Abstract
Paclitaxel is a chemotherapeutic agent widely used for treating carcinomas. Patients receiving paclitaxel often develop neuropathic pain and have a reduced quality of life which hinders the use of this life-saving drug. In this study, we determined the role of GABA transporters in the genesis of paclitaxel-induced neuropathic pain using behavioral tests, electrophysiology, and biochemical techniques. We found that tonic GABA receptor activities in the spinal dorsal horn were reduced in rats with neuropathic pain induced by paclitaxel. In normal controls, tonic GABA receptor activities were mainly controlled by the GABA transporter GAT-1 but not GAT-3. In the spinal dorsal horn, GAT-1 was expressed at presynaptic terminals and astrocytes while GAT-3 was only expressed in astrocytes. In rats with paclitaxel-induced neuropathic pain, the protein expression of GAT-1 was increased while GAT-3 was decreased. This was concurrently associated with an increase of global GABA uptake. The paclitaxel-induced attenuation of GABAergic tonic inhibition was ameliorated by blocking GAT-1 but not GAT-3 transporters. Paclitaxel-induced neuropathic pain was significantly attenuated by the intrathecal injection of a GAT-1 inhibitor. These findings suggest that targeting GAT-1 transporters for reversing disinhibition in the spinal dorsal horn may be a useful approach for treating paclitaxel-induced neuropathic pain.
Keywords: taxol, nociception, neurotoxicity, γ-aminobutyric acid, IPSCs, patch clamp
Introduction
Paclitaxel (taxol) is a potent anti-tumor drug used for the treatment of carcinomas in a wide range of organs including lung, breast, ovaries, prostate and others. The clinical application of this life-saving agent is hampered by paclitaxel-induced neuropathic pain (P-INP). Currently, effective treatments for P-INP are not available. It is known that pathological pain originates from aberrant neuronal activities along the pain signalling pathway, including peripheral nociceptors, neurons in the spinal dorsal horn and supraspinal pain centres. Homeostasis between excitatory and inhibitory receptor activities is crucial to maintain normal neuronal activities in the central nervous system (CNS). In the spinal dorsal horn, attenuation in glycinergic and/or γ-aminobutyric acid (GABA) inhibitory system contributes to the genesis of pathological pain. For example, reduction of glycinergic receptor activities in the spinal superficial dorsal horn is associated with inflammatory pain (Harvey et al. 2004, Bonin & De Koninck 2013) while impairment in GABAergic inhibitory synaptic activities in the spinal dorsal horn is an important mechanism contributing to the genesis of neuropathic pain induced by nerve injury (Coull et al. 2003, Coull et al. 2005, Bonin & De Koninck 2013, Moore et al. 2002). Currently, whether and how the spinal inhibitory system is altered in paclitaxel-induced neuropathic pain remains inadequately understood.
GABA is the major inhibitory neurotransmitter released from GABAergic interneurons in the spinal dorsal horn (Bardoni et al. 2013, Bonin & De Koninck 2013). GABA exerts its inhibitory effects through acting on ionotropic GABAA receptors and metabotropic GABAB receptors at presynaptic terminals to reduce presynaptic glutamate release (Bardoni et al. 2013). Activation of presynaptic GABAB receptors with baclofen can ameliorate pathological pain (Gaillard et al. 2014, Fukuhara et al. 2013). GABA also acts on GABAA receptors at postsynaptic neurons to cause influx of Cl- and membrane hyperpolarization in postsynaptic neurons (Bardoni et al. 2013). Activation of synaptic GABAA receptors by GABA released presynaptically produces phasic inhibition, while activation of extrasynaptic GABAA receptors by ambient GABA is related to tonic inhibition of neurons (Belelli et al. 2009, Lee & Maguire 2014). Studies of GABAergic receptor activities in the spinal dorsal horn have mainly concentrated on understanding the fast synaptic (phasic) inhibition. Little is known about the regulation of GABAergic tonic inhibition in the spinal dorsal horn in normal and pathological pain conditions.
One important factor that regulates the clearance and maintenance of the homeostasis of extracellular inhibitory transmitters is the GABA transporter system (Zhou & Danbolt 2013). GABA transporters are located on the plasma membrane in neurons and astrocytes, which transport extracellular GABA into the cell as it is not metabolized extracellularly (Zhou & Danbolt 2013). In the CNS, there are mainly two types of GABA transporters, GABA transporter-1 (GAT-1) and GABA transporter-3 (GAT-3). Studies in the forebrain show that GABAergic tonic inhibition is controlled by GABA transporters. The regulation of GABA receptor activities by GAT-1 and GAT-3 and cellular types expressing GAT-1 and GAT-3 are region-specific (Park et al. 2009, Kersante et al. 2013, Belelli et al. 2009, Lee & Maguire 2014). Previous studies have suggested that the protein expression of GABA transporters is altered in animals with pathological pain induced by inflammation or nerve injury (Ng & Ong 2001, Ng & Ong 2002, Daemen et al. 2008). Currently, it is unclear whether changes of tonic GABAergic inhibition and GABA transporters contribute to the genesis of paclitaxel-induced neuropathic pain.
In this study, we revealed, for the first time, that GABAergic tonic inhibition in the spinal dorsal horn of rats with P-INP is reduced. We defined the cellular location of GAT-1 and GAT-3 and the role of these transporters in GABAergic tonic inhibition in normal and P-INP. We demonstrated that blocking GAT-1 in the spinal dorsal horn is a powerful approach to ameliorating P-INP.
Methods and materials
Animals
Adult male Sprague-Dawley rats (body weight: 170-220 g, Harlan Laboratories) were used. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Georgia and were fully compliant with the National Institutes of Health Guidelines for the Use and Care of Laboratory Animals.
Paclitaxel-induced neuropathic pain model in rats
P-INP was induced in rats by intraperitoneal (i.p.) injection of paclitaxel (Taxol, Bristol-Myers Squibb) at a dose of 2 mg/kg on four alternate days (days 1, 3, 5, and 7) with a cumulative dose of 8 mg/kg. Paclitaxel and vehicle were prepared and injected as previously described (Gao et al. 2013).
Behavioral tests
All the behavioral testing were conducted in a quiet room with room temperature at 22 °C (Weng et al. 2003). To test mechanical sensitivity, rats were placed on a wire mesh, loosely restrained under a plexiglass cage (12 × 20 × 15 cm3) and allowed to acclimate for at least 30 min. Hind paw withdrawal response thresholds to mechanical stimuli were defined with a set of von Frey monofilaments (bending force from 0.58 to 14.68 g), which were applied in an ascending order to evoked a 50% or greater withdrawal responses (Yan et al. 2014). This value was later averaged across all animals in each group to yield the group response threshold. To determine thermal sensitivity, rats were placed on a smooth glass plate pre-heated at 30 °C. A radiant heat beam (diameter 5 mm2) was directed onto the mid-plantar surface of the hind paw from beneath (Hargreaves et al. 1988). The withdrawal latency was recorded as previously described (Maixner et al. 2015). When the behavioral tests and i.p. injection took place on the same day, the i.p. injection was made after the behavioral tests. The experimenters conducting the behavior tests were blinded to the type of treatments given to the animals.
To determine whether the tested agents cause impairment in motor functions or sedation, the rotarod test was conducted as described previously (Stone et al. 2014, Hara et al. 2014). Animals were placed on a rotating drum and the drum was set to rotate from 4 to 40 rpm over a period of 5 min. The period of time in seconds at which the animal fell from the drum was recorded. The mean time for each treatment group of animals was taken for statistical analysis.
Intrathecal catheter implantation
A polyethylene (PE-10) catheter that ended at the spinal L4 segment was intrathecally placed as previously described (Yaksh & Rudy 1976). Briefly, rats were anesthetized with 2-3% isoflurane. A PE-10 catheter was carefully inserted into an opening at the atlanto-occipital membrane and advanced to the lumber enlargement. Behavior tests and intrathecal drug administration were conducted 7 days after the intrathecal implantation of the catheter. At the end of the behavior experiments, 50 μL of 2% lidocaine was injected into the catheter. If hind paw paralysis did not occur after the lidocaine injection, rats were omitted from the data analysis.
Western blotting
Animals were deeply anesthetized with urethane (1.3–1.5 g/kg, i.p.). The L4-L5 spinal segment was exposed and removed from the rats. The dorsal half of the spinal segment was isolated and quickly frozen in liquid nitrogen and stored at -80 °C for later use. Frozen tissues were homogenized and protein was isolated and quantitated as previously described (Gao et al. 2013, Maixner et al. 2015). Protein samples (40 μg) were electrophoresed in 10 % SDS polyacrylamide gels and transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA). The membranes were blocked with 5% milk or 5% BSA, and then incubated respectively overnight at 4 °C with polyclonal rabbit anti- GAT-1 (1:1000, Abcam) polyclonal rabbit anti-GAT-3 (1:1000, Abcam) primary antibodies, or a monoclonal mouse anti-β-actin (1:2000, Sigma-Aldrich, St. Louis, USA) primary antibody as a loading control. Then the blots were incubated for 1 hr at room temperature with corresponding HRP-conjugated secondary antibodies (1:5000; Santa Cruz Biotechnology, CA, USA), visualized in ECL solution (Super Signal West Pico Chemiluminescent Substrate, Pierce, Rockford, IL, USA) for 1 min, and exposed onto FluorChem HD2 System. The intensity of immunoreactive bands was quantified as previously described (Gao et al. 2013). Levels of each biomarker were expressed as the ratio to the loading control protein (β-actin).
GABA uptake assay
GABA uptake activity in the lumbar spinal dorsal horn of rats was measured using synaptosome preparations according to previously established protocols with modifications (Wonnemann et al. 2000, Hu et al. 2003, Ozkan et al. 1997, Yan et al. 2014). The L4-L5 spinal segment was removed from the rat anesthetized with urethane (1.3–1.5 g/kg, i.p) and the dorsal half of the spinal cord was isolated. Synaptosome preparations were prepared immediately in Syn Per TM synaptic protein extraction reagent (Thermo scientific) and quantified by the BCA assay (Thermo scientific). Briefly the homogenates were centrifuged at 10,500 × g for 10 min at 4 °C, and the supernatant was collected. The remaining pellets were re-suspended in the same solution and re-centrifuged at 9,200 × g for 10 min at 4°C. The two supernatant were combined and centrifuged again at 9,200 × g for 10 min at 4°C to obtain the synaptosomal pellets, which contained both neuronal and glial GABA transporters. The synaptosome protein (50 μg) was incubated in Locke's buffer solution containing: 0.5 mM EDTA, 0.5 mM EGTA, 0.2 mM phenylmethylsulfonyl fluoride, 0.32 M sucrose, 5 μg/ml pepstatin, 5 μg/ml aprotinin, 20 μg/ml trypsin inhibitor, 4 μg/ml leupeptin, and 0.01 M phosphate-buffered saline. GABA uptake activity was determined by incubating the synaptosome preparation with a solution containing 0.4 μCi of - Aminobutyric Acid (GABA), γ-[2,3-3H(N)] (Perkin Elmer Life Sciences, Boston, MA) at room temperature for 10 min. The reaction was terminated by filtering the synaptosomes through a Whatman GF/B filter presoaked with the same buffer solution. The filter was washed two times with ice-cold Locke's buffer (2ml) and was then transferred to a vial containing a scintillation cocktail. The radioactivity of the final samples was measured by a liquid scintillation counter (Beckman, LS6500).
Immunohistochemical Analysis
Male Sprague-Dawley rats were deeply anesthetized with urethane (1.3-1.5 g/kg,i.p.) and the L4 and L5 spinal cord was removed, fixed, and cyrosectioned as previously described (Weng et al. 2014). Sections were incubated overnight at 4 °C in 2% normal goat serum and 0.3% Triton X-100 in 0.1 M PBS containing primary antibodies against the following targets: rabbit anti- GAT-1 (1:200, Abcam), rabbit anti-GAT-3 (1:200, Abcam), mouse anti-GFAP (a marker for astrocytes, 1:500, Cell Signaling), rat anti-OX-42 (a marker for microglia, 1:500, AbD Serotec), and mouse anti MAP2 (a marker for neuronal cytoskeleton 1:500, Cell Signaling) antibodies. After washing three times with 0.1 M PBS, the sections were incubated for 2 hr at room temperature with the corresponding Texas Red antibody (1:500 Vector Laboratories), Alexa Fluor 488 antibody (1:500 Life Technologies), or the Mouse Anti-NeuN Alexa Fluor 488 conjugated antibody (the neuronal cell body marker, 1:200, Millipore). After rinsing three times with 0.1M PBS, the sections were mounted onto gelatin-coated slides, air-dried, and cover-slipped with Vectashield mounting medium (Vector Laboratories). Non-adjacent sections were selected randomly, and the immunostaining for each antibody were viewed under an Olympus BX43 microscope with an Olympus U-CMAD3 camera. Images were processed using Olympus-cellSens Dimensions.
Recording of GABAergic currents
Spinal slices were obtained from rats as previously described (Weng et al. 2007). Briefly, rats were deeply anesthetized under isoflurane, and a laminectomy was then performed to remove the lumbar spinal cord. The L4 to L5 spinal segment was placed in ice-cold sucrose-based artificial cerebrospinal fluid (aCSF) pre-saturated with 95% O2 and 5% CO2. The sucrose-based aCSF contained 234 mM sucrose, 3.6 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.2 mM NaH2PO4, 12.0 mM glucose, and 25.0 mM NaHCO3. Transverse spinal cord slices (400 μm thick) were cut in the ice-cold sucrose aCSF and then pre-incubated for at least 2 hours in Krebs solution oxygenated with 95% O2 and 5% CO2 at 35 °C before they were transferred to the recording chamber. The Krebs solution contained 117.0 mM NaCl, 3.6 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.2 mM NaH2PO4, 11.0 mM glucose, and 25.0 mM NaHCO3. Following pre-incubation, a rat spinal slice was placed in the recording chamber (1.5 ml in volume), perfused with Krebs solution at 35 °C, and saturated with 95% O2 and 5% CO2. Borosilicate glass recording electrodes (resistance, 3–5 MΩ) were made and filled with an internal solution containing 110 mM Cs2SO4, 5 mM KCl, 2.0 mM MgCl2, 0.5 mM CaCl2, 5.0 mM HEPES, 5.0 mM EGTA, 5.0 mM ATP-Mg, 0.5mM Na-GTP, and 10 mM lidocaine N-ethyl bromide (QX314), adjusted to pH 7.2–7.4 with 1 M CsOH (290–300 mOsm) (Jiang et al. 2012). The recording electrodes were directed to the out layer of spinal dorsal horn lamina II. Whole-cell configurations were established by applying moderate negative pressure after electrode contact (Weng et al. 2007). A seal resistance of ≥ 2 GΩ and an access resistance of about 20 MΩ were considered acceptable. GABAergic currents were recorded in the presence of 0.5 μM strychnine (a glycine receptor inhibitor), 10 μM DNQX (an AMPA/kainate receptor inhibitor) and 25 μM D-AP5 (NMDA receptor inhibitor) at a holding potential of 0 mV (Moore et al. 2002). The depth of the recorded cell in the slice (about 50 μm below the surface) was kept constant across all experiments.
Materials
NO-711 and SNAP5114 were obtained from Sigma-Aldrich (St. Louis, MO).
Data analysis
All data are presented as the mean ± SEM. One way or two way ANOVA with repeated measures over time or different treatments was used to detect differences on paw-withdrawal response thresholds and latencies, followed by the Bonferroni post hoc test to determine sources of differences. Student's t-tests were used to make comparison between groups (non-paired t) and within the same group (paired t). A p value less than 0.05 was considered statistically significant.
Results
In this study, rats used for the mass spectrometry, electrophysiology, and Western blot experiments were assigned into two groups, paclitaxel treated group and vehicle treated group. Paclitaxel (2 mg/kg. i.p.) or vehicle was respectively injected into animals in the paclitaxel group or vehicle group on days 1, 3, 5, and 7. Hind paw withdrawal response thresholds to mechanical and thermal stimuli were measured on days 1, 5 and 10. Consistent with previous findings by us (Gao et al. 2013) and others (Polomano et al. 2001, Okubo et al. 2011), rats receiving paclitaxel in this scheme developed mechanical allodynia and thermal hyperalgesia. These were evident by a significant reduction (51 vehicle-treated rats versus 48 paclitaxel-treated rats) of mechanical thresholds (F (2, 46) = 196, P < 0.001) and latencies (F (2, 46) = 98.65, P < 0.001) of hind paw withdrawal responses on day 5 and day 10 after the first injection (Fig.1). Electrophysiology and Western blot experiments were performed on rats 10 days after the first i.p. injection, when all rats treated with paclitaxel developed mechanical allodynia and thermal hyperalgesia.
Figure 1. Rats treated with paclitaxel develop mechanical allodynia and thermal hyperalgesia.
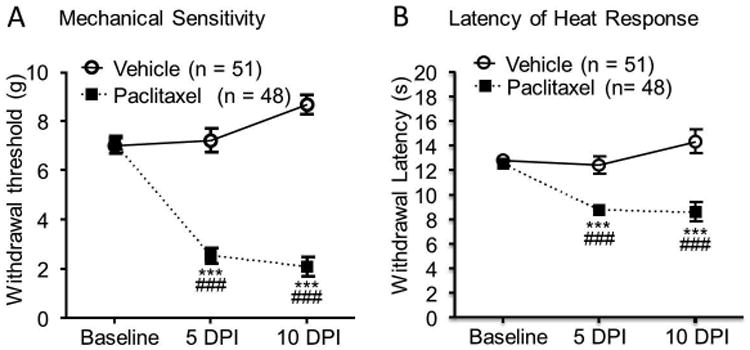
Rats were injected (i.p.) with either vehicle or paclitaxel (2.0 mg/kg) on four alternate days (days 1, 3, 5 and 7). The mean (± SEM) mechanical thresholds of hind paw withdrawal responses (A) and latencies of the withdrawal response to heat stimuli (B) prior to the i.p. injection (Baseline), and on 5 days (5 DPI) and 10 days (10 DPI) post the first injection were plotted. Comparisons of data between baseline and each time point in the paclitaxel group are labeled with *. Comparisons of data between the vehicle group and the paclitaxel group at each time point are indicated with #. Three symbols, P < 0.001.
GABAergic tonic inhibition in the spinal dorsal horn is weakened in paclitaxel-treated rats
To determine whether altered GABAergic receptor activities in the spinal cord contribute to the genesis of P-INP, we compared GABAergic tonic inhibition in spinal dorsal horn neurons in rats treated with paclitaxel and rats treated with vehicle. Following previously published protocols (Bai et al. 2001, Jia et al. 2005, Clarkson et al. 2010), we analyzed GABAergic tonic inhibition by bath-perfusing the GABAA receptor inhibitor bicuculline (25 μM) and measuring changes of holding currents when GABAergic currents in the spinal dorsal horn neurons were recorded. We found that perfusion of bicuculline into the recording chamber blocked spontaneous GABAergic IPSCs and revealed a shift in the holding currents in slices taken from vehicle control rats (tested in 11 neurons) (Fig. 2A) and paclitaxel-induced neuropathic rats (tested in 11 neurons) (Fig. 2B). The changes of holding currents induced by bicuculline in rats with P-INP (23.93 ± 2.76 pA) were significantly less (Fig. 2C, P < 0.001) than those in vehicle control rats (63.93 ± 3.53 pA) (Fig. 2C). These data indicated that GABAergic tonic inhibition is reduced in the spinal dorsal horn of rats with P-INP. Thus, enhancing GABAergic tonic inhibition may be an effective approach to alleviate P-INP.
Figure 2. GABAergic tonic inhibition in the spinal dorsal horn is weakened in the paclitaxel-treated rats.
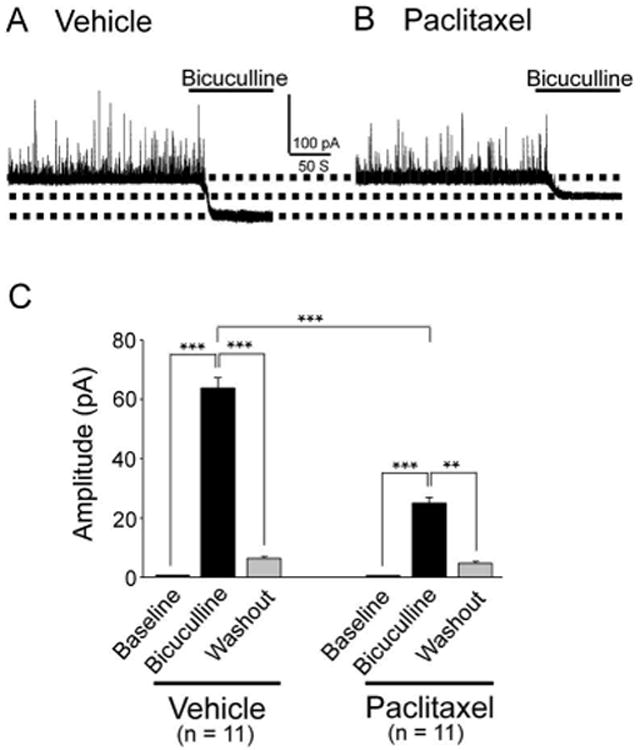
Raw data show recordings of GABAergic currents before and after blocking of GABAA receptors with bicuculline (25 μM) obtained from vehicle (A) and paclitaxel (B) treated rats. (C) Shows the mean (± SEM) changes of holding currents induced by bicuculline in vehicle and paclitaxel treated rats. ** P < 0.01; *** P < 0.001.
Tonic GABA receptor activities in the spinal dorsal horn are mainly regulated by GAT-1 under normal conditions
Studies in the forebrain have shown that tonic GABAergic inhibition is regulated both by GABA synthesis (Nishikawa et al. 2011, Fatemi et al. 2005) and by GABA transporters (Semyanov et al. 2004, Wu et al. 2006, Smith et al. 2007, Kirmse et al. 2008, Nishikawa et al. 2011). To determine the role of GABA transporters in the regulation of GABAergic receptor activities in the spinal dorsal horn, we first determined the cellular location of the GABA transporters GAT-1 and GAT-3 in the spinal dorsal horn of normal control animals using immunohistochemistry technique. As shown in Figure 3A and B, GAT-1 and GAT-3 were widely expressed in the entire spinal dorsal horn with more dense expression in the superficial dorsal horn (laminae I and II). Furthermore, expression of GAT-1 was co-localized with a marker for neuronal cytoskeleton (MAP2) and the astrocyte marker (GFAP), but not with the neuronal cell body marker (NeuN) or microglia marker (OX42) (Fig. 3C). In contrast, GAT-3 was predominantly co-localized with GFAP, but not OX42, MAP2, or NeuN (Fig. 3D). These data indicate that: a) GAT-1 is expressed in neuronal presynaptic terminals and astrocytes; b) GAT-3 is only present in astrocytes; c) neither GAT-1 nor GAT-3 is expressed in microglia.
Figure 3. GAT-1 is expressed in neurons and astrocytes and GAT-3 only is present in astrocytes.
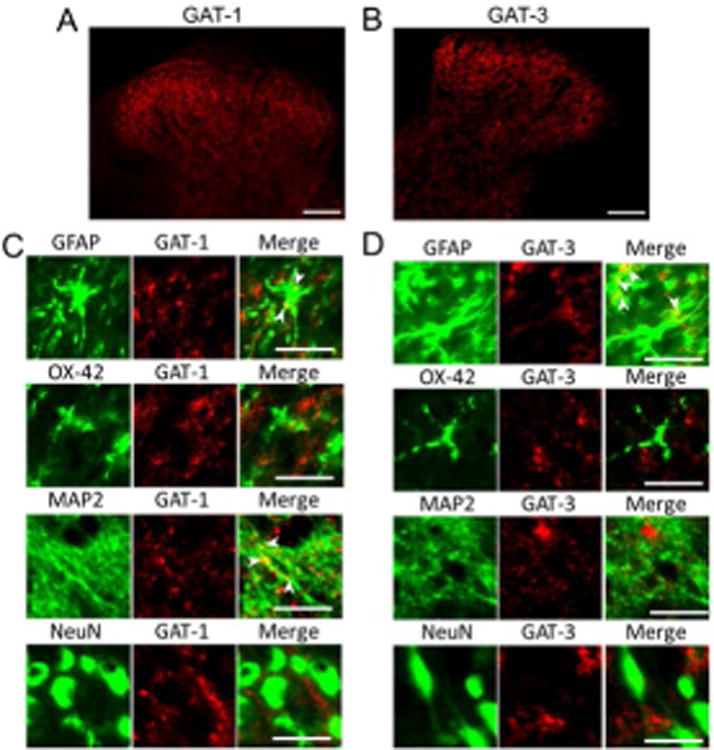
Samples were obtained from the spinal dorsal horn of normal control rats. (A) and (B) respectively show staining of GAT-1 and GAT-3 in the spinal dorsal horn, note higher expressions of GAT-1 and GAT-3 at the superficial dorsal horn (Scale bar = 100 μM). (C) Shows that expression of GAT-1 is colocalized with MAP2 (a marker for neuronal cytoskeleton) and GFAP (an astrocyte marker), but not with NeuN (a neuronal cell body marker) or OX42 (a microglia marker) (Scale bar = 20 μM). (D) Shows that GAT-3 is predominantly colocalized with GFAP, but not OX42, MAP2, or NeuN (Scale bar = 20 μM).
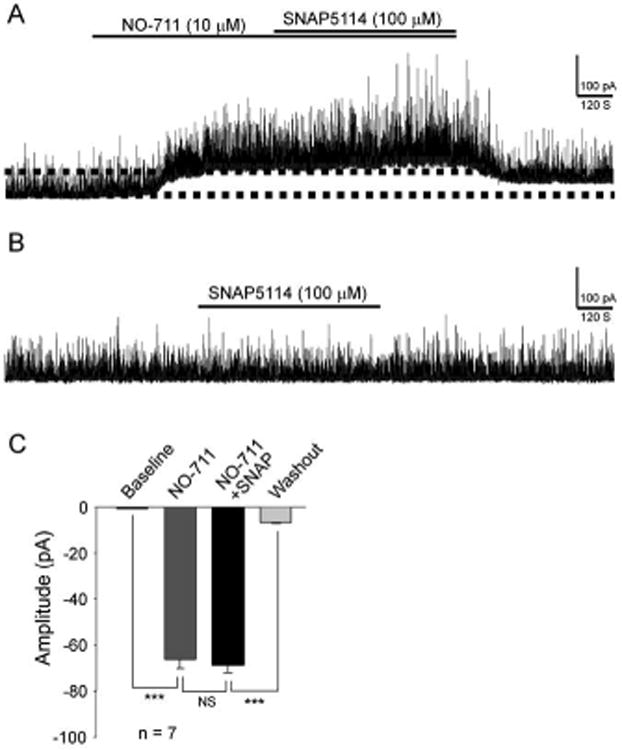
We next determined whether GABAergic tonic inhibition in the spinal dorsal horn is regulated by GAT-1 and GAT-3 in normal controls. NO-711 and SNAP5114 are widely used to selectively block GAT-1 and GAT-3 respectively (Nusser & Mody 2002, Rossi et al. 2003, Keros & Hablitz 2005). We recorded GABAergic tonic currents before and during bath-perfusion of the GAT-1 inhibitor (NO-711, 10 μM). As shown in Figure 4A and C, the GAT-1 inhibitor significantly (P < 0.001) increased the holding currents (which reflects tonically active GABA conductance) by -37.05 ± 2.36 pA (n = 8). Under the condition when GAT-1 was inhibited by NO-711 (10 μM), the addition of the GAT-3 inhibitor SNAP5114 (100 μM) resulted in a further increase of holding currents (n = 8, P < 0.001) (Fig. 4A and C). Interestingly, when GAT-3 was blocked alone by bath-perfusion containing SNAP5114 (100 μM), we did not observe a significant change in the holding currents (n = 9, P = 0.58) (Fig. 4B). These data indicated that GAT-1 is critical in maintaining tonic GABA inhibition in the spinal dorsal horn.
Figure 4. Tonic GABA receptor activities in the spinal dorsal horn are mainly regulated by GAT-1 under normal conditions.
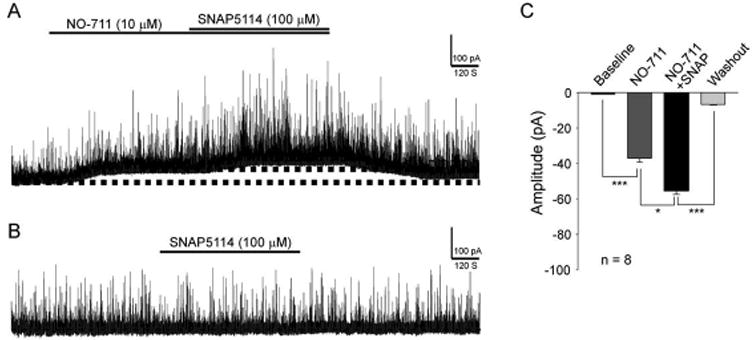
Data were obtained from normal control rats. (A) Shows recordings of GABAergic currents before and after blocking of GAT-1 with NO-711 (10 μM) and then further inhibition of GAT-3 with SNAP5114 (100 μM). (B) Shows recordings of GABAergic currents before and after blocking of GAT-3 alone with SNAP5114 (100 μM). (C) Shows the mean (± SEM) changes of holding currents induced by NO-711 and then NO-711 plus SNAP5114. ** P < 0.01; *** P < 0.001.
Expression of GAT-1 is increased while expression of GAT-3 is reduced in the spinal dorsal horn in rats with P-INP, which is concurrently associated with increased GABA uptake activities
We then determined whether altered functions of GAT-1 and GAT-3 are implicated in the genesis of P-INP. We examined protein expressions of GAT-1 and GAT-3 in the dorsal half of the L4 to L5 spinal segment in the paclitaxel group or vehicle group. As shown in Figure 5A, in comparison with rats treated with vehicles (n = 10), the expression of GAT-1 in the spinal dorsal horn was significantly increased (n = 11, P < 0.001) while the expression of GAT-3 in the spinal dorsal horn was significantly reduced in rats with P-INP. We next determined the global GABA uptake activities carried by both GAT-1 and GAT-3 in the spinal dorsal horn of rats with P-INP and rats receiving vehicle treatment using synaptosome preparations (Mitrovic et al. 1999, Sung et al. 2003). We found that GABA uptake activities were significantly increased in rats with P-INP (n = 4, P < 0.001) in comparison with rats (n = 3) in the vehicle group (Fig. 5B). These data indicate that in the spinal dorsal horn of rats with P-INP GABA uptake activities are increased, which is ascribed to the increased GAT-1 function.
Figure 5. Rats with P-INP have an increased protein expression of GAT-1 and reduced protein expression of GAT-3, which was accompanied with increased GABA uptake activities.
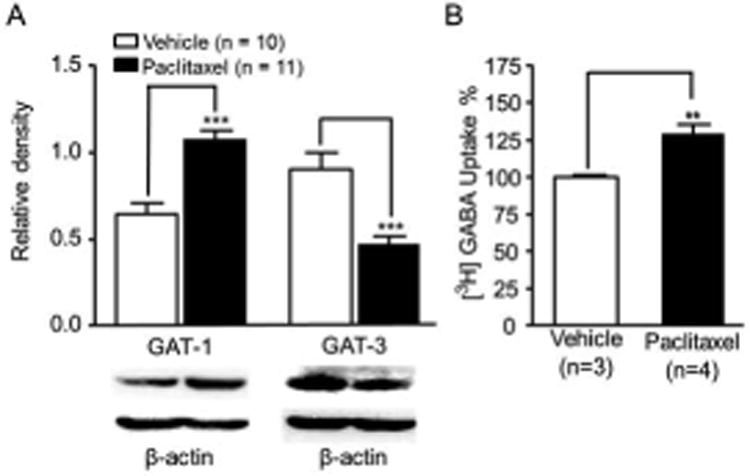
(A) Shows the mean (± SEM) relative density of GAT-1 and GAT-3 to β-Actin in the spinal dorsal horn of rats treated with vehicle and paclitaxel. Samples of GAT-1 and GAT-3 expressions in the spinal dorsal horn at the L4 to L5 segment in paclitaxel and vehicle treated rats are shown. (B) Shows the mean (± SEM) GABA uptake activities obtained from the spinal dorsal horn of rats receiving vehicle and paclitaxel treatment. The GABA uptake activities in the synaptosome preparation from rats treated with paclitaxel treatment were normalized with those treated with vehicle measured at the same time. ** P < 0.01; *** P < 0.001.
The paclitaxel-induced GABAergic disinhibition is ameliorated by blocking GAT-1 but not GAT-3
We then investigated whether the paclitaxel-induced suppression of GABAergic tonic inhibition in the spinal dorsal horn can be ameliorated by blocking GAT-1 and GAT-3. As shown in Figure 6A and C, the GAT-1 inhibitor (NO-711, 10 μM) significantly increased the holding currents. The degree of increases induced by the GAT-1 inhibitor on the holding currents in the paclitaxel-treated rats (-66.27 ± 3.80 pA, n = 7) (Fig. 6C) was significantly (P < 0.001) higher than those in vehicle treated rats (-37.05 ± 2.36 pA, n = 8) (Fig. 4C). Furthermore, we did not observe significant (P < 0.05) alterations in the holding current in paclitaxel treated rats when the GAT-3 inhibitor (SNAP5114, 100 μM) was perfused into the bath in the presence of the GAT-1 inhibitor (NO-711, 10 μM) (Fig. 6A and C). The holding current was not significantly altered by the GAT-3 inhibitor alone (Fig. 6B) (n = 9, P = 0.39). These data indicate that inhibition of GAT-1 is a potent approach to restore the paclitaxel-induced suppression of GABA receptor tonic activities in the spinal dorsal horn.
Figure 6. The paclitaxel-induced GABAergic disinhibition is ameliorated by blocking GAT-1 but not GAT-3.
The original recordings in (A) and (B) were obtained from rats with P-INP. (A) Shows recordings of GABAergic currents before and after blocking of GAT-1 with NO711 (10 μM) and then further inhibition of GAT-3 with SNAP5114 (100 μM). (B) Shows recordings of GABAergic currents before and after blocking of GAT-3 alone with SNAP5114 (100 μM). (C) Shows mean (± SEM) changes of holding currents induced by NO-711 and then NO-711 plus SNAP5114 in rats treated with paclitaxel. ** P < 0.01; *** P < 0.001.
Mechanical allodynia and thermal hyperalgesia in rats with P-INP are attenuated by intrathecal injection of GAT-1 inhibitors
Finally, we determined whether inhibition of GAT-1 can ameliorate mechanical allodynia and thermal hyperalgesia induced by paclitaxel. Rats pre-implanted with intrathecal catheters were assigned into 4 groups: paclitaxel+saline group, paclitaxel+GAT-1 inhibitor group, vehicle+saline group, and vehicle+GAT-1 inhibitor group. After taking baseline withdrawal response thresholds to radiant heat and mechanical stimuli, rats received either 4 injection of paclitaxel (2 mg/kg, i.p) or vehicle on alternative days. Ten days following the first injection, mechanical allodynia and thermal hyeralgesia were confirmed by behavioral tests using von Frey monofilament (Fig. 7A) and radiant heat stimuli (Fig. 7B). Meanwhile mechanical and thermal thresholds in the vehicle+saline group and naïve+GAT-1 inhibitor group remained stable. We then topically applied the GAT-1 inhibitor NO-711 (10 μg in a volume of 10 μl) onto the spinal lumbar enlargement in the paclitaxel+GAT-1 inhibitor group and naïve+GAT-1 inhibitor group through the pre-implanted intrathecal (i.t.) catheter. Rats in the paclitaxel+saline group and vehicle+saline group received 10 μl of saline via intrathecal injection. Changes in thermal and mechanical sensitivities were determined at 0.5, 1, 3, and 24 hours after the i.t. injection. The paclitaxel-induced mechanical allodynia and thermal hyperalgesia were significantly attenuated by a single intrathecal injection of the GAT-1 inhibitor NO-711(10 μg). As shown in Fig. 7A, NO-711 significantly increased the mechanical thresholds in the paclitaxel+GAT-1 group at 30 min (10.00 ± 0.01 g) and 60 min (8.00 ± 0.89 g) in comparison with their own values (4.00 ± 0.89 g) before the NO-711 injection (F (4,20) = 30.18, P < 0.001), or in comparison with their counterparts in the paclitaxel+saline group at 30 min (5.2 ± 0.87 g) and 60 min (3.6 ± 0.97 g) (main effects of drug: F (1,45) = 12.39, P < 0.01; main effect of time: F(4,45) = 8.90, P < 0.001; interaction: F(4,45) = 4.30, P < 0.005; 5 to 6 rats/group). Similarly, latencies of withdrawal responses to radiant heat stimuli in the paclitaxel+GAT-1 group were also significantly increased at 30 min (13.50 ± 1.07 s) and 60 min (11.51 ± 0.87 s) after the injection of NO-711(10 μg) in comparison with the values (7.45 ± 0.50 s) prior to the NO-711 injection (F (4,20) = 14.33, P < 0.001), and their counterparts in the paclitaxel+saline group at 30 min (8.13 ± 0.38 s) and 60 min (7.97 ± 0.77 s, Fig. 7B) (main effects of drug: F (1,45) = 19.97, P < 0.001; main effect of time: F(4,45) = 5.52, P < 0.01; interaction: F (4,45) = 6.46, P < 0.003; 5 to 6 rats/group). The effects of GAT-1 inhibitors on mechanical and thermal thresholds disappeared by 3 hours after drug administration. These results indicated that the blockade of GAT-1 activities is a powerful approach to reverse P-INP. In contrast, administration of saline in the vehicle+saline group or the GAT-1 inhibitor (10 μg in a volume of 10 μl) in the naive+GAT-1 inhibitor group did not significantly alter mechanical or thermal thresholds in these two groups (Fig. 7A and B). To further determine whether the GAT-1 inhibitor at the tested dose (10 μg) cause impairment on motor functions and/or sedation, the rotarod test was performed. Retention time on an accelerating rotarod is a widely used index to monitor motor functions and/or sedation in animals, since sedation also causes the animals to fall (Stone et al. 2014, Hara et al. 2014). We found that in comparison with naïve rats (n = 8) receiving intrathecal saline injection (10 μl), intrathecal administration of the GAT-1 inhibitor NO-711 (10 μg in a volume of 10 μl) to naïve rats (n = 8) did not significantly (P = 0.22 to 0.77) alter the retention time on the rotarod over the observation period (0.5 to 24 hours after the intrathecal injection) (data not shown). These results indicate that NO-711 at a dose of 10 μg does not cause motor impairment or sedation.
Figure 7. Mechanical allodynia and thermal hyperalgesia in rats with P-INP are attenuated by an intrathecal injection of a GAT-1 inhibitor.
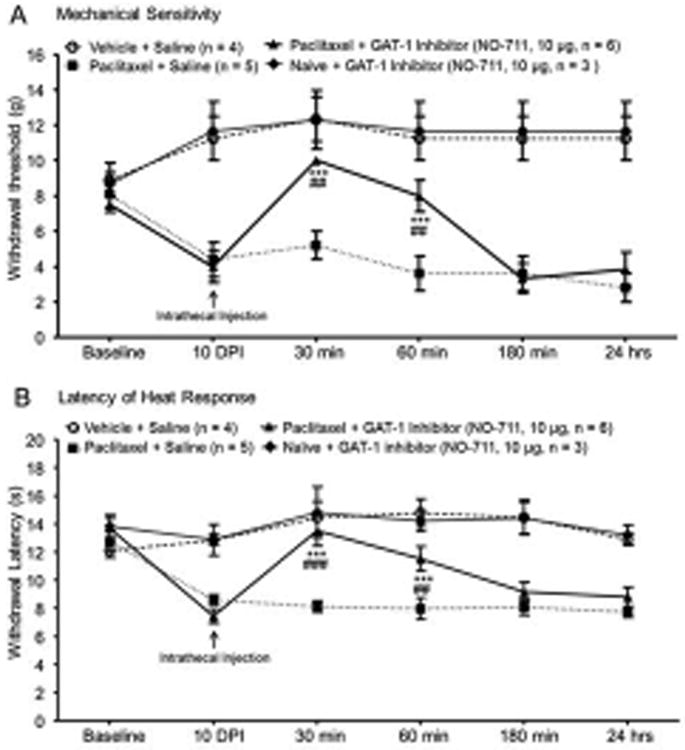
Line plots show measurements of mechanical thresholds of hind paw withdrawal responses (A) and latencies of the withdrawal response to heat stimuli (B) collected at baseline, 10 days post-i.p. vehicle or paclitaxel injection (10 DPI), and then 30, 60, 180 min and 24 hours after the intrathecal administration of the tested agent. Baseline indicates the measurement before animals received i.p. vehicle or paclitaxel injection. Comparisons between data collected on 10 DPI and at following each time point are indicated with * for the paclitaxel+GAT-1 inhibitor group. Comparisons between the paclitaxel+saline group and paclitaxel+GAT-1 inhibitor group are labeled with #. Two symbols: P < 0.01, three symbols: P < 0.001, data shows mean (± SEM).
Discussion
Mechanisms underlying paclitaxel-induced neuropathic pain
Pathological changes in both peripheral nerves and the spinal dorsal horn have been reported in animals with P-INP. For example, P-INP in rats is associated with the degeneration of intraepidermal terminal arbors of sensory neurons and activation of Langerhans cells in the skin (Siau et al. 2006, Boyette-Davis et al. 2011). Furthermore, the incidence of swollen and vacuolated axonal mitochondria is increased in myelinated and unmyelinated sensory axons (Xiao & Bennett 2012, Zheng et al. 2011). Agents that enhance mitochondrial function have been shown to be effective in the prevention or attenuation of P-INP (Jin et al. 2008). The protein and mRNA expressions of TRPV1 in dorsal root ganglion sensory neurons are increased in rats receiving repeated paclitaxel treatments (Hara et al. 2013). More recently, it was reported that chronic paclitaxel significantly increases the protein expression of the chemokine CX3CL1 in A-fiber primary sensory neurons and infiltration of macrophages into the dorsal root ganglion (DRG) in rats, which contributes to the paclitaxel-induced DRG neuronal apoptosis of A fibers and pathological pain (Huang et al. 2014). At the spinal cord level, we have previously shown that wide-dynamic range neurons in the spinal dorsal horn in rats with paclitaxel-induced neuropathic pain display a significant increase in both spontaneous activities (action potentials) and responses to noxious mechanical, heating, and cooling stimuli, as well as an abnormal wind-up to transcutaneous electrical stimuli (Cata et al. 2006). Loss of homeostasis between excitatory and inhibitory inputs to neurons is a major factor leading to exaggerated neuronal activation. In this regard, the down-regulation of glial glutamate transporters in the spinal dorsal horn has been demonstrated to be causal in the genesis of P-INP (Weng et al. 2005, Doyle et al. 2012, Gao et al. 2013). Glial glutamate transporters control neuronal activation by promptly clearing the excitatory neuronal transmitter glutamate released from presynaptic terminals in the spinal dorsal horn (Nie & Weng 2010, Weng et al. 2007, Nie & Weng 2009). Improving glial glutamate transporter functions by reducing levels of peroxynitrite (Doyle et al. 2012) or suppression of GSK3β activities (Gao et al. 2013) in the spinal dorsal horn effectively prevents and attenuates P-INP. It was recently reported that up-regulation of NKCC1 in the spinal dorsal horn causes a depolarizing shift and reduces GABA-induced membrane hyperpolarization in GABAergic neurons following P-INP (Chen et al. 2014). Whether the tonic GABAergic inhibition is altered in P-INP has not been explored. Our current study provided direct evidence that GABAergic tonic disinhibition in the spinal dorsal horn is implicated in the genesis of P-INP.
Regulation of GABAergic receptor activities in the spinal dorsal horn in pathological pain conditions
Disinhibition in the spinal dorsal horn is a critical factor leading to excessive neuronal activation in animals with neuropathic pain induced by mechanical injury. Extracellular GABA level in the lumbar dorsal horn is reduced by nerve injury (Stiller et al. 1996, Castro-Lopes et al. 1993). In neuropathic rats, amplitudes and frequencies of GABAA receptor-mediated IPSCs in neurons in the superficial dorsal horn are attenuated (Moore et al. 2002). Additionally, the spinal application of GABA agonists attenuate mechanical allodynia and thermal hyperalgesia induced by nerve injury (Malan et al. 2002). It is known that GABAergic receptor activities are governed by at least the following factors: the amount of GABA released from presynaptic terminals, the function of GABA transporters, and the function of GABA receptors at the postsynaptic neuron (Zhou & Danbolt 2013). GABA produces phasic inhibition by acting on GABAA receptors inside the synapse, and tonic inhibition by acting on extrasynaptic GABAA receptors (Belelli et al. 2009). Studies of GABA receptor activities in the spinal dorsal horn have been mainly focused on the phasic GABA inhibition. For example, synaptic GABAergic activities (phasic inhibition) are reduced when the release probability of GABA neurotransmitters is decreased by activation of presynaptic A1 adenosine-receptors or GABAB receptors (Hugel & Schlichter 2003, Yang et al. 2004). Activation of neuronal acetylcholine receptors (Takeda et al. 2003) or M3 muscarinic acetylcholine receptors (Zhang et al. 2006) at presynaptic terminals increases the GABA release from the presynaptic terminals and GABAergic phasic inhibition. Reduction of GABA synthesis through the glutamate-glutamine cycle at the presynaptic terminals also decreases GABAergic phasic inhibition (Jiang et al. 2012). At the post-synaptic site, phasic inhibition induced by the activation of GABA receptors are reduced by the down-regulation of the K+-Cl− cotransporter KCC2, which disrupts Cl− homeostasis in neurons (Coull et al. 2003, Coull et al. 2005).
Previous studies have shown that GABAergic tonic inhibition is present in the spinal dorsal horn (Ataka & Gu 2006, Takahashi et al. 2006, Maeda et al. 2010). It is generally believed that extrasynaptic GABAA receptors (which contain the δ subunit) are responsible for the generation of GABAergic tonic inhibition. It has been demonstrated that selective activation of the extrasynaptic GABAA receptors with the δGABAA receptor-preferring agonist 4,5,6,7-tetrahydroisoxazolo [5,4-c]pyridine-3-ol (THIP) increases the tonic GABA inhibition, suppresses neuronal excitability in the spinal dorsal horn, and acute nociception in mice (Bonin et al. 2011). Currently, whether GABAergic tonic inhibition in the spinal dorsal horn is regulated by GABA transporters remains unexplored. Our study filled this gap by demonstrating that tonic GABA receptor activities in the spinal dorsal horn are regulated by GABA transporters both in normal and neuropathic pain induced by paclitaxel treatment.
Role of GABA transporters in the regulation of GABA receptor activities in the CNS
GABA transporters play an important role in the clearance and homeostasis of extracellular GABA in the CNS because no enzymes are available in the extracellular space to convert GABA into a biologically inert molecule (Zhou & Danbolt 2013). Among the four GABA transporters, GAT-1 and GAT-3 are widely expressed in the CNS (Melone et al. 2013, Guastella et al. 1990). Expression of GAT-1 and GAT-3 transporters is region-specific. In the forebrain area, GAT-1 is abundantly expressed in terminals of cortical neurons but not in thalamic, Purkinje and striatonigral synapses where GAT-3 is expressed mainly in astrocytes (Zhou & Danbolt 2013). Studies on the cellular location of GAT-1 and GAT-3 in the spinal dorsal horn are limited. In one study on the rat spinal trigeminal nucleus, GAT-1 and GAT-3 are expressed in astrocytes (Ng & Ong 2001). Expressions of GAT-1 in presynaptic terminals and GAT-3 in astrocytes in the mouse spinal dorsal horn were recently reported (Kim et al. 2014). Our current study on rats further confirmed that: a) GAT-1 is positioned at the presynaptic terminals but not at the neuronal cell body; b) GAT-1 is also expressed in astrocytes; c) GAT-3 is only expressed in astrocytes; d) neither GAT-1 nor GAT-3 is expressed in microglia.
Regulation of GABAergic tonic inhibition by GAT-1 and GAT-3 varies depending on different regions. For example, pharmacological inhibition of GAT-1 increases GABAergic tonic currents in the hippocampus (Nusser & Mody 2002) and cerebellum (Rossi et al. 2003) but not in the sensorimotor cortex (Keros & Hablitz 2005). When GAT-3 is inhibited, GABAergic tonic currents are increased in the supraoptic nucleus (Park et al. 2006) but not in the sensorimotor cortex (Keros & Hablitz 2005). A combined inhibition of GAT-1 and GAT-3 is required to significantly enhance tonic inhibition in the sensorimotor cortex (Keros & Hablitz 2005). The regulation of tonic inhibition in the spinal dorsal horn is unknown. Our study showed that under normal conditions, GABAergic tonic inhibition in the spinal dorsal horn was increased when GAT-1 but not GAT-3 was inhibited. Only under the condition when GAT-1 had been inhibited, further inhibition of GAT-3 increased GABAergic tonic inhibition. These data suggest that in comparison with GAT-3, GAT-1 is positioned in proximity to the presynaptic terminals, and the GABA uptake by GAT-1 is more powerful, which may result from the higher efficacy or number of GAT-1 transporters. Consistent with this notion, GAT-1 was found to be expressed in presynaptic terminals by us (Fig. 3) and others (Kim et al. 2014), and the global GABA uptake is increased in rats with the up-regulation of GAT-1 protein expression and down-regulation of GAT-3 expression (Fig. 5).
Protein expressions of GAT-1 and GAT-3 in the spinal dorsal horn are altered under different pathological pain conditions but controversy remains. Animals with neuropathic pain induced by chronic constriction of the sciatic nerve or spared nerve injury have an increased protein expression of GAT-1 in the spinal dorsal horn (Daemen et al. 2008) or the gracile nucleus (Gosselin et al. 2010). Using the same animal model, others have reported that the protein expression of GAT-1 in the same area is reduced (Shih et al. 2008, Miletic et al. 2003). Expressions of GAT-1 and GAT-3 transporters are increased in the spinal trigeminal nucleus in rats with inflammatory pain induced by carrageenan injection (Ng & Ong 2001). In another inflammatory pain model induced by formalin injection, global GABA uptake in the mouse spinal cord is increased at 20 min and 120 min after formalin injection (Hu et al. 2003). Our present study demonstrates that GAT-1 protein expression is increased while GAT-3 protein expression is reduced in the spinal dorsal horn in rats with P-INP, and these changes are concurrently associated with an increase of global GABA uptake at the same region. These data suggest that enhanced GABA uptake may in part contribute to the reduced GABAergic tonic inhibition in rats treated with paclitaxel. Targeting GABA transporters to produce analgesic effects has been reported. It was reported that latencies in the tail flick reflex (Hu et al. 2003) or the hot plate test (Kubo et al. 2009) were prolonged in mice receiving intraperitoneal injection of NO-711 (3 to 10 mg/kg). However, others showed that intrathecal application of GAT-1 inhibitor NO-711 (up to 100 μg) did not produce analgesic effects in the sham-operated control rats (Li et al. 2011). In agreement with this study, our findings show that intrathecal application of NO-711 (10 μg) does not alter mechanical and thermal thresholds of paw withdrawal responses, or motor functions in rats. Systemic or intrathecal administration of GAT-1 inhibitors ameliorates neuropathic pain induced by chronic constriction of the sciatic nerve (Daemen et al. 2008, Li et al. 2011), and the second phase nociceptive behaviors in the formalin pain model (Hu et al. 2003). Similarly, spinal inhibition of GAT-3 suppresses the second-phase response in the formalin pain model and attenuates mechanical allodynia induced by chronic constriction injury in rats (Kataoka et al. 2013). Currently, the regulation of GABAergic tonic inhibition by GABA transporters in pathological pain conditions has not been investigated. In this study, we found that the attenuation of GABAergic tonic inhibition in the spinal dorsal horn of rats with P-INP is significantly reversed by the GAT-1 inhibitor but not GAT-3 inhibitor. In intact animals, we demonstrated that inhibition of spinal GAT-1 ameliorates mechanical allodynia and thermal hyperalgesia induced by paclitaxel treatment.
Conclusions
In this study, we found that disinhibition in the spinal dorsal horn contributes to the genesis of P-INP. This is at least in part due to enhanced GABA uptake by GAT-1 in the spinal dorsal horn. Suppression of GAT-1 activities reverses disinhibition in the spinal dorsal horn and attenuates mechanical allodynia and thermal hyperalgesia induced by paclitaxel treatment. Together, these results suggest targeting GAT-1 activity may be useful for the development of therapeutics in P-INP.
Acknowledgments
This project was supported by the National Institute of Neurological Disorders and Stroke grant RO1NS064289 to H.R. Weng.
Abbreviations
- P-INP
Paclitaxel induced neuropathic pain
- IPSCs
Inhibitory postsynaptic currents
- GAT-1
GABA transporter-1
- GAT-3
GABA transporter-3
- DPI
Days post-intraperitoneal injection
- i.p.
Intra-peritoneal
- MAP2
Microtubule-associated protein 2
- GFAP
glial fibrillary acidic protein
- NeuN
Neuronal Nuclei
- OX42
CD11b -cluster of differentiation molecule 11B
- L4
Lumbar 4
- L5
Lumbar 5
- TRPV1
transient receptor potential cation channel, subfamily V, member 1
- CX3CL1
chemokine (C-X3-C motif) ligand 1
- DRG
Dorsal root ganglion
- GSK3β
Glycogen synthase kinase 3 beta
- KCC2
K-Cl cotransporter
Footnotes
The authors declare that there are no conflicts of interest.
References
- Ataka T, Gu JG. Relationship between tonic inhibitory currents and phasic inhibitory activity in the spinal cord lamina II region of adult mice. Molecular pain. 2006;2:36. doi: 10.1186/1744-8069-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Takazawa T, Tong CK, Choudhury P, Scherrer G, Macdermott AB. Pre- and postsynaptic inhibitory control in the spinal cord dorsal horn. Ann N Y Acad Sci. 2013;1279:90–96. doi: 10.1111/nyas.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, De Koninck Y. Restoring ionotropic inhibition as an analgesic strategy. Neuroscience letters. 2013;557 Pt A:43–51. doi: 10.1016/j.neulet.2013.09.047. [DOI] [PubMed] [Google Scholar]
- Bonin RP, Labrakakis C, Eng DG, Whissell PD, De Koninck Y, Orser BA. Pharmacological enhancement of delta-subunit-containing GABA(A) receptors that generate a tonic inhibitory conductance in spinal neurons attenuates acute nociception in mice. Pain. 2011;152:1317–1326. doi: 10.1016/j.pain.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of Taxol-induced hyperalgesia and can be prevented by treatment with minocycline. PAIN. 2011;152:308–313. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Lopes JM, Tavares I, Coimbra A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain research. 1993;620:287–291. doi: 10.1016/0006-8993(93)90167-l. [DOI] [PubMed] [Google Scholar]
- Cata JP, Weng HR, Chen JH, Dougherty PM. Altered discharges of spinal wide dynamic range neurons and down-regulation of glutamate transporter expression in rats with paclitaxel-induced hyperalgesia. Neuroscience. 2006;138:329–338. doi: 10.1016/j.neuroscience.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Chen SR, Zhu L, Chen H, Wen L, Laumet G, Pan HL. Increased Spinal Cord Na+-K+-2Cl- Cotransporter-1 (NKCC1) Activity Contributes to Impairment of Synaptic Inhibition in Paclitaxel-induced Neuropathic Pain. J Biol Chem. 2014;289:31111–31120. doi: 10.1074/jbc.M114.600320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Coull JAM, Boudreau D, Bachand K, Prescott SA, Nault F, S¡k A, DeKoninck P, DeKoninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Daemen MA, Hoogland G, Cijntje JM, Spincemaille GH. Upregulation of the GABA-transporter GAT-1 in the spinal cord contributes to pain behaviour in experimental neuropathy. Neuroscience letters. 2008;444:112–115. doi: 10.1016/j.neulet.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Doyle T, Chen Z, Muscoli C, et al. Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6149–6160. doi: 10.1523/JNEUROSCI.6343-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res. 2005;72:109–122. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Gao M, Yan X, Weng HR. Inhibition of glycogen synthase kinase 3beta activity with lithium prevents and attenuates paclitaxel-induced neuropathic pain. Neuroscience. 2013;254:301–311. doi: 10.1016/j.neuroscience.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin RD, Bebber D, Decosterd I. Upregulation of the GABA transporter GAT-1 in the gracile nucleus in the spared nerve injury model of neuropathic pain. Neuroscience letters. 2010;480:132–137. doi: 10.1016/j.neulet.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel MC, Davidson N, Lester HA, Kanner BI. Cloning and expression of a rat brain GABA transporter. Science (New York, NY) 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Hara K, Haranishi Y, Kataoka K, Takahashi Y, Terada T, Nakamura M, Sata T. Chlorogenic acid administered intrathecally alleviates mechanical and cold hyperalgesia in a rat neuropathic pain model. Eur J Pharmacol. 2014;723:459–464. doi: 10.1016/j.ejphar.2013.10.046. [DOI] [PubMed] [Google Scholar]
- Hara T, Chiba T, Abe K, Makabe A, Ikeno S, Kawakami K, Utsunomiya I, Hama T, Taguchi K. Effect of paclitaxel on transient receptor potential vanilloid 1 in rat dorsal root ganglion. Pain. 2013 doi: 10.1016/j.pain.2013.02.023. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Depner UB, Wassle H, et al. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- Hu JH, Yang N, Ma YH, Zhou XG, Jiang J, Duan SH, Mei ZT, Fei J, Guo LH. Hyperalgesic effects of gamma-aminobutyric acid transporter I in mice. Journal of neuroscience research. 2003;73:565–572. doi: 10.1002/jnr.10677. [DOI] [PubMed] [Google Scholar]
- Huang ZZ, Li D, Liu CC, Cui Y, Zhu HQ, Zhang WW, Li YY, Xin WJ. CX3CL1-mediated macrophage activation contributed to paclitaxel-induced DRG neuronal apoptosis and painful peripheral neuropathy. Brain Behav Immun. 2014;40:155–165. doi: 10.1016/j.bbi.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Hugel S, Schlichter R. Convergent control of synaptic GABA release from rat dorsal horn neurones by adenosine and GABA autoreceptors. The Journal of physiology. 2003;551:479–489. doi: 10.1113/jphysiol.2003.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. Journal of neurophysiology. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Jiang E, Yan X, Weng HR. Glial glutamate transporter and glutamine synthetase regulate GABAergic synaptic strength in the spinal dorsal horn. J Neurochem. 2012;121:526–536. doi: 10.1111/j.1471-4159.2012.07694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HW, Flatters SJ, Xiao WH, Mulhern HL, Bennett GJ. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Experimental neurology. 2008;210:229–237. doi: 10.1016/j.expneurol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K, Hara K, Haranishi Y, Terada T, Sata T. The antinociceptive effect of SNAP5114, a gamma-aminobutyric acid transporter-3 inhibitor, in rat experimental pain models. Anesth Analg. 2013;116:1162–1169. doi: 10.1213/ANE.0b013e318282dda7. [DOI] [PubMed] [Google Scholar]
- Keros S, Hablitz JJ. Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. Journal of neurophysiology. 2005;94:2073–2085. doi: 10.1152/jn.00520.2005. [DOI] [PubMed] [Google Scholar]
- Kersante F, Rowley SC, Pavlov I, Gutierrez-Mecinas M, Semyanov A, Reul JM, Walker MC, Linthorst AC. A functional role for both -aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus. The Journal of physiology. 2013;591:2429–2441. doi: 10.1113/jphysiol.2012.246298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kosaka Y, Shimizu-Okabe C, Niizaki A, Takayama C. Characteristic development of the GABA-removal system in the mouse spinal cord. Neuroscience. 2014;262:129–142. doi: 10.1016/j.neuroscience.2013.12.066. [DOI] [PubMed] [Google Scholar]
- Kirmse K, Dvorzhak A, Kirischuk S, Grantyn R. GABA transporter 1 tunes GABAergic synaptic transmission at output neurons of the mouse neostriatum. The Journal of physiology. 2008;586:5665–5678. doi: 10.1113/jphysiol.2008.161943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K, Nishikawa K, Ishizeki J, Hardy-Yamada M, Yanagawa Y, Saito S. Thermal hyperalgesia via supraspinal mechanisms in mice lacking glutamate decarboxylase 65. The Journal of pharmacology and experimental therapeutics. 2009;331:162–169. doi: 10.1124/jpet.109.156034. [DOI] [PubMed] [Google Scholar]
- Lee V, Maguire J. The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Frontiers in neural circuits. 2014;8:3. doi: 10.3389/fncir.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gu P, Fu B, Liu F, Li E. Analgesic effect of intrathecally gamma-aminobutyric acid transporter-1 inhibitor NO-711 administrating on neuropathic pain in rats. Neuroscience letters. 2011;494:6–9. doi: 10.1016/j.neulet.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Maeda A, Katafuchi T, Oba Y, Shiokawa H, Yoshimura M. Enhancement of GABAergic tonic currents by midazolam and noradrenaline in rat substantia gelatinosa neurons in vitro. Anesthesiology. 2010;113:429–437. doi: 10.1097/ALN.0b013e3181e19bd4. [DOI] [PubMed] [Google Scholar]
- Maixner DW, Yan X, Gao M, Yadav R, Weng HR. Adenosine Monophosphate-activated Protein Kinase Regulates Interleukin-1beta Expression and Glial Glutamate Transporter Function in Rodents with Neuropathic Pain. Anesthesiology. 2015 doi: 10.1097/ALN.0000000000000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TP, Mata HP, Porreca F. Spinal GABA A and GABAB receptor pharmacology in a model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- Melone M, Ciappelloni S, Conti F. A quantitative analysis of cellular and synaptic localization of GAT-1 and GAT-3 in rat neocortex. Brain structure & function. 2013 doi: 10.1007/s00429-013-0690-8. [DOI] [PubMed] [Google Scholar]
- Miletic G, Draganic P, Pankratz MT, Miletic V. Muscimol prevents long-lasting potentiation of dorsal horn field potentials in rats with chronic constriction injury exhibiting decreased levels of the GABA transporter GAT-1. Pain. 2003;105:347–353. doi: 10.1016/s0304-3959(03)00250-1. [DOI] [PubMed] [Google Scholar]
- Mitrovic AD, Maddison JE, Johnston GA. Influence of the oestrous cycle on L-glutamate and L-aspartate transport in rat brain synaptosomes. Neurochem Int. 1999;34:101–108. doi: 10.1016/s0197-0186(98)00066-7. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. Journal of Neuroscience. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CH, Ong WY. Increased expression of gamma-aminobutyric acid transporters GAT-1 and GAT-3 in the spinal trigeminal nucleus after facial carrageenan injections. Pain. 2001;92:29–40. doi: 10.1016/s0304-3959(00)00468-1. [DOI] [PubMed] [Google Scholar]
- Ng CH, Ong WY. Increased synaptosomal [3H] GABA uptake in the rat brainstem after facial carrageenan injections. Pain. 2002;98:259–268. doi: 10.1016/S0304-3959(01)00491-2. [DOI] [PubMed] [Google Scholar]
- Nie H, Weng HR. Glutamate transporters prevent excessive activation of NMDA receptors and extrasynaptic glutamate spillover in the spinal dorsal horn. Journal of neurophysiology. 2009;101:2041–2051. doi: 10.1152/jn.91138.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Weng HR. Impaired glial glutamate uptake induces extrasynaptic glutamate spillover in the spinal sensory synapses of neuropathic rats. Journal of neurophysiology. 2010;103:2570–2580. doi: 10.1152/jn.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Kubo K, Obata H, Yanagawa Y, Saito S. The influence of manipulations to alter ambient GABA concentrations on the hypnotic and immobilizing actions produced by sevoflurane, propofol, and midazolam. Neuropharmacology. 2011;61:172–180. doi: 10.1016/j.neuropharm.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. Journal of neurophysiology. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Okubo K, Takahashi T, Sekiguchi F, et al. Inhibition of T-type calcium channels and hydrogen sulfide-forming enzyme reverses paclitaxel-evoked neuropathic hyperalgesia in rats. Neuroscience. 2011;188:148–156. doi: 10.1016/j.neuroscience.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Ozkan ED, Lee FS, Ueda T. A protein factor that inhibits ATP-dependent glutamate and gamma-aminobutyric acid accumulation into synaptic vesicles: purification and initial characterization. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4137–4142. doi: 10.1073/pnas.94.8.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Jo JY, Zheng H, Patel KP, Stern JE. Regulation of tonic GABA inhibitory function, presympathetic neuronal activity and sympathetic outflow from the paraventricular nucleus by astroglial GABA transporters. The Journal of physiology. 2009;587:4645–4660. doi: 10.1113/jphysiol.2009.173435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Skalska S, Stern JE. Characterization of a novel tonic gamma-aminobutyric acidA receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology. 2006;147:3746–3760. doi: 10.1210/en.2006-0218. [DOI] [PubMed] [Google Scholar]
- Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. The Journal of physiology. 2003;548:97–110. doi: 10.1113/jphysiol.2002.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shih A, Miletic V, Miletic G, Smith LJ. Midazolam administration reverses thermal hyperalgesia and prevents gamma-aminobutyric acid transporter loss in a rodent model of neuropathic pain. Anesth Analg. 2008;106:1296–1302. doi: 10.1213/ane.0b013e318164f1e9. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siau C, Xiao W, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Experimental neurology. 2006;201:507–514. doi: 10.1016/j.expneurol.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CG, Bowery NG, Whitehead KJ. GABA transporter type 1 (GAT-1) uptake inhibition reduces stimulated aspartate and glutamate release in the dorsal spinal cord in vivo via different GABAergic mechanisms. Neuropharmacology. 2007;53:975–981. doi: 10.1016/j.neuropharm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Stiller CO, Cui JG, O'Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of y-aminobutyric acid in the dorsal horn and suppression of tactile alloydynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery. 1996;39 doi: 10.1097/00006123-199608000-00026. [DOI] [PubMed] [Google Scholar]
- Stone LS, German JP, Kitto KF, Fairbanks CA, Wilcox GL. Morphine and clonidine combination therapy improves therapeutic window in mice: synergy in antinociceptive but not in sedative or cardiovascular effects. PLoS One. 2014;9:e109903. doi: 10.1371/journal.pone.0109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. Journal of Neuroscience. 2003;23:2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Mashimo T, Uchida I. GABAergic tonic inhibition of substantia gelatinosa neurons in mouse spinal cord. Neuroreport. 2006;17:1331–1335. doi: 10.1097/01.wnr.0000230515.86090.bc. [DOI] [PubMed] [Google Scholar]
- Takeda D, Nakatsuka T, Papke R, Gu JG. Modulation of inhibitory synaptic activity by a non-alpha4beta2, non-alpha7 subtype of nicotinic receptors in the substantia gelatinosa of adult rat spinal cord. Pain. 2003;101:13–23. doi: 10.1016/s0304-3959(02)00074-x. [DOI] [PubMed] [Google Scholar]
- Weng HR, Gao M, Maixner DW. Glycogen synthase kinase 3 beta regulates glial glutamate transporter protein expression in the spinal dorsal horn in rats with neuropathic pain. Experimental neurology. 2014;252:18–27. doi: 10.1016/j.expneurol.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng HR, Aravindan N, Cata JP, Chen JH, Shaw AD, Dougherty PM. Spinal glial glutamate transporters downregulate in rats with taxol-induced hyperalgesia. Neurosci Lett. 2005;386:18–22. doi: 10.1016/j.neulet.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Weng HR, Chen JH, Pan ZZ, Nie H. Glial glutamate transporter 1 regulates the spatial and temporal coding of glutamatergic synaptic transmission in spinal lamina II neurons. Neuroscience. 2007;149:898–907. doi: 10.1016/j.neuroscience.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Weng HR, Cordella JV, Dougherty PM. Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. Pain. 2003;103:131–138. doi: 10.1016/s0304-3959(02)00445-1. [DOI] [PubMed] [Google Scholar]
- Wonnemann M, Singer A, Muller WE. Inhibition of Synaptosomal Uptake of 3H-L-glutamate and 3H-GABA by Hyperforin, a Major Constituent of St. John's Wort: The Role of Amiloride Sensitive Sodium Conductive Pathways. Neuropsychopharmacology. 2000;23:188–197. doi: 10.1016/S0893-133X(00)00102-0. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson GB. The transmembrane sodium gradient influences ambient GABA concentration by altering the equilibrium of GABA transporters. Journal of neurophysiology. 2006;96:2425–2436. doi: 10.1152/jn.00545.2006. [DOI] [PubMed] [Google Scholar]
- Xiao WH, Bennett GJ. Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain. 2012;153:704–709. doi: 10.1016/j.pain.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science (New York, NY) 1976;192:1357–1358. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- Yan X, Yadav R, Gao M, Weng HR. Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia. 2014;62:1093–1109. doi: 10.1002/glia.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Fujita T, Kumamoto E. Adenosine inhibits GABAergic and glycinergic transmission in adult rat substantia gelatinosa neurons. J Neurophysiol. 2004;92:2867–2877. doi: 10.1152/jn.00291.2004. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Chen SR, Matsui M, Gautam D, Wess J, Pan HL. Opposing functions of spinal M2, M3, and M4 receptor subtypes in regulation of GABAergic inputs to dorsal horn neurons revealed by muscarinic receptor knockout mice. Mol Pharmacol. 2006;69:1048–1055. doi: 10.1124/mol.105.018069. [DOI] [PubMed] [Google Scholar]
- Zheng H, Xiao WH, Bennett GJ. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Experimental neurology. 2011;232:154–161. doi: 10.1016/j.expneurol.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Danbolt NC. GABA and Glutamate Transporters in Brain. Front Endocrinol (Lausanne) 2013;4:165. doi: 10.3389/fendo.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]


