Abstract
In mammalian cells the cytosolic concentration of free Zn2+ ions is extremely low (nM–fM range) and unlikely to provide an adequate pool for the uptake and accumulation of zinc in mitochondria. We previously identified a mitochondrial uptake transport process that effectively transports zinc directly from low molecular weight zinc ligands independent of and in the absence of available free Zn2+ ions. Since metallothionein (MT) is an important ligand form of cellular zinc, we determined if Zn7-MT was an effective chaperone and donor for delivery and uptake of zinc by prostate and liver mitochondria. The results reveal that both intact mitochondria and mitoplasts effectively accumulated zinc from Zn7-MT. The study confirms and extends our previous report that the putative zinc transporter is associated with the inner mitochondrial membrane and involves a direct exchange of zinc from the ligand to the transporter. The ventral prostate cells contain no detectable MT; so that ligands (such as citrate, aspartate) other than MT are zinc donors for mitochondrial zinc accumulation. However, in liver and perhaps other cells, Zn7-MT is probably important in the cytosolic trafficking of zinc to the mitochondria for the uptake of zinc into the mitochondrial matrix by the putative zinc uptake transporter.
Keywords: Zinc uptake transporter, Mitochondria, Zinc metallothionein, Prostate, Liver
1. Introduction
We previously reported the kinetic identification of a putative zinc uptake transporter in prostate and liver mitochondria [1]. The transporter is associated with the inner mitochondrial membrane, exhibits the characteristics of a facilitative transport process, does not require a free Zn2+ ion pool for transport; and effectively transports zinc from zinc ligands with log Kf values up to ~11, but not from ligands with log Kf values equal to or greater than 13. The transporter exhibits an apparent log Kf ~12. The Km ranges from about 30–80 μM zinc, depending upon the ligand and the source of mitochondria. Because the free Zn2+ ion concentration of cytosol is negligible (nanomolar to femtomolar range) [2], we postulated that low molecular weight zinc ligands (ZnLigands) comprise the major cytosolic pool of zinc for delivery to and transport into the mitochondria by this putative zinc uptake transporter.
It was essential to determine if zinc metallothionein might also be an important donor form for mitochondrial zinc uptake. Recently we obtained two different Zn7-MT sources with which we determined zinc transport into mitochondria. Rabbit liver Zn7-MT-2, prepared as described in Halthout et al. [3], was kindly provided by Dr. C. Fenselau at the University of Maryland, College Park, MD. Recombinant human Zn7-MT, described in Hong and Maret [4], was generously provided by Dr. Maret at the University of Texas Medical Branch, Galveston, Texas. Both preparations were devoid of free Zn2+ ions. A poly-clonal antibody against human and rat MT-1 and -2 proteins was generously provided by Dr. P.C. Huang at Johns Hopkins University, Baltimore, MD [5]. The methods for prostate and liver mitochondria preparation, the conditions for zinc accumulation, and all other assays were the same as previously described [1]. Although the experimental protocol was limited by the availability Zn7-MT, the results obtained were apparent, conclusive and consistent with our earlier report [1].
Zinc uptake from 30 μM purified rabbit liver Zn7-MT-2 (MT-2: 30 μM; total bound zinc: 210 μM) by intact mitochondria was compared to that from 30 μM ZnCysteine (zinc: 30 μM; Cysteine: 90 μM). The latter is included because we previously showed that it is as effective as free Zn2+ ions as a donor form for mitochondrial uptake and because its log Kf ~10 is close to Zn7-MT-2 (~11–12). Fig. 1(a) shows that mitochondrial zinc accumulation from rabbit liver Zn7-MT-2 was as effective as from ZnCysteine in both liver and ventral prostate mitochondrial preparations. The results with ZnCysteine also confirms our previous observation that the liver and prostate mitochondria have the capacity to accumulate similar levels of zinc. In order to determine if zinc accumulation was due to the mitochondrial accumulation of Zn7-MT-2, the levels of the mitochondrial MT were determined by Western blot. Fig. 1(b) shows that the presence of endogenous ~12 kDa MT in liver mitochondria, but no significant MT change was observed during incubation with rabbit liver Zn7-MT-2. In the ventral prostate mitochondria, no detectable MT was found in the presence or absence of 30 μM rabbit liver Zn7-MT-2. Therefore, zinc accumulation involves the transfer of zinc from the ligand, which confirms our previous observation [1] with other ZnLigands.
Fig. 1.
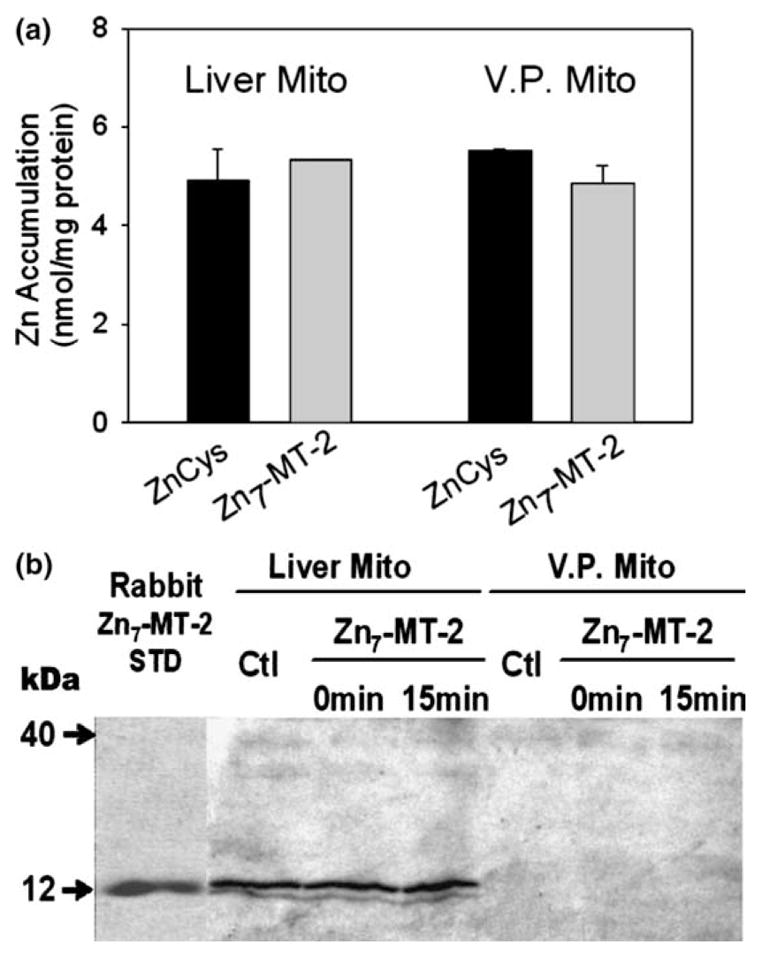
Liver and VP mitochondrial zinc accumulation from rabbit liver Zn7-MT-2: (a) comparison of zinc accumulation from ZnCys and Zn7-MT-2 in liver and ventral prostate mitochondria by atomic absorption assay. ZnCys contains 30 μM zinc and 90 μM ligand. Zn-MT contains 30 μM MT and 210 μM zinc. Zinc uptake was performed for 10 min incubation at room temperature. (VP Zn7-MT: n = 2; liver Zn7-MT: n = 1; ZnCys: n = 3); (b) western blot detection of MT-2 imported into isolated liver and ventral prostate mitochondria. Aliquots of 50 μg mitochondrial protein were used for western blot. Purified rabbit liver MT-2 (0.1 μg) served as a MT standard.
Zinc accumulation was also determined from recombinant Zn7-MT (Maret preparation) in liver mitoplast preparation. Fig. 2(a) shows that significant zinc accumulation resulted from Zn7-MT; but, unlike in Fig. 1(a), somewhat less than the accumulation from ZnCysteine. The absence of zinc accumulation from ZnEDTA shows that the inner membrane is in tact, and that ZnEDTA is not a zinc donor form due to its high binding affinity (log Kf ~16) as we previously reported [1]. The absence of MT accumulation in the mitoplasts (Fig. 2(b)) confirms that zinc is transferred from the ligand in the transport process, and confirms that the putative transporter is associated with the inner membrane. An apparent difference in the recombinant preparation is its detection as a ~40 kDa doublet for which no explanation can be provided at this time (personal communication with Dr. Maret). This might also account for the lower zinc accumulation with this preparation. Nevertheless, when combined with the results in Fig. 1, it is apparent that Zn7-MT-2 is an effective zinc donor for the putative zinc uptake transporter involved in the transport of zinc across the inner membrane and into the mitochondrial matrix. The results are also consistent with our earlier conclusion that the putative zinc uptake transporter has an apparent log Kf ~ 12.
Fig. 2.
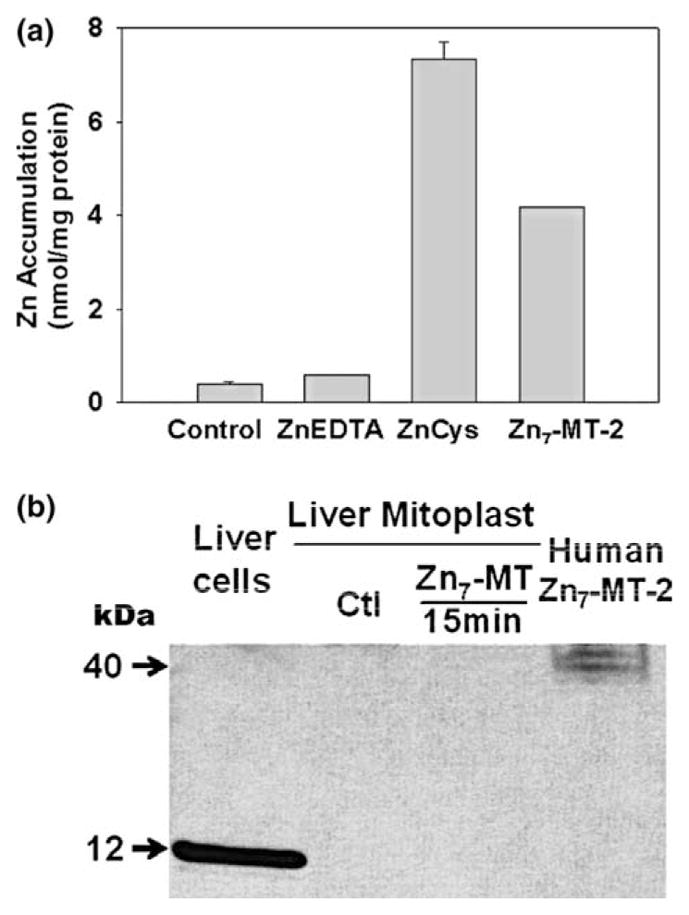
Zinc accumulation from recombinant human Zn7-MT-2 by liver mitoplast: (a) zinc accumulation from 30 μM ZnCys, Zn7-MT-2 and ZnEDTA by liver mitoplast by atomic absorption assay. Conditions were the same as Fig. 1(a). (Zn7-MT: n = 2; others: n = 3); (b) western blot detection of Zn7-MT-2 imported into liver mitoplast. Aliquots of 50 μg mitoplast protein were used for western blot. Fifty microgram liver cell protein extract and 0.1 μg recombinant human MT-2 served as MT standards.
Previous studies indicated that oxidants can induce zinc release from MT by oxidizing the sulfur ligands in zinc/thiolate cluster [6–9]. Ye et al. [10] reported that cytosolic Zn7-MT-2 could enter the intermembrane space where oxidative conditions and/or lower pH cause the release of zinc that inhibits terminal oxidation. This raises the possibility that released free Zn2+ ions from Zn7-MT-2 traversed the inner membrane into the mitochondrial matrix. However, our zinc uptake studies employed non-respiring oxidatively inactive in tact mitochondria and mitoplasts. Therefore the uptake of zinc from Zn7-MT-2 did not require a release of free Zn2+ ions for transport into the mitochondria, as we previously reported [1] for other ligands. This is consistent with the observation that zinc is directly transferred from Zn7-MT-2 to acceptor protein without the release of free Zn2+ ion [3]. Although a total of seven zinc binds to one MT molecule, previous studies demonstrated that Zn7-MT does not transfer all of its zinc to other proteins, but one zinc in the Zn3-β cluster is more prone to transfer than the others[3,8,11]. This might account for the observation (Fig. 1) that the accumulation of zinc from Zn7-MT-2 that contains 210 μM zinc/30 μM MT-2 was similar to zinc accumulation from 30 μM ZnCysteine.
In the rat ventral prostate cells, the high levels of citrate and aspartate are the apparent important ZnLigands that serve as cytosolic zinc-donors for the characteristic high mitochondrial uptake and accumulation of zinc [1] since metallothioneins are not detectable in these cells [unpublished information; 11–14]. In liver, and likely other mammalian cells, Zn7-MT-2 could be an important chaperone for delivery to and transport of zinc by the mitochondria. Most importantly, the mounting evidence supports the concept that, in the absence of a sufficient cytosolic free Zn2+ pool, loosely bound ZnLigands (including Zn7-MT-2) are effective donors of zinc for the mitochondrial transport of zinc.
Acknowledgments
We thank Dr. P.C. Huang for kindly providing the metallothionein antibody, and Dr. Wolgang Maret and Dr. Catherine Fenselau for generously providing the metallothionein for this study and for their helpful comments and discussions. These studies were supported by NIH grants CA79903 and CA71207 and DOD grant PC001174.
References
- 1.Guan Z, Kukoyi B, Feng P, Kennedy MC, Franklin RB, Costello LC. J Inorg Biochem. 2003;97:199–206. doi: 10.1016/s0162-0134(03)00291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Outten CE, O’Halloran TV. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 3.Hathout Y, Fabrisa D, Fenselau C. Internat J Mass Spectrom. 2001;204:1–6. [Google Scholar]
- 4.Hong SH, Maret W. Proc Natl Acad Sci USA. 2003;100:2255–2260. doi: 10.1073/pnas.0438005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danielson KG, Ohi S, Huang PC. Proc Natl Acad Sci USA. 1982;79:2301–2304. doi: 10.1073/pnas.79.7.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maret W. Proc Natl Acad Sci USA. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacob C, Maret W, Vallee BL. Proc Natl Acad Sci USA. 1998;95:3489–3494. doi: 10.1073/pnas.95.7.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce LL, Gandley RE, Han W, Wasserloos K, Stitt M, Kanai AJ, McLaughlin MK, Pitt BR, Levitan ES. Proc Natl Acad Sci USA. 2000;97:477–482. doi: 10.1073/pnas.97.1.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turan B, Fliss H, Desilets M. Am J Physiol. 1997;272:H2095–H2106. doi: 10.1152/ajpheart.1997.272.5.H2095. [DOI] [PubMed] [Google Scholar]
- 10.Ye B, Maret W, Vallee BL. Proc Natl Acad Sci USA. 2001;98:2317–2322. doi: 10.1073/pnas.041619198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bataineh ZM, Heidger PM, Jr, Thompson SA, Timms BG. Prostate. 1986;9:397–410. doi: 10.1002/pros.2990090409. [DOI] [PubMed] [Google Scholar]
- 12.Coogan TP, Shiraishi N, Waalkes MP. Environ Health Perspect. 1994;102(Suppl 3):137–139. doi: 10.1289/ehp.94102s3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghatak SP, Oliveria P, Kaplan P, Ho SM. Prostate. 1996;29:91–100. doi: 10.1002/(SICI)1097-0045(199608)29:2<91::AID-PROS4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Waalkes MP, Perantoni A. Toxicol Appl Pharmacol. 1989;101:83–94. doi: 10.1016/0041-008x(89)90214-7. [DOI] [PubMed] [Google Scholar]


