Abstract
Recombinant adeno-associated viral vectors (rAAVs) have been widely used for gene delivery in animal models, and are currently evaluated for human gene therapy after successful clinical trials in the treatment of inherited, degenerative or acquired diseases such as Leber congenital amaurosis, Parkinson disease, or heart failure. However, limitations in vector tropism, such as limited tissue specificity and insufficient transduction efficiencies of particular tissues and cell types, still preclude therapeutic applications in certain tissues.
Wild-type AAVs are defective viruses that require the presence of a helper virus to complete their life cycle. One the one hand, this unique property makes AAV vectors one of the safest available viral vectors for gene delivery. On the other hand, it also represents a potential obstacle because rAAV vectors have to overcome several biological barriers in the absence of a helper virus to transduce successfully a cell. Consequently, a better understanding of the cellular roadblocks that limit rAAV gene delivery is crucial and, during the last 15 years, numerous studies resulted in an expanding body of knowledge of the intracellular trafficking pathways of rAAV vectors. This review describes our current understanding of the mechanisms involved in rAAV attachment to target cells, endocytosis, intracellular trafficking, capsid processing, nuclear import and genome release with an emphasis on the most recent discoveries in the field and the emerging strategies used to improve the efficiency of AAV-derived vectors.
Keywords: adeno-associated virus, AAV, intracellular trafficking
INTRODUCTION
Gene therapy consists of the introduction of a therapeutic nucleic acid into a target cell in order to i) replace the function of a defective gene, ii) increase the expression of a down-regulated gene or iii) reduce detrimental levels of a protein, via RNA interference or antisense technology. Gene transfer is considered the last and only option to treat certain life-threatening or otherwise debilitating diseases for which no other therapeutic strategy is available such as, for instance, genetic disorders, drug-resistant cancers, heart failure or neurodegenerative diseases. Despite significant progress in the field of non-viral DNA transfer1, in vivo delivery of unprotected nucleic acids has proven difficult, since naked DNA is prone to rapid extracellular degradation and in the cell cytoplasm. For successful transfection of a cell the DNA has to be readily transported across the plasma membrane, escape from endocytic vesicles and finally cross the nuclear membrane. The transfection reagents developed so far have achieved considerable efficiencies in in vitro transduction of cell lines but the efficiency in in vivo transduction remains limited. Animal viruses, on the other hand, have evolved over millions of years in order to optimize the delivery of their genetic material into host cells and, despite potential issues relating to their clinical safety and/or their immunogenicity, viruses are still considered the most effective and promising vectors for in vivo gene delivery. Among the variety of viruses used as vectors for gene transfer (retroviruses, herpesviruses, adenoviruses, poxviruses, etc...), the most impressive therapeutic successes so far have been obtained with retrovirus vectors in the treatment of children suffering from X-linked Severe Combined Immunodeficiency Disease (SCID)2-4 and with adeno-associated virus-derived vectors in the treatment of Leber congenital amaurosis, an inherited eye disease leading to retinal degeneration5-7. Adeno-associated viruses (AAV) are small non-enveloped DNA viruses first discovered as contaminants of adenovirus preparations8. They belong to the genus dependovirus of the sub-family parvovirinae and the family parvoviridae and require co-infection with a helper DNA virus (adenovirus, herpesvirus or papillomavirus) to complete their productive replication cycle9. AAVs are nonpathogenic in humans or animals, and show a low immunogenicity in comparison to other viruses10. AAVs have an icosahedral capsid (22-26 nm in diameter) containing a single-stranded DNA genome of approximately 4.7 kilobases that is flanked by two T-shaped inverted terminal repeats (ITRs). The left-hand of the viral genome contains the rep gene, encoding the nonstructural proteins rep78, 68, 52 and 40 that are required for DNA replication and packaging. The right-hand of the AAV genome contains the cap ORF, encoding the capsid proteins VP1, VP2 and VP3 and a newly identified chaperone, the so-called assembly-activating protein (AAP), that is necessary for capsid formation11. The isolation of an infectious clone of AAV212, 13 and the demonstration that the AAV2 backbone could be used to express foreign proteins in cultured cells14, 15 paved the way for using AAVs as gene delivery vectors. In contrast to other viruses such as first and second generation adenoviruses, which require several viral genes in cis for viral vector production, the ITRs are the only cis-elements of the AAV genome necessary for DNA replication and packaging, and the entire rep and cap ORFs can be replaced by any sequence of interest within a size limit of approximately 5kb16. Furthermore, any transgene flanked by AAV2 ITRs can be packaged into the capsid of any of the nine AAV serotypes (AAV1-9) currently evaluated for in vivo transduction17, 18. This is of particular importance since different AAV serotypes show very different tissue tropisms19-21 (summarized in Table 1). AAV-derived vector DNA rarely integrates in the host cell genome but can lead to sustained, long-term transgene expression in non-dividing cells22, which makes them particularly suitable for gene therapy of largely post-mitotic tissues like the brain, the retina, the liver, skeletal muscles or the heart.
Table 1.
AAV receptors and preferential tissue tropism
| Virus | Glycan Receptor | Co-receptor/other | Tissue tropisma |
|---|---|---|---|
| AAV1 | N-linked Sialic acid122, 123 | Unknown | SMb124, CNS125, Retina126, Pancreas127 |
| AAV2 | HSPG25 | FGFR126, HGFR30, LamR29, CD9 tetraspanin128 | VSMC129, SM130, CNS131, Liver132, Kidney133 |
| AAV3 | HSPG18 | FGFR1134, HGFR135, LamR29 | Hepatocarcinoma136, SM124 |
| AAV4 | O-linked Sialic acid137 | Unknown | CNS138, Retina139 |
| AAV5 | N-linked Sialic acid137, 140 | PDGFR141 | SM124, CNS138, Lung142, Retina126 |
| AAV6 | N-linked Sialic acid123, HSPG122 | EGFR143 | SM144, SM (IV)145, Heart24, Lung146 |
| AAV7 | Unknown | Unknown | SM23, Retina147, CNS148 |
| AAV8 | Unknown | LamR29 | Liver23, SM23, 149, CNS148, Retina147, Pancreas150, Heart149 |
| AAV9 | N-linked galactose151 | LamR29 | Liver152, Heart (I.V.)152, 153, Brain (I.V.)154, SM (I.V.)155, Lungs156, Pancreas153, Kidney (I.V.)156 |
| BAAV | Ganglioside GM137 | Unknown | Unknown |
Preferential tissue tropism following local delivery, unless otherwise indicated (I.V.=Intravenous injection)
Abbreviations : SM, Skeletal Muscle; CNS, Central Nervous System; VSMC, Vascular Smooth Muscle Cells
CELLULAR BARRIERS TO AAV TRANSDUCTION
Despite the great potential of rAAV for in vivo gene transfer and the availability of several serotypes and a large number of capsid variants23, AAV-derived vectors display insufficient transduction efficiency in certain tissues and their relatively low organ specificity can be problematic for specific therapeutic applications. For instance, serotypes 1, 6 and 9, which are often referred to as “cardiotropic” due to their efficient transduction of cardiomyocytes, also transduce the liver to a significant extent following intravenous injection. Conversely, overall transduction levels are comparatively low for all serotypes in organs such as the brain, the pancreas or the kidneys following systemic injection20, 24. Like all non-enveloped DNA viruses, AAVs must balance the need for a high capsid stability to prevent degradation in the extracellular environment as well as within the cell (e.g., by lysosomal proteases or the proteasome) with the capacity to readily release their genome in the nucleus of the target cell. Thus, AAV-based vectors must overcome several limiting and complex steps between the cell membrane and the nucleus, where they ultimately deliver their genome for successful transduction. First, the virion must bind to the surface of the target cell via one or more receptors/co-receptors. The second step consists in the uptake of the virus-receptor complex by endocytosis following invagination of the cell membrane. The next steps include the maturation of virus-containing vesicles into more acidic endocytic compartments, either late endosomes, recycling tubular endosomes or lysosomes, followed by retrograde transport to the trans-Golgi, medial-Golgi, cis-Golgi, ER-Golgi intermediate compartment (ERGIC, also called cis-Golgi network) and/or the endoplasmic reticulum. Those stages, which can be collectively characterized as “endosomal trafficking”, can trigger irreversible modifications of the viral capsid both due to the lower pH and through the (enzymatic) action of resident proteins of these compartments. Retrograde transport is followed by escape of the viral particle into the cytoplasm. This is most likely followed by the nuclear import of the intact viral particle through the nuclear pore complex (NPC; see below). After nuclear translocation, single-stranded DNA is released by capsid uncoating and converted into double-stranded DNA to allow transgene expression.
Although receptor expression pattern and viral attachment are obviously important in determining the tropism of AAV vectors, there is now strong evidence that post-attachment steps, such as endosomal escape or nuclear import, can drastically alter transduction efficiency. In fact, almost each AAV trafficking stage, from endocytosis to nuclear import, can constitute a rate-limiting step in a serotype- and cell type-dependent manner. While many details of AAV trafficking remain to be determined, the use of both small drug and genetic inhibitors and enhancers (Table 2) combined with fluorescence microscopy have greatly contributed to our understanding of AAV trafficking. Importantly, this increasing knowledge has also resulted in the development of novel strategies that might help in overcoming some of the limitations that AAV trafficking poses to successful transduction.
Table 2.
Inhibitors and enhancers of AAV trafficking/transduction
| Virus | Drug/Protein/Mutation | Effect | Fold enh/inhiba | Possible Target |
|---|---|---|---|---|
| AAV2 | Heparin25 | Inhibitor | - 100-fold | Blocks attachment |
| AAV2 | Bafilomycin A139, 51 | Inhibitor | - 10-fold | Blocks endosomal acidification/trafficking |
| AAV2 | Brefeldin A51 | Inhibitor | - 100-fold | Disrupts the Golgi apparatus, blocks EE-LE transport and Golgi-ER transport/trafficking |
| AAV2 | Chloroquine65 | Inhibitor | - 100-fold | Blocks endosomal acidification/trafficking |
| AAV2 | Wortmannin43 | Inhibitor | - 4-fold | Blocks EE-EE and CLIC/GEEC-EE fusion/trafficking |
| AAV2-8 | Cathepsin B-L inhibitors84 | Inhibitor | - 3-fold | Blocks endosomal capsid processing |
| AAV2 | Rac1 T17N43 | Inhibitor | - 2-fold | Blocks macropinocytosis/entry |
| AAV2 | Dynamin1 K44A39, 40 | Inhibitor | - 2-3-fold | Blocks clathrin-mediated endocytosis/entry |
| AAV2 | Rab7 siRNA67 | Inhibitor | - 2-fold | Blocks late endosome-Golgi transport/trafficking |
| AAV2 | Rab11 siRNA67 | Inhibitor | - 40% | Blocks recycling endosome-Golgi transport/trafficking |
| AAV2 | AAV HD/ANb mutant53, 75 | Inhibitor | - 500-fold | Endosomal escape |
| AAV2 | AAV BC3 mutant65, 79 | Inhibitor | - 10000-fold | Viral NLS mutant, blocks nuclear import |
| AAV2 | AAV BR3K mutant53 | Inhibitor | - 500-fold | Blocks mobilization from the nucleolus |
| AAV2 | Capsid phosphorylation111 | Inhibitor | - 3-fold | Signal for capsid ubiquitination? |
| AAV2-5 | MG13251, 97 | Enhancer | + 10-100-fold | Proteasome inhibitor/capsid protection? Trafficking?/2nd strand synthesis? |
| AAV2-5 | LLnL97 | Enhancer | + 10-200-fold | Proteasome inhibitor, similar to MG132 |
| AAV2-5 | Doxorubicin157, 158 | Enhancer | + 10-100-fold | Topoisomerase inhibitor |
| AAV2 | E3 Ub ligase inhibitor96 | Enhancer | + 3-fold | Blocks cellular/capsid ubiquitination? |
| AAV2 | hydroxyurea159-161 | Enhancer | + 10-100-fold | Triggers DNA damage response/Additive effect with MG132 |
| AAV2 | Tyrphostin-2398 | Enhancer | + 20-40-fold | Blocks capsid phosphorylation/ubiquitination? similar to MG132 |
| AAV2 | Adenovirus109 | Enhancer | + 10-20-fold | Multiple/Unknown, non-additive with MG132) |
| AAV2 | Misfolded CFTR protein mutant | Enhancer | +10-fold | ER stress/Misfolded Protein Response inducer |
| AAV2 | Heat shock99 | Enhancer | + 10-fold | 2nd strand synthesis? Hsp70 induction? ER stress? Synergistic effect with Tyrphostin23 |
| AAV2 | TC-PTP162 | Enhancer | + 16-fold | Blocks capsid phosphorylation/ubiquitination? |
| AAV2 | PP5110 | Enhancer | + 6-fold | Blocks capsid phosphorylation/ubiquitination? |
| AAV2-6 | AAV Tyrosine Mutants114, 115, 163, 164 | Enhancer | + 10-100-fold | Blocks capsid phosphorylation/ubiquitination? Non-additive with MG132 |
Transduction data are expressed as ratio to control infections and may represent experiments performed in various cell lines/animals/time points.
Abbreviations : HD/AN, VP1 double mutant (75HD>AN) with no PLA2 activity; BC3, VP1/2 basic cluster 3 168RK>NN mutant; BR3K, highly basic VP1/2 basic cluster 3 167A>K mutant; CFTR, Cystic fibrosis transmembrane conductance regulator; TC-PTP, T cell protein tyrosine phosphatase; PP5, serine/threonine protein phosphatase 5.
AAV ATTACHMENT : RECEPTORS AND CORECEPTORS
Despite our rapidly growing knowledge about novel AAV serotypes, the vast majority of studies on AAV biology have been performed using AAV2 as a model, for reasons that are both historical (first AAV infectious clone12) and practical (ability to transduce efficiently common laboratory cell lines such as HeLa or HEK-293T15). Hence, AAV2 was the first serotype whose attachment receptor was investigated and identified as heparan sulfate proteoglycan (HSPG) by competition and overexpression assays25, 26. This finding was followed by the identification of several putative proteinaceous co-receptors such as fibroblast growth factor receptor 1 (FGFR1)26, integrin αVβ527, integrin α5β128, laminin receptor29 or hepatocyte growth factor receptor (HGFR)30, suggesting that AAV2 infection requires a primary glycan receptor together with a co-receptor for optimal attachment and internalization. However, independent studies have challenged the role of integrin αVβ5 in AAV2 transduction31 and, more recently, a high throughput siRNA screen failed to confirm a role of FGFR1 or HGFR in AAV2 transduction of human aortic endothelial cells32. Interestingly, naturally occurring AAV2 variants recovered from human subjects do not use HSPG as an attachment receptor33, which could suggest that AAV2 ability to bind HSPG constitutes a tissue culture adaptation, positively selected via multiple passages of AAV-contaminated adenovirus preparations in HEK293 cells. AAV2 capsid mutants deficient in HSPG binding show a markedly reduced transduction of mouse liver but retain the ability to transduce cardiac muscle34, indicating that AAV2 can use alternative receptors. Overall, the identification of receptors used by many AAV serotypes has reinforced the general view that AAVs use one or more proteoglycan conjugate as a primary receptor (HSPG for AAV2, AAV3 and AAV6, O-linked 2,3-sialic acid for AAV4, N-linked sialic acid for AAV1, AAV5 and AAV6, N-linked galactose for AAV9) together with a proteinaceous receptor (FGFR1, integrins and/or HGFR for AAV2, HGFR for AAV3, EGFR for AAV6, PDGFR for AAV5, LamR for AAV2, AAV3, AAV 8 and AAV9) for efficient binding and endocytosis (Table 1). Dual receptor requirement is frequently observed for other viruses35 and is reminiscent of ligand-mediated activation and subsequent endocytosis of receptors such as FGFR1, which require HSPG binding36. One exception to this rule would be bovine AAV (BAAV), which requires a sialylated glycolipid, GM1 ganglioside, for transduction37. Most interestingly, recent data indicate that binding of AAV2 to HSPG not only allows virus attachment, but also induces a conformational change of the capsid38. This could suggest that the binding of AAV to its proteoglycan primary receptor “locks” the virion in a transition state that may increase its affinity for the secondary receptor and consequently trigger endocytosis.
AAV ENDOCYTOSIS
Following attachment to surface receptors, AAV internalization occurs via endocytosis, a process that starts with the invagination of the plasma membrane domains containing virus-receptor complexes and is followed by the scission and release of the newly formed vesicle into the cell. In the case of AAV2, this process is fast and efficient, since the totality of virions bound to the cell surface can be internalized in about 30 minutes39, 40. Therefore, virus internalization per se is likely not a rate-limiting step for AAV2 transduction, at least in tissue culture. Mammalian cells have developed multiple mechanisms of endocytosis aimed at targeting and sorting membrane-bound or extracellular cargos towards specific intracellular compartments. Those pathways include, but are not restricted to, clathrin-mediated endocytosis, caveolae-mediated endocytosis, macropinocytosis, phagocytosis, or several ill-characterized clathrin- and dynamin-independent pathways41, 42. Early studies showed a partial (50 to 70%) inhibition of AAV2 uptake and transduction in cells overexpressing a dominant-negative mutant of dynamin, a protein involved in the scission of clathrin-coated pits and caveolae39, 40, suggesting that clathrin-mediated uptake was the default pathway of AAV2 endocytosis. Later studies showed that inhibition of the Rac1 GTPase strongly decreased cellular uptake of AAV243 and that expression of a constitutively active mutant of Rac1 dramatically increased transduction44. These observations were surprising because Rac1 is the major effector of macropinocytosis, a dynamin-independent fluid-phase endocytic pathway distinct from clathrin-mediated endocytosis45, and there is evidence that constitutively active Rac1 inhibits clathrin-mediated endocytosis46, 47. In addition, internalized AAV2 virions showed little or no co-localization with a fluorescently labeled adenovirus type 540, which is known to enter target cells via clathrin-mediated endocytosis48.
Recently, our group revisited the nature of the endocytic mechanism of AAV2 endocytosis and we could not confirm a clear involvement of clathrin, dynamin or Rac1 in AAV2 transduction but instead found that viral infectious entry is dependent on membrane cholesterol, actin, Cdc42, Arf1 and GRAF1, which together define the recently characterized Clathrin-Independent Carriers/GPI-Enriched Endocytic Compartment (CLIC/GEEC) endocytic pathway49. Similarly, a genome-wide siRNA screen has identified several components of CLIC/GEEC as strong inhibitors of AAV2 transduction in human aortic endothelial cells32 and recent experiments show that the internalization mechanism of HSPG, the primary receptor for AAV2, shares many features of CLIC/GEEC50. In our study CLIC/GEEC endocytosis was required for efficient transduction, whereas inhibition of dynamin activity by dynasore or by dominant-negative dynamin mutants had no effect. There is currently no clear explanation to this discrepancy with previous studies. One possibility is that the use of adenoviral vectors for protein overexpression in some of the aforementioned studies39, 43, 44 could modify general cell homeostasis. Pre-infection with first generation adenoviral expression vectors (that retain E2, E4 and VA regions) shows a substantial cytopathic effect (our unpublished observations) and increases AAV2 transduction by at least one order of magnitude51-53, and it has been reported that adenovirus infection enhances trafficking of AAV2 to the nucleus54. Furthermore, Adenovirus type 5 E4 region enhances the expression of adhesion proteins on the cell surface55, and E4orf1 partially localizes to clathrin vesicles56 and modulates Rac1 and PI3K activity57. Therefore, it cannot be excluded that the use of Adenoviral vectors modulates with AAV2 trafficking.
In our studies, inhibition of either dynamin or CLIC/GEEC only partially inhibited virus internalization, but simultaneous inhibition of both pathways completely blocked viral entry, which indicates that AAV2 can enter cells via at least two distinct pathways49. These observations are not restricted to AAV2, since a similar dichotomy has been suggested for AAV558, 59, although this serotype reportedly uses a different endocytic mechanism60. Importantly, in the case of AAV2, only CLIC/GEEC is required for successful transduction, suggesting that viral particles internalized via dynamin-dependent endocytosis are directed towards a “dead-end” compartment and seldom contribute to transduction. Thus, efficient endocytosis is necessary but not sufficient for AAV transduction. This notion is supported by the fact that NIH3T3 cells can internalize AAV2 virions as efficiently as HeLa or 293T cells, but they are not permissive to transduction due to impaired post-entry trafficking61. In vivo, experiments performed with several AAV serotypes show no strict correlation between intracellular viral DNA accumulation and transgene expression in various organs20, 24, 62. Another striking example of the coexistence of infectious and non-infectious endocytic pathways within the same cell was provided by Di Pasquale and colleagues, who showed that intact AAV4, AAV5 or BAAV (but not AAV2 or AAV6) viral particles could cross monolayers of polarized cells when applied on the apical side, by a process known as transcytosis63. Interestingly, drugs known to inhibit transcytosis strongly increased cell transduction, suggesting that polarized cells can redirect attached virions towards two separate endocytic pathways, one of which leads to transcytosis, and the other results in nuclear transport and gene expression. It is yet unclear whether this dichotomy reflects the use of a different receptor, but recent experiments by DiPasquale and colleagues indicate that BAAV uses a distinct receptor for transcytosis versus transduction64. Taken together, all these studies indicate that AAV can be internalized through a variety of mechanisms, namely clathrin-mediated endocytosis, caveolae-mediated endocytosis, via the CLIC/GEEC pathway, or possibly through transcytosis (Figure 1) the nature of which will then commit the virions to either an infectious pathway (by ultimately delivering the virion to the nucleus) or to a non-infectious pathway (by targeting the virion to lysosome/proteasome system for degradation or by transporting it to the extracellular medium by transcytosis). Thus, even though endocytosis per se is not necessarily rate-limiting from a strictly quantitative point, it constitutes a crucial step in irreversibly determining the fate of incoming AAV virions.
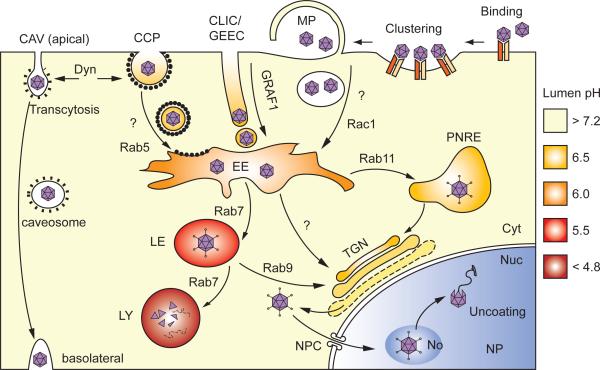
Figure 1.
Model of Entry and Intracellular Trafficking of AAV Vectors. Following binding to a receptor/co-receptor complex, rAAV enters target cell through endocytosis via one or more of the following pathways: CLIC/GEEC endocytosis, clathrin-mediated endocytosis (CCP) or caveolar endocytosis (CAV). Virions are sorted towards the trans-Golgi network (TGN) along a retrograde transport pathway presumably involving trafficking via early endosomes (EE), followed by late endosomes (LE), perinuclear recycling endosomes (PNRE) or both. Capsid conformation changes and exposure of the PLA2 domain (spikes) allows cytoplasmic release from the Golgi apparatus or the Endoplasmic Reticulum (ER), and nuclear import via the nuclear pore complex (NPC). After nuclear import, intact capsids accumulate in the nucleolus (No) before mobilization into the nucleoplasm (NP) and genome release by partial uncoating. Some of the steps indicated are hypothetical and have not been conclusively proven yet.
POST-ENDOCYTIC TRAFFICKING
In contrast to virus internalization, several lines of evidence indicate that intracellular trafficking of AAV is a rate-limiting, slow and inefficient process. In cultured cells, only a small fraction of viral particles access the nucleus within 16-20 hours, while the majority accumulate in a perinuclear compartment40. AAV2 transduction can be efficiently blocked by lysosomotropic drugs like bafilomycin A1, chloroquine or ammonium chloride, that buffer endosomal pH, indicating that endosome acidification is necessary for AAV2 processing40, 51, 65 and inhibition of transduction by brefeldin A suggests that transport steps involving the Golgi apparatus are involved51. The retrograde transport intermediates used by AAV are not yet completely defined, but at least for AAV2 and AAV5 incoming virions appear to accumulate in the Golgi apparatus before their release in the cytoplasm53, 59, 66. Previous studies indicate that a fraction of AAV2 virions transit via late and/or recycling endosomes enriched in Rab7 and Rab11, respectively67. AAV retrograde transport from an early endosomal compartment towards the trans-Golgi network is possibly similar to previously described pathways involved in trafficking of bacterial toxins68, 69 or other non-fusogenic DNA viruses such as SV40 or murine polyomavirus70-72. The transport of AAVs within the endomembrane system induces profound changes in capsid conformation that are required for efficient transduction73. Perhaps the most convincing evidence for the necessity of endosomal processing of AAV came from a study by Sonntag et al.65, who showed that microinjection of intact virus in the cytoplasm - and even in the nucleus - of HeLa cells resulted in very low transduction. In the same study, the authors also observed that transduction could be blocked by cytoplasmic injection of antibodies targeted to the intact capsids or to the N-terminal part of VP1 and VP2. In conclusion, these experiments unequivocally demonstrate that i) the AAV2 capsid must undergo a conformational change in the endocytic system, ii) passage of the virions through the cytoplasm is an obligatory stage in transduction, iii) endosomal processing exposes the VP1 and VP2 N-terminal domains outside of the capsid and iv) the presence of capsids with externalized VP1 and/or VP2 N-termini in the cytoplasm is required for transduction. The N-terminal domains of VP1, which is buried inside the capsid in intact virions74, contains a phospholipase A2 (PLA2) domain that has been shown to be necessary for endosomal escape and transduction75-78 as well as three basic clusters (BC1, 2 and 3) that presumably act as nuclear localization signals (NLS) for the viral capsid65, 79. Taken together, these observations suggest that within the endocytic system a conformational change exposes the VP1 and VP2 N-termini outside the capsid, which then allows the virus to escape the endomembrane compartment into the cytoplasm. Such process has been observed for several non-enveloped viruses, including papillomavirus80, polyomavirus81 or adenovirus82. Once in the cytoplasm, the basic residue clusters of VP1 or VP2 would then mediate the nuclear import of full virions via the NPC. AAV2 virions carrying a mutation in either the PLA2 domain or the first basic cluster in VP1 accumulate in the Golgi apparatus, which suggests that i) exposure of the VP1 PLA2 domain is not necessary for endosome-to-Golgi retrograde transport and ii) that the endosomal release may occur from the Golgi apparatus or the ER-Golgi intermediate compartment (ERGIC)53, 83. In vitro exposure of AAV2 to acidic pH (pH 4-5) does not induce VP1/2 N-termini externalization65. This indicates that while endosomal acidification is necessary for AAV2 processing, endosomal acidification alone is insufficient for VP1/2 externalization. Thus, endosomal acidification could be necessary for vesicular trafficking and/or dissociation of the virus/receptor complex, but insufficient for capsid conformational modifications and endosomal escape. Recent studies indicate that endosomal cathepsins B and L are necessary for transduction by “priming” the capsids by partial proteolysis84, but it is yet uncertain whether this interaction modulates endosomal escape or a later step. Strikingly, cathepsin digestion of AAV2 and AAV8 showed a different cleavage pattern, which could be partially responsible for the fact that AAV2 and AAV8 show different uncoating kinetics85. AAV5 was not sensitive to cathepsin proteolysis indicating that this process is not conserved among all AAV serotypes84.
NUCLEAR IMPORT
The mechanism allowing AAV particles to cross the nuclear membrane is still poorly understood, but converging evidence suggest that intact particles are translocated into the nucleus before DNA release. Intact virions can still be detected inside the nucleus by immunofluorescence microscopy40, 43, 53, 86, nuclear microinjection of antibodies against intact capsids blocks transduction65, and fully infectious virions can be recovered from infected cell nuclei86. Although Kleinschmidt and colleagues suggested that AAV2 nuclear import was inefficient and rate-limiting, based on poor infectivity of virions with exposed VP1/2 N- termini following cytoplasmic injection65, this phenomenon could reflect an incomplete processing of the capsid rather than a low rate of nuclear import. Given the complexity of capsid modifications in the endomembrane system, as discussed earlier, it is very difficult to evaluate the efficiency and kinetics of AAV nuclear import per se.
The basic clusters BC2 and BC3, shared by the N-termini of VP1 and VP2, are necessary for transduction65, 79 and together confer nuclear localization to a heterologous fusion protein65, 87, which strongly suggests that the BC2-3 domain form a bipartite NLS that allows virus nuclear import via the NPC. AAV2 basic clusters 2 and 3 are separated by a 23-amino acids linker, which classifies them as a non-classical NLS. Therefore it is likely that AAV2 nuclear import relies on a direct interaction between the VP1/2 N-terminus and a member of the importin-β family, rather than with a classical importinα/importinβ complex.88-90
Once the virus has gained access to the nucleus, it is readily transported to the nucleolus, in which it is maintained as an intact particle until its egress into the nucleoplasm86. The process leading to AAV2 nucleolar localization is unknown, but it should be mentioned that AAV2 capsid has been reported to interact directly with nucleolin91 and B23/nucleophosmin92, two major nucleolar proteins. The exact role of nucleolar transport in AAV biology is uncertain, but the observation that nucleolin or nucleophosmin knock-down by RNA interference strongly increase AAV2 transduction86 suggests that nucleolar transit may be dispensable or even detrimental for AAV2 uncoating and gene expression. The process of uncoating itself is not completely understood, but it is probable, as observed with autonomous parvoviruses, that DNA can be released without complete disassembly of the capsid93, 94. The kinetics of DNA release appear to be cell- and serotype-dependent. In cells from cardiac origin, AAV6-packaged genomes have been reported to be released more efficiently than their AAV2-packaged counterparts, but the opposite trend is observed in HeLa cells95. Similarly, although AAV2, 6 and 8 show similar uptake and nuclear import rates during murine liver transduction, AAV6 and 8 appear to release their genome earlier than AAV2. Surprisingly, intact AAV2 virions are completely and rapidly dissociated following incubation with a liver nuclear extract85. Despite an apparent contradiction with the in vivo data, this observation could be explained by the phenomenon of nucleolar sequestration suggested by Johnson and colleagues, as discussed earlier; if AAV2 sequestration in the nucleolus prevents uncoating and genome release in vivo, one could expect the uncoating to be more efficient in an in vitro reaction, in which virions are directly exposed to nucleoplasmic factors. These observations also mirror those by Miao and colleagues, who described a striking discrepancy between substantial nuclear accumulation of AAV2 genomes and poor transduction in mouse liver62. It will be very interesting to investigate further the possible relationship between nucleolar mobilization and the serotype- and tissue-specific differences in AAV transduction.
PROTEASOME INHIBITORS AND CAPSID UBIQUITINATION
Pioneer experiments by Douar and colleagues showed that the proteasome inhibitor MG132 dramatically enhanced AAV2 transduction in cultured cells and delayed viral DNA decay51. In addition, AAV2 and AAV5 capsids can be ubiquitinated both in vivo and in vitro96, 97, and transduction is increased, albeit to a lesser extent, following treatment with a E3 ubiquitin ligase inhibitor96. This led to the hypothesis that a significant fraction of incoming virions were targeted to proteasome degradation by ubiquitin conjugation of the capsid during cytoplasmic trafficking. However, this model was later challenged by several observations: i) other proteasome inhibitors, such as LLnL, enhance transduction to the same extent as MG132 with no visible effect on viral DNA degradation and ii) the accumulation of viral DNA following MG132 treatment (about 2-3 fold 24h post-infection) cannot account for the increase in transduction observed at the same time point (50-fold). Altogether, these observations clearly indicate the dramatic increase in transduction caused by MG132 cannot be explained by an inhibition of proteasome-mediated virus degradation alone. Along those lines, it was proposed that proteasome inhibitors increase AAV trafficking to the nucleus by a yet unexplained mechanism, in a cell- and serotype-specific manner96-98.
Interestingly, proteasome inhibitors are strong activators of the ER stress/misfolded protein response pathway, and recent studies have shown that ER stress induction could potentiate AAV2 transduction by an order of magnitude83. Consistently, heat-shock treatment99 and alteration of cell redox status100, both known as potent ER stress inducers, increase AAV2 transduction to a comparable extent. Proteasome inhibitors also induce the formation of large nucleolar stress bodies enriched in proteasome, ubiquitinated cellular proteins, nucleoplasmic proteasome targets and heat-shock proteins101-103, reminiscent of the strong nucleolar accumulation of AAV2 observed after MG132 treatment53, 86.
The exact role of capsid ubiquitination in AAV transduction is still poorly understood. For instance, it is not certain whether ubiquitination is beneficial or detrimental for AAV2 transduction. In the studies by Duan et al., transduction enhancement observed after E3 ligase inhibition might result from indirect pleiotropic effects of the drug on the cell metabolism. Mutagenesis of lysines residues exposed on the surface of the AAV2 capsid, the potential targets of ubiquitination, showed no improvement in infectivity34, 104, 105. Interestingly, in vitro capsid ubiquitination is much more efficient on denatured capsid proteins than on intact virions97, which led the authors to formulate the hypothesis that ubiquitination in vivo requires a conformational change of the capsid and the exposure of normally inaccessible lysine residues. Consistent with this view, bioinformatical analysis indicates that most of the potentially ubiquitinated lysine residues localize on the N-termini of VP1 and VP2 in AAV serotypes 1 to 12 (our unpublished observations). Hence, it will be interesting to study the function of these residues in AAV trafficking and transduction.
CAPSID PHOSPHORYLATION AND TYROSINE MUTANTS
A fascinating development in our understanding of the cellular roadblocks to AAV transduction came from a series of studies on the role of tyrosine phosphorylation in viral trafficking and gene expression. Initial reports from Qing and colleagues showed that a cellular phosphoprotein, ssD-BP (single-strand D-sequence binding protein) could bind to the ITR of the AAV2 genome and block complementary strand synthesis106. General inhibition of tyrosine phosphorylation by genistein or tyrphostin-23 (Tyr23), but also treatment with the DNA-damaging drug hydroxyurea or expression of adenovirus E4orf6 protein, dephosphorylated ssD-BP and showed a strong concomitant increase in AAV2 transduction107-109. In parallel, T-cell protein tyrosine phosphatase (TC-PTP) or protein phosphatase 5 (PP5) expression dramatically increased transduction110.
Later studies from the same group demonstrated that phosphorylation inhibitors also had strong positive effects on virus transport to the nucleus98. Intriguingly, Tyr23 did not show a synergistic or additional effect with MG132, which suggests that both drugs enhance transduction via a common mechanism. Simultaneously, Zhong et al. found that Tyr23 in the intact AAV2 capsids could be specifically phosphorylated in vitro by the EGFR-protein tyrosine kinase (EGFR-PTK). Most interestingly, in vitro phosphorylated AAV2 showed a 3-fold reduction in transduction, independently of second-strand DNA synthesis. A possible explanation was that exposed phosphotyrosines acted as a positive signal for capsid ubiquitination111. Consistently, mutagenesis of highly conserved exposed tyrosine residues (Y444F, Y500F or Y730F) on AAV2 capsids enhanced transduction up to 10-fold in HeLa cells and 30-fold in mouse liver. Transduction by tyrosine mutants was not further enhanced by adenovirus co-infection, MG132 or Tyrphostin-23 treatment, which again suggests that all these distinct treatments target a common step of AAV transduction, involving capsid phosphorylation and possibly ubiquitination. Since then, single or combined tyrosine mutants of AAV2 have been successfully tested in vitro in fibroblasts and mesenchymal stem cells112 and in vivo in murine hepatocytes113, 114, and retina115, 116. All studies so far showed a marked increase (10- to 100-fold) of transduction, successful transduction of non-permissive cells and an additive effect of multiple (up to three) tyrosine mutations. Improved transduction of mouse skeletal muscle was also obtained with tyrosine mutants of AAV6 vectors, and interestingly showed similarly enhanced long-term persistence of intracellular viral DNA117.
CONCLUDING REMARKS
The last decade has seen considerable progress in our understanding of AAV biology and the rapid identification of novel serotypes with different tissue tropism is opening new avenues for gene therapy applications. It is now well recognized that intracellular trafficking of AAV vectors constitutes a major limiting step in transduction. Not surprisingly, our knowledge of this highly complex, multistep process is still incomplete and future research is needed to improve our understanding of AAV capsid processing, endosomal escape, nuclear import and uncoating. This might be a challenging quest and it could prove difficult to isolate all of these steps from each other since early capsid modifications in the endosomal system could have major consequences on every downstream event, such as, for instance, the uncoating in the nucleus. The recent investigations about AAV capsid phosphorylation, which culminated in the discovery of tyrosine mutant vectors demonstrate that every discovery in the field of AAV trafficking can potentially translate into rapid and major improvements in gene therapy applications since it could allow to reduce dramatically the vector doses111 or to transduce cell types refractory to wild-type capsid vectors112. Although it may seem counter-intuitive that targeted modification of evolutionary conserved surface residues improves viral transduction, one should keep in mind that AAV vectors are defective viruses. AAVs have evolved to co-infect target cells with a helper virus, and they are likely not optimized for autonomous infection. Almost every step of AAV transduction, from endosomal processing to transgene expression, might be enhanced by adenovirus co-infection. This means that AAV vectors offer room for improvement and might be “perfectible” by genetic manipulation of the capsid. This notion is supported by a growing body of evidence showing that directed evolution of AAV capsid from random mutants or shuffling libraries can create vectors displaying much higher transduction efficiency and/or specificity for selected cell types (as reviewed by Xiao Xiao and David Schaffer in this issue). At first sight, AAV may seem an inefficient virus with recombinant AAV exhibiting a fairly high particle-to-infectivity ratio118, but recent studies indicate that in the presence of Adenovirus, infectivity of wild-type AAV2 can approach a physical-to-infectious ratio of one, defining it as a near-perfect virus119. Recombinant AAV2 is nearly 100-fold less efficient than its wild-type counterpart in the same assay, tantalizingly opening the possibility that the sequence or secondary structure of the DNA packaged into AAV capsid may also influence transduction. If this hypothesis was confirmed, determination of sequence-specific DNA-capsid interactions could have great consequences for the design of AAV gene therapy vectors, as it could influence both the production and the infectivity of recombinant viruses, as previously observed for autonomous parvoviruses120, 121.
ACKNOWLEDGEMENTS
The authors apologize to all those whose work has not been cited as a result of space limitations. This work was supported by US National Institutes of Health Grants HL077322 (to T.W.), and HL100396 and HL088434 (to Roger J. Hajjar, Mount Sinai School of Medicine, New York, NY).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Kaneda Y. Update on non-viral delivery methods for cancer therapy: possibilities of a drug delivery system with anticancer activities beyond delivery as a new therapeutic tool. Expert Opin Drug Deliv. 2010;7(9):1079–93. doi: 10.1517/17425247.2010.510511. [DOI] [PubMed] [Google Scholar]
- 2.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288(5466):669–72. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 3.Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364(9452):2181–7. doi: 10.1016/S0140-6736(04)17590-9. [DOI] [PubMed] [Google Scholar]
- 4.Kohn DB, Candotti F. Gene therapy fulfilling its promise. N Engl J Med. 2009;360(5):518–21. doi: 10.1056/NEJMe0809614. [DOI] [PubMed] [Google Scholar]
- 5.Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18(3):643–50. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374(9701):1597–605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herzog RW, Cao O, Srivastava A. Two decades of clinical gene therapy--success is finally mounting. Discov Med. 2010;9(45):105–11. [PMC free article] [PubMed] [Google Scholar]
- 8.Hoggan MD, Blacklow NR, Rowe WP. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966;55(6):1467–74. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerr JR. In: Parvoviruses. Arnold Hodder., editor. Distributed in the United States of America by Oxford University Press; London New York: 2006. [Google Scholar]
- 10.Sun JY, Anand-Jawa V, Chatterjee S, Wong KK. Immune responses to adeno-associated virus and its recombinant vectors. Gene Ther. 2003;10(11):964–76. doi: 10.1038/sj.gt.3302039. [DOI] [PubMed] [Google Scholar]
- 11.Sonntag F, Schmidt K, Kleinschmidt JA. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc Natl Acad Sci U S A. 2010;107(22):10220–5. doi: 10.1073/pnas.1001673107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samulski RJ, Berns KI, Tan M, Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc Natl Acad Sci U S A. 1982;79(6):2077–81. doi: 10.1073/pnas.79.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laughlin CA, Tratschin JD, Coon H, Carter BJ. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene. 1983;23(1):65–73. doi: 10.1016/0378-1119(83)90217-2. [DOI] [PubMed] [Google Scholar]
- 14.Hermonat PL, Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1984;81(20):6466–70. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tratschin JD, West MH, Sandbank T, Carter BJ. A human parvovirus, adeno-associated virus, as a eucaryotic vector: transient expression and encapsidation of the procaryotic gene for chloramphenicol acetyltransferase. Mol Cell Biol. 1984;4(10):2072–81. doi: 10.1128/mcb.4.10.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 1996;7(17):2101–12. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 17.Duan D, Yan Z, Yue Y, Ding W, Engelhardt JF. Enhancement of muscle gene delivery with pseudotyped adeno-associated virus type 5 correlates with myoblast differentiation. J Virol. 2001;75(16):7662–71. doi: 10.1128/JVI.75.16.7662-7671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76(2):791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimm D, Kay MA. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther. 2003;3(4):281–304. doi: 10.2174/1566523034578285. [DOI] [PubMed] [Google Scholar]
- 20.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16(6):1073–80. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 21.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14(3):316–27. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Podsakoff G, Wong KK, Jr., Chatterjee S. Efficient gene transfer into nondividing cells by adeno-associated virus-based vectors. J Virol. 1994;68(9):5656–66. doi: 10.1128/jvi.68.9.5656-5666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(18):11854–9. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zincarelli C, Soltys S, Rengo G, Koch WJ, Rabinowitz JE. Comparative cardiac gene delivery of adeno-associated virus serotypes 1-9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin Transl Sci. 2010;3(3):81–9. doi: 10.1111/j.1752-8062.2010.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72(2):1438–45. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5(1):71–7. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 27.Summerford C, Bartlett JS, Samulski RJ. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5(1):78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 28.Asokan A, Hamra JB, Govindasamy L, Agbandje-McKenna M, Samulski RJ. Adeno-associated virus type 2 contains an integrin alpha5beta1 binding domain essential for viral cell entry. J Virol. 2006;80(18):8961–9. doi: 10.1128/JVI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80(19):9831–6. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kashiwakura Y, Tamayose K, Iwabuchi K, Hirai Y, Shimada T, Matsumoto K, et al. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J Virol. 2005;79(1):609–14. doi: 10.1128/JVI.79.1.609-614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu J, Brown KE. Integrin alphaVbeta5 is not involved in adeno-associated virus type 2 (AAV2) infection. Virology. 1999;264(2):436–40. doi: 10.1006/viro.1999.0010. [DOI] [PubMed] [Google Scholar]
- 32.Wallen AJ, Barker GA, Fein DE, Jing H, Diamond SL. Enhancers of Adeno-associated Virus AAV2 Transduction via High Throughput siRNA Screening. Mol Ther. 2011;19(6):1152–60. doi: 10.1038/mt.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen CL, Jensen RL, Schnepp BC, Connell MJ, Shell R, Sferra TJ, et al. Molecular characterization of adeno-associated viruses infecting children. J Virol. 2005;79(23):14781–92. doi: 10.1128/JVI.79.23.14781-14792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kern A, Schmidt K, Leder C, Muller OJ, Wobus CE, Bettinger K, et al. Identification of a heparin-binding motif on adeno-associated virus type 2 capsids. J Virol. 2003;77(20):11072–81. doi: 10.1128/JVI.77.20.11072-11081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalia M, Jameel S. Virus entry paradigms. Amino Acids. 2009 doi: 10.1007/s00726-009-0363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kan M, Wang F, Xu J, Crabb JW, Hou J, McKeehan WL. An essential heparin-binding domain in the fibroblast growth factor receptor kinase. Science. 1993;259(5103):1918–21. doi: 10.1126/science.8456318. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt M, Chiorini JA. Gangliosides are essential for bovine adeno-associated virus entry. J Virol. 2006;80(11):5516–22. doi: 10.1128/JVI.02393-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy HC, Bowman VD, Govindasamy L, McKenna R, Nash K, Warrington K, et al. Heparin binding induces conformational changes in Adeno-associated virus serotype 2. J Struct Biol. 2009;165(3):146–56. doi: 10.1016/j.jsb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan D, Li Q, Kao AW, Yue Y, Pessin JE, Engelhardt JF. Dynamin is required for recombinant adeno-associated virus type 2 infection. J Virol. 1999;73(12):10371–6. doi: 10.1128/jvi.73.12.10371-10376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartlett JS, Wilcher R, Samulski RJ. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J Virol. 2000;74(6):2777–85. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603–12. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 43.Sanlioglu S, Benson PK, Yang J, Atkinson EM, Reynolds T, Engelhardt JF. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation. J Virol. 2000;74(19):9184–96. doi: 10.1128/jvi.74.19.9184-9196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanlioglu AD, Karacay B, Benson PK, Engelhardt JF, Sanlioglu S. Novel approaches to augment adeno-associated virus type-2 endocytosis and transduction. Virus Res. 2004;104(1):51–9. doi: 10.1016/j.virusres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70(3):401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 46.Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382(6587):177–9. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- 47.Malecz N, McCabe PC, Spaargaren C, Qiu R, Chuang Y, Symons M. Synaptojanin 2, a novel Rac1 effector that regulates clathrin-mediated endocytosis. Curr Biol. 2000;10(21):1383–6. doi: 10.1016/s0960-9822(00)00778-8. [DOI] [PubMed] [Google Scholar]
- 48.Meier O, Greber UF. Adenovirus endocytosis. J Gene Med. 2004;6(Suppl 1):S152–63. doi: 10.1002/jgm.553. [DOI] [PubMed] [Google Scholar]
- 49.Nonnenmacher M, Weber T. Adeno-Associated Virus 2 Infection Requires Endocytosis through the CLIC/GEEC Pathway. Cell Host Microbe. 2011;10(6):563–76. doi: 10.1016/j.chom.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wittrup A, Zhang SH, Svensson KJ, Kucharzewska P, Johansson MC, Morgelin M, et al. Magnetic nanoparticle-based isolation of endocytic vesicles reveals a role of the heat shock protein GRP75 in macromolecular delivery. Proc Natl Acad Sci U S A. 2010;107(30):13342–7. doi: 10.1073/pnas.1002622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Douar AM, Poulard K, Stockholm D, Danos O. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J Virol. 2001;75(4):1824–33. doi: 10.1128/JVI.75.4.1824-1833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson WD, Westphal H. A cascade of adenovirus early functions is required for expression of adeno-associated virus. Cell. 1981;27(1 Pt 2):133–41. doi: 10.1016/0092-8674(81)90367-6. [DOI] [PubMed] [Google Scholar]
- 53.Johnson J, Li C, Diprimio N, Weinberg M, McCown T, Samulski R. Mutagenesis of adeno-associated virus type 2 capsid protein VP1 uncovers new roles for basic amino acids in trafficking and cell-specific transduction. J Virol. 2010;84(17):8888–902. doi: 10.1128/JVI.00687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao W, Warrington KH, Jr., Hearing P, Hughes J, Muzyczka N. Adenovirus-facilitated nuclear translocation of adeno-associated virus type 2. J Virol. 2002;76(22):11505–17. doi: 10.1128/JVI.76.22.11505-11517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rafii S, Dias S, Meeus S, Hattori K, Ramachandran R, Feuerback F, et al. Infection of endothelium with E1(-)E4(+), but not E1(-)E4(-), adenovirus gene transfer vectors enhances leukocyte adhesion and migration by modulation of ICAM-1, VCAM-1, CD34, and chemokine expression. Circ Res. 2001;88(9):903–10. doi: 10.1161/hh0901.089884. [DOI] [PubMed] [Google Scholar]
- 56.Chung SH, Frese KK, Weiss RS, Prasad BV, Javier RT. A new crucial protein interaction element that targets the adenovirus E4-ORF1 oncoprotein to membrane vesicles. J Virol. 2007;81(9):4787–97. doi: 10.1128/JVI.02855-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas MA, Broughton RS, Goodrum FD, Ornelles DA. E4orf1 limits the oncolytic potential of the E1B-55K deletion mutant adenovirus. J Virol. 2009;83(6):2406–16. doi: 10.1128/JVI.01972-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bantel-Schaal U, Braspenning-Wesch I, Kartenbeck J. Adeno-associated virus type 5 exploits two different entry pathways in human embryo fibroblasts. J Gen Virol. 2009;90(Pt 2):317–22. doi: 10.1099/vir.0.005595-0. [DOI] [PubMed] [Google Scholar]
- 59.Bantel-Schaal U, Hub B, Kartenbeck J. Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment. J Virol. 2002;76(5):2340–9. doi: 10.1128/jvi.76.5.2340-2349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keiser NW, Yan Z, Zhang Y, Lei-Butters DC, Engelhardt JF. Unique Characteristics of AAV1, 2, and 5 Viral Entry, Intracellular Trafficking, and Nuclear Import Define Transduction Efficiency in HeLa Cells. Hum Gene Ther. 2011 doi: 10.1089/hum.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansen J, Qing K, Kwon HJ, Mah C, Srivastava A. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J Virol. 2000;74(2):992–6. doi: 10.1128/jvi.74.2.992-996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miao CH, Nakai H, Thompson AR, Storm TA, Chiu W, Snyder RO, et al. Nonrandom transduction of recombinant adeno-associated virus vectors in mouse hepatocytes in vivo: cell cycling does not influence hepatocyte transduction. J Virol. 2000;74(8):3793–803. doi: 10.1128/jvi.74.8.3793-3803.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Pasquale G, Chiorini J. AAV transcytosis through barrier epithelia and endothelium. Mol Ther. 2006;13(3):506–16. doi: 10.1016/j.ymthe.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 64.Di Pasquale G, Kaludov N, Agbandje-McKenna M, Chiorini JA. BAAV transcytosis requires an interaction with beta-1-4 linked-glucosamine and gp96. PLoS ONE. 2010;5(3):e9336. doi: 10.1371/journal.pone.0009336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sonntag F, Bleker S, Leuchs B, Fischer R, Kleinschmidt JA. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J Virol. 2006;80(22):11040–54. doi: 10.1128/JVI.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pajusola K, Gruchala M, Joch H, Luscher TF, Yla-Herttuala S, Bueler H. Cell-type-specific characteristics modulate the transduction efficiency of adeno-associated virus type 2 and restrain infection of endothelial cells. J Virol. 2002;76(22):11530–40. doi: 10.1128/JVI.76.22.11530-11540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding W, Zhang LN, Yeaman C, Engelhardt JF. rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion. Mol Ther. 2006;13(4):671–82. doi: 10.1016/j.ymthe.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7(8):568–79. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 69.Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135(7):1175–87. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3(5):473–83. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 71.Mannova P, Forstova J. Mouse polyomavirus utilizes recycling endosomes for a traffic pathway independent of COPI vesicle transport. J Virol. 2003;77(3):1672–81. doi: 10.1128/JVI.77.3.1672-1681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spooner RA, Smith DC, Easton AJ, Roberts LM, Lord JM. Retrograde transport pathways utilised by viruses and protein toxins. Virol J. 2006;3:26. doi: 10.1186/1743-422X-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nam HJ, Gurda BL, McKenna R, Potter M, Byrne B, Salganik M, et al. Structural studies of adeno-associated virus serotype 8 capsid transitions associated with endosomal trafficking. J Virol. 2011;85(22):11791–9. doi: 10.1128/JVI.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kronenberg S, Bottcher B, von der Lieth CW, Bleker S, Kleinschmidt JA. A conformational change in the adeno-associated virus type 2 capsid leads to the exposure of hidden VP1 N termini. J Virol. 2005;79(9):5296–303. doi: 10.1128/JVI.79.9.5296-5303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Girod A, Wobus CE, Zadori Z, Ried M, Leike K, Tijssen P, et al. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J Gen Virol. 2002;83(Pt 5):973–8. doi: 10.1099/0022-1317-83-5-973. [DOI] [PubMed] [Google Scholar]
- 76.Grieger JC, Johnson JS, Gurda-Whitaker B, Agbandje-McKenna M, Samulski RJ. Surface-exposed adeno-associated virus Vp1-NLS capsid fusion protein rescues infectivity of noninfectious wild-type Vp2/Vp3 and Vp3-only capsids but not that of fivefold pore mutant virions. J Virol. 2007;81(15):7833–43. doi: 10.1128/JVI.00580-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stahnke S, Lux K, Uhrig S, Kreppel F, Hösel M, Coutelle O, et al. Intrinsic phospholipase A2 activity of adeno-associated virus is involved in endosomal escape of incoming particles. Virology. 2011;409(1):77–83. doi: 10.1016/j.virol.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 78.Stahnke S, Lux K, Uhrig S, Kreppel F, Hosel M, Coutelle O, et al. Intrinsic phospholipase A2 activity of adeno-associated virus is involved in endosomal escape of incoming particles. Virology. 2011;409(1):77–83. doi: 10.1016/j.virol.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 79.Grieger JC, Snowdy S, Samulski RJ. Separate basic region motifs within the adeno-associated virus capsid proteins are essential for infectivity and assembly. J Virol. 2006;80(11):5199–210. doi: 10.1128/JVI.02723-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richards RM, Lowy DR, Schiller JT, Day PM. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc Natl Acad Sci U S A. 2006;103(5):1522–7. doi: 10.1073/pnas.0508815103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Magnuson B, Rainey EK, Benjamin T, Baryshev M, Mkrtchian S, Tsai B. ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding. Mol Cell. 2005;20(2):289–300. doi: 10.1016/j.molcel.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 82.Wang K, Guan T, Cheresh DA, Nemerow GR. Regulation of adenovirus membrane penetration by the cytoplasmic tail of integrin beta5. J Virol. 2000;74(6):2731–9. doi: 10.1128/jvi.74.6.2731-2739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson JS, Gentzsch M, Zhang L, Ribeiro CM, Kantor B, Kafri T, et al. AAV Exploits Subcellular Stress Associated with Inflammation, Endoplasmic Reticulum Expansion, and Misfolded Proteins in Models of Cystic Fibrosis. PLoS Pathog. 2011;7(5):e1002053. doi: 10.1371/journal.ppat.1002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akache B, Grimm D, Shen X, Fuess S, Yant SR, Glazer DS, et al. A two-hybrid screen identifies cathepsins B and L as uncoating factors for adeno-associated virus 2 and 8. Mol Ther. 2007;15(2):330–9. doi: 10.1038/sj.mt.6300053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thomas CE, Storm TA, Huang Z, Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78(6):3110–22. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson J, Samulski R. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J Virol. 2009;83(6):2632–44. doi: 10.1128/JVI.02309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoque M, Ishizu K, Matsumoto A, Han SI, Arisaka F, Takayama M, et al. Nuclear transport of the major capsid protein is essential for adeno-associated virus capsid formation. J Virol. 1999;73(9):7912–5. doi: 10.1128/jvi.73.9.7912-7915.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gorlich D, Kostka S, Kraft R, Dingwall C, Laskey RA, Hartmann E, et al. Two different subunits of importin cooperate to recognize nuclear localization signals and bind them to the nuclear envelope. Curr Biol. 1995;5(4):383–92. doi: 10.1016/s0960-9822(95)00079-0. [DOI] [PubMed] [Google Scholar]
- 89.Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–60. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 90.Lee BJ, Cansizoglu AE, Suel KE, Louis TH, Zhang Z, Chook YM. Rules for nuclear localization sequence recognition by karyopherin beta 2. Cell. 2006;126(3):543–58. doi: 10.1016/j.cell.2006.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qiu J, Brown KE. A 110-kDa nuclear shuttle protein, nucleolin, specifically binds to adeno-associated virus type 2 (AAV-2) capsid. Virology. 1999;257(2):373–82. doi: 10.1006/viro.1999.9664. [DOI] [PubMed] [Google Scholar]
- 92.Bevington JM, Needham PG, Verrill KC, Collaco RF, Basrur V, Trempe JP. Adeno-associated virus interactions with B23/Nucleophosmin: identification of sub-nucleolar virion regions. Virology. 2007;357(1):102–13. doi: 10.1016/j.virol.2006.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cotmore SF, D'Abramo A M, Jr., Ticknor CM, Tattersall P. Controlled conformational transitions in the MVM virion expose the VP1 N-terminus and viral genome without particle disassembly. Virology. 1999;254(1):169–81. doi: 10.1006/viro.1998.9520. [DOI] [PubMed] [Google Scholar]
- 94.Ros C, Baltzer C, Mani B, Kempf C. Parvovirus uncoating in vitro reveals a mechanism of DNA release without capsid disassembly and striking differences in encapsidated DNA stability. Virology. 2006;345(1):137–47. doi: 10.1016/j.virol.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 95.Sipo I, Fechner H, Pinkert S, Suckau L, Wang X, Weger S, et al. Differential internalization and nuclear uncoating of self-complementary adeno-associated virus pseudotype vectors as determinants of cardiac cell transduction. Gene Ther. 2007;14(18):1319–29. doi: 10.1038/sj.gt.3302987. [DOI] [PubMed] [Google Scholar]
- 96.Duan D, Yue Y, Yan Z, Yang J, Engelhardt JF. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest. 2000;105(11):1573–87. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan Z, Zak R, Luxton GW, Ritchie TC, Bantel-Schaal U, Engelhardt JF. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol. 2002;76(5):2043–53. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhong L, Zhao W, Wu J, Li B, Zolotukhin S, Govindasamy L, et al. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol Ther. 2007;15(7):1323–30. doi: 10.1038/sj.mt.6300170. [DOI] [PubMed] [Google Scholar]
- 99.Zhong L, Qing K, Si Y, Chen L, Tan M, Srivastava A. Heat-shock treatment-mediated increase in transduction by recombinant adeno-associated virus 2 vectors is independent of the cellular heat-shock protein 90. J Biol Chem. 2004;279(13):12714–23. doi: 10.1074/jbc.M310548200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sanlioglu S, Engelhardt JF. Cellular redox state alters recombinant adeno-associated virus transduction through tyrosine phosphatase pathways. Gene Ther. 1999;6(8):1427–37. doi: 10.1038/sj.gt.3300967. [DOI] [PubMed] [Google Scholar]
- 101.Latonen L, Moore HM, Bai B, Jaamaa S, Laiho M. Proteasome inhibitors induce nucleolar aggregation of proteasome target proteins and polyadenylated RNA by altering ubiquitin availability. Oncogene. 2011;30(7):790–805. doi: 10.1038/onc.2010.469. [DOI] [PubMed] [Google Scholar]
- 102.Condemine W, Takahashi Y, Le Bras M, de The H. A nucleolar targeting signal in PML-I addresses PML to nucleolar caps in stressed or senescent cells. J Cell Sci. 2007;120(Pt 18):3219–27. doi: 10.1242/jcs.007492. [DOI] [PubMed] [Google Scholar]
- 103.Mattsson K, Pokrovskaja K, Kiss C, Klein G, Szekely L. Proteins associated with the promyelocytic leukemia gene product (PML)-containing nuclear body move to the nucleolus upon inhibition of proteasome-dependent protein degradation. Proc Natl Acad Sci U S A. 2001;98(3):1012–7. doi: 10.1073/pnas.031566998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Opie SR, Warrington KH, Jr., Agbandje-McKenna M, Zolotukhin S, Muzyczka N. Identification of amino acid residues in the capsid proteins of adeno-associated virus type 2 that contribute to heparan sulfate proteoglycan binding. J Virol. 2003;77(12):6995–7006. doi: 10.1128/JVI.77.12.6995-7006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu P, Xiao W, Conlon T, Hughes J, Agbandje-McKenna M, Ferkol T, et al. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J Virol. 2000;74(18):8635–47. doi: 10.1128/jvi.74.18.8635-8647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qing K, Khuntirat B, Mah C, Kube DM, Wang XS, Ponnazhagan S, et al. Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J Virol. 1998;72(2):1593–9. doi: 10.1128/jvi.72.2.1593-1599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qing K, Wang XS, Kube DM, Ponnazhagan S, Bajpai A, Srivastava A. Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc Natl Acad Sci U S A. 1997;94(20):10879–84. doi: 10.1073/pnas.94.20.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mah C, Qing K, Khuntirat B, Ponnazhagan S, Wang XS, Kube DM, et al. Adeno-associated virus type 2-mediated gene transfer: role of epidermal growth factor receptor protein tyrosine kinase in transgene expression. J Virol. 1998;72(12):9835–43. doi: 10.1128/jvi.72.12.9835-9843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fisher KJ, Gao GP, Weitzman MD, DeMatteo R, Burda JF, Wilson JM. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70(1):520–32. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jayandharan GR, Zhong L, Li B, Kachniarz B, Srivastava A. Strategies for improving the transduction efficiency of single-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2008;15(18):1287–93. doi: 10.1038/gt.2008.89. [DOI] [PubMed] [Google Scholar]
- 111.Zhong L, Li B, Jayandharan G, Mah C, Govindasamy L, Agbandje-McKenna M, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381(2):194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li M, Jayandharan G, Li B, Ling C, Ma W, Srivastava A, et al. High-efficiency transduction of fibroblasts and mesenchymal stem cells by tyrosine-mutant AAV2 vectors for their potential use in cellular therapy. Hum Gene Ther. 2010;21(11):1527–43. doi: 10.1089/hum.2010.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jayandharan GR, Zhong L, Sack BK, Rivers AE, Li M, Li B, et al. Optimized adeno-associated virus (AAV)-protein phosphatase-5 helper viruses for efficient liver transduction by single-stranded AAV vectors: therapeutic expression of factor IX at reduced vector doses. Hum Gene Ther. 2010;21(3):271–83. doi: 10.1089/hum.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Markusic DM, Herzog RW, Aslanidi GV, Hoffman BE, Li B, Li M, et al. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther. 2010;18(12):2048–56. doi: 10.1038/mt.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Petrs-Silva H, Dinculescu A, Li Q, Deng W-T, Pang J-J, Min S-H, et al. Novel Properties of Tyrosine-mutant AAV2 Vectors in the Mouse Retina. Mol Ther. 2010 doi: 10.1038/mt.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Petrs-Silva H, Dinculescu A, Li Q, Min S-H, Chiodo V, Pang J-J, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17(3):463–71. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qiao C, Zhang W, Yuan Z, Shin JH, Li J, Jayandharan GR, et al. Adeno-associated virus serotype 6 capsid tyrosine-to-phenylalanine mutations improve gene transfer to skeletal muscle. Hum Gene Ther. 2010;21(10):1343–8. doi: 10.1089/hum.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grimm D, Kern A, Pawlita M, Ferrari F, Samulski R, Kleinschmidt J. Titration of AAV-2 particles via a novel capsid ELISA: packaging of genomes can limit production of recombinant AAV-2. Gene Ther. 1999;6(7):1322–30. doi: 10.1038/sj.gt.3300946. [DOI] [PubMed] [Google Scholar]
- 119.Zeltner N, Kohlbrenner E, Clement N, Weber T, Linden RM. Near-perfect infectivity of wild-type AAV as benchmark for infectivity of recombinant AAV vectors. Gene Ther. 2010;17(7):872–9. doi: 10.1038/gt.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chapman MS, Rossmann MG. Single-stranded DNA-protein interactions in canine parvovirus. Structure. 1995;3(2):151–62. doi: 10.1016/s0969-2126(01)00146-0. [DOI] [PubMed] [Google Scholar]
- 121.Lang SI, Boelz S, Stroh-Dege AY, Rommelaere J, Dinsart C, Cornelis JJ. The infectivity and lytic activity of minute virus of mice wild-type and derived vector particles are strikingly different. J Virol. 2005;79(1):289–98. doi: 10.1128/JVI.79.1.289-298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ng R, Govindasamy L, Gurda BL, McKenna R, Kozyreva OG, Samulski RJ, et al. Structural characterization of the dual glycan binding adeno-associated virus serotype 6. J Virol. 2010;84(24):12945–57. doi: 10.1128/JVI.01235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu Z, Miller E, Agbandje-McKenna M, Samulski RJ. Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol. 2006;80(18):9093–103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, Walsh CE. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther. 2000;2(6):619–23. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- 125.Wang C, Wang CM, Clark KR, Sferra TJ. Recombinant AAV serotype 1 transduction efficiency and tropism in the murine brain. Gene Ther. 2003;10(17):1528–34. doi: 10.1038/sj.gt.3302011. [DOI] [PubMed] [Google Scholar]
- 126.Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM. Novel AAV serotypes for improved ocular gene transfer. J Gene Med. 2008;10(4):375–82. doi: 10.1002/jgm.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loiler SA, Tang Q, Clarke T, Campbell-Thompson ML, Chiodo V, Hauswirth W, et al. Localized gene expression following administration of adeno-associated viral vectors via pancreatic ducts. Mol Ther. 2005;12(3):519–27. doi: 10.1016/j.ymthe.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 128.Kurzeder C, Koppold B, Sauer G, Pabst S, Kreienberg R, Deissler H. CD9 promotes adeno-associated virus type 2 infection of mammary carcinoma cells with low cell surface expression of heparan sulphate proteoglycans. Int J Mol Med. 2007;19(2):325–33. [PubMed] [Google Scholar]
- 129.Richter M, Iwata A, Nyhuis J, Nitta Y, Miller AD, Halbert CL, et al. Adeno-associated virus vector transduction of vascular smooth muscle cells in vivo. Physiol Genomics. 2000;2(3):117–27. doi: 10.1152/physiolgenomics.2000.2.3.117. [DOI] [PubMed] [Google Scholar]
- 130.Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101(8):2963–72. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 131.Bartlett JS, Samulski RJ, McCown TJ. Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum Gene Ther. 1998;9(8):1181–6. doi: 10.1089/hum.1998.9.8-1181. [DOI] [PubMed] [Google Scholar]
- 132.Koeberl DD, Alexander IE, Halbert CL, Russell DW, Miller AD. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc Natl Acad Sci U S A. 1997;94(4):1426–31. doi: 10.1073/pnas.94.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takeda S, Takahashi M, Mizukami H, Kobayashi E, Takeuchi K, Hakamata Y, et al. Successful gene transfer using adeno-associated virus vectors into the kidney: comparison among adeno-associated virus serotype 1-5 vectors in vitro and in vivo. Nephron Exp Nephrol. 2004;96(4):e119–26. doi: 10.1159/000077378. [DOI] [PubMed] [Google Scholar]
- 134.Blackburn SD, Steadman RA, Johnson FB. Attachment of adeno-associated virus type 3H to fibroblast growth factor receptor 1. Arch Virol. 2006;151(3):617–23. doi: 10.1007/s00705-005-0650-6. [DOI] [PubMed] [Google Scholar]
- 135.Ling C, Lu Y, Kalsi JK, Jayandharan GR, Li B, Ma W, et al. Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum Gene Ther. 2010;21(12):1741–7. doi: 10.1089/hum.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Glushakova LG, Lisankie MJ, Eruslanov EB, Ojano-Dirain C, Zolotukhin I, Liu C, et al. AAV3-mediated transfer and expression of the pyruvate dehydrogenase E1 alpha subunit gene causes metabolic remodeling and apoptosis of human liver cancer cells. Mol Genet Metab. 2009;98(3):289–99. doi: 10.1016/j.ymgme.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol. 2001;75(15):6884–93. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci U S A. 2000;97(7):3428–32. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weber M, Rabinowitz J, Provost N, Conrath H, Folliot S, Briot D, et al. Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol Ther. 2003;7(6):774–81. doi: 10.1016/s1525-0016(03)00098-4. [DOI] [PubMed] [Google Scholar]
- 140.Walters RW, Yi SM, Keshavjee S, Brown KE, Welsh MJ, Chiorini JA, et al. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem. 2001;276(23):20610–6. doi: 10.1074/jbc.M101559200. [DOI] [PubMed] [Google Scholar]
- 141.Di Pasquale G, Davidson BL, Stein CS, Martins I, Scudiero D, Monks A, et al. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med. 2003;9(10):1306–12. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- 142.Seiler MP, Miller AD, Zabner J, Halbert CL. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum Gene Ther. 2006;17(1):10–9. doi: 10.1089/hum.2006.17.10. [DOI] [PubMed] [Google Scholar]
- 143.Weller ML, Amornphimoltham P, Schmidt M, Wilson PA, Gutkind JS, Chiorini JA. Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nat Med. 2010;16(6):662–4. doi: 10.1038/nm.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL, et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10(4):671–8. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 145.Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10(8):828–34. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Halbert CL, Allen JM, Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J Virol. 2001;75(14):6615–24. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Allocca M, Mussolino C, Garcia-Hoyos M, Sanges D, Iodice C, Petrillo M, et al. Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J Virol. 2007;81(20):11372–80. doi: 10.1128/JVI.01327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, et al. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther. 2007;18(3):195–206. doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- 149.Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23(3):321–8. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 150.Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y, Kay MA. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005;79(1):214–24. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shen S, Byrant KD, Brown SM, Randell SH, Asokan A. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J Biol Chem. 2011 doi: 10.1074/jbc.M110.210922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vandendriessche T, Thorrez L, Acosta-Sanchez A, Petrus I, Wang L, Ma L, et al. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J Thromb Haemost. 2007;5(1):16–24. doi: 10.1111/j.1538-7836.2006.02220.x. [DOI] [PubMed] [Google Scholar]
- 153.Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14(1):45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27(1):59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yue Y, Shin JH, Duan D. Whole body skeletal muscle transduction in neonatal dogs with AAV-9. Methods Mol Biol. 2011;709:313–29. doi: 10.1007/978-1-61737-982-6_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bostick B, Ghosh A, Yue Y, Long C, Duan D. Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther. 2007;14(22):1605–9. doi: 10.1038/sj.gt.3303029. [DOI] [PubMed] [Google Scholar]
- 157.Yan Z, Zak R, Zhang Y, Ding W, Godwin S, Munson K, et al. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J Virol. 2004;78(6):2863–74. doi: 10.1128/JVI.78.6.2863-2874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang T, Hu J, Ding W, Wang X. Doxorubicin augments rAAV-2 transduction in rat neuronal cells. Neurochem Int. 2009;55(7):521–8. doi: 10.1016/j.neuint.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 159.Hansen J, Qing K, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: altered endocytic processing enhances transduction efficiency in murine fibroblasts. J Virol. 2001;75(9):4080–90. doi: 10.1128/JVI.75.9.4080-4090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ju XD, Lou SQ, Wang WG, Peng JQ, Tian H. Effect of hydroxyurea and etoposide on transduction of human bone marrow mesenchymal stem and progenitor cell by adeno-associated virus vectors. Acta Pharmacol Sin. 2004;25(2):196–202. [PubMed] [Google Scholar]
- 161.Russell DW, Alexander IE, Miller AD. DNA synthesis and topoisomerase inhibitors increase transduction by adeno-associated virus vectors. Proc Natl Acad Sci U S A. 1995;92(12):5719–23. doi: 10.1073/pnas.92.12.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qing K, Li W, Zhong L, Tan M, Hansen J, Weigel-Kelley KA, et al. Adeno-associated virus type 2-mediated gene transfer: role of cellular T-cell protein tyrosine phosphatase in transgene expression in established cell lines in vitro and transgenic mice in vivo. J Virol. 2003;77(4):2741–6. doi: 10.1128/JVI.77.4.2741-2746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhong L, Li B, Mah C, Govindasamy L, Agbandje-McKenna M, Cooper M, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105(22):7827–32. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kauss MA, Smith LJ, Zhong L, Srivastava A, Wong KK, Chatterjee S. Enhanced long-term transduction and multilineage engraftment of human hematopoietic stem cells transduced with tyrosine-modified recombinant adeno-associated virus serotype 2. Hum Gene Ther. 2010;21(9):1129–36. doi: 10.1089/hum.2010.016. [DOI] [PMC free article] [PubMed] [Google Scholar]