Abstract
Anabolic androgenic steroids (AASs) represent a large group of synthetic derivatives of testosterone, produced to maximize anabolic effects and minimize the androgenic ones. AAS can be administered orally, parenterally by intramuscular injection and transdermally. Androgens act by binding to the nuclear androgen receptor (AR) in the cytoplasm and then translocate into the nucleus. This binding results in sequential conformational changes of the receptor affecting the interaction between receptor and protein, and receptor and DNA.
Skeletal muscle can be considered as the main target tissue for the anabolic effects of AAS, which are mediated by ARs which after exposure to AASs are up-regulated and their number increases with body building. Therefore, AASs determine an increase in muscle size as a consequence of a dose-dependent hypertrophy resulting in an increase of the cross-sectional areas of both type I and type II muscle fibers and myonuclear domains. Moreover, it has been reported that AASs can increase tolerance to exercise by making the muscles more capable to overload therefore shielding them from muscle fiber damage and improving the level of protein synthesis during recovery.
Despite some therapeutic use of AASs, there is also wide abuse among athletes especially bodybuilders in order to improve their performances and to increase muscle growth and lean body mass, taking into account the significant anabolic effects of these drugs.
The prolonged misuse and abuse of AASs can determine several adverse effects, some of which may be even fatal especially on the cardiovascular system because they may increase the risk of sudden cardiac death (SCD), myocardial infarction, altered serum lipoproteins, and cardiac hypertrophy.
The aim of this review is to focus on deaths related to AAS abuse, trying to evaluate the autoptic, histopathological and toxicological findings in order to investigate the pathophysiological mechanism that underlines this type of death, which is still obscure in several aspects. The review of the literature allowed us to identify 19 fatal cases between 1990 and 2012, in which the autopsy excluded in all cases, extracardiac causes of death.
Keywords: Anabolic Androgenic Steroids (AAS), cardiovascular effects, sudden cardiac death, toxicity
INTRODUCTION
Anabolic androgenic steroids (AASs) represent a large group of synthetic derivatives of testosterone, produced to maximize anabolic effects and minimize the androgenic ones [1, 2].
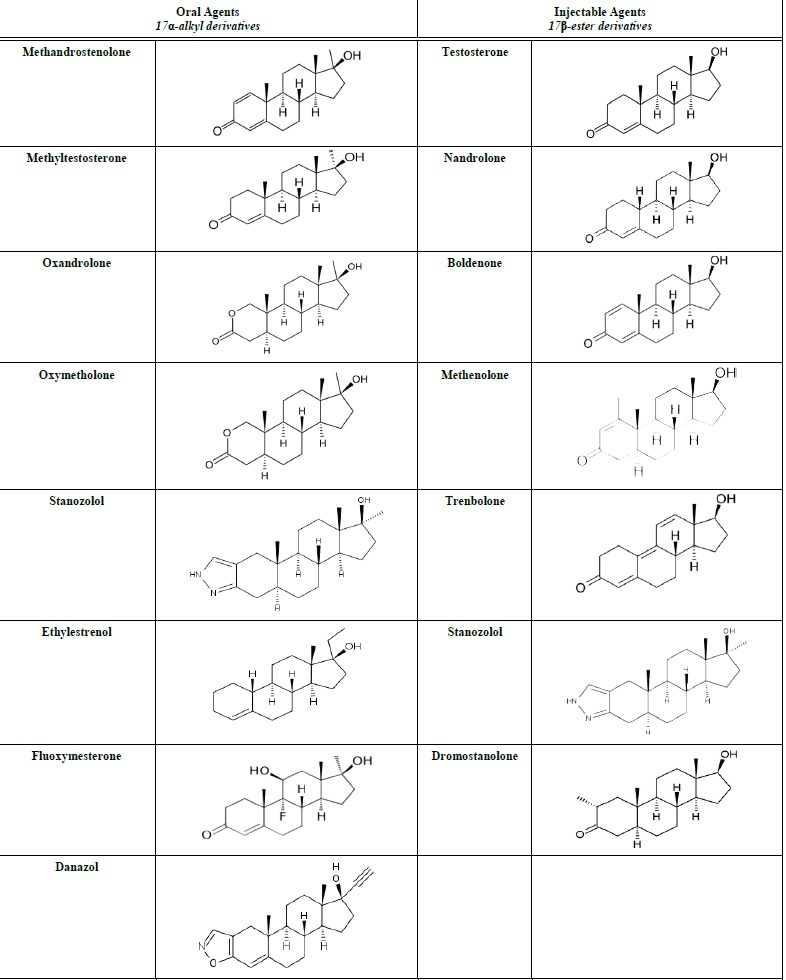
AASs can be administered orally, parenterally by intramuscular injection and transdermally. The most common oral and injectable AASs with their chemical structures are reported in Table 1. Androgens act by binding to the nuclear androgen receptor (AR) in the cytoplasm and then translocate into the nucleus. This binding results in sequential conformational changes of the receptor affecting the interaction between receptor and protein, and receptor and DNA [3].
Table 1.
AASs most commonly abused (oral and injectable formulations).
 |
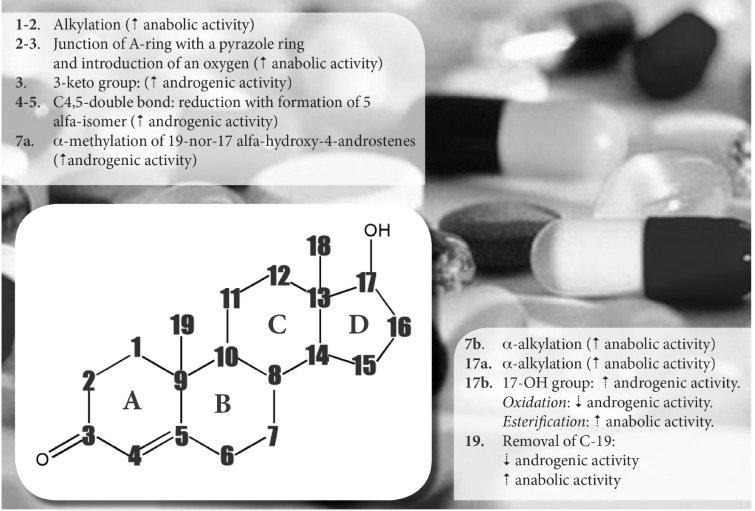
The basic structure of all steroids is a perhydro-cyclopentano phenanthrene ring system that can be modified in order to obtain several designed chemical modifications [3]. The most important chemical modification, in which the basic structure can undergo, is reported in Fig. 1.
Fig. (1).
The basic structure of steroids can be changed in order to obtain several designed chemical modifications
The name “anabolic androgenic steroids” already suggests their “anabolic” (from Greek ἀναβολή “throw upward”) and “androgenic” (Greek ἀνδρός “of a man” + -γενής “born”) properties.
Anabolism is defined by Kuhn [4] as “any state in which nitrogen is differentially retained in lean body mass, either through stimulation of protein synthesis and/or decreased breakdown of protein anywhere in the body”. Skeletal muscle can be considered as the main target tissue for the anabolic effects of AAS, which are mediated by ARs which after exposure to AASs are up-regulated and their number increases with body building [5]. Therefore, AASs determine an increase in muscle size as a consequence of a dose-dependent hypertrophy resulting in an increase of the cross-sectional areas of both type I and type II muscle fibers and myonucleardomains [6]. Moreover, it has been reported that AASs can increase tolerance to exercise by making the muscles more capable to overload therefore shielding them from muscle fiber damage and improving the level of protein synthesis during recovery [7].
Despite some therapeutic use of AASs (severe burns, primary or secondary hypogonadism, short stature, HIV wasting syndrome etc.) there is also wide abuse among athletes especially bodybuilders in order to improve their performances and to increase muscle growth and lean body mass, taking into account the significant anabolic effects above reported. Not by chance, these substances fall within the vast group of “performance-enhancing drugs”, which also include: stimulants, painkillers, sedatives and anxiolytics, diuretics, blood boosters and masking drugs. A high-dose regimen is “stacked” by combining numerous oral and injectable AASs, which are self-administered in drug “cycles” which last from 4 to 12 weeks [7-9].
Furthermore, AAS users frequently associate other substances to AASs, the so called “steroid-accessory drugs”, such as ephedrine, growth hormone, insulin, diuretics, GHB etc [10-15] for several reasons.
The prolonged misuse and abuse of AASs can determine several adverse effects, some of which may be even fatal especially on the cardiovascular system because they may increase the risk of sudden cardiac death (SCD), myocardial infarction, altered serum lipoproteins, and cardiac hypertrophy [7]. The most frequent cardiovascular adverse effects due to AASs are summarized in Fig. 2.
Fig. (2).
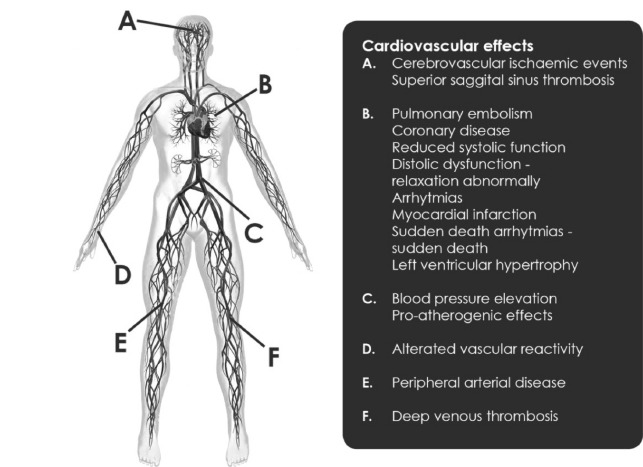
Cardiovascular adverse effects due to a prolonged use of AASs
The aim of this review is to focus on deaths related to AAS abuse, trying to evaluate the autoptic, histopathological and toxicological findings in order to investigate the pathophysiological mechanism that underlines this type of death, which is still obscure in several aspects.
MATERIALS AND METHODS
Some databases, from 1975 to June 2014, were searched: Medline, Cochrane Central, Scopus, Web of Science, Science Direct, EMBASE and Google Scholar, using the following keywords: Anabolic Androgenic Steroid, death, cardiovascular effects, toxicity, side/adverse effects. The main key word “Anabolic Androgenic Steroid” was individually searched in association to each of the others. The 189 sources found after the initial screening in order to exclude duplicate sources and retrospective studies, were selected according to the “inclusion criteria”, which allowed the identification of 10 sources. A comprehensive flow diagram with inclusion criteria is reported in Fig. 3.
Fig. (3).
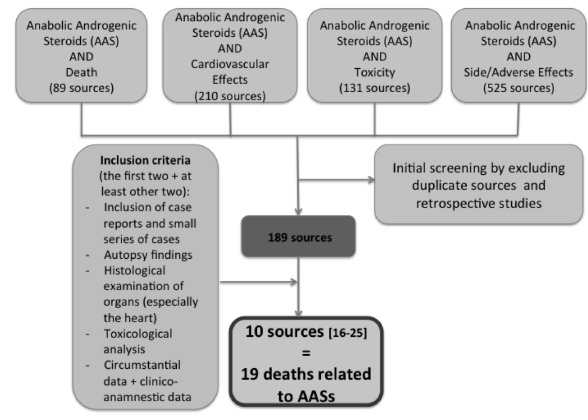
Flow diagram with inclusion criteria for the selection of sources for the purpose of the review
RESULTS
The review of the literature using the flow diagram reported in Fig. 3 allowed us to identify 19 fatal cases between 1990 and 2012. The most important autoptic, histopathological findings and circumstantial data are reported in Table 2, while the toxicological findings are reported in Table 3.
Table 2.
Autoptic, macroscopic and histological findings in 19 AAS related deaths.
| Study | Number of Cases | Age (yrs), Sex, Height and Weight | Heart Dimensions Wall Thickness (mm) |
Cardiac Macroscopic and Histopathological Findings | Other Findings | |||
|---|---|---|---|---|---|---|---|---|
| Weight(g) | LV | RV | IS | |||||
| Luke etal (1990) [16] | 1 | 27 M 96.6kg | 530 | 16 | 4 | -- | Marked cardiac hypertrophy with regional myocardial fibrosis. Contraction band necrosis, lymphocytic infiltration | Renal hypertrophy, hepatosplenomegaly |
| Ferenchick G.S. (1991) [17] | 1 | 22 M | --- | --- | --- | --- | Occlusive thrombus in left coronary artery | |
| Kennedy et al (1993) [18] | 2 | 18 M 24 M |
410 440 |
13 17 |
4 6 |
17 12 |
Hypertrophic cardiomyopathy, myocarditis, disarray, focal fibrosis. Focal fibrosis, lymphocytic infiltration |
|
| Dickerman et al (1996) [19] |
1 | 26 M 182cm 136kg | 440 | ---- | ---- | --- | Left ventricular hypertrophy | Bilateral pulmonary embolism from deep venous thrombus of lower extremities |
| Hausmann et al (1998) [20] |
1 | 23 M | 500 | ---- | ---- | --- | Right ventricle dilatation, focal endocardial induration. Enlargement and nuclear polymorphism of left ventricle muscle fibres; disseminated focal necrosis with loss of nuclear staining, dehiscence of intercalated discs and interstitial fibrosis |
Liver parenchyma soft and fragile, cerebral edema, acute vascular congestion in liver spleen and kidney. Capillary hyperemia, platelet aggregation, several fibrinous clots in lungs, liver and kidneys. Nuclear fat free vacuoles, pielosis hepatis. |
| Fineschi et al (2001) [21] | 2 | 32 M 189cm 90kg
29 M 166cm 72kg |
450
390 |
14
19 |
4
6 |
14
13 |
One grayish zone in internal half of the anterior-lateral wall of the left ventricle which corresponded histologically to a typical infarct necrosis, dead hyperdistended myocardial cells with sarcomeres in registered order. Occasional foci of contraction band necrosis and few fibrotic microfoci in internal portion of left ventricle and interventricular septum. Occasional isolated myocardial cells with contraction bands and segmentation of myocardial cells |
|
| Weight(g) | LV | RV | IS | |||||
| Di Paolo et al (2007) [22] | 4 | 29 M 190cm 127kg 27 M 190cm 100kg 37 F 162cm 71kg 31 M 175cm 79kg |
490 360 310 400 |
13 11 10 12 |
---- ---- ---- ---- |
--- --- --- --- |
Small vessels disease, severe interstitial and epicardial fibrosis, hypotrofic myocytes in fibrosis areas, hypetrophy in non fibrosis areas, focal fatty substitution in of the anterior LV wall, mild focal intimal hyperplasia in coronary arteries, myocardial bridge Small vessels disease, focal interstitial and epicardial fibrosis, hypotrofic myocytes in fibrosis areas, mild focal intimal hyperplasia in coronary arteries Focal interstitial and epicardial fibrosis, hypotrofic myocytes in fibrosis areas, unique granulation tissue focus, normal coronary arteries. Small vessels disease, focal interstitial fibrosis and moderate/ severe epicardial fibrosis, myocytes hypertrophy, occasional basophilic degeneration, mild intimal hyperplasia in coronary arteries |
|
| Fineschi et al (2007) [23] |
2 | 29 M 166 cm 72kg 30 M 178 cm 90kg |
380 400 |
---- ---- |
---- ---- |
--- --- |
Foci of contract band necrosis, two microfoci of fibrosis (subendocardial anterior left ventricle, interventricular septum) segmentation of myocardial cells, widening of intercalated discs, bundles of contracted myocardium alterning with bundles of distended myocardium with granular disruption of myocytes. Coronary scattered fatty streaks, focal myocardial fibrosis |
Cholestasis pielosis hepatis |
| Thiblin et al (2009) [24] | 1 | 29 F 172 cm 76kg | 331 | ---- | ---- | --- | Isolated flat area of fatty thickening 0.5 x 0.3 cm in proximal part of left anterior descending coronary artery (LAD), few small foci of granulation tissue, lymphocytic infiltration around several middle size and small intramural vessels | Adrenal diminished of cortex and medulla. Uterus slightly larger and ovaries slightly smaller. Internal organ abnormally heavy (liver 2298g; kidneys 394g; lungs 1500g) Lung congested with multiples area of erythrocytes-containing alveoli |
| Weight(g) | LV | RV | IS | |||||
| Montisci et al (2012) [25] | 4 | 32 M 180cm 110kg 31 M 172cm 120kg 32 M 178cm 94 kg 25 M 185cm 125kg |
450 900 580 390 |
15 15 16 13 |
4 5 5 3.5 |
16 20 18 16 |
Concentric left ventricular hypertrophy. Focal disarray with interstitial and replacement type fibrosis foci of lymphoplasmacellular infiltrates (CD3+) edema and patchy necrosis. Biventricular eccentric hypertrophy and mild atrial dilatation, mural endocardial thrombosis anterior left ventricular wall. Hypertrophic myocytes, interstitial and replacement fibrosis colliquative myocytolysis. Hypertrophy biventricular dilatation. Hypertrophied myocytes with bizarre nuclei, foci of myocytolysis with a mild inflammatory reaction and active fibrosis, fibrofatty replacement LAD stenosis 50%. Diffuse interstitial edema, multiple foci of polymorphous inflammatory infiltrates with eosinophils. |
Multiorgan congestion, liver steatosis Multiorgan congestion, acute pulmonary edema, liver steatosis (2700 g), testicular atrophy Multiorgan congestion, lung hemorrhagic infarction, acute hepatic congestion Multiorgan congestion |
Table 3.
Toxicological findings and circumstantial data in 19 AAS related deaths.
| Study | Number of Cases (n=19) |
Age (yrs), Sex, Height and Weight | Circumstances | History of Abuse and Route of Administration | Method of Detention | Toxicological Findings |
|---|---|---|---|---|---|---|
| Luke et al (1990) [16] | 1 | 27 M 96.6kg | Collapse during a bench press workout | He had taken anabolic androgenic steroids parenterally for several months previously. |
URINE Nandrolone (19-nor-testosterone) and metabolites | |
| Ferenchick G.S. (1991) [17] | 1 | 22 M | ||||
| Kennedy et al (1993) [18] | 2 | 18 M 24 M |
cardiac arrests during training sessions | URINE Oxymesterone URINE Oxymesterone |
||
| Dickerman et al (1996) [19] | 1 | 26 M 182cm 136kg | Collapse while moving furniture | History of anabolic steroid use | ||
| Hausmann et al (1998) [20] | 1 | 23 M 192cm 94kg | Found dead at home | He had taken anabolic steroid for 9 months. In his apartment were found: -Testosterone cyclopentilpropionate 250 i.m. -Methenolone enantate 100 mg i.m. -Liothyronin hydrochloride 100 ug tablets -Spironolactone 100 mg tablets -Clomifen 25 mg capsules -Clenbuterol hydrochloride 0.02 mg tablets |
EIA and GC-MS after derivatisation | URINE Mesterolone, Methandienone Testosterone, Nandrolone and Clenbuterol T/E ratio 64:1 |
| Fineschi et al (2001) [21] |
2 | 32 M 189cm 90kg 29 M 166cm 72kg |
Sudden death during a weight lifting workout at the gymnasium Found unconscious in bed |
For several months he had been taking testosterone propionate (700mg/wk) and nandrolone (200 mg/wk) parenterally and stanozolol (70 mg/wk) per os He had used anabolic steroids parenterally ( nandrolone 250mg/wk and stanazolol 350 mg/wk) for several months |
Urine screening, GC-MS Urine screening, GC-MS |
URINE 19-nor-androsterone; 19-nor-etiocholanolone, nor-epiandrosterone (metabolites of Nandrolone) 3-idrossi-stanozolol 3-idrossi-17-epistazonozolol (metabolities of Stanozolol) URINE 19-nor-androsterone; 19-nor-etiocholanolone, nor-epiandrosterone (metabolites of nandrolone) 3-idrossi-stanozolol 3-idrossi-17-epistazonozolol (metabolities of stanozolol) |
| Di Paolo et al (2007) [22] |
4 | 29 M 190cm 127kg 27 M 190cm 100kg 37 F 162cm 71kg 31 M 175cm 79kg |
Loss of consciousness during spin bike lesson Sudden death while in a night club Found dead in her car Found dead in his bedroom |
History of AAS abuse History of AAS abuse History of AAS abuse History of AAS abuse |
Testosterone GC-MS Stanozolol ELISA HPLC/MS Testosterone GC-MS Stanozolol ELISA HPLC/MS Testosterone GC-MS Stanozolol ELISA HPLC/MS Testosterone GC-MS Stanozolol ELISA HPLC/MS |
URINE Negative URINE Stanozolol Testosterone URINE Negative URINE Stanozolol |
| Fineschi et al (2007) [23] |
2 | 29 M 166 cm 72kg 30 M 78 cm 90kg |
Collapsed and died after dinner in his apartment Collapsed and died at home |
He had been taking testosterone, nandrolone e stanozolol parenterally for several years Had taken AAS 6 months before. In a ashtray near the body a 2-ml of nandrolone decanoate was found. The apartment contained a veritable arsenal of drug |
Screening, GC-MS-MS Screening, GC-MS-MS |
URINE Nandrolone (non misurable) Stanozolol 43 µg/l T/E ratio= 28.7 BLOOD Nandrolone 19 ng/ml URINE Norandrosterone 208.4 ng/ml T/E ratio=42 |
| Thiblin et al (2009) [24] |
1 | 29 F 172 cm 76kg | Found dead at home | She had used nine different AASs in various combinations during the previous 8 months. Moreover, ephedrine, tadalafil metandienon, mestanolon stanozolol pills and clenbuterol tablets were found |
Immunological screening, GC-MS | BLOOD Ephedrine 0.4 µg/l and Norephedrine 0.1 µg per g blood URINE Testosterone 31.4 µg/ml; OH-stanozolol 29.3 ng/ml; 16βOH-stanozolol 16.5 ng/ml; Boldenon 2109 ng/ml T/E ratio=28.3 |
| Montisci et al (2012) [25] |
4 | 32 M 180cm 110kg 31 M 172cm 120kg 32 M 178cm 94 kg 25 M 185cm 125kg |
Found dead in his bed Collapsed during training. Heart failure during hospitalization Death after a dentistry visit Death while sleeping |
He had been taking AAS parenterally for 7 years Consumption of AAS (boldenone dromostanolone metanolone enantato stanazolol tranbolone) for several years He had been taking AAS for several years Several drugs (AAS and anorectics) were found in the apartment |
Screening with EMIT-ELISA LC-MS/MS Screening with EMIT-ELISA LC-MS/MS Screening with EMIT-ELISA LC-MS/MS Screening with EMIT-ELISA LC-MS/MS |
Negative HAIR Stanozolol 5.0 pg/ng Negative URINE Testosterone 94 ng/ml Epitestosterone 9 ng/ml Nortestosterone 3500 ng/ml |
Of the 19 cases, 17 (89.5%) were males whereas only 2 (10.5%) were females. The age ranged from 18 to 37 years (mean age: 28 ± 4.4). Among the 19 fatal cases, in 14 bodies
(12 males and 2 females) the data available allowed to calculate the body mass index (BMI) and the results are reported in Table 4. In none of the cases the BMI was lower than 24.9, which is considered the upper limit of the normal healthy weight.
Table 4.
Autoptic, macroscopic and histological findings in 19 AAS related deaths.
| Authors | Age (yrs) | Sex | Height (cm) | Weight (kg) | BMI |
|---|---|---|---|---|---|
| Dickerman et al (1996) [19] | 26 | M | 182 | 136 | 41.1 |
| Fineschi et al (2001) [21] | 32 | M | 189 | 90 | 25.2 |
| 29 | M | 166 | 72 | 26.1 | |
| Di Paolo et al (2007) [22] | 29 | M | 190 | 127 | 35.2 |
| 27 | M | 190 | 100 | 27.7 | |
| 37 | F | 162 | 71 | 27.1 | |
| 31 | M | 175 | 79 | 25.8 | |
| Fineschi et al (2007) [23] | 29 | M | 166 | 72 | 26.1 |
| 30 | M | 178 | 90 | 28.4 | |
| Thiblin et al (2009) [24] | 29 | F | 172 | 76 | 25.7 |
| Montisci et al (2012) [25] | 32 | M | 180 | 110 | 33.9 |
| 31 | M | 172 | 120 | 40.6 | |
| 32 | M | 178 | 94 | 29.7 | |
| 25 | M | 185 | 125 | 36.5 |
BMI = 18.5-24.9, Normal
BMI = 25.0-29.9, Overweight
BMI = 30.0-34.9, Moderately Obese
BMI = 35.0-39.9, Severely Obese
BMI = ≥40, Very Severely Obese
For all cases the autopsy excluded extracardiac causes of death, only in one case a bilateral pulmonary embolism from deep venous thrombus of lower extremities was found (Table 2).
Toxicological investigation performed mainly on urine samples but also in blood and hair samples, by using several screening tests and analytical methods revealed in 12 cases [16, 18, 20-25] the presence of AASs and/or their metabolites in urine specimens; in one case [23] nandrolone was detected in blood, whereas in another case [25] stanozolol was found in hair. In the remaining 6 cases in which the toxicological analysis was negative, circumstantial data and evidences reported by relatives and friends of the deceased highlighted a previous prolonged use of AASs.
DISCUSSION AND CONCLUSIONS
The chronic use of AASs can cause various pathological alterations, which are related to dose, frequency and patterns of use. Taking into account that numerous organs and apparatus are the target of AASs, several adverse effects can involve the liver, cardiovascular, reproductive, musculoskeletal, endocrine, renal, immunologic and hematological systems as well as some psychological effects; a schematic representation is reported in Fig. 4.
Fig. (4).
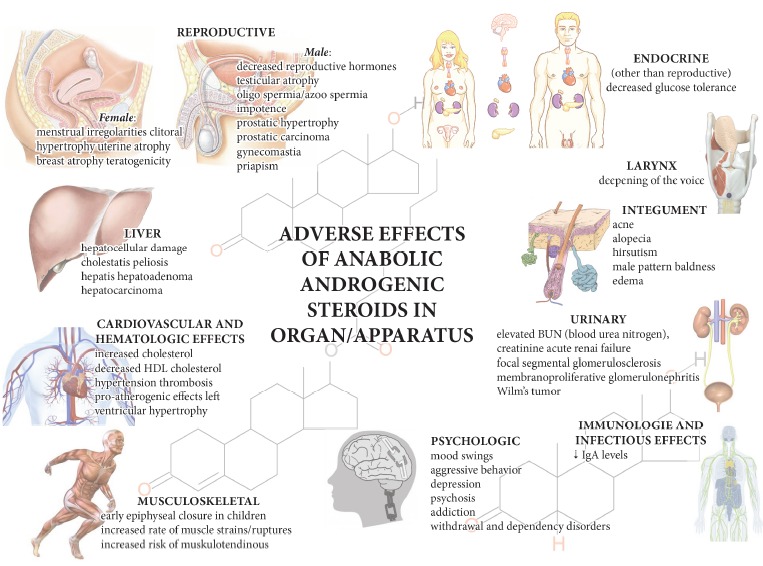
Adverse effects due to AASs which can affect numerous organs and apparatus
Here 19 fatal cases are reported; although only single case report or small series of cases were included, whereas retrospective studies and other papers that did not fulfill the inclusion criteria were not taken into account, some consideration can be formulated; in all cases the autopsy findings together with the histological examination have highlighted cardiac causes of death. Only in one case [19] a mechanical cardiovascular cause of death was found (a bilateral pulmonary embolism from deep venous thrombus of lower extremities). In numerous cases [16, 18, 19, 22, 25], a common finding was a left ventricular hypertrophy, frequently associated with fibrosis and myocytolisis. A myocardial hypertrophy was not found in the 4 cases reported by Fineschi et al in two different reports [21, 23].
What is the significance that could be attributed to the myocardial hypertrophy? A vigorous training in young athletes can determine a left ventricularhypertrophy, independently of the use of AASs (the so called “athlete's heart”) [25-28].
Melchert and Welder [29] categorized the effects of AAS on the cardiovascular system into four groups of activities: vasospastic, atherogenic, thrombotic and direct myocardial injuries. AAS can induce adverse cardiovascular effects such as left ventricular hypertrophy (LVH), hypertension, impaired diastolic filling, arrhythmia, erythrocytosis, thrombosis and altered lipoprotein profiles [30]. Abnormalities in cardio-vascular reflex control of the cardiovascular system [31-35] and in vascular reactivity [36-40] have also been reported.
Studies on isolated hearts from rats treated chronically with nandrolonedecanoate (ND) have also shown a raise in myocardial susceptibility to ischemia/reperfusion injuries [41, 42].
Nandrolone abuse combined with vigorous exercise training may lead to impaired diastolic function and concentric hypertrophy of the left ventricular (LV) wall [43]. Vigorous weight lifting itself would also cause LV wall mass and thickness increase but cardiac function would not be affected. However, when combined with AAS abuse pathological cardiac hypertrophy could be caused [44]. In another study, rats were treated with ND for 6 weeks (total dose 30 mg /kg). ND stimulated cardiomegaly that reversed after the end of treatment [45].
Rocha et al. [46] studied the effects on cardiac function in rats undergoing swimming training and those not undergoing it. They investigated that swimming training combined with high doses of nandrolone (5 mg/kg per injection, equal to 10 mg/kg per week) sharpens cardiac hypertrophy with interstitial fibrosis. Without a doubt these findings explain the high propensity to the onset and continuance of malignant cardiac arrhythmias. An explanation might be the change of the sympathetic autonomic activity modulated by the renin-angiotensin-system (RAS). Experiments have shown that RAS plays a significant role in the development of LVH and myocardial fibrosis.
Angiotensin II type 1 receptor’s (AT1R’s) stimulation is associated with the regulation of cell growth and proliferation of vascular smooth muscle cells, cardiomyocytes and endothelial cells involved in endothelial dysfunction, atherosclerotic vascular phenomena, congestive heart failure and myocardial infarction.
Marques Neto et al. [47] pointed out that the treatment with supraphysiological, chronic doses of AASs induce cardiac parasympathetic disturbances in ventricular depolarization in both exercised and sedentary rats. Unambiguously, it has been shown that the blockage of the RAS, and in particular of AT1R by losartan, obstructs QT prolongation.
Down-regulation of ion channel subunits, KChIP2, Kv1.4 and Kv4.3, could explain the autonomic dysfunction and cardiac repolarization disturbances caused by chronic treatment with supraphysiological doses of ND. Moreover, prolonged QT intervals and ventricular action potential could be explained by the reduced density of the transient outward potassium [48]. No augmentation in tissue collagen content or in the mRNA expression of types I and III collagens have been shown by histological analysis. However, Rocha et al. [46] found an increment in heart collagen content but not in mRNA expression. The previously mentioned unconformity could be attributed to the duration of treatment with ND and the age of rats used. Participation of the potassium (K) current in the generation of prolonged QT and potential action duration has been noticed. Ito is the transient outward K+ current which is one of the main repolarizing currents in the mammalian myocardium and is generally believed to flow through Kv1.4, Kv4.2 and Kv4.3 channels in rats [49, 50]. In heart hypertrophic cases Ito is down-regulated [51-53].
Low Ito density, Kv1.4 and Kv4.3 down-regulation in the left ventricle and prolonged action was noticed in the group treated with nandrolone compared to the control group. Homogenous distribution of Kv4.3 channel in the rat’s ventricular wall, higher Kv4.2 in the epicardial and lower in the endocardial ventricular wall have been observed [49, 50]. These differences may partially explain the up-regulation of Kv4.2 and prolonged QTc interval and action potential. The expression of KChIP2 is considerably decreased in heart failure and hypertrophy [54, 55]. KChIP2 was found to be significant for Ito expression in the human heart and the correlation between KChIP2 absence and a total loss of Ito together with an increased susceptibility to ventricular arrhythmias in mice has been shown [56].
According to Riezzo et al. [57] the following effects were produced in physically trained mice intramuscularly treated with ND: moderate increase of heart weight, morphologically extensive cardiac hypertrophy and a wide colliquative myocytolysis which together could result in a significant heart failure. The increase of the heart weight suggested enhanced heart protein synthesis.
Medei et al. [58] found approximately 25% less nuclei and higher cardiomyocyte nuclei diameter in the ventricles of the group treated with ND. Lower nuclei suggests a toxic effect of ND which may involve a pro-apoptotic mechanism [59].
Tanno et al [60] found that ND treatment whether combined with resistance training or not induced pathological concentric hypertrophy, re-expression of fetal genes, systolic and diastolic function impairment and an incremented myocardial collagen content leading to LVH.
Increased relative left ventricle wall thickness (RWT) was observed as a consequence of intensive physical training in rats treated with ND compared to the respective non-trained ones. In addition, the non-trained nandrolone treated group also produced higher RWT compared to the non-trained treated group. Increased interventricular septum thickness in the end-diastole (IVSDia) was noticed in both the non-trained nandrolone treated and trained vehicle-treated groups, compared to the non-trained vehicle treated rats.
A considerable lower ratio of maximum early to late transmitral flow velocity (E/A ratio) was observed in the trained groups, in comparison with non-trained groups. Nandrolone-treated groups (both trained and non-trained) showed lower E/A ratio in comparison with the respective vehicle-treated groups. Moreover, significant decrease in the expression of alpha-myosin heavy chain (α-MHC) mRNA and beta-myosin heavy chain (β-MHC) mRNA in the left ventricle was induced by nandrolone and resistance training respectively. Penna C. et al. [61] found that short-term ND treatment induces an overexpression of β2-adrenoceptors without cardiac hypertrophy.
Increment in cardiovascular mortality has been associated to an imbalance of (ANS) activity [54].
AASs can acutely inhibit the reuptake of catecholamines into extraneuronal tissue [62] and consequently the increment of catecholamine concentrations at receptor sites occurs. Although, the neuronal catecholamine transporter is normally responsible for the reuptake of noradrenaline, it has also been proved responsible for nonexocytotic release of noradrenaline from sympathetic nerve terminals during ischemia. An increased release of noradrenaline has been implicated in ischemia-induced arrhythmia [63, 64].
Tylicki [65] investigated the short-term effects of ND in rats’ cardiac system. An increased activity of 6-phosphogluconate dehydrogenases and glucose-6-phosphate was observed in rat hearts, also ND activated isocitrate dehydrogenase and malic enzyme, which are other NADP-linked dehydrogenases. During the same study a significantly increased heart weight was also observed 10 days after nandrolone administration. It was shown that treatment with ND causes small QRS complex extension that might slightly reduce the spreading rate of the action potential through the heart ventricles, possibly because of the greater heart mass.
It is known that administration of doses higher than normal (supraphysiological) of ND impair exercise-induced cardioprotection in treadmill-exercised rats. Chaves et al. [66] were the first to say that enhanced levels of antioxidant enzyme levels produced after exercise are impaired with ND treatment (10mg/kg for 8 weeks), a fact which is well correlated to the cardiac injurious effects of the drug. It was observed that the hearts of animals treated with nandrolone and having undergone training (DT group) showed lower glutathione peroxidase (GPx), superoxide dismutase (SOD) and glutathione reductase (GR) activities compared with controls and trained groups of animals (CT). The latter observation indicates that nandrolone could act through blocking or down regulating the mechanisms implicated in the improvement of antioxidant defenses in DT animals, which might explain the lower percentage of left ventricular developed pressure and augmented infarct size in DT group.
It has been shown, in other researches on rats that exercise training improves myocardial resistance to reperfusion injury/ischemia [67-69] since physiological cardiac hypertrophy amends the sensitiveness of that heart making it more resistant to the previously mentioned disorders in vivo rat hearts [70]. Notwithstanding the strenuous research efforts, the molecular mechanism(s) involved in exercise-induced cardio protection is still debatable.
The numerous studies above reported in animal models, especially in rats, have called into question several pathophysiological mechanisms, which may explain some of the macroscopic and microscopic finding regarding the 19 cases here reported; however, we have to underline that these cases are single case report or small series of cases and not experimental studies. Moreover, the users of these substances frequently associate numerous steroids, in different forms, singularly and in several temporal combinations and cycles, and commonly, various steroid-accessory drugs are also used. Therefore, the interpretation of the postmortem findings is particularly difficult and no comprehensive conclusions can be done.
Finally, a brief remark must be placed regarding the BMI which was in all cases (12 males and 2 females) higher than 25. Although according to the BMI ranges from an “overweight” (9 cases) to a “very severely obese” (2 cases) were found, however, the BMI is not a direct measure of body fatness and BMI is calculated from an individual's weight which includes both muscle and fat. As a result, some subjects such as highly trained athletes may have a high BMI because of increased muscularity rather than increased body fatness; therefore, these results should be evaluated with caution.
The relationship between AAS abuse, vigorous exercise training, and cardiac death can be evaluated only by the application of an investigative protocol, which must include a rigorous methodology covering:
A complete autopsy with a special regard to AAS target organs and apparatus (the cardiovascular system in primis).
Histological and immunohistochemical analysis of AAS target organs.
A broad toxicological investigation, preceded by a careful evaluation of clinico-anamnestic data, in order to confirm an AAS consumption (including type of AASs, concentration and interval of exposure) and the possible detection of other substances which could have contributed to the fatal outcome. For this purpose, different matrices can be used; urine is the most common, because it provides a prolonged detection time window, but also several other matrices such as: blood, serum, plasma, hair, oral fluid and nails can be used.
The comparison of the cases reported here, allows us to support the hypothesis that the combined effects of strong workout, the prolonged/chronic or previous abuse of AASs in different forms and combinations, have predisposed these subjects to develop different patterns of myocardial injuries and consequent sudden cardiac death [21, 23].
Therefore, the authors would strengthen the “warning” already expressed in previous reports [21, 23] against the use/abuse of these substances among professional and non-professional athletes. Moreover, only through a careful examination of all suspicious cases of AAS related deaths with the application of a rigorous investigative protocol, these cases can be identified and they could provide further information and data that may increase the knowledge of this type of deaths.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
References
- 1.Yesalis C.E., Bahrke M.S. Anabolic-androgenic steroids. Current issues. Sports Med. 1995;19(5):326–340. doi: 10.2165/00007256-199519050-00003. [DOI] [PubMed] [Google Scholar]
- 2.Evans N.A. Current concepts in anabolic-androgenic steroids. Am. J. Sports Med. 2004;32(2):534–542. doi: 10.1177/0363546503262202. [DOI] [PubMed] [Google Scholar]
- 3.Fragkaki A.G., Angelis Y.S., Koupparis M., Tsantili-Kakoulidou A., Kokotos G., Georgakopoulos C. Structural characteristics of anabolic androgenic steroids contributing to binding to the androgen receptor and to their anabolic and androgenic activities. Applied modifications in the steroidal structure. Steroids. 2009;74(2):172–197. doi: 10.1016/j.steroids.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn C.M. Anabolic steroids. Recent Prog. Horm. Res. 2002;57:411–434. doi: 10.1210/rp.57.1.411. [DOI] [PubMed] [Google Scholar]
- 5.Kadi F., Bonnerud P., Eriksson A., Thornell L.E. The expression of androgen receptors in human neck and limb muscles: effects of training and self-administration of androgenic-anabolic steroids. Histochem. Cell Biol. 2000;113(1):25–29. doi: 10.1007/s004180050003. [DOI] [PubMed] [Google Scholar]
- 6.Sinha-Hikim I., Artaza J., Woodhouse L., Gonzalez-Cadavid N., Singh A.B., Lee M.I., Storer T.W., Casaburi R., Shen R., Bhasin S. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am. J. Physiol. Endocrinol. Metab. 2002;283(1):E154–E164. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- 7.Turillazzi E., Perilli G., Di Paolo M., Neri M., Riezzo I., Fineschi V. Side effects of AAS abuse: an overview. Mini Rev. Med. Chem. 2011;11(5):374–389. doi: 10.2174/138955711795445925. [DOI] [PubMed] [Google Scholar]
- 8.Reardon C.L., Creado S. Drug abuse in athletes. Subst. Abuse Rehabil. 2014;5:95–105. doi: 10.2147/SAR.S53784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breuner C.C. Performance-enhancing substances. Adolesc. Med. State Art Rev. 2014;25(1):113–125. [PubMed] [Google Scholar]
- 10.Evans N.A. Gym and tonic: a profile of 100 male steroid users. Br. J. Sports Med. 1997;31(1):54–58. doi: 10.1136/bjsm.31.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkinson A.B., Evans N.A. Anabolic androgenic steroids: a survey of 500 users. Med. Sci. Sports Exerc. 2006;38(4):644–651. doi: 10.1249/01.mss.0000210194.56834.5d. [DOI] [PubMed] [Google Scholar]
- 12.Dumestre-Toulet V., Cirimele V., Ludes B., Gromb S., Kintz P. Hair analysis of seven bodybuilders for anabolic steroids, ephedrine, and clenbuterol. J. Forensic Sci. 2002;47(1):211–214. [PubMed] [Google Scholar]
- 13.Bertol E., Mari F., Vaiano F., Romano G., Zaami S., Baglìo G., Busardò F.P. Determination of GHB in human hair by HPLC-MS/MS: Development and validation of a method and application to a study group and three possible single exposure cases. Drug Test. Anal. doi: 10.1002/dta.1679. in press. [DOI] [PubMed] [Google Scholar]
- 14.Van Cauter E., Plat L., Scharf M.B., Leproult R., Cespedes S., L’Hermite-Balériaux M., Copinschi G. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men. J. Clin. Invest. 1997;100(3):745–753. doi: 10.1172/JCI119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busardò F.P., Bertol E., Vaiano F., Baglio G., Montana A., Barbera N., Zaami S., Romano G. Post mortem concentrations of endogenous gamma hydroxybutyric acid (GHB) and in vitro formation in stored blood and urine samples. Forensic Sci. Int. 2014;243:144–148. doi: 10.1016/j.forsciint.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Luke J.L., Farb A., Virmani R., Sample R.H. Sudden cardiac death during exercise in a weight lifter using anabolic androgenic steroids: pathological and toxicological findings. J. Forensic Sci. 1990;35(6):1441–1447. [PubMed] [Google Scholar]
- 17.Ferenchick G.S. Anabolic/androgenic steroid abuse and thrombosis: is there a connection? Med. Hypotheses. 1991;35(1):27–31. doi: 10.1016/0306-9877(91)90079-E. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy M.C., Lawrence C. Anabolic steroid abuse and cardiac death. Med. J. Aust. 1993;158(5):346–348. doi: 10.5694/j.1326-5377.1993.tb121797.x. [DOI] [PubMed] [Google Scholar]
- 19.Dickerman R.D., McConathy W.J., Schaller F., Zachariah N.Y. Cardiovascular complications and anabolic steroids. Eur. Heart J. 1996;17(12):1912. doi: 10.1093/oxfordjournals.eurheartj.a014812. [DOI] [PubMed] [Google Scholar]
- 20.Hausmann R., Hammer S., Betz P. Performance enhancing drugs (doping agents) and sudden death--a case report and review of the literature. Int. J. Legal Med. 1998;111(5):261–264. doi: 10.1007/s004140050165. [DOI] [PubMed] [Google Scholar]
- 21.Fineschi V., Baroldi G., Monciotti F., Paglicci R.L. [DOI] [PubMed]; Fineschi V., Baroldi G., Monciotti F., Paglicci Reattelli L., Turillazzi E. Anabolic steroid abuse and cardiac sudden death: a pathologic study. Arch. Pathol. Lab. Med. 2001;125(2):253–255. doi: 10.1043/0003-9985(2001)125<0253:ASAACS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Di Paolo M., Agozzino M., Toni C., Luciani A.B., Molendini L., Scaglione M., Inzani F., Pasotti M., Buzzi F., Arbustini E. Sudden anabolic steroid abuse-related death in athletes. Int. J. Cardiol. 2007;114(1):114–117. doi: 10.1016/j.ijcard.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 23.Fineschi V., Riezzo I., Centini F., Silingardi E., Licata M., Beduschi G., Karch S.B. Sudden cardiac death during anabolic steroid abuse: morphologic and toxicologic findings in two fatal cases of bodybuilders. Int. J. Legal Med. 2007;121(1):48–53. doi: 10.1007/s00414-005-0055-9. [DOI] [PubMed] [Google Scholar]
- 24.Thiblin I., Mobini-Far H., Frisk M. Sudden unexpected death in a female fitness athlete, with a possible connection to the use of anabolic androgenic steroids (AAS) and ephedrine. Forensic Sci. Int. 2009;184(1-3):e7–e11. doi: 10.1016/j.forsciint.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Montisci M., El Mazloum R., Cecchetto G., Terranova C., Ferrara S.D., Thiene G., Basso C. Anabolic androgenic steroids abuse and cardiac death in athletes: morphological and toxicological findings in four fatal cases. Forensic Sci. Int. 2012;217(1-3):e13–e18. doi: 10.1016/j.forsciint.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery H.E., Clarkson P., Dollery C.M., Prasad K., Losi M.A., Hemingway H., Statters D., Jubb M., Girvain M., Varnava A., World M., Deanfield J., Talmud P., McEwan J.R., McKenna W.J., Humphries S. Association of angiotensin-converting enzyme gene I/D polymorphism with change in left ventricular mass in response to physical training. Circulation. 1997;96(3):741–747. doi: 10.1161/01.CIR.96.3.741. [DOI] [PubMed] [Google Scholar]
- 27.Pluim B.M., Zwinderman A.H., van der Laarse A., van der Wall E.E. The athlete’sheart. A meta-analysis of cardiac structure and function. Circulation. 2002;101:336–344. doi: 10.1161/01.CIR.101.3.336. [DOI] [PubMed] [Google Scholar]
- 28.Haykowsky M.J., Dressendorfer R., Taylor D., Mandic S., Humen D. Resistance training and cardiac hypertrophy: unravelling the training effect. Sports Med. 2002;32(13):837–849. doi: 10.2165/00007256-200232130-00003. [DOI] [PubMed] [Google Scholar]
- 29.Melchert R.B., Welder A.A. Cardiovascular effects of androgenic-anabolic steroids. Med. Sci. Sports Exerc. 1995;27(9):1252–1262. doi: 10.1249/00005768-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Kutscher E.C., Lund B.C., Perry P.J. Anabolic steroids: a review for the clinician. Sports Med. 2002;32(5):285–296. doi: 10.2165/00007256-200232050-00001. [DOI] [PubMed] [Google Scholar]
- 31.El-Mas M.M., Afify E.A., Mohy El-Din M.M., Omar A.G., Sharabi F.M. Testosterone facilitates the baroreceptor control of reflex bradycardia: role of cardiac sympathetic and parasympathetic components. J. Cardiovasc. Pharmacol. 2001;38(5):754–763. doi: 10.1097/00005344-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 32.El-Mas M.M., Afify E.A., Omar A.G., Sharabi F.M. Cyclosporine adversely affects baroreflexes via inhibition of testosterone modulation of cardiac vagal control. J. Pharmacol. Exp. Ther. 2002;301(1):346–354. doi: 10.1124/jpet.301.1.346. [DOI] [PubMed] [Google Scholar]
- 33.Beutel A., Bergamaschi C.T., Campos R.R. Effects of chronic anabolic steroid treatment on tonic and reflex cardiovascular control in male rats. J. Steroid Biochem. Mol. Biol. 2005;93(1):43–48. doi: 10.1016/j.jsbmb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Ward G.R., Abdel-Rahman A.A. Effect of testosterone replacement or duration of castration on baroreflex bradycardia in conscious rats. BMC Pharmacol. 2005;5:9. doi: 10.1186/1471-2210-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward G.R., Abdel-Rahman A.A. Orchiectomy or androgen receptor blockade attenuates baroreflex-mediated bradycardia in conscious rats. BMC Pharmacol. 2006;6:2. doi: 10.1186/1471-2210-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sader M. A., Griffiths K. A., Skilton M. R., Wishart S. M., Handelsman D. J., Celermajer D. S. Physiological testosterone replacement and arterial endothelial function in men. 2003. [DOI] [PubMed]
- 37.Gonzales R.J., Krause D.N., Duckles S.P. Testosterone suppresses endothelium-dependent dilation of rat middle cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2004;286(2):H552–H560. doi: 10.1152/ajpheart.00663.2003. [DOI] [PubMed] [Google Scholar]
- 38.Cunha T. S., Moura M. J., Bernardes C. F., Tanno A. P., Marcondes F. K. Vascular sensitivity to phenylephrine in rats submitted to anaerobic training and nandrolone treatment. Hypertension. 2005;46(4):1010–5. doi: 10.1161/01.HYP.0000174600.51515.e7. [DOI] [PubMed] [Google Scholar]
- 39.Lane H.A., Grace F., Smith J.C., Morris K., Cockcroft J., Scanlon M.F., Davies J.S. Impaired vasoreactivity in bodybuilders using androgenic anabolic steroids. Eur. J. Clin. Invest. 2006;36(7):483–488. doi: 10.1111/j.1365-2362.2006.01667.x. [DOI] [PubMed] [Google Scholar]
- 40.Dorsett-Martin W.A., Hester R.L. Sex hormones and aortic wall remodeling in an arteriovenous fistula. Gend. Med. 2007;4(2):157–169. doi: 10.1016/S1550-8579(07)80029-5. [DOI] [PubMed] [Google Scholar]
- 41.Du Toit E.F., Rossouw E., Van Rooyen J., Lochner A. Proposed mechanisms for the anabolic steroid-induced increase in myocardial susceptibility to ischaemia/reperfusion injury. Cardiovasc. J. S. Afr. 2005;16(1):21–28. [PubMed] [Google Scholar]
- 42.Phillis B.D., Abeywardena M.Y., Adams M.J., Kennedy J.A., Irvine R.J. Nandrolone potentiates arrhythmogenic effects of cardiac ischemia in the rat. Toxicol. Sci. 2007;99(2):605–611. doi: 10.1093/toxsci/kfm186. [DOI] [PubMed] [Google Scholar]
- 43.Urhausen A., Hölpes R., Kindermann W. One- and two-dimensional echocardiography in bodybuilders using anabolic steroids. Eur. J. Appl. Physiol. Occup. Physiol. 1989;58(6):633–640. doi: 10.1007/BF00418510. [DOI] [PubMed] [Google Scholar]
- 44.Dickerman R.D., Schaller F., Prather I., McConathy W.J. Sudden cardiac death in a 20-year-old bodybuilder using anabolic steroids. Cardiology. 1995;86(2):172–173. doi: 10.1159/000176867. [DOI] [PubMed] [Google Scholar]
- 45.Pesola M.K. Reversibility of the haemodynamic effects of anabolic steroids in rats. Eur. J. Appl. Physiol. Occup. Physiol. 1988;58(1-2):125–131. doi: 10.1007/BF00636615. [DOI] [PubMed] [Google Scholar]
- 46.Rocha F.L., Carmo E.C., Roque F.R., Hashimoto N.Y., Rossoni L.V., Frimm C., Anéas I., Negrão C.E., Krieger J.E., Oliveira E.M. Anabolic steroids induce cardiac renin-angiotensin system and impair the beneficial effects of aerobic training in rats. Am. J. Physiol. Heart Circ. Physiol. 2007;293(6):H3575–H3583. doi: 10.1152/ajpheart.01251.2006. [DOI] [PubMed] [Google Scholar]
- 47.Marques Neto S.R., da H Silva A., dos Santos M.C., Ferraz E.F., Nascimento J.H. The blockade of angiotensin AT1 and aldosterone receptors protects rats from synthetic androgen-induced cardiac autonomic dysfunction. Acta Physiol. 2013;208(2):166–171. doi: 10.1111/apha.12056. [DOI] [PubMed] [Google Scholar]
- 48.Benjamin I.J., Jalil J.E., Tan L.B., Cho K., Weber K.T., Clark W.A. Isoproterenol-induced myocardial fibrosis in relation to myocyte necrosis. Circ. Res. 1989;65(3):657–670. doi: 10.1161/01.RES.65.3.657. [DOI] [PubMed] [Google Scholar]
- 49.Wickenden A.D., Jegla T.J., Kaprielian R., Backx P.H. Regional contributions of Kv1.4, Kv4.2, and Kv4.3 to transient outward K+ current in rat ventricle. Am. J. Physiol. 1999;276(5 Pt 2):H1599–H1607. doi: 10.1152/ajpheart.1999.276.5.H1599. [DOI] [PubMed] [Google Scholar]
- 50.Casis O., Iriarte M., Gallego M., Sánchez-Chapula J.A. Differences in regional distribution of K+ current densities in rat ventricle. Life Sci. 1998;63(5):391–400. doi: 10.1016/S0024-3205(98)00287-2. [DOI] [PubMed] [Google Scholar]
- 51.Knollmann B.C., Knollmann-Ritschel B.E., Weissman N.J., Jones L.R., Morad M. Remodelling of ionic currents in hypertrophied and failing hearts of transgenic mice overexpressing calsequestrin. J. Physiol. 2000;525(Pt 2):483–498. doi: 10.1111/j.1469-7793.2000.t01-1-00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gidh-Jain M., Huang B., Jain P., el-Sherif N. Differential expression of voltage-gated K+ channel genes in left ventricular remodeled myocardium after experimental myocardial infarction. Circ. Res. 1996;79(4):669–675. doi: 10.1161/01.RES.79.4.669. [DOI] [PubMed] [Google Scholar]
- 53.Kaprielian R., Wickenden A.D., Kassiri Z., Parker T.G., Liu P.P., Backx P.H. Relationship between K+ channel down-regulation and [Ca2+]i in rat ventricular myocytes following myocardial infarction. J. Physiol. 1999;517(Pt 1):229–245. doi: 10.1111/j.1469-7793.1999.0229z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz P.J., La Rovere M.T., Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation. 1992;85(1) Suppl.:I77–I91. [PubMed] [Google Scholar]
- 55.Kobayashi T., Yamada Y., Nagashima M., Seki S., Tsutsuura M., Ito Y., Sakuma I., Hamada H., Abe T., Tohse N. Contribution of KChIP2 to the developmental increase in transient outward current of rat cardiomyocytes. J. Mol. Cell. Cardiol. 2003;35(9):1073–1082. doi: 10.1016/S0022-2828(03)00199-8. [DOI] [PubMed] [Google Scholar]
- 56.Kuo H.C., Cheng C.F., Clark R.B., Lin J.J., Lin J.L., Hoshijima M., Nguyêñ-Trân V.T., Gu Y., Ikeda Y., Chu P.H., Ross J., Giles W.R., Chien K.R. A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell. 2001;107(6):801–813. doi: 10.1016/S0092-8674(01)00588-8. [DOI] [PubMed] [Google Scholar]
- 57.Riezzo I., De Carlo D., Neri M., Nieddu A., Turillazzi E., Fineschi V. Heart disease induced by AAS abuse, using experimental mice/rats models and the role of exercise-induced cardiotoxicity. Mini Rev. Med. Chem. 2011;11(5):409–424. doi: 10.2174/138955711795445862. [DOI] [PubMed] [Google Scholar]
- 58.Medei E., Marocolo M., Rodrigues Dde.C., Arantes P.C., Takiya C.M., Silva J., Rondinelli E., Goldenberg R.C., de Carvalho A.C., Nascimento J.H. Chronic treatment with anabolic steroids induces ventricular repolarization disturbances: cellular, ionic and molecular mechanism. J. Mol. Cell. Cardiol. 2010;49(2):165–175. doi: 10.1016/j.yjmcc.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 59.Fanton L., Belhani D., Vaillant F., Tabib A., Gomez L., Descotes J., Dehina L., Bui-Xuan B., Malicier D., Timour Q. Heart lesions associated with anabolic steroid abuse: comparison of post-mortem findings in athletes and norethandrolone-induced lesions in rabbits. Exp. Toxicol. Pathol. 2009;61(4):317–323. doi: 10.1016/j.etp.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Tanno A.P., das Neves V.J., Rosa K.T., Cunha T.S., Giordano F.C., Calil C.M., Guzzoni V., Fernandes T., de Oliveira E.M., Novaes P.D., Irigoyen M.C., Moura M.J., Marcondes F.K. Nandrolone and resistance training induce heart remodeling: role of fetal genes and implications for cardiac pathophysiology. Life Sci. 2011;89(17-18):631–637. doi: 10.1016/j.lfs.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Penna C., Abbadessa G., Mancardi D., Spaccamiglio A., Racca S., Pagliaro P. 2007. [PubMed]
- 62.Salt P.J. Inhibition of noradrenaline uptake 2 in the isolated rat heart by steroids, clonidine and methoxylated phenylethylamines. Eur. J. Pharmacol. 1972;20(3):329–340. doi: 10.1016/0014-2999(72)90194-X. [DOI] [PubMed] [Google Scholar]
- 63.Du X.J., Dart A.M. Mechanisms of noradrenaline release in the anoxic heart of the rat. Cardiovasc. Res. 1993;27(11):2011–2015. doi: 10.1093/cvr/27.11.2011. [DOI] [PubMed] [Google Scholar]
- 64.Du X.J., Woodcock E.A., Little P.J., Esler M.D., Dart A.M. Protection of neuronal uptake-1 inhibitors in ischemic and anoxic hearts by norepinephrine-dependent and -independent mechanisms. J. Cardiovasc. Pharmacol. 1998;32(4):621–628. doi: 10.1097/00005344-199810000-00015. [DOI] [PubMed] [Google Scholar]
- 65.Tylicki A., Kawalko A., Sokolska J., Strumilo S. Effect of anabolic steroid nandrolone decanoate on the properties of certain enzymes in the heart, liver, and muscle of rats, and their effect on rats’ cardiac electrophysiology. Horm. Metab. Res. 2007;39(4):268–272. doi: 10.1055/s-2007-973094. [DOI] [PubMed] [Google Scholar]
- 66.Chaves E.A., Pereira-Junior P.P., Fortunato R.S., Masuda M.O., de Carvalho A.C., de Carvalho D.P., Oliveira M.F., Nascimento J.H. Nandrolone decanoate impairs exercise-induced cardioprotection: role of antioxidant enzymes. J. Steroid Biochem. Mol. Biol. 2006;99(4-5):223–230. doi: 10.1016/j.jsbmb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Bowles D.K., Farrar R.P., Starnes J.W. Exercise training improves cardiac function after ischemia in the isolated, working rat heart. Am. J. Physiol. 1992;263(3 Pt 2):H804–H809. doi: 10.1152/ajpheart.1992.263.3.H804. [DOI] [PubMed] [Google Scholar]
- 68.Libonati J.R., Gaughan J.P., Hefner C.A., Gow A., Paolone A.M., Houser S.R. Reduced ischemia and reperfusion injury following exercise training. Med. Sci. Sports Exerc. 1997;29(4):509–516. doi: 10.1097/00005768-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 69.Margonato V., Milano G., Allibardi S., Merati G., de Jonge R., Samaja M. Swim training improves myocardial resistance to ischemia in rats. Int. J. Sports Med. 2000;21(3):163–167. doi: 10.1055/s-2000-8876. [DOI] [PubMed] [Google Scholar]
- 70.Ji L.L., Fu R.G., Mitchell E.W., Griffiths M., Waldrop T.G., Swartz H.M. Cardiac hypertrophy alters myocardial response to ischaemia and reperfusion in vivo. Acta Physiol. Scand. 1994;151(3):279–290. doi: 10.1111/j.1748-1716.1994.tb09747.x. [DOI] [PubMed] [Google Scholar]