Abstract
Although we usually report 5-year cancer survival using population-based cancer registry data, nowadays many cancer patients survive longer and need to be followed-up for more than 5 years. Long-term cancer survival figures are scarce in Japan. Here we report 10-year cancer survival and conditional survival using an established statistical approach. We received data on 1 387 489 cancer cases from six prefectural population-based cancer registries in Japan, diagnosed between 1993 and 2009 and followed-up for at least 5 years. We estimated the 10-year relative survival of patients who were followed-up between 2002 and 2006 using period analysis. Using this 10-year survival, we also calculated the conditional 5-year survival for cancer survivors who lived for some years after diagnosis. We reported 10-year survival and conditional survival of 23 types of cancer for 15–99-year-old patients and four types of cancer for children (0–14 years old) and adolescent and young adults (15–29 years old) patients by sex. Variation in 10-year cancer survival by site was wide, from 5% for pancreatic cancer to 95% for female thyroid cancer. Approximately 70–80% of children and adolescent and young adult cancer patients survived for more than 10 years. Conditional 5-year survival for most cancer sites increased according to years, whereas those for liver cancer and multiple myeloma did not increase. We reported 10-year cancer survival and conditional survival using population-based cancer registries in Japan. It is important for patients and clinicians to report these relevant figures using population-based data.
Keywords: Cancer, cancer registry, conditional survival, period analysis, survival
Usually, population-based cancer registries report 5-year relative survival of cancer patients. Nowadays, however, many patients with a variety of cancers can live more than 5 years and thus need more information about their long-term prognosis. Clinicians and medical staff also need information about how long they should follow up their cancer patients and when they can assume patients are cured of cancer. This type of data, based on nationwide databases, was scarce in Japan. Using conventional methods (cohort approach) to calculate cancer survival, we need to follow-up for a certain period (e.g. 5 or 10 years) after diagnosis. Ten-year survival using conventional methods is based on the data of patients who were diagnosed more than 10 years ago; both patients and clinicians need information that is more up-to-date. To solve the problem, an alternative method (period approach) has recently been applied to estimate more up-to-date long-term survival in other countries.(1–5)
Using 10-year survival, we can also report the conditional 5-year survival, as this is known to be a useful statistic for cancer patients, especially for long-term cancer survivors. Conditional survival is a survival estimate based on data of patients who have survived 1 or more years. As they provide more relevant information for cancer patients, their families, and clinicians, some countries have started to report these figures.(6–9)
Our research project (J-CANSIS, the Japanese CANcer Survival Information for Society), supported by Grant-In-Aid from the Ministry of Health, Labor and Welfare of Japan in the financial year 2013, aimed to analyze recent trends in cancer survival and report long-term survival based on population-based cancer registry data in Japan.
In this study, we aimed to report the latest 10-year survival of cancer patients applying established statistical methods, and demonstrate conditional survival as relevant information for cancer survivors.
Methods
Study design
A total of 1 387 489 cancer cases were provided by the population-based cancer registries of six prefectures (Yamagata, Miyagi, Fukui, Niigata, Osaka, and Nagasaki) in Japan. These prefectural cancer registries have cancer records with high data quality (% of death certificate only = 3.9–17.7, Table S1.1) and have been used to estimate national statistics for cancer survival in Japan for a long time. This study was approved by the ethical committee of the Osaka Medical Center for Cancer and Cardiovascular Diseases (Osaka, Japan) in September 2013, and use of the data was approved by the six prefectural cancer registries.
Data excluded
We excluded data that were registered by death certificate only and in situ cases from the analysis. Numbers of submitted and excluded cases from analysis are shown in Table S1.1. We analyzed 789 600 cases with first, primary, and invasive malignant tumor in a total of six prefectural cancer registries (Table S1.2).
Follow-up of patients
In our research project, we used data of cancer patients who were followed-up for at least 5 years post-diagnosis. Follow-up methods, years of diagnosis, and follow-up for each registry are shown in Table S2. All cancer registries adopted linkage to the death certificate database in the prefecture to confirm the vital status of patients. Patients without linkage to the prefecture death certificate database are considered as alive based on this method. This assumption will be biased and cause overestimation of survival, because if patients die in a prefecture other than that in which they were diagnosed, their death will not be noted. Registries of Yamagata, Fukui, Osaka (for the whole period), and Nagasaki (partial period) additionally confirm the vital status of patients who were considered as alive 5 (and 10) years after diagnosis using linkage to the residential database from the death certificate. This method can complement data on patients who moved outside the prefecture where they were registered. In total, the percentage of lost to follow-up was <4%. We used a subset of the study period in which all prefectures had covered years of diagnosis and follow-up, shown in Figure 1.
Fig 1.
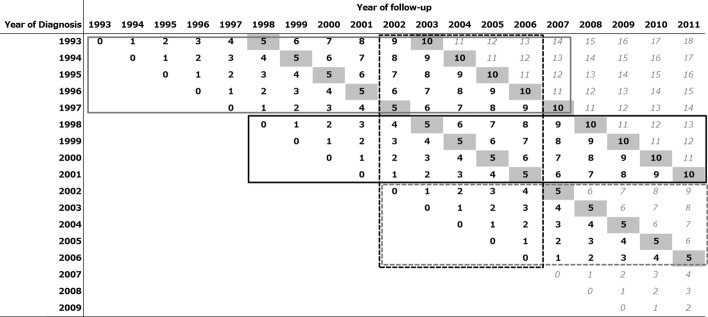
Diagnosed and followed-up years of submitted patient data from six Japanese prefectural cancer registries. Bold black figures indicate data from all six prefectures; gray figures mean a limited number of registries have provided data. The solid gray line box shows the data used to calculate 10-year survival for patients diagnosed between 1993 and 1997 using conventional methods (cohort approach). The solid black line box shows the data used to calculate 10-year survival for patients diagnosed between 1998 and 2001 using the cohort approach. The dashed gray line box shows the data to calculate 5-year survival for patients diagnosed between 2002 and 2006 using the cohort approach. The dashed black line box shows the data for period approach we applied in this study.
We calculated relative survival by sex and cancer site: 23 types for 15–99-year-old patients and four types for childhood and adolescent and young adult (AYA) cancer.
Statistical analysis
In the original research project, we analyzed all the data in Figure 1 to examine trends in cancer survival. Using conventional approaches, we calculated 10-year survival for patients diagnosed between 1993 and 1997 (Fig. 1, solid gray line box) and between 1998 and 2001 (Fig. 1, solid black line box), 5-year survival for patients diagnosed between 2002 and 2006 (Fig. 1, dashed gray line box). In addition, we estimated the 10-year survival for patients followed-up between 2002 and 2006 using the period approach (dashed black line box). In this paper, we report the 10-year survival for patients followed-up between 2002 and 2006 using the period approach and the conditional survival based on the 10-year survival, due to limitations of space. The whole report of this research project, which includes all statistics of 10-year survival by period, sex, and cancer site and the latest 10-year survival and conditional survival by sex, cancer site, age group, and stage at diagnosis is available on the website: http://www.mc.pref.osaka.jp/ocr/data/data2/j-cansis.html (in Japanese).
Relative survival
Relative survival is one of the standard methods to adjust competing causes of death, which is used when we report cancer survival from population-based cancer registry data; the ratio of the observed survival (overall survival) and the expected survival estimated by background mortality (obtained from life tables). We used the complete (single-year-of-age) national population life tables by sex to derive the background mortality of cancer patients.(10) In this study, we applied the maximum likelihood method(11) to estimate relative survival using the strel command in the publicly available Stata program.(12) The concept of relative survival is explained in the Document S1.
Period approach to estimate 10-year survival
We applied the period approach(13–16) to estimate 10-year survival. Usually we use a conventional method (cohort approach) to report cancer survival. However, long-term survival using the conventional method would be outdated, because we need to wait a long time to follow-up, up to 10 years after diagnosis. The period approach was developed to solve the problem and enabled us to estimate up-to-date long-term survival using recently followed-up data (Fig. 1, dashed black line box). Using the period approach, we only used data on patients who were alive at some point during 2002–2006, and the cumulative survival was estimated as the product of interval-specific relative survival values for cohorts of patients who were diagnosed in earlier years (1993–2006). In this study, we estimated the 10-year relative survival of patients who were followed-up between 2002 and 2006.
Conditional survival: figures for cancer survivors
Using the latest 10-year survival estimates, we also calculated conditional 5-year survival, which was 5-year survival with the precondition of having already survived a certain length of time (0–5 years in this report). Conditional 5-year survival for x-year survival is calculated as follows: divide the (x + 5)-year cumulative survival rate by the x-year cumulative survival, or calculate (x + 5)-year cumulative survival, limited to the x-year survivors, in accordance with other studies.(6–9) We show how the conditional survival estimate was obtained in Document S2, together with examples.
All statistical analyses were carried out using the standard statistical package Stata version 13.1.(17)
Results
We calculated 10-year relative survival based on patients who were followed-up between 2002 and 2006 by sex and cancer site (Table 1, Fig. S1a,b, and Table S3.1–3.3 in detail). For both sexes, over 85% of patients with thyroid and skin cancer survived for more than 10 years. Ten-year survival rates of pancreas and liver cancer patients were <10%. For men, prostate cancer patients also had a good prognosis; 10-year survival was 78%. For women, 10-year survival of breast cancer patients was approximately 80%. Ten-year survival rates of lung, oral cavity, esophageal, thyroid cancer, and malignant lymphoma for women were 8–13% higher than for men. On the other hand, men with stomach, colon, gallbladder, and bladder cancer survived longer than women.
Table 1.
Ten-year relative survival in Japanese cancer patients followed-up between 2002 and 2006 (period approach) and conditional 5-year survival of 5-year survivors (15–99 years old)
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Site (ICD-10 code) | n | Ten-year relative survival, % (95% CI) | Conditional 5-year survival of 5-year survivors, % (95% CI) | n | Ten-year relative survival, % (95% CI) | Conditional 5-year survival of 5-year survivors, % (95% CI) |
| Lip, oral cavity, and pharynx (C00–C14) | 4214 | 41.4 (39.4–43.5) | 83.3 (80.4–85.9) | 1857 | 53.6 (50.5–56.5) | 89.3 (85.8–92.0) |
| Esophagus (C15) | 8265 | 24.0 (22.7–25.4) | 74.7 (71.5–77.6) | 1540 | 32.4 (29.2–35.5) | 86.2 (79.8–90.6) |
| Stomach (C16) | 42 930 | 61.3 (60.7–62.0) | 96.8 (96.2–97.3) | 20 778 | 58.2 (57.3–59.0) | 96.5 (95.8–97.1) |
| Colon (C18) | 18 514 | 68.9 (67.9–70.0) | 97.2 (96.3–98.0) | 16 907 | 62.8 (61.8–63.8) | 96.1 (95.3–96.8) |
| Rectum (C19–C20) | 11 922 | 60.8 (59.5–62.0) | 92.7 (91.5–93.8) | 6866 | 63.2 (61.7–64.7) | 94.4 (93.1–95.5) |
| Liver (C22) | 14 230 | 9.6 (9.0–10.3) | 38.0 (35.8–40.2) | 6945 | 9.1 (8.2–10.0) | 38.4 (35.1–41.7) |
| Gallbladder etc. (C23–C24) | 4436 | 18.5 (16.9–20.1) | 85.9 (80.6–89.8) | 5064 | 15.5 (14.3–16.8) | 87.6 (83.2–90.9) |
| Pancreas (C25) | 6310 | 4.6 (3.9–5.4) | 78.8 (70.2–85.2) | 5318 | 4.8 (4.1–5.6) | 81.6 (72.4–88.0) |
| Larynx (C32)† | 2297 | 73.8 (70.8–76.6) | 93.2 (90.3–95.3) | – | – | – |
| Lung (C33–C34) | 30 537 | 18.1 (17.4–18.7) | 79.4 (77.4–81.3) | 12 525 | 31.2 (30.1–32.3) | 84.2 (82.1–86.2) |
| Skin (C43–C44) | 2213 | 86.6 (83.0–89.4) | 96.5 (92.6–98.4) | 2431 | 90.4 (87.5–92.6) | 97.6 (94.7–98.9) |
| Breast (C50) | – | – | – | 28 301 | 79.3 (78.6–79.9) | 90.5 (90.0–91.1) |
| Cervix uteri (C53) | – | – | – | 5106 | 66.1 (64.5–67.7) | 95.4 (94.2–96.4) |
| Corpus uteri (C54) | – | – | – | 4097 | 75.6 (73.7–77.3) | 96.2 (94.6–97.3) |
| Ovary (C56) | – | – | – | 4163 | 43.9 (42.0–45.7) | 85.6 (83.3–87.6) |
| Prostate (C61) | 19 519 | 78.0 (75.8–79.9) | 89.2 (86.9–91.1) | – | – | – |
| Kidney, renal pelvis, ureter etc. (C64–C66, C68) | 4725 | 59.3 (57.1–61.4) | 90.5 (88.1–92.5) | 2374 | 57.1 (54.2–59.8) | 91.5 (88.4–93.9) |
| Bladder (C67) | 5937 | 74.6 (72.6–76.5) | 94.3 (92.4–95.8) | 1928 | 62.8 (59.5–65.8) | 95.3 (91.8–97.3) |
| Brain and CNS (C70–C72, C75.1–C75.3)‡ | 921 | 21.5 (18.6–24.6) | 75.0 (68.1–80.6) | 785 | 24.4 (21.1–27.8) | 84.1 (77.1–89.0) |
| Thyroid (C73) | 1077 | 87.1 (83.2–90.2) | 97.9 (92.9–99.4) | 3713 | 94.8 (93.5–95.9) | 99.3 (98.1–99.7) |
| Malingant lymphoma (C81–C85, C96) | 4577 | 43.1 (41.0–45.1) | 86.9 (84.0–89.4) | 3925 | 50.6 (48.4–52.7) | 87.1 (84.4–89.4) |
| Multiple myeloma (C88–C90) | 1153 | 11.4 (8.9–14.3) | 41.2 (32.7–49.5) | 1090 | 14.3 (11.6–17.2) | 48.4 (40.2–56.1) |
| Leukemia (C91–C95) | 2599 | 20.5 (18.6–22.5) | 80.4 (75.2–84.7) | 1894 | 20.5 (18.4–22.7) | 77.4 (71.9–81.9) |
Both sexes combined. ‡;Malignant cases only. –, Not applicable; CI, confidence interval; CNS, central nervous system.
For both child and AYA patients, 10-year survival in males was lower than females. Ten-year survival of leukemia, acute lymphoblastic leukemia, and malignant lymphoma was higher among children than AYAs (Table 2, Fig. S1c,d, and Table S3.1 in detail).
Table 2.
Ten-year relative survival in Japanese cancer patients followed-up between 2002 and 2006 (period approach) and conditional 5-year survival of 5-year survivors: Children (0–14 years old) and adolescents and young adults (AYAs, 15–29 years old)
| Children (0–14 years old) |
AYAs (15–29 years old) |
|||||
|---|---|---|---|---|---|---|
| Types of cancer (ICD-10 code) | n | Ten-year relative survival, % (95% CI) | Conditional 5-year survival of 5-year survivors, % (95% CI) | n | Ten-year relative survival, % (95% CI) | Conditional 5-year survival of 5-year survivors, % (95% CI) |
| All sites (C00–C96) | ||||||
| male | 762 | 73.2 (69.8–76.3) | 94.9 (92.7–96.5) | 1060 | 66.0 (62.9–68.9) | 94.5 (92.4–96.0) |
| female | 621 | 79.3 (75.8–82.3) | 96.8 (94.7–98.1) | 1396 | 75.3 (72.8–77.7) | 94.9 (93.2–96.2) |
| Leukemia (C91–C95) | 470 | 76.5 (72.2–80.3) | 96.1 (93.4–97.7) | 277 | 52.5 (46.1–58.6) | 92.3 (83.6–96.4) |
| ALL | 310 | 78.6 (73.3–83.0) | 96.8 (94.7–98.1) | 97 | 36.9 (26.4–47.4) | 87.4 (60.7–96.4) |
| Malignant lymphoma (C81–C85, C96) | 125 | 88.6 (81.4–93.1) | 89.7 (82.6–94.0) | 262 | 73.4 (66.7–78.9) | 93.6 (87.2–96.9) |
| Brain and CNS (C70–C72, C75)† | 271 | 58.0 (51.3–64.2) | 95.5 (90.5–97.9) | 170 | 58.9 (50.8–66.1) | 83.5 (75.2–89.3) |
Malignant cases only. ALL, acute lymphoblastic leukemia (ICD O3-M 9811-9818, 9826, 9835-9837); CI, confidence interval; CNS, central nervous system.
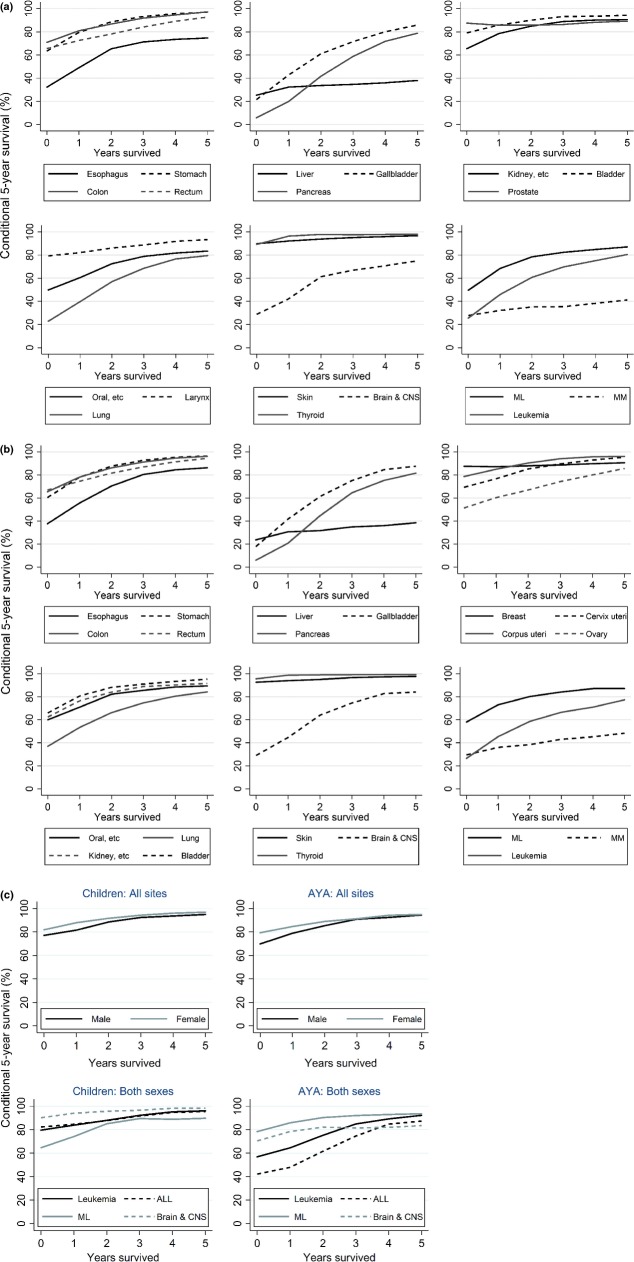
Conditional survival showed different patterns according to cancer site (Fig. 2a,b, Table 1, and Table S4.1–4.3 in detail). Most cancer sites, such as stomach, colorectum gallbladder, and kidney, showed that after surviving 2–3 years post diagnosis, the conditional 5-year survival approached 100%. Conditional survival of liver cancer and multiple myeloma patients did not increase; even 5 years post diagnosis, conditional 5-year survival was <50%. Prostate and breast cancer patients achieved high 5-year survival from the initial phase after diagnosis; however, survival among those with conditional 5-year survival did not increase. This means that a small proportion of those cancer patients continued to die after diagnosis. For thyroid and skin cancer, 5-year survival at diagnosis was approximately 90%, and conditional 5-year survival of survivors some years after diagnosis was approaching 100%. This means that patients of those cancers generally did not die from those cancers for a long time.
Fig 2.
Conditional 5-year survival for male (a) and female (b) cancer patients in Japan followed-up in 2002–2006. (c) Conditional 5-year survival for childhood and adolescent and young adult (AYA) cancer patients followed-up in 2002–2006. ALL, acute lymphoblastic leukemia; CNS, central nervous system; ML, malignant lymphoma; MM, multiple myeloma.
For both male and female childhood cancer, conditional 5-year survival increased over the years (Fig. 2c, Table 2, and Table S4.1–4.3 in detail). Conditional 5-year survival reached more than 95% 5 years after diagnosis. For AYA cancer patients, although the 5-year survival rates at diagnosis were lower than those of children, conditional 5-year survival for 5-year survivors approached 95%.
Discussion
We reported 10-year cancer survival and conditional survival using population-based cancer registry data from six prefectures with high quality and a long history within Japan. These statistics have been required by cancer patients and clinicians in order to know their prognosis for a long time. Nowadays, many patients can be cured of cancer due to improvements in cancer management (early detection and treatment). However, some sites of cancer patients need to be medically followed-up, because of the possibility of recurrence of disease. Publishing this type of statistical data is one way to support cancer patients and clinicians.
Ten-year survival
Ten-year survival rates of thyroid, skin and breast cancer were higher than 85–90%. This means that these patients have a very low possibility of death from those cancers after diagnosis. Cancer at sites that can be diagnosed earlier, such as prostate, thyroid, breast, cervix uteri, colon, rectum, stomach, and bladder, have a relatively better prognosis. In contrast, 10-year survival rates of some cancer sites that cannot be diagnosed early or for which there is no curative treatment, such as pancreas and liver cancer and multiple myeloma, are very low.
The advantage of survival for lung, oral cavity, and esophageal cancers in females may be partly explained by the differences in smoking prevalence, which was known as a prognostic factor.(18)
Comparing Japanese data with Korean data(3) (1999–2007), 10-year survival of some cancer sites in patients from Korea and Japan was similar. For esophagus, stomach, lung, and prostate cancer, 10-year survival rates in Japan was higher than those in Korea. Long-term survival of thyroid, cervical, corpus, ovarian cancer, and leukemia was higher in Korea than in Japan. These differences may be partly related to variations in the system of early detection and cancer care in both countries.
Compared with Swedish data(2) (period approach of 2005–2009), 10-year survival for most sites of cancer in Japan were higher than in Sweden, especially esophageal, stomach, colorectal, lung, ovarian, cervical, and thyroid cancer which were much higher. However, 10-year survival of multiple myeloma in Japan was slightly lower than in Sweden.
For childhood cancer, compared with the SEER report (US data),(19) although the age range was slightly different (US, 0–19 years; Japan, 0–14 years), 10-year survival was similar for leukemia, ALL, and all sites, except for brain and central nervous system, for which survival was slightly lower in Japan.
Further research is needed to investigate the mechanisms of differences in cancer survival between countries, by comparing distribution of stage at diagnosis and treatment, based on a strictly controlled protocol, as implemented by some international collaborative studies.(20,21)
Conditional survival
We presented conditional survival using up-to-date long-term survival, which was a relatively new approach to demonstrate cancer survival figures for survivors. Using this approach, we were able to provide more relevant information than conventional survival figures. As shown in the supporting information Doc S2 (example 1: stomach cancer), conditional 5-year survival of cancer of digestive organs increased according to years survived and mostly reached 100%. This means that patients who have an unfavorable status (stage) died shortly after diagnosis, and remaining patients who survived more than 5 years have almost the same probability as the general population. They could therefore be considered as cured.
On the other hand, as we show in example 2 in the supporting information Doc S2, conditional 5-year survival of liver cancer and multiple myeloma did not increase, even some years post diagnosis. This type of figure indicates that a certain number of cancer patients continue to die during follow-up years. Liver cancer patients have a high possibility of recurrence, or die from liver cirrhosis or liver failure related to the hepatitis B or C virus. There is essentially no chance of cure in patients with multiple myeloma so conditional survival remains low even after 5 years from diagnosis.
Although breast cancer showed high survival, a small proportion of survivors continue to die from the cancer, probably due to a recurrence (example 3 in the supporting information Doc S2). Similar figures were shown for prostate cancer. In total, prostate cancer patients had a favorable prognosis as most patients were diagnosed at an early stage by prostate-specific antigen testing. However, some patients who were diagnosed at an advanced stage received hormonal therapy and the treatment was effective for a few years; some patients subsequently developed resistance to the treatment and died after some years.
Limitation of the study
At the time this study was implemented, there were a limited number of prefectural cancer registries that could provide the data to estimate long-term survival using the period approach. Timeliness of registration and follow-up of patients still lagged behind North American and northern European countries. In Japan, the Cancer Registry Law was enacted in December 2013, with the aim of promoting the effective use of cancer registry data for cancer control. The law encourages improvement in the quality of population-based cancer registry data and provision of the research results for practical use by cancer patients, their families, oncologists, and public health workers. In addition, as all prefectures established prefectural cancer registries in 2012, the quality of cancer registry will improve considerably. In the near future, we will be able to estimate more up-to-date long-term cancer survival using data from many more prefectures in Japan.
We reported 10-year cancer survival and conditional survival using six prefectural population-based cancer registries in Japan. It is important for cancer patients and clinicians to report these relevant figures in succession using unbiased population-based data.
Acknowledgments
We thank the Yamagata, Miyagi, Fukui, Niigata, Osaka, and Nagasaki Cancer Registries for understanding our research concept and providing data and all medical institutes that cooperated by submitting data to population-based cancer registries. We also extend appreciation to Drs Akira Oshima, Nobuhiro Saruki, Tomotaka Sobue, Hideo Tanaka, Midori Soda, and Akiko Ikeda who gave us relevant suggestions on how to present the results, and Drs Hiroji Iwata, Masahiko Yano, and Fumiaki Imamura who commented on the work from a clinical viewpoint. This work was supported by the Ministry of Health, Labour and Welfare of Japan through a Health and Labour Sciences Research Grant for the Third Term Comprehensive Control Research for Cancer, No. H25-008 (for Young Researchers) to Y.I., H.I., T.M. and A.I.
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Table S1.1. Submitted data and data excluded from analysis.
Table S1.2. Analyzed cases diagnosed between 1993 and 2006 by sex and site.
Table S2. Years of diagnosis, follow-up method, and period of submitted data from each Japanese prefectural cancer registry.
Table S3.1. One-, 3-, 5- and 10-year relative survival of cancer patients by sex and cancer sites: Six selected prefectures in Japan, followed-up in 2002–2006 (all patients: all ages, all stages).
Table S3.2. One-, 3-, 5- and 10-year relative survival of cancer patients by sex and cancer sites: Six selected prefectures in Japan, followed-up in 2002–2006 (by age group: 15–64/65–74/75–99 years or 15–44/45–64/65–99 years).
Table S3.3. One-, 3-, 5- and 10-year relative survival of cancer patients by sex and cancer sites: Six selected prefectures in Japan, followed-up in 2002–2006 (by stage: localised/regional/distant).
Table S4.1. Conditional 5-year survival (%) of 0- to 5-year survivors in Japan (six selected prefectures), patients followed-up in 2002 and 2006 (All patients: all ages, all stages). [Correction added on 7 November 2014, after first online publication: Some data under Childhood Cancer for ALL, Malignant lymphoma, and Brain and CNS have been corrected.]
Table S4.2. Conditional 5-year survival (%) of 0- to 5-year survivors in Japan (six selected prefectures), patients followed-up in 2002 and 2006 (By age group: 15–64/65–74/75–99 or 15–44/45–64/65–99).
Table S4.3. Conditional 5-year survival (%) of 0- to 5-year survivors in Japan (six selected prefectures), patients followed-up in 2002 and 2006 (By Stage).
Fig. S1a. Ten-year relative survival of patients followed-up in 2002–2006 using period analysis, based on data from six selected prefectural population-based cancer registries in Japan (men, 15–99 years old).
Fig. S1b. Ten-year relative survival of patients followed-up in 2002–2006 using period analysis, based on data from six selected prefectural population-based cancer registries in Japan (women, 15–99 years old).
Fig. S1c. Ten-year relative survival of childhood cancer patients followed-up in 2002–2006 using period analysis, based on data from six selected prefectural population-based cancer registries in Japan (both sexes, 0–14 years old).
Fig. S1d. Ten-year relative survival of cancer patients of adolescent and young adults followed-up in 2002–2006 using period analysis, based on data from six selected prefectural population-based cancer registries in Japan (both sexes, 15–29 years old).
Doc. S1. Additional explanations of relative survival (net survival): Why do we use the relative survival approach for population-based cancer registry data?
Doc. S2. Additional explanations of conditional survival: Relationship between conventional relative survival curves and conditional 5-year survival curves.
References
- 1.Allemani C, Minicozzi P, Berrino F, et al. Predictions of survival up to 10 years after diagnosis for European women with breast cancer in 2000–2002. Int J Cancer. 2013;132:2404–12. doi: 10.1002/ijc.27895. [DOI] [PubMed] [Google Scholar]
- 2.Talback M, Dickman PW. Predicting the survival of cancer patients recently diagnosed in Sweden and an evaluation of predictions published in 2004. Acta Oncol. 2012;51:17–27. doi: 10.3109/0284186X.2011.626444. [DOI] [PubMed] [Google Scholar]
- 3.Lee JY, Jung KW, Park S, et al. Long-term survival of cancer patients in Korea, 1993–2007: National Cancer Registry Study. Asian Pac J Cancer Prev. 2010;11:1459–64. [PubMed] [Google Scholar]
- 4.Arndt V, Kaatsch P, Steliarova-Foucher E, Peris-Bonet R, Brenner H. Up-to-date monitoring of childhood cancer long-term survival in Europe: central nervous system tumours. Ann Oncol. 2007;18:1734–42. doi: 10.1093/annonc/mdm188. [DOI] [PubMed] [Google Scholar]
- 5.Zuccolo L, Dama E, Maule MM, Pastore G, Merletti F, Magnani C. Updating long-term childhood cancer survival trend with period and mixed analysis: good news from population-based estimates in Italy. Eur J Cancer. 2006;42:1135–42. doi: 10.1016/j.ejca.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y, Nakayama T, Miyashiro I, Ioka A, Tsukuma H. Conditional survival for longer-term survivors from 2000–2004 using population-based cancer registry data in Osaka, Japan. BMC Cancer. 2013;13:304. doi: 10.1186/1471-2407-13-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu XQ, Baade PD, O'Connell DL. Conditional survival of cancer patients: an Australian perspective. BMC Cancer. 2012;12:460. doi: 10.1186/1471-2407-12-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellison LF, Bryant H, Lockwood G, Shack L. Conditional survival analyses across cancer sites. Health Rep. 2011;22:21–5. [PubMed] [Google Scholar]
- 9.Merrill RM, Hunter BD. Conditional survival among cancer patients in the United States. Oncologist. 2010;15:873–82. doi: 10.1634/theoncologist.2009-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ministry of Health, Labour and Welfare. Abridged Life Tables in Japan for 1962–2011. Tokyo, Japan: Center for Cancer Co/ntrol and Information Services, National Cancer Center; 2011. [Google Scholar]
- 11.Esteve J, Benhamou E, Croasdale M, Raymond L. Relative survival and the estimation of net survival: elements for further discussion. Stat Med. 1990;9:529–38. doi: 10.1002/sim.4780090506. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Research UK Cancer Survival Group, London School of Hygiene and Tropical Medicine. 2009. Strel computer program version 1.2.7 for cancer survival analysis. [Cited 11 Jun 2014.] Available from URL: http://www.lshtm.ac.uk/ncdeu/cancersurvival/tools/index.htm.
- 13.Brenner H, Gefeller O. Deriving more up-to-date estimates of long-term patient survival. J Clin Epidemiol. 1997;50:211–6. doi: 10.1016/s0895-4356(97)00280-1. [DOI] [PubMed] [Google Scholar]
- 14.Brenner H, Gefeller O, Hakulinen T. Period analysis for ‘up-to-date’ cancer survival data: theory, empirical evaluation, computational realisation and applications. Eur J Cancer. 2004;40:326–35. doi: 10.1016/j.ejca.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Brenner H, Hakulinen T. Up-to-date long-term survival curves of patients with cancer by period analysis. J Clin Oncol. 2002;20:826–32. doi: 10.1200/JCO.2002.20.3.826. [DOI] [PubMed] [Google Scholar]
- 16.Brenner H, Soderman B, Hakulinen T. Use of period analysis for providing more up-to-date estimates of long-term survival rates: empirical evaluation among 370,000 cancer patients in Finland. Int J Epidemiol. 2002;31:456–62. [PubMed] [Google Scholar]
- 17.StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 18.Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132:401–10. doi: 10.1002/ijc.27617. [DOI] [PubMed] [Google Scholar]
- 19.Ward E, Desantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 20.Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–38. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.London School of Hygiene and Tropical Medicine. CONCORD programme. Grobal surveillance of cancer survival. [Cited 11 Jun 2014.] Available from URL: http://www.lshtm.ac.uk/eph/ncde/cancersurvival/research/concord/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.1. Submitted data and data excluded from analysis.
Table S1.2. Analyzed cases diagnosed between 1993 and 2006 by sex and site.
Table S2. Years of diagnosis, follow-up method, and period of submitted data from each Japanese prefectural cancer registry.
Table S3.1. One-, 3-, 5- and 10-year relative survival of cancer patients by sex and cancer sites: Six selected prefectures in Japan, followed-up in 2002–2006 (all patients: all ages, all stages).
Table S3.2. One-, 3-, 5- and 10-year relative survival of cancer patients by sex and cancer sites: Six selected prefectures in Japan, followed-up in 2002–2006 (by age group: 15–64/65–74/75–99 years or 15–44/45–64/65–99 years).
Table S3.3. One-, 3-, 5- and 10-year relative survival of cancer patients by sex and cancer sites: Six selected prefectures in Japan, followed-up in 2002–2006 (by stage: localised/regional/distant).
Table S4.1. Conditional 5-year survival (%) of 0- to 5-year survivors in Japan (six selected prefectures), patients followed-up in 2002 and 2006 (All patients: all ages, all stages). [Correction added on 7 November 2014, after first online publication: Some data under Childhood Cancer for ALL, Malignant lymphoma, and Brain and CNS have been corrected.]
Table S4.2. Conditional 5-year survival (%) of 0- to 5-year survivors in Japan (six selected prefectures), patients followed-up in 2002 and 2006 (By age group: 15–64/65–74/75–99 or 15–44/45–64/65–99).
Table S4.3. Conditional 5-year survival (%) of 0- to 5-year survivors in Japan (six selected prefectures), patients followed-up in 2002 and 2006 (By Stage).
Fig. S1a. Ten-year relative survival of patients followed-up in 2002–2006 using period analysis, based on data from six selected prefectural population-based cancer registries in Japan (men, 15–99 years old).
Fig. S1b. Ten-year relative survival of patients followed-up in 2002–2006 using period analysis, based on data from six selected prefectural population-based cancer registries in Japan (women, 15–99 years old).
Fig. S1c. Ten-year relative survival of childhood cancer patients followed-up in 2002–2006 using period analysis, based on data from six selected prefectural population-based cancer registries in Japan (both sexes, 0–14 years old).
Fig. S1d. Ten-year relative survival of cancer patients of adolescent and young adults followed-up in 2002–2006 using period analysis, based on data from six selected prefectural population-based cancer registries in Japan (both sexes, 15–29 years old).
Doc. S1. Additional explanations of relative survival (net survival): Why do we use the relative survival approach for population-based cancer registry data?
Doc. S2. Additional explanations of conditional survival: Relationship between conventional relative survival curves and conditional 5-year survival curves.