Supplemental Digital Content is available in the text.
Keywords: acute otitis media, etiology, HIV, viruses, viral–bacterial interactions
Abstract
Background:
Bacteria and respiratory viruses are implicated in the pathogenesis of acute otitis media (AOM); however, data from low–middle income countries are sparse. We investigated the etiology of AOM in HIV-infected (HIV+), HIV-uninfected (HIV−) and HIV-exposed clinically asymptomatic for HIV-infection (HEU) South African children.
Methods:
Children ≥3 months to <5 years of age with AOM were enrolled between May 2009 and April 2010 (NCT01031082). Middle ear fluid samples were cultured for bacteria; antibacterial susceptibility was done and serotyping undertaken for Streptococcus pneumoniae and Haemophilus influenzae. Nasopharyngeal aspirates were analyzed for respiratory viruses using immunofluorescence assay and polymerase chain reaction.
Results:
Of 260 AOM episodes (HIV+:15; HIV−:182; HEU:63), bacteria were found in 54.6%, including Haemophilus influenzae (30.8%), 98.8% of which were nontypeable, and Streptococcus pneumoniae (20.4%), Staphylococcus aureus (15.8%), Moraxella catarrhalis (5.0%) and Streptococcus pyogenes (1.5%). Nonsusceptibility of Streptococcus pneumoniae to penicillin was 64.2%. Respiratory viruses were detected in 74.2% of cases. Human rhinovirus was most frequently detected (37.7%), followed by adenovirus (14.2%) and human bocavirus (11.5%) overall and irrespective of HIV status. Respiratory viruses were identified concurrently with S. pneumoniae, H. influenzae, M. catarrhalis (76.9–78.8%) and Staphylococcus aureus (63.4%) cultured from middle ear fluid, as well as in 72.0% of episodes negative for any bacteria.
Conclusion:
The study suggests that respiratory viruses and pathogenic bacteria play an important role in the development of AOM in children. A similar spectrum of pathogens was observed independently of HIV status. Vaccines targeting both nontypeable Haemophilus influenzae and S. pneumoniae may have a broad impact on AOM in South Africa.
Acute otitis media (AOM) causes significant morbidity in children and is the most common reason for outpatient antibiotic therapy.1,2 Although AOM is usually mild, if untreated or unresolved, it may lead to complications, such as chronic suppurative otitis media,3 which is very prevalent in Africa.4 Pathogenesis of AOM typically begins with a viral respiratory tract infection, which may facilitate certain bacterial colonizers of the nasopharynx to enter the middle ear.5 Streptococcus pneumoniae and nontypeable Haemophilus influenzae (NTHi) are important etiological agents of AOM.6 Moraxella catarrhalis and Streptococcus pyogenes may also be found in a smaller proportion of AOM cases.6 Viruses that have been implicated in AOM include respiratory syncytial virus (RSV), human rhinovirus (hRV), influenza viruses and parainfluenza viruses.7–10 Moreover, bacterial and viral co-infections, which have been observed in 28–70% of AOM cases9 were associated with prolonged clinical illness.11
Children infected with HIV are known to be at greater risk for bacterial and viral infections, including AOM.12 There are limited data from developing countries on the etiology of uncomplicated-AOM and generally limited global data on the association of respiratory viral–bacterial co-infections in AOM. With both antibiotics and pneumocococcal conjugate vaccines (PCV) available for treatment and prophylaxis,13 understanding the etiology of AOM in Africa is important to better inform clinical management.
This study investigated the bacterial and viral etiology of AOM and the antibacterial susceptibility in HIV-infected (HIV+), HIV-uninfected (HIV−) and HIV-exposed clinically presumed-uninfected (HEU) South African children.
MATERIALS AND METHODS
Study Design and Population
Identification of potential study participants into this prospective study between May 2009 and April 2010 (NCT01031082) was undertaken at a primary health care clinic (Lilian Ngoyi Clinic), with suspected AOM cases being referred to the Respiratory and Meningeal Pathogens Research Unit (RMPRU) based at the neighboring Chris Hani-Baragwanath Academic Hospital, Soweto, South Africa. The study adhered to Good Clinical Practice guidelines, including the Declaration of Helsinki and the country’s local rules and regulations. The local Independent Ethics Committee reviewed and approved all study-related documents. Parents/guardians provided written informed consent before enrollment.
Children 3 months to <5 years of age with AOM, as confirmed by an ear–nose–throat specialist were enrolled in the study. AOM was diagnosed based on the onset of at least one of the following symptoms within 3 days: otalgia/irritability, conjunctivitis, fever and either Paradise’s criteria (bulging, diffused or localized inflamed tympanic membranes) or spontaneous otorrhea (ie, perforations occurring within 24 hours of the hospital visit). A case was considered a new episode if there was a 30-day symptom-free interval since the resolution of the preceding episode.
Children were excluded from the study in the event of: hospitalization either during AOM diagnosis or during treatment; otitis externa or otitis media with effusion; pressure equalization tube; treatment with oral or intravenous antibiotics for other conditions within 72 hours before study entry; or the administration of prophylactic antibiotics for recurrent AOM (defined as ≥3 episodes in the previous 6 months, or ≥4 episodes in the previous 12 months).
HIV Testing
HIV testing was mainly undertaken at the discretion of the attending physicians; children <18 months of age were evaluated using HIV-polymerase chain reaction (PCR) and HIV-infection status of children >18 months was based on enzyme linked immunosorbent assay positivity. Children were categorized as: confirmed HIV-infected (HIV+) when they tested HIV positive either previously through the preventing mother to child HIV transmission programs in place or when during the AOM episode they presented with clinical stigmata of HIV and the attending-physician requested an HIV test; confirmed HIV-uninfected (HIV−) when they were not tested because they were born to HIV-uninfected mothers and had no clinical symptoms of HIV, or when independently of the HIV status of the mother at the time of the AOM episode they presented with clinical stigmata of HIV and the attending-physician requested an HIV test that was negative; and HIV-exposed clinically presumed-uninfected (HEU) if they were born to HIV-infected mothers, but who were asymptomatic and who did not undergo HIV testing by the attending physician.
Bacterial and Respiratory Virus Identification
Middle ear fluid (MEF) samples were collected either by tympanocentesis or by careful sampling of spontaneous otorrhea by needle insertion into the perforated tympanic membrane. The selection of spontaneous otorrhea cases was limited to 20%. The samples were inoculated onto Amies transport medium14 and processed at RMPRU within 16 hours of collection. Samples were inoculated onto chocolate agar (otorrhea samples were inoculated onto chocolate agar with bacitracin) and blood agar with gentamycin. S. pneumoniae, Haemophilus influenzae, M. catarrhalis, S. pyogenes and Staphylococcus aureus were identified by standard microbiological procedures.15 S. pneumoniae serotypes were identified by Quellung method and H. influenzae serotypes by real-time PCR16 and confirmed with monovalent antisera. The isolates were further tested for antibacterial susceptibility. Minimal inhibitory concentrations were obtained with E-tests (bioMérieux, Craponne, France) and interpreted using the criteria published by the Clinical and Laboratory Standard Institute in 2009.17
At the same visit, nasopharyngeal aspirates (NPA) were collected in viral transport media as previously described18 and analyzed for RSV, influenza A and B, parainfluenza viruses 1–3, and adenovirus by immunofluorescence assay and real-time reverse transcriptase-PCR (rRT-PCR). If the NPA were rRT-PCR-positive for influenza A, a subsequent H1N1-2009 pandemic (A[H1N1]pdm09) subtyping rRT-PCR was performed. rRT-PCR was also undertaken for human metapneumovirus (hMPV), human bocavirus (hBoV), 2 polyomaviruses (PyV-KI and PyV-WU) and hRV. Extraction of total nucleic acids from NPAs and rRT-PCR was undertaken as previously described.19 The primers and probes used are listed in Table, Supplemental Digital Content 1, http://links.lww.com/INF/C125. Internal controls were included to check the extraction step and the presence of assay inhibitors; positive and negative controls were included in each experiment.
Statistical Analysis
Children meeting all eligibility criteria, complying with protocol-defined procedures and for whom laboratory results were available were included in the analyses. The frequency of detection of the different etiological agents was calculated, including co-detections. Antibacterial susceptibility of S. pneumoniae, H. influenzae, M. catarrhalis and Staphylococcus aureus was determined and history of previous vaccination with PCV was recorded. Seven-valent PCV vaccine-serotypes included serotypes 4, 6B, 9V, 14, 18C, 19F and 23F. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC) and Microsoft Excel.
RESULTS
A total of 260 AOM episodes (HIV+: 15; HIV−: 182 and HEU: 63) corresponding to 248 children (236 reported 1 episode and 12 reported 2 episodes) were included in the analyses. Children were excluded for: violating the protocol (n = 2), using antibiotics before sample collection (n = 1) and for unsuccessful MEF sample collection (n = 2). The overall median age was 14 months (range: 4–58 months) and 53.6% were male. Demographic characteristics were similar among HIV+, HIV− and HEU children.
Overall, there were 100 unilateral episodes (for which a sample was collected from 1 ear) and 160 bilateral episodes including: 10 versus 5, respectively in HIV+, 69 versus 113 in HIV− and 21 versus 42 in HEU children. The majority of samples were collected by tympanocentesis (245/260; 94.2%). The greatest proportion of AOM episodes was recorded among children aged 3–11 months (n = 108; 41.5%) followed by children aged 12–23 months old (n = 85; 32.7%), 24–35 months old (n = 43; 16.5%), 36–47 months old (n = 17; 6.5%) and 48–59 months old (n = 7; 2.7%).
Bacterial Etiology, Serotype Distribution and Antibacterial Susceptibility
At least 1 bacterial pathogen was found in 54.6% (142/260) of AOM episodes. Overall, H. influenzae [30.8% (95% confidence interval {CI}: 25.2–36.8)] and S. pneumoniae [20.4% (95% CI: 15.7–25.8)] were the most commonly isolated bacteria. Bacterial etiology was similar among HIV+, HIV− and HEU children (Table 1). Fifteen episodes were positive for both S. pneumoniae and H. influenzae (HIV+: 1; HIV−: 14). H. influenzae and S. pneumoniae were similarly distributed across age groups (Fig. 1A). Staphylococcus aureus was identified in 15.8% (95% CI: 11.6–20.8) of cases and in 48.8% (n = 20) of which was the only bacterial pathogen identified; 88% of all Staphylococcus aureus detections were cultured from tympanocentesis samples.
TABLE 1.
Bacterial Etiology for AOM by HIV Status (N = 260)
FIGURE 1.
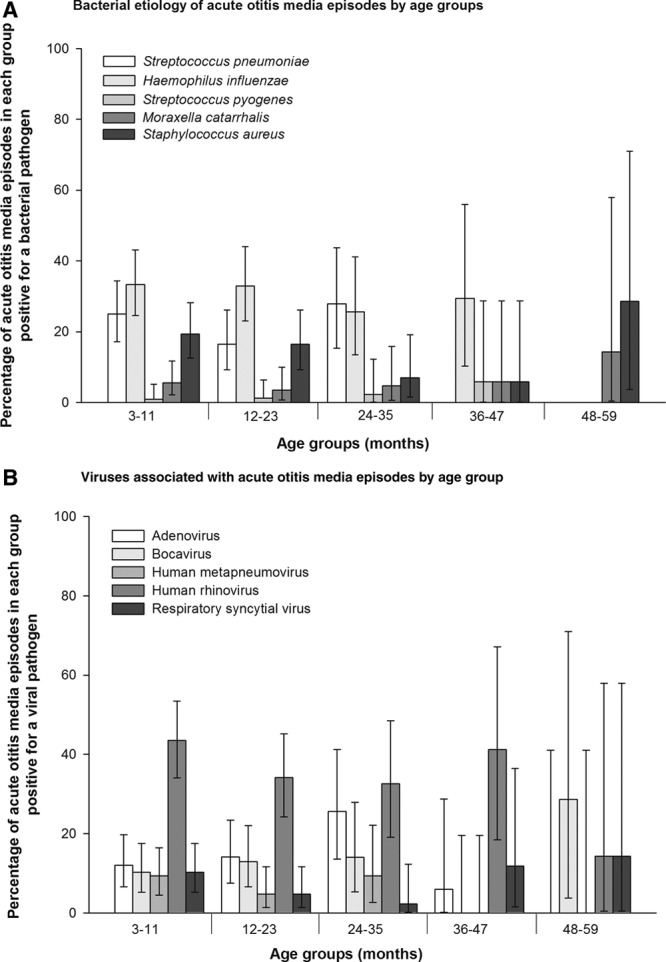
A, Bacterial etiology of acute otitis media episodes by age groups. B, Viral etiology of acute otitis media episodes by age groups.
The majority of H. influenzae episodes were NTHi (79/80; 98.8%). The most frequently isolated S. pneumoniae serotypes were 19F (12/53; 22.6%) followed by 19A and 15B (each accounting for 6/53; 11.3%; Table, Supplemental Digital Content 2, http://links.lww.com/INF/C126).
The prevalence of S. pneumoniae nonsusceptibility to penicillin was 64.2% (34/53) and was noted for vaccine-serotypes, as well as nonvaccine serotypes 15B, 15C and 16 (Fig., Supplemental Digital Content 3, http://links.lww.com/INF/C127). For other antibiotics, 45.3% (24/53) were resistant to azithromycin, 28.3% (15/53) to trimethoprim/sulfamethoxazole, 18.9% (10/53) to erythromycin, 15.1% (8/53) to tetracycline, 9.4% (5/53) to cefuroxime and 1.9% (each accounting for 1/53) to amoxicillin/clavulanate and levofloxacin, no resistance to cefotaxime was detected.
Among 79 NTHi-positive episodes, 60.8% (n = 48) were resistant to trimethoprim/sulfamethoxazole, 13.9% (n = 11) to ampicillin and 2.5% (n = 2 each) to amoxicillin/clavulanate, cefuroxime and tetracycline (Fig., Supplemental Digital Content 4, http://links.lww.com/INF/C128). M. catarrhalis was resistant to penicillin (12/13; 92.3%), cefotaxime (6/13; 46.2%), trimethoprim/sulfamethoxazole (5/13; 38.5%) and with lower resistance to azithromycin, cefuroxime, levofloxacin and tetracycline (each accounting for 7.7%; 1/13). The prevalence of Staphylococcus aureus resistant to penicillin was 97.6% (40/41), to gentamycin 24.4% (10/41) and showed low prevalence of resistance to tetracycline (3/41; 7.3%) and levofloxacin (1/41; 2.4%). No methicillin resistance was detected. Antibacterial susceptibility was similar among HIV+, HIV− and HEU children.
Pneumococcal Conjugate Vaccination History
Overall, 16.1% (40/248) of children received at least 1 dose of PCV, of which 27.5% (11/40) received 3 doses and 2.5% (1/40) received 4 doses. Vaccination status was similar irrespective of HIV status. Of 102 children in the age group 3–11 months, 37.3% (n = 38) had a history of at least 1 dose of PCV: 21.1% (n = 8) of which received 1 dose, 52.6% (n = 20) received 2 doses, 23.7% (n = 9) received 3 doses and 2.6% (n = 1) received 4 doses. No significant differences were seen in the prevalence of S. pneumoniae between PCV-vaccinated (10.0%; 95% CI: 2.8–23.7) and unvaccinated children [22.3% (95% CI: 17.0–28.4); P = 0.08] or in the prevalence of H. influenzae [35.0% (95% CI: 20.6−51.7) vs. 30.0% (95% CI: 24.0−36.5), respectively; P = 0.53]. S. pneumoniae serotypes observed in vaccinated children were 14, 18C, 21 and 6A (each accounting for one episode). Serotypes 1, 6B, 14, 15B, 15C, 16, 19A, 19F, 21, 23F, 3, 4, 5, 6A, 6B, 9N and 9V (each accounting for at least 1 episode) were detected in unvaccinated children. No statistically significant difference was detected in the prevalence of PCV7-serotypes between PCV-vaccinated [5.0% (95% CI: 0.6–16.9)] and unvaccinated children [12.7% (95% CI: 8.6–17.9); P = 0.28). Of 80 H. influenzae-positive episodes, 65 in the PCV-unvaccinated group and 14 in the PCV-vaccinated group were NTHi.
Viral Pathogens and Viral–bacterial Co-detection
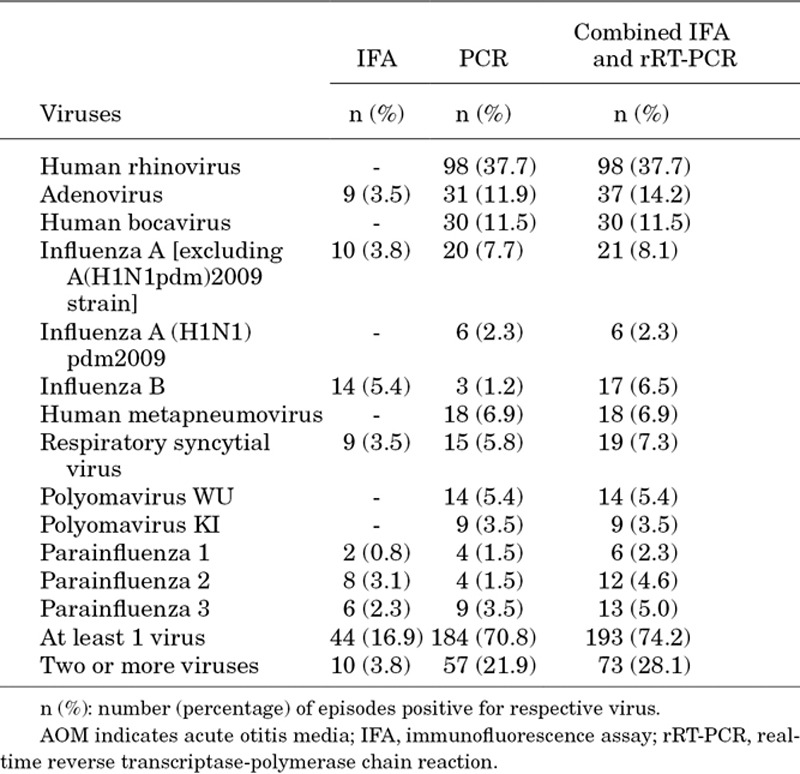
NPA were collected from all 260 AOM episodes. At least 1 respiratory virus was identified in 74.2% (193/260) of episodes when analyzed by combined immunofluorescence assay and rRT-PCR, including 66.7% (10/15) in HIV+, 75.8% (138/182) in HIV− and 71.4% (45/63) in HEU children. The most commonly identified virus was hRV (n = 98; 37.7%) followed by adenovirus (n = 37; 14.2%), hBoV (n = 30; 11.5%), influenza viruses (A + B; n = 44; 16.9%), RSV (n = 19; 7.3%) and hMPV (n = 18; 6.9%; Table 2). hRV was also the most frequent virus irrespective of HIV status (HIV+: 33.3%, HIV−: 38.5% and HEU children: 36.5%) and was frequently isolated from AOM episodes in children aged 3–11 (43.5%), 12–23 (34.1%), 24–35 (32.6%) and 36–47 (41.2%) months (Fig. 1B). In 28.1% (n = 73) of AOM episodes, more than 1 virus was detected; the percentage of episodes with concomitant detection of more than 1 virus is shown in Table, Supplemental Digital Content 5, http://links.lww.com/INF/C129.
TABLE 2.
Viral Pathogens Identified in AOM Episodes by IFA and rRT-PCR (N = 260)
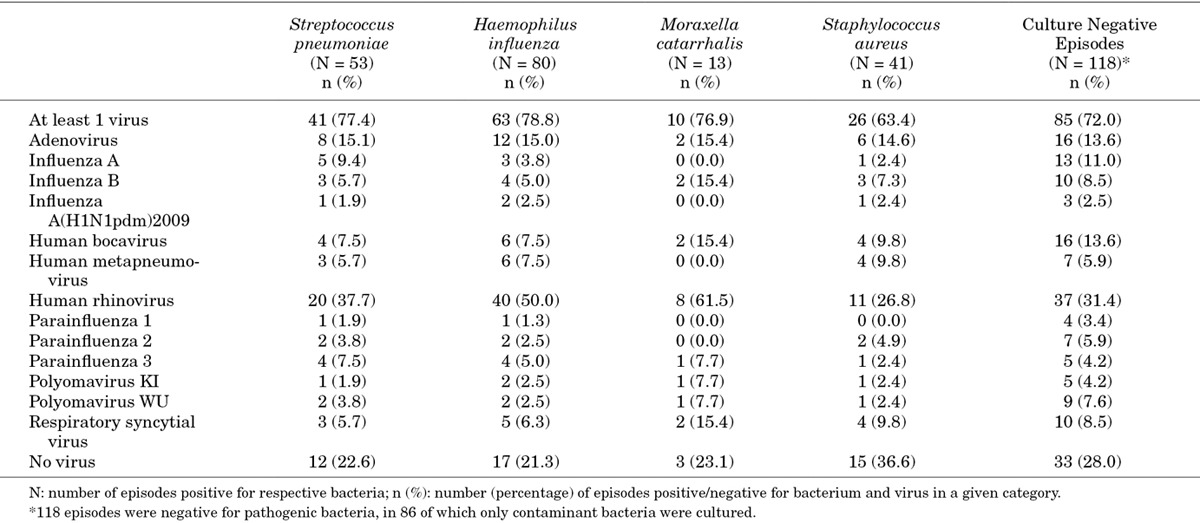
Co-infections with at least 1 virus and bacteria were observed frequently (range: 76.9–78.8%) among episodes positive for S. pneumoniae, H. influenzae and M. catarrhalis and in 63.4% of Staphylococcus aureus-positive episodes and in 25.0% of S. pyogenes-positive episodes (Table 3). At least 2 viruses were found in 22.6% of S. pneumoniae-positive episodes, 22.5% of H. influenzae-positive episodes, 53.8% of M. catarrhalis-positive episodes and 24.4% of Staphylococcus aureus-positive episodes. At least 1 virus was identified in 72.0% (85/118) of episodes negative for pathogenic bacteria, mainly hRV and hBoV.
TABLE 3.
Co-infection Between Bacteria and Viruses: Combined IFA and rRT-PCR
Seasonality
The pattern of AOM episodes due to bacterial and viral pathogens, and the monthly detection of individual respiratory viruses are illustrated in Figure 2. Adenovirus was observed throughout the year peaking in March, hBoV peaked in April, whereas hRV occurred perennially with peak activity in September and April. RSV peaked in March and April and hMPV occurred from June to September peaking in July and August. Influenza A was detected from May to August with 2 peaks in June and August. Influenza-A(H1N1)pdm09, parainfluenza viruses 1–3, and the 2 polyomaviruses had reduced activity compared with the other viruses.
FIGURE 2.
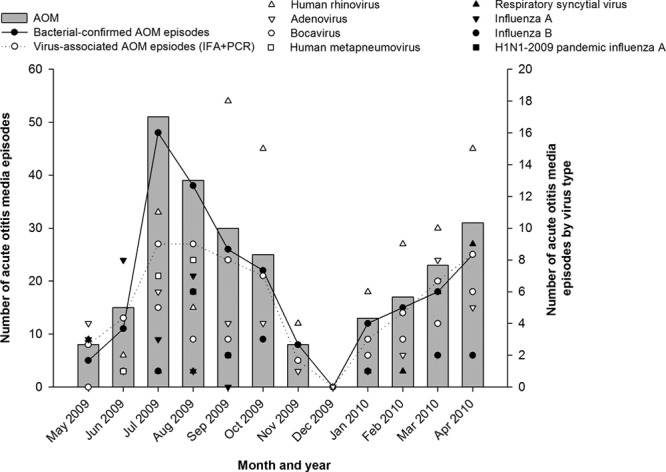
Seasonal distribution of acute otitis media episodes (N = 260). IFA indicates immunofluorescence assay; PCR, polymerase chain reaction.
Both S. pneumoniae and H. influenzae were observed throughout the year with peak activity between July and October. H. influenzae peaked for the second time in April.
DISCUSSION
To the best of our knowledge, this is the first study in Africa to provide the characterization of the bacterial and viral pathogens associated with uncomplicated-AOM in HIV-infected and HEU children. We found that a high percentage of AOM episodes (54.6%) cultured-positive for at least 1 bacterial otopathogen under examination. Overall, H. influenzae (30.8%) and S. pneumoniae (20.4%) were the most common bacterial agents, which is in accordance with the etiology of AOM in Costa Rica, Mexico, Colombia, Venezuela, Germany, France and Israel.20–27 A previous South African study conducted over a decade ago, also implicated H. influenzae and S. pneumoniae as the leading causes of complicated-AOM in South African children.28 We also detected a broad spectrum of respiratory viruses in 74.2% of the NPA collected at the time of AOM episodes. The similarity of our results across HIV+, HIV− and HEU children suggests that there was no meaningful difference in the etiology of AOM by HIV status. Notably, we identified a high prevalence of bacteria-respiratory virus co-infections, suggesting a possible synergistic role between bacteria and viruses in the pathogenesis of AOM in these setting.
Interestingly, we observed a higher number of bilateral versus unilateral episodes, a previous study suggested that bilateral AOM normally presents with more severe clinical characteristics compared with unilateral AOM.29 In our study, however, HIV+ children were more likely to have unilateral than bilateral AOM compared with HIV− and HEU children, an observation that requires further confirmation.
NTHi was responsible for the majority (98.8%) of H. influenzae-positive episodes, as previously seen in other countries.20–26 The most prevalent pneumococcal serotypes were 19F, 19A, 23F, 4, 6A and 6B, which is in agreement with the most common serotypes observed globally.30 Indeed, 19F was previously documented as the most frequent serotype in AOM episodes in South Africa.28 The presence of vaccine serotypes in vaccinated and unvaccinated children reflects the low coverage of PCV in this population due to the early period of 7-valent PCV (Prevnar/Prevenar, Pfizer/Wyeth, New York, NY; PCV7) introduction. In South Africa, PCV7 has been available since April 2009 through the public national immunization program to an estimated birth cohort of 1.2 million children, where 3 doses of PCV7 are administered to children at 6, 14 weeks and 9 months.31 Other PCVs available in the private market are 10-valent PCV (Synflorix, GlaxoSmithKline, Belgium; PHiD-CV) and 13-valent PCV (Prevnar 13/Prevenar 13, Pfizer/Wyeth, USA; PCV13) licensed in 2008 and 2009, respectively.32
In our study, Staphylococcus aureus was isolated from 16% of all AOM episodes. The prevalence of Staphylococcus aureus has been reported is several studies on AOM and varied from very infrequent in uncomplicated AOM in the United States, to 10.4–27% in Italian and Taiwanese children with otorrhea, as well as 17% in a study involving recurrent AOM in the Netherlands.33–36 The majority (88%) of the Staphylococcus aureus episodes in our study were identified from tympanocentesis samples. However, as we did not systematically evaluate for response to empiric treatment which would not necessarily have optimally covered for Staphylococcus aureus,37 we were unable to determine whether identification of Staphylococcus aureus in this setting was truly causative or reflected contamination from the auditory canal. Further studies are warranted to clarify the role of Staphylococcus aureus in AOM in African children which may shed light on whether this could be contributing to the high burden of suppurative AOM in Africa.
While high resistance levels to antibiotics is expected in South Africa given the overuse of antibiotics in the public and private sectors,38 we observed a moderate level of antibiotic resistance overall and by HIV status. The resistance of S. pneumoniae serotype 19A to penicillin was mainly intermediate, thereby highlighting the lack of resistance from this usually penicillin-resistant serotype.39 These findings are consistent with recent reports from other countries,23,24 and underscore the observed change in antibiotic resistance to penicillin over the past decade, particularly because a high percentage of samples were resistant to penicillin in the previous South African study.28 Similarly, most other vaccine serotypes were also intermediate in their resistant to penicillin. Our study also indicated that NTHi-positive episodes were resistant to trimethoprim/sulfamethoxazole (60.8%) and ampicillin (13.9%), consistent with previous reports from South Africa.24,28 Furthermore, the low resistance for S. pneumoniae and NTHi to amoxicillin/clavulanate supports its continued use in the treatment of AOM as recommended in South Africa.37 In addition to antibiotic treatment for AOM, vaccination indicated against AOM may help control the associated disease burden.40,41 Because more than 50% of the isolated serotypes in our study were PCV7-serotypes and the non-PCV7-serotypes among unvaccinated children were 3, 5, 6A and 19A in conjunction to the burden of NTHi, it may be useful to consider the potential value of PCVs in their protection against AOM in South Africa.
A study in Finland between 2006 and 2009 found respiratory viruses in 87% of nasopharyngeal samples from children aged 6–36 months with AOM.42 The prevalence of respiratory viruses was, however, lower in studies from Turkey and Japan.43,44 The Turkish study included children 6 months–12 years old and identified respiratory viruses in 32.5% of MEF samples43 and the Japanese study in children <10 years old detected respiratory viruses in 33% of nasopharyngeal secretions.44 Possible reasons for the higher detection in our study may be related to differences on site of sample collection (middle ear or nasopharynx), our experimental approach as rRT-PCR is more sensitive than conventional viral diagnostics and detects viral nucleic acids even in the absence of acute illness11 or that our study included children <5 years who are at greater risk of developing AOM. Another plausible reason for our higher viral detection may be due to the testing of more recently discovered respiratory viruses.45–48
hRV was the most common virus (37.7%), which is corroborated by previous reports;42 in addition, hRV was frequently detected irrespective of HIV status. A retrospective study in the USA between 2002 and 2010 demonstrated temporal associations among RSV, hMPV and influenza in individuals aged <18 years, but showed no association between AOM and hRV or adenovirus.49 This calls into question the causal role of hRV and adenovirus which tend to recur in their circulation having no seasonal pattern contrary to RSV, hMPV and influenza which are likely to be more seasonal as illustrated by us. Moreover, the uncertainty of specific viruses as etiological agents for recurrent AOM has been suggested in a study that found similar frequency of respiratory viruses in both children with recurrent AOM and healthy children.50
Viral–bacterial interactions among AOM episodes tend to be associated with more severe AOM.51 Animal models, tissue culture experiments and population-wide epidemiological studies have suggested interactions between S. pneumoniae and respiratory viruses in the pathogenesis of AOM,52 the most well-known synergistic effect being that of influenza and S. pneumoniae.53 We detected influenza viruses (A + B) in combination with S. pneumoniae in 8 episodes and RSV and S. pneumoniae in 3 episodes. A high viral RSV load in combination with S. pneumoniae has been associated with an increased risk of AOM, and the same study also showed an increased risk of AOM during co-infections with hBoV and H. influenzae,54 combination found in 6 episodes in our study. Further studies to better understand the mechanisms of viral–bacterial co-detection in the nasopharynx and the development of AOM in this region are required.
The role of the identified respiratory viruses in the pathogenesis of AOM must be interpreted with caution given certain limitations. MEF samples in this study were not analyzed for respiratory viruses due to the insufficient sample volume. The lack of a control group of healthy children restricts this study from addressing whether viral identification was co-incidental or causally related to AOM. Other limitations of our study are that the number of HIV+ children enrolled was lower than the number of HIV− and HEU children; besides HEU were presumed to be HIV-uninfected without a confirmatory HIV test, and the HIV− group included children born to HIV-infected and HIV-uninfected mothers. The presence of some viruses was only accessed by PCR while for others PCR and immunofluorescence assay were used, so comparisons were limited. Results from a single center should also be interpreted cautiously when considering potential national implications. In addition, our study did not examine the association of NTHi with recurrent AOM cases, which has been recognized previously.55 Furthermore, the lack of a difference in bacterial etiology between vaccinated and unvaccinated children was possibly due to the low vaccination coverage at the time of the study, whereas such differences have been noted elsewhere following introduction of PCV7.56 A follow-up etiology study in the context of high vaccination coverage should be performed.
The results of our study suggest that bacteria and respiratory viruses may play an important role in the development of AOM in children. We also confirmed that the HIV status had no impact on the etiology of AOM in the country which may be useful in the clinical management of AOM in the region. Furthermore, vaccines targeting both NTHi and S. pneumoniae may be an important tool in reducing the burden of AOM in South Africa.
ACKNOWLEDGMENTS
The authors thank all the investigators involved in this trial. The authors also thank the parents and children who participated in this study. The authors acknowledge Mrs. Linda de Gouveia for assistance in serotyping the isolates and Dr. Maurice Hockman, who helped train doctors on the tympanocentesis procedure. In addition, the following employees of the GlaxoSmithKline group of companies are acknowledged – Rodrigo DeAntonio Suarez for critical review of the manuscript and his expert input; Ashmita Ravishankar and Mark Franco for manuscript writing; Geetha Subramanyam and Manjula K for publication management.
Supplementary Material
Footnotes
SAM, NG, NvN, CLC, PVA and MCN declare that their institution received grants to conduct trials from the GlaxoSmithKline group of companies/various pharmaceutical companies; SAM has undertaken consultancy and received payment for lectures including service on speakers bureaus for various pharmaceutical companies; KD declares no conflict of interest; RD and MVD are/were employed by the GlaxoSmithKline group of companies at the time of this study. MVD also holds stock ownership from the sponsoring company. This study was sponsored and funded by GlaxoSmithKline Biologicals S.A., Rixensart, Belgium. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis, and also took charge of all costs associated with the development and the publishing of the manuscript.
All authors participated in the design or implementation or analysis, interpretation of the study and the development of this manuscript. All authors had full access to the data and gave final approval before submission. SAM was the Principal investigator and together with co-investigators KD, NG, CLC, MCN, PVA and Lab Manager NvN was responsible for the conduct of the trial. In addition, the following authors were involved in specific activities: CLC and PVA: Provision of study materials or subjects; SAM: Choice/recruitment of centers/recruitment of investigators; SAM, KD, NvN, MCN, PVA: Administrative, technical or logistic support; NvN, CLC, PVA: Contribution to collection and assembly of data (eg, center coordination, data extraction, quality check); NvN: Laboratory/serology testing; SAM, CLC, MCN, PVA: Supervision of the study/research group. MVD was the Epidemiologist from GlaxoSmithKline Vaccines, and RD was the Biostatistician involved in the analysis and providing statistical inputs.
Trademarks: Prevnar/Prevenar and Prevnar13/Prevenar13 are trademarks of Pfizer/Wyeth, USA. Synflorix is a trademark of the GlaxoSmithKline group of companies.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Rovers MM. The burden of otitis media. Vaccine. 2008;26(Suppl 7):G2–G4. doi: 10.1016/j.vaccine.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Monasta L, Ronfani L, Marchetti F, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7:e36226. doi: 10.1371/journal.pone.0036226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris PS, Leach AJ. Acute and chronic otitis media. Pediatr Clin North Am. 2009;56:1383–1399. doi: 10.1016/j.pcl.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acquin J. Chronic suppurative otitis media: burden of illness and management options. Geneva: World Health Organization; 2004. Available at: http://www.who.int/pbd/deafness/activities/hearing_care/otitis_media.pdf. Accessed February 21, 2014. [Google Scholar]
- 5.Bakaletz LO. Immunopathogenesis of polymicrobial otitis media. J Leukoc Biol. 2010;87:213–222. doi: 10.1189/jlb.0709518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leibovitz E, Jacobs MR, Dagan R. Haemophilus influenzae: a significant pathogen in acute otitis media. Pediatr Infect Dis J. 2004;23:1142–1152. [PubMed] [Google Scholar]
- 7.Heikkinen T, Chonmaitree T. Importance of respiratory viruses in acute otitis media. Clin Microbiol Rev. 2003;16:230–241. doi: 10.1128/CMR.16.2.230-241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massa HM, Cripps AW, Lehmann D. Otitis media: viruses, bacteria, biofilms and vaccines. Med J Aust. 2009;191(9 Suppl):S44–S49. doi: 10.5694/j.1326-5377.2009.tb02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chonmaitree T, Revai K, Grady JJ, et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46:815–823. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chonmaitree T, Ruohola A, Hendley JO. Presence of viral nucleic acids in the middle ear: acute otitis media pathogen or bystander? Pediatr Infect Dis J. 2012;31:325–330. doi: 10.1097/INF.0b013e318241afe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnett ED, Klein JO, Pelton SI, et al. Otitis media in children born to human immunodeficiency virus-infected mothers. Pediatr Infect Dis J. 1992;11:360–364. doi: 10.1097/00006454-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964–e999. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 14.Horn VK, Tóth C, Wegienek J. ASM 98th General Meeting. 1998. Viability of microorganisms in four swab systems. p. C436. [Google Scholar]
- 15.Murray P. Manual of Clinial Microbiology. 6th ed. Washington, DC: American Society of Microbiology; 1995. [Google Scholar]
- 16.Maaroufi Y, De Bruyne JM, Heymans C, et al. Real-time PCR for determining capsular serotypes of Haemophilus influenzae. J Clin Microbiol. 2007;45:2305–2308. doi: 10.1128/JCM.00102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Nineteenth informational supplement M100-S19. Wayne, PA: Clinical and Laboratory Standards Institute; 2009. [Google Scholar]
- 18.Madhi SA, Schoub B, Simmank K, et al. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1. J Pediatr. 2000;137:78–84. doi: 10.1067/mpd.2000.105350. [DOI] [PubMed] [Google Scholar]
- 19.Nunes MC, Kuschner Z, Rabede Z, et al. Clinical epidemiology of bocavirus, rhinovirus, two polyomaviruses and four coronaviruses in HIV-infected and HIV-uninfected South African children. PLoS One. 2014;9:e86448. doi: 10.1371/journal.pone.0086448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arguedas A, Dagan R, Soley C, et al. Microbiology of otitis media in Costa Rican children, 1999 through 2001. Pediatr Infect Dis J. 2003;22:1063–1068. doi: 10.1097/01.inf.0000101189.81501.e9. [DOI] [PubMed] [Google Scholar]
- 21.Aguilar L, Alvarado O, Soley C, et al. Microbiology of the middle ear fluid in Costa Rican children between 2002 and 2007. Int J Pediatr Otorhinolaryngol. 2009;73:1407–1411. doi: 10.1016/j.ijporl.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Parra MM, Aguilar GM, Echaniz-Aviles G, et al. Bacterial etiology and serotypes of acute otitis media in Mexican children. Vaccine. 2011;29:5544–5549. doi: 10.1016/j.vaccine.2011.04.128. [DOI] [PubMed] [Google Scholar]
- 23.Sierra A, Lopez P, Zapata MA, et al. Non-typeable Haemophilus influenzae and Streptococcus pneumoniae as primary causes of acute otitis media in colombian children: a prospective study. BMC Infect Dis. 2011;11:4. doi: 10.1186/1471-2334-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naranjo L, Suarez JA, DeAntonio R, et al. Non-capsulated and capsulated Haemophilus influenzae in children with acute otitis media in Venezuela: a prospective epidemiological study. BMC Infect Dis. 2012;12:40. doi: 10.1186/1471-2334-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grevers G, Wiedemann S, Bohn JC, et al. Identification and characterization of the bacterial etiology of clinically problematic acute otitis media after tympanocentesis or spontaneous otorrhea in German children. BMC Infect Dis. 2012;12:312. doi: 10.1186/1471-2334-12-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couloigner V, Levy C, François M, et al. Pathogens implicated in acute otitis media failures after 7-valent pneumococcal conjugate vaccine implementation in France: distribution, serotypes, and resistance levels. Pediatr Infect Dis J. 2012;31:154–158. doi: 10.1097/INF.0b013e3182357c8d. [DOI] [PubMed] [Google Scholar]
- 27.Leibovitz E, Raiz S, Piglansky L, et al. Resistance pattern of middle ear fluid isolates in acute otitis media recently treated with antibiotics. Pediatr Infect Dis J. 1998;17:463–469. doi: 10.1097/00006454-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Huebner RE, Wasas AD, Hockman M, et al. ENT Study Group. Bacterial aetiology of non-resolving otitis media in South African children. J Laryngol Otol. 2003;117:169–172. doi: 10.1258/002221503321192430. [DOI] [PubMed] [Google Scholar]
- 29.Leibovitz E, Asher E, Piglansky L, et al. Is bilateral acute otitis media clinically different than unilateral acute otitis media? Pediatr Infect Dis J. 2007;26:589–592. doi: 10.1097/INF.0b013e318060cc19. [DOI] [PubMed] [Google Scholar]
- 30.Rodgers GL, Arguedas A, Cohen R, et al. Global serotype distribution among Streptococcus pneumoniae isolates causing otitis media in children: potential implications for pneumococcal conjugate vaccines. Vaccine. 2009;27:3802–3810. doi: 10.1016/j.vaccine.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 31.Madhi SA, Cohen C, von Gottberg A. Introduction of pneumococcal conjugate vaccine into the public immunization program in South Africa: translating research into policy. Vaccine. 2012;30(Suppl 3):C21–C27. doi: 10.1016/j.vaccine.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 32.Johnson HL, Deloria-Knoll M, Levine OS, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29:304–309. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchisio P, Bianchini S, Baggi E, et al. A retrospective evaluation of microbiology of acute otitis media complicated by spontaneous otorrhea in children living in Milan, Italy. Infection. 2013;41:629–635. doi: 10.1007/s15010-012-0371-1. [DOI] [PubMed] [Google Scholar]
- 35.Chen YJ, Hsieh YC, Huang YC, et al. Clinical manifestations and microbiology of acute otitis media with spontaneous otorrhea in children. J Microbiol Immunol Infect. 2013;46:382–388. doi: 10.1016/j.jmii.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Veenhoven R, Bogaert D, Uiterwaal C, et al. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. Lancet. 2003;361:2189–2195. doi: 10.1016/S0140-6736(03)13772-5. [DOI] [PubMed] [Google Scholar]
- 37.Brink AJ, Cotton MF, Feldman C, et al. Working Group of the Infectious Diseases Society of South Africa. Guideline for the management of upper respiratory tract infections. S Afr Med J. 2004;94(6 Pt 2):475–483. [PubMed] [Google Scholar]
- 38.Global Antibiotic Resistance Partnership. Situation analysis: Antibiotic use and resistance in South Africa. 2011;101:549–596. Available at: http://www.cddep.org/publications/situation_analysis_antibiotic_use_and_resistance_south_africa. Accessed February 21, 2014. [Google Scholar]
- 39.Dagan R, Givon-Lavi N, Leibovitz E, et al. Introduction and proliferation of multidrug-resistant Streptococcus pneumoniae serotype 19A clones that cause acute otitis media in an unvaccinated population. J Infect Dis. 2009;199:776–785. doi: 10.1086/597044. [DOI] [PubMed] [Google Scholar]
- 40.Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–409. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 41.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Ruohola A, Pettigrew MM, Lindholm L, et al. Bacterial and viral interactions within the nasopharynx contribute to the risk of acute otitis media. J Infect. 2013;66:247–254. doi: 10.1016/j.jinf.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bulut Y, Güven M, Otlu B, et al. Acute otitis media and respiratory viruses. Eur J Pediatr. 2007;166:223–228. doi: 10.1007/s00431-006-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yano H, Okitsu N, Hori T, et al. Detection of respiratory viruses in nasopharyngeal secretions and middle ear fluid from children with acute otitis media. Acta Otolaryngol. 2009;129:19–24. doi: 10.1080/00016480802032777. [DOI] [PubMed] [Google Scholar]
- 45.Allander T, Andreasson K, Gupta S, et al. Identification of a third human polyomavirus. J Virol. 2007;81:4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaynor AM, Nissen MD, Whiley DM, et al. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3:e64. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ricour C, Goubau P. Human bocavirus, a newly discovered parvovirus of the respiratory tract. Acta Clin Belg. 2008;63:329–334. doi: 10.1179/acb.2008.064. [DOI] [PubMed] [Google Scholar]
- 49.Stockmann C, Ampofo K, Hersh AL, et al. Seasonality of acute otitis media and the role of respiratory viral activity in children. Pediatr Infect Dis J. 2013;32:314–319. doi: 10.1097/INF.0b013e31827d104e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiertsema SP, Chidlow GR, Kirkham LA, et al. High detection rates of nucleic acids of a wide range of respiratory viruses in the nasopharynx and the middle ear of children with a history of recurrent acute otitis media. J Med Virol. 2011;83:2008–2017. doi: 10.1002/jmv.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marom T, Nokso-Koivisto J, Chonmaitree T. Viral-bacterial interactions in acute otitis media. Curr Allergy Asthma Rep. 2012;12:551–558. doi: 10.1007/s11882-012-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore DP, Dagan R, Madhi SA. Respiratory viral and pneumococcal coinfection of the respiratory tract: implications of pneumococcal vaccination. Expert Rev Respir Med. 2012;6:451–465. doi: 10.1586/ers.12.32. [DOI] [PubMed] [Google Scholar]
- 53.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pettigrew MM, Gent JF, Pyles RB, et al. Viral-bacterial interactions and risk of acute otitis media complicating upper respiratory tract infection. J Clin Microbiol. 2011;49:3750–3755. doi: 10.1128/JCM.01186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy TF, Faden H, Bakaletz LO, et al. Nontypeable Haemophilus influenzae as a pathogen in children. Pediatr Infect Dis J. 2009;28:43–48. doi: 10.1097/INF.0b013e318184dba2. [DOI] [PubMed] [Google Scholar]
- 56.Coker TR, Chan LS, Newberry SJ, et al. Diagnosis, microbial epidemiology, and antibiotic treatment of acute otitis media in children: a systematic review. JAMA. 2010;304:2161–2169. doi: 10.1001/jama.2010.1651. [DOI] [PubMed] [Google Scholar]


