Abstract
An antibacterial monomer 12-methacryloyloxydodecylpyridinum bromide (MDPB)-containing experimental, chemically cured primer was prepared to develop a new resin-based root canal filling system. This study investigated the antibacterial effects of the MDPB-containing primer (experimental primer [EP]) against Enterococcus faecalis and assessed the in vitro bonding and sealing abilities of the filling system, consisting of EP and a Bis-GMA-based sealer resin. Antibacterial effects of EP were evaluated by contact with planktonic or adherent bacteria for 30 or 60 sec, and the viable bacterial number was counted. The antibacterial effects against E. faecalis in dentinal tubules were also assessed, according to a root canal infection model. Bonding and sealing abilities of the experimental filling system were examined by microtensile bond strength tests and leakage tests based on fluid filtration methods. Significantly greater reduction in viable bacteria in planktonic and adherent form was obtained by short-period contact with EP compared with the control primer (without MDPB) or with the proprietary (Epiphany) primer (p < .05). Significantly greater bactericidal effects of the EP inside the dentinal tubule of root, as opposed to the control primer or Epiphany primer, were confirmed according to a root canal infection model (p < .05), and 100% killing of E. faecalis could be obtained by the application of EP after irrigation with a 5% sodium hypochlorite solution. The experimental endodontic filling system demonstrated significantly greater bond strength to root dentin than Epiphany sealer system (Epiphany primer and Epiphany Root Canal Sealant; p < .05), showing formation of resin tags and a hybridized layer. Leakage tests clarified that the experimental system provided excellent sealing. This study confirmed that the MDPB-containing experimental antibacterial primer has the ability to effectively disinfect the root canal. Additionally, the experimental root canal filling system employing this primer and the Bis-GMA-based sealer resin is useful for achieving good sealing, suggesting its possible benefit for successful endodontic treatments.
Keywords: root canal filling materials, antibacterial agents, resins, quaternary ammonium compounds, endodontics, Enterococcus faecalis
Introduction
For successful endodontic treatment, eradication of bacteria inside the root canal is fundamental. However, complete elimination of bacterial infection in the root canal is difficult, even by mechanical instrumentation, irrigation (Matsuo et al., 2003), or medication (Siqueira & Lopes, 1999). Therefore, it is considered to be beneficial to provide endodontic filling materials with antibacterial activity.
The antibacterial monomer 12-methacryloyloxydodecylpyridinium bromide (MDPB) is a resin monomer synthesized by combining a quaternary ammonium compound with a methacryloyl group (Imazato et al., 1994). MDPB has a strong antibacterial activity against various caries-related bacteria before polymerization (Imazato et al., 1994, 1999). In addition, the antibacterial component is immobilized in a polymer network by polymerization of MDPB, and such immobilized antimicrobial does not leach out from cured resins. Employing an MDPB-containing antibacterial self-etching primer, the world’s first adhesive system for restoration with antibacterial effects was successfully commercialized (Imazato, 2009).
Izutani et al. (2010) investigated antibacterial activity of MDPB against endodontic pathogenic bacteria in biofilm form and found that these bacteria were killed by application of MDPB in a short period. Given such findings, we hypothesized that the MDPB-containing root canal filling system demonstrates an antibacterial effect against endodontic pathogens and provides excellent sealing as endodontic filling materials. Therefore, a new MDPB-containing primer was developed for disinfection of root canal dentin and filling of root canals in combination with a sealer resin. In this study, in vitro antibacterial effects of this experimental primer (EP) and its sealing effectiveness in combination with a Bis-GMA-based sealer resin were assessed to test the hypothesis.
Materials & Methods
Primers and Bacteria Used
The EP is a chemically cured, 2-bottled-type self-etching primer based on HEMA. Liquid A contains 10% MDPB, and the concentration of MDPB in EP after mixing liquids A and B is 5%. MDPB-free control primer (CP)—with similar components to EP, except for inclusion of MDPB—and the proprietary primer (PP; Epiphany Primer, Pentron, Wallingford, CT, USA) were included for comparison (Table 1).
Table 1.
Materials Used by Group
| Material (Code): Manufacturer | Components |
|---|---|
| EP–SA sealer | |
| Primer: Experimental primer containing 5% MDPB (EP)a | Liquid A: HEMA, MDP, MDPB (10%) Liquid B: methacrylate monomer, water |
| Sealer: SA cement (SA sealer); Kuraray Noritake Dental (Tokyo, Japan) | Paste A: Bis-GMA, TEGDMA, methacrylate monomer, MDP, filler (barium glass, silica), dl-camphorquinone, benzoyl peroxide, initiator Paste B: Bis-GMA, methacrylate monomer, filler (barium glass, silica, sodium fluoride), surface treated sodium fluoride, accelerators |
| Point: Resilon; Pentron (Wallingford, CT, USA) | Polyester difunctional methacrylate resin, bioactive glass, radiopaque filler |
| CP–SA sealer | |
| Primer: control primer (CP)a | Liquid A: HEMA, MDP Liquid B: methacrylate monomer, water |
| Sealer: SA cement (SA sealer); Kuraray Noritake Dental | |
| Point: Resilon; Pentron | |
| PP–Epiphany sealer | |
| Primer: Epiphany Primer (PP); Pentron | HEMA, AMPS, camphorquinone, water |
| Sealer: Epiphany Root Canal Sealant (Epiphany sealer); Pentron | UDMA, PEGDMA, EBPADMA, Bis-GMA, barium borosilicate glasses, barium sulfate, silica, calcium hydroxide, bismuth oxychloride with amines, peroxide, photo initiator |
| Point: Resilon; Pentron | |
| ZOE sealer | |
| Sealer: Pulp Canal Sealer EWT (ZOE sealer); SybronEndo (Orange, CA, USA) | Powder: zinc oxide, silver powder, thymol iodide, dimeric resin Liquid: 4-allyl-2-methoxyphenol, balsam resin, water |
| Point: Gutta Percha Points (gutta-percha point); GC (Tokyo, Japan) | Gutta-percha, zinc oxide, wax, resin, sulfate |
AMPS, 2-acrylamide-2-methylpropane sulfonate; Bis-GMA, 2,2-bis[4-(3-mathacryloxy-2-hydroxypropoxy)phenyl] propane; EBPADMA, ethoxylated bisphenol A dimethacrylate; HEMA, 2-hydroxyethyl methacrylate; MDP, 10-methacryloxydecyl dihydrogen phosphate; MDPB, 12-methacryloyloxydodecylpyridinium bromide; PEGDMA, poly (ethylene glycol) dimethacrylate; TEGDMA, triethyleneglycol dimethacrylate; UDMA, urethane dimethacrylate.
All components of EP and CP were given from Kuraray Noritake Dental.
As representative bacteria isolated from the root with periapical lesion (Gomes et al., 2004), Enterococcus faecalis was used. E. faecalis SS497 was cultured for 12 hr in brain-heart infusion (BHI; Becton Dickinson, Sparks, MD, USA) broth and used for the experiments.
Bactericidal Effects against Planktonic Bacteria
E. faecalis was adjusted to approximately 1 × 106 colony-forming units (CFU) per milliliter, and 10 µL of each primer or distilled water for the control was added to 90 µL of bacterial suspension. After agitation for 30 or 60 sec to keep the bacteria in contact with each primer, the suspension was diluted 100 times by adding 0.01 M phosphate-buffered saline (pH 7.4) to reduce the concentration of the primer far below the minimum inhibitory concentration values. The diluted suspensions were inoculated onto BHI agar plates, and the number of viable bacteria (CFU) was counted after 48 hr of incubation at 37°C in an anaerobic chamber.
Bactericidal Effects against Adherent Bacteria
A collagen disc (Ø13.5 mm, Sumilon cell tight C-1 cell disc LF, Akita Sumitomo Bake Co., Akita, Japan) was placed in a well of a 12-well microplate, and 3 mL of E. faecalis suspension at approximately 1 × 103 CFU/mL was inoculated. The microplate was incubated anaerobically for 6 hr at 37°C to enable bacteria attach to the disc.
Fifty microliters of each primer or distilled water for the control was applied for 30 or 60 sec to the disc with adherent bacteria. The primer solution was removed gently after adding small amount of water, and the disc was put into a tube containing 10 mL of broth. The tube was ultrasonicated in an ice-cold bath for 10 min to collect bacteria. The suspensions were inoculated onto BHI agar plates, and the viable bacterial number was counted.
Assessment of Bactericidal Effects Based on a Root Canal Infection Model
The effectiveness of the EP inside the dentinal tubule of root was evaluated with the method described by Haapasalo and Ørstavik (1987), with some modification.
Extracted human, sound, single-rooted teeth were obtained from patients at Osaka University Dental Hospital under the protocol approved by the Ethics Committee of the Osaka University Graduate School of Dentistry (No. H21-E19). The teeth were cut with a low-speed diamond saw under water cooling, and 4-mm-thick slices were obtained from the upper part of the roots. The slices were prepared into an approximate external diameter of 6 mm using a grinding polisher (Ecomet III, Buehler). The canals of the specimens were then enlarged with round carbide burs with a 2.3-mm diameter (ISO 023, Beldenta Supply Inc., Hyogo, Japan), mounted in a low-speed hand piece. A smear layer was removed by placing the specimens in 18% EDTA, followed by 5% sodium hypochlorite (NaOCl), for 10 min with ultrasonication. The absence of a smear layer and the presence of open dentinal tubules were confirmed via a scanning electron microscope (SEM; JSM-6390LV, JEOL, Tokyo, Japan).
The specimens were autoclaved and incubated in BHI broth for 48 hr to confirm sterilization. Then, 1 root specimen was placed into 5 mL of E. faecalis suspension (approximately 1 × 108 CFU/mL) and incubated for 7 d, replacing 2 mL of suspension with fresh broth every 2 d. Bacterial penetration into the dentinal tubules was confirmed by Brown and Brenn staining.
The 12 root specimens with bacterial contamination were divided randomly into 4 groups (n = 3). The intracanal dentin of the infected specimen was treated by each primer for 30 sec or left untreated for the control. After drying with sterile paper points, the dentin chips were harvested from the canal lumens by drilling with the sterile round burs mounted to a low-speed hand piece. Sequential sampling of dentine to a depth of 400 µm was performed through burs with increasing diameters (ISO 025, 027, 029, and 031; Beldenta Supply Inc.). The dentin chips obtained (approximately 18 mg) were collected into a tube containing 5 mL of BHI broth, and the viable bacterial number was counted.
Another 15 infected specimens were divided into 5 groups and treated as follows:
Group 1: no treatment (control)
Group 2: irrigation with 5% NaOCl for 10 sec
Group 3: irrigation with 5% NaOCl for 10 sec, drying, and treatment with EP for 30 sec
Group 4: irrigation with 5% NaOCl for 30 sec
Group 5: irrigation with 5% NaOCl for 30 sec, drying, and treatment with EP for 30 sec
After each treatment, the bacteria were collected as described above, and the viable bacterial number was counted.
Bonding Ability of Experimental Sealer System
For the sealer resin to be combined with EP, dual cured Bis-GMA-based resin (SA Cement, Kuraray Noritake Dental, Tokyo, Japan; i.e., SA sealer) was employed (Table 1). Human incisors within 6 mo of extraction were sectioned at 9.5 mm from the apical end. A canal was prepared to ISO size 60 and rinsed with 5 mL of 5% NaOCl and 18% EDTA, followed by 5 mL of distilled water, and dried with paper points. Sixteen roots were divided randomly into 2 groups:
Group 1: apply EP for 30 sec, dry, and fill with SA sealer
Group 2: apply PP for 30 sec, dry, and fill with Epiphany Root Canal Sealant (Pentron; i.e., Epiphany sealer)
After obturation, the roots were light irradiated for 40 sec and stored in 100% humidity at 37°C. After 24 hr, from the cervical half of the roots, 3 disc-shaped slabs with a thickness of approximately 1.0 mm were obtained by slicing perpendicular to the tooth axis. An hourglass-shaped specimen, which included the 2 sides of the bonding interfaces, was trimmed from each slab as described previously (Wu et al., 2009). The specimen was then tested with a tabletop materials testing machine (EZ Test, Shimadzu, Kyoto, Japan) with a crosshead speed of 1.0 mm/min. The microtensile bond strength (MTBS) was calculated according to a unit of stress (megapascal [MPa]) based on the area of the fracture surface. The interfacial fracture surface area was calculated according to the formula used in the previous study (Bouillaguet et al., 2003).
To observe the interfacial morphology, the root canal prepared as described above was filled with a resin point Resilon (Pentron), with EP and SA sealer by the single-cone obturation technique (n = 3). The coronal surface of the root filling was irradiated for 40 sec, and the specimens were stored in 100% humidity at 37°C for 24 hr. The surface of specimens sectioned longitudinally into halves was polished and observed through SEM after dehydration in an ascending ethanol series, freeze-drying, and platinum sputter coating. For micromorphologic analysis of the bonding interface, the polished surfaces were treated with 50% phosphoric acid for 2 min, then 12% NaOCl for 4 min, and observed by SEM.
Leakage Tests Based on Fluid Filtration Methods
Twenty-four roots were prepared as described and divided randomly into 4 groups. Groups 1, 2, and 3 were obturated with EP–SA sealer, CP–SA sealer, or PP–Epiphany sealer, respectively. Filling with gutta-percha point (Gutta Percha Points, GC Co., Tokyo, Japan), based on zinc oxide eugenol sealer (Pulp Canal Sealer EWT, SybronEndo Co., Orange, CA, USA; i.e., ZOE sealer), was employed as the fourth group.
After 1 or 4 wk of storage in water at 37°C, microleakage of the filled roots was evaluated by a fluid filtration method (Raina et al., 2007). A pressure of 0.0689 MPa (10 psi) was applied, and the fluid flow rate was expressed in microliters per minute based on the air bubble movement.
Statistical Analysis
The results for bacterial counts and leakage tests were statistically analyzed by analysis of variance and Student-Newman-Keuls’s post hoc test, with a significance level of p < .05. The results for MTBS tests were statistically analyzed by the Student’s t test with a significance level of p < .05.
Results
Bactericidal Effects
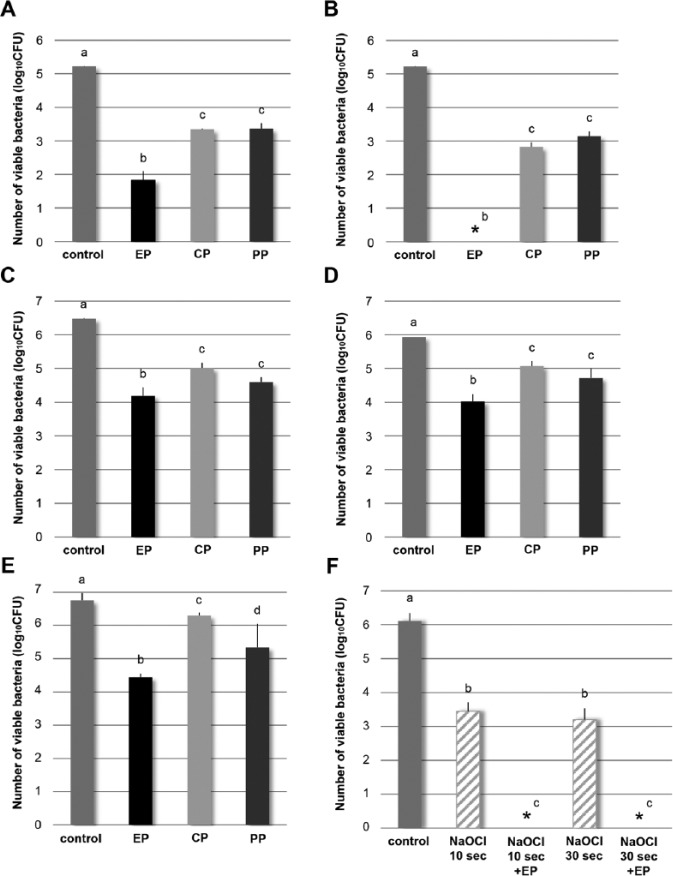
For both planktonic and adherent bacteria, significantly greater reduction in viable bacteria was obtained by EP than CP or PP (p < .05). By contact with EP, 99.9% killing of planktonic bacteria was obtained after 30 sec and 100% killing after 60 sec (Fig. 1A, 1B). For adherent bacteria, EP killed more than 98% after 30 sec and 99% after 60 sec (Fig. 1C, 1D). For bacteria in dentinal tubules, EP demonstrated significantly greater reduction than CP or PP (p < .05) (Fig. 1E), showing 99.5% killing.
Figure 1.
Number of viable Enterococcus faecalis after contact with the primer. (A, B) Planktonic bacteria and (C, D) adherent bacteria. Contact times: 30 sec (A, C), 60 sec (B, D). (E, F) Infected root model. (F) Treatment with 5% NaOCl alone or 5% NaOCl + EP. *No bacteria were recovered. The bar represents the standard deviation of 3 replicates. No significant differences between the same letters (i.e., a-d; analysis of variance and Student-Newman-Keuls’s test; p < .05). CP, control primer; EP, experimental primer; PP, proprietary primer.
Irrigation with NaOCl reduced viable bacteria in the dentinal tubules, but complete elimination could not be obtained. However, application of EP after NaOCl irrigation resulted in 100% killing for both irrigation times by NaOCl (Fig. 1F).
Bonding Ability
The EP–SA sealer group demonstrated significantly greater MTBS than the PP–Epiphany sealer group (p = .001; Fig. 2A). By SEM observation of the interface, close contact of SA sealer with root dentin was observed, and no gap was found in any part of the root (Fig. 2B). Resin tags penetrated inside the dentinal tubules for a depth of up to approximately 10 µm. Formation of a thin hybridized zone, less than 0.5 µm thick, was seen (Fig. 2C).
Figure 2.
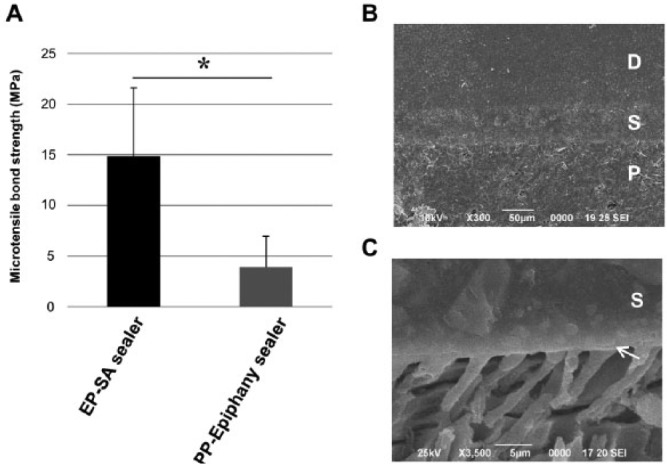
Bonding ability of experimental sealer system. (A) Microtensile bond strength of EP–SA sealer and PP–Epiphany sealer. The bar represents the standard deviation of 24 specimens. Three disc-shaped slabs (approximately 1.0 mm thick) were obtained by slicing filled root perpendicular to the tooth axis, and an hourglass-shaped specimen was trimmed from each slab with a diamond point. Microtensile bond strength tests were conducted with a crosshead speed of 1.0 mm/min. Pretesting failure, resulting in 0 MPa, was seen in 1 of 24 specimens of the EP–SA sealer group and 10 of 24 specimens of the PP–Epiphany sealer group. For the EP–SA sealer group, adhesive failure at the interface between dentin and sealer was seen in 15 of 24 specimens, and 6 of 24 specimens showed mixed adhesive failure and cohesive failure within the dentin or within the sealer. In the PP–Epiphany group, 21 of 24 specimens showed adhesive failure, and the rest showed a combination of adhesive failure and cohesive failure within the sealer. *Significant differences (Student’s t test; p = .001). (B) Scanning electron microscope image of a section of root filled with EP–SA sealer. (C) Scanning electron microscope image of the bonding interface of the EP–SA sealer to root dentin observed after treatment with 50% phosphoric acid for 2 min and 12% NaOCl for 4 min. Formation of resin tags and a hybridized zone (arrow) can be seen. D, dentin; EP, experimental primer; MPa, megapascal; P, resin point; PP, proprietary primer; S, SA sealer.
Fluid Filtration Tests
After 1 wk, the fluid flow rate was null for the EP–SA sealer group, and those of the EP–SA sealer and CP–SA sealer groups were significantly lower than the PP–Epiphany sealer and ZOE sealer groups (p < .05) (Fig. 3A). After 4 wk, although the values of all groups increased, EP–SA sealer and CP–SA sealer groups showed significantly lower values than the PP–Epiphany sealer group (p < .05) (Fig. 3B).
Figure 3.
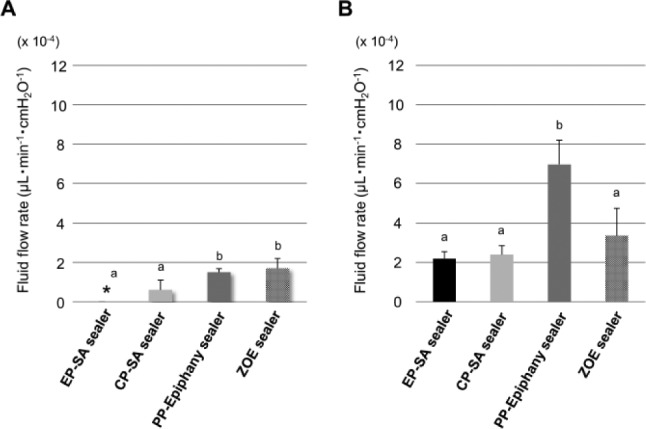
Fluid flow rates of EP–SA sealer, CP–SA sealer, PP–Epiphany sealer, and ZOE sealer. (A) After 1 wk of storage and (B) after 4 wk of storage. The bar represents the standard deviation of 3 replicates. *No fluid flow was observed. No significant differences between the same letters (i.e., a, b; analysis of variance and Student-Newman-Keuls’s test, p < .05). CP, control primer; EP, experimental primer; PP, proprietary primer.
Discussion
Many studies on various properties of the HEMA-based, light-cured self-etching primer containing 5% MDPB for composite restoration are available, including demonstration of its strong antibacterial activity against caries-related bacteria (Imazato et al., 1997, 2004, 2014; Özer et al., 2003; Carvalho et al., 2012). It has also been reported, through in vitro and in vivo studies, that incorporation of MDPB into the light-cured primer up to 5% had no adverse influences on the bonding ability to dentin (Imazato et al., 1997, 2007). Therefore, an experimental chemically cured primer containing 5% MDPB for root canal filling (EP) was developed, expecting maximum antibacterial effects and high sealing ability. All 3 primers tested showed antibacterial effects due to their acidic composition to some extent. However, EP showed the highest antibacterial activity of all against planktonic and adherent bacterial during a short-period contact. This indicates that MDPB incorporated in chemically cured primer can attack bacteria in a rapid manner based on the killing mechanism of quaternary ammonium compounds (McDonnell and Russell, 1999). It has been reported that the light-cured MDPB-containing primer for composite restorations had the ability to penetrate deeply into dentin in vitro (Schmalz et al., 2004) and in vivo (Imazato et al., 2004). The ability of MDPB-containing EP to penetrate and kill bacteria in root canal dentin was clarified by via the infected root model.
E. faecalis is relevant to secondary and persistent root canal infection (Siqueira and Rôças, 2005). Complete elimination of E. faecalis in the infected root model was not achieved by irrigation with 5% NaOCl. In the model used, bacterial penetration into the dentinal tubules reaching up to approximately 300-µm depth was observed by Brown and Brenn staining (Appendix Figure), which is consistent with the previous report (Haapasalo & Ørstavik 1987). NaOCl may not have been able to reach the deeper part of tubules even when application time was prolonged. However, application of EP was effective to kill residual bacteria after NaOCl irrigation, and all E. faecalis could be eradicated. Elimination of bacteria in the shallow part by NaOCl irrigation enabled complete killing of bacteria by EP treatment in the deeper part of the dentinal tubules. Therefore, the new treatment strategy to use MDPB-containing primer for root canal filling is expected to contribute to successful endodontic treatment. To visualize the antibacterial effects of EP against the bacteria in dentinal tubules, further observation of bacterial viability in infected root is under progress through confocal laser scanning microscopy as reported previously (Ma et al., 2011).
Many studies indicated the presence of interfacial gaps between dentin and the Epiphany system (Tay et al., 2005; Costa et al., 2010). The MTBS value of the EP–SA sealer group was 3 times higher than that of PP–Epiphany sealer. In addition, the EP–SA sealer group demonstrated a lower chance of adhesive failure than PP–Epiphany sealer and cohesive failure within the sealer or dentin. These findings support high bonding ability of the EP–SA sealer group to root dentin. By observing the interfacial morphology, close contact of the filling with root dentin was found when EP and SA sealer were used. The effective bonding of EP–SA sealer to root dentin is clear from the results of observation of the interface with SEM to show formation of resin tags and a hybridized zone. The self-etching primer EP contains MDP (10-methacryloxydecyl dihydrogen phosphate), and chemical binding of MDP to hydroxyapatite (Yoshida et al., 2004) in addition to hybridization is considered to contribute to the high bonding ability of EP–SA sealer.
Many recent studies used the fluid filtration method to assess the sealing ability of root fillings (Biggs et al., 2006; Paqué & Sirtes, 2007). After both 1 and 4 wk of storage, the EP–SA sealer and CP–SA sealer groups showed better sealing abilities than the PP–Epiphany sealer group. It has been reported that the sealing ability of root canal sealer correlates with bonding ability (Neelakantan et al., 2011). The superior bonding ability of EP–SA sealer, as demonstrated by MTBS tests, was effective at improving the sealing ability. Epiphany sealer was reported to show extensive calcium release and high solubility because of the erosion of filler particles (Versiani et al., 2006). Even when the SA sealer in combination with EP or CP showed deterioration of sealing after 4 wk, it was found to have better sealing ability than others.
Antibacterial monomers, including MDPB, have been shown to demonstrate bacteriostatic effects after polymerization (Imazato et al., 1994, 2014; Li et al., 2009; Antonucci et al., 2012). Therefore, it is possible that EP inhibits bacterial growth after being cured by immobilized MDPB. The long-term antibacterial effects shown by endodontic filling materials contribute to better clinical results, and such usefulness of the experimental MDPB-containing primer for root canal filling is of further interest.
Conclusions
This in vitro study demonstrated that an experimental antibacterial root canal filling system has the ability to effectively disinfect the root canal and is useful for achieving good sealing, indicating its possible benefit for successful endodontic treatments.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported in part by a Grant-in-Aid for Scientific Research (nos. 19209060, 25893121, 26293409) from the Japan Society for the Promotion of Science.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. (2012). Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent Mater 28:219-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs SG, Knowles KI, Ibarrola JL, Pashley DH. (2006). An in vitro assessment of the sealing ability of resilon/epiphany using fluid filtration. J Endod 32:759-761. [DOI] [PubMed] [Google Scholar]
- Bouillaguet S, Troesch S, Wataha JC, Krejci I, Meyer JM, Pashley DH. (2003). Microtensile bond strength between adhesive cements and root canal dentin. Dent Mater 19:199-205. [DOI] [PubMed] [Google Scholar]
- Carvalho FG, Puppin-Rontani RM, Fúcio SB, Negrini Tde C, Carlo HL, Garcia-Godoy F. (2012). Analysis by confocal laser scanning microscopy of the MDPB bactericidal effect on S. mutans biofilm CLSM analysis of MDPB bactericidal effect on biofilm. J Appl Oral Sci 20:568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JA, Rached-Júnior FA, Souza-Gabriel AE, Silva-Sousa YT, Sousa-Neto MD. (2010). Push-out strength of methacrylate resin-based sealers to root canal walls. Int Endod J 43:698-706. [DOI] [PubMed] [Google Scholar]
- Gomes BP, Pinheiro ET, Gadê-Neto CR, Sousa EL, Ferraz CC, Zaia AA, et al. (2004). Microbiological examination of infected dental root canals. Oral Microbiol Immunol 19:71-76. [DOI] [PubMed] [Google Scholar]
- Haapasalo M, Ørstavik D. (1987). In vitro infection and disinfection of dentinal tubules. J Dent Res 66:1375-1379. [DOI] [PubMed] [Google Scholar]
- Imazato S. (2009). Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dent Mater J 28:11-19. [DOI] [PubMed] [Google Scholar]
- Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. (1994). Incorporation of bacterial inhibitor into resin composite. J Dent Res 73:1437-1443. [DOI] [PubMed] [Google Scholar]
- Imazato S, Kinomoto Y, Tarumi H, Torii M, Russell RR, McCabe JF. (1997). Incorporation of antibacterial monomer MDPB into dentin primer. J Dent Res 76:768-772. [DOI] [PubMed] [Google Scholar]
- Imazato S, Ebi N, Tarumi H, Russell RR, Kaneko T, Ebisu S. (1999). Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials 20:899-903. [DOI] [PubMed] [Google Scholar]
- Imazato S, Kaneko T, Takahashi Y, Noiri Y, Ebisu S. (2004). In vivo antibacterial effects of dentin primer incorporating MDPB. Oper Dent 29:369-375. [PubMed] [Google Scholar]
- Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. (2007). An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dent Mater 23:170-176. [DOI] [PubMed] [Google Scholar]
- Imazato S, Ma S, Chen JH, Xu HH. (2014). Therapeutic polymers for dental adhesives: loading resins with bio-active components. Dent Mater 30:97-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izutani N, Imazato S, Noiri Y, Ebisu S. (2010). Antibacterial effects of MDPB against anaerobes associated with endodontic infections. Int Endod J 43:637-645. [DOI] [PubMed] [Google Scholar]
- Li F, Chai ZG, Sun MN, Wang F, Ma S, Zhang L, et al. (2009). Anti-biofilm effect of dental adhesive with cationic monomer. J Dent Res 88:372-376. [DOI] [PubMed] [Google Scholar]
- Ma J, Wang Z, Shen Y, Haapasalo M. (2011). A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J Endod 37:1380-1385. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Shirakami T, Ozaki K, Nakanishi T, Yumoto H, Ebisu S. (2003). An immunohistological study of the localization of bacteria invading root pulpal walls of teeth with periapical lesions. J Endod 29:194-200. [DOI] [PubMed] [Google Scholar]
- McDonnell G, Russell AD. (1999). Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan P, Subbarao C, Subbarao CV, De-Deus G, Zehnder M. (2011). The impact of root dentine conditioning on sealing ability and push-out bond strength of an epoxy resin root canal sealer. Int Endod J 44:491-498. [DOI] [PubMed] [Google Scholar]
- Özer F, Karakaya Ş, Ünlü N, Erganiş O, Kav K, Imazato S. (2003). Comparison of antibacterial activity of two dentin bonding systems using agar well technique and tooth cavity model. J Dent 31:111-116. [DOI] [PubMed] [Google Scholar]
- Paqué F, Sirtes G. (2007). Apical sealing ability of Resilon/Epiphany versus gutta-percha/AH Plus: immediate and 16-months leakage. Int Endod J 40:722-729. [DOI] [PubMed] [Google Scholar]
- Raina R, Loushine RJ, Weller RN, Tay FR, Pashley DH. (2007). Evaluation of the quality of the apical seal in Resilon/Epiphany and Gutta-Percha/AH Plus-filled root canals by using a fluid filtration approach. J Endod 33:944-947. [DOI] [PubMed] [Google Scholar]
- Schmalz G, Ergücü Z, Hiller KA. (2004). Effect of dentin on the antibacterial activity of dentin bonding agents. J Endod 30:352-358. [DOI] [PubMed] [Google Scholar]
- Siqueira JF, Jr, Lopes HP. (1999). Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J 32:361-369. [DOI] [PubMed] [Google Scholar]
- Siqueira JF, Jr, Rôças IN. (2005). Exploiting molecular methods to explore endodontic infections: part 2. Redefining the endodontic microbiota. J Endod 31:488-498. [DOI] [PubMed] [Google Scholar]
- Tay FR, Loushine RJ, Weller RN, Kimbrough WF, Pashley DH, Mak YF, et al. (2005). Ultrastructural evaluation of the apical seal in roots filled with a polycaprolactone-based root canal filling material. J Endod 31:514-519. [DOI] [PubMed] [Google Scholar]
- Versiani MA, Carvalho-Junior JR, Padilha MI, Lacey S, Pascon EA, Sousa-Neto MD. (2006). A comparative study of physicochemical properties of AH Plus and Epiphany root canal sealants. Int Endod J 39:464-471. [DOI] [PubMed] [Google Scholar]
- Wu H, Hayashi M, Okamura K, Koytchev EV, Imazato S, Tanaka S, et al. (2009). Effects of light penetration and smear layer removal on adhesion of post–cores to root canal dentin by self-etching adhesives. Dent Mater 25:1484-1492. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, et al. (2004). Comparative study on adhesive performance of functional monomers. J Dent Res 83:454-458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.