Summary
Neurite branching is essential for correct assembly of neural circuits, yet remains a poorly understood process. For example, the neural cell adhesion molecule KAL-1/anosmin-1, which is mutated in Kallmann Syndrome regulates neurite branching through mechanisms largely unknown. Here we show that KAL-1/anosmin-1 mediates neurite branching as an autocrine co-factor with EGL-17/FGF through a receptor complex consisting of the conserved cell adhesion molecule SAX-7/L1CAM and the fibroblast growth factor receptor EGL-15/FGFR. This protein complex, which appears conserved in humans, requires the immunoglobulin (Ig) domains of SAX-7/L1CAM and the FN(III) domains of KAL-1/anosmin-1 for formation in vitro as well as function in vivo. The kinase domain of the EGL-15/FGFR is required for branching, and genetic evidence suggests that ras-mediated signaling downstream of EGL-15/FGFR is necessary to effect branching. Our studies establish a molecular pathway that regulates neurite branching during development of the nervous system.
Introduction
The nervous system presents as a network of neurons that are interconnected in specific and reproducible patterns. During development neurons often form branches to increase the possibilities for synaptic input and output (Schmidt and Rathjen, 2010; Gibson and Ma, 2011). Moreover, branching has been mechanistically linked to synapse formation (Chia et al., 2014) suggesting that it is an essential process during neural circuit assembly. However, the molecular pathways that govern neuronal branching remain poorly understood and only a limited number of branching factors have been identified (Schmidt and Rathjen, 2010; Gibson and Ma, 2011). One of these, KAL-1/anosmin-1, encodes a putative secreted cell adhesion molecule, which when mutated causes Kallmann Syndrome (KS) in humans (Franco et al., 1991; Legouis et al., 1991). KS is characterized by anosmia and infertility, presumably as a result of neuronal targeting and migration defects (Lutz et al., 1993). KAL-1/anosmin-1 comprises a WAP (whey acidic protein) like protease inhibitor domain and four fibronectin type III (FN(III)) domains (Fig. 1A). Experiments in vitro and in vivo suggest that KAL1/anosmin-1 plays a role in neurite branching both in vertebrates and invertebrates (Bülow et al., 2002; Rugarli et al., 2002; Soussi-Yanicostas et al., 2002). Yet, how KAL1/anosmin-1 functions to mediate branching in vivo has remained largely elusive.
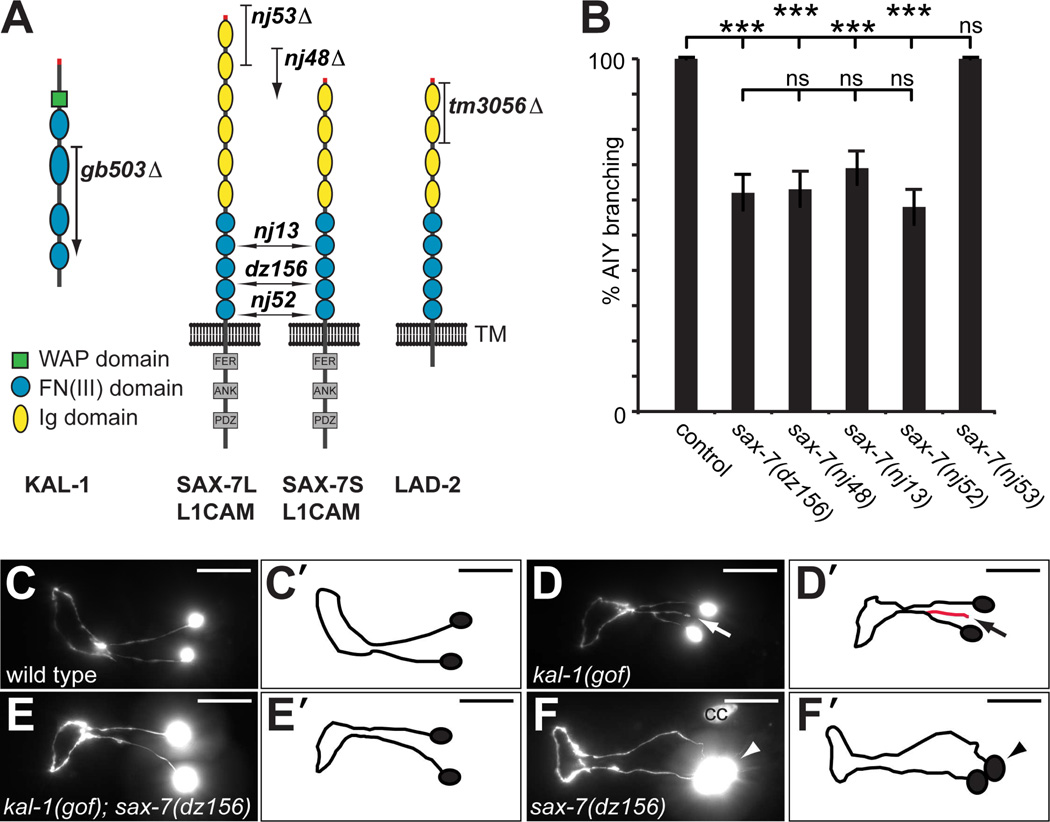
Fig. 1. SAX-7/L1CAM is required for kal-1-dependent axon branching.
A Schematic of the SAX-7/L1CAM and LAD-2 cell adhesion molecules with alleles indicated: nj53 removes the long isoform SAX-7L; nj48, nj13, nj52 and dz156 are deletions, insertions or nonsense mutations and result in premature termination of both SAX-7L and SAX-7S. The tm3056 deletion allele results in a frameshift and removes all LAD-2 isoforms (Wang et al., 2008). Ig: immunoglobulin domain, FN: fibronectin domain III, FER: conserved FERM domain, ANK: ankyrin binding domain, PDZ: PDZ domain.
B Quantification of animals with kal-1/anosmin-1-dependent branches in AIY neurons (% AIY branching) of the genotypes indicated. Generally, AIY interneurons were visualized with mgIs18 (Is[Pttx-3::GFP]) and kal-1 expressed in AIY from the transgenes otIs35X (Is[Pttx-3::kal-1; rol-6(d)]) or otIs76IV (Is[Pttx-3::kal-1; Pcc::GFP]). Statistical significance is indicated: ns: not significant, **: P < 0.005, ***: P < 0.0005. See Dataset S1 for full primary data.
C – F Ventral/sublateral views of adult animals showing the pair of AIY interneurons. Overexpression of kal-1 specifically in AIY neurons results in a branching gain of function phenotype (kal-1(gof), arrow, D,D’) that is not seen in wild type animals (C,C’)(Bülow et al., 2002). This kal-1-dependent branching is suppressed by loss of sax-7 function (E,E’). Loss of sax-7 also results in a cell body positioning defect (arrowhead, F,F’). The nj48 allele of sax-7/L1CAM failed to complement dz156 with regard to the cell body positioning phenotype (Fig. S1A). In all panels, anterior is to the left and scale bars indicate 20µm. cc: coelomocyte.
Results
The Kallmann Syndrome gene kal-1/anosmin-1 genetically interacts with the neural cell adhesion molecule SAX-7/L1CAM
KAL-1/anosmin-1 has been shown to be important for neurite branching in both vertebrates and invertebrates (Bülow et al., 2002; Rugarli et al., 2002; Soussi-Yanicostas et al., 2002). For example, misexpression of KAL-1/anosmin-1 in several classes of neurons in Caenorhabditis elegans, including AIY interneurons, causes kal-1/anosmin-1-dependent neurite branching (Fig. 1A–D)(Bülow et al., 2002). In a genetic screen for loci required for this kal-1/anosmin-1-dependent branching paradigm we identified dz156, an allele that introduces a premature stop codon after 885 amino acids in the neural cell adhesion molecule SAX-7 (Fig. 1A,B)(Díaz-Balzac et al., 2014). SAX-7 is a single pass transmembrane protein with extracellular Ig and FN(III) domains and, the nematode ortholog of the conserved neural cell adhesion molecule L1CAM (Chen et al., 2001). SAX-7/L1CAM exists in two extracellular variants, a short SAX-7S and a long SAX-7L form (Fig. 1A)(Wang et al., 2005). The dz156 allele is predicted to truncate both isoforms in the fourth FN(III) domain and is likely a strong if not complete loss of function allele.
Both dz156 and other loss of function alleles of sax-7/L1CAM suppressed kal-1/anosmin-1-dependent branching in AIY neurons (Fig. 1B,E). In addition, sax-7/L1CAM mutants displayed a cell-positioning defect of AIY neurons, similar to defects described for other neurons in C. elegans (Fig. 1F, Fig. S1A)(Sasakura et al., 2005; Pocock et al., 2008). To determine which isoform of SAX-7/L1CAM was required for kal-1/anosmin-1-dependent branching, we tested the sax-7(nj53) allele, which specifically removes the SAX-7L long isoform (Sasakura et al., 2005). We found that loss of SAX-7L did not suppress kal-1/anosmin-1-dependent branching in AIY. Moreover, loss of the paralogous L1CAM-like cell adhesion molecule LAD-2 (Fig. 1A)(Wang et al., 2008) affected kal-1-dependent branching in AIY neurons neither in a genetic wild type nor sax-7/L1CAM mutant background (Fig. 1B, Fig. S1B). These data suggest that kal-1/anosmin-1-dependent branching in AIY neurons requires specifically the short form SAX-7S/L1CAM whereas LAD-2 serves no obvious functions in this context.
sax-7/L1CAM acts genetically in a pathway with kal-1/anosmin-1, heparan sulfate and the egl-15/FGFR-egl-17/FGF receptor-ligand pair
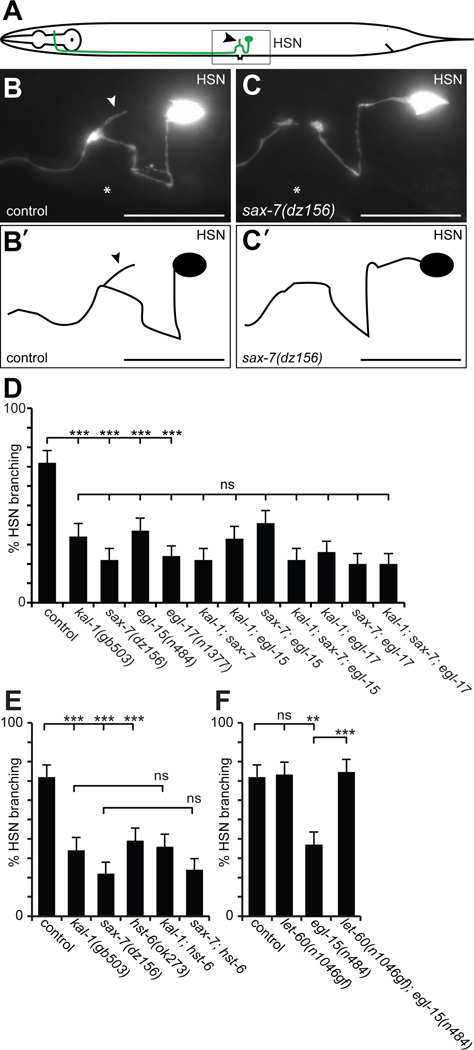
Our results suggested that kal-1/anosmin-1 and sax-7/L1CAM act genetically in the same pathway, at least for kal-1/anosmin-1-dependent branching of AIY neurons. To investigate this notion in a genetic loss of function context, we turned to the hermaphrodite-specific motor neurons HSN (Fig. 2A). The HSN neurons are a pair of cells located laterally in the midbody region of the worm. Each sends a process ventrally into the nerve cord where it travels anteriorly to the nerve ring (White et al., 1986)(Fig. 2A). In the vicinity of the vulva, the processes send a branch that innervates the vulval muscles (Garriga et al., 1993) and require both kal-1/anosmin-1 and the heparan sulfate 3-O-sulfotransferase hst-3.2 (Tecle et al., 2013). We found that formation of HSN branches also required sax-7/L1CAM (Fig. 2A–C). This phenotype was not enhanced in a kal-1; sax-7 double null mutant (Fig. 2D), demonstrating that both genes act genetically in the same pathway.
Fig. 2. kal-1/anosmin-1, sax-7/L1CAM, egl-15/FGFR, and EGL-17/FGF act in a genetic pathway.
A Schematic with the position and morphology of the hermaphrodite specific neuron (HSN) indicated. Only the left HSN is shown for simplicity. An arrowhead indicates the characteristic branch formed near the vulva and a box the approximate extent of the images shown in (B) and (C).
B – C Lateral views with schematics (B’,C’) of adult animals showing the hermaphrodite-specific serotonergic neuron HSN. HSN neurons were visualized with zdIs13 (Is[Ptph-1::GFP]). Anterior is to the left, asterisks indicate the location of the vulva and a scale bar 10 µm.
D – F Quantification of HSNs with branches in the genotypes indicated. Statistical significance is shown as: **: P<0.005, ***: P<0.0005, ns: not significant. There is no statistically significant difference in any pairwise comparison between single and multiple mutants in D. In F, the data for control and egl-15(n484) is identical to D and E and shown for comparison only. See Dataset S1 for full primary data.
Since mutations in human fibroblast growth factor receptor FGFR1 and its ligand FGF8 have also been shown to cause Kallmann Syndrome (Dodé et al., 2003; Falardeau et al., 2008), we tested if the sole fibroblast growth factor receptor in C. elegans encoded by egl-15/FGFR and its fibroblast growth factor ligand egl-17/FGF are required for branch formation in HSN neurons. Indeed, the formation of HSN branches required both a specific splice variant of EGL-15/FGFR, named EGL-15A, and its canonical ligand EGL-17/FGF (Fig. 2D). Moreover, complete loss of EGL-15A/FGFR or EGL-17/FGF, respectively, was not further enhanced by concomitant genetic removal of KAL-1/anosmin-1, SAX-7/L1CAM, or both, suggesting that all four genes act genetically in the same pathway to mediate branch formation of HSN motor neurons (Fig. 2D).
Heparan sulfate proteoglycans are a class of extracellular glycans of great molecular complexity that have been implicated in the function of KAL-1/anosmin-1 in cell culture, C. elegans, and humans (Soussi-Yanicostas et al., 1996; Soussi-Yanicostas et al., 1998; Bülow et al., 2002; Bülow and Hobert, 2004; Hudson et al., 2006; Bhattacharya et al., 2009; Tornberg et al., 2011; Tecle et al., 2013). We found that formation of HSN branches also required the heparan sulfate HS 6-O-sulfotransferase hst-6/HS6ST1 (Fig. 2E), a gene involved in introducing molecular diversity in heparan sulfate and, shown to be mutant in some patients with Kallmann Syndrome (Tornberg et al., 2011). The hst-6/HS6ST1 loss of function phenotype was not enhanced by concomitant genetic removal of kal-1/anosmin-1 or sax-7/L1CAM. Thus, hst-6/HS6ST1 acts genetically in a pathway with kal-1/anosmin-1 and sax-7/L1CAM and, by inference, with egl-15A/FGFR1 and egl-17/FGF to mediate branch formation of HSN motor neurons (Fig. 2). Similarly, double mutant analyses between HS genes and sax-7/L1CAM in the context of kal-1/anosmin-1-dependent branching of AIY neurons also suggested that SAX-7/L1CAM acts in concert with heparan sulfate (Fig. S1C).
The secreted cell adhesion molecule KAL-1/anosmin-1 acts as an autocrine cofactor of SAX-7/L1CAM-EGL-15/FGFR mediated neurite branching
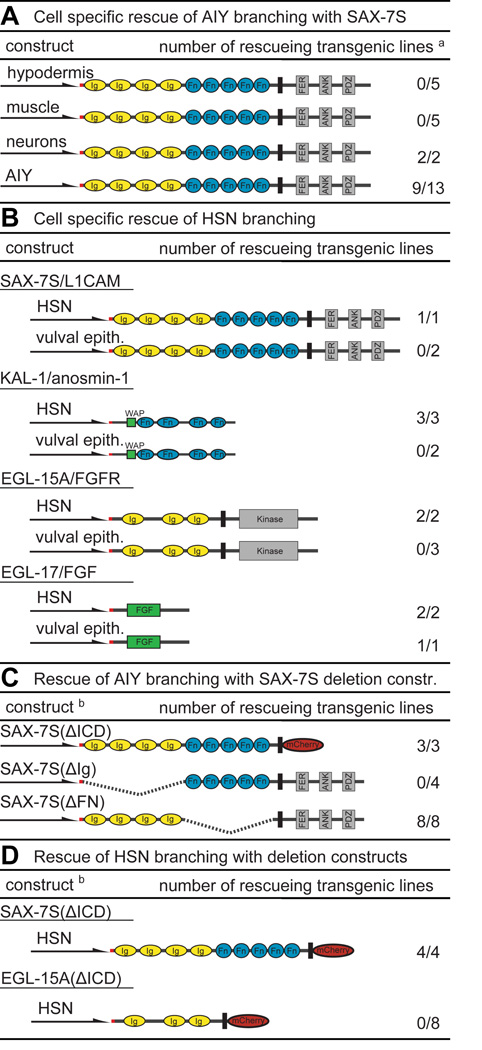
We next determined where SAX-7/L1CAM, KAL-1/anosmin-1, EGL-15/FGFR and EGL-17/FGF act to mediate neurite branching. We found that expression of the SAX-7S short isoform in the hypodermis or in muscle failed to rescue the suppression of kal-1/anosmin-1-dependent branching in AIY neurons due to loss of sax-7/L1CAM (Fig. 3A). In contrast, expression specifically in AIY neurons or, pan-neuronally rescued the suppression of branching, suggesting that SAX-7S/L1CAM acts cell-autonomously in AIY neurons to mediate branching (Fig. 3A). Surprisingly, the long isoform SAX-7L/L1CAM could only rescue AIY branching non-autonomously (Fig. S1D). This is in contrast to cell positioning defects of AIY which, in accordance with previous studies (Sasakura et al., 2005), were rescued only by pan-neuronal expression of the short isoform SAX-7S/L1CAM (Fig. S1E). These data suggest that branching and cell positioning in AIY interneurons require molecularly distinct mechanisms of SAX-7/L1CAM function.
Fig. 3. Summary of heterologous transgenic rescue experiments.
A Cell specific rescue of kal-1/anosmin-1-dependent branching in AIY neurons of sax-7/L1CAM mutants. Schematically shown are the constructs, promoters used (Pdpy-7/hypodermal, Pmyo-3/muscle, Punc-14/pan-neuronal, Pttx-3/AIY) and the number of rescuing out of the total number of lines. Color coding and abbreviation as in Fig. 1. a Rescue in all panels was defined as restoration of branches in transgenic animals and had to be statistically significant (P<0.05) when compared to nontransgenic siblings. N = 100 in all assays. See Dataset S2 for full primary rescue data of all panels.
B Cell specific rescue of branching in HSN motoneurons. An HSN specific promoter (Punc-86)(Shen and Bargmann, 2003) and a promoter that is expressed in the vulval epithelium (Pegl-17)(Burdine et al., 1998) were used to drive expression in the respective tissues. Both promoters have previously been used to determine cell-autonomy of function for genes involved in HSN development (Shen and Bargmann, 2003; Shen et al., 2004).
C Structure/function analyses of SAX-7S/L1CAM in kal-1/anosmin-1-dependent branching in AIY neurons using the Punc-14 pan-neuronal promoter. b In panels (C) and (D), deletions are indicated as dashed lines or by replacement of domains with mCherry: SAX-7S(ΔICD), SAX-7S(ΔIg), SAX-7S(ΔFn) and, EGL-15(ΔICD) which delete the intracellular domains (ΔICD), Ig domains (ΔIg) or FN(III) domains (ΔFN).
D Structure/function analyses of SAX-7S/L1CAM and EGL-15A/FGFR in HSN motoneurons using the Punc-86 HSN specific promoter.
The short form SAX-7S/L1CAM also cell autonomously rescued HSN branching defects of sax-7/L1CAM mutants, but not when expressed non-autonomously in the vulval epithelium (Fig. 3B), a tissue previously shown to control synapse formation of HSN neurons cell non-autonomously (Shen and Bargmann, 2003). Moreover, EGL-15A/FGFR-dependent branching in HSN was restored by expression of EGL-15/FGFR in HSN neurons but not in the vulval epithelium (Fig. 3B). Intriguingly, expression of KAL-1/anosmin-1 in HSN neurons, but not in the vulval epithelium rescued the HSN mutant phenotype, also suggesting a cell autonomous autocrine role for KAL-1/anosmin-1 in HSN branching (Fig. 3B) – despite the fact that a kal-1 reporter is expressed in both the vulval epithelium and in HSN (Bülow et al., 2002). In contrast, expression of EGL-17/FGF rescued the HSN branching defects when expressed in either HSN or the vulval epithelium (Fig. 3B). Since an egl-17 reporter is expressed in the vulval epithelium (Burdine et al., 1998), these findings argue strongly for a non-autonomous mode of action of the FGF ligand.
To determine which domains of SAX-7S/L1CAM and the receptor tyrosine kinase EGL-15A/FGFR are required for branching we created deletion constructs. We found that replacing the intracellular domain of SAX-7S/L1CAM with fluorescent mCherry (SAX-7S(ΔICD)) retained full rescuing activity in both AIY and HSN branching contexts, suggesting that the conserved intracellular domain of SAX-7S/L1CAM is dispensable for function during neurite branching (Fig. 3C,D). However, the intracellular domain of SAX-7S/L1CAM was required for cell positioning (Fig. S1F), further corroborating that SAX-7 functions through distinct pathways in branching and cell positioning. Lastly, the Ig domains but not the FN(III) domains of SAX-7S/L1CAM were necessary for both kal-1-dependent branching and cell positioning of AIY neurons (Fig. 3C, Fig. S1F).
In a last set of experiments, we found that a construct in which the intracellular domain of EGL-15A was replaced by mCherry (EGL-15A(ΔICD)) and which was previously shown to be functional in other cellular contexts (Bülow et al., 2004), failed to rescue the HSN defects (Fig. 3D), suggesting that intracellular signaling of EGL-15/FGFR is required for branching. To further investigate this notion, we asked whether constitutively active LET-60/RAS signaling could suppress loss of EGL-15A/FGFR as previously shown for neuronal and non-neuronal phenotypes of egl-15/FGFR mutants (DeVore et al., 1995; Bülow et al., 2004). We found that constitutively activated LET-60/RAS completely suppressed the HSN branching defects due to loss of EGL-15A/FGFR (Fig. 2F), suggesting that RAS-signaling downstream of the EGL-15A/FGFR receptor is required to mediate neuronal branching in HSN neurons. Taken together these experiments suggest that (1) SAX-7S/L1CAM serves cell-autonomously with the EGL-15A/FGFR receptor in HSN neurons, that (2) KAL-1/anosmin-1 functions as an autocrine co-factor in HSN and that (3) the EGL-17/FGF ligand functions as a non-autonomous ligand that is likely derived from the vulval epithelium. Moreover, ras-signaling downstream of the receptor tyrosine kinase EGL-15/FGFR is required, whereas the intracellular domain of the cell adhesion molecule SAX-7/L1CAM is dispensable for neurite branching but not cell positioning.
SAX-7/L1CAM forms a complex with KAL-1/anosmin-1 and EGL-15/FGFR
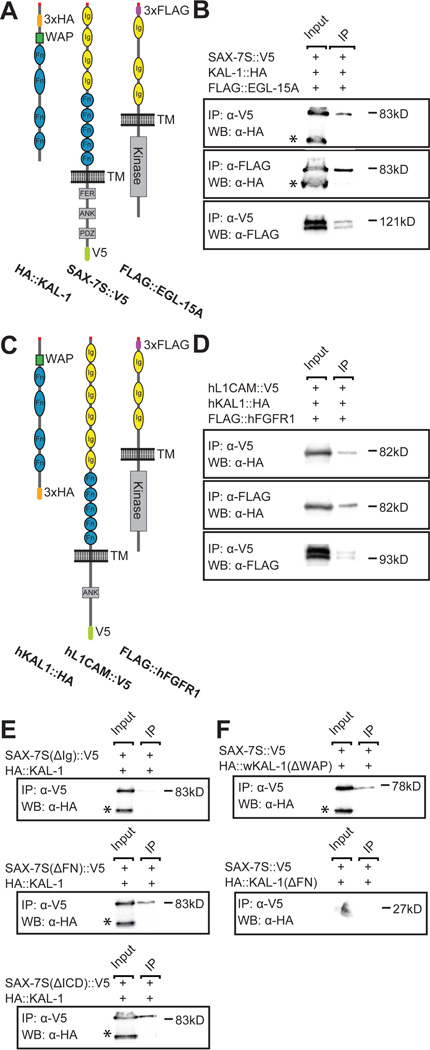
The genetic interactions between kal-1/anosmin-1, sax-7/L1CAM, egl-15/FGFR prompted us to test whether these factors can form a complex. We transiently expressed tagged versions of the C. elegans proteins KAL-1/anosmin-1, SAX-7/L1CAM or EGL-15/FGFR alone and in combination in human embryonic kidney cells (Fig. 4A) and, following cell lysis, conducted co-immunoprecipitation experiments. We found that KAL-1/anosmin-1 co-immunoprecipitated with an antibody against SAX-7/L1CAM and, vice versa, that SAX-7/L1CAM co-immunoprecipitated with an antibody against KAL-1/anosmin-1 (Fig. 4B, and data not shown). Moreover, KAL-1/anosmin-1 was coprecipitated with an antibody against EGL-15/FGFR (Fig. 4B) and, EGL-15/FGFR co-immunoprecipitated with an antibody against SAX-7/L1CAM (Fig. 4B), suggesting that all three proteins are part of the same complex. We found similar molecular interactions between the human proteins hKAL1, hL1CAM and hFGFR1 (Fig. 4C,D). Together with reports showing a physical interaction between purified hKAL1 and FGFR in vitro (Hu et al., 2009), this data strongly suggests evolutionary conservation of the tripartite complex from worms to humans.
Fig. 4. KAL-1/anosmin-1 forms a complex with SAX-7S/L1CAM and EGL-15A/FGFR.
A Schematic of the C. elegans protein used in transfection assays with tags indicated (approximately drawn to scale). All constructs appear functional in transgenic assays (data not shown).
B Input (total cell lysate) and immunoprecipitates (IP) from cells cotransfected with HA::KAL-1, SAX-7S::V5 and FLAG::EGL-15A, and detected by antibodies in Western Blots (WB) as indicated. The α-HA antibody always recognizes an additional band of lower molecular weight (asterisk) that is not coimmunopreciptated by interacting proteins. This fragment could constitute a proteolytically cleaved product that is unable to interact with the proteins tested here because it lacks the larger portion of the FN(III) domains. The α-FLAG antibody recognizes characteristic doublet bands of the EGL-15/FGFR, both of which are precipitated by interacting SAX-7/L1CAM. Control Western Blots to show expression of all C. elegans proteins in the same lysates used for IP experiments is shown in Fig. S2A. kDa, kilodalton in all panels.
C Schematic of the human proteins used in transfection assays with tags indicated (approximately to scale).
D Input (total cell lysate) and immunoprecipitates (IP) from cells cotransfected with human hKAL1::HA, hL1CAM::V5 and FLAG::hFGFR1, and detected by antibodies in Western Blots (WB) as indicated. The α-FLAG antibody also recognizes a doublet of the hFGFR1, both of which are precipitated by interacting hL1CAM. A control Western Blot showing expression of human proteins in the same lysates used for IP experiments is shown in Fig. S2B.
E Input (total cell lysates) and immunoprecipitates (IP) from cells cotransfected with SAX-7S(DIg)::V5, SAX-7S(DICD)::V5, or SAX-7S(DFN)::V5, respectively with 3xHA::KAL-1 and detected with antibodies in Western Blots (WB) as indicated.
F Input (total cell lysates) and immunoprecipitates (IP) from cells cotransfected with SAX-7S::V5 with HA::KAL-1(DWAP) or HA::KAL-1(DFN), respectively and detected with antibodies in Western Blots (WB) as indicated.
Since the Ig domains of SAX-7/L1CAM are required for branching activity in vivo (Fig. 3C), we determined if the Ig domains were also necessary for complex formation between SAX-7/L1CAM and KAL-1/anosmin-1. We found that a protein that lacked all Ig domains (SAX-7SΔIg) was unable to co-immunoprecipitate KAL-1/anosmin-1 (Fig. 4E). In contrast, proteins without the intracellular domain of SAX-7/L1CAM (SAX-7SΔICD) or the FN(III) domains of SAX-7/L1CAM (SAX-7SΔFN) still precipitated KAL-1/anosmin-1. This suggested that KAL-1/anosmin-1 binds SAX-7S/L1CAM through the Ig domains of SAX-7S/L1CAM independently of the FN(III) or intracellular domain and that this interaction is important for function.
Finally, we tested whether the interaction between the Ig domains of SAX-7S/L1CAM required the whey acidic protein (WAP) domain or the FN(III) repeats in KAL-1/anosmin-1. These experiments established that the FN(III) domains but not he WAP domain of KAL-1/anosmin-1 are required for the complex with SAX-7/L1CAM (Fig. 4F). Taken together, these data suggest that SAX-7/L1CAM, KAL-1/anosmin-1 and EGL-15/FGFR are part of a conserved complex in vitro and, that the Ig domains of SAX-7/L1CAM and the FN(III) of KAL-1/anosmin-1 are important for its formation.
Discussion
Here, we identified a molecular mechanism for neurite branching involving the neural cell adhesion molecule KAL-1/anosmin-1. Branching is mediated by a conserved multiprotein receptor complex consisting of SAX-7/L1CAM and EGL-15/FGFR and requires the autocrine activity of KAL-1/anosmin-1, and the non-autonomous activation by the EGL-17/FGF ligand. Importantly, the intracellular domain of EGL-15/FGFR but not SAX-7/L1CAM is necessary, and genetic evidence suggests that ras-signaling downstream of EGL-15/FGFR is required to mediate branching.
KAL-1/anosmin-1 as an autocrine co-factor for the EGL-15/FGFR-SAX-7/L1CAM receptor complex
Several lines of evidence suggest that KAL-1/anosmin-1 acts in an autocrine fashion to mediate neurite branching through the putative receptor complex EGL-15/FGFR and SAX-7/L1CAM. First, KAL-1/anosmin-1 can only rescue the mutant phenotype in HSN neurons when expressed in HSN neurons, but not when expressed in other tissues that are in physical contact with the HSN neuron. Second, previous experiments showed that misexpression of KAL-1/anosmin-1 even in cells that form direct physical contacts resulted in strictly cell-autonomous branching (Bülow et al., 2002). Two other examples of autocrine signaling have been described for axon outgrowth and branching, including brain derived neurotrophic factor (BDNF) and non-canonical Wnt signaling (Cheng et al., 2011; Ryu et al., 2013) suggesting that this signaling mode could present a more general mechanism. What could be the functional relevance of autocrine signaling? Both branching and outgrowth are examples of localized growth. An autocrine co-factor may serve as a positive feedback amplifier that facilitates a branching decision by first creating a bistable state. The fact that exogenous addition of KAL-1/anosmin-1 in chicken embryos increased FGF8 expression is also consistent with a positive feedback loop (Endo et al., 2012). Alternatively, and not mutually exclusive, an autocrine co-factor could create a high affinity complex that sensitizes the response to a diffusible ligand such as EGL-17/FGF. KAL-1/anosmin-1 could also act as an environmental sensor that allows the branching process to be initiated at a particular anatomical location along the path of axon migration in response to an extrinsic signal. Further experiments will be required to distinguish between these possibilities for KAL-1/anosmin-1 function in branching.
SAX-7 as a multifunctional cofactor of different signaling pathways
SAX-7/L1CAM appears widely expressed in C. elegans and is localized to the cell membrane in places of cell-cell contact (Chen et al., 2001). In addition to non-neuronal functions (Grana et al., 2010; Jafari et al., 2010; Lynch et al., 2012), sax-7/L1CAM is important for development and maintenance of different aspects of the nervous system (Sasakura et al., 2005; Wang et al., 2005; Pocock et al., 2008; Dong et al., 2013; Salzberg et al., 2013; Opperman et al., 2015). Interestingly, distinct domains of the protein are necessary for different processes. The branching function of SAX-7/L1CAM is dependent on the Ig domain but independent of the FN(III) repeats and the intracellular domain (this study). In contrast, the role of SAX-7/L1CAM in dendrite development relies on the FN(III), but not the intracellular or Ig domains (Dong et al., 2013; Salzberg et al., 2013) whereas maintenance of cell body position requires the Ig domains and the intracellular domain, but not the FN(III) domains (this study). Thus, SAX-7/L1CAM may functions as a scaffolding molecule that through interactions with other factors facilitates the assembly of multiprotein complexes in a cell specific fashion. Different interaction partners of SAX-7/L1CAM may provide specificity for the binding properties of the resulting complexes with other molecules whereas SAX-7/L1CAM may provide spatial specificity to such complexes through subcellular localization of SAX-7/L1CAM (Dong et al., 2013; Salzberg et al., 2013). The diverse functions played by SAX-7/L1CAM in different pathways could provide an explanation for the pleiotropic defects seen in patients with CRASH-syndrome (corpus callosum hypoplasia, mental retardation, adducted thumbs, spastic paraplegia, and hydrocephalus) as a result of different mutations in L1CAM (reviewed in (Fransen et al., 1997; Weller and Gärtner, 2001)).
A function for EGL-15A/FGFR in neurite branching
FGFR function is essential for development of non-neuronal and neuronal tissues. Studies in mice have shown that FGF8 signaling through the FGFR1 is required for neurogenesis and morphogenesis of the olfactory epithelium (Hébert et al., 2003; Kawauchi et al., 2005). Moreover, FGFR is important for neurite outgrowth in both vertebrates and invertebrates (Williams et al., 1994; Bülow et al., 2004). Our genetic data establishes a function of FGF signaling in branching and suggests that this function of EGL-15A/FGFR in HSN neurons requires let-60/ras-dependent signaling downstream of the FGF receptor. This is not unprecedented as let-60/ras-dependent signaling functions downstream of the EGL-15/FGFR in C. elegans during neurite extension (Bülow et al., 2004) and development (DeVore et al., 1995). Additionally, mice overexpressing KAL1/anosmin-1 display elevated levels of activated ERK1/2 in oligodendrocyte precursors, which is also consistent with ras-dependent signaling (Murcia-Belmonte et al., 2015). However, in different contexts of CAM-dependent signaling, a phospholipase gamma dependent cascade has been suggested to act downstream of the FGFR (reviewed in (Doherty and Walsh, 1996)). Thus, it will be important to determine how signaling downstream of the EGL-15/FGFR is regulated.
Experimental Procedures
C. elegans strains and imaging
All strains were maintained using standard methods (Brenner, 1974). All experiments were performed at 20°C and worms were scored as 1-d ay-old adults unless otherwise specified. For a full strain list see Supplemental Experimental Procedures. Fluorescent images were captured in live C. elegans using a Plan-Apochromat 40×/1.4 objective on a Zeiss Axioimager Z1 Apotome. Worms were immobilized using 10 mM sodium azide and Z stacks were collected. Maximum intensity projections were used for further analysis.
Molecular biology and transgenesis
To assemble tissue specific expression constructs for rescue of loss of function phenotypes, the respective cDNAs were cloned under control of tissue specific promoters. All plasmids contained the unc-54 3’UTR. For expression in cell culture, the respective cDNAs of C. elegans and human SAX-7S/L1CAM, KAL-1/anosmin-1 and EGL-15A/FGFR were tagged and cloned into pcDNA3.1a (for SAX-7S/L1CAM and KAL-1/anosmin-1) or pCMV8 (for EGL-15A/FGFR). For all transgenic rescue experiments, DNA constructs were injected at 5 ng/µl together with an injection marker at 50 ng/µl. For detailed information on plasmid construction and transgenic strains see Supplemental Experimental Procedures.
Statistical analysis
For all proportions statistical significance was calculated using the z-test whereas averages were compared using the two-tailed Student’s T-test. The Bonferroni correction for multiple comparisons was used where applicable. Statistical significance is indicated as: ns: not significant, *: P < 0.05, **: P < 0.005, ***: P < 0.0005.
Cell culture and immunoprecipitation
HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum and Antibiotic-Antimycotic (Gibco). Cells were transfected with plasmids DNA using Lipofectamine 2000 in accordance with the manufacturer’s instructions (Life technologies). After 48 hours, cells were lysed using RIPA lysis buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris/HCl [pH 7.4], and HALT protease inhibitor cocktail [Thermo scientific]). For immunoprecipitations, the lysates were precleared with Protein A/G agarose (Santa Cruz Biotechnology) and subsequently incubated with the following antibodies: rat monoclonal anti-HA (Roche), mouse monoclonal anti-FLAG M2 (Sigma-Aldrich), and mouse monoclonal anti-V5 (Life technologies). After 1 hour of incubation, Protein A/G agarose (Santa Cruz Biotechnology) was added to the lysates and incubated overnight. The following day the lysates were washed twice with RIPA lysis buffer, re-suspended in PBS (Sigma-Aldrich) and analyzed by SDS-PAGE and Western Blotting.
Supplementary Material
Highlights.
KAL-1/anosmin-1 controls branching as an autocrine co-factor with EGL-17/FGF
KAL-1/anosmin-1, SAX-7/L1CAM and EGL-15/FGFR form a conserved biochemical complex
Ras signaling downstream of EGL-15/FGFR mediates KAL-1-dependent branching
The results delineate a molecular pathway that regulates neurite branching
Acknowledgments
We thank T. Boulin, J. Hébert, R. Townley and members of the Bülow laboratory for comments on the manuscript and for discussion during the course of this work; J. Culotti, O. Hobert, K. Shen and, R. Pocock for DNA clones, the Caenorhabditis Genetics Center (CGC) for strains; M. Akabas for use of his cell culture facility. This work was funded in part through the NIH (R01HD055380 and R01GM101313 to H.E.B; T32GM007288 and F31HD066967 to C.A.D.B.; P30HD071593 to Albert Einstein College of Medicine; P40 OD010440 to CGC) and a Human Genome Pilot Project from Albert Einstein College of Medicine. H.E.B. is an Alfred P. Sloan and Irma T. Hirschl/Monique Weill-Caulier research fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bhattacharya R, Townley RA, Berry KL, Bülow HE. The PAPS transporter PST-1 is required for heparan sulfation and is essential for viability and neural development in C. elegans. J Cell Sci. 2009;122:4492–4504. doi: 10.1242/jcs.050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülow HE, Berry KL, Topper LH, Peles E, Hobert O. Heparan sulfate proteoglycan-dependent induction of axon branching and axon misrouting by the Kallmann syndrome gene kal-1. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6346–6351. doi: 10.1073/pnas.092128099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülow HE, Boulin T, Hobert O. Differential functions of the C. elegans FGF receptor in axon outgrowth and maintenance of axon position. Neuron. 2004;42:367–374. doi: 10.1016/s0896-6273(04)00246-6. [DOI] [PubMed] [Google Scholar]
- Bülow HE, Hobert O. Differential sulfations and epimerization define heparan sulfate specificity in nervous system development. Neuron. 2004;41:723–736. doi: 10.1016/s0896-6273(04)00084-4. [DOI] [PubMed] [Google Scholar]
- Burdine RD, Branda CS, Stern MJ. EGL-17(FGF) expression coordinates the attraction of the migrating sex myoblasts with vulval induction in C. elegans. Development. 1998;125:1083–1093. doi: 10.1242/dev.125.6.1083. [DOI] [PubMed] [Google Scholar]
- Chen L, Ong B, Bennett V. LAD-1, the Caenorhabditis elegans L1CAM homologue, participates in embryonic and gonadal morphogenesis and is a substrate for fibroblast growth factor receptor pathway-dependent phosphotyrosine-based signaling. J Cell Biol. 2001;154:841–856. doi: 10.1083/jcb.200009004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PL, Song AH, Wong YH, Wang S, Zhang X, Poo MM. Self-amplifying autocrine actions of BDNF in axon development. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18430–18435. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia PH, Chen B, Li P, Rosen MK, Shen K. Local F-actin network links synapse formation and axon branching. Cell. 2014;156:208–220. doi: 10.1016/j.cell.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVore DL, Horvitz HR, Stern MJ. An FGF receptor signaling pathway is required for the normal cell migrations of the sex myoblasts in C. elegans hermaphrodites. Cell. 1995;83:611–620. doi: 10.1016/0092-8674(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Díaz-Balzac CA, Lázaro-Peña MI, Tecle E, Gomez N, Bülow HE. Complex Cooperative Functions of Heparan Sulfate Proteoglycans Shape Nervous System Development in Caenorhabditis elegans. G3. 2014;4:1859–1870. doi: 10.1534/g3.114.012591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodé C, Levilliers J, Dupont JM, De Paepe A, Le Du N, Soussi-Yanicostas N, Coimbra RS, Delmaghani S, Compain-Nouaille S, Baverel F, et al. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet. 2003;33:463–465. doi: 10.1038/ng1122. [DOI] [PubMed] [Google Scholar]
- Doherty P, Walsh FS. CAM-FGF Receptor Interactions: A Model for Axonal Growth. Molecular and cellular neurosciences. 1996;8:99–111. doi: 10.1006/mcne.1996.0049. [DOI] [PubMed] [Google Scholar]
- Dong X, Liu OW, Howell AS, Shen K. An extracellular adhesion molecule complex patterns dendritic branching and morphogenesis. Cell. 2013;155:296–307. doi: 10.1016/j.cell.2013.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Ishiwata-Endo H, Yamada KM. Extracellular matrix protein anosmin promotes neural crest formation and regulates FGF, BMP, and WNT activities. Developmental cell. 2012;23:305–316. doi: 10.1016/j.devcel.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, et al. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008;118:2822–2831. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, Carrozzo R, Maestrini E, Pieretti M, Taillon-Miller P, et al. A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991;353:529–536. doi: 10.1038/353529a0. [DOI] [PubMed] [Google Scholar]
- Fransen E, Van Camp G, Vits L, Willems PJ. L1-associated diseases: clinical geneticists divide, molecular geneticists unite. Human molecular genetics. 1997;6:1625–1632. doi: 10.1093/hmg/6.10.1625. [DOI] [PubMed] [Google Scholar]
- Garriga G, Desai C, Horvitz HR. Cell interactions control the direction of outgrowth, branching and fasciculation of the HSN axons of Caenorhabditis elegans. Development. 1993;117:1071–1087. doi: 10.1242/dev.117.3.1071. [DOI] [PubMed] [Google Scholar]
- Gibson DA, Ma L. Developmental regulation of axon branching in the vertebrate nervous system. Development. 2011;138:183–195. doi: 10.1242/dev.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana TM, Cox EA, Lynch AM, Hardin J. SAX-7/L1CAM and HMR-1/cadherin function redundantly in blastomere compaction and non-muscle myosin accumulation during Caenorhabditis elegans gastrulation. Developmental biology. 2010;344:731–744. doi: 10.1016/j.ydbio.2010.05.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert JM, Lin M, Partanen J, Rossant J, McConnell SK. FGF signaling through FGFR1 is required for olfactory bulb morphogenesis. Development. 2003;130:1101–1111. doi: 10.1242/dev.00334. [DOI] [PubMed] [Google Scholar]
- Hu Y, Guimond SE, Travers P, Cadman S, Hohenester E, Turnbull JE, Kim SH, Bouloux PM. Novel mechanisms of fibroblast growth factor receptor 1 regulation by extracellular matrix protein anosmin-1. The Journal of biological chemistry. 2009 doi: 10.1074/jbc.M109.049155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson ML, Kinnunen T, Cinar HN, Chisholm AD. C. elegans Kallmann syndrome protein KAL-1 interacts with syndecan and glypican to regulate neuronal cell migrations. Developmental biology. 2006;294:352–365. doi: 10.1016/j.ydbio.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Jafari G, Burghoorn J, Kawano T, Mathew M, Morck C, Axang C, Ailion M, Thomas JH, Culotti JG, Swoboda P, et al. Genetics of extracellular matrix remodeling during organ growth using the Caenorhabditis elegans pharynx model. Genetics. 2010;186:969–982. doi: 10.1534/genetics.110.120519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Shou J, Santos R, Hébert JM, McConnell SK, Mason I, Calof AL. Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132:5211–5223. doi: 10.1242/dev.02143. [DOI] [PubMed] [Google Scholar]
- Legouis R, Hardelin JP, Levilliers J, Claverie JM, Compain S, Wunderle V, Millasseau P, Le Paslier D, Cohen D, Caterina D, et al. The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell. 1991;67:423–435. doi: 10.1016/0092-8674(91)90193-3. [DOI] [PubMed] [Google Scholar]
- Lutz B, Rugarli EI, Eichele G, Ballabio A. X-linked Kallmann syndrome. A neuronal targeting defect in the olfactory system? FEBS Lett. 1993;325:128–134. doi: 10.1016/0014-5793(93)81428-3. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Grana T, Cox-Paulson E, Couthier A, Cameron M, Chin-Sang I, Pettitt J, Hardin J. A genome-wide functional screen shows MAGI-1 is an L1CAM-dependent stabilizer of apical junctions in C. elegans. Current biology : CB. 2012;22:1891–1899. doi: 10.1016/j.cub.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia-Belmonte V, Esteban PF, Martinez-Hernandez J, Gruart A, Lujan R, Delgado-Garcia JM, de Castro F. Anosmin-1 over-expression regulates oligodendrocyte precursor cell proliferation, migration and myelin sheath thickness. Brain structure & function. 2015 doi: 10.1007/s00429-014-0977-4. [DOI] [PubMed] [Google Scholar]
- Opperman K, Moseley-Alldredge M, Yochem J, Bell L, Kanayinkal T, Chen L. A Novel Nondevelopmental Role of the SAX-7/L1CAM Cell Adhesion Molecule in Synaptic Regulation in Caenorhabditis elegans. Genetics. 2015;199:497–509. doi: 10.1534/genetics.114.169581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock R, Benard CY, Shapiro L, Hobert O. Functional dissection of the C. elegans cell adhesion molecule SAX-7, a homologue of human L1. Molecular and cellular neurosciences. 2008;37:56–68. doi: 10.1016/j.mcn.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Rugarli EI, Di Schiavi E, Hilliard MA, Arbucci S, Ghezzi C, Facciolli A, Coppola G, Ballabio A, Bazzicalupo P. The Kallmann syndrome gene homolog in C. elegans is involved in epidermal morphogenesis and neurite branching. Development. 2002;129:1283–1294. doi: 10.1242/dev.129.5.1283. [DOI] [PubMed] [Google Scholar]
- Ryu YK, Collins SE, Ho HY, Zhao H, Kuruvilla R. An autocrine Wnt5a-Ror signaling loop mediates sympathetic target innervation. Developmental biology. 2013;377:79–89. doi: 10.1016/j.ydbio.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg Y, Diaz-Balzac CA, Ramirez-Suarez NJ, Attreed M, Tecle E, Desbois M, Kaprielian Z, Bülow HE. Skin-Derived Cues Control Arborization of Sensory Dendrites in Caenorhabditis elegans. Cell. 2013;155:308–320. doi: 10.1016/j.cell.2013.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakura H, Inada H, Kuhara A, Fusaoka E, Takemoto D, Takeuchi K, Mori I. Maintenance of neuronal positions in organized ganglia by SAX-7, a Caenorhabditis elegans homologue of L1. Embo J. 2005;24:1477–1488. doi: 10.1038/sj.emboj.7600621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Rathjen FG. Signalling mechanisms regulating axonal branching in vivo. Bioessays. 2010;32:977–985. doi: 10.1002/bies.201000054. [DOI] [PubMed] [Google Scholar]
- Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 2003;112:619–630. doi: 10.1016/s0092-8674(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- Soussi-Yanicostas N, de Castro F, Julliard AK, Perfettini I, Chedotal A, Petit C. Anosmin-1, defective in the X-linked form of Kallmann syndrome, promotes axonal branch formation from olfactory bulb output neurons. Cell. 2002;109:217–228. doi: 10.1016/s0092-8674(02)00713-4. [DOI] [PubMed] [Google Scholar]
- Soussi-Yanicostas N, Faivre-Sarrailh C, Hardelin JP, Levilliers J, Rougon G, Petit C. Anosmin-1 underlying the X chromosome-linked Kallmann syndrome is an adhesion molecule that can modulate neurite growth in a cell-type specific manner. J Cell Sci. 1998;111:2953–2965. doi: 10.1242/jcs.111.19.2953. [DOI] [PubMed] [Google Scholar]
- Soussi-Yanicostas N, Hardelin JP, Arroyo-Jimenez MM, Ardouin O, Legouis R, Levilliers J, Traincard F, Betton JM, Cabanie L, Petit C. Initial characterization of anosmin-1, a putative extracellular matrix protein synthesized by definite neuronal cell populations in the central nervous system. J Cell Sci. 1996;109:1749–1757. doi: 10.1242/jcs.109.7.1749. [DOI] [PubMed] [Google Scholar]
- Tecle E, Diaz-Balzac CA, Bülow HE. Distinct 3-O-sulfated heparan sulfate modification patterns are required for kal-1-dependent neurite branching in a context-dependent manner in Caenorhabditis elegans. G3. 2013;3:541–552. doi: 10.1534/g3.112.005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornberg J, Sykiotis GP, Keefe K, Plummer L, Hoang X, Hall JE, Quinton R, Seminara SB, Hughes V, Van Vliet G, et al. Heparan sulfate 6-O-sulfotransferase 1, a gene involved in extracellular sugar modifications, is mutated in patients with idiopathic hypogonadotrophic hypogonadism. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11524–11529. doi: 10.1073/pnas.1102284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kweon J, Larson S, Chen L. A role for the C. elegans L1CAM homologue lad-1/sax-7 in maintaining tissue attachment. Developmental biology. 2005;284:273–291. doi: 10.1016/j.ydbio.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang W, Cheever T, Schwarz V, Opperman K, Hutter H, Koepp D, Chen L. The C. elegans L1CAM homologue LAD-2 functions as a coreceptor in MAB-20/Sema2 mediated axon guidance. J Cell Biol. 2008;180:233–246. doi: 10.1083/jcb.200704178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S, Gärtner J. Genetic and clinical aspects of X-linked hydrocephalus (L1 disease): Mutations in the L1CAM gene. Human mutation. 2001;18:1–12. doi: 10.1002/humu.1144. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London B Biological Sciences. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.