Abstract
Liver failure is a clinical syndrome of various etiologies, manifesting as jaundice, encephalopathy, coagulopathy and circulatory dysfunction, which result in subsequent multiorgan failure. Clinically, liver failure is classified into four categories: acute, subacute, acute-on-chronic and chronic liver failure. Massive hepatocyte death is considered to be the core event in the development of liver failure, which occurs when the extent of hepatocyte death is beyond the liver regenerative capacity. Direct damage and immune-mediated liver injury are two major factors involved in this process. Increasing evidence has suggested the essential role of immune-mediated liver injury in the pathogenesis of liver failure. Here, we review the evolved concepts concerning the mechanisms of immune-mediated liver injury in liver failure from human and animal studies. Both innate and adaptive immunity, especially the interaction of various immune cells and molecules as well as death receptor signaling system are discussed. In addition, we highlight the concept of “immune coagulation”, which has been shown to be related to the disease progression and liver injury exacerbation in HBV related acute-on-chronic liver failure.
Keywords: immune, innate immunity, adaptive immunity, humoral immunity, cytokines, liver failure
Introduction
Liver failure is a severe inability of the liver to perform its normal synthetic and metabolic function, as clinically characterized by jaundice, ascites, encephalopathy and coagulopathy, which may lead to multiple organ failures and death. Liver failure can develop as acute liver failure (ALF) in the absence of any pre-existing liver disease, acute-on-chronic liver failure (ACLF) in the presence of known or unknown chronic liver disease, or chronically as a decompensation of pre-existing end-stage liver disease (Sarin et al., 2009[70]).
Liver failure may result from various etiologies, among which drugs and long-term alcohol consumption are the respective leading causes of acute (subacute) and chronic liver failure in western countries. Nevertheless, hepatitis virus infections, primarily hepatitis B, as well as other non-hepatotropic viruses are the main causes of liver failure in the Asian and developing countries (Nanda et al., 1994[56]; Tibbs and Williams, 1995[83]; Williams, 1996[91]; Tandon et al., 1999[78]; Chu et al., 1999[12]).
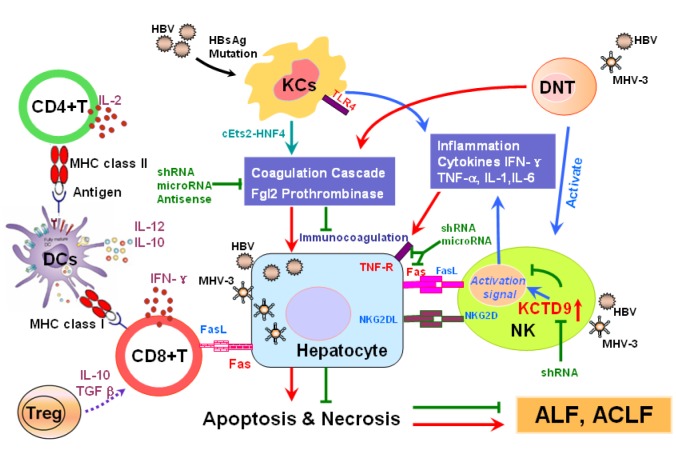
In the development of liver failure, massive hepatocellular death is considered to be the core event, as accompanied by the inflammatory cell infiltration and hepatic ischemic injury. Liver failure occurs when the rate and extent of hepatocellular death exceeds the regenerative capacity of the liver. The direct injury and immune mediated liver injury both contribute to these pathological processes. More and more evidence suggested that the immune response plays a pivotal role in the pathogenesis of liver failure, especially the activation of immune cells, including kuppfer cells, dendritic cells (DCs), natural killer (NK) cells, cytotoxic T lymphocytes (CTLs), regulatory T (Treg) cells, etc., and their production of cytokines. In the early phase of virus induced ALF/ACLF, kuppfer cells produce quantities of proinflammatory cytokines, including tumor necrosis factor-alfa (TNF-α), interleukin-1 (IL-1) and interferon-gama (IFN-γ), which lead to hepatocellular injury. Additionally, kuppfer cells that express procoagulant fibrinogen-like protein (FGL2)/fibroleukin prothrombinase also promote hepatocyte death by initiating “immune coagulation”. NK/NKT cells are also involved in this stage, eliminating the infected hepatocytes by either mediating apoptosis through Fas/ FasL and natural killer group 2D (NKG2D)/ NKG2DL pathway, or secreting IFN-γ and perforin. DCs is responsible for presenting the antigens to T helper cells, and the activated lymphocytes are recruited to liver by certain chemokines. In the later period, adaptive immune response takes priority. CTLs directly kill the infected hepatocytes through Fas/FasL or perforin pathway, or clear the virus by secreting antiviral agents. Tregs, which are characterized by the production of IL-10 and TGF-β, tend to inhibit the proliferation and function of effector T cells and modulate the immune response (Figure 1(Fig. 1)). However, the mechanisms involved are complex and still remain not fully understood.
Figure 1. Proposed mechanism of hepatitis virus-induced and immune-mediated liver injury. The significant role of the innate and adaptive immune response, including macrophages and CD4-CD8- double negative T cells expressing fgl2, natural killer cells and their production of KCTD9, as well as the interaction between dendritic cells and T lymphocytes is shown. KCs, Kupffer cells; NK, natural killer cells; Treg, Regulatory T Cell; DNT, double negative T cell; DCs, dendritic cells; IFN-γ, Interferon γ; TGF-β, transforming growth factor-β; MHV-3, Murine Hepatitis Virus Strain 3; shRNA, short hairpin RNA; TNF-R, Tumor Necrosis Factor receptor; KCTD9, Potassium Channel Tetramerisation Domain Containing 9, cEts2-HNF4, cEts2-Hepatocyte Nuclear Factor 4;TLR 4, Toll Like Receptor 4; MHC, Major Histocompatibility Complex.
1. Innate immunity
Both innate and adaptive immunity participate in the immune-mediated liver injury that leads to liver failure. The innate immune system is activated prior to the adaptive immune system, and enables the highly specialized adaptive immune system to confer long-term immunological memory. The innate immune system acts as the first-line of defense against invading pathogens and other potential threats to the host. Exquisite coordination of multiple innate immune cells is crucial to efficiently destroy and clear the invading pathogens and other molecular threats. Liver is highly enriched in innate immune cells, such as kupffer cells, natural killer (NK) cells, NKT cells and dendritic cells (DC), which result in unique immune responses against microbial pathogens (Thomson and Knolle, 2010[82]). There is increasing evidence suggesting that liver plays a pivotal role in innate immune responses and acts more as an immune organ (Crispe, 2009[13]; Gao et al., 2008[21]; Racanelli and Rehermann, 2006[64]).
1.1 Macrophages / immune coagulation system
Macrophages display a remarkable plasticity and can differentiate functionally into M1 and M2 subsets (Yang et al., 2012[97]). Activated M1 macrophages are associated with type I cytokines (e.g., TNF-α, IL-6 and IFN-γ) and exhibits the potent pro-inflammatory effects, which promote Th1 immune response. On the contrary, activated M2 macrophages release several immunoregulatory mediators (e.g., IL-4, IL-10 and IL-13) that suppress inflammation and promote tissue repair. (Laskin, 2009[40]; Benoit et al., 2008[6]; Montaner, 1999[51]; Gordon and Martinez, 2010[24]). Kupffer cells are the liver resident macrophages constituting the majority of tissue macrophages of the body. Normally, naïve KCs is likely to differentiate into M2 phenotype, maintaining immune tolerance in the liver. At the early stage of acute liver failure, activated KCs display as the classical M1 phenotype and promote liver injury. At the resolution stage of ALF, KCs exhibit more like M2 macrophages, which produce anti-inflammatory and regenerative cytokines (Antoniades et al., 2012[3]). Several studies have demonstrated the correlation between the functional states of KCs and the extent of liver injury. In a mouse model of ALF induced by concanavalin A (con A), the apoptosis and dysfunction of KCs were observed and accompanied by the increased production of macrophage-related inflammatory cytokines. These observations indicated that the deactivation and apoptosis of hepatic macrophages may be a potential link between inflammation and immunoparesis in ALF (Yang et al., 2013[98]). It is reported that during ALF, the activated KCs resulted in a significant increase in sCD163, which highly correlated with fatal outcome (Hiraoka et al., 2005[27]; Møller et al., 2007[50]). Mita et al. (2005[47]) examined 24 patients with fulminant hepatic failure who underwent liver transplantation and discovered that FasL was expressed predominantly on liver macrophages, which contribute to the severe liver injury through Fas/FasL pathway.
Activation of the immune coagulation system has been implicated in the pathogenesis of fulminant liver failure. Our previous studies have shown the importance of activated macrophages and its expression of fibrinogen-like protein-2 (fgl2) either in a murine hepatitis virus strain 3 (MHV-3) induced fulminant hepatitis animal model or in patients with hepatitis B-related acute-on-chronic liver failure. Fgl2 is able to directly cleave prothrombin into thrombin, resulting in intravascular fibrin deposition within the liver and culminating in widespread hepatocyte necrosis (Levy et al., 2000[41]; Zhu et al., 2005[113], 2006[112]). After m-fgl2 antisense administration, the overall survival rate increased from 0 to 33.33 % in mice with MHV-3-induced fulminant hepatitis. Furthermore, the survival rate in mice receiving dual mfgl2 and mTNFR1 interference was superior to that of mice receiving single-target interference (Gao et al., 2010[22]). HBV-induced human fgl2 (hfgl2) transcription is dependent on c-Ets-2 and mitogen-activated protein (MAP) kinase signaling. A positive correlation was observed between hfgl2 expression and the degree of liver injury, as indicated by the bilirubin levels. The hfgl2 expression in peripheral blood mononuclear cells (PBMC) may also be used as a biomarker for monitoring the severity of acute-on-chronic hepatitis B and hfgl2 could be a potential target for therapeutic intervention (Han et al., 2008[26]; Wu et al., 2010[93]).
1.2 Natural killer/natural killer T cells
Natural killer (NK) cells account for a large part of the liver lymphocytes and are considered as the first-line effector against pathogens (Dong et al., 2007[15], 2004[16]; Zhang et al., 2005[103]). NK cells with expression of TRAIL showed strong cytotoxicity against primary hepatocytes in the liver injury (Ochi et al., 2004[61]). The activated NK cells produce plenty of IFN-γ, but the liver injury was absolutely independent on IFN-γ. Perforin and FasL were also confirmed to be two dispensable effectors in the NK cell-mediated liver injury (Dong et al., 2007[15]). A variety of groups have reported the critical role of NK cells in the pathogenesis of liver injury. Zou et al. (2010[114]) in our lab has investigated the role of liver NK cells in the development of hepatocyte necrosis in both fulminant hepatic failure (FHF) and acute-on-chronic liver failure (ACLF) due to viral infection. After MHV-3 infection, a large number of NK cells were recruited into the liver from peripheral blood, spleen and bone marrow. Meanwhile, the cytotoxic activity of NK cells was also significantly enhanced with an increasing intracellular IFN-γ and TNF-α production. Both Fas/FasL and NKG2D/NKG2DL pathways were found to contribute to liver NK cell-mediated hepatocyte toxicity in the FHF model. In patients with acute-on-chronic hepatitis B liver failure (ACHBLF), the accumulation of liver NK cells and increased expression of FasL and cytotoxicity receptors (NKp30 and NKp46) on peripheral NK cells were correlated with disease progression (Zou et al., 2010[114]). Our colleagues also observed a newly identified potassium channel tetramerisation domain containing 9 (KCTD9), which was highly expressed in peripheral and hepatic NK cells from ACHBLF patients, and its expression had a positive correlation with the severity of liver injury. Inhibition of KCTD9 by shRNA resulted in reduced cytotoxic function. These results suggest the involvement of KCTD9 in NK cell activation.
Natural killer T cells are a unique subset of T cells expressing surface markers of both T cells and NK cells. NKT cells are considered to be an important role in Con A-induced liver injury by producing IFN-γ and IL-4 as well as Fas-mediated hepatocyte apoptosis (Tiegs, 2007[84]). Zhao et al. (2011[111]) demonstrated a novel role of Vγ4 γδ T cells in conducting a protective effect against Con A-induced fulminant hepatitis through negatively regulating the function of NKT cells in an IL-17A-dependent manner. This finding indirectly confirmed the role of NKT cells involved in the pathogenesis of liver injury. Kakimi et al. (2000[31]) found that activated intrahepatic NKT cells participate in the controlling of HBV replication by secreting antiviral cytokines (IFN-γ and IFN-α/β) in HBV transgenic mice.
1.3 Dendritic cells
Dendritic cells (DC) are a kind of specific antigen-presenting cells (APC) and comparatively abundant in the liver. DC constitutes the body's primary source of type I interferon (IFN), which regulate the local immune responses in liver. Recent studies have indicated that the number and function of DCs are closely associated with the prognosis of HBV-associated ACLF patients with different therapies. Zhang et al. (2008[107]) reported that activated pDCs accumulated in large numbers in liver of the ACHBLF patients, and produced IFN-α, which subsequently induced IL-12 and IL-10 production via toll-like receptor-9 ligation in liver-infiltrating lymphocytes. Zhao et al. (2012[109]) indicated that higher mDC numbers at baseline and the recovery of mDC number at the end of treatment may represent a prognostic marker for favorable response to corticosteroid treatment in ACHBLF patients. Khanam et al. (2014[32]) have found that the frequencies of circulating and intrahepatic myeloid (mDCs) and plasmacytoid (pDCs) dendritic cells (DCs) increased significantly post-G-CSF treatment in ACLF patients along with improved clinical parameters serum bilirubin and international normalized ratio (INR).
1.4 Toll-like receptors (TLRs)
Toll-like receptors (TLRs) play a key role in the innate immune response to invading pathogens. The Toll-like receptor 4 (TLR4) expresses mainly on macrophages and plays an important role in mediating macrophage activation and subsequent production of pro-inflammatory cytokine, which leads to the progression of severe liver injury. Increasing studies have focused on the potential mechanisms of TLR4 in the development of liver failure as well as the effect of its antagonists for treatment. In a recent study, a TLR4 antagonist (E5564) was used to treat the rats with d-galactosamine (d-galactosamine) and lipopolysaccharide (LPS)-induced acute liver failure, and remarkably reduced the liver injury and improved the overall survival rate of these rats. The TLR4 antagonist may work through blocking endotoxin-induced TNF-α overproduction of macrophages (Kitazawa et al., 2010[34]). Another study also suggested that TLR4 in the liver and extrahepatic organs were activated in acetaminophen (APAP)-induced acute liver failure and lead to the progression of ALF and multiorgan dysfunction (MOD). And the administration of the TLR4 antagonist STM28 prevented liver injury and associated MOD (Shah et al., 2013[71]). Fisher et al. (2013[18]) compared wild-type mice with TLR4-mutant, E5564 (a TLR4 antagonist) -treated, and KC-depleted mice in liver pathology and survival rate after APAP administration. All groups had significant liver injury but only wild-type mice had markedly worse survival. These results also pointed to a link between TLR4 and KCs in the APAP induced ALF and confirmed the efficiency of TLR4 antagonism in treating ALF. Except TLR 4, TLR3 is also found to be important in innate immunity and is responsible for recognizing viral pathogens. Rong et al. (2013[66]) revealed that the prevalence of TLR3 1234CT genotype and T allele was significantly increased in CHB patients with ACLF than those without ACLF. TLR3 C1234T polymorphism could be a risk factor for the development of chronic HBV infection, especially the CHB-related ACLF.
2. Adaptive immunity
2.1 Cellular immunity
It is commonly accepted that the adaptive immunity plays an essential role in the pathogenesis of HBV related ACLF. The clearance of virus depends primarily on two ways: the direct killing of infected hepatocytes by cytotoxic T lymphocytes (CTLs) through Fas/FasL or perforin pathway, or by the release of IFN-γ (Guidotti et al., 1999[25]; Thimme et al., 2003[81]; Bertoletti et al., 2003[8]; Kondo et al., 2004[36]).
2.1.1 T cells
T cell responses, especially T helper cell and CTL responses are of considerable importance in viral control and immune-mediated liver damage. The reduction of CD4+T lymphocytes, predominantly conventional CD4+T cells, was observed in ACHBLF patients. And the development of suppressive CD4+ Tregs greatly prevails over conventional CD4+T cells, constituting important characteristics of adaptive immune dysfunction of HBV related ACLF (Dong et al., 2013[14]). CD8+ CTLs are the predominant effector T lymphocytes that contribute to the extensive hepacellular death in virus induced liver failure. The elevated PD-1 expression could promote CD8+ T cells apoptosis and pathogenic CD8+ T-cell responses and liver damage. It is found that in ACHBLF patients, the expression of PD-1 and PD-L1 was positively correlated with disease progression, and the PD-1 expression on effector CD8+ T cells was lower than those on other CD8+ T cells, which maybe accounted for the liver failure (Zhang et al., 2008[106]; Liu et al., 2010[43][44]).
2.1.2 Regulatory T cells (Tregs)
CD4+CD25+ regulatory T cells (Tregs) are able to suppress the proliferation and function of effector T cells and maintain immunological tolerance to self and foreign antigens (Stoop et al., 2005[75]; Franzese et al., 2005[19]). However, the relationship between the frequency and function of CD4+CD25+ Tregs and the severity of ACLF has not reached broad consensus. Yang et al. (2012[96]) showed that the CD4(+)CD25(high) Tregs prevalence in peripheral blood is highly increased and positively correlated with HBV DNA load, suggesting its indicative of disease severity in ACLF or CHB patients. A study showed activated T cells and chemokine receptors could induce Tregs and recruit other inflammatory cells in liver, but the increased expression of regulatory T cells in liver could not effectively downregulate the activation of inflammatory cells in ACLF (Khanam et al., 2010[33]). High mobility group box-1 (HMGB1) protein, which is involved in the process of endotoxemia, can weaken the immune activity of Treg cells and favors liver failure in patients with chronic HBV infection (Wang et al., 2010[88]). Fibrinogen-like protein 2 (Fgl2) was also an important effector molecular for Tregs that contributed to the susceptibility to MHV-3-induced fulminant hepatitis (Shalev et al., 2009[72]). CD3+ CD4-CD8-double negative T cells (DNT) are a novel subset of regulatory T cells, which exacerbate the disease progression in MHV-3-induced fulminant hepatitis by expressing Fgl2 (Wu et al., 2012[92]). The number of newly identified CD39+FoxP3+CD4+ Treg cells weas detected significantly less in chronic active hepatitis B and ACLF patients than those in asymptomatic HBV carriers. The increased proportions of circulating CD39+ Tregs were positively correlated with serum viral load, but inversely correlated with serum ALT level, indicating that these CD39+ Tregs may be associated with the HBV disease progression (Tang et al., 2012[79]). Recently, Zhang et al. (2012[105]) has identified the heterogeneity of human CD4(+)Foxp3(+) T cells. The circulating and intrahepatic CD4(+)CD45RA(-)Foxp3(hi)-activated Tregs (aTregs) were selectively increased in patients with active CHB and ACLF. In both peripheral blood mononuclear cells (PBMCs) and livers, ACLF patients showed a dramatic elevation of interleukin 17A-secreting CD45RA(-)Foxp3(lo) non-suppressive T cells (non-Tregs), which were shown to be associated with severe liver damage.
2.1.3 Th17 cells
Th17 cells, which are characterized by the production of IL-17 and IL-21, have recently been identified as the third distinct subset of effector T cells. IL-21 could promote Th17 differentiation in a STAT3-dependent manner, and induce the release of cytokines such as IL-17 and IFN-γ, which may involved in the pathogenesis of ACLF (Wei et al., 2007[90]; Hu et al., 2011[29]). Th17 cells and Treg cells are with reciprocally regulated differentiation and function. TGF-β can induce Treg-specific transcription factor Foxp3, while the addition of IL-6 to TGF-β can inhibit the generation of Treg cells and induce Th17 cells. Frequency and function of these two cell subsets vary in patients with chronic hepatitis B. The fine balance between Th17 and Treg cells is crucial for maintenance of immune homeostasis (Zhao et al., 2010[110]; Ye et al., 2010[99]). In recent years, the points on the ratio of Th-17/Treg cells in ACLF patients and its relationship with the disease prognosis remain controversial. Zhai et al. (2011[101]) demonstrated both Th17 and FoxP3(+) Treg cells in the peripheral blood were increased in ACLF patients and the ratio of Th-17 to Treg cells was associated inversely with the survival of ACLF patients. Niu et al. found that liver IL-17(+) T cells increased markedly in the ACLF patients, while the Foxp3+ T cells did not show a significant difference between ACLF and CLF patients. In addition, the ACLF group showed a dramatically higher IL-17(+)/ Foxp3(+) ratio than the CLF group, and the imbalance between IL-17(+) and Foxp3(+) T cells in the liver may lead to progression of the disease (Niu et al., 2011[59]). Restoring the Treg cell/Th17 cell ratio during the follow-up phase of ACLF could maintain the immune system at a steady state, which favours good prognosis (Niu et al., 2013[60]). Nevertheless, Zhang et al. (2013[102]) made a different conclusion that Th17 cells increased while Treg cells decreased in the recovery phase of ACLF, and a high ratio of Th-17 to Treg cells leads to a better prognosis. Thus, more investigations are needed to carry out on this issue.
2.2 Humoral immunity
HBV-associated acute liver injury is believed to be prominently mediated by the cellular immune response (Thimme et al., 2003[81]) and the role of humoral immune response in this process is poorly understood. The viral clearance and antibody response are more rapid and active in fulminant hepatitis B than in traditional cases of acute hepatitis B (Trepo et al., 1976[86]; Tabor et al., 1976[76]; Gimson et al., 1983[23]). A recent study analyzing liver tissues from two patients show that HBV-associated ALF is characterized by an overwhelming B cell response centered in the liver with massive intrahepatic production of IgG and IgM by infiltrating plasma cells against the hepatitis B core antigen (HBcAg) of HBV, implicating a major role of B cell immunity in the pathogenesis of HBV-associated ALF (Farci et al., 2010[17]). Thus, the dynamic changes of humoral immunity in the development of HBV-associated ALF and underlying mechanisms need further investigation.
3. Cytokines
In the development of liver failure, a prominent phenomenon of host immune response is the cascade activation of proinflammatory cytokines, displaying as“sepsis-like”immune paralysis (Wasmuth et al., 2005[89]). Proinflammatory and anti-inflammatory cytokines in the pathogenesis of liver injury were extensively investigated, and cytokines including TNF-α, IFN-γ and IL-6 etc were identified to be of considerable importance (Galun et al., 2002[20]; Yin et al., 2003[100]; Odeh et al., 2004[62]; Rutherford et al., 2007[67]; Nagaki et al., 2008[55]).
TNF-α is considered exhibiting different effects in different phases. In the early phase of liver injury, high level of TNF-α protects hepatocytes from apoptosis, favors their repairing and regeneration. Whereas continuous increase of TNF-α induces the apoptosis and necrosis of hepatocytes. The increase of blood TNF-α as well as overexpression of TNF-α receptor 1 is directly correlated to the mortality of patients with liver failure (Tokushige et al., 2000[85]; Nagaki et al., 2000[54]). In D-GalN/LPS-induced murine fulminant hepatic failure, TNF-α expression was also enhanced remarkably (Lian et al., 2010[42]).
IFN-γ is primarily produced by NK cells and activated T cells, and induces macrophage activation and a delayed-type hypersensitivity response that damages the liver (Ando et al., 1993[1]). The vital role of IFN-γ in the pathogenesis of HBV or ConA/LPS induced ALF were proved by several studies (Nicoletti et al., 2000[58]; Shimizu et al., 2002[73]). TNF-α and IFN-γ were both highly expressed in the liver of ACLF patients compared with those in CHB patients. The increase of IFN-γ was due to the recruitment of CD4+ and CD8+ T lymphocytes while the upregulation of TNF-α is associated with the enhancement of Kupffer cells (Zou et al., 2009).
IL-6 is also produced mainly by macrophages and T cells, possessing pleiotropic characteristics of both anti-inflammatory and pro-inflammatory cytokine. A number of studies have suggested the relationship between increased hepatic and circulating IL-6 expression and the severity of liver injury in acute/subacute liver failure in human (Jin et al., 2006[30]). Another animal study also suggested the Con A-induced liver injury is mediated by CD4+ T cells acting within the liver, and at least in part through the secretion of IL-6 (Cao et al., 1998[9]). In ACHBLF patients receiving glucocorticoid treatment, IL-6 and TNF-α levels were statistically lower, while the SOCS1 transcription level was higher with better prognosis (Zhang et al., 2014[104]). Nonetheless, some investigators discovered IL-6 was protective for liver damage induced by ConA, acetaminophen, carbon tetrachloride (CCL4) or other stimulus. This effect is probably mediated by the induction of anti-apoptotic proteins including Bcl-xL, Bcl-2 and FLIP, which activate the signal transducer and activator of transcription 3 (STAT3) pathway and inhibit IFN-γ secretion (Mizuhara et al., 1994[48], 1996[49]; Kovalovich et al., 2001[38], 2000[37]; Hong et al., 2002[28]; Masubuchi et al., 2003[46]; Taub, 2003[80]; Klein et al., 2005[35]). In an anti-CD137 mAb-induced liver injury mice model, ectopic IL-6 expression prevented CD8+ T cell-mediated liver damage by increasing accumulation of Gr-1+CD11b+ myeloid derived suppressor cells (MDSCs) in the liver (Cheng et al., 2011[11]).
IL-17, the characteristic cytokine produced by Th 17 cellsis involved in the mobilization, recruitment and activation of immune cells. IL-17 induces IL-8 mediated neurophils recruitment in liver, resulting in the liver injury due to HBV infection (Ye et al., 2010[99]). In ACHBLF patients, the increased serum IL-17 level contributes to the immune activation and disease aggravation (Yang et al., 2013[94]).
The anti-inflammatory cytokine IL-10 can inhibit the activation of monocytes by downregulating NF-κB, and reduce the production of pro-inflammatory cytokines. However, the overexpression of IL-10 mediates the immunoparesis contributing to prognosis in ALF. Berry et al. showed that admission levels and early changes in serum IL-10 are predictive of poor outcome as well as the pro-inflammatory cytokine (TNF-α and IL-6) in acute liver failure (Berry et al., 2010[7]; Antoniades et al., 2008[2]).
4. Death receptor signaling
Death receptor-induced hepatocyte apoptosis plays a large part in the development of acute liver failure. Death receptors that are expressed on hepatocytes include Fas (CD95), tumour necrosis factor receptor 1 (TNF-R1), tumor necrosis factor-related apoptosis inducing ligand receptors 1 and 2 (TRAIL-R1/TRAIL-R2), death receptor 3 and 6. These death receptors can trigger intracellular signalling pathways when bound to their respective ligands (Yin and Ding, 2010[100]; Malhi et al., 2006[45]).
Fas can be expressed on a variety of cells in liver, including hepatocytes, stellate cells, kupffer cells, endothelial cells and cholangiocytes (Ni et al., 1994[57]; Saile et al., 1997[69]; Muschen et al., 1998[53]; Cardier et al., 1999[10]; Ueno et al., 2000[87]). The liver is very sensitive to Fas-induced apoptosis. Experimental studies have suggested that apoptosis via the Fas/FasL signaling pathway may play an important role in the development of acute liver failure. In mice, administration anti-Fas agonistic antibody causes rapid death due to fulminant hepatitis (Ogasawara et al., 1993[63]). In human fulminant hepatic failure, Fas was found to be highly expressed on a large number of hepatocytes, while FasL was detected expressing on both liver and peripheral lymphocytes, and the soluble FasL in serum significantly increased as well (Ryo et al., 2000[68]; Tagami et al., 2003[77]). As previously mentioned, Fas/FasL pathway also contributes to the liver NK cell-mediated hepatocyte injury in both mouse FHF and human HBV-ACLF (Zou et al., 2010[114]).
TNF-R1 is highly expressed on hepatocytes. Activation of TNF-R1 can mediate NF-κB activation, apoptosis or necroptosis, and leads to different outcomes, which are determined by distinct TNF receptor-associated signaling complexes (Bantel and Schulze-Osthoff, 2012[4]). TNF-α has been shown to be an important mediator of ALF due to CCL4 or hepatic ischemia-reperfusion injury, but not acetaminophen induced injury. Accumulating evidence suggests that oxidative stress may sensitize the ischemic liver to TNF-α, leading to potentially massive apoptosis (Riordan and Williams, 2003[65]). TNF receptor gene deletion could delay disease progression and attenuate disease severity in experimental acute liver failure (Bémeur et al., 2010[5]). Anti-TNF-α polyclonal antibody was beneficial to protect the rats in human serum albumin (HSA) and D-Gal/LPS induced ACLF (Yang et al., 2014[95]). It has been shown in vitro that TNF-α can enhance hepatocyte FasL-mediated cytotoxicity, and in turn, TNF-α induced apoptosis was associated with Fas and FasL upregulation (Kuhla et al., 2008[39]). The serum TNF-α and soluble FasL can be used as biomarkers in non-acetaminophen-induced acute liver failure (Singhal et al., 2009[74]).
TRAIL has several receptors, among which TRAIL-R1 and TRAIL-R2 are so-called full functional receptors. When engaged, they initiate a similar cascade as Fas does, recruiting FADD and initiator caspase-8 or caspase-10 to the receptor complex, and induce hepatocyte apoptosis (Yin and Ding, 2003[100]). Data are conflicting about the hepatotoxicity of TRAIL. Hepatocytes are sensitive for TRAIL-mediated apoptosis in vitro, but TRAIL induces no apoptosis in healthy livers in vivo. It has been shown that TRAIL enables the organisms to specifically kill virus-infected hepatocytes (Mundt et al., 2003[52]). However, little data suggests a direct correlation between TRAIL and liver failure.
Summary
The pivotal role of immune mediated liver injury in the pathogenesis of liver failure has been commonly accepted. The activation of various immune cells and secretion of cytokines initiate immune response in the early phase and cause massive hepatocyte death. A number of immune cells and molecules modulate the balance of pro- and anti-inflammatory factors through different pathways. Once the balance is broken, the host immune disorder leads to uncontrolled immune responses and subsequent severe liver damage that beyond liver regeneration. The mechanisms involved in the immune mediated liver injury, especially the interaction among different immune cells are complex and need further investigation. These novel theories would provide new strategies to control the unbalanced immune response and thus beneficial for managing patients with liver failure.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC81030007, NSFC81171558), National twelfth “five years” project in Science and Technology (2013ZX10002003), Hubei Province's Outstanding Medical Academic Leader Program, and Program for Innovative Research Team of Chinese Ministry of Education (IRT1131).
References
- 1.Ando K, Moriyama T, Guidotti LG, Wirth S, Schreiber RD, Schlicht HJ, et al. Mechanisms of class I restricted immunopathology. J Exp Med. 1993;178:1541–1554. doi: 10.1084/jem.178.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniades CG, Berry PA, Wendon JA, Vergani D. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008;49:845–861. doi: 10.1016/j.jhep.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, et al. Source and characterisation of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. 2012;56:735–46. doi: 10.1002/hep.25657. [DOI] [PubMed] [Google Scholar]
- 4.Bantel H, Schulze-Osthoff K. Mechanisms of cell death in acute liver failure. Front Physiol. 2012;3:79. doi: 10.3389/fphys.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bémeur C, Qu H, Desjardins P, Butterworth RF. IL-1 or TNF receptor gene deletion delays onset of encephalopathy and attenuates brain edema in experimental acute liver failure. Neurochem Int. 2010;56:213–215. doi: 10.1016/j.neuint.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–9. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 7.Berry PA, Antoniades CG, Hussain MJ, McPhail MJ, Bernal W, Vergani D, et al. Admission levels and early changes in serum interleukin-10 are predictive of poor outcome in acute liver failure and decompensated cirrhosis. Liver Int. 2010;30:733–740. doi: 10.1111/j.1478-3231.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- 8.Bertoletti A, Maini M, Williams R. Role of hepatitis B virus specific cytotoxic T cells in liver damage and viral control. Antiviral Res. 2003;60:61–6. doi: 10.1016/j.antiviral.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Cao Q, Batey R, Pang G, Russell A, Clancy R. IL-6, IFN-gamma and TNF-alpha production by liver-associated T cells and acute liver injury in rats administered concanavalin A. Immunol Cell Biol. 1998;76:542–549. doi: 10.1046/j.1440-1711.1998.00779.x. [DOI] [PubMed] [Google Scholar]
- 10.Cardier JE, Schulte T, Kammer H, Kwak J, Cardier M. Fas (CD95,APO-1) antigen expression and function in murine liver endothelial cells: implications for the regulation of apoptosis in liver endothelial cells. FASEB J. 1999;13:1950–1960. doi: 10.1096/fasebj.13.14.1950. [DOI] [PubMed] [Google Scholar]
- 11.Cheng L, Wang J, Li X, Xing Q, Du P, Su L, et al. Interleukin-6 induces Gr-1+CD11b+ myeloid cells tos CD8+ T cell-mediated liver injury in mice. PLoS One. 2011;6:e17631. doi: 10.1371/journal.pone.0017631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu CM, Yeh CT, Liaw YF. Viral superinfection in previously unrecognized chronic carriers of hepatitis B virus with superimposed acute fulminant versus nonfulminant hepatitis. J Clin Microbiol. 1999;37:235. doi: 10.1128/jcm.37.1.235-237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–63. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 14.Dong X, Gong Y, Zeng H, Hao Y, Wang X, Hou J, et al. Imbalance between circulating CD4+ regulatory T and conventional T lymphocytes in patients with HBV-related acute-on-chronic liver failure. Liver Int. 2013;33:1517–1526. doi: 10.1111/liv.12248. [DOI] [PubMed] [Google Scholar]
- 15.Dong Z, Wei H, Sun R, Tian Z. The roles of innate immune cells in liver injury and regeneration. Cell Mol Immunol. 2007;4:241–52. [PubMed] [Google Scholar]
- 16.Dong ZJ, Wei HM, Sun R, Tian ZG, Gao B. Isolation of murine hepatic lymphocytes using mechanical dissection for phenotypic and functional analysis of NK1.1+ cells. World J Gastroenterol. 2004;10:1928–1933. doi: 10.3748/wjg.v10.i13.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farci P, Diaz G, Chen Z, Govindarajan S, Tice A, Agulto L, et al. B cell gene signature with massive intrahepatic production of antibodies to hepatitis B core antigen in hepatitis B virus-associated acute liver failure. Proc Natl Acad Sci U S A. 2010;107:8766–8771. doi: 10.1073/pnas.1003854107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher JE, McKenzie TJ, Lillegard JB, Yu Y, Juskewitch JE, Nedredal GI, et al. Role of Kupffer cells and toll-like receptor 4 in acetaminophen-induced acute liver failure. J Surg Res. 2013;180:147–155. doi: 10.1016/j.jss.2012.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzese O, Kennedy PT, Gehring AJ, Gotto J, Williams R, Maini MK, et al. Modulation of the CD8 + -T-cell response by CD4 + CD25 + regulatory T cells in patients with hepatitis B virus infection. J Virol. 2005;79:3322–3328. doi: 10.1128/JVI.79.6.3322-3328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galun E, Axelrod JH. The role of cytokines in liver failure and regeneration: potential new molecular therapies. Biochim Biophys Acta. 2002;1592:345–358. doi: 10.1016/s0167-4889(02)00326-9. [DOI] [PubMed] [Google Scholar]
- 21.Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2008;47:729–36. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 22.Gao S, Wang M, Ye H, Guo J, Xi D, Wang Z, et al. Dual interference with novel genes mfgl2 and mTNFR1 ameliorates murine hepatitis virus type 3-induced fulminant hepatitis in BALB/cJ mice. Hum Gene Ther. 2010;21:969–977. doi: 10.1089/hum.2009.177. [DOI] [PubMed] [Google Scholar]
- 23.Gimson AE, Tedder RS, White YS, Eddleston AL, Williams R. Serological markers in fulminant hepatitis B. Gut. 1983;24:615–7. doi: 10.1136/gut.24.7.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 26.Han M, Yan W, Guo W, Xi D, Zhou Y, Li W, et al. Hepatitis B virus-induced hFGL2 transcription is dependent on c-Ets-2 and MAPK signal pathway. J Biol Chem. 2008;283:32715–29. doi: 10.1074/jbc.M806769200. [DOI] [PubMed] [Google Scholar]
- 27.Hiraoka A, Horiike N, Akbar SM, Michitaka K, Matsuyama T, Onji M. Soluble CD163 in patients with liver diseases: very high levels of soluble CD163 in patients with fulminant hepatic failure. J Gastroenterol. 2005;40:52–56. doi: 10.1007/s00535-004-1493-8. [DOI] [PubMed] [Google Scholar]
- 28.Hong F, Kim WH, Tian Z, Jaruga B, Ishac E, Shen X, et al. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: involvement of induction of Bcl-2 and Bcl-x(L) proteins. Oncogene. 2002;21:32–43. doi: 10.1038/sj.onc.1205016. [DOI] [PubMed] [Google Scholar]
- 29.Hu X, Ma S, Huang X, Jiang X, Zhu X, Gao H, et al. Interleukin-21 is upregulated in hepatitis B-related acute-on-chronic liver failure and associated with severity of liver disease. J Viral Hepat. 2011;18:458–467. doi: 10.1111/j.1365-2893.2011.01475.x. [DOI] [PubMed] [Google Scholar]
- 30.Jin X, Zimmers TA, Perez EA, Pierce RH, Zhang Z, Koniaris LG. Paradoxical effects of short-and long-term interleukin-6 exposure on liver injury and repair. Hepatology. 2006;43:474–484. doi: 10.1002/hep.21087. [DOI] [PubMed] [Google Scholar]
- 31.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–30. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khanam A, Trehanpati N, Garg V, Kumar C, Garg H, Sharma BC, et al. Altered frequencies of dendritic cells and IFN-c-secreting T cells with granulocyte colony-stimulating factor (G-CSF) therapy in acute-on-chronic liver failure. Liver Int. 2014;34:505–513. doi: 10.1111/liv.12415. [DOI] [PubMed] [Google Scholar]
- 33.Khanam A, Trehanpati N, Shrivastava S, Garg V, Sakhuja P, Sarin SK, et al. Increased expression of regulatory T cells in liver ineffective in downregulating the inflammatory activation of cells in acute on chronic liver failure (ACLF) Hepatology. 2010;52(4 Suppl):1105A. [Google Scholar]
- 34.Kitazawa T, Tsujimoto T, Kawaratani H, Fukui H. Salvage effect of E5564, Toll-like receptor 4 antagonist on d-galactosamine and lipopolysaccharide-induced acute liver failure in rats. J Gastroenterol Hepatol. 2010;25:1009–1012. doi: 10.1111/j.1440-1746.2009.06145.x. [DOI] [PubMed] [Google Scholar]
- 35.Klein C, Wüstefeld T, Assmus U, Roskams T, Rose-John S, Müller M, et al. The IL-6-gp130-STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. J Clin Invest. 2005;115:860–9. doi: 10.1172/JCI200523640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondo Y, Kobayashi K, Asabe S, Shiina M, Niitsuma H, Ueno Y, et al. Vigorous response of cytotoxic T lymphocytes associated with systemic activation of CD8 T lymphocytes in fulminant hepatitis B. Liver Int. 2004;24:561–7. doi: 10.1111/j.1478-3231.2004.0982.x. [DOI] [PubMed] [Google Scholar]
- 37.Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 2000;31:149–9. doi: 10.1002/hep.510310123. [DOI] [PubMed] [Google Scholar]
- 38.Kovalovich K, Li W, DeAngelis R, Greenbaum LE, Ciliberto G, et al. Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J Biol Chem. 2001;276:26605–13. doi: 10.1074/jbc.M100740200. [DOI] [PubMed] [Google Scholar]
- 39.Kuhla A, Eipel C, Siebert N, Abshagen K, Menger MD, Vollmar B. Hepatocellular apoptosis is mediated by TNFalpha-dependent Fas/FasLigand cytotoxicity in a murine model of acute liver failure. Apoptosis. 2008;13:1427–1438. doi: 10.1007/s10495-008-0269-7. [DOI] [PubMed] [Google Scholar]
- 40.Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem Res Toxicol. 2009;22:1376–85. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy GA, Liu M, Ding J, Yuwaraj S, Leibowitz J, Marsden PA, et al. Molecular and functional analysis of human prothrombinase gene (HFGL2) and its role in viral hapetitis. Am J Pathol. 2000;156:1217–1225. doi: 10.1016/S0002-9440(10)64992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lian LH, Jin X, Wu YL, Cai XF, Lee JJ, Nan JX. Hepatoprotective effects of Sedum sarmentosum on D-galactosamine/lipopolysaccharide-induced murine fulminant hepatic failure. J Pharmacol Sci. 2010;114:147–157. doi: 10.1254/jphs.10045fp. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Shi F, Tien P, Wang FS, Wang HF. Sustained overexpression of PD-1 on CD8 + T cells was significantly associated with poor prognosis in patients with HBV-related acute-on-chronic liver failure. Hepatology. 2010;52(4 Suppl):1085A. [Google Scholar]
- 44.Liu XY, Shi F, Zhao H, Wang HF. Research of PD-1 expression in CD8+ T cell of peripheral blood with HBV-associated acute-on-chronic liver failure. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2010;24:125–127. [PubMed] [Google Scholar]
- 45.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43(Suppl 1):S31–44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 46.Masubuchi Y, Bourdi M, Reilly TP, Graf ML, George JW, Pohl LR. Role of interleukin-6 in hepatic heat shock protein expression and protection against acetaminophen-induced liver disease. Biochem Biophys Res Commun. 2003;304:207–12. doi: 10.1016/s0006-291x(03)00572-2. [DOI] [PubMed] [Google Scholar]
- 47.Mita A, Hashikura Y, Tagawa Y, Nakayama J, Kawakubo M, Miyagawa S. Expression of fas ligand by hepatic macrophages in patients with fulminant hepatic failure. Am J Gastroenterol. 2005;100:2551–2559. doi: 10.1111/j.1572-0241.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 48.Mizuhara H, O'Neill E, Seki N, Ogawa T, Kusunoki C, Otsuka K, et al. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med. 1994;179:1529–37. doi: 10.1084/jem.179.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizuhara H, Uno M, Seki N, Yamashita M, Yamaoka M, Ogawa T, et al. Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology. 1996;23:1608–1615. doi: 10.1053/jhep.1996.v23.pm0008675184. [DOI] [PubMed] [Google Scholar]
- 50.Møller HJ, Grønbaek H, Schiødt FV, Holland-Fischer P, Schilsky M, Munoz S, et al. Soluble CD163 from activated macrophages predicts mortality in acute liver failure. J Hepatol. 2007;47:671–676. doi: 10.1016/j.jhep.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montaner LJ, da Silva RP, Sun J, Sutterwala S, Hollinshead M, Vaux D, et al. Type 1 and type 2 cytokine regulation of macrophage endocytosis: differential activation by IL-4/IL-13 as opposed to IFN-g or IL-10. J Immunol. 1999;162:4606–13. [PubMed] [Google Scholar]
- 52.Mundt B, Kühnel F, Zender L, Paul Y, Tillmann H, Trautwein C. Involvement of TRAIL and its receptors in viral hepatitis. FASEB J. 2003;17:94–96. doi: 10.1096/fj.02-0537fje. [DOI] [PubMed] [Google Scholar]
- 53.Muschen M, Warskulat U, Douillard P, Gilbert E, Haussinger D. Regulation of CD95 (APO-1/Fas) receptor and ligand expression by lipopolysaccharide and dexamethasone in parenchymal and nonparenchymal rat liver cells. Hepatology. 1998;27:200–208. doi: 10.1002/hep.510270131. [DOI] [PubMed] [Google Scholar]
- 54.Nagaki M, Iwai H, Naiki T, Ohnishi H, Muto Y, Moriwaki H. High levels of serum interleukin-10 and tumor necrosis factor are associated with fatality in fulminant hepatitis. J Infect Dis. 2000;182:1103–1108. doi: 10.1086/315826. [DOI] [PubMed] [Google Scholar]
- 55.Nagaki M, Moriwaki H. Implication of cytokines: roles of tumor necrosis factor-alpha in liver injury. Hepatol Res. 2008;38(S1):S19–S28. doi: 10.1111/j.1872-034X.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- 56.Nanda SK, Yalcinkaya K, Panigrahi AK, Acharya SK, Jameel S, Panda SK. Etiological role of hepatitis E virus in sporadic fulminant hepatitis. J Med Virol. 1994;42:133. doi: 10.1002/jmv.1890420207. [DOI] [PubMed] [Google Scholar]
- 57.Ni R, Tomita Y, Matsuda K, Ichihara A, Ishimura K, Ogasawara J, et al. Fas-mediated apoptosis in primary cultured mouse hepatocytes. Exp Cell Res. 1994;215:332–337. doi: 10.1006/excr.1994.1349. [DOI] [PubMed] [Google Scholar]
- 58.Nicoletti F, Zaccone P, Xiang M, Magro G, Di Mauro M, Di Marco R, et al. Essential pathogenetic role for interferon (IFN)-gamma in concanavalin A-induced T cell-dependent hepatitis: exacerbation by exogenous IFN-gamma and prevention by IFN-gamma receptor-immunoglobulin fusion protein. Cytokine. 2000;12:315–23. doi: 10.1006/cyto.1999.0561. [DOI] [PubMed] [Google Scholar]
- 59.Niu Y, Liu H, Yin D, Yi R, Chen T, Xue H, et al. The balance between intrahepatic IL-17(+) T cells and Foxp3(+)regulatory T cells plays an important role in HBV-related end-stage liver disease. BMC Immunol. 2011;12:47. doi: 10.1186/1471-2172-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niu YH, Yin DL, Liu HL, Yi RT, Yang YC, Xue HA, et al. Restoring the Treg cell to Th17 cell ratio may alleviate HBV-related acute-on-chronic liver failure. World J Gastroenterol. 2013;19:4146–4154. doi: 10.3748/wjg.v19.i26.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ochi M, Ohdan H, Mitsuta H, Onoe T, Tokita D, Hara H, et al. Liver NK cells expressing TRAIL are toxic against self hepatocytes in mice. Hepatology. 2004;39:1321–31. doi: 10.1002/hep.20204. [DOI] [PubMed] [Google Scholar]
- 62.Odeh M, Sabo E, Srugo I, Oliven A. Serum levels of tumor necrosis factor-alpha correlate with severity of hepatic encephalopathy due to chronic liver failure. Liver Int. 2004;24:110–116. doi: 10.1111/j.1478-3231.2004.0894.x. [DOI] [PubMed] [Google Scholar]
- 63.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 64.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(Suppl 1):S54–62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 65.Riordan SM, Williams R. Mechanisms of hepatocyte injury, multiorgan failure, and prognostic criteria in acute liver failure. Semin Liver Dis. 2003;23:203–215. doi: 10.1055/s-2003-42639. [DOI] [PubMed] [Google Scholar]
- 66.Rong Y, Song H, You S, Zhu B, Zang H, Zhao Y, et al. Association of Toll-like receptor 3 polymorphisms with chronic hepatitis B and hepatitis B-related acute-on-chronic liver failure. Inflammation. 2013;36:413–418. doi: 10.1007/s10753-012-9560-4. [DOI] [PubMed] [Google Scholar]
- 67.Rutherford AE, Hynan LS, Borges CB, Forcione DG, Blackard JT, Lin W, et al. Serum apoptosis markers in acute liver failure: a pilot study. Clin Gastroenterol Hepatol. 2007;5:1477–1483. doi: 10.1016/j.cgh.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 68.Ryo K, Kamogawa Y, Ikeda I, Yamauchi K, Yonehara S, Nagata S, et al. Significance of Fas antigen-mediated apoptosis in human fulminant hepatic failure. Am J Gastroenterol. 2000;95:2047–2055. doi: 10.1111/j.1572-0241.2000.02268.x. [DOI] [PubMed] [Google Scholar]
- 69.Saile B, Knittel T, Matthes N, Schott P, Ramadori G. CD95/CD95L-mediated apoptosis of the hepatic stellate cell. A mechanism terminating uncontrolled hepatic stellate cell proliferation during hepatic tissue repair. Am J Pathol. 1997;151:1265–1272. [PMC free article] [PubMed] [Google Scholar]
- 70.Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2009;3:269–82. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shah N, Montes de Oca M, Jover-Cobos M, Tanamoto K, Muroi M, Sugiyama K, et al. Role of toll-like receptor 4 in mediating multiorgan dysfunction in mice with acetaminophen induced acute liver failure. Liver Transpl. 2013;19:751–761. doi: 10.1002/lt.23655. [DOI] [PubMed] [Google Scholar]
- 72.Shalev I, Wong KM, Foerster K, Zhu Y, Chan C, Maknojia A, et al. The novel CD4+CD25+regulatory T cell effector molecule fibrinogen-like protein 2 contributes to the outcome of murine fulminant viral hepatitis. Hepatology. 2009;49:387–97. doi: 10.1002/hep.22684. [DOI] [PubMed] [Google Scholar]
- 73.Shimizu Y, Margenthaler JA, Landeros K, Otomo N, Doherty G, Flye MW. The resistance of P. acnes-primed interferon g-deficient mice to low dose lipopolysaccharide-induced acute liver injury. Hepatology. 2002;35:805–814. doi: 10.1053/jhep.2002.32484. [DOI] [PubMed] [Google Scholar]
- 74.Singhal S, Chakravarty A, Das BC, Kar P. Tumour necrosis factor-alpha and soluble Fas ligand as biomarkers in non-acetaminophen-induced acute liver failure. Biomarkers. 2009;14:347–353. doi: 10.1080/13547500903013664. [DOI] [PubMed] [Google Scholar]
- 75.Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, et al. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771–778. doi: 10.1002/hep.20649. [DOI] [PubMed] [Google Scholar]
- 76.Tabor E, Gerety RJ, Hoofnagle JH, Barker LF. Immune response in fulminant viral hepatitis, type B. Gastroenterology. 1976;71:635–40. [PubMed] [Google Scholar]
- 77.Tagami A, Ohnishi H, Hughes RD. Increased serum soluble Fas in patients with acute liver failure due to paracetamol overdose. Hepatogastroenterology. 2003;50:742–745. [PubMed] [Google Scholar]
- 78.Tandon BN, Bernauau J, O'Grady J, Gupta SD, Krisch RE, Liaw YF, et al. Recommendation of the international association for the study of the liver subcommittee on nomenclature of acute and subacute liver failure. J Gastroenterol Hepatol. 1999;14:403. doi: 10.1046/j.1440-1746.1999.01905.x. [DOI] [PubMed] [Google Scholar]
- 79.Tang Y, Jiang L, Zheng Y, Ni B, Wu Y. Expression of CD39 on FoxP3+ T regulatory cells correlates with progression of HBV infection. BMC Immunol. 2012;13:17. doi: 10.1186/1471-2172-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taub R. Hepatoprotection via the IL-6/Stat3 pathway. J Clin Invest. 2003;112:978–80. doi: 10.1172/JCI19974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, et al. CD8(1) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 83.Tibbs C, Williams R. Viral causes and managenant of acute liver failure. J Hepatol. 1995;22(Suppl 1):68. [PubMed] [Google Scholar]
- 84.Tiegs G. Cellular and cytokine-mediated mechanisms of inflammation and its modulation in immune-mediated liver injury. Z Gastroenterol. 2007;45:63–70. doi: 10.1055/s-2006-927397. [DOI] [PubMed] [Google Scholar]
- 85.Tokushige K, Yamaguchi N, Ikeda I, Hashimoto E, Yamauchi K, Hayashi N. Significance of soluble TNF receptor-1 in acute-type fulminant hepatitis. Am J Gastroenterol. 2000;95:2040–2046. doi: 10.1111/j.1572-0241.2000.02270.x. [DOI] [PubMed] [Google Scholar]
- 86.Trepo CG, Robert D, Motin J, Trepo D, Sepetjian M, Prince AM. Hepatitis B antigen (HBSAg) and/or antibodies (anti-HBS and anti-HBC) in fulminant hepatitis: Pathogenic and prognostic significance. Gut. 1976;17:10–13. doi: 10.1136/gut.17.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ueno Y, Ishii M, Yahagi K, Mano Y, Kisara N, Nakamura N, et al. Fas-mediated cholangiopathy in the murine model of graft versus host disease. Hepatology. 2000;31:966–974. doi: 10.1053/he.2000.5764. [DOI] [PubMed] [Google Scholar]
- 88.Wang LW, Chen H, Gong ZJ. High mobility group box-1 protein inhibits regulatory T cell immune activity in liver failure in patients with chronic hepatitis B. Hepatobiliary Pancreat Dis Int. 2010;9:499–507. [PubMed] [Google Scholar]
- 89.Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E, et al. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol. 2005;42:195–201. doi: 10.1016/j.jhep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 90.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Williams R. Classification, etiology and consideration of outcome in acute liver failure. Semin Liver Dis. 1996;16:343. doi: 10.1055/s-2007-1007247. [DOI] [PubMed] [Google Scholar]
- 92.Wu D, Wang HW, Chen T, Yan WM, Xi D, Luo XP, et al. A disparate subset of double negative t cells contributes to the outcome of murine fulminant viral hepatitis via effector molecule fibrinogen-like protein 2. Hepatology. 2012;56(Suppl):118A. doi: 10.1007/s12026-015-8727-0. [DOI] [PubMed] [Google Scholar]
- 93.Wu ZG, Han MF, Chen T, Yan W, Ning Q. Acute liver failure: mechanisms of immune-mediated liver injury. Liver Int. 2010;30:782–794. doi: 10.1111/j.1478-3231.2010.02262.x. [DOI] [PubMed] [Google Scholar]
- 94.Yang B, Wang Y, Zhao C, Yan W, Che H, Shen C, et al. Increased Th17 cells and interleukin-17 contribute to immune activation and disease aggravation in patients with chronic hepatitis B virus infection. Immunol Lett. 2013;149:41–49. doi: 10.1016/j.imlet.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 95.Yang F, Li X, Wang LK, Wang LW, Han XQ, Zhang H, et al. Inhibitions of NF-κB and TNF-α result in differential effects in rats with acute on chronic liver failure induced by d-Gal and LPS. Inflammation. 2014;37:848–857. doi: 10.1007/s10753-013-9805-x. [DOI] [PubMed] [Google Scholar]
- 96.Yang J, Yi P, Wei L, Xu Z, Chen Y, Tang L, et al. Phenotypes and clinical significance of circulating CD4(+)CD25(+) regulatory T cells (Tregs) in patients with acute-on-chronic liver failure (ACLF) J Transl Med. 2012;10:193. doi: 10.1186/1479-5876-10-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang Q, Shi Y, He J, Chen Z. The evolving story of macrophages in acute liver failure. Immun Lett. 2012;147:1–9. doi: 10.1016/j.imlet.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 98.Yang Q, Shi Y, Yang Y, Chen Z. Deactivation and apoptosis of hepatic macrophages are involved in the development of concanavalin A induced acute liver failure. Mol Med Rep. 2013;8:757–762. doi: 10.3892/mmr.2013.1575. [DOI] [PubMed] [Google Scholar]
- 99.Ye Y, Xie X, Yu J, Zhou L, Xie H, Jiang G, et al. Involvement of Th17 and Th1 effector responses in patients with Hepatitis B. J Clin Immunol. 2010;30:546–555. doi: 10.1007/s10875-010-9416-3. [DOI] [PubMed] [Google Scholar]
- 100.Yin XM, Ding WX. Death receptor activation-induced hepatocyte apoptosis and liver injury. Curr Mol Med. 2003;3:491–508. doi: 10.2174/1566524033479555. [DOI] [PubMed] [Google Scholar]
- 101.Zhai S, Zhang L, Dang S, Yu Y, Zhao Z, Zhao W, et al. The ratio of Th-17 to Treg cells is associated with survival of patients with acute-on-chronic hepatitis B liver failure. Viral Immunol. 2011;24:303–310. doi: 10.1089/vim.2010.0135. [DOI] [PubMed] [Google Scholar]
- 102.Zhang GL, Xie DY, Lin BL, Xie C, Ye YN, Peng L, et al. Imbalance of interleukin-17-producing CD4 T cells/regulatory T cells axis occurs inremission stage of patients with hepatitis B virus-related acute-on-chronic liver failure. J Gastroenterol Hepatol. 2013;28:513–521. doi: 10.1111/jgh.12082. [DOI] [PubMed] [Google Scholar]
- 103.Zhang JH, Dong ZJ, Zhou RB, Luo DM, Wei HM, Tian ZG. Isolation of lymphocytes and their innate immune characterizations from liver, intestine, lung and uterus. Cell Mol Immunol. 2005;2:271–280. [PubMed] [Google Scholar]
- 104.Zhang JJ, Fan YC, Zhao ZH, Yang Y, Dou CY, Gao S, et al. Prognoses of patients with acute-on-chronic hepatitis B liver failure are closely associated with altered SOCS1 mRNA expression and cytokine production following glucocorticoid treatment. Cell Mol Immunol. 2014;11:396–404. doi: 10.1038/cmi.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang M, Zhou J, Zhao T, Huang G, Tan Y, Tan S, et al. Dissection of a circulating and intrahepatic CD4(+) Foxp3(+) T-cell subpopulation in chronic hepatitis B virus (HBV) infection: a highly informative strategy for distinguishing chronic HBV infection states. J Infect Dis. 2012;205:1111–1120. doi: 10.1093/infdis/jis011. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008;134:1938–1949. doi: 10.1053/j.gastro.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Z, Zou ZS, Fu JL, Cai L, Jin L, Liu YJ, et al. Severe dendritic cell perturbation is actively involved in the pathogenesis of acute-on-chronic hepatitis B liver failure. J Hepatol. 2008;49:396–406. doi: 10.1016/j.jhep.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Z, Zou ZS, Fu JL, Cai L, Jin L, Liu YJ, et al. Severe dendritic cell perturbation is actively involved in the pathogenesis of acute-on-chronic hepatitis B liver failure. J Hepatol. 2008;49:396–406. doi: 10.1016/j.jhep.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 109.Zhao J, Zhang JY, Yu HW, He YL, Zhao JJ, Li J, et al. Improved survival ratios correlate with myeloid dendritic cell restoration in acute-on-chronic liver failure patients receiving methylprednisolone therapy. Cell Mol Immunol. 2012;9:417–422. doi: 10.1038/cmi.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao L, Qiu de K, Ma X. Th17 cells: the emerging reciprocal partner of regulatory T cells in the liver. J Dig Dis. 2010;11:126–133. doi: 10.1111/j.1751-2980.2010.00428.x. [DOI] [PubMed] [Google Scholar]
- 111.Zhao N, Hao J, Ni Y, Luo W, Liang R, Cao G, et al. Vγ4 γδ T cell-derived IL-17A negatively regulates NKT cell function in Con A-induced fulminant hepatitis. J Immunol. 2011;187:5007–5014. doi: 10.4049/jimmunol.1101315. [DOI] [PubMed] [Google Scholar]
- 112.Zhu C, Sun Y, Luo X, Yan W, Xi D, Ning Q. Novel mfgl2 antisense plasmid inhibits murine fgl2 expression and ameliorates murine hepatitis virus type 3-induced fulminant hepatitis in BALB/cJ mice. Hum Gene Ther. 2006;17:589–600. doi: 10.1089/hum.2006.17.589. [DOI] [PubMed] [Google Scholar]
- 113.Zhu CL, Yan WM, Zhu F, Zhu YF, Xi D, Tian DY, et al. Fibrinogen-like protein 2 fibroleukin expression and its correlation with disease progression in murine hepatitis virus type 3-induced fulminant hepatitis and in patients with severe viral hepatitis B. World J Gastroenterol. 2005;11:6936–6940. doi: 10.3748/wjg.v11.i44.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zou Y, Chen T, Han M, Wang H, Yan W, Song G, et al. Increased killing of liver NK cells by Fas/Fas ligand and NKG2D/NKG2D ligand contributes to hepatocyte necrosis in virus-induced liver failure. J Immunol. 2010;184:466–75. doi: 10.4049/jimmunol.0900687. [DOI] [PubMed] [Google Scholar]
- 115.Zou Z, Li B, Xu D, Zhang Z, Zhao JM, Zhou G, et al. Imbalanced intrahepatic cytokine expression of interferon-gamma, tumor necrosis factor-alpha, and interleukin-10 in patients with acute-on-chronic liver failure associated with hepatitis B virus infection. J Clin Gastroenterol. 2009;43:182–190. doi: 10.1097/MCG.0b013e3181624464. [DOI] [PubMed] [Google Scholar]