Abstract
Nandrolone is included in the class II of anabolic androgenic steroids (AAS) which is composed of 19-nor-testosterone-derivates. In general, AAS is a broad and rapidly increasing group of synthetic androgens used both clinically and illicitly. AAS in general and nandrolone decanoate (ND) in particular have been associated with several behavioral disorders. The purpose of this review is to summarize the literature concerning studies dealing with ND exposure on animal models, mostly rats that mimic human abuse systems (i.e. supraphysiological doses). We have focused in particular on researches that have investigated how ND alters the function and expression of neuronal signaling molecules that underlie behavior, anxiety, aggression, learning and memory, reproductive behaviors, locomotion and reward.
Keywords: Nandrolone decanoate (ND), Anabolic androgenic steroids (AAS), neurological effects, anxiety, aggression, learning and memory
INTRODUCTION
Nandrolone is included in the class II of anabolic androgenic steroids (AAS) which is composed of 19-nor-testosterone-derivates. In general, AAS is a broad and rapidly increasing group of synthetic androgens used both clinically and illicitly. Nandrolone is applicable in clinical practice, burns, radiation therapy, surgery, trauma and various forms of anemia [1]. Moreover, it has also been used to treat chronic debilitating diseases: human immuno-deficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS)-associated wasting syndrome [2]. However, due to its beneficial properties such as tissue-building booster, maintenance of strength and muscle mass, libido and bone health it has become very popular among athletes in their attempt to increase their strength, to accelerate muscle development, to promote recovery and enhance aggression [3]. The compound is famous not only among adults but also adolescents because of its anabolic, muscle building properties [4-7]. It is considered worldwide to be one of the most commonly abused AAS [8, 9]. Despite the previously mentioned desired effects there are also many harmful effects on the central nervous system (CNS) which have been stated in literature.
Structurally, nandrolone and compounds belonging to the class II of AAS, differ from testosterone at the C19 position where a methyl group (CH3) is substituted by a hydrogen. The previously mentioned substitution leads to the half-time extension beyond that contributed by esterification alone. Esterification of the hydroxy group (OH) at the C17 position of nandrolone with decanoic acid yields ND [10]. It is worth saying that despite the retention of the C4–C5 double bond in ND, the androgenic activity of the compound is reduced at the androgen receptor compared to dihydrotestosterone and is more anabolic than testosterone [10-12].
The chemical structures of nandrolone decanoate, testosterone and 5β-dihydrotestosterone are reported in Fig. 1.
Fig. (1).
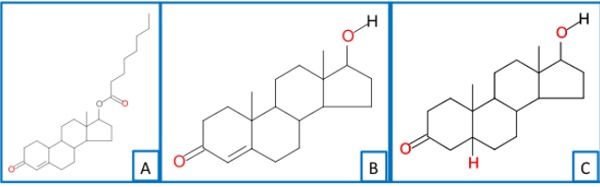
Chemical structure of Nandrolone Decanoate (A), Testosterone (B) and 5β-dihydrotestosterone (C).
Despite the fact that AAS are banned substances by professional sport organizations and illegal drugs by the DEA, they are used and abused extensively, especially by teenagers and young athletes or non-athletes either for cosmetic or recreational purposes. Users may even take from 10 to 100 times higher doses than the physiological one [7].
MATERIALS AND METHODS
The following databases (from 1980 to January 2014) Medline, Cochrane Central, Scopus, Web of Science and Science Direct were used, searching the following key words: Nandrolone decanoate (ND), Anabolic androgenic steroids (AAS), neurological effects, behavior, anxiety, aggression, learning, memory, reproductive behaviors, locomotion, reward and endocrine system. The first two key words, ND and AAS, were searched singularly and associated individually to each of the other keywords. Among the 3084 sources found, 113 were considered as appropriate for the purpose of the paper.
RESULTS
Aggression
There is a correlation between administration of nandrolone decanoate (ND) and aggressive behavior, in fact increased aggressive behavior [13] has been shown in many studies but contradictory outcomes when using different methodological approaches no effect on aggression [14-18] have been reported as well.
Breuer et al. [14] investigated the effects on Long-Evans rats after being treated with ND, testosterone propionate or stanozolol (5 mg/kg 5 times/week for 12 weeks) concluding that aggressive behavior had increased in rats treated with testosterone propionate compared to controls. In contrast, ND administered rats and control groups demonstrated similar aggressive levels.
Findings of Breuer et al [14] are consistent with Albert et al. [19] who showed that both ND treated rats and controls exposed few lateral attacks when undergoing a competitive test situation.
McGinnis et al [16] carried out tests to find whether AAS - enhanced aggression is reversible. They assessed aggression in male rats following withdrawal from ND, stanozolol, ortestosterone propionate; 5 mg/kg 5 days a week for 12 weeks. Rats receiving ND showed similar aggressive behavior to controls. On the other hand, Long et al. [13] stated that Sprague–Dawley rats treated with ND (2 mg/day or 20 mg/week for 4 weeks) showed increased levels of aggression compared to controls. Discrepancies between the findings of Long et al. and later studies [14, 15] could not be fully explained, but may be attributed to differences in rat strain (Long- Evans versus Sprague–Dawley) and/or particular testing conditions.
Studies carried out subsequently failed to find aggression in either adult or adolescent male rats after ND administration [20, 21]. However, the results from Long et al. may be attributed to experience since the nadrolone-treated males used for this specific study were given two weeks of fight training prior to the initiation of ND administration. Males in the other studies had no former aggressive experience.
Rats in McGinnis et al. [15]. The fact that the effect (no effect) of ND on intermale aggression was replicated [14, 15] demonstrates that under particular testing conditions ND does not promote aggression in Long-Evans male rats.
Lindqvis et al. [22] administered rats with ND ((15 mg/kg) for 14 days) to examine competitive aggression. Starting from one week after the ultimate ND injection, the Wistar rats were acclimated in new cages and with new cage-mates for 3 days. On the fourth day, water intake was restricted and competitive aggressive behaviors demonstrated by AAS and vehicle-treated rats were recorded upon introduction of an active waterspout. Baseline levels of water intake were determined after the test when rats were placed in individual cages. Baseline levels of drinking were similar among the groups. However, during the test AAS-treated rats spent more time drinking. Therefore it is difficult to interpret these findings since the test was carried out a week after the last ND administration and the clearance rate of ND is unknown.
Series of experiments have been conducted by Melloni and colleagues [23-25] on gonadally intact adolescent male hamsters after their treatment with a cocktail of ND, testosterone cypionate and boldenone undecylenate. Hamsters treated for 14 days with AAS demonstrated more attacks and bites and a reduced latency to bite the intruder compared to controls. Similar results observed in subsequent studies by the same lab on hamsters treated with the AAS coctail for 30 days [26-28].
Male Syrian hamsters were treated daily with a combination containing ND, testosterone cypionate and boldenone undecylenate (2, 2, 1mg/kg, respectively) either during adolescence or adulthood for 14 days. The day after the treatment ended, males were tested for either sexual behavior or agonistic behavior with a receptive female or a male intruder, respectively. Adult AAS – treated males displayed lower levels of sexual behavior and no heightened aggression compared to controls whereas, the corresponding adolescent males showed significant increase in aggressive and sexual behavior compared to controls [29].
For experimental purposes rats were stimulated by a mild tail pinch by the experimenter. Reactivity to such stimuli was tested using ND-treated animals and controls after the cessation of the ND treatment period and up to six to eight weeks afterwards. It has been shown that animals displayed enhanced reactivity concerning air puffs, flank prods and capturing, on both test occasions. McGinnis et al. exported the same results as both studies enhanced reactivity a few weeks after the end of the noted nandrolone-treatment. McGinnis et al tested the behavioral responses to tail pinches 12 weeks after the cessation of the steroid treatment and observed no enhanced aggressive behaviors.
Rossbach et al. [30] demonstrated that AAS can increase excitatory neurotransmission in adult rodents via augmented phosphorylation of the NMDA receptor glutamate which results in an increment of aggression, impulsiveness and irritability in adult and adolescent rats.
Interaction between ND and NMDA sites as well as sigma receptors has been considered. For the purposes of the study, male rats were daily administered ND for 14 days. Presuming that ND might react on the brain through interaction with neurosteroids. Radiolabelled [3H]ifenprodil was employed in the binding experiments as it shows a significant affinity for both NMDA and sigma receptors. It was reported that [3H]ifenprodil binds to both sigma-1 and sigma-2 sites and can be displaced to some extent from both sites by the neurosteroids dehydroepiandrosterone sulphate (DHEAS), pregnenolone sulphate (PS), and pregnanolone sulphate (3a5bS). The remaining [3H]ifenprodil was displaced from the site of sigma-1 by the (þ)-SKF 10,047, which is a sigma-1 receptor-selective ligand. Chronic treatment with nandrolone altered the sigma-1 receptor affinity with the above mentioned neurosteroids but sigma–2 receptor remained unchangeable. Moreover it was noted that NMDA receptor subunit NR2B was not affected by treatment with ND.
Arginin Vasopressin (AV) has been implicated in inducing aggressive behavior. Harrison et al. [28] have demonstrated that treatment of preadolescent male hamsters with a cocktail including ND for a 30 days period resulted in increased aggression compared to controls. It was also noticed that the average area innervated by AV immuno-reactive fibers in the anterior hypothalamus and AV content were increased.
The region most affected in the expression of aggressive behavior is the hypothalamus. N-methyl-D-aspartate receptor (NMDA) involved in the inductance of some types of aggressive response [31]. The NMDA receptor subunit mRNA expression levels are significantly decreased after 14 days of treatment with AAS [32].
According to Tamaki T. et al [33] elevated levels of adrenergic and serotonergic amines, nandrolone-stimulated, in the hypothalamus could bring about an improved physical performance but also aggressive behavior.
The androgen action is related to its ability to bind and activate specific androgen receptors. The immunoreactivity of substance P (SP), which is a peptidergic factor associated with enhanced aggression in several brain regions, amygdala, hypothalamus, periaqueductal gray area and striatum, [34] has been shown to increase after ND administration. ND has also been shown to react on the SP system at several levels, including receptor densities, peptide concentrations and enzymatic processing [35, 36]. Chronic treatment with ND has been associated with impact on both opioid concentrations and the tachykinin levels in brain areas connected with the control of emotional behavior such as depression, aggression and reward.
Enhanced c-fos expression has been noted in limbic brain regions involving the behavioral, stress and reward systems, in the guinea pig after being treated with ND [37]. Hypothalamic vasopressin levels increased and offensive aggression has been facilitated in adolescent male Syrian hamsters after repeated treatment with AAS [26].
According to Carrillo et al and Fisher et al [38, 39] exposure to nandrolone during adolescence alters the normal activity of the glutamatergic system in the latero anterior hypothalamus (LAH), brain regions involved in the expression of aggressive behavior. Other studies [40-42] showed that nandrolone induces aggression in mice only at high anabolic doses through experiments conducted on the gastrocnemius muscle which is hormone-sensitive.
Anxiety, Fear and Stress
Anxiogenic [43] and anxiolytic effects [44] have been associated with nandrolone. Kouvelas D. et al [45] suggest that chronic treatment with high doses of ND induces anxiolytic-type behavior, an impaired social memory, possible spatial learning and recall performance via activation of the central androgenic receptors (ARs), while the acquisition of information remains unaltered. In rats administered with ND (15 mg/kg subcutaneously) and flutamide (FL), an AR antagonist, reduction of anxiety was abolished. Contrarily, in a former study when FL was applied to the hippocampus, an increased anxiety-like behavior was noted, possibly as a result of interaction between the drug and targets other than ARs [46]. Interaction of ND and FL with the ARs in brain areas, is known to contribute to anxiety-related behavior such as hypothalamus, basal ganglia, amygdala, septum, hippocampus, motor nuclei in the brain stem, cerebellum, spinal cord, andcerebral cortex, by interfering with serotonergic, glutamatergic, dopaminergic pathways, which may lead to anxiety reduction and memory impairment.
On the other hand, Minkin et al. [43] noticed that Long-Evans rats (gonadally intact or gonadectomized) following ND treatment (10 or 50 mg/week for 8 weeks) spent more time compared to controls in the margins of the open-field (i.e. thigmotaxis), suggesting an increased level of anxiety. Same results were reported by Minkin and Meyer since anxiogenic response was induced in rats treated with ND during the open field task.
Nandrolone enhances synaptic currents mediated by the GABAA receptor in the ventromedial nucleus of the hypothalamus, but diminishes them in the pre-optic area [47]. These effects depend on the subunit composition of the GABAA receptor. In the dorsomedial hypothalamus, blockade and enhancement of GABAA-mediated currents are associated to anxiogenic responses [48] and anxiolysis [49], respectively.
Reward and Dependence
Psychostimulants, such as cocaine and d-amphetamine, elevate extracellular dopamine (DA) concentrations by the reuptake inhibition of the DA transporters [50]. Up-regulation of the DA transporters in the caudatum/putamen has been noticed after treatment with ND [51, 52] suggesting to reflect a response of an enhanced DA activity [53]. The effects of d-amphetamine (0.5 mg/kg 15 min prior to test) on brain stimulation reward were studied before and after the rats were administered with a cocktail containing ND, testosterone cypionate and boldenone undecylenate (2, 2, 1 mg/ kg daily for 15 weeks) [54]. It was suggested that although ND has no direct effect on the brain reward system, it potentiates the rewarding effects of amphetamine.
Santa Kailanto et al. [55] suggest that prolonged changes in rats’ brain reward circuits associated to drug dependence could be produced after ND treatment (20 mg/kg intramuscularly (i.m.) every other day over a 10-day period). The high concentrations of extracellular DA and serotonin (5-HT), evoked by the cocaine were attenuated by pretreatment with ND. Changes noticed in DA and 5-HT systems after ND treatment, were still persistent after a significantly long period after the cessation of the administration.
Kurling S. et al. suggest that pretreatment with ND could modulate the rewarding effects evoked by MDMA and amphetamines. [50, 56]. Taking into account that activation of the dopaminergic neuronal system in mesolimbic brain areas, is believed to play a crucial role in mediating the reward-related effects of drugs of abuse the results provided here are in accordance with studies reporting that ND has the ability to decrease morphine reward [57] and alter some of the effects of cannabinoids when used chronically [58]. Moreover, its chronic administration could blunt the subjective effects of cocaine in rats
Kurling-Kailanto S. et al [59] suggest that AASs can produce some alterations in brain reward systems that contribute to the maintaining of drug dependence. Realistically, ND showed an important action at the doses tested, on the satisfying effects of cocaine. It was indicated that subchronic pretreatment with ND weakens dose-dependently the reward-related behavioral and neuro-chemical results of acute cocaine use. Both behavioral and neurochemical effects of ND were apparent for at least 28 days after the cessation of the treatment.
Few possible mechanisms have been suggested explaining how ND can react on dopaminergic response to cocaine. Activation of monoamine oxidase (MAO) could lead to elevation of the metabolism of both DA and 5-HT. However, the latter is not supported by the outcomes of Thiblin et al. [60] since MAO activity was not changed by ND. Pretreatment with ND also weakened the cocaine-induced increase of extracellular 5-HT in the nucleus accumbens (NAc) dose-dependency.
In summing up, we can say that ND dose-dependency inhibits both the DA and 5-HT outflow in NAc which is caused by cocaine and decreases cocaine-induced behavioral effects in experimental animals. Taking into account that total outflow of both DA and 5-HT is considered to be related to the pleasing stimulant drugs-induced effects, it suggests that ND may modulate the delectable effects of cocaine.
Animal studies showed that AAS can promote dependency and a link between the effects of AAS and opioids has been reported [61]. It is difficult to distinguish between the direct and the indirect AAS rewards which result in an increased strength in humans. It has been showed in animal studies that AAS reward and athletic performance are irrelevant. Chronic treatment with ND in rats heightened the levels of endogenous opioids and their receptors in select limbic regions, including elevation in beta-endorphin in the ventral tegmental area (VTA) [62]. However, we must remember that, dissimilar with many drugs of abuse, AASs do not acutely stimulate the dopamine release in the NAc [63].
Learning, Memory and Work Capacity
According to Magnusson K. et al [64] nandrolone administered male rats displayed memory function impairment, possibly via dynorphinergic mechanisms in the hippocampus. The hippocampus is a brain region associated with cognitive function since the limbic brain is linked to several types of learning and memory functions. This region displays a relatively high density of androgen receptors in rats, which suggests a relationship between the androgen receptor and cognitive function [65-69].
Locomotion and Physical Activity
McGinnisM Y et al [70] studied the wheel running activity and circadian rhythmicity in gonadal intact male rats after treatment with three different AAS (stanozolol, testosterone and nadrolone). Rats exposed to nandrolone showed a significant decrease when running on the wheel whereas none of the AASs altered phase response and circadian rhythmicity measures.
Nandrolone effect on locomotor activity is controversial. Some studies found that nandrolone had no effect on locomotion [71] whereas other findings suggest that nandrolone phenylpropionate-treated rats displayed increased running endurance compared to controls [72].
Impact of ND on the Endocrine System: Effects on the HPAA (Hypothalamic—Pituitary— Adrenal Axis)
The effect of ND on HPAA has been shown in some studies. Effects on the corticosterone, adrenocorticotropin hormone, proopiomelanocortin, corticotropin releasing factor (CRF) and CRF receptor1 (CRF R1) mRNA in the pituitary, hypothalamus and amygdala of rats have been noted. Moreover effects of ND on adiponectin, insulin, ghrelin, leptin and corticosterone (CORT), and cortical serotoninergic system (CSS) have been observed [73-75].
Racca S. et al. [73] evaluated the effects of subchronic administration of ND (once a day for 14 days) on HPAA and CSS response to acute restraint stress (RS). Acute RS produced the following effects: increase in adrenocorticotrophin (ACTH) (both in blood and in pituitary corticotropes) and CORT (in blood), glucocorticoid receptor (GR) reduction in the hippocampus and hypothalamus cytosol and GR translocation in hippocampus nuclear fraction, stimulation of the cortical serotonin re-uptake and activation of hippocampus cytosolic ERK2. ND itself, i.e. in non-stressed rats, had no effect on these parameters, apart from a raise in hippocampus cytosolic phospho-ERK1/2 (hippocampal extracellular signal related kinase) and a decrease of plasma CORT and ACTH levels. In contrast, in stressed ND-treated rats stress-induced plasma ACTH increase and all other above mentioned stress effects were prevented, apart from an increment in pituitary ACTH positive cell density. Although, prolonged supratherapeutical doses of ND administration in rats, did not notably affect HPAA and serotonin transporter activity under no-stress conditions, they may deregulate the stress-induced hormonal cascade which plays a crucial role in depressive psychopathology.
Considerable reduction in CORT plasma levels in rats after ND treatment (15 mg/kg for 14 days) has been noted by Alsio et al [74]. Decreased expression of two key enzymes involved in CORT synthesis (5a-reductase I and 11b-OHase) in adrenals of rats identified after prolonged ND treatment, suggesting that chronic treatment with ND could impair CORT adrenal release by acting on the gene transcription of enzymes involved in CORT synthesis.
Moreover, nandrolone treatment increased HMGCR (steroid synthesising enzymes in adrenal gland) expression in the adrenals and reduced expression levels of the b3-adrenoceptor in the adipose tissue. Indeed, nandrolone may reduce adiponectin via a direct effect on the adipocyte, as indicated by cell culture experiments [76].
It is worth saying that the expression of adiponectin is affected by plasma CORT and that the reduced levels of adiponectin in the present study may result from the simultaneous reduction in CORT [77]. Cardiovascular and metabolic unhealthiness correlated with ND abuse is mediated by reduced adiponectin levels [78-80], since adiponectin has both anti-atherogenic and anti-diabetic effects [81]. Moreover cardiovascular disease has been associated with reduced circulating levels of adiponectin [82].
Schlussman et al. [75] suggested, after experiments with ND treated rats (either 45 mg/kg for 14 days or 15 mg/kg ND for 3 days), that ND action on stress response is dose and/or time dependent. A decrease in circulating CORT levels 24 h after the cessation of long term – treatment (14 days) and a considerable increase in circulating CORT levels 1 h after the end of short term– treatment (3 days) and a return to the control levels 24h later was noted. Differences in time course of ND effects were observed in mRNA levels of various components of the HPA. No alterations in mRNA levels of hypothalamic CRF, anterior pituitary proopiomelanocortin (POMC) or anterior pituitary CRF R1 were observed. However, a considerable decrease in both anterior pituitary POMC and hypothalamic CRF have been reported in a chronic study with ND.
Nandrolone and Neurotransmitters: γ-Aminobutyric Acid Type A (GABAA) Receptors
Studies have demonstrated that chronic treatment with ND changes GABAergic transmission in neural circuits that play a crucial role in the expression of aggression. ND enhances synaptic currents mediated by the GABAA receptor in the ventromedial nucleus of the hypothalamus, but diminishes them in the pre-optic area [83].
Specifically, there was a semantically increased release of neurons from the central amygdala (CeA) projecting to the nucleus bed of the stria terminalis (BnST) and to the GABAA receptor-mediated inhibition which target BnST neurons. [84] GABAergic transmission in the mammalian forebrain has been associated to aggression [85], reproductive behavior, stress [88] and anxiety expression.
Effect of the AAS on ion channels in the CNS has been observed both in social isolation [89] and chronic AAS exposure [90]. Increased expression of the α5 subunit of the GABAA receptor in forebrain regions associated with aggression and anxiety development [91] (including hippocampus, amyglada, [92] medial pre optic area [93, 94], and the frontal cortex) [95] has been noted.
Although AASs have been shown to alter GABAA receptor expression after chronic treatment [94], when given acutely they allosterically modulate the GABAA receptor function [96].
The GABAA receptor subunit gene expression is regulated by both 17b-estradiol [97] and testosterone [98], possibly by signaling receptors mediated by nuclear androgen and estrogen. AAS may mimic these gonadal steroids by acting on these receptors [99].
According to studies, AASs alter the synthesis of endogenous neurosteroids and of GABA synthesizing enzymes. Such alteration in endogenous neurosteroid levels may affect the brain and all behaviors associated with AAS abuse.
5-Hydroxytryptamine (5-HT) and 5-HT Receptors
The serotonergic, 5-HT, pathways originate in the midbrain, the raphe nuclei and innervate the ventral tegmental area, [100] the substantia nigra, [83] the nucleus accumbens and the striatum [101]. Serotonergic activity regulates sexual behavior, aggression, fear, anxiety and reward [102].
Attenuation of the 5-HT system has been involved with increased aggression in humans and animals [103, 104].
According to Kurling S et al [105] supraphysiological doses of ND (20 mg/kg) considerably increased the concentrations of 5-HT in the hippocampus, hippocampus and cerebral cortex.
Thiblin et al. [60] evaluated the effects of different AASs including among others ND and testosterone propionate on Sprague–Dawley rats. They found that 5-HT metabolism (measured as a ratio of 5-HT to 5-hydroxyindoleacetic acid) was enhanced in the hippocampus, but 5-HT synthesis rate was not significantly increased. Conflicting results presented in earlier studies suggested that treatment with testosterone propionate resulted in decreased levels of 5-HT in the diencephalon [106] and in the hippocampus [107].
Racca S et al [73] evaluated the effect of ND on HPAA and cortical serotoninergic system activity in response to acute restrain (RS). In non-stressed ND treated animals no effect on 5-HT re-uptake was observed while it entirely impaired the raise of serotonin transporter (SERT) activity caused by acute RS, probably as a consequence of the stress-induced increase of 5-HT levels in the synaptic cleft.
Dopamine and Dopamine Receptors
Young Syrian hamsters treated with AAS produced increased levels of dopamine (DA) and dopamine D2 receptor (DA D2R) expression in the anterior hypothalamus [108, 109]. DA D2Rs are androgen sensitive and AAS treatment considerably increases D2 mRNA expression in the brain [110]. Increased D2R immune-reactivity in the hypothalamus (AH) has been noticed after treatment with AAS in young hamsters by Ricci et al. [111]. As the activation of D2Rs enhance neuronal inhibition, a rise in DA and D2R expression in the AH indicates that hypothalamic DA enhances aggression. Another theory implicates disinhibitory mechanism for enhanced expression of aggressiveness. In this way, a blockade of D2Rs would allow an increased GABAergic inhibition on the excitatory AH neurons. It is assumed that these D2-GABAergic neurons synapse on vasopressin (VP) neurons within the AH. In addition, GABA was previously considered to be able to modulate VP activity in the AH [112]. Hypothalamic VP activity has been proved to play a crucial role in aggression control.
Opioids and Opioid Receptors
Alterations in the endogenous opioid system, because of AAS administration, were first reported in 1995 [92]. Dynorphin B (Dyn B) and met-enkephalin-Arg6Phe7 (MEAP) are opioids, which serve as markers for two genetically different opioid peptide system activities.
ND affects both enkephalin and dynorphin systems in striatum, AH and periaqueductal gray (PAG). Moreover, chronic administration induces an imbalance in nucleus accumbens (structures associated with reward mechanisms) [113].
CONCLUSIONS
ND is able to cause several psychological effects, a graphical representation is reported in Fig. 2. Many of the effects and the associated mechanisms have been explained in detail in the present review.
Fig. (2).
Possible psychological effects caused by ND.
To sum up, serotonin, glutamate and dopamine systems, activation of GABAA and NMDA receptors as well as activation of steroid receptors such as estrogen, mineralocorticoid, progesterone and glucocorticoid receptors could all contribute to the altered behaviors described.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
References
- 1.Pardridge W.M. Serum bioavailability of sex steroid hormones. Clin. Endocrinol. Metab. 1986;15(2):259–278. doi: 10.1016/S0300-595X(86)80024-X. [DOI] [PubMed] [Google Scholar]
- 2.Basaria S., Wahlstrom J.T., Dobs A.S. Clinical review 138: Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J. Clin. Endocrinol. Metab. 2001;86(11):5108–5117. doi: 10.1210/jcem.86.11.7983. [DOI] [PubMed] [Google Scholar]
- 3.Riezzo I., Turillazzi E., Bello S., Cantatore S., Cerretani D., Di Paolo M., Fiaschi A.I., Frati P., Neri M., Pedretti M., Fineschi V. Chronic nandrolone administration promotes oxidative stress, induction of pro-inflammatory cytokine and TNF-α mediated apoptosis in the kidneys of CD1 treated mice. Toxicol. Appl. Pharmacol. 2014;280(1):97–106. doi: 10.1016/j.taap.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Bahrke M.S., Yesalis C.E., III, Wright J.E. Psychological and behavioural effects of endogenous testosterone and anabolic-androgenic steroids. An update. Sports Med. 1996;22(6):367–390. doi: 10.2165/00007256-199622060-00005. [DOI] [PubMed] [Google Scholar]
- 5.Buckley W.E., Yesalis C.E., III, Friedl K.E., Anderson W.A., Streit A.L., Wright J.E. Estimated prevalence of anabolic steroid use among male high school seniors. JAMA. 1988;260(23):3441–3445. doi: 10.1001/jama.1988.03410230059028. [DOI] [PubMed] [Google Scholar]
- 6.DuRant R.H., Rickert V.I., Ashworth C.S., Newman C., Slavens G. Use of multiple drugs among adolescents who use anabolic steroids. N. Engl. J. Med. 1993;328(13):922–926. doi: 10.1056/NEJM199304013281304. [DOI] [PubMed] [Google Scholar]
- 7.Kopera H. The history of anabolic steroids and a review of clinical experience with anabolic steroids. Acta Endocrinol. Suppl. (Copenh.) 1985;271:11–18. doi: 10.1530/acta.0.109s00011. [DOI] [PubMed] [Google Scholar]
- 8.Eklöf A.C., Thurelius A.M., Garle M., Rane A., Sjöqvist F. The anti-doping hot-line, a means to capture the abuse of doping agents in the Swedish society and a new service function in clinical pharmacology. Eur. J. Clin. Pharmacol. 2003;59(8-9):571–577. doi: 10.1007/s00228-003-0633-z. [DOI] [PubMed] [Google Scholar]
- 9.Verroken M. Ethical aspects and the prevalence of hormone abuse in sport. J. Endocrinol. 2001;170(1):49–54. doi: 10.1677/joe.0.1700049. [DOI] [PubMed] [Google Scholar]
- 10.Shahidi N. T. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin. Ther. 2001;23(9):1355–90. doi: 10.1016/S0149-2918(01)80114-4. [DOI] [PubMed] [Google Scholar]
- 11.Ryan K.J. Biological aromatization of steroids. J. Biol. Chem. 1959;234(2):268–272. [PubMed] [Google Scholar]
- 12.Winters S.J. Androgens: endocrine physiology and pharmacology. NIDA Res. Monogr. 1990;102:113–130. [PubMed] [Google Scholar]
- 13.Long S.F., Wilson M.C., Sufka K.J., Davis W.M. The effects of cocaine and nandrolone co-administration on aggression in male rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1996;20(5):839–856. doi: 10.1016/0278-5846(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 14.Breuer M.E., McGinnis M.Y., Lumia A.R., Possidente B.P. Aggression in male rats receiving anabolic androgenic steroids: effects of social and environmental provocation. Horm. Behav. 2001;40(3):409–418. doi: 10.1006/hbeh.2001.1706. [DOI] [PubMed] [Google Scholar]
- 15.McGinnis M.Y., Lumia A.R., Breuer M.E., Possidente B. Physical provocation potentiates aggression in male rats receiving anabolic androgenic steroids. Horm. Behav. 2002;41(1):101–110. doi: 10.1006/hbeh.2001.1742. [DOI] [PubMed] [Google Scholar]
- 16.McGinnis M.Y., Lumia A.R., Possidente B.P. Effects of withdrawal from anabolic androgenic steroids on aggression in adult male rats. Physiol. Behav. 2002;75(4):541–549. doi: 10.1016/S0031-9384(02)00657-1. [DOI] [PubMed] [Google Scholar]
- 17.Farrell S.F., McGinnis M.Y. Effects of pubertal anabolic-androgenic steroid (AAS) administration on reproductive and aggressive behaviors in male rats. Behav. Neurosci. 2003;117(5):904–911. doi: 10.1037/0735-7044.117.5.904. [DOI] [PubMed] [Google Scholar]
- 18.Wesson D.W., McGinnis M.Y. Stacking anabolic androgenic steroids (AAS) during puberty in rats: a neuroendocrine and behavioral assessment. Pharmacol. Biochem. Behav. 2006;83(3):410–419. doi: 10.1016/j.pbb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Albert D.J., Walsh M.L., Jonik R.H. Aggression in humans: what is its biological foundation? Neurosci. Biobehav. Rev. 1993;17(4):405–425. doi: 10.1016/S0149-7634(05)80117-4. [DOI] [PubMed] [Google Scholar]
- 20.McGinnis M.Y. Anabolic androgenic steroids and aggression: studies using animal models. Ann. N. Y. Acad. Sci. 2004;1036:399–415. doi: 10.1196/annals.1330.024. [DOI] [PubMed] [Google Scholar]
- 21.Farrell S.F., McGinnis M.Y. Long-term effects of pubertal anabolic-androgenic steroid exposure on reproductive and aggressive behaviors in male rats. Horm. Behav. 2004;46(2):193–203. doi: 10.1016/j.yhbeh.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Lindqvist A.S., Johansson-Steensland P., Nyberg F., Fahlke C. Anabolic androgenic steroid affects competitive behaviour, behavioural response to ethanol and brain serotonin levels. Behav. Brain Res. 2002;133(1):21–29. doi: 10.1016/S0166-4328(01)00408-9. [DOI] [PubMed] [Google Scholar]
- 23.Melloni R. H., Jr, Ferris C. F. Adolescent anabolic steroid use and aggressive behavior in golden hamsters. Ann. N. Y. Acad. Sci. 1996;794:372–5. doi: 10.1111/j.1749-6632.1996.tb32546.x. [DOI] [PubMed] [Google Scholar]
- 24.Melloni R.H., Jr, Connor D.F., Hang P.T., Harrison R.J., Ferris C.F. Anabolic-androgenic steroid exposure during adolescence and aggressive behavior in golden hamsters. Physiol. Behav. 1997;61(3):359–364. doi: 10.1016/S0031-9384(96)00373-3. [DOI] [PubMed] [Google Scholar]
- 25.Clark A.S., Henderson L.P. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci. Biobehav. Rev. 2003;27(5):413–436. doi: 10.1016/S0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 26.DeLeon K.R., Grimes J.M., Melloni R.H., Jr Repeated anabolic-androgenic steroid treatment during adolescence increases vasopressin V(1A) receptor binding in Syrian hamsters: correlation with offensive aggression. Horm. Behav. 2002;42(2):182–191. doi: 10.1006/hbeh.2002.1802. [DOI] [PubMed] [Google Scholar]
- 27.Grimes J.M., Melloni R.H., Jr Serotonin modulates offensive attack in adolescent anabolic steroid-treated hamsters. Pharmacol. Biochem. Behav. 2002;73(3):713–721. doi: 10.1016/S0091-3057(02)00880-8. [DOI] [PubMed] [Google Scholar]
- 28.Harrison R.J., Connor D.F., Nowak C., Nash K., Melloni R.H., Jr Chronic anabolic-androgenic steroid treatment during adolescence increases anterior hypothalamic vasopressin and aggression in intact hamsters. Psychoneuroendocrinology. 2000;25(4):317–338. doi: 10.1016/S0306-4530(99)00057-8. [DOI] [PubMed] [Google Scholar]
- 29.Grimes J.M., Ricci L.A., Melloni R.H., Jr Plasticity in anterior hypothalamic vasopressin correlates with aggression during anabolic-androgenic steroid withdrawal in hamsters. Behav. Neurosci. 2006;120(1):115–124. doi: 10.1037/0735-7044.120.1.115. [DOI] [PubMed] [Google Scholar]
- 30.Rossbach U. L., Steensland P., Nyberg F., Le Greves P. Nandrolone-induced hippocampal phosphorylation of NMDA receptor subunits and ERKs. 2007. [DOI] [PubMed]
- 31.Lu C.L., Shaikh M.B., Siegel A. Role of NMDA receptors in hypothalamic facilitation of feline defensive rage elicited from the midbrain periaqueductal gray. Brain Res. 1992;581(1):123–132. doi: 10.1016/0006-8993(92)90351-9. [DOI] [PubMed] [Google Scholar]
- 32.Le Grevès P., Huang W., Johansson P., Thörnwall M., Zhou Q., Nyberg F. Effects of an anabolic-androgenic steroid on the regulation of the NMDA receptor NR1, NR2A and NR2B subunit mRNAs in brain regions of the male rat. Neurosci. Lett. 1997;226(1):61–64. doi: 10.1016/S0304-3940(97)00244-9. [DOI] [PubMed] [Google Scholar]
- 33.Tamaki T., Shiraishi T., Takeda H., Matsumiya T., Roy R.R., Edgerton V.R. Nandrolone decanoate enhances hypothalamic biogenic amines in rats. Med. Sci. Sports Exerc. 2003;35(1):32–38. doi: 10.1097/00005768-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Hallberg M., Johansson P., Kindlundh A.M., Nyberg F. Anabolic-androgenic steroids affect the content of substance P and substance P(1-7) in the rat brain. Peptides. 2000;21(6):845–852. doi: 10.1016/S0196-9781(00)00218-7. [DOI] [PubMed] [Google Scholar]
- 35.Pernow B. Distribution of substance P in the central and peripheral nervous system. Nature. 1953;171(4356):746. doi: 10.1038/171746a0. [DOI] [PubMed] [Google Scholar]
- 36.Chang M.M., Leeman S.E., Niall H.D. Amino-acid sequence of substance P. Nat. New Biol. 1971;232(29):86–87. doi: 10.1038/newbio232086a0. [DOI] [PubMed] [Google Scholar]
- 37.Kindlundh A.M., Lindblom J., Bergström L., Wikberg J.E., Nyberg F. The anabolic-androgenic steroid nandrolone decanoate affects the density of dopamine receptors in the male rat brain. Eur. J. Neurosci. 2001;13(2):291–296. doi: 10.1046/j.0953-816X.2000.01402.x. [DOI] [PubMed] [Google Scholar]
- 38.Carrillo M., Ricci L.A., Melloni R.H., Jr Adolescent anabolic androgenic steroids reorganize the glutamatergic neural circuitry in the hypothalamus. Brain Res. 2009;1249:118–127. doi: 10.1016/j.brainres.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 39.Fischer S.G., Ricci L.A., Melloni R.H., Jr Repeated anabolic/androgenic steroid exposure during adolescence alters phosphate-activated glutaminase and glutamate receptor 1 (GluR1) subunit immunoreactivity in Hamster brain: correlation with offensive aggression. Behav. Brain Res. 2007;180(1):77–85. doi: 10.1016/j.bbr.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisenberg E., Gordan G.S. The levator ani muscle of the rat as an index of myotrophic activity of steroidal hormones. J. Pharmacol. Exp. Ther. 1950;99(1):38–44. [PubMed] [Google Scholar]
- 41.Roselli C.E. The effect of anabolic-androgenic steroids on aromatase activity and androgen receptor binding in the rat preoptic area. Brain Res. 1998;792(2):271–276. doi: 10.1016/S0006-8993(98)00148-6. [DOI] [PubMed] [Google Scholar]
- 42.Orlando R., Caruso A., Molinaro G., Motolese M., Matrisciano F., Togna G., Melchiorri D., Nicoletti F., Bruno V. Nanomolar concentrations of anabolic-androgenic steroids amplify excitotoxic neuronal death in mixed mouse cortical cultures. Brain Res. 2007;1165:21–29. doi: 10.1016/j.brainres.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 43.Minkin D.M., Meyer M.E., van Haaren F. Behavioral effects of long-term administration of an anabolic steroid in intact and castrated male Wistar rats. Pharmacol. Biochem. Behav. 1993;44(4):959–963. doi: 10.1016/0091-3057(93)90031-N. [DOI] [PubMed] [Google Scholar]
- 44.Aikey J. L., Nyby J. G., Anmuth D. M., James P. J. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm. Behav. 2002;42(4):448–60. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- 45.Kouvelas D., Pourzitaki C., Papazisis G., Dagklis T., Dimou K., Kraus M.M. Nandrolone abuse decreases anxiety and impairs memory in rats via central androgenic receptors. Int. J. Neuropsychopharmacol. 2008;11(7):925–934. doi: 10.1017/S1461145708008754. [DOI] [PubMed] [Google Scholar]
- 46.Edinger K.L., Frye C.A. Intrahippocampal administration of an androgen receptor antagonist, flutamide, can increase anxiety-like behavior in intact and DHT-replaced male rats. Horm. Behav. 2006;50(2):216–222. doi: 10.1016/j.yhbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 47.McCarthy M.M., Frank A. Frank A. Beach Award. Functional significance of steroid modulation of GABAergic neurotransmission: analysis at the behavioral, cellular, and molecular levels. Horm. Behav. 1995;29(2):131–140. doi: 10.1006/hbeh.1995.1010. [DOI] [PubMed] [Google Scholar]
- 48.Shekhar A. GABA receptors in the region of the dorsomedial hypothalamus of rats regulate anxiety in the elevated plus-maze test. I. Behavioral measures. Brain Res. 1993;627(1):9–16. doi: 10.1016/0006-8993(93)90742-6. [DOI] [PubMed] [Google Scholar]
- 49.Shekhar A., Katner J.S. Dorsomedial hypothalamic GABA regulates anxiety in the social interaction test. Pharmacol. Biochem. Behav. 1995;50(2):253–258. doi: 10.1016/0091-3057(94)00307-5. [DOI] [PubMed] [Google Scholar]
- 50.Giros B., Jaber M., Jones S.R., Wightman R.M., Caron M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 51.Kindlundh A. M., Rahman S., Lindblom J., Nyberg F. Increased dopamine transporter density in the male rat brain following chronic nandrolone decanoate administration. Neurosci. Lett. 2004;356 (2):131–6. doi: 10.1016/j.neulet.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 52.Kindlundh A.M., Bergström M., Monazzam A., Hallberg M., Blomqvist G., Långström B., Nyberg F. Dopaminergic effects after chronic treatment with nandrolone visualized in rat brain by positron emission tomography. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26(7-8):1303–1308. doi: 10.1016/S0278-5846(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 53.Jaber M., Jones S., Giros B., Caron M. G. The dopamine transporter: a crucial component regulating dopamine transmission. Mov. Disord. 1997;12(5):629–33. doi: 10.1002/mds.870120502. [DOI] [PubMed] [Google Scholar]
- 54.Clark A.S., Lindenfeld R.C., Gibbons C.H. Anabolic-androgenic steroids and brain reward. Pharmacol. Biochem. Behav. 1996;53(3):741–745. doi: 10.1016/0091-3057(95)02082-9. [DOI] [PubMed] [Google Scholar]
- 55.Kailanto S., Kankaanpää A., Seppälä T. Subchronic steroid administration induces long lasting changes in neurochemical and behavioral response to cocaine in rats. Steroids. 2011;76(12):1310–1316. doi: 10.1016/j.steroids.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 56.Kurling S., Kankaanpää A., Seppälä T. Sub-chronic nandrolone treatment modifies neurochemical and behavioral effects of amphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in rats. Behav. Brain Res. 2008;189(1):191–201. doi: 10.1016/j.bbr.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 57.Célérier E., Yazdi M.T., Castañé A., Ghozland S., Nyberg F., Maldonado R. Effects of nandrolone on acute morphine responses, tolerance and dependence in mice. Eur. J. Pharmacol. 2003;465(1-2):69–81. doi: 10.1016/S0014-2999(03)01462-6. [DOI] [PubMed] [Google Scholar]
- 58.Celerier E., Ahdepil T., Wikander H., Berrendero F., Nyberg F., Maldonado R. Influence of the anabolic-androgenic steroid nandrolone on cannabinoid dependence. 2006. [DOI] [PubMed]
- 59.Kurling-Kailanto S., Kankaanpää A., Seppälä T. Subchronic nandrolone administration reduces cocaine-induced dopamine and 5-hydroxytryptamine outflow in the rat nucleus accumbens. Psychopharmacology (Berl) 2010;209(3):271–281. doi: 10.1007/s00213-010-1796-9. [DOI] [PubMed] [Google Scholar]
- 60.Thiblin I., Finn A., Ross S.B., Stenfors C. Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. Br. J. Pharmacol. 1999;126(6):1301–1306. doi: 10.1038/sj.bjp.0702412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wood R.I. Anabolic-androgenic steroid dependence? Insights from animals and humans. Front. Neuroendocrinol. 2008;29(4):490–506. doi: 10.1016/j.yfrne.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johansson P., Ray A., Zhou Q., Huang W., Karlsson K., Nyberg F. Anabolic androgenic steroids increase beta-endorphin levels in the ventral tegmental area in the male rat brain. Neurosci. Res. 1997;27(2):185–9. doi: 10.1016/S0168-0102(96)01141-8. [DOI] [PubMed] [Google Scholar]
- 63.Triemstra J.L., Sato S.M., Wood R.I. Testosterone and nucleus accumbens dopamine in the male Syrian hamster. Psychoneuroendocrinology. 2008;33(3):386–94. doi: 10.1016/j.psyneuen.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magnusson K., Hånell A., Bazov I., Clausen F., Zhou Q., Nyberg F. Nandrolone decanoate administration elevates hippocampal prodynorphin mRNA expression and impairs Morris water maze performance in male rats. Neurosci. Lett. 2009;467(3):189–193. doi: 10.1016/j.neulet.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 65.Kerr J.E., Allore R.J., Beck S.G., Handa R.J. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136(8):3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- 66.Svensson J., Diez M., Engel J., Wass C., Tivesten A., Jansson J.O., Isaksson O., Archer T., Hökfelt T., Ohlsson C. Endocrine, liver-derived IGF-I is of importance for spatial learning and memory in old mice. J. Endocrinol. 2006;189(3):617–627. doi: 10.1677/joe.1.06631. [DOI] [PubMed] [Google Scholar]
- 67.McDaniel K.L., Mundy W.R., Tilson H.A. Microinjection of dynorphin into the hippocampus impairs spatial learning in rats. Pharmacol. Biochem. Behav. 1990;35(2):429–435. doi: 10.1016/0091-3057(90)90180-P. [DOI] [PubMed] [Google Scholar]
- 68.Sandin J., Nylander I., Georgieva J., Schött P.A., Ogren S.O., Terenius L. Hippocampal dynorphin B injections impair spatial learning in rats: a kappa-opioid receptor-mediated effect. Neuroscience. 1998;85(2):375–382. doi: 10.1016/S0306-4522(97)00605-2. [DOI] [PubMed] [Google Scholar]
- 69.Daumas S., Betourne A., Halley H., Wolfer D.P., Lipp H.P., Lassalle J.M., Francés B. Transient activation of the CA3 Kappa opioid system in the dorsal hippocampus modulates complex memory processing in mice. Neurobiol. Learn. Mem. 2007;88(1):94–103. doi: 10.1016/j.nlm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 70.McGinnis M.Y., Lumia A.R., Tetel M.J., Molenda-Figueira H.A., Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiol. Behav. 2007;92(5):1010–1018. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salvador A., Moya-Albiol L., Martínez-Sanchis S., Simón V.M. Lack of effects of anabolic-androgenic steroids on locomotor activity in intact male mice. Percept. Mot. Skills. 1999;88(1):319–328. doi: 10.2466/pms.1999.88.1.319. [DOI] [PubMed] [Google Scholar]
- 72.Van Zyl C.G., Noakes T.D., Lambert M.I. Anabolic-androgenic steroid increases running endurance in rats. Med. Sci. Sports Exerc. 1995;27(10):1385–1389. doi: 10.1249/00005768-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Racca S., Piccione F., Spaccamiglio A., Carriero V.M., De Francia S., Cangemi L., Esculapio P., Papotti M., Migliaretti G., Portaleone P., Di Carlo F., Abbadessa G. Effects of sub-chronic nandrolone administration on hormonal adaptive response to acute stress in rats. Psychoneuroendocrinology. 2012;37(8):1234–1247. doi: 10.1016/j.psyneuen.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 74.Alsiö J., Birgner C., Björkblom L., Isaksson P., Bergström L., Schiöth H.B., Lindblom J. Impact of nandrolone decanoate on gene expression in endocrine systems related to the adverse effects of anabolic androgenic steroids. Basic Clin. Pharmacol. Toxicol. 2009;105(5):307–314. doi: 10.1111/j.1742-7843.2009.00439.x. [DOI] [PubMed] [Google Scholar]
- 75.Schlussman S.D., Zhou Y., Johansson P., Kiuru A., Ho A., Nyberg F., Kreek M.J. Effects of the androgenic anabolic steroid, nandrolone decanoate, on adrenocorticotropin hormone, corticosterone and proopiomelanocortin, corticotropin releasing factor (CRF) and CRF receptor1 mRNA levels in the hypothalamus, pituitary and amygdala of the rat. Neurosci. Lett. 2000;284(3):190–194. doi: 10.1016/S0304-3940(00)01016-8. [DOI] [PubMed] [Google Scholar]
- 76.Nishizawa H., Shimomura I., Kishida K., Maeda N., Kuriyama H., Nagaretani H., Matsuda M., Kondo H., Furuyama N., Kihara S., Nakamura T., Tochino Y., Funahashi T., Matsuzawa Y. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51(9):2734–2741. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 77.Makimura H., Mizuno T.M., Bergen H., Mobbs C.V. Adiponectin is stimulated by adrenalectomy in ob/ob mice and is highly correlated with resistin mRNA. Am. J. Physiol. Endocrinol. Metab. 2002;283(6):E1266–E1271. doi: 10.1152/ajpendo.00227.2002. [DOI] [PubMed] [Google Scholar]
- 78.Dickerman R.D., McConathy W.J., Zachariah N.Y. Testosterone, sex hormone-binding globulin, lipoproteins, and vascular disease risk. J. Cardiovasc. Risk. 1997;4(5-6):363–366. doi: 10.1097/00043798-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 79.Lenders J.W., Demacker P.N., Vos J.A., Jansen P.L., Hoitsma A.J., van ’t Laar A., Thien T. Deleterious effects of anabolic steroids on serum lipoproteins, blood pressure, and liver function in amateur body builders. Int. J. Sports Med. 1988;9(1):19–23. doi: 10.1055/s-2007-1024972. [DOI] [PubMed] [Google Scholar]
- 80.Maravelias C., Dona A., Stefanidou M., Spiliopoulou C. Adverse effects of anabolic steroids in athletes. A constant threat. Toxicol. Lett. 2005;158(3):167–175. doi: 10.1016/j.toxlet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 81.Nishida M., Funahashi T., Shimomura I. Pathophysiological significance of adiponectin. Med. Mol. Morphol. 2007;40(2):55–67. doi: 10.1007/s00795-007-0366-7. [DOI] [PubMed] [Google Scholar]
- 82.Giannessi D., Maltinti M., Del Ry S. Adiponectin circulating levels: a new emerging biomarker of cardiovascular risk. Pharmacol. Res. 2007;56(6):459–467. doi: 10.1016/j.phrs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 83.Masonis A.E., McCarthy M.P. Direct effects of the anabolic/androgenic steroids, stanozolol and 17 alpha-methyltestosterone, on benzodiazepine binding to the. gamma-aminobutyric acid(a) receptor. Neurosci. Lett. 1995;189(1):35–38. doi: 10.1016/0304-3940(95)11445-3. [DOI] [PubMed] [Google Scholar]
- 84.Oberlander J.G., Henderson L.P. Corticotropin-releasing factor modulation of forebrain GABAergic transmission has a pivotal role in the expression of anabolic steroid-induced anxiety in the female mouse. Neuropsychopharmacology. 2012;37(6):1483–1499. doi: 10.1038/npp.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siegel A., Roeling T.A., Gregg T.R., Kruk M.R. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci. Biobehav. Rev. 1999;23(3):359–389. doi: 10.1016/S0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 86.Jones B.L., Whiting P.J., Henderson L.P. Mechanisms of anabolic androgenic steroid inhibition of mammalian epsilon-subunit-containing GABAA receptors. J. Physiol. 2006;573(Pt 3):571–593. doi: 10.1113/jphysiol.2006.106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin Y. S., Li X. F., Shao B. The role of GABAergic signalling in stress-induced suppression of gonadotrophin-releasing hormone pulse generator frequency in female rats. J. Neuroendocrinol. 2012;24(3):477–88. doi: 10.1111/j.1365-2826.2011.02270.x. [DOI] [PubMed] [Google Scholar]
- 88.Wilson M.A. GABA physiology: modulation by benzodiazepines and hormones. Crit. Rev. Neurobiol. 1996;10(1):1–37. doi: 10.1615/CritRevNeurobiol.v10.i1.10. [DOI] [PubMed] [Google Scholar]
- 89.Pinna G., Agis-Balboa R.C., Zhubi A., Matsumoto K., Grayson D.R., Costa E., Guidotti A. Imidazenil and diazepam increase locomotor activity in mice exposed to protracted social isolation. Proc. Natl. Acad. Sci. USA. 2006;103(11):4275–4280. doi: 10.1073/pnas.0600329103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Penatti C.A., Porter D.M., Henderson L.P. Chronic exposure to anabolic androgenic steroids alters neuronal function in the mammalian forebrain via androgen receptor- and estrogen receptor-mediated mechanisms. J. Neurosci. 2009;29(40):12484–12496. doi: 10.1523/JNEUROSCI.3108-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leshner A.I., Koob G.F. Drugs of abuse and the brain. Proc. Assoc. Am. Physicians. 1999;111(2):99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- 92.Menard C.S., Hebert T.J., Dohanich G.P., Harlan R.E. Androgenic-anabolic steroids modify beta-endorphin immunoreactivity in the rat brain. Brain Res. 1995;669(2):255–262. doi: 10.1016/0006-8993(94)01266-K. [DOI] [PubMed] [Google Scholar]
- 93.Penatti C.A., Costine B.A., Porter D.M., Henderson L.P. Effects of chronic exposure to an anabolic androgenic steroid cocktail on alpha5-receptor-mediated GABAergic transmission and neural signaling in the forebrain of female mice. Neuroscience. 2009;161(2):526–537. doi: 10.1016/j.neuroscience.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McIntyre K.L., Porter D.M., Henderson L.P. Anabolic androgenic steroids induce age-, sex-, and dose-dependent changes in GABA(A) receptor subunit mRNAs in the mouse forebrain. Neuropharmacology. 2002;43(4):634–645. doi: 10.1016/S0028-3908(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 95.Pibiri F., Nelson M., Carboni G., Pinna G. Neurosteroids regulate mouse aggression induced by anabolic androgenic steroids. Neuroreport. 2006;17(14):1537–1541. doi: 10.1097/01.wnr.0000234752.03808.b2. [DOI] [PubMed] [Google Scholar]
- 96.Jorge-Rivera J.C., McIntyre K.L., Henderson L.P. Anabolic steroids induce region- and subunit-specific rapid modulation of GABA(A) receptor-mediated currents in the rat forebrain. J. Neurophysiol. 2000;83(6):3299–3309. doi: 10.1152/jn.2000.83.6.3299. [DOI] [PubMed] [Google Scholar]
- 97.Herbison A.E., Fénelon V.S. Estrogen regulation of GABAA receptor subunit mRNA expression in preoptic area and bed nucleus of the stria terminalis of female rat brain. J. Neurosci. 1995;15(3 Pt 2):2328–2337. doi: 10.1016/j.brainres.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang L., Chang Y.H., Feldman A.N., Ma W., Lahjouji F., Barker J.L., Hu Q., Maric D., Li B.S., Li W., Rubinow D.R. The expression of GABA(A) receptor alpha2 subunit is upregulated by testosterone in rat cerebral cortex. Neurosci. Lett. 1999;265(1):25–28. doi: 10.1016/S0304-3940(99)00193-7. [DOI] [PubMed] [Google Scholar]
- 99.Mehta A.K., Ticku M.K. An update on GABAA receptors. Brain Res. Brain Res. Rev. 1999;29(2-3):196–217. doi: 10.1016/S0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- 100.Hervé D., Pickel V.M., Joh T.H., Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res. 1987;435(1-2):71–83. doi: 10.1016/0006-8993(87)91588-5. [DOI] [PubMed] [Google Scholar]
- 101.Azmitia E.C., Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 1978;179(3):641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- 102.Bonasera S.J., Tecott L.H. Mouse models of serotonin receptor function: toward a genetic dissection of serotonin systems. Pharmacol. Ther. 2000;88(2):133–142. doi: 10.1016/S0163-7258(00)00087-5. [DOI] [PubMed] [Google Scholar]
- 103.Matte A.C., Tornow H. Parachlorophenylalanine produces dissociated effects on aggression “emotionality” and motor activity. 1978. [DOI] [PubMed]
- 104.Miczek K.A., Fish E.W., De Bold J.F., De Almeida R.M. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology (Berl) 2002;163(3-4):434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- 105.Kurling S., Kankaanpaa A., Ellermaa S., Karila T., Seppala T. The effect of sub-chronic nandrolone decanoate treatment on dopaminergic and serotonergic neuronal systems in the brains of rats. Brain Res. 2005;1044(1):67–75. doi: 10.1016/j.brainres.2005.02.071. [DOI] [PubMed] [Google Scholar]
- 106.Martinez-Conde E., Leret M.L., Diaz S. The influence of testosterone in the brain of the male rat on levels of serotonin (5-HT) and hydroxyindole-acetic acid (5-HIAA). Comp. Biochem. Physiol. C. Comp. Pharmacol. Toxicol. 1985;80(2):411–414. doi: 10.1016/0742-8413(85)90077-5. [DOI] [PubMed] [Google Scholar]
- 107.Bonson K.R., Winter J.C. Reversal of testosterone-induced dominance by the serotonergic agonist quipazine. Pharmacol. Biochem. Behav. 1992;42(4):809–813. doi: 10.1016/0091-3057(92)90034-D. [DOI] [PubMed] [Google Scholar]
- 108.Ferris C.F., Melloni R.H., Jr, Koppel G., Perry K.W., Fuller R.W., Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J. Neurosci. 1997;17(11):4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwartzer J.J., Melloni R.H., Jr Anterior hypothalamic dopamine D2 receptors modulate adolescent anabolic/androgenic steroid-induced offensive aggression in the Syrian hamster. Behav. Pharmacol. 2010;21(4):314–322. doi: 10.1097/FBP.0b013e32833b10f1. [DOI] [PubMed] [Google Scholar]
- 110.Birgner C., Kindlundh-Högberg A.M., Alsiö J., Lindblom J., Schiöth H.B., Bergström L. The anabolic androgenic steroid nandrolone decanoate affects mRNA expression of dopaminergic but not serotonergic receptors. Brain Res. 2008;1240:221–228. doi: 10.1016/j.brainres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 111.Ricci L.A., Schwartzer J.J., Melloni R.H., Jr Alterations in the anterior hypothalamic dopamine system in aggressive adolescent AAS-treated hamsters. Horm. Behav. 2009;55(2):348–355. doi: 10.1016/j.yhbeh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 112.Ghuman S. P., Prabhakar S., Smith R. F., Dobson H. gammaamino butyric acid control of arginine vasopressin release from the ewe hypothalamus in vitro: sensitivity to estradiol. Reprod. Domest. Anim. 2007;42(5):527–35. doi: 10.1111/j.1439-0531.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 113.Herz A. Opioid reward mechanisms: a key role in drug abuse? Can. J. Physiol. Pharmacol. 1998;76(3):252–258. doi: 10.1139/y98-017. [DOI] [PubMed] [Google Scholar]


