Abstract
Rapidly cycling fetal and neonatal hematopoietic stem cells (HSCs) generate a pool of quiescent adult HSCs after establishing hematopoiesis in the bone marrow. We report an essential role for the trithorax group gene absent, small, or homeotic 1-like (Ash1l) at this developmental transition. Emergence and expansion of Ash1l-deficient fetal/neonatal HSCs were preserved; however, in young adult animals, HSCs were profoundly depleted. Ash1l-deficient adult HSCs had markedly decreased quiescence and reduced cyclin-dependent kinase inhibitor 1b/c (Cdkn1b/1c) expression and failed to establish long-term trilineage bone marrow hematopoiesis after transplantation to irradiated recipients. Wild-type HSCs could efficiently engraft when transferred to unirradiated, Ash1l-deficient recipients, indicating increased availability of functional HSC niches in these mice. Ash1l deficiency also decreased expression of multiple Hox genes in hematopoietic progenitors. Ash1l cooperated functionally with mixed-lineage leukemia 1 (Mll1), as combined loss of Ash1l and Mll1, but not isolated Ash1l or Mll1 deficiency, induced overt hematopoietic failure. Our results uncover a trithorax group gene network that controls quiescence, niche occupancy, and self-renewal potential in adult HSCs.
Keywords: Hematology, Stem cells, Transplantation
Introduction
Fetal hematopoietic stem cells (HSCs) proliferate rapidly and contribute to massive expansion of the HSC pool (1, 2). In contrast, adult HSCs divide rarely in steady-state conditions, as a majority of cells reside in the quiescent G0 phase of the cell cycle and return to quiescence after periods of proliferation (3–8). In mice, transition from the fetal/neonatal to the adult HSC program occurs in the bone marrow (BM) niche within weeks after birth and coincides with establishment of HSC quiescence, functional and phenotypic changes, and downregulated expression of the transcription factor Sox17 and other fetal HSC genes (1, 9–12). Failure to establish or maintain quiescence has been linked to adult HSC depletion, increased sensitivity to myeloablation, and reduced engraftment in transplantation assays (1, 13–16). However, the regulatory mechanisms controlling quiescence and successful initiation of the adult HSC program in the BM remain poorly understood.
The trithorax group (TrxG) is a diverse family of epigenetic regulators originally identified to control body patterning in Drosophila (17). In flies, individual TrxG members cooperate to promote expression of homeobox (Hox) genes, and combined heterozygous TrxG gene mutations act as dominant enhancers of one another (18, 19). Mixed-lineage leukemia 1 (Mll1) is the trithorax homolog and founding TrxG member in mammals. In humans, MLL1 was first identified as a recurrent translocation partner in acute leukemias characterized by upregulated HOX gene expression (20–23). Endogenous Mll1 regulates expression of Hox genes and other targets as well as the function of normal HSCs (24–26). Mll1 encodes a large protein containing a C-terminal Su(var)3-9/enhancer-of-zeste/trithorax (SET) domain with H3K4 histone methyltransferase activity (27). MLL1 functions as a part of a multiprotein complex that includes RBBP5, WDR5, and ASH2L (28–31). Limited information is available about cooperative interactions among mammalian TrxG members, in particular with proteins in which an association with the MLL complex has not been as yet identified.
The TrxG gene absent, small, or homeotic 1 (ash1) was first discovered in genetic screens seeking regulators of Drosophila imaginal discs (32). ash1 encodes a large protein containing an internal SET domain with putative histone methyltransferase activity (33). Its mammalian homolog Ash1l also encodes a SET domain–containing protein that can associate with actively transcribed loci, including at several Hox genes (34–36). However, unlike the much smaller protein ASH2L, ASH1L has not been identified so far as a member of the MLL protein complex. Recently, the ASH1L SET domain was reported to have intrinsic H3K36 dimethyltransferase activity using in vitro biochemical assays (37–39). Neither the physiological significance of Ash1l nor the idea of cooperativity with Mll1 and other TrxG members has been evaluated in vivo.
In this report, we describe an essential role for the TrxG member Ash1l in the maintenance and function of adult HSCs but not in the in vivo expansion of fetal or neonatal hematopoietic progenitors. Ash1l was essential for the establishment of quiescence at the fetal to adult HSC transition in the BM within weeks after birth. Ash1l deficiency led to profound depletion of adult HSCs and multipotent progenitors as well as to a lack of functional HSCs capable of long-term BM reconstitution in transplantation assays. Unlike in wild-type recipients, after nonmyeloablative transplantation of normal HSCs, cells could efficiently engraft the BM and establish durable hematopoiesis in Ash1l-deficient mice, suggesting poor competition of residual HSCs for niche space. Despite profound defects in transplantation assays, Ash1l-deficient mice exhibited no overt hematopoietic failure in steady-state conditions, with evidence of increased self-renewal in progenitors downstream of HSCs. However, combining Ash1l deficiency with inactivation of the TrxG gene Mll1 or its cofactor gene Men1 led to rapid BM failure. These data reveal an essential physiological function for the TrxG gene Ash1l in adult HSCs and represent the first genetic demonstration of cooperativity between TrxG members in mammals.
Results
Ash1l-deficient mice have normal numbers of fetal and neonatal HSCs but profound depletion of adult HSCs.
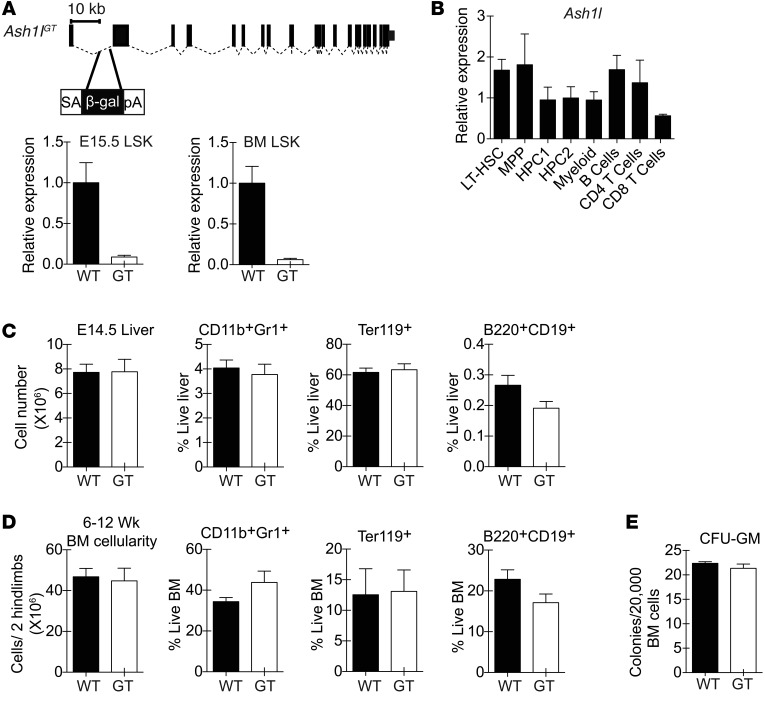
To examine the function of Ash1l in hematopoiesis, we used a gene trap insertion allele containing a splice-acceptor cassette in the first Ash1l intron (Figure 1A). This strategy resulted in a >90% reduction of full-length Ash1l transcripts in fetal liver and BM lineage–SCA1+c-KIT+ (LSK) HSCs and hematopoietic progenitor cells. Ash1l was expressed in all subpopulations of LSK progenitors and in selected mature cell subsets (Figure 1B). Homozygosity for the Ash1lGT allele preserved fetal liver and BM cellularity as well as myeloid, erythroid, and B lineage cells in Ash1lGT/GT mice compared with that in Ashl+/+ littermates (Figure 1, C and D). The capacity to form myeloid colonies was normal in Ash1l-deficient BM, consistent with preserved progenitor numbers and function (Figure 1E).
Figure 1. Preserved overall fetal and adult hematopoietic output in Ash1lGT/GT mice.
(A) Generation of the Ash1lGT allele by insertion of a splice-acceptor gene trap cassette into the first Ash1l intron. Homozygosity led to >90% reduction in wild-type transcripts in fetal (E15.5) and adult LSK progenitors, as shown by quantitative RT-PCR (qRT-PCR) with primers amplifying cDNA across the exon 1–2 boundary (mean ± SD). (B) qRT-PCR analysis of Ash1l expression normalized to Hprt1 in selected hematopoietic populations (LT-HSC, LSK CD150+CD48– LT-HSCs; MPP, LSK MPPs; HPC1, LSK CD150–CD48+ hematopoietic progenitor cells; HPC2, LSK CD150+CD48+ hematopoietic progenitor cells; myeloid, CD11b+Gr1+ myeloid cells; B cells, B220+AA4.1– B cells; CD4 T cells, TCRβ+CD4+ cells; CD8 T cells, TCRβ+CD8+ T cells) (mean ± SD). (C) Cellularity and percentage of myeloid, erythroid, and B lineage cells in E14.5 wild-type and Ash1lGT/GT (GT) fetal liver (n ≥ 4 per genotype from 2 independent experiments; mean ± SEM). (D) Cellularity and percentage of myeloid, erythroid, and B lineage cells in young adult (6- to 12-week-old) wild-type and Ash1lGT/GT BM (n ≥ 6 per genotype from >2 independent experiments; mean ± SEM). (E) Myeloid colony formation by wild-type and Ash1lGT/GT BM in CFU-GM assays (mean ± SEM, representative of 2 experiments). No statistically significant differences by t test.
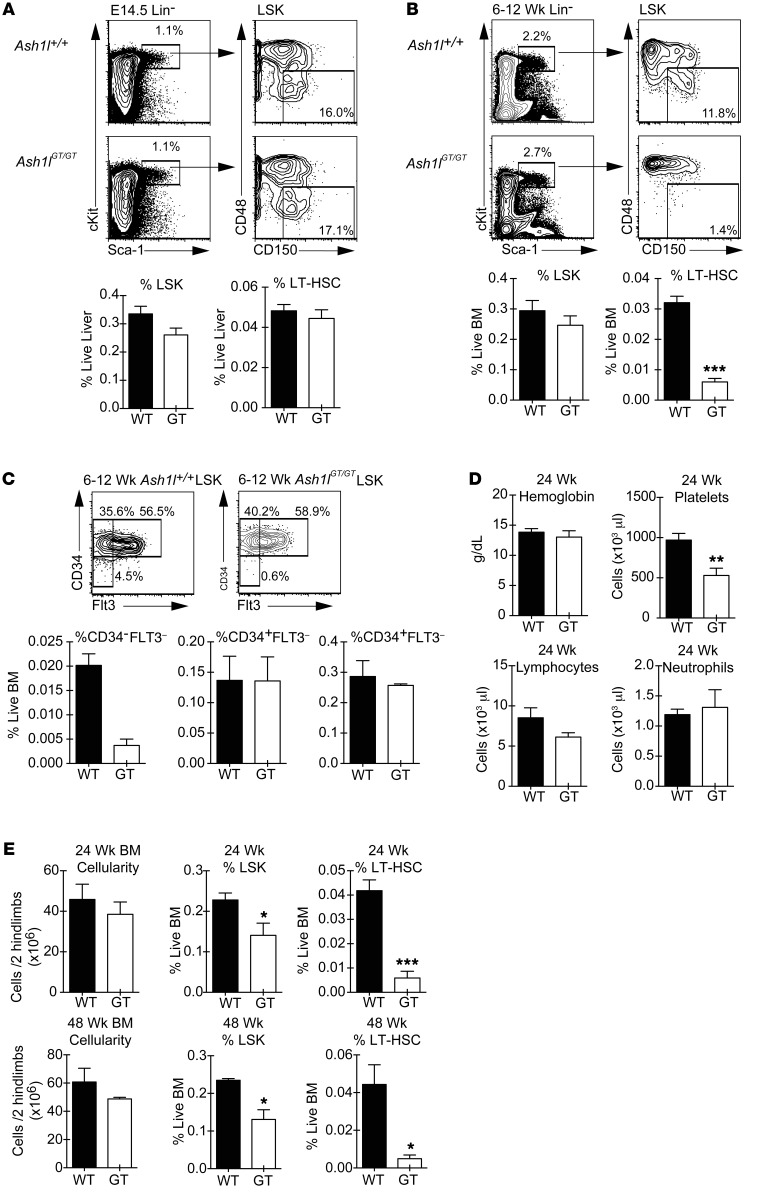
We quantified HSCs as the CD150+CD48– fraction of LSK progenitors, a definition that identifies both fetal and adult long-term HSCs (LT-HSCs) (40, 41). Ash1l-deficient LT-HSCs were present at normal frequency in the fetal liver, showing that phenotypically defined fetal LT-HSCs emerged and expanded normally in these mice (Figure 2A). HSC numbers were also preserved in the neonatal liver (data not shown). However, by 6 to 12 weeks after birth, LT-HSCs had decreased by 5- to 10-fold in Ash1lGT/GT BM as compared with BM of wild-type littermates (Figure 2B). A similar profound reduction was observed using the CD34–FLT3–LSK phenotype as an alternative definition of LT-HSCs, with preserved frequencies of CD34+FLT3– and CD34+FLT3+ downstream progenitors (Figure 2C and refs. 42, 43). We next fractionated LSK progenitors using CD150/CD48 expression, as the functional potential of these subpopulations was recently characterized in detail (44). Both LSK CD150+CD48– LT-HSCs and CD150–CD48– multipotent progenitors (MPPs), but not CD48+LSK hematopoietic progenitor cells (HPC1/2), were decreased in Ash1lGT/GT mice (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI78124DS1). Despite persistent LT-HSC depletion, 24-week-old Ash1l-deficient mice maintained normal blood counts, except for modest thrombocytopenia (Figure 2D). Overall BM cellularity remained preserved at 24 weeks as well as at 48 weeks of life. Flow cytometric analysis showed persistent profound LT-HSC depletion, while numbers of LSK progenitors were reduced only by about 30% at 24 weeks and 50% at 48 weeks (Figure 2E). These data suggest a specific role of Ash1l in the maintenance of adult BM HSCs, with downstream compensatory mechanisms preserving overall hematopoietic function in steady-state conditions.
Figure 2. Young adult Ash1lGT/GT mice show profound LT-HSC depletion, as defined phenotypically.
(A) Frequency of E14.5 fetal liver LSK and LT-HSC (CD150+CD48–LSK) cells in wild-type and Ash1lGT/GT fetuses (wild-type n = 4, Ash1lGT/GT n = 8 from 2 experiments; mean ± SEM). (B) Flow cytometric analysis showing >5-fold reduced frequency of LT-HSCs in young adult (6- to 12-week-old) Ash1lGT/GT mice (wild-type n = 6, Ash1lGT/GT n = 8 from 3 experiments; mean ± SEM ). (C) Reduced CD34–FLT3–LSK, but not CD34+FLT3–LSK or CD34+FLT3+LSK, progenitors in young adult Ash1lGT/GT mice (n = 4 mice per genotype from 2 experiments; mean ± SEM). (D) Complete blood counts of 24-week-old mice, showing reduced platelets but preserved lymphocytes, neutrophils, and hemoglobin contents (n = 6 mice per genotype; mean ± SEM). (E) BM cellularity and progenitor contents in 24-week-old mice (n = 6 mice per genotype from 3 experiments; mean ± SEM) and 48-week-old mice (n = 3 per genotype from 2 experiments; mean ± SEM). Flow cytometric analysis demonstrates a reduction in LSK progenitors and persistent 5- to 10-fold reduction in LT-HSCs. *P < 0.05, **P < 0.01, ***P < 0.001, t test.
To evaluate whether the hematopoietic phenotype of Ash1l-deficient mice was associated with mobilization of BM HSCs and hematopoietic progenitor cells into the periphery, we assessed LSK and LT-HSC frequency as well as colony-forming activity in the spleen (Supplemental Figure 2). The frequency of spleen LSK/LT-HSCs and granulocyte-macrophage CFU (CFU-GM) colonies was decreased in Ash1lGT/GT mice. These findings indicate the absence of a mobilization phenotype in these mice and suggest that the maintenance of adult Ash1l-deficient hematopoietic progenitors was relatively more impaired outside as compared with that within the BM.
Ash1l-deficient mice lack HSCs capable of long-term BM reconstitution after transplantation.
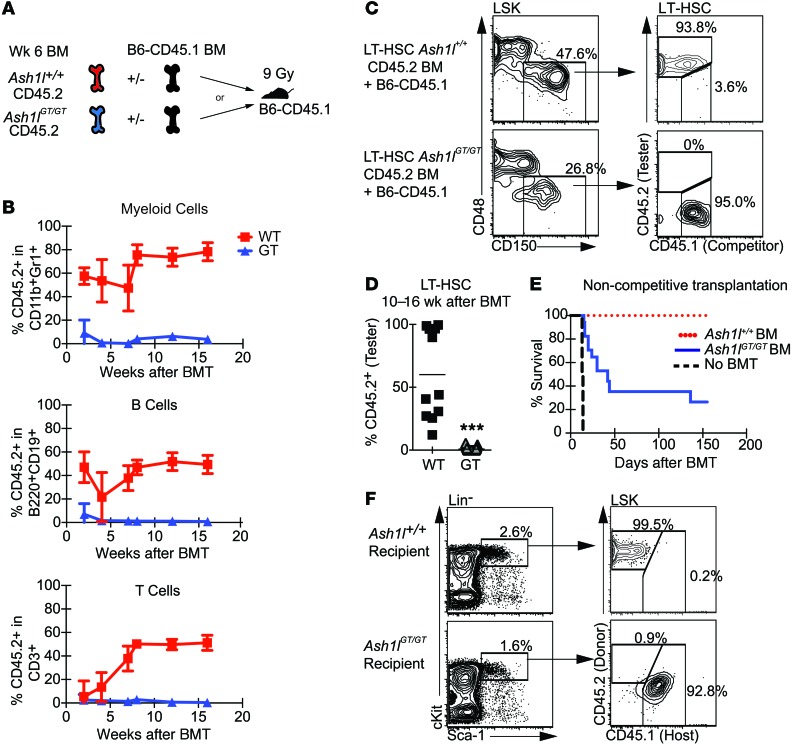
As the gold standard definition of LT-HSCs is based on their function (45, 46), we studied the long-term reconstitution potential of Ash1l-deficient progenitors in transplantation assays (Figure 3A). This strategy allowed us to evaluate LT-HSC function irrespective of surface phenotype. We transplanted lethally irradiated CD45.1+ recipient mice with CD45.2+ Ash1lGT/GT or Ash1l+/+ BM and an equal number of CD45.1+ competitor BM cells. Although transient output was observed 2 weeks after transplantation, Ash1lGT/GT BM failed to support long-term trilineage reconstitution of the myeloid, B, and T cell compartments (Figure 3B). Analysis of BM LT-HSCs 10–16 weeks after transplantation showed a complete absence of Ash1lGT/GT LT-HSCs (Figure 3, C and D). The lack of significant reconstitution beyond a few weeks was consistent with the depletion of both LT-HSCs and multipotent progenitors in Ash1l-deficient mice, as defined using CD150/CD48 expression (43). To examine the relative contribution of CD48–LSK cells (containing LT-HSCs and multipotent progenitors) and CD48+LSK cells (containing HPC1/2 progenitors) to transient reconstitution after transplant, we competitively transferred sort-purified CD48–LSK and CD48+LSK progenitors to lethally irradiated recipients (Supplemental Figure 3). Consistent with past data (44), only transient hematopoietic output was detected from wild-type HPC1/2 cells, while LSK MPP/LT-HSC sustained long-term reconstitution. This pattern of activity was preserved with Ash1l-deficient progenitors at early time points after transplantation, with the bulk of short-term reconstitution coming from the CD48–LSK fraction (2–4 weeks after transplant). As observed with transplantation of total BM, however, phenotypically defined MPP/LT-HSCs were functionally unable to sustain long-term reconstitution. These data suggest that Ash1l-deficient CD48–LSK cells can home to the BM and provide initial progeny but lack long-term self-renewal potential.
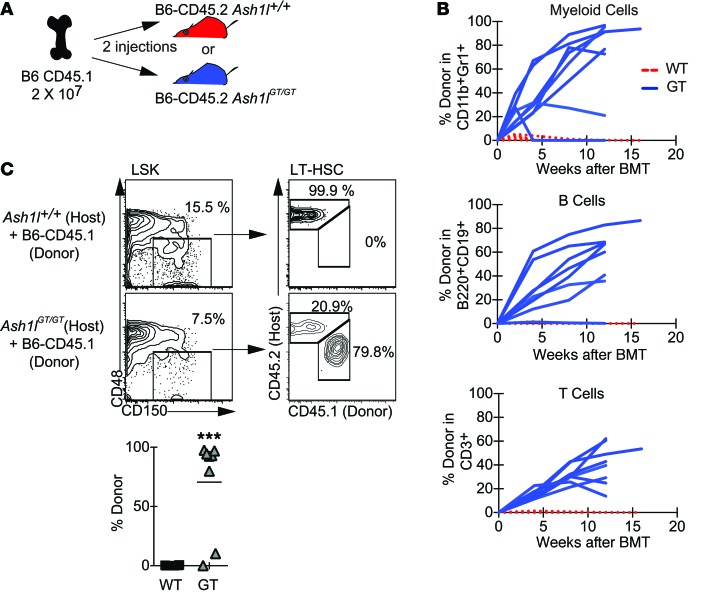
Figure 3. Competitive and noncompetitive transplantation assays reveal a lack of Ash1l-deficient HSCs capable of long-term hematopoietic reconstitution.
(A) Experimental strategy: Ash1l+/+or Ash1lGT/GT B6-CD45.2 BM was injected into irradiated (9 Gy) B6-CD45.1 recipients, with or without B6-CD45.1 competitor BM (5 × 105 cells each for competitive transplantation and 106 cells for noncompetitive transplantation). (B) Peripheral blood analysis 2–16 weeks after competitive transplantation, showing a profound reduction of Ash1lGT/GT BM contribution to myeloid, B, and T lineage reconstitution (wild-type n = 11, Ash1lGT/GT n = 23; mean ± SD from 2 experiments). BMT, BM transplantation. (C and D) CD45.2/CD45.1 chimerism in BM LT-HSCs 10–16 weeks after transplantation, showing absence of Ash1lGT/GT LT-HSCs (wild-type n = 11, Ash1lGT/GT n = 22; data from individual mice, with mean shown, pooled from 2 experiments, ***P < 0.001, t test). (E) Survival after noncompetitive BM transplantation, showing only partial radioprotection by Ash1lGT/GT BM (n = 17 mice per genotype from 2 experiments; 8 mice for no BM transplantation control). (F) Flow cytometric analysis of surviving Ash1lGT/GT recipients, showing exclusively host-derived LSK cells. Control Ash1l+/+ recipients were reconstituted with CD45.2+ donor-derived progenitors (representative of 3 mice per genotype from 2 experiments).
We next examined Ash1lGT/GT LT-HSC function after transplantation without competition. The majority of irradiated Ash1lGT/GT BM recipients died within 10 to 150 days after transplantation, consistent with transient radioprotection but suggestive of LT-HSC dysfunction (Figure 3E). Surviving Ash1lGT/GT BM recipients exhibited host CD45.1+ reconstitution and no Ash1lGT/GT LT-HSCs (Figure 3F). Thus, Ash1lGT/GT BM did not house transplantable LT-HSCs, even as defined by noncompetitive transplantation.
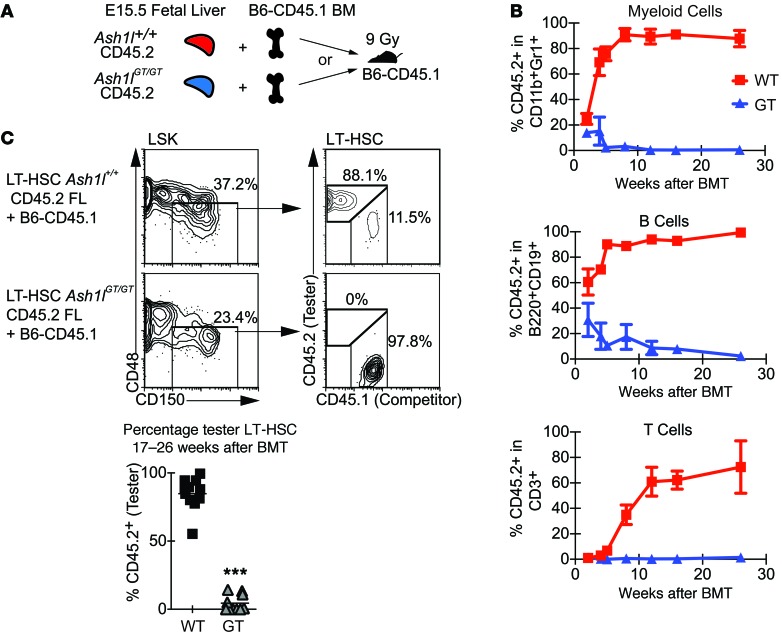
We then performed transplantation using fetal liver as the donor source, thereby normalizing phenotypic LT-HSC frequency and eliminating the possibility that stromal BM defects contributed to LT-HSC dysfunction (Figure 4A). Ash1lGT/GT fetal liver progenitors were incapable of sustaining myeloid, B, and T lineage reconstitution after transplantation (Figure 4B). Analysis of the BM at 17 to 26 weeks after transplantation showed no detectable Ash1lGT/GT LT-HSCs (Figure 4C). Thus, fetal Ash1lGT/GT LT-HSCs were present in normal numbers, as defined phenotypically, but could not establish durable hematopoiesis in the BM after transplantation, indicating that they were functionally defective once transferred into the postnatal environment.
Figure 4. Ash1l-deficient fetal liver cells do not sustain long-term BM reconstitution after competitive transplantation.
(A) Experimental strategy: Ash1l+/+or Ash1lGT/GT B6-CD45.2 E15.5 fetal liver was mixed with wild-type B6-CD45.1 competitor BM (1:1 ratio; 2.5 × 105 cells each) and injected into lethally irradiated (9 Gy) B6-CD45.1 recipients. (B) Analysis of peripheral blood 2–26 weeks after transplantation, showing a profound reduction of Ash1lGT/GT contribution to myeloid, B, and T lineage reconstitution (n = 10–11 mice per genotype; mean ± SD from 2 experiments). (C) CD45.2/CD45.1 chimerism in CD150+CD48–LSKs (LT-HSCs) 17–26 weeks after transplantation, showing markedly decreased fetal liver-derived Ash1lGT/GT LT-HSCs (wild-type n = 11, Ash1lGT/GT n = 10 mice, ***P < 0.001, t test; horizontal bars show the mean).
To evaluate the homing capacity of Ash1l-deficient progenitors, we analyzed recipient BM 24 hours after transplantation into nonirradiated mice (Supplemental Figure 4A). Equivalent numbers of donor-derived wild-type and Ash1lGT/GT cells were recovered (Supplemental Figure 4B). Similar observations were made in irradiated recipients (data not shown). Altogether, our findings reveal profound LT-HSC dysfunction that becomes apparent in the context of the adult BM niche after transplantation, despite preserved initial homing and even when fetal cells are used as a source of progenitors.
The Ash1l-deficient BM supports wild-type HSC engraftment in the absence of myeloablation.
Since Ash1lGT/GT BM had reduced LT-HSCs that were nonfunctional in transplantation assays, we reasoned that available niche space and/or defective progenitors could allow engraftment of donor HSCs without myeloablation. We infused 2 boluses of wild-type CD45.1+ BM (or B6-GFP BM in selected experiments) into CD45.2+ Ash1l+/+ or Ash1lGT/GT mice without prior irradiation (Figure 5A). Few, if any, donor-derived cells engrafted in wild-type recipients, as expected. In contrast, the majority (7 of 8) of Ash1lGT/GT recipients achieved stable or steadily rising trilineage output from infused BM, as shown by the 20% to 90% donor-derived contribution to blood lineages for ≥12 weeks (Figure 5B). At completion of the experiment, donor LT-HSCs were present in the Ash1lGT/GT BM (Figure 5C). Thus, the Ash1lGT/GT BM niche supported long-term stable engraftment by wild-type HSCs, while residual Ash1lGT/GT HSCs and hematopoietic progenitor cells competed poorly for niche space.
Figure 5. Ash1l deficiency allows engraftment of wild-type HSCs in the absence of myeloablation.
(A) Experimental strategy: B6-CD45.2 Ash1l+/+ or Ash1lGT/GT mice received 2 × 107 B6-CD45.1 or B6-GFP BM cells (2 doses 1 week apart i.v.), without prior irradiation. (B) Analysis of peripheral blood, showing myeloid, B, and T cell output from CD45.1+ donor BM in 7 of 8 Ash1lGT/GT recipients versus 0 of 6 Ash1l+/+ recipients (data from 3 experiments). Lines represent individual recipients. Reconstitution was sustained for ≥12 weeks. (C) Flow cytometric analysis of LT-HSCs 12 to 21 weeks after infusion, showing donor LT-HSC engraftment in 7 of 8 Ash1lGT/GT recipients versus 0 of 6 wild-type recipients (pooled from 3 experiments, with horizontal bars showing the mean; ***P < 0.001, t test).
Neonatal Ash1lGT/GT HSCs home to the BM but fail to establish a quiescent adult HSC pool.
As LT-HSC loss was first observed in young adult Ash1lGT/GT BM, we studied BM HSCs within 1 to 3 weeks after birth. At this stage, Ash1lGT/GT mice had a normal frequency of phenotypically defined LT-HSCs in the BM, indicating preserved initial homing from the liver (Figure 6, A and B, and data not shown). However, P19 Ash1lGT/GT HSCs showed increased expression of CD34 (Figure 6B). In mice, CD34 is upregulated in cycling HSCs and marks fetal but not the most quiescent adult mouse LT-HSCs (3, 10). To test whether Ash1lGT/GT BM HSCs aberrantly maintained a fetal program, we used a Sox17-GFP reporter to study expression of Sox17, a master regulator of fetal HSCs (11). Ash1lGT/GT HSCs were Sox17-GFP+ in the fetal liver and extinguished Sox17 expression by 2 weeks after birth (Figure 6C). A similar pattern was observed for Lin28b transcripts, which were detected in fetal HSCs, as previously reported (12), but absent in both wild-type and Ash1lGT/GT adult HSCs (Figure 6C). Thus, Ash1l deficiency did not result in the maintenance of a global fetal HSC state.
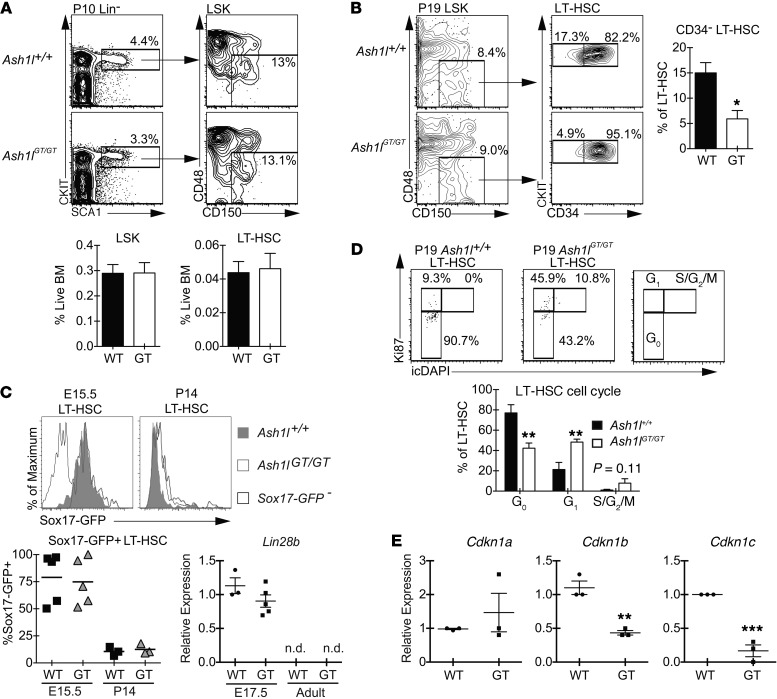
Figure 6. Ash1lGT/GT LT-HSCs home to the BM but fail to establish normal quiescence, despite appropriate extinction of the fetal HSC program.
(A) Flow cytometric analysis of P10 BM, showing comparable frequencies of phenotypically defined LT-HSCs (CD150+CD48–LSK) in Ash1lGT/GT and Ash1l+/+ littermates (≥9 mice per genotype; mean ± SEM). (B) Decreased percentage of quiescent CD34– cells in Ash1lGT/GT LT-HSCs (P19) (4 mice per genotype; mean ± SEM). (C) Sox17-GFP expression in E15.5 fetal liver and P14 BM LT-HSCs (CD150+CD48–LSK cells). Sox17-GFP was present in fetal LT-HSCs but extinguished in wild-type and Ash1lGT/GT P14 BM (n ≥ 3 per genotype, 4 experiments; symbols show individual mice and the bars show the mean). qRT-PCR analysis shows similar Lin28b gene expression in Ash1lGT/GT and Ash1l+/+ fetal LSK progenitors, with loss of expression in adult progenitors of both genotypes (n = 3–5 per group; mean ± SEM) (n.d., not detectable). (D) Flow cytometry plots (Ki67 vs. DAPI) showing decreased G0 (quiescent fraction) and increased distribution into G1 and S/G2/M phases of the cell cycle in P19 Ash1lGT/GT LT-HSCs (5 mice per genotype; mean ± SEM). (E) Reduced Cdkn1b and Cdkn1c expression relative to Hprt1 in P10 Ash1lGT/GT LSK progenitors (qRT-PCR, n = 3 per group, mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001, t test.
We next assessed cell cycle status in Ash1lGT/GT HSCs. As shown by Ki67/DAPI staining, P19 Ash1lGT/GT HSCs had a markedly reduced G0 fraction, increased entry into G1, and a trend for more HSCs in S/G2/M phases of the cell cycle (Figure 6D). Since most HSCs exit the cell cycle in the BM between P14 and P21 (1), this suggested that Ash1l-deficient HSCs failed to establish a quiescent stem cell pool at the fetal to adult transition. P19 Ash1lGT/GT LT-HSCs but not total BM or c-KIThiSCA1hi cells also showed increased BrdU incorporation (Supplemental Figure 5). Recent work showed that BM HSC quiescence is dependent on combined effects of the cyclin-dependent kinase inhibitors p27 (encoded by Cdkn1b) and p57 (encoded by Cdkn1c) (15, 47). Ash1lGT/GT BM LSK cells had markedly decreased expression of these two critical regulators (Figure 6E). Expression of p21 (encoded by Cdkn1a) was not altered. These data suggest that Ash1l directly or indirectly regulates p27 and p57 expression during establishment of quiescence in young adult HSCs.
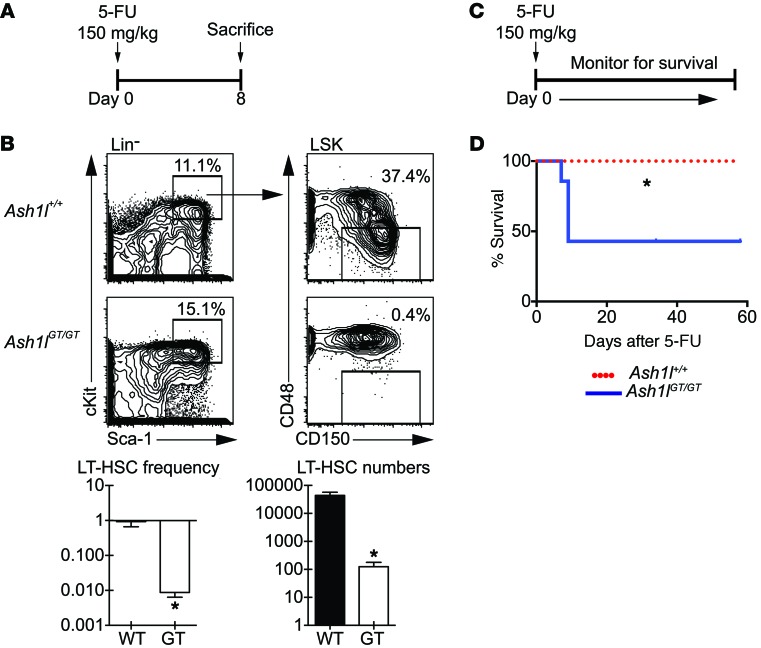
We next challenged Ash1lGT/GT and control mice with 5-fluorouracil (5-FU) (Figure 7A). 5-FU treatment is toxic to dividing cells but spares quiescent cells, including many normal LT-HSCs. A single 5-FU treatment resulted in a 2-log reduction of Ash1lGT/GT LT-HSCs compared with controls, thereby worsening LT-HSC loss in Ash1lGT/GT mice by 10- to 20-fold (Figure 7B). This was consistent with increased sensitivity of Ash1lGT/GT HSCs to 5-FU, due, at least in part, to decreased quiescence. Consistent with these findings, 5-FU administration was associated with impaired survival of Ash1l-deficient mice, even after administration of a single dose (Figure 7, C and D).
Figure 7. Ash1lGT/GT mice are more sensitive to 5-FU challenge than wild-type mice.
(A) Experimental strategy: mice of indicated genotypes were injected with 150 mg/kg 5-FU and sacrificed 8 days later. (B) Flow cytometric analysis of LT-HSCs (CD150+CD48–LSK cells), showing 2-log reduction in frequency and 2.5-log reduction in LT-HSC numbers in Ash1lGT/GT mice as compared with control mice after 5-FU exposure (n ≥ 4 mice per genotype; mean ± SEM, *P < 0.05, t test). (C) Experimental strategy: mice were injected with 150 mg/kg 5-FU and monitored for survival. (D) Survival of mice after 5-FU challenge (7 mice per genotype, representative of 2 experiments, *P < 0.05, log-rank Mantel-Cox test).
Increased cumulative proliferation of hematopoietic progenitors compensates for HSC loss in Ash1l-deficient mice.
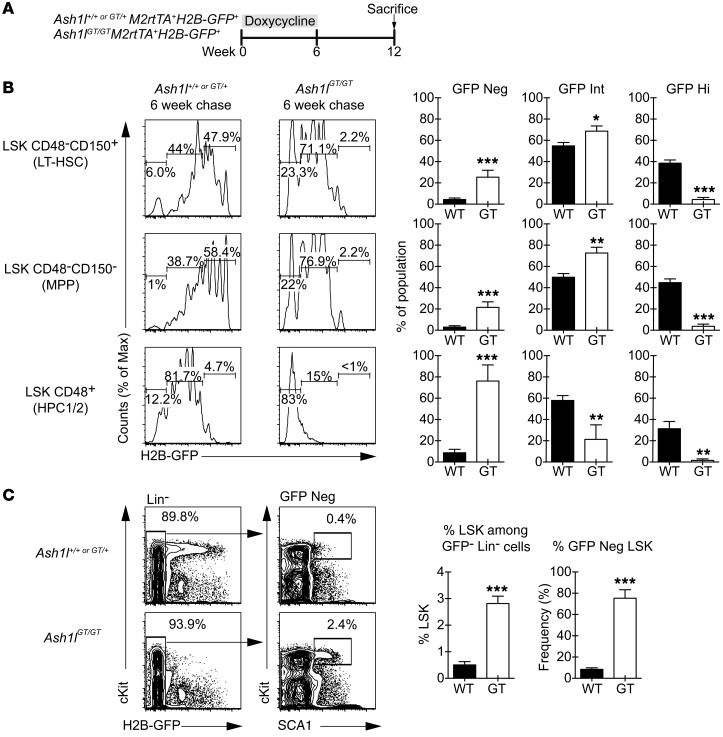
HSC loss and profound defects in transplantation assays contrasted with preservation of hematopoietic output under steady-state conditions in Ash1l-deficient mice. To explore this paradox, we used a pulse-chase H2B-GFP–labeling system to study proliferation history of phenotypically defined HSCs as well as downstream progenitors that normally have limited self-renewal potential (Figure 8A and ref. 8). This strategy allows tetracycline-induced expression of H2B-GFP in all cells (pulse), followed by dilution of GFP fluorescence upon cell division after tetracycline withdrawal (chase). Ash1lGT/GT LT-HSCs had increased proliferation throughout the 6-week chase period, as indicated by enhanced GFP dilution (Figure 8B). The entire Ash1lGT/GT LSK compartment had markedly increased proliferation as compared with control LSK cells. We could not detect a residual population of LSK or Lin– cells with high GFP fluorescence in Ash1l-deficient mice, consistent with the absence of highly quiescent progenitors (Figure 8B). In contrast, we observed a markedly increased proportion of Ash1lGT/GT Lin–GFP– cells that had preserved a primitive LSK phenotype after 6 weeks of chase (Figure 8C). These findings suggest that increased self-renewal of progenitors downstream of HSCs could compensate for LT-HSC loss and sustain hematopoietic output in Ash1lGT/GT mice.
Figure 8. In vivo proliferation history shows decreased quiescence of Ash1l-deficient HSCs but increased persistence of proliferating downstream progenitors.
(A) Experimental strategy: Ash1l+/+ or Ash1lGT/GT mice with M2rtTA and H2B-GFP transgenes were maintained on doxycycline for 6 weeks to label hematopoietic cells with GFP. GFP dilution was monitored by flow cytometry after a 6-week chase period. (B) Flow cytometric analysis after chase, showing significantly increased GFP dilution in Ash1lGT/GT LSK CD150+CD48– cells (containing LT-HSCs), LSK CD150–CD48– (MPP), and LSK CD48+ progenitors (HPC1/2) compared with controls, consistent with increased cell division (≥6 mice per genotype; mean ± SEM). (C) Flow cytometric analysis, demonstrating that the H2B-GFP–negative fraction of Lin– cells was enriched for primitive LSK progenitors in Ash1lGT/GT versus Ash1l+/+ mice (≥ 4 mice per genotype; mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001, t test.
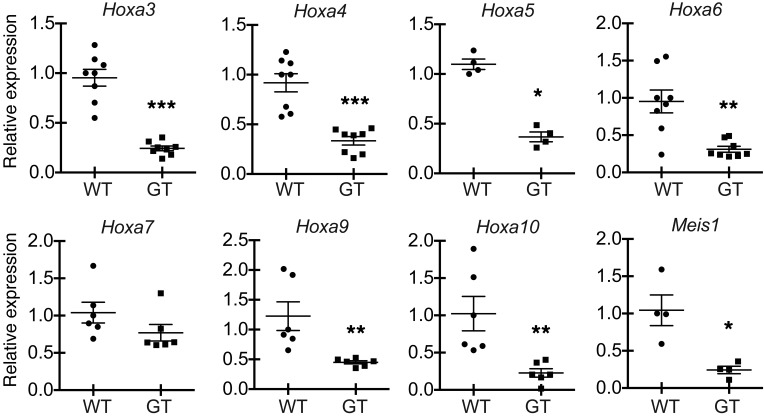
Ash1l regulates the expression of multiple Hox genes.
In flies, TrxG members collaborate to maintain Hox gene expression during development. We reasoned that, if mammalian TrxG members cooperated with one another, they might also share target genes and functional effects. Mll1, the mammalian homolog of fly trithorax, regulates expression of multiple Hoxa genes and Hox-related genes, such as Meis1. Ash1lGT/GT LSK progenitors had reduced expression of Hoxa3, Hoxa4, Hoxa5, Hoxa6, Hoxa9, Hoxa10, and Meis1 (Figure 9). Thus, Ash1l and Mll1 might share multiple target genes, including Hox genes (25, 48–50). Of note, the expression of Mll1 itself and that of other genes previously associated with HSC homing and niche retention (Rac1, Rac2, Dnmt1) was not changed (refs. 51, 52, and data not shown).
Figure 9. Ash1l regulates expression of Hox and Hox-related genes in hematopoietic progenitors.
Relative abundance of Hoxa and Meis1 normalized to Hprt1 transcripts in sort-purified adult Ash1lGT/GT LSK progenitors, as assessed by qRT-PCR (triplicate analysis of 4 to 8 individual biological samples per genotype, mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, t test).
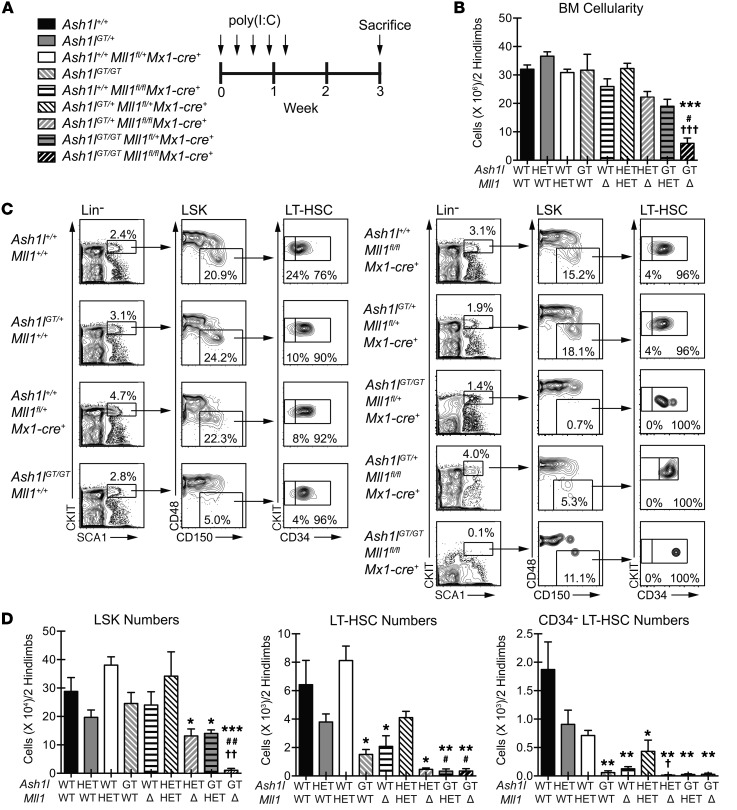
Ash1l cooperates with Mll1 and Men1 to maintain hematopoiesis.
To further explore the functional cooperativity of Ash1l and Mll1 in hematopoiesis, we bred Ash1lGT/+ mice with Mll1fl/flMx1-Cre+ mice, using a conditional Mll1 allele reported to induce only mild hematopoietic defects upon inactivation in steady-state conditions (26). This strategy allowed deletion of one or both Mll1 alleles via poly(I:C) injection in the presence of graded Ash1l doses (Figure 10A). Combined Mll1 and Ash1l deficiency resulted in rapid and profound reduction in BM cellularity, suggesting hematopoietic failure (Figure 10B). Flow cytometric analysis of LT-HSCs and hematopoietic progenitors showed that Ash1lGT/GT Mll1fl/flMx1-Cre+ mice had profound reductions in both LT-HSCs and LSK progenitors (Figure 10, C and D). In addition, we observed a unique sensitivity of LT-HSCs to the combined Mll1 and Ash1l gene dose, as numbers of LT-HSCs decreased more profoundly when homozygous Ash1l or Mll1 inactivation was combined with heterozygous inactivation of Mll1 or Ash1l, respectively (Figure 10C). CD34– LT-HSCs, a highly quiescent adult HSC subset identified with surface markers in mice, were particularly sensitive to inactivation of an increasing number of Ash1l and Mll1 alleles (Figure 10D), suggesting that Ash1l and Mll1 cooperate to maintain quiescent HSCs. However, multipotent progenitors and overall BM cellularity were only depleted upon targeting of both Mll1 and Ash1l alleles (Figure 10, B–D). Finally, we assessed the combined effects of Ash1l deficiency and inactivation of Men1, encoding the MLL1 cofactor menin (Supplemental Figure 6). Menin is essential for recruitment of the MLL1 complex, at least to a subset of target genes, and was previously shown to regulate HSC homeostasis (53–55). Profound thrombocytopenia, BM hypocellularity, and loss of all hematopoietic progenitors were observed upon combined deficiency of both Ash1l and Men1 alleles but not when even one of the four alleles was maintained (Supplemental Figure 6, B–D). LT-HSCs were particularly sensitive to graded loss of Ash1l and Men1 alleles. (Supplemental Figure 6D). Although only some Mll1 target genes are sensitive to Men1 loss (50), these genes may be important for the cooperative effects of Ash1l and Mll1 in hematopoiesis. Together, these findings reveal cooperativity between Ash1l and Mll1 or Men1 in HSCs and hematopoietic progenitor cells, uncovering a unique sensitivity of quiescent adult HSCs to regulation by the TrxG family.
Figure 10. Combined Ash1l and Mll1 deficiency induces overt hematopoietic failure and profound depletion of LT-HSCs and LSK progenitors.
(A) Experimental strategy: mice of indicated genotypes were injected with poly(I:C) (20 μg every 2 days 5 times). (B) Reduced BM cellularity in Ash1lGT/GT Mll1fl/flMx1-Cre+mice (≥2 mice per genotype; mean ± SEM). (C) Flow cytometric analysis showing severe reduction in CD34– LT-HSCs, LT-HSCs, and LSK progenitors in Ash1lGT/GT Mll1fl/flMx1-Cre+mice and reduced CD34–LT-HSC and LT-HSC frequency with cumulative inactivation of Ash1l and Mll1 alleles. The representative plot for Ash1lGT/+ Mll1fl/flMx1-Cre+ BM is derived from a separate experiment, thus gating definitions differ from the other samples. (D) LSK and LT-HSC numbers, reflecting hematopoietic failure in Ash1lGT/GT Mll1fl/flMx1-Cre+ mice and LT-HSC sensitivity to loss of Ash1l and Mll1 alleles (≥2 mice per genotype; mean ± SEM), and CD34– LT-HSC numbers in 2 hind legs, showing high sensitivity of this population to regulation by TrxG members. *P < 0.05, **P < 0.01, ***P < 0.001, compared with wild type; #P < 0.05, ##P < 0.01, compared with Ash1lGT/GT; †P < 0.05, ††P < 0.01, †††P < 0.001, compared with induced Mllfl/fl Mx1-Cre+, t test.
Discussion
Our findings uncover an essential function for the TrxG gene Ash1l in the regulation of adult HSCs and reveal functional cooperativity of TrxG members in hematopoiesis. When Ash1l levels were profoundly reduced in Ash1lGT/GT mice, the ability of young adult LT-HSCs to establish and maintain quiescence was impaired. Ash1l-deficient HSCs developed and expanded normally in the fetal liver and initially seeded the BM. Once in the BM, these LT-HSCs did not upregulate expression of Cdkn1b/c, which was associated with reduced numbers of quiescent HSCs. Because fetal HSCs are known to divide rapidly, these results could have arisen from the persistence of a fetal program upon reaching the BM. However, expression of Sox17 and Lin28b, two essential regulators of the fetal HSC transcriptional program, was properly extinguished in BM Ash1lGT/GT LT-HSCs, indicating that their reduced ability to establish quiescence occurred despite transition from fetal to adult state. Altogether, we believe that Ash1l is a novel key regulator of changes associated with the adaptation of HSCs to the BM niche at the fetal to adult transition or upon transplantation of HSCs into an adult recipient.
Ash1lGT/GT BM had profoundly reduced LT-HSC numbers but maintained relatively normal mature hematopoietic cell output. This paradox did not result from the presence of phenotypically abnormal but functional HSCs residing in the Ash1lGT/GT BM, as young adult Ash1lGT/GT BM failed to achieve long-term reconstitution in irradiated recipients after competitive and noncompetitive transplantation. Based on this gold standard approach of HSC biology, we could not detect LT-HSC function in the Ash1lGT/GT BM, indicating that the presence of functional HSCs with an alternative phenotype was unlikely. Furthermore, we could also not detect functional LT-HSCs after transplantation of Ash1lGT/GT fetal liver progenitors, which had LT-HSC frequency comparable to that of Ash1l+/+ mice by phenotypic criteria. Thus, despite their normal in vivo expansion in the fetal/neonatal liver, Ash1lGT/GT LT-HSCs could not establish durable hematopoiesis in the BM niche after transplantation, indicating that they were functionally abnormal, even if they expanded normally in the fetal environment. As normal numbers of Ash1lGT/GT HSCs were initially present in the neonatal BM, our data are consistent with preserved HSC homing to the BM in these mice but also with failure to respond to niche factors that are required for the establishment of quiescence and subsequent stable engraftment. Among putative essential niche-derived factors in the BM, thrombopoietin (TPO) and TGF-β have been linked to induction of quiescence through Cdkn1b/c upregulation (14, 56, 57). Furthermore, decreased expression of multiple posterior Hoxa genes was observed in young adult HSCs of TPO-deficient mice (14). Thus, Ash1l may be necessary to establish a normal transcriptional response downstream of TPO or other essential BM niche factors required for adult HSC quiescence, maintenance, and function. As genetic inactivation of Hoxa9 and other Hoxa genes was shown to cause HSC defects, we speculate that decreased expression of multiple Hoxa genes accounts for part of the defects in Ash1l-deficient HSCs, although other factors are likely involved as well (58, 59).
Ash1l-deficient mice did not progress to frank hematopoietic failure, despite severe depletion of phenotypically defined LT-HSCs and lack of transplantable HSCs. A possible explanation for these findings is that few residual LT-HSCs could maintain steady-state hematopoiesis through a compensatory increase in proliferation. Using H2B-GFP in a pulse-chase system (8), we indeed observed increased cumulative proliferation of all primitive Ash1lGT/GT hematopoietic progenitors, consistent with the absence of a slowly cycling progenitor pool. A striking feature of Ash1l-deficient hematopoiesis, however, was the large increase in cells that maintained a primitive LSK phenotype, despite the complete loss of GFP at the end of the chase period. Thus, Ash1l-deficient progenitors downstream of LT-HSCs showed evidence of increased self-renewal in vivo in the absence of a normal HSC compartment. This phenomenon is reminiscent of recent observations that early thymocyte progenitors acquire extended self-renewal in vivo when the normal input of blood-derived T lineage progenitors is impaired, suggesting that self-renewal potential can be modulated in individual hematopoietic progenitors as an adaptation to pathological conditions (60, 61). It is interesting to speculate about why compensatory changes in Ash1l-deficient mice were not sustained after transplantation into irradiated recipients. Emerging evidence indicates that steady-state hematopoiesis and stress hematopoiesis after transplantation are regulated by different mechanisms (62). After transplantation, hematopoiesis is driven by a small number of LT-HSCs that outcompete other progenitors, while steady-state hematopoiesis can be maintained for extended periods of time by a broader range of downstream progenitors with more restricted potential. Thus, it is possible that Ash1l-deficient mice were competent for this type of hematopoiesis but selectively deficient in LT-HSC function after transplant.
Only a few genetic models of HSC dysfunction that support nonablative transplantation have been reported in hematopoietic biology (52, 63). In Ash1lGT/GT mice, successful engraftment in nonablative transplantation experiments suggested that the Ash1l-deficient BM niche was not defective, as it could support maintenance of wild-type LT-HSCs. Stable wild-type LT-HSC engraftment suggested that niche spaces were available due to LT-HSC depletion and/or that remaining Ash1lGT/GT LT-HSCs competed poorly for niche space. Either scenario is consistent with extreme LT-HSC dysfunction when Ash1l levels are reduced. Of note, the full effects of Ash1l could be even more profound than observed in Ash1lGT/GT mice, as these mice had low residual levels of normal Ash1l transcripts, consistent with Ash1lGT being a severely hypomorphic but not a null allele.
Cooperative effects of Mll1 and Ash1l in hematopoiesis highlight an evolutionarily conserved feature of TrxG members, a phenomenon identified in flies but not previously described in mammals. Loss of a single Ash1lGT allele amplified LT-HSC depletion in Men1-deficient and Mll1-deficient mice, while complete hematopoietic failure was observed only when all 4 alleles were deficient. This dominant phenotypic enhancement is reminiscent of criteria used to identify TrxG members in Drosophila. To date, the biochemical mechanisms underlying this genetic observation remain poorly understood. Given that both Mll1 and Ash1l are required for Hox gene expression, it is possible that MLL1 and ASH1L proteins act nonredundantly to promote transcription at a shared subset of target genes. Indeed, both Ash1lGT/GT and Mll1- or Men1-deficient hematopoietic progenitors displayed a reduced, but not absent, expression of posterior Hoxa genes, suggesting multifactorial regulation at this locus (25, 50, 54). Since MLL1 and ASH1L SET domains have H3K4 and H3K36 methyltransferase activity, two histone marks that are involved in transcription initiation and elongation steps, respectively, it can be envisioned that they target different regulatory steps of transcription. In this scenario, either Mll1 or Ash1l inactivation can lead to compromised Hoxa gene expression. It also remains to be determined whether the cooperative interaction between Mll1 and Ash1l is limited to the regulation of Hoxa genes in LT-HSCs, especially since Mll1 was reported to control multiple other genes in normal hematopoiesis (50). Future work should explore the detailed molecular mechanisms by which TrxG members cooperatively regulate quiescent adult HSCs.
Methods
Mice.
C57BL/6.Ptprca (B6-SJL, CD45.1+) mice were from the National Cancer Institute. B6-GFP transgenic mice expressing GFP under the control of the human ubiquitin C promoter were from The Jackson Laboratory (64). Ash1lGT/+ embryonic stem cells were obtained from Sanger Institute. After blastocyst injection, Ash1lGT/+ mice were generated by the University of Michigan Transgenic Animal Core and intercrossed. In most experiments, Ash1lGT/+ mice were backcrossed to the C57BL/6 background (B6, CD45.2+) for at least 6 generations. In selected experiments, Ash1lGT/+ mice were bred with Sox17GFP/+ mice, with transgenic mice containing both Rosa26-rtTA and TetOP-H2B-GFP, with Men1fl/flMx1-Cre+ mice or with Mll1fl/flMx1-Cre+ mice (8, 11, 26, 65, 66). To label hematopoietic cells with H2B-GFP, Rosa26-rtTA TetOP-H2B-GFP mice were maintained on doxycycline (2 mg/ml in drinking water) for 6 weeks. Mice were maintained on normal water for a chase period (6 weeks) to allow GFP dilution in dividing cells. Men1 or Mll1 excision was achieved with poly(I:C) (Amersham; 50 μg i.p. every 2 days for 5 doses).
Flow cytometry.
Single cell suspensions were prepared from fetal liver, BM, or blood, followed by red blood cell lysis (ACK buffer, Cambrex). The following antibodies were from BioLegend: anti-CD3, anti-CD4, anti-CD8, anti-CD11b, anti-CD11c, anti-CD19, anti-CD48, anti-CD150, anti-Gr1/Ly-6G, anti-B220, anti-NK1.1, anti-TCRβ, anti-TCRγδ, and anti-cKIT. Anti-SCA1 was from eBiosciences. We used the following antibody cocktail to exclude lineage+ cells: anti-CD11b, anti-Gr1, anti-CD11c, anti-B220, anti-CD19, anti-CD3, anti-TCRβ, anti-TCRγδ, anti-CD8, anti-NK1.1, and anti-Ter119. In fetal samples, CD11b was omitted. BrdU analysis was performed using a BrdU Labeling Kit (BD Biosciences). Ki67 staining was achieved using the BD Ki67 Set (BD Biosciences). Analysis was performed on a FACSCanto and sorting was performed on a FACSAria II/III (BD Biosciences). Dead cells were excluded with 4′6-diamidino-2-phenylindole (Sigma-Aldrich). Flow cytometry files were analyzed with FlowJo (TreeStar).
BM and fetal liver cell transplantation.
Six- to eight-week-old B6-SJL (CD45.1+) mice were lethally irradiated (900 Gy, 37Cs source). Four hours after irradiation, mice were transplanted with 6- to 10-week-old donor BM or E15.5 fetal liver cells via tail vein injection. For competitive transplantation, we mixed equal numbers of competitor B6-SJL BM and tester CD45.2+ BM or fetal liver cells. For nonablative transplantation, 5- to 8-week-old Ash1lGT/GT mice or wild-type controls were injected twice at 1-week intervals with 2.0 × 107 B6-SJL (CD45.1+) or B6-GFP (CD45.2+) BM cells. For BM homing studies, recipients were analyzed 24 hours after transplantation.
Complete blood counts.
Blood was obtained through retroorbital bleeding and transferred to EDTA-treated tubes. Complete blood counts were determined using the Advia 120 Hematology System (Siemens).
CFU-GM.
20,000 BM cells or 200,000 spleen cells were plated per ml of MethoCult GF M3534 (Stem Cell Technologies). Colonies were scored 7 to 10 days later.
Quantitative real-time PCR.
For gene expression analyses, at least 5,000 cells from each population of interest were sort purified directly into Trizol (Invitrogen). After RNA extraction, cDNA was generated using the SuperScript III First-Strand Synthesis Kit (Invitrogen) or the Nugen Ovation PicoSL WTA System (Nugen Technologies). Relative gene expression was measured using TaqMan primers and probe sets from Applied Biosystems (Hoxa5: Mm00439362_m1; Hoxa7: Mm00657963_m1; Hoxa9: Mm00439364_m1; Hoxa10: Mm00433966_m1; Meis1: Mm00487664_m1; Cdkn1a: Mm00432448_m1; Cdkn1b: Mm00438168_m1; Cdkn1c: Mm00438170_m1) or SYBR Green (Fisher) with the following primer pairs: Hoxa3, AAGCGACCTACTACGACAG and TGGCATTGTAAGCGAACC; Hoxa4, CAGAACCGGAGAATGAAGTG and CGAGGCAGTGTTGGAAGA; and Hoxa6, TTACCAACAGTCCAACTC and GGTCTTTATCAGAATAGAAACA. Reactions were carried out on a Mastercycler realplex (Eppendorf). Relative expression was calculated after normalization with Hprt1 expression using the ΔΔCT method.
Statistics.
Comparison of two means was performed with 2-tailed unpaired Student’s t test. Where indicated, we used a log-rank Mantel-Cox test.
Study approval.
The University of Michigan Committee on Use and Care of Animals approved all experiments.
Supplementary Material
Acknowledgments
We are grateful to Hugh J.M. Brady for Mll1fl/fl mice, Sean Morrison for Sox17-GFP mice, David Ginsburg for B6-GFP mice, and Hanno Hock for Rosa26-rtTA and TetOP-H2B-GFP mice. This work was supported by the Sidney Kimmel Cancer Research Foundation, the University of Michigan Comprehensive Cancer Center, the D. Dan and Betty Kahn Foundation, and the NIH (RO1-AI091627 to I. Maillard, R37-HD30428 to S.A. Camper). Individual support included T32 training grants from the University of Michigan’s Medical Scientist Training Program (GM07863 to M. Jones), Center for Organogenesis (HD007505 to M. Jones and J. Chase), and Cellular and Molecular Biology Program (GM007315 to J. Chase). Flow cytometry was partially supported by a core grant from the NIH to the University of Michigan Cancer Center (P30-CA46592).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2015;125(5):2007–2020. doi:10.1172/JCI78124.
References
- 1.Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116(10):2808–2816. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ema H, Nakauchi H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood. 2000;95(7):2284–2288. [PubMed] [Google Scholar]
- 3.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 4.Takizawa H, Regoes RR, Boddupalli CS, Bonhoeffer S, Manz MG. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J Exp Med. 2011;208(2):273–284. doi: 10.1084/jem.20101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venezia TA, et al. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2004;2(10):e301. doi: 10.1371/journal.pbio.0020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96(6):3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature. 2010;465(7299):793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foudi A, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2009;27(1):84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowie MB, et al. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci U S A. 2007;104(14):5878–5882. doi: 10.1073/pnas.0700460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito T, Tajima F, Ogawa M. Developmental changes of CD34 expression by murine hematopoietic stem cells. Exp Hematol. 2000;28(11):1269–1273. doi: 10.1016/S0301-472X(00)00535-X. [DOI] [PubMed] [Google Scholar]
- 11.Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130(3):470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335(6073):1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming WH, Alpern EJ, Uchida N, Ikuta K, Spangrude GJ, Weissman IL. Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells. J Cell Biol. 1993;122(4):897–902. doi: 10.1083/jcb.122.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian H, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1(6):671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Zou P, et al. p57(Kip2) and p27(Kip1) cooperate to maintain hematopoietic stem cell quiescence through interactions with Hsc70. Cell Stem Cell. 2011;9(3):247–261. doi: 10.1016/j.stem.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9(2):115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 17.Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- 18.Shearn A. The ash-1, ash-2 and trithorax genes of Drosophila melanogaster are functionally related. Genetics. 1989;121(3):517–525. doi: 10.1093/genetics/121.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tripoulas NA, Hersperger E, La Jeunesse D, Shearn A. Molecular genetic analysis of the Drosophila melanogaster gene absent, small or homeotic discs1 (ash1). Genetics. 1994;137(4):1027–1038. doi: 10.1093/genetics/137.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong SA, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30(1):41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 21.Gu Y, et al. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992;71(4):701–708. doi: 10.1016/0092-8674(92)90603-A. [DOI] [PubMed] [Google Scholar]
- 22.Tkachuk DC, Kohler S, Cleary ML. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992;71(4):691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- 23.Ziemin-van der Poel S, et al. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci U S A. 1991;88(23):10735–10739. doi: 10.1073/pnas.88.23.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378(6556):505–508. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
- 25.Jude CD, Climer L, Xu D, Artinger E, Fisher JK, Ernst P. Unique and independent roles for MLL in adult hematopoietic stem cells and progenitors. Cell Stem Cell. 2007;1(3):324–337. doi: 10.1016/j.stem.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMahon KA, et al. Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell. 2007;1(3):338–345. doi: 10.1016/j.stem.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Milne TA, et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell. 2002;10(5):1107–1117. doi: 10.1016/S1097-2765(02)00741-4. [DOI] [PubMed] [Google Scholar]
- 28.Dou Y, et al. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol. 2006;13(8):713–719. doi: 10.1038/nsmb1128. [DOI] [PubMed] [Google Scholar]
- 29.Southall SM, Wong PS, Odho Z, Roe SM, Wilson JR. Structural basis for the requirement of additional factors for MLL1 SET domain activity and recognition of epigenetic marks. Mol Cell. 2009;33(2):181–191. doi: 10.1016/j.molcel.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 30.Steward MM, Lee JS, O’Donovan A, Wyatt M, Bernstein BE, Shilatifard A. Molecular regulation of H3K4 trimethylation by ASH2L, a shared subunit of MLL complexes. Nat Struct Mol Biol. 2006;13(9):852–854. doi: 10.1038/nsmb1131. [DOI] [PubMed] [Google Scholar]
- 31.Ruthenburg AJ, et al. Histone H3 recognition and presentation by the WDR5 module of the MLL1 complex. Nat Struct Mol Biol. 2006;13(8):704–712. doi: 10.1038/nsmb1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shearn A, Rice T, Garen A, Gehring W. Imaginal disc abnormalities in lethal mutants of Drosophila. Proc Natl Acad Sci U S A. 1971;68(10):2594–2598. doi: 10.1073/pnas.68.10.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripoulas N, LaJeunesse D, Gildea J, Shearn A. The Drosophila ash1 gene product, which is localized at specific sites on polytene chromosomes, contains a SET domain and a PHD finger. Genetics. 1996;143(2):913–928. doi: 10.1093/genetics/143.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregory GD, et al. Mammalian ASH1L is a histone methyltransferase that occupies the transcribed region of active genes. Mol Cell Biol. 2007;27(24):8466–8479. doi: 10.1128/MCB.00993-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka Y, Kawahashi K, Katagiri Z, Nakayama Y, Mahajan M, Kioussis D. Dual function of histone H3 lysine 36 methyltransferase ASH1 in regulation of Hox gene expression. PLoS One. 2011;6(11):e28171. doi: 10.1371/journal.pone.0028171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka Y, Nakayama Y, Taniguchi M, Kioussis D. Regulation of early T cell development by the PHD finger of histone lysine methyltransferase ASH1. Biochem Biophys Res Commun. 2008;365(3):589–594. doi: 10.1016/j.bbrc.2007.10.159. [DOI] [PubMed] [Google Scholar]
- 37.An S, Yeo KJ, Jeon YH, Song JJ. Crystal structure of the human histone methyltransferase ASH1L catalytic domain and its implications for the regulatory mechanism. J Biol Chem. 2011;286(10):8369–8374. doi: 10.1074/jbc.M110.203380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan W, Xu M, Huang C, Liu N, Chen S, Zhu B. H3K36 methylation antagonizes PRC2-mediated H3K27 methylation. J Biol Chem. 2011;286(10):7983–7989. doi: 10.1074/jbc.M110.194027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka Y, Katagiri Z, Kawahashi K, Kioussis D, Kitajima S. Trithorax-group protein ASH1 methylates histone H3 lysine 36. Gene. 2007;397(1):161–168. doi: 10.1016/j.gene.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 40.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 41.Kim I, He S, Yilmaz OH, Kiel MJ, Morrison SJ. Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood. 2006;108(2):737–744. doi: 10.1182/blood-2005-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, et al. Identification of Lin(-)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105(7):2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 43.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98(25):14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13(1):102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112(9):3543–3553. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossi L, et al. Less is more: unveiling the functional core of hematopoietic stem cells through knockout mice. Cell Stem Cell. 2012;11(3):302–317. doi: 10.1016/j.stem.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto A, et al. p57 is required for quiescence and maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2011;9(3):262–271. doi: 10.1016/j.stem.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 48.Milne TA, et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci U S A. 2005;102(3):749–754. doi: 10.1073/pnas.0408836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milne TA, Dou Y, Martin ME, Brock HW, Roeder RG, Hess JL. MLL associates specifically with a subset of transcriptionally active target genes. Proc Natl Acad Sci U S A. 2005;102(41):14765–14770. doi: 10.1073/pnas.0503630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Artinger EL, et al. An MLL-dependent network sustains hematopoiesis. Proc Natl Acad Sci U S A. 2013;110(29):12000–12005. doi: 10.1073/pnas.1301278110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11(8):886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 52.Trowbridge JJ, Snow JW, Kim J, Orkin SH. DNA methyltransferase 1 is essential for and uniquely regulates hematopoietic stem and progenitor cells. Cell Stem Cell. 2009;5(4):442–449. doi: 10.1016/j.stem.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123(2):207–218. doi: 10.1016/j.cell.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 54.Maillard I, et al. Menin regulates the function of hematopoietic stem cells and lymphoid progenitors. Blood. 2009;113(8):1661–1669. doi: 10.1182/blood-2009-01-135012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen YX, et al. The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression. Proc Natl Acad Sci U S A. 2006;103(4):1018–1023. doi: 10.1073/pnas.0510347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshihara H, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1(6):685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 57.Yamazaki S, Iwama A, Takayanagi S, Eto K, Ema H, Nakauchi H. TGF-beta as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood. 2009;113(6):1250–1256. doi: 10.1182/blood-2008-04-146480. [DOI] [PubMed] [Google Scholar]
- 58.Lawrence HJ, et al. Loss of expression of the Hoxa-9 homeobox gene impairs the proliferation and repopulating ability of hematopoietic stem cells. Blood. 2005;106(12):3988–3994. doi: 10.1182/blood-2005-05-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di-Poi N, Koch U, Radtke F, Duboule D. Additive and global functions of HoxA cluster genes in mesoderm derivatives. Dev Biol. 2010;341(2):488–498. doi: 10.1016/j.ydbio.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Martins VC, et al. Thymus-autonomous T cell development in the absence of progenitor import. J Exp Med. 2012;209(8):1409–1417. doi: 10.1084/jem.20120846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peaudecerf L, et al. Thymocytes may persist and differentiate without any input from bone marrow progenitors. J Exp Med. 2012;209(8):1401–1408. doi: 10.1084/jem.20120845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun J, et al. Clonal dynamics of native haematopoiesis. Nature. 2014;514(7522):322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z, Li G, Tse W, Bunting KD. Conditional deletion of STAT5 in adult mouse hematopoietic stem cells causes loss of quiescence and permits efficient nonablative stem cell replacement. Blood. 2009;113(20):4856–4865. doi: 10.1182/blood-2008-09-181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivo. Cell Immunol. 2001;214(2):110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- 65.Crabtree JS, et al. Of mice and MEN1: Insulinomas in a conditional mouse knockout. Mol Cell Biol. 2003;23(17):6075–6085. doi: 10.1128/MCB.23.17.6075-6085.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269(5229):1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.