Abstract
Background
Focal cartilage lesions in the knee joint have limited capacity to heal. Current animal experiments show that incisions of the deep zone of a cartilage allograft allow acceptable integration for the graft.
Questions/Purposes
We performed this clinical study to determine (1) if the multiply incised cartilage graft is surgically applicable for focal cartilage lesions, (2) whether this allograft has a potential to integrate to the repair site, and (3) if patients show clinical improvement.
Patients and Methods
Seven patients with 8 chondral lesions were enrolled into the study. Symptomatic lesions between 2 and 8 cm2 were accepted. Additional injuries were allowed but were addressed simultaneously. Grafts were tailored to match and the deep zone of the cartilage was multiply incised to augment the basal integration before securing in place. Rigorous postoperative physiotherapy followed. At 12 and 24 months the patients’ satisfaction were measured and serial magnetic resonance imaging (MRI) was performed in 6 patients.
Results
Following the implantations no adverse reaction occurred. MRI evaluation postoperatively showed the graft in place in 5 out of 6 patients. In 1 patient, MRI suggested partial delamination at 1 year and graft degeneration at 2 years. Short Form–36 health survey and the Lysholm knee score demonstrated a significant improvement in the first year; however, by 2 years there was a noticeable drop in the scores. Conclusions. Multiply incised pure chondral allograft used for cartilage repair appears to be a relatively safe method. Further studies are necessary to assess its potential in cartilage repair before its clinical use.
Keywords: cartilage repair, chondral lesion, allograft, clinical study
Introduction
Focal full-thickness cartilage lesions are not uncommon in young patients,1 and the articular hyaline cartilage has only very limited capacity to repair in mature individuals. In earlier studies, articular cartilage was demonstrated to have limited capacity to heal to the subchondral bone once detached.2,3 The idea of transplanting pure cartilage onto the subchondral bone has been considered unorthodox. This long-standing dogma has been challenged recently and it appears that the concept of cartilage as an indifferent and passive part of the reintegration process is to be reevaluated. In the past few years, 2 distinct methods have been introduced, that deliver small pure cartilage particulates (<2 mm3 in size) to the defect site.4,5 These techniques provide “large cell-clusters” in their original extracellular matrix, and as the original tissue is minced it can be shaped freely to match the dimensions of the lesion. The clinical results of these repair methods are encouraging; however most orthopedic surgeons would still express their skepticism about the idea of implanting large cartilage graft without subchondral bone (the “ultra-thin” shell graft).
In a previous animal study, we reported acceptable healing between the subchondral bone and pure cartilage allograft when a simple cartilage surface augmentation method was employed before the implantation6 (multiple incisions in the deep zone of full thickness articular cartilage graft without compromising the superficial layer). The clear difference between the methods using particulated cartilage for repair4,5 and our multiply incised graft6 is the integrity of the superficial cartilage layer providing good primary strength to a much larger, incised—but not morselized graft. Macroscopic assessment of the repair tissue 6 months after implantation in the porcine model showed good defect fill, acceptable marginal integration; the histological scores were similar to the results of autologous chondrocyte implantation and superior to microfracturing.6 We hypothesized that this technique could be feasible in the human knee joint when using fresh pure chondral allografts.
The present study was designed to investigate whether multiply incised pure cartilage allograft is a safe method to use for focal cartilage lesions allowing potential future clinical trials.
Methods
Patient Enrollment
All individuals with symptomatic focal cartilage lesions in the knee joint were provisionally enrolled into the study regardless of the mechanism of the injury and informed consent was obtained. Final screening was performed intraoperatively, and Outerbridge classification7,8 grade 3 to 4 focal lesions of size 2 to 8 cm2 were accepted. Additional injuries in the same joint (e.g., meniscal tear, anterior cruciate ligament [ACL] deficiency) were allowed, but all were addressed simultaneously. Patients with (1) cartilage lesions on opposing articular surfaces (kissing lesion), (2) cartilage involvement in all 3 compartments, (3) advanced osteoarthritic signs, or (4) focal osteonecrotic lesions were excluded from the study. Two female and 5 male patients (age 14-44 years) were enrolled into the study in a 1-year period; a total of 8 cartilage lesions were repaired using cartilage allograft (1 patient with 2 distinct cartilage lesions). The baseline characteristics of the patients and the cartilage lesions are presented in Table 1. The patient selection criteria, the treatment and follow-up protocol were approved by the local institutional review board as well as the National Ethical Committee for Science and Research and the study was conducted with the principles of the Declaration of Helsinki.
Table 1.
Demographics of the Patients Treated with Processed Pure Cartilage Allograft.
| Case No. | Sex | Age | BMI | Location of defect | Defect size | Additional procedure |
|---|---|---|---|---|---|---|
| 1 | F | 14 | 28 | Patella: central inferior part | 4.4 cm2 | None |
| 2 | M | 34 | 25 | MFC: central extension WBS | 3.2 cm2 | ACL reconstruction |
| 3 | M | 44 | 31 | MFC: lateral extension WBS | 5.3 cm2 | Partial medial meniscectomy |
| Trochlea: central inferior part | 4.0 cm2 | |||||
| 4 | M | 36 | 25 | MFC: lateral extension WBS | 2.0 cm2 | ACL reconstruction |
| 5 | M | 31 | 29 | MFC: central extension WBS | 3.5 cm2 | ACL reconstruction |
| 6 | M | 32 | 31 | MFC: lateral extension WBS | 3.4 cm2 | ACL reconstruction |
| 7 | F | 35 | 34 | MFC: lateral extension WBS | 5.4 cm2 | Partial medial meniscectomy |
ACL = anterior cruciate ligament; BMI = body mass index; MFC = medial femoral condyle; WBS = weightbearing surface.
Harvest and Storage of the Cartilage Graft
Fresh human knee joints from the local tissue bank were used as the source for the pure cartilage graft. The age of the donors was between 20 and 45 years. The medial and the lateral femoral condyles were the primary sources; 6 to 8 cm2 pure chondral graft was obtained from each condyle. Full-thickness cartilage grafts were removed with a curvilinear cutting blade (congruent with the articular surface) (Fig. 1). The harvested grafts were 1.5 to 2.0 mm in thickness; special care was taken not to incorporate the subchondral bone: All grafts were assessed macroscopically and all osseal remnants were removed using blades and a high-speed burr. In addition to the serological screening of the donor, each specimen was tested for bacterial contamination according to the national regulations for musculoskeletal tissue transplantation. The pure cartilage grafts were stored at 4 °C in Dulbecco Modified Eagle Medium with HEPES (N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid) buffer (Sigma-Aldrich, Munich, Germany) until implantation. The storage time slightly varied but in all cases it was less than 28 days.
Figure 1.
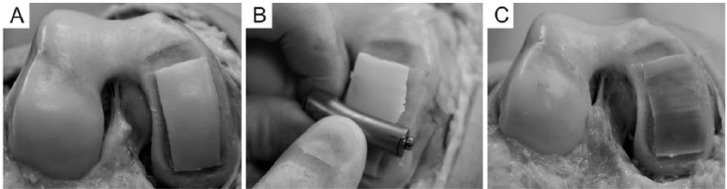
Cartilage shell harvesting from cadaveric knee joint. (A) The donor surface is prepared by removing the surrounding cartilage tissue to allow easier removal of the cartilage graft. (B) A curvilinear harvester device is used to peel the cartilage from the subchondral bone. (C) The donor surface after successful harvesting; full thickness cartilage is removed from the medial femoral condyle.
Cartilage Graft Surface Augmentation
To reduce cellular damage the grafts were slowly warmed to room temperature. Because of the nature of the procedure, the donor and the recipient knee joints did not have to be size-matched preoperatively. Prior to implantation the deep zone of the cartilage graft was incised (Fig. 2) using a specially designed incisor. This device comprises of a base and a cutting handle holding numerous cutting blades aligned parallel to each other. It is designed so that the incisions do not penetrate the cartilage tissue leaving the superficial zone untouched. The cutting distance between the blades was set for 700 µm, which converted the rigid specimens into a pliable graft increasing the accessible surface for basal integration. The depth limiter of the cutting device was set to 600 µm; thus, the superficial layer of the hyaline cartilage was left intact and the graft remained firm allowing easy handling and secure fixation during surgery (Fig. 3).
Figure 2.
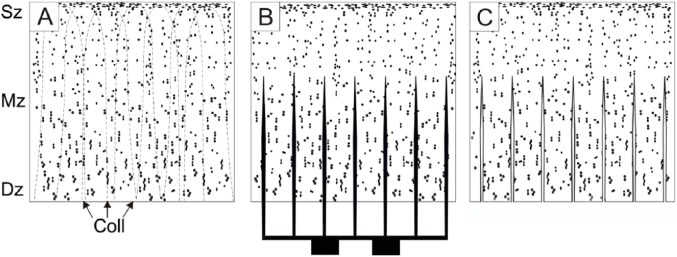
A schematic drawing to illustrate the production of multiply incised pure chondral graft. Dots represent lacunae, the 3 zones of hyaline cartilage are labeled Sz (superficial zone), Mz (middle zone), and Dz (deep zone). (A) Cross-section of the full-thickness articular cartilage specimen harvested from a donor surface. Collagen fiber pattern is shown with broken lines (Coll). (B) Incision of the graft using multiple parallel blades. The cuts go through the deep and middle zone of cartilage but leave the superficial zone uncompromised. Please note, the incisions run parallel with the collagen fibers (Coll), thus, collagen network is left considerably intact. (C) Processed chondrograft ready to be implanted.
Figure 3.
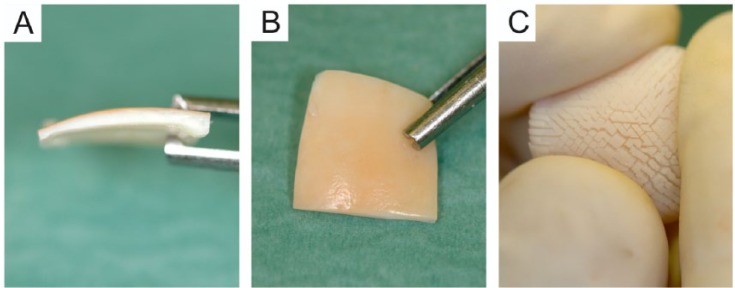
Macroscopic appearance of the multiply incised pure cartilage allograft. (A) A side view of the graft showing the cartilage graft without subchondral bone (1.5-2 mm in thickness). (B) The superficial (articular) side of the graft; please note that this surface of the graft remains intact providing the graft with good tensile strength. (C) The “hedgehog” appearance of the deep zone of the graft after folding it to a concave shape. The picture demonstrates how the incisions of the deep zone render the sample to an easily pliable implant with increased basal surface.
Surgical Procedure
The cartilage defect size and the grade of degeneration were assessed arthroscopically. Lesions suitable for cartilage graft implantation were approached by mini-arthrotomy (except case 1 with patellar eversion). Vertical walls around the lesions were created using a blade and ring curette; the remaining cartilage was completely removed from the underlying subchondral bone. Using a sterile tin foil replica of the lesion the surface-enhanced chondrograft was appropriately sized, laid upon the defect with the incised side facing the subchondral bone and secured in place with 6/0 vicryl interrupted sutures grasping about 3 mm from each cartilage side (Fig. 4). Surface level difference between the surrounding cartilage and the graft was accepted up to 0.5 mm. The gap (<1 mm) between the graft and the host tissue was sealed with fibrin glue (Tissucol, Baxter, Halle, Germany) to enhance peripheral integration. The implanted and the recipient cartilage tissues were frequently irrigated throughout the surgery to minimize cell damage. Additional injuries of the joint (meniscal tear, ACL deficiency) were addressed simultaneously (prior to the cartilage repair).
Figure 4.
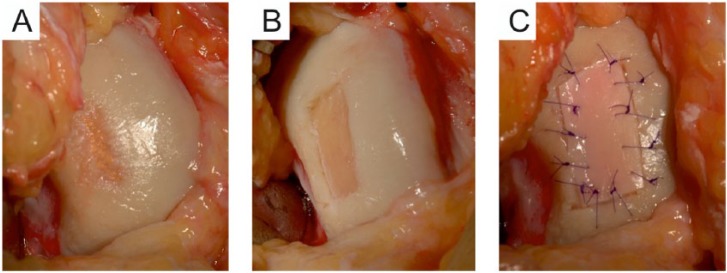
Intraoperative stages of the implantation. (A) After confirmatory arthroscopy the focal cartilage lesion of the medial femoral condyle is approached via mini arthrotomy. (B) The lesion is prepared with blade and ring curette by removing the affected cartilage, forming solid vertical shoulder around the defect. (C) The multiply incised cartilage graft is tailored to the shape and size of the lesion and secured to the lesion with the intact superficial zone facing outward.
Rehabilitation Protocol
The rehabilitation protocol focused on the protection of the grafts from overload and extreme shear forces. Besides daily continuous passive motion (CPM) patients were managed with toe-touch weight bearing for a minimum of 2 weeks following surgery and were only allowed partial weight bearing from the third week progressing to full weight bearing after 6 weeks. The protocol varied individually depending on the location of the lesion and on the concurrent ligament repair.
In the case of the patellar lesion, full weight bearing was allowed immediately with a hinged brace locked in extension for 6 weeks. CPM between 0° and 40° was applied daily in the first 3 weeks, progressively increasing to 0° to 90° in the following 3 weeks.
Clinical Outcome Measures
Knee function and a general quality-of-life evaluation was carried out prospectively at 0, 12, and 24 months postoperatively using the Lysholm Scoring Scale system9 and the Short Form Health Survey (SF-36)10,11 standardized outcome assessment tools. All data were collected by an independent colleague.
Magnetic Resonance Imaging
MRI scans were performed postoperatively in 6 out of 7 patients. MR images were obtained and evaluated 12 and 24 months after surgery. In 5 patients postoperative images were performed using a 3-T Magnetom TIM Trio whole-body MRI scanner (Siemens AG, Erlangen, Germany) with the use of an 8-channel transmit/receive phased-array knee coil (Invivo, Orlando, FL). After the standard 3-plane calibration scan, the following sequences were performed during a single imaging session to investigate the repair response of cartilage allograft: (1) sagittal, coronal proton density–weighted turbo spin-echo; (2) coronal T1-weighted turbo spin-echo; and (3) axial T2-weighted turbo spin-echo. Proton density–weighted images were acquired with and without fat suppression. In 1 of the 6 patients, only 1.5-T MR images were obtained because of implantable cardioverter defibrillator (case 3 in Table 1). One patient refused to be assessed with MRI because of claustrophobia (case 7 in Table 1). All MR images were postoperatively assessed by an experienced musculoskeletal radiologist and scrutinized for graft delamination, displacement, graft morphology (flush, protruding, depressed), and for the presence of the interface gap. The cartilage signal was noted as isointense, hypointense, or hyperintense and the subchondral bone characteristics were determined (fat, edema, or fibrous) in all cases.
Statistical Analyses
The data was analyzed using SPSS statistical package (SPSS v20, IBM, Armonk, NY). Paired T test and nonparametric Wilcoxon test were used for comparison between the data before and after the graft implantation. Statistically significant effects were those for which the null value of the effect lay outside the 95% confidence interval (i.e., P < 0.05).
Results
Graft Quality and Adverse Reaction
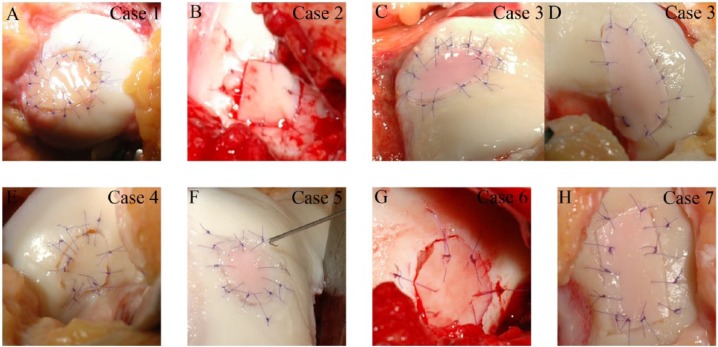
We found that the preparation of the cartilage graft was quick and simple; the resulting processed cartilage was firm but very moldable, hence easy to handle. The graft held the stitches well even after the multiple incisions and once the fibrin glue was set the graft appeared solid and fixed in its place (Fig. 5). There was no major adverse reaction in the follow-up period and none of the patients experienced joint locking or catching.
Figure 5.
Macroscopic appearances of the implanted cartilage grafts. Each case represents a different patient, case numbers correspond to the numbers in Table 1. C and D demonstrate 2 distinct location of the same knee joint.
Magnetic Resonance Imaging
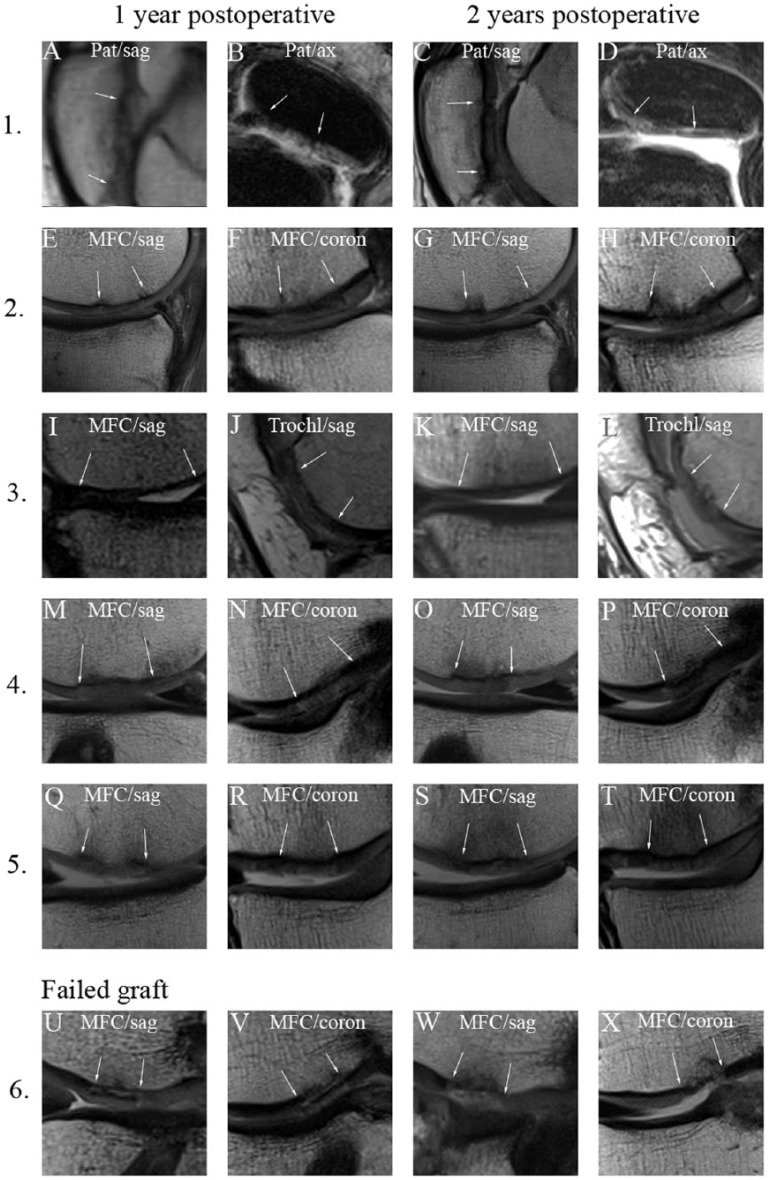
At 12 months postoperatively in 5 out of 6 cases, the analysis of the MR images demonstrated that, the implanted cartilage graft had normal cartilage signal intensity and the thickness of the implanted allograft was maintained (Fig. 6). When the signals of the implanted graft and the surrounding tissue were compared, the graft signal properties appeared isointense. The subchondral plate remained intact and no subchondral cyst or intralesional osteophytes were identified. The edema of the subchondral bone decreased and often only mild edematic changes were found in the subchondral bone at the border of the original graft after 1 year suggesting incomplete marginal integration. The MRIs at 2 years showed that the signal quality of the implant appeared isointense and the thickness of the graft was maintained (Fig. 6). In one case, a possible delamination was identified on the first MRI scan 12 months postoperatively. Two years after surgery, the graft was absent from the site of the lesion and the edema in the affected subchondral bone persisted (Fig. 6U-X).
Figure 6.
Postoperative proton density–weighted images of the cartilage lesions repaired with multiply incised pure cartilage allograft. The repair sites are shown 1 year (1-2. columns) and 2 years (3-4. columns) postoperatively. The white arrows mark the edge of the graft. Numbers for each row correspond to the case numbers in Table 1. Case 3 shows 2 distinct location of the same knee joint. While the first 5 cases show acceptable integration case 6 represents a failed graft. Pat = patella; MFC = medial femoral condyle; Trochl = trochlea; sag = sagittal plane; ax = axial plane; coron = coronal plane.
Clinical Outcome Scores
Because of the low number of patients statistical analysis of the functional outcome scores is of limited clinical relevance. The Lysholm knee score and the physical health assessment with SF-36 showed significant improvement in all patients at 1 year. Although at 2 years, the scores were higher than the baseline scores in all but one patient, there is a clear drop of the scores in many patients after the first year (Fig. 7). Considering the limited number of patients involved in the study, we were not able to correlate patient body mass index, age, location of the lesion, or size of the lesion with the functional scores.
Figure 7.
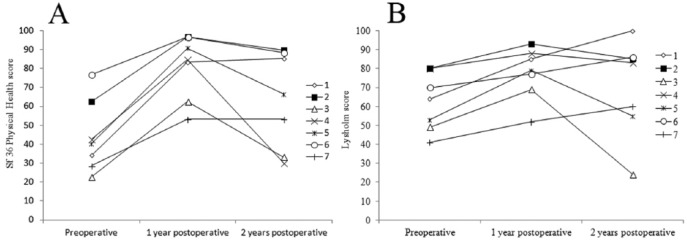
Short Form–36 (SF-36) physical health score (A) and Lysholm knee score (B) preoperatively, 1 and 2 years postoperatively. Each patient is shown as a separate line and the numbers correspond with the case numbers shown in Table 1.
Second-Look Arthroscopy
In one case (case 4 in Table 1) the ACL graft failed 9 months postoperatively following a small accident. In this case, the second-look arthroscopy 14 months postoperatively demonstrated that the cartilage graft was in place with acceptable basal and marginal integrity. The macroscopic appearance of the repaired area was smooth and flush with the adjacent cartilage with somewhat softer consistency than the surrounding cartilage tissue when tried with arthroscopic probe (Fig. 8).
Figure 8.
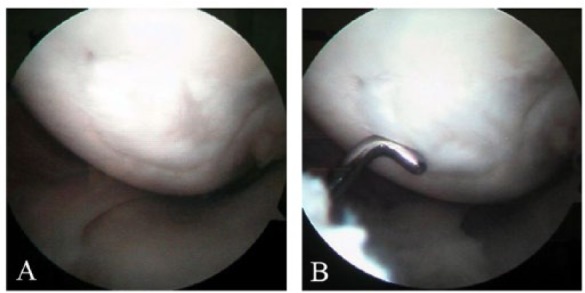
Macroscopic pictures from second-look arthroscopy in case 4. The arthroscopy was performed 14 months after the implantation of the graft. The border of the graft on the lateral aspect of the medial femoral condyle is still evident.
Discussion
To date, most orthopedic surgeons have believed that cartilage has very little potential to heal back once separated from the subchondral bone. The reason why cartilage has such limited potential in vertical and horizontal integration is still a matter of debate. Many factors have been suspected, such as the impaired collagen network crosslinking12,13 or proteoglycan components (lubricin) in the synovial fluid14 that would prevent integration. Chondrocyte immobility in the extracellular matrix has also been speculated to be a significant limiting factor in the repair capacity. The concept of cartilage as an indifferent and passive part of the reintegration process, however has been challenged.15-17 Several studies demonstrated that cartilage cells are rather motile and can move through the dense extracellular matrix15,16 once adequate signals are present or even migrate across the integration site.17 Incision through the cartilage tissue has been shown to induce chondrocyte motility and cells even evade the original cartilage tissue18 producing neocartilage around the edges, which varies depending on the zone (superficial vs. deep) and on the age of the cartilage (young vs. adult).18 As more evidence accumulated proving certain intrinsic capacity of pure cartilage to repair lesions, new pure cartilage-based techniques have emerged. Cartilage Autograft Implantation System (CAIS, DePuy Mitek, Raynham, MA) is one of the new repair procedures, where cartilage tissue obtained from the non-weightbearing surface of the affected joint is processed in situ resulting in an autologous cartilage fragment-loaded scaffold that is applied to the lesion of the weightbearing area. This method is still in the premarketing clinical trial phase, but the 2-year follow-up data of the prospective clinical safety trial are very encouraging.4 Another cartilage-based repair method is the DeNovo NT repair system (Zimmer, Warsaw, IN/ISTO, St Louis, MO), which is based on minced cadaveric juvenile cartilage. The articular hyaline cartilage is cut into 1-mm3 cartilage particles and fibrin glue is used to secure the evenly distributed tissue particles on the defected surface. Although the data from the postmarketing clinical trial is very promising, the available literature is still very limited.5,19
Considering the promising results from these two pure hyaline cartilage tissue-based repair techniques (CAIS, DeNovo NT) the idea of transplanting larger cartilage samples peeled off the subchondral bone may appear less radical. In a previous animal study, we successfully transplanted large (2 cm2) cartilage allografts in a porcine model.6 The stability of the graft was convincing 6 and 24 weeks postoperatively, and the resulting tissue appeared to be hyaline or hyaline-like cartilage. Our grafts contained all 3 zones of the hyaline cartilage. The deep zone of the cartilage graft is multiply incised to augment the basal integration (increased surface)6 and the resulting graft (often referred to as the “hedgehog-graft” because of its appearance when turned inside-out) becomes flexible and can be adjusted to most of the surfaces without difficulties. This graft represents, in a way, transition between osteochondral shell allografts and particulated cartilage auto- or allografts. Since the cartilage thickness and the joint loading in the porcine model are comparable to human knee joints we hypothesized that this technique could also be used in human chondral lesions; therefore, we conducted this small clinical study.
The main limitation of this study is the small number of patients enrolled and the various coexistent injuries and reconstructive surgeries in the affected joints. Second-look arthroscopy was only performed in 1 patient. Although high-resolution MR images were obtained, the MRI sequences did not allow us to use any validated MRI scoring system and biochemical and immune-histological analysis of the grafts would be essential to assess the condition of the cartilage grafts (cell migration, changes in the collagen and proteoglycan network). In spite of the 2-year minimum follow-up in this study, delayed degeneration of the cartilage implant is still possible.
We found that multiple incision of hyaline cartilage is a simple process that preserves the mechanical integrity of the tissue graft. It does not sacrifice the underlying bone tissue and we found no major adverse reaction postoperatively. Interestingly, the patient with graft delamination/degeneration (case 6 in Table 1) did not experience any joint pain, loss of function, or a clicking or locking sensation.
Several factors before the actual implantation have impact on the endurance of the cartilage tissue graft. Any sharp trauma in the cartilage tissue may generate significant chondrocyte damage in the adjacent zone.20,21 That is probably true for the mincing cartilage procedures as well as for the multiple parallel incisions that we carried out. The exact effect of tissue glue on the freshly cut surfaces is largely unknown; however, fibrin glue appears to promote cell migration and increase cell viability =22 and it is widely used in various other cartilage repair procedures.4,5,23 Further in-depth viability and migration studies should investigate the significance of the mechanical injuries and the tissue adhesives. Allograft storage also plays a pivotal role in cartilage cell survival. Although there is evidence that in caprine osteochondral allografts chondrocytes show better viability during prolonged storage at 37 °C,24 most of the current human constructs and tissue banks use fresh grafts stored at 4 °C and recommend implantation within 21 to 28 days of harvest. It has been shown that after 28 days, the metabolism and viability of the chondrocytes in osteochondral allografts stored at 4 °C significantly declines25,26 and so we implanted our cartilage allografts within 4 weeks of procurement.
The MRI results are encouraging, as the thickness and the appearance of the cartilage graft seems to be preserved in most patients. The patients’ physical status showed improvement in all cases at 1 year and in 6 out of 7 patients at 2 years (please note that this patient sustained an additional knee injury after the surgery). It is crucial, however, to note that there is a clear drop in the functional scores in more than half of the patients, which might also be an indicator of a potential early failure of the graft.
Multiply incised cartilage allograft has certain advantages when compared to conventional osteochondral allografting: since all bony parts are meticulously removed before implantation the graft consists of pure hyaline cartilage and it may be less antigenic than bone. Furthermore, the “hedgehog graft” is easily moldable, it can be tailored to any size and shape as needed, therefore relatively forgiving in terms of differences between the donor and recipient surface geometry. On the other hand, this technique has its downsides. In addition to the common drawbacks of any allogeneic tissue transplantation (i.e., possible disease transmission, relative shortage of donors, expensive preparation, limited time interval for implantation) immunogenicity is a major concern. Earlier studies have shown that isolated chondrocytes can elicit immune response but intact hyaline cartilage is rather immunoprivileged.27 It is likely because of the dense extracellular matrix structure of hyaline cartilage that prevents effective immune-surveillance in the deeper layers of the tissue.28 Morselization or multiple incisions of the intact hyaline cartilage, however, might alter this inert immune status and so further research should be conducted to investigate its clinical significance. Furthermore, when using pure cartilage grafts the resulting primary stability of the graft is inferior to any method using press-fit osteochondral graft. The strength of fixation for larger grafts, however, could possibly be improved with additional fixations (e.g., transosseal resorbable staples).29 Since the graft integration occurs mainly on the basal surface, as long as the fixation of the graft is appropriate multiply incised cartilage grafts could be applied for cases with larger lesions
In this study, we demonstrated that using multiply incised cartilage allograft for focal cartilage lesions in the knee joint is a relatively safe repair modality. Nevertheless, additional studies must be carried out to assess the performance of this graft in the long term and to learn more about the possible complications and limitations of this technique.
Footnotes
Acknowledgments and Funding: We thank Prof. Tamas Illes for his support and helpful suggestions during the research, Orsolya Boda for her professional assistance, and Anna Wilkinson for expert linguistic support. The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflict of Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by our institutional review board.
References
- 1. Aroen A, Loken S, Heir S, Alvik E, Ekeland A, Granlund OG, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211-5. [DOI] [PubMed] [Google Scholar]
- 2. Bentley G, Greer RB., 3rd Homotransplantation of isolated epiphyseal and articular cartilage chondrocytes into joint surfaces of rabbits. Nature. 1971;230:385-8. [DOI] [PubMed] [Google Scholar]
- 3. Pap K, Krompecher S. Arthroplasty of the knee: experimental and clinical experiences. J Bone Joint Surg Am. 1961;43:523-37. [Google Scholar]
- 4. Cole BJ, Farr J, Winalski CS, Hosea T, Richmond J, Mandelbaum B, et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39:1170-9. [DOI] [PubMed] [Google Scholar]
- 5. Farr J, Yao JQ. Chondral defect repair with particulated juvenile cartilage allograft. Cartilage. 2012;4:346-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bardos T, Farkas B, Mezes B, Vancsodi J, Kvell K, Czompoly T, et al. : Osteochondral integration of multiply incised pure cartilage allograft: repair method of focal chondral defects in a porcine model. Am J Sports Med. 2009;37(Suppl 1):50S-57S. [DOI] [PubMed] [Google Scholar]
- 7. Cameron ML, Briggs KK, Steadman JR. Reproducibility and reliability of the outerbridge classification for grading chondral lesions of the knee arthroscopically. Am J Sports Med. 2003;31:83-6. [DOI] [PubMed] [Google Scholar]
- 8. Outerbridge RE: The etiology of chondromalacia patellae. J Bone Joint Surg Br 1961;43-B:752-7. [DOI] [PubMed] [Google Scholar]
- 9. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-49. [PubMed] [Google Scholar]
- 10. Kosinski M, Keller SD, Hatoum HT, Kong SX, Ware JE., Jr. The SF-36 Health Survey as a generic outcome measure in clinical trials of patients with osteoarthritis and rheumatoid arthritis: tests of data quality, scaling assumptions and score reliability. Med Care. 1999;37:MS10-22. [DOI] [PubMed] [Google Scholar]
- 11. Kosinski M, Keller SD, Ware JE, Jr, Hatoum HT, Kong SX. The SF-36 Health Survey as a generic outcome measure in clinical trials of patients with osteoarthritis and rheumatoid arthritis: relative validity of scales in relation to clinical measures of arthritis severity. Med Care. 1999;37:MS23-39. [DOI] [PubMed] [Google Scholar]
- 12. DiMicco MA, Sah RL. Integrative cartilage repair: adhesive strength is correlated with collagen deposition. J Orthop Res. 2001;19:1105-12. [DOI] [PubMed] [Google Scholar]
- 13. DiMicco MA, Waters SN, Akeson WH, Sah RL. Integrative articular cartilage repair: dependence on developmental stage and collagen metabolism. Osteoarthritis Cartilage 2002;10:218-25. [DOI] [PubMed] [Google Scholar]
- 14. Schaefer DB, Wendt D, Moretti M, Jakob M, Jay GD, Heberer M, et al. Lubricin reduces cartilage–cartilage integration. Biorheology. 2004;41:503-8. [PubMed] [Google Scholar]
- 15. Morales TI. Chondrocyte moves: clever strategies? Osteoarthritis Cartilage 2007;15:861-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lyman JR, Chappell JD, Morales TI, Kelley SS, Lee GM. Response of chondrocytes to local mechanical injury in an ex vivo model. Cartilage. 2012;3:58-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Theodoropoulos JS, De Croos JN, Park SS, Pilliar R, Kandel RA. Integration of tissue-engineered cartilage with host cartilage: an in vitro model. Clin Orthop Relat Res. 2011;469:2785-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bos PK, Kops N, Verhaar JA, van Osch GJ. Cellular origin of neocartilage formed at wound edges of articular cartilage in a tissue culture experiment. Osteoarthritis Cartilage. 2008;16:204-11. [DOI] [PubMed] [Google Scholar]
- 19. Kruse DL, Ng A, Paden M, Stone PA. Arthroscopic De Novo NT® juvenile allograft cartilage implantation in the talus: a case presentation. J Foot Ankle Surg. 2012;51:218-21. [DOI] [PubMed] [Google Scholar]
- 20. Redman SN, Dowthwaite GP, Thomson BM, Archer CW. The cellular responses of articular cartilage to sharp and blunt trauma. Osteoarthritis Cartilage. 2004;12:106-16. [DOI] [PubMed] [Google Scholar]
- 21. Huntley JS, Bush PG, McBirnie JM, Simpson AH, Hall AC. Chondrocyte death associated with human femoral osteochondral harvest as performed for mosaicplasty. J Bone Joint Surg Am 2005;87:351-60. [DOI] [PubMed] [Google Scholar]
- 22. Gille J, Meisner U, Ehlers EM, Muller A, Russlies M, Behrens P. Migration pattern, morphology and viability of cells suspended in or sealed with fibrin glue: a histomorphologic study. Tissue Cell. 2005;37:339-48. [DOI] [PubMed] [Google Scholar]
- 23. Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259-71. [DOI] [PubMed] [Google Scholar]
- 24. Pallante AL, Bae WC, Chen AC, Görtz S, Bugbee WD, Sah RL. Chondrocyte viability is higher after prolonged storage at 37 degrees C than at 4 degrees C for osteochondral grafts. Am J Sports Med. 2009;37(Suppl 1):24S-32S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85-A:2111-20. [DOI] [PubMed] [Google Scholar]
- 26. Strauss EJ, Sershon R, Barker JU, Kercher J, Salata M, Verma NN. The basic science and clinical applications of osteochondral allografts. Bull NYU Hosp Jt Dis. 2012;70:217-23. [PubMed] [Google Scholar]
- 27. Langer F, Gross AE. Immunogenicity of allograft articular cartilage. J Bone Joint Surg Am. 1974;56:297-304. [PubMed] [Google Scholar]
- 28. Maroudas A, Bullough P, Swanson SA, Freeman MA. The permeability of articular cartilage. J Bone Joint Surg Br 1968;50:166-77. [PubMed] [Google Scholar]
- 29. Zheng N, Lu Y, Boock R, Conrad B, Binette F. Retention of scaffold with staple fixation for cartilage repair during cyclic motion of human cadaveric knees. J Musculoskeletal Res. 2008;11:151-9. [Google Scholar]