adequate symptom relief despite receiving an adequate course of cognitive-behavioral therapy (CBT) and at least 2 adequate trials of selective serotonin reuptake inhibitors (SSRI) (or clomipramine). For a more extensive review of the assessment and treatment of uncomplicated pediatric OCD, please refer to the recently published AACAP practice parameters.2
METHOD
We searched PUBMED (December 9, 2013) using the medical subject heading (MeSH) term “obsessive-compulsive disorder” and using the search filters (1) “all child (ages 0–18)” and (2) “randomized controlled trials” or “meta-analysis.” Furthermore, several recent evidence-based treatment guidelines and systematic reviews in OCD (across all ages) were scanned for additional references and guidelines.2–8 Finally, evidence and recommendations from the recent AACAP practice parameters in OCD were included in this review.2 Recommendations made in this review are accompanied by a statement regarding the strength of the underlying support that are based on the criteria utilized in AACAP Practice Guidelines (clinical standard, clinical guidance, clinical opinion, or not endorsed).9 Additionally, the evidence level behind each of the recommendations is specified with particular attention in regards to whether studies were conducted in pediatric and/or adult populations.
RESULTS
First-Line Treatments
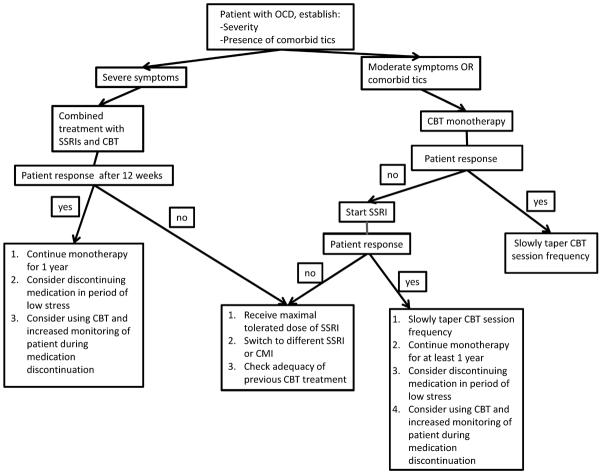
Figure 1 depicts a clinical algorithm for choosing optimal treatment for treatment-naïve children with OCD. Cognitive-behavioral therapy (CBT) and pharmacological treatment with selective serotonin reuptake inhibitors (SSRIs) are first-line treatments for OCD (clinical standard: meta-analysis and randomized controlled trials in children and adults). When OCD first presents, families should be advised to start CBT alone or in combination with medication (clinical standard: meta-analysis and randomized controlled trials in children).
Figure 1.
Algorithm for the management of pediatric obsessive-compulsive disorder (OCD).
Note: CBT = cognitive-behavioral therapy; CMI = clomipramine; SSRI = selective serotonin reuptake inhibitors.
Cognitive-Behavioral Therapy
CBT is a time-limited, present-oriented form of behavioral psychotherapy that teaches children and family members tools to adaptively respond to obsessive-compulsive symptoms. CBT represents a joint therapist and patient effort that together forms a collaborative team to address symptoms. Unless clinically indicated, parents should play a substantial and active role in treatment (i.e., learning to be the child's “coach”) to foster overall gains, homework compliance, and generalization of skills. There are three primary treatment components: psychoeducation, cognitive therapy, and exposure and response prevention.
Methodologically rigorous studies among children and adolescents with OCD have established the superiority of CBT to placebo/waitlist, attention-control conditions, and SSRI medications.1,10–14 A recent meta-analysis demonstrated that the effect size for CBT with (d=1.7) and without (d=1.203) SSRI therapy was superior to SSRI treatment alone (d=0.75).15 In one of the most rigorous studies to date, the Pediatric OCD Treatment Study (POTS I, 2004) prospectively examined the efficacy of combined therapy relative to CBT alone, finding that combined treatment was superior to CBT and sertraline alone (Cohen's d=1.40 vs. 0.97 and 0.67).1 Findings from the POTS II study, which demonstrated the superiority of medication management plus CBT with experienced providers compared to medication management alone or medication management plus instructions in CBT, further emphasize the importance of having CBT treatment conducted by practitioners with experience and high fidelity to all the treatment elements, particularly exposure sessions.16 Indeed, based on efficacy data and patient preferences for non-pharmacological approaches, current practice parameters recommend that clinicians begin with CBT alone for mild to moderate OCD presentations and use combined CBT-SSRI treatment in moderate to severe presentations.17
Selective Serotonin Reuptake Inhibitors
In contrast to the trial data in pediatric depression, there exists nearly indisputable evidence that SSRIs are effective in the treatment of pediatric OCD.4,18,19 Every published, placebo-controlled trial conducted on SSRIs for pediatric OCD demonstrates a significant benefit compared to placebo.5 This data include trials of fluoxetine, fluvoxamine, sertraline, and paroxetine.1,20–25 SSRI doses in these trials typically involved starting at the low end of the US Food and Drug Administration (FDA) dose guidelines and titrating up to the maximum tolerated dose (typically the FDA maximum recommended dose) over 3 to 4 weeks. The FDA recommended target doses for SSRIs in pediatric OCD are fluoxetine 20–60mg, fluvoxamine 100–300mg, sertraline 100–200mg, and paroxetine 20–60mg. The duration of these SSRI trials in pediatric OCD was 10 to 16 weeks. Meta-analyses of these trials suggest that SSRIs for pediatric OCD have a medium-to-large effect size (ES=0.5) and a small number needed to treat (NNT=5) compared to placebo.4,5
What is the most effective dose of SSRIs in pediatric OCD?
In contrast to similar literature in major depressive disorder, meta-analysis of fixed-dose trials in adults with OCD has demonstrated a significant dose response relationship.26 High doses of SSRIs were associated with a significantly greater improvement in OCD symptoms (as measured by Yale-Brown Obsessive Compulsive Scale [Y-BOCS]) and likelihood of treatment response compared to lower doses of SSRIs. Although statistically significant, the incremental treatment gains of using high doses of SSRIs were modest. High-dose SSRI treatment was associated with a 1.4-point greater Y-BOCS improvement compared to low-dose SSRI treatment. Furthermore, the NNT=16–17 favoring high-dose compared to low-dose SSRI treatment was similarly modest. Fixed-dose trials comparing the efficacy of different dosing strategies in pediatric OCD are absent. Randomized, placebo-controlled trials of SSRI agents in pediatric OCD have almost exclusively employed dosing strategies that targeted the maximum FDA recommended dose.
Within fixed-dose SSRI trials in adult OCD, higher doses of SSRIs were associated with an increased rate of dropouts “due to side-effects” but not an increased rate of all-cause dropout when compared to lower doses of SSRIs.26 Given the slightly differing side effects of SSRIs in pediatric populations, one should use caution, especially when generalizing the dose-related tolerability from adults with OCD to children. The specific side effects associated with SSRIs that are increasingly likely in pediatric compared to adult patients include treatment-emergent suicidal ideation, behavioral activation, and mania.4,27 Sexual dysfunction is a common, dose-dependent side effect of SSRI medication that represents a particularly challenging treatment issue in adolescents and adults with OCD.28
Overall, meta-analysis data from fixed-dose SSRI trials in adults with OCD suggest 2 possible dosing strategies: (1) Maximize Tolerability: Start individuals with OCD at the lower end of the FDA recommended dose range for SSRI and wait an adequate duration of time (8–12 weeks) to assess symptom improvement; or (2) Maximize Efficacy: Titrate an individual to the maximum FDA-recommended dose of an SSRI over the course of 3–4 weeks and monitor for symptom improvement. Particularly in children, where there is (1) an absence of data to support a dose-response relationship and (2) a less benign side-effect profile for SSRIs, opting for a dosing strategy to maximize the tolerability of SSRIs initially is prudent (clinical opinion: adult meta-analysis data).26 Nonetheless, in children who do not or only partially respond to minimum-dose SSRI therapy, titrating the dose upward towards the maximum tolerated range is advisable. Escalating SSRI dose upward has evidence of efficacy in OCD and is associated with a much more benign side-effect profile compared to other pharmacological options in pediatric OCD.
How long should SSRI pharmacotherapy be continued?
Current treatment guidelines recommend the continuation of SSRIs for at least one year following successful treatment in SSRI-responsive individuals.6,8 This recommendation is based on several relapse-prevention studies that examined the efficacy of SSRIs compared to placebo in preventing relapse in treatment-responsive adults with OCD. Meta-analysis of the 5 placebo-controlled SSRI discontinuation trials (after acute response) suggests that adult participants randomly assigned to discontinue SSRI pharmacotherapy (placebo) are twice as likely to relapse as those assigned to continued SSRI pharmacotherapy.29 For reference, the likelihood of relapse over the next 6 months with continued SSRI pharmacotherapy in participants who received an acute treatment response is 25–40%.30,31
In contrast to adult OCD, discontinuation trials in pediatric OCD are nonexistent. Children with OCD represent a particularly challenging group in which to make this clinical decision, as a non-trivial proportion of pediatric cases appear to experience a natural remission of symptoms during adolescence that may be unrelated to pharmacotherapy.32,33 Furthermore, there exists a complete absence of child-centered data on which to make this decision. Given the observation that a large proportion of pediatric OCD cases remit during adolescence, SSRI discontinuation is a reasonable treatment strategy in children who have had minimal OCD symptoms for an extended period of time (e.g. 6–12 months) (clinical guidance: adult meta-analysis and randomized controlled trial data). Discontinuation is particularly reasonable in children who are experiencing physical side effects or psychological discomfort from taking the medication. We recommend that these patients should be warned that the time period corresponding to SSRI discontinuation represents a high-risk period for relapse (clinical guidance: adult meta-analysis and randomized controlled trial data). Additionally, when discontinued, SSRIs should be tapered off slowly (so as to minimize any discontinuation symptoms that may provoke OCD relapse), and attempted during periods of low stress where a temporary relapse of symptoms could be most tolerated (clinical opinion: clinical experience across the lifespan). Also, children and their families should be monitored closely and educated on possible warning signs that their OCD may be worsening during the discontinuation period (clinical opinion: clinical experience across the lifespan). In children who have also benefited from CBT, booster sessions during the SSRI discontinuation period may be helpful.
Criteria for Treatment Outcomes in OCD
The greatest challenge in generalizing treatment outcomes across trials is that these terms are inconsistently defined across trials. For instance, treatment response has been defined as a 25%, 30%, or even a 35% reduction in (C)Y-BOCS score (plus/minus being “very much” or “much” improved on the CGI).34 The OCD field appears to be moving towards a consensus where partial treatment response is defined as a 25–35% improvement in OCD symptoms measured by the (C)Y-BOCS, and full treatment response is defined as a greater than 35% improvement in OCD symptoms.
Treatment-refractory OCD is another commonly used term in research and clinical practice without an agreed-upon definition. Perhaps among the most useful concepts in the field is that “treatment-refractory OCD” can be viewed along a continuum in stages from simply not improving sufficiently on a single first-line intervention (CBT or SSRI) to being severely symptomatic despite treatment with multiple SSRIs and/or clomipramine (≥3), CBT, and other augmentation strategies.35 For the sake of this review, a “patient with treatment-refractory OCD” is defined as failing to achieve adequate symptom relief despite receiving an adequate course of CBT treatment and at least 2 adequate trials of SSRI medications (including clomipramine).
Treatment-Refractory OCD
Is my patient really treatment refractory?
Evaluation for treatment-refractory OCD in children should focus on: (1) proper diagnosis; (2) optimal delivery of first-line treatments for pediatric OCD; and (3) moderating factors that might influence treatment delivery and efficacy. Common psychiatric conditions that are commonly misdiagnosed as OCD in younger children include elements of normal development, insistence on sameness, perseverative behaviors and restricted interests associated with autism spectrum disorder, and complex tics.36 Among older children and adolescents, ruminations associated with depressive disorders, prodromal psychotic symptoms, complex tics, and autism spectrum disorder behaviors can be commonly misdiagnosed as OCD.36,37 Another crucial aspect of a thorough evaluation of treatment-refractory OCD is determining whether evidence-based first-line treatments have been optimally delivered. For SSRI pharmacotherapy, this involves determining whether a child has received an adequate dose and duration of prior treatment and whether the family has been compliant with that treatment. A child should not be considered treatment-refractory until he has received two SSRIs at an adequate dose, the maximum tolerated dose, for an adequate duration of treatment (12 weeks total and at least 8 weeks at the maximal dose) (clinical guidance: meta-analysis and randomized controlled trial data in adults). Furthermore, in children who have not been able to tolerate an SSRI at minimum-recommended FDA dose either due to side effects or parental anxiety, these patients should be viewed as treatment-intolerant rather than treatment-refractory. There currently exists no evidence that subtherapeutic SSRI doses are effective in children with OCD. If CBT has already been properly exhausted as a treatment option, then every effort should be made to increase the likelihood of SSRI tolerability, including improved parental education and communication regarding side effects, more gradual dosing titration of SSRI, and prescription of concomitant medication to help with side effects. Lastly, it is recommended that medication compliance should be verified in cases that are not responsive to initial SSRI treatment (clinical opinion: clinical experience across the lifespan).
The adequacy of prior CBT needs to be assessed thoroughly (clinical standard: meta-analysis and randomized controlled trials across the lifespan). Most critically, it needs to be determined whether children and their families have received a sufficient dose of the most effective element of CBT—exposure and response prevention—for an adequate number of sessions and duration of time. Often clinicians say that they are providing CBT but are not incorporating exposure and response prevention sufficiently or at all. Helpful questions to ask the child, parent, and/or previous therapist to determine adequacy of past CBT trials are: (1) did the child ever receive homework from the therapist?; (2) did the child ever make a list of OCD symptoms and rate them in severity?; (3) was the child exposed to a situation designed to reproduce the anxiety associated with the OCD symptoms and asked to resist performing compulsions?; and (4) were the parent(s) involved in the CBT sessions and/or homework? If the answer to any of these questions is in doubt, then repeating CBT trial is advisable.
The last aspect of a thorough evaluation for treatment-refractory OCD is assessing for important moderators of treatment effects that affect treatment delivery and efficacy. The four moderators that appear particularly important in pediatric OCD are: (1) presence of comorbid tic disorders; (2) prominent hoarding symptoms; (3) level of insight into OCD symptoms; and (4) level of parental accommodation. These moderators of OCD treatment effects are discussed below.
Factors Associated With Treatment Outcome
Comorbid Tic Disorders
Tic-related OCD has been added as a specifier for an OCD diagnosis in DSM-5. Tic-related OCD is defined as having “a current or past history of tic disorder.38 Children with Tourette syndrome or chronic tic disorders are at high risk of developing future OCD symptoms, with approximately one-third of children with Tourette syndrome (TS) experiencing OCD at some point during their lives. The onset of OCD symptoms in children with tic disorders appears to occur around the ages of 12–13 years, when tics typically reach their worst-ever severity.32 Tic-related OCD cases tend to have a male predominance and an earlier age of onset compared to non tic-related OCD cases. Additionally, tic-related OCD is associated with a higher proportion of symmetry-related, “just-right,” and forbidden thoughts OCD symptoms.39,40 There is some evidence that children with OCD and comorbid tic disorders may have a good long-term prognosis, whereby OCD symptoms may follow the developmental trajectory of comorbid tic symptoms and improve during adolescence.32 By contrast, in adulthood, there is some evidence that comorbid tics may be associated with poor long-term outcome in OCD cases.41 These data suggest that there may be a substantial portion of children with OCD thatimprove during adolescence, but when that improvement does not occur, OCD symptoms may be particularly resistant to traditional treatments.
Comorbid tic symptoms appear to be an important moderator of SSRI but not CBT effects in OCD. CBT appears equally effective in patients with and without comorbid tics and should be considered the first-line treatment in these patients.42,43 By contrast, SSRI pharmacotherapy appears less effective in reducing OCD symptoms in children with comorbid tics. Pediatric OCD trials of paroxetine and sertraline have demonstrated a poorer response in children with comorbid tics.43 Meta-analysis of antipsychotic augmentation trials in adults with treatment-refractory OCD suggests that OCD patients with comorbid tics are significantly more likely to respond to this intervention.44 The NNT for response of OCD symptoms to antipsychotic augmentation in patients with comorbid tic disorders was 2 compared to 6 in participants without comorbid tic disorders.44 The improved efficacy of antipsychotic augmentation in treating OCD symptoms in patients with comorbid tic disorders is not surprising, since antipsychotic medications are the most effective pharmacological agent in the treatment of tics.45
In children with OCD and comorbid tic disorders, CBT should be encouraged as an initial treatment given the evidence of its improved comparative efficacy relative to medications (clinical guidance: pediatric randomized controlled trial data). SSRIs are still recommended to be utilized as the initial pharmacological treatment in children with OCD, given the worse side-effect profile and the lack of evidence supporting the use of antipsychotic agents for treatment-refractory pediatric OCD (clinical standard: pediatric randomized controlled trial data).
Prominent Hoarding Symptoms
Hoarding disorder is newly recognized as a separate entity from OCD in the DSM-5. Hoarding disorder is characterized by persistent difficulty discarding or parting with possessions, regardless of their actual value. This difficulty must be associated with significant distress or impairment and an excessive accumulation of objects,38 although clinical judgment must be considered in working with youth, as accumulation may be more modest than in adults.46
Prominent hoarding symptoms in children with OCD appear to be associated with poor long-term outcome.32 Additionally, meta-analysis suggests that OCD patients with predominant hoarding symptoms have poor short-term response to traditional OCD treatments.47 OCD patients with prominent hoarding symptoms appear to be at least 1/3 less likely to respond to SSRI pharmacotherapy, behavioral therapy for OCD, or their combination.47 Hoarding's poor prognosis was evident across both pediatric and adult OCD populations.47 Pediatric OCD patients with prominent hoarding symptoms may benefit from CBT with a psychotherapist with particular expertise in the area of hoarding (clinical opinion: clinical experience across the lifespan).
Insight into OCD Symptoms
Approximately 30–40% of youth with OCD present with poor or limited insight into the degree to which they perceive their obsessive-compulsive symptoms as absurd, excessive, and senseless.48–50 This presentation complicates the nature of assessment and treatment, as many youth with poor insight will not consider their symptoms to be problematic and thus resist engaging in treatment. Indeed, presence of poor insight has been linked to reduced CBT and pharmacological treatment outcome.48,51 Clinically, some evidence in adult studies suggests that insight may be improved through successful treatment.52 In those with poor or limited insight, treatment may last for a longer duration and/or move at a slower pace relative to children with good insight.
Family Accommodation
Family accommodation of the child's obsessive-compulsive symptoms occurs in many families of children with OCD.53,54 Family accommodation refers to the degree to which family members accommodate a child's obsessive-compulsive symptoms and the level of distress/impairment that the family members and patient experience as a result of accommodation. This includes actions taken by family members to facilitate rituals (e.g., provide items needed for rituals, hear “confessions”), provide reassurance about fears, acquiesce to the child's demands (e.g., not touching something), decrease child day-to-day responsibilities, or assist with/complete tasks for the child. Family accommodation is usually well intentioned and guided by the family members' desire to reduce the child's level of distress, impairment, and symptom severity.55 However, family accommodation functions in the same manner as a ritual in that obsessive-compulsive distress is reduced, thereby negatively reinforcing further symptom engagement.56
Family accommodation occurs across a range of presentations. It is not strongly linked to obsessive-compulsive symptom severity but has been linked to functional impairment, family conflict/stress, disruptive behavior, and presence of cleaning rituals/contamination fears.55–58 Family accommodation at pretreatment has been negatively associated with treatment response, with data suggesting that the change in family accommodation during treatment was related to posttreatment outcomes.51,59 For these reasons, substantial family involvement is recommended in which the parent is included in treatment sessions with the goal of fostering their understanding of CBT and being able to serve as the child's therapy “coach” (clinical standard: component of randomized controlled trials of CBT in children).
Treatment Strategies for Treatment-Refractory OCD
Data to guide evidence-based treatment in pediatric OCD are severely lacking once first-line interventions are completed. Even the data guiding treatment for adults with refractory OCD are often suboptimal or incomplete. Many commonly utilized interventions for treatment-refractory OCD do not have proven efficacy in double-blind, placebo-controlled trials (e.g. clomipramine augmentation, riluzole augmentation, benzodiazepine use). Furthermore, many interventions for treatment-refractory OCD have only shown efficacy in placebo-controlled trials in single-site, small pilot trials. These trials are in desperate need of replication, as small pilot trials are highly prone to bias (publication bias and reporting bias as well as detection, performance, and selection bias if there are problems with concealment). Rigorous pharmacological research into treatment strategies for treatment-refractory pediatric OCD in the published literature is absent. The goal of this review is to be transparent about the evidence base utilized in the treatment of children with OCD.
Cognitive-Behavioral Therapy
Cognitive-behavioral therapy has demonstrated robust outcomes for augmenting partial or non-response to SSRIs or clomipramine, and thus should always be considered as an initial augmentation approach (clinical standard: randomized controlled trials across the lifespan). Similar to findings in adults with OCD, the addition of weekly CBT to a stable antidepressant in youth with OCD was associated with significantly improved response rates (68.6%) relative to youth who received brief instructional CBT together with continued antidepressant treatment (34.0%) or continued antidepressant treatment alone (30.0%; d = 0.85).16,60,61 Intensive CBT (i.e., daily sessions for three weeks' duration) was also associated with significantly improved outcomes among youth who remained symptomatic following antidepressant treatment.62 Eighty percent of youth responded to intervention with a corresponding large effect on the primary outcome (d = 2.37). The intensive CBT study provides support that a more concentrated course of intervention may have particular benefit among youth who have not responded to prior first-line interventions.62,63 Additionally, studies from the adult literature suggest that using motivational interviewing may be a useful tool to improve outcome or enhance adherence to evidence-based treatments of any kind, including CBT (clinical opinion: randomized controlled trials in adults).64
Raising SSRI Dose
Raising SSRI medications to the maximal tolerated dose within the FDA-recommended dose range is an evidence-based treatment strategy as discussed earlier. Clinical research in adult OCD has also focused on examining whether supratherapeutic dosing, raising SSRI doses above the therapeutic range, confers additional treatment benefits in SSRI-resistant OCD. A randomized, double-blind, controlled trial compared the efficacy of continued sertraline treatment at the maximum recommended dose (200mg) to high-dose sertraline therapy (maximum of 400mg daily) in 66 adults who had significant residual OCD symptoms despite 16-week treatment with sertraline 200mg daily.65 High-dose sertraline therapy was associated with a statistically significant, albeit modest (ES=0.5; NNT for treatment response=15) further improvement in OCD symptoms compared to continued sertraline therapy at the FDA maximum recommended dose. There were no differences in side-effects or dropout rates between the two treatment arms. Escalating SSRI dose above the FDA range in children has been insufficiently studied. The safety profile of supratherapeutic-dose SSRI treatment remains largely unknown, especially in pediatric patients. However, the scant evidence that exists and pediatric clinical experience suggests that it represents a reasonable treatment strategy that is sometimes successful and reasonable in patients with a partial response to maximal-dose SSRI therapy of sufficient duration who have experienced minimal side effects from their medications (clinical opinion: randomized controlled trial in adults).
Clomipramine Augmentation of SSRI
Meta-analyses of placebo-controlled trials in both pediatric and adult OCD suggest that clomipramine may be significantly more effective than SSRIs in the treatment of OCD.5,66 The superiority of clomipramine is robust to adjustment for both publication year and placebo response rates across studies. Despite robustness to any degree of statistical adjustment, it remains possible that clomipramine's apparent superiority across clinical trials may be due to (1) a more SRI-naïve population in earlier studies; (2) improved trial methodology in later SSRI trials (better methodology to ensure blinding, intention-to-treat analysis, etc.); or possibly, (3) clomipramine may indeed be more effective than SSRIs.
Regardless of whether clomipramine is more, equally, or less effective than other SSRIs in pediatric OCD, the authors currently reserve the use of clomipramine until children have not responded to 1 or 2 adequate trials of SSRI medications because of the more significant side-effect profile (clinical opinion: tolerability data in pediatric randomized controlled trials), an adequate SSRI trial being defined as maximal tolerated dose SSRI treatment for 8–12 weeks). The side effects of clomipramine that relegate it to second-line treatment behind SSRIs include increased risk of fatal overdose, cardiac arrhythmia, and lowering of the seizure threshold. More common side effects of clomipramine include weight gain, sedation, and dry mouth. Additionally, clomipramine requires electrocardiogram (EKG) monitoring in children due to potential arrythmogenic effects and blood levels at higher doses due to seizure risk,67 although this monitoring becomes much less substantial when children reach a stable dose of clomipramine.
The addition of clomipramine to maximal dose SSRI therapy is a treatment strategy advocated by some of the most experienced and respected clinicians in OCD treatment (clinical opinion: randomized controlled trials in adults).3,8 Currently, no randomized controlled trials have demonstrated that clomipramine augmentation is more effective than SSRI treatment at the maximum tolerated dose. However, two clinical trials have begun to assess whether clomipramine augmentation can confer a benefit above SSRI monotherapy (albeit at starting doses below the maximum range). In one trial of 16 adult patients with OCD refractory to fluoxetine and clomipramine, patients were randomly assigned to 90 days of treatment with either citalopram (40mg) alone or a combination of citalopram (40mg) and clomipramine (150mg) after a 4 week washout period.68 The citalopram+clomipramine arm showed significantly greater improvement in this unblinded trial. Another trial conducted by the Brazilian OCD Consortium compared the efficacy of 3 treatment strategies in 54 adult OCD patients that did not respond to fluoxetine 40mg after a sufficient duration of treatment. These patients were randomized to either the addition of clomipramine (75mg daily), quetiapine (200mg daily), or raising the fluoxetine dose up to 80mg.69 Patients receiving either the addition of clomipramine or an increase in fluoxetine dosing did significantly better than those receiving quetiapine augmentation. Patients receiving clomipramine augmentation did nominally but not statistically significantly worse than those who received an increase in fluoxetine dose. Neither of the current trials testing clomipramine augmentation began with SSRI doses at the maximum efficacy point. This decision was probably made because of potential concerns with serotonin syndrome resulting from combining SRI medications above the maximum limit. Regardless, adding a small dose of clomipramine (≤75mg) is an augmentation strategy commonly used in clinical practice and deserves further study against other commonly used treatment strategies (continued SSRI monotherapy, supratherapeutic SSRI pharmacotherapy, and antipsychotic augmentation). Trial data concerning the use of clomipramine augmentation in children are nonexistent. Additionally, the use of SSRI augmentation of clomipramine is another sometimes-advocated treatment strategy with a limited evidence base in either adults or children.70 This strategy may be especially useful in patients who cannot tolerate higher doses of clomipramine.
Given the evidence of efficacy for clomipramine in OCD, many clinicians assume that serotonin-norepinephrine reuptake inhibitors (SNRIs), which act by a similar mechanism, may be more effective than SSRIs in treating OCD (not supported: randomized controlled trial data in adults). There exist no published randomized, placebo-controlled trials of SNRIs in adults or children with OCD. Furthermore, the limited data that does exist comparing SSRIs to SNRIs does not support this conclusion. A 12-week randomized controlled trial comparing venlafaxine (300mg/day) and paroxetine (60mg/day) failed to demonstrate a significant difference between treatments, although both groups demonstrated a significant improvement with time.71 Forty-three non-responders in this trial were switched to the other antidepressant for an additional 4 weeks. Patients with OCD switched from venlafaxine to paroxetine had a higher rate of response to the alternative antidepressant (53%) compared to patients with OCD being switched from paroxetine to venlafaxine (19%).72
Antipsychotic Augmentation
Antipsychotic augmentation is by far the most studied intervention for treatment-refractory OCD in adults. Antipsychotic augmentation involves adding a low-dose of an antipsychotic medication (similar to those employed in tic disorders) to continuing SSRI (or clomipramine) pharmacotherapy. Antipsychotic augmentation, like other treatment strategies for treatment-refractory OCD, should be initiated only after patients have failed to improve sufficiently on SSRI pharmacotherapy at the maximum tolerated dose for an appropriate duration of treatment (10–12 weeks). Meta-analysis has demonstrated a significant benefit of antipsychotic augmentation compared to continuing SSRI monotherapy in randomized, placebo-controlled trials in adults with OCD.44 The NNT was 4–5 for inducing a treatment response with antipsychotic augmentation compared to continuing SSRI monotherapy. Within the 9 trials included in the meta-analysis, there was no evidence that one particular antipsychotic is more effective than any other.31,73–80 Risperidone, haloperidol, quetiapine, and olanzapine trials were included in the meta-analysis.81,82 Subsequently conducted randomized, placebo-controlled trials of aripiprazole also suggest that this antipsychotic agent may be useful as a treatment strategy in refractory OCD.74,75 Small, underpowered clinical trials involving head-to-head comparisons between antipsychotic agents have similarly been unable to detect significant differences in efficacy between treatments.83,84 Doses of antipsychotics used in augmentation trials for OCD have been fairly modest compared to acute psychosis/mania trials, and trials usually span at least 6 weeks in duration. Further data from meta-analysis suggest that it is important to wait at least 12 weeks after starting maximal dose SSRI pharmacotherapy before starting antipsychotic augmentation.
Research into the efficacy of antipsychotic augmentation in pediatric OCD is lacking. Circumstantial data from adult studies, strong evidence for efficacy, and evidence of increased efficacy in adult patients with comorbid tics (which constitute a modestly greater proportion of childhood cases) may suggest that antipsychotic augmentation could be effective in childhood OCD. Therefore, antipsychotic augmentation is a reasonable treatment option in children with treatment-refractory OCD (clinical guidance: uncontrolled trials in children and meta-analysis of randomized controlled trials in adults). Nonetheless, clinicians treating children with OCD and child psychiatric clinic researchers have been appropriately hesitant to use/study antipsychotic agents for OCD because of the poor side-effective profile, especially in children. Extrapolating from the adult antipsychotic augmentation data optimistically, only 1 in 6 patients with OCD and 1 in 2 patients with OCD and comorbid tics will benefit from this intervention. Therefore, given the long-term metabolic side effects of antipsychotic agents in children, it is imperative to discontinue these medications if a child has not achieved noticeable improvement after 6–12 weeks and to initiate these medications only after children have failed to achieve adequate symptom relief with multiple adequate SSRI trials and CBT of adequate duration and intensity (clinical guidance: adult randomized controlled trials). Further caution is warranted in expediting antipsychotic augmentation in any OCD treatment algorithm, as CBT outperformed antipsychotic augmentation in a recent randomized blinded trial in adults with OCD.17
Glutamate Modulating Agents
Converging lines of evidence from multiple areas of study have in recent years suggested that glutamate abnormalities may be important in the pathogenesis of OCD.85–88 Cerebral spinal fluid studies have demonstrated higher levels of glutamate in the cerebrospinal fluid in treatment-naïve adults with OCD compared to healthy controls. Neuroimaging studies using magnetic resonance spectroscopy have similarly demonstrated higher levels of glutamate in the caudate nucleus in treatment-naïve children with OCD.89 Several genetic studies have demonstrated an association between S1C1A1 polymorphisms, a glutamate transporter gene, and a diagnosis of OCD. Animal models have also suggested the importance of the glutamate system in OCD. SAPAP3 knockout mice demonstrate compulsive grooming and anxiety behaviors that are reversible with acute fluoxetine treatment.90 SAPAP3 codes for an NMDA receptor scaffolding protein.90 More recently, a mouse model using optogenetic techniques to stimulate glutamatergic projections from the orbitofrontal cortex to striatum demonstrated that chronic (but not acute) stimulation of these glutamatergic neurons led to compulsive grooming behaviors and an anxious phenotype. These symptoms were reduced with chronic fluoxetine administration.91 This converging evidence has led to increasing research on the glutamatergic system in OCD and led to increasing focus on the potential efficacy of glutamate-modulating agents in its treatment.
Riluzole
Riluzole is a glutamate-modulating agent that is FDA approved for amyotrophic lateral sclerosis (ALS). Uncontrolled studies have suggested that riluzole may be effective in the treatment of OCD at doses of 50mg twice daily in both children and adults with OCD.92–95 However, a recent double-blind, placebo-controlled add-on trial in 60 children with OCD failed to demonstrate a benefit of riluzole compared to placebo. Sixteen percent of patients in the riluzole group and 18% of participants in the placebo group responded to treatment.96 It should be noted that a little over a quarter of the pediatric OCD sample had a comorbid autism spectrum disorder. A placebo-controlled riluzole trial in adults with treatment-refractory OCD is ongoing. Although well tolerated by the majority of patients, riluzole's common side effects include tiredness, dizziness, and nausea. Riluzole has also been associated with hepatotoxicity such that liver function needs to be tested every 3 weeks at the beginning of its use and then less frequently thereafter.97 Also, case reports in pediatric OCD have associated its use with pancreatitis in several children taking multiple concomitant medications.94,96 Riluzole should only be a pharmacological option in children who have failed to improve on other more evidence-based treatments for OCD (clinical opinion: positive uncontrolled studies in pediatric populations and negative, underpowered randomized controlled trial in children).
Ketamine
Ketamine is a potent NMDA receptor antagonist that is FDA approved as a pediatric anesthetic agent at higher doses. Numerous studies have also demonstrated that ketamine given intravenously at a dose of .5mg/kg over 40 minutes leads to potent antidepressant effects that peak 1–3 days following infusion and dissipate 1–2 weeks following initial infusion. Remission rates of depressive symptoms in these trials are consistently above 50% within the first week of treatment.98 The antidepressant effects of ketamine have been replicated in multiple treatment trials including some with active controls. Uncontrolled studies have suggested that the short-term antidepressant effects of ketamine can be maintained with repeated infusions, although the side effects of long-term ketamine use in this dosage regimen is unknown.98
Two trials have examined the acute effects of ketamine in adults with treatment-refractory OCD. The first open trial examined the effects of intravenous ketamine (0.5mg/kg over 40 minutes) in 10 adults with treatment-refractory OCD as an add-on to their existing medications.99 An extremely short-lived benefit of ketamine was observed for OCD during the first 3 hours following infusion. However, none of the 10 patients exhibited a response to ketamine 1–7 days following infusion. Additionally, in the 7 patients with comorbid depression, ketamine significantly reduced comorbid depressive symptomology to a greater extent than OCD symptoms from 1–7 days following infusion. Four of 7 patients were judged to be responders to ketamine in terms of their depression symptomology.99 A second, saline-controlled crossover trial in 15 adult patients with OCD but washed off their OCD medications as outpatients demonstrated a significant benefit of ketamine compared to placebo during the first week after ketamine infusion.100 Fifty percent of patients with OCD (compared to 0% on placebo) responded to ketamine infusion.100 Additionally, there were significant carryover effects with ketamine such that the benefits of treatment lasted beyond 1 week. The differing results between these two studies may be attributable to (1) the differences between concomitant medications received by participants between studies; (2) difference in underlying severity between patient populations (the former involved inpatients with refractory OCD whereas the latter involved refractory patients with OCD who could be washed off current medications as outpatients); and (3) issues with blinding inherent with the short-term psychotomimetic side effects of ketamine infusion. Future studies will be needed to definitely establish the efficacy of ketamine in adult populations. Intravenous and intranasal ketamine is commonly used in pediatric populations for pediatric sedation in short-term procedures, but its safety profile for mental disorders is unknown. Given the possible but rare cardiovascular side effects associated with ketamine infusion, it should only be practiced by experienced physicians in appropriate facilities, if at all in children.
Memantine
Memantine is a non-competitive NMDA receptor antagonist that is FDA approved for the treatment of Alzheimer's disease. A single, randomized, double-blind, placebo-controlled trial in 38 adults with OCD examined the efficacy of concurrent addition of memantine (20 mg/day titrated up over 1 week) or placebo to fluvoxamine 200mg/day.101 Fluvoxamine+memantine (89% response rate) was demonstrated to be superior to fluvoxamine+placebo (32% response rate) in this 8-week trial. No placebo-controlled augmentation trials have examined the efficacy in treatment-refractory OCD. However, a flexible-dose case-controlled study suggested modestly greater improvement with memantine compared to patients in a matched control group.102 Case report-level data have anecdotally suggested the efficacy of memantine in children with treatment-refractory OCD.103 Memantine represents a potential pharmacological option in children who have failed all evidence-based treatments for OCD (clinical opinion: randomized controlled trials in adults).
Topiramate
Topiramate is an antiepileptic agent that acts by directly inhibiting (AMPA)/kainate glutamate receptors. A double-blind, placebo-controlled trial in 49 adults with OCD who failed to respond to at least 1 SSRI trial of adequate dose and duration demonstrated efficacy of topiramate.104 Topiramate was increased weekly in 25mg increments to a maximum dose of 200mg daily. Fifty percent of patients receiving topiramate had a treatment response compared to 0 out of 25 patients in the placebo group. A second randomized, placebo-controlled study of topiramate (up to 400mg/day) in 36 adults with treatment-refractory OCD failed to detect a significant difference between topiramate and placebo.105 In secondary analysis, topiramate was superior to placebo in reducing Y-BOCS compulsions. However, topiramate was poorly tolerated in this trial, with 28% of patients receiving topiramate discontinuing due to tolerability and 39% requiring a dose reduction. Little data exist regarding the efficacy of topiramate in children with OCD. However, the safety profile of topiramate in children is well known from trials of epilepsy and migraines. Tiredness, drowsiness, dizziness, loss of coordination, concentration difficulties, and speech and language problems are known side effects of topiramate. Topiramate represents a pharmacological option in children who have failed to improve on other, more evidence-based treatments for OCD (clinical opinion: conflicting randomized controlled trials in adults).
N-Acetylcysteine
N-acetylcysteine (NAC) is a naturally occurring amino acid that has been used safely for decades as an antioxidant agent and as an antidote for acetaminophen overdose. Due to its benign safety profile, NAC is available as an over-the-counter medication at a cost of less than 25 cents a day. More recently, NAC has been demonstrated to be a glutamate modulating agent.
A randomized, placebo-controlled trial in 48 adults with OCD who failed to respond to 12 weeks of SSRI monotherapy compared the efficacy of NAC (2,400mg/day) compared to placebo. NAC was started at 600mg/day and then doubled weekly to a final dose of 2,400mg/day (divided into 2 doses). NAC was superior to placebo after 12 weeks of treatment, with 53% of patients responding in the NAC group compared to 15% in the placebo group. Clinical trials are currently underway studying the efficacy of NAC in pediatric OCD. NAC has been demonstrated to be quite well tolerated in previous pediatric trials in autism, trichotillomania, and cannabis dependence. 106,107 NAC currently represents a reasonable treatment option in children who have failed evidence-based treatments for OCD or in families opposed to using prescription medication in their children (clinical opinion: randomized controlled trial in adults).
Benzodiazepines
Benzodiazepines have demonstrated efficacy in the short-term treatment of anxiety disorders in adults.108 By contrast, double-blind, placebo-controlled trials in adults with OCD have consistently failed to demonstrate efficacy of benzodiazepines compared to placebo. A small, double-blind, placebo-controlled monotherapy trial of 27 adults with OCD failed to demonstrate a benefit of clonazepam compared to placebo on any primary (Y-BOCS, proportion of treatment responders) or secondary (depression and anxiety ratings) outcome measures. 109 Another small, placebo-controlled trial in 37 adults with OCD compared the concurrent addition of clonazepam or placebo to sertraline over 12 weeks.110 This trial also failed to demonstrate a benefit of clonazepam compared to placebo. The use of benzodiazepines in pediatric OCD is currently not supported by the literature (not supported: adult randomized controlled trials).
Neurosurgical and Neurostimulatory Techniques for Treatment-Refractory OCD
Neurosurgical techniques have been used for decades in treatment of adults with treatment-refractory OCD that causes severe impairment and distress. The current role of neurosurgical techniques in pediatric OCD should be nonexistent given the substantial proportion of pediatric OCD cases that remit over the long-term and the unknown efficacy of these techniques in adults, let alone children (clinical opinion: clinical experience across the lifespan). The common ablative procedures commonly practiced in psychosurgery for OCD include anterior cingulatomy, capsulotomy, subcaudate tractotomy, and limbic leucotomy. The specifics of each procedure are covered in other reviews in the area.111,112 Currently, no published controlled studies have demonstrated the efficacy of neurosurgery for the treatment of OCD. Reviews of open studies of neurosurgical procedures in OCD have suggested 50–60% response rates observed after 6–24 months.112 The increasing use of gamma knife surgery has made the procedure more precise and less invasive over recent years.
Deep brain stimulation (DBS) involves the surgical implantation of electrodes and the introduction of targeted electrical stimulation to specific brain regions through the implanted electrodes. DBS in OCD typically targets the ventral capsule/striatum or subthalamic nucleus.113 Crossover trials comparing when the implanted electrodes are “on” compared to “off” have demonstrated the efficacy of DBS for both brain regions. Advantages of DBS compared to neurosurgery include the fact that the procedure is potentially reversible, a better side effect profile, and the adjustability of the stimulation parameters. Disadvantages include expense, need for battery replacement, and extensive follow-up time to manage stimulus parameters. Adverse effects of DBS are rare but have included intracranial hemorrhage, infection, electrode breakage, and stimulation-induced adverse effects such as hypomania.
Repetitive transcranial magnetic stimulation (fTMS) is a non-invasive technique that uses a magnetic field applied to the scalp to induce electrical activity in the underlying cortex. The advantages of rTMS are that it is completely non-invasive with few side effects, especially when low-frequency stimulation is used. Side effects of rTMS include headaches, focal muscle aches, and, less commonly, seizures and syncope. The disadvantages of rTMS are that it can only directly stimulate the outer 6 cm of cortex and cannot directly target deeper structures (e.g. striatum, orbitofrontal cortex). Additionally, the sham-control is far from optimal in ensuring blinding in trials due to local effects of stimulation and the time-consuming nature of the procedure—typically an hour, 5 days a week, for several weeks. rTMS targeting the supplemental motor cortex has demonstrated efficacy in a single, sham-controlled trial in 18 treatment-refractory adults with OCD.114 Another randomized controlled trial of 20 adults with OCD studied sequential administration of low-frequency rTMS over right dorsolateral prefrontal cortex and bilateral SMA and demonstrated no significant difference between active and sham treatment.115 Further, rTMS trials targeting left and right dorsolateral prefrontal cortex have failed to demonstrated efficacy compared to sham-controls.116
DISCUSSION
Although the majority of children respond to first-line treatments for pediatric OCD, there exists a substantial proportion of children who do not achieve adequate, or even any, OCD symptom relief through evidence-based interventions. Although the long-term prognosis for children with OCD is good, with over half achieving remission of symptoms over the long term with treatment, many of these children with refractory OCD experience lifelong impairment related to OCD symptoms. As (1) research on strategies to combat treatment-refractory OCD in children is scarce; (2) data on pharmacological treatments for treatment-refractory OCD in adults suggest that most refractory patients with OCD will still not respond to pharmacological treatments; and (3) the side-effect profile in children for many pharmacological agents used in treatment-refractory OCD is problematic, every effort should be made to make sure children have had optimal first-line treatment for OCD before progressing to augmentation strategies. Further research is needed to ensure that optimal first-line treatment for OCD, especially CBT, is disseminated to all children. Children with treatment-refractory OCD represent a modest but challenging and often heartrending fraction of the patients any one clinician treats, no matter how specialized. Only through working together can we discover more effective treatments for children and families suffering with treatment-refractory OCD.
Acknowledgments
The authors acknowledge the support of the National Institutes of Health (NIH; 1K23MH091240; to M.H.B.), the National Alliance for Research on Schizophrenia and Depression (NARSAD; M.H.B.), the Patterson Foundation (M.H.B.), the Rembrandt Foundation (M.H.B.), and UL1 RR024139 from the National Center for Research Resources, a component of NIH, and NIH Roadmap for Medical Research (M.H.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr. Bloch has received grant or research support from the NIH K23 Award, the Trichotillomania Learning Center, the Tourette Syndrome Association, the Patterson Foundation, NARSAD, the Rembrandt Foundation, and the American Academy of Child and Adolescent Psychiatry Research Initiative Junior Investigator Award. Dr. Storch has received grant funding from NIH, the Centers for Disease Control and Prevention, the Agency for Healthcare Research and Quality, the International OCD Foundation, and Ortho-McNeil Scientific Affairs Pharmaceuticals. He has received textbook honorarium from Springer, the American Psychological Association, Wiley Publishers, and Lawrence Erlbaum Associates. He is an educational consultant for Rogers Memorial Hospital. He serves as a consultant for Prophase, Inc. and CroNos, Inc., and serves on the Speaker's Bureau and Scientific Advisory Board of the International OCD Foundation. He has received research support from the All Children's Hospital Guild Endowed Chair.
REFERENCES
- 1.Pediatric OCD Treatment Study Team Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969–1976. doi: 10.1001/jama.292.16.1969. [DOI] [PubMed] [Google Scholar]
- 2.Practice Parameter for the Assessment and Treatment of Children and Adolescenets with Obsessive-Compulsive Disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):98–113. doi: 10.1016/j.jaac.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Arumugham SS, Reddy JY. Augmentation strategies in obsessive-compulsive disorder. Expert review of neurotherapeutics. 2013;13(2):187–202. doi: 10.1586/ern.12.160. quiz 203. [DOI] [PubMed] [Google Scholar]
- 4.Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- 5.Geller DA, Biederman J, Stewart SE, et al. Which SSRI? A meta-analysis of pharmacotherapy trials in pediatric obsessive-compulsive disorder. The American journal of psychiatry. 2003;160(11):1919–1928. doi: 10.1176/appi.ajp.160.11.1919. [DOI] [PubMed] [Google Scholar]
- 6.Fineberg NA, Brown A, Reghunandanan S, Pampaloni I. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2012;15(8):1173–1191. doi: 10.1017/S1461145711001829. [DOI] [PubMed] [Google Scholar]
- 7.Stein DJ, Koen N, Fineberg N, et al. A 2012 evidence-based algorithm for the pharmacotherapy for obsessive-compulsive disorder. Current psychiatry reports. 2012;14(3):211–219. doi: 10.1007/s11920-012-0268-9. [DOI] [PubMed] [Google Scholar]
- 8.Work Group on Obsessive-Compulsive Disorder . Practice Guideline for the Treatment of Patients With Obsessive-Compulsive Disorder. American Psychiatric Association; Arlington, VA: 2007. [PubMed] [Google Scholar]
- 9.Zarin DA, Seigle L, Pincus HA, McIntyre JS. Evidence-based practice guidelines. Psychopharmacology bulletin. 1997;33(4):641–646. [PubMed] [Google Scholar]
- 10.Barrett P, Healy-Farrell L, March JS. Cognitive-behavioral family treatment of childhood obsessive-compulsive disorder: a controlled trial. J Am Acad Child Adolesc Psychiatry. 2004;43(1):46–62. doi: 10.1097/00004583-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Freeman JB, Garcia AM, Coyne L, et al. Early childhood OCD: Preliminary findings from a family-based cognitive-behavioral approach. J Am Acad Child Adolesc Psychiatry. 2008;47(5):593–602. doi: 10.1097/CHI.0b013e31816765f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piacentini J, Bergman RL, Chang S, et al. Controlled Comparison of Family Cognitive Behavioral Therapy and Psychoeducation/Relaxation Training for Child Obsessive-Compulsive Disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(11):1149–1161. doi: 10.1016/j.jaac.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Balkom AJ, de Haan E, van Oppen P, Spinhoven P, Hoogduin KA, van Dyck R. Cognitive and behavioral therapies alone versus in combination with fluvoxamine in the treatment of obsessive compulsive disorder. J Nerv Ment Dis. 1998;186(8):492–499. doi: 10.1097/00005053-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 14.van Balkom AJ, de Haan E, van Oppen P, Spinhoven P, Hoogduin KA, van Dyck R. Cognitive and behavioral therapies alone versus in combination with fluvoxamine in the treatment of obsessive compulsive disorder. The Journal of nervous and mental disease. 1998;186(8):492–499. doi: 10.1097/00005053-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Meca J, Rosa-Alcazar AI, Iniesta-Sepulveda M, Rosa-Alcazar A. Differential efficacy of cognitive-behavioral therapy and pharmacological treatments for pediatric obsessive-compulsive disorder: A meta-analysis. Journal of anxiety disorders. 2013;28(1):31–44. doi: 10.1016/j.janxdis.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Franklin ME, Sapyta J, Freeman JB, et al. Cognitive behavior therapy augmentation of pharmacotherapy in pediatric obsessive-compulsive disorder: the Pediatric OCD Treatment Study II (POTS II) randomized controlled trial. JAMA. 2011;306(11):1224–1232. doi: 10.1001/jama.2011.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson HB, Foa EB, Liebowitz MR, et al. Cognitive-behavioral therapy vs risperidone for augmenting serotonin reuptake inhibitors in obsessive-compulsive disorder: a randomized clinical trial. JAMA psychiatry. 2013;70(11):1190–1199. doi: 10.1001/jamapsychiatry.2013.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. The New England journal of medicine. 2008;358(3):252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- 19.Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet. 2004;363(9418):1341–1345. doi: 10.1016/S0140-6736(04)16043-1. [DOI] [PubMed] [Google Scholar]
- 20.Geller DA, Hoog SL, Heiligenstein JH, et al. Fluoxetine treatment for obsessive-compulsive disorder in children and adolescents: a placebo-controlled clinical trial. J Am Acad Child Adolesc Psychiatry. 2001;40(7):773–779. doi: 10.1097/00004583-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Geller DA, Wagner KD, Emslie G, et al. Paroxetine treatment in children and adolescents with obsessive-compulsive disorder: a randomized, multicenter, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2004;43(11):1387–1396. doi: 10.1097/01.chi.0000138356.29099.f1. [DOI] [PubMed] [Google Scholar]
- 22.Liebowitz MR, Turner SM, Piacentini J, et al. Fluoxetine in children and adolescents with OCD: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2002;41(12):1431–1438. doi: 10.1097/00004583-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 23.March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752–1756. doi: 10.1001/jama.280.20.1752. [DOI] [PubMed] [Google Scholar]
- 24.Riddle MA, Reeve EA, Yaryura-Tobias JA, et al. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomized, controlled, multicenter trial. J Am Acad Child Adolesc Psychiatry. 2001;40(2):222–229. doi: 10.1097/00004583-200102000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Riddle MA, Scahill L, King RA, et al. Double-blind, crossover trial of fluoxetine and placebo in children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 1992;31(6):1062–1069. doi: 10.1097/00004583-199211000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, Pittenger C. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Molecular psychiatry. 2010;15(8):850–855. doi: 10.1038/mp.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin A, Young C, Leckman JF, Mukonoweshuro C, Rosenheck R, Leslie D. Age effects on antidepressant-induced manic conversion. Archives of pediatrics and adolescent medicine. 2004;158(8):773–780. doi: 10.1001/archpedi.158.8.773. [DOI] [PubMed] [Google Scholar]
- 28.Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. Journal of clinical psychopharmacology. 2009;29(3):259–266. doi: 10.1097/JCP.0b013e3181a5233f. [DOI] [PubMed] [Google Scholar]
- 29.Fineberg NA, Pampaloni I, Pallanti S, Ipser J, Stein DJ. Sustained response versus relapse: the pharmacotherapeutic goal for obsessive-compulsive disorder. International clinical psychopharmacology. 2007;22(6):313–322. doi: 10.1097/YIC.0b013e32825ea312. [DOI] [PubMed] [Google Scholar]
- 30.Fineberg NA, Tonnoir B, Lemming O, Stein DJ. Escitalopram prevents relapse of obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2007;17(6–7):430–439. doi: 10.1016/j.euroneuro.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Hollander E, Baldini Rossi N, Sood E, Pallanti S. Risperidone augmentation in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2003;6(4):397–401. doi: 10.1017/S1461145703003730. [DOI] [PubMed] [Google Scholar]
- 32.Bloch MH, Craiglow BG, Landeros-Weisenberger A, et al. Predictors of early adult outcomes in pediatric-onset obsessive-compulsive disorder. Pediatrics. 2009;124(4):1085–1093. doi: 10.1542/peds.2009-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart SE, Geller DA, Jenike M, et al. Long-term outcome of pediatric obsessive-compulsive disorder: a meta-analysis and qualitative review of the literature. Acta psychiatrica Scandinavica. 2004;110(1):4–13. doi: 10.1111/j.1600-0447.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- 34.Storch EA, Lewin AB, De Nadai AS, Murphy TK. Defining treatment response and remission in obsessive-compulsive disorder: a signal detection analysis of the Children's Yale-Brown Obsessive Compulsive Scale. J Am Acad Child Adolesc Psychiatry. 2010;49(7):708–717. doi: 10.1016/j.jaac.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Pallanti S, Quercioli L. Treatment-refractory obsessive-compulsive disorder: methodological issues, operational definitions and therapeutic lines. Prog neuropsychopharmacol biol psychiatry. 2006;30(3):400–412. doi: 10.1016/j.pnpbp.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 36.Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):98–113. doi: 10.1016/j.jaac.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 37.American Psychiatric Association . Treatment of Patients with Obsessive-Compulsive Disorder. American Psychiatric Association; Arlington, VA: 2007. [PubMed] [Google Scholar]
- 38.Diagnostic and statistical manual of mental disorders : DSM-5. American Psychiatric Association; Arlington, VA: 2013. American Psychiatric Association and the American Psychiatric Association DSM Task Force. [Google Scholar]
- 39.Leckman JF, Grice DE, Barr LC, et al. Tic-related vs. non-tic-related obsessive compulsive disorder. Anxiety. 1994;1(5):208–215. [PubMed] [Google Scholar]
- 40.Prado HS, Rosario MC, Lee J, Hounie AG, Shavitt RG, Miguel EC. Sensory phenomena in obsessive-compulsive disorder and tic disorders: a review of the literature. CNS spectrums. 2008;13(5):425–432. doi: 10.1017/s1092852900016606. [DOI] [PubMed] [Google Scholar]
- 41.Wewetzer C, Jans T, Muller B, et al. Long-term outcome and prognosis of obsessive-compulsive disorder with onset in childhood or adolescence. European child and adolescent psychiatry. 2001;10(1):37–46. doi: 10.1007/s007870170045. [DOI] [PubMed] [Google Scholar]
- 42.Geller DA, Biederman J, Stewart SE, et al. Impact of comorbidity on treatment response to paroxetine in pediatric obsessive-compulsive disorder: is the use of exclusion criteria empirically supported in randomized clinical trials? J Child Adolesc Psychopharmacol. 2003;13(Suppl 1):S19–29. doi: 10.1089/104454603322126313. [DOI] [PubMed] [Google Scholar]
- 43.March JS, Franklin ME, Leonard H, et al. Tics moderate treatment outcome with sertraline but not cognitive-behavior therapy in pediatric obsessive-compulsive disorder. Biological psychiatry. 2007;61(3):344–347. doi: 10.1016/j.biopsych.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 44.Bloch MH, Landeros-Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Molecular psychiatry. 2006;11(7):622–632. doi: 10.1038/sj.mp.4001823. [DOI] [PubMed] [Google Scholar]
- 45.Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH. Systematic review: pharmacological treatment of tic disorders--efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neuroscience and biobehavioral reviews. 2013;37(6):1162–1171. doi: 10.1016/j.neubiorev.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Storch EA, Rahman O, Park JM, Reid J, Murphy TK, Lewin AB. Compulsive hoarding in children. Journal of clinical psychology. 2011;67(5):507–516. doi: 10.1002/jclp.20794. [DOI] [PubMed] [Google Scholar]
- 47.Bloch MH, Bartley CA, Zipperer L, et al. Meta-analysis: hoarding symptoms associated with poor treatment outcome in obsessive-compulsive disorder. Molecular psychiatry. 2014;19:1025–1030. doi: 10.1038/mp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Storch EA, Milsom VA, Merlo LJ, et al. Insight in pediatric obsessive-compulsive disorder: associations with clinical presentation. Psychiatry research. 2008;160(2):212–220. doi: 10.1016/j.psychres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Lewin AB, Bergman RL, Peris TS, Chang S, McCracken JT, Piacentini J. Correlates of insight among youth with obsessive-compulsive disorder. Journal of child psychology and psychiatry, and allied disciplines. 2010;51(5):603–611. doi: 10.1111/j.1469-7610.2009.02181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Storch EA, De Nadai AS, Jacob ML, et al. Phenomenology and correlates of insight in pediatric obsessive-compulsive disorder. Comprehensive psychiatry. 2014;55(3):613–620. doi: 10.1016/j.comppsych.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Garcia AM, Sapyta JJ, Moore PS, et al. Predictors and moderators of treatment outcome in the Pediatric Obsessive Compulsive Treatment Study (POTS I) J Am Acad Child Adolesc Psychiatry. 2010;49(10):1024–1033. doi: 10.1016/j.jaac.2010.06.013. quiz 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisen JL, Rasmussen SA, Phillips KA, et al. Insight and treatment outcome in obsessive-compulsive disorder. Comprehensive psychiatry. 2001;42(6):494–497. doi: 10.1053/comp.2001.27898. [DOI] [PubMed] [Google Scholar]
- 53.Bipeta R, Yerramilli SS, Pingali S, Karredla AR, Ali MO. A cross-sectional study of insight and family accommodation in pediatric obsessive-compulsive disorder. Child and adolescent psychiatry and mental health. 2013;7(1):20. doi: 10.1186/1753-2000-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lebowitz ER, Woolston J, Bar-Haim Y, et al. Family accommodation in pediatric anxiety disorders. Depression and anxiety. 2013;30(1):47–54. doi: 10.1002/da.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caporino NE, Morgan J, Beckstead J, Phares V, Murphy TK, Storch EA. A structural equation analysis of family accommodation in pediatric obsessive-compulsive disorder. Journal of abnormal child psychology. 2012;40(1):133–143. doi: 10.1007/s10802-011-9549-8. [DOI] [PubMed] [Google Scholar]
- 56.Storch EA, Lack CW, Merlo LJ, et al. Clinical features of children and adolescents with obsessive-compulsive disorder and hoarding symptoms. Compr Psychiatry. 2007;48(4):313–318. doi: 10.1016/j.comppsych.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Peris TS, Bergman RL, Langley A, Chang S, McCracken JT, Piacentini J. Correlates of accommodation of pediatric obsessive-compulsive disorder: parent, child, and family characteristics. J Am Acad Child Adolesc Psychiatry. 2008;47(10):1173–1181. doi: 10.1097/CHI.0b013e3181825a91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Storch EA, Jones AM, Lack CW, et al. Rage attacks in pediatric obsessive-compulsive disorder: phenomenology and clinical correlates. J Am Acad Child Adolesc Psychiatry. 2012;51(6):582–592. doi: 10.1016/j.jaac.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 59.Merlo LJ, Lehmkuhl HD, Geffken GR, Storch EA. Decreased family accommodation associated with improved therapy outcome in pediatric obsessive-compulsive disorder. J Consult Clin Psychol. 2009;77(2):355–360. doi: 10.1037/a0012652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simpson HB, Foa EB, Liebowitz MR, et al. A randomized, controlled trial of cognitive-behavioral therapy for augmenting pharmacotherapy in obsessive-compulsive disorder. The American journal of psychiatry. 2008;165(5):621–630. doi: 10.1176/appi.ajp.2007.07091440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simpson HB, Marcus SM, Zuckoff A, Franklin M, Foa EB. Patient adherence to cognitive-behavioral therapy predicts long-term outcome in obsessive-compulsive disorder. The Journal of clinical psychiatry. 2012;73(9):1265–1266. doi: 10.4088/JCP.12l07879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Storch EA, Lehmkuhl HD, Ricketts E, Geffken GR, Marien W, Murphy TK. An open trial of intensive family based cognitive-behavioral therapy in youth with obsessive-compulsive disorder who are medication partial responders or nonresponders. J Clin Child Adolesc Psychol. 2010;39(2):260–268. doi: 10.1080/15374410903532676. [DOI] [PubMed] [Google Scholar]
- 63.Storch EA, Geffken GR, Merlo LJ, et al. Family-based cognitive-behavioral therapy for pediatric obsessive-compulsive disorder: comparison of intensive and weekly approaches. J Am Acad Child Adolesc Psychiatry. 2007;46(4):469–478. doi: 10.1097/chi.0b013e31803062e7. [DOI] [PubMed] [Google Scholar]
- 64.Simpson HB, Zuckoff A. Using Motivational Interviewing to Enhance Treatment Outcome in People With Obsessive-Compulsive Disorder. Cognitive and behavioral practice. 2011;18:28–37. doi: 10.1016/j.cbpra.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ninan PT, Koran LM, Kiev A, et al. High-dose sertraline strategy for nonresponders to acute treatment for obsessive-compulsive disorder: a multicenter double-blind trial. The Journal of clinical psychiatry. 2006;67(1):15–22. doi: 10.4088/jcp.v67n0103. [DOI] [PubMed] [Google Scholar]
- 66.Ackerman DL, Greenland S. Multivariate meta-analysis of controlled drug studies for obsessive-compulsive disorder. Journal of clinical psychopharmacology. 2002;22(3):309–317. doi: 10.1097/00004714-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 67.DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31(1):45–49. doi: 10.1097/00004583-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 68.Pallanti S, Quercioli L, Paiva RS, Koran LM. Citalopram for treatment-resistant obsessive-compulsive disorder. European psychiatry : the journal of the Association of European Psychiatrists. 1999;14(2):101–106. doi: 10.1016/s0924-9338(99)80725-1. [DOI] [PubMed] [Google Scholar]
- 69.Diniz JB, Shavitt RG, Pereira CA, et al. Quetiapine versus clomipramine in the augmentation of selective serotonin reuptake inhibitors for the treatment of obsessive-compulsive disorder: a randomized, open-label trial. Journal of psychopharmacology. 2010;24(3):297–307. doi: 10.1177/0269881108099423. [DOI] [PubMed] [Google Scholar]
- 70.Marazziti D, Golia F, Consoli G, et al. Effectiveness of long-term augmentation with citalopram to clomipramine in treatment-resistant OCD patients. CNS spectrums. 2008;13(11):971–976. doi: 10.1017/s1092852900014024. [DOI] [PubMed] [Google Scholar]
- 71.Denys D, van der Wee N, van Megen HJ, Westenberg HG. A double blind comparison of venlafaxine and paroxetine in obsessive-compulsive disorder. Journal of clinical psychopharmacology. 2003;23(6):568–575. doi: 10.1097/01.jcp.0000095342.32154.54. [DOI] [PubMed] [Google Scholar]
- 72.Denys D, van Megen HJ, van der Wee N, Westenberg HG. A double-blind switch study of paroxetine and venlafaxine in obsessive-compulsive disorder. The Journal of clinical psychiatry. 2004;65(1):37–43. doi: 10.4088/jcp.v65n0106. [DOI] [PubMed] [Google Scholar]
- 73.Bystritsky A, Ackerman DL, Rosen RM, et al. Augmentation of serotonin reuptake inhibitors in refractory obsessive-compulsive disorder using adjunctive olanzapine: a placebo-controlled trial. The Journal of clinical psychiatry. 2004;65(4):565–568. doi: 10.4088/jcp.v65n0418. [DOI] [PubMed] [Google Scholar]
- 74.Carey PD, Vythilingum B, Seedat S, Muller JE, van Ameringen M, Stein DJ. Quetiapine augmentation of SRIs in treatment refractory obsessive-compulsive disorder: a double-blind, randomised, placebo-controlled study [ISRCTN83050762] BMC psychiatry. 2005;5:5. doi: 10.1186/1471-244X-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Denys D, de Geus F, van Megen HJ, Westenberg HG. A double-blind, randomized, placebo-controlled trial of quetiapine addition in patients with obsessive-compulsive disorder refractory to serotonin reuptake inhibitors. The Journal of clinical psychiatry. 2004;65(8):1040–1048. doi: 10.4088/jcp.v65n0803. [DOI] [PubMed] [Google Scholar]
- 76.Erzegovesi S, Guglielmo E, Siliprandi F, Bellodi L. Low-dose risperidone augmentation of fluvoxamine treatment in obsessive-compulsive disorder: a double-blind, placebo-controlled study. European neuropsychopharmacol. 2005;15(1):69–74. doi: 10.1016/j.euroneuro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 77.Fineberg NA, Sivakumaran T, Roberts A, Gale T. Adding quetiapine to SRI in treatment-resistant obsessive-compulsive disorder: a randomized controlled treatment study. International clinical psychopharmacology. 2005;20(4):223–226. doi: 10.1097/00004850-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 78.McDougle CJ, Epperson CN, Pelton GH, Wasylink S, Price LH. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Archives of general psychiatry. 2000;57(8):794–801. doi: 10.1001/archpsyc.57.8.794. [DOI] [PubMed] [Google Scholar]
- 79.McDougle CJ, Goodman WK, Leckman JF, Lee NC, Heninger GR, Price LH. Haloperidol addition in fluvoxamine-refractory obsessive-compulsive disorder. A double-blind, placebo-controlled study in patients with and without tics. Archives of general psychiatry. 1994;51(4):302–308. doi: 10.1001/archpsyc.1994.03950040046006. [DOI] [PubMed] [Google Scholar]
- 80.Shapira NA, Ward HE, Mandoki M, et al. A double-blind, placebo-controlled trial of olanzapine addition in fluoxetine-refractory obsessive-compulsive disorder. Biological psychiatry. 2004;55(5):553–555. doi: 10.1016/j.biopsych.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 81.Muscatello MR, Bruno A, Pandolfo G, et al. Effect of aripiprazole augmentation of serotonin reuptake inhibitors or clomipramine in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. Journal of clinical psychopharmacology. 2011;31(2):174–179. doi: 10.1097/JCP.0b013e31820e3db6. [DOI] [PubMed] [Google Scholar]
- 82.Sayyah M, Sayyah M, Boostani H, Ghaffari SM, Hoseini A. Effects of aripiprazole augmentation in treatment-resistant obsessive-compulsive disorder (a double blind clinical trial) Depression and anxiety. 2012;29(10):850–854. doi: 10.1002/da.21996. [DOI] [PubMed] [Google Scholar]
- 83.Li X, May RS, Tolbert LC, Jackson WT, Flournoy JM, Baxter LR. Risperidone and haloperidol augmentation of serotonin reuptake inhibitors in refractory obsessive-compulsive disorder: a crossover study. The Journal of clinical psychiatry. 2005;66(6):736–743. doi: 10.4088/jcp.v66n0610. [DOI] [PubMed] [Google Scholar]
- 84.Maina G, Pessina E, Albert U, Bogetto F. 8-week, single-blind, randomized trial comparing risperidone versus olanzapine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2008;18(5):364–372. doi: 10.1016/j.euroneuro.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 85.Grados MA, Specht MW, Sung HM, Fortune D. Glutamate drugs and pharmacogenetics of OCD: a pathway-based exploratory approach. Expert opinion on drug discovery. 2013;8(12):1515–1527. doi: 10.1517/17460441.2013.845553. [DOI] [PubMed] [Google Scholar]
- 86.Kariuki-Nyuthe C, Gomez-Mancilla B, Stein DJ. Obsessive compulsive disorder and the glutamatergic system. Current opinion in psychiatry. 2014;27(1):32–37. doi: 10.1097/YCO.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 87.Pittenger C, Krystal JH, Coric V. Glutamate-modulating drugs as novel pharmacotherapeutic agents in the treatment of obsessive-compulsive disorder. NeuroRx. 2006;3(1):69–81. doi: 10.1016/j.nurx.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu K, Hanna GL, Rosenberg DR, Arnold PD. The role of glutamate signaling in the pathogenesis and treatment of obsessive-compulsive disorder. Pharmacology, biochemistry, and behavior. 2012;100(4):726–735. doi: 10.1016/j.pbb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosenberg DR, Keshavan MS, O'Hearn KM, et al. Frontostriatal measurement in treatment-naive children with obsessive-compulsive disorder. Archives of general psychiatry. 1997;54(9):824–830. doi: 10.1001/archpsyc.1997.01830210068007. [DOI] [PubMed] [Google Scholar]
- 90.Welch JM, Lu J, Rodriguiz RM, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448(7156):894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahmari SE, Spellman T, Douglass NL, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340(6137):1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coric V, Taskiran S, Pittenger C, et al. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biological psychiatry. 2005;58(5):424–428. doi: 10.1016/j.biopsych.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 93.Grant P, Lougee L, Hirschtritt M, Swedo SE. An open-label trial of riluzole, a glutamate antagonist, in children with treatment-resistant obsessive-compulsive disorder. Journal of child and adolescent psychopharmacology. 2007;17(6):761–767. doi: 10.1089/cap.2007.0021. [DOI] [PubMed] [Google Scholar]
- 94.Grant P, Song JY, Swedo SE. Review of the use of the glutamate antagonist riluzole in psychiatric disorders and a description of recent use in childhood obsessive-compulsive disorder. Journal of child and adolescent psychopharmacology. 2010;20(4):309–315. doi: 10.1089/cap.2010.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pittenger C, Kelmendi B, Wasylink S, Bloch MH, Coric V. Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: a series of 13 cases, with long-term follow-up. Journal of clinical psychopharmacology. 2008;28(3):363–367. doi: 10.1097/JCP.0b013e3181727548. [DOI] [PubMed] [Google Scholar]
- 96.Grant PJ, Joseph LA, Farmer CA, et al. 12-week, placebo-controlled trial of add-on riluzole in the treatment of childhood-onset obsessive-compulsive disorder. Neuropsychopharmacology. 2014;39(6):1453–1459. doi: 10.1038/npp.2013.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4(3):191–206. [PubMed] [Google Scholar]
- 98.Aan Het Rot M, Zarate CA, Jr, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biological psychiatry. 2012;72(7):537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bloch MH, Wasylink S, Landeros-Weisenberger A, et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biological psychiatry. 2012;72(11):964–970. doi: 10.1016/j.biopsych.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rodriguez CI, Kegeles LS, Levinson A, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38(12):2475–2483. doi: 10.1038/npp.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghaleiha A, Entezari N, Modabbernia A, et al. Memantine add-on in moderate to severe obsessive-compulsive disorder: randomized double-blind placebo-controlled study. Journal of psychiatric research. 2013;47(2):175–180. doi: 10.1016/j.jpsychires.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 102.Stewart SE, Jenike EA, Hezel DM, et al. A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. Journal of clinical psychopharmacology. 2010;30(1):34–39. doi: 10.1097/JCP.0b013e3181c856de. [DOI] [PubMed] [Google Scholar]
- 103.Hezel DM, Beattie K, Stewart SE. Memantine as an augmenting agent for severe pediatric OCD. The American journal of psychiatry. 2009;166(2):237. doi: 10.1176/appi.ajp.2008.08091427. [DOI] [PubMed] [Google Scholar]
- 104.Mowla A, Khajeian AM, Sahraian A, Chohedri AH, Kashkoli F. Topiramate Augmentation in Resistant OCD: A Double-Blind Placebo-Controlled Clinical Trial. CNS Spectrums. 2010;15(11):613–7. doi: 10.1017/S1092852912000065. [DOI] [PubMed] [Google Scholar]
- 105.Berlin HA, Koran LM, Jenike MA, et al. Double-blind, placebo-controlled trial of topiramate augmentation in treatment-resistant obsessive-compulsive disorder. The Journal of clinical psychiatry. 2011;72(5):716–721. doi: 10.4088/JCP.09m05266gre. [DOI] [PubMed] [Google Scholar]
- 106.Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. The American journal of psychiatry. 2012;169(8):805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hardan AY, Fung LK, Libove RA, et al. A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biological psychiatry. 2012;71(11):956–961. doi: 10.1016/j.biopsych.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koen N, Stein DJ. Pharmacotherapy of anxiety disorders: a critical review. Dialogues in clinical neuroscience. 2011;13(4):423–437. doi: 10.31887/DCNS.2011.13.4/nkoen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hollander E, Kaplan A, Stahl SM. A double-blind, placebo-controlled trial of clonazepam in obsessive-compulsive disorder. World J Biol Psychiatry. 2003;4(1):30–34. doi: 10.3109/15622970309167908. [DOI] [PubMed] [Google Scholar]
- 110.Crockett BA, Churchill E, Davidson JR. A double-blind combination study of clonazepam with sertraline in obsessive-compulsive disorder. Ann Clin Psychiatry. 2004;16(3):127–132. doi: 10.1080/10401230490486972. [DOI] [PubMed] [Google Scholar]
- 111.Lopes AC, de Mathis ME, Canteras MM, Salvajoli JV, Del Porto JA, Miguel EC. [Update on neurosurgical treatment for obsessive compulsive disorder] Revista brasileira de psiquiatria. 2004;26(1):62–66. doi: 10.1590/s1516-44462004000100015. [DOI] [PubMed] [Google Scholar]
- 112.Greenberg BD, Rauch SL, Haber SN. Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology. 2010;35(1):317–336. doi: 10.1038/npp.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blomstedt P, Sjoberg RL, Hansson M, Bodlund O, Hariz MI. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World neurosurgery. 2013;80(6):e245–253. doi: 10.1016/j.wneu.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 114.Mantovani A, Lisanby SH, Pieraccini F, Ulivelli M, Castrogiovanni P, Rossi S. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette's syndrome (TS) Int J Neuropsychopharmacol. 2006;9(1):95–100. doi: 10.1017/S1461145705005729. [DOI] [PubMed] [Google Scholar]
- 115.Kang JI, Kim CH, Namkoong K, Lee CI, Kim SJ. A randomized controlled study of sequentially applied repetitive transcranial magnetic stimulation in obsessive-compulsive disorder. The Journal of clinical psychiatry. 2009;70(12):1645–1651. doi: 10.4088/JCP.08m04500. [DOI] [PubMed] [Google Scholar]
- 116.Sachdev PS, Loo CK, Mitchell PB, McFarquhar TF, Malhi GS. Repetitive transcranial magnetic stimulation for the treatment of obsessive compulsive disorder: a double-blind controlled investigation. Psychological medicine. 2007;37(11):1645–1649. doi: 10.1017/S0033291707001092. [DOI] [PubMed] [Google Scholar]