Abstract
Background and aim
Morbid obesity represents a proinflammatory and pro-oxidative state associated with dysregulation of adipokines. We aimed to evaluate the circulating levels of chemerin and omentin-1 in morbidly obese (MO) patients and to investigate the relationship between these two adipokines and between each of them and anthropometric, metabolic, oxidative stress and chronic inflammatory parameters.
Material and methods
32 MO patients and 20 controls were investigated in this study. Anthropometric, metabolism parameters, inflammatory markers, oxidative stress indicators as well as chemerin and omentin-1 were measured.
Results
Serum levels of chemerin were increased while omentin-1 levels were decreased in MO patients when compared with controls. Chemerin correlated positively with insulin, HOMA-IR, LDL cholesterol and negatively with total antioxidant response. Omentin-1 correlated negatively with tumor necrosis factor alpha and total cholesterol. In a multiple linear stepwise regression analysis we learnt that only HOMA-IR (β=0.70, p<0.001), total cholesterol (β=0.42, p<0.001) and triglycerides (β=0.31, p<0.05) remained significantly associated with chemerin changes. Using the same analysis we noticed that total cholesterol (β=−0.71, p<0.001), fasting glucose (β= −0.40, p<0.05) and body mass index (BMI) (β= −0.38, p<0.05) were considered to be significant predictors for omentin-1 changes.
Conclusions
Chemerin and omentin-1 synthesis was dysregulated in MO patients. Chemerin might play a role in insulin resistance and oxidative stress. Chemerin changes seemed to be predicted mainly by insulin resistance. Omentin-1 levels were inversely associated with chronic inflammation and dyslipidemia while the main modulating factors seemed to be dyslipidemia, hyperglycemia and BMI.
Keywords: morbid obesity, adipokines, oxidative stress, chronic inflammation
Introduction
Obesity is one of the most important concerns of the 21st century, and it is considered to be a major public health issue worldwide. Morbid obesity defined as body mass index (BMI) over 40 kg/m2 represents a stage in which lifestyle changes, diet and pharmaceutical agents fail to obtain and sustain a significant weight loss [1]. A cluster of diseases such as type 2 diabetes mellitus, dyslipidemia, hypertension, coronary heart disease, obstructive sleep apnea, nonalcoholic fatty liver disease, gastroesophageal reflux disease and several malignancies are associated with obesity and threat to reduce life expectancy [2]. Adipose tissue is considered to be an active endocrine organ that produces a various number of adipokines which are dysregulated in obesity and induce a state of chronic inflammation, oxidative stress and insulin resistance thereby promoting metabolic complications [3,4].
Chemerin is one of the novel adipokines secreted by adipose tissue in an inactive form as prochemerin which is then activated through proteolytic cleavage by serine proteases of the coagulation, fibrinolytic and inflammatory cascades [5]. Recent investigations point out chemerin as a regulator of adipogenesis, glucose metabolism and inflammation as it plays an important role in macrophage infiltration into the adipose tissue [6,7]. The body of evidence demonstrates that obesity is associated with elevated levels of chemerin that might influence dysregulation of glucose metabolism. On the other hand, obese patients with type 2 diabetes often have hyperinsulinemia, which seems to increase serum chemerin levels [8]. The implication of chemerin in metabolism and inflammation might provide a link between obesity and its related disorders, such as type 2 diabetes and cardiovascular diseases [9–12].
Omentin-1 is a fat depot-specific secretory protein produced by the stromal vascular cells from the adipose tissue. Omentin-1 enhances insulin-stimulated glucose uptake in adipocytes but has no intrinsic insulin mimicking activity [13]. Omentin-1 plasma levels and the adipose tissue gene expression are decreased in obesity and there is a positive correlation with the plasma adiponectin and high-density lipoprotein and an inverse correlation with waist circumference, BMI, and insulin resistance [14,15].
Controversy has arisen concerning the regulatory mechanisms that modulate chemerin and omentin-1 expression and function, namely obesity [14,16–19], chronic inflammation [15,20,21], insulin resistance [21–23], or oxidative stress. Further studies are necessary in morbidly obese (MO) patients to explain the involvement of chemerin and omentin-1 in glucose homeostasis, inflammation, and oxidative stress as well as to clarify whether these two novel adipokines are dysregulated in response to increased adipose tissue, insulin resistance, chronic inflammation, or oxidative stress. Therefore the present study was designed to investigate the levels of chemerin and omentin-1, to analyze the link between the two novel adipokines and between each of them and MO patient obesity, metabolic state, chronic inflammation, and oxidative stress.
Materials and Methods
2.1. Study design, setting and ethics statements
This was a case-control study in which the period of data collection was between November 2011 and February 2013. The study was conducted at the Physiology and Patophysiology Departments from “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania. Morbidly obese patients were recruited from Bariatric Center of Excellence (BariXL) of “Ponderas” (Delta) Hospital, Bucharest and were all eligible for bariatric surgery. The study protocol was reviewed and approved by the Ethics Committee of “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania. The present study was conducted in accordance with the ethical principles presented in the Declaration of Helsinki [24]. All participants were informed about the aims and methods of the study. Written informed consent was obtained from all participants.
2.2. Subjects
Two groups of subjects one consisting of 32 MO patients (12 male and 20 female) aged between 20 and 63 and the other formed out of 20 normal weight healthy controls matched for age and gender were investigated in this study. The inclusion criteria for the MO group was BMI over 40 kg/m2 and agreement to participate in the study. The exclusion criteria were psychiatric disorders (schizophrenia); severe endocrine diseases other than diabetes; acute infectious and inflammatory diseases; cardiac, hepatic, or renal failure; cancer or systemic diseases (systemic autoimmune diseases) smoking, alcohol, or drug addiction. There were no patients under insulin treatment. For the control group the inclusion criteria were the same age and gender as in the case of the MO group and BMI between 18.5 and 24.9 kg/m2. All controls were clinically and biochemically free from any disease and under no medication.
2.3 Anthropometric measurements, blood collection and laboratory analysis
Anthropometric measurements were performed and BMI was calculated by dividing the weight into kilograms by the square of the height in meters.
Blood samples were collected after overnight fast and serum was separated after centrifugation. The samples were assayed immediately or stored until analysis at −80°C for biochemical tests and at −20°C for enzyme-linked immunosorbent assays (ELISAs). Serum fasting glucose, total cholesterol and triglycerides were measured using the standard enzymatic colorimetric method (kit Clini-Lab Diagnosticum Hungary). Serum high-density lipoprotein-cholesterol (HDL cholesterol) was determined using the precipitation method (kit Clini-Lab Diagnosticum Hungary). Serum low-density lipoprotein-cholesterol (LDL cholesterol) concentration was calculated by Friedewald formula [25]. Fasting serum insulin, chemerin and omentin-1 levels were assayed by commercially ELISA kit (Millipore, U.S.A) according to the manufacturer’s protocol. Insulin resistance was estimated according to the homeostasis model assessment of insulin resistance (HOMA-IR). The following formula was used in its calculation HOMA-IR = (fasting insulin μU/ml × fasting glucose mg/dl/)/22.5×18 [26]. A normal value was considered to be <2.5. C reactive protein (CRP) was measured using an ELISA kit from Abcam, Cambridge, UK. Serum tumor necrosis factor alpha (TNF-α) value was quantified using the ELISA kit from Thermo Scientific, U.S.A. All ELISA procedures were performed using TECAN device.
Serum nitric oxide (NO) concentration was evaluated using a colorimetric method. Nitrite and nitrate (NOx) were determined as final stabile products. The principle of this method is the reduction of nitrate by vanadium (III) combined with detection by Griess reaction. The Griess reaction was used as a measure of nitric oxide synthase (NOS) activity and of NO synthesis. Serum samples were passed through 10-kDa filters (Sartorius AG, Goettingen, Germany) and deproteinized by methanol/diethylether (3/1, v/v) (sample: methanol/diethylether, 1:9, v/v) [27]. 100 μLVCl3 (8 mg/mL) were added to 100 μL of the supernatant for reduction of nitrate to nitrite, followed by addition of 100 μL Griess reagents (50 μLSULF and 50 μLNEDD). (0.1%). Absorbance was read at 540 nm after 30 min of incubation at 37°C. Serum NOx was expressed as nitrite μmol/l [28].
Total oxidative status (TOS) and total antioxidant response (TAR) were evaluated as oxidative stress markers. We used a colorimetric method for determination of serum TOS. The assay is based on the oxidation of ferrous to ferric ion in the presence of various oxidant species in acidic medium and the measurement of the ferric ion by xylenol orange. Calibration was done with hydrogen peroxide (H2O2), and the results were expressed in μmol H2O2 equiv./l[29]. Serum TAR was measured using a colorimetric method as well. Fenton reaction produces hydroxyl radical, and the rate of reactions is monitored by following the absorbance of colored dianisidyl radicals. The oxidative reactions initiated by the hydroxyl radicals present in the reaction medium are suppressed by the antioxidant components of the serum thereby preventing the color change and providing an effective measure of the total antioxidant capacity of the serum. The assay is calibrated with Trolox, and the results are expressed as mmol trolox equiv./l [30]. Absorbance of the samples was measured in a Cecil 3000 spectrophotometer.
The percent ratio of the total oxidative status to the total antioxidant response gave the oxidative stress index (OSI), an indicator of the degree of oxidative stress [31]. OSI was calculated with the formula: OSI (Arbitrary Unit) = TOS (μmol H2O2 Equiv. /L) / TAC (mmol Trolox Equiv./L).
2.4. Statistical analysis
The univariate normality of the data from the MO patients and control subjects, respectively, was analyzed using the Shapiro–Wilk test, graphical method (Q–Q plot), and evaluation of skewness and kurtosis together with their standard errors. The descriptive statistics were calculated as median and interquartile range for non-Gaussian variables and as mean ± standard deviation for Gaussian distribution. The comparison between groups was performed using the Student’s t test coupled with the Levene test for determining the homogeneity of the variance for the normally distributed data. Otherwise the Mann–Whitney U-test was applied for skewed data. Relationships between variables were verified by the Spearman’s test. Any skewed data were logarithmically transformed before performing simple and multiple linear stepwise regression analyses. Levels of statistical significance were set at P <0.05. The statistical analysis was performed with the SPSS v.19 software.
Results
The descriptive characteristics of the studied groups were presented in table I. Median age was similar between groups. No statistically significant differences were observed between HDL cholesterol when comparing the two groups. Regarding BMI, triglycerides, fasting glucose, fasting insulin, HOMA-IR, TOS, OSI, NOx, CRP, TNF-α and chemerin values they were significantly increased in MO patients as compared with control subjects. TAR and omentin-1 were found to be decreased statistically significant in MO patients when compared with normal weight healthy subjects.
Table I.
Anthropometric and biochemical characteristics of the groups
| Control Subjects (n=20) | MO Patients (n=32) | P | |
|---|---|---|---|
| Age | 39 (31–41) | 41.5 (31.75–46.50) | NS |
| Weight (kg) | 57 (51–59) | 127 (110–148.50) | p<0.001 |
| BMI (kg/m2) | 19.72 (19.19–24.60) | 46.29 (40.72–50.23) | p<0.001 |
| Total Cholesterol (mg/dl) | 151.71±24.08 | 197.90±51.35 | p<0.05 |
| Triglycerides (mg/dl) | 82 (63–94) | 140.5 (115.5–187.25) | p<0.001 |
| HDL cholesterol (mg/dl) | 39.71±9.30 | 44.00±10.53 | NS |
| LDL cholesterol (mg/dl) | 95.95±19.83 | 126.79±46.50 | p<0.05 |
| Fasting Glucose (mg/dl) | 87 (83–98) | 111.5 (90.25–127) | p<0.05 |
| Fasting Insulin (μU/ml) | 5.31 (4.38–9.75) | 10.82(5.62–13.75) | p<0.05 |
| HOMA-IR | 1.32 (0.9–1.97) | 2.55 (1.40–3.86) | p<0.05 |
| TOS (μmol H2O2 equiv./l) | 19.91 (16.39–26.43) | 106.35 (67.37–120.04) | p<0.001 |
| TAR (mmol trolox equiv./l) | 0.65 (0.62–0.73) | 0.58 (0.51–0.63) | p<0.05 |
| OSI | 33.14±8.85 | 170.75±60.98 | p<0.001 |
| NOx (μmol/l) | 47.70±5.28 | 66.89±11.81 | p<0.001 |
| CRP (pg/ml) | 0.30 (0.17–0.50) | 12.94 (8.58–17.17) | p<0.001 |
| TNF-α (pg/ml) | 19.52 (8.49–26.87) | 28.09 (18.90–33.92) | p<0.05 |
| Chemerin (ng/ml) | 25.45 (19.75–30.10) | 74.20 (58.31–116.90) | p<0.001 |
| Omentin-1 (ng/ml) | 106.38±28.44 | 79.72±28.08 | P<0.05 |
The results are expressed as mean±s.d for parameters with Gaussian distribution and median (25–75 percentiles) for non Gaussian distribution; p-values were calculated by the Student t-test (normally distributed variables; total cholesterol, HDL cholesterol, LDL cholesterol, NOx, OSI, omentin-1) or the nonparametric Mann–Whitney U-test (not normally distributed variables; weight, BMI, triglycerides, fasting glucose, insulin, HOMA-IR, TOS, TAR, CRP, TNF-α, chemerin).
BMI: body mass index, HDL cholesterol: high-density lipoprotein-cholesterol, LDL cholesterol: low-density lipoprotein-cholesterol, HOMA-IR: homeostasis model assessment-insulin resistance, TOS: total oxidant status, TAR: total antioxidant response, OSI: oxidative stress index, NOx: NO metabolites nitrite/nitrate, CRP: C-reactive protein, TNF-α: tumor necrosis factor α
Table II revealed that chemerin correlated positively with fasting insulin, HOMA-IR and negatively with TAR. Omentin-1 correlated negatively with total cholesterol and TNF-α. No correlation between chemerin and omentin-1 was noticed.
Table II.
Bivariate correlation (Spearman) of chemerin and omentin-1 with other variables in MO patients
| Parameter | Chemerin | Omentin-1 | ||
|---|---|---|---|---|
| r | p | r | p | |
| BMI (kg/m2) | 0.86 | 0.641 | 0.034 | 0.884 |
| Weight (kg) | 0.178 | 0.331 | 0.065 | 0.780 |
| Total Cholesterol (mg/dl) | 0.228 | 0.210 | −0.559 | 0.008 |
| Triglycerides (mg/dl) | 0.098 | 0.595 | −0.27 | 0.907 |
| HDL cholesterol (mg/dl) | 0.231 | 0.267 | −0.261 | 0.253 |
| LDL cholesterol (mg/dl) | 0.503 | 0.009 | −0.256 | 0.263 |
| Fasting Glucose (mg/dl) | 0.305 | 0.090 | 0.235 | 0.306 |
| Fasting Insulin (μU/ml) | 0.629 | 0.000 | 0.130 | 0.575 |
| HOMA-IR | 0.578 | 0.001 | 0.281 | 0.218 |
| TOS (μmol H2O2 equiv./l) | 0.157 | 0.392 | 0.155 | 0.504 |
| TAR (mmol trolox equiv./l) | −0.480 | 0.005 | 0.154 | 0.505 |
| OSI | 0.148 | 0.420 | 0.061 | 0.793 |
| NOx (μmol/l) | 0.126 | 0.492 | 0.103 | 0.658 |
| CRP (pg/ml) | 0.260 | 0.150 | 0.104 | 0.654 |
| TNF-α (pg/ml) | 0.115 | 0.531 | −0.498 | 0.022 |
| Chemerin (ng/ml) | - | - | 0.099 | 0.670 |
| Omentin-1 (ng/ml) | 0.099 | 0.670 | - | - |
BMI: body mass index, HDL cholesterol: high-density lipoprotein-cholesterol, LDL cholesterol: low-density lipoprotein-cholesterol, HOMA-IR: homeostasis model assessment-insulin resistance, TOS total oxidant status, TAR total antioxidant response, OSI: oxidative stress index NOx: NO metabolites nitrite/nitrate, CRP: C-reactive protein, TNF-α tumor necrosis factor α
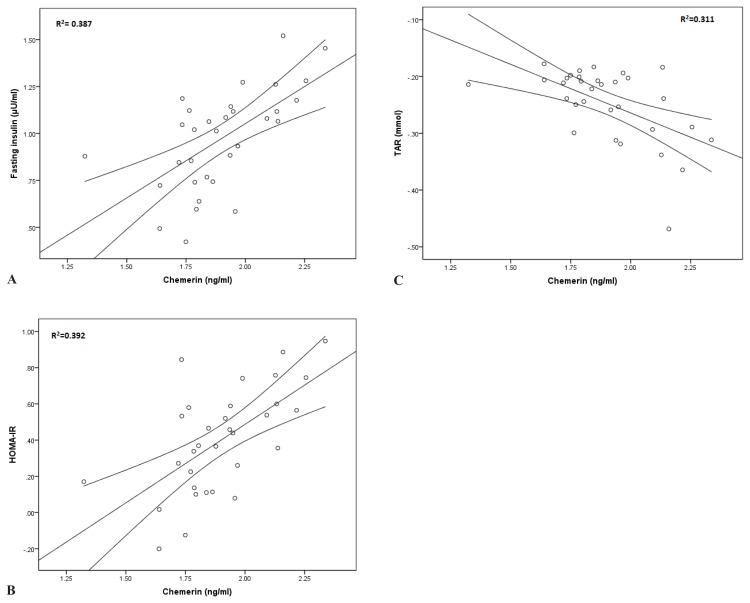
In a simple linear regression we observed that serum chemerin levels correlated positively with fasting insulin (β=0.622, p<0.001), HOMA-IR (β=0.626, p<0.001), LDL cholesterol (β=0.552, p<0.05) and negatively with TAR (β=−0.558, p<0.001) (Figure 1). Serum omentin-1 levels correlated negatively with TNF-α (β=−0.558, p<0.05) and total cholesterol (β=−0.633, p<0.05).
Figure 1.
Simple linear regression between chemerin and markers of glucose homeostasis and oxidative stress in MO patients. (A) Chemerin as an independent parameter and fasting insulin. (B) Chemerin as an independent parameter and HOMA-IR. (C) Chemerin as an independent parameter and TAR.
In a multiple linear stepwise regression analysis with serum chemerin as a dependent variable and weight, BMI, total cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, fasting glucose, fasting insulin, HOMA-IR, TOS, TAR, NOx, CRP, TNF-α and omentin-1 respectively as independent variables only HOMA-IR (β=0.70, p<0.001), total cholesterol (β=0.42, p<0.001) and triglycerides (β=0.31, p<0.05) remained significantly associated with chemerin (table III).
Table III.
Multiple linear regression analyses with serum chemerin as a dependent variable in MO patients
| Chemerin | Unstandardized coefficients | Standardized Coefficients | t | Significance | |
|---|---|---|---|---|---|
| B | s.e. | β | |||
| HOMA-IR | 0.54 | 0.09 | 0.70 | 6.02 | 0.00 |
| Total cholesterol | 0.00 | 0.00 | 0.42 | 3.60 | 0.00 |
| Triglycerides | 0.41 | 0.15 | 0.31 | 2.64 | 0.01 |
| Model | R | R2 | Adjusted R2 | Standard error of the estimate | |
| 0.847 | 0.718 | 0.677 | 0.13 | ||
Beta is a standardized regression coefficient which shows the significance of each independent variable in the multiple linear regression analyses. Adjusted R2 express the significant and independent contribution of HOMA-IR, total cholesterol and triglycerides to circulating chemerin in MO patients
Using the same analysis with omentin-1 as a dependent variable and weight, BMI, total cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, fasting glucose, fasting insulin, HOMA-IR, TOS, TAR, NOx, CRP, TNF-α and chemerin respectively as independent variables we noticed that only total cholesterol (β=−0.71, p<0.001), fasting glucose (β=−0.40, p<0.05) and BMI (β=−0.38, p<0.05) were considered to be significant predictors for serum omentin-1 changes (table IV).
Table IV.
Multiple linear regression analyses with serum omentin-1 as a dependent variable in MO patients
| Omentin-1 | Unstandardized coefficients | Standardized Coefficients | t | Significance | |
|---|---|---|---|---|---|
| B | s.e. | β | |||
| Total cholesterol | −0.36 | 0.07 | −0.71 | −4.86 | 0.00 |
| Fasting glucose | −107.66 | 38.58 | −0.40 | −2.79 | 0.01 |
| BMI | −146.74 | 57.19 | −0.38 | −2.56 | 0.02 |
| Model | R | R2 | Adjusted R2 | Standard error of the estimate | |
| 0.809 | 0.654 | 0.593 | 17.91 | ||
Beta is a standardized regression coefficient which shows the significance of each independent variable in the multiple linear regression analyses. Adjusted R2 express the significant and independent contribution of total cholesterol, fasting glucose and BMI to circulating omentin-1 in MO patients
Discussions
The purpose of the present study was to evaluate the circulating levels of chemerin and omentin-1 in MO patients as compared to normal weight healthy controls. Additionally, we aimed to investigate the relationship between the two novel adipokines and between each of them and anthropometric, metabolic, oxidative stress and chronic inflammatory parameters.
Several features of obesity such as insulin resistance, dyslipidemia, oxidative stress and chronic inflammation lead to metabolic and cardiovascular diseases. There is an important interrelation between oxidative stress and chronic inflammation which augments free radicals production and vice versa. Nitric oxide (NO) overproduced in MO by the TNF-α stimulated macrophages reacts with superoxide, a free radical induced by nicotinamide adenine dinucleotide phosphate (NADPH) and leads to the formation of peroxynitrite, a very aggressive reactive species which induces oxidation of carbohydrates, lipids and proteins [32,33]. Thereby, NO can be considered both a proinflammatory and an oxidative stress marker. Obesity-induced insulin resistance and type 2 diabetes are determined also by the dysregulation of some adipokines such as TNF-α, chemerin and omentin-1 [3,13]. However, the relationship between chemerin, omentin-1, insulin resistance, oxidative stress and chronic inflammation respectively are not completely elucidated.
Chemerin is a novel adipokine that has been suggested to represent the link between obesity and the development of type 2 diabetes. In our study we demonstrated that circulating chemerin levels were increased in MO patients, as shown by other authors as well [34,35]. However we found no significant correlation between chemerin and BMI in accordance with some authors [11] and in contrast with others [16,17,18,35], and this could be due to the fact that BMI is not considered to be the best tool for adipose tissue evaluation. We also demonstrated that chemerin correlated positively with fasting insulin and HOMA-IR and negatively with total oxidative response, suggesting that chemerin might induce dysregulation of glucose homeostasis and oxidative stress. Thereby, chemerin could be a link between insulin resistance and oxidative stress in MO patients. Other authors reported chemerin to be associated with the cluster of features of metabolic syndrome such as high fasting glucose [11,21], triglycerides [11,16,21], low HDL cholesterol [11,16], elevated blood pressure [11,33] and insulin resistance [11,17,21,34] in obese, overweight but also in lean subjects. On the other hand, Bozaoglu K et al. noted and explained the lack of associations between chemerin levels and metabolic syndrome phenotypes by the dysregulation of metabolic processes associated with the development of type 2 diabetes which might have a disruptive effect [35]. Regarding chronic inflammation we revealed elevated levels of TNF-α, CRP and NOx, but no significant correlations between chemerin and the aforementioned parameters. Chemerin is a proinflammatory marker produced by the adipocytes and preadipocytes as it acts as a chemoattractant for leukocytes to sites of inflammation. Thereby, it might be possible that it plays a role in chronic inflammation that occurs in obesity [7]. Chronic inflammation induces free radicals production and in our study we noticed increased levels of TOS and OSI, markers of oxidative stress. Other authors revealed that in obese patients with type 2 diabetes chemerin correlated positively with 8-iso prostaglandin F2α (8-iso-PGF2α), another marker of oxidative stress [36].
Another important problem to elucidate was whether serum chemerin levels are elevated in obesity mainly due to adipose tissue excess, chronic inflammation, insulin resistance or dyslipidemia. To address this issue we used a multiple regression analysis and we observed that the main independent contributor to chemerin increase was HOMA-IR. Our finding was similar to Chakaroun et al. who demonstrated that both insulin resistance and chronic inflammation were BMI-independent predictors of elevated serum chemerin concentrations and in contrast with Sledzinski et al. who concluded that BMI was the main predictor of serum chemerin concentration [18,21]. According to our findings we could speculate that chemerin is more related to glucose metabolism and insulin resistance than excess of adipose tissue. In this respect some authors revealed increased level of chemerin in lean, overweight, and obese patients with type 2 diabetes independently of BMI while others concluded that the increase of serum chemerin was related to the amount of total percentage of body fat in patients with no previous history or evidence of cardiovascular disease, diabetes, hypertension or dyslipidemia [37,38]. On the other hand, when analyzing a group of obese type 2 diabetic patients Weingert et al. underlined that elevated chemerin levels were more associated with inflammation rather than BMI, thereby suggesting that chemerin might be more like a marker of inflammation than obesity [20].
Omentin-1 is a new adipokine involved in insulin mediated glucose uptake and has been demonstrated to be decreased in obesity, insulin resistance and type 2 diabetes [15,19]. Our results regarding levels of omentin-1 were in agreement with other studies that have demonstrated reduced values of this novel adipokine in MO patients as compared to normal weight healthy subjects [39]. In our group of patients no correlation was found between omentin-1 and BMI and this finding goes hand in hand with Auguet et al. and in disagreement with Moreno-Navarette et al. who studied two groups of obese men and women [39,40]. Decreased levels of omentin-1 might explain the reduced insulin mediated glucose uptake in insulin sensitive tissues and might contribute to development of type 2 diabetes in obese patients. Auguet et al. demonstrated in a group of MO women that plasma omentin levels inversely correlated with glucose metabolism parameters [39]. We failed to demonstrate a negative correlation between omentin-1 and glucose homeostasis parameters, but when analyzing using a multiple linear regression we observed that fasting glucose was an independent contributor for circulating omentin-1 level, suggesting that increased serum glucose might reduce omentin-1 production in MO patients. From a simple linear regression we learnt that omentin-1 correlated inversely with TNF-α implying that decreased levels of omentin-1 as found in our group of patients might be involved in promoting and sustaining chronic inflammation, as revealed by other authors as well [41]. Obesity is associated with insulin resistance and chronic inflammation, but omentin-1 seems to be a positive marker involved in preventing the development of obesity-associated diseases.
A multiple linear regression analysis revealed that the independent contributors to circulating levels of omentin-1 were total cholesterol, fasting glucose and BMI in a percentage of 59.3%. This explains that in MO patients decreased levels of omentin-1 are mainly determined by three parameters of metabolic syndrome. Moreno-Navarette et al. showed in a multiple linear regression analyses that BMI and sex were the two independent contributors to circulating omentin variance after adjusting by fasting insulin [42]. When studied according to sex, BMI was the only independent contributor to circulating omentin-1 variance after adjusting by fasting insulin [42].
Conclusions
Chemerin levels were significantly increased in MO patients and were related to insulin resistance and oxidative stress. Chemerin changes seemed to be predicted mainly by insulin resistance. Interestingly, we found no relation between chemerin and anthropometric parameters and this merits further investigation. Omentin-1 levels were decreased in MO, were inversely associated with chronic inflammation and dyslipidemia and the main modulating factors seemed to be dyslipidemia, hyperglicemia and BMI.
Acknowledgements
This study was performed as part of a financed grant from the University of Medicine and Pharmacy “Iuliu Hatieganu”, Cluj-Napoca (Romania), no. 27020/39/15.11.2011.
References
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric Surgery A Systematic Review and Meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Bullo M, Casas-Agustench P, Amigo-Correig P, Aranceta J, Salas-Salvado J. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutrition. 2007;10(10A):1164–1172. doi: 10.1017/S1368980007000663. [DOI] [PubMed] [Google Scholar]
- 3.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, et al. Inflammation, Oxidative Stress, and Obesity. Int J Mol Sci. 2011;12:3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Nakayama T. Inflammation, a Link between Obesity and Cardiovascular Disease. Hindawi Publishing Corporation Mediators of Inflammation. 2010:1–17. doi: 10.1155/2010/535918. ID 535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.John H, Hierer J, Haas O, Forssmann WG. Quantification of angiotensin-converting-enzymemediated degradation of human chemerin 145–154 in plasma by matrix-assisted laser desorption/ionization-timeof-flight mass spectrometry. Anal Biochem. 2007;362(1):117–125. doi: 10.1016/j.ab.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Rourke JL, Dranse HJ, Sinal CJ. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes Rev. 2013;14(3):245–262. doi: 10.1111/obr.12009. [DOI] [PubMed] [Google Scholar]
- 8.Roman AA, Parlee SD, Sinal CJ. Chemerin: a potential endocrine link between obesity and type 2 diabetes. Endocrine. 2012;42:243–251. doi: 10.1007/s12020-012-9698-8. [DOI] [PubMed] [Google Scholar]
- 9.Tan BK, Chen J, Farhatullah S, et al. Insulin and metformin regulate circulating and adipose tissue chemerin. Diabetes. 2009;58:1971–1977. doi: 10.2337/db08-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehrke M, Becker A, Greif M, et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol. 2009;161(2):339–344. doi: 10.1530/EJE-09-0380. [DOI] [PubMed] [Google Scholar]
- 11.Wang LY, Wei L, Yu HY, et al. Relationship of serum Chemerin to obesity and type 2 diabetes mellitus. Zhonghua Yi Xue Za Zhi. 2009;89(4):235–238. [PubMed] [Google Scholar]
- 12.Stejskal D, Karpisek M, Hanulova Z, Svestak M. Chemerin is an independent marker of the metabolic syndrome in a Caucasian population—a pilot study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152(2):217–221. doi: 10.5507/bp.2008.033. [DOI] [PubMed] [Google Scholar]
- 13.Singh B, Arora S, Goswami B, Mallika V. Metabolic syndrome: A review of emerging markers and management. Diabetes & Metabolic Syndrome:Clinical Research & Reviews. 2009;3(4):240–254. [Google Scholar]
- 14.Zhuang XF, Zhao MM, Weng CL, Sun NL. Adipocytokines: A bridge connecting obesity and insulin resistance. Med Hypotheses. 2009;73(6):981–985. doi: 10.1016/j.mehy.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 15.de Souza Batista CM, Yang RZ, Lee MJ, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56(6):1655–1661. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 16.Tan BK, Adya R, Randeva HS. Omentin: A Novel Link Between Inflammation, Diabesity, and Cardiovascular Disease. Trends Cardiovasc Med. 2010;20:143–148. doi: 10.1016/j.tcm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Bozaoglu K, Bolton K, McMillan J, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148(10):4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 18.Sell H, Divoux A, Poitou C, et al. Chemerin Correlates with Markers for Fatty Liver in Morbidly Obese Patients and Strongly Decreases after Weight Loss Induced by Bariatric Surgery. J Clin Endocrinol Metab. 2010;95(6):2892–2896. doi: 10.1210/jc.2009-2374. [DOI] [PubMed] [Google Scholar]
- 19.Sledzinski T, Korczynska J, Hallmann A, et al. The increase of serum chemerin concentration is mainly associated with the increase of BMI in obese, non-diabetic subjects. J Endocrinol Invest. 2013;36(6):428–434. doi: 10.3275/8770. [DOI] [PubMed] [Google Scholar]
- 20.Pan HY, Guo L, Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract. 2010;88(1):29–33. doi: 10.1016/j.diabres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Weigert J, Neumeier M, Wanninger J, et al. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol (Oxf) 2010;72(3):342–348. doi: 10.1111/j.1365-2265.2009.03664.x. [DOI] [PubMed] [Google Scholar]
- 22.Chakaroun R, Raschpichler M, Klöting N, et al. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism. 2012;61(5):706–714. doi: 10.1016/j.metabol.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Gürsoy G, Kırnap NG, Eşbah O, et al. The relationship between plasma omentin-1 levels and insulin resistance in newly diagnosed type 2 diabetıc women. Clinical Reviews and Opinions. 2010;2(4):49–54. [Google Scholar]
- 24.Yan P, Liu D, Long M, Ren Y, Pang J, Li R. Changes of serum omentin levels and relationship between omentin and adiponectin concentrations in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2011;119(4):257–263. doi: 10.1055/s-0030-1269912. [DOI] [PubMed] [Google Scholar]
- 25.World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79(4):373–374. [PMC free article] [PubMed] [Google Scholar]
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative centrifuge. Clin Chem. 1972;18:499–500. [PubMed] [Google Scholar]
- 27.Mattheus DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessement:insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Ghasemi A, Hedayati M, Biabani H. Protein precipitation methods evaluated for determination of serum nitric oxide end products by the Griess assay. J Med Sci Res. 2007;2:29–32. [Google Scholar]
- 29.Miranda K, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 30.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37(2):112–119. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Harma M, Harma M, Erel O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med Wkly. 2003;133(41–42):563–566. doi: 10.4414/smw.2003.10397. [DOI] [PubMed] [Google Scholar]
- 33.Cătoi AF, Pârvu A, Galea RF, Pop ID, Mures̃an A, Cătoi C. Nitric Oxide, Oxidant Status and Antioxidant Response in Morbidly Obese Patients: the Impact of 1-Year Surgical Weight Loss. Obesity Surgery. 2013;23:1858–1863. doi: 10.1007/s11695-013-0968-1. [DOI] [PubMed] [Google Scholar]
- 34.Hopps E, Not 3o D, Caimi G, Averna MR. A novel component of the metabolic syndrome: The oxidative stress. Nutrition, Metabolism and Cardiovascular Diseases. 2010;20:72–77. doi: 10.1016/j.numecd.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Jialal I, Devaraj S, Kaur H, Adams-Huet B, Bremer AA. Increased chemerin and decreased omentin-1 in both adipose tissue and plasma in nascent metabolic syndrome. J Clin Endocrinol Metab. 2013;98(3):E514–E517. doi: 10.1210/jc.2012-3673. [DOI] [PubMed] [Google Scholar]
- 36.Bozaoglu K, Segal D, Shields KA, et al. Chemerin Is Associated with Metabolic Syndrome Phenotypes in a Mexican-American Population. J Clin Endocrinol Metab. 2009;94(8):3085–3088. doi: 10.1210/jc.2008-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shan YU, Ying Z, Meizhen L, et al. Chemerin and apelin are positively correlated with inflammation in obese type 2 diabetic patients. Chinese Medical Journal. 2012;125(19):3440–3444. [PubMed] [Google Scholar]
- 38.Neuparth MJ, Proenc̃a JB, Santos-Silva A, Coimbra S. Hindawi Publishing Corporation ISRN Obesity. 2013. Adipokines, Oxidized Low-Density Lipoprotein, and C-Reactive Protein Levels in Lean, Overweight, and Obese Portuguese Patients with Type 2 Diabetes; pp. 1–7. ID 142097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fatima S, Bozaoglu K, Rehman R, Alam F, Memon AS. Elevated Chemerin Levels in Pakistani Men: An Interrelation with Metabolic Syndrome Phenotypes. PLOS ONE. 2013;8(2):1–7. doi: 10.1371/journal.pone.0057113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auguet T, Quintero Y, Riesco D, et al. New adipokines vaspin and omentin. Circulating levels and gene expression in adipose tissue from morbidly obese women. BMC Medical Genetics. 2011;12:60. doi: 10.1186/1471-2350-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreno-Navarrete JM, Catalán V, Ortega F, et al. Circulating omentin concentration increases after weight loss. Nutr Metab (Lond) 2010;7:27. doi: 10.1186/1743-7075-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Mesallamy HO, El-Derany MO, Hamd NM. Serum omentin-1 and chemerin levels are interrelated in patients with Type 2 diabetes mellitus with or without ischaemic heart disease. Diabet Med. 2011;28(10):1194–1200. doi: 10.1111/j.1464-5491.2011.03353.x. [DOI] [PubMed] [Google Scholar]
- 43.Moreno-Navarrete JM, Francisco Ortega F, Castro A, Sabater M, Ricart W, Fernández-Real JM. Circulating Omentin as a Novel Biomarker of Endothelial Dysfunction. Obesity Journal. 2011;19(8):1552–1559. doi: 10.1038/oby.2010.351. [DOI] [PubMed] [Google Scholar]