Significance
The dopamine D2 receptor (D2R), a G protein-coupled receptor, can initiate signaling events through both activation of G proteins and interactions with β-arrestins. To begin to understand the contribution of these events in the physiology of the dopamine system, D2R was mutated to be functionally selective for each signaling pathway. The engineered receptors are functional in vitro and in vivo. Furthermore, both functions are essentially dissociable and mediate different physiological and pharmacological responses. These tools provide an additional but previously unavailable approach to elucidate the role of biased signaling in the multiple physiological actions of the dopamine system.
Keywords: dopamine, GPCR, functional selectivity, G protein, β-arrestin
Abstract
The neuromodulator dopamine signals through the dopamine D2 receptor (D2R) to modulate central nervous system functions through diverse signal transduction pathways. D2R is a prominent target for drug treatments in disorders where dopamine function is aberrant, such as schizophrenia. D2R signals through distinct G-protein and β-arrestin pathways, and drugs that are functionally selective for these pathways could have improved therapeutic potential. How D2R signals through the two pathways is still not well defined, and efforts to elucidate these pathways have been hampered by the lack of adequate tools for assessing the contribution of each pathway independently. To address this, Evolutionary Trace was used to produce D2R mutants with strongly biased signal transduction for either the G-protein or β-arrestin interactions. These mutants were used to resolve the role of G proteins and β-arrestins in D2R signaling assays. The results show that D2R interactions with the two downstream effectors are dissociable and that G-protein signaling accounts for D2R canonical MAP kinase signaling cascade activation, whereas β-arrestin only activates elements of this cascade under certain conditions. Nevertheless, when expressed in mice in GABAergic medium spiny neurons of the striatum, the β-arrestin–biased D2R caused a significant potentiation of amphetamine-induced locomotion, whereas the G protein-biased D2R had minimal effects. The mutant receptors generated here provide a molecular tool set that should enable a better definition of the individual roles of G-protein and β-arrestin signaling pathways in D2R pharmacology, neurobiology, and associated pathologies.
G protein-coupled receptors (GPCRs) are the largest receptor family and transmit the physiological effects of numerous biologically active molecules. GPCR signal transduction cascades account for diverse genomic, biochemical, cellular, and behavioral responses including cell fate determination, developmental reprogramming, olfactory, taste and light sensation, as well as complex behaviors mediated by neuromodulators (1). The diversity of responses to a particular hormone or neuromodulator is dictated not only by its cognate receptor but also by the ability of that receptor to engage distinct signaling pathways. For a number of GPCRs, their propensity to activate distinct G proteins can elicit diverse responses depending on the cellular environment (2). However, an even more subtle but intriguing mode of signaling has been attributed to the ability of a receptor to activate signaling pathways independent of G-protein activation, through the scaffolding of signaling complexes by β-arrestin, a component of the GPCR desensitization and internalization machinery (3). These two signaling modes harbor distinct functional properties, and in instances the same ligand can act as an agonist for one pathway but antagonist at the other. The selective or biased activation of a given pathway is commonly referred to as “functional selectivity” and can be easily demonstrated in heterologous systems especially when biased small molecule ligands are available (4). Biased GPCR ligands may have high therapeutic potential as these receptors represent the largest targets of drugs on the market. However, determining the functional contributions of G-protein and β-arrestin signaling pathways to the biological actions of an endogenous ligand acting upon its receptor still remains a challenging undertaking.
Dopamine (DA) is a neuromodulator that is known to regulate movement, reward, cognition, emotion, and affect. The dopamine D2 receptor (D2R) is a prominent GPCR that mediates the actions of DA. All typical antipsychotics, such as haloperidol, are potent D2R blockers (5), whereas atypical antipsychotics, such as aripiprazole and clozapine, have unique pharmacology, exhibiting weak partial agonist activity at D2R or reduced antagonist efficacy, respectively (6). Previous studies have demonstrated the ability of D2Rs to engage different signal transduction pathways depending on the cellular complement of G proteins as well as their ability to regulate different physiological processes (7–9). β-arrestin 2 knockout mice provided robust behavioral and biochemical evidence for a critical D2R/β-arrestin signaling pathway in the striatum (10). Furthermore, neuronal selective deletion of GSK3β, a putative D2R/β-arrestin 2 effector, could reproduce the pharmacological blockade of D2Rs with antipsychotics (11). Although these studies suggest that D2Rs, like many other GPCRs, use pleiotropic signaling pathways to mediate their effects, the brain DA system is uniquely complex, as diverse responses may also rely upon many other determinants. One well-documented variable is the mode of stimulation of DA receptors, which is a function of the tonic or phasic release of DA (12). The expression profile of D2R is also complex, being expressed not only in DA synthesizing neurons of the substantia nigra and ventral tegmental area where they function as presynaptic autoreceptors but also in GABAergic medium spiny neurons (MSNs), cholinergic interneurons of the striatum, and cortical neurons (13), where they function as postsynaptic receptors. Thus, understanding the contributions of functional selectivity at D2R in intact biological systems is a challenge that cannot be elucidated in heterologous systems alone. To develop tools where this challenge can begin to be addressed, the Evolutionary Trace (ET) (14) approach was used to engineer D2R mutants that selectively interact with either G proteins or β-arrestins, designated [Gprot]D2R and [βarr]D2R, respectively. These mutants show separation of G-protein and β-arrestin interactions, and expression of these mutants in vivo in the mouse striatum provides proof-of-concept for their biological activity and discrete functions.
Results
Evolutionary Trace-Guided Mutagenesis of D2R.
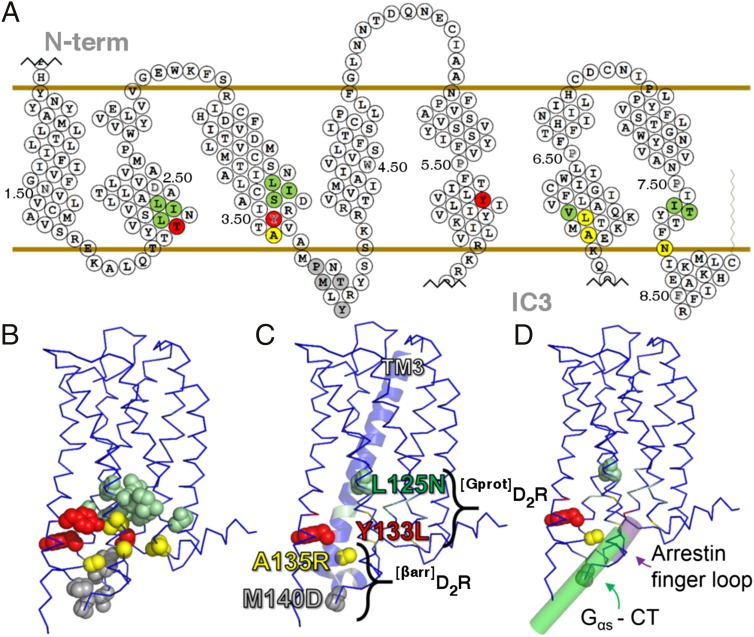
The ET method identifies amino acids that determine the function of a protein and map its functional sites when a structure is available (15, 16). ET algorithms exploit protein orthologs and paralogs to correlate sequence variations with phylogenetic divergences and determine whether substitutions at a particular residue are likely to produce a functional change in the protein (17). The predictive power of ET is further enhanced when specific crystal structures (18) and more sophisticated models of the evolution of structure and function can be applied (16, 19). Here a combination of these approaches (cocrystals of receptors and signaling molecules as well as more sophisticated algorithms; Fig. S1) was used to identify residues in D2R that could be mutated to achieve functional selectivity. The residues that were identified as being potentially critical for functional selectivity are mapped onto a snake-like plot of D2R (Fig. 1A) and the crystal structure of D3R [Fig. 1B; Protein Data Bank (PDB) ID 3PBL] (20), which shows the physical proximity of each residue to each other as well as to the cytosolic side of the receptor.
Fig. 1.
Generation of functionally selective D2R mutants. (A) Snake-like plot of D2R with each round of mutagenesis color-coded according to Table S1 and Fig. S1. Red residues, derived from TYY; green spheres, predicted from piET algorithm; yellow spheres, predicted from proximity to rhodopsin/transducin Gα subunit C-terminal fragment cocrystal; gray spheres, identified residues from β2AR/Gαβγ cocrystal in intracellular loop two. Ballesteros–Weinstein numbering identified for each transmembrane domain. The long N terminus (N-term) and intracellular loop three (IC3) were abridged. The same color scheme was used to highlight the residues on the structure of D3R (20) because D3R is the most closely related GPCR to D2R with an available crystal structure (81% sequence identity for transmembrane domains). (B) D3R structure is represented as a blue ribbon, and ET-identified residues are spheres. (C) The biased mutants all occur within 20 amino acids of the DRY motif on transmembrane domain three (TM3). (D) D3R aligned to β2AR in complex with Gαβγ (26) (green cylinder PDB ID 3SN6) as well as rhodopsin in complex with the finger-loop domain of visual arrestin (51) (purple cylinder PDB ID 4PXF). D3R to β2AR alignment yielded an RMSD = 1.8 and D3R alignment to rhodopsin RMSD = 2.7 using pymol MatchAlign command.
To achieve specific and robust separation of G protein- and β-arrestin–dependent interactions, Evolutionary Action (EA) (21) was used to predict residue changes. EA models the evolutionary relationship between genotype and phenotype as a smooth process upon which a mutation causes a small perturbation. Explicitly, if γ is the genotype sequence and φ is the fitness phenotype, EA postulates an evolutionary function f between them exists, such that
| [1] |
and f is differentiable so that the Evolutionary Action point mutation Δγ on fitness is
| [2] |
In practice, f remains unknown, but its derivative (or gradient) f′ is given by ET, and Δγ is given by substitution odds. The Evolutionary Action Eq. 2 is thus generally solvable for coding mutation of proteins and quantifies the effect of mutations over multiple scales, spanning molecular, clinical, and population genetics effects (21–24). Here, for each residue identified in Fig. 1 A and B, mutations were predicted and scored by EA according to how likely they would produce a phenotype (Table S1). Each point mutation was tested for G-protein activity by cAMP inhibition and β-arrestin 2 recruitment by bioluminescent resonance energy transfer (BRET) (25) and fidelity of plasma membrane trafficking as well as lack of constitutive activity. These mutants were binned into four categories: (i) β-arrestin–biased, (ii) G protein-biased, (iii) deficient at both pathways, or (iv) unaffected at either pathway (Fig. S2A). Residues that retained the desired phenotype were further combined into double (Fig. S2B), triple (Fig. S2C), quadruple, and quintuple (Fig. S2D) point mutations. This initial characterization yielded a robust landscape of unique functionally selective mutants.
The two mutants that showed the greatest functional separation are designated [Gprot]D2R (L125N Y133L) and [βarr]D2R (A135R M140D). Each of these mutations occurs within 20 amino acids of the DRY motif of TM3 (Fig. 1C). [Gprot]D2R mutations are more distal from the interacting regions of the recently resolved cocrystals of receptors and G proteins or arrestin fragments, whereas [βarr]D2R mutations are more proximal (Fig. 1D). The [Gprot]D2R mutant is derived from the more sophisticated ET algorithms (TYY and piET; Fig. S1), whereas [βarr]D2R arose from residues identified in the more specific crystal structures of receptors and G proteins (26, 27).
[Gprot]D2R and [βarr]D2R Display Distinct but Expected Properties.
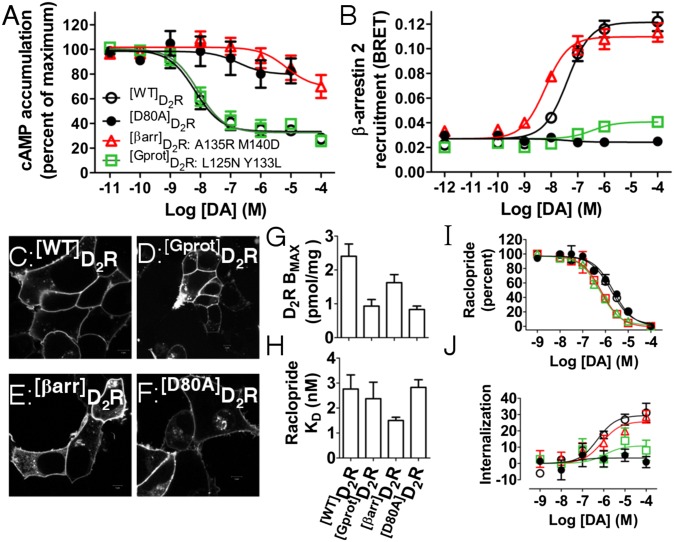
Each receptor was profiled for G-protein and β-arrestin activity. In addition, a negative control point mutation, [D80A]D2R (28), was included in all experiments because this mutant has been shown to bind ligands and traffic to the plasma membrane but is deficient in signaling. As shown in Fig. 2A, [Gprot]D2R retained full efficacy and potency at cAMP inhibition compared with [WT]D2R, whereas [βarr]D2R and [D80A]D2R are severely deficient. In contrast, β-arrestin 2 recruitment is retained and even enhanced in [βarr]D2R whereas both efficacy and potency are either lost or markedly reduced in [D80A]D2R and [Gprot]D2R as determined by BRET (Fig. 2B).
Fig. 2.
Biased D2R mutants derived from Evolutionary Trace. (A) Inhibition of cAMP as determined by GloSensor compared with [WT]D2R positive control and [D80A]D2R negative control. (B) β-arrestin 2 recruitment determined by BRET for the same receptors as in A. All points are SEM of n = 3–7 done in duplicate. Confocal images of (C) [WT]D2R, (D) [Gprot]D2R, (E) [βarr]D2R, and (F) [D80A]D2R expressed in live cells. (G) BMAX (with SEM) determined from n = 3 radioligand binding experiments. (H) KD from BMAX determination experiments. (I) DA competition binding experiments to determine KI. (J) D2R internalization assessed by live cell HA antibody staining of D2R (SEM, n = 5 done in triplicate).
Point mutations in GPCRs, especially ones that affect signaling, are notorious for inducing unstable proteins (29). To address this concern, the membrane localization of [WT]D2R (Fig. 2C) in live cells was compared with [Gprot]D2R (Fig. 2D), [βarr]D2R (Fig. 2E), and [D80A]D2R (Fig. 2F). Each mutant was more similar to [WT]D2R than two typical D2R mutants that display membrane localization deficiencies ([DRY]D2R and [TYY]D2R; Fig. S3 C and D). To quantitatively assess the expression of these mutants, traditional radioligand determinations of BMAX (Fig. 2G) and KD (Fig. 2H) were performed. When transiently transfected into HEK 293T cells, all mutated receptors expressed between 1 and 1.5 pmol/mg protein, and their levels were 30–50% lower than the [WT]D2R under the same conditions (Fig. 2G). However, the KD for the antagonist raclopride was virtually identical (Fig. 2H), and the KI for DA was also unchanged (Fig. 2I). Receptor internalization, as assessed by cell surface ELISA on live cells (30), demonstrates predictable internalization patterns: [βarr]D2R and [WT]D2R internalize to the same degree (30%) as previously reported (31), whereas [Gprot]D2R and [D80A]D2R are severely deficient (Fig. 2J).
The separation in apparent affinity for the endogenous ligand dopamine for cAMP inhibition, β-arrestin 2 recruitment, and internalization is 100- to 1,000-fold between the two engineered receptors, whereas the KD of raclopride remains unchanged. Similarly, the response of each receptor mutant would be greater than 90% distinct even at the highest physiological levels of dopamine (100 µM in Fig. 2 A, B, and J and Table S2). Additionally, the slight differences in expression levels of the various D2R mutants does not seem to affect their coupling potencies as increasing the amounts of transfected D2Rs has the same effects for [WT]D2R and [Gprot]D2R. Thus, these ET-derived mutants display a robust but selective disruption in D2R function.
In Vitro Functional Selectivity Between [Gprot]D2R and [βarr]D2R.
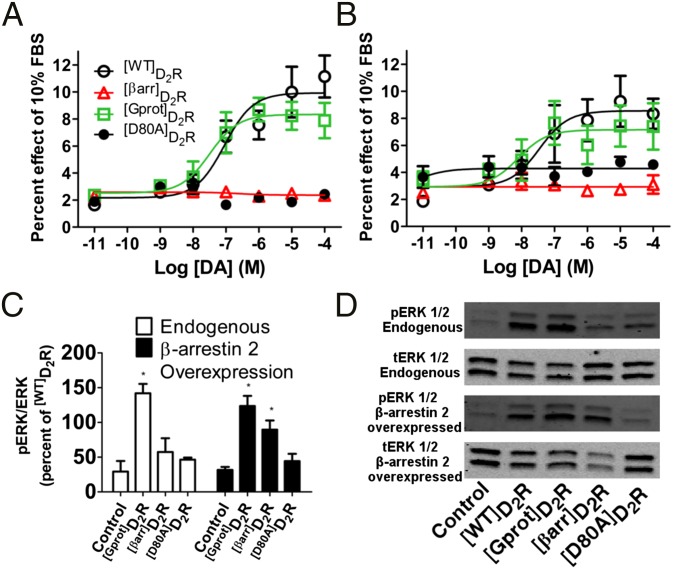
The relationship between GPCR G protein- and β-arrestin–dependent signaling is complex. G protein-mediated signaling is rapid and transient, and engagement of β-arrestin inhibits the G-protein pathways. In addition, formation of the GPCR/β-arrestin complex normally depends upon phosphorylation of the receptor (32). Moreover, G proteins and β-arrestins can engage the same pathway but with distinct cellular consequences. One well-documented example of this is the MAP kinase cascade (33). To address this relationship, two related transcriptional reporters for MAP kinase signaling were transfected along with the mutated D2Rs in HEK 293T cells. As shown in Fig. 3 A and B, a dose-dependent DA activation of MAP kinase transcription was observed in [WT]D2R and [Gprot]D2R but absent in [βarr]D2R and [D80A]D2R as well as [WT]D2R treated with pertussis toxin (Fig. S3B). Probing ERK phosphorylation through Western blot analysis revealed activation by [βarr]D2R compared with [D80A]D2R or untransfected cells only when β-arrestin 2 is overexpressed (Fig. 3 C and D). In contrast, [Gprot]D2R activated ERK phosphorylation regardless of β-arrestin expression, compared with [D80A]D2R. This indicates that D2R activates canonical MAP kinase activity through G proteins, whereas β-arrestin may produce noncanonical ERK activity under conditions of enhanced β-arrestin or kinase expression, as observed in other GPCR systems (34).
Fig. 3.
Assessment of MAP kinase activity at D2R. (A) SRF and (B) SRE MAP kinase transcriptional promoter mediated expression of luciferase (SEM, n = 5–6 done in triplicate). (C) Western blot analysis of ERK (*P < 0.05 Newman–Keuls post hoc compared with [D80A]D2R or untransfected after one-way ANOVA P < 0.05, SEM, n = 3–6) with and without β-arrestin 2 overexpression. (D) Representative blot for the data presented in C.
[Gprot]D2R and [βarr]D2R Are Biologically Active and Display Functional Differences.
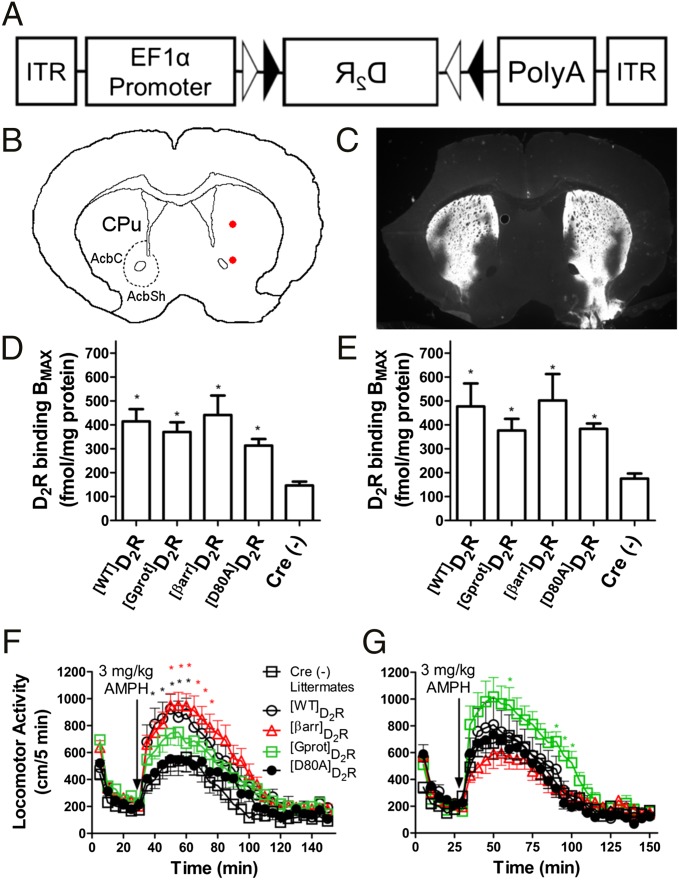
The biological activity and functional properties of [Gprot]D2R and [βarr]D2R were tested with a virally mediated in vivo overexpression approach. Adeno-associated viral (AAV) vectors containing a double-floxed inverted ORF (DIO) of each HA-tagged D2R transgene driven by the housekeeping gene EF1α promoter (35) were synthesized and packaged into viral particles (Fig. 4A). Each D2R construct was injected bilaterally into the dorsal striatum (caudate putamen) and the ventral striatum (nucleus accumbens) (Fig. 4B). Extent of viral transduction was assessed by staining for the HA epitope tag on the N terminus of D2Rs (Fig. 4C). Neuronal specificity of expression was achieved using the Adora2A-Cre mouse line, which selectively expresses Cre in D2R-expressing medium spiny neurons (MSNs) but not presynaptic DA projection cells (36), and these Adora2A-Cre mice were also crossed to a mouse strain with β-arrestin 2 floxed to allow for specific deletion of β-arrestin 2 in indirect pathway MSNs. [WT]D2R, [Gprot]D2R, [βarr]D2R, or [D80A]D2R yielded a twofold to fourfold increase in striatal D2R expression as measured by ligand binding (Fig. 4D, expression in Adora2A-Cre, and Fig. 4E, expression when β-arrestin 2 is genetically deleted). Overexpression of the [WT]D2R led to a ∼1.5-fold potentiation in the amphetamine induced locomotor response (Fig. 4F). [βarr]D2R overexpression led to a similar potentiation, whereas the [Gprot]D2R overexpression was much less effective. However, to demonstrate construct validity, the same experimental design was carried out in Adora2A-Cre::β-arrestin 2flox/flox mice. As shown in Fig. 4G, although overexpression of [Gprot]D2R produced a slight increase in the amphetamine response, the robust increase previously observed with both [WT]D2R and [βarr]D2R was completely absent. Note the differences in control responses to amphetamine between Fig. 4 F and G because these mice are on a different background. This suggests that the enhanced amphetamine response of [βarr]D2R is dependent upon β-arrestin 2. These findings demonstrate that the functionally selective engineered D2R mutants are biologically active in vivo and mediate distinct functions.
Fig. 4.
The physiological relevance of D2R functional selectivity. (A) Viral transgene packaged into AAV, which allowed for Cre-dependent expression of D2R through a double-floxed inverted ORF (DIO). (B) 0.75 µL of virus was injected bilaterally into the dorsal and ventral striatum with each injection site indicated by the red dots, and a total of 3 µL was injected into the striatum of each mouse. CPu, caudate putamen; AcbC, nucleus accumbens, core; AcbSh, nucleus accumbens, shell. (C) Representative staining pattern of the N-terminal HA tagged D2R shows transduction of a majority of the dorsal striatum and at least 50% of the ventral striatum with variable transduction in the olfactory tubercle. Radioligand binding revealed a twofold to fourfold overexpression of each receptor as determined from membranes prepared from striatal dissections from Adora2A-Cre (D) and Adora2A-Cre::β-arrestin 2 flox (E) mice (*P < 0.05 Newman–Keuls post hoc compared with Cre (−) controls after one-way ANOVA P < 0.05, SEM, n = 4–6). (F) Potentiation of amphetamine-induced locomotion in mice when D2R is overexpressed (*P < 0.05 bonferroni post hoc compared with [D80A]D2R after repeated measures two-way ANOVA P < 0.05 for receptor expression type SEM, n = 11–12, color coded for receptor type). (G) The amphetamine response potentiation of [WT]D2R and [βarr]D2R is abolished when β-arrestin 2 is genetically deleted from D2R-expressing medium spiny neurons (*P < 0.05 bonferroni post hoc compared with [D80A]D2R after repeated measures two-way ANOVA P < 0.05 for receptor expression type SEM, n = 8–13).
Discussion
DA is an important regulator of both CNS and peripheral physiological homeostasis. Disruptions in the function of DA have been associated with schizophrenia, depression, mania, attention deficit disorders, drug abuse, and Parkinson’s disease in the CNS and hypertension and prolactinemia in the periphery (37). DA exerts its function through two major GPCRs: D2R and D1R (as well as the D1R-like D5R and D2R-like D3R and D4R receptors). The results described here provide a functional template to begin to investigate the in vivo pharmacological, biochemical, and neuronally selective actions of D2R. Precise molecular control was achieved by engineering D2Rs specifically designed to interact with either G proteins or β-arrestins, and these receptors can be reconstituted in cell culture or in specific neuronal populations in vivo.
Over the last several years, state-of-the-art optogenetic (38) and pharmacogenetic (39) approaches have been developed to map brain pathways and cellular functions of neuronal populations. However, these approaches are not amenable to the elucidation of molecular mechanisms because they are not designed to manipulate the specific biochemical mechanisms of an endogenous ligand through its cognate receptor. Additionally, optogenetic or pharmacogenetic control of intracellular signaling cascades, such as G proteins (40) or ERK (41), do not allow for the interrogation of endogenous ligand dynamic changes or the effect of therapeutics to the system. Understanding the biology of D2R will require determinants such as the contextual influence of phasic and tonic DA release (12) and the monitoring of therapeutics, such as antipsychotics. Although less widely applicable, functionally selective GPCRs are a desirable alternative because they resolve many of the limitations of the more general approaches.
Previously, biochemical studies in mice carrying complete deletion of β-arrestin 2 (10) or cell type-specific genetic deletion of GSK3β (11) have provided evidence for the importance of the β-arrestin 2-mediated D2R signaling pathway in the actions of DA. However, evidence from such studies is limited by the fact that β-arrestin 2 interacts with multiple GPCRs and GSK3β is a signaling hub downstream of many signaling networks, including both G proteins (42) and β-arrestin 2 (10). The current approach more specifically targets D2R, and these pathway-specific mutant D2Rs were developed to begin to elucidate the contribution of individual pathways to physiological and pharmacological DA responses. Biased mutants similar to those in other GPCRs like the β2-adrenergic (TYY) and angiotensin 1A (DRY) receptors (43, 44) generated unstable proteins when engineered into D2R. Additionally, several D2R mutants have also been generated and shown to affect β-arrestin and GPCR kinase interactions (45, 46), postendocytic trafficking (31), desensitization (47), and resensitization (48). Although these D2R mutants have informed various aspects of function and regulation of D2Rs, consideration of some of these mutants for the work described here did not fulfill all necessary inclusion criteria.
The pharmacological fidelity (trafficking, ligand binding and signal transduction) of [Gprot]D2R and [βarr]D2R revealed robust and specific engagement of each pathway. Through sequential iterations (Fig. S2), each mutation converged on transmembrane domain three (TM3), an alpha helix critical for the transmission of conformational changes from ligand binding to signaling molecules (49). These changes in signal transduction allowed for the elucidation of complex signaling paradigms. MAP kinase cascades have previously been shown to be activated downstream of G proteins and β-arrestins (25, 26), but as shown here, [Gprot]D2R is responsible for a major component of the ERK signaling cascade with the normal complement of kinases and β-arrestins present in HEK 293T cells. However, overexpression of β-arrestin 2 revealed the ability of [βarr]D2R to couple to ERK. It is interesting to note that although [Gprot]D2R did not significantly enhance the transcriptional activity, there was a small potentiation in pERK observed compared with [WT]D2R. Taken together, these data indicate that receptor transducer elements, such as MAP kinases, may also exhibit functional selectivity. Finally, to assess the in vivo function of the engineered receptors we used a neuronally selective overexpression approach, which carries the caveat of assessing function in the presence of the normal signaling of endogenous receptors. Despite this limitation, expression of mutant D2Rs in D2R+MSNs revealed marked differences in their ability to affect responses to the psychotropic drug amphetamine. The [βarr]D2R was more effective at enhancing the amphetamine response than the [Gprot]D2R. Although the extent of the separation was surprising, it is consistent with previous genetic manipulation studies, which have predicted an important role for the D2R/β-arrestin 2 pathway in vivo as genetic deletion of β-arrestin 2 has been shown to decrease the locomotor response to amphetamine (8, 10). [Gprot]D2R only slightly potentiated the amphetamine response, and this trend was enhanced by genetic deletion of β-arrestin 2 in D2R expressing MSNs. In contrast, [WT]D2R and [βarr]D2R lost their potentiation of the amphetamine response when β-arrestin 2 was deleted. These data demonstrate the complexity of even basic GPCR signaling events and should allow for insights into the biased actions of the endogenous neurotransmitter.
In summary, functionally selective or biased signaling engineered GPCRs can display in vivo biological activity and mediate distinct pharmacological responses. The robust separation of signal achieved with [Gprot]D2R and [βarr]D2R will allow for direct elucidation of more complex functional selectivity principles when applied to diverse D2R systems. These mutants differ from [WT]D2R by only two amino acids and yet have specific D2R functions disrupted. Functional selectivity has considerable therapeutic potential, but the molecular details have been obscured by the complexity of receptor activation. Furthermore, some signaling events can only be understood in the context of the in vivo architecture (50). [Gprot]D2R and [βarr]D2R are unique tools that should allow for a better understanding of the molecular, cellular, and physiological actions of dopamine as well as provide a template for the development of small molecules with therapeutic predictive value.
Materials and Methods
Evolutionary Trace.
Multiple rounds of ET-guided mutagenesis were conducted on D2R. Each round took advantage of enhancements to the Evolutionary Trace method and GPCR crystallography. The previously reported (43) β-adrenergic 2A receptor TYY served as a starting point for D2R mutagenesis. β2AR-TYY was previously shown to signal through β-arrestins but not G proteins. The first round targeted these homologous positions in D2R (T69, Y133, and Y209). Based on the initial results of TYY mutations, new targets were added based on ET importance and structural location. Substitutions for targeted positions were based on homology in the multiple sequence alignment. In order for D2R to be functional, mutations to cognate amino acids found in other GPCRs at the equivalent sequence position were selected.
Due to the variation in the GPCR loop regions, the transmembrane domains and loops were analyzed separately. The multiple sequence alignment of the transmembrane region was made up of 2512 Class A GPCRs. These sequences were gathered from GPCRDB, aligned, and filtered for the 195 gapless seven transmembrane helix residues. We used the updated pair interaction ET algorithm (piET) (16), which achieves greater accuracy by taking into account the residue contacts seen in a structure, here the crystal structure of rhodopsin in complex with the C terminus peptide of the endogenous G protein (27). The residues targeted for mutation were selected based on their evolutionarily importance (top 5%) and proximity to the C terminus peptide (within 12 Angstroms, the residues in DRY and NPXXY motifs being ignored). The Evolutionary Action algorithm (21) was used to identify substitutions with varying harshness.
An analysis specific to D2R was used to identify the key ET residues in the second intracellular loop region. The crystal structure of β2AR in complex with Gαs (26) was also used to narrow down to the crucial residues for G-protein activation. The multiple sequence alignment for D2R entire sequence (including loops) was made of 66 homologs extracted from a BLAST analysis of the National Center for Biotechnology Information (NCBI) Reference Sequence database where we filtered based on protein length (90% of the query protein) and sequence identity (>60%). This was to ensure we use the most relevant information for ET analysis. Substitutions for targeted positions were also identified with the Evolutionary Action algorithm.
Mutagenesis PCR.
The Agilent Technologies QuikChange mutagenesis kit was used to carry out all mutagenesis according to manufacturer’s instructions. Primers were designed as instructed, with the minimum amount of nucleotide changes required to achieve a mutation. Multiple point mutations were created by using the same primers for single point mutations on already mutated constructs. All constructs were confirmed to have no coding errors by sequencing.
Cell Culture and Transfections.
HEK 293T American Type Culture Collection (ATCC) cells were cultured and transfected as previously reported (25). Please see SI Materials and Methods for a description of the receptor activity assays presented in Figs. 2 and 3.
Mouse Lines.
All mouse studies were conducted in accordance with the National Institutes of Health Guidelines for Animal Care and Use and with an approved animal protocol from the Duke University Animal Care and Use Committee. Please see SI Materials and Methods for more detailed description of the mouse work presented in Fig. 4.
Data Handling.
All dose–response curves were analyzed using the nonlinear regression function Y = Bottom + (Top − Bottom)/(1 + 10^((LogEC50 − X))) of GraphPad Prism 5. All binding curves were fit to Y = Bmax*X/(Kd + X). Statistical analyses were performed as reported in figure legends using GraphPad Prism 5.
Supplementary Material
Acknowledgments
The authors thank Drs. Bernard Masri and Joshua C. Snyder for helpful discussions during the course of this work as well as Wendy Roberts, XuiQin Zhang, and Benjamin Phillips expert technical assistance in the maintenance of mouse colonies. This work was supported in part by the National Institutes of Health Grants 5R37MH073853 (to M.G.C.) and K01DA024763 (to C.E.B.). O.L. gratefully acknowledges support from the National Institutes of Health (GM066099 and GM079656) and from the National Science Foundation (DBI-1356569). T.F.P. is supported by an award from the Ruth K. Broad Biomedical Research Foundation. The continued support of the Pall Family Foundation is greatly appreciated.
Footnotes
Conflict of interest statement: M.G.C. has received compensation from Lundbeck as a member of their Psychopharmacology Advisory Board and is a consultant for Omeros Corp. M.G.C. also owns equity in Acadia Pharmaceuticals.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502742112/-/DCSupplemental.
References
- 1.Bjarnadóttir TK, et al. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics. 2006;88(3):263–273. doi: 10.1016/j.ygeno.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320(1):1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 3.Shenoy SK, Lefkowitz RJ. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32(9):521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Free RB, et al. Discovery and characterization of a G protein-biased agonist that inhibits β-arrestin recruitment to the D2 dopamine receptor. Mol Pharmacol. 2014;86(1):96–105. doi: 10.1124/mol.113.090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192(4238):481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 6.Mailman RB, Murthy V. Third generation antipsychotic drugs: Partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488–501. doi: 10.2174/138161210790361461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83(7):1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 8.Baik JH, et al. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377(6548):424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 9.Jiang M, et al. Multiple neurological abnormalities in mice deficient in the G protein Go. Proc Natl Acad Sci USA. 1998;95(6):3269–3274. doi: 10.1073/pnas.95.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Urs NM, Snyder JC, Jacobsen JP, Peterson SM, Caron MG. Deletion of GSK3β in D2R-expressing neurons reveals distinct roles for β-arrestin signaling in antipsychotic and lithium action. Proc Natl Acad Sci USA. 2012;109(50):20732–20737. doi: 10.1073/pnas.1215489109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai HC, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(5930):1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76(1):33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkins A, Erdin S, Lua R, Lichtarge O. Evolutionary trace for prediction and redesign of protein functional sites. Methods Mol Biol. 2012;819:29–42. doi: 10.1007/978-1-61779-465-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichtarge O, Bourne HR, Cohen FE. An evolutionary trace method defines binding surfaces common to protein families. J Mol Biol. 1996;257(2):342–358. doi: 10.1006/jmbi.1996.0167. [DOI] [PubMed] [Google Scholar]
- 16.Wilkins AD, et al. Accounting for epistatic interactions improves the functional analysis of protein structures. Bioinformatics. 2013;29(21):2714–2721. doi: 10.1093/bioinformatics/btt489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez GJ, Yao R, Lichtarge O, Wensel TG. Evolution-guided discovery and recoding of allosteric pathway specificity determinants in psychoactive bioamine receptors. Proc Natl Acad Sci USA. 2010;107(17):7787–7792. doi: 10.1073/pnas.0914877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granier S, Kobilka B. A new era of GPCR structural and chemical biology. Nat Chem Biol. 2012;8(8):670–673. doi: 10.1038/nchembio.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katsonis P, et al. Single nucleotide variations: Biological impact and theoretical interpretation. Protein Sci. 2014;23(12):1650–1666. doi: 10.1002/pro.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chien EY, et al. Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science. 2010;330(6007):1091–1095. doi: 10.1126/science.1197410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsonis P, Lichtarge O. A formal perturbation equation between genotype and phenotype determines the Evolutionary Action of protein-coding variations on fitness. Genome Res. 2014;24(12):2050–2058. doi: 10.1101/gr.176214.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neskey DM, et al. Evolutionary Action score of TP53 (EAp53) identifies high-risk mutations associated with decreased survival and increased distant metastases in head and neck cancer. Cancer Res. 2015;75(7):1527–1536. doi: 10.1158/0008-5472.CAN-14-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osman AA, et al. Evolutionary Action score of TP53 coding variants (EAp53) is predictive of platinum response in head and neck cancer patients. Cancer Res. 2015;75(7):1205–1215. doi: 10.1158/0008-5472.CAN-14-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osman AA, et al. Wee-1 kinase inhibition overcomes cisplatin resistance associated with high-risk TP53 mutations in head and neck cancer through mitotic arrest followed by senescence. Mol Cancer Ther. 2015;14(2):608–619. doi: 10.1158/1535-7163.MCT-14-0735-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masri B, et al. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci USA. 2008;105(36):13656–13661. doi: 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasmussen SG, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477(7366):549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheerer P, et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455(7212):497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 28.Neve KA, Cox BA, Henningsen RA, Spanoyannis A, Neve RL. Pivotal role for aspartate-80 in the regulation of dopamine D2 receptor affinity for drugs and inhibition of adenylyl cyclase. Mol Pharmacol. 1991;39(6):733–739. [PubMed] [Google Scholar]
- 29.Wilbanks AM, Laporte SA, Bohn LM, Barak LS, Caron MG. Apparent loss-of-function mutant GPCRs revealed as constitutively desensitized receptors. Biochemistry. 2002;41(40):11981–11989. doi: 10.1021/bi020275m. [DOI] [PubMed] [Google Scholar]
- 30.Espinoza S, et al. Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol Pharmacol. 2011;80(3):416–425. doi: 10.1124/mol.111.073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Namkung Y, Dipace C, Javitch JA, Sibley DR. G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor. J Biol Chem. 2009;284(22):15038–15051. doi: 10.1074/jbc.M900388200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nobles KN, et al. Distinct phosphorylation sites on the β(2)-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci Signal. 2011;4(185):ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tohgo A, et al. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem. 2003;278(8):6258–6267. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- 34.Lee MH, El-Shewy HM, Luttrell DK, Luttrell LM. Role of beta-arrestin-mediated desensitization and signaling in the control of angiotensin AT1a receptor-stimulated transcription. J Biol Chem. 2008;283(4):2088–2097. doi: 10.1074/jbc.M706892200. [DOI] [PubMed] [Google Scholar]
- 35.Cardin JA, et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc. 2010;5(2):247–254. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80(6):1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 38.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 39.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104(12):5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Neill PR, Gautam N. Subcellular optogenetic inhibition of G proteins generates signaling gradients and cell migration. Mol Biol Cell. 2014;25(15):2305–2314. doi: 10.1091/mbc.E14-04-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toettcher JE, Weiner OD, Lim WA. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell. 2013;155(6):1422–1434. doi: 10.1016/j.cell.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mannoury la Cour C, Salles MJ, Pasteau V, Millan MJ. Signaling pathways leading to phosphorylation of Akt and GSK-3β by activation of cloned human and rat cerebral D2and D3 receptors. Mol Pharmacol. 2011;79(1):91–105. doi: 10.1124/mol.110.065409. [DOI] [PubMed] [Google Scholar]
- 43.Shenoy SK, et al. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281(2):1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 44.Wei H, et al. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100(19):10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lan H, Liu Y, Bell MI, Gurevich VV, Neve KA. A dopamine D2 receptor mutant capable of G protein-mediated signaling but deficient in arrestin binding. Mol Pharmacol. 2009;75(1):113–123. doi: 10.1124/mol.108.050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Namkung Y, Dipace C, Urizar E, Javitch JA, Sibley DR. G protein-coupled receptor kinase-2 constitutively regulates D2 dopamine receptor expression and signaling independently of receptor phosphorylation. J Biol Chem. 2009;284(49):34103–34115. doi: 10.1074/jbc.M109.055707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Celver J, Sharma M, Thanawala V, Christopher Octeau J, Kovoor A. Arrestin-dependent but G-protein coupled receptor kinase-independent uncoupling of D2-dopamine receptors. J Neurochem. 2013;127(1):57–65. doi: 10.1111/jnc.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho D, et al. Agonist-induced endocytosis and receptor phosphorylation mediate resensitization of dopamine D(2) receptors. Mol Endocrinol. 2010;24(3):574–586. doi: 10.1210/me.2009-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venkatakrishnan AJ, et al. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494(7436):185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- 50.Anzalone A, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023–9034. doi: 10.1523/JNEUROSCI.0918-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szczepek M, et al. Crystal structure of a common GPCR-binding interface for G protein and arrestin. Nat Commun. 2014;5:4801. doi: 10.1038/ncomms5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.