Abstract
Many mammalian organs undergo branching morphogenesis to create highly arborized structures with maximized surface area for specialized organ function. Cooperative cell-cell and cell-matrix adhesions that sculpt the emerging tissue architecture are guided by dynamic basement membranes. Properties of the basement membrane are reciprocally controlled by the interacting epithelial and mesenchymal cell populations. Here we discuss how basement membrane remodeling is required for branching morphogenesis to regulate cell-matrix and cell-cell adhesions that are required for cell patterning during morphogenesis and how basement membrane impacts morphogenesis by stimulation of cell patterning, force generation, and mechanotransduction. We suggest that in addition to creating mature epithelial architecture, remodeling of the epithelial basement membrane during branching morphogenesis is also essential to promote maturation of the stromal mesenchyme to create mature organ structure. Recapitulation of developmental cell-matrix and cell-cell interactions are of critical importance in tissue engineering and regeneration strategies that seek to restore organ function.
Keywords: branching morphogenesis, cell-matrix adhesions, cell-cell adhesions, heterotypic, mechanotransduction, tissue engineering
Introduction
Branching morphogenesis creates organs having a large internal surface area for specialized organ function within a compact space. Many mammalian organs undergo branching morphogenesis, including the lungs, kidneys, pancreas, prostate, submandibular salivary glands (salivary glands), and mammary glands. During branching morphogenesis, the basement membrane orchestrates many epithelial cell behaviors, including cell proliferation, cell polarization, and migration that require dynamic cell-matrix adhesions with the basement membrane (Costantini and Kopan, 2010; Daley and Yamada, 2013; Gray et al., 2010; Hsu and Yamada, 2010; Kim and Nelson, 2012; Sequeira et al., 2010; Warburton et al., 2010; Yurchenco, 2011). Basement membranes are dense interconnected protein and glycoprotein networks associated with the basal epithelial surfaces that form boundaries between the epithelial and mesenchymal tissue compartments and have structural, mechanical, and chemical signaling properties that influence their associated epithelia. Integrins are the principal cell surface basement membrane receptors that mediate basement membrane assembly and cell-matrix adhesion, and basement membrane assembly is initiated by assembly of a laminin scaffold requiring integrin β1 function on the basolateral epithelial cell membrane. A collagen IV lattice is cross-linked to the laminin-based scaffold via nidogen and other proteins, including agrican and perlecan (Aumailley et al., 2000; Li et al., 2003; Yurchenco, 2011). Other diverse proteins and proteoglycans are then differentially integrated into basement membranes to confer tissue-type and physiology-dependent compositions that impact morphogenesis and subsequently, homeostasis.
Although most studies have focused on the epithelial cell population during branching morphogenesis, there is a growing appreciation of the interdependent nature of the epithelial and stromal compartments during organogenesis. Assembly, remodeling, and maintenance of basement membranes requires the cooperation of both the epithelial and mesenchymal tissue compartments at the boundary interface, and aberrations of basement membrane in disease states can originate in either tissue compartment. In this review, we discuss how heterotypic cell control of basement membrane dynamics regulates adhesions to drive branching morphogenesis, and how mechanical properties of the assembled matrices impact morphogenesis. We suggest that in addition to creating mature epithelial architecture, remodeling of the epithelial basement membrane during branching morphogenesis is also essential to promote maturation of the stromal mesenchyme and facilitate organization of the mesenchymal fibroblasts, vasculature, and innervation with the arborized epithelial structure. Finally, we discuss how recapitulation of this heterotypic cell control of basement membrane is an important consideration in effective tissue engineering and regenerative medicine approaches.
Epithelial-stromal interactions sculpt basement membranes at tissue interfaces
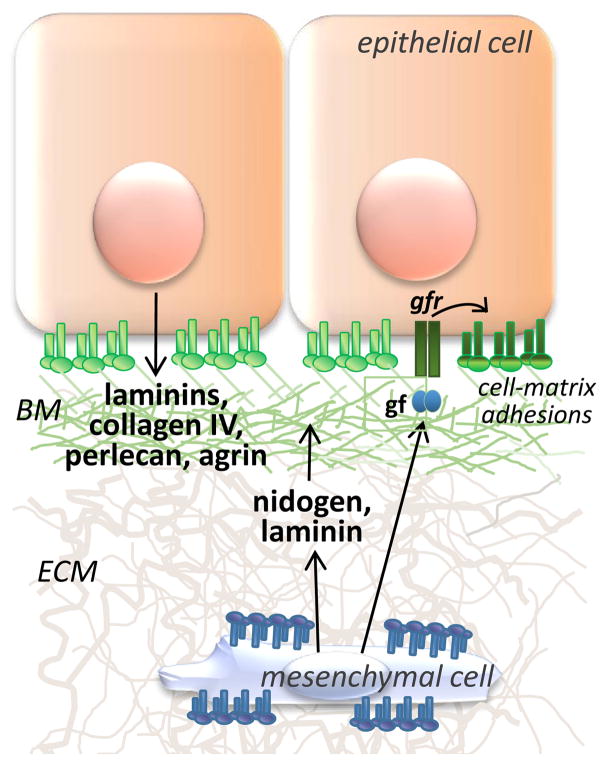
Tissue recombination experiments, where the epithelium and mesenchyme of branched organs are separated and recombined, have demonstrated a requirement for mesenchyme in branching morphogenesis (Gittes et al., 1996; Lawson, 1974). Although isolated epithelia can undergo limited branching in the absence of mesenchyme, this mesenchyme-free epithelial growth requires integrin-mediated adhesion to basement membrane proteins together with exogenous growth factors that substitute for the mesenchyme (Koyama et al., 2009; Nogawa and Takahashi, 1991). In vivo, epithelial-mesenchymal cooperation is required to orchestrate basement membrane assembly and remodeling during embryogenesis (see Fig. 1) (reviewed in (Yurchenco, 2011). In the developing kidney (Lee et al., 1993), lung (Thomas and Dziadek, 1994), pancreas (Crisera et al., 2000), mammary gland (Keely et al., 1995), and salivary gland (Kadoya et al., 1995), the stromal mesenchyme cells synthesize laminin that is required for assembly of the epithelial basement membrane at the epithelial-stromal interface and to drive epithelial integrin-mediated organ-dependent epithelial cleft formation and ductal morphogenesis (Crisera et al., 2000). Additionally, stabilization and retention of the basement membrane at the epithelial-stromal interface is facilitated by nidogen that is secreted solely from the mesenchyme that condenses around the epithelium and is required for branching morphogenesis in several organs (Ekblom et al., 1994; Kadoya et al., 1997). Stromally produced growth factors can also promote regulated adhesion of epithelial cells with the nascent basement membrane during branching morphogenesis, and epithelial integrin binding to laminin is required for stromal HGF-induced mammary gland morphogenesis (Klinowska et al., 1999; Yang et al., 1995), and stromal FGF-induced salivary gland morphogenesis (Rebustini et al., 2007).
Fig. 1. Heterotypic cell contributions to basement membrane assembly.
Epithelial cells produce a diverse group of basement membrane (BM) proteins (i.e. laminins, collagen IV, agrin, perlecan, etc) and other basement membrane components that are anchored to the cell membrane via integrins and other matrix receptor proteins. Mesenchymal cells, enclosed within and attached to their own ECM via integrins, synthesize some laminin and also nidogen that crosslinks laminins with collagen IV. Epithelial cell adherence to the assembled BM can be regulated by mesenchymal growth factors that activate epithelial growth factor receptors.
Remodeling of the basement membrane at the epithelial-stromal interface is required for branching morphogenesis
Continual basement membrane remodeling is required for branching morphogenesis, where dynamic changes in the composition and spatial distribution of the basement membrane modulate the activities of the basement membrane-adherent epithelial cells. Incorporation of fibronectin into basement membrane is essential for branching morphogenesis in the salivary gland (Sakai et al., 2003) and lung (De Langhe et al., 2005). In the developing salivary gland, fibronectin drives the formation of narrow clefts between epithelial cells of premature buds that are required for the formation of nascent proacinar and ductal structures (Larsen et al., 2006; Sakai et al., 2003), which form through replacement of epithelial cell-cell adhesions with cell-matrix adhesions as basement membrane is translocated rearward towards the ducts, accumulating at the base of clefts and in newly forming ducts (Harunaga et al., 2014; Hsu et al., 2013; Larsen et al., 2006). Fibronectin promotes cell proliferation and cleft progression in an integrin-dependent signaling cascade that creates a feed-forward mechanism for cleft progression, requiring active integrin β1 on the epithelial cell membrane (Daley et al., 2009; Daley et al., 2011; Sakai et al., 2003). The rapid synthesis and turnover of basement membrane has been highlighted by time-lapse imaging studies in which labeled basement membrane proteins can be tracked over time (Harunaga et al., 2014; Hsu et al., 2013; Larsen et al., 2006). The mechanisms through which rapid basement membrane remodeling occurs remain poorly understood. However, it is clear that matrix metalloproteases (MMPs) produced by the epithelium and the mesenchyme contribute to basement remodeling, promote epithelial invasion and epithelial-mesenchyme transition (EMT), and are required for branching morphogenesis in diverse organs (Bruni-Cardoso et al., 2010; Oblander et al., 2005; Rebustini et al., 2009; Tan et al., 2014; Wiseman et al., 2003). In addition to structural remodeling of the basement membrane, another important consequence of protease activity is to liberate bioactive fragments of structural proteins and growth factors from the basement membrane, as with MMP15-dependent liberation of the collagen IV NC1 fragment, which activates signaling in the epithelial cells to promote salivary gland branching (Rebustini et al., 2009), and many other examples (Horejs et al., 2014; Koshikawa et al., 2010; Loffek et al., 2011; Maller et al., 2010; McCawley and Matrisian, 2001; Mott and Werb, 2004). Importantly, heterotypic control of cell adhesions and basement membrane remodeling at the epithelial-mesenchymal boundary are also required for adult organ homeostasis, including maintenance of epithelial stem cell niches, as reviewed in (Glukhova and Streuli, 2013; Hsu et al., 2014; O’Brien and Bilder, 2013). Understanding the mechanisms required to maintain organ homeostasis are significant since defective basement membrane and extracellular matrix (ECM) homeostasis is a contributing factor to many diseases, including cancer and fibrotic diseases (Cox and Erler, 2011).
Spatiotemporal control of epithelial cell-cell adhesions creates critical cell subpopulations during morphogenesis
Branching morphogenesis and the establishment of mature organ structure require complex spatio-temporal regulation of cell-cell adhesions (Hsu and Yamada, 2010; Nelson and Gleghorn, 2012; Walker et al., 2008). Cell-cell adhesions confer integrity and functional coupling in mature epithelial cells; however, these adhesions are dynamically coordinated during branching morphogenesis. Recent studies indicate that a transient loss of cell-cell adhesions is required at specific times in specific cells during the course of branching morphogenesis. In the embryonic salivary gland, a discreet loss of epithelial cell adhesion occurs at the base of progressing clefts in response to fibronectin-induced activation of btbd7. Btbd7 induces a partial EMT including transcriptional activation of slug/snail2 and stimulation of a transient loss of E-cadherin containing adhesions to allow for redistribution of the epithelial cells and the basement membrane at the base of the nascent clefts (Onodera et al., 2010). In the mammary gland, the tip cells of the end buds also undergo a partial EMT during branching (Lee et al., 2011), and precisely timed repression of the EMT by the transcription factor, ovo-like zinc finger 2 (Ovol2), is critical for proper epithelial morphogenesis (Watanabe et al., 2014). Additionally, luminal epithelial cells migrate collectively through the mammary myoepithelial cell layer to initiate new branches (Ewald et al., 2008), and FGF-2 stimulated branching in an vitro model of mammary epithelial branching requires disruption of desmosomal cell-cell adhesions, which can be inhibited by activation of aryl hydrocarbon receptor (AHR) (Basham et al., 2013). In embryonic lung epithelium, tight junctions and adherens junctions, maintained by Scribble, are required for normal morphogenesis (Yates et al., 2013). However, tight junctions are generally formed at the apical surfaces at late stages of branching morphogenesis both as a requirement for tubulogenesis of the ducts (Zegers, 2014) and to stimulate epithelial cell polarity and subsequent epithelial cell function (reviewed in (Garrido-Urbani et al., 2014; Gonzalez-Mariscal et al., 2014; Rodriguez-Boulan and Macara, 2014). Thus, delineation of the molecular controls regulating cell-cell adhesions and how these adhesions synergize with cell-matrix adhesions for organogenesis and homeostasis is an important and active area of current research.
Topology and compliance of the basement membrane and extracellular matrix regulate branching morphogenesis
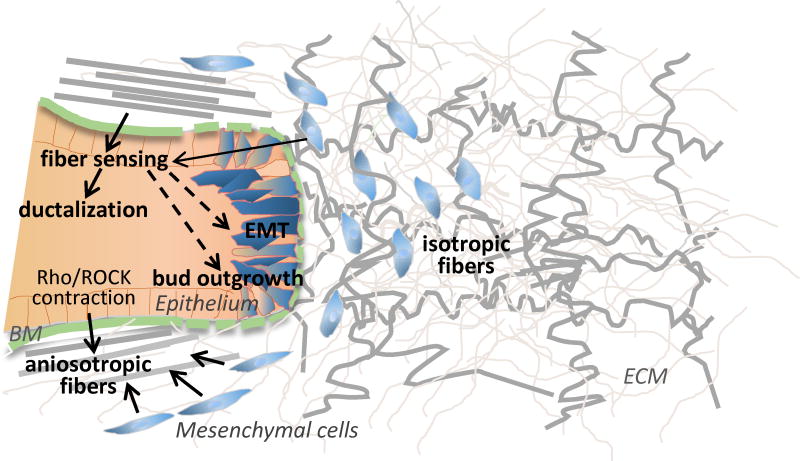
Several lines of evidence indicate that the topology and compliance of ECM and basement membranes facilitate cell adhesion-based forces that sculpt organ form (see Fig. 2). In mammary gland development, stromal collagen fiber alignment can dictate patterning of the branching epithelium by creating local anisotropy and directing epithelial branching (Brownfield et al., 2013). Although previous studies indicate the mesenchyme cells position the fibers (Grinnell, 2003; Hieda and Nakanishi, 1997), cell culture studies with the non-invasive mammary epithelial cell line, MCF-10A, show that these epithelial cells themselves can also organize a supplied collagen matrix and subsequently respond to the organized matrix (Barnes et al., 2014). In vivo, heterotypic cell interactions may be required for fiber orientation. Interestingly, ductal structures formed adjacent to the anisotropic fibers and acinar structures near the isotropic fibers (Barnes et al., 2014), suggesting fiber structure and forces imparted by the assembled matrix can regulate cell differentiation. How matrix receptors recognize the isotropic and anisotropic matrix is not fully understood. However, Rho/ROCK-mediated contraction was reported not to be required for sensing collagen fiber orientation, but instead was implicated in enhancing collagen I fiber orientation, which would reinforce branching directional decisions (Brownfield et al., 2013). In several branching organs, thinning or transient disruption of the basement membrane at the epithelial tip invading the surrounding stroma is compromised, allowing the basement membrane structural integrity to create directional forces that guide epithelial expansion and branch orientation (Gjorevski and Nelson, 2011; Harunaga et al., 2014; Hsu et al., 2013; Hsu and Yamada, 2010). These studies reflect current interests in elucidating the mechanisms by which ECM and basement membrane dynamics are transduced to create morphogenetic forces, and how such forces control organ development and homeostasis.
Fig. 2. Topology and compliance of the basement membrane and extracellular matrix regulate branching morphogenesis.
In developing mammary gland, cells at the tip of an epithelial end bud extend into the surrounding anisotropic ECM and undergo a local EMT. Expansion and extension of the bud is facilitated by breaks in the basement membrane, as are detected in other branching organs. In a 3D mammary model system, axially aligned anisotropic collagen I fibers are sensed by the the epithelium and stimulate ductalization while isotropic fibers facilitate acinar formation and may also facilitate bud outgrowth and EMT (dotted lines). Mesenchyme cells can align ECM and epithelial cells can also contribute to fiber alignment through Rho/ROCK pathway-mediated contraction.
One way that ECM and basement membrane dynamics participate in generation of morphogenetic forces is by modulating micro-environmental compliance. Extracellular compliance controls cell phenotype via integrin and focal adhesion signaling and regulation of cell contractility (Discher et al., 2005; Engler et al., 2006; Pelham and Wang, 1997). In the developing salivary gland, branching morphogenesis and epithelial differentiation were shown to be inhibited by culture of submandibular gland organ explants on gels of aberrantly high stiffness/low compliance (Miyajima et al., 2011; Peters et al., 2014a). The mechanisms driving these compliance-mediated differences in glandular development are unknown, but significant differences in the organization of the ECM and basement membrane were observed in glands cultured at low vs high compliance (Peters et al., 2015). The hippo effector protein, Yap, which is known to be mechanosensitive (Low et al., 2014), is critical in forming airway epithelium in the developing lung (Mahoney et al., 2014), whereas activation of the hippo effector, Taz, is required for ductal morphogenesis in the salivary gland (Enger et al., 2013). Aberrant extracellular stiffness disrupts branching behavior of mammary cells in 3D cultures (Chaudhuri et al., 2014; Gomez et al., 2010; Wozniak et al., 2003), and is associated with cancer progression (Chaudhuri et al., 2014; Paszek and Weaver, 2004; Rubashkin et al., 2014).
Recent work indicates that one mechanism through which cells respond to stiffness is through regulation of the activation state and levels of integrins (Friedland et al., 2009; Huebsch et al., 2010; Puklin-Faucher and Sheetz, 2009; Schwartz, 2010). Loss of activated integrin β1 was detected at the periphery of the salivary gland epithelium in developing glands grown at aberrantly low compliance that was associated with a decrease in myoepithelial differentiation, consistent with integrin β1 functioning as a compliance sensor in branching morphogenesis (Peters et al., 2015). Recent work reveals that integrin endocytosis and the recycling of integrins back to the cell surface can be controlled by microenvironmental stiffness (Du et al., 2011; Huebsch et al., 2010) to regulate the number of available integrins, which may occur in branching organs. In the related process of sprouting angiogenesis, dynamin 2 (DNM2)-regulated endocytosis of vascular endothelial growth factor receptor 2 (VEGFR2) is required for endothelial focal adhesion and integrin β1 turnover, in that loss of VEGFR2 turnover prevents focal adhesion disassembly and causes accumulation of integrin β1 at sites of failed angiogenesis (Lee et al., 2014). In isolated fibroblasts sensing compliance via fibronectin, αv-class integrins were found to activate a GEF-H1-RhoA pathway that is coupled to the formin mDia1 but not to non-muscle myosin type II, and α5β1 integrins activated a RhoA-ROCK-non-muscle-myosin II pathway (Schiller et al., 2013). Future studies will need to elucidate the interplay between these cellular mechanisms for compliance-mediated signaling and effects on cell phenotype. Since the properties of the ECM and basement membrane are impacted by the global mesenchymal tissue organization, studies that address the role of compliance in intact organs with native ECM and basement membrane assemblies will be essential to understand how modulation of the compliance of the ECM and basement membrane networks themselves affects branching morphogenesis and organ function.
Co-patterning of heterotypic cell populations is an inherent requirement of branching morphogenesis
A common emerging theme during morphogenesis is that heterotypic cell interactions at the basement membrane interface create co-patterning of different tissue types to promote mature tissue architecture. Stromal collagens are important for linking the basement membrane to the underlying stroma for tissue integrity during organogenesis in general (Yurchenco, 2011). For example, at the skin dermal-epidermal junction, dermal fibroblasts and epidermal keratinocytes cooperate to make basement membrane (Benny et al., 2014), and fibroblast contribution of collagen VII is particularly important for structural integrity. Co-patterning of interacting cell populations by shared basement membranes also contributes to establishment and maintenance of mature tissue architecture. For example, during angiogenesis, interaction of mesenchymal smooth muscle with endothelium stabilizes the endothelial basement membrane and cellular tube. Remodeling of the endothelial basement membrane and cellular architecture are required for sprouting angiogenesis, a process similar to epithelial branching morphogenesis (Loibl et al., 2014; Stratman et al., 2009). Innervation patterning and neurovascular co-alignment can also be promoted by shared basement membrane, as in the developing pancreatic islets where the vascular basement membrane is required to guide islet innervation (Reinert et al., 2014). Interestingly, recent data show that basement membranes are functionally asymmetric, with the epithelial side of the basement membrane being laminin-enriched, stiffer, and preferentially binding epithelial cells (Halfter et al., 2013). Implantation of basement membranes in vivo demonstrated that human basement membranes have an instructive role in organizing the neuronal cell and mesenchymal cell populations on opposite sides of an assembling spinal cord (Halfter and Yip, 2014). Whether asymmetric basement membrane assembly and adhesion is general mechanism for heterotypic cell population co-patterning in solid branching organs will be an interesting topic for future studies.
Several recent studies point to the interdependence of epithelial branching morphogenesis with mesenchymal cell subsets. As discussed previously, mesenchymal fibroblasts are essential for assembly and remodeling of the basement membrane and for production of growth factors that regulate epithelial cell behaviors, including cell adhesion for the elaboration of mature tissue architecture. Additionally, recent data reveal an instructive role for developing innervation in epithelial patterning. Acetylcholine signaling from parasympathetic innervation ingressing into maturing clefts expands the cytokeratin 5-positive epithelial progenitor cell population in the developing salivary gland and prostate (Knox et al., 2010). Parasympathetic innervation sustains this function in naïve (Knox et al., 2010) and damaged (Knox et al., 2013) adult salivary gland, suggesting that parasympathetic innervation is an important component of basal progenitor cell homeostasis. Parasympathetic innervation also controls ductal elongation and lumen formation in the developing salivary gland, via a distinct molecular cascade requiring vasoactive intestinal peptide (VIP) signaling (Nedvetsky et al., 2014). Interestingly, perfusion-independent vascular signaling is required for the development and co-patterning of the epithelium in several branched organs. In the embryonic pancreas, endothelial cells have an instructive role in epithelial differentiation and patterning (Lammert et al., 2001, 2003; Magenheim et al., 2011), and proper patterning of lung epithelium during branching morphogenesis requires vascular endothelium (Lazarus et al., 2011). Endothelial cells can also promote branching and partial EMT of mammary cells in a 3D in vitro assay, which is regulated by the receptor tyrosine kinase modulator sprouty-2 (Sigurdsson et al., 2013), while a role for endothelium in salivary gland branching has not yet been described. In liver, VEGFR2-expressing vascular endothelial cells are required for specification of the liver endoderm and expansion of the liver into the surrounding mesenchyme prior to the completion of vasculogenesis and onset of circulation (Matsumoto et al., 2001). Taken together, these data indicate that despite a conserved requirement for co-patterning of the epithelium and endothelium in branching morphogenesis, there are organ-specific differences in the epithelial-stromal co-patterning. Definition of the mechanisms driving co-patterning of the epithelium and stromal vasculature and nerves is a critical unmet need, and will likely require organ-specific considerations for therapeutic development.
Implications of heterotypic cell interactions in tissue engineering and regenerative medicine
To engineer a branched organ, three general strategies are possible to generate a compact organ containing a large surface area: 1) use a natural bifunctionally organized decellularized scaffold that contains inherent instructive signals, 2) engineer a complex scaffold that in some way replicates the complexity of the native organ using natural, synthetic, or a combination of materials, or 3) provide an environment in which the cells can self-organize and generate their own scaffold as they undergo the developmental program of branching morphogenesis or something resembling it. All three strategies and combinations of these strategies are being pursued by different groups with distinct approaches. Currently, natural, decellularized scaffolds out-perform any synthetic organ scaffolds for solid organ engineering (Faulk et al., 2014; Song and Ott, 2011). Such natural scaffolds include complex, spatially organized extracellular signals to stimulate cell-matrix adhesion, cell-cell adhesion, and cellular self-organization of the diverse cell types required for mature organ function that engineers have not yet been able to fully recapitulate. Thus, stimulating heterotypic cell self-organization and matrix production in engineered constructs is a current goal of many tissue engineers. For example, recent work aimed at improving the quality of the basement membrane at the dermal/epidermal boundary in engineered skin revealed that it was the dermal fibroblasts that contributed the most to the enhanced basement membrane, and basement membrane production by these cells was in turn stimulated by natural scaffolds (Benny et al., 2014). Some combination of deliberate engineering and self-organization of heterotypic cell populations may ultimately succeed.
Current efforts in tissue engineering and regenerative medicine are increasingly focused on the need to support and maintain parenchymal tissue using the interacting stromal cell populations both for organ generation and for sustained organ function. With the recognition that multiple cooperating cell types are required for organogenesis and epithelial basement membrane assembly and remodeling, engineered scaffolds must promote survival and self-organization of both epithelial and mesenchymal cell populations, including the mesenchymal fibroblasts that support the epithelial-stromal interface and the integrated vasculature and innervation. Additionally, engineered scaffolds must provide an appropriate environmental compliance or stiffness to allow tensional homeostasis and regulation of integrin-based cell adhesions (Du et al., 2011; Huebsch et al., 2010) and support functional differentiation and retention of complex interacting cell populations (Bissell and Bilder, 2003; Hu et al., 2008; Peters et al., 2015). Ultimately, scaffolds that best support dynamic homotypic and heterotypic cell interactions and encourage the cells to deposit and remodel their own extracellular matrices and basement membranes may be required to engineer functional and sustainable organs. Eventual development of tissue-engineered constructs capable of long-term maintenance of homeostasis will require recapitulation of a niche for renewing progenitor cell populations (Dziasko et al., 2014; Fujiwara et al., 2011; Marthiens et al., 2010; Menezes et al., 2014). In regenerating tissues, similar considerations for strategies to promote recapitulation of the complex developmental programs and restoration of the microenvironment and progenitor cell niches are required.
Conclusion
Concepts and examples discussed here illustrate the importance of heterotypic cell control of basement membrane dynamics and cell adhesion for elaboration and maintenance of parenchymal cell function in branched organs. Additionally, we suggest that while branching morphogenesis has long been recognized to create patterned epithelial tissue, epithelial-stromal co-patterning is critical during organogenesis, and thus will be essential to create functional and effectively host-integrated neo-organs. These themes highlight the need to provide scaffolds of appropriate compliance with organized chemical signals to facilitate appropriately localized and dynamic remodeling of basement membrane and cell-matrix adhesions for effective therapeutic restoration of impaired organ function. Application of knowledge regarding control of basement membrane dynamics and cell patterning by heterotypic cell populations will enable improved tissue engineering and regenerative medicine approaches by defining the required cell populations and characteristics of their environments to both create organ architecture and to be able maintain it. As the requirements for organ development and maintenance may be distinct, a significant future challenge will be to integrate knowledge of these processes into concrete strategies for effective organ regeneration and engineering.
Highlights.
Epithelial-stromal interactions sculpt basement membranes at tissue interfaces.
Adhesion dynamics create epithelial cell subpopulations during morphogenesis.
Basement membrane and extracellular matrix properties influence tissue architecture.
Heterotypic cell co-patterning is an inherent requirement of branching morphogenesis.
How basement membrane dynamics drive development can inform regenerative medicine.
Acknowledgments
The authors thank Drs. Sarah B. Peters, Livingston Van De Water, and Michael J. Gerdes for helpful discussions. Supported by NIH/NIDCR grants DE02184101, DE019244, and DE022467 and a grant from the New York Research Alliance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aumailley M, Pesch M, Tunggal L, Gaill F, Fassler R. Altered synthesis of laminin 1 and absence of basement membrane component deposition in (beta)1 integrin-deficient embryoid bodies. Journal of cell science. 2000;113(Pt 2):259–268. doi: 10.1242/jcs.113.2.259. [DOI] [PubMed] [Google Scholar]
- Barnes C, Speroni L, Quinn KP, Montevil M, Saetzler K, Bode-Animashaun G, McKerr G, Georgakoudi I, Downes CS, Sonnenschein C, Howard CV, Soto AM. From single cells to tissues: interactions between the matrix and human breast cells in real time. PloS one. 2014;9:e93325. doi: 10.1371/journal.pone.0093325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basham KJ, Kieffer C, Shelton DN, Leonard CJ, Bhonde VR, Vankayalapati H, Milash B, Bearss DJ, Looper RE, Welm BE. Chemical genetic screen reveals a role for desmosomal adhesion in mammary branching morphogenesis. The Journal of biological chemistry. 2013;288:2261–2270. doi: 10.1074/jbc.M112.411033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benny P, Badowski C, Lane B, Raghunath M. Making More Matrix: Enhancing the deposition of dermal-epidermal junction components in vitro and accelerating organotypic skin culture development, using macromolecular crowding. Tissue engineering. Part A. 2014 Oct 9; doi: 10.1089/ten.tea.2013.0784. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Bilder D. Polarity determination in breast tissue: desmosomal adhesion, myoepithelial cells, and laminin 1. Breast cancer research: BCR. 2003;5:117–119. doi: 10.1186/bcr579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownfield DG, Venugopalan G, Lo A, Mori H, Tanner K, Fletcher DA, Bissell MJ. Patterned collagen fibers orient branching mammary epithelium through distinct signaling modules. Curr Biol. 2013;23:703–709. doi: 10.1016/j.cub.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni-Cardoso A, Rosa-Ribeiro R, Pascoal VD, De Thomaz AA, Cesar CL, Carvalho HF. MMP-2 regulates rat ventral prostate development in vitro. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239:737–746. doi: 10.1002/dvdy.22222. [DOI] [PubMed] [Google Scholar]
- Chaudhuri O, Koshy ST, Branco da Cunha C, Shin JW, Verbeke CS, Allison KH, Mooney DJ. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nature materials. 2014;13:970–978. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Developmental cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Disease models & mechanisms. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisera CA, Kadison AS, Breslow GD, Maldonado TS, Longaker MT, Gittes GK. Expression and role of laminin-1 in mouse pancreatic organogenesis. Diabetes. 2000;49:936–944. doi: 10.2337/diabetes.49.6.936. [DOI] [PubMed] [Google Scholar]
- Daley WP, Gulfo KM, Sequeira SJ, Larsen M. Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Developmental biology. 2009;336:169–182. doi: 10.1016/j.ydbio.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Kohn JM, Larsen M. A focal adhesion protein-based mechanochemical checkpoint regulates cleft progression during branching morphogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240:2069–2083. doi: 10.1002/dvdy.22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Yamada KM. ECM-modulated cellular dynamics as a driving force for tissue morphogenesis. Curr Opin Genet Dev. 2013;23:408–414. doi: 10.1016/j.gde.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Langhe SP, Sala FG, Del Moral PM, Fairbanks TJ, Yamada KM, Warburton D, Burns RC, Bellusci S. Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Developmental biology. 2005;277:316–331. doi: 10.1016/j.ydbio.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Du J, Chen X, Liang X, Zhang G, Xu J, He L, Zhan Q, Feng XQ, Chien S, Yang C. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9466–9471. doi: 10.1073/pnas.1106467108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziasko MA, Armer HE, Levis HJ, Shortt AJ, Tuft S, Daniels JT. Localisation of epithelial cells capable of holoclone formation in vitro and direct interaction with stromal cells in the native human limbal crypt. PloS one. 2014;9:e94283. doi: 10.1371/journal.pone.0094283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P, Ekblom M, Fecker L, Klein G, Zhang HY, Kadoya Y, Chu ML, Mayer U, Timpl R. Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Development. 1994;120:2003–2014. doi: 10.1242/dev.120.7.2003. [DOI] [PubMed] [Google Scholar]
- Enger TB, Samad-Zadeh A, Bouchie MP, Skarstein K, Galtung HK, Mera T, Walker J, Menko AS, Varelas X, Faustman DL, Jensen JL, Kukuruzinska MA. The Hippo signaling pathway is required for salivary gland development and its dysregulation is associated with Sjogren’s syndrome. Laboratory investigation; a journal of technical methods and pathology. 2013;93:1203–1218. doi: 10.1038/labinvest.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Developmental cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk DM, Johnson SA, Zhang L, Badylak SF. Role of the extracellular matrix in whole organ engineering. Journal of cellular physiology. 2014;229:984–989. doi: 10.1002/jcp.24532. [DOI] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Urbani S, Bradfield PF, Imhof BA. Tight junction dynamics: the role of junctional adhesion molecules (JAMs) Cell and tissue research. 2014;355:701–715. doi: 10.1007/s00441-014-1820-1. [DOI] [PubMed] [Google Scholar]
- Gittes GK, Galante PE, Hanahan D, Rutter WJ, Debase HT. Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development. 1996;122:439–447. doi: 10.1242/dev.122.2.439. [DOI] [PubMed] [Google Scholar]
- Gjorevski N, Nelson CM. Integrated morphodynamic signalling of the mammary gland. Nature reviews. 2011;12:581–593. doi: 10.1038/nrm3168. [DOI] [PubMed] [Google Scholar]
- Glukhova MA, Streuli CH. How integrins control breast biology. Current opinion in cell biology. 2013;25:633–641. doi: 10.1016/j.ceb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez EW, Chen QK, Gjorevski N, Nelson CM. Tissue geometry patterns epithelial-mesenchymal transition via intercellular mechanotransduction. Journal of cellular biochemistry. 2010;110:44–51. doi: 10.1002/jcb.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Dominguez-Calderon A, Raya-Sandino A, Ortega-Olvera JM, Vargas-Sierra O, Martinez-Revollar G. Tight junctions and the regulation of gene expression. Seminars in cell & developmental biology. 2014 Aug 23; doi: 10.1016/j.semcdb.2014.08.009. pii: S1084-9521(14)00245-6 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Gray RS, Cheung KJ, Ewald AJ. Cellular mechanisms regulating epithelial morphogenesis and cancer invasion. Current opinion in cell biology. 2010;22:640–650. doi: 10.1016/j.ceb.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends in cell biology. 2003;13:264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Halfter W, Monnier C, Muller D, Oertle P, Uechi G, Balasubramani M, Safi F, Lim R, Loparic M, Henrich PB. The bi-functional organization of human basement membranes. PloS one. 2013;8:e67660. doi: 10.1371/journal.pone.0067660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Yip J. An organizing function of basement membranes in the developing nervous system. Mechanisms of development. 2014;133:1–10. doi: 10.1016/j.mod.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Harunaga JS, Doyle AD, Yamada KM. Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Developmental biology. 2014;394:197–205. doi: 10.1016/j.ydbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieda Y, Nakanishi Y. Epithelial morphogenesis in mouse embryonic submandibular gland: its relationships to the tissue organization of epithelium and mesenchyme. Development, growth & differentiation. 1997;39:1–8. doi: 10.1046/j.1440-169x.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- Horejs CM, Serio A, Purvis A, Gormley AJ, Bertazzo S, Poliniewicz A, Wang AJ, DiMaggio P, Hohenester E, Stevens MM. Biologically-active laminin-111 fragment that modulates the epithelial-to-mesenchymal transition in embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:5908–5913. doi: 10.1073/pnas.1403139111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JC, Koo H, Harunaga JS, Matsumoto K, Doyle AD, Yamada KM. Region-specific epithelial cell dynamics during branching morphogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2013;242:1066–1077. doi: 10.1002/dvdy.24000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JC, Yamada KM. Salivary gland branching morphogenesis--recent progress and future opportunities. Int J Oral Sci. 2010;2:117–126. doi: 10.4248/IJOS10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20:847–856. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, Richardson A, Violette S, Nikolskaya T, Nikolsky Y, Bauerlein EL, Hahn WC, Gelman RS, Allred C, Bissell MJ, Schnitt S, Polyak K. Regulation of in situ to invasive breast carcinoma transition. Cancer cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature materials. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya Y, Kadoya K, Durbeej M, Holmvall K, Sorokin L, Ekblom P. Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. The Journal of cell biology. 1995;129:521–534. doi: 10.1083/jcb.129.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya Y, Salmivirta K, Talts JF, Kadoya K, Mayer U, Timpl R, Ekblom P. Importance of nidogen binding to laminin gamma1 for branching epithelial morphogenesis of the submandibular gland. Development. 1997;124:683–691. doi: 10.1242/dev.124.3.683. [DOI] [PubMed] [Google Scholar]
- Keely PJ, Wu JE, Santoro SA. The spatial and temporal expression of the alpha 2 beta 1 integrin and its ligands, collagen I, collagen IV, and laminin, suggest important roles in mouse mammary morphogenesis. Differentiation; research in biological diversity. 1995;59:1–13. doi: 10.1046/j.1432-0436.1995.5910001.x. [DOI] [PubMed] [Google Scholar]
- Kim HY, Nelson CM. Extracellular matrix and cytoskeletal dynamics during branching morphogenesis. Organogenesis. 2012;8:56–64. doi: 10.4161/org.19813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinowska TC, Soriano JV, Edwards GM, Oliver JM, Valentijn AJ, Montesano R, Streuli CH. Laminin and beta1 integrins are crucial for normal mammary gland development in the mouse. Developmental biology. 1999;215:13–32. doi: 10.1006/dbio.1999.9435. [DOI] [PubMed] [Google Scholar]
- Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, Wilson AJ, Hoffman MP. Parasympathetic stimulation improves epithelial organ regeneration. Nature communications. 2013;4:1494. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science. 2010;329:1645–1647. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa N, Mizushima H, Minegishi T, Iwamoto R, Mekada E, Seiki M. Membrane type 1-matrix metalloproteinase cleaves off the NH2-terminal portion of heparin-binding epidermal growth factor and converts it into a heparin-independent growth factor. Cancer research. 2010;70:6093–6103. doi: 10.1158/0008-5472.CAN-10-0346. [DOI] [PubMed] [Google Scholar]
- Koyama N, Hayashi T, Gresik EW, Kashimata M. Role of alpha 6 integrin subunit in branching morphogenesis of fetal mouse submandibular gland: investigation by mesenchyme-free epithelial culture system. The journal of medical investigation: JMI. 2009;56(Suppl):247–249. doi: 10.2152/jmi.56.247. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Role of endothelial cells in early pancreas and liver development. Mechanisms of development. 2003;120:59–64. doi: 10.1016/s0925-4773(02)00332-5. [DOI] [PubMed] [Google Scholar]
- Larsen M, Wei C, Yamada KM. Cell and fibronectin dynamics during branching morphogenesis. Journal of cell science. 2006;119:3376–3384. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- Lawson KA. Mesenchyme specificity in rodent salivary gland development: the response of salivary epithelium to lung mesenchyme in vitro. Journal of embryology and experimental morphology. 1974;32:469–493. [PubMed] [Google Scholar]
- Lazarus A, Del-Moral PM, Ilovich O, Mishani E, Warburton D, Keshet E. A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Development. 2011;138:2359–2368. doi: 10.1242/dev.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Gjorevski N, Boghaert E, Radisky DC, Nelson CM. Snail1, Snail2, and E47 promote mammary epithelial branching morphogenesis. The EMBO journal. 2011;30:2662–2674. doi: 10.1038/emboj.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LK, Pollock AS, Lovett DH. Asymmetric origins of the mature glomerular basement membrane. Journal of cellular physiology. 1993;157:169–177. doi: 10.1002/jcp.1041570122. [DOI] [PubMed] [Google Scholar]
- Lee MY, Skoura A, Park EJ, Landskroner-Eiger S, Jozsef L, Luciano AK, Murata T, Pasula S, Dong Y, Bouaouina M, Calderwood DA, Ferguson SM, De Camilli P, Sessa WC. Dynamin 2 regulation of integrin endocytosis, but not VEGF signaling, is crucial for developmental angiogenesis. Development. 2014;141:1465–1472. doi: 10.1242/dev.104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Edgar D, Fassler R, Wadsworth W, Yurchenco PD. The role of laminin in embryonic cell polarization and tissue organization. Developmental cell. 2003;4:613–624. doi: 10.1016/s1534-5807(03)00128-x. [DOI] [PubMed] [Google Scholar]
- Loffek S, Schilling O, Franzke CW. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: a critical balance. The European respiratory journal. 2011;38:191–208. doi: 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- Loibl M, Binder A, Herrmann M, Duttenhoefer F, Richards RG, Nerlich M, Alini M, Verrier S. Direct cell-cell contact between mesenchymal stem cells and endothelial progenitor cells induces a pericyte-like phenotype in vitro. BioMed research international. 2014;2014:395781. doi: 10.1155/2014/395781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS letters. 2014;588:2663–2670. doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Magenheim J, Ilovich O, Lazarus A, Klochendler A, Ziv O, Werman R, Hija A, Cleaver O, Mishani E, Keshet E, Dor Y. Blood vessels restrain pancreas branching, differentiation and growth. Development. 2011;138:4743–4752. doi: 10.1242/dev.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Developmental cell. 2014;30:137–150. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller O, Martinson H, Schedin P. Extracellular matrix composition reveals complex and dynamic stromal-epithelial interactions in the mammary gland. Journal of mammary gland biology and neoplasia. 2010;15:301–318. doi: 10.1007/s10911-010-9189-6. [DOI] [PubMed] [Google Scholar]
- Marthiens V, Kazanis I, Moss L, Long K, Ffrench-Constant C. Adhesion molecules in the stem cell niche--more than just staying in shape? Journal of cell science. 2010;123:1613–1622. doi: 10.1242/jcs.054312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Current opinion in cell biology. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Menezes K, Nascimento MA, Goncalves JP, Cruz AS, Lopes DV, Curzio B, Bonamino M, de Menezes JR, Borojevic R, Rossi MI, Coelho-Sampaio T. Human mesenchymal cells from adipose tissue deposit laminin and promote regeneration of injured spinal cord in rats. PloS one. 2014;9:e96020. doi: 10.1371/journal.pone.0096020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima H, Matsumoto T, Sakai T, Yamaguchi S, An SH, Abe M, Wakisaka S, Lee KY, Egusa H, Imazato S. Hydrogel-based biomimetic environment for in vitro modulation of branching morphogenesis. Biomaterials. 2011;32:6754–6763. doi: 10.1016/j.biomaterials.2011.05.072. [DOI] [PubMed] [Google Scholar]
- Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Current opinion in cell biology. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedvetsky PI, Emmerson E, Finley JK, Ettinger A, Cruz-Pacheco N, Prochazka J, Haddox CL, Northrup E, Hodges C, Mostov KE, Hoffman MP, Knox SM. Parasympathetic innervation regulates tubulogenesis in the developing salivary gland. Developmental cell. 2014;30:449–462. doi: 10.1016/j.devcel.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Gleghorn JP. Sculpting organs: mechanical regulation of tissue development. Annual review of biomedical engineering. 2012;14:129–154. doi: 10.1146/annurev-bioeng-071811-150043. [DOI] [PubMed] [Google Scholar]
- Nogawa H, Takahashi Y. Substitution for mesenchyme by basement-membrane-like substratum and epidermal growth factor in inducing branching morphogenesis of mouse salivary epithelium. Development. 1991;112:855–861. doi: 10.1242/dev.112.3.855. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Bilder D. Beyond the niche: tissue-level coordination of stem cell dynamics. Annual review of cell and developmental biology. 2013;29:107–136. doi: 10.1146/annurev-cellbio-101512-122319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblander SA, Zhou Z, Galvez BG, Starcher B, Shannon JM, Durbeej M, Arroyo AG, Tryggvason K, Apte SS. Distinctive functions of membrane type 1 matrix-metalloprotease (MT1-MMP or MMP-14) in lung and submandibular gland development are independent of its role in pro-MMP-2 activation. Developmental biology. 2005;277:255–269. doi: 10.1016/j.ydbio.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Onodera T, Sakai T, Hsu JC, Matsumoto K, Chiorini JA, Yamada KM. Btbd7 regulates epithelial cell dynamics and branching morphogenesis. Science. 2010;329:562–565. doi: 10.1126/science.1191880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. Journal of mammary gland biology and neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SB, Naim N, Nelson DA, Mosier AP, Cady NC, Larsen M. Biocompatible tissue scaffold compliance promotes salivary gland morphogenesis and differentiation. Tissue engineering Part A. 2014a;20:1632–1642. doi: 10.1089/ten.tea.2013.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters Sarah B, Nelson Deirdre A, Kwon Hae Ryong, Koslow Matthew, DeSantis Kara A, Larsen Melinda. TGFβ signaling promotes matrix assembly during mechanosensitive embryonic salivary gland restoration. Matrix Biology. 2015 doi: 10.1016/j.matbio.2015.01.020. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. Journal of cell science. 2009;122:179–186. doi: 10.1242/jcs.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebustini IT, Myers C, Lassiter KS, Surmak A, Szabova L, Holmbeck K, Pedchenko V, Hudson BG, Hoffman MP. MT2-MMP-dependent release of collagen IV NC1 domains regulates submandibular gland branching morphogenesis. Developmental cell. 2009;17:482–493. doi: 10.1016/j.devcel.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebustini IT, Patel VN, Stewart JS, Layvey A, Georges-Labouesse E, Miner JH, Hoffman MP. Laminin alpha5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through beta1 integrin signaling. Developmental biology. 2007;308:15–29. doi: 10.1016/j.ydbio.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert RB, Cai Q, Hong JY, Plank JL, Aamodt K, Prasad N, Aramandla R, Dai C, Levy SE, Pozzi A, Labosky PA, Wright CV, Brissova M, Powers AC. Vascular endothelial growth factor coordinates islet innervation via vascular scaffolding. Development. 2014;141:1480–1491. doi: 10.1242/dev.098657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nature reviews. 2014;15:225–242. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubashkin MG, Cassereau L, Bainer R, DuFort CC, Yui Y, Ou G, Paszek MJ, Davidson MW, Chen YY, Weaver VM. Force engages vinculin and promotes tumor progression by enhancing PI3K activation of phosphatidylinositol (3,4,5)-triphosphate. Cancer research. 2014;74:4597–4611. doi: 10.1158/0008-5472.CAN-13-3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- Schiller HB, Hermann MR, Polleux J, Vignaud T, Zanivan S, Friedel CC, Sun Z, Raducanu A, Gottschalk KE, Thery M, Mann M, Fassler R. beta1- and alphav-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nature cell biology. 2013;15:625–636. doi: 10.1038/ncb2747. [DOI] [PubMed] [Google Scholar]
- Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harbor perspectives in biology. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira SJ, Larsen M, DeVine T. Extracellular matrix and growth factors in salivary gland development. Frontiers of oral biology. 2010;14:48–77. doi: 10.1159/000313707. [DOI] [PubMed] [Google Scholar]
- Sigurdsson V, Ingthorsson S, Hilmarsdottir B, Gustafsdottir SM, Franzdottir SR, Arason AJ, Steingrimsson E, Magnusson MK, Gudjonsson T. Expression and functional role of sprouty-2 in breast morphogenesis. PloS one. 2013;8:e60798. doi: 10.1371/journal.pone.0060798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends in molecular medicine. 2011;17:424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Buache E, Alpy F, Daguenet E, Tomasetto CL, Ren GS, Rio MC. Stromal matrix metalloproteinase-11 is involved in the mammary gland postnatal development. Oncogene. 2014;33:4050–4059. doi: 10.1038/onc.2013.434. [DOI] [PubMed] [Google Scholar]
- Thomas T, Dziadek M. Expression of collagen alpha 1(IV), laminin and nidogen genes in the embryonic mouse lung: implications for branching morphogenesis. Mechanisms of development. 1994;45:193–201. doi: 10.1016/0925-4773(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Walker JL, Menko AS, Khalil S, Rebustini I, Hoffman MP, Kreidberg JA, Kukuruzinska MA. Diverse roles of E-cadherin in the morphogenesis of the submandibular gland: insights into the formation of acinar and ductal structures. Developmental dynamics: an official publication of the American Association of Anatomists. 2008;237:3128–3141. doi: 10.1002/dvdy.21717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR, Jesudason E. Lung organogenesis. Current topics in developmental biology. 2010;90:73–158. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Villarreal-Ponce A, Sun P, Salmans ML, Fallahi M, Andersen B, Dai X. Mammary morphogenesis and regeneration require the inhibition of EMT at terminal end buds by Ovol2 transcriptional repressor. Developmental cell. 2014;29:59–74. doi: 10.1016/j.devcel.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. The Journal of cell biology. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. The Journal of cell biology. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Spitzer E, Meyer D, Sachs M, Niemann C, Hartmann G, Weidner KM, Birchmeier C, Birchmeier W. Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. The Journal of cell biology. 1995;131:215–226. doi: 10.1083/jcb.131.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates LL, Schnatwinkel C, Hazelwood L, Chessum L, Paudyal A, Hilton H, Romero MR, Wilde J, Bogani D, Sanderson J, Formstone C, Murdoch JN, Niswander LA, Greenfield A, Dean CH. Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung. Developmental biology. 2013;373:267–280. doi: 10.1016/j.ydbio.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harbor perspectives in biology 3. 2011 Feb 1;3(2) doi: 10.1101/cshperspect.a004911. pii: a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers MM. 3D in vitro cell culture models of tube formation. Seminars in cell & developmental biology. 2014;31:132–140. doi: 10.1016/j.semcdb.2014.02.016. [DOI] [PubMed] [Google Scholar]