Abstract
Perturbations in dynamic properties of mitochondria including fission, fusion, and movement lead to disruption of energy supply to synapses contributing to neuropathology and cognitive dysfunction in Alzheimer’s disease (AD). The molecular mechanisms underlying these defects are still unclear. Previously, we have shown that ERβ is localized in the mitochondria and ERβ knock down disrupts mitochondrial functions. Because a selective ERβ modulator (DPN) can activate PKA, and localized PKA signaling in the mitochondrial membrane regulates mitochondrial structure and functions, we reasoned that ERβ signaling in the mitochondrial membrane rescues many of the mitochondrial defects caused by soluble Aβ oligomer. We now report that DPN treatment in primary hippocampal neurons attenuates soluble Aβ-oligomer induced dendritic mitochondrial fission and reduced mobility. Additionally, Aβ treatment reduced the respiratory reserve capacity of hippocampal neuron and inhibited phosphorylation of Drp1 at its PKA site, which induces excessive mitochondrial fission, and DPN treatment ameliorates these inhibitions Finally, we discovered a direct interaction of ERβ with a mitochondrial resident protein AKAP1, which induces the PKA-mediated local signaling pathway involved in increased oxidative phosphorylation and inhibition of mitochondrial fission. Taken together, our findings highlight the possibility that ERβ signaling pathway may be a useful mitochondria-directed therapeutic target for AD.
Keywords: Estrogen receptor β, Alzheimer’s disease, AKAP1, PKA, mitochondrial fission and fusion, mitochondrial movement
1. Introduction
Brain executes cognitive functions by computing time-dependent pre-and post-synaptic spiking (Izhikevich, 2006). Neuronal computation is energetically expensive. Energy supply needed for executing cognitive function by spiking neurons is maximized by mechanisms that increase mitochondrial ATP production in response to synaptic activity and trafficking of mitochondria to active synapses (Attwell and Gibb, 2005). Thus, mitochondria are essential for synaptic function and spike transmission through normal mitochondrial energy production, biogenesis, and movement between soma and the synaptic zone (Li et al., 2004) In vitro studies and AD mice models have shown that amyloid β (Aβ) directly perturbs mitochondrial function, causes decreased ATP production, increased Ca2+, excessive fragmentation, and inhibition of trafficking of mitochondria in axons (Suen et al., 2008; Rhein et al., 2009; Du et al., 2010; Manczak and Reddy, 2012). Perturbations in dynamic properties of mitochondria including fission, fusion, trafficking and turnover, can lead to synaptic dysfunction (Li et al., 2004), apoptosis (Suen et al., 2008) and necroptosis (Wang et al., 2012) seen in AD. AD pathogenesis believed to be driven by β-amyloid peptide (Aβ) causes impairment of oxidative phosphorylation (Rhein et al., 2009), and deceleration of movement of synaptic mitochondria (Suen et al., 2008).
In addition, mitochondrial fission protein Drp1 interacts with Aβ and phosphorylated tau in AD neurons (Manczak and Reddy, 2012). Aβ overproduction in neuroblastoma cell line (Xinglong Wang et al., 2008) and Aβ peptide treatment in mouse hippocampal neurons (Calkins MJ, and Reddy PH., 2011), cause abnormal mitochondrial dynamics as a result of modulation of mitochondrial fission or fusion proteins. Impaired mitochondrial dynamics and synaptic degeneration also found in a mouse model of AD (Calkins et al., 2011). Further, it has been reported that in AD patients neurons expression of mitochondrial fission genes Drp1 and Fis1 is increased and mitochondrial fusion genes Mfn1, Mfn2, and Opa1 is decreased (Manczak M et al., 2011). In addition, mitochondrial fission protein Drp1 interacts with Aβ and phosphorylated tau in AD neurons (Manczak and Reddy, 2012). Thus, abnormal mitochondrial dynamics and excessive mitochondrial fission could lead to bioenergetics dysfunction in AD neurons. In fact, bioenergetics dysfunction, as measured by changes in oxygen consumption, respiratory coupling and glucose utilization has been observed in the mitochondria from AD and mild cognitive impairment (MCI) patients (Silva et al., 2013) and in the mitochondria from AD mice (Yao et al., 2009). It has also been reported that defective mitochondrial biogenesis contribute to mitochondrial abnormalities in AD could be rescued by cAMP dependent PKA/CREB activation pathway in Aβ overexpressed cells (Sheng et al., 2012). Therefore, amelioration of mitochondrial dysfunction is a potential therapeutic target for AD.
Although we are beginning to understand the nature of Aβ-induced mitochondrial defects, the molecular mechanisms underlying these defects are still unclear. A better understanding of how Aβ causes defects in mitochondria will be invaluable both in understanding synaptic dysfunction and in ameliorating mitochondrial defects in AD. Important clues have been provided by studies showing that, i) Aβ inactivates PKA activity in AD brains and in cultured hippocampal neurons (Vitolo et al., 2002). ii) Estrogen and selective estrogen receptor modulators activate PKA and induces PKA signaling cascades (Liu et al., 2008). iii) ERβ resides in the outer mitochondrial membrane and knock down of ERβ affect mitochondrial function (Yang et al., 2009). iv) Potentiation of brain mitochondrial function by estrogen receptor β-selective ligands (Yao et al., 2013). v) Mitochondrial outer membrane protein kinase A/A kinase anchoring protein 1 (PKA/AKAP1) and phosphatase (Feliciello et al., 2005; Cardone et al., 2004) complex, by signaling locally control fission and fusion of mitochondria, mitochondrial network integrity, movement of mitochondria, cell survival and oxidative phosphorylation (Livigni et al., 2006), and mitochondrial biogenesis in a reversible manner of phosphorylation-dephosphorylation of Drp1, thereby increasing oxidative phosphorylation by phosphorylating respiratory chain enzymes (Chang and Blackstone, 2007; Carlucci et al., 2008; Dickey and Strack, 201). And vi) dephosphorylation of Drp1 at a highly conserved Ser residue in the c-terminal GTPase effector domain (Ser 617, Ser 637, Ser 656) by calcineurin (protein phosphatase 2B) promote mitochondrial fragmentation (Cribbs and Strack, 2007; Cereghetti et al., 2008). Protein kinase A (PKA)-mediated phosphorylation at the same site inhibits Drp1 to elongate mitochondria via unopposed fusion (Chang and Blackstone, 2007; Cribbs and Strack, 2007). Vii) PKA is targeted to the outer mitochondrial membrane (OMM) by A kinase anchoring protein 1 (AKAP1, also known as D-AKAP1, AKAP121, AKAP149, and s-AKAP84) (Carlucci et al., 2008) enhancing Drp1 phosphorylation, mitochondrial elongation, and neuroprotection (Merrill et al., 2011).
Based on these clues, we reasoned that mitochondrial PKA is inhibited by mitochondrial accumulated Aβ that leads to uninhibited dynamine-related protein 1 (Drp1) activity causing excessive mitochondrial fragmentation and that estrogens and mitochondrial membrane ERβ through interaction with PKA/AKAP1 complex, form local signal transduction units and ameliorates inactivation of PKA-mediated Drp1 phosphorylation and Aβ-induced defects in mitochondrial structure and function.
2. Results
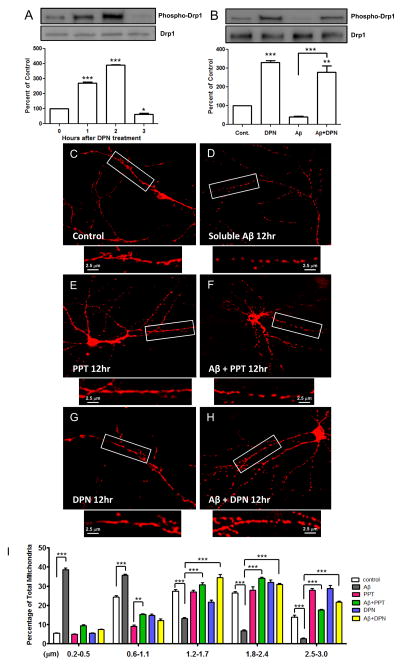
It has been shown that soluble Aβ-oligomers inhibit PKA (Vitolo et al., 2002), whereas ERβ agonist induces activation of PKA-CREB pathway (Liu et al., 2008). Also, Aβ accumulates in the mitochondria through the interaction with the mitochondrial resident protein alcohol dehydrogenase ABAD (Lustbader et al., 2004). Because both PKA and ERβ resides in the mitochondrial membrane (Yang et al., 2009; Dickey and Strack, 2011), and phosphorylated Drp1 at PKA site inhibits mitochondrial fission (Chang and Blackstone, 2007), we reasoned that mitochondrial PKA is inhibited by mitochondrial accumulated Aβ, leading to uninhibited excessive fission and estrogens by ERβ-mediated activation of PKA phosphorylate Drp1, ameliorates Aβ-induced mitochondrial excessive fragmentation. To determine whether inhibition of Drp1 phosphorylation by Aβ can be ameliorated by estrogens, we applied ERβ agonist DPN and soluble Aβ-oligomers to rat hippocampal primary neurons. To this end, we first sought to determine the effects of ERβ specific ligand, DPN on the phosphorylation status of Drp1 in normal and soluble Aβ-oligomer treated primary hippocampal neuron. Treatment of 50nM DPN in E18 rat hippocampal neuron cultured for 15 days in vitro rapidly induced phosphorylation of Drp1 at PKA sites when normalized with total Drp1 (Fig 1A). Soluble synthetic Aβ (1-42) oligomer (500nM) treatment for 2 hrs partially inhibits but DPN along with Aβ treatment resulted in amelioration of Aβ-induced inhibition (Fig 1B).
Fig. 1.
Estrogens rapidly enhance phosphorylation of Drp1 (ser637), rescue Aβ-induced inhibition of Drp1 phosphorylation and reduce mitochondrial fission in dendrites of primary rat hippocampal neuron. A) Effects of DPN (50 nM) on Drp1 phosphorylation. Expression levels were normalized to actin and presented as mean ± SD compared to the vehicle (**p< 0.01 versus vehicle; n = 4). B) Effects of Aβ (Aβ1-42 oligomers 200 nM) and DPN (50 nM) on Drp1 phosphorylation. Expression levels were normalized to actin (mean ± SD, ***p< 0.001 DPN versus vehicle; and ***p< 0.001 versus the connected group, n= 4). C–H) Effects of Aβ, PPT, DPN and their combination on mitochondrial length. I) Lengths of dendritic mitochondria. **p< 0.01 and ***p< 0.001 versus the connected groups. N = 600 (Vehicle), 450 (Aβ), 400 (PPT), 480 (Aβ + PPT), 550 (DPN) and 500 (Aβ + DPN).
We next asked whether estrogen signaling could inhibit Aβ-induced mitochondrial fragmentation. Because both ERβ and ERβ are present in the mitochondria, we used both receptors ligands to evaluate the changes in mitochondrial morphology by measuring average dendritic branches mitochondrial lengths as visualized by mitoDS red expression plasmid transfected hippocampal neurons. We selected dendritic branches on the basis of neuronal morphology for quantitative analysis. Representative confocal micrographs showed that 200nM Aβ results in severe fragmentation (Fig 1D) compared to control (Fig 1C), and both ERβ agonist PPT (Fig 1F) and ERβ agonist DPN (Fig 1H) ameliorates Aβ effects as evident in more elongated mitochondrial structure compared to Aβ alone. Quantitative analysis (Fig 1I) revealed that there was a significantly decreased average mitochondrial length following soluble Aβ-oligomer treatment as compared with control. Both PPT and DPN treatment attenuates Aβ-induced fragmentation as evident by an increasing percentage of longer mitochondria compared to Aβ alone. These results suggest that estrogen signaling prevents Aβ-induced dendritic mitochondrial fragmentation.
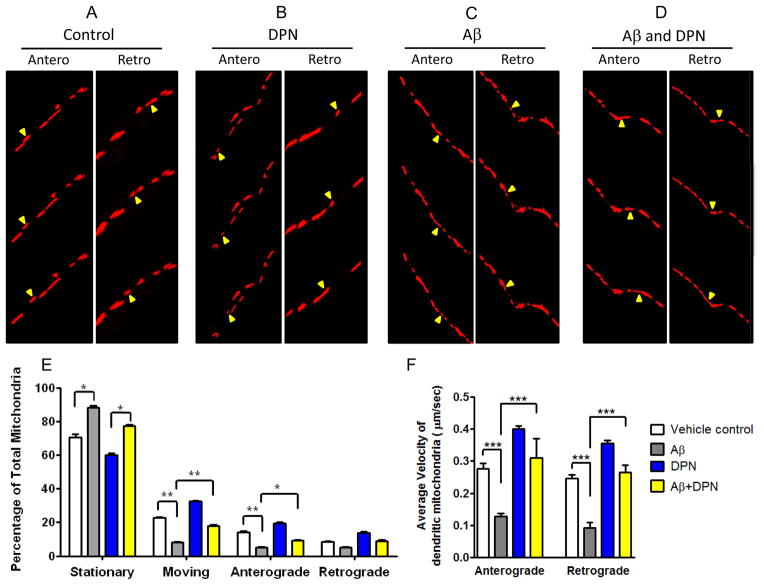
Mitochondrial transport is regulated by a series of molecular adaptors that mediate the attachment of mitochondria to molecular motors. Specifically, connection of mitochondria to kinesin motor involves the outer mitochondrial protein Miro, which indirectly attaches to kinesin heavy chain via the adaptor protein Milton. Mitochondrial transport is influenced by fission protein Drp1, which directly or indirectly interacts with the Miro/Milton complex (Saotome et al., 2008). It has been shown that Aβ-induced fragmented mitochondria moves at a slower rate in axons (Du et al., 2010). We monitored mitochondrial movement within dendritic branches. Quantitatively, two mobility characteristics were recorded for each mitochondrion: (i) whether it moved and (ii) whether movement was in the anterograde or retrograde direction. Subsequently, the relative percentage of stationary, mobile, and anterograde and retrograde moving mitochondria were calculated. Representative videos of moving mitochondria (Supplemental Video1–4) and Fig 2A–D show that Aβ treatment resulted in reduction in both total number of moving mitochondria and their velocity, whereas 2 hrs treatment with 50nM DPN to the same Aβ treated dendritic region increased both the number of moving mitochondria as well as their velocity. For quantitative analysis, we calculated the relative percentage of stationary, mobile, and anterograde and retrograde moving mitochondria. Fig 2E indicates that for Aβ-treated neurons, total number of moving mitochondria was decreased. DPN treatment alone resulted in increased movement compared to control, and Aβ+DPN treatment increased total movement and anterograde movement but had no significant effect on retrograde movement. When calculated separately the Aβ treatment resulted in lowering average velocity of anterograde and retrograde moving mitochondria whereas Aβ+DPN treatment increased the average velocity. Taken together, these studies raise the possibilities that DPN signaling through the interaction of mitochondrial resident ERβ with the mitochondrial resident PKA inactivates Drp1 activity by PKA site phosphorylation and exert an important role in ameliorating defects of dynamic properties of mitochondria in the dendritic branches of primary hippocampal neurons.
Fig. 2.
Ameliorating effects of DPN on Aβ-induced defects in mitochondrial movement in dendrites of primary rat hippocampal neuron. A–D). Representative snapshot images from time lapse video showing mitochondrial movement in vehicle (A) Aβ (B), DPN (C), and Aβ + DPN (D) treatment groups. (E) Percentage of stationary and moving mitochondria. Depicted are mean ± SD. *p< 0.05 vs. the connected groups, **p< 0.01 vs. the connected groups. N = 600 (Vehicle), 450 (Aβ), 550 (DPN) and 500 (Aβ + DPN) mitochondria. (F) Analysis of mean anterograde and retrograde velocity of movable mitochondria (μm/sec) is shown. Depicted are mean ± SD. ***p< 0.001 vs. the groups connected. N= 58 (Vehicle), 61 (Aβ), 62 (DPN) and 62 (Aβ + DPN) dendrites.
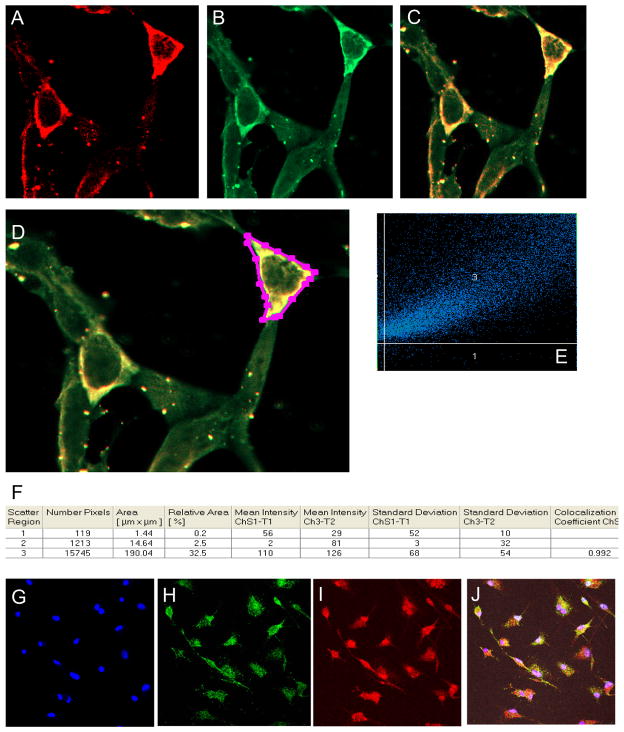
Next, we reasoned that if ERβ mediated PKA/AKAP1 signaling occurs locally in the mitochondrial membrane then both ERβ and AKAP1 have to interact either in the mitochondrial membrane or in the cytoplasm first then transport to the mitochondria, because ERβ does not possess mitochondrial localization signal. In order to test this possibility, first we ectopically expressed HA-tagged fulllength ERβ and DDK-tagged AKAP1 protein by transiently transfecting respective expression vector in HEK 293 cells and assessed colocalization by immunoflurescence analysis. Photomicrographs of cells expressing DDK- and HA-tagged proteins shown in Fig 3A–3F were analyzed for colocalization of AKAP1 and ERβ, using Zeiss LSM software. We observed a strong colocalization of 99.2% between the two proteins, suggesting a molecular interaction. Very high ectopic expression of both AKAP1 and ERβ in HEK 293 cells may or may not represent true colocalzation of endogenous AKAP1 and ERβ in primary neurons. Thus, we determined localization of endogenous AKAP1 and ERβ in primary rat cortical neurons by sequential immunostaining. Photomicrographs of primary neurons shown in Fig 3G–3J clearly indicate that both proteins are colocalized.
Fig. 3.
Localization of AKAP1 and ERβ in the mitochondria. HEK 293 cells were transfected with equimolar concentrations of C-terminal DDK fused to AKAP1 and C-terminal HA fused to ERβ expression vector (both GeneCopoeia) plasmid DNA, using lipofectamine 2000 reagent (Invitrogen, CA, USA). Forty eight hours after transfection, cells were immunostained with anti-DDK mouse and anti-HA rabbit primary antibody followed by Alexa 633 anti-mouse and Alexa 488 anti-rabbit secondary antibody. Cells were then visualized by Zeiss LSM510 confocal microscope. Photomicrographs were analyzed for colocalization of AKAP1 and ERβ, using Zeiss LSM software. A) Red, DDK antibody, B) Green, HA antibody, C) Merged, and D) Marked cell used for co-localization analysis as shown in (E) and in the tabular form in F. For the localization of endogenous AKAP1 and ERβ, rat primary neurons were sequentially immunostained with anti-AKAP1 and anti-ERβ primary antibody. Photomicrographs were shown in blue for DAPI (G), in red for ERβ (H), in green for AKAP1 (I) and merged (J).
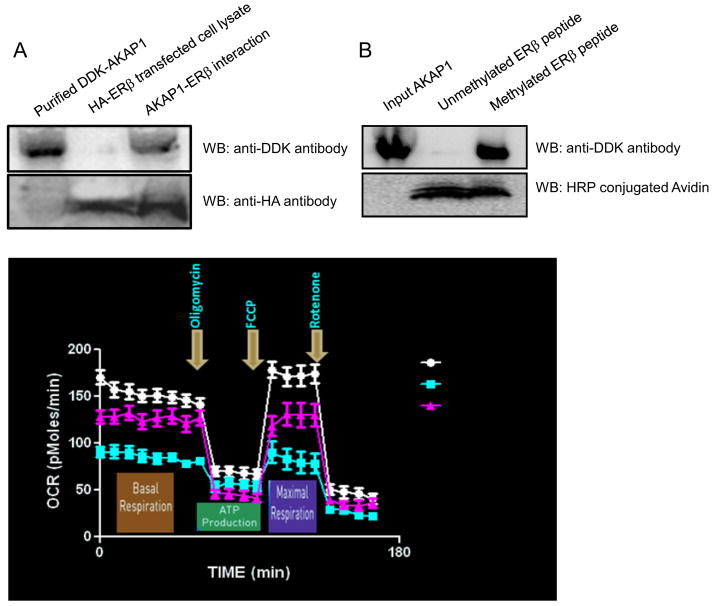
To test for a molecular interaction of AKAP1 with ERβ, we expressed the respective tagged AKAP1 and ERβ genes in HEK 293 cells, purified and their physical interaction was determined by pull-down assay (see Supplemental Fig 1) followed by western blot analysis. Purified and immobilized DDK-AKAP1 protein in anti-DDK antibody conjucated agarose beads when allowed to interact with HA-ERβ transfected HEK 293 cell lysate, eluted protein fraction showed both protein in western blot analysis (Fig 4A). For further analysis of the specific domain of PKA involved with ERβ interaction, we reasoned that because AKAP1 contains a tudor domain and it binds arginine dimethylated proteins (Côté and Richard, 2005) and dimethylated ERβ triggers its interaction with various kinases (Le Romancer et al., 2008), AKAP1 interacts with arginine methylated ERβ. To test this possibility, we analysed the synthetic arginine dimethylated and unmethylated ERβ peptide and full length purified DDK-AKAP1 protein interaction by pull-down assay (see Supplemental Fig 2). 100nM of respective peptide were allowed to interact with the same ammount of immobilized DDK-AKAP1 in agarose beads and after extensive washing, interactant was analysed by western blot. Fig 4B shows that methylated but not unmethylated ERβ specific peptide strongly binds with purified full length tudor domain containing AKAP1 protein. Taken together these results strongly indicate that dimetylated ERβ binds to AKAP1.
Fig. 4.
Mitochondrial resident AKAP1 protein (AKAP149) interacts with ERβ protein. (A) Top panel, immunoblot of pull-down assay with anti-DDK antibody; bottom panel, same blot after stripping and probing with anti-HA antibody. (B) AKAP1 protein interacts with arginine methylated domain containing ERβ peptide with high affinity. Top panel, immunoblot of pull-down assay with anti-DDK antibody; bottom panel same blot without stripping and probing with anti-Avidin-HRP antibody. (C) Aβ treated hippocampal neurons exhibit decreased respiration and DPN ameliorates Aβ-induced inhibition of respiration. Arrows indicate time of addition of oligomycin (1 μM), FCCP (1 mM), and rotenone (1 μM). Oxygen consumption rates (OCR) in Aβ treated primary neurons are lower than control and DPN treatment ameliorates both basal and maximal OCR. N= 4 and data are expressed as mean ± SD.
Finally, we ask whether amelioration of structural defects by DPN is reflected in improved mitochondrial function. We assessed mitochondrial function by measuring oxygen consumption rate in hippocampal neuron. Fig 4C shows that Aβ treatment inhibited both basal as well as maximum oxygen consumption rate when compared to vehicle control, whereas DPN improved oxygen consumption rate in Aβ treated hippocampal neurons.
3. Discussion
Neuronal mitochondria are vital for fueling the intense energy demands associated with synaptic transmission. Energy supply is maximized by mechanisms that increase mitochondrial ATP production in response to synaptic activity and targeting mitochondria to active synapses (Attwell and Gibb, 2005). It has been shown that synaptic activity modulates the motility and fusion/fission balance of mitochondria and controls mitochondrial distribution in dendrites and spines (Li et al., 2004).
Also, molecular manipulation of genes important for fission, Drp1 and fusion, OPA1 results in reduction of dendritic mitochondria content and loss of synapses and dendritic spines, whereas increasing dendritic mitochondrial content or mitochondrial activity enhances the number and plasticity of spines and synapses (Li et al., 2004). In AD mice, studies indicate the impairment of mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in neurons (Calkins et al., 2011). Recent studies indicate that levels of Drp1 activity significantly increased in the cortex tissues from AD patients compared to age matched control and also in the cerebral cortex from the AD mice (Manczak and Reddy, 2012) due to increased interaction of hyperphosphorylated tau and Aβ with Drp1. This enhanced Drp1 activity could lead to excessive fragmentation of mitochondria in AD neurons. Thus, perturbations in dynamic properties of mitochondria occur in AD, and consequently reversing the defects might provide a mitochondria-directed therapy.
In this study, we demonstrated that soluble synthetic Aβ1-42 inhibited phosphorylation of mitochondrial fission inducing protein, Drp1 at its protein kinase A (PKA)-dependent phosphorylation site. The inhibition of Drp1 phosphorylation was partially restored by treatment of estrogen receptor β agonist which is known to be involved in activating PKA-mediated signaling pathways (Liu et al., 2008). These results suggest that Aβ-induced inhibition of phosphorylation of Drp1 may lead to unimpeded mitochondrial fission resulting in inefficient ATP generation. Our results also raise the possibility that activation of PKA signaling by estrogens in hippocampal neurons inhibits Aβ-induced mitochondrial fragmentation due to uninhibited fission.
High efficiency ATP producing mitochondria are continuously generated in active neuron by the controlled collective processes such as fission, fusion, movement, and biogenesis. Also, activated mitochondrial resident PKA by phosphorylating mitochondrial fission protein Drp1 generates mitochondria that have high efficiency ATP producing capacity due to unopposed fusion. PKA mediated phosphorylation of respiratory chain complex enzymes promotes high rates ATP synthesis (Carlucci et al., 2008). In the present study, we have observed that estrogen signaling ameliorates Aβ-induced dendritic mitochondrial fragmentations. Because, the ERβ activation by DPN induces phosphorylation of Drp1 in hippocampal neurons and phosphorylation of Drp1 inhibits mitochondrial fission, our results are the first which showed that estrogen signaling via inducing PKA signaling cascades can ameliorate Aβ-induced excessive mitochondrial fragmentation.
Mitochondrial transport is regulated by a series of molecular adaptors that mediate the attachment of mitochondria to molecular motors. Specifically, connection of mitochondria to kinesin motor involves the outer mitochondrial protein Miro, which indirectly attaches to kinesin heavy chain via the adaptor protein Milton (Fransson et al., 2006). Fission protein Drp1 and fusion protein Mitofusion 2 directly or indirectly interacts with the Miro/Milton complex (Saotome et al., 2008). It has been shown that Aβ-induced fragmented mitochondria moves at a slower rate in axons (Du et al., 2010). The transport of mitochondria in dendrites and mitochondrial distribution at synapses are critical for neurotransmission, synaptic plasticity, and axonal outgrowth (Li et al., 2004). Mutations in proteins that regulate mitochondrial dynamics compromise synaptic function and plasticity (Li et al., 2004; Verstreken, et al., 2005) and defective mitochondrial trafficking and dynamics are implicated in AD (Chang et al., 2006; Rui et al., 2006; Wang et al., 2009). Our findings clearly demonstrate that soluble Aβ oligomer impaired mitochondrial mobility in dendrites of hippocampal neurons and ERβ activation by DPN in hippocampal neurons partially restores both the retrograde and anterograde movements in the dendrites.
A decline in mitochondrial respiration and enzymes required for bioenergetics in vivo occurred in 3xTg-AD mice as early as 3 months of age (Yao et al., 2009). We observed that Aβ-induced structural/dynamic defects influences mitochondrial function in primary hippocampal neuron as Aβ treatment inhibited basal and maximum oxygen consumption rate (OCR), whereas DPN partially restored OCR.
Recently, we and others have demonstrated the functional importance of mitochondrial resident ERs in neurons and in breast cancer cells (Pedram et al., 2006; Yang et al., 2009). However, we have not hitherto demonstrated a molecular mechanism(s) by which localized mitochondrial ERs and not the plasma membrane-associated ERs mediated signaling could lead to neuroprotection or breast cancer cell survival. Our observation clearly demonstrate that ectopically expressed ERβ and mitochondrial resident AKAP1 protein in HEK-293 cells colocalize in the mitochondria and arginine methylation site spanning ERβ domain peptides interacts strongly with mitochondrial resident AKAP1 protein. Taken together, these results strongly indicate that dimethylated ERβ binds to AKAP1. Further, DPN ligand-ERβ-PKA-AKAP1 interaction induced signaling phosphorylates Drp1 and attenuate Aβ-induced mitochondrial fragmentation and inhibition of dynamic properties of mitochondria in the dendrites of neurons. Thus, our findings that the ERβ specific ligand, DPN, acting on mitochondrial resident ERβ can attenuate the mitochondrial defects caused by Aβ treatment, highlight the possibility that this pathway may be a useful mitochondria-directed therapeutic target for Alzheimer’s disease.
4. Experimental procedure
4.1. Primary Neuronal Cultures
At embryonic day 18 (E18), pregnant rats were anesthetized and cervically dislocated. The brains of pups were removed and placed into magnesium (Mg2+) free Hank’s balance salt solution (HBSS). Cortices and hippocampi were removed under a dissecting microscope, washed, and placed into neurobasal culture media (without phenol red) supplemented with B27 and pen-strep (all from Gibco, Carlsbad, CA). The hippocampi were triturated using a graded series of fine polished Pasteur pipettes, and then filtered through a 40 μm nylon cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ). The neurons were plated on poly-L-lysine coated 100mm dishes and glass coverslips, and cultured in vitro in 95% humidity and 5% CO2 atmosphere for 15 days. At day 2 cells were treated with 5 μM 1-beta-D- arabinofuranosylcytosine (AraC) to inhibit glial cell growth.
4.2. Preparation of Soluble Oligomer
Synthetic Aβ1–42 (Tocris, Ellisville, Missouri) oligomer was prepared without the fibrillar component according to the published method (Barghorn et al. 2005). In brief, Aβ1–42 was dissolved in 1,1,1,3,3,3- hexafluoro-2-propanol to 1 mM. The clear solution was then evaporated to dryness. Dried peptide was diluted in DMSO to 5 mM and sonicated for 10 min in bath sonicator. The peptide solution was resuspended in cold Neuro basal medium and immediately vortexed. The solution was then incubated at 4 °C for 24 h. After high speed centrifugation the supernatant was collected and it was comprised of fibrillar-free oligomers, as well as monomers as visualized by polyacrylamide gel electrophoresis and silver staining.
4.3. Western Blot Analysis
After the respective treatments, primary neurons were homogenized in 100 μL of ice cold buffer containing 50 mM Tris, 10 mM Mg2+, 1 mM EDTA, 1 mM EGTA, 10 mM benzamide, 100 ng/ml leupeptin, 100 ng/ml aproteinin, 0.08 mM sodium molybdate, 0.01% tritonX-100, 10 μM okadoic acid, and 2 mM sodium pyrophosphate, pH 7.4. Aliquots of the lysed and sonicated homogenate were taken to determine protein concentration using protein assay reagent (Bio-Rad Laboratories, Hercules, CA). Samples containing 30 μg of protein were electrophoresed on a SDS/PAGE gel. The protein was transferred onto PVDF membrane (Millipore, Billerica, MA), blocked for 1 hour with PBS containing 4% non-fat dried milk, and probed overnight at 4 °C with primary antibody. Primary antibodies rabbit mAb phospho-Drp1(Ser 637) (Cell Signaling Technology, Danvers, MA), mouse monoclonal β-Actin (Santa Cruz, CA), anti-DDK monoclonal antibody (OriGene Technologies, Rockville, MD), HA-Tag rabbit polyclonal (OriGene Technologies), streptavidin-HRP (Cell Signaling) were used at a dilution of 1:1000. After washing 3 times with PBS, the membranes were further incubated at room temperature with horseradish peroxidase conjugated secondary antibodies (Bio-Rad Laboratories, Hercules, CA) at a dilution of 1:1000. The proteins were visualized with supersignal chemiluminesence (Pierce Biotechnology, Rockford, IL) using UVP digital camera (Upland, CA), and densitometric analysis was done by UVP software system. Densitometric data from at least three independent experiments were subjected to one way ANOVA, followed by Tukey’s Multiple Comparison Test.
4.4. Dendritic Mitochondrial Length Measurement
Dendritic mitochondria were visualized by the transfection of pDsRed2-mito (Clontech) in neurons at days in vitro (DIV) 15 using lipofectamine LTX and plus reagent (Invitrogen) according to the manufacturer’s protocol. Three days after transfection, neurons were used for experiments. Dendritic branches of pyramidal neurons were determined by morphological characteristics. Particles with strong labeling (compared with background) and clear edges confined in dendritic branches were considered to be mitochondria. Lengths of particles with strong pDsRed2-mito labeling and clear edges confined in dendrites were measured using the filament and the particle tracing system, Imaris XT software (Bitplane, Saint Paul, MN). Data were collected from 600, 450, 550, and 500 mitochondria of vehicle, Aβ, DPN, and DPN+Aβ groups, respectively, in 5 independent experiments. The data was subjected to two-way ANOVA, followed by Bonferroni Test, for the assessment of group differences, and is presented as a bar graph depicting the average ± SD, using GraphPad Prism software (La Jolla, CA).
4.5. Dendritic Mitochondrial Movement Recording and Data Analysis
Dendritic mitochondria were visualized by the transfection of pDsRed2-mito (Clontech) in neurons at days in vitro (DIV) 15 using lipofectamine LTX and plus reagent (Invitrogen) according to the manufacturer’s protocol. Three days after transfection, neurons were used for experiments. Dendritic branches of pyradimal neorons were determined by morphological characteristics. Particles with strong labeling (compared with background) and clear edges confined in dendritic branches were considered to be mitochondria. An organelle was considered to be nonmobile if it remained stationary for the entire 2 minute recording period; movement was counted only if the displacement was more than the length of the mitochondrion. The net direction of resumed movement was recorded as either stationary or moved, and it was determined by comparing the net displacement between the initial and final positions relative to the cell body. Time-lapse images were captured under an inverted one-photon laser scanning microscopy using Zeiss LSM510 Zeiss microscope with a stage-based chamber (5% CO2, 37°C). Images of region of interest (ROI) were taken every 3 s for a total of 2 min under 40× magnification. Mitochondrial movement data were analyzed using the particle tracking system Imaris XT software (Bitplane, Saint Paul, MN). Mitochondrial movement toward the distal end of the dendrite is considered to be anterograde, whereas that toward the proximal end is considered to be retrograde. The resulting coordinates were used to calculate the average velocity (μm/s) of all mobile mitochondria for each experimental condition. Data were collected from 620, 546, 590, and 563 mitochondria from 58, 61, 62 and 62 dendrites of vehicle, Aβ, DPN and Aβ+DPN groups, respectively, in three independent experiments. Aβ+DPN group represents the same Aβ treated dendrites as we first treated the neuron with Aβ then made the time lapse videos for Aβ group then we added DPN to measure the effects of DPN in the same Aβ treated dendritic region. The data was subjected to two-way ANOVA, followed by Bonferroni Test, for the assessment of group differences, and was presented as a bar graph depicting the average ± SD, using GraphPad Prism software (La Jolla, CA).
4.6. Immunocytochemistry
HEK 293 cells grown in coverslips were transfected with equimolar concentrations of C-terminal DDK fused to AKAP1 and C-terminal HA fused to ERβ expression vector (both from GeneCopoeia) plasmid DNA, using lipofectamine 2000 reagent (Invitrogen, CA, USA). Forty eight hours after transfection, cells were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) for 20 min. After 1 hour block with 5% bovine serum albumin (BSA), coverslips were incubated with anti DDK mouse and anti HA rabbit primary antibody followed by Alexa 633 anti-mouse and Alexa 488 anti-rabbit secondary antibody (Molecular Probes, now Life technologies, USA). Cells were then visualized by Zeiss LSM510 confocal microscope. Photomicrographs were analyzed for colocalization of AKAP1 and ERβ, using Zeiss LSM software.
For the localization of endogenous AKAP1 and ERβ, rat primary neurons (7DIV) were sequentially immunostained by first incubating neurons with anti-AKAP1 primary followed by Alexa 488 secondary then anti-ERβ primary antibody followed by TRITC conjugated secondary antibody. Cells were then visualized by Zeiss LSM510 confocal microscope.
4.7. Pull-Down Assay for AKAP1 protein/ERβ protein interaction and AKAP1 protein/arginine methylated ERβ peptide interaction
Shown are schematics of two pull-down assays used to demonstrate direct interaction between AKAP1 and ERβ (Supplemental Schematic 1) and between AKAP1 and arginine methylated ERβ peptide (Supplemental Schematic 2). Steps involved for Schematic 1 include: 1) DDK-tagged AKAP1 expression plasmid transfection in HEK293 cells; 2) affinity purification of AKAP1 protein by first binding HEK293 cell lysate with Anti-DDK antibody conjugated agarose beads (ORIGINE) and then after repeated washing, eluted with buffer contain 0.1 M glycine HCl at pH 3.5 and collected in a tube containing buffer1M Tris-HCl, pH8. Eluted fraction was analyzed as shown in Coomassie blue stained SDS-PAGE gel; 3) HA-ERβ expression plasmid transfection in HEK293 cells; 4) 24 hrs after transfection, cell lysis in RIPA buffer; 5) immobilization of DDK-AKAP1 protein in antiDDK-Ab conjugated agarose beads; 6) Binding of HA-ERβ protein containing HEK 293 cell lysate; 7) washed away unbound proteins; and 8) eluted and analyzed for AKAP1-ERβ interaction by western blot analysis as shown in the western blot in the Schematic 1. Shown in the Schematic 2 are the steps for direct interaction between DDK-AKAP1 and biotinylated arginine methylated or unmethylated ERβ peptide. Steps involved for Schematic 2 include: 1) binding of biotinylated peptide unmethylated and methylated with avidin conjugated agarose beads; 2) after repeated washing the beads, binding of affinity purified DDK-AKAP1 protein to immobilized ERβ peptide; and 3) washing, eluting, and analyzing by western blot analysis, as shown in the western blot in the Schematic 2.
4.8. Seahorse XF-24 Metabolic Flux Analysis
Primary hippocampal neurons were cultured on Seahorse XF-24 plates at a density of 50,000 cells per well. Neurons were grown in Neurobasal Medium with B27 supplement for 14 days before experiment. On the day of metabolic flux analysis, cells were changed to unbuffered DMEM (DMEM base medium supplemented with 25 mM glucose, 1 mM sodium pyruvate, 31 mM NaCl, 2 mM GlutaMax, pH 7.4) and incubated at 37 °C in a non-CO2 incubator for 1 h. All medium and injection reagents were adjusted to pH 7.4 on the day of assay. Four baseline measurements of OCR were taken before sequential injection of mitochondrial active agents. Three readings were taken after each addition of mitochondrial active agent. The mitochondrial agents were used oligomycin (1 μM), FCCP (1 μM), and rotenone (1 μM). OCR was automatically calculated and recorded by the Seahorse XF-24 software. After the assays, plates were saved and protein readings were measured for each well to confirm equal cell numbers per well.
Supplementary Material
Video S1. Movement of control mitochondria in the region of interest of dendritic branch of primary neuron.
Video S2. Movement of mitochondria in soluble Aβ treated dendritic branch of primary neuron.
Video S3. Movement of mitochondria in ERβ agonist, DPN treated dendritic branch of primary neuron.
Video S4. Movement of mitochondria after 2 hours of DPN treatment in the same soluble Aβ treated dendritic branch shown in video (S2).
Fig. S1. Schematics of experimental strategy for AKAP1-ERβ interaction.
Fig. S2. Schematics of experimental strategy for AKAP1 and methylated ERβ peptide interaction.
ERβ agonist, DPN reverse Oligomeric Aβ mediated mitochondrial dysfunction.
The effects of DPN are due to its activation of PKA, which phopshorylates Drp1.
PKA binding protein, AKAP1 interacts with methylated ERβ Protein.
DPN mediated signaling pathway regulates mitochondrial fission/fusion dynamics.
Acknowledgments
The authors acknowledge the support for this project of NIH grants P01 AG022550, P01 AG027956, P20 GM109098 and U54GM104942.
Abbreviations
- AD
Alzheimer’s Disease
- Aβ
amyloid β
- ERβ
estrogen receptor β
- Drp1
dynamine-related protein 1
- PKA/AKAP1
protein kinase A/A kinase anchoring protein 1
- DPN
Diarylpropionitrile (2,3-bis(4-Hydroxyphenyl)-propionitrile
Footnotes
Author Contribution: SS and JWS designed studies; SS and SJ performed research; SS and JS analyzed data; SS and JWS wrote the paper.
The Authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci. 2005;6:841–849. doi: 10.1038/nrn1784. [DOI] [PubMed] [Google Scholar]
- Barghorn S, Nimmrich V, Stribinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hilen H. Globular amyloid β-peptide1-42 oligomer-a homogenous and stable neuropathological protein in Alzheimer’s disease. J Neurochem. 2005;95:834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, Zhu X. Impaired Mitochondrial Biogenesis Contributes to Mitochondrial Dysfunction in Alzheimer’s disease. J Neurochem. 2012;120:419–429. doi: 10.1111/j.1471-4159.2011.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins MJ, Reddy PH. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer’s disease neurons. Biochim Biophys Acta. 2011;1812:507–513. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone L, Carlucci A, Affaitati A, Livigni A, DeCristofaro T, Garbi C, Varrone S, Ullrich A, Gottesman ME, Avvedimento EV, Feliciello A. Mitochondrial AKAP121 binds and targets protein tyrosine phosphatase D1, a novel positive regulator of src signaling. Mol Cell Biol. 2004;24:4613–26. doi: 10.1128/MCB.24.11.4613-4626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlucci A, Lignitto L, Feliciello A. Control of mitochondria dynamics and oxidative metabolism by cAMP, AKAPs and the proteasome. Trends in Cell Biology. 2008;18:604–613. doi: 10.1016/j.tcb.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Castellani R, Hirai K, Aliev G, Drew KL, Nunomura A, Takeda A, Cash AD, Obrenovich ME, Perry G, Smith MA. Role of mitochondrial dysfunction in Alzheimer’s disease. J Neurosci Res. 2002;70:357–60. doi: 10.1002/jnr.10389. [DOI] [PubMed] [Google Scholar]
- Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26:7035–45. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté J, Richard S. Tudor domains bind symmetrical dimethylated arginines. J Biol Chem. 2005;280:28476–28483. doi: 10.1074/jbc.M414328200. [DOI] [PubMed] [Google Scholar]
- Dickey AS, Strack S. PKA/AKAP1 and PP2A/Bβ2 Regulate Neuronal Morphogenesis via Drp1 Phosphorylation and Mitochondrial Bioenergetics. J Neurosci. 2011;31:15716–15726. doi: 10.1523/JNEUROSCI.3159-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc Natl Acad Sci USA. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciello A, Gottesman ME, Avvedimento EV. cAMP-PKA signaling to the mitochondria: protein scaffolds, mRNA and phosphatases. Cell Signal. 2005;17:279–87. doi: 10.1016/j.cellsig.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Fransson S, Ruusala A, Aspenström P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun. 2006;344:500–10. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM. Polychronization: Computation with Spikes. Neural Computation. 2006;18:245–282. doi: 10.1162/089976606775093882. [DOI] [PubMed] [Google Scholar]
- Le Romancer M, Treilleux I, Leconte N, Robin-Lespinasse Y, Sentis S, Bouchekioua-Bouzaghou K, Goddard S, Gobert-Gosse S, Corbo L. Regulation of Estrogen Rapid Signaling through Arginine Methylation by PRMT1. Molecular Cell. 2008;31:212–221. doi: 10.1016/j.molcel.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muñiz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelly C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Livigni A, Scorziello A, Agnese S, Adornetto A, Carlucci A, Garbi C, Castaldo I, Annunziato L, Avvedimento EV, Feliciello A. Mitochondrial AKAP121 links cAMP and src signaling to oxidative metabolism. Mol Biol Cell. 2006;17:263–71. doi: 10.1091/mbc.E05-09-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Reddy PH. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum Mol Genet. 2012;21:2538–2547. doi: 10.1093/hmg/dds072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Wallace DC, Levin ER. Functional estrogen receptors in the mitochondria of breast cancer cells. Mol Biol Cell. 2006;17:2125–37. doi: 10.1091/mbc.E05-11-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Dröse S, Brandt U, Savaskan E, Czech C, Götz J, Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci USA 2009. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J Neurosci. 2006;26:10480–7. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnóczky G. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci USA. 2008;105:20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva DF, Selfridge JE, Lu J, EL, Roy N, Hutfles L, Burns JM, Michaelis EK, Yan S, Cardoso SM, Swerdlow RH. Bioenergetic flux, mitochondrial mass and mitochondrial morphology dynamics in AD and MCI cybrid cell lines. Hum Mol Genet. 2013;22:3931–3946. doi: 10.1093/hmg/ddt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–90. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–78. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: Reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci USA. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29:9090–103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Wang X, Sua B, Siedlak SL, Moreirab PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-β overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci USA. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Sarkar SN, Liu R, Perez EJ, Wang X, Wen Y, Yan LJ, Simpkins JW. Estrogen Receptor β as a mitochondrial vulnerability factor. J Biol Chem. 2009;284:9540–9548. doi: 10.1074/jbc.M808246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Zhao L, Mao Z, Chen S, Wong KC, To J, Brington RD. Potentiation of brain mitochondrial function by S- equol and R/S-equol estrogen receptor β-selective phytoSERM treatments. Brain Res. 2013;1514:128–141. doi: 10.1016/j.brainres.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Movement of control mitochondria in the region of interest of dendritic branch of primary neuron.
Video S2. Movement of mitochondria in soluble Aβ treated dendritic branch of primary neuron.
Video S3. Movement of mitochondria in ERβ agonist, DPN treated dendritic branch of primary neuron.
Video S4. Movement of mitochondria after 2 hours of DPN treatment in the same soluble Aβ treated dendritic branch shown in video (S2).
Fig. S1. Schematics of experimental strategy for AKAP1-ERβ interaction.
Fig. S2. Schematics of experimental strategy for AKAP1 and methylated ERβ peptide interaction.