Abstract
During adolescence, considerable social and biological changes occur that interact with functional brain maturation, some of which are sex-specific. The amygdala is one brain area that has displayed sexual dimorphism, specifically in socio-affective (superficial amygdala [SFA]), stress (centromedial amygdala [CMA]), and learning and memory (basolateral amygdala [BLA]) processing. The amygdala has also been implicated in mood and anxiety disorders which also display sex-specific features, most prominently observed during adolescence. Using functional magnetic resonance imaging (fMRI), the present study examined the interaction of age and sex on resting state functional connectivity (RSFC) of amygdala sub-regions, BLA and SFA, in a sample of healthy adolescents between the ages 10-16 years (n=122, 71 boys). Whole-brain, voxel-wise partial correlation analyses were conducted to determine RSFC of bilateral BLA and SFA seed regions, created using the Eickhoff-Zilles maximum probability maps based on cytoarchitectonic mapping and FMRIB's Integrated Registration and Segmentation Tool (FIRST). Monte Carlo simulation was implemented to correct for multiple comparisons (threshold of 53 contiguous voxels with a z-value ≥ 2.25). Results indicated that with increasing age, there was a corresponding decrease in RSFC between both amygdala sub-regions and parieto-occipital cortices, with a concurrent increase in RSFC with medial prefrontal cortex (mPFC). Specifically, boys and girls demonstrated increased coupling of mPFC and left and right SFA with age, respectively; however, neither sex showed increased connectivity between mPFC and BLA, which could indicate relative immaturity of fronto-limbic networks that is similar across sex. A dissociation in connectivity between BLA- and SFA- parieto-occipital RSFC emerged, in which girls had weaker negative RSFC between SFA and parieto-occipital regions and boys had weaker negative RSFC of BLA and parieto-occipital regions with increased age, both standing in contrast to adult patterns of amygdala sub-regional RSFC. The present findings suggest relative immaturity of amygdala sub-regional RSFC with parieto-occipital cortices during adolescence, with unique patterns in both sexes that may support memory and socio-affective processing in boys and girls, respectively. Understanding the underlying normative functional architecture of brain networks associated with the amygdala during adolescence may better inform future research of the neural features associated with increased risk for internalizing psychopathology.
Keywords: resting state functional connectivity, amygdala, adolescence, sex differences
1. Introduction
Structural and functional connectivity between the amygdala and cortical brain regions undergoes dramatic maturation across adolescence (Qin et al., 2012, Gabard-Durnam et al., 2014). Resting state functional connectivity (RSFC) refers to the coupling of spontaneous blood oxygen level-dependent (BOLD) signal, as measured with functional magnetic resonance imaging (fMRI), in discrete brain regions or networks. Positive functional connectivity between regions is thought to reflect patterns of synchronous activity or increased communication. Limbic structures, including the amygdala, demonstrate emerging functional and structural maturity by early adolescence (Giedd et al., 1996, Ostby et al., 2009, Wierenga et al., 2014); however, prefrontal cortical brain regions display a protracted rate of development that extends into the third decade of life (Giedd et al., 1996, Gogtay et al., 2004). As such, it is believed that amygdalar functioning is not down-regulated effectively by medial prefrontal cortex (mPFC) and rostral anterior cingulate cortex (rACC) during adolescence (i.e. Dual Systems Model), which can manifest as heightened emotional reactivity typical of this developmental period (Hare et al., 2008, Perlman and Pelphrey, 2011, McRae et al., 2012, Gee et al., 2013). An imbalance in maturity of frontal and limbic brain regions is likely insufficient, however, to explain the range of behavior in adolescents. Additionally, there are sex differences in the structural development of the amygdala during adolescence (Giedd et al., 1996) in which males demonstrate significant increases in volume that females do not (Giedd et al., 1996), as well as in prefrontal cortices, with girls peaking in gray matter volume approximately two years earlier than boys (Lenroot et al., 2007). These sex-specific structural developmental trajectories may impact concomitant functional connectivity of these brain regions. Previous studies in adults have reported sex differences in amygdala sub-region shape and volume (Kim et al., 2011, Kim et al., 2012) and in RSFC of non-limbic brain regions using a variety of analytic methods (Kilpatrick et al., 2006, Biswal et al., 2010, Tian et al., 2011, Casanova et al., 2012, Satterthwaite et al., 2014). Furthermore, atypical functional connectivity of amygdala-mPFC neurocircuitry has been shown to underlie disrupted emotional and cognitive ability during psychopathologic states, such as schizophrenia, bipolar disorder, and mood disorders (Anand et al., 2005, Das et al., 2007, Henry et al., 2008, Wang et al., 2009, Berking and Wupperman, 2012, Cisler and Olatunji, 2012), many of which emerge in late adolescence and display sex-specific onset and progression of illness.
Examination of the functional interactions of amygdala-cortical neurocircuitry is complicated by the fact that the amygdala is not one homogenous structure (LeDoux, 2003, Price, 2003, Amunts et al., 2005). The amygdala can be subdivided into basolateral (BLA), centromedial (CMA) and superficial (SFA) nuclei, each with distinct functional connections to the cortex supporting different brain functions. The BLA facilitates associative learning processes, like fear conditioning, through afferent projections from the frontal cortex and other subcortical regions (LeDoux, 2003, Phelps and LeDoux, 2005). The CMA is critical in the generation of behavioral responses through projections to the brainstem, striatum, and regions of the cortex (LeDoux, 2003). Finally, the SFA is relevant for olfactory (Price, 2003, Heimer and Van Hoesen, 2006) and affective processes (Bzdok et al., 2013a). Previous work has found distinct functional connectivity patterns, as measured with RSFC, across amygdalar nuclei (Roy et al., 2009, Li et al., 2012, Qin et al., 2012, Gabard-Durnam et al., 2014), specifically different patterns of age-dependent positive connectivity between amygdalar nuclei and ventromedial PFC (vmPFC), temporal, and subcortical regions, as well as negative connectivity with parietal and occipital cortices (Qin et al., 2012, Gabard-Durnam et al., 2014). However, only one study performed a secondary analysis to examine sex differences in adolescents, which did not yield a significant effect of sex (Gabard-Durnam et al., 2014).
The current study examined sex differences in age-dependent RSFC of amygdalar nuclei in a relatively large adolescent sample. Previous studies attempting to characterize developmental differences in amygdalar RSFC have appropriately used samples with broad age ranges spanning childhood and adulthood (Qin et al., 2012, Gabard-Durnam et al., 2014); however, sex differences may be obscured when collapsing data across a variety of developmental stages. Given the dynamic nature of adolescent brain development and sex differences in amygdalar and frontal lobe gray matter maturation (Giedd et al., 1996, Lenroot et al., 2007), additional examination of amygdalar RSFC during adolescence may better address whether sex differences in amygdala sub-nuclei RSFC exist over the span of this period. Previous research has shown coupling of mPFC and all amygdala sub-regions with increasing age (Roy et al., 2009, Qin et al., 2012, Gabard-Durnam et al., 2014), while different studies have also shown protracted prefrontal cortical development through adolescence, with girls showing relative maturity as compared to boys (Giedd et al., 1996, Lenroot et al., 2007). In light of this research, we hypothesized a positive relationship between age and RSFC of amygdalar sub-regions and mPFC across the sampled age range that would be stronger in girls, compared to boys. Sex differences in the developmental trajectory of RSFC of amygdala sub-regions may provide insight on the mechanisms that support sex differences in the onset and progression of mental illness.
2. Materials and Methods
2.1. Participants
Data from an ongoing adolescent neurodevelopment protocol were used for this study. Participants with anatomical magnetic resonance imaging (MRI) and resting state functional (RSFC) MRI data, acceptable amygdala sub-nuclei region of interest (ROI) masks (see 2.5 Definition of amygdala sub-region ROIs) and limited head movement (see 2.4 Motion Correction) were included in functional connectivity analyses. The total sample included 122 adolescents (boys = 71) between the ages of 10 and 16 years. A restricted age range was employed to capture developmental effects and sex differences in amygdalar functional connectivity specific to adolescence.
Written assent and consent from children and their parents, respectively, were obtained in accordance with the Oregon Health & Science University (OHSU) Institutional Review Board. Exclusionary criteria included current (past 12 month) diagnosis of DSM-IV psychiatric disorders, significant substance use (>10 lifetime alcoholic drinks or >2 drinks/occasion, >5 uses of marijuana, any other drug use, or >4 cigarettes per day), neurological illness, significant head trauma, chronic medical problems affecting the central nervous system, prenatal exposure to drugs or alcohol, reported history of psychotic disorders in biological parents, current pharmacological treatment that may affect neural function (e.g. psychoactive medication), the inability of a parent to provide family history information, left-handedness (Edinburgh Handedness Inventory, (Oldfield, 1971)), pregnancy, and MRI contraindications (e.g. braces, irremovable ferrous material).
Once eligibility was established, youth were administered the Wechsler Abbreviated Scale of Intelligence (Weschler, 1999) – short form and the self-rated Pubertal Development Scale (PDS) (Petersen, 1988). Self-report on the PDS has been shown to correlate moderately with other measurements of pubertal status, like Tanner's Sexual Maturation Scale (Bond et al., 2006). Parents of youth were administered the Hollingshead Index of Social Position (Hollingshead, 1975) to determine socioeconomic status (SES), which is based on occupation and educational attainment of each parent.
2.2 Image acquisition
Participants were scanned on a Siemens Trim Trio 3.0 Tesla MRI scanner at the Advanced Imaging Research Center at OHSU. One high-resolution T1-weighted MPRAGE sequence of 9 minutes and 14 seconds was acquired (TR = 2300 ms, TE = 3.58 ms, orientation = sagittal, 256×256 matrix, resolution = 13 mm). BOLD-weighted functional images were collected (along the anterior-posterior commissure) using T2*-weighted echo planar imaging (TR = 2500 ms, TE = 30 ms, flip angle = 90 degrees, FOV = 240 mm2, 36 slices covering the entire brain, slice thickness = 3.8 mm, resolution = 3.75 × 3.75 × 3.8 mm). Two runs of 4 minutes and 17 seconds of resting state BOLD data were acquired, during which participants were instructed to stay still and fixate on a white cross in the center of a black screen projected from the head of the scanner and viewed with a mirror mounted on 12-channel head coil. The resting state runs were separated by a 10-minute task that was the same for every participant. Following completion of the scan, youth confirmed wakefulness during resting state scans.
2.3 Image processing
Data processing followed commonly used procedures to reduce spurious noise and artifacts (Fair et al., 2007, Fair et al., 2009, Fair et al., 2012, Mills et al., 2012, Costa Dias et al., 2013). In order, these steps included slice time correction, debanding, rigid body head motion correction with regression of 3 translational and 3 rotational parameters, and signal normalization to a mode value of 1000. Resting state runs were then concatenated and underwent subsequent processing together. Anatomical images were transformed into 3 mm3 voxels in standard Talairach space (Talairach and Tournoux, 1988) and used for co-registration of functional data into the same atlas space. Proper co-registration was confirmed by visual inspection (A.C.). Functional data underwent further processing, including temporal band-pass filtering to remove high-frequency noise (0.009 Hz < f < 0.08 Hz), detrending, regression of white matter and ventricular signal from amygdalar ROIs, global signal regression from the whole brain, and regression of white matter, ventricular and whole-brain signal derivatives.
2.5 Definition of amygdala sub-region ROIs
ROIs of amygdala sub-regions were created using cytoarchitectonic maps implemented in the Analysis of Functional NeuroImages (AFNI) suite (Cox, 1996). Bilateral BLA, CMA and SFA ROIs were created in AFNI, based on the Eickhoff-Zilles maximum probability map from post-mortem analysis (Amunts et al., 2005). Although cytoarchitectonic maps are precise microstructural tools, they are based on adult samples that may not be representative of adolescent populations. Therefore, to account for developmental, as well as individual differences, a model-based segmentation method was implemented using FMRIB's Integrated Registration and Segmentation Tool (FIRST) (Patenaude et al., 2011) to create subject-based amygdala ROIs based on grey and white matter boundaries present on participant T1-weighted images. Bilateral ROIs of the entire amygdala were created and visually inspected to confirm accurate segmentation (M.D.R. and G.A.). Final ROIs constituted the overlap from maximum probability maps and FIRST amygdala masks to confirm precise localization of amygdala sub-regions. No voxel corresponded to more than one ROI. On average, BLA ROIs had larger voxel counts (905.1 ± 232.4), followed by SFA (180.8 ± 85.6) and CMA (0.9 ± 2.2), corresponding to anatomical size. However, most CMA voxel counts were quite small and in many cases zero, so we chose not to include CMA ROIs in functional connectivity analyses. BLA size, based on voxel count, was larger in left compared to right hemisphere (t120 = 33.41, p < 0.001), while right SFA voxel count was larger than left SFA (t120 = −10.71, p < 0.001). Further, voxel counts were not statistically different by sex for any sub-region (all t120 < 0.48, p > 0.05). Functional connectivity maps were created from processed RSFC data by correlating average BOLD signal from each sub-region with every voxel in the brain.
2.4 Motion correction
Strict motion correction procedures were applied to resting state functional maps, due to the sensitivity of RSFC data to head motion (Power et al., 2012, Satterthwaite et al., 2012, Van Dijk et al., 2012). TRs with signal intensities exceeding absolute values of 8 (or 0.8% signal change), as measured by the variance of the signal change from the average signal (DVAR), were excluded to limit the effect of head movement on MRI signal (Shannon et al., 2011, Power et al., 2012). DVAR values, which are based in BOLD signal intensity, differ across datasets for a variety of reasons (e.g. blurring kernel size, frequency filter or data acquisition characteristics) (Power et al., 2014), effectively eliminating the need for a standard threshold. Upon examination of the present data and processing methods, which excluded blurring altogether, a threshold of 0.8% was determined to be appropriate. Based on the DVAR algorithm, frames were removed using a threshold of 40%, such that participants with greater than 40% of frames removed (80 of 200 TRs), were excluded from analyses entirely (Fair et al., 2012, Power et al., 2012, Cservenka et al., 2014). This threshold was employed to ensure that all participants had a minimum of 120 TRs, or approximately 5 minutes of resting state data for analyses, shown to yield sufficiently high sensitivity (77%) for detecting true functional connections (Smith et al., 2011). A final measure, fframewise displacement (FD) (Fair et al., 2012, Power et al., 2012), was measured post-hoc to confirm the remaining volumes had equivalent head movement between males and females. The FD method indexes head movement relative to adjacent volumes and is based on the following scalar formula: FDi =|Δdix|+|Δdiy|+|Δdiz|+|Δαi|+|Δβi|+|Δγi|), where Δdix =d(i−1)x –dix. Using this formula, an FD remaining mean variable was calculated for every individual, representing the degree of micro-movement (in the range of millimeters) of the previously scrubbed (DVAR) data. Sex differences in FD remaining mean were assessed with an independent samples t-test and correlations between FD remaining mean and age were determined with Pearson's correlation. To further confirm our findings were not confounded by head movement, we performed a supplementary analysis using the FD measurement itself for motion censoring (see Supplementary Methods).
2.6 Data analysis
2.6.1 Demographic data
Demographic data were examined for normality and outliers (2.5 standard deviations from the mean) within the whole sample and by sex using IBM SPSS Statistics 20 (Armonk, NY: IBM Corp.). No variables exceeded absolute skew/kurtosis values of 2.0, and thus were considered normally distributed. Sex differences for IQ, SES, and age were assessed with independent samples t-tests, and PDS sex differences were examined with a Mann-Whitney U test.
2.6.2 Imaging data
Using functional connectivity maps, a whole-brain, voxel-wise partial correlation that included age, sex (dummy coded), and sex-by-age was implemented with in-house software using 4dfp tools developed at Washington University as previously utilized (Fair et al., 2012) to assess amygdala sub-region RSFC. Due to a residual correlation between FD remaining mean and age, this variable was included as a covariate in the analyses (see 3. Results). A Monte Carlo simulation was implemented to account for multiple comparisons (threshold of 53 contiguous voxels with a z-value ≥ 2.25). This analysis was conducted four times, once for each sub-region (bilateral BLA and SFA). For each amygdala sub-region, the outcome of these analyses provided effects of a sex-by-age interaction (with age, sex, and FD remaining mean in the model). Values from significant clusters in sex-by-age analyses for each sub-region were extracted and plotted to confirm the interaction effect. The unique effects of age (controlling for sex, sex-by-age, and FD remaining mean) and sex (controlling for age, sex-by-age, and FD remaining mean), were also obtained and are reported in Supplementary Data.
2.6.3 Post-hoc Analyses
To account for potential confounds of puberty, post-hoc analyses were conducted using Fisher's Z-transformed correlation coefficients extracted from cortical brain regions that were functionally connected with amygdala sub-regions. The partial correlation models used in the RSFC analyses (2.6.2 Imaging data) were reconstructed in SPSS Statistics 20 (Armonk, NY: IBM Corp.); puberty was then included in the models as an additional covariate to determine if RSFC results remained significant.
3. Results
Boys and girls did not differ statistically by age, IQ or SES, but did differ in pubertal development, although on average, boys and girls reported the same pubertal stage (see Table 1). Age distribution by sex was also statistically similar (see Supplementary Figure 6). Prior to data scrubbing, FD values were statistically similar in boys and girls (t120 = −1.91, p = 0.06) and FD was negatively correlated with age (r2 = −0.22, p = 0.02). Following data scrubbing, there were no sex differences in FD (i.e. FD remaining mean) (t120 = 0.27, p = 0.79), but age was still negatively correlated with FD (r2 = −0.31, p = 0.001); therefore, FD remaining mean values were included as a covariate in RSFC analyses.
Table 1.
Demographics
| Girls (n=51) | Boys (n=71) | Statistic | |
|---|---|---|---|
| Age (years) | 13.8 (1.4) | 14.1 (1.2) | t = 0.90 |
| aIQ | 113.3 (9.3) | 113.3 (11.0) | t = 0.04 |
| bSES | 27.6 (12.3) | 29.6 (13.3) | t = 0.85 |
| cPuberty | 3.8 (0.8) | 3.1 (0.9) | U = 4.80** |
Wechsler Abbreviated Scale of Intelligence
Hollingshead Index of Social Position; larger values indicate lower socioeconomic status (middle class corresponds to 32-47 range)
Crockett Pubertal Development Scale; Values range from 1-5, with larger values referring to more advanced pubertal development.
Indicates p < .001.
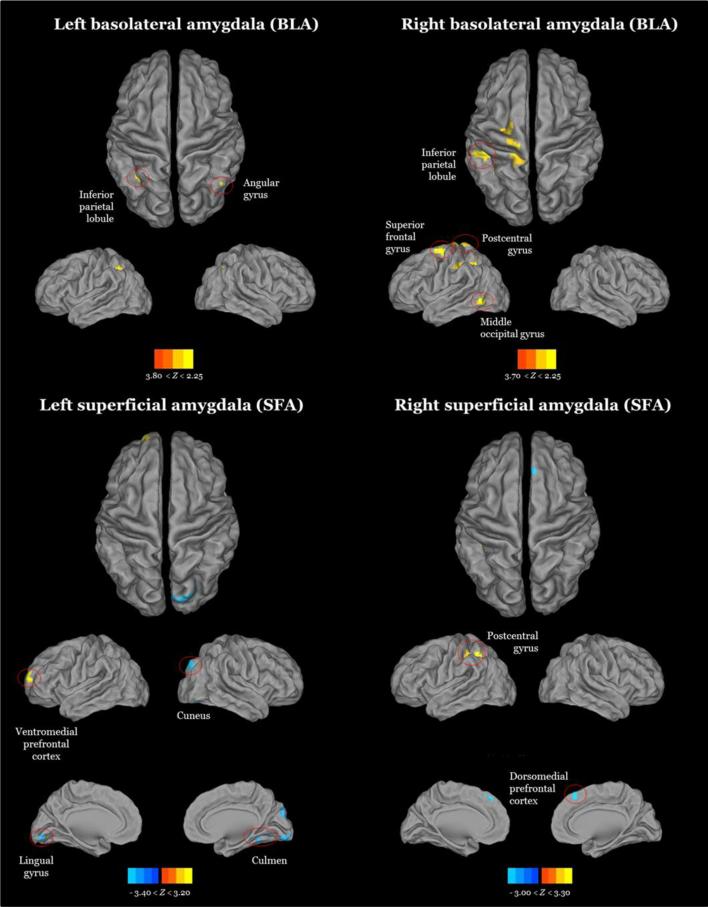
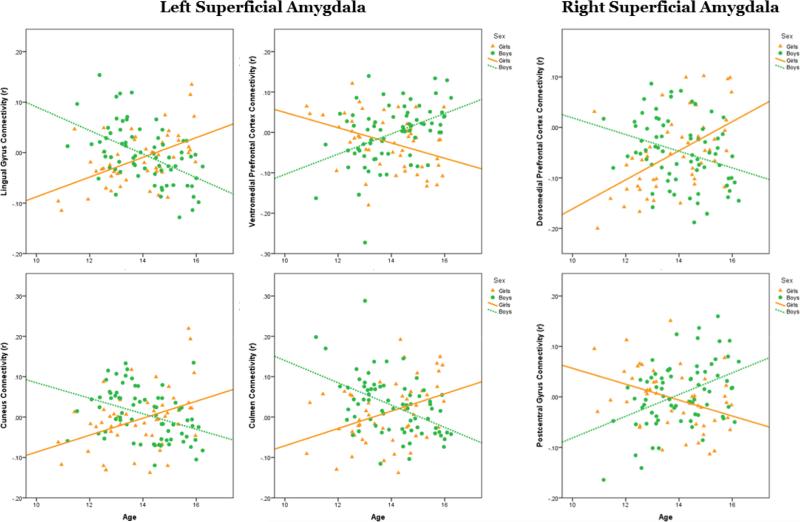
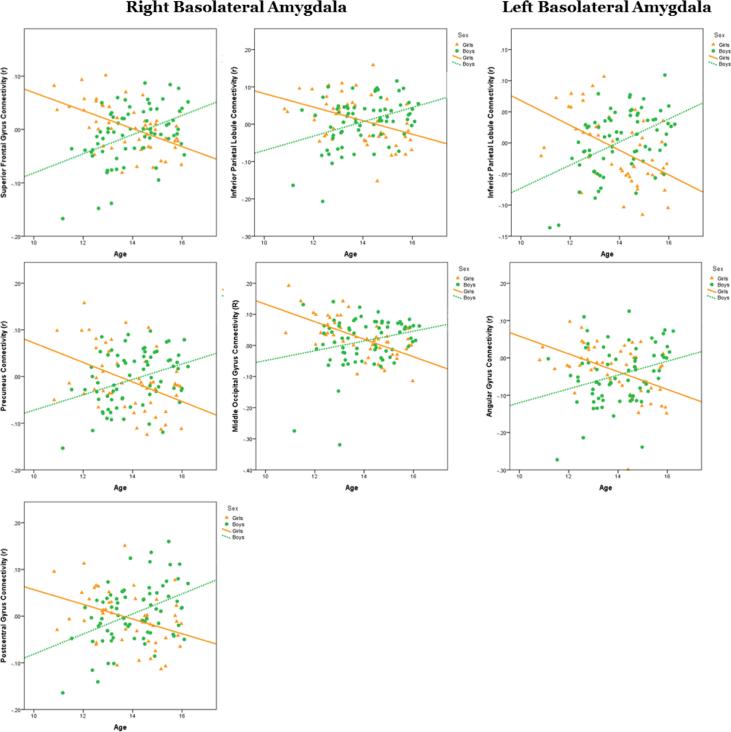
Although examination of group differences by sex is informative, given the age range of our sample, the interactions between age and sex in amygdala sub-region RSFC was pursued. This examination revealed a dissociation of RSFC of BLA, in which girls had positive RSFC at younger ages that decreased over adolescent development into negative RSFC, while boys had negative RSFC at younger ages that increased over development to positive RSFC. Several sex-by-age interactions in amygdala-cortical connectivity were identified. In left BLA, an interaction of sex and age was seen in left inferior parietal lobule (IPL) and right angular gyrus (AG) that displayed the pattern described above: increased positive connectivity with age in boys and increased negative connectivity with age in girls. In right BLA, an interaction was observed in left superior frontal gyrus (SFG), IPL, precuneus (PC), middle occipital gyrus (MOG) and postcentral gyrus with the same dissociation as left BLA RSFC patterns across the sexes. Patterns of RSFC in the sex-byage interaction of SFA with cortical brain regions diverged, however, in some cases. In left SFA an interaction was seen in right lingual gyrus (LG), left SFG, right cuneus and right culmen, and in all these regions, except left vmPFC, girls had increased positive connectivity with age, and boys had increased negative connectivity with age. Connectivity between left SFA and vmPFC followed the patterns observed in RSFC of BLA with cortical brain regions. Finally, in right SFA, connectivity with left postcentral gyrus also followed the same patterns of RSFC observed with BLA, while connectivity with left dorsomedial PFC (dmPFC) showed the opposite pattern, with girls having increased positive connectivity with age and boys having increased negative connectivity with age (Figures 1-3 and Table 2).
Figure 1.
Dorsal and lateral surface mapped results showing significant age-by-sex interactions (blue: negative, yellow: positive) in the coupling of amygdala sub-nuclei and other brain regions. Top left: Age-by-sex interaction with the left basolateral amygdala. Top right: Age-by-sex interaction with the right basolateral amygdala. Bottom left: Age-by-sex interaction with the left superficial amygdala. Bottom right: age-by-sex interaction with the left basolateral amygdala. All findings underwent Monte Carlo multiple comparisons correction (z ≥ 2.25).
Figure 3.
Fisher's Z transformed R-values from brain regions with significant functional connectivity age-by-sex interactions with left superficial amygdala (left) and right superficial amygdala (right) were extracted and plotted against age and by sex.
Table 2.
Age-by-Sex changes in functional connectivity (RSFC) with amygdala sub-regions.
| Structure | BA | Voxels (mm3) | Peak Talairach coordinates (x, y, z) | Boys Direction of positive FC change with increasing age | Boys Direction of negative FC change with increasing age | Girls Direction of positive FC change with increasing age | Girls Direction of negative FC change with increasing age | Z-score |
|---|---|---|---|---|---|---|---|---|
| Left Basolateral | ||||||||
| L Inferior Parietal Lobule | 7 | 132 | −32, −69, 21 | ↑ | ↑ | 2.66 | ||
| R Angular Gyrus | 39 | 57 | 34, −51, 30 | ↑ | ↑ | 2.67 | ||
| Right Basolateral | ||||||||
| L Superior Frontal Gyrus | 6 | 161 | −32, −9, 39 | ↑ | ↑ | 2.71 | ||
| L Precuneus | 7 | 86 | −28, −51, 39 | ↑ | ↑ | 2.79 | ||
| L Middle Occipital Gyrus | 19 | 67 | −56, −57, −18 | ↑ | ↑ | 2.63 | ||
| L Inferior Parietal Lobule | 40 | 107 | −32, −27, 30 | ↑ | ↑ | 2.73 | ||
| L Postcentral Gyrus | 57 | −16, −21, 69 | ↑ | ↑ | 2.67 | |||
| Left Superficial | ||||||||
| R Lingual Gyrus | 18 | 110 | 32, −81, −21 | ↑ | ↑ | −2.58 | ||
| L Ventromedial Prefrontal Cortex | 10 | 70 | −20, 57, 12 | ↑ | ↑ | 2.69 | ||
| R Cuneus | 19 | 123 | 4, −87, 18 | −2.58 | ||||
| R Culmen | 88 | 16, −39, −21 | ↑ | ↑ | −2.61 | |||
| Right Superficial | ||||||||
| L Postcentral Gyrus | 3 | 129 | −28, −27, 30 | ↑ | ↑ | 2.63 | ||
| R Dorsomedial Prefrontal Cortex | 8 | 70 | 2, 39, 39 | ↑ | ↑ | −2.91 |
L=Left, R=Right.
3.1 Post-hoc Analyses
The effect of puberty on amygdala sub-regional RSFC was examined separately post-hoc. Introducing puberty into partial correlation analyses did not significantly change the aforementioned RSFC results (all r2 ≥ 0.52, p ≤ 0.001).
4. Discussion
Analysis of positive and negative resting state functional connectivity (RSFC) in an early- to mid-adolescent sample demonstrated significant age-by-sex interactions in RSFC between amygdala sub-regions and other cortical brain regions. Both SFA and BLA sub-regions showed decreased connectivity with parieto-occipital cortices and increased connectivity with mPFC, which corresponds with adult RSFC patterns of amygdala sub-regions (Roy et al., 2009, Qin et al., 2012, Gabard-Durnam et al., 2014), albeit with varying effects by sex. Girls and boys exhibited opposing RSFC connectivity patterns that only partially reflect adult functional connectivity of parieto-occipital cortices with sub-regions of the amygdala, in which SFA RSFC is more mature in girls, while BLA RSFC is more mature in boys. Additionally, in contrast to our hypothesis, functional connectivity between amygdala sub-regions and mPFC was not more mature in girls. Rather, with age, boys showed coupling between vmPFC and BLA, while girls showed coupling between dmPFC and SFA, which may indicate relative immaturity of both male and female adolescent fronto-limbic networks in comparison to adults.
4.1 Age-by-sex effects
Analysis of the interactions of sex and age on RSFC of amygdala sub-regions and other cortical brain regions revealed a consistent pattern that differentiated male and female adolescents. Specifically, at younger ages, girls had more positive RSFC between BLA and posterior-occipital cortex that diminished to negative connectivity by the age of 14 years, reflecting increased decoupling of these regions with age. On the other hand, boys showed increased coupling between similar regions with age, with a shift from negative to positive connectivity, also around the age of 14 years. The opposite pattern was observed with functional connectivity of SFA and parieto-occipital brain regions: with age, boys had more robust negative RSFC, while girls had more positive RSFC between SFA and parieto-occipital cortex. Although the groups were not matched on pubertal status, the effect of puberty was examined post-hoc, and this maturational gap did not statistically affect the results of the present analyses. Negative functional connectivity, or segregation and specialization of networks (Rubinov and Sporns, 2010), has been observed between amygdalar sub-regions and more dorsal and posterior brain regions in both adults (Roy et al., 2009) and in a cross-sectional sample spanning childhood and early adulthood (Gabard-Durnam et al., 2014). Therefore, the present results support an interpretation of increased maturity of limbic-parietal-occipital networks supported by BLA in girls and SFA in boys that may underlie distinct cognitive and behavioral profiles.
In contrast, functional connectivity of mPFC-amygdala RSFC appears to be similarly integrated in male and female adolescents, with a few notable distinctions. Specifically, increased positive connectivity between left SFA and left vmPFC with age was observed in boys, while girls showed increased positive connectivity between right SFA and a more dorsal mPFC region. These findings are in line with the Dual Systems Model that posits increasing top-down regulation of the limbic system via mPFC with age (Galvan et al., 2006, Steinberg, 2010); however, the regional specificity of the present findings slightly deviate from this model. Our findings are specific to SFA and lateralized by sex, with functional connections to different regions of the mPFC. Many studies in rodents and humans have functionally differentiated the dorsal and ventral mPFC, and there is general concensus that dmPFC supports cognitive processing, while vmPFC supports more emotional processing (Euston et al., 2012, Bzdok et al., 2013b) or internally-directed activity (Raichle et al., 2001). The vmPFC is a component of the “ventral affective system” described in the Dual Systems Model, which posits an imbalance in access to affective versus cognitive control brain regions leading to ineffective top-down regulation of the affective system by the cognitive control system (Steinberg, 2010). The relatively delayed maturation of cognitive control regions favors emotional processing by regions like the amygdala and vmPFC in early adolescence; however, with increasing age and maturity, cognitive control brain regions like dorsolateral PFC and dmPFC more effectively regulate limbic regions. Increased coupling between SFA and dmPFC in girls may indicate a more mature pattern of connectivity facilitating top-down regulation by dmPFC. An alternative interpretation is that RSFC between SFA and both ventral and dorsal mPFC support different components of social cognition. The mPFC has been strongly implicated in social cognition and also displays functional segregation along the dorsal-ventral axis (Amodio and Frith, 2006, Pfeifer and Allen, 2012, Bzdok et al., 2013b); therefore, distinct RSFC of SFA sub-regions with vmPFC and dmPFC in boys and girls may functionally relate to different aspect of social cognition. A recent study comparing dmPFC and vmPFC function with meta-analytic connectivity modeling found that vmPFC is primarily involved in bottom-up, approach/avoidance, and evaluation-related processing, while dmPFC was mostly involved in top-down and metacognition-related processing in social cognition (Bzdok et al., 2013b). More research is necessary to confirm behavioral relevance of the RSFC patterns observed in the present study.
Connectivity of the amygdala, as well as its sub-regions, has been studied in human and animal models (Pitkanen et al., 2000, Cunningham et al., 2002, Roy et al., 2009, Bach et al., 2011, Gabard-Durnam et al., 2014), and more recently in children (Qin et al., 2012) and across adolescence (Gabard-Durnam et al., 2014). These and other studies suggest that the amygdala is positively connected with subcortical and limbic regions, and that functional connectivity between these brain regions is stabilized early in childhood development (Gabard-Durnam et al., 2014); however, functional coupling between other cortical regions may be delayed. Previous research examining RSFC of amygdala sub-regions found increased positive connectivity with age (10 – 25 years) between all sub-regions and the mPFC (Gabard-Durnam et al., 2014), which may indicate that integration of the fronto-limbic network is part of a general developmental course. Due to the lack of coupling between BLA and mPFC, the current sample may be displaying some immaturity in fronto-limbic connectivity. The age range of the current sample (10-16 years) is more representative of the adolescent period, therefore he lack of RSFC between mPFC and BLA may also suggest that fronto-limbic connectivity is primarily supported by RSFC between mPFC and SFA during adolescence. Connectivity between mPFC and BLA may be delayed and strengthen later in adolescence or early adulthood. Given that the SFA supports processing of emotional, olfactory and social stimuli (Goossens et al., 2009), its coupling with the mPFC may confer an advantage that supersedes coupling of mPFC with BLA, which supports emotional learning and memory processes, during adolescence. This is interpretation is speculative, however, and requires additional study to verify.
Although all sub-regions are relevant for emotional processing (Ball et al., 2007, Hurlemann et al., 2008, Bzdok et al., 2013a), BLA is additionally important for memory function (Bzdok et al., 2013a); therefore, males demonstrating positive RSFC between parieto-occipital and BLA may report problems with memory functions. An alternative, and more parsimonious interpretation, is that males rely on a functional coupling between BLA and parieto-occipital brain regions for memory functions during this early- to mid-adolescent period, which is supported by research in adults and adolescents (Darki and Klingberg, 2014, Hill et al., 2014). Cortical regions functionally coupled with bilateral BLA include areas that are important for memory, such as the IPL (Rama et al., 2004) and PC (Lundstrom et al., 2005). Furthermore, SFA is more functionally connected with parieto-occipital cortical regions in females as compared to males. The SFA is the most conserved amygdala sub-region and it is thought to play an important role in social communication via olfactory (Bzdok et al., 2013a) and social cues (Goossens et al., 2009, Bzdok et al., 2013a); therefore, increasing RSFC between this sub-region and the parieto-occipital cortex could indicate that adolescent girls have relatively compromised socio-emotional processing. This interpretation is supported by studies conducted in adults showing functional connectivity of parieto-occipital cortex with SFA is linked with childhood behavioral inhibition (Roy et al., 2014) and current social inhibition (Blackford et al., 2014). A different study showed that administration of allopregnanolone reduced connectivity between the amygdala and parietal cortex, which was also correlated with reductions in negative affect (Sripada et al., 2014). However, these effects have not been confirmed in adolescents. Notably, studies that have examined amygdalar RSFC in children and adolescents with major depressive disorder have found increased connectivity of the amygdala with parietal and occipital cortices (Cullen et al., 2014, Jacobs et al., 2014, Pannekoek et al., 2014); therefore, it is possible that functional coupling of SFA and parieto-occipital regions is present in healthy girls, but may partly account for the increased risk for depression in females. Alternatively, girls may rely on regions such as the cuneus and LG, which are implicated in emotional face processing (Bremner et al., 2004, Kitada et al., 2010, Kret et al., 2011), for socio-emotional processing as a distinct mechanism supporting neural functional connectivity patterns that in turn manifest into sexually dimorphic social behavior. One study in adults found that negative connectivity between SFA and the temporoparietal junction is positively related to harm avoidance and that this relationship is more robust in women, compared to men (Li et al., 2012), demonstrating that functional decoupling of SFA and parietal cortex is relevant for social communication and behavior, but that behavioral outcomes may differ depending on sex. If decoupling of SFA and parieto-occipital cortices is indeed the norm in adulthood as some studies suggest (Roy et al., 2009, Gabard-Durnam et al., 2014), then the sexually dimorphic trajectories of amygdala sub-regional connectivity observed in the current sample may give way to mature patterns observed in adults; however, research comparing sex differences in adolescents and adults will be necessary to confirm this hypothesis.
4.2 Strengths and limitations
The present study carefully examined the interaction between age and sex in RSFC of superficial and basolateral amygdalae in a sample of adolescents. The intersection of sex and age is particularly relevant during this period of development, as striking biological and social changes take place that influence neurophysiologic function, often in a sexually dimorphic fashion. The large sample size, careful delineation of amygdala sub-regions, and meticulous assessment of head motion and subsequent data scrubbing (see 2.4 Motion Correction and Supplementary Data) inspires confidence in the reported results. However, some limitations of the study should be noted. For example, although the data processing and motion censoring approach employed by the current study is appropriate for the stated aims, we tested the robustness of our findings by adjusting the functional connectivity processing steps and employing a different method of motion censoring in a supplementary analysis (see Supplementary Methods), as suggested by prior reports (Hallquist et al., 2013, Power et al., 2014). The results of this analysis show consistency with the reported findings, albeit some findings, potentially due to reduced power, remained trend like and did not reach significance. Second, due to our strict delineation of amygdala sub-regions (see 2.5 Definition of amygdala sub-region ROIs), we could not confidently ascertain that CMA ROIs indeed corresponded to anatomical CMA. Because the CMA is the smallest nucleus of the amygdala, many participants had CMA ROIs with as little as one voxel and as many as ten corresponding to this region; the majority of participants had zero voxels that confidently corresponded to the CMA (48% in left CMA and 100% in right CMA). As such, RSFC analyses were not conducted with either left or right CMA seed regions, which limits direct comparisons with existing amygdala sub-region RSFC studies (Roy et al., 2009, Li et al., 2012, Qin et al., 2012, Gabard-Durnam et al., 2014). Of these studies, two used the same Eickhoff-Zilles maximum probability maps (Amunts et al., 2005) to determine sub-regions; however, they did not account for developmental and individual variation in anatomy by masking with model-based (Patenaude et al., 2011) segmented amygdalar ROIs (Qin et al., 2012, Gabard-Durnam et al., 2014), as was done in the current study. Accounting for developmental differences in brain maturation is crucial when studying young populations. Previous studies have shown that throughout the span of adolescence, different brain regions reach peak gray matter volume at distinct rates (Giedd, 2004, Gogtay et al., 2004, Creze et al., 2014), with concurrent changes in neurophysiology (Whitford et al., 2007). Due to the dynamic nature of brain development, we believe a more stringent classification of amygdala sub-regions outweigh the benefits of including a CMA ROI in our analyses.
An additional limitation of this study is its cross sectional design, which is not ideal for capturing developmental effects. While the current study included a relatively large sample, future research should attempt to utilize longitudinal designs to confirm the present findings. Direct comparisons with adult samples may also provide valuable insight about RSFC patterns that are unique to the adolescent period. The current study design also limits conclusions about causal relationships of age and sex on functional connectivity of the brain. Our measurements reflect a snapshot of brain RSFC over a sample of male and female adolescents at varying ages, which provide information about the developmental course of functional connectivity as a function of sex. Lastly, our measure of puberty shares only modest concordance with clinician evaluations of pubertal girls (Brooks-Gunn et al., 1987), which we did not have access to, and a moderate agreement with self-reported Tanner staging (Bond et al., 2006), limiting our interpretation of true pubertal effects. However, we can conclude that sex differences in our measure of puberty did not statistically impact age-by-sex RSFC results.
4.3 Conclusions
The current study demonstrated sex-specific trajectories of amygdala sub-region RSFC in a large sample of early- to mid-adolescents. Our hypothesis predicting increased coupling between fronto-limbic regions in girls was not supported. Rather, both sexes showed comparable patterns of functional coupling, which is in accordance with a healthy developmental trajectory (Roy et al., 2009, Qin et al., 2012, Gabard-Durnam et al., 2014). However, there was a laterality effect that differentiated the sexes in frontal-limbic RSFC. Specifically, boys showed functional coupling between left SFA and vmPFC, while girls showed coupling between right SFA and dmPFC. With increasing age, boys also displayed more mature decoupling of SFA and parieto-occipital cortex, compared to girls who showed immature functional coupling between these regions. Conversely, girls had more mature decoupling of BLA and parieto-occipital cortical regions, while boys showed immature functional coupling between these same areas. Overall, these results indicate a sex-specific dissociation in amygdala sub-region RSFC with cortical brain regions, which underscores the importance of both the examination of sex differences in adolescent samples and of amygdalar function by sub-region, potentially in the contexts of socio-emotional processing and with populations at risk for, or suffering from, depression.
Supplementary Material
Highlights.
Amygdalar functional coupling with parieto-occipital cortex decreases with age.
Amygdalar functional coupling with medial frontal cortex increases with age.
Boys have more integration of basolateral amygdala and parieto-occipital cortex.
Girls have more integration of superficial amygdala and parieto-occipital cortex.
Adolescents show integration of superficial amygdalae and medial frontal cortex.
Figure 2.
Fisher's Z transformed R-values from brain regions with significant functional connectivity age-by-sex interactions with left basolateral amygdala (left) and right basolateral amygdala (right) were extracted and plotted against age and by sex.
Acknowledgements
Gratitude is extended to Eric Earl for his helpful discussions about functional connectivity processing and motion censoring and to the members of the Developmental Brain Imaging Lab at Oregon Health & Science University for their efforts in data collection and processing. Financial support for data collection, data analysis and preparation of this manuscript was provided by the National Institute on Alcohol Abuse and Alcoholism (R01 AA017664 & T32 AA007468-24).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and embryology. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Bach DR, Behrens TE, Garrido L, Weiskopf N, Dolan RJ. Deep and superficial amygdala nuclei projections revealed in vivo by probabilistic tractography. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:618–623. doi: 10.1523/JNEUROSCI.2744-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T, Rahm B, Eickhoff SB, Schulze-Bonhage A, Speck O, Mutschler I. Response properties of human amygdala subregions: evidence based on functional MRI combined with probabilistic anatomical maps. PloS one. 2007;2:e307. doi: 10.1371/journal.pone.0000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berking M, Wupperman P. Emotion regulation and mental health: recent findings, current challenges, and future directions. Current opinion in psychiatry. 2012;25:128–134. doi: 10.1097/YCO.0b013e3283503669. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Clauss JA, Avery SN, Cowan RL, Benningfield MM, VanDerKlok RM. Amygdala-cingulate intrinsic connectivity is associated with degree of social inhibition. Biological psychology. 2014;99:15–25. doi: 10.1016/j.biopsycho.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond L, Clements J, Bertalli N, Evans-Whipp T, McMorris BJ, Patton GC, Toumbourou JW, Catalano RF. A comparison of self-reported puberty using the Pubertal Development Scale and the Sexual Maturation Scale in a school-based epidemiologic survey. Journal of adolescence. 2006;29:709–720. doi: 10.1016/j.adolescence.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Vaccarino V, Charney DS. Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. The American journal of psychiatry. 2004;161:637–645. doi: 10.1176/appi.ajp.161.4.637. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child development. 1987;58:829–841. [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Human brain mapping. 2013a;34:3247–3266. doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Engemann DA, Laird AR, Fox PT, Eickhoff SB. Segregation of the human medial prefrontal cortex in social cognition. Frontiers in human neuroscience. 2013b;7:232. doi: 10.3389/fnhum.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova R, Whitlow CT, Wagner B, Espeland MA, Maldjian JA. Combining graph and machine learning methods to analyze differences in functional connectivity across sex. The open neuroimaging journal. 2012;6:1–9. doi: 10.2174/1874440001206010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Olatunji BO. Emotion regulation and anxiety disorders. Current psychiatry reports. 2012;14:182–187. doi: 10.1007/s11920-012-0262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, Stevens CA, Musser ED, Carpenter SD, Grayson DS, Mitchell SH, Nigg JT, Fair DA. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23:33–45. doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Creze M, Versheure L, Besson P, Sauvage C, Leclerc X, Jissendi-Tchofo P. Age- and gender-related regional variations of human brain cortical thickness, complexity, and gradient in the third decade. Human brain mapping. 2014;35:2817–2835. doi: 10.1002/hbm.22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Casimo K, Fair DA, Nagel BJ. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res. 2014;221:210–219. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, Lim KO. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA psychiatry. 2014;71:1138–1147. doi: 10.1001/jamapsychiatry.2014.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. The Journal of comparative neurology. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Darki F, Klingberg T. The Role of Fronto-Parietal and Fronto-Striatal Networks in the Development of Working Memory: A Longitudinal Study. Cereb Cortex. 2014 doi: 10.1093/cercor/bht352. [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Flynn G, Harris AW, Liddell BJ, Whitford TJ, Peduto A, Gordon E, Williams LM. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophrenia research. 2007;90:284–294. doi: 10.1016/j.schres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS computational biology. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, Schlaggar BL, Mennes M, Gutman D, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang YF, Mostofsky S, Castellanos FX, Milham MP. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Hare T, Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23 years: A cross-sectional study. NeuroImage. 2014;95C:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. The Journal of comparative neurology. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L, Kukolja J, Onur OA, Fink GR, Maier W, Griez E, Schruers K, Hurlemann R. Selective processing of social stimuli in the superficial amygdala. Human brain mapping. 2009;30:3332–3338. doi: 10.1002/hbm.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neuroscience and biobehavioral reviews. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Henry JD, Rendell PG, Green MJ, McDonald S, O'Donnell M. Emotion regulation in schizophrenia: affective, social, and clinical correlates of suppression and reappraisal. Journal of abnormal psychology. 2008;117:473–478. doi: 10.1037/0021-843X.117.2.473. [DOI] [PubMed] [Google Scholar]
- Hill AC, Laird AR, Robinson JL. Gender differences in working memory networks: a BrainMap meta-analysis. Biological psychology. 2014;102:18–29. doi: 10.1016/j.biopsycho.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AA. Four-factor index of social status. 1975 [Google Scholar]
- Hurlemann R, Rehme AK, Diessel M, Kukolja J, Maier W, Walter H, Cohen MX. Segregating intra-amygdalar responses to dynamic facial emotion with cytoarchitectonic maximum probability maps. Journal of neuroscience methods. 2008;172:13–20. doi: 10.1016/j.jneumeth.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Jacobs RH, Jenkins LM, Gabriel LB, Barba A, Ryan KA, Weisenbach SL, Verges A, Baker AM, Peters AT, Crane NA, Gotlib IH, Zubieta JK, Phan KL, Langenecker SA, Welsh RC. Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PloS one. 2014;9:e104366. doi: 10.1371/journal.pone.0104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim N, Kim S, Hong S, Park K, Lim S, Park JM, Na B, Chae Y, Lee J, Yeo S, Choe IH, Cho SY, Cho G. Sex differences in amygdala subregions: Evidence from subregional shape analysis. Neuroimage. 2012;60:2054–2061. doi: 10.1016/j.neuroimage.2012.02.025. [DOI] [PubMed] [Google Scholar]
- Kim N, Kim HJ, Hwang J, Yoon SJ, Cho HB, Renshaw PF, Lyoo IK, Kim JE. Amygdalar shape analysis method using surface contour aligning, spherical mapping, and probabilistic subregional segmentation. Neuroscience letters. 2011;488:65–69. doi: 10.1016/j.neulet.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada R, Johnsrude IS, Kochiyama T, Lederman SJ. Brain networks involved in haptic and visual identification of facial expressions of emotion: an fMRI study. NeuroImage. 2010;49:1677–1689. doi: 10.1016/j.neuroimage.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Kret ME, Pichon S, Grezes J, de Gelder B. Similarities and differences in perceiving threat from dynamic faces and bodies. An fMRI study. NeuroImage. 2011;54:1755–1762. doi: 10.1016/j.neuroimage.2010.08.012. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and molecular neurobiology. 2003;23:727–738. doi: 10.1023/a:1025048802629. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Qin W, Jiang T, Zhang Y, Yu C. Sex-dependent correlations between the personality dimension of harm avoidance and the resting-state functional connectivity of amygdala subregions. PloS one. 2012;7:e35925. doi: 10.1371/journal.pone.0035925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. NeuroImage. 2005;27:824–834. doi: 10.1016/j.neuroimage.2005.05.008. [DOI] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Gabrieli JD, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social cognitive and affective neuroscience. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Bathula D, Dias TG, Iyer SP, Fenesy MC, Musser ED, Stevens CA, Thurlow BL, Carpenter SD, Nagel BJ, Nigg JT, Fair DA. Altered cortico-striatal-thalamic connectivity in relation to spatial working memory capacity in children with ADHD. Frontiers in psychiatry / Frontiers Research Foundation. 2012;3:2. doi: 10.3389/fpsyt.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek JN, van der Werff SJ, Meens PH, van den Bulk BG, Jolles DD, Veer IM, van Lang ND, Rombouts SA, van der Wee NJ, Vermeiren RR. Aberrant resting-state functional connectivity in limbic and salience networks in treatment-naive clinically depressed adolescents. Journal of child psychology and psychiatry, and allied disciplines. 2014 doi: 10.1111/jcpp.12266. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA. Developing connections for affective regulation: age-related changes in emotional brain connectivity. Journal of experimental child psychology. 2011;108:607–620. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in cognitive sciences. 2012;16:322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Annals of the New York Academy of Sciences. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Annals of the New York Academy of Sciences. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Qin S, Young CB, Supekar K, Uddin LQ, Menon V. Immature integration and segregation of emotion-related brain circuitry in young children. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7941–7946. doi: 10.1073/pnas.1120408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama P, Poremba A, Sala JB, Yee L, Malloy M, Mishkin M, Courtney SM. Dissociable functional cortical topographies for working memory maintenance of voice identity and location. Cereb Cortex. 2004;14:768–780. doi: 10.1093/cercor/bhh037. [DOI] [PubMed] [Google Scholar]
- Roy AK, Benson BE, Degnan KA, Perez-Edgar K, Pine DS, Fox NA, Ernst M. Alterations in amygdala functional connectivity reflect early temperament. Biological psychology. 2014 doi: 10.1016/j.biopsycho.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, Gennatas ED, Elliott MA, Smith A, Hakonarson H, Verma R, Davatzikos C, Gur RE, Gur RC. Linked Sex Differences in Cognition and Functional Connectivity in Youth. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon BJ, Raichle ME, Snyder AZ, Fair DA, Mills KL, Zhang D, Bache K, Calhoun VD, Nigg JT, Nagel BJ, Stevens AA, Kiehl KA. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proc Natl Acad Sci U S A. 2011;108:11241–11245. doi: 10.1073/pnas.1108241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JF, Pillai A, Chen K, Horwitz B. Effective Connectivity Modeling for fMRI: Six Issues and Possible Solutions Using Linear Dynamic Systems. Frontiers in systems neuroscience. 2011;5:104. doi: 10.3389/fnsys.2011.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Welsh RC, Marx CE, Liberzon I. The neurosteroids allopregnanolone and dehydroepiandrosterone modulate resting-state amygdala connectivity. Human brain mapping. 2014;35:3249–3261. doi: 10.1002/hbm.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental psychobiology. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain : 3-dimensional proportional system : an approach to cerebral imaging. Georg Thieme; Stuttgart ; New York: 1988. [Google Scholar]
- Tian L, Wang J, Yan C, He Y. Hemisphere- and gender-related differences in small-world brain networks: a resting-state functional MRI study. NeuroImage. 2011;54:191–202. doi: 10.1016/j.neuroimage.2010.07.066. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, Tie K, Gong G, Shah MP, Jones M, Uderman J, Constable RT, Blumberg HP. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biological psychiatry. 2009;66:516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D. Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]
- Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Human brain mapping. 2007;28:228–237. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga L, Langen M, Ambrosino S, van Dijk S, Oranje B, Durston S. Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. NeuroImage. 2014;96:67–72. doi: 10.1016/j.neuroimage.2014.03.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.