Abstract
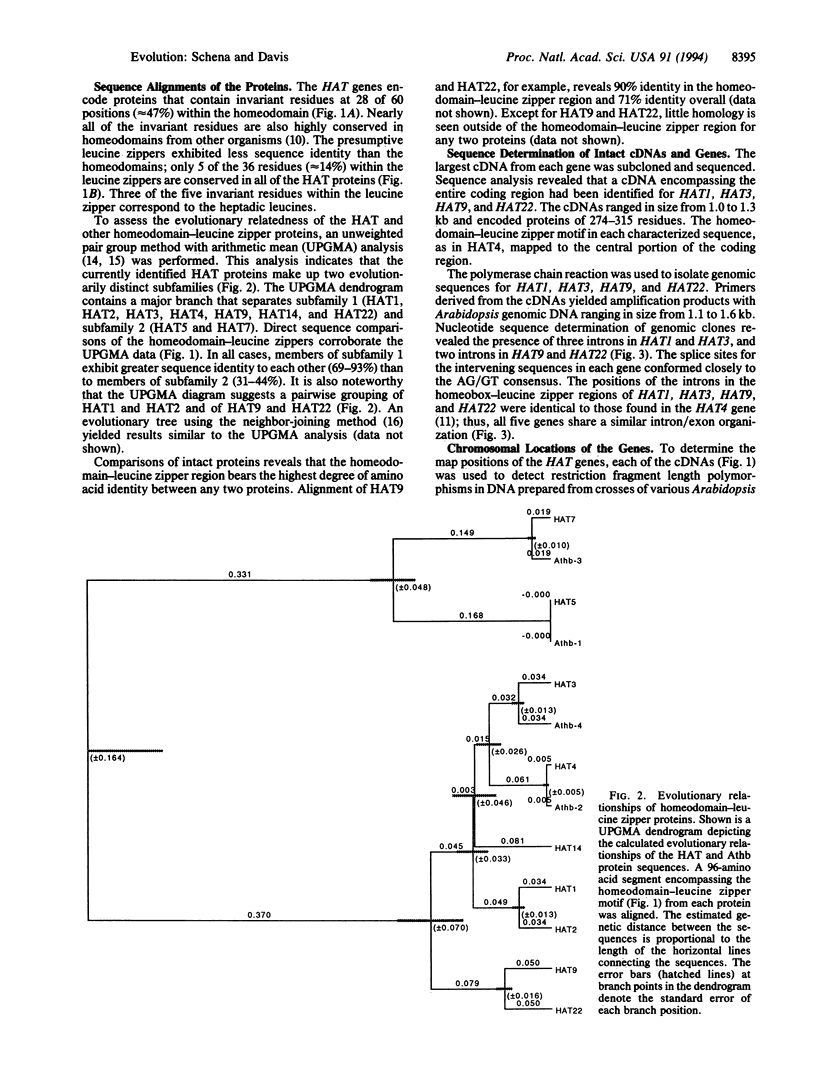
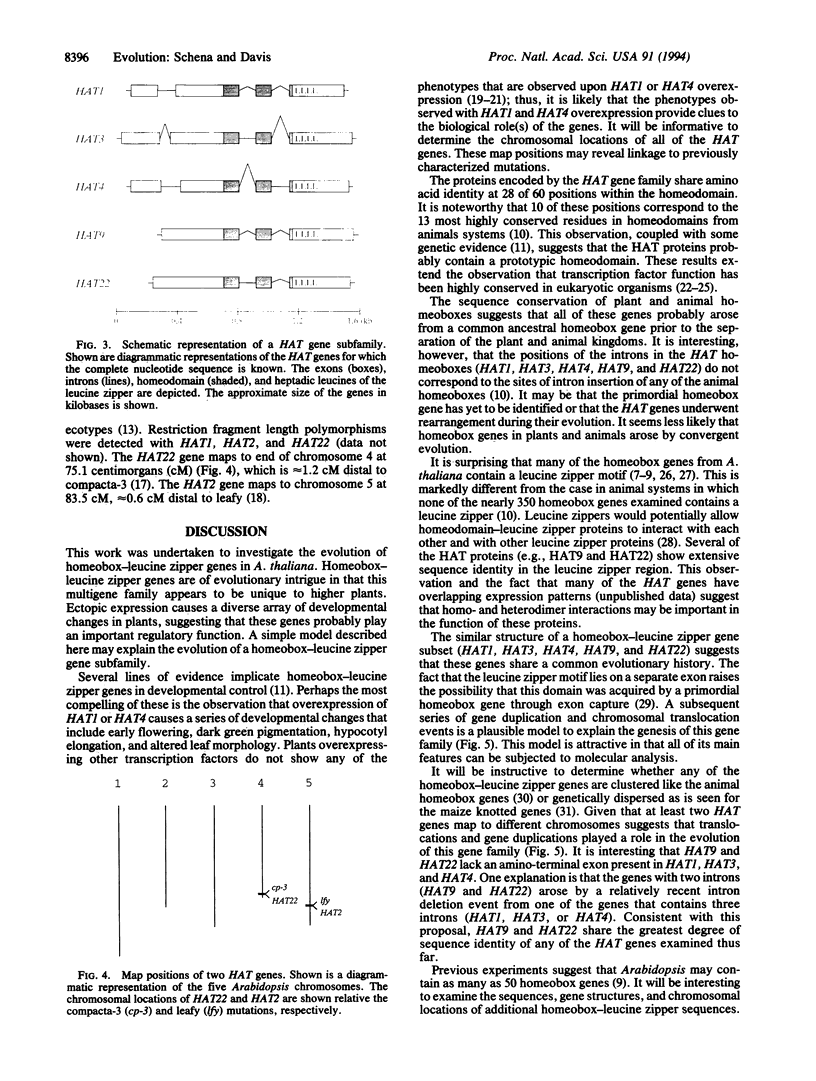
Homeobox genes are present in both plants and animals. Homeobox-leucine zipper genes, however, have been identified thus far only in the small mustard plant Arabidopsis thaliana. This observation suggests that homeobox-leucine zipper genes evolved after the divergence of plants and animals, perhaps to mediate specific regulatory events. To better understand this gene family, we isolated several sequences containing the homeobox-leucine zipper motif and carried out a comparative analysis of nine homeobox-leucine zipper genes (HAT1, HAT2, HAT3, HAT4, HAT5, HAT7, HAT9, HAT14, and HAT22). Gene structures, sequence comparisons, and chromosomal locations suggest a simple model for the evolution of these genes. The model postulates that a primordial homeobox gene acquired a leucine zipper by exon capture. The nascent homeobox-leucine zipper gene then appears to have undergone a series of gene duplication and chromosomal translocation events, leading to the formation of the HAT gene family. This work has general implications for the evolution of regulatory genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carabelli M., Sessa G., Baima S., Morelli G., Ruberti I. The Arabidopsis Athb-2 and -4 genes are strongly induced by far-red-rich light. Plant J. 1993 Sep;4(3):469–479. doi: 10.1046/j.1365-313x.1993.04030469.x. [DOI] [PubMed] [Google Scholar]
- Chang C., Bowman J. L., DeJohn A. W., Lander E. S., Meyerowitz E. M. Restriction fragment length polymorphism linkage map for Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6856–6860. doi: 10.1073/pnas.85.18.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorit R. L., Schoenbach L., Gilbert W. How big is the universe of exons? Science. 1990 Dec 7;250(4986):1377–1382. doi: 10.1126/science.2255907. [DOI] [PubMed] [Google Scholar]
- Groot P. C., Mager W. H., Henriquez N. V., Pronk J. C., Arwert F., Planta R. J., Eriksson A. W., Frants R. R. Evolution of the human alpha-amylase multigene family through unequal, homologous, and inter- and intrachromosomal crossovers. Genomics. 1990 Sep;8(1):97–105. doi: 10.1016/0888-7543(90)90230-r. [DOI] [PubMed] [Google Scholar]
- Hightower R. C., Meagher R. B. The molecular evolution of actin. Genetics. 1986 Sep;114(1):315–332. doi: 10.1093/genetics/114.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M., Hanhart C. J., van der Veen J. H. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet. 1991 Sep;229(1):57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Duquette M., Urry L., McHenry K., Smith T. F. The evolution of the thrombospondin gene family. J Mol Evol. 1993 Jun;36(6):509–516. doi: 10.1007/BF00556355. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Ichikawa H., Saito A., Tada Y., Fujimura T., Kano-Murakami Y. Expression of a rice homeobox gene causes altered morphology of transgenic plants. Plant Cell. 1993 Sep;5(9):1039–1048. doi: 10.1105/tpc.5.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J., Söderman E., Svenson M., Borkird C., Engström P. A new homeobox-leucine zipper gene from Arabidopsis thaliana. Plant Mol Biol. 1992 Mar;18(5):1019–1022. doi: 10.1007/BF00019223. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Mizukami Y., Ma H. Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsis plants alters floral organ identity. Cell. 1992 Oct 2;71(1):119–131. doi: 10.1016/0092-8674(92)90271-d. [DOI] [PubMed] [Google Scholar]
- Ohta T. How gene families evolve. Theor Popul Biol. 1990 Feb;37(1):213–219. doi: 10.1016/0040-5809(90)90036-u. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Gann A. A. Activators and targets. Nature. 1990 Jul 26;346(6282):329–331. doi: 10.1038/346329a0. [DOI] [PubMed] [Google Scholar]
- Ruberti I., Sessa G., Lucchetti S., Morelli G. A novel class of plant proteins containing a homeodomain with a closely linked leucine zipper motif. EMBO J. 1991 Jul;10(7):1787–1791. doi: 10.1002/j.1460-2075.1991.tb07703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schena M., Davis R. W. HD-Zip proteins: members of an Arabidopsis homeodomain protein superfamily. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3894–3898. doi: 10.1073/pnas.89.9.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena M., Lloyd A. M., Davis R. W. A steroid-inducible gene expression system for plant cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10421–10425. doi: 10.1073/pnas.88.23.10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena M., Lloyd A. M., Davis R. W. The HAT4 gene of Arabidopsis encodes a developmental regulator. Genes Dev. 1993 Mar;7(3):367–379. doi: 10.1101/gad.7.3.367. [DOI] [PubMed] [Google Scholar]
- Schena M., Yamamoto K. R. Mammalian glucocorticoid receptor derivatives enhance transcription in yeast. Science. 1988 Aug 19;241(4868):965–967. doi: 10.1126/science.3043665. [DOI] [PubMed] [Google Scholar]
- Schindler U., Beckmann H., Cashmore A. R. HAT3.1, a novel Arabidopsis homeodomain protein containing a conserved cysteine-rich region. Plant J. 1993 Jul;4(1):137–150. doi: 10.1046/j.1365-313x.1993.04010137.x. [DOI] [PubMed] [Google Scholar]
- Sinha N. R., Williams R. E., Hake S. Overexpression of the maize homeo box gene, KNOTTED-1, causes a switch from determinate to indeterminate cell fates. Genes Dev. 1993 May;7(5):787–795. doi: 10.1101/gad.7.5.787. [DOI] [PubMed] [Google Scholar]
- Struhl K. Duality of TBP, the universal transcription factor. Science. 1994 Feb 25;263(5150):1103–1104. doi: 10.1126/science.8108728. [DOI] [PubMed] [Google Scholar]
- Vollbrecht E., Veit B., Sinha N., Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. 1991 Mar 21;350(6315):241–243. doi: 10.1038/350241a0. [DOI] [PubMed] [Google Scholar]
- Weigel D., Alvarez J., Smyth D. R., Yanofsky M. F., Meyerowitz E. M. LEAFY controls floral meristem identity in Arabidopsis. Cell. 1992 May 29;69(5):843–859. doi: 10.1016/0092-8674(92)90295-n. [DOI] [PubMed] [Google Scholar]
- Wines D. R., Brady J. M., Southard E. M., MacDonald R. J. Evolution of the rat kallikrein gene family: gene conversion leads to functional diversity. J Mol Evol. 1991 Jun;32(6):476–492. doi: 10.1007/BF02102650. [DOI] [PubMed] [Google Scholar]




