Abstract
Objective:
To determine whether resection of areas with evidence of intense, synchronized neural firing during seizures is an accurate indicator of postoperative outcome.
Methods:
Channels meeting phase-locked high gamma (PLHG) criteria were identified retrospectively from intracranial EEG recordings (102 seizures, 46 implantations, 45 patients). Extent of removal of both the seizure onset zone (SOZ) and PLHG was correlated with seizure outcome, classified as good (Engel class I or II, n = 32) or poor (Engel class III or IV, n = 13).
Results:
Patients with good outcomes had significantly greater proportions of both SOZ and the first 4 (early) PLHG sites resected. Improved outcome classification was noted with early PLHG, as measured by the area under the receiver operating characteristic curves (PLHG 0.79, SOZ 0.68) and by odds ratios for resections including at least 75% of sites identified by each measure (PLHG 9.7 [95% CI: 2.3–41.5], SOZ 5.3 [95% CI: 1.2–23.3]). Among patients with resection of at least 75% of the SOZ, 78% (n = 30) had good outcomes, increasing to 91% when the resection also included at least 75% of early PLHG sites (n = 22).
Conclusions:
This study demonstrates the localizing value of early PLHG, which is comparable to that provided by the SOZ. Incorporation of PLHG into the clinical evaluation may improve surgical efficacy and help to focus resections on the most critical areas.
Surgery for pharmacoresistant focal epilepsy relies on identifying the seizure source,1 but this is often difficult to judge from invasive EEG recordings. The fundamental characteristic of the “core” of seizing brain is intense population firing aligned with powerful depolarization.2,3 Traditionally, the core has been presumed to be identifiable from visualized initial ictal EEG changes, i.e., the seizure onset zone (SOZ).1 However, our previous work has revealed that the core occupies only a portion of the area in which ictal EEG waveforms are seen.4 Surrounding the core is a rapidly expanding “penumbra” in which inhibitory currents act against local seizure invasion by restraining pyramidal cell firing.4–7 Penumbral synaptic currents cannot be reliably differentiated from the depolarizations in the core with standard visual EEG interpretation.8 Previously, we proposed that the core can be identified using a measure based on high gamma (80–150 Hz) activity phase-locked to low-frequency EEG discharges (PLHG).8 The existing literature on high-frequency oscillations supports this view.9–11 Sites demonstrating increased high-frequency power during seizures12,13 or at seizure onset14–17 are often located within the SOZ, and resection of these sites has been associated with improved postoperative seizure control.18
We hypothesized that the extent of resection of the earliest PLHG-generating sites correlates with good postoperative seizure control and compares favorably with SOZ resection. We tested this hypothesis with a retrospective analysis of seizures recorded intracranially from surgical patients with epilepsy.
METHODS
Standard protocol approvals, registrations, and patient consents.
Consecutive epilepsy surgery procedures meeting study criteria (figure 1) were identified at Columbia University Medical Center (CUMC, 2005–2012) and the National Hospital for Neurology and Neurosurgery in London (NHNN, 2011–2013). The study was approved by the Institutional Review Board at CUMC, and by the National Research Ethics Service at NHNN.
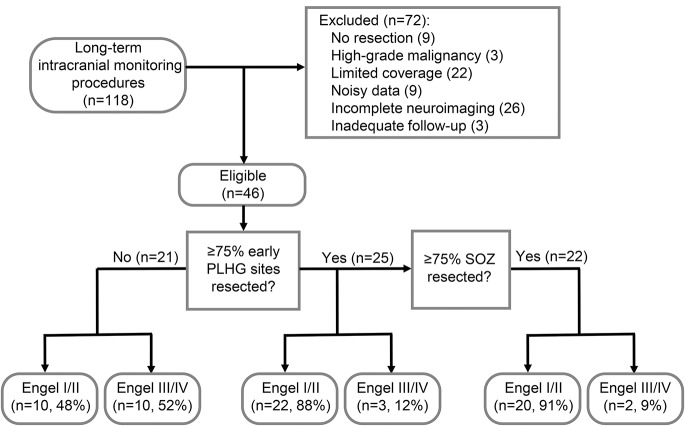
Figure 1. Diagnostic algorithm using the SOZ and PLHG metrics, and resulting surgical outcome classification.
The chart combines data from both the contributing centers (Columbia University Medical Center and University College London). “Limited coverage” indicates exclusion of wide-area (e.g., bihemispheric) surveys, and “noisy data” refers to cases with too few usable channels or reference noise that could not be removed with blind source separation. Classification was based on resection extent of 75% of electrode sites for each measure, averaged across analyzed seizures for each patient. The best results were achieved by combining criteria based on the SOZ and the early-recruited (first 4 channels) PLHG sites. PLHG = phase-locked high gamma; SOZ = seizure onset zone.
Data analysis.
Electrode configurations included both subdural electrodes (3.0-mm diameter) with 0.5- or 1-cm center-to-center spacing, and depth (2.3-mm length) electrodes (Ad-Tech, Racine, WI). Data were recorded with standard clinical video EEG systems (XLTek at CUMC, Nicolet One at NHNN; Natus Medical Inc., Oakville, Canada) sampled at 500 or 1,000 Hz per channel, with bandpass filtering between 0.5 Hz and 1/4 sampling rate, 24/16 bit precision, and 0.31/0.15 µV resolution, respectively). Postoperative seizure outcome was classified using the Engel scale,19 with good outcomes defined as Engel class I or II (seizure-free and rare disabling seizures, respectively) and poor outcomes as class III or IV (worthwhile improvement or no improvement, respectively). The SOZ was determined by visual EEG review (typically 1- to 70-Hz bandpass filter, 10 seconds per screen) by the treating epileptologists at the time of the evaluation as the sites of the earliest departure from interictal patterns leading to sustained seizure activity1 and including rapid spread within 1 second. Herald spikes if present were considered as supportive data. Although high-frequency (>80 Hz) data were acquired before the time of surgery, they were not accessible in the clinical review software, and their evaluation was not standard clinical practice at either center during the study period.
EEG signal analysis.
The first 3 seizures recorded from each patient, including atypical seizures, were truncated to 4 minutes and analyzed. The PLHG measure was implemented as described in our previous study.8 Briefly, artifact was addressed either by excluding channels with visualized excess 80- to 150-Hz noise, or removing noise using blind source separation with independent component analysis (EEGLAB, University of California San Diego).20 Instantaneous high gamma (80–150 Hz, 500th order symmetric finite impulse response) amplitude was derived from the Hilbert transform, normalized to a 30-second preictal baseline, and weighted with the simultaneous phase-locking value computed from the low-frequency (4–30 Hz) phase. Herald spikes, i.e., interictal-appearing discharges occurring immediately before the electrographic seizure onset, were excluded from both the seizure and the preictal baseline to avoid bias. PLHG values were computed in 333-millisecond windows and averaged across 20 overlapping windows. The threshold for definition of a PLHG site was determined for each seizure independently, based on the PLHG value distribution. If the distribution was bimodal, with clear separation between core and penumbral activity, the threshold was defined as half the coefficient of variation less than the mean of the higher-valued distribution. If the distribution was unimodal, with core sites indicated by positive outliers, the threshold was defined as 3 coefficients of variation over the mean. Line length21 (2–25 Hz, normalized to 30-second preictal baseline with 2.5-SD threshold8,22) was used as an objective measure approximating seizure spread as viewed in EEG. All calculations were fully automated and performed blinded to outcome using custom software (MATLAB; MathWorks, Natick, MA).
Classification of electrodes.
To define the resection boundaries and the position of implanted electrodes, preoperative volumetric MRIs (1.5T or 3T) were coregistered to postimplantation CT scans and postresection MRIs using the Advanced Normalization Tools,23 C3D,24 FSL (Oxford, UK), and AMIRA (FEI Burlington, MA). Intraoperative photographs, detailed operative notes, and EEG reports were used to confirm the final array placements. Following coregistration, 3-dimensional images were manually reviewed to identify the electrodes positioned within the resection boundaries.
Statistical analysis.
The locations of SOZ and PLHG electrodes were determined regarding the resection cavity. The proportion of resected sites for each measure was then calculated. PLHG sites were subdivided into 3 groups: the first 4 channels (“early PLHG”), first 8 channels (“late PLHG”), and all channels (“entire”). The choice of the first 4 electrodes was based on the average size of the SOZ (4.8 ± 0.4 channels). For SOZ, the resection ratio was calculated using all of the designated channels. Receiver operating characteristic (ROC) curves were constructed for the SOZ and early PLHG tests for 25%, 50%, 75%, and 100% resected cutoff values. True positives, defined as the proportion of patients with good outcomes meeting the cutoff values, were plotted vs false positives, defined as the proportion of patients with poor outcomes meeting cutoff values. The quality of outcome classification was assessed using area under the ROC curve (AUROC) and odds ratios. The Wilcoxon rank sum test was used to compare resection volumes, channel counts, and seizure stereotypy calculated using Levenshtein distance, a measure of the differences in channel sequences.25 Standard error measurements were used throughout.
RESULTS
Ninety-five consecutive epilepsy procedures with chronic intracranial recordings were identified from CUMC and 33 from NHNN. Of these, 36 procedures from CUMC and 10 from NHNN were included (figure 1). In all, there were 102 seizures from 46 implantations in 45 patients, with 9 seizures in 6 patients lasting longer than 4 minutes. One patient underwent 2 implantation procedures performed 9 months apart (table e-1 on the Neurology® Web site at Neurology.org). Brain MRI scans for 24 patients (54%) lacked clearly localizing structural abnormalities, and 23 patients (52%) had nonspecific tissue pathology findings including gliosis. Good surgical outcomes were seen in 32 patients, and poor outcomes in 14 implantations in 13 patients. The proportion of good outcomes did not differ significantly between lesional (73%) and nonlesional (67%) patients. Excluding 10 patients in whom volumetric data were not available, there was no difference in resection volumes between outcome groups (26.0 ± 4.3 cm3 [n = 24 Engel class I/II], 25.4 ± 7.0 cm3 [n = 12 Engel class III/IV], p = 0.67, Wilcoxon rank sum test). Mean follow-up time was 2.4 ± 0.3 years, with a range of 0.75 to 6.5 years (table e-1). Of the 4 patients with less than 1 year follow-up, all had poor (Engel class IV) surgical outcomes, and 2 underwent subsequent epilepsy surgery procedures within 1 year.
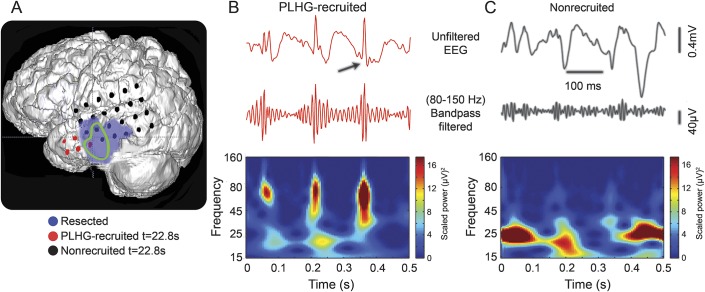
Figure 2 illustrates the analysis in a patient (16) with recurrent postoperative seizures following a wide resection that included a well-defined left temporal lesion and the complete SOZ. PLHG was seen beginning 22 seconds after seizure onset (figure 2, A and B, red disks and trace) in a small number of anterior temporal electrodes just anterior to the lesion and outside of the resection cavity. EEG traces from electrodes in the SOZ (green trace) not meeting PLHG criteria (black disks) demonstrated an unequivocal EEG rhythm but no discernable high gamma bursting (figure 2C, black trace).
Figure 2. Surgically sparing early ictal PLHG sites is associated with seizure recurrence.
(A) Three-dimensional brain reconstruction of patient 16 who had an Engel class III outcome following resection (purple) of a left temporal lesion and the complete SOZ (green trace). PLHG sites identified by 22.8 seconds after seizure onset are shown in red, and the remainder of the lateral temporal grid electrodes in black. (B) EEG segment from an early PLHG channel (top, red) demonstrating high gamma oscillations phase locked to the negative peaks of the ictal discharges (black arrow). The increased high gamma signal is evident in the corresponding 80- to 150-Hz filtered trace (peak amplitude of approximately 100 μV), and in the Morlet wavelet spectrogram calculated from the unfiltered data segment and demonstrating islands of increased power in the high gamma band. (C) EEG segment from an SOZ electrode that did not meet PLHG recruitment criteria (top, black) exhibiting an ictal rhythm of similar amplitude but lacking discrete high gamma oscillations, as is evident in the corresponding filtered high gamma trace and spectrogram. PLHG = phase-locked high gamma; SOZ = seizure onset zone.
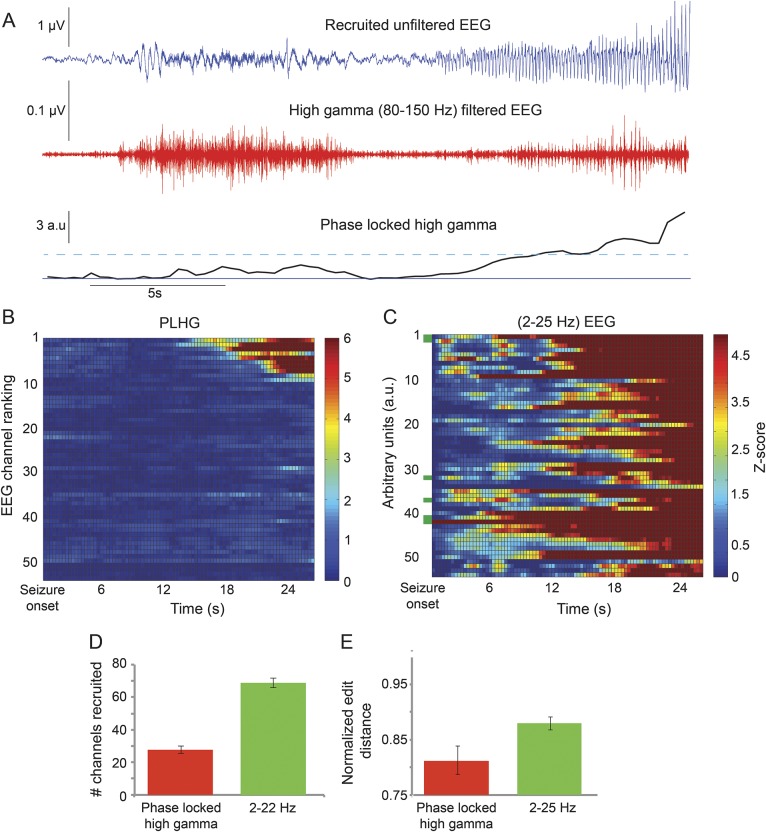
PLHG was identified in all but 2 patients, both of whom had Engel class IV outcomes. Many channels exhibited increased high gamma amplitude (figure 3A) without meeting PLHG criteria. The typical PLHG channel showed a brief attenuation in the high gamma filtered trace, followed by repetitive bursts aligned with the rhythmic waveforms seen in the raw EEG trace. PLHG values increased only with this latter amplitude-modulated pattern. The average time of earliest PLHG recruitment was 14.2 ± 2.4 seconds after seizure onset.
Figure 3. Delayed onset and limited extent of amplitude-modulated high gamma activity.
(A) Seizure recorded from patient 16 (EEG, blue, top) and the corresponding 80- to 150-Hz filtered trace (red, middle) shows the contrast between early, steady high gamma activity and late-onset bursting activity that tracks the low-frequency rhythm. The PLHG time series metric (black, bottom) does not increase past the recruitment threshold (cyan dotted line) until shortly after high gamma bursting begins. (B) Line scan of ictal PLHG values for the same seizure, with the top 14 channels ranked in their recruitment sequence. Note the sharp demarcation between recruited and nonrecruited channels, the equally sharp transition to recruitment, and the delayed appearance (17 seconds after seizure onset) of the earliest suprathreshold PLHG values. (C) Corresponding line scan of line-length values calculated from the 2- to 25-Hz bandpass-filtered EEG signals, demonstrating more diffuse activity. Seizure onset zone channels are marked by green bars along the y-axis. (D) Across all implantations and seizures, fewer channels were recruited over the entire seizure duration based on the PLHG measure, compared with 2- to 25-Hz line length (n = 102, p < 0.001, paired t test). (E) The PLHG recruitment sequence demonstrated a greater degree of stereotypy (i.e., decreased normalized edit distance) than was the case for line length, across all implantations in which multiple seizures were recorded (n = 31 implantations, 86 seizures, p < 0.01, Wilcoxon rank sum test). PLHG = phase-locked high gamma.
The spatiotemporal evolution of PLHG sites indicated a sharply demarcated, expanding region located within the area demonstrating low-frequency rhythmic activity (figure 3B). Across all nonsecondarily generalized seizures, the maximum number of PLHG electrodes was 21.0 ± 1.6 by seizure termination (or 4 minutes, if seizure duration was longer), compared with 64.8 ± 2.9 sites identified using line length (figure 3D, n = 87 seizures, p < 0.001, Wilcoxon rank sum test). In patients with multiple seizures, the PLHG recruitment sequence also demonstrated greater stereotypy among seizures in an individual patient than did line length (figure 3E, n = 31, p < 0.05, Wilcoxon rank sum test).
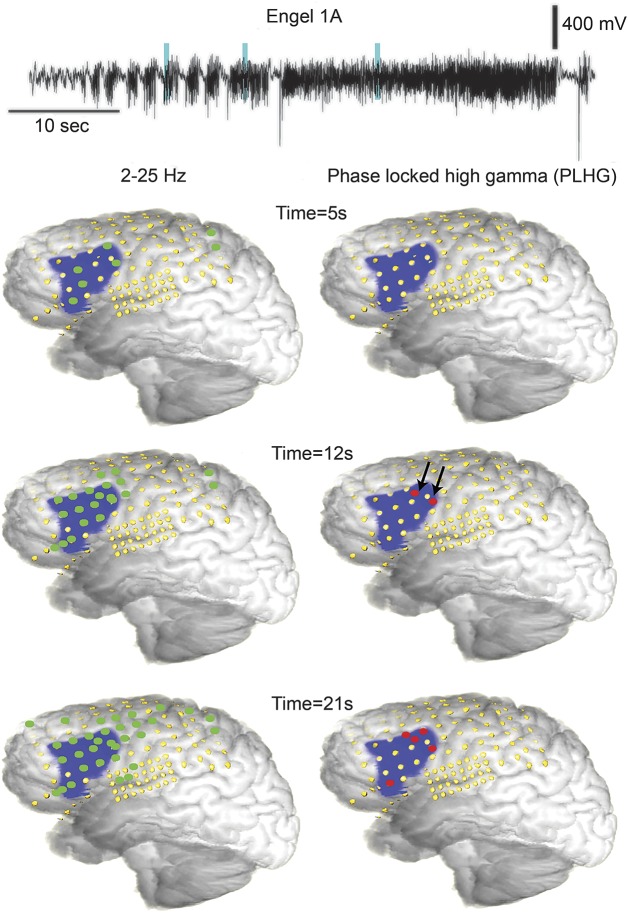
Figure 4 illustrates the contrast between traditional visual EEG analysis (as indexed by the 2- to 25-Hz line-length measure) and PLHG during seizure evolution, in a patient (42) with Engel class I outcome. At 12 seconds after seizure onset, PLHG was evident in depth electrode recordings from within the surgical margins (black arrows). By 21 seconds, PLHG had spread to neighboring subdural electrodes, also within the margins of the resection. In contrast, by 12 seconds, rhythmic low-frequency activity extended well outside the resection area.
Figure 4. Delayed spread of PLHG sites positioned inside resection boundaries.
(A) Three-dimensional brain reconstructions including electrode sites (yellow) and resection (blue) for a patient (42) with Engel class 1A outcome. The wideband EEG trace from an electrode positioned within the SOZ demonstrated an initial pattern of increased repetitive spiking (top). Cyan vertical lines indicate the times of the 3 activity snapshots below, shown as 3-dimensional brain reconstructions with superimposed electrode positions (yellow) and resected region (blue). Line length recruited sites (left images, green disks) quickly spread to the entire resection area and beyond. Early PLHG (right images, red disks) was first seen in proximal depth electrodes (black arrows) inside the SOZ, with spreading PLHG recruitment remaining within the resection boundaries through 21 seconds after seizure onset. PLHG = phase-locked high gamma; SOZ = seizure onset zone.
Unlike the SOZ, the seizure core is not a static entity, but rather expands as the seizure propagates. As expected, resection of PLHG appearing early in the seizure was superior to late-appearing PLHG as an outcome classifier (figure 5A). Subsequent analysis was therefore focused on the early (first 4) PLHG sites. Early PLHG was seen in an average of 45% of the SOZ channels. Both SOZ and early PLHG resection correlated with postoperative outcome classification. In patients with good outcomes, 91.5% ± 2.6% of the SOZ was resected, while in patients with poor outcomes, 65.0% ± 10.5% of the SOZ was resected (figure 5A, n = 46, p < 0.05, Wilcoxon rank sum test). Similarly, 72.7% ± 5.1% of early PLHG sites were resected in patients with good outcomes, compared with 45.4% ± 8.6% in patients with poor outcomes (figure 4A, n = 46, p < 0.01). Recognizing that our definition of early PLHG is necessarily arbitrary, we repeated the classification analysis using a time-based criterion of PLHG identification within 30 seconds after seizure onset, and again found a significant correlation with outcome (n = 46, p < 0.02, Wilcoxon rank sum test). To underscore the importance of phase locking, we duplicated the analysis using high gamma (80–150 Hz) amplitude irrespective of phase, again focusing on early sites. The correlation with outcome classification was not statistically significant (p = 0.06, Wilcoxon rank sum test, figure e-1).
Figure 5. Surgical outcome classification by extent of SOZ vs early PLHG resection.
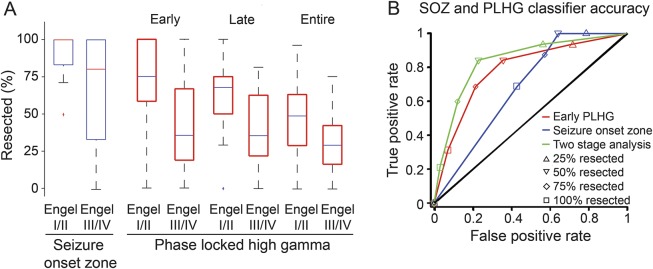
(A) Box plots of the percentage of electrode sites resected according to surgical outcome for the SOZ (left, blue) and early, late, and total PLHG (red, right). There was a significant difference in the proportion of SOZ and early PLHG (first 4 channels) resection between patients with good outcome (Engel class I/II) and poor outcome (Engel class III/IV). This difference was less evident when late PLHG (first 8 channels) and the full set of PLHG channels were considered. (B) Receiver operating characteristic curves comparing the sensitivity and specificity of seizure outcome classification using SOZ (blue), early PLHG (red), and a 2-stage analysis using both measures (green). Results at each data point were based on resections exceeding the corresponding cutoff proportion for each measure (inset). AUROCs for SOZ and early PLHG were 0.68 and 0.79, respectively. The solid black line indicates random performance, with an AUROC of 0.5. The differences in the SOZ and early PLHG curves are most striking at cutoff values of 75% and higher. Combining both measures resulted in a measurable improvement in outcome classification, with an AUROC of 0.86. AUROC = area under the receiver operating characteristic curve; PLHG = phase-locked high gamma; SOZ = seizure onset zone.
ROC curves were used to examine the effects of incomplete SOZ and PLHG resection (figure 5B). The AUROC values were 0.68 and 0.79, respectively. The relatively low specificity of the SOZ was especially notable: among implantation procedures with poor outcomes, the SOZ was completely resected in 6 (46%), and 75% or more of the SOZ was resected in 8 (62%). In contrast, 75% or more of early PLHG sites were resected in 3 cases (23%) with poor outcomes. The odds ratio for good outcome in the 35 patients with 75% or more of the SOZ resected was 5.3 (95% CI: 1.2–23.3), compared with 9.7 (95% CI: 2.3–41.5) for the 25 patients with resection of at least 75% of early PLHG sites. However, the difference between the two measures did not reach statistical significance.
We next asked whether sequential 2-stage testing using both SOZ and early PLHG information would improve the accuracy of outcome classification. The 2-stage AUROC improved to 0.86 (figure 5B, green curve). Of the 22 patients meeting the 75% cutoff value for both SOZ and early PLHG, 91% had good outcomes, while poor outcomes were limited to just 9% (figure 1). Fourteen of the patients (64%) became seizure-free. Among patients with clearly localizing lesions on preoperative MRI scans, 91% (n = 11) had good outcomes, vs 92% (n = 12) in nonlesional cases, with 64% and 58% of patients becoming seizure-free, respectively. Among the 36 cases with resection of at least 75% of the SOZ, 78% had good outcomes and 53% became seizure-free, with no difference between lesional (79%, n = 19) and nonlesional groups (76%, n = 17). No difference was found for resection volumes between the 75% SOZ resection group (29.0 ± 4.3 cm3, n = 29) and the group with 75% of both SOZ and early PLHG electrode sites resected (28.8 ± 5.1 cm3, n = 20, p > 0.05, Wilcoxon rank sum test).
To assess whether intact early PLHG sites can be the nidus for recurrent seizures, we evaluated follow-up scalp and intracranial EEG recordings, available for 16 of the 23 patients with recurrent seizures (including Engel class II outcomes). Early PLHG sites were left intact in 12 of these cases (75%). The follow-up studies demonstrated interictal discharges in 8 patients and seizures in 5 patients whose localization was consistent (or not inconsistent) with the intact early PLHG sites. In 3 patients, recurrent interictal discharges and/or seizures localized to sites not sampled by the original intracranial electrodes. Of note, at least one of the seizures recorded intracranially before resection demonstrated no PLHG positive sites in 2 of these 3 patients. Patient 3 underwent a second implantation that revealed PLHG sites adjacent to the edge of the prior resection, corresponding to an area where the first implantation demonstrated a row of early PLHG sites just inside the resection boundary.
DISCUSSION
Only 30% to 50% of patients who undergo tailored resections based on invasive EEG recordings are seizure-free after 1 year,26,27 compared with 60% to 80% of appropriately selected patients following anteromesial temporal lobectomy or lesionectomy.28,29 We had hypothesized that the wide-ranging penumbra confounds SOZ identification, that this may be in part responsible for the relatively poor outcomes in EEG-guided procedures, and that the use of phase-locked high-frequency information can increase the accuracy of traditional visual EEG interpretation. We found that the extent of resection of both the SOZ and early PLHG sites correlates with seizure outcome. In contrast to the wide distribution typically seen during visual EEG interpretation, the seizure core as identified by the PLHG measure can be detected beginning several seconds after seizure onset, and has both a limited extent and stereotypical spread pattern.
Our previous work details a theoretical framework for the spatial structure of seizures that provides a useful context for the interpretation of ictal high-frequency oscillations. As EEG low frequencies correlate poorly with neuronal firing, it is difficult to differentiate the seizure core and penumbra using standard visual interpretation. However, it is possible to do so using high gamma band signals as a surrogate for the synchronized, intense multiunit firing associated with paroxysmal depolarizing shifts2,3 in the sampled cortical tissue.8 Phase coupling to the low-frequency rhythm is therefore essential; indeed, we found that resection of sites with increased high gamma amplitude irrespective of phase was a worse predictor than resection of the SOZ. Equally important is the delayed appearance of PLHG after seizure onset, due to the slow propagation of the ictal wavefront and time required for rhythmic excitatory barrages to synchronize across the large territory sampled by clinical electrodes.8 This implies that the SOZ is almost always delineated based on the early inhibitory response to the seizure.
The region initially invaded by the seizure, as identified by early PLHG, is of greatest interest for the purposes of epilepsy surgery, and indeed we found that the predictive value of PLHG resection for surgical outcome was dependent on early appearance. Thus, core areas that are invaded late may be less capable of triggering a seizure, although the clinical significance of late seizure invasion requires further study. Propagation of the core to noncontiguous sites, if it occurs, may also be an important consideration.
In contrast to previous reports,18,30 our study found that SOZ resection is an effective classifier of seizure outcome. The larger size of our study may have made it possible to reach statistical significance, although differences in clinical methodology including patient selection may have contributed. The high rate of incomplete SOZ resection that we observed (39%), comparable to rates previously reported,31 deserves further investigation, as it appears to be an important factor limiting outcomes after electrode implantation.26,27 Of note, the presence of a localizing lesion did not appear to have a significant effect on outcome. Because the patients were not deemed to be candidates for single-stage lesionectomy, they are not likely to be representative of populations in prior surgical outcome studies of lesional syndromes.
Our concept of the ictal penumbra predicts that resecting regions displaying strong synaptic currents paired with unstructured, minimally increased firing would not contribute to control of seizures. While not definitive, this study provides evidence to support this prediction. Resection of early PLHG sites correlated with surgical outcome regardless of the extent of SOZ resection, and sparing up to 25% of the SOZ did not have a significant impact on outcomes. Caution should be exercised, however, before applying this observation to surgical procedures. The spatial specificity of high gamma activity is both its great advantage and its greatest weakness. Because electrode coverage of the convoluted cortical surface is necessarily sparse, it is theoretically possible for PLHG sites to go undetected even if they lie within the covered regions. Furthermore, high gamma activity is still only a surrogate marker of the seizure core. It will therefore always be necessary to consider high gamma data in the context of the spatial and temporal evolution of low-frequency EEG, as well as the broader clinical picture.
With the context and methods we have provided, incorporation of high-frequency activity into intracranial EEG interpretation can help to focus resection or other targeted therapies32 onto the most critical regions. Furthermore, our study suggests that it is feasible for EEG-guided resections to achieve outcomes comparable to those achieved by standard temporal lobectomy for mesial temporal lobe syndromes, and by resection of well-defined structural lesions.26 Addressing the limitations of EEG-guided epilepsy surgery highlighted in this study may make the procedure more effective.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the treating epileptologists, neurosurgeons, and clinical fellows at Columbia University Medical Center and the National Hospital for Neurology and Neurosurgery in London.
GLOSSARY
- AUROC
area under the receiver operating characteristic curve
- CUMC
Columbia University Medical Center
- NHNN
National Hospital for Neurology and Neurosurgery
- PLHG
phase-locked high gamma
- ROC
receiver operating characteristic
- SOZ
seizure onset zone
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
S.A.W.: study concept and design, analysis and interpretation of data, statistical analysis, drafting and revision of the manuscript. A.L.: analysis and interpretation of the data, revision of the manuscript. R.C.: study design, analysis and interpretation of data, revision of the manuscript. G.P.B.: study design, analysis, and interpretation of data, revision of the manuscript. G.M.M.: analysis and interpretation of the data. R.R.G.: analysis and interpretation of the data. B.Z.: analysis and interpretation of data. C.G.F.: analysis and interpretation of data. R.G.E.: study concept and design, revision of the manuscript. A.J.T.: study concept. L.M.B.: interpretation of data, revision of the manuscript. M.N.: analysis and interpretation of data. R.R.: analysis and interpretation of data. B.D.: analysis and interpretation of the data, drafting and revision of the manuscript. A.W.M.: analysis and interpretation of the data. M.C.W.: analysis and interpretation of the data, drafting and revision of the manuscript. C.A.S.: study concept and design, analysis and interpretation of data, statistical analysis, revision of the manuscript, study supervision.
STUDY FUNDING
US funding sources included NIH/National Institute of Neurological Disorders and Stroke (R01-NS084142, C.A.S. and R25-10416928, S.A.W.) and the Epilepsy Foundation (R.C.). This study was partly undertaken at UCLH/UCL who received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. The authors thank Epilepsy Research UK for grant support.
DISCLOSURE
S. Weiss reports research support from NIH (R25 10416928); US patent application 14/507432, “Methods and systems to identify phase-locked high-frequency oscillations in the brain.” A. Lemesiou reports no disclosures relevant to the manuscript. R. Connors reports research support from Epilepsy Foundation (Research and Training Fellowships for Clinicians). G. Banks and G. McKhann report no disclosures relevant to the manuscript. R. Goodman is an advisory board member with compensation received at NeuroPace Inc. B. Zhao reports no disclosures relevant to the manuscript. C. Filippi is a consultant with Regeneron Pharmaceuticals and Syntactx, and has research support from the Coulter Foundation, Irving Foundation Pilot Grant, and NIH (R01 CA161404). M. Nowell reports research support from Wellcome Trust and UK Department of Heath (HICF-T4-275, programme grant 97914). R. Rodionov reports research support from Wellcome Trust and UK Department of Heath (HICF-T4-275, programme grant 97914). B. Diehl is an editorial board member for Seizure, European Journal of Epilepsy, and Nervenarzt, and reports research support from Epilepsy Research UK and NIH (NS090407/RES509426 and NS076965/RES508546). A. McEvoy reports honoraria from UCB, Essai, Baxter, and Fannin. M. Walker has research support from Epilepsy Research UK. A. Trevelyan has research support from MRC, Wellcome Trust, and NIH (R01 NS084142). L. Bateman has research support from NIH (U01 NS090407) and CURE (Henry Lapham Memorial Award). R. Emerson consults for Persyst Development Corp., and has research support from NIH (R01 NS084142). C. Schevon consults for Persyst Development Corp., and has research support from NIH (R01 NS084142). Catherine Schevon also shares the CURE Henry Lapham Memorial Award with Lisa Bateman. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Luders H, Rosenow F. Presurgical evaluation of epilepsy. Brain 2001;124:1683–1700. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto H, Marsan CA. Cortical cellular phenomena in experimental epilepsy: ictal manifestations. Exp Neurol 1964;9:305–326. [DOI] [PubMed] [Google Scholar]
- 3.Goldensohn ES, Purpura DP. Intracellular potentials of cortical neurons during focal epileptogenic discharges. Science 1963;139:840–842. [DOI] [PubMed] [Google Scholar]
- 4.Schevon CA, Weiss SA, McKhann GJ, et al. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun 2012;3:1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trevelyan A, Sussillo D, Watson B, Yuste R. Modular propagation of epileptiform activity: evidence for an inhibitory veto in neocortex. J Neurosci 2006;26:12447–12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trevelyan A, Sussillo D, Yuste R. Feedforward inhibition contributes to the control of epileptiform propagation speed. J Neurosci 2007;27:3383–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trevelyan AJ, Schevon CA. How inhibition influences seizure propagation. Neuropharmacology 2012;69:45–54. [DOI] [PubMed] [Google Scholar]
- 8.Weiss SA, Banks GP, McKhann GM, et al. Ictal high frequency oscillations distinguish two types of seizure territories in humans. Brain 2013;136:3796–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim GM, Wong SM, Anderson RA, et al. Dynamic modulation of epileptic high frequency oscillations by the phase of slower cortical rhythms. Exp Neurol 2014;251:30–38. [DOI] [PubMed] [Google Scholar]
- 10.Zijlmans M, Jiruska P, Zelmann R, Leijten FSS, Jefferys JGR, Gotman J. High-frequency oscillations as a new biomarker in epilepsy. Ann Neurol 2012;71:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bragin A, Engel J, Jr, Wilson C, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus 1999;9:137–142. [DOI] [PubMed] [Google Scholar]
- 12.Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. 2004;127:1496–1506. [DOI] [PubMed] [Google Scholar]
- 13.Modur PN, Zhang S, Vitaz TW. Ictal high-frequency oscillations in neocortical epilepsy: implications for seizure localization and surgical resection. Epilepsia 2011;52:1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alarcon G, Binnie C, Elwes R, Polkey C. Power spectrum and intracranial EEG patterns at seizure onset in partial epilepsy. Electroencephalogr Clin Neurophysiol 1995;94:326–337. [DOI] [PubMed] [Google Scholar]
- 15.Traub R, Whittington M, Buhl E, et al. A possible role for gap junctions in generation of very fast EEG oscillations preceding the onset of, and perhaps initiating, seizures. Epilepsia 2001;42:153–170. [DOI] [PubMed] [Google Scholar]
- 16.Fisher R, Webber W, Lesser R, Arroyo S, Uematsu S. High-frequency EEG activity at the start of seizures. J Clin Neurophysiol 1992;9:441–448. [DOI] [PubMed] [Google Scholar]
- 17.Jirsch J, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain 2006;129:1593. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs J, Zijlmans M, Zelmann R, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol 2010;67:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engel J., Jr Outcome with respect to epileptic seizures. In: Engel J, Jr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987:535–571. [Google Scholar]
- 20.Amari SI, Cichocki A, Yang HH. A new learning algorithm for blind signal separation. In: Touretzky DS, Mozer MC, Hasselmo ME, editors. Advances in Neural Information Processing Systems 8. Cambridge, MA: The MIT Press; 1996:757–763. [Google Scholar]
- 21.Esteller R, Echauz J, Tcheng T, Litt B, Pless B. Line length: an efficient feature for seizure onset detection. In: Proceedings of the 23rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2001;2:1707–1710.
- 22.Schindler K, Leung H, Elger C, Lehnertz K. Assessing seizure dynamics by analysing the correlation structure of multichannel intracranial EEG. Brain 2007;130:65. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Davis K, Azarion A, et al. Brain parcellation aids in electrode localization in epileptic patients. In: Linte CA, Moore J, Chen E, Holmes D, III, editors. Augmented Environments for Computer-Assisted Interventions. Berlin: Springer Berlin Heidelberg; 2012:130–137. [Google Scholar]
- 24.Azarion AA, Wu J, Davis KA, et al. An open-source automated platform for three-dimensional visualization of subdural electrodes using CT-MRI coregistration. Epilepsia 2014;55:2028–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schauerte B, Fink GA. Focusing computational visual attention in multi-modal human-robot interaction. In: ICMI-MLMI '10. New York: ACM Press; 2010:1. [Google Scholar]
- 26.Tellez-Zenteno JF, Dhar R, Hernandez-Ronquillo L, Wiebe S. Long-term outcomes in epilepsy surgery: antiepileptic drugs, mortality, cognitive and psychosocial aspects. Brain 2007;130:334–345. [DOI] [PubMed] [Google Scholar]
- 27.Cohen-Gadol AA, Wilhelmi BG, Collignon F, et al. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal lobe sclerosis. J Neurosurg 2006;104:513–524. [DOI] [PubMed] [Google Scholar]
- 28.Téllez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res 2010;89:310–318. [DOI] [PubMed] [Google Scholar]
- 29.Spencer SS, Berg AT, Vickrey BG, et al. Predicting long-term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology 2005;65:912–918. [DOI] [PubMed] [Google Scholar]
- 30.Haegelen C, Perucca P, Châtillon CE, et al. High-frequency oscillations, extent of surgical resection, and surgical outcome in drug-resistant focal epilepsy. Epilepsia 2013;54:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry MS, Dunoyer C, Dean P, et al. Predictors of seizure freedom after incomplete resection in children. Neurology 2010;75:1448–1453. [DOI] [PubMed] [Google Scholar]
- 32.Morrell MJ; RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011;77:1295–1304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.