Introduction
NeuroAIDS is a disease that incorporates both infectious and degenerative pathophysiologic pathways. It has a known cause, has several animal models, and is under investigation and treatment using multiple avenues, in vivo and in vitro [1–7]. Select highlights from the spectrum of NeuroAIDS research predominantly related to human immunodeficiency virus-1 clade B (HIV-1B) are reviewed below.
Clinical
Diagnosis, neurology, and neuroimaging
HIV-1 invades the central nervous system (CNS) early and can cause persistent infection and inflammation. Although early infection is typically asymptomatic, cerebrospinal fluid (CSF) analysis and magnetic resonance spectroscopy (MRS) imaging can detect CNS abnormalities during this period [8–11].
Chronic HIV-1 infection can result in neurodegenerative disease, overall termed NeuroAIDS. The clinical expression of this process includes neurocognitive impairments such as decreased attention/concentration, psychomotor speed, memory, learning, information processing, and executive function. There is also motor slowing, incoordination, and tremor, that may progress to disabling weakness, spasticity, extrapyramidal movement disorders, and paraparesis [12,13]. In addition, there may be behavioral effects such as apathy and irritability. Psychomotor retardation (associated with damage to the frontal-striatal systems) also may occur [14,15]. The clinical severity of this process ranges from asymptomatic neurocognitive impairment (ANI), to a mild neuro-cognitive disorder (MND), to full-blown HIV-associated dementia (HAD) [16]. The clinical nosology applied to this process has evolved, from the original AIDS dementia complex (ADC) [13], to HIV-associated cognitive-motor complex (HIVCMC) [17], and most recently to HIV-associated neurocognitive disorders (HAND) [16].
HIV-associated neurocognitive disorders were first identified in individuals with advanced AIDS, who had for example opportunistic infections, high viral loads, and elevated markers of immune activation. The development of HAND is influenced by host and viral characteristics, comorbid factors, substance abuse, and antiretroviral therapy. Although older literature focuses on HAD, milder forms of HAND (MND, ANI) were recently reported to be common in highly active antiretroviral therapy (HAART)-treated individuals with partial immune reconstitution, higher CD4+ cell counts, and suppressed viral loads. This is consistent with the decline in the incidence per 1000 person-years of HAD from 6.49 in 1997 to 0.66 2006 and associated with the introduction of HAART in 1996 [18]. More recently, HAND showed decreased association with immune activation and had a more diffuse range of neuropsychological deficits that may overlap with other brain diseases [18–21].
In-vivo studies of HAND have utilized neuroimaging techniques including MRS, functional MRI (fMRI), and morphometry. MRS measures brain biochemistry, fMRI measures changes in blood flow related to neural activity in the brain, and morphometry models quantitative changes in neuroanatomical structures. These techniques also have been used to detect changes in the brains of asymptomatic HIV-1-positive patients [10,14,22].
Aging and neuropathology
In the US, the percentage of HIV-1-positive individuals, 50 years of age and older, increased from 19% to more than 25% between 2001 and 2005, primarily due to HAART-related increased survival. Seniors often present with advanced HIV-1 disease because an AIDS diagnosis was overlooked [18]. Aging adversely affects both cell-mediated and humoral immunity. Older HIV-1-positive persons tend to progress to AIDS and to die more rapidly, and increased age is a strong risk factor for HAND. Comorbid conditions, the cognitive changes that occur in ‘normal’ aging, and the increased risk for neurode-generative diseases all present major difficulties in the assessment of neurocognitive impairment in older HIV-1-positive adults [23,24]. The diagnostic and neuro-pathological characteristics of some of these conditions and those of HAND are summarized in Table 1 [25–44].
Table 6.
Animal models.
| Animal model system | Advantage | Disadvantage | References |
|---|---|---|---|
| Mice Transgenic for gp120, g160, or Tat | Can determine specific effects of individual proteins | Cannot study effects of virus replication | [200–202] |
| SCID mice injected intracerebrally with HIV-1-infected macrophages | Utilizes replication-competent virus infection | Direct injection into CNS required in absence of systemic infection | [203] |
| FIV infection of cats | Utilizes replication competent virus/host system, can be used for behavioral testing | Cellular tropism of FIV differs from that of HIV-1 so effects may be different | [204–206] |
| SIV-infected microglia in macaques | Virus infection in natural virus/host system, viral tropism skewed towards CNS | Complicated and tedious system to produce infected microglia | [208] |
| Combination of two SIV isolates for infection of macaques | Rapid, consistent development of AIDS and neurological dysfunction | Combination of viruses makes it difficult to ascertain the effects of specific viral genetics on neuropathogenesis | [209] |
| Single SIV isolate infection of macaques | High incidence (>90%) of neuropathogenesis | SIV, although an excellent model, still differs from HIV-1 | [210] |
| SIV infection of macaques coupled with CD8 cell depletion | Rapid development of AIDS and neuropathogenic disease | Eliminates a key component of the immune response, which may have effects on other immune mediators such that pathogenesis may be altered as compared with natural infection | [211] |
CNS, central nervous system; FIV, feline immunodeficiency virus; SCID, severe combined immune deficiency; SIV, simian immunodeficiency virus.
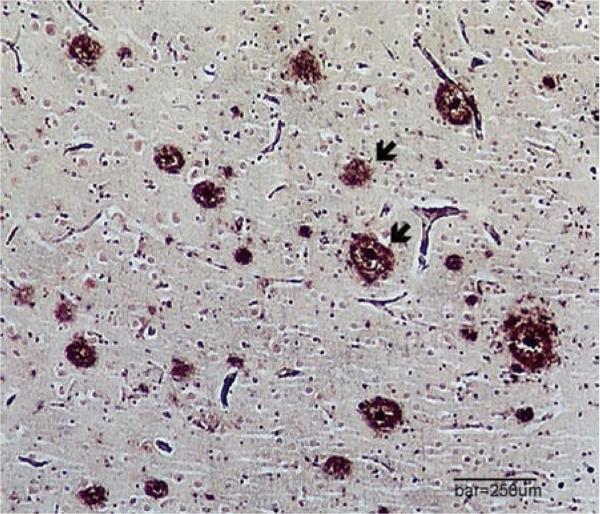
Immune dysfunction, inflammation, and hyperlipidemia are features of HIV-1 infection (and its treatment) and are risk factors for Alzheimer's disease in the elderly. Likewise, increased amyloid has been reported in HIV-1-positive brain tissue (Fig. 1), possibly because HIV-1 Tat protein inhibits amyloid degradation. Moreover, Tat production, HAART, and aging may also result in increased phosphorylation of tau protein in the hippo-campus. Expression of alpha-synuclein is increased in the substantia nigra of some HIV-positive brains. In the setting of HIV-1, these findings of the accumulation of abnormal proteins may point to common pathways activated in multiple brain diseases, possibly by brain inflammation [45–50].
Fig. 1. Amyloid-containing plaques (arrows) in the frontal cortex of a demented 69-year-old HIV-1-positive woman.
Clinically, HIV-associated neurocognitive disorder was only one factor contributing to her dementia. At death, her brain contained numerous amyloid plaques and tau-neurofibrillary tangles (not shown), typical of AD (modified Bielschowsky silver stain, bar equals 250 μm). AD, Alzheimer's disease.
Drug abuse
Drug abusers with HIV-1 infection have higher viral loads, increased immunosuppression, more severe cognitive impairment, and neuropathological changes. However, causality is difficult to ascertain because drug abusers (commonly poly-drug abusers) are often non-compliant with medications and ambiguous in self-report [51,52].
In-vitro studies show that opiates, cocaine, and meth-amphetamine potentiate HIV-1 replication and synergize with HIV-1 proteins to cause glial cell activation, neurotoxicity, and breakdown of the blood–brain barrier [53]. The opioid system has dichotomous effects on HIV-1 replication. Mu receptor stimulation increases HIV-1 infection via increased expression of chemokine receptors (CCR3, CCR5, and CXCR4), that are also co-receptors for HIV-1 [54], whereas kappa receptor activation decreases CCR5 expression [55]. Opiates activate HIV-1 replication in latently infected macrophages and cocaine increases HIV-1 replication in macrophages via production of IL-10 [56,57]. In addition, cocaine increases HIV-1 replication in astrocytes and in dendritic cells (by up-regulation of DC-SIGN) [58,59] and methamphetamine increases HIV-1 infection via dopa-mine receptor signaling leading to up-regulation of CCR5 [60]. Moreover, cocaine and methamphetamine may both accelerate the development of HAND through increased dopamine, macrophage infiltration, and HIV-1 replication [61].
Cocaine and methamphetamine enhance monocyte migration across the blood–brain barrier involving disruption of endothelial cell tight junctions. Cocaine increases adhesion molecule expression [53], whereas methamphetamine up-regulates inflammatory genes in endothelial cells [62]. Morphine alone does not alter the blood–brain barrier; however, in combination with HIV-1-Tat protein, it alters tight junction expression [63]. Morphine withdrawal and associated stress also damages the blood–brain barrier [64]. Morphine and cocaine separately are not toxic to striatal neurons, but they each substantially potentiate Tat neurotoxicity [65]. For example, in a Tat transgenic mouse model, morphine caused shortening of dendrites [66]. Cocaine potentiates Tat-mediated glial cell activation and oxidative stress [65,67]. In post-mortem human brains, selective degeneration of pyramidal neurons and interneurons in the neocortex and limbic system have been associated with cognitive alterations in methamphetamine and cocaine users [68]. Loss of calbindin and parvalbumin interneurons correlates with memory deficits in HIV-1-infected methamphetamine users [69].
Treatment
Goals for treatment of NeuroAIDS include suppressing HIV through optimizing HAARTand treating associated psychiatric, neurological, and neuropsychological dys-functions including mood disorders, substance abuse, and painful peripheral neuropathies. Whereas it is commonly accepted that antiretroviral therapy ameliorates HAND, there is debate about the best regimen for HAND. Moreover, whether brain and CSF penetration play a role in treatment efficacy is under investigation as well. One study reported that neuropsychological test scores improved after a HAART regimen with high penetration and another study indicated no improvement, irrespective of blood–brain barrier penetration [70,71]. Additional studies suggested antiretroviral drugs with better CSF penetration improved outcomes [72–76]. Such studies include brain assessment by examination of CSF when CNS proteins and metabolites can be found, as well as by direct imaging techniques including MRI [72,75].
Palliative and adjunctive therapy of HAND [palliative therapy ameliorates symptoms without affecting the disease and adjunctive therapy does not treat the known cause of HIV-1 by reducing viral replication, but treats the downstream effects (e.g. neurodegeneration)] may improve the patient's ability to function. For example, since depression may be an important confounder in the diagnosis of HAND and reduces quality of life, HIV-1-positive individuals should be screened for mood disorders, and these can be treated with antidepressants [3]. In addition, methylphenidate and other psychostimulants, often used for attention deficit disorders, may help to reduce apathy and improve psychomotor slowing in HAND. Several drugs were investigated as potential neuroprotective agents. In a phase II randomized controlled trial within the Adult AIDS Clinical Trials Group (ACTG), adults with mild to severe ADC, receiving stable antiretroviral therapy and memantine treatment, showed improvement of MRS parameters in the brain; however, there was no significant clinical improvement [77]. Likewise, a trial of selegiline, a monoamine oxidase (MAO)-B inhibitor with antioxidant and neurotrophic properties, passed safety assessment in phase II studies in cognitively impaired HIV-1-positive patients, but failed to demonstrate cognitive or functional improvement [78]. An anticonvulsant (and mood-stabilizing) drug, sodium valproate (VPA) inhibits the activity of the glycogen synthase kinase-3-beta (GSK-3β) pathway [79]. Preliminary clinical studies using VPA suggested this drug might treat symptoms related to HAND by reversing HIV-1-induced damage to gray matter. Patients treated with VPA showed improved neuropsychological function compared to placebo; however, there were concerns related to toxicity, drug interactions, and lack of long-term benefit to reduce the size of the latent HIV-1 reservoir [80–82].
Molecular
Brain virus load
Comprehensive quantification of HIV-1 load in brain parenchyma can utilize at least three different types of measurements: proviral DNA, unspliced viral RNA, and multispliced viral RNA (HIV-1-multispliced RNA indicates viral replication; unspliced HIV-1 RNA indicates persistence with incomplete or dysfunctional replication). In brain virus load studies, several brain regions generally are sampled with the underlying hypothesis that increased brain HIV-1 viral load should be associated with premortem neurocognitive impairment such as HAND or HAD. However, this is not always the case and may be associated with several factors including variations in previous duration of infection, sampling across brain regions, irreparable neuronal damage, persistence of inflammation, as well as variations in HAART [83–88]. However, HAART probably reduces RNA viral load more effectively than proviral DNA load. Indeed, in some studies the circulating peripheral HIV proviral DNA load in macrophage/ monocytes (CD14/CD16) was associated with HAD and minor cognitive motor disorder, independent of HIV RNA levels; the majority of these patients were treated with HAART [89–91]. Additional factors that may require attention include HAART resistance mutations, the particular mutational profile of the peripheral and CSF viruses that may influence HIV-1 neurovirulence, epigenetic mechanisms, and micro-(mi-) RNA expression. Factors including elevated plasma lipo-polysaccharide (LPS) as well as co-infections including HCV may be important in groups such as injection drug and alcohol abusers [91–96].
Brain virus evolution
In NeuroAIDS, HIV-1 evolution (HIV-1 evolution refers to the increased and wide phylogenetic and sequence diversity that occurs over time and place. The different groups of sequences are referred to as swarms or quasispecies. The term, HIV-1 evolution, is also used to typify their wide variations of sequence diversity and phylogenetically clustered groups in the human brain in NeuroAIDS [97–99]) is hypothesized to modulate HIV-1 neuroinvasiveness and subsequent pathogenesis, for example strains with increased neurovirulence may have specific changes in the Nef protein associated with neurodegeneration in vitro [100]. In corollary, HIV-1 evolution may occur separately in compartmentalized brain regions, CSF, blood, and spleen and this paradigm has stood the test of 12 years of research. Compartmentalization may also occur also at the cellular level in astrocytes, macrophages, and multinucleated giant cells. In addition, a feline model, using feline immunodeficiency virus (FIV) produced supporting evidence of this precept [97,98,101–107]. Overall, this paradigm has been demonstrated for several HIV-1 genes including Env, Pol, Gag, and Nef [108]. However, one phylogenetic study did not conform to the compartmentalization paradigm [109]. In such studies, the conclusions depend heavily on the reliability of the sequences analyzed. Moreover, maintaining criteria for sequence database veracity continues to be important but should be systematically applied [110–113].
Genetics of susceptibility and progression
Genes associated with immune and neurobiological functioning may play a role in NeuroAIDS progression and susceptibility to HAND. Candidate gene studies have identified a number of polymorphisms that modify the function or expression of immunologic factors and neurotransmitters thought to be involved in HIV-1 infection and NeuroAIDS progression (Table 2) [114–128].
Table 2.
Genetics of susceptibility.
| Gene | Explanation | References |
|---|---|---|
| Chemokine (C-C motif) receptor-5 (CCR5) | A deletion in the CCR5 HIV-1 co-receptor gene, resulting in the CCR5-Δ-32 allele, confers resistance to infection and slower disease progression. Some studies found an association of this polymorphism with lower risk for HAND, whereas other studies did not. | [114–118] |
| CCR2 | A SNP in the CCR2 minor HIV-1 co-receptor gene, resulting in the CCR2-V64I allele, is associated with slower cognitive decline. | [119] |
| Apolipoprotein E (ApoE) | Some studies found a positive relationship between the ε4 allele and HAND, whereas other studies did not find an association between ε4 and HAND or encephalitis. Some studies suggested an age-modified association of older individuals who possess the ε4 allele with greater risk for HAND. | [24,117,120–124] |
| Macrophage inflammatory protein-1 alpha (MIP-1α, CCL3) | In one cross-sectional study, the minor allele resulting from SNP rs113071 was associated with a two-fold increased risk of HAND. | [125] |
| Stromal cell-derived factor-1 (SDF-1) | One study found that among a sample of primarily African American children, homozygosity for the SDF1-3′-A allele was associated with faster progression and decline in neurocognitive ability. A more recent study found no association between this allele and neurocognitive deficits in a sample of Chinese adults. | [116,117] |
| Monocyte chemotactic protein-1 (MCP-1, CCL2) | One study reported homozygosity for the MCP-1-2578G allele to be associated with 50% reduction in risk for HIV-1 infection. However, once infected, the same genotype was associated with accelerated disease progression and a 4.5-fold increased risk of HAD. Another SNP resulting in the MCP-1–2518G allele was found to be marginally associated with CNS impairment in HIV-1-positive children, but not associated with cognitive deficits in adults. | [117,126,127] |
| Tumor necrosis factor-alpha (TNF-α) | Two studies found an association between the TNF-α 308A allele and HAD. However, no association was found between this SNP and postmortem signs of HIV-encephalitis in a separate study. | [120,121,128] |
| Mannose-binding lectin (protein C)-2 (MBL2) | Homozygosity for an MBL2 allele (termed O/O), resulting in essentially nonfunctional MBL2, was associated with faster cognitive decline over 12 months in an HIV-1-positive Chinese cohort. | [117] |
CNS, central nervous system; HAND, HIV-associated neurocognitive disorder; SNP, single-nucleotide polymorphism.
A deletion within the CCR5 gene, for example, confers resistance to HIV-1 infection and an early study found that a disproportionately small number of heterozygous carriers of this allele were detected among a sample of HAD patients [114,115]. Since those early studies, this and other polymorphisms within genes associated with immune functioning have been associated with risk for and progression to HAND [e.g. chemokine (C-C motif) receptor-2, chemokine (C-C motif) ligand-3, stromal cell-derived factor-1, monocyte chemo-attractant protein-1, and tumor necrosis factor-1 alpha (TNF-1α)] [116,117,119–121,125,129–132], although few findings have been replicated. The ε4 allele of apolipoprotein E (ApoE), long implicated in the pathogenesis of Alzheimer's disease, has been examined as a risk factor for HAND, also with equivocal findings thus far [24,117,123,124,126,133]. Notably, in addition to a greater incidence of HAND among ApoE-4 carriers, Spector et al. [117] found the mannose-binding lectin (protein C)-2 O/O genotype to be associated with increased risk for progressive cognitive decline over 12 months among a Chinese cohort, whereas none of the aforementioned immunologic factors were associated with incidence of HAND or cognitive decline.
Dopaminergic pathway genes including dopamine transporter and catechol-O-methyltransferase (COMT) are also of interest in the neuropathogenesis of HAND, as dopamine pathway dysfunction appears to underlie many neurobehavioral deficits seen in HIV-1-infected indi viduals [24,134,135]. Recent studies have begun to examine the role of COMT polymorphisms in HAND, although much work remains to determine if genotype and HIV confer an additive or synergistic effect on neurocognitive functioning. The recent application of genome-wide association studies may reveal previously unknown factors involved in HIV-1-related neuro-pathogenesis. This method, which requires very large cohorts, previously has been successful in revealing novel genes associated with viral set point and HIV-1 progression [118].
Host gene expression
HIV-1 infection activates macrophage/microglia and astrocytes, which produces several neurotoxins including pro-inflammatory cytokines including TNF-α, IL-1β, and interferon (IFN)-γ. These inflammatory mediators promote inflammatory signaling cascades in NeuroAIDS with a cumulative result of neuronal toxicity [49,136–138]. TNF-α levels increase in the CNS of patients with HAND and in-vitro exposure of macrophages and microglia to gp120 or Tat induces TNF-1α expression [139]. TNF-1α up-regulates the expression of inter-cellular adhesion molecule-1 (ICAM-1), vascular endothelium adhesion molecule-1 (VCAM-1), and surface E-selectin on astrocytes and cerebral endothelial cells, with consequent transendothelial migration of activated macrophages from the periphery into the CNS. TNF-1a up-regulates monocyte chemoattractant-1 and fractalkine expression [140,141], and neuronal expression of genes for calbindin, macrophage and astrocyte expression of macrophage inflammatory protein (MIP)-α, regulated on activation normal T cell expressed and secreted (RANTES), and MIP-1β. TNF-α also increases the release of glutamate by astrocytes and microglia [142], and decreases the uptake of neuro-toxic glutamate by macrophage/microglia [143]. These effects are mediated by over-stimulation of NMDA receptors, which results in excessive calcium influx and generation of nitric oxide and superoxide anions, which are neurotoxic [144]. In human neuron cultures, exposure to gp120 and Tat causes increased expression of sphingomyelin and ceramide that is likely mediated by TNF-α and IL-1β. In human astrocyte cultures, activation of indoleamine-2,3-dioxygenase (IDO) results in elevation of tryptophan catabolism and the production of neurotoxins including kynurenine due to Tat protein for HIV-1B and not C. The authors conclude from the latter experiment that the prevalence of HAD may correlate with epidemiological differences in HIV-1B vs. C [139,145–148]. Finally, microarray technology has been applied to NeuroAIDS to measure simultaneous expression of large numbers of genes including recently discovered micro-(mi)-RNAs, as reviewed in detail elsewhere (Table 3) [49,50,96,136,138,140,141,145–154].
Table 3.
Gene expression in NeuroAIDS.
| Cells | Comments | References |
|---|---|---|
| Macrophage/microglia | HIV-1 infection of these cells causes up-regulation of the expression of: TNF-α, IFN-γ, IL-1β, MCP-1, fractalkine, MIP-1a, and MIP-1b. | [49,136,138,140,141] |
| GLUT5 and HCgp39 | [155] | |
| Cerebral endothelial cells | ICAM-1, VCAM-1, and surface E-selectin | [136,138] |
| vWf | [155] | |
| Astrocytes | ICAM-1, VCAM-1 | [136] |
| Up-regulation of interferon antiviral responses, intercellular contacts, cell adhesion and signaling | [136] | |
| Neurons | Sphingomyelin and ceramide | [145–148] |
| Mixed glial cultures | IL-1β, TIMP-1, Caspase 9, S100A8 and 9, MMP12, IL-8, MCP1, MRC-1, and IL-6 | [151] |
| Macrophage/monocytes | CCL2(MCP1), CCL7, CXCL5, TNFSF14, kinases, and phosphatases | [153] |
A few select cytokines, chemokines, and other molecules involved in pathogenesis of NeuroAIDS are tabulated. The expression of these genes is up-regulated compared to normal tissue. In addition, many of these molecules are down-regulated; a complete list is extensive and beyond our scope here. Tumor necrosis factor-alpha (TNF-α); interferon-gamma (IFN-γ); interleukin-1β (IL-1β); monocyte chemoattractant-1 (MCP-1); macrophage inflammatory protein (MIP)-1a and MIP-1b; human cartilage glycoprotein 39 (HCgp39); intercellular adhesion molecule-1 (ICAM-1); vascular endothelium adhesion molecule-1 (VCAM-1); von Willebrand factor (vWf); human S100A8 (S100A8), matrix metalloproteinase-12 (MMP-12), interleukin-8 (IL-8); monocyte chemotactic protein 1 (MCP-1); mannose receptor, C type 1 (MRC-1); interleukin 6 (IL-6); chemokine C-C motif ligand 2 (CCL2); chemokine C-C motif ligand 7 (CCL7); matrix metalloproteinase-12 (MMP-12); chemokine C-X-C motif 5 (CXCL5), tumor necrosis factor ligand superfamily member 14 (TNFSF14).
Promising modes of therapeutic gene delivery
Few drugs treat NeuroAIDS effectively in part because of incomplete CNS penetration. However, noninvasive intra-nasal delivery of therapeutic drugs to the CNS can bypass the blood–brain barrier via the olfactory nerve [155]. Novel CNS delivery systems can blunt induction of apoptosis by using recombinant SV40-derived vectors to deliver antioxidant enzymes [e.g. Cu/Zn-superoxide dismutase (SOD1) and glutathione peroxidase (GPx1)]. This protects cells from free radical-mediated gp120-induced apoptosis by detoxifying oxygen free radicals, and leads to a 10-fold reduction of neuronal apoptosis. Further testing in animal models and in clinical trials for treatment of HAND is now needed [156,157]. However, these protocols carry safety concerns because of the direct connection of olfactory nerves into the brain.
Transgene expression, another brain-targeted treatment, is improved with the systemic injection of the polyol sugar, mannitol. Mannitol, though not as well tolerated as originally thought, opens the blood–brain barrier transiently, with a concomitant increase in the number of brain cells exposed to experimental treatments. Thus, mannitol can be used for delivery of vectors (to produce trangenes) and the amyloid-beta homologue (Gd-DTPA-K6Abeta1–30) to image plaques [158–160].
Adenoviruses, lentiviruses, onco-retroviruses, and herpes simplex virus serve as transgene vectors, but often are associated with inflammation that reduces the effectiveness of the gene transfer. Adeno-associated virus (AAV) causes a mild immune response while it incorporates its genome in dividing and nondividing cells, and AAV-2 strains carry extensive gene loads with enhanced infectivity of neurons via axonal entry and retrograde transport [161]. AAV yields successful gene transfer in animal models of Alzheimer's disease, PD, and other CNS disorders [162], and in phase I trials for PD. However, the use of AAV is not without caveats as AAV-mediated gene transfer can induce tumors in animal models [163].
Lentiviruses have a long incubation period and delayed immunogenicity. They deliver a significant quantity of genetic material into the host cell's DNA, and can infect neighboring cells, without producing extracellular particles. The efficiency of their CNS uptake was enhanced with mannitol and the vasodilator, bradykinin. However, lentiviruses have distinct immune and biologic properties that need further investigation (Tables 4 and 5 and Fig. 2) [53,137,155–189].
Table 4.
Therapy that penetrates the blood–brain barrier.
| Therapeutic Intervention | Brief description | References |
|---|---|---|
| Viral modes of administration | Noninvasive intra-nasal delivery via the olfactory nerve | [155] |
| Recombinant SV40-derived vectors | Of antioxidant enzymes to blunt induction of apoptosis (e.g. Cu/Zn-superoxide dismutase (SOD1), glutathione peroxidase (GPx1) | [156,157] |
| Systemic mannitol-aided transgene expression | Open the blood–brain barrier transiently | [158–160] |
| Virus-mediated modes of administration | Virus-induced mild immune and inflammatory response that fosters incorporation of the viral genome in dividing and nondividing cells | [161–164] |
| Adenovirus AAV-2 strains | Via axonal entry and retrograde transport | [161,162] |
| Lentiviruses | With or without enhancement by mannitol and bradykinin | [164] |
Table 5.
Drugs that penetrate the BBB.
| Drug class | Drug | CPE 2010 rank | Transporter | References |
|---|---|---|---|---|
| Nucleoside/nucleotide reverse transcriptase inhibitors | Zidovudine | 4 | P-gp, BCRP, ENT, CNT, OAT | [167–170] |
| Abacavir | 3 | P-gp | [167] | |
| Emtricitabine | 3 | MRP | [165,171] | |
| Didanosine | 2 | ENT, CNT | [172,173] | |
| Lamivudine | 2 | OCT, MRP | [170,174] | |
| Stavudine | 2 | CNT | [167,170] | |
| Tenofovir | 1 | MRP | [165,171] | |
| Zalcitabine | 1 | OAT, ENT, CNT | [170,173,174] | |
| Nonnucleoside reverse transcriptase inhibitors | Nevirapine | 4 | P-gp, MRP | [165,171] |
| Delavirdine | 3 | P-gp, MRP | [171] | |
| Efavirenz | 3 | P-gp, MRP | [171] | |
| Etravirine | 2 | Unknown | - | |
| Protease inhibitors | Indinavir/ritonavir | 4 | P-gp | [167,175] |
| Darunavir/ritonavir | 3 | MRP | [176,177] | |
| Fosamprenavir/ritonavir | 3 | P-gp | [178] | |
| Indinavir | 3 | P-gp | [175] | |
| Lopinavir/ritonavir | 3 | MRP | [179] | |
| Atazanavir | 2 | P-gp | [180] | |
| Atazanavir/ritonavir | 2 | P-gp | [180] | |
| Fosamprenavir | 2 | P-gp | [178] | |
| Nelfinavir | 1 | P-gp, BCRP | [175,181] | |
| Ritonavir | 1 | P-gp, BCRP | [181,182] | |
| Saquinavir | 1 | P-gp, MRP, BCRP | [175,179,181] | |
| Saquinavir/ritonavir | 1 | P-gp, MRP, BCRP | [175,179,181] | |
| Tipranavir/rritonavir | 1 | P-gp | [183] | |
| CCR-5 inhibitor | Maraviroc | 3 | P-gp | [184] |
| Fusion inhibitor | Enfuvirtide | 1 | Unknown | [184] |
| Integrase inhibitor | Raltegravir | 3 | Unknown | [185] |
This table shows the CNS-Penetration effectiveness (CPE) ranking of antiretrovirals. A higher number estimates better penetration in the CNS. Thus, 4 indicates highest penetration and 1 indicates lowest penetration. The initial CPE rank was based on physiochemical characteristics, CSF concentrations, and efficacy data [165]. However, recent cross-sectional data that included CSF vs. plasma viral load studies from the CHARTER Cohort led to a revised CPE ranking system. The new system reflects stronger associations with CSF viral load analysis by incorporating recent pharmacokinetic and pharmacodynamic data [166]. Drug transporters involved in each antiretroviral distribution at the BBB are mentioned. The transporters are ATP-binding cassette (ABC) superfamily including P-Glycoprotein (P-gp), Multidrug resistance associated protein (MRP) and breast cancer resistance protein (BCRP). Solute Carrier superfamily (SLC) includes organic anion-transporting polypeptide (OATP), organic anion transporter (OAT), and organic cation transporter (OCT). Nucleoside transporters such as equilibrative nucleoside transporter (ENT), concentrative nucleoside transporter (CNT). Unknown, transporter is not characterized. –, no information available. Roche discontinued manufacturing Hivid (ddC) due to availability of newer antiretrovirals (http://www.nelm.nhs.uk/en/NeLM-Area/Other-Lib-Updates/SPC-Changes/501482/, last accessed 7/6/2010).
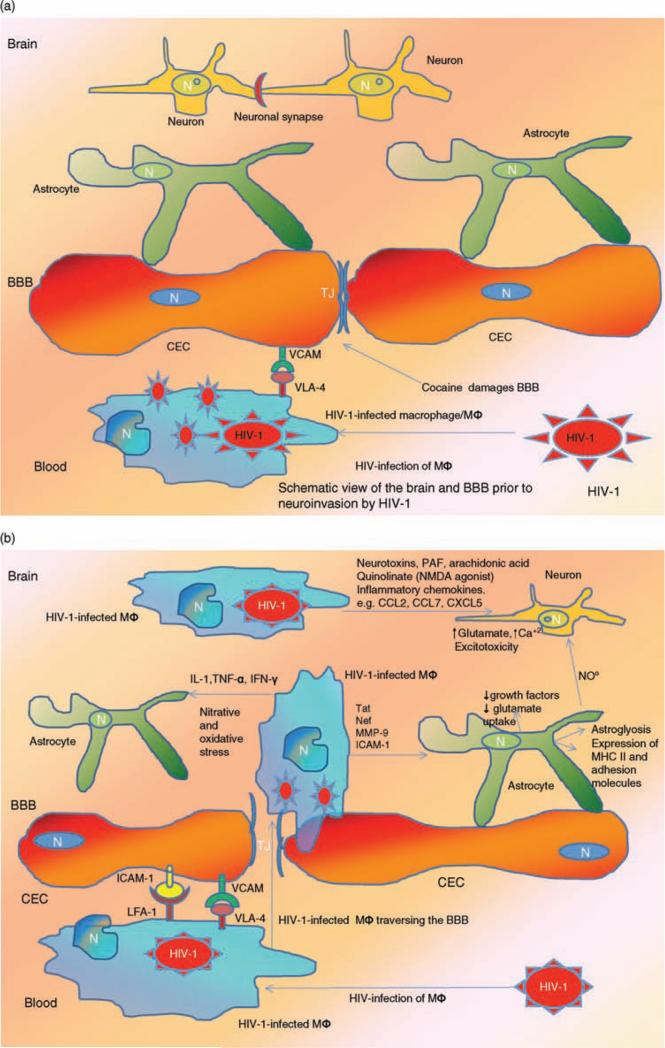
Fig. 2. Blood–brain barrier in NeuroAIDS.
(a) Blood–brain barrier (BBB) prior to infection. Normally, the human BBB, which is composed of interacting adjacent cerebral endothelial cells (CECs) and astrocytes, effectively separates the CNS from cellular and molecular components of the peripheral circulation and creates a unique reservoir environment for functioning neurons and astrocytes among other cells in the brain. Adjacent CECs contain tight junctions (TJs) that serve as the anatomic and functional barrier against harmful molecules from the blood stream. The process of transendothelial migration of HIV-1-infected monocytes involves interactions among adhesion molecules on CECs [e.g. vascular endothelial adhesion molecule (VCAM)] and their ligands on the activated leukocyte [very late antigen-4 (VLA-4)]. During the course of NeuroAIDS, HIV-1-infected macrophage/monocytes from the blood, traverse the TJs of the CECs and infiltrate the brain parenchyma. Once within this milieu, they differentiate into macrophage/microglia (MΦ). Drugs including cocaine and methamphetamine damage the BBB and further facilitate ingress of the HIV-1-infected monocytes into the brain. (b) BBB postinfection. Once within the brain parenchyma, HIV-1-infected MΦ secrete elevated levels of pro-inflammatory cytokines including interleukin-β (IL-β), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α), which in turn activate uninfected macrophage/microglia and adversely affect astrocytes. These pro-inflammatory cytokines also increase BBB permeability and induce further expression of adhesion molecules including VCAM and inter-cellular adhesion molecule (ICAM) by activated CECs, which in turn promote further ingress of HIV-1-infected monocytes into the CNS. In addition to cytokines, inflammatory chemokines contribute to brain inflammation [e.g. chemokine ligand 2 (CCL2); chemokine ligand 7 (CCL7); C-X-C motif chemokine 5 (CXCL5)]. Within the CNS, MFs produce nitric oxide (NO) and promote oxidative injury that affects both astrocytes and CECs. HIV-1-infected MΦs also release HIV-1 Tat protein that induces expression of the adhesion molecules by CECs. Neuronal injury in the context of NeuroAIDS is also promoted by processes including the activation of the N-methyl-D-aspartate receptor (NMDAR)-coupled ion channels, which in turn leads to massive influx of Ca+2 ions. This process then leads to activation of several enzymes including matrix metallo-proteinases, generation of free radicals, and release of glutamate, which cause neuronal apoptosis. Other inflammatory mediators such as platelet activating factor (PAF), arachidonate, and quinolinate (an NMDA agonist) also participate in neuronal injury. HIV-1 infection of astrocytes also leads to astroglyosis and inflammatory cascades. Reproduced with permission from [53,137,186–189].
Vaccines: classical and nonclassical
To produce a vaccine against NeuroAIDS, an effective HIV-1 vaccine must first be developed and the design and development of HIV-1 vaccines is still in progress [190]. The STEP HIV vaccine trial failure [STEP, an international HIV-1 vaccine clinical trial that commenced in 2004, is also known as the HVTN 502 or Merck V520-023 study, cosponsored by NIAID and Merck Corp. The trial tests three recombinant adenovirus-5 (rAD5) vectors, each expressing an HIV-1 gene: Ad5-gag, Ad5-pol and Ad5-Nef (http://www.hvtn.org/media/pr/step111307.html)] and this led to further intense debate related to the utility of the vaccine approach [191]. Some of the major concerns associated with the development of HIV/AIDS vaccines are viral diversity (viral mutation), HIV-1-host molecular mimicry (sequence level homology among viral and human genes), and HLA polymorphism among ethnic groups (including Black, Hispanic, Oriental, Pacific Islander, American Indian, Australian aboriginal, and Caucasian). The latest IMGT/HLA database (http://www.ebi.ac.uk/imgt/hla/) update (as of 3-06-2010) has 4447 HLA alleles displaying sequence polymorphisms among these ethnicities. Nevertheless, efforts are underway using a multifaceted strategy for vaccine development. Four priming injections of a recombinant canary pox vector (ALVAC-HIV) and two booster injections of gp120 subunit (AIDSVAX-B/E) in a community-based, randomized, multicenter, double-blind, placebo-controlled efficacy trial (NCT00223080) in Thailand, showed some encouraging results for future research in that there was a trend (P = 0.08) to prevent HIV-1 infection among vaccine recipients (efficacy 26.4%). However, the vaccine did not affect the degree of viremia or the CD4+ T-cell count in patients who later seroconverted [192]. Nonetheless, recent data from sequence to structure relationship studies [193–195] with corresponding immunological findings [196] to HIV-1 gp120 provide encouraging insights to continue use HIV-1 Env as a potential target for vaccine development.
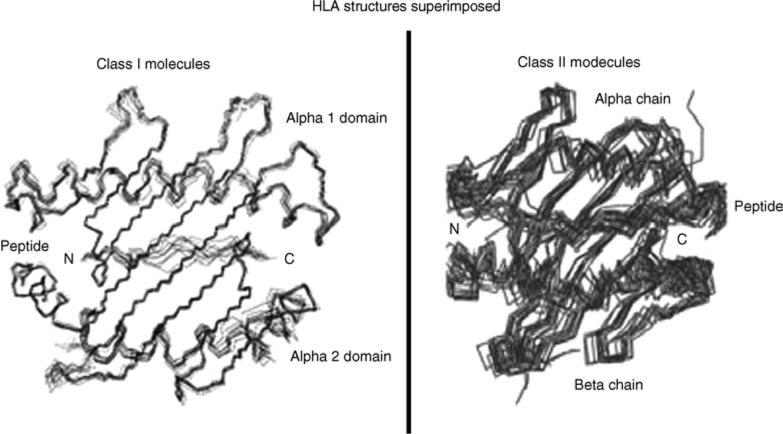
A novel approach uses population-specific HLA sequences to design short HIV-1 oligopeptide-specific T-cell-based epitopes in the context of NeuroAIDS [197]. An HIV/AIDS oligopeptide vaccine consists of a cocktail of short synthetic antigenic oligopeptides (8–20 residues) [197,198]. These oligopeptides exhibit either CD8+ or CD4+ T-cell immunity by specifically binding to respective class I or class II HLA alleles (that is illustrated in Fig. 3) [199]. The LANL/HIV database (http://www.hiv.lanl.gov/content/immunology/index.html) documents a range of HIV-1-specific oligopeptides exhibiting T-cell immunity, with known HLA specificity. Cocktails of these oligopeptides are administered in conjunction with incomplete Freund's adjuvant (water-in-oil emulsion) to stimulate specific T-cell response [197,198]. Moreover, short oligopeptides are more capable of entering the brain than larger envelope and other viral proteins and hence are promising vaccine paradigm candidates for HIV/AIDS and NeuroAIDS [197].
Fig. 3. HLA structures (class I and II) superimposed with peptides in complex form using a set of known HLA-peptide structures determined by X-ray crystallography.
The illustration shows structural features of superimposed multiple short peptides binding to HLA molecules, which is a prerequisite to stimulate T-cell immunity. HLA molecules are often polymorphic across ethnic groups and hence peptide binding specificity varies for each population. Reproduced with permission from [199].
Animal models
Several animal model systems have been developed to investigate HIV-1-induced CNS disease. Transgenic mice were utilized to generate strains expressing HIV-1-encoded neurotoxic proteins, including Tat, under the control of a doxycycline promoter, HIV-1 gp120 under control of the glial fibrillary acidic protein (GFAP) promoter (for expression in astrocytes), and gp160 under the control of neurofilament promoters (for expression in neurons), to investigate the role of these HIV-1 proteins in neuropathogenesis. Additional technologies exploited the severe combined immune deficient/human (SCID/hu) mouse system when SCID mice were inoculated intracranially with HIV-1-infected human monocytes. FIV (FIV is a cat lentivirus related to HIV-1) infection was also utilized as a model for HIV-1 infection and this FIV/cat system proved useful to investigate cognitive and behavioral deficits in an infection-based model [200–206].
Simian immunodeficiency virus (SIV) is also known to induce neuropathologic changes in infected macaques [207]; however, several approaches were developed to circumvent the relatively low incidence of neuropathogenic infection. Passage in microglial cell culture generated a virus that replicates more efficiently within the CNS and results in increased incidence of neuropathological infection [208]. A multivirus combination (pathogenic and neurovirulent viruses together) yields a significant increase in the incidence of CNS disease in macaques. For example, co-inoculation of the SIV/DeltaB670 and SIV/17E-Fr precipitated neuropathogenic infection in pigtailed macaques [209]. Subsequently, a single virus system induced neuropathogenic infection in greater than 90% of infected pigtailed macaques [210]. The latter two systems offer the desired high incidence of neuropatho-genesis and disease pattern that are remarkably similar to NeuroAIDS. Depletion of CD8+ T cells increased the incidence of CNS disease for more traditional SIV isolates (Table 6) [200–206,208–211]. These model systems help demonstrate pathogenic mechanisms in HIV-1-induced CNS disease. Continued improvements should enable a comprehensive understanding of HIV-1-induced neurological disease and the development of effective therapies.
Conclusion
Since the earliest studies in NeuroAIDS, nearly 30 years ago, many different approaches and models for Neuro-AIDS evolved. We conclude with key current issues dealt with in this review. CNS infection by HIV-1 can result in NeuroAIDS. The infection is chronic and subsequent neurodegeneration leads to several domains of neurocognitive impairment. NeuroAIDS is increased in older HIV-1+ adults and associated drug abuse exacerbates these impairments, synergizes with virus replication, and increases virus load and immunosuppression. In the brain, HIV-1 load and evolution affect these outcomes. However, HAART greatly reduces virus load by decreasing viral replication but has little effect on the viral reservoirs in the brain. Despite the use of HAART, milder forms of HAND are present in nearly 50% of HIV-1-positive adults. Thus palliative and adjunctive therapy are necessary to improve the patient's ability to function, although to date most clinical trials with such approaches have shown minimal or no efficacy. At the genetic level, immunologic, neuro-transmitter host gene polymorphisms, and perturbed gene expression may influence NeuroAIDS progression and susceptibility; however, these tests currently lack the sensitivity and specificity for use in clinical practice for prediction of susceptibilityof NeuroAIDS. Novel therapies that deliver genes into the CNS using AAV and other viruses as vectors may be a potential therapeutic approach to combat destructive processes such as apoptosis and inflammation thereby reducing brain damage in Neuro-AIDS. Great dexterity is provided by animal models including transgenic mice, felines, and nonhuman primates to investigate all these aspects of viral infection and disease. These animal models also have great potential for the development of therapeutics for NeuroAIDS. Finally, HIV/AIDS therapeutic vaccines still require development for overall use and effectiveness let alone for the brain. The obstacles to vaccines include viral diversity, HIV-1-host molecular mimicry, and HLA polymorphism among ethnic groups. The use of oligopeptide vaccines is a step in a more effective direction. Directions of future studies that attack HIV/NeuroAIDS include greater sophistication in manipulation to target specific signaling and molecular processes. Specific developments in medications and vaccines that cross the blood–brain barrier are crucial as well.
Table 1.
Diagnosis (clinical and neuropathology).
| Clinical condition | Selected key clinical diagnosis/signs/symptoms | Selected key findings on pathological examination of post mortem brain | References |
|---|---|---|---|
| HAND | At this time, there is no single, agreed-upon, diagnostic biomarker of HIV-associated neurocognitive disorders (HAND). Rather, HAND remains a clinical diagnosis, made in those HIV-1-positive individuals who manifest cognitive decline and in whom other conditions can be excluded by clinical and laboratory tests, such as other CNS infections, metabolic diseases, and depression. This requires a holistic appreciation of the individual's virologic and immunologic status, other medications/recreational drugs, and comorbid diseases, as well as the neurological and neurocognitive examination. In general, HAND is not associated with a decreased or fluctuating level of consciousness, focal findings such as hemiparesis, hemianopsia, or aphasia, or systemic signs of meningismus or fever. The neurological examination may be normal, or show diffuse, symmetrical abnormalities in the pyramidal and extrapyramidal motor systems, hyper-reflexia, and frontal release signs. Similarly, key neurocognitive findings are nonlateralizing. Key findings include motor and psychomotor slowing, decreased attention and concentration, decreased executive function, information processing, learning and memory. In particular, working (short-term) memory is affected early on, and is consistent with a mixed encoding and retrieval profile. In contrast, the memory loss in Alzheimer's disease consists of rapid forgetting due to inability to process novel information. | The neuropathologic examination is in large part dictated by the clinical setting and by what is easily seen using tissue sections stained with hematoxylin and eosin (H&E). Unfortunately, many changes are not strictly associated with a particular clinical diagnosis, are nonspecific, and do not lead to a diagnosis. For example, the neuropathologic evaluation in the case of HIV-1-positive individuals with neurocognitive deficits, seeks to identify positive evidence of HIV-1 in the CNS as well as any conditions clinically expected or otherwise that may be causing or contributing to the deficits. However, particularly given the widespread use of antiretrovirals, the presence of HIV-1 in the brain at autopsy is neither necessary nor sufficient for the clinical diagnosis of any level of neurocognitive damage in HIV-1-infected patients. | [16,25,26] |
| Asymptomatic neurocognitive impairment (ANI) | Normal level of consciousness. Neurological examination is nonfocal. Subclinical neurocognitive impairment as diagnosed by neuropsychological testing (e.g. global rating <5), and no significant impairment of activities of daily living. | Usually minimal and nondiagnostic and/or related to treatment or comorbid conditions. | [16,25,26] |
| Minor neurocognitive disorder (MND) | Normal level of consciousness. Neurological examination is nonfocal. Mild decline in cognition in addition to mild everyday functioning impairment and affects the more difficult activities of daily living. | Usually minimal and nondiagnostic and/or related to treatment or comorbid conditions. | [16,25,26] |
| HIV-associated dementia (HAD) | Normal level of consciousness. Neurological examination is nonfocal, or may demonstrate spasticity and/or extrapyramidal signs. Significant decline in cognition (e.g. global rating >7) along with major functional impairment, that affects routine activities of daily living. | In untreated or inadequately treated cases, findings may include multinucleated giant cells and/or immunoreactivity for HIV-1 antigens in the brain. These findings indicate the presence of HIV-1 in the brain but the correlation with HAD is not one-to-one and only nonspecific atrophy and gliosis may be seen, especially with ART exposure. | [16,25,26] |
| Other neurodegenerative conditions | |||
| Mild cognitive impairment (MCI) | Clinically pertinent in age-appropriate HIV-1-positive and those with genetic markers for familial AD. Characterized by subjective cognitive complaints that are not age-appropriate. There may be evidence for mild impairments via neuropsychological testing, but not to the severity of dementia. Activities of daily living are essentially normal. | When MCI is ‘amnestic’, then it usually evolves into AD and would likely have some pathology. However, MCI is also used to describe ‘nonamnestic’ conditions, and would therefore be a potential precursor to non-AD conditions. Possibly, one might expect to detect the beginnings of neuropathological changes in patients with MCI of a nonamnestic nature as with amnestic changes. Indeed, neuropathological changes are variable in MCI ranging from no significant changes to changes seen in degenerative diseases including AD and diffuse Lewy body disease. Moreover, MRI scans of patients with MCI can detect progressive brain gray matter loss in some of these patients. | [27–30] |
| AD | Clinically pertinent in age-appropriate HIV-1-positive and those with genetic markers for familial AD. Presence of an early and significant episodic memory impairment that includes gradual and progressive change in memory function over more than 6 months; objective evidence (e.g. neuropsychological testing) of significantly impaired episodic memory functioning; the episodic memory impairment can be isolated or associated with other cognitive changes. | Cortical atrophy with relative sparing of primary sensory and motor cortices; amyloid plaques and neurofibrillary tangles. | [31] |
| Diffuse Lewy body (DLB) disease | Dementia denied as progressive cognitive decline sufficient to interfere with daily functioning. Memory deficits may not be prominent. In addition, two or more of the following for diagnosis of probable DLB, or one for diagnosis of possible DLB. Fluctuating cognition with pronounced variations in attention and alertness; recurrent visual hallucinations that are typically well formed and detailed; spontaneous features of Parkinsonism. | Alpha-synuclein positive Lewy bodies in the cerebral cortex and other areas of the brain. | [32] |
| Vascular dementia | Clinically pertinent in age-appropriate HIV-1-positive individuals that manifest focal clinical signs and symptoms, stepwise or fluctuating pattern, with historical, clinical or laboratory evidence of significant vascular disease, evidence of cerebrovascular disease or stroke on imaging. Dementia, as defined as a decline in cognitive functioning, including memory impairment and at least two other cognitive domains. A clear temporal relationship between vascular disease and dementia. | Loss of brain tissue due to infarction, often multifocal, intimal and medial thickening with and without atherosclerosis cerebral blood vessels. | [33,34] |
| Other CNS infections | |||
| Neurosyphilis | Symptoms include cognitive deficits, meningitis, and stroke. Diagnosis confirmed by positive CSF VDRL, but some cases may require empiric treatment due to the low sensitivity of this test. | Chronic, plasma cell rich meningo-encephalitis; spirochetes identified by various methods. | [35,36] |
| CNS tuberculosis | Symptoms of chronic meningo-encephalitis, such as altered level of consciousness, cognitive impairment, headache, small vessel stroke. Diagnosis confirmed by CSF acid-fast bacterial culture or PCR. | Granulomas usually basal lepto-meningeal; acid-fast bacilli identified by culture or special stain. | [36] |
| Chronic fungal meningitides | Usually present as subacute meningitis or meningo-encephalitis with fever, malaise, headache, and impaired cognition. Diagnosis is confirmed by positive CSF India ink (Cryptococcus), fungal cultures, and/or serology. In some cases, diagnosis is made empirically, when there is documented extra-neural infection and no other cause for neurological symptoms. | Inflammatory infiltrate varies; identification of organism by culture or special stain. | [36,37] |
| CNS toxoplasmosis | Definitive diagnosis can be made by brain biopsy; probable diagnosis can be established in patients with encephalitis, enhancing brain lesions surrounded by edema on MRI or CT, positive IgG toxoplasma serology and clinical response to antitoxoplasmosis treatment. | Inflammation variable: identification of organism by routine examination or immunohistochemistry. | [37,38] |
| PML | Progressive focal neurologic deficits, such as aphasia, hemiparesis, hemianopsia, usually corresponding to space-occupying white matter lesions on MRI. Definitive diagnosis can be made in patients with symptomatic brain lesions by brain biopsy or positive CSF JCV PCR; however, the latter is relatively insensitive. | Demyelination with viral inclusions in oligodendroglia cells: may confirm with immunohistochemical stain for polyoma virus. | [38,39] |
| CMV encephalitis | Presenting CNS syndromes include cognitive impairment, delirium, cranial neuropathies, ataxia, myelitis, and polyradiculopathy. The demonstration of CMV by CSF PCR or in brain tissue in a patient with a compatible clinical syndrome establishes the diagnosis. | Various lesions, often ependymitis: identification of organism by characteristic inclusions or by immunohistochemistry. | [38,40] |
| CNS tumor | |||
| Primary CNS lymphoma | Symptoms (such as headache, personality changes, cognitive dysfunction, seizures or focal motor signs) are referable to one or more brain mass lesions, typically contrast enhancing; biopsy remains the standard of diagnosis. Most patients have very low (<50 cells/μl) CD4+ cell count. Detection of Epstein–Barr virus by CSF PCR is highly suggestive. | Identification of neoplastic lymphoid infiltrate, usually high-grade B cell, by immunohistochemistry and other methods. | [36,38] |
| Toxic-metabolic encephalopathies | |||
| Hepatic failure | Fluctuating level of consciousness, disturbed sleep, personality changes, delirium; extrapyramidal signs in advanced cases; abnormal psychometric tests, slow EEG with triphasic waveforms, elevated serum ammonia. | May see Alzheimer type II gliosis. | [41,42] |
| Chronic hypoxemia | Symptoms of headache, disorientation, somnolence, diagnosed by low pO2, acidosis, and elevated pCO2. | Hypoxia/ischemia can accompany some toxic-metabolic conditions in which case there may be neuronal loss most frequently of vulnerable cells such as hippocampal pyramidal cells. | [43,44] |
| Depression | Complaints of memory loss are out of proportion to actual loss of function as measured by neuropsychological testing and/or activities of daily living. No associated neurological signs or abnormal imaging. | No identified pathological variable in HIV-1-positive. | [26] |
This table shows selected key clinical diagnoses, signs, and symptoms in NeuroAIDS and potential neuropathological correlates (when they occur). Other comorbid and complicating neurodegenerative conditions, infections and toxic encephalopathies are mentioned as well. AD, Alzheimer's disease; CMV, cytomegalovirus; CNS, central nervous system; CSF, cerebrospinal fluid; CT, computed tomography; EEG, electroencephalography; IgG, immunoglobulin G; PML, progressive multifocal leukoencephalopathy; VDRL, venereal disease research laboratory.
Acknowledgements
Raisa Avezova (Division of Oral Biology and Medicine, UCLA School of Dentistry, Los Angeles, California, USA) and Philippe Grisel, MD, PhD (Cardiology Service, University Hospital of Geneva, Geneva, Switzerland) are credited as co-authors, as there is a limitation of 12 authors.
We apologize to our colleagues in that although there is a wealth of critical contributions to NeuroAIDS research, many could not be included because of limitations in the scope of this review. We also wish to express our appreciation to the Editors for their advice. Gopichandran Sowmya (Biomedical Informatics Society, India) is acknowledged for her assistance. Support for this work included NIH Grants: F.C. – Alzheimer Association, University of California Senate, NIH AI07126, CA16042, DA07683, and DA10442; E.S., A.L., D.C., and P.S. –NIH NS038841, ES-NIH MH083500, AL-NIH DA026099.
Footnotes
Authors and contributions: P.S. – introduction, brain virus load and evolution, Conclusion, editing manuscript, and corresponding author.
P.K. – vaccines, Figure 3.
R.K.F. – brain virus load.
D.C. – diagnosis, neurology, and neuroimaging; aging and neuropathology, Figure 1, Table 1.
F.C. – gene therapy, Table 4.
E.S. – diagnosis, neurology, and neuroimaging, Table 1.
A.J.L. – genetics, Table 2.
A.M. – gene expression, Figure 2, Table 3.
F.J.N. – animal models, Table 6.
C.S. – treatment, Table 5.
A.N. – drug abuse.
J.T.S. – treatment, Table 5.
In addition, all authors contributed to correcting and editing the text.
Raisa Avezova and Philippe Grisel, MD, PhD contributed to gene therapy.
The authors report no financial conflicts.
References
- 1.Singer EJ, Valdes-Sueiras M, Commins D, Levine AJ. Neurologic presentations of AIDS. Neurol Clin. 2010;28:253–275. doi: 10.1016/j.ncl.2009.09.018. doi: 10.1016/j.ncl.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minagar A, Shapshak P. HIV associated dementia: clinical features and pathogenesis. In: Minagar A, Shapshak P, editors. NEURO-AIDS. Nova Science Publ; Hauppauge, New York: 2006. [Google Scholar]
- 3.Fernandez F, Ruiz P, editors. Psychiatric aspects of HIV/AIDS. Lippincott Williams and Wilkins; Philadelphia, PA: 2006. [Google Scholar]
- 4.Goodkin K. Virology, Immunology, Transmission, and disease stage. In: Fernandez F, Ruiz P, editors. Psychiatric aspects of HIV/AIDS. Lippincott Williams and Wilkins; Philadelphia, PA: 2006. pp. 11–22. [Google Scholar]
- 5.Goodkin K, Verma A, Shapshak P, editors. The spectrum of NeuroAIDS disorders: pathophysiology, diagnosis, and treatment. ASM Press; Washington, DC: 2008. [Google Scholar]
- 6.Petito C, Kerza-Kwiatecki AP, Gendelman HE, McCarthy M, Nath A, Podack ER, et al. Neuronal injury in HIV infection. J Neurovirol. 1999;5:327–341. doi: 10.3109/13550289909029474. [DOI] [PubMed] [Google Scholar]
- 7.Ironson G, Weiss SM, Lydston D, Tobin J, Lechner S, Ishii M, et al. The impact of improved self-efficacy on viral-load in culturally diverse women living with AIDS: the SMART/EST Women's Project. AIDS Care. 2005;17:222–236. doi: 10.1080/09540120512331326365. [DOI] [PubMed] [Google Scholar]
- 8.Resnick L, Berger JR, Shapshak P, Tourtellotte WW. Early penetration of the blood-brain-barrier by HTLV-III/LAV. Neurology. 1988;38:9–15. doi: 10.1212/wnl.38.1.9. [DOI] [PubMed] [Google Scholar]
- 9.Arendt G, Nolting T, Frisch C, Husstedt IW, Gregor N, Koutsilieri E, et al. Intrathecal viral replication and cerebral deficits in different stages of human immunodeficiency virus disease. J Neurovirol. 2007;13:225–232. doi: 10.1080/13550280701315355. [DOI] [PubMed] [Google Scholar]
- 10.Tarasów E, Wiercińska-Drapało A, Kubas B, Dzienis W, Orzechowska-Bobkiewicz A, Prokopowicz D, et al. Cerebral MR spectroscopy in neurologically asymptomatic HIV-infected patients. Acta Radiol. 2003;44:206–212. doi: 10.1080/j.1600-0455.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 11.Minagar A, Commins D, Alexander JS, Hoque R, Chiappelli F, Singer EJ, et al. Diagnostics for NeuroAIDS: present and future. Molec Diagn Ther. 2008;12:25–43. doi: 10.1007/BF03256266. [DOI] [PubMed] [Google Scholar]
- 12.Power C, Boissé L, Rourke S, Gill MJ. NeuroAIDS: an evolving epidemic. Can J Neurol Sci. 2009;36:285–295. doi: 10.1017/s0317167100007009. [DOI] [PubMed] [Google Scholar]
- 13.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 14.Klunder AD, Chiang MC, Dutton RA, Lee SE, Toga AW, Lopez OL, et al. Mapping cerebellar degeneration in HIV/AIDS. Neuroreport. 2008;19:1655–1659. doi: 10.1097/WNR.0b013e328311d374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole MA, Castellon SA, Perkins AC, Ureno OS, Robinet MB, Reinhard MJ, et al. Relationship between psychiatric status and frontal-subcortical systems in HIV-infected individuals. J Int Neuropsychol Soc. 2007;13:549–554. doi: 10.1017/S135561770707066X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. Epub 2007 Oct 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AAN (American Academy Neurology) Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 1991;41:778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- 18.Bhaskaran K, Mussini C, Antinori A, Walker AS, Dorrucci M, Sabin C, et al. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008;63:213–221. doi: 10.1002/ana.21225. [DOI] [PubMed] [Google Scholar]
- 19.McArthur JC, McDermott MP, McClernon D, C St. Hillaire K, Conant K, Marder, et al. Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch Neurol. 2004;61:1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- 20.Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre and posthighly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10:350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- 21.Simioni S, Cavassini M, Annoni JM, Rimbault A, Abraham AR, Bourquin Isabelle, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 22.Descamps M, Hyare H, Stebbing J, Winston A. Magnetic resonance imaging and spectroscopy of the brain in HIV disease. J HIV Ther. 2008;13:55–58. [PubMed] [Google Scholar]
- 23.Babiker AG, Peto T, Porter K, Walker AS, Darbyshire JH. Age as a determinant of survival in HIV infection. J Clin Epidemiol. 2001;54(Suppl 1):S16–S21. doi: 10.1016/s0895-4356(01)00456-5. [DOI] [PubMed] [Google Scholar]
- 24.Valcour VC, Shikuma C, Shiramizu G, Watters M, Poff P, Selnes O, et al. Age, Apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV cohort. J Neuroimmunol. 2004;157:197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, et al. Inter-rater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 26.Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, et al. Clinico-neuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 2009;15:360–370. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62:1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- 28.Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. doi: 10.1001/archneur.63.5.665. PMID 16682536. [DOI] [PubMed] [Google Scholar]
- 29.Saito Y, Murayama S. Neuropathology of mild cognitive impairment. Neuropathology. 2007;6:58–84. doi: 10.1111/j.1440-1789.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- 30.Whitwell JL, Shiung MM, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70:512–520. doi: 10.1212/01.wnl.0000280575.77437.a2. doi: 10.1212/01.wnl.0000280575.77437.a2. PMID 17898323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer's disease’: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 32.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 33.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 34.Wiederkehr S, Simard M, Fortin C, van Reekum R. Validity of the clinical diagnostic criteria for vascular dementia: a critical review. Part II. J Neuropsychiatry Clin Neurosci. 2008;20:162–177. doi: 10.1176/jnp.2008.20.2.162. [DOI] [PubMed] [Google Scholar]
- 35.Marra CM, Critchlow CW, Hook EW, 3rd, Collier AC, Lukehart SA. Cerebrospinal fluid treponemal antibodies in untreated early syphilis. Arch Neurol. 1995;52:68–72. doi: 10.1001/archneur.1995.00540250072015. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. quiz CE1-4. [PubMed] [Google Scholar]
- 37.Portegies P, Solod L, Cinque P, Chaudhuri A, Begovac J, Everall I, et al. Guidelines for the diagnosis and management of neurological complications of HIV infection. Eur J Neurol. 2004;11:297–304. doi: 10.1111/j.1468-1331.2004.00856.x. [DOI] [PubMed] [Google Scholar]
- 38.Petito CK. Neuropathology of acquired immunodeficiency syndrome. In: Nelson JS, Mena H, Parisi JS, Schochet SS, editors. The principles, practice of neuropathology. 2nd ed. Oxford University Press; New York: 2003. pp. 95–111. [Google Scholar]
- 39.Wang Y, Kirby JE, Qian Q. Effective use of JC virus PCR for diagnosis of progressive multifocal leukoencephalopathy. J Med Microbiol. 2009;58:253–255. doi: 10.1099/jmm.0.004432-0. [DOI] [PubMed] [Google Scholar]
- 40.Cinque P, Bossolasco S, Bestetti A, Sala S, Pierotti C, Lazzarin A. Molecular studies of cerebrospinal fluid in human immunodeficiency virus type 1-associated opportunistic central nervous system diseases–: an update. J Neurovirol. 2002;8(Suppl 2):122–128. doi: 10.1080/13550280290167957. [DOI] [PubMed] [Google Scholar]
- 41.Cash WJ, McConville P, McDermott E, McCormick PA, Callender ME, McDougall NI. Current concepts in the assessment and treatment of hepatic encephalopathy. QJM. 2010;103:9–16. doi: 10.1093/qjmed/hcp152. [DOI] [PubMed] [Google Scholar]
- 42.Harper C, Butterworth R. Nutritional and metabolic disorders. In: Graham DI, Lantos PL, editors. Greenfield's neuropathology. 7th ed. Arnold Publishing; London: 2002. pp. 607–651. [Google Scholar]
- 43.Dreibelbis JE, Jozefowicz RF. Neurologic complications of respiratory disease. Neurol Clin. 2010;28:37–43. doi: 10.1016/j.ncl.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Auer RN, Sutherland GR. Hypoxia and related conditions. In: Graham DI, Lantos PL, editors. Greenfield's neuropathology. 7th ed. Arnold Publishing; London: 2002. pp. 233–280. [Google Scholar]
- 45.Xu J, Ikezu T. The comorbidity of HIV-associated neurocognitive disorders and Alzheimer's disease: a foreseeable medical challenge in post-HAART era. J Neuroimmune Pharmacol. 2009;4:200–212. doi: 10.1007/s11481-008-9136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pulliam L. HIV regulation of amyloid beta production. J Neuroimmune Pharmacol. 2009;4:213–217. doi: 10.1007/s11481-009-9151-9. [DOI] [PubMed] [Google Scholar]
- 47.Anthony IC, Ramage SN, Camie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active antiretroviral therapy. Acta Neuropathol. 2006;111:529–538. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 48.Khanlou N, Moore DJ, Chana G, Cherner M, Lazzaretto D, Dawes S, et al. Increased frequency of alpha-synuclein in the substantia nigra in human immunodeficiency virus infection. J Neurovirol. 2009;15:131–138. doi: 10.1080/13550280802578075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minagar A, Shapshak P, Duran EM, Kablinger AS, Alexander JS, Kelley RE, et al. HIV-associated dementia, Alzheimer's disease, multiple sclerosis, and schizophrenia: gene expression review. J Neurol Sci. 2004;224(1–2):3–17. doi: 10.1016/j.jns.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202(1–2):13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- 51.Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral-load, and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121:2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- 52.Kim MT, Hill MN. Validity of self-report of illicit drug use in young hypertensive urban African-American males. Addict Beh. 2003;28:795–802. doi: 10.1016/s0306-4603(01)00277-5. doi: 10.1016/S0306-4603(01)00277-5. [DOI] [PubMed] [Google Scholar]
- 53.Fiala M, Eshleman AJ, Cashman J, Lin J, Lossinsky AS, Suarez V, et al. Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. J Neurovirol. 2005;11:281–291. doi: 10.1080/13550280590952835. [DOI] [PubMed] [Google Scholar]
- 54.Peterson PK, Gekker G, Hu S, Lokensgard J, Portoghese PS, Chao CC. Endomorphin-1 potentiates HIV-1 expression in human brain cell cultures: implication of an atypical muopioid receptor. Neuropharmacology. 1999;38:273–278. doi: 10.1016/s0028-3908(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 55.Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 2003;24:116–121. doi: 10.1016/s1471-4906(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Wang X, Tian S, Guo CJ, Douglas SD, Ho WZ. Methadone enhances human immunodeficiency virus infection of human immune cells. J Infect Dis. 2002;185:118–122. doi: 10.1086/338011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dhillon NK, Williams R, Peng F, Tsai YJ, Dhillon S, Nicolay B, et al. Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J Neurovirol. 2007;13:483–495. doi: 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- 58.Nair MP, Mahajan SD, Schwartz SA, Reynolds J, Whitney R, Bernstein Z, et al. Cocaine modulates dendritic cell-specific C type intercellular adhesion molecule-3-grabbing nonintegrin expression by dendritic cells in HIV-1 patients. J Immunol. 2005;174:6617–6626. doi: 10.4049/jimmunol.174.11.6617. [DOI] [PubMed] [Google Scholar]
- 59.Reynolds JL, Mahajan SD, Bindukumar B, Sykes D, Schwartz SA, Nair MP. Proteomic analysis of the effects of cocaine on the enhancement of HIV-1 replication in normal human astrocytes (NHA). Brain Res. 2006;1123:226–236. doi: 10.1016/j.brainres.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, et al. Methamphetamine enhances HIV infection of macrophages. Am J Pathol. 2008;172:1617–1624. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol. 2009;175:1148–1159. doi: 10.2353/ajpath.2009.081067. doi: 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee YW, Hennig B, Yao J, Toborek M. Methamphetamine induces AP-1 and NF-kappaB binding and transactivation in human brain endothelial cells. J Neurosci Res. 2001;66:583–591. doi: 10.1002/jnr.1248. [DOI] [PubMed] [Google Scholar]
- 63.Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Fernandez SF, et al. Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability. J Clin Immunol. 2008;28:528–541. doi: 10.1007/s10875-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 64.Sharma HS, Ali SF. Alterations in blood-brain barrier function by morphine and methamphetamine. Ann N Y Acad Sci. 2006;1074:198–224. doi: 10.1196/annals.1369.020. [DOI] [PubMed] [Google Scholar]
- 65.Turchan J, Anderson C, Hauser KF, Sun Q, Zhang J, Liu Y, et al. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2001;2:3–11. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, et al. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrod SB, Mactutus CF, Fitting S, Hasselrot U, Booze RM. Intra-accumbal Tat1-72 alters acute and sensitized responses to cocaine. Pharmacol Biochem Behav. 2008;90:723–729. doi: 10.1016/j.pbb.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuczenski R, Everall IP, Crews L, Adame A, Grant I, Masliah E. Escalating dose-multiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Exp Neurol. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, et al. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67:1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- 70.Sacktor N, Tarwater PM, Skolasky RL, McArthur JC, Selnes OA, Becker J, et al. CSF antiretroviral drug penetrance and the treatment of HIV-associated psychomotor slowing. Neurology. 2001;57:542–555. doi: 10.1212/wnl.57.3.542. [DOI] [PubMed] [Google Scholar]
- 71.Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, et al. AIDS Clinical Trials Group 736 Study Team Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marra CM, Lockhart D, Zunt JR, Perrin M, Coombs RW, Collier AC. Changes in CSF and plasma HIV-1 RNA and cognition after starting highly active antiretroviral therapy. Neurology. 2003;60:1388–1395. doi: 10.1212/01.wnl.0000058768.73358.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Foudraine NA, Hoetelmans RM, Lange JM, de Wolf F, van Benthem BH, Maas JJ, et al. Cerebrospinal-fluid HIV-1 RNA and drug concentrations after treatment with lamivudine plus zidovudine or stavudine. Lancet. 1998;351:1547–1550. doi: 10.1016/S0140-6736(98)07333-4. [DOI] [PubMed] [Google Scholar]
- 74.Antinori A, Perno CF, Giancola ML, Forbici F, Ippolito G, Hoetelmans RM, et al. Efficacy of Cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis. 2005;41:1787–1793. doi: 10.1086/498310. [DOI] [PubMed] [Google Scholar]
- 75.Letendre SL, van den Brande G, Hermes A, Woods SP, Durelle J, Beck JM, et al. Lopinavir with ritonavir reduces the HIV RNA level in cerebrospinal fluid. Clin Infect Dis. 2007;45:1511–1517. [Google Scholar]
- 76.Couzigou C, Seang S, Morand-Joubert L, Roque-Afonso AM, Escaut L, Vittecoq D. Efficacy of etravirine for treatment of acute HIV meningoencephalitis. Clin Infect Dis. 2009;48:e62–e65. doi: 10.1086/597109. [DOI] [PubMed] [Google Scholar]
- 77.Schifitto G, Navia BA, Yiannoutsos CT, Marra CM, Chang L, Ernst T, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS. 2007;21:1877–1886. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- 78.Schifitto G, Zhang J, Evans SR, Sacktor N, Simpson D, Millar LL, et al. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology. 2007;69:1314–1321. doi: 10.1212/01.wnl.0000268487.78753.0f. [DOI] [PubMed] [Google Scholar]
- 79.Dewhurst S, Maggirwar SB, Schifitto G, Gendelman HE, Gelbard HA. Glycogen synthase kinase 3 beta (GSK-3 beta) as a therapeutic target in NeuroAIDS. J Neuroimmune Pharmacol. 2007;2:93–96. doi: 10.1007/s11481-006-9051-1. [DOI] [PubMed] [Google Scholar]
- 80.Schifitto G, Peterson DR, Zhong J, Ni H, Cruttenden K, Gaugh M, et al. Valproic acid adjunctive therapy for HIV-associated cognitive impairment: a first report. Neurology. 2006;66:919–921. doi: 10.1212/01.wnl.0000204294.28189.03. Epub 2006 Mar 1. [DOI] [PubMed] [Google Scholar]
- 81.Lee K, Vivithanaporn P, Siemieniuk RA, Krentz HB, Maingat F, Gill MJ, et al. Clinical outcomes and immune benefits of antiepileptic drug therapy in HIV/AIDS. BMC Neurol. 2010;10:44–48. doi: 10.1186/1471-2377-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sagot-Lerolle N, Lamine A, Chaix ML, Boufassa F, Aboulker JP, Costagliola D, et al. ANRS EP39 study. Prolonged valproic acid treatment does not reduce the size of latent HIV reservoir. AIDS. 2008;22:1125–1129. doi: 10.1097/QAD.0b013e3282fd6ddc. [DOI] [PubMed] [Google Scholar]
- 83.Fujimura RK. Minagar A, Shapshak P, editors. Viral-load and HIV-l associated dementia: neuropathology and drug efficacy. Chapter 5 in NeuroAIDS. 2005. pp. 101–119. [ISBN: 1-59454-610-X]
- 84.Fujimura RK, Goodkin K, Petito C, Douyon R, Feaster DJ, Concha M, et al. HIV-1 proviral DNA load across neuroanatomic regions of individuals with evidence for HIV-1-associated dementia. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:146–152. doi: 10.1097/00042560-199711010-00002. [DOI] [PubMed] [Google Scholar]
- 85.Fujimura RK, Khamis I, Shapshak P, Goodkin K. Regional quantitative comparison of multispliced to unspliced ratios of HIV-1 RNA copy number in infected human brain. J NeuroAIDS. 2004;2:45–60. doi: 10.1300/J128v02n04_04. [DOI] [PubMed] [Google Scholar]
- 86.Zhao L, Galligan DC, Lamers SL, Yu S, Shagrun L, Salemi M, et al. High-level HIV-1 DNA concentrations in brain tissues differentiate patients with post-HAART AIDS dementia complex or cardiovascular disease from those with AIDS. Sci China C Life Sci. 2009;52:651–656. doi: 10.1007/s11427-009-0085-5. [DOI] [PubMed] [Google Scholar]
- 87.Petito C, Hexin C, Angeline RM, Torres-Munoz J, Roberts B, Wood C. HIV infection of choroid plexus in AIDS and asymptomatic HIV-infected patients suggests that the choroid plexus may be a reservoir of productive infection. J NeuroVirol. 1999;5:670–677. doi: 10.3109/13550289909021295. [DOI] [PubMed] [Google Scholar]
- 88.Zhou L, Ng T, Yuksel A, Wang B, Dwyer DE, Saksena NK. Short communication: absence of HIV infection in the choroid plexus of two patients who died rapidly with HIV-associated dementia. AIDS Res Hum Retroviruses. 2008;24:839–843. doi: 10.1089/aid.2007.0147. [DOI] [PubMed] [Google Scholar]
- 89.Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, et al. Circulating proviral HIV DNA and HIV-associated dementia. AIDS. 2005;19:45–52. doi: 10.1097/00002030-200501030-00005. PubMed: 15627032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shiramizu B, Williams AE, Shikuma C, Valcour V. Amount of HIV DNA in peripheral blood mononuclear cells is proportional to the severity of HIV-1-associated neurocognitive disorders. J Neuropsychiatry Clin Neurosci. 2009;21:68–74. doi: 10.1176/appi.neuropsych.21.1.68. doi: 10.1176/appi.neuropsych.21.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Valcour VG, Shiramizu BT, Sithinamsuwan P, Nidhinandana S, Ratto-Kim S, Ananworanich J, et al. HIV DNA and cognition in Thai longitudinal HAART initiation cohort: SEARCH 001 cohort study. Neurol. 2009;72:992–998. doi: 10.1212/01.wnl.0000344404.12759.83. doi: 10.1212/01.wnl.0000344404.12759.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hightower GK, Letendre SL, Cherner M, Gibson SA, Ellis RJ, Wolfson TJ, et al. Select resistance-associated mutations in blood are associated with lower CSF viral-loads and better neuropsychological performance. Virology. 2009 Sep 15;394:243–248. doi: 10.1016/j.virol.2009.08.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ancuta P, Kamat A, Kunstman KJ, Kim E-Y, Autissier P, Wurcel A, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chiappelli F, Shapshak P, Commins D, Singer E, Minagar A, Oluwadara O, et al. Molecular epigenetics, chromatin, and NeuroAIDS/HIV. I. Immunopathological implications. Bioinformation. 2008;3:47–52. doi: 10.6026/97320630003047. PMID: 19052666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shapshak P, Chiappelli F, Commins D, Singer E, Levine AJ, Somboonwit C, et al. Molecular epigenetics, chromatin, and NeuroAIDS/HIV. II. Translational implications. Bioinformation. 2008;3:53–57. doi: 10.6026/97320630003053. PMID: 19052667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tatro ET, Scott ER, Nguyen TB, Salaria S, Banerjee S, Moore DJ, et al. Evidence for alteration of gene regulatory networks through microRNAs of the HIV-infected brain: novel analysis of retrospective cases. PLoS One. 2010;5:e10337. doi: 10.1371/journal.pone.0010337. [doi:10.1371/journal.pone.0010337] ( www.plosone.org) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zárate S, Kosakovsky-Pond SL, Shapshak P, Frost SDW. A comparative study of methods for detecting sequence compartmentalization in HIV-1. J Virol. 2007;61:6643–6651. doi: 10.1128/JVI.02268-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smit TK, Brew BJ, Tourtellotte W, Morgello S, Gelman BB, Saksena NK. Independent evolution of human immunodeficiency virus (HIV) drug resistance mutations in diverse areas of the brain in HIV-infected patients, with and without dementia, on antiretroviral treatment. J Virol. 2004;78:10133–10148. doi: 10.1128/JVI.78.18.10133-10148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burkala EJ, He J, West JT, Wood C, Petito C. Compartmentalization of HIV-1 in the central nervous system: role of the choroid plexus. AIDS. 2005;19:675–684. doi: 10.1097/01.aids.0000166090.31693.aa. [DOI] [PubMed] [Google Scholar]
- 100.van Marle G, Henry S, Todoruk T, Sullivan A, Silva C, Rourke SB, et al. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology. 2004;329:302–318. doi: 10.1016/j.virol.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 101.Chang J, Jozwiak R, Wang B, Ng T, Ge YC, Bolton W, et al. Unique HIV type 1 V3 region sequences derived from six different regions of brain: region-specific evolution within host-determined quasispecies. AIDS Res Hum Retr. 1998;14:25–30. doi: 10.1089/aid.1998.14.25. [DOI] [PubMed] [Google Scholar]
- 102.Shapshak P, Segal DM, Crandall K, Fujimura RK, Zhang BT, Xin KQ, et al. Independent evolution of HIV-1 in different brain regions. AIDS Res Hum Retr. 1999;15:811–820. doi: 10.1089/088922299310719. [DOI] [PubMed] [Google Scholar]
- 103.Caragounis EC, Gisslén M, Lindh M, Nordborg C, Westergren S, Hagberg L, et al. Comparison of HIV-1 pol and env sequences of blood, CSF, brain and spleen isolates collected ante-mortem and postmortem. Acta Neurol Scand. 2008;117:108–116. doi: 10.1111/j.1600-0404.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 104.Pillai SK, Pond SL, Liu Y, Good BM, Strain MC, Ellis RJ, et al. Genetic attributes of cerebrospinal fluid-derived HIV-1 env. Brain. 2006;129(Pt 7):1872–1883. doi: 10.1093/brain/awl136. Epub 2006 May 30. [DOI] [PubMed] [Google Scholar]
- 105.Salemi M, Lamers SL, Yu S, de Oliveira T, Fitch WM, McGrath MS. Phylodynamic analysis of human immunodeficiency virus type 1 in distinct brain compartments provides a model for the neuropathogenesis of AIDS. J Virol. 2005;79:11343–11352. doi: 10.1128/JVI.79.17.11343-11352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu P, Hudson LC, Tompkins MB, Vahlenkamp TW, Meeker RB. Compartmentalization and evolution of feline immunodeficiency virus between the central nervous system and periphery following intracerebroventricular or systemic inoculation. J Neurovirol. 2006;12:307–321. doi: 10.1080/13550280600889575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thompson KA, Churchill MJ, Gorry PR, Sterjovski J, Oelrichs RB, Wesselingh SL, et al. Astrocyte specific viral strains in HIV dementia. Ann Neurol. 2004;56:873–877. doi: 10.1002/ana.20304. [DOI] [PubMed] [Google Scholar]
- 108.Agopian K, Wei BL, Garcia JV, Gabuzda D. CD4 and MHC-I downregulation are conserved in primary HIV-1 Nef alleles from brain and lymphoid tissues, but Pak2 activation is highly variable. Virology. 2007;358:119–135. doi: 10.1016/j.virol.2006.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ohagen A, Devitt A, Kunstman KJ, Gorry PR, Rose PP, Korber B, et al. Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. J Virol. 2003;77:12336–12345. doi: 10.1128/JVI.77.22.12336-12345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Korber BT, Learn GH, Jr, Mullins JI, Hahn BH, Wolinsky S. Protecting HIV databases. Nature. 1995;378:242–244. doi: 10.1038/378242a0. [DOI] [PubMed] [Google Scholar]
- 111.Learn GH, Jr, Korber BT, Foley B, Hahn BH, Wolinsky SM, Mullins JI. Maintaining the integrity of human immunodeficiency virus sequence databases. J Virol. 1996;70:5720–5730. doi: 10.1128/jvi.70.8.5720-5730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Herbeck JT, Nickle DC, Learn GH, Jr, Gottlieb GS, Curlin ME, Heath L, et al. Human immunodeficiency virus type 1 env evolves toward ancestral states upon transmission to a new host. J Virol. 2006;80:1637–1644. doi: 10.1128/JVI.80.4.1637-1644.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mefford ME, Gorry PR, Kunstman K, Wolinsky SM, Gabuzda D. Bioinformatic prediction programs underestimate the frequency of CXCR4 usage by R5X4 HIV type 1 in brain and other tissues. AIDS Res Hum Retroviruses. 2008;24:1215–1220. doi: 10.1089/aid.2008.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 115.Boven LA, van der Bruggen T, van Asbecka BS, Marxa JJM, Nottet HSLM Potential role of CCR5 polymorphism in the development of AIDS dementia complex. FEMS Immunol Med Microbiol. 1999;26(3–4):243–247. doi: 10.1111/j.1574-695X.1999.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 116.Singh KK, Barroga CF, Hughes MD, Chen J, Raskino C, McKinney RE, Spector SA. Genetic influence of CCR5, CCR2, and SDF1 variants on human immunodeficiency virus 1 (HIV-1)-related disease progression and neurological impairment, in children with symptomatic HIV-1 infection. J Infect Dis. 2003;188:1461–1472. doi: 10.1086/379038. [DOI] [PubMed] [Google Scholar]
- 117.Spector SA, Singh KK, Gupta S, Cysique LA, Jin H, Letendre S, et al. Heaton RK, HNRC Group APOE epsilon4 and MBL-2 O/O genotypes are associated with neurocognitive impairment in HIV-infected plasma donors. AIDS. 2010;24:1471–1479. doi: 10.1097/QAD.0b013e328339e25c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fellay J, Ge D, Shianna KV, Colombo S, Ledergerber B, Cirulli ET, et al. Immunology NCfHAV. Common genetic variation and the control of HIV-1 in humans. PLoS Genet. 2009;5:e1000791. doi: 10.1371/journal.pgen.1000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singh KK, Ellis RJ, Letendre S, Heaton R, Grant I, Spector SA. CCR2 polymorphisms affect neuropsychological impairment in HIV-1-infected adults. J Neuroimmunol. 2004;157(1–2):185–192. doi: 10.1016/j.jneuroim.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 120.Diaz-Arrastia R, Gong Y, Kelly CJ, Gelman BB. Host genetic polymorphisms in human immunodeficiency virus-related neurologic disease. J Neurovirol. 2004;10(Suppl 1):67–73. doi: 10.1080/753312755. [DOI] [PubMed] [Google Scholar]
- 121.Pemberton LA, Stone E, Price P, van Bockxmeer F, Brew BJ. The relationship between ApoE, TNFA, IL1a, IL1b and IL12b genes and HIV-1-associated dementia. HIV Med. 2008;9:677–680. doi: 10.1111/j.1468-1293.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 122.Corder EH, Robertson K, Lannfelt L, Bogdanovic N, Eggertsen G, Wilkins J, et al. HIV-infected subjects with the (4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med. 1998;4:1182–1184. doi: 10.1038/2677. [DOI] [PubMed] [Google Scholar]
- 123.Burt TD, Agan BK, Marconi VC, He W, Kulkarni H, Mold JE, et al. Apolipoprotein (Apo) (4 enhances HIV-1 cell entry in vitro, and the APOE (4/(4 genotype accelerates HIV disease progression. Proc Natl Acad Sci US. 2008;105:8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dunlop O, Goplen AK, Liestøl K, Myrvang B, Rootwelt H, Christophersen B, et al. HIV dementia and apolipoprotein E. Acta Neurol Scand. 1997;95:315–318. doi: 10.1111/j.1600-0404.1997.tb00217.x. [DOI] [PubMed] [Google Scholar]
- 125.Levine AJ, Singer EJ, Sinsheimer JS, Hinkin CH, Papp J, Dandekar S, et al. CCL3 genotype and current depression increase risk of HIV-associated dementia. Neurobeh HIV Med. 2009;1:1–7. doi: 10.2147/nbhiv.s6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummidi S, et al. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci USA. 2002;99:13795–13800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Singh KK, Hughes MD, Chen J, Spector SA. Impact of MCP-1-2518-G allele on the HIV-1 disease of children in the United States. AIDS. 2006;20:475–478. doi: 10.1097/01.aids.0000200540.09856.58. [DOI] [PubMed] [Google Scholar]
- 128.Quasney MW, Zhang Q, Sargent S, Mynatt M, Glass J, McArthur J. Increased frequency of the tumor necrosis factor-alpha-308 A allele in adults with human immunodeficiency virus dementia. Ann Neurol. 2001;50:157–162. [PubMed] [Google Scholar]
- 129.Winkler C, Modi W, Smith MW, Nelson GW, Wu X, Carrington M, et al. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. ALIVE Study, Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC). Science. 1998;279:389–393. doi: 10.1126/science.279.5349.389. [DOI] [PubMed] [Google Scholar]
- 130.Shrestha S, Strathdee SA, Galai N, Oleksyk T, Fallin MD, Mehta S, et al. Behavioral risk exposure and host genetics of susceptibility to HIV-1 infection. J Infect Dis. 2006;193:16–26. doi: 10.1086/498532. [DOI] [PubMed] [Google Scholar]
- 131.Price P, James I, Fernandez S, French MA. Alleles of the gene encoding IL-1alpha may predict control of plasma viraemia in HIV-1 patients on highly active antiretroviral therapy. AIDS. 2004;18:1495–1501. doi: 10.1097/01.aids.0000131352.06784.c6. [DOI] [PubMed] [Google Scholar]
- 132.Levine AJ, Singer EJ, Shapshak P. The role of host genetics in the susceptibility for HIV-associated neurocognitive disorders. AIDS Behav. 2009;13:118–132. doi: 10.1007/s10461-008-9360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pomara N, Belzer KD, Silva R, Cooper TB, Sidtis JJ. The apolipoprotein E (4 allele and memory performance in HIV-1 seropositive subjects: differences at baseline but not after acute oral lorazepam challenge. Psychopharmacology. 2008;201:125–135. doi: 10.1007/s00213-008-1253-1. [DOI] [PubMed] [Google Scholar]
- 134.Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bousman CA, Cherner M, Atkinson JH, Grant I, Tsuang MT, Everall IP. Impact of the catechol-O-methyl-transferase Val158Met polymorphism on executive functioning in the context of HIV-infection and methamphetamine dependence. Neurobehav HIV Med. 2010;2:1–11. doi: 10.2147/NBHIV.S8245. PMCID: PMC2804107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Borjabad A, Brooks AI, Volsky DJ. Gene expression profiles of HIV-1-infected glia and brain: toward better understanding of the role of astrocytes in HIV-1-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2009 doi: 10.1007/s11481-009-9167-1. [Epub ahead of print] PubMed PMID: 19697136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 138.Shapshak P, Duncan D, Minagar D, Rodriguez de la Vega P, Stewart R, Petito CK. Elevated expression of IFN-γ in the HIV-1 infected brain. Front Biosci. 2004;9:1073–1081. doi: 10.2741/1271. [DOI] [PubMed] [Google Scholar]
- 139.Nicolini A, Ajmone-Cat MA, Bernardo A, Levi G, Minghetti L. Human immunodeficiency virus type-1 Tat protein induces nuclear factor (NF)-kappaB activation and oxidative stress in microglial cultures by independent mechanisms. J Neurochem. 2001;79:713–716. doi: 10.1046/j.1471-4159.2001.00568.x. [DOI] [PubMed] [Google Scholar]
- 140.Hurwitz AA, Lyman WD, Berman JW. Tumor necrosis factor alpha and transforming growth factor beta upregulated astrocyte expression of monocyte chemo-attractant protein-1. J Neuroimmunol. 1995;57:193–198. doi: 10.1016/0165-5728(95)00011-p. [DOI] [PubMed] [Google Scholar]
- 141.Erichsen D, Lopez AL, Peng H, Niemann D, Williams C, Bauer M, et al. Neuronal injury regulates fractalkine: relevance for HIV-1 associated dementia. J Neuroimmunol. 2003;138:144–155. doi: 10.1016/s0165-5728(03)00117-6. [DOI] [PubMed] [Google Scholar]
- 142.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, et al. CXCR-4 activated astrocyte glutamate release via TNF-alpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 143.Fine SM, Angel RA, Perry SW, Epstein LG, Rothstein JD, Dewhurst S, et al. Tumor necrosis factor alpha inhibits glutamate uptake by primary human astrocytes. Implications for pathogenesis of HIV-1 dementia. J Biol Chem. 1996;271:15303–15306. doi: 10.1074/jbc.271.26.15303. [DOI] [PubMed] [Google Scholar]
- 144.Yeh MW, Kaul M, Zheng J, Nottet HS, Thylin M, Gendelman HE, et al. Cytokine-stimulated, but not HIV-infected, human monocyte-derived macrophages produce neurotoxic levels of L-cysteine. J Immunol. 2000;164:4265–4270. doi: 10.4049/jimmunol.164.8.4265. [DOI] [PubMed] [Google Scholar]
- 145.Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- 146.Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- 147.Viviani B, Corsini E, Binaglia M, Galli CL, Marinovich M. Reactive oxygen species generated by glia are responsible for neuron death induced by human immunodeficiency virus-glycoprotein 120 in vitro. Neuroscience. 2001;107:51–58. doi: 10.1016/s0306-4522(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 148.Samikkannu T, Saiyed ZM, Rao KV, Babu DK, Rodriguez JW, Papuashvili MN, et al. Differential regulation of indoleamine-2,3-dioxygenase (IDO) by HIV type 1 clade B and C Tat protein. AIDS Res Hum Retroviruses. 2009;25:329–335. doi: 10.1089/aid.2008.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Geschwind DH. DNA microarrays: translation of the genome from laboratory to clinic. Lancet Neurol. 2003;2:275–282. doi: 10.1016/s1474-4422(03)00379-x. [DOI] [PubMed] [Google Scholar]
- 150.Shapshak P, Minagar A, Duran EM, Ziegler F, Davis W, Seth R, et al. Gene expression in HIV associated dementia. In: Minagar A, Alexander JS, editors. Inflammatory disorders of the nervous system, clinical aspects, pathogenesis, and management. Humana Press; Totowa, NJ: 2005. [Google Scholar]
- 151.Albright AV, González-Scarano F. Microarray analysis of activated mixed glial (microglia) and monocyte-derived macrophage gene expression. J Neuroimmunol. 2004;157:27–38. doi: 10.1016/j.jneuroim.2004.09.007. PubMed PMID: 15579277. [DOI] [PubMed] [Google Scholar]
- 152.Galey D, Becker K, Haughey N, Kalehua A, Taub D, Woodward J, et al. Differential transcriptional regulation by human immunodeficiency virus type 1 and gp120 in human astrocytes. J Neurovirol. 2003;9:358–371. doi: 10.1080/13550280390201119. PubMed PMID: 12775419. [DOI] [PubMed] [Google Scholar]
- 153.Roberts ES, Zandonatti MA, Watry DD, Madden LJ, Henriksen SJ, Taffe MA, et al. Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol. 2003;162:2041–2057. doi: 10.1016/S0002-9440(10)64336-2. PubMed PMID: 12759259; PubMed Central PMCID: PMC1868118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Masliah E, Robert ES, Langford D, Everall I, Crews L, Adame A, et al. Patterns of gene dysregulation in the frontal cortex of patients with HIV encephalitis. J Neuroimmun. 2004;157:163–175. doi: 10.1016/j.jneuroim.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 155.Hanson LR, Frey WH. Strategies for intranasal delivery of therapeutics for the prevention and treatment of NeuroAIDS. J Neuroimmune Pharmacol. 2007;2:81–86. doi: 10.1007/s11481-006-9039-x. [DOI] [PubMed] [Google Scholar]
- 156.Agrawal L, Louboutin JP, Reyes BA, Van Bockstaele EJ, Strayer DS. Antioxidant enzyme gene delivery to protect from HIV-1 gp120-induced neuronal apoptosis. Gene Ther. 2006;13:1645–1656. doi: 10.1038/sj.gt.3302821. [DOI] [PubMed] [Google Scholar]
- 157.Zink MC. Translational research models and novel adjunctive therapies for NeuroAIDS. J Neuroimmune Pharmacol. 2007;2:14–19. doi: 10.1007/s11481-006-9062-y. [DOI] [PubMed] [Google Scholar]
- 158.Louboutin JP, Agrawal L, Reyes BA, Van Bockstaele EJ, Strayer DS. Protecting neurons from HIV-1 gp120-induced oxidant stress using both localized intracerebral and generalized intraventricular administration of antioxidant enzymes delivered by SV40-derived vectors. Gene Ther. 2007;14:1650–1661. doi: 10.1038/sj.gt.3303030. [DOI] [PubMed] [Google Scholar]
- 159.Infanti JL. Challenging the gold standard: should mannitol remain our first-line defense against intracranial hypertension? J Neurosci Nurs. 2008;40:362–368. [PubMed] [Google Scholar]
- 160.Sigurdsson EM, Wadghiri YZ, Mosconi L, Blind JA, Knudsen E, Asuni A, et al. T. A nontoxic ligand for voxel-based MRI analysis of plaques in AD transgenic mice. Neurobiol Aging. 2008;29:836–847. doi: 10.1016/j.neurobiolaging.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xu J, Ma C, Bass C, Terwilliger EF. A combination of mutations enhances the neurotropism of AAV-2. Virology. 2005;341:203–214. doi: 10.1016/j.virol.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 162.Terzi D, Zachariou V. Adeno-associated virus-mediated gene delivery approaches for the treatment of CNS disorders. Biotechnol J. 2008;3:1555–1563. doi: 10.1002/biot.200800284. [DOI] [PubMed] [Google Scholar]
- 163.Romano G. Current development of adeno-associated viral vectors. Drug News Perspect. 2005;18:311–316. doi: 10.1358/dnp.2005.18.5.917326. [DOI] [PubMed] [Google Scholar]
- 164.Lu Y. GFP-lentiviral vectors targeting for NeuroAIDS. Methods Mol Biol. 2009;515:177–197. doi: 10.1007/978-1-59745-559-6_12. [DOI] [PubMed] [Google Scholar]
- 165.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, et al. CHARTER Group Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Letendre S, Ellis R, Ances B, McCutchan Neurologic complications if HIV disease and their treatment. Topics HIV Med. 2010;18:45–55. [PMC free article] [PubMed] [Google Scholar]
- 167.DeLuca A, Ciancio B, Larussa D, Murri R, Cingolani A, Rizzo MG, et al. Correlates of independent HIV-1 replication in the CNS and of its control by antiretrovirals. Neurology. 2002;59:342–347. doi: 10.1212/wnl.59.3.342. [DOI] [PubMed] [Google Scholar]
- 168.Gollapudi S, Gupta S. Human immunodeficiency virus I-induced expression of P-glycoprotein. Biochem Biophys Res Commun. 1990;171:1002–1007. doi: 10.1016/0006-291x(90)90783-j. [DOI] [PubMed] [Google Scholar]
- 169.Yao SY, Ng AM, Sundaram M, Cass CE, Baldwin SA, Young JD. Transport of antiviral 3’-deoxy-nucleoside drugs by recombinant human and rat equilibrative, nitro-benzyl-thio-inosine (NBMPR)-insensitive (ENT2) nucleoside transporter proteins produced in Xenopus oocytes. Mol Membr Biol. 2001;18:161–167. doi: 10.1080/09687680110048318. [DOI] [PubMed] [Google Scholar]
- 170.Minuesa G, Purcet S, Erkizia I, Molina-Arcas M, Bofill M, Izquierdo-Useros N, et al. Expression and functionality of antihuman immunodeficiency virus and anticancer drug up-take transporters in immune cells. J Pharmacol Exp Ther. 2008;324:558–567. doi: 10.1124/jpet.107.131482. Epub 2007 Nov 27. [DOI] [PubMed] [Google Scholar]
- 171.Sturmer E, von Moltke LL, Perloff MD, Greenblatt DJ. Differential modulation of P-glycoprotein expression and activity by nonnucleoside HIV-1 reverse transcriptase inhibitors in cell culture. Pharm Res. 2002;19:1038–1045. doi: 10.1023/a:1016430825740. [DOI] [PubMed] [Google Scholar]
- 172.Gibbs JE, Jayabalan P, Thomas SA. Mechanisms by which 2’,3’-dideoxyinosine (ddI) crosses the guinea-pig CNS barriers; relevance to HIV therapy. J Neurochem. 2003;84:725–734. doi: 10.1046/j.1471-4159.2003.01560.x. [DOI] [PubMed] [Google Scholar]
- 173.Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–743. doi: 10.1007/s00424-003-1103-2. Epub 2003 Jun 28. [DOI] [PubMed] [Google Scholar]
- 174.Jung N, Lehmann C, Rubbert A, Knispel M, Hartmann P, van Lunzen J, et al. Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab Dispos. 2008;36:1616–1623. doi: 10.1124/dmd.108.020826. Epub 2008 May 19. [DOI] [PubMed] [Google Scholar]
- 175.Kim AE, Dintaman JM, Waddell DS, Silverman JA. Saquinavir, an HIV protease inhibitor, is transported by P-glycoprotein. J Pharmacol Exp Ther. 1998;286:1439–1445. [PubMed] [Google Scholar]
- 176.Lee LS, Soon GH, Shen P, Yong EL, Flexner C, Pham P. Darunavir/ritonavir and Efavirenz exert differential effects on MRP1 transporter expression and function in healthy volunteers. Antivir Ther. 2010;15:275–279. doi: 10.3851/IMP1505. [DOI] [PubMed] [Google Scholar]
- 177.Fujimoto H, Higuchi M, Watanabe H, Koh Y, Ghosh AK, Mitsuya H, et al. P-glycoprotein mediates efflux transport of darunavir in human intestinal Caco-2 and ABCB1 gene-transfected renal LLC-PK1 cell lines. Biol Pharm Bull. 2009;32:1588–1593. doi: 10.1248/bpb.32.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vierling P, Greiner J. Prodrugs of HIV protease inhibitors. Curr Pharm Des. 2003;9:1755–1770. doi: 10.2174/1381612033454441. [DOI] [PubMed] [Google Scholar]
- 179.Eilers M, Roy U, Mondal D. MRP (ABCC) transporters-mediated efflux of anti-HIV drugs, Saquinavir and zidovudine, from human endothelial cells. Exp Biol Med (Maywood) 2008;233:1149–1160. doi: 10.3181/0802-RM-59. Epub 2008 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zastre JA, Chan GN, Ronaldson PT, Ramaswamy M, Couraud PO, Romero IA, et al. Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J Neurosci Res. 2009;87:1023–1036. doi: 10.1002/jnr.21898. [DOI] [PubMed] [Google Scholar]
- 181.Gupta A, Zhang Y, Unadkat JD, Mao Q. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2). J Pharmacol Exp Ther. 2004;310:334–341. doi: 10.1124/jpet.104.065342. Epub 2004 Mar 8. [DOI] [PubMed] [Google Scholar]
- 182.Perloff MD, von Moltke LL, Fahey JM, Greenblatt DJ. Induction of P-glycoprotein expression and activity by ritonavir in bovine brain microvessel endothelial cells. J Pharm Pharmacol. 2007;59:947–953. doi: 10.1211/jpp.59.7.0006. [DOI] [PubMed] [Google Scholar]
- 183.Dumond JB, Vourvahis M, Rezk NL, Patterson KB, Tien HC, White N, et al. A phenotype-genotype approach to predicting CYP450 and P-glycoprotein drug interactions with the mixed inhibitor/inducer tipranavir/ritonavir. Clin Pharmacol Ther. 2010;87:735–742. doi: 10.1038/clpt.2009.253. Epub 2010 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Walker DK, Bowers SJ, Mitchell RJ, Potchoiba MJ, Schroeder CM, Small HF. Preclinical assessment of the distribution of Maraviroc to potential human immunodeficiency virus (HIV) sanctuary sites in the central nervous system (CNS) and gut-associated lymphoid tissue (GALT). Xenobiotica. 2008;38:1330–1339. doi: 10.1080/00498250802447409. [DOI] [PubMed] [Google Scholar]
- 185.Price RW, Parham R, Kroll JL, Wring SA, Baker B, Sailstad J, et al. Enfuvirtide cerebrospinal fluid (CSF) pharmacokinetics and potential use in defining CSF HIV-1 origin. Antivir Ther. 2008;13:369–374. [PMC free article] [PubMed] [Google Scholar]
- 186.Morris KA, Davies NWS, Brew BJ. A guide to interpretation of neuroimmunological biomarkers in the combined antiretroviral therapy-era of HIV central nervous system disease. Neurobehav HIV Med. 2010;2:59–72. doi: 10.2147/NBHIV.S7167. [Google Scholar]
- 187.Diesing TS, Swindells S, Gelbard H, Gendelman HE. HIV-1-associated dementia: a basic science and clinical perspective. AIDS Read. 2002;12:358–368. [PubMed] [Google Scholar]
- 188.Smits HA, Boven LA, Pereira CF, Verhoef J, Nottet HS. Role of macrophage activation in the pathogenesis of Alzheimer's disease and human immunodeficiency virus type 1-associated dementia. Eur J Clin Invest. 2000;30:526–535. doi: 10.1046/j.1365-2362.2000.00661.x. [DOI] [PubMed] [Google Scholar]
- 189.Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28–35. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Alter G, Ananworanich J, Pantophlet R, Rybicki EP, Buonaguro L. Report on the AIDS vaccine 2008 conference. Hum Vaccine. 2009;5:119–125. doi: 10.4161/hv.5.3.7557. [DOI] [PubMed] [Google Scholar]
- 191.Bass E, Feuer C, Warren M. AIDS vaccine research and advocacy: an update. BETA. 2009;21:24–30. [PubMed] [Google Scholar]
- 192.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 193.Koff WC, Berkley SF. The renaissance in HIV vaccine development–: future directions. N Engl J Med. 2010 doi: 10.1056/NEJMp1007629. [DOI] [PubMed] [Google Scholar]
- 194.Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010 doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zolla-Pazner S, Cardozo T. Structure–function relationships of HIV-1 envelope sequence-variable regions refocus vaccine design. Nature Rev Immunol. 2010;10:527–535. doi: 10.1038/nri2801. doi: 10.1038/nri2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, et al. Rational design of envelope surface identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kangueane P, Kayathri R, Kishore M, Flower DR, Sadler K, Chiappelli F, et al. Designing HIV gp120 peptide vaccines: rhetoric or reality for NeuroAIDS, Chapter 9. In: Goodkin K, Shapshak P, Verma A, editors. The spectrum of NeuroAIDS disorders: pathophysiology, diagnosis, and treatment. ASM Press; Washington, DC: 2008. pp. 105–119. [Google Scholar]
- 198.DeGroot AS, Marcon L, Bishop EA, Rivera D, Kutzler M, Weiner DB, et al. HIV vaccine development by computer-assisted design: the GAIA vaccine. Vaccine. 2005;23:2136–2148. doi: 10.1016/j.vaccine.2005.01.097. [DOI] [PubMed] [Google Scholar]
- 199.Mohanapriya A, Nandagond S, Shapshak P, Kangueane U, Kangueane P. A HLA-DRB supertype chart with potential overlapping peptide binding function. Bioinformation. 2010;4:300–309. doi: 10.6026/97320630004300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 201.Michaud J, Fajardo R, Charron G, Sauvageau A, Berrada F, Ramla D, et al. Neuropathology of NFHgp160 transgenic mice expressing HIV-1 env protein in neurons. J Neuropathol Exp Neurol. 2001;60:574–587. doi: 10.1093/jnen/60.6.574. [DOI] [PubMed] [Google Scholar]
- 202.Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tyor WR, Power C, Gendelman HE, Markham RB. A model of human immunodeficiency virus encephalitis in SCID mice. PNAS, USA. 1993;90:8658–8662. doi: 10.1073/pnas.90.18.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hartman K. Feline immunodeficiency virus infection: an overview. Vet J. 1998;155:123–137. doi: 10.1016/S1090-0233(98)80008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fox HS, Phillips TR. FIV and NeuroAIDS. J Neurovirol. 2002;8:155–157. doi: 10.1080/13550280290049714. [DOI] [PubMed] [Google Scholar]
- 206.Manigat F, Vivithanaporn P, Zhu Y, Taylor A, Baker G, Pearson K, et al. Neurobehavioral performance in feline immunodeficiency virus infection: integrated analysis of viral burden, neuroinflammation, and neuronal injury in cortex. J Neurosci. 2009;29:8429–8437. doi: 10.1523/JNEUROSCI.5818-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ward JM, O'Leary TJ, Baskin GB, Benveniste R, Harris CA, Nara PL, et al. Immunohistochemical localization of human and simian immunodeficiency viral antigens in fixed tissue sections. Am J Pathol. 1987;127:199–205. [PMC free article] [PubMed] [Google Scholar]
- 208.Watry D, Lane TE, Streb M, Fox HS. Transfer of neuropathogenic simian immunodeficiency virus with naturally infected microglia. Am J Pathol. 1995;146:914–923. [PMC free article] [PubMed] [Google Scholar]
- 209.Zink MC, Suryanarayana K, Mankowski JL, Shen A, Piatak M, Jr, Spelman JP, et al. High viral-load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Novembre FJ, DeRosayro J, O'Neil SP, Anderson DC, Klumpp SA, McClure HM. Isolation and characterization of a neuropathogenic simian immunodeficiency virus derived from a sooty mangabey. J Virol. 1998;72:8841–8851. doi: 10.1128/jvi.72.11.8841-8851.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, et al. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV NeuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]