Abstract
Objective:
Successful repair of defects in the avascular zone of meniscus remains a challenge in orthopedics. This proof of concept study aimed to investigate a guided tissue regeneration approach for treatment of tears in meniscus avascular zone in a goat model.
Design:
Full-depth longitudinal tear was created in the avascular zone of the meniscus and sutured. In the two treatment groups, porcine collagen membrane was wrapped around the tear without (CM) or with injection of expanded autologous chondrocytes (CM+cells), whereas in the control group the tear remained only sutured. Gait recovery was evaluated during the entire follow-up period. On explantation at 3 and 6 months, macroscopic gross inspection assessed healing of tears, degradation of collagen membrane, potential signs of inflammation, and osteoarthritic changes. Microscopic histology scoring criteria were developed to evaluate healing of tears, the cellular response, and the inflammatory response.
Results:
Gait recovery suggested protective effect of collagen membrane and was supported by macroscopical evaluation where improved tear healing was noted in both treated groups. Histology scoring in CM compared to suture group revealed an increase in tear margins contact, newly formed connective tissue between margins, and cell formations surrounded with new matrix after 3 months yet not maintained after 6 months. In contrast, in the CM+cells group these features were observed after 3 and 6 months.
Conclusions:
A transient, short-term guided tissue regeneration of avascular meniscal tears occurred upon application of collagen membrane, whereas addition of expanded autologous chondrocytes supported more sustainable longer term tear healing.
Keywords: animal models, meniscus, biomaterials, chondrocytes, histology scoring system
Introduction
Meniscus is a fibrocartilagenous tissue that functions to transmit load, absorb shock, and stabilize the knee joint. Meniscal injuries—including tears and tissue loss—are frequently diagnosed in orthopedics and lead to pain, joint dysfunction, and cartilage degeneration.1-3 The peripheral, vascularized zone of the meniscus can regenerate spontaneously and the defects within that area are successfully repaired.4,5 Unfortunately, the majority of meniscal defects occur in the inner, avascular zone, where the lack of blood supply limits the healing process.6,7 In the past, avascular defects were treated by total or partial meniscectomy mainly to alleviate the pain and discomfort. As the amount of resected meniscal tissue has been correlated to the occurrence of osteoarthritis,3,8 current treatments aim to maximally preserve the meniscus tissue.7,9,10
In the past years, tissue engineering approaches—combining biomaterials, cells, and growth factors—have been investigated for the treatment of meniscus defects in the avascular zone.11-15 Different natural and synthetic biomaterials were employed for meniscal repair in animal models including hydrogels in rabbit and sheep16,17; polycaprolacton-polyurethane, subintestinal submucosa, and polyurethane in dogs18-20; and HYAFF/polycaprolacton in sheep.21,22 Combinations of cells seeded on biomaterials, grown in vitro and subsequently implanted in different animal models, were also investigated.23-26 However, the reliably successful treatment is still unavailable.
Guided tissue regeneration has been successfully used to regenerate various tissues, including bone and nerve. In the present study, we investigated whether this approach would prove successful for the treatment of meniscus tears in the avascular zone in a goat model. Our study included three groups: (a) control group, where the tear in the avascular zone was only sutured; (b) a treatment group CM (collagen membrane), where the collagen membrane was “wrapped” around the sutured tear; and (c) a treatment group CM+cells, where the expanded autologous chondrocytes were injected underneath the “wrapped” membrane. We hypothesized that collagen membrane improves regeneration over simple suturing and that the addition of chondrocytes—cells with chondrogenic potential, resembling fibrochondrocytes (meniscus cells)—would further facilitate the healing process.
Material and Methods
Study Design
All procedures were approved by the local ethical committee for animal studies (Canton Bern, Approval Number 78-05). The study consisted of two treatment groups and a control group. A horizontal tear was created in the avascular zone of the medial meniscus in 36 skeletally mature goats (55 ± 3kg). Two treatment approaches comprised single suture of the tear followed by either wrapping of a Chondro-Gide derivative cross-linked collagen I/III membrane around the tear (CM group, n = 12), or wrapping a collagen membrane with additional injection of expanded autologous articular chondrocytes (CM+cells group, n = 12). In a control group (n = 12), tears were only sutured as performed in human clinical practice for similar indications. Goats were sacrificed 3 and 6 months after surgeries.
Surgical Procedure and Clinical Follow-Up
Surgery was performed under general anesthesia. A full-depth 6-mm longitudinal tear was created in the avascular zone of the medial anterior meniscus in a controlled fashion (blade no. 15). In all groups a single vertical suture was introduced according to the inside-out technique27 (Fig. 1A). In the control group, no additional interventions were performed. In the CM group, a collagen membrane was wrapped—with the cells’ porous side toward the meniscus surface—and sutured to completely cover the tear (Fig. 1B). In the second treatment group, 15 million autologous chondrocytes were injected into the tear upon wrapping and securing the collagen membrane (Fig. 1C). Fibrin glue (Tissucol Duo S, Baxter, Volketswil, Switzerland) was used to seal the membrane as performed in the ACI technique for cartilage repair. Autologous chondrocytes were isolated from a biopsy taken 3 weeks prior surgeries as previously described,28 plated at the density of 10,000 cells/cm2 in DMEM/F-12/10% fetal bovine serum (Biosera, East Sussex, United Kingdom), 100 U/mL penicillin + 100 µg/mL streptomycin, and 50 µg/mL ascorbic acid, and passaged four times at 80% confluence. All goats received full-limb cast for 4 weeks. Upon cast removal the level of lameness was scored at monthly intervals throughout the follow-up period using an adapted scoring system.29
Figure 1.
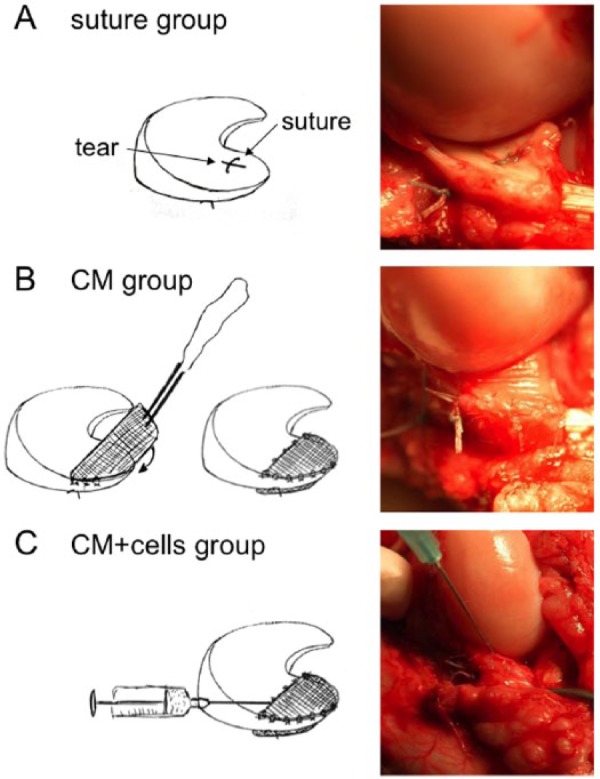
Treatments of meniscal tears. In the control group (A), a single horizontal suture was applied to hold tear margins in tight contact. In the CM group (B), upon suturing, the collagen membrane was wrapped around the meniscus and secured on both surfaces. In the CM+cells group (C), upon suturing the tear and securing the collagen membrane, expanded autologous chondrocytes were injected into the tear and under the collagen membrane.
Gross Inspection
After sacrifice, knee joints were opened and the following parameters were evaluated: evidence of tear healing from both meniscal sides, presence and degree of collagen membrane degradation and tear coverage, and synovial membrane inflammation based on tissue color changes, increased vascularity, and swelling. Tear repair and cartilage degeneration was evaluated upon application of Indian ink. Signs of osteoarthritis (OA) were assessed on femoral condyles and tibial plateau using the modified Outerbridge score30: Grade 0 = normal cartilage; Grade 1 = softening; Grade 2 = superficial fibrillation; Grade 3 = deep fibrillation (fissuring); Grade 4 = erosion (size <4 mm); Grade 5 = erosion (size 4-10 mm); Grade 6 = erosion (size >10 mm).
Histology
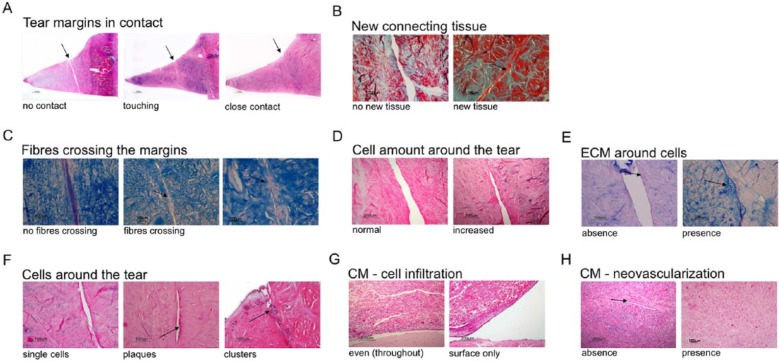
Menisci were fixed in 4% formaldehyde and embedded in paraffin. The tears were sampled at five levels, each 500 µm apart (Fig. 2), and 3 µm thin sequential histology sections from each level were stained with (a) hematoxylin and eosin (H&E) for general cell and tissue morphology, (b) Masson Trichrome for detection of collagens, and (c) Alcian Blue for proteoglycan content. Each slide was subdivided into four microscopic zones/sectors and graded independently by three blinded scientists. Grading was based on the presence or absence of the designated parameter. The following three groups of criteria were analyzed (Fig. 3): (1) criteria assessing healing: (A) tear margins in contact, (B) presence of newly formed connecting tissue inside the tear, (C) presence of collagens fibers crossing the defect; (2) criteria evaluating cellular response: (D) an increase in cell amount around the tear margins compared to the surrounding meniscus tissue, (E) presence of extracellular matrix (proteoglycans) around cells close to tear margins, (F) cell organizations around the tear defined as either single cells, or “groups of cells” defined as cells aligned along the defect (plaques) or cells forming clusters; (3) criteria indicative of inflammatory response, that is, a cellular response to the collagen membrane): (G) cellular infiltration and (H) vascularization. Only five samples were evaluated in the control group—6 months—and treatment group CM+cells—3 months—due to artefacts during histology processing.
Figure 2.
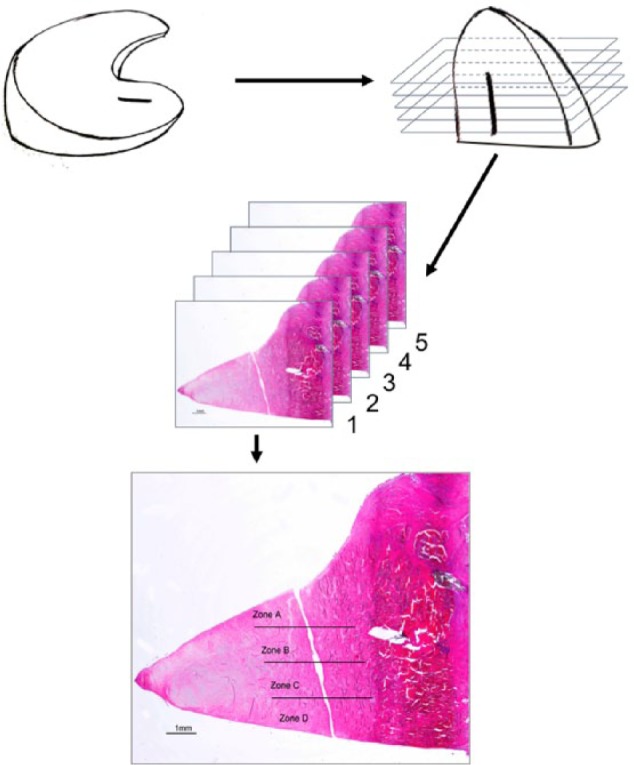
Histology evaluation approach. Samples were cut in five serial sections, each section was subdivided into four microscopic zone/sectors (A-D) and graded by three blinded scientists.
Figure 3.
Histology scoring approach. Parameter “tear margins in contact” (A) distinguished between “no contact,” “touching,” and “close contact,” where “touching” and “close contact” were considered as indicative of healing (H&E); parameter “new connecting tissue” (B) evaluated the absence or presence of new tissue (Masson Trichrome); parameter “fibers crossing the tear” assessed absence or presence of fibers bridging the two tear margins (Alcian blue); parameter “cell amount around the tear” (D) evaluated no increase (normal) or increase in cell numbers around the tear compared to the surrounding meniscus tissue (H&E); parameter “ECM around cells” (E) evaluated the absence or presence of extracellular matrix proteoglycans produced by cells (Alcian blue); parameter “cell organizations around the tear” (F) distinguished between single cells and groups of cells consisting of clusters and/or plaques (H&E); parameter “CM cell infiltration” (G) assessed cellular infiltration of either only the surface or throughout (even) the entire collagen membrane (H&E); parameter “CM neovascularization” (H) evaluated the absence or presence of blood vessels (indicated with an arrow) within collagen membrane (H&E stain). H&E = hemotoxylin–eosin.
Statistical Analysis
Values for lameness and macroscopic OA evaluation of femoral condyles and tibial plateau are reported as mean ± SEM. A two-tailed, unpaired Student’s t-test was used to determine the significance of changes between control (suture) and treatment groups. P values < 0.05 were considered statistically significant. Data for the analysis of histology criteria were submitted to a nonparametric multiway crosstabs analysis to test associations and crossover relationships between variables controlling for treatment, time, cut, and area/sector. Post hoc analysis using the Pearson chi-square statistic, likelihood ratio, and odds ratio was used to test significance at P < 0.05.
Results
Clinical Evaluation
All goats could stand on the operated leg immediately after surgery. Signs of stiffness or inflammation were not detected. All goats recovered a nearly normal gait within 3 months postoperatively. In both treatment groups the gait further improved until 6 months, with significant difference in the CM+cells group compared to the control group after 5 and 6 months (Table 1, Fig. 4).
Table 1.
Gait Recovery During the Follow-Up Period.
| Treatment Group | 1 Month | 2 Months | 3 Months | 4 Months | 5 Months | 6 Months |
|---|---|---|---|---|---|---|
| Suture | 3.7 ± 0.3 | 1.6 ± 0.3 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| CM | 3.3 ± 0.2 | 1.4 ± 0.2 | 1.0 ± 0.1 | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 |
| CM+cells | 3.1 ± 0.2 | 1.3 ± 0.2 | 0.8 ± 0.1 | 0.3 ± 0.2 | 0.2 ± 0.2* | 0.2 ± 0.2* |
The degree of lameness was scored from 5 for bad lameness to 0 for full weight bearing. Data are shown as mean ± SEM; n = 6 per group. Statistical significance is indicated with an asterisk.
Figure 4.
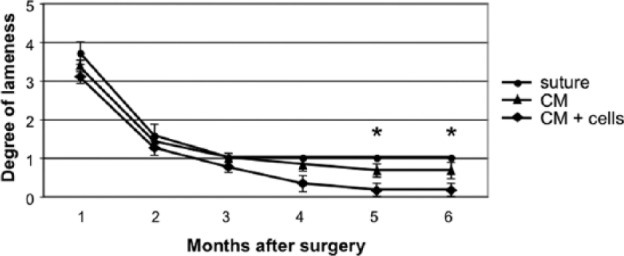
Gait recovery during the follow-up period. The degree of lameness was scored from 5 for bad lameness to 0 for full weight bearing. Data are shown as mean ± SEM. Significant difference between groups is indicated with an asterisk.
Macroscopic Analysis
Gross inspection of synovial membranes revealed minor synovitis in the CM+cells group at 6 months, without any clinical relevance. The evaluation of cartilage surface after 3 months revealed the highest OA score in femoral condyles in the CM group (2.7) (Table 2). In tibial plateau, the highest OA score after 3 months was noted in the CM group (2.7) and the CM+cells group (3.2), significantly different to the control group (1.8). However, the scores between the CM and CM+cells groups were similar to suture after 6 months. Overall, only minor development of OA was observed.
Table 2.
Evaluation of Osteoarthritis on Femoral Condyles and Tibial Plateau Based on the Modified Outerbridge Score.
| Femoral Condyle |
Tibial Plateau |
|||
|---|---|---|---|---|
| Treatment Group | 3 Months | 6 Months | 3 Months | 6 Months |
| Control | 1.7 ± 1.1 | 2.3 ± 0.9 | 1.8 ± 0.2 | 2.0 ± 0.0 |
| CM | 2.7 ± 1.2 | 2.2 ± 0.3 | 2.7 ± 1.2* | 1.7 ± 0.2 |
| CM+cells | 0.2 ± 0.2 | 1.2 ± 0.2 | 3.2 ± 1.3* | 2.3 ± 0.6 |
Data are presented as mean ± SEM; n = 6 for each group. Statistical significance is indicated with an asterisk.
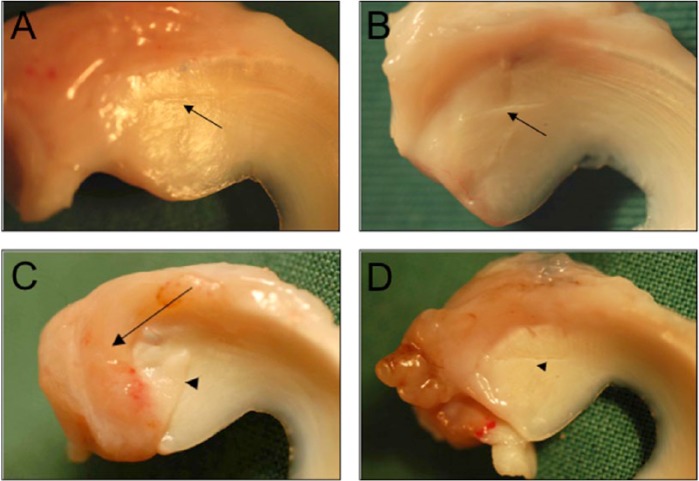
Gross inspection of the tear status—on both proximal and distal meniscus sides—comprised assessment of the contact between tear margins and the presence/absence of the collagen membrane reflecting tear coverage or exposure (Fig. 5A, B; Table 3). Contact between tear margins was observed in 4/6 of the goats in suture and CM groups and in 5/6 goats in CM+cells group at 3 months. However, in contrast to the suture group, where the tear margins’ contact decreased to 1/6 goat after 6 months, it remained stable (4/6 of the goats) in the CM and CM+cells groups. Remnants of the collagen membrane were found in all CM and CM+cells treated goats, attached to the cranial and peripheral meniscus edges, and integrated in the surrounding tissue. Depending on the level of collagen membrane degradation, tears were partially or completely exposed (Fig. 5C, D; Table 3). In CM group, tear exposure slightly increased from 3 to 6 months, whereas tear exposure in 4/6 of the goats in the CM+cells group after 3 months further increased to tear exposure in all goats at 6 months.
Figure 5.
Macroscopic evaluation of tear appearance and tear coverage with collagen membrane. Tears in contact were considered as either healed, with visible connecting tissue between the margins (A), or not healed, with a visible gap between the margins (B), indicated with arrowheads. The partial or complete degradation of collagen membrane allowed for partial tear coverage (C), indicated with an arrow, or complete tear exposure (D).
Table 3.
Macroscopical Evaluation of the Tear Status.
| Suture |
CM |
CM+Cells |
||||
|---|---|---|---|---|---|---|
| Tear Status | 3 Months | 6 Months | 3 Months | 6 Months | 3 Months | 6 Months |
| Contact | 4/6 | 1/6 | 4/6 | 4/6 | 5/6 | 4/6 |
| No contact | 2/6 | 5/6 | 2/6 | 2/6 | 1/6 | 2/6 |
| Partially covered | na | na | 3/6 | 2/6 | 2/6 | 0/6 |
| Exposed | na | na | 3/6 | 4/6 | 4/6 | 6/6 |
Contact between tear margins and the tear exposure associated with the tear coverage by the collagen membrane were assessed. na = not applicable.
Microscopic Analysis: Histology Evaluation
Histology analysis confirmed the location of tears within the avascular zone in all samples. The scoring results were first examined for four independent variables: time, treatment, five consecutive cuts/sections (assessing longitudinal healing along the tear), and four zones/sectors (assessing healing within each cut/section (A, B, C, D; see Fig. 2). Given that five consecutive cuts showed the same results for different treatments over time, this variable was not taken for further analysis. In contrast, different sectors—reflecting depth of the tear from surface to bottom—showed significant differences for several criteria in relation to treatments and time, and were therefore analyzed separately (Fig. 6). The criteria were grouped into categories reflecting (1) healing (margins in contact, new connective tissue in tears, and fibers crossing the tear margins; Fig. 6A), (2) cellular response (amount of cells around the tear, ECM around cells, and cell formations; Fig. 6B), and (3) inflammatory response (cellular infiltration of the collagen membrane; Fig. 6C). Neovascularization within CM did not show any differences for either time or treatment and was not analyzed further.
Figure 6.
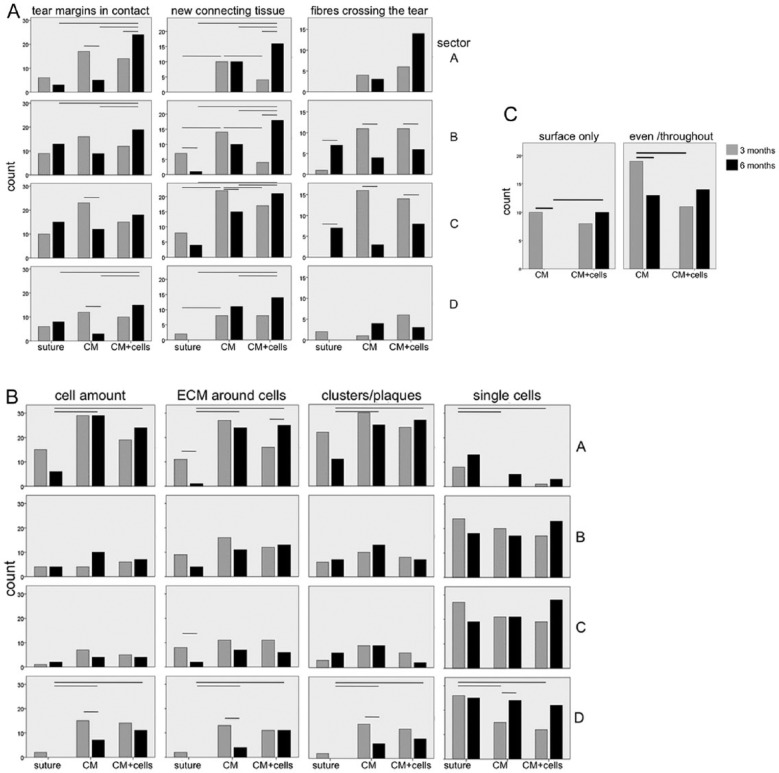
Analysis of histology evaluation criteria. The criteria were grouped into categories reflecting: (A) healing—comprising tear margins in contact, new connecting tissue in tears, and fibers crossing the tear margins; (B) cellular response—comprising amount of cells around the tear, ECM around cells, and cell formations, that is, clusters/plaques or single cells; and (C) inflammatory response—comprising cellular infiltration of the collagen membrane. Graphs for each evaluation parameter are presented per sector (tear depth from surface to bottom A, B, C, D). The number of events (counts) on the y axis of the assessed criterion per treatment group at 3 and 6 months (x axis) is indicated. Statistically significant differences are outlined above the corresponding columns (P < 0.05).
Histology results investigating healing indicate that at 3 months, contact of tear margins was mainly noted in the CM group and found significantly higher compared to 6 months in sectors A, C, and D. At 6 months, most pronounced contact of tear margins was observed in the CM+cells group, significantly higher to the same group at 3 months in sector A, and significantly higher in sectors A, B, and D in comparison to control (suture) and CM groups. Similarly, at 3 months new connecting tissue was mainly observed in the CM group (significantly higher to other groups in sectors A, B, and C). At 6 months most connecting tissue was found in CM+cells, compared to 3 months (significantly higher in sectors A and B) and CM and control groups (significant in all sectors). At 3 months fibers crossing the tear were observed in CM and CM+cells groups (significantly different compared to 6 months in sectors B and C), with only few detected in the suture group. At 6 months most fibers crossing was noted in the CM+cells group in sector A (albeit not significant) whereas the numbers increased in suture group at 6 months compared to 3 months (significant in sectors B and C). Overall, both treatment groups showed better results at 3 months for tear margins in contact and new connective tissue compared to suture, whereas the CM+cells group showed better results after 6 months compared to the other groups in all sectors. While the same result was obtained for fibers crossing in sector A, most fibers bridging the tear margins were observed in the CM and CM+cells groups after 3 months compared to 6 months in sectors B and C.
The results investigating the cellular response revealed similar patterns for cell amount, presence of ECM around cells, and formation of clusters and plaques around the tear. At 3 months similar cell amounts were found in the CM group in sector A compared to 6 months, whereas higher cell amount was found in sector D. At 6 months higher cell amount was observed in the CM and CM+cells groups compared to suture (significant in sectors A and D). The highest amount of cells was noted in sector A in all groups at both time points. Similar results were obtained for ECM presence around the cells: at 3 months in suture and CM groups more matrix deposition was observed compared to 6 months (significant in sectors A and C for suture, and D for CM). At 6 months more ECM around cells was seen in CM+cells compared to both other treatments (significant in sector A). Complementary observations were also seen for cell formations (clusters/plaques), with more groups of cells after 6 months in CM and CM+cells groups compared to suture (significant in sectors A and D). Single cells were mainly observed in suture at 3 months (significant in sectors A and D to both other groups). At 6 months, more single cells were seen in CM and CM+cells (significant for CM in sector D). Overall, cellular response was mainly observed in sector A, particularly in CM after 3 months and in CM+cells after 6 months.
Analysis of superficial (surface only) or deep (even/throughout) cellular infiltration of the CM membrane indicated that there was significantly more deep cellular infiltration in the CM group at 3 months compared to 6 months and compared to CM+cells after 3 months. There was more surface cellular infiltration in CM+cells after 6 months, whereas the even cellular infiltration remained unchanged from 3 to 6 months. The lack of any difference for neovascularization for any time point or treatment indicated that blood vessels formation was not affected by any treatment.
Discussion
Untreated injuries of the meniscus progressively destabilize the knee articulation and ultimately lead to degenerative osteoarthritic changes; meniscus preservation and regeneration remains therefore essential to maintain the functional integrity of the knee joint.9,10 In this study, we investigated whether application of the guided tissue regeneration approach alone or in combination with autologous chondrocytes would enhance healing of a tear introduced in the avascular portion of the meniscus in a goat model. Our macroscopical and histology results indicate a transient healing process of the tears upon sole application of the collagen membrane, pronounced after 3 months but not sustained after 6 months. In contrast, combination of the collagen membrane with cells allows for sustained tear healing after 6 months. Both treatment groups showed better results compared to the control (suture) group.
Regeneration of injuries in the vascularized portion of the meniscus occurs via typical wound healing where the wound hematoma (fibrin clot) acts as a scaffold for cellular ingrowth as well as a source of chemotactic and mitogenic stimuli.31 Sutures, meniscal arrows, fibrin sealants, and laser welding were shown to promote healing in the vascular zone. In contrast, injuries in the avascular zone heal poorly and still represent a challenge in knee surgery. First attempts to repair injuries located in the avascular zone relied on vascular induction, where fibrin clot formation represented the first “scaffold” and provided a cytokine-rich milieu to assist the reparative processes.32 An alternative repair technique included the transplantation of a vascularized synovial flap.33-35 Because of unsatisfactory results, particularly in complex tear cases, other approaches have been developed and employed either as a scaffold/implant alone or combined with cells.11,13,14 Our approach comprised guided tissue regeneration by wrapping of the membrane around the tear located in the avascular zone, use of cross-linked collagen membrane due to proven prolongation of the degradation time on collagen fiber cross-linking,36 and injection of autologous chondrocytes due to their described similarities to meniscus fibrochondrocytes,37 extensive characteri-zation,28 and approved clinical application in cartilage repair.38,39
All goats treated with collagen membrane recovered normal gait faster compared to the control group, suggesting a protective effect of the collagen membrane. Gross evaluation also revealed better healing in all CM groups, further supporting the beneficial effect of the collagen membrane, possibly through mechanical protection and creation of a favorable secluded microenvironment. However, CM+cells treatment resulted in collagen membrane degradation and tear exposure in all goats after 6 months, suggesting that injected chondrocytes could have contributed to the membrane degradation process. Osteoarthritic changes were overall minor, more pronounced on the tibial plateau and different only in the CM+cells group at 3 months compared to CM and suture. The long-term effect of joint protection, that is, prevention of an OA development has to be addressed in a longer follow-up study.
Suturing the tear was insufficient to promote sustainable healing, in agreement with previous data.40-42 Less efficient healing as well as lower cellular response in comparison to either CM group was particularly obvious in the upper part of the meniscus (sector A) and could be possibly explained by the direct tear exposure to mechanical impact and/or friction forces. An increase in fibers crossing of the tear margins in the inner part after 6 months could indicate an intrinsic attempt toward healing.
Compared to suturing only, wrapping the tear with collagen membrane improved the healing process and cellular response after 3 months but neither was maintained after 6 months. These results indicate guided tissue regenerative albeit transient effect of the collagen membrane. For avascular defects, the anatomical distance between the tear and synovium could represent a limiting factor. The high number of fibroblast-like cells observed within the remnants of the collagen membrane—mainly after 3 months—could indicate that the membrane stimulated intrinsic cellular response of residing fibrochondrocytes, progenitor cells, and synovial fibroblasts resulting in cell migration, proliferation, and differentiation. An increased number of cells was observed around the tear margins in all CM groups, forming cell clusters and/or plaques concomitant with increased ECM production. Although clusters represent a hallmark of osteoarthritic cartilage, recent studies suggest their presence during cartilage repair process,43,44 and a similar mechanism could be envisioned during the meniscus healing process. Recently identified CD34+ meniscus cells, considered as progenitor cells, residing in the meniscus superficial zone could also contribute to the healing process.45 Finally, mesenchymal cells present in different tissues within the joint have also been hypothesized to migrate into the meniscal lesion, proliferate, and synthesize matrix components.1,31,46,47
Compared to wrapping the membrane only, the additional injection of expanded autologous chondrocytes under the collagen membrane improved healing of tears after 6 months, in accordance with previously demonstrated contribution of cells for meniscus repair in preclinical studies.48 However, cellular response was not more pronounced in the CM+cells group compared to the CM group after 6 months, suggesting that externally injected cells did not further contribute to cellular activity. The contribution of chondrocytes in synthesizing neo-fibrocartilaginous matrix has been previously demonstrated in a study where devitalized meniscal scaffolds, preseeded with articular chondrocytes, were sutured into avascular lesions of pigs.24 In our study, chondrocytes could have contributed to the healing process either directly or as trophic mediators. Given that an increase in cellular response (formation of clusters and plaques) was noted in all groups—albeit more prominently in CM and CM+cells groups—it appears that the applied chondrocytes acted as trophic mediators, releasing growth factors, cytokines, and chemotactic molecules, as previously described for mesenchymal stem cells.49,50
A recent clinical study on 30 patients where a collagen membrane Chondro-Gide was applied with a similar “wrapping” technique on different tear types in the meniscus demonstrated an improvement upon a follow-up of an average of 2.5 years.51 These observations are consistent with a recent literature review describing differences of meniscal treatment results in preclinical studies and clinical studies: in animals, addition of cells leads to better results, whereas in patients sole application of scaffolds results in healing.48
In summary, after 3 months the tissue guided regeneration—enhanced by wrapping of a collagen membrane—demonstrated an improved healing of tears in the meniscal avascular zone in comparison to suture. However, an additional injection of autologous chondrocytes proved necessary for the maintenance of the repaired new tissue after 6 months.
Footnotes
Acknowledgments and Funding: We would like to thank the Surgical Research Unit, Department of Clinical Research and Clinic for Large Animals, University of Bern, for their invaluable assistance during surgeries; Verena Winkelmann for preparation of histology; Kathryn Stok for statistical analysis; and José Diaz-Romero for valuable comments during manuscript preparation. The study was supported by the Commission for Technology and Innovation (CTI), Bern, Switzerland (Grant No. 7630.1).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BS is an employee of Geistlich Pharma GA.
Ethical Approval: All procedures were approved by the local ethical committee for animal studies (Canton Bern, Approval Number 78-05).
References
- 1. Koski JA, Ibarra C, Rodeo SA, Warren RF. Meniscal injury and repair: clinical status. Orthop Clin North Am. 2000;31(3):419-36. [DOI] [PubMed] [Google Scholar]
- 2. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-69. [DOI] [PubMed] [Google Scholar]
- 3. McDermott ID, Amis AA. The consequences of meniscectomy. J Bone Joint Surg Br. 2006;88(12):1549-56. [DOI] [PubMed] [Google Scholar]
- 4. Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10(2):90-5. [DOI] [PubMed] [Google Scholar]
- 5. Cooper DE, Arnoczky SP, Warren RF. Meniscal repair. Clin Sports Med. 1991;10(3):529-48. [PubMed] [Google Scholar]
- 6. Miller DW, Warner JJP, Harner CD. Meniscal repair. In: Fu FH, Harner CD, Vince KG, editors. Knee repair. Philadelphia: Lippincott Williams & Wilkins; 1994. [Google Scholar]
- 7. Starke C, Kopf S, Petersen W, Becker R. Meniscal repair. Arthroscopy. 2009;25(9):1033-44. [DOI] [PubMed] [Google Scholar]
- 8. Petty CA, Lubowitz JH. Does arthroscopic partial meniscectomy result in knee osteoarthritis? A systematic review with a minimum of 8 years’ follow-up. Arthroscopy. 2011;27(3):419-24. [DOI] [PubMed] [Google Scholar]
- 9. Arnold MP, Hirschmann MT, Verdonk PC. See the whole picture: knee preserving therapy needs more than surface repair. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):195-6. [DOI] [PubMed] [Google Scholar]
- 10. Cook JL. The current status of treatment for large meniscal defects. Clin Orthop Relat Res. 2005;(435):88-95. [DOI] [PubMed] [Google Scholar]
- 11. Anz AW, Rodkey WG. Biological enhancement of meniscus repair and replacement. Sports Med Arthrosc. 2012;20(2):115-20. [DOI] [PubMed] [Google Scholar]
- 12. Buma P, van Tienen T, Veth R. The collagen meniscus implant. Expert Rev Med Devices. 2007;4(4):507-16. [DOI] [PubMed] [Google Scholar]
- 13. Schoenfeld AJ, Landis WJ, Kay DB. Tissue-engineered meniscal constructs. Am J Orthop (Belle Mead NJ). 2007;36(11):614-20. [PubMed] [Google Scholar]
- 14. Scotti C, Hirschmann MT, Antinolfi P, Martin I, Peretti GM. Meniscus repair and regeneration: review on current methods and research potential. Eur Cell Mater. 2013;26:150-70. [DOI] [PubMed] [Google Scholar]
- 15. Sweigart MA, Athanasiou KA. Toward tissue engineering of the knee meniscus. Tissue Eng. 2001;7(2):111-29. [DOI] [PubMed] [Google Scholar]
- 16. Kelly BT, Robertson W, Potter HG, Deng XH, Turner AS, Lyman A, et al. Hydrogel meniscal replacement in the sheep knee: preliminary evaluation of chondroprotective effects. Am J Sports Med. 2007;35(1):43-52. [DOI] [PubMed] [Google Scholar]
- 17. Kobayashi M, Chang YS, Oka M. A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials. 2005;26(16):3243-8. [DOI] [PubMed] [Google Scholar]
- 18. Cook JL, Fox DB, Malaviya P, Tomlinson JL, Farr J, Kuroki K, et al. Evaluation of small intestinal submucosa grafts for meniscal regeneration in a clinically relevant posterior meniscectomy model in dogs. J Knee Surg. 2006;19(3):159-67. [DOI] [PubMed] [Google Scholar]
- 19. Heijkants RG, van Calck RV, De Groot JH, Pennings AJ, Schouten AJ, van Tienen TG, et al. Design, synthesis and properties of a degradable polyurethane scaffold for meniscus regeneration. J Mater Sci Mater Med. 2004;15(4):423-7. [DOI] [PubMed] [Google Scholar]
- 20. Welsing RT, van Tienen TG, Ramrattan N, Heijkants R, Schouten AJ, Veth RP, et al. Effect on tissue differentiation and articular cartilage degradation of a polymer meniscus implant: a 2-year follow-up study in dogs. Am J Sports Med. 2008;36(10):1978-89. [DOI] [PubMed] [Google Scholar]
- 21. Chiari C, Koller U, Dorotka R, Eder C, Plasenzotti R, Lang S, et al. A tissue engineering approach to meniscus regeneration in a sheep model. Osteoarthritis Cartilage. 2006;14(10):1056-65. [DOI] [PubMed] [Google Scholar]
- 22. Kon E, Chiari C, Marcacci M, Delcogliano M, Salter DM, Martin I, et al. Tissue engineering for total meniscal substitution: animal study in sheep model. Tissue Eng Part A. 2008;14(6):1067-80. [DOI] [PubMed] [Google Scholar]
- 23. Martinek V, Ueblacker P, Braun K, Nitschke S, Mannhardt R, Specht K, et al. Second generation of meniscus transplantation: in-vivo study with tissue engineered meniscus replacement. Arch Orthop Trauma Surg. 2006;126(4):228-34. [DOI] [PubMed] [Google Scholar]
- 24. Peretti GM, Gill TJ, Xu JW, Randolph MA, Morse KR, Zaleske DJ. Cell-based therapy for meniscal repair: a large animal study. Am J Sports Med. 2004;32(1):146-58. [DOI] [PubMed] [Google Scholar]
- 25. Weinand C, Peretti GM, Adams SB, Jr, Bonassar LJ, Randolph MA, Gill TJ. An allogenic cell-based implant for meniscal lesions. Am J Sports Med. 2006;34(11):1779-89. [DOI] [PubMed] [Google Scholar]
- 26. Zellner J, Mueller M, Berner A, Dienstknecht T, Kujat R, Nerlich M, et al. Role of mesenchymal stem cells in tissue engineering of meniscus. J Biomed Mater Res A. 2010;94(4):1150-61. [DOI] [PubMed] [Google Scholar]
- 27. Rubman MH, Noyes FR, Barber-Westin SD. Arthroscopic repair of meniscal tears that extend into the avascular zone. A review of 198 single and complex tears. Am J Sports Med. 1998;26(1):87-95. [DOI] [PubMed] [Google Scholar]
- 28. Diaz-Romero J, Gaillard JP, Grogan SP, Nesic D, Trub T, Mainil-Varlet P. Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol. 2005;202(3):731-42. [DOI] [PubMed] [Google Scholar]
- 29. Erkert RS, MacAllister CG, Payton ME, Clarke CR. Use of force plate analysis to compare the analgesic effects of intravenous administration of phenylbutazone and flunixin meglumine in horses with navicular syndrome. Am J Vet Res. 2005;66(2):284-8. [DOI] [PubMed] [Google Scholar]
- 30. Outerbridge HK, Outerbridge AR, Outerbridge RE. The use of a lateral patellar autologous graft for the repair of a large osteochondral defect in the knee. J Bone Joint Surg Am. 1995;77(1):65-72. [DOI] [PubMed] [Google Scholar]
- 31. Arnoczky SP, Warren RF. The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am J Sports Med. 1983;11(3):131-41. [DOI] [PubMed] [Google Scholar]
- 32. Arnoczky SP, Warren RF, Spivak JM. Meniscal repair using an exogenous fibrin clot. An experimental study in dogs. J Bone Joint Surg Am. 1988;70(8):1209-17. [PubMed] [Google Scholar]
- 33. Gershuni DH, Skyhar MJ, Danzig LA, Camp J, Hargens AR, Akeson WH. Experimental models to promote healing of tears in the avascular segment of canine knee menisci. J Bone Joint Surg Am. 1989;71(9):1363-70. [PubMed] [Google Scholar]
- 34. Kimura M, Shirakura K, Hasegawa A, Kobuna Y, Niijima M. Second look arthroscopy after meniscal repair. Factors affecting the healing rate. Clin Orthop Relat Res. 1995;(314):185-91. [PubMed] [Google Scholar]
- 35. Kobuna Y, Shirakura K, Niijima M. Meniscal repair using a flap of synovium. An experimental study in the dog. Am J Knee Surg. 1995;8(2):52-5. [PubMed] [Google Scholar]
- 36. Rothamel D, Schwarz F, Sager M, Herten M, Sculean A, Becker J. Biodegradation of differently cross-linked collagen membranes: an experimental study in the rat. Clin Oral Implants Res. 2005;16(3):369-78. [DOI] [PubMed] [Google Scholar]
- 37. Benjamin M, Ralphs JR. Biology of fibrocartilage cells. Int Rev Cytol. 2004;233:1-45. [DOI] [PubMed] [Google Scholar]
- 38. Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. Arthroscopy. 2007;23(4):381-7. [DOI] [PubMed] [Google Scholar]
- 39. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boyd KT, Myers PT. Meniscus preservation; rationale, repair techniques and results. Knee. 2003;10(1):1-11. [DOI] [PubMed] [Google Scholar]
- 41. Heatley FW. The meniscus—can it be repaired? An experimental investigation in rabbits. J Bone Joint Surg Br. 1980;62(3):397-402. [DOI] [PubMed] [Google Scholar]
- 42. Seil R, Kohn D. Meniscus reconstruction. Established and innovative methods. Unfallchirurg. 2001;104(4):274-87. [DOI] [PubMed] [Google Scholar]
- 43. Lotz MK, Otsuki S, Grogan SP, Sah R, Terkeltaub R, D’Lima D. Cartilage cell clusters. Arthritis Rheum. 2010;62(8):2206-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mainil-Varlet P, Van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010;38(5):880-90. [DOI] [PubMed] [Google Scholar]
- 45. Van der Bracht H, Verdonk R, Verbruggen R, Elewaut D, Verdonk P. Cell-based meniscus tissue engineering. In: Ashammakhi N, Reis R, Chiellini E, editors. Topics in tissue engineering. Vol. 3; 2007. p. 1-13. [Google Scholar]
- 46. Horie M, Driscoll MD, Sampson HW, Sekiya I, Caroom CT, Prockop DJ, et al. Implantation of allogenic synovial stem cells promotes meniscal regeneration in a rabbit meniscal defect model. J Bone Joint Surg Am. 2012;94(8):701-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klompmaker J, Veth RP, Jansen HW, Nielsen HK, de Groot JH, Pennings AJ, et al. Meniscal repair by fibrocartilage in the dog: characterization of the repair tissue and the role of vascularity. Biomaterials. 1996;17(17):1685-91. [DOI] [PubMed] [Google Scholar]
- 48. Pereira H, Frias AM, Oliveira JM, Espregueira-Mendes J, Reis RL. Tissue engineering and regenerative medicine strategies in meniscus lesions. Arthroscopy. 2011;27(12):1706-19. [DOI] [PubMed] [Google Scholar]
- 49. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076-84. [DOI] [PubMed] [Google Scholar]
- 50. Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464-74. [DOI] [PubMed] [Google Scholar]
- 51. Jacobi M, Jakob RP. Meniscal repair: enhancement of healing process. In: Philippe Beaufils RV, editor. The meniscus. 1st ed Berlin: Springer Verlag; 2010. p. 129-35. [Google Scholar]