Abstract
A characteristic feature of the human airway epithelium is the presence of ciliated cells bearing motile cilia, specialized cell surface projections containing axonemes comprised of microtubules and dynein arms, which provide ATP-driven motility. In the airways, cilia function in concert with airway mucus to mediate the critical function of mucociliary clearance, cleansing the airways of inhaled particles and pathogens. The prototypical disorder of respiratory cilia is primary ciliary dyskinesia, an inherited disorder that leads to impaired mucociliary clearance, repeated chest infections, and progressive destruction of lung architecture. Numerous acquired lung diseases are also marked by abnormalities in both cilia structure and function. In this review we summarize current knowledge regarding airway ciliated cells and cilia, how they function to maintain a healthy epithelium, and how disorders of cilia structure and function contribute to inherited and acquired lung disease.
Keywords: Airway, epithelium, cilia, mucociliary escalator
Introduction
The human airway, a dichotomous hollow tube branching structure of up to 23 generations from trachea to the alveoli, is lined by a continuous layer of pseudostratified epithelium comprised of approximately 1010 cells covering a surface area of 2500 cm2 (1). The airway epithelium is composed of 4 major cell types lining a continuous basement membrane, including ciliated, secretory, undifferentiated intermediate and basal cells (2). The basal cells function as the stem/progenitor cells, responding to cell senescence and injury by generating intermediate undifferentiated cells, which then differentiate to ciliated and secretory cells in a ratio of 7–8 to 1 in both the large (0 to 5 generations) and small airways (≥6 generations).
The ciliated and secretory cells are the first line of defense against inhaled pathogens and particulates. This includes tight junctions linking the cells, providing a physical barrier; receptors that sense the environment signaling the cells to secrete defense-related molecules in response to inhaled pathogens, particulates and xenobiotics; and importantly, the mucociliary escalator, a layer of fluid and mucins lining the epithelium that functions to clean the airways by moving continuously from the lower respiratory tract cephalad, where it is insensibly swallowed (2–4). The effectiveness of the mucociliary escalator depends on hydration, mucins produced by secretory cells and on the coordinated function of the ciliated cells that provides the force and direction of the escalator (4). When there is dysfunction of the mucociliary escalator, the defenses of the epithelium are markedly weakened, resulting in lung disease.
The focus of this review is on the role of ciliated cells and cilia per se in the normal function of the airway epithelium and how abnormalities in the structure and function of airway cilia result in disease. To do so, we will first summarize what is known about human airway ciliated cells and cilia, provide an overview of the role of the ciliated cells in the mucociliary escalator and detail how airway cilia structure and function are assessed in humans. This will provide the background to describe the current state of knowledge of the inherited and acquired disorders of airway cilia dysfunction.
Airway Ciliated Cells
The airway ciliated cell, the dominant cell type of the airway epithelium has a columnar shape that tapers toward the surface resting on the basement membrane (Figure 1A, B). The percentage of ciliated cells increases with airway branching, from 47±2% in trachea to 73±1% in the small airway epithelium (5). Ciliated cells are easily distinguishable from other cell types by the presence of cilia (~200 to 300 cilia per cell) on the luminal surface, each 0.2 to 0.3 μm in diameter and ranging from 6 to 7 μm in length in the upper airways, to 4 μm in the smaller airways (6). The apical surface of ciliated cells also contains numerous microvilli, which play a role in the transepithelial movement of fluid and electrolytes (6). Adjacent ciliated cells are connected via tight junctions, specialized multiprotein structures that regulate the passage of solutes and ions across the epithelial barrier and separate the apical and basolateral epithelial compartments, and E-cadherin-based adherens junctions that provide firm cell-to-cell adhesion (3, 7). At the basolateral pole, ciliated cells connect to the airway epithelial basement membrane directly or through desmosome-mediated attachment to basal cells (8).
Figure 1.
The human airway epithelium. A. Histology of the large airway epithelium (4th–5th generation of bronchi) from a healthy nonsmoker. Shown are ciliated cells, secretory cells, intermediate undifferentiated cells, and basal cells. Hematoxylin and eosin, scale bar = 20 μm. B. Ciliated cells isolated from the human small airway epithelium (10th–12th generation of bronchi) obtained by bronchoscopic brushings from a normal healthy individual. Diff-Quik, scale bar = 10 μm. C. Role of cilia in airway mucociliary clearance. The mucociliary escalator is composed of the mucus gel layer, periciliary layer, and ciliated cells. The gel-forming mucins produced by mucous secretory (“goblet”) cells are major constituents of the mucus gel layer, which entraps microorganisms and other inhaled particles and transports them out of the lung through cilia beating. The membrane-bound mucins form a brush-like pericellular niche around the cilia that controls the distribution of water between the 2 layers. During the power forward stroke, the ciliary tips extend upward into the mucus gel layer, propelling the mucus forward. During the slow return stroke, the cilia recede and are contained completely in the periciliary layer. Normal cilia length (4 to 7 μm, depending on the airway region) is critical for effective mucociliary clearance.
The major function of airway ciliated cells is to mediate propulsion of the mucus gel layer in a cephalad direction, thus maintaining the mucociliary escalator (Figure 1C) (4, 9). The ciliated cells accomplish this by the highly coordinated in-plane beating of cilia across multiple cells, generating a wave-like movement across the epithelial surface (4). The forward stroke of the cilia is rapid and powerful to allow penetration into the mucus layer and propulsion of the mucus gel layer in a cephalad direction (4).
Generation of Airway Ciliated Cells
Airway ciliated cells are terminally differentiated cells incapable of self-renewal (10). The ciliated cells turn over slowly, estimated at 1–4 months in humans (11). They are replenished by the basal cells which function as stem/progenitor cells for airway epithelial ciliated and secretory cells (Figure 2A) (12). This process is accelerated in response to injury, to which ciliated cells are sensitive (11). In human subjects who underwent mechanical injury of large airways with a cytology brush, the ciliated cell population was replenished by 14 days after injury (13). Exposure of basal cell stem/progenitor cells to the luminal air is critical for basal cell differentiation toward the ciliated cell lineage (14). This can be modeled in vitro; when purified human airway basal cells are cultured on type IV basement membrane collagen with the apical surface exposed to air, the basal cells differentiate to a mucociliated epithelium (Figure 2B).
Figure 2.
Ciliated cell differentiation in the human airway epithelium. A. Differentiation pathways. The basal stem/progenitor cells are capable of self-renewal and are responsible for generation of intermediate progenies, which, upon distinct activation programs, differentiate into ciliated or secretory cells. Ciliated cell differentiation depends on transcription factor forkhead box J1 (FOXJ1), whereas generation of secretory cells requires activation of Notch signaling and/or SAM pointed domain containing ETS transcription factor (SPDEF). B. Differentiation of human airway basal cells (day 0) into ciliated airway epithelium (day 28) in air-liquid interface culture. Appearance of ciliated cells is demonstrated by expression of β-tubulin IV (red signal, immunofluorescence). Cell nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI; blue signal). Scale bar = 10 μm.
Basal cell-derived progenitors mature into fully differentiated ciliated cells as a result of activation of the transcription factor forkhead box J1 (FOXJ1) with contribution from the regulatory factor X (RFX) family (15–17). FOXJ1 specifically supports formation of ciliated cells, whereas Notch signaling and activation of the SAM pointed domain containing ETS transcription factor (SPDEF) are critical for secretory cell differentiation (18, 19).
Airway Cilia Structure
Cilia are evolutionarily conserved hair-like cellular organelles that project from the cell surface (20). The basic structure consists of a centriole-derived, microtubule core termed the axoneme that protrudes continuously from the plasma membrane (Figure 3) (21). Cilia are subdivided into two classes, immotile or motile, based on the physical ultrastructural characteristics of the axoneme (21–23). Airway cilia are in the motile cilia class, as are cilia found on ciliated cells of sinuses, brain ventricle ependyma, oviducts and epididymal ducts (24–26). Immotile cilia (referred to as “primary cilia”) are solitary structures on most cell types where they function to sense the environment (20, 24, 27).
Figure 3.
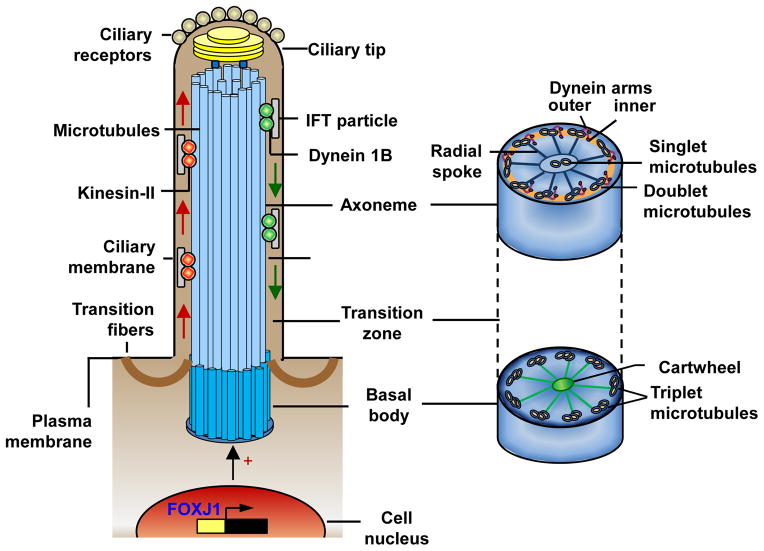
Structure and maintenance of human airway cilia. Each airway cilium is comprised of a “9+2” axoneme with 9 doublet microtubules and a central pair of microtubule singlets, surrounded by a specialized ciliary membrane. The ciliary membrane is contiguous with, but distinct from, the plasma membrane and harbors a number of receptors, critical for sensing environmental signals. Cilia formation is initiated and coordinated by a distinct gene expression program, primarily by activation of the transcription factor forkhead box J1 (FOXJ1). Cilia assembly begins with formation of the basal body from the centrosome, which migrates to and docks on the cell surface. The basal body is a specialized centriole with a “9+3” microtubule structure and a cartwheel embedded in pericentriolar material and anchored to the plasma membrane by transition fibers. Axonemal microtubule doublets arise from the inner 2 microtubules of the basal body microtubule triplets, extend from the basal body and form the ciliary membrane by pushing out an extension of the plasma membrane. Axonemal microtubules are elongated distally via in-traflagellar transport (IFT) of proteins, which are synthesized in the cell and moved as IFT particles by kinesin-2 motors from the basal body to the ciliary tip (anterograde IFT, left side) and by the cytoplasmic dynein motors back to the basal body (retrograde IFT, right side). The ciliary tip contains the microtubule “+” ends, from which the axonemes grow, and a number of signaling components responsible for the sensory function of cilia. The force needed for cilia beating is produced by the outer and inner dynein arms of the axonemal microtubule doublets connected to the central pair of microtubules by radial spokes.
Airway cilia have components typical for motile cilia (see Figure 3 for an overview and Supplemental Information for further details). Proteomic analysis of cilia isolated from in vitro generated human airway epithelial cells on air-liquid interface culture identified >200 axonemal proteins (28). Some, such as α and β tubulin, are conserved with axonemal proteins identified in other motile cilia, but some are unique, including the sperm or testis associated proteins SPA17 and SPAG6 and retinitis pigmentosa protein 1 known to associate with photoreceptor axonemal structures (28). Characterization of the transcriptome of murine tracheal epithelial cells identified similarities among components of motile and primary cilia (29), and many genes identified as part of the mouse ciliated cell transcriptome correspond to human airway cilia proteins, suggesting conservation across species.
Cilia Growth and Maintenance
Ciliogenesis is a complex, multistage process coupled to the cell cycle. Cilia cannot synthesize protein, and all proteins required for ciliogenesis and cilia maintenance are moved into and out of cilia by intraflagellar transport. Cilia length, a process affected by smoking, is regulated by multiple genes (see Figure 3 and Table I for an overview and Supplemental Information for details).
Table I.
Genes Encoding Major Components of Airway Motile Cilia1
| Category and gene name2 | Gene symbol | Associated with PCD3 | Smoking effect4 |
|---|---|---|---|
| Axoneme – outer dynein arm | |||
| Dynein, axonemal, heavy chain 5 | DNAH5 | + | ↓ |
| Dynein, axonemal, heavy chain 9 | DNAH9 | + | ↓ |
| Dynein, axonemal, heavy chain 11 | DNAH11 | + | ↓ |
| Dynein, axonemal, intermediate chain 1 | DNAI1 | + | |
| Dynein, axonemal, intermediate chain 2 | DNAI2 | + | |
| Dynein, axonemal, light chain 1 | DNAL1 | + | |
| Axoneme – inner dynein arm | |||
| Dynein, axonemal, heavy chain 3 | DNAH3 | ||
| Dynein, axonemal, heavy chain 6 | DNAH6 | ||
| Dynein, axonemal, heavy chain 12 | DNAH12 | ||
| Dynein, axonemal, light intermediate chain 1 | DNALI1 | ↓ | |
| WD repeat domain 63 | WDR63 | ||
| WD repeat domain 78 | WDR78 | ||
| Dynein assembly and docking | |||
| Dynein, axonemal, assembly factor 1 | DNAAF1 | + | |
| Dynein, axonemal, assembly factor 2 | DNAAF2 | ||
| Dynein, axonemal, assembly factor 3 | DNAAF3 | + | |
| Dyslexia susceptibility 1 candidate 1 | DYX1C1 | + | |
| Armadillo repeat containing 4 | ARMC4 | + | |
| Coiled-coil domain containing 39 | CCDC39 | + | |
| Coiled-coil domain containing 40 | CCDC40 | + | |
| Coiled-coil domain containing 114 | CCDC114 | + | |
| Dynein regulatory complex subunit 1 homolog | DRC1 | + | |
| Leucine rich repeat containing 6 | LRRC6 | + | |
| HEAT repeat containing 2 | HEATR2 | + | |
| Zinc finger, MYND-type containing 10 | ZMYND10 | + | |
| Central pair | |||
| HYDIN, axonemal central pair apparatus protein | HYDIN | + | |
| Sperm associated antigen 6 | SPAG6 | ↓ | |
| Sperm associated antigen 16 | SPAG16 | ||
| Sperm associated antigen 17 | SPAG17 | + | |
| Sperm associated antigen 1 | SPAG1 | + | |
| Primary ciliary dyskinesia protein 1 | PCDP1 | ||
| Radial spoke | |||
| Radial spoke head 1 homolog | RSPH1 | + | |
| Radial spoke head 3 homolog | RSPH3 | ||
| Radial spoke head 4a homolog | RSPH4A | + | |
| Radial spoke head 9 homolog | RSPH9 | + | |
| Tubulins and other microtubule-associated | |||
| Tubulin, alpha 1A | TUB1A1 | ||
| Tubulin, alpha 3 | TUBA3 | ||
| Tubulin, beta 2C | TUBB2C | ||
| Tubulin, beta 4 | TUBB4 | ||
| Tektin 1 | TEKT1 | ↓ | |
| NME/NM23 family member 8 | NME8 | + | |
| Anterograde intraflagellar transport (IFT) | |||
| Kinesin family member 3A | KIF3A | ||
| Kinesin family member 3B | KIF3B | ||
| IFT protein 20 homolog | IFT20 | ||
| IFT protein 46 homolog | IFT46 | ||
| IFT protein 52 homolog | IFT52 | ||
| IFT protein 57 homolog | IFT57 | ↓ | |
| IFT protein 80 homolog | IFT80 | ↓ | |
| IFT protein 81 homolog | IFT81 | ||
| IFT protein 88 homolog | IFT88 | ||
| IFT protein 172 homolog | IFT172 | ↓ | |
| Clusterin associated protein 1 | CLUAP1 | ↓ | |
| RAB, member RAS oncogene family-like 5 | RABL5 | ||
| TNF receptor-associated factor 3 interacting protein 1 | TRAF3IP1 | ↓ | |
| Tetratricopeptide repeat domain 26 | TTC26 | ↓ | |
| Tetratricopeptide repeat domain 30B | TTC30B | ||
| Retrograde IFT | |||
| Dynein, cytoplasmic 2, heavy chain 1 | DYNC2H1 | ↓ | |
| Dynein, cytoplasmic 2, light intermediate chain 1 | DYNC2LI1 | ||
| IFT protein 122 homolog | IFT122 | ||
| IFT protein 140 homolog | IFT140 | ||
| Tetratricopeptide repeat domain 21B | TTC21B | ||
| WD repeat domain 19 | WDR19 | ↓ | |
| WD repeat domain 35 | WDR35 | ↓ | |
| Basal body and BBSome5 | |||
| Bardet-Biedl syndrome 1 | BBS1 | ||
| Bardet-Biedl syndrome 2 | BBS2 | ||
| Bardet-Biedl syndrome 4 | BBS4 | ||
| Bardet-Biedl syndrome 5 | BBS5 | ↓ | |
| Bardet-Biedl syndrome 6 | BBS6 | ||
| Bardet-Biedl syndrome 7 | BBS7 | ||
| Bardet-Biedl syndrome 8 | BBS8 | ||
| Bardet-Biedl syndrome 9 | BBS9 | ↓ | |
| BBSome interacting protein 1 | BBIP1 | ↓ | |
| Oral-facial-digital syndrome 1 | OFD1 | + | ↓ |
| Outer dense fiber of sperm tails 2 | ODF2 | ↓ | |
| Ezrin | EZR | ↓ | |
| Transition zone | |||
| Nephronophthisis 1 (juvenile) | NPHP1 | ↓ | |
| Retinitis pigmentosa GTPase regulator | RPGR | + | |
| Transmembrane protein 67 | TMEM67 | ↓ | |
| Receptors, ion channels and signaling molecules | |||
| Taste receptor, type 2, member 4 | TAS2R4 | ||
| Taste receptor, type 2, member 38 | TAS2R38 | ||
| Taste receptor, type 2, member 43 | TAS2R43 | ||
| Taste receptor, type 2, member 46 | TAS2R46 | ||
| Sodium channel, non-voltage-gated 1 alpha subunit | SCNN1A | ||
| Nitric oxide synthase 3 (endothelial cell) | NOS3 | + | |
| Protein kinase, cGMP-dependent, type I | PRKG1 | ||
| A kinase (PRKA) anchor protein 14 | AKAP14 | ||
| Progesterone receptor | PGR | ||
| Serum response factor | SRF | ||
| Transcription factors – inducers of airway cilia formation | |||
| Forkhead box J1 | FOXJ1 | ||
| Regulatory factor X, 3 | RFX3 | ||
| V-myb avian myeloblastosis viral oncogene | MYB | ||
| Multiciliate differentiation and DNA synthesis associated cell cycle protein | MCIDAS | ||
For detailed references regarding individual genes associated with primary ciliary dyskinesia and genes down-regulated by smoking, see Supplemental Table I.
Genes encoding the major structural components of airway motile cilia and/or relevant to airway ciliogenesis and regulation of ciliated cell function based on the available literature are included.
PCD, primary ciliary dyskinesia; genes known to cause PCD or associated with PCD pathogenesis based on the available literature are indicated (+).
Effect of cigarette smoking on the expression of individual genes related to airway motile cilia structure and/or function based on the available literature is shown; ↓ down-regulation.
BBSome, a complex of Bardet–Biedl syndrome (BBS) proteins.
Transcriptional Control of Cilia Genes
It is well documented that FOXJ1 is central to airway epithelial ciliogenesis, with the RFX family transcription factors contributing. However, the transcriptional control of ciliogenesis and the maintenance of human airway cilia is not well understood, and likely involves a complex interaction of several transcription factors (see Supplemental Information for further details
Cilia Function
In the healthy human lung, cilia beat at 12 to 15 Hz in coordinated waves of metachronal motion that propel mucus cephalad at 4 to 20 mm/min (30). Cilia tips contact the mucus layer only on the forward stroke; on the reverse stroke, a bend in the cilia shaft causes the tip to pass underneath the mucus layer, causing the cilia to be propelled only in the forward direction on the forward stroke (Figure 1C) (31, 32). The contents are propelled through the vocal cords into the pharynx where an estimated 30 ml of respiratory mucus are expectorated or swallowed daily (30).
Multiple signaling molecules, including cAMP, Ca2+ and nitric oxide, and progesterone regulate airway cilia beat frequency (33–36). In an important observation, Welsh and co-workers (37) identified sensory bitter taste receptors (T2R) on cilia of differentiated human airway epithelium, providing a mechanism by which airway cilia sense the environment. Several other environment airway cilia sensing receptors have been identified. Ciliated cells also express structures that maintain normal periciliary fluid osmolarity, including epithelial sodium channels and the cystic fibrosis transmembrane conductance regulator (38, 39) (see Supplemental Information of further details).
Cilia Function in the Mucociliary Escalator
Mucociliary clearance requires highly coordinated synchronized beating of cilia across multiple ciliated cells. Under normal conditions, the ciliary machinery functions in two modes to generate two different rates (slow and high) of beat frequency. The slow beat frequency is modulated by the inherent dynein ATPase activity of the axoneme, whereas the high beat frequency involves increasing dynein ATPase activity in response to specific signaling molecules (35, 36, 40). The mechanisms controlling the switch from a slow to high rate of frequency are not fully elucidated, but extensive post-translational modifications play a role, including phosphorylation and dephosphorylation of axonemal components (35). In addition to cAMP, Ca2+ and nitric oxide, mechanical forces (e.g., shear stress) can also regulate cilia beat frequency by modulating the levels of apical ATP release and subsequent influx of Ca2+ into cells (41, 42). The mechanisms regulating synchronization of the beat across multiple cilia are less well understood and no common control mechanism has been identified (35, 36, 40).
The majority of human in vivo studies of cilia beat frequency have been limited to the nasal epithelium. These studies have demonstrated that cilia beat frequency is affected by age, exercise and environmental stimuli such as cigarette smoke (43–46). Ciliary beat frequency and mucociliary clearance slow with aging (43, 44, 47).
Methods of Evaluating Cilia Structure and Function
Several methods can be used to evaluate cilia structure and function in humans. Samples of airway ciliated cells for study can be obtained post-mortem or from living subjects directly from the lung via bronchoscopic brushing or biopsy, or using nasal samples as a proxy (48). Light microscopy can be used to assess cilia length (49, 50) and phase contrast microscopy to examine cilia beating (51). Transmission electron microscopy is used to evaluate cilia ultrastructure for features including orientation of the central microtubule pair, number and location of dynein arms, and orientation of peripheral tubules (51). Electron tomography uses transmission electron microscopy images for 3D visualization of structures (52). The 3D structure of the respiratory epithelium can also be assessed by scanning electron microscopy for presence and number of cilia per cell and ciliary orientation (51).
Ciliary beat frequency can be assessed by cine photography (32, 53), photoelectric methods (54, 55), or by measuring light reflected by the moving cilia via microphotometry (56). Methods using optical coherence tomography to measure cilia activity have been described; this allows for visualization of cilia movement (57). Optical flow analysis has also been used to evaluate cilia motion in cultured human cells (58).
Integrated assessment of mucociliary clearance can be evaluated by placing a saccharine tablet or solution behind the inferior turbinate and measuring the time until a sweet taste is perceived by the subject (59). This may also be done in conjunction with the placement of a drop of blue indigocarmine solution, allowing measurement of the time until a blue line can be observed on the posterior pharyngeal wall (59). Other methods involve measuring the clearance of labeled particles from the airways using scintigraphy with technetium-labeled albumin mini microspheres or macroaggregates (60, 61), aerosolized Fe2SO3 particles containing technetium (62), or Teflon particles tagged with technetium, indium or gold (63, 64). The movement of unlabeled Teflon particles can be visualized using fiberoptic bronchoscopy videos (65).
Inherited Disorders of Cilia Dysfunction
The most important inherited disorders of airway cilia dysfunction are primary cilia dyskinesia and cystic fibrosis; other inherited cilia-related disorders are very rare.
Primary Ciliary Dyskinesia
The classic disorder of respiratory cilia dysfunction is primary ciliary dyskinesia (PCD), an autosomal recessive disorder of motile cilia (Table I) (25). The symptoms and signs of this disorder were recognized long before the mechanism was understood (66). It was first referred to as Kartagener syndrome, the triad of chronic sinusitis, bronchiectasis, and situs inversus (25, 66, 67). Subsequently, male infertility was noted to be associated with Kartagener syndrome, and dynein arm defects were observed in both the spermatozoa and respiratory epithelial cells, leading to the syndrome being named “immotile cilia syndrome” (68). The disorder was renamed PCD when it was recognized that a subgroup of patients with ciliary motility but ineffective mucociliary clearance manifest the same clinical syndrome (67, 69). The clinical manifestations of PCD include chronic otitis media, transient hearing loss/speech delays, nasal congestion, chronic sinusitis, recurrent lower respiratory tract infection, bronchiectasis, male infertility, defects in organ laterality (50% of cases) and in newborns, neonatal respiratory disorders.
Organ laterality in embryogenesis is determined by the normal rotary motion of a single specialized cilium found on each of the cells in the ventral node which defines right-left symmetry in the developing embryo (25). Without normal directional motion of this specialized cilium, organ placement is random, which is why situs inversus is found in approximately 50% of individuals with PCD.
Cilia structural defects
A variety of cilia structural defects observed by electron microscopy are associated with the PCD clinical phenotype. The most common are absent or short outer dynein arms, or an outer dynein arm and inner dynein arm defect (25). Isolated inner dynein arm defects are uncommon and when causative are typically associated with abnormalities in the central apparatus of the cilia such as microtubular disorganization or abnormally placed outer doublets (25).
PCD is also associated with reduced cilia beat frequency, most prominent in patients with dynein arm defects (70). Ciliary wave form is dyskinetic in PCD, further contributing to the lack of effective mucociliary transport (70). Airway particle clearance is prolonged to 1 wk in PCD patients as compared to 12 hr in normal nonsmokers (71).
Genetic basis of PCD
The genetic basis can be identified in approximately 65% of PCD patients (Table I) (25). Many mutations lead to defects in all cilia, but others produce structural abnormalities in only a fraction of cilia or exhibit no ultrastructural defects. A commercial genetic test for 60 mutant alleles of DNAI1 and DNAH5 is available (72). Most (85%) mutations implicated in PCD are loss-of-function mutations (25). The majority are rare variants found only in a single family or patient (25).
The typical causative genes for PCD encode ciliary components with mutations in specific genes having predictable effects on cilia ultrastructure. Genes coding for protein components of the outer or inner dynein arm lead to defects in those structures, and mutations in genes encoding cytoplasmic proteins that participate in ciliary assembly lead to defects in both the outer and inner dynein arms. Because the dynein arms function as ATP-dependent motors for ciliary movement, defects in these structures result in ciliary dyskinesia. Genes coding for components of the central pair microtubules and radial spokes lead to defects in those structures, which can lead to abnormalities in both ciliary beat frequency and beat coordination (73).
Because the specialized cilium present in the ventral node that controls organ laterality has no central complex, mutations leading to central complex defects do not produce laterality abnormalities. A mutation in cyclin O (CCNO) is causative of defective mucociliary clearance and bronchiectasis (74). CCNO mutant cells are defective in centriole generation and placement and affected individuals have reduced numbers of airway cilia. The cilia that were present did contain axonemal proteins and affected individuals did not exhibit laterality defects.
Clinical manifestations
Diagnosis is challenging due to heterogeneity in clinical symptoms and severity, cilia ultrastructural abnormalities identified by electron microscopy, and by variability in the phenotype associated with the causative mutations (67). Typical initial screening tests include nasal epithelial assessment of ciliary motion and mucociliary transport using a saccharine taste test or radioisotope clearance (67, 75). The diagnosis of PCD is conventionally confirmed by identification of ciliary ultrastructural defects by electron microscopy. However, 30% of patients exhibit normal ultrastructure (25). Other diagnostic modalities include assessment of nasal ciliary beat frequency and motility and measurements of nasal nitric oxide, which is low in PCD (25). Fluorescent labeled antibodies may be used to evaluate for absence of specific cilia proteins (25). Caution must be taken to carry out functional and structural studies when the affected individual has had no recent infection to avoid false positive findings due to secondary ciliary dyskinesia (67).
Lung disease in PCD results from defective mucociliary clearance and worsens over time due to repeated respiratory tract infections (25). Nearly all adults develop bronchiectasis. Lung function testing generally reveals an obstructive ventilatory defect with or without air trapping; mixed obstructive and restrictive patterns are also observed (67). Management centers on airway clearance therapies, use of antibiotics for lung infections, routine immunizations, and avoidance of tobacco smoke exposure (25). Surgical resection of severe localized disease is rarely indicated and lung transplant is an option for end-stage disease (67).
Cystic Fibrosis
Cystic fibrosis (CF) is an inherited disorder due to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The disorder predominantly affects Caucasians with an incidence of 1 in 2500 newborns of Northern European descent (76). Nearly 2000 CFTR mutations have been identified (76, 77). Although affected infants are born with seemingly normal lungs, chronic lung disease develops as a result of abnormal mucociliary clearance, leading to repeated infections and the development of bronchiectasis (78).
The CFTR gene encodes a cAMP-regulated chloride channel expressed apically in epithelial cells (76). The channel modulates chloride secretion and also regulates other membrane proteins including the epithelial sodium channel (ENaC) (76). Because both CFTR and ENaC control water movement through the epithelium, CFTR dysfunction leads to increased fluid absorption, dehydration of the epithelial surface, and altered mucin concentration in abnormal airway mucus (76, 79). A “gel-on-brush” model of the periciliary layer postulates that airway mucus sits atop a dense “brush” of tethered macromolecules in the periciliary layer that function to prevent mucus from penetrating the periciliary space (9). In this model, if the airway surface is sufficiently dehydrated, as in CF, the mucus layer compresses the periciliary brush and cilia, interfering with mucus clearance. Alterations in CFTR function may also contribute to lung disease via abnormal modulation of epithelial inflammation and altered bicarbonate transport (76).
The abnormal mucociliary clearance in individuals with CF results from the abnormal biophysical properties of airway mucus, not primarily from ciliopathy (76, 80). Experimental animal data suggest that the most important factor controlling mucus clearance efficiency is airway surface hydration (76). In CF, the increased absorption of Na+ and reduced secretion of Cl− lead to reduced water content in both the mucus layer and the periciliary layer (79). This leads to a mucus layer that is highly adhesive (79, 80). The failure to clear these abnormal secretions is evident shortly after birth with bronchiolar mucus plugs detected within 48 hr of birth in CF neonates (81). In addition to the abnormal hydration status, mucus hypersecretion eventually occurs in response to recurrent infection and persistent inflammation; this further worsens the physical properties and the clearance of mucus (79). CF epithelial cells alone, without submucosal glands, generate abnormal airway surface liquid; this may be an explanation for the presence of CF lung disease even in distal airways that lack submucosal glands (82).
Electron microscopy of airway cilia from patients with CF shows alterations similar to those seen in a control group of individuals with chronic bronchitis, including compound cilia, excess cytoplasmic matrix, and an abnormal number or arrangement of microtubular doublets (83). With progressive disease, the airways develop squamous metaplasia and dysplasia, regions of missing cilia, cilia with missing inner dynein arms, abnormal numbers and location of microtubular doublets, compound cilia, single microtubules in place of normal doublets, multiple cilia occupying the central area, and detachment of the axonemal membrane from the groups of cytoplasmic filaments (84).
CF was historically considered a fatal childhood disease, but with improved therapies, including antibiotics and airway clearance treatments, the average life expectancy is now 37 years (76). Because of the abnormal mucus characteristics underlying the disease, treatments to reduce mucus adhesivity are efficacious in CF (80). In part, the “sticky” nature of CF mucus results from high concentrations of DNA derived from neutrophils recruited in response to the chronic infection (85). The use of aerosol recombinant DNAse as an effective therapy in CF is directed toward reducing the adhesive properties of the mucus layer (85). In the subset of CF caused by genotype G551D, a missense mutation found in 4 to 5% of CF patients that affects the function of CFTR channels at the cell surface (86), treatment with a CFTR potentiator, ivacaftor, leads to sustained improvements in lung function, weight, and sweat chloride concentration (a measure of CFTR activity), and decreased exacerbations and chest symptoms. Ivacaftor works by increasing the amount of time that activated CFTR channels at the cell surface remain open and increases the chloride transport activity of G551D-CFTR protein (86).
Other Inherited Disorders
Individuals with α1-antitrypsin deficiency with “pure” emphysema have normal mucociliary clearance (63, 87), although α1-antitrypsin deficiency is associated with bronchiectasis (88). In Usher syndrome, a rare autosomal recessive disorder characterized by sensorineural deafness, vestibular dysfunction, and retinitis pigmentosa with progressive visual loss, case reports have noted bronchiectasis with impaired mucociliary clearance but no ultrastructural ciliary abnormalities (89).
Disorders of primary cilia may also have lung manifestations. Jeune syndrome (asphyxiating thoracic dystrophy), is an autosomal recessive disease with skeletal abnormalities as well as variable hepatic, pancreatic and retinal manifestations (90, 91). Respiratory failure is the most common cause of death due to restrictive disease caused by the chest wall abnormalities. While the genetics are incompletely understood, mutations in both DYNC1H1, a dynein heavy chain gene, and IFT80, involved in intraflagellar transport, have been linked to Jeune syndrome (90, 91). Roifman syndrome, a rare complex of bone dysplasia, growth retardation, retinal dystrophy and humoral immunodeficiency associated with recurrent respiratory infections, has been suggested as a possible ciliopathy of primary cilia (92).
Acquired Disorders of Airway Cilia
Abnormalities of mucociliary clearance, and consequent reduced host defenses of the lung, are a common theme in many acquired lung disorders. In many of these disorders the airway cilia demonstrate acquired structural and/or functional abnormalities with associated abnormalities in mucociliary clearance.
Cigarette Smoking
Smoking has long been recognized to suppress mucociliary clearance in most smokers and there is documented slowing of mucociliary clearance immediately after smoking cigarettes (93, 94). Individuals with bronchitis have reduced mucociliary clearance (95). Smoking cessation improves measurements of nasal mucociliary clearance compared to baseline values obtained prior to smoking cessation (96).
Examination of the airway ciliated cells of both male and female smokers shows patches of atypical nuclei and missing cilia (97). These abnormalities increase with increasing intensity of smoking behavior, and are more frequent in smokers using high tar/nicotine cigarettes (98). Smoking induces expression of epidermal growth factor (EGF) in ciliated cells, which may shift basal cell fate toward a squamous phenotype and suppress ciliated cell differentiation (99). Healthy smokers have shorter cilia in the large and small airways compared to nonsmokers, with further shortening observed in smokers with COPD (49, 50). Smoking is associated with suppression of a number of genes in the airway epithelium, likely contributing to slowing the process of regenerating cilia (Table I).
Electron microscopic assessment of cilia ultrastructure demonstrates that smokers with chronic bronchitis have greater numbers of ciliary abnormalities compared to nonsmokers, including compound cilia and giant cilia, and other abnormalities in the microtubules and the axonemal 9 + 2 pattern of organization and in cilia orientation (100–102). These abnormalities may persist after smoking cessation (103).
Data on the effect of smoking on cilia beat frequency are conflicting. Exposure to cigarette smoke extract (104) or direct cigarette smoke (105) leads to reduced cilia beat frequency in in vitro models of human airway epithelium. In contrast, nasal epithelium grown in culture demonstrated increased cilia beat frequency in samples from smokers or those with passive exposure compared to nonsmokers (106). In one study utilizing freshly obtained nasal samples, there was no difference in cilia beat frequency in smokers, nonsmokers, or nonsmokers who acutely smoked two cigarettes (107). No difference was found in cilia beat frequency of resected tissue from cancer surgery in smokers vs nonsmokers (108) or in samples obtained by biopsy (109). A study including smokers with lung disease found that cilia beat frequency was unaltered in healthy smokers compared to nonsmokers, though decreased in smokers with moderate to severe COPD (46). However, a contradictory study found increased cilia beat frequency in nasal biopsies from both active and passive smokers compared to nonsmokers (110).
There is a reduction in exhaled NO observed in smokers and smoking cessation is associated with an increase in exhaled NO; given that NO is important for normal ciliary beating, this may be one mechanism for the effect of cigarette smoking on ciliary motion (111). In vitro, cigarette smoke up-regulates airway epithelial expression of IL-8, which does not decrease ciliary beat frequency directly, but does abrogate the increase in ciliary beat frequency induced by beta-agonists (112).
Passive smoke exposure may also be associated with ciliary abnormalities. Children undergoing sinus surgery who were exposed to environmental tobacco smoke were found to have reduced regrowth of cilia following the procedure compared to children without passive smoke exposure (113). Examination of nasal mucosa in children passively exposed to smoke showed both patchy and generalized loss of cilia on a background of other epithelial abnormalities (114). Adenoid explants from children with exposure to secondhand smoke showed a blunted cilia beat frequency response to ciliary stimulants ex vivo (115). This is consistent with data showing an increased risk of respiratory disease in children exposed to passive cigarette smoke (113). Adults exposed to passive smoking have reduced nasal mucociliary clearance compared to nonsmokers, though to a lesser extent than active smokers (116).
Use of Alternative Tobacco Products and Illicit Drugs
Limited data exist on cilia abnormalities in users of alternative tobacco products and illicit drugs. Users of marijuana more frequently have regions of cilia loss and goblet cell hyperplasia, which may correlate with increased mucus secretion and altered mucociliary clearance, than do nonsmokers (117, 118). Cocaine use is also associated with focal loss of airway cilia (117). The effects of alternative tobacco product use, including shisha (waterpipe, hookah) or electronic cigarettes, on cilia structure or function are not known.
Environmental Pollutants
Several studies link environmental exposure to airborne pollutants to cilia abnormalities and dysfunction. Nasal biopsies from Mexico City residents exposed to high levels of air pollution showed patches of short cilia and regions of cilia loss (119). Healthy nonsmokers exposed to ozone at 0.4 ppm or sulfur dioxide at 0.75 ppm showed no abnormalities in cilia structure assessed in nasal samples, though experimental animals exposed to substantially higher concentrations of ozone (4 ppm) demonstrated blebbing and vesiculation of ciliary membranes as well as disruption of trachea cilia structure (120). Exposure of cultured human bronchial epithelial cells to diesel exhaust particles, but not to NO2, reduces cilia beat frequency (121). Compounds present in indoor air pollution, including formaldehyde, acrolein, and ammonia have effects on cilia beating and structure as well as mucus flow (122). Workplace exposures have also been linked to cilia dysfunction, including cadmium (reduced cilia beat frequency), nickel (reduced cilia beat frequency, cell damage and disorganized cilia), hairspray (reduced mucociliary clearance in the nose and trachea of hairdressers), and wood dust (decreased nasal mucociliary clearance and loss of ciliated epithelium) (122).
COPD
Mucociliary clearance is impaired in COPD in association with increased vulnerability to respiratory tract infections (87). This decrease in clearance is attributed to a shortening of cilia caused by cigarette smoke as well as airway epithelial dysfunction (123). Respiratory cilia are shorter in healthy smokers than in nonsmokers, and even shorter in smokers with COPD than in smokers without evidence of airway disease (49, 50). Cilia beating is impaired in nasal cilia from individuals with COPD (46), even after smoking cessation (124).
A new concept in COPD cilia dysfunction is that of ciliophagy, the consumption of cilia components by autophagic mechanisms (125). Lam and colleagues (123) demonstrated increased lung autophagic flux in the setting of cigarette smoke, and that mice deficient in autophagy mechanisms resisted smoke-induced shortening of cilia and smoke-induced impairment of mucociliary clearance. Cigarette smoke exposure also increased the turnover of cilia proteins by autophagy, a process mediated by cytosolic deacetylase HDAC6 (123). In this model, administration of tubastatin A, an inhibitor of HDAC6, or 4-phenyl butyric acid, a chemical chaperone, prior to cigarette smoke exposure protected mice from cigarette smoke-induced reduction in mucociliary clearance (123, 125). Similarly, blockage of autophagy enhances primary cilia growth and cilia-associated signaling during normal nutritional conditions (126) and autophagic degradation of a ciliopathy protein, OFD1 (oral-facial-digital syndrome 1), at centriolar satellites promotes primary cilium biogenesis (127).
Bronchiectasis
Bronchiectasis is characterized by a localized, irreversible dilation of segments of the bronchial tree in association with loss of airway smooth muscle and elastic fibers (128). The most common cause is infection, but it is also associated with inhalation of toxic gases, aspiration of stomach contents and drug use (128). Interestingly, other than bronchiectasis associated with PCD, the incidence of cilia ultrastructural defects is not significantly different in idiopathic bronchiectasis compared to normal controls (129). Cilia orientation in individuals with idiopathic bronchiectasis is typically not different compared to controls (129, 130). Conflicting data exist regarding whether ciliary beat frequency in idiopathic bronchiectasis is reduced compared to normal controls (129, 131). The addition of sputum from patients with bronchiectasis to nasal epithelial fragments suspended in tissue culture medium results in reduced ciliary beat frequency, but improves after treatment with antibiotics (132). It has been proposed that defective signaling of sensory proteins in motile cilia may lead to ciliopathy and development of bronchiectasis (133).
Asthma
Mucociliary clearance is impaired in asthma (134). Autopsy specimens from cases of fatal asthma show loss of cilia, shedding of the bronchial epithelium and failure of clearance of mucus with bronchial plugging (134, 135). Many functional studies in asthma subjects have documented impaired mucociliary clearance (136–139), and abnormal clearance of secretions is clinically apparent to asthma patients (136).
While alterations in characteristics of mucus play a role, changes in cilia structure and function also contribute to reduced mucociliary clearance in asthma. The shedding of ciliated epithelium observed at autopsy (134, 135) is also observed in cells obtained via biopsy and cells shed from the airway epithelium and recovered by bronchoalveolar lavage (140). Electron microscopy of epithelial biopsies in both children and adults with asthma shows damage to ciliated cells, with vacuolization of the endoplasmic reticulum and mitochondria, loss of cilia, and abnormal cilia structure (141, 142). In contrast, ultrastructural examination of nasal biopsies from patients with aspirin-exacerbated respiratory disease (aspirin sensitivity, nasal polyposis and asthma) shows no ciliary abnormalities (143). Ciliary beat frequency is reduced in moderate and severe asthma compared to controls (144) and the ciliary beat direction is disorganized (142). Subjects with moderate and severe asthma have more dyskinetic and immotile cilia than controls, while cilia length is unchanged in asthmatics as compared to controls (144). Related to these abnormalities in function, subjects with severe asthma have more ciliary disorientation, ciliary depletion, and microtubular defects compared to both normal controls and subjects with mild asthma (144). Sputum from asthma patients has an inhibitory effect on cilia beating in bronchial epithelial explants (145) A number of mediators implicated in asthma, including prostaglandins, bradykinin, prostacyclin and leukotriene D4 increase cilia beat frequency (56, 146), while varying effects on ciliary beat frequency have been reported for histamine and leukotriene C4 (56, 146, 147).
The Th2-type cytokine interleukin-13 (IL-13) is found at increased levels in the airways of asthmatics and is a key mediator of the epithelial abnormalities of asthma (148). In culture models of mucociliary differentiation, IL-13 promotes goblet cell differentiation and reduced numbers of ciliated cells (149). IL-13 is associated with modulation of expression and localization of ezrin, which anchors basal bodies to the apical cytoskeleton, and IL-13 exposure interferes with the apical localization of ezrin, leading to reduced numbers of basal bodies (16, 149, 150). The addition of IL-13 to differentiated airway epithelium in culture reduces both the number of ciliated cells and the number of cilia per cell (16). In cultured airway epithelium, IL-13 also decreases and eventually eliminates ciliary beat frequency (149). These effects appear to be mediated by an IL-13-induced decrease in expression of FoxJ1, via a decrease in FoxJ1 promoter activity, and of ezrin (16). Using a candidate gene approach, Kovacic et al (151) found that variants in KIF3A, a member of the kinesin family of microtubular motors critical to intraflagellar transport, were significantly associated with asthma. Kim and colleagues (152) found an association in a Korean population between KIF3A polymorphisms and aspirin-associated respiratory disease.
Acute and Chronic Infection
Both infectious microorganisms and the immune/inflammatory response to infection can alter airway cilia function, leading to impaired mucociliary clearance and retained secretions (153). Recurrent bronchitis is associated with loss of ciliated cells in children (154). A number of microorganisms impair cilia function by mechanisms including reducing ciliary beat frequency, disrupting ciliary coordination and inducing ciliary dyskinesia (155, 156). Some bacterial pathogens specifically target ciliated cells for adherence, including Actinobacillus pleuropneumonia, Pseudomonas aeruginosa, Moraxella catarrhalis, Mycoplasma pneumonia, Mycoplasma hyopneumoniae, and Bordatella species (155). Epithelial samples from chronically infected patients with bronchiectasis show a disruption of ciliary orientation associated with decreased mucociliary clearance although cilia ultrastructure and ciliary beat frequency remain normal (157). Lung samples from patients infected with respiratory syncytial virus show epithelial damage and loss of cilia associated with decreased expression of FoxJ1, findings replicated in a mouse model (156).
In addition to the effect of the microorganism itself, the host response to infection may also contribute to deficient mucociliary clearance. Human neutrophil elastase causes epithelial disruption and at high concentrations reduces ciliary beat frequency (153). Reactive oxygen species generated by polymorphonuclear leukocytes, especially H2O2, decrease ciliary beat frequency (158).
Interstitial Lung Disease
Limited data are available on cilia structure and function in interstitial lung disease. Mucociliary clearance is normal in subjects with asbestosis, sarcoidosis, and pulmonary fibrosis (159, 160). Transcriptional profiling of lung tissue samples from 119 subjects with idiopathic pulmonary fibrosis (IPF) categorized these subjects into two distinct groups, defined by expression of cilia-related genes, a finding validated in an independent cohort of 111 IPF patients (161). Interestingly, patients with high expression of these cilia-related genes had more microscopic honeycombing, but not fibroblastic foci, and had higher expression of MUC5B and MMP7.
Lung Transplantation
Infection plays an important role in early death after lung transplant and may also contribute to the development of bronchiolitis obliterans syndrome, a leading cause of later deaths post-transplant (162). Mucociliary clearance is impaired early after lung transplant (163, 164). Studies of ciliary beat frequency in adults have had mixed findings, with some studies showing reduced ciliary beat frequency in transplanted bronchi (165) while others did not (163, 166, 167). In a study in children post-lung transplant for cystic fibrosis as well as for non-suppurative lung disease, significant ultrastructural abnormalities in the epithelium were observed 7 to 12 months post-transplant below the anastomosis as compared to above, including loss of ciliated cells, ciliated cells with loss of cilia, and ciliary disorientation (168).
Bone Marrow Transplant
Bronchiolitis obliterans is a common pulmonary complication following stem cell transplantation and an important cause of death following transplant (169). In a study of nasal cilia morphology and function in 36 Chinese patients after allogeneic stem cell transplant, reduced ciliary beat frequency was observed in the transplant patients compared to controls, with a greater reduction in ciliary beat frequency in patients with bronchiolitis obliterans (170). Assessment of 19 Chinese patients both before and after stem cell transplant showed reduced ciliary beat frequency both before and after transplant compared to age- and sex-matched controls but showed no correlation between ciliary abnormalities and pulmonary dysfunction (171).
Mechanical Ventilation
The use of invasive mechanical ventilation for the treatment of respiratory failure may induce airway epithelial injury and cilia dysfunction. In acutely intubated patients, reduced mucociliary clearance was associated with duration of mechanical ventilation, smoking status, and isolation of pathogenic bacteria in the tracheobronchial tree (172). Airway epithelial injury including cilia loss is found in experimental animals undergoing various modes of mechanical ventilation, with some differences in pattern of injury depending on ventilator mode (173, 174). In contrast to invasive mechanical ventilation, in a group of patients using nasal continuous positive airway pressure for obstructive sleep apnea, no decrement in nasal clearance was observed (175).
Shock, Sepsis
Little is known about cilia function in patients with sepsis or shock. Absence of ciliary motility has been reported post-mortem in patients dying of sepsis or multiorgan system failure (176).
Effect of Drugs
A variety of drugs have an effect on airway cilia function. Activation of axonemal cAMP-dependent protein kinase A (PKA) increases cilia beat frequency by phosphorylation of dynein light chains (35). β-agonists, including salmeterol and salbutamol, raise intracellular cAMP levels and thereby increase cilia beat frequency (177). Consistent with this observation, inhalation of salbutamol increases mucociliary clearance in normals and those with chronic bronchitis (178). Similarly, methylxanthines (theophylline, aminophylline) inhibit phosphodiesterase and increase cAMP, leading to ciliary stimulation (179). The PDE4 inhibitor roflumilast increases cilia beat frequency in vitro and reverses the decrease in cilia beat frequency observed with treatment with cigarette smoke extract (180). The cholinergic agents acetylcholine and pilocarpine increase cilia beat frequency (181) and conversely, anticholinergic drugs reduce cilia beat frequency (182). Topical application of corticosteroids to airway epithelium in culture results in a small increase in cilia beat frequency (181). Prolonged inhaled steroid treatment in asthmatics has been shown to reverse epithelial damage seen in the same subjects prior to steroid treatment, including areas of epithelium that showed loss of cilia (183). Inhaled beclomethasone in patients with COPD does not affect mucociliary clearance (184). Sisson and colleagues (185) found that alcohol rapidly stimulates cilia beating through both nitric oxide- and cAMP-dependent mechanisms and have suggested that this pathway is downregulated by chronic alcohol exposure leading to chronic cilia dysfunction.
Aspirin decreases mucociliary clearance, although whether this effect is due to changes in water transport and airway secretions, or to changes in ciliary beating and coordination, is not clear (186). Volatile anesthetics depress cilia beat frequency in cultured rat tracheal cells, with halothane and isoflurane having a greater effect than sevoflurane (187). N-acetylcysteine reduces cilia beat frequency in cultured nasal epithelium but no effect was seen in nasal epithelium of CF patients taking the drug (188).
Lung Cancer
Because of the role of primary cilia in cell cycle regulation, primary cilia may be important in tumorigenesis, and genes important in cilia formation and function exhibit dysregulated expression in multiple tumor types (189). Few data exist, however, on airway cilia structure and function in the setting of lung cancer, though the histologic progression from normal histology to dysplasia to lung cancer is characterized by changes including loss of cilia (190). Less commonly, lung cancer variants with ciliated epithelium are reported (191–194). Loss of ciliary function does not appear to predispose to lung cancer and lung cancer in primary ciliary dyskinesia is rare (195). Mucociliary clearance has been reported to be slower in individuals with lung cancer than in those with chronic bronchitis and no cancer, matched for smoking history (196). Expression of ciliated cell genes has been found to be markedly decreased in a “basal cell-high” lung adenocarcinoma subset, which is characterized by increased expression of airway basal cell-related genes and associated with smoking status, higher frequency of KRAS mutations and a particularly aggressive clinical phenotype (197).
HIV
Most data on cilia function in the setting of HIV infection is focused on nasal cilia. Nasal mucociliary clearance was evaluated in a cohort of HIV+ individuals compared to HIV− controls; no subjects had active nasal symptoms or sinusitis at the time of the study. Findings included reduced mucociliary clearance in HIV+ individuals, with a trend toward worsening mucociliary clearance with progression of HIV infection to AIDS as well as a trend toward worsened mucociliary clearance in those subjects with a history of recurrent sinus infections (198). A subsequent study corroborated altered mucociliary clearance and documented abnormalities in cilia axonemal structure in HIV+ individuals, but the majority of the individuals studied had respiratory infections at the time of evaluation, which may have impacted the results, since acute infection is known to reversibly affect cilia structure and function (199). HIV+ individuals have reduced nasal NO compared to controls, which may correlate with reduced cilia function (200). Guiafenesin treatment in HIV+ individuals may reduce nasal symptoms but is not associated with improvement in mucociliary clearance (201).
Future Directions
While there is no question regarding the critical role of airway ciliated cells and cilia per se on human health, there are many areas where new insights will help understand the role of ciliated cells/cilia in the pathogenesis of human disease. First, although much is known about the control of ciliated cell differentiation and the structure and maintenance of cilia in many model systems and in the murine lung, there are limited molecular/biologic studies of ciliated cells/cilia in human airways. These studies would be greatly accelerated by the development of methods to isolate pure populations of airway ciliated cells in health and disease. Second, while a great deal of progress has been made on the identification of genetic variants associated with primary ciliary dyskinesia, there has been little attention focused on genetic variation and what are thought of as the acquired disorders of airway cilia dysfunction. In this regard, there may be mild variants in the genes controlling airway cilia structure and function that contribute to the increased susceptibility to disease associated with environmental stress, particularly cigarette smoking. Finally, most of the data regarding airway cilia-related abnormalities in acquired lung disorders are descriptive, without the underlying mechanisms clearly defined. Based on the knowledge of abnormal mucociliary clearance in many acquired lung disorders, new classes of pharmaceutical agents to reverse/prevent abnormal mucociliary clearance cannot be developed until the dysfunctional biology responsible for mucociliary dysfunction can be understood.
Supplementary Material
Acknowledgments
We thank Suzanne Cloonan for critical review and N. Mohamed for help in preparing this manuscript. These studies were supported, in part, by, R01HL107882, R01HL118857, and U01HL121828. AET is supported, in part, by K23 HL103837; and RS is supported, in part, by The Parker B. Francis Foundation.
References
- 1.Mercer RR, Russell ML, Roggli VL, Crapo JD. Cell number and distribution in human and rat airways. Am J Respir Cell Mol Biol. 1994;10:613–24. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- 2.Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc. 2008;5:772–7. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8:432–46. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 4.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–7. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raman T, O’Connor TP, Hackett NR, Wang W, Harvey BG, et al. Quality control in microarray assessment of gene expression in human airway epithelium. BMC Genomics. 2009;10:493. doi: 10.1186/1471-2164-10-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang MMJ, Shih L, Wu R. Pulmonary Epithelium: Cell Types and Functions. In: Proud D, editor. The Pulmonary Epithelium in Health and Disease. Chichester, UK: John Wiley & Songs, Ltd; 2008. pp. 1–16. [Google Scholar]
- 7.Shaykhiev R, Otaki F, Bonsu P, Dang DT, Teater M, et al. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell Mol Life Sci. 2011;68:877–92. doi: 10.1007/s00018-010-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res. 2001;27:401–15. doi: 10.1080/019021401300317125. [DOI] [PubMed] [Google Scholar]
- 9.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–41. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci U S A. 2007;104:410–7. doi: 10.1073/pnas.0610770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breeze RG, Wheeldon EB. The cells of the pulmonary airways. Am Rev Respir Dis. 1977;116:705–77. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- 12.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–5. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heguy A, Harvey BG, Leopold PL, Dolgalev I, Raman T, Crystal RG. Responses of the human airway epithelium transcriptome to in vivo injury. Physiol Genomics. 2007;29:139–48. doi: 10.1152/physiolgenomics.00167.2006. [DOI] [PubMed] [Google Scholar]
- 14.Gerovac BJ, Valencia M, Baumlin N, Salathe M, Conner GE, Fregien NL. Sub- mersion and Hypoxia Inhibit Ciliated Cell Differentiation in a Notch Dependent Manner. Am J Respir Cell Mol Biol. 2014 doi: 10.1165/rcmb.2013-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Didon L, Zwick RK, Chao IW, Walters MS, Wang R, et al. RFX3 modulation of FOXJ1 regulation of cilia genes in the human airway epithelium. Respir Res. 2013;14:70. doi: 10.1186/1465-9921-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomperts BN, Kim LJ, Flaherty SA, Hackett BP. IL-13 regulates cilia loss and foxj1 expression in human airway epithelium. Am J Respir Cell Mol Biol. 2007;37:339–46. doi: 10.1165/rcmb.2006-0400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You Y, Huang T, Richer EJ, Schmidt JE, Zabner J, et al. Role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L650–L657. doi: 10.1152/ajplung.00170.2003. [DOI] [PubMed] [Google Scholar]
- 18.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–48. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–24. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt-Dias M. Evolution: Tracing the origins of centrioles, cilia, and flagella. J Cell Biol. 2011;194:165–75. doi: 10.1083/jcb.201011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12:222–34. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 22.Broekhuis JR, Leong WY, Jansen G. Regulation of cilium length and intraflagellar transport. Int Rev Cell Mol Biol. 2013;303:101–38. doi: 10.1016/B978-0-12-407697-6.00003-9. [DOI] [PubMed] [Google Scholar]
- 23.Satir P, Christensen ST. Structure and function of mammalian cilia. Histochem Cell Biol. 2008;129:687–93. doi: 10.1007/s00418-008-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choksi SP, Lauter G, Swoboda P, Roy S. Switching on cilia: transcriptional net-works regulating ciliogenesis. Development. 2014;141:1427–41. doi: 10.1242/dev.074666. [DOI] [PubMed] [Google Scholar]
- 25.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188:913–22. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi D, Takeda H. Ciliary motility: the components and cytoplasmic preassembly mechanisms of the axonemal dyneins. Differentiation. 2012;83:S23–S29. doi: 10.1016/j.diff.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–33. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 28.Ostrowski LE, Blackburn K, Radde KM, Moyer MB, Schlatzer DM, et al. A proteomic analysis of human cilia: identification of novel components. Mol Cell Proteomics. 2002;1:451–65. doi: 10.1074/mcp.m200037-mcp200. [DOI] [PubMed] [Google Scholar]
- 29.Hoh RA, Stowe TR, Turk E, Stearns T. Transcriptional program of ciliated epithelial cells reveals new cilium and centrosome components and links to human disease. PLoS One. 2012;7:e52166. doi: 10.1371/journal.pone.0052166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–47. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–15. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 32.Sleigh MA, Blake JR, Liron N. The propulsion of mucus by cilia. Am Rev Respir Dis. 1988;137:726–41. doi: 10.1164/ajrccm/137.3.726. [DOI] [PubMed] [Google Scholar]
- 33.Jain R, Ray JM, Pan JH, Brody SL. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Cell Mol Biol. 2012;46:446–53. doi: 10.1165/rcmb.2011-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiao J, Wang H, Lou W, Jin S, Fan E, et al. Regulation of ciliary beat frequency by the nitric oxide signaling pathway in mouse nasal and tracheal epithelial cells. Exp Cell Res. 2011;317:2548–53. doi: 10.1016/j.yexcr.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401–22. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 36.Schmid A, Salathe M. Ciliary beat coordination by calcium. Biol Cell. 2011;103:159–69. doi: 10.1042/BC20100120. [DOI] [PubMed] [Google Scholar]
- 37.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–4. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enuka Y, Hanukoglu I, Edelheit O, Vaknine H, Hanukoglu A. Epithelial sodium channels (ENaC) are uniformly distributed on motile cilia in the oviduct and the respiratory airways. Histochem Cell Biol. 2012;137:339–53. doi: 10.1007/s00418-011-0904-1. [DOI] [PubMed] [Google Scholar]
- 39.Ostrowski LE, Hutchins JR, Zakel K, O’Neal WK. Targeting expression of a transgene to the airway surface epithelium using a ciliated cell-specific promoter. Mol Ther. 2003;8:637–45. doi: 10.1016/s1525-0016(03)00221-1. [DOI] [PubMed] [Google Scholar]
- 40.Braiman A, Priel Z. Efficient mucociliary transport relies on efficient regulation of ciliary beating. Respir Physiol Neurobiol. 2008;163:202–7. doi: 10.1016/j.resp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Button B, Boucher RC. Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Respir Physiol Neurobiol. 2008;163:189–201. doi: 10.1016/j.resp.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis CW, Lazarowski E. Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling. Respir Physiol Neurobiol. 2008;163:208–13. doi: 10.1016/j.resp.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agius AM, Smallman LA, Pahor AL. Age, smoking and nasal ciliary beat frequency. Clin Otolaryngol Allied Sci. 1998;23:227–30. doi: 10.1046/j.1365-2273.1998.00141.x. [DOI] [PubMed] [Google Scholar]
- 44.Ho JC, Chan KN, Hu WH, Lam WK, Zheng L, et al. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001;163:983–8. doi: 10.1164/ajrccm.163.4.9909121. [DOI] [PubMed] [Google Scholar]
- 45.Muns G, Singer P, Wolf F, Rubinstein I. Impaired nasal mucociliary clearance in long-distance runners. Int J Sports Med. 1995;16:209–13. doi: 10.1055/s-2007-972993. [DOI] [PubMed] [Google Scholar]
- 46.Yaghi A, Zaman A, Cox G, Dolovich MB. Ciliary beating is depressed in nasal cilia from chronic obstructive pulmonary disease subjects. Respir Med. 2012;106:1139–47. doi: 10.1016/j.rmed.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Svartengren M, Falk R, Philipson K. Long-term clearance from small airways decreases with age. Eur Respir J. 2005;26:609–15. doi: 10.1183/09031936.05.00002105. [DOI] [PubMed] [Google Scholar]
- 48.Lee RM, Rossman CM, O’Brodovich H. Assessment of postmortem respiratory ciliary motility and ultrastructure. Am Rev Respir Dis. 1987;136:445–7. doi: 10.1164/ajrccm/136.2.445. [DOI] [PubMed] [Google Scholar]
- 49.Hessel J, Heldrich J, Fuller J, Staudt MR, Radisch S, et al. Intraflagellar transport gene expression associated with short cilia in smoking and COPD. PLoS One. 2014;9:e85453. doi: 10.1371/journal.pone.0085453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leopold PL, O’Mahony MJ, Lian XJ, Tilley AE, Harvey BG, Crystal RG. Smoking is associated with shortened airway cilia. PLoS One. 2009;4:e8157. doi: 10.1371/journal.pone.0008157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veerman AJ, van DL, Feenstra L, Leene W. The immotile cilia syndrome: phase contrast light microscopy, scanning and transmission electron microscopy. Pediatrics. 1980;65:698–702. [PubMed] [Google Scholar]
- 52.Shoemark A, Hogg C. Electron tomography of respiratory cilia. Thorax. 2013;68:190–1. doi: 10.1136/thoraxjnl-2012-202938. [DOI] [PubMed] [Google Scholar]
- 53.Luk CK, Dulfano MJ. Effect of pH, viscosity and ionic-strength changes on ciliary beating frequency of human bronchial explants. Clin Sci (Lond) 1983;64:449–51. doi: 10.1042/cs0640449. [DOI] [PubMed] [Google Scholar]
- 54.Low PM, Luk CK, Dulfano MJ, Finch PJ. Ciliary beat frequency of human respiratory tract by different sampling techniques. Am Rev Respir Dis. 1984;130:497–8. doi: 10.1164/arrd.1984.130.3.497. [DOI] [PubMed] [Google Scholar]
- 55.Yager JA, Ellman H, Dulfano MJ. Human ciliary beat frequency at three levels of the tracheobronchial tree. Am Rev Respir Dis. 1980;121:661–5. doi: 10.1164/arrd.1980.121.4.661. [DOI] [PubMed] [Google Scholar]
- 56.Cyrus CB, Yang B, McCaffrey TV. Leukotrienes C4 and D4 increase the ciliary beat frequency in human upper airway mucosa in vitro. Otolaryngol Head Neck Surg. 1998;118:472–7. doi: 10.1177/019459989811800407. [DOI] [PubMed] [Google Scholar]
- 57.Oldenburg AL, Chhetri RK, Hill DB, Button B. Monitoring airway mucus flow and ciliary activity with optical coherence tomography. Biomed Opt Express. 2012;3:1978–92. doi: 10.1364/BOE.3.001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parrilla E, Armengot M, Mata M, Cortijo J, Riera J, et al. Ciliary motility activity measurement using a dense optical flow algorithm. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:4446–9. doi: 10.1109/EMBC.2013.6610533. [DOI] [PubMed] [Google Scholar]
- 59.Braat JP, Ainge G, Bowles JA, Richards DH, Van RD, et al. The lack of effect of benzalkonium chloride on the cilia of the nasal mucosa in patients with perennial allergic rhinitis: a combined functional, light, scanning and transmission electron microscopy study. Clin Exp Allergy. 1995;25:957–65. doi: 10.1111/j.1365-2222.1995.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 60.Weiss T, Dorow P, Felix R. Regional mucociliary removal of inhaled particles in smokers with small airways disease. Respiration. 1983;44:338–45. doi: 10.1159/000194566. [DOI] [PubMed] [Google Scholar]
- 61.Zwas ST, Katz I, Belfer B, Baum GL, Aharonson E. Scintigraphic monitoring of mucociliary tracheo-bronchial clearance of technetium-99m macroaggregated albumin aerosol. J Nucl Med. 1987;28:161–7. [PubMed] [Google Scholar]
- 62.Foster WM, Bergofsky EH, Bohning DE, Lippmann M, Albert RE. Effect of ad-renergic agents and their mode of action on mucociliary clearance in man. J Appl Physiol. 1976;41:146–52. doi: 10.1152/jappl.1976.41.2.146. [DOI] [PubMed] [Google Scholar]
- 63.Mossberg B, Philipson K, Camner P. Tracheobronchial clearance in patients with emphysema associated with alpha1-antitrypsin deficiency. Scand J Respir Dis. 1978;59:1–7. [PubMed] [Google Scholar]
- 64.Stahlhofen W, Gebhart J, Heyder J, Philipson K, Camner P. Intercomparison of regional deposition of aerosol particles in the human respiratory tract and their long-term elimination. Exp Lung Res. 1981;2:131–9. doi: 10.3109/01902148109052309. [DOI] [PubMed] [Google Scholar]
- 65.Wood RE, Wanner A, Hirsch J, Farrell PM. Tracheal mucociliary transport in patients with cystic fibrosis and its stimulation by terbutaline. Am Rev Respir Dis. 1975;111:733–8. doi: 10.1164/arrd.1975.111.6.733. [DOI] [PubMed] [Google Scholar]
- 66.Kartagener M. Zur Pathogenese der Bronchiektasien: Bronchiektasien bei Situs viscerum inversus. Beitrage zur Klinik der Tuberkulose. 1933;83:489–501. [Google Scholar]
- 67.Cowan MJ, Gladwin MT, Shelhamer JH. Disorders of ciliary motility. Am J Med Sci. 2001;321:3–10. doi: 10.1097/00000441-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–9. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- 69.Rossman CM, Forrest JB, Lee RM, Newhouse MT. The dyskinetic cilia syndrome. Ciliary motility in immotile cilia syndrome. Chest. 1980;78:580–2. doi: 10.1378/chest.78.4.580. [DOI] [PubMed] [Google Scholar]
- 70.Rossman CM, Lee RM, Forrest JB, Newhouse MT. Nasal ciliary ultrastructure and function in patients with primary ciliary dyskinesia compared with that in normal subjects and in subjects with various respiratory diseases. Am Rev Respir Dis. 1984;129:161–7. doi: 10.1164/arrd.1984.129.1.161. [DOI] [PubMed] [Google Scholar]
- 71.Moller W, Haussinger K, Ziegler-Heitbrock L, Heyder J. Mucociliary and long- term particle clearance in airways of patients with immotile cilia. Respir Res. 2006;7:10. doi: 10.1186/1465-9921-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell SD, et al. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med. 2009;11:473–87. doi: 10.1097/GIM.0b013e3181a53562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kott E, Legendre M, Copin B, Papon JF, stot-Le MF, et al. Loss-of-function mutations in RSPH1 cause primary ciliary dyskinesia with central-complex and radial-spoke defects. Am J Hum Genet. 2013;93:561–70. doi: 10.1016/j.ajhg.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wallmeier J, Al-Mutairi DA, Chen CT, Loges NT, Pennekamp P, et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Genet. 2014 doi: 10.1038/ng.2961. [DOI] [PubMed] [Google Scholar]
- 75.Verra F, Fleury-Feith J, Boucherat M, Pinchon MC, Bignon J, Escudier E. Do nasal ciliary changes reflect bronchial changes? An ultrastructural study. Am Rev Respir Dis. 1993;147:908–13. doi: 10.1164/ajrccm/147.4.908. [DOI] [PubMed] [Google Scholar]
- 76.Ehre C, Ridley C, Thornton DJ. Cystic fibrosis: An inherited disease affecting mucin-producing organs. Int J Biochem Cell Biol. 2014 doi: 10.1016/j.biocel.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 78.Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–58. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 79.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–70. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 80.Rubin BK. Mucus structure and properties in cystic fibrosis. Paediatr Respir Rev. 2007;8:4–7. doi: 10.1016/j.prrv.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 81.Zuelzer WW, Newton WA., Jr The pathogenesis of fibrocystic disease of the pancreas; a study of 36 cases with special reference to the pulmonary lesions. Pediatrics. 1949;4:53–69. [PubMed] [Google Scholar]
- 82.Derichs N, Jin BJ, Song Y, Finkbeiner WE, Verkman AS. Hyperviscous airway periciliary and mucous liquid layers in cystic fibrosis measured by confocal fluorescence photobleaching. FASEB J. 2011;25:2325–32. doi: 10.1096/fj.10-179549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katz SM, Holsclaw DS., Jr Ultrastructural features of respiratory cilia in cystic fibrosis. Am J Clin Pathol. 1980;73:682–5. doi: 10.1093/ajcp/73.5.682. [DOI] [PubMed] [Google Scholar]
- 84.Piorunek T, Marszalek A, Biczysko W, Gozdzik J, Cofta S, Seget M. Correlation between the stage of cystic fibrosis and the level of morphological changes in adult patients. J Physiol Pharmacol. 2008;59(Suppl 6):565–72. [PubMed] [Google Scholar]
- 85.Hubbard RC, McElvaney NG, Birrer P, Shak S, Robinson WW, et al. A preliminary study of aerosolized recombinant human deoxyribonuclease I in the treatment of cystic fibrosis. N Engl J Med. 1992;326:812–5. doi: 10.1056/NEJM199203193261207. [DOI] [PubMed] [Google Scholar]
- 86.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–72. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Camner P, Mossberg B, Philipson K. Tracheobronchial clearance and chronic obstructive lung disease. Scand J Respir Dis. 1973;54:272–81. [PubMed] [Google Scholar]
- 88.Shin MS, Ho KJ. Bronchiectasis in patients with alpha 1-antitrypsin deficiency. A rare occurrence? Chest. 1993;104:1384–6. doi: 10.1378/chest.104.5.1384. [DOI] [PubMed] [Google Scholar]
- 89.Bonneau D, Raymond F, Kremer C, Klossek JM, Kaplan J, Patte F. Usher syndrome type I associated with bronchiectasis and immotile nasal cilia in two brothers. J Med Genet. 1993;30:253–4. doi: 10.1136/jmg.30.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–9. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- 91.Schmidts M, Vodopiutz J, Christou-Savina S, Cortes CR, Inerney-Leo AM, et al. Mutations in the gene encoding IFT dynein complex component WDR34 cause Jeune asphyxiating thoracic dystrophy. Am J Hum Genet. 2013;93:932–44. doi: 10.1016/j.ajhg.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gray PE, Sillence D, Kakakios A. Is Roifman syndrome an X-linked ciliopathy with humoral immunodeficiency? Evidence from 2 new cases. Int J Immunogenet. 2011;38:501–5. doi: 10.1111/j.1744-313X.2011.01041.x. [DOI] [PubMed] [Google Scholar]
- 93.Albert RE, Lippmann M, Briscoe W. The characteristics of bronchial clearance in humans and the effects of cigarette smoking. Arch Environ Health. 1969;18:738–55. doi: 10.1080/00039896.1969.10665482. [DOI] [PubMed] [Google Scholar]
- 94.Pavia D, Thomson ML, Pocock SJ. Evidence for temporary slowing of mucociliary clearance in the lung caused by tobacco smoking. Nature. 1971;231:325–6. doi: 10.1038/231325a0. [DOI] [PubMed] [Google Scholar]
- 95.Foster WM, Langenback EG, Bergofsky EH. Disassociation in the mucociliary function of central and peripheral airways of asymptomatic smokers. Am Rev Respir Dis. 1985;132:633–9. doi: 10.1164/arrd.1985.132.3.633. [DOI] [PubMed] [Google Scholar]
- 96.Ramos EM, De Toledo AC, Xavier RF, Fosco LC, Vieira RP, et al. Reversibility of impaired nasal mucociliary clearance in smokers following a smoking cessation programme. Respirology. 2011;16:849–55. doi: 10.1111/j.1440-1843.2011.01985.x. [DOI] [PubMed] [Google Scholar]
- 97.Auerbach O, Stout AP, Hammond EC, Garfinkel L. Changes in bronchial epithelium in relation to sex, age, residence, smoking and pneumonia. N Engl J Med. 1962;267:111–9. doi: 10.1056/NEJM196207192670301. [DOI] [PubMed] [Google Scholar]
- 98.Auerbach O, Hammond EC, Garfinkel L. Changes in bronchial epithelium in relation to cigarette smoking, 1955–1960 vs. 1970–1977. N Engl J Med. 1979;300:381–5. doi: 10.1056/NEJM197902223000801. [DOI] [PubMed] [Google Scholar]
- 99.Shaykhiev R, Zuo WL, Chao I, Fukui T, Witover B, et al. EGF shifts human airway basal cell fate toward a smoking-associated airway epithelial phenotype. Proc Natl Acad Sci U S A. 2013;110:12102–7. doi: 10.1073/pnas.1303058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fox B, Bull TB, Oliver TN. The distribution and assessment of electron- microscopic abnormalities of human cilia. Eur J Respir Dis Suppl. 1983;127:11–8. [PubMed] [Google Scholar]
- 101.Lungarella G, Fonzi L, Ermini G. Abnormalities of bronchial cilia in patients with chronic bronchitis. An ultrastructural and quantitative analysis. Lung. 1983;161:147–56. doi: 10.1007/BF02713856. [DOI] [PubMed] [Google Scholar]
- 102.McDowell EM, Barrett LA, Harris CC, Trump BF. Abnormal cilia in human bronchial epithelium. Arch Pathol Lab Med. 1976;100:429–36. [PubMed] [Google Scholar]
- 103.Verra F, Escudier E, Lebargy F, Bernaudin JF, de CH, Bignon J. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med. 1995;151:630–4. doi: 10.1164/ajrccm/151.3_Pt_1.630. [DOI] [PubMed] [Google Scholar]
- 104.Ballenger JJ. Experimental effect of cigarette smoke on human respiratory cilia. N Engl J Med. 1960;263:832–5. doi: 10.1056/NEJM196010272631704. [DOI] [PubMed] [Google Scholar]
- 105.Dalhamn T. The effect of cigarette smoke on ciliary activity in the upper respiratory tract. AMA Arch Otolaryngol. 1959;70:166–8. doi: 10.1001/archotol.1959.00730040172003. [DOI] [PubMed] [Google Scholar]
- 106.Carson JL, Lu TS, Brighton L, Hazucha M, Jaspers I, Zhou H. Phenotypic and physiologic variability in nasal epithelium cultured from smokers and non-smokers exposed to secondhand tobacco smoke. In Vitro Cell Dev Biol Anim. 2010;46:606–12. doi: 10.1007/s11626-010-9310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stanley PJ, Wilson R, Greenstone MA, MacWilliam L, Cole PJ. Effect of cigarette smoking on nasal mucociliary clearance and ciliary beat frequency. Thorax. 1986;41:519–23. doi: 10.1136/thx.41.7.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clary-Meinesz C, Mouroux Jüé, Huitorel P, Cosson J, Schoevaert D, Blaive B. CIliary beat frequency in human bronchi and bronchioles. CHEST Journal. 1997;111:692–7. doi: 10.1378/chest.111.3.692. [DOI] [PubMed] [Google Scholar]
- 109.Dulfano MJ, Luk CK, Beckage M, Wooten O. Ciliary beat frequency in human respiratory explants. Am Rev Respir Dis. 1981;123:139–40. doi: 10.1164/arrd.1981.123.1.139. [DOI] [PubMed] [Google Scholar]
- 110.Zhou H, Wang X, Brighton L, Hazucha M, Jaspers I, Carson JL. Increased nasal epithelial ciliary beat frequency associated with lifestyle tobacco smoke exposure. Inhal Toxicol. 2009;21:875–81. doi: 10.1080/08958370802555898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kharitonov SA, Robbins RA, Yates D, Keatings V, Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med. 1995;152:609–12. doi: 10.1164/ajrccm.152.2.7543345. [DOI] [PubMed] [Google Scholar]
- 112.Allen-Gipson DS, Romberger DJ, Forget MA, May KL, Sisson JH, Wyatt TA. IL-8 inhibits isoproterenol-stimulated ciliary beat frequency in bovine bronchial epithelial cells. J Aerosol Med. 2004;17:107–15. doi: 10.1089/0894268041457138. [DOI] [PubMed] [Google Scholar]
- 113.Atef A, Zeid IA, Qotb M, El Rab EG. Effect of passive smoking on ciliary regeneration of nasal mucosa after functional endoscopic sinus surgery in children. J Laryngol Otol. 2009;123:75–9. doi: 10.1017/S0022215108003678. [DOI] [PubMed] [Google Scholar]
- 114.Elwany S, Ibrahim AA, Mandour Z, Talaat I. Effect of passive smoking on the ultrastructure of the nasal mucosa in children. Laryngoscope. 2012;122:965–9. doi: 10.1002/lary.23246. [DOI] [PubMed] [Google Scholar]
- 115.Wang LF, White DR, Andreoli SM, Mulligan RM, Discolo CM, Schlosser RJ. Cigarette smoke inhibits dynamic ciliary beat frequency in pediatric adenoid explants. Otolaryngol Head Neck Surg. 2012;146:659–63. doi: 10.1177/0194599811431414. [DOI] [PubMed] [Google Scholar]
- 116.Habesoglu M, Demir K, Yumusakhuylu AC, Yilmaz AS, Oysu C. Does passive smoking have an effect on nasal mucociliary clearance? Otolaryngol Head Neck Surg. 2012;147:152–6. doi: 10.1177/0194599812439004. [DOI] [PubMed] [Google Scholar]
- 117.Fligiel SE, Roth MD, Kleerup EC, Barsky SH, Simmons MS, Tashkin DP. Tracheobronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Chest. 1997;112:319–26. doi: 10.1378/chest.112.2.319. [DOI] [PubMed] [Google Scholar]
- 118.Roth MD, Arora A, Barsky SH, Kleerup EC, Simmons M, Tashkin DP. Airway inflammation in young marijuana and tobacco smokers. Am J Respir Crit Care Med. 1998;157:928–37. doi: 10.1164/ajrccm.157.3.9701026. [DOI] [PubMed] [Google Scholar]
- 119.Calderon-Garciduenas L, Rodriguez-Alcaraz A, Villarreal-Calderon A, Lyght O, Janszen D, Morgan KT. Nasal epithelium as a sentinel for airborne environmental pollution. Toxicol Sci. 1998;46:352–64. doi: 10.1006/toxs.1998.2549. [DOI] [PubMed] [Google Scholar]
- 120.Carson JL, Collier AM, Fernald GW, Hu SC. Microtubular discontinuities as acquired ciliary defects in airway epithelium of patients with chronic respiratory diseases. Ultrastruct Pathol. 1994;18:327–32. doi: 10.3109/01913129409023201. [DOI] [PubMed] [Google Scholar]
- 121.Riechelmann H, Kienast K, Schellenberg J, Mann WJ. An in vitro model to study effects of airborne pollutants on human ciliary activity. Rhinology. 1994;32:105–8. [PubMed] [Google Scholar]
- 122.Pedersen M. Ciliary activity and pollution. Lung. 1990;168(Suppl):368–76. doi: 10.1007/BF02718154. [DOI] [PubMed] [Google Scholar]
- 123.Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, et al. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest. 2013;123:5212–30. doi: 10.1172/JCI69636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koblizek V, Tomsova M, Cermakova E, Papousek P, Pracharova S, et al. Impairment of nasal mucociliary clearance in former smokers with stable chronic obstructive pulmonary disease relates to the presence of a chronic bronchitis phenotype. Rhinology. 2011;49:397–406. doi: 10.4193/Rhino11.051. [DOI] [PubMed] [Google Scholar]
- 125.Cloonan SM, Lam HC, Ryter SW, Choi AM. “Ciliophagy”: The consumption of cilia components by autophagy. Autophagy. 2014;10:532–4. doi: 10.4161/auto.27641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pampliega O, Orhon I, Patel B, Sridhar S, az-Carretero A, et al. Functional interaction between autophagy and ciliogenesis. Nature. 2013;502:194–200. doi: 10.1038/nature12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang Z, Lin MG, Stowe TR, Chen S, Zhu M, et al. Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature. 2013;502:254–7. doi: 10.1038/nature12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McShane PJ, Naureckas ET, Tino G, Strek ME. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2013;188:647–56. doi: 10.1164/rccm.201303-0411CI. [DOI] [PubMed] [Google Scholar]
- 129.de Iongh RU, Rutland J. Ciliary defects in healthy subjects, bronchiectasis, and primary ciliary dyskinesia. Am J Respir Crit Care Med. 1995;151:1559–67. doi: 10.1164/ajrccm.151.5.7735615. [DOI] [PubMed] [Google Scholar]
- 130.Tsang KW, Tipoe G, Sun J, Tan KC, Leung R, et al. Clinical value of ciliary assessment in bronchiectasis. Lung. 2005;183:73–86. doi: 10.1007/s00408-004-2520-5. [DOI] [PubMed] [Google Scholar]
- 131.Rutland J, Cole PJ. Nasal mucociliary clearance and ciliary beat frequency in cystic fibrosis compared with sinusitis and bronchiectasis. Thorax. 1981;36:654–8. doi: 10.1136/thx.36.9.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smallman LA, Hill SL, Stockley RA. Reduction of ciliary beat frequency in vitro by sputum from patients with bronchiectasis: a serine proteinase effect. Thorax. 1984;39:663–7. doi: 10.1136/thx.39.9.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jain R, Javidan-Nejad C, exander-Brett J, Horani A, Cabellon MC, et al. Sensory functions of motile cilia and implication for bronchiectasis. Front Biosci (Schol Ed) 2012;4:1088–98. doi: 10.2741/s320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hilding AC. The relation of ciliary insufficiency to death from asthma and other respiratory diseases. Ann Otol Rhinol Laryngol. 1943;52:5–19. [Google Scholar]
- 135.Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol. 1960;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mezey RJ, Cohn MA, Fernandez RJ, Januszkiewicz AJ, Wanner A. Mucociliary transport in allergic patients with antigen-induced bronchospasm. Am Rev Respir Dis. 1978;118:677–84. doi: 10.1164/arrd.1978.118.4.677. [DOI] [PubMed] [Google Scholar]
- 137.Mossberg B, Strandberg K, Philipson K, Camner P. Tracheobronchial clearance in bronchial asthma: response to beta-adrenoceptor stimulation. Scand J Respir Dis. 1976;57:119–28. [PubMed] [Google Scholar]
- 138.Bateman JR, Pavia D, Sheahan NF, Agnew JE, Clarke SW. Impaired tracheobronchial clearance in patients with mild stable asthma. Thorax. 1983;38:463–7. doi: 10.1136/thx.38.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ahmed T, Greenblatt DW, Birch S, Marchette B, Wanner A. Abnormal mucociliary transport in allergic patients with antigen-induced bronchospasm: role of slow reacting substance of anaphylaxis. Am Rev Respir Dis. 1981;124:110–4. doi: 10.1164/arrd.1981.124.2.110. [DOI] [PubMed] [Google Scholar]
- 140.Beasley R, Roche WR, Roberts JA, Holgate ST. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989;139:806–17. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- 141.Cokugras H, Akcakaya N, Seckin I, Camcioglu Y, Sarimurat N, Aksoy F. Ultra-structural examination of bronchial biopsy specimens from children with moderate asthma. Thorax. 2001;56:25–9. doi: 10.1136/thorax.56.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Laitinen LA, Heino M, Laitinen A, Kava T, Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985;131:599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- 143.Lewis FH, Beals TF, Carey TE, Baker SR, Mathews KP. Ultrastructural and functional studies of cilia from patients with asthma, aspirin intolerance, and nasal polyps. Chest. 1983;83:487–90. doi: 10.1378/chest.83.3.487. [DOI] [PubMed] [Google Scholar]
- 144.Thomas B, Rutman A, Hirst RA, Haldar P, Wardlaw AJ, et al. Ciliary dysfunction and ultrastructural abnormalities are features of severe asthma. Journal of Allergy and Clinical Immunology. 2010;126:722–9. doi: 10.1016/j.jaci.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 145.Dulfano MJ, Luk CK, Beckage M, Wooten O. Ciliary inhibitory effects of asthma patients’ sputum. Clin Sci (Lond) 1982;63:393–6. doi: 10.1042/cs0630393. [DOI] [PubMed] [Google Scholar]
- 146.Joki S, Saano V, Koskela T, Toskala E, Bray MA, Nuutinen J. Effect of leukotriene D4 on ciliary activity in human, guinea-pig and rat respiratory mucosa. Pulm Pharmacol. 1996;9:231–8. doi: 10.1006/pulp.1996.0029. [DOI] [PubMed] [Google Scholar]
- 147.Schuil PJ, van Gelder JM, ten BM, Graamans K, Huizing EH. Histamine and leukotriene C4 effects on in vitro ciliary beat frequency of human upper respiratory cilia. Eur Arch Otorhinolaryngol. 1994;251:325–8. doi: 10.1007/BF00171538. [DOI] [PubMed] [Google Scholar]
- 148.Ingram JL, Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol. 2012;130:829–42. doi: 10.1016/j.jaci.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 149.Laoukili J, Perret E, Willems T, Minty A, Parthoens E, et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest. 2001;108:1817–24. doi: 10.1172/JCI13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thavagnanam S, Parker JC, McBrien ME, Skibinski G, Heaney LG, Shields MD. Effects of IL-13 on mucociliary differentiation of pediatric asthmatic bronchial epithelial cells. Pediatr Res. 2011;69:95–100. doi: 10.1203/PDR.0b013e318204edb5. [DOI] [PubMed] [Google Scholar]
- 151.Kovacic MB, Myers JM, Wang N, Martin LJ, Lindsey M, et al. Identification of KIF3A as a novel candidate gene for childhood asthma using RNA expression and population allelic frequencies differences. PLoS One. 2011;6:e23714. doi: 10.1371/journal.pone.0023714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim JH, Cha JY, Cheong HS, Park JS, Jang AS, et al. KIF3A, a cilia structural gene on chromosome 5q31, and its polymorphisms show an association with aspirin hypersensitivity in asthma. J Clin Immunol. 2011;31:112–21. doi: 10.1007/s10875-010-9462-x. [DOI] [PubMed] [Google Scholar]
- 153.Amitani R, Wilson R, Rutman A, Read R, Ward C, et al. Effects of Human Neutrophil Elastase and Pseudomonas aeruginosa Proteinases on Human Respiratory Epithelium. Am J Respir Cell Mol Biol. 1991;4:26–32. doi: 10.1165/ajrcmb/4.1.26. [DOI] [PubMed] [Google Scholar]
- 154.Gaillard D, Jouet JB, Egreteau L, Plotkowski L, Zahm JM, et al. Airway epithelial damage and inflammation in children with recurrent bronchitis. Am J Respir Crit Care Med. 1994;150:810–7. doi: 10.1164/ajrccm.150.3.8087356. [DOI] [PubMed] [Google Scholar]
- 155.Balder R, Krunkosky TM, Nguyen CQ, Feezel L, Lafontaine ER. Hag mediates adherence of Moraxella catarrhalis to ciliated human airway cells. Infect Immun. 2009;77:4597–608. doi: 10.1128/IAI.00212-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Look DC, Walter MJ, Williamson MR, Pang L, You Y, et al. Effects of paramyxoviral infection on airway epithelial cell Foxj1 expression, ciliogenesis, and mucociliary function. Am J Pathol. 2001;159:2055–69. doi: 10.1016/S0002-9440(10)63057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rayner CFJ, Rutman A, Dewar A, Cole PJ, Wilson R. Ciliary Disorientation in Patients with Chronic Upper Respiratory Tract Inflammation. Am J Respir Crit Care Med. 1995;151:800–4. doi: 10.1164/ajrccm.151.3.7881674. [DOI] [PubMed] [Google Scholar]
- 158.Kantar A, Oggiano N, Giorgi PL, Braga PC, Fiorini R. Polymorphonuclear leukocyte-generated oxygen metabolites decrease beat frequency of human respiratory cilia. Lung. 1994;172:215–22. doi: 10.1007/BF00164438. [DOI] [PubMed] [Google Scholar]
- 159.Isawa T, Teshima T, Hirano T, Ebina A, Konno K. Mucociliary clearance mechanism in interstitial lung disease. Tohoku J Exp Med. 1986;148:169–78. doi: 10.1620/tjem.148.169. [DOI] [PubMed] [Google Scholar]
- 160.Thomson ML, Short MD. Mucociliary function in health, chronic obstructive airway disease, and asbestosis. J Appl Physiol. 1969;26:535–9. doi: 10.1152/jappl.1969.26.5.535. [DOI] [PubMed] [Google Scholar]
- 161.Yang IV, Coldren CD, Leach SM, Seibold MA, Murphy E, et al. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax. 2013;68:1114–21. doi: 10.1136/thoraxjnl-2012-202943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Knoop C, Estenne M. Chronic allograft dysfunction. Clin Chest Med. 2011;32:311–26. doi: 10.1016/j.ccm.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dolovich M, Rossman C, Chambers C, Grossman RF, New-house M, Maurer J. Mucociliary function in patients following single lung or lung/heart transplantation. Am Rev Respir Dis. 1987;135:7. [Google Scholar]
- 164.Shankar S, Fulsham L, Read RC, Theodoropoulos S, Cole PJ, et al. Mucociliary function after lung transplantation. Transplant Proc. 1991;23:1222–3. [PubMed] [Google Scholar]
- 165.Veale D, Glasper PN, Gascoigne A, Dark JH, Gibson GJ, Corris PA. Ciliary beat frequency in transplanted lungs. Thorax. 1993;48:629–31. doi: 10.1136/thx.48.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Norgaard MA, Andersen CB, Pettersson G. Airway epithelium of transplanted lungs with and without direct bronchial artery revascularization. Eur J Cardiothorac Surg. 1999;15:37–44. doi: 10.1016/s1010-7940(98)00292-9. [DOI] [PubMed] [Google Scholar]
- 167.Read RC, Shankar S, Rutman A, Feldman C, Yacoub M, et al. Ciliary beat frequency and structure of recipient and donor epithelia following lung transplantation. Eur Respir J. 1991;4:796–801. [PubMed] [Google Scholar]
- 168.Thomas B, Aurora P, Spencer H, Elliott M, Rutman A, et al. Persistent disruption of ciliated epithelium following paediatric lung transplantation. Eur Respir J. 2012;40:1245–52. doi: 10.1183/09031936.00174711. [DOI] [PubMed] [Google Scholar]
- 169.Uhlving HH, Buchvald F, Heilmann CJ, Nielsen KG, Gormsen M, Muller KG. Bronchiolitis obliterans after allo-SCT: clinical criteria and treatment options. Bone Marrow Transplant. 2012;47:1020–9. doi: 10.1038/bmt.2011.161. [DOI] [PubMed] [Google Scholar]
- 170.Au WY, Ho JC, Lie AK, Sun J, Zheng L, et al. Respiratory ciliary function in bone marrow recipients. Bone Marrow Transplant. 2001;27:1147–51. doi: 10.1038/sj.bmt.1703049. [DOI] [PubMed] [Google Scholar]
- 171.Au WY, Ho JC, Lie AK, Sun J, Zheng L, et al. A prospective study of respiratory ciliary structure and function after stem cell transplantation. Bone Marrow Transplant. 2006;38:243–8. doi: 10.1038/sj.bmt.1705430. [DOI] [PubMed] [Google Scholar]
- 172.Konrad F, Schiener R, Marx T, Georgieff M. Ultrastructure and mucociliary transport of bronchial respiratory epithelium in intubated patients. Intensive Care Med. 1995;21:482–9. doi: 10.1007/BF01706201. [DOI] [PubMed] [Google Scholar]
- 173.Mammel MC, Ophoven JP, Lewallen PK, Gordon MJ, Sutton MC, Boros SJ. High- frequency ventilation and tracheal injuries. Pediatrics. 1986;77:608–13. [PubMed] [Google Scholar]
- 174.Ophoven JP, Mammel MC, Boros SJ. Tracheobronchial injury with high-frequency oscillatory ventilation. J Pediatr. 1988;112:845–6. doi: 10.1016/s0022-3476(88)80717-0. [DOI] [PubMed] [Google Scholar]
- 175.Bossi R, Piatti G, Roma E, Ambrosetti U. Effects of long-term nasal continuous positive airway pressure therapy on morphology, function, and mucociliary clearance of nasal epithelium in patients with obstructive sleep apnea syndrome. Laryngoscope. 2004;114:1431–4. doi: 10.1097/00005537-200408000-00022. [DOI] [PubMed] [Google Scholar]
- 176.Romanelli MC, Gelardi M, Fiorella ML, Tattoli L, Di VG, Solarino B. Nasal ciliary motility: a new tool in estimating the time of death. Int J Legal Med. 2012;126:427–33. doi: 10.1007/s00414-012-0682-x. [DOI] [PubMed] [Google Scholar]
- 177.Devalia JL, Sapsford RJ, Rusznak C, Toumbis MJ, Davies RJ. The effects of salmeterol and salbutamol on ciliary beat frequency of cultured human bronchial epithelial cells, in vitro. Pulm Pharmacol. 1992;5:257–63. doi: 10.1016/0952-0600(92)90068-r. [DOI] [PubMed] [Google Scholar]
- 178.Lafortuna CL, Fazio F. Acute effect of inhaled salbutamol on mucociliary clearance in health and chronic bronchitis. Respiration. 1984;45:111–23. doi: 10.1159/000194607. [DOI] [PubMed] [Google Scholar]
- 179.Wanner A. Effects of methylxanthines on airway mucociliary function. The American Journal of Medicine. 1985;79:16–21. doi: 10.1016/0002-9343(85)90082-8. [DOI] [PubMed] [Google Scholar]
- 180.Milara J, Armengot M, Banuls P, Tenor H, Beume R, et al. Roflumilast N-oxide, a PDE4 inhibitor, improves cilia motility and ciliated human bronchial epithelial cells compromised by cigarette smoke in vitro. Br J Pharmacol. 2012;166:2243–62. doi: 10.1111/j.1476-5381.2012.01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Iravani J, Melville GN. Effects of drugs and environmental factors on ciliary movement (author’s transl) Respiration. 1975;32:157–64. doi: 10.1159/000193645. [DOI] [PubMed] [Google Scholar]
- 182.Corssen G, Allen CR. Acetylcholine: its significance in controlling ciliary activity of human respiratory epithelium in vitro. J Appl Physiol. 1959;14:901–4. doi: 10.1152/jappl.1959.14.6.901. [DOI] [PubMed] [Google Scholar]
- 183.Lundgren R, Soderberg M, Horstedt P, Stenling R. Morphological studies of bronchial mucosal biopsies from asthmatics before and after ten years of treatment with inhaled steroids. Eur Respir J. 1988;1:883–9. [PubMed] [Google Scholar]
- 184.Fazio F, Lafortuna CL. Beclomethasone dipropionate does not affect mucociliary clearance in patients with chronic obstructive lung disease. Respiration. 1986;50:62–5. doi: 10.1159/000194908. [DOI] [PubMed] [Google Scholar]
- 185.Sisson JH, Pavlik JA, Wyatt TA. Alcohol stimulates ciliary motility of isolated airway axonemes through a nitric oxide, cyclase, and cyclic nucleotide-dependent kinase mechanism. Alcohol Clin Exp Res. 2009;33:610–6. doi: 10.1111/j.1530-0277.2008.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gerrity TR, Cotromanes E, Garrard CS, Yeates DB, Lourenco RV. The effect of aspirin on lung mucociliary clearance. N Engl J Med. 1983;308:139–41. doi: 10.1056/NEJM198301203080306. [DOI] [PubMed] [Google Scholar]
- 187.Matsuura S, Shirakami G, Iida H, Tanimoto K, Fukuda K. The effect of sevoflurane on ciliary motility in rat cultured tracheal epithelial cells: a comparison with isoflurane and halothane. Anesth Analg. 2006;102:1703–8. doi: 10.1213/01.ane.0000216001.36932.a3. [DOI] [PubMed] [Google Scholar]
- 188.Stafanger G, Koch C. N-acetylcysteine in cystic fibrosis and Pseudomonas aeruginosa infection: clinical score, spirometry and ciliary motility. Eur Respir J. 1989;2:234–7. [PubMed] [Google Scholar]
- 189.Shpak M, Goldberg MM, Cowperthwaite MC. Cilia gene expression patterns in cancer. Cancer Genomics Proteomics. 2014;11:13–24. [PubMed] [Google Scholar]
- 190.Auerbach O, Stout AP. Histopathological aspects of occult cancer of the lung. Ann N Y Acad Sci. 1964;114:803–10. [PubMed] [Google Scholar]
- 191.Imai T, Suga M, Kaimori M, Hiyama M, Yokoyama K, Kurotaki H. Peripheral pulmonary papillary adenocarcinoma with prominent cilia: report of a rare case that was difficult to diagnose preoperatively. Acta Cytol. 2010;54:949–57. [PubMed] [Google Scholar]
- 192.Nakamura S, Koshikawa T, Sato T, Hayashi K, Suchi T. Extremely well differentiated papillary adenocarcinoma of the lung with prominent cilia formation. Acta Pathol Jpn. 1992;42:745–50. doi: 10.1111/j.1440-1827.1992.tb03225.x. [DOI] [PubMed] [Google Scholar]
- 193.Park WY, Kim MH, Shin DH, Lee JH, Choi KU, et al. Ciliated adenocarcinomas of the lung: a tumor of non-terminal respiratory unit origin. Mod Pathol. 2012;25:1265–74. doi: 10.1038/modpathol.2012.76. [DOI] [PubMed] [Google Scholar]
- 194.Sato S, Koike T, Homma K, Yokoyama A. Ciliated muconodular papillary tumour of the lung: a newly defined low-grade malignant tumour. Interact Cardiovasc Thorac Surg. 2010;11:685–7. doi: 10.1510/icvts.2009.229989. [DOI] [PubMed] [Google Scholar]
- 195.Inoue Y, Suga A, Sekido Y, Yamada S, Iwazaki M. A case of surgically resected lung cancer in a patient with Kartagener’s syndrome. Tokai J Exp Clin Med. 2011;36:21–4. [PubMed] [Google Scholar]
- 196.Matthys H, Vastag E, Kohler D, Daikeler G, Fischer J. Mucociliary clearance in patients with chronic bronchitis and bronchial carcinoma. Respiration. 1983;44:329–37. doi: 10.1159/000194565. [DOI] [PubMed] [Google Scholar]
- 197.Fukui T, Shaykhiev R, gosto-Perez F, Mezey JG, Downey RJ, et al. Lung adenocarcinoma subtypes based on expression of human airway basal cell genes. Eur Respir J. 2013;42:1332–44. doi: 10.1183/09031936.00144012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Milgrim LM, Rubin JS, Small CB. Mucociliary clearance abnormalities in the HIV-infected patient: a precursor to acute sinusitis. Laryngoscope. 1995;105:1202–8. doi: 10.1288/00005537-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 199.Armengot M, Climent B, Carda C, Ortega E, Basterra J. Clinical and ultrastructural evaluation of nasal mucociliary function in HIV-positive patients. Preliminary investigation. Acta Otorrinolaringol Esp. 1997;48:27–30. [PubMed] [Google Scholar]
- 200.Palm J, Lidman C, Graf P, Alving K, Lundberg J. Nasal nitric oxide is reduced in patients with HIV. Acta Otolaryngol. 2000;120:420–3. doi: 10.1080/000164800750000676. [DOI] [PubMed] [Google Scholar]
- 201.Rosen EJ, Calhoun KH. Alterations of nasal mucociliary clearance in association with HIV infection and the effect of guaifenesin therapy. Laryngoscope. 2005;115:27–30. doi: 10.1097/01.mlg.0000150678.83602.d4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.