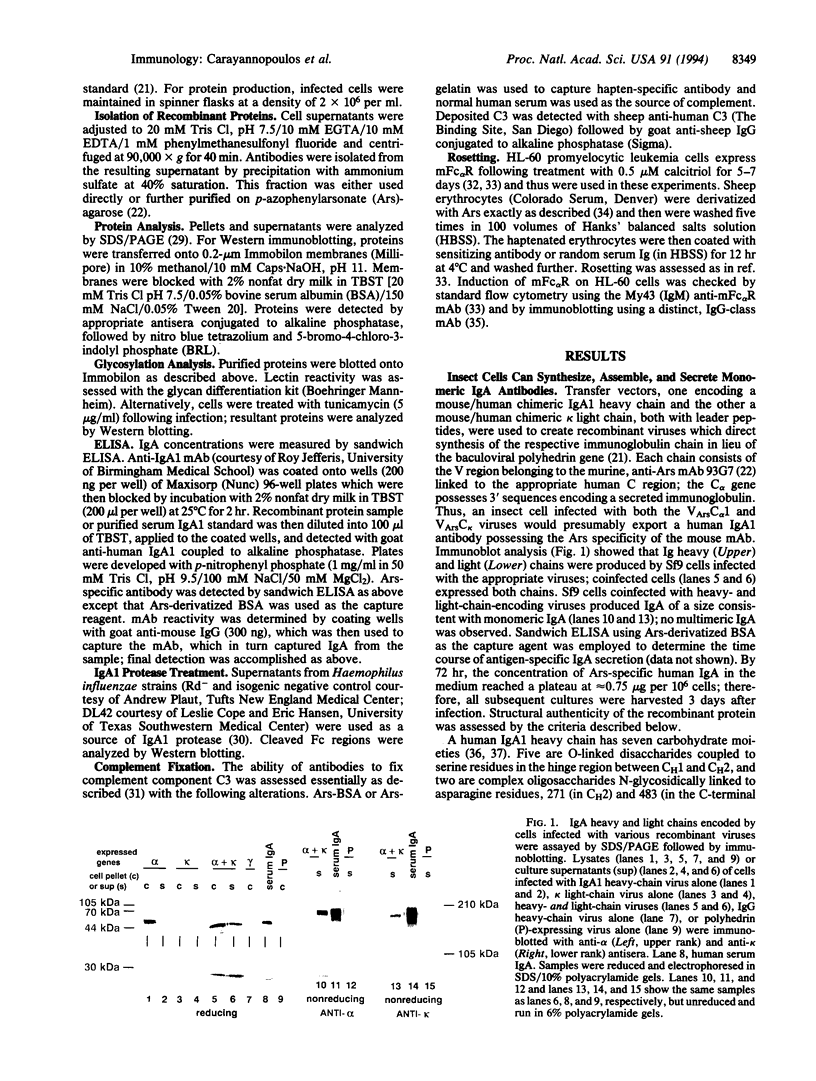
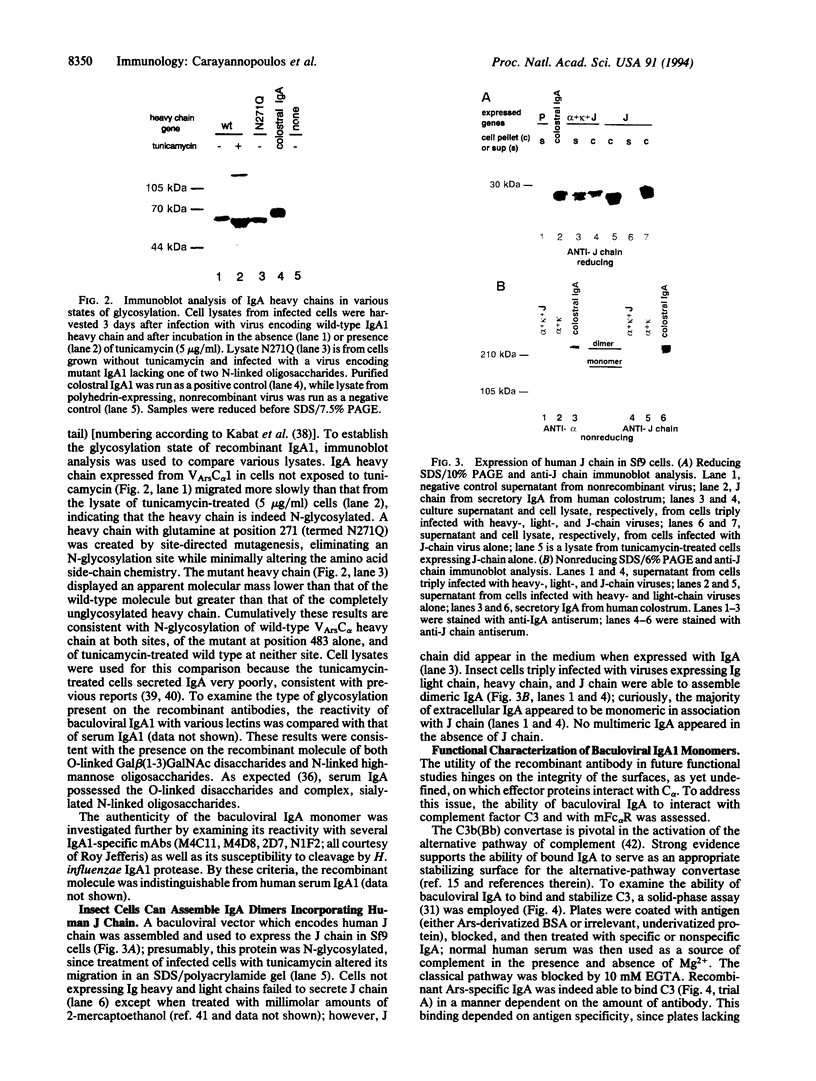
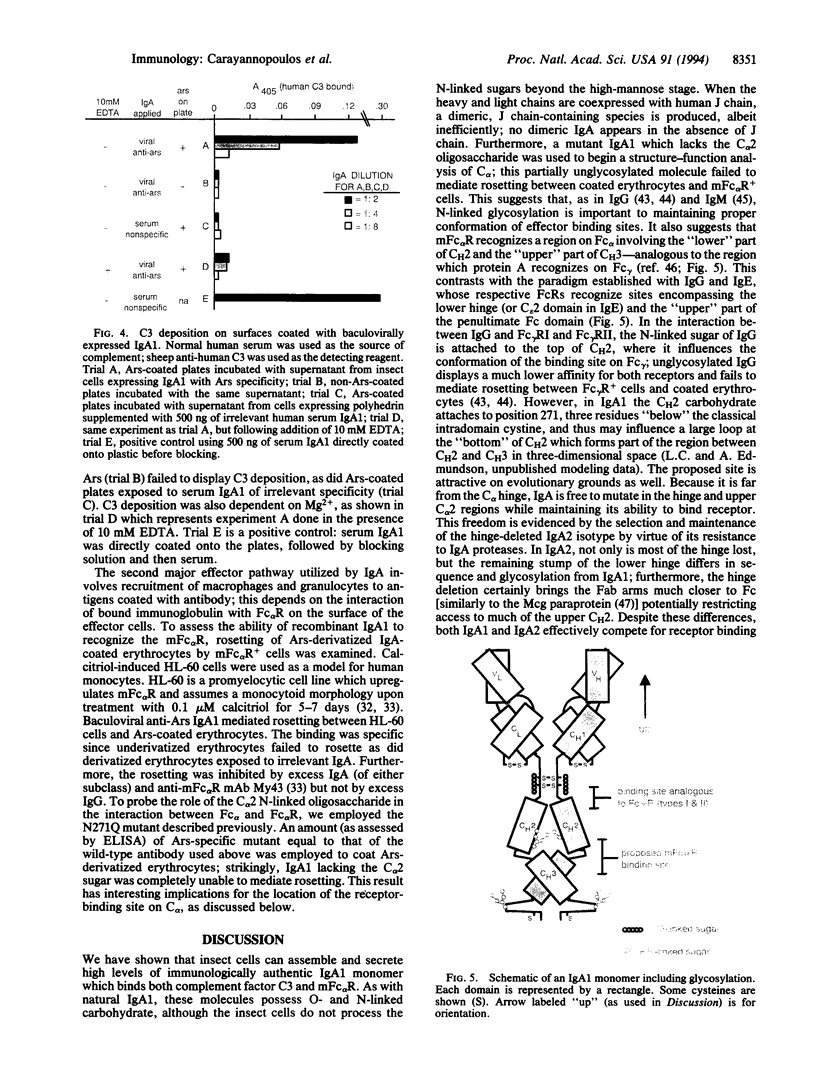
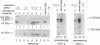Abstract
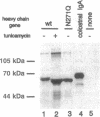
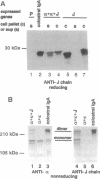
IgA serves as the first line of humoral defense at all mucosal surfaces and is present in large quantities in serum. To map the sites of interaction of immune effector molecules with the IgA constant region (C alpha), we have expressed soluble, chimeric human IgA in insect cells using recombinant baculoviruses. This antibody is correctly assembled into heavy chain/light chain heterodimers, N-glycosylated, and secreted by the insect cells; further, when coexpressed with a human J chain, the antibodies can assemble into dimers. The recombinant protein is authentic by a number of criteria, including antigen-binding, recognition by monoclonal antibodies, complement fixation via the alternative pathway, and specific binding to the monocyte IgA Fc receptor. We have also constructed viruses which encode structurally altered IgA heavy chains. Using one of these variant viruses, we have shown that glycosylation of the second domain of C alpha is required for interaction with the monocyte IgA Fc receptor. This system should prove useful in further characterization of the structure-function relationships in human C alpha.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberini C. M., Bet P., Milstein C., Sitia R. Secretion of immunoglobulin M assembly intermediates in the presence of reducing agents. Nature. 1990 Oct 4;347(6292):485–487. doi: 10.1038/347485a0. [DOI] [PubMed] [Google Scholar]
- Bachovchin W. W., Plaut A. G., Flentke G. R., Lynch M., Kettner C. A. Inhibition of IgA1 proteinases from Neisseria gonorrhoeae and Hemophilus influenzae by peptide prolyl boronic acids. J Biol Chem. 1990 Mar 5;265(7):3738–3743. [PubMed] [Google Scholar]
- Baenziger J., Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974 Nov 25;249(22):7270–7281. [PubMed] [Google Scholar]
- Brüggemann M., Williams G. T., Bindon C. I., Clark M. R., Walker M. R., Jefferis R., Waldmann H., Neuberger M. S. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987 Nov 1;166(5):1351–1361. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D. R., Woof J. M. Human antibody effector function. Adv Immunol. 1992;51:1–84. doi: 10.1016/s0065-2776(08)60486-1. [DOI] [PubMed] [Google Scholar]
- Clarkson A. R., Woodroffe A. J., Bannister K. M., Lomax-Smith J. D., Aarons I. The syndrome of IgA nephropathy. Clin Nephrol. 1984 Jan;21(1):7–14. [PubMed] [Google Scholar]
- Davis A. C., Roux K. H., Pursey J., Shulman M. J. Intermolecular disulfide bonding in IgM: effects of replacing cysteine residues in the mu heavy chain. EMBO J. 1989 Sep;8(9):2519–2526. doi: 10.1002/j.1460-2075.1989.tb08389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981 Apr 28;20(9):2361–2370. [PubMed] [Google Scholar]
- Dietzschold B., Kao M., Zheng Y. M., Chen Z. Y., Maul G., Fu Z. F., Rupprecht C. E., Koprowski H. Delineation of putative mechanisms involved in antibody-mediated clearance of rabies virus from the central nervous system. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7252–7256. doi: 10.1073/pnas.89.15.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzych J. M., Grezel D., Xu C. B., Neyrinck J. L., Capron M., Ouma J. H., Butterworth A. E., Capron A. IgA antibodies to a protective antigen in human Schistosomiasis mansoni. J Immunol. 1993 Jan 15;150(2):527–535. [PubMed] [Google Scholar]
- Guddat L. W., Herron J. N., Edmundson A. B. Three-dimensional structure of a human immunoglobulin with a hinge deletion. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4271–4275. doi: 10.1073/pnas.90.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasemann C. A., Capra J. D. High-level production of a functional immunoglobulin heterodimer in a baculovirus expression system. Proc Natl Acad Sci U S A. 1990 May;87(10):3942–3946. doi: 10.1073/pnas.87.10.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S., Kornfeld S. Effect of tunicamycin on IgM, IgA, and IgG secretion by mouse plasmacytoma cells. J Immunol. 1978 Sep;121(3):990–996. [PubMed] [Google Scholar]
- Hickman S., Wong-Yip Y. P. Re-expression of nonglycosylated surface IgA in trypsin-treated MOPC 315 plasmacytoma cells. J Immunol. 1979 Jul;123(1):389–395. [PubMed] [Google Scholar]
- Horton R. M., Cai Z. L., Ho S. N., Pease L. R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990 May;8(5):528–535. [PubMed] [Google Scholar]
- Jonard P. P., Rambaud J. C., Dive C., Vaerman J. P., Galian A., Delacroix D. L. Secretion of immunoglobulins and plasma proteins from the jejunal mucosa. Transport rate and origin of polymeric immunoglobulin A. J Clin Invest. 1984 Aug;74(2):525–535. doi: 10.1172/JCI111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Russell M. W. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev. 1988 Jun;52(2):296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani S., Ito K., Miyamoto T. IgG, IgA, and IgM antibodies to mite in sera and sputa from asthmatic patients. Ann Allergy. 1985 Oct;55(4):612–620. [PubMed] [Google Scholar]
- Koshland M. E. The coming of age of the immunoglobulin J chain. Annu Rev Immunol. 1985;3:425–453. doi: 10.1146/annurev.iy.03.040185.002233. [DOI] [PubMed] [Google Scholar]
- Kurita T., Kiyono H., Komiyama K., Grossi C. E., Mestecky J., McGhee J. R. Isotype-specific immunoregulation; characterization and function of Fc receptors on T-T hybridomas which produce murine IgA-binding factor. J Immunol. 1986 Jun 1;136(11):3953–3960. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucisano Valim Y. M., Lachmann P. J. The effect of antibody isotype and antigenic epitope density on the complement-fixing activity of immune complexes: a systematic study using chimaeric anti-NIP antibodies with human Fc regions. Clin Exp Immunol. 1991 Apr;84(1):1–8. doi: 10.1111/j.1365-2249.1991.tb08115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliszewski C. R., March C. J., Schoenborn M. A., Gimpel S., Shen L. Expression cloning of a human Fc receptor for IgA. J Exp Med. 1990 Dec 1;172(6):1665–1672. doi: 10.1084/jem.172.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Korsmeyer S. J. Human J chain gene. Structure and expression in B lymphoid cells. J Exp Med. 1985 Apr 1;161(4):832–849. doi: 10.1084/jem.161.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Monteiro R. C., Cooper M. D., Kubagawa H. Molecular heterogeneity of Fc alpha receptors detected by receptor-specific monoclonal antibodies. J Immunol. 1992 Mar 15;148(6):1764–1770. [PubMed] [Google Scholar]
- Monteiro R. C., Hostoffer R. W., Cooper M. D., Bonner J. R., Gartland G. L., Kubagawa H. Definition of immunoglobulin A receptors on eosinophils and their enhanced expression in allergic individuals. J Clin Invest. 1993 Oct;92(4):1681–1685. doi: 10.1172/JCI116754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro R. C., Kubagawa H., Cooper M. D. Cellular distribution, regulation, and biochemical nature of an Fc alpha receptor in humans. J Exp Med. 1990 Mar 1;171(3):597–613. doi: 10.1084/jem.171.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton H. C., Atkin J. D., Owens R. J., Woof J. M. Purification and characterization of chimeric human IgA1 and IgA2 expressed in COS and Chinese hamster ovary cells. J Immunol. 1993 Nov 1;151(9):4743–4752. [PubMed] [Google Scholar]
- Mostov K. E., Blobel G. A transmembrane precursor of secretory component. The receptor for transcellular transport of polymeric immunoglobulins. J Biol Chem. 1982 Oct 10;257(19):11816–11821. [PubMed] [Google Scholar]
- Popper H., Pongratz M., Lanzer G. IgA2-alveolitis and eosinophilic pneumonia--a possibly virus-triggered allergy. Allergol Immunopathol (Madr) 1982 May-Jun;10(3):177–184. [PubMed] [Google Scholar]
- Rigby W. F., Shen L., Ball E. D., Fanger M. W. 1,25-Dihydroxyvitamin D3 induces a myelomonocytic phenotype with enhanced effector cell function in the HL-60 promyelocytic leukemia cell line. Mol Immunol. 1985 May;22(5):567–572. doi: 10.1016/0161-5890(85)90180-4. [DOI] [PubMed] [Google Scholar]
- Schneiderman R. D., Lint T. F., Knight K. L. Activation of the alternative pathway of complement by twelve different rabbit-mouse chimeric transfectoma IgA isotypes. J Immunol. 1990 Jul 1;145(1):233–237. [PubMed] [Google Scholar]
- Shen L., Lasser R., Fanger M. W. My 43, a monoclonal antibody that reacts with human myeloid cells inhibits monocyte IgA binding and triggers function. J Immunol. 1989 Dec 15;143(12):4117–4122. [PubMed] [Google Scholar]
- Shen L., Lasser R., Fanger M. W. My 43, a monoclonal antibody that reacts with human myeloid cells inhibits monocyte IgA binding and triggers function. J Immunol. 1989 Dec 15;143(12):4117–4122. [PubMed] [Google Scholar]
- Tao M. H., Morrison S. L. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol. 1989 Oct 15;143(8):2595–2601. [PubMed] [Google Scholar]
- Tonnelle C., DeMars R., Long E. O. DO beta: a new beta chain gene in HLA-D with a distinct regulation of expression. EMBO J. 1985 Nov;4(11):2839–2847. doi: 10.1002/j.1460-2075.1985.tb04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraño A., Tsuzukida Y., Liu Y. S., Putnam F. W. Location and structural significance of the oligosaccharides in human Ig-A1 and IgA2 immunoglobulins. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2301–2305. doi: 10.1073/pnas.74.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuaillon N., Taylor L. D., Lonberg N., Tucker P. W., Capra J. D. Human immunoglobulin heavy-chain minilocus recombination in transgenic mice: gene-segment use in mu and gamma transcripts. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3720–3724. doi: 10.1073/pnas.90.8.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underdown B. J., Schiff J. M. Immunoglobulin A: strategic defense initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389–417. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]
- Vaughn J. L., Goodwin R. H., Tompkins G. J., McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro. 1977 Apr;13(4):213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- Walker M. R., Lund J., Thompson K. M., Jefferis R. Aglycosylation of human IgG1 and IgG3 monoclonal antibodies can eliminate recognition by human cells expressing Fc gamma RI and/or Fc gamma RII receptors. Biochem J. 1989 Apr 15;259(2):347–353. doi: 10.1042/bj2590347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. F., Shulman M. J., Isenman D. E., Painter R. H. C1 binding by mouse IgM. The effect of abnormal glycosylation at position 402 resulting from a serine to asparagine exchange at residue 406 of the mu-chain. J Biol Chem. 1990 Jun 25;265(18):10506–10513. [PubMed] [Google Scholar]