Abstract
Despite considerable progress being made in understanding pancreatic cancer (PC) pathogenesis, it still remains the 10th most often diagnosed malignancy in the world and 4th leading cause of cancer related deaths in the United States with a five year survival rate of only 6%. The aggressive nature, lack of early diagnostic and prognostic markers, late clinical presentation, and limited efficacy of existing treatment regimens makes PC a lethal cancer with high mortality and poor prognosis. Therefore, novel reliable biomarkers and molecular targets are urgently needed to combat this deadly disease. MicroRNAs (miRNAs) are short (19–24 nucleotides) non-coding RNA molecules implicated in the regulation of gene expression at post-transcriptional level and play significant roles in various physiological and pathological conditions. Aberrant expression of miRNAs has been reported in several cancers including PC and is implicated in PC pathogenesis and progression, suggesting their utility in diagnosis, prognosis and therapy. In this review, we summarize the role of several miRNAs that regulate various oncogenes (KRAS) and tumor suppressor genes (p53, p16, SMAD4 etc) involved in PC development, their prospective roles as diagnostic and prognostic markers and their therapeutic targets.
1. Introduction
Pancreatic cancer (PC) is a lethal malignancy and remains a major clinical challenge. Due to its early metastatic nature, more than 80% of PC patients have invasive disease at the time of diagnosis, which makes surgical and medical intrusions mostly unsuccessful, resulting in high mortality and poor prognosis [1, 2]. With equal incidence to mortality ratio and a five year survival rate of less than 6%, PC is considered to be the most deadliest and aggressive cancer compared to other malignancies [3]. Limitations of the current multimodality therapeutic regimens for PC highlights the urgent need to understand the molecular mechanism/pathway(s) governing initiation, progression and metastasis for discovering novel diagnostic, prognostic and therapeutic targets for better management of this lethal disease. Although several research efforts are directed to discover specific bio-molecules with their utility in early diagnosis, prognosis and therapy for PC, these currently used biomolecules do not have adequate sensitivity and specificity to detect PC in its early stages, nor can they be used to target PC.
The advent of microRNAs (miRNAs) research has opened new avenues to understand the gene regulatory mechanisms at the post-transcriptional level. miRNAs are double stranded small non-coding RNA molecules of 19–24 nucleotides (nt) in length, regulating gene expression at the post-transcriptional level either by degradation or translational inhibition of their target mRNA [4, 5]. Details about the miRNA biogenesis, role in physiology, development and miRNA mediated pathological conditions have already been discussed elsewhere [5–13]. Association of miRNAs role in cancer was first described in Chronic Lymphocytic Leukemia (CLL) [14] and subsequently several miRNAs were reported to be aberrantly expressed in several other cancers including PC [12, 15–22]. They play significant roles in initiation, progression, metastasis, and therapeutic resistance [23]. Although miRNAs represent only 3% of the human genome, they regulate 20–30% of the protein coding genes [24, 25]. Recent studies have shown that 50% of miRNA genes are located in genomic instability regions that are generally associated with neoplastic transformation [26]. Therefore, miRNA signatures can provide better patho-genomic information about tumors than their transcriptome profiles [27]. Moreover, expression patterns of miRNAs are tissue specific [28, 29] and unique to tumor type and tissue of origin [12, 27, 30], thus making miRNAs valuable tools for diagnosis, prognosis and therapy. Pancreatic cancer pathogenesis is a multistep process that involves a compendium of sequential genetic alterations in oncogenes like KRAS and several tumor suppressors including p53, p16, SMAD4 etc [31]. In this review article, we have summarized the role of miRNAs in PC pathogenesis with specific emphasis on their role in regulating KRAS, p53, p16, and SMAD4 expression, along with their utility as early detection/prognostic markers and therapeutic targets.
1.1 Deregulated miRNAs in Pancreatic Cancer
Several research studies have proposed an association between altered miRNA expression and PC. Identification of miRNA(s) at various stages of PC progression is critical to understand as their biological functions will help us develop unique diagnostic/prognostic markers and therapeutic targets. In this context, miRNA profiling of tumor samples, pancreatic juice, serum and cyst fluid engenders a potential avenue for miRNA-based biomarker development. The first miRNA expression study was done by Poy et al, where the expression of miR-375 and miR-376 was observed in mouse pancreas (pancreas specific miRNAs) but not in the brain, heart, and liver tissues [32]. Later, high throughput analysis identified several miRNA signatures that were able to precisely classify tumors and differentiate PC from normal pancreas and pancreatitis [33]. In addition, profiling of PC tissue or desmoplasia revealed several precursor miRNAs that were aberrantly expressed and localized to tumor cells including miR-221, -376a, and -301 [34]. Further, extensive miRNA expression profiling in healthy individuals, pancreatitis, precursor pancreatic intraepithelial neoplasm (PanIN) lesions, and pancreatic ductal adenocarcinoma (PDAC) patients have revealed their diagnostic and prognostic utility [11, 35–37]. Subsequently, over expression of miR-376 was reported in human Panc-1 PC cells compared to lung, breast, head and neck, colorectal, prostate, and hematopoietic cancer cells [38], although no difference in expression of miR-375 was observed [12, 38]. While expression of miR-216 and 217 (pancreas specific miRNAs), and the lack of miR-133a are characteristic of normal pancreatic tissues [33], over expression of miR-103 and miR-107, and downregulation of miR-155 are characteristic of PC specimens [39]. Furthermore, up regulation of miR-21 and downregulation of miR-150, miR-30d in PC tissues compared to normal pancreas was reported [40]. The miR-221, miR-376a and miR-301 are present specifically only in the tumor cells of PC and are among the top differentially expressed miRNAs [34]. Recently, miRNA microarray analyses in a panel of 15 PC cell lines revealed overexpression of miR-10a, miR-92, and miR-17-5p [41]. In addition to cell lines, several studies have reported differential expression of miRNAs that can distinguish between PDAC from chronic pancreatitis and normal pancreas [33, 42–44] and a cluster of miR30a-3p, miR-105, miR-127, miR-187, miR-452, and miR-518a-2 was also associated with better prognosis of lymph node positive patients [33]. The miR-203 was over expressed in PC and associated with poor prognosis of patients with no residual tumors [45]. Yu et al profiled 700 miRNAs in PanIN lesions and observed an overexpression of let-7f/g, miR-18a, -15b, -21, -29a/b/c, -31, -93, -95, -101, -103, -106b, 146a, -155, -182, -190, -193b, -194, -196b, -200a/b, -203, -222, 338-3p, -429, and 486-3p, and no or weak expression of miR-107, -139-3p/5p, -216a/b, -217, -218 and -483-5p in PanIN-3 lesions [46]. A similar miRNA expression profile using 10 PC cell lines and 17 pairs of PC/normal tissues has revealed significant up-regulation of miR-196a, miR-190, miR-186, miR-221, miR-222, miR-200b, miR-15b, and miR-95 [47]. In addition, miR-376a, miR-301, miR-132 and miR-212 were reported to be over expressed specifically in PC compared to normal or benign adjacent pancreas [34, 48]. On the contrary, miR-132 was also found to be down regulated in PC tissues compared to normal and benign tissues [49].
The miRNA deregulation is not only observed in human PC cell lines and tissues but is also evident in the circulation of PC patients. Many studies have identified differential up-regulation of miRNAs including miR-642b, miR-885-5p, miR-22, miR-20a, miR-21, miR-24, miR-25, miR-99a, miR-185, miR-191, miR-155, miR-196a, and miR-18a in blood of PC patients and these miRNAs could differentiate PC patients from healthy individuals [50]. In addition, miR-2 was over expressed in serum [51], whereas, over expression of miR-196a, miR-217, miR-451, and miR-486-5p [52] and down regulation of let-7c, let-7d, let-7f, and miR-200c was observed in the fine needle aspiration samples (FNA) of PC patients [52].
2. miRNA and KRAS in pancreatic cancer
KRAS is a membrane bound Guanosine Triphosphate (GTP) binding protein from the RAS family of proteins that regulates several signal transduction pathways like RAF, MAP2K, MAPK Tiam1, Ral-GEF and PI3K AKT upon receiving extracellular stimuli. Usually, RAS associated signaling activation is short lived due to its intrinsic GTPase activity, switching off the RAS signaling cascades. However, KRAS is mostly mutated (KrasG12D) in 90% of PC [53] that results in gain of function and hyper activation of downstream signaling cascades involved in the change of transcriptional dynamics, cell survival and proliferation facilitating tumor progression and metastasis [54]. In spite of establishing KRASG12D as a potent oncogene, attempts to inhibit the hyper activity or identifying various factors that induce this mutation have failed. Recent discoveries have identified several miRNAs that target KRASG12D by binding to the 3′UTR of the KRAS mRNA transcript and lead to its degradation or translational inhibition. In this section we have attempted to elucidate the role of miR-96, -126, -143/145, -217 and Let-7 mediated regulation of oncogenic KRAS in PC.
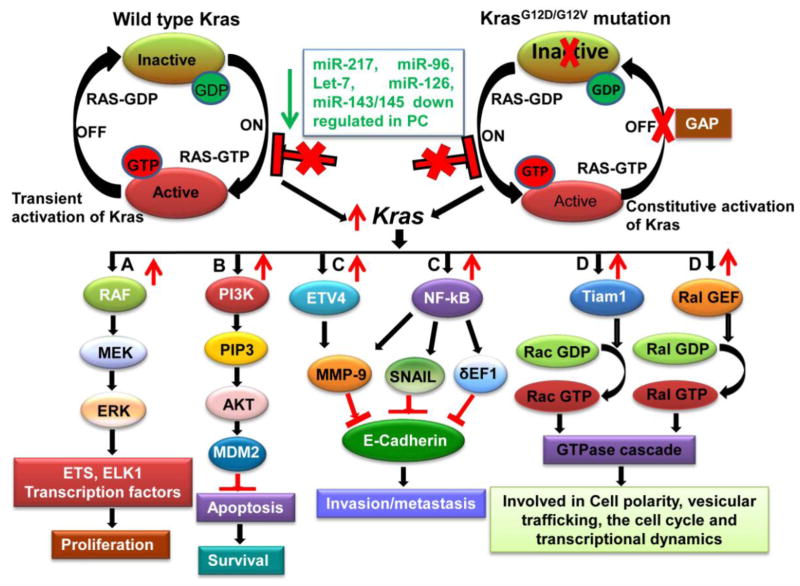
Recently, Yu et al. has observed that miR-96, which is usually lost in PC tissues compared to normal pancreas targets KRAS by perfect base-pairing with its 3′UTR [55]. Overexpression of miR-96 in PC cell lines resulted in decreased cell migration, invasion, and proliferation, indicating its tumor suppressive potential [55]. Similarly, miR-126 was also found to be down regulated in PDAC compared to benign cystic tumors and was identified to inhibit translation of KRAS by binding to its two putative seed regions on the 3′ UTR suggesting that re-expression of miR-126 can be a powerful therapeutic strategy for KRAS mediated oncogenesis [56]. Not only do miRNAs target wild type/mutated KRAS gene expression, KRASG12D also modulates miRNA(s) expression during cellular transformation. RAS-responsive element-binding protein (RREB1) binds to the promoter of miR-143/145 cluster and negatively regulates their expression. Surprisingly, oncogenic KRASG12D induces RREB1 expression in PC as means to check expression of the miR-143/145 cluster for enhanced tumorigenesis [57]. While constitutive miR-143/145 cluster expression targets RREB1 protein to inhibit a feed forward circuit of KRAS signals via RREB1, the KRASG12D mediated over expression of RREB1 in turn represses the miR-143/145 cluster expression and therefore facilitates further KRAS mediated signaling [57]. Besides the miR-143/145 cluster, another study using in situ hybridization technique revealed down regulation of miR-217 in 76% of PC tissues [58]. The same study also reported that over expression of this miR-217 in PC cells decreased KRAS expression and phosphorylated AKT levels, suggesting that miR-217 not only downregulates KRAS expression but also affects the downstream signaling molecules involved in cell survival and proliferation [58]. Let-7 family miRNAs have multiple target sites on the 3′ UTR of KRAS and downregulate its expression [59]. Several studies have shown significant down-regulation of let-7 expression in PC samples compared to cancer adjacent normal tissues, and PC patients with loss of let-7 expression were ineligible for surgery due to aggressiveness of the disease [60]. They also observed that ectopic over-expression of let-7 in PC cells significantly downregulates KRAS expression and inhibits MAPK with concomitant decrease in cell proliferation. However, let-7 over-expression fails to inhibit tumor growth and progression [60]. In addition to affecting KRAS expression in PC, miRNAs also modulate KRAS expression in breast and lung cancer. Kopp et al., observed an inverse correlation between KRAS expression and miR-200c expression in a panel of breast cancer cell lines [61]. Furthermore, they also observed that miR-200c directly regulates KRAS activity and additionally affects other tumorigenic pathways inhibiting tumor progression and resistance to therapy. Therefore, miRNAs definitely play a key role in modulating PC as they regulate the expression of key oncogenes such as KRAS which has been shown to be the initiating event for PC and important for tumor maintenance (Figure 1).
Figure 1. Therapeutic potential of miRNA-mediated KRAS down-regulation in pancreatic cancer.
More than 90% of pancreatic cancer (PC) patients have mutations (G12D/G12V) in codon 12 of KRAS that results in its constitutive activation and hyper activation of its downstream signaling pathways accompanied by increased proliferation, motility and survival of PC cells. KRAS targeting miRNAs like miR-217, -96, -126, -143/145 and Let-7 are significantly down regulated in PC patients that results in increased KRAS expression. Restoration of these miRNAs expression may result in abrogation of KRAS mediated signaling pathways such as (A) RAF/MEK/ERK that will result in decreased PC cell proliferation, (B) PI3K/AKT pathway abrogation will decrease PC cell survival, C) Inhibition of NF-κB and ETV4 will result in decreased cell invasion and metastasis by up regulating E-Cadherin expression and (D) Down regulation of Tiam1 and Ral-GEF will effect cell polarity, vesicular trafficking, cell cycle and transcriptional dynamics in pancreatic cancer (miRNAs in color in green indicates downregulated in PC). RAF: Raf/mil family of serine/threonine protein kinases; MEK: Mitogenactivated protein kinase; ERK: Extracellular signal-regulated kinases; ETS: E-twenty-six family transcription factors; ELK1: ELK1, member of ETS oncogene family; PI3K: Phosphatidylinositol-4,5-bisphosphate 3-kinase; PIP3: Phosphatidylinositol (3,4,5)-trisphosphate; AKT: v-akt murine thymoma viral oncogene homolog; MDM2: Mouse double minute 2 homolog; ETV4: Human ETS translocation variant 4; NF-κB: Nuclear Factor Kappa B; MMP9: Matrix Metalloproteinase 9; SNAIL: Snail family zinc finger 1; δ-EF1: translational elongation factor 1 delta; TIAM1: T-cell lymphoma invasion and metastasis 1; Ral-GEFs: Ral guanine nucleotide exchange factors.
3. miRNA(s) and the p53 pathway
Inactivation of tumor suppressor genes is commonly observed in the multistep progression of various cancers. The Trp53 (p53), referred to as the guardian of the genome is located on chromosome 17p and plays an important role in controlling cellular responses involving cell cycle arrest and programmed cell death due to various physiological stress [62]. Wild type p53 binds to DNA and induces p21 expression to negatively regulate cell division-stimulating proteins including cyclin D1 and cyclin-dependent kinase-2 resulting in growth arrest [63]. However, more than 50% of adult human tumors have inactivating mutations or deletions in the p53 gene leading to loss of its function without affecting its translation [64]. Inactivation of p53 due to somatic mutations is observed in 50–75% of PDAC [65]. Gain of function mutations (R175, G245, R248, R249, R273 and R282) in the DNA binding domain of p53 leads to loss of its function associated with uncontrolled cell growth, cell survival, and genetic instability [64]. More precisely, R175/R248/R273 mutations of p53 are mainly observed at late PanINs with dysplasia and occurs mostly in single allelic loss coupled with an intragenic mutation of the second allele, leading to uncontrolled cell proliferation and inhibition of apoptosis [66]. These mutations may be structural affecting folding of the protein or they can affect p53-DNA interactions, thereby altering transcriptional regulation of its target genes [67–69]. However, mutant p53 recognizes and binds to transcription factors/cofactors/p63/p73 proteins that leads to transcriptional upregulation of genes involved in uncontrolled growth [70–73]. Besides in vitro studies, recently transforming capabilities of mutant p53 were successfully demonstrated using genetically engineered mouse models (GEMs) for various mutant p53 [65, 74, 75]. Recently, a co-operative association of mutant p53 (p53R172H) and other genes was observed in cellular signaling pathways and cell fate decisions by regulating cell proliferation, motility and invasion, apoptosis, inflammation and angiogenesis either in the cancer cell itself or in the surrounding microenvironment during the tumorigenesis and metastasis [76]. In addition, the co-operative role of mutant p53R172H and KrasG12D has also been investigated during PC pathogenesis using GEM models [77].
3.1 p53 mediated regulation of miRNAs
Besides regulating proteins involved in cell cycle and apoptosis, wild type p53 has recently been shown to modulate expression of several miRNAs in cancer cells, while siRNA mediated knockdown of p53 abolished effects on miRNA expression [78]. Surprisingly, global sequence analysis has revealed that >46% of miRNA promoters have p53 binding sites suggesting that p53 can be a master regulator of miRNA expression [78] and subsequently other studies also showed transactivation of several miRNAS by p53 [79]. miR-34 family members are evolutionary conserved between species and are transcribed from two different gene loci [80]. While miR-34a is located at chromosome 1p36 and is expressed in wide variety of tissues, miR-34b and miR-34c are localized to chromosome 11q23 and mainly expressed in lung tissues [81]. Recently p53 has been shown to bind to p53 responsive elements on the promoters of miR-34 family (miR-34a/b/c) of miRNAs, thereby inducing cell cycle arrest and apoptosis [82–87]. Ectopic overexpression of miR-34 leads to G1 phase cell-cycle arrest [82, 84, 87], inhibition of proliferation, colony formation [83], increased apoptosis [86–88], and induced cellular senescence in human diploid fibroblasts by targeting several of its putative target genes like CDK4, BCL-2, Cyclin E, CDK6, c-MET, SIRT1, c-MYC, N-MYC, Axl, SNAIL and LDH etc [84]. However, several cancers including PC harbor CpG methylation on promoters of miR-34a/b/c and this significantly inhibits their transcription by p53 [89, 90]. PC cells have a low or undetectable level of miR-34a [91], however their low/loss of expression was neither associated with loss of heterozygosity (LOH), p53 mutation, nor with promoter CpG methylation [82].
Several reports have shown that mutant p53 also regulates transcription of miR-130b, miR-155, and miR-205 and thus alter the expression of their target genes like ZEB1 and ZNF652 either by inhibiting/degrading translation of their mRNA transcripts, thereby influencing invasive and metastatic potential of cells [92–94]. The mutant p53 regulates these miRNAs in either p63 dependent (miR-155 and miR-205) or p63-independent manner (miR-130b) [92–94]. The miR-15a/16-1 family is another transcriptional target of p53 that is significantly down regulated in PC [15]. Ectopic expression of miR-15a was shown to down-regulate WNT3A and FGF7 and results in reduced survival and proliferation of PC cells [95]. Furthermore, the tumor suppressor functions of this cluster was confirmed both in vitro and in vivo, whereby it downregulated ALDOA and TPI1 expression, affecting glycolysis and cell metabolism [15]. By inducing miR-145 expression, p53 downregulates c-MYC expression and impedes tumor growth both in vitro and in vivo [96]. However, miR-145 expression was significantly down regulated in PC tissues as compared to healthy control groups [43]. The p53 regulated miR-107 expression inhibits hypoxia inducible factor-1β (HIF-1β) expression, that on its interaction with HIF-1α forms HIF-1 complex and induces genes that are involved in the pathogenesis of cancer [97]. However, miR-107 is up regulated in many solid tumors including PC [12, 97]. Besides PC, overexpressed miR-107 in breast cancer negatively regulates expression of tumor suppressor let-7 miRNA [97]. Ectopic expression of miR-107 destabilizes let-7, and thereby upregulates let-7 target genes that are involved in progression of breast cancer [97]. The miR-200 family members (miR-200c/141, miR-200a/b/429) inhibit expression of Zeb1 and Zeb2 transcription factors favoring mesenchymal to epithelial transition and reduced tumor growth [98, 99]. Transcription factor Zeb1, by upregulating Jag1, mastermind-like co-activators Maml2 and Maml3 promotes pancreatic cancer progression through notch signaling. p53 has been shown to induce expression of miR-200 family members accompanied by downregulation of Zeb1 and Zeb2 expression that resulted in decreased tumor progression. Furthermore, knockdown of p53 expression resulted in abrogation of miR-200 family members associated with up regulation of Zeb1 and Zeb2. However, Soubani et al. has recently shown that chemo-resistant PC cell lines have downregulated expression of miR-200 family members including miR-200a/b/c suggesting deregulated p53 signaling in these cells [100]. In addition, upregulated expression of Zeb1 and Jag1 was also associated with decreased miR-200 family members in PC [101]. Similarly Kim et al. has shown that p53 also induces the expression of miR-192 family members (miR-192/194/215) that target Zeb1 expression and inhibit epithelial to mesenchymal transition and induces cell cycle arrest by targeting MAD2L1, CUL5, and CDC7 genes [98, 99, 102]. Contrary, expression of miR-192 was high in PDAC tissues as compared to normal pancreas [103]. Besides transcriptional regulation, p53 also regulates miRNA maturation post-transcriptionally by modulating the function of drosha that is involved in the processing of pri-miRNA to pre-miRNA in the nucleus. To achieve this process, it forms a complex with DEAD box RNA helicases p68 and p72 and interacts with drosha to process pri-miRNA to pre-miRNA (miR-16-1, miR-143 and miR-145) [104]. Significant downregulation of miR-145 and miR-16-1 as seen in PC [43, 95] speculates that point mutations like R175H/R273H in p53 may decrease the maturation of these miRNAs. Further, Dnmt3a directly interacts with p53 and inhibits p53 mediated transcription of p21 and other tumor suppressor genes in the genome [105].
3.2 miRNAs regulating p53 expression
Several in silico analysis tools have predicted potential binding sites on the 3′UTR of p53 mRNA for multiple miRNAs. Recently, A study revealed that ectopic expression of miR-491-5p in SW-1990 PC cells down regulates p53 expression suggesting that p53 not only has the potential to modulate miRNA expression, but in turn miRNAs can also regulate p53 expression for cooperative normal functioning of cells [106]. Besides effecting p53 expression, miR-491-5p overexpression also abrogated several signaling pathways like Jak/Stat3, PI3K/Akt accompanied by decreased cell proliferation and induction of apoptosis [106]. Tumor suppressor miR-34 cluster is also involved in inhibiting cell proliferation, migration, invasion, and epithelial to mesenchymal transition [89, 107]. It has been shown that p53, by up regulating miR-34a expression, represses NAD-dependent deacetylases that in turn increases acetylated p53 (lysine 382) and transcriptionally upregulates p21 and PUMA expression [108, 109]. Furthermore, miR-34a also represses HDM4, a negative regulator of p53 and therefore creates a positive feedback loop on p53 expression [110]. Therefore, any imbalance in this positive feedback mechanism decreases p53 activity accompanied by enhanced cell proliferation and cell survival. Unfortunately, due to CpG island promoter hypermethylation, miR-34s are frequently silenced in a variety of cancers including PC [107]. But ectopic expression of miR-34a in PC cells has been shown to induce cellular senescence and cell cycle arrest by targeting CDK6. Another miRNA, miR-214 is over expressed in PC [12] and is involved in cancer progression, metastasis and chemo-resistance [111, 112]. This overexpressed miR-214 targets p53 and relieves negative inhibition on Nanog expression thereby enriching the stem cell population in ovarian cancer cells [113]. While miR-125b is over expressed in PC [33], it negatively regulates p53 expression during stress, its ectopic expression also downregulates p53 expression and inhibits apoptosis in lung fibroblast and human neuroblastoma cells [114].
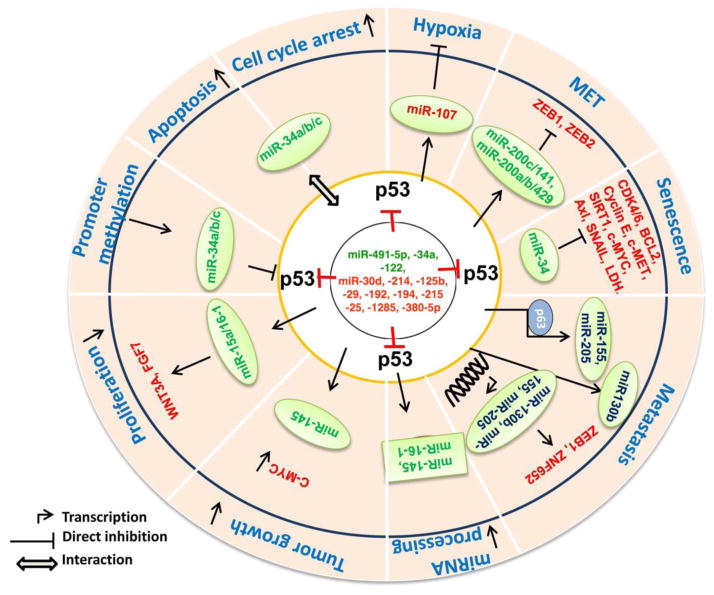
The p85α is a regulatory subunit of the PI3K complex that negatively regulates p53 expression and plays a critical role in cell survival and apoptosis [115]. However, miR-29 mediated p85α downregulation has been shown to activate p53 signaling pathway and induces apoptosis in Hela cells [116]. Interestingly, overexpression of miR-29a/b/c was observed in PC tissue and the PC cell lines MiaPaCa-2, PANC-1, and BxPC-3 as compared to normal tissues and HPDE cells [47]. In contrast, miR-504 downregulates p53 expression and inhibits p53 mediated apoptosis, cell cycle arrest that leads to tumor promotion in vivo [117]. miR-122 by modulating cyclin G1/PP2A and MDM2 expression activates and stabilizes p53 protein expression in hepatocellular carcinoma cells [118] but it is significantly down regulated in PC [43]. Whereas other miRNAs like miR-33, miR-1285, miR-380-5p, miR-25, miR-133 and miR-30d directly bind to the 3′UTR of p53 and repress its expression [119], out of which miR-133b is down regulated, whereas miR-25 is overexpressed in mouse PC [120]. P53 also modulates MDM2 by negatively regulating miRNAs such as miR-192/194/215 and miR-605 as a feedback loop mechanism to check p53 activity physiologically [121, 122], however, miR-192 is significantly upregulated in the PC patients [103, 123]. The overall role of p53 mediated miRNA transcription and its regulation by miRNAs is shown in Figure 2.
Figure 2. miRNA mediated signaling of p53 in pancreatic cancer.
DNA damage and stress signals like starvation, hypoxia, oncogene hyperactivation etc. by upregulating p53 expression transcriptionally modulate several miRNAs (miR-107, -34a/b/c, miR-34) that induce cell cycle arrest, apoptosis cellular senescence and inhibits hypoxia. p53 mediated overexpression of miR-15a/16-1 clusture inhibits proliferation of pancreatic cancer cells by downregualting WNT3A and FGF7 proteins. By upregulating miR-145 expression, p53 down-regulates c-Myc expression thereby decreases cell survival in vitro and impedes tumor growth in vivo. Similarly, p53 by regulating miR-200a/b/c, miR-141 and miR-429 inhibits ZEB1 and ZEB2 transcription factors that favor mesenchymal to epithelial transition thereby impeding the tumor growth. However, mutated p53 negatively regulates miR-155, miR-205 (p63 dependent manner) and miR-130b, (p63 independent) that targets ZEB1 and ZNF652 expression thereby enhance the invasion, migration and metastatic potential of pancreatic cancer cells. Missense mutations in p53 also interferes miRNAs (miR-145 and miR-16-1) maturation resulting in enhanced cell proliferation, invasion and migration. Certain miRNAs like miR-491-5p, -34a, -122, miR-30d, miR-214, -125b, -29, -192, -194, -215, -25, -1285, -380-5p regulate p53 expression by binding to its 3’UTR. Through feedback mechanism, CpG promoter hypermethylation of miR-34a/b/c could also influence p53 transcription thereby hindering p53 mediated biological functions. Abbreviations: P53: Tumor protein p53; BCL2: B cell leukemia/lymphoma 2; c-MYC: v-Myc avian myelocytomatosis viral oncogene homolog; CDK4/6: Cyclin-Dependent Kinase 4/6; SNAIL: Snail Family Zinc Finger 1; c-MET: Met Proto-Oncogene; AXL: AXL Receptor Tyrosine Kinase; SIRT-1: Sirtuin1; ZEB1/2: Zinc Finger E-Box Binding Homeobox 1/2; LDH: Lactate Dehydrogenase; ZNF652: Zinc Finger Protein 652; WNT3A: Wingless-Type MMTV Integration Site Family, Member 3A; FGF7: Fibroblast Growth Factor 7.
4. p16 and miRNA(s) in pancreatic cancer
The INK4A/p16, also known as cyclin dependent kinase inhibitor 2A (CDKN2A) is a tumor suppressor gene involved in cell cycle regulation and cellular senescence [124, 125]. However, promoter methylation, missense mutation and deletions of the p16 gene leads to its inactivation in most of the cancers including PC where its function is lost in around 95% of cases [126]. In addition, miRNA mediated regulation of p16 has been documented in many cancers including PC. Recent studies have shown that miR-24 was up regulated in PC [11, 127], and other study have shown that it binds to both the coding and 3′UTR regions of p16 mRNA that results in its translation inhibition associated with decreased cell proliferation [128]. Similarly, miR-10b, also overexpressed in PC [129], post-transcriptionally regulates p16 expression by binding to its 3′UTR region, whereas inhibition of this miRNA in glioblastoma resulted in inhibition of tumor growth through activation of cell cycle arrest and apoptotic pathway [130]. In addition, several other miRNAs like miR-128a upregulate p16 expression by targeting its negative regulator Bmi-1, resulting in decreased growth of medullablastoma cells [131]. Similarly, miR-20a also upregulates p16 expression and inhibits tumorigenesis [132]. DNA methyltransferase (DNMT1) has been shown to negatively regulates p16 expression, and silencing of its regulators like miR-148a and miR-152 leads to decreased p16 expression and enhanced cholangiocarcinoma growth [133]. Not only do miRNAs target p16 expression, indeed p16 signaling has been shown to regulate expression of miRNA(s). In this context, ectopic overexpression of p16 in breast and glioma cells increased miR-410 and miR-650 expression and reduced CDK1 protein levels [134]. In addition, UV induced DNA damage initiates association of p16 with sp1 transcription factor and CDK4 (cyclin dependent kinase) causing subsequent upregulation of miR-141 and miR-146b-5p [135].
5. TGF-β/SMAD signaling regulates miRNAs in PC
SMAD proteins are the backbone of the transforming growth factor beta (TGF-β) family of cytokines [136]. Binding of ligand to TGF-β Receptor II (TGFβRII) results in its heterodimerisation with TGF-β Receptor I (TGFβRI) with their subsequent trans-phosphorylation and activation of their substrate SMADs. The human genome encodes eight different SMAD proteins among which SMAD-1, SMAD-2, SMAD-3, SMAD-5 and SMAD-8 are regulated by the TGF-β family of receptors commonly referred as R-SMADs. A common SMAD protein, SMAD-4 is a co-mediator that translates extracellular signals into response. Activated SMAD-2 and/or SMAD-3 heterodimerises with SMAD-4 protein and translocates into the nucleus, along with other co-regulators to engage in transcriptional regulation of various target genes. Whereas, SMAD-6 and SMAD-7 are inhibitory SMADs (I-SMADs), which act as a negative regulator of R-SMADs [137]. Previous studies have shown the existence of signaling cross talks between SMAD proteins and other non-SMAD pathways such as extracellular signal related kinase (ERK), MAPK, protein kinase C (PKC). These intracellular kinases can phosphorylate R-SMADs at potential serine residues preventing accumulation of R-SMADs in the nucleus [138]. In a normal epithelial cells, TGF-β acts as a potent tumor suppressor eliciting growth inhibition through downregulating transcription factor c-Myc and up regulating cell cycle inhibitor proteins such as p15 and p21 [139, 140]. In cancer cells, the TGF-β/SMAD signaling is modulated and loss of some components of the TGF-β/SMAD pathway leads to impairment of TGF-β/SMAD mediated growth arrest [141].
Several in vitro and in vivo findings have revealed that a variety of miRNAs are controlled and modulated under the influence of R-SMAD proteins [142–145] and the TGF-β signaling pathway. For example, TGF-β signaling induces maturation of miR-21 through various post transcriptional modifications of primary miR-21 into mature miR-21 through a microprocessor complex consisting of DROSHA and RNA helicase p68. Thus ligand specific activation of SMAD protein may be involved in the biosynthesis of miRNAs such as miR-21, which in turn downregulate the tumor suppressor genes PDCD4 and PTEN, enhancing tumor invasion and metastasis [144]. Kong et al. have demonstrated that TGF-β/SMAD-4 signaling can induce miR-155 expression and its restoration inhibits cell migration and invasion in normal murine mammary epithelial cells [145]. Naito et al. have identified the presence of increased miR-143 expression in scirrhous type gastric cancer but not in non scirrhous type gastric cancer specimens. Further, several studies have revealed that miR-143 is expressed mainly by stromal fibroblast cells, not by gastric cancer cells and this expression is under the influence of TGF-β/SMAD signaling activation [146]. Recently, a functional screening approach was done to identify miRNAs that can regulate SMAD-4 expression in gastric cancer cells. Using a luciferase reporter assay supported by bioinformatics analysis they identified that miR-199a directly targets SMAD-4 expression, suggesting the implication of miR-199a as a negative regulator of TGF-β/SMAD signaling [147]. In addition, Liu et al. have shown that the 130a/301a/454 of family of miRNAs regulates TGF-β signaling by repressing the SMAD-4 expression by directly binding to its 3′-UTR sequence [148], further, this cluster is upregulated in PC [149, 150]. Among the SMAD family of tumor suppressor genes, SMAD-4 (DPC-4) is frequently inactivated in pancreatic, bile duct adenocarcinoma, and colorectal cancer. Studies have explored that SMAD-4 gene is inactivated in 55% of PC patients either by deletion in both the alleles (35%) or by intra-genic mutation in one allele coupled with LOH (20%) [151]. In the classical PC progression model, SMAD-4 gene inactivation occurs at the later stages of PanINs, thus loss of SMAD tumor suppressor was correlated with disease advancement or metastasis [152]. A recent study demonstrated that co-expression of SMAD-6 and/or SMAD-7 along with SMAD-4 in patients with PDAC could be one possible mechanism of SMAD-4 inactivation [153].
Traditional small molecular inhibitors like SD-208, SB431542 and LY-2157299, specific for TGF-β receptor abrogated SMAD mediated responses like its anti-invasive effect in PC cells [154] and inhibited cancer advancement and metastasis in xenograft mouse models [155]. Treatment of mouse preantral granulosa cells with recombinant TGF-β leads to upregulation of miR-224. Further this upregulated miR-224 was diminished upon treatment of above cells with SB-431542 (a TGF-β type I receptor inhibitor), indicating that anti-TGF-β therapies could block the canonical TGF-β/SMAD signaling pathway and their respective miRNA biogenesis and regulation [156]. miRNAs play a critical role in cell mediated immune response by controlling the dendritic cells function in cancer patients. Recently, Du et al. identified that miRNA-146a is aberrantly expressed in human CD14+ monocyte-derived dendritic cells from PC patients in vitro. Further, miR-146a expression is correlated with impairment of differentiation and inhibition of the antigen presentation function of dendritic cells through the mechanism of SMAD-4 repression. Thus, miRNAs are one of the factors involved in the regulation of immune response indirectly by modulating dendritic cells [157]. Interestingly, overexpression of other miRNAs can also directly influence SMAD-4. The miR-483-3p and miR-421 are involved in PC progression by directly regulating the tumor suppressor DPC4/SMAD4. Specifically, the expression level of miR-421 is inversely correlated with DPC4/SMAD-4 expression in PC specimens. Through various in vitro studies, it has been identified that miR-421 can also play a dual role by targeting DPC4/SMAD4 along with its downstream effectors p21 and p15 in PC [158, 159]. More recently, Li et al had identified the negative regulatory role of miR-494 on transcriptional activator FOXM1 on pancreatic cancer cells. Clinically, miR 494 expression levels were demonstrated to be higher in adjacent normal pancreatic tissues compared to PDAC specimens, suggesting that reduced expression of miR 494 as a critical factor in pancreatic cancer progression and development. By restoration of miR-494, the authors have showed decreased pancreatic cancer cell growth and metastasis through various in vitro and in vivo assays. Finally, they correlated the reduced expression of miR-494 with increased expression of FOXM1 and enhanced nuclear translocation of betacatenin. They also identified the molecular mechanism and association of SMAD4/miR-494/FOXM1/Betacatenin signaling cascade and its biological impact on pancreatic cancer pathogenesis. In particular, miR-494 is the novel microRNA identified with tumorsuppressive function in PDAC [160]. Along similar lines, miR-182-5p was reported to be overexpressed in bladder cancer, which could potentially target SMAD-4 expression. The repressed SMAD-4 can influence nuclear transport of β-catenin thereby resulting in activation of WNT signaling cascade in cancer cells [19]. Also, another study by Geraldo et al. has shown that ectopic overexpression of miR-146b-5p in rat follicular thyroid carcinoma cells resulted in significant increase in cell proliferation and development of resistance to TGF-β mediated cell cycle arrest. Thus, miR-146b-5p regulates the TGF-β mediated signaling pathway by repressing SMAD4 in thyroid cancer cells [161]. Overall these studies provide evidence that SMAD proteins are (i) involved in miRNA biogenesis, (ii) lost in expression in various cancers, and (iii) downregulated by miRNAs (Figure 3).
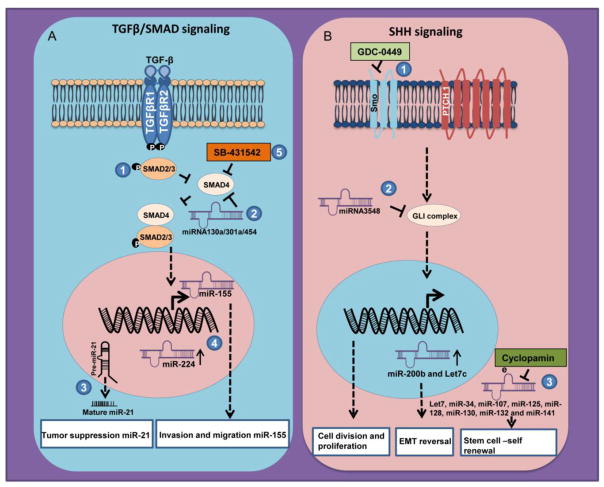
Figure 3. MiRNAs involved in TGF-β/SMAD and SHH signaling and their targeting approaches for pancreatic cancer pathogenesis.
(A) Binding of TGF-β to its cognate receptor leads to phosphorylation and therefore activation of SMAD signaling. (1) Phosphorylated SMADs (SMAD2 and SMAD3) form a complex with co-mediator SMAD4 and translocates to the nucleus for active transcription of the target genes. (2) Many of the miRNAs are involved in the positive and negative regulation of TGF-β/SMAD signaling cascade. miRNA130a/301a/454 abrogate TGF-β/SMAD mediated signaling by blocking SMAD4 activation. (3) TGF-β/SMAD signaling is also involved in miRNA biogenesis e.g miR-21. (4) Certain other miRNAs like miR-155 and miR-224 can also be influenced by TGF-β/SMAD mediated signaling cascade. (5) Pharmacological inhibition of SMAD4 protein by small molecule inhibitor SB-431542 leads to modulation of miRNAs downstream of TGF-β/SMAD signaling. (B) In the presence of SHH ligand, the PTCH1-SMO association gets remitted, resulting in enhanced downstream transcriptional activation of GLI family of proteins leading to target gene expression. (1) Use of small molecule inhibitor, Vismodegib (GDC-0449) inhibits hedgehog signaling pathway thereby interfering with regulation of miR-200b and let-7c, that are critical regulators of EMT and drug resistance (2) Whereas indirect inhibition of SHH activation by synthetic miRNA (GLI-1-miR-3548) targets GLI family of transcription factors that resulted in inhibition of pancreatic cancer cell division and proliferation. (3) Cyclopamine also appears to interrupt SHH signaling through regulating miRNAs (let7, miR-34, miR-107, miR-125, miR-128, miR-130, miR-132 and miR-141) responsible for stem cell self -renewal process.
6. Sonic Hedgehog signaling and miRNA activation in pancreatic cancer
The Sonic Hedgehog (SHH) signal transduction pathway is critically important during embryonic development and is activated by binding of Shh to its cognate Patched-1 (PTCH1) receptor. Following PTCH1 activation, transmembrane G-protein coupled receptor (GPCR)-like protein Smoothened (SMO) is freed and activated resulting in nuclear translocation of the GLI1 family of transcription factors for target gene activation [162]. In addition, through GLI1, SHH mediated cellular responses are also coordinated through MAPK and the phosphatidylinositol-3 kinase (PI3K) signaling cascades for enhanced proliferation and cell survival [163]. Besides being re-activated in injured adult tissues, SHH signaling is also aberrantly activated in several malignancies promoting resistance to chemotherapeutics. While there is no expression in normal pancreas, Thayer et al. have reported aberrant expression of SHH in 70% of PC specimens and in precursor PanIN lesions (PanIN1 to PanIN3), suggesting its importance in the initiation and maintenance of PC pathogenesis [164]. SHH also contributes to the acceleration of desmoplastic response in PC [165]. Earlier studies have shown that miRNAs regulate SHH signaling either by targeting proteins upstream of SHH or by directly targeting the components of SHH pathway members. For example, miR-196 and miR-452 by regulating Shh signaling effects limb development [8] and epithelial to mesenchymal transition in enriched neural crest cells respectively [166]. In addition, the miR-17~92 cluster (miR-19a, miR-20 and miR-92) was overexpressed and coordinated with the Shh signaling activation in murine and human brain cancer (medulloblastoma) [167]. Specific to PC, synthetic miR-3548 that targets GLI1 transcription factor resulted in effective inhibition of cell division and cell proliferation [168]. Not only do miRNA regulate SHH signaling, SHH signaling also effects miRNA expression, as evidenced by the fact that use of pharmacological hedgehog inhibitor vismodegib (GDC-0449) upregulates the expression of miR-200b and let-7c that results in reversal of EMT phenotype and attenuation of drug resistance in NSCLC cells [169]; whereas cyclopamine, a SHH inhibitor in PC cells significantly inhibited expression of let7, miR-34, miR-107, miR-125, miR-128, miR-130, miR-132, and miR-141 [170]. Therefore, identification of a complete miRNA profile that exist to regulate various components of SHH signaling, will be helpful in identifying the central mechanism of sustained SHH activation in cancer cells. The miRNAs identified through this mechanistic approach would be suitable candidates for therapeutic targets in pancreatic and other cancers.
7. The impact of miRNAs on cell cycle and proliferation of pancreatic cancer cells
Deregulated cell proliferation due to aberrant regulation of cyclins and cyclin dependent kinases (CDKs) is a major hallmark of cancer [171]. An efficient way through which cancer cells manipulate cell cycle regulatory proteins is by altering the miRNA(s) expression. Usually, miRNAs that downregulate cyclins, CDKs and other cell cycle progression proteins are silenced, whereas miRNAs targeting inhibitors of cell cycle progression are over expressed in lung cancers and PC [172–174]. Sequence homology analysis of seed regions of several differentially regulated miRNAs and 3′ UTRs of mRNA indicate that the above strategy is employed by many cancers [175]. In PC, proliferation and cell cycle regulatory proteins like CCND1, CDKN1A (p21 WAF1), CDKN1B (p27 Kip1), E2F1, Rb1, CDK4, CDK6, CDKN1C (p57 Kip2), ABL-1, EZH2, c-Myc, KRAS, CDC25B, PTEN, LATS2, Spry2, RREB1, AKT and STAT3 are all affected by aberrantly regulated miRNA [48, 55, 60, 90, 172–191]. Deregulation of these proteins leads to more rapid progression through the cell cycle and intern accelerated cell proliferation through various signaling pathways. For example miR-34a that downregulates CCND1, CDK4 and CDK6 is frequently lost in PC resulting in increased cell proliferation [173, 174]. CDK6 is also targeted by miR-107, which like several other miRNAs is epigenetically silenced in PC cells [172]. The miR-148a is also frequently downregulated in PC and targets the CDC25B phosphatase that otherwise activates CDK1/cyclinB complex for increased cell proliferation [185]. Conversely PC cells aberrantly upregulate miRNAs that target inhibitors of cyclins and CDKs [175]. Over expression of miR-106 targets p21 thereby preventing the G1 cell cycle block for uninterrupted cell cycle progression [183]. Similarly miR-221/222 and miR-106b family miRNAs allows cancer cells to progress through the cell cycle by down regulating CDKN1B (p27 Kip1) and CDKN1C (p57 Kip2) circumventing the restriction point [179, 181, 183, 189]. Inhibition of TGF-β/SMAD-4, p16, p53, and others tumor suppressors expression by miRNAs is a tactic frequently employed by PC to deregulate cell proliferation [48, 175, 188]. Overexpression of the miR-106b-25 cluster was reported in various cancers including PC and this cluster impairs the TGF-β signaling by targeting its downstream effectors like cell cycle inhibitor CDKN1A (p21) and the proapoptotic gene BCL2L11 (BIM) [192] leading to increased cell proliferation. Further, this cluster also activates TGF-β signaling by targeting SMAD7, which result in a tumor promoting role of TGF-β [193]. However, p53 can also have gain of function mutations that can increase cell proliferation and other tumorigenic traits; in this case miRNA against p53 can have beneficial effects [65, 194, 195]. Aberrantly expressed miR-132 and miR-212 suppress Rb1 expression in PC, allowing E2F transcription factor to activate the transcription of several cyclins for enhanced cell cycle progression [48]. In addition, overexpressed miR-21 significantly downregulates PTEN expression allowing uncontrolled PC cell proliferation. This downregulation of PTEN was reversed upon antisense inhibition of miR-21, suggesting a useful therapeutic approach to combat PC [48, 175, 188]. Overactive oncogenes such as KRAS commonly drive cell proliferation in PC, and the miRNAs (miR-217, miR-96, miR-126, and miR-143/145 cluster) capable of downregulating KRAS, are commonly downregulated in PC [55–58]. Key players from aberrantly overexpressed oncogenic pathways alter miRNA expression. Overexpressed Notch-1 in PC induces EMT by upregulating miR-21 and downregulating expression of Let-7a, Let-7b, Let7c, miR-200b and miR-200c [176].
8. Potential role of miRNAs in pancreatic cancer diagnosis
Traditionally, PC diagnosis and staging is performed by using tissues biopsies and ultrasound guided FNAs. Serum CA19.9 (carbohydrate antigen 19.9) is the only gold standard biomarker approved by Food and Drug administration for PC, but it lacks specificity and sensitivity. Although widespread research has been focused to identify early detection markers, none has yielded a biomarker specific to PC. Because of high stability, tissue specificity and ease of availability of miRNAs, recent studies are focused on performing miRNA expression profiling of PC tissues, serum/plasma, and other body fluids to identify suitable biomarker(s) for early diagnosis of this deadly disease. Several studies have revealed that altered miRNA expression profiles in various cancers, and their association with various clinical parameters including disease progression, response to therapy, survival, lymph node metastasis etc. and their utility as prognostic and diagnostic markers [33, 46, 47, 47, 103]. Therefore, it is very important to accurately determine the miRNA expression profile with high specificity and sensitivity in a given tissue or speci c cell type. But accurate profiling of individual miRNAs with high specificity and sensitivity is technically challenging as mature miRNAs are very short (20–25 bp), differ in their GC content, lack mutual sequence features in mature miRNAs like poly-A tails and single nucleotide difference within miRNA families. However, recent rapid technological advancements like deep sequencing/parallel sequencing [196], oligonucleotide microarrays [197–200], northern blotting with radiolabelled probes [201], qPCR-based detection of mature miRNAs (TaqMan assays) [202–204], single molecule detection in liquid phase [205] and in situ hybridization [206, 207] have made it feasible to profile miRNAs in various tissue/cell types with high accuracy. miRNA microarray platform is a high-throughput method to compare differential miRNA expression profiles between the disease and the control states, whereas, deep sequencing methods involves generation of cDNA libraries from small RNA population followed by deep sequencing using different methods [208, 209]. The advantages of deep sequencing includes (i) its inexpensive and helps in the discovery of novel miRNAs, (ii) detects mature miRNAs with variation in length as well as modifications [210] and (iii) helps to identify low-abundance miRNAs with modest expression differences [208, 211–213]. Differentially expressed miRNAs as identified by either microarray or deep sequencing and are further validated by real time Q-PCR using TaqMan probed assays with high sensitivity and specificity. In addition, in situ hybridization is used to view and localize miRNAs with in cells and also help us to pinpoint their distribution in tumor/stromal cell compartment/cell type-specific expression [204]. The advantage of using northern blot techniques is that it does not require technical knowledge and special equipment but the disadvantage of this technique is poor sensitivity and more time consumption. But less time consumption and high specificity of miRNA detection was reported with the use of LNA-modified oligonucleotides [214].
Several studies have shown differential expression of miRNAs in body fluids of PC patients compared to normal healthy controls [50, 215, 216]. Using miRNA microarray analysis, a cohort of three miRNAs (miR-642b, miR-885-5p, and miR-22) were identified from blood that can identify early stage PC patients from healthy control group with a sensitivity and specificity of 91% [AUC= 0.97 (p < 0.001)] [217]. Similarly, Frampton et al. also identified a signature of seven upregulated (miR-21, -23a, -31, -100, -143, -155, and miR-221) and three downregulated (miR-148a, -217 and miR-375) miRNAs that can identify PC patients from healthy controls [218]. Furthermore, overexpression of miR-21 and miR-31, and downregulation of miR-375 in tumor tissues were associated with poor PC patient survival [218]. Recently, Danish BIOPAC (Biomarkers in patients with Pancreatic Adenocarcinoma) identified 2 diagnostic panels of circulating miRNAs (panel I: miR-145, miR-150, miR-223, miR-636 with sensitivity of 85% and specificity of 64% with AUC of 0.86; while panel II includes miR-636, miR-26b, miR-223, miR-122, miR-150, miR-145, miR-505 miR-34a, miR-885.5p, miR-126* having sensitivity and specificity of 85% with AUC of 0.93. Both these panels can significantly distinguish PC patients from healthy individuals [219]. Li et al. analyzed expression profile of 735 miRNAs in the serum and identified miR-1290 that distinguished PC patients from healthy controls with sensitivity 81% and specificity of 80% with AUC of 0.96 [220]. Furthermore, they observed that increased serum miR-1290 was better than CA19.9 in distinguishing early stage PC patients from controls [220]. Additionally, combination of CA19.9 with miR-27a-3p [221] and miR-16 and miR-196a [222] expression can also precisely discriminate PC patients from healthy controls. In a similar study, a panel of seven miRNAs (miR-24, miR-20a, miR-25, miR-21, miR-185, miR-99a, and miR-191) in serum was used to accurately diagnose PC patients from the healthy individuals, and can differentiate different stages of PDAC patients (stage I patients with 96.2% and Stage II with 91.7) with high sensitivity and specificity [223]. Similarly, increased levels of serum miR-192 can distinguish PC from healthy controls with a sensitivity of 76% and specificity of 55% [103]. In addition, overexpression of miR-192 in PANC-1 PC cells results in an increase in cell proliferation, migration, inhibition of apoptosis along with induced the progression of cell cycle from the G0/G1 to the S phase [103]. Plasma miR-221 level was significantly higher in PC patients and was used as indicative of non-resectable status of the PC patients and distant metastasis [225]. In a similar study, a panel of seven miRNAs including miR-20a, miR-21, miR-24, miR-25, miR-99a, miR-185, and miR-191 in serum can accurately (83.6%) diagnose PC patients [223]. In addition to body fluids, Bauer et al. reported that deregulated miRNAs from tissues can discriminate between PC and pancreatitis [226]. Analysis of fine needle aspirations (FNAs) revealed that increased expression of miR-196a and miR-217 can accurately discriminate PC from benign pancreatic lesions with a sensitivity of 90% and specificity of 100% [227]. Similarly, down regulation of let-7 was observed in the FNA from PC patients [60]. In addition, many other studies have reported significantly higher serum concentration of miR-210, miR-18a, and miR-155 in PC patients compared to healthy controls [50, 215, 216] and concentration of blood circulating miR-155, miR-21 and miR-210 was significantly higher in rats with PC [228]. Although, differentially expressed miR-27a-3p in PBMCs can sufficiently discriminate PC from benign pancreatic/peri-pancreatic diseases (BPD) with a sensitivity of 82.2% and specificity of 76.7% (AUC= 0.840), combination of miR-27a-3p expression in PBMC and CA19.9 serum levels had significantly higher diagnostic accuracy than either of them alone with a sensitivity of 85.3% and specificity of 81.6% (AUC= 0.886) [221]. However, it has been shown that blood based miRNA expression profiles could not distinguish pancreatitis from PC [226].
Recently, a global miRNA expression study in pancreatic juice (exocrine pancreatic secretions) from 6 PC and 6 non-pancreatic, non-healthy (NPNH) patients was performed using miRNA microarray. The top differentially expressed miRNAs (miR-205, miR-210, miR-492, and miR-1247) were further confirmed in pancreatic juice from 50PC, 19 CP patients and 19 non-pancreatic, non-healthy (NPNH) controls, which showed marked difference in their expression from PDAC patients compared to those without pancreatic disease. These four miRNAs can diagnose PDAC with a sensitivity and specificity of 87% and 88% respectively, but inclusion of CA19.9 enhanced the sensitivity and the specificity to 91% and 100% respectively. Over expression of the above mentioned four miRNAs in pancreatic juice was further associated with reduced overall survival (OS) and lymph node metastasis [229]. Not only in humans, PC bearing rats also showed increased blood levels of miR-155, miR-21 and miR-210 compared to normal control [228]. Similarly, high levels of circulating miR-196a and -196b was observed in KPC (KrasG12D;Trp53R172H;Pdx1-Cre) mice with PanIN2/3 lesions as well as PDAC as compared to contemporary littermate control or KPC mice with PanIN1 lesions. Further, expression of these miRNAs were significantly higher in the serum of PC patients with sporadic/hereditary or individuals at risk (IAR) with multifocal PanIN2/3 lesions compared to patients with neuroendocrine pancreatic tumors or chronic pancreatitis, IAR with PanIN1 or no PanIN lesions and healthy controls with a sensitivity of 100% and specificity of 90% (AUC = 0.99). However, it was observed only in few samples, therefore, further validation of miR-196a and -196b should be carried out to test their utility in PC diagnosis [230]. A meta-analysis study was carried out on 18 articles with a total of 2,036 patients and 1,444 controls to determine the role miRNAs in early diagnosis and reported that pooled sensitivity of 82 % (95 % CI, 78–86 %); and specificity of 77 % (95 % CI, 73–81 %) with AUC of 0.86 (95 % CI, 0.83–0.89), suggesting the potential diagnostic value of miRNAs (inclusion of multiple miRNAs for diagnosis) to discriminate PC patients from healthy controls with high sensitivity and specificity [231].
9. miRNAs as therapeutic agents in pancreatic cancer
Contribution of miRNAs in the initiation and progression of PC suggest that miRNAs can be exploited for the development of novel therapeutic strategies. The strategies followed to develop miRNA based therapies are similar to that of other gene targeted therapies. The main rationale for developing miRNA based therapies over targeting a single oncogene is due to the ability of a single miRNA to target many genes and signaling pathways at once. Therefore, targeting of a single miRNA can produce dramatic results due to its ability to affect numerous cellular processes. Primarily, miRNA targeted therapeutics can be classified into miRNA mimics and miRNA antagonists. The miRNA mimics double-stranded RNAs that are chemically modified in vitro and used to restore the expression of miRNAs that are lost during the disease process and functionally mimic the endogenous miRNAs. On the other hand, miRNA antagonists are developed to target endogenous miRNAs that have shown a gain-of-function in diseased tissues. Previously, we have extensively discussed various methods that are used to deliver miRNA based therapeutics [233], however here we will briefly describe some currently used methods to deliver miRNA therapeutics.
The miRNA antagonists are chemically synthesized single stranded oligonucleotides complementary to specific a miRNA, with specific chemical modification (2′-O-methoxyethyl (2′-MOE) and 2′-fluoro (2′-F) and locked nucleic acid (LNA) chemistries) [234–239]. These modifications provide extreme stability, high binding affinity to target miRNAs, resistance to nuclease degradation, leading to the sequestration of target miRNA within the RISC, inhibiting it from being processed or degraded. These modified antisense oligonucleotides (2′-MOE, LNA, and morpholinos) are utilized to antagonize miRNA activity and its processing. These oligos are frequently complementary to the miRNA guide strand, blocking its activity. They can often produce similar effects by sterically blocking only the seed region due to increased binding strength. These oligos can also antagonize miRNAs by interfering with miRNA processing by binding to the Drosha and Dicer guide sequences.
A previous study has shown that successful targeting of miR-122 in liver by intravenous injection of antagomir, regulates genes essential for cholesterol biosynthesis, leading to a decline in concentration of miR-122 and reducing circulating levels of cholesterol [240]. Similarly, cardiac fibrosis and hypertrophy was significantly reduced after targeting miR-21 and mir-133 respectively [241, 242]. Further, chimpanzees chronically infected with Hepatitis C were protected against viremia and liver pathology after targeting miR-122 with the LNA-antimiR [243]. The major drawbacks of anti-miR oligonucleotides are off target effects and affect endogenous RNA species other than the intended target miRNAs. However, these off target effects can be minimized by conjugating antagomir with ligands for target organ/specific cell surface receptors. Besides antagomirs, several studies have successfully employed small molecule (low molecular weight compounds) inhibitors to target miRNAs such as miR-21, miR-122 and miR-27a [232, 244, 245] in vitro. Recent study has shown that morpholinos are capable of antagonizing miRNA by binding to the miRNA response element. They are totally immune to nucleases and at appropriate concentrations can only interfere with translation by binding to the 5′ end of mRNA near the start codon. This approach is useful when a miRNA has both pro-cancer and anti-cancer targets simultaneously, because at appropriate lengths it can protect key anti-cancer miRNA targets while not interfering with the downregulation of the pro-cancer targets. In order to reach effective concentrations of synthetic oligos (in vivo) at the target cells, it is beneficial to conjugate them to cholesterol or arginine rich peptides to increase cellular uptake and diffusion out of endosomes [246]. A preclinical study involving administration of anti-miR-221 modified with cholesterol significantly enhanced liver tissue distribution, inhibiting tumor growth leading to increased survival compared to unmodified anti-miR-221 in a mouse model of hepatocellular carcinoma [247]. Similarly, substantial inhibition of tumor metastasis was achieved after therapeutic targeting of miR-10b with cholesterol-modified anti-miR-10b in a mouse mammary tumor model [248]. Another alternative method of antagonizing miRNA is the miRNA sponge, produced by a transgene. miRNA sponges have 4–10 miRNA binding sites with small spacer nucleotides in-between [249]. Efficient miRNA sponges are designed with imperfect complementarity; this hinders sponge degradation and results in longer miRNA sequestration. Sponges are typically complementary only to the seed region allowing targeting of an entire family of miRNAs. Silencing the miRNAs in miR-17-92 cluster by employing miRNA sponges harboring multiple binding sites for these family members demonstrates the proof of principle for this approach [250]. In addition, additional methods are being investigated, such as, small molecules that interfere with dicer function [232].
miRNA mimics are identical, or near identical to the guide strand of endogenous miRNA. Double stranded oligos are 100–1000 fold more effective than single stranded ones at mimicking miRNA, as they are present as duplexes of the guide and the passenger strand [251]. Although the passenger strand is usually modified with cholesterol at the 3′ end to increase cellular uptake [252], miRNA mimics are still susceptible to nuclease degradation, can be targeted by the innate immune system, and also affect non target tissue. In light of the above, miRNA mimics have limitations with regard to therapeutic applications. To further enhance therapeutic delivery, lipid based delivery systems have been developed. Liposomes of smaller diameter (<100nm) allow for a high drug to lipid ratio. Liposomes have been successfully used to deliver miR-34a mimic intravenously in a mouse model of non-small cell lung carcinoma resulting in reduction of tumor growth, while not having significant renal or hepatic toxicity or triggering an immune response [253]. In order to develop precise targeting of anti-cancer miRNAs, tumor specific promoters can be utilized to specifically express the reintroduced miRNA in the tumor cells [254]. An example of this is the T-VISA-miR-34a construct that utilizes the human telomerase reverse transcriptase promoter, which is activated only in cancerous tissue, to express miR-34a [254]. As with other gene therapies, adeno-associated viruses can be used to enhance miRNA construct delivery [255]. Adeno-associated viruses have different serotypes that have differing affinity for various organs aiding in the targeting of miRNA, and has already proven effective in various murine cancer models [255].
Pancreatic cancer
Some preclinical studies have shown that targeting mature miRNAs or their precursors by using synthetic or chemically modified antisense oligonucleotides, and/or overexpression of some miRNAs in PC results in decreased tumor burden [207]. The oncomir miR-21 is over expressed in the early stages of PDAC [256], and it has been shown that PC cells are addicted to miR-21 [257]. Lentiviral based targeting of miR-21 by its antagonists resulted in increased angiogenesis, strong inhibition of PDAC derived cell lines proliferation both in vitro and in vivo as well. Further, combinational treatment involving miR-21 antagonists and gemcitabine (125 mg/kg) leads to significant regression of tumor growth in vivo. Therefore, delivery of miR-21 antagonists may be a useful therapeutic intervention in several cancers including PC because of its up regulation in multiple cancer types [258]. The phase-I clinical trial (NCT01274455) involving 24 PDA patients with advanced disease treated with intra tumoral injection of CYL-02 (Gene therapy product) using non-viral vector mediated delivery such as endoscopic ultrasound followed by gemcitabine treatment showed the feasibility and safety of intra tumor injection. A recent study has shown that co-delivery of a nanosystem comprising of anti-miR oligonucleotide and human serum albumin-1-palmitoyl-2-oleoyl-sn-glycero-3-ethylphosphocholine:cholesterol resulted in effective silencing of individually overexpressed miRNAs (miR-21, miR-221, miR-222, and miR-10) in PC cells leading to upregulation of their target genes. Further, treatment of PC cells with the above nanosystem containing anti-miR-21 and chemotherapeutic drug sunitinib has produced a strong synergistic antitumor effect, therefore, the above combination therapy may have potential therapeutic value in PDACs [259]. Arora et al developed a poly (D, L-lactide-co-glycolide) (PLGA)-based nanoformulation of miR-150 (miR-150-NF) having high encapsulation efficiency (~78%) and sustained release capacity. When PC cells were treated with miR-150-NF, it resulted in effective intracellular concentration of miR-150 mimics leading to down regulation of its target gene (MUC4), which resulted in the inhibition of cell growth, clonogenicity, motility, and invasion [260]. Further, intra-peritoneal injection of LNA-based miR-21 antagonist resulted in down regulation of oncomir miR-21 leading to decreased splenomegaly in mice with systemic lupus erythematous [261]. In contrast, over expression of oncogenic miR-21 and miR-221 enhanced the malignant phenotype of PC cells [187]. However, inhibition of miR-21 and miR-221 with the use of antisense oligonucleotides resulted in decreased proliferation and increased apoptosis of PC cells by increasing expression of PTEN, RECK, and p27 [187]. In combination with gemcitabine, use of antisense oligonucleotides also synergistically killed PC cells [187]. In addition to oligonucleotides, targeting miR-10a expression by using retinoic acid receptor (RAR) antagonist repressed PC metastasis [209].
Over expression of miR-143 [208] and the viral mediated delivery of miR-143 or miR-145 resulted in decreased PC metastasis and reduced tumor formation in PC cells [57]. Restoration of tumor suppressor miRNAs like let-7, miR-96, and miR-150 in PC cells led to decreased tumorigenesis and cell proliferation [60]. Furthermore, restoration of let-7 led to KRAS downregulation and decreased mitogen-activated protein kinase activation [60]. The use of oncogenic miR-34 mimics or its virus mediated infection led to apoptosis, cell cycle arrest, decreased clonogenicity, invasion, and increased chemo/radiation sensitivity of PC cells by targeting Bcl-2 and Notch1/2 [210]. Several studies have shown that Let-7 and miR-34 are significantly down regulated in various solid tumors as well as in cancer stem cells. Administration of let-7 and miR-34 resulted in changes in cancer stem cell phenotypic properties. Therefore, therapeutic delivery of let-7 and miR-34 may result in decreased tumor growth as well as number of viable cancer stem cells [262, 263]. Further, restoration of let-7 either in the form of a let-7 mimic or a viral mediated therapeutic delivery resulted in significant reduction of tumor growth xenograft model for human non-small cell lung cancer as well as in the KrasG12D transgenic mouse model [264]. Similarly, restoration of Let-7b by intra-nasal inhalation of adenovirus carrying Let-7b in a spontaneous Kras model for lung cancer lad to a significant decrease in tumor growth compared to mice injected with only the vector [265], whereas, intra-tumor injection of Let-7 carrying plasmid into xenograft tumors produced by orthotopic implantation of Capan1 cells did not show any change in tumor growth/proliferation compared to control mice [60]. Growth of prostate and lung cancer was significantly inhibited after systemic delivery of a miR-34 mimic in lung and prostate cancer mouse models [266, 267]. Several studies have also revealed that administration of tumor suppressor miRNAs mimics in mouse models revealed no toxicity in normal tissues, and therefore are well endured [255, 265, 268]. Further, delivery of miR-34a mimics in combination with lipid-containing formulation also did not elicit any non-specific immune response [267]. All of these studies provide a rationale to use miRNAs for therapeutic intervention of lethal PC.
Not only in PC, several miRNAs including miR-208/499/195 are currently under development as therapeutic agents [269] in the area of cardiovascular disease. miR-208 is a heart muscle specific miRNA and it stimulates hypertrophy of cardiomyocyte, fibrosis and β-MHC expression under stress and hypoxia conditions. The miR-208 knockout mice were shown to be highly resilient to cardiomyocyte hypertrophy and fibrosis due to stress or hypoxia, therefore, targeting of miR-208 may significantly ameliorate the chronic heart disease [270]. Recently, miRagen Therapeutics in collaboration with Danish Santaris Pharma developed a major LNA-based anti-miR-208 therapeutic (MGN-9103 antimir) to target miR-208 expression for treatment of chronic heart failure. In addition, they also developed two more LNA-based antagomirs to target miR-15, miR-195 (MGN-1374 antimir) and miR-451 (MGN-4893 antimir) for the treatment of myocardial infarction [271, 272] and polycythemia vera [273] respectively. With another miRNA miR-195, which is overexpressed in cardiac hypertrophy, the transgenic mice over expressing this miRNA resulted in cardiac failure and death of the animal [270], therefore anti-miR-195 can be used to treat cardiac disease.
Santaris Pharma has recently developed an LNA-modified antagomir Miravirsen (or SPC3649) against miR-122 that is important for induction of viral transcription of Hepatitis C virus (HCV) for Hepatitis C treatment [243, 274]. Treatment of chimpanzees having chronic HCV with SPC3649 has shown decreased viral RNA in serum without any adverse effects [243]. Currently, Miravirsen is under phase IIa clinical trials, and has provided a significant protection to patients with HCV [275]. Regulus Therapeutics (San Diego USA) has generated several antagomirs (miR-10b, miR-21, miR-103/107, miR-182, miR-380-5p) to target miRNAs over expressed in different conditions (Glioblastoma, hepatocellular carcinoma, atherosclerosis, kidney fibrosis, and HCV infection) but none of the above therapeutics has reached clinical phase. Later on they employed anti-miR-21/anti-miR-380-5p/anti-miR-182 to prevent metastasis and invasion of glioma [276], neuroblastoma [277] and melanoma [278] respectively. Mirna Therapeutics has developed miRNA mimics to restore the expression of specific miRNAs, which are lost during the course of tumorigenesis. They developed MRX34, the first miR-34a mimic compound that entered into Phase I clinical trials (NCT01829971). Administration of MRX34 (restoration of miR-34) produced potent anti-tumor effects in several mice cancer models [266]. Further, pre-clinical testing of the combination of MRX34 and liposomes in mouse models of hepatocellular carcinoma have shown very promising outcomes with minimal toxicity (NCT01829971) [279].
10. Conclusion and future perspectives
Although the involvement of miRNAs in disease pathogenesis is still emerging, deregulated miRNA expression in PC suggests their critical roles in its development and progression. Many studies have shown their involvement in a wide variety of biological processes including cell survival, proliferation, invasion/metastasis, apoptosis, and drug resistance of PC [55, 83, 91, 113, 158, 174, 181, 280]. Furthermore, differential expression profiles of circulating miRNAs among PC patients and healthy individuals render them potential biomarkers for diagnosis and prognosis. Besides the fact that a single miRNA targets multiple genes, several miRNAs frequently share the same target transcript, which makes miRNA actions more additive resulting in large changes in disease state (Table 1). In light of the above, it is apparent that cancer cells frequently deregulate miRNA expression to shortcut the selection process during the evolution of benign to malignant state. In this process these tumor cells may develop addiction to either aberrantly over expressed oncogenic or downregulated tumor suppressor miRNAs. Therefore, it seems logical that restoring tumor suppressor miRNAs at the pre-cancer levels [173, 188] or use of miRNA inhibitors may hold promise for therapeutic intervention against cancers. Tumor promoting effects have been directly countered in experimental models primarily in two ways; (a) by nanoparticle/virally delivered miRNA, used to restore tumor suppressive miRNAs [173] and (b) by antisense blocking oligos against oncogenic miRNAs. Pancreatic cancer is composed of dense stroma, also called the desmoplastic reaction that result in poor penetration of therapeutic agents resulting in reduced efficacy due to lower available drug concentration in the center of the tumor. Use of higher amounts of miRNAs is associated with off target effects that results in higher toxicity and thus miRNA based therapy is a major challenge for PC therapy. For considering miRNAs as therapeutic targets in PC, several challenges need to be addressed. First specific delivery of the miRNA to PC tumors in vivo presents a significant challenge. In order to address this, nano-particles carrying the miRNAs for delivery can be coated with ligands or antibody fragments specific to up-regulated cell surface receptors on the cancer cells to increase tumor specific miRNA delivery that will enhance efficacy and reduce targeting to normal cells. Furthermore, antisense molecules do not readily cross cell membranes and this therefore limits their use. However, recently intravenous injection of morpholino-oligos against miRNAs have shown some promise [281]. These morpholinos are absorbed by endocytosis and can diffuse through endosomes with the aid of either a octaguanidinium dendrimer or arginine-rich cell-penetrating peptides (CPP) linked to their 3′ or 5′ ends [281]. Furthermore, target specific delivery of these morpholino-oligos may be enhanced by either altering the CPP structure or by attaching a ligand specific to an upregulated receptor on the surface of PC cells.
Table 1.
Therapeutically important miRNAs in pancreatic cancer pathogenesis
| S. NO | Target gene | % Target gene mutations in PC | Regulation of target genes by miRNAs | Reference |
|---|---|---|---|---|
| 1 | Kras | 90–95% | [53] | |
| miR-96, | [55] | |||
| miR-126, | [56] | |||
| miR-143/145 | [57] | |||
| miR-217 | [58] | |||
| Let-7 | [59] | |||
| 2 | P53 | 50–75% | [65] | |
| p53 mediated transcription of various miRNAs | miR-34a, miR-34b/c | [82–87] | ||
| miR-130b, miR-155 and miR-205 | [92–94] | |||
| miR-15a/16-1 | [15] | |||
| miR-145 | [96] | |||
| miR-107 | [109] | |||
| miR-200a/b/c, miR-141 miR-429 |
[98, 99] | |||
| miR-192, miR-194 miR-215 |
[102] | |||
| miR-16-1, miR-143, miR-145 | [104] | |||
| miRNAs regulates p53 gene expression by binding to its 3′ UTR region | miR-491-5p | [106] | ||
| miR-34a | [89, 108] | |||
| miR-214 | [111] | |||
| miR-125b | [114] | |||
| miR-29 | [116] | |||
| miR-504 | [117] | |||
| miR-122 | [118] | |||
| miR-33, miR-1285, miR-380-5p, miR-25, miR-133 and miR-30d | [119] | |||
| miR-192/194/215 and miR-605 | [121, 122] | |||
| 4 | INK4A/p16 | 90–95% | [282] | |
| miRNAs that regulate p16 expression | miR-24 | [283] | ||
| miR-10b | [284] | |||
| miR-128a | [285] | |||
| miR-20a | [286] | |||
| miR-148a and miR-152 | [287] | |||
| miRNAs regulated by p16 | miR-410 and miR-650 | [288] | ||
| miR-141 and miR-146b-5p | [289] | |||
| 5 | TGF-β/Smad | |||
| TGF-β/Smad4 signaling mediated miRNA induction and maturation | miR-21 | [144] | ||
| miR-155 | [145] | |||
| miR-143 | [146] | |||
| 6 | SMAD4/DPC4 | inactivated in 55% PC cases | [151] | |
| miRNAs directly targeting SMAD4 | miR-199a | [147] | ||
| 130a/301a/454 | [148] | |||
| miR-146a | [157] | |||
| miR-483-3p | [158] | |||
| miR-421 | [159] | |||
| miR-146b-5p | [161] | |||
| miR-182-5p | [19] | |||
| 7 | SHH | Up regulation of Shh in 70% of PDAC specimens | ||
| miRNAs targeting SHH | miR-196 and miR-452 | [8, 166] | ||
| 8 | Gli 1 | miR-3548 | [168] | |
Most PC patients have multiple mutations that either inactivate tumor suppressors like p16, p53 or activate oncogenes, most importantly KRAS. Therefore, for better management of PC, inhibition of miRNA miR-491-5p, -34a, -214, -125b, -29, -504, -122, -33, -1285, -380-5p, -25 -133 and miR-30d to increase p53 expression; miR-24 and miR-10b to increase p16 expression; miR-130a/301a/454 to increase Smad4 expression; miR-106 to increase p21; miR-221/222 for p27 upregulation and miR-132 and miR-212 expression to increase Rb1 expression seems reasonable to combat this deadly disease. In addition, reintroduction of miRNA that suppress oncogenes and inhibit cell cycle progression like miR-217, miR-96, Let-7, miR-126, miR-143/145 to decrease KRAS expression; miR-192, -194, -215, -15a/16-1, -34a/b/c, 145, -200a/b/c, -141, -429 and miR-107 to down regulate c-Myc and cyclins expression can have significant effect on inhibiting PC growth (Table 1). miRNAs that target KRAS expression are not specific to the mutant form and therefore use of a single miRNA may not have sufficient downregulation of mutant KRAS to be effectual in vivo. It may however be of interest to utilize a cocktail of several miRNAs to downregulate mutated KRAS expression in PC. However, use of a cocktail of miRNAs at high doses to counteract the effects of oncogenic KRAS may have undesirable effects on healthy adult cells. To this end, improvements in delivery techniques and targeted delivery of miRNA cocktails may provide a larger window before toxicity becomes a problem. In addition, it will be worthless to use antisense-oligos against miRNAs targeting mutated p16 and/or p53 in a subset of PC patients. It may even be counterproductive in the cases where p53 has gain of function mutations. Therefore, it will be of interest to genotype PC tumors to identify aberrantly expressed miRNAs, mutations of key tumor suppressors and oncogenes for effective decision making with regard to which miRNAs should be blocked or reintroduced.
Acknowledgments
The authors of this work are supported, in part, by grants from the National Institutes of Health (RO1 CA183459, TMEN U54 CA163120, EDRN UO1 CA111294, SPORE P50 CA127297, and RO3 CA167342).
Abbreviations
- AKT
protein kinase B
- ALDOA
Aldolase A, fructose-bisphosphate
- Axl
AXL Receptor tyrosine kinase
- BCL2
B-cell CLL/lymphoma 2
- c-abl oncogene 1
non-receptor tyrosine kinase
- CA19.9
Carbohydrate antigen 19.9
- CCND1
Cyclin D1
- CDC25B
Cell division cycle 25B, ABL-1
- CDKN1C
Cyclin-dependent kinase inhibitor 1C
- CDC7
Cell division cycle 7
- CDK4
Cyclin-Dependent Kinase 4
- CDK6
Cyclin-Dependent Kinase 6
- CLL
Chronic Lymphocytic Leukemia
- c-Met
Met proto-oncogene
- c-MYC
v-Myc avian myelocytomatosis viral oncogene homolog
- CUL5
Cullin 5
- DNMT1
DNA methyltransferase
- E2F1
E2F transcription factor 1
- EMT
Epithelial-mesenchymal transition
- EZH2
enhancer of zeste 2 polycomb repressive complex 2 subunit
- FGF7
Fibroblast growth factor 7
- GEM models
Genetically engineered mouse models
- GLI1
GLI family zinc finger 1
- GTP
Guanosine Triphosphate
- HDM4
Mouse Double Minute 4, Human Homolog Of P53-Binding Protein
- HIF-1α/β
Hypoxia inducible factor-1 α/β
- HPDE
Human pancreatic ductal epithelial cells
- INK4A (p16)
Cyclin-dependent kinase inhibitor 2A
- Jag1
Jagged 1
- Jak2
Janus kinase 2
- Kras
V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog
- LATS2
large tumor suppressor kinase 2
- LDH
Lactate dehydrogenase
- LOH
Loss of heterozygosity
- MAD2L1
MAD2 mitotic arrest deficient-like 1 (yeast)
- Maml1/2
mastermind-like co-activators ½
- MEK
Mitogen –activated protein kinase
- miRNA
microRNA
- MDM2
Mouse Double Minute 2
- Nanog
Nanog homeobox
- n-MYC
v-Myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog
- NSCLC
Non-small cell lung cancer
- PanIN
Pancreatic Intraepithelial Neoplasm
- P15
Cyclin-dependent kinase inhibitor 2B
- p21
Cyclin-dependent kinase inhibitor 1A
- PC
Pancreatic cancer
- PDAC
Pancreatic Ductal Adenocarcinoma
- PDCD4
Programmed cell death 4
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- PIP3
Phosphatidylinositol (3,4,5)-trisphosphate
- PKC
Protein kinase C
- PTEN
Phosphatase and tensin homolog
- PP2A
Serine/threonine protein phosphatase 2A
- PUMA
P53 Up-Regulated Modulator Of Apoptosis
- RAF
Raf/mil family of serine/threonine protein kinases
- RREB1
RAS-responsive element-binding protein
- Shh
Sonic Hedgehog
- Spry2
Sprouty homolog 2
- FNAs
fine needle aspirations
- STAT3
Signal transducer and activator of transcription 3
- SIRT1
Sirtuin 1
- SMAD4
SMAD family member 4
- SNAIL
Snail family zinc finger 1
- TGF-β
Transforming growth factor beta
- TGFβRI
Transforming growth factor beta Receptor I
- TGFβRII
Transforming growth factor beta Receptor II
- TPI1
Triosephosphate isomerase 1
- TP53
Tumor suppressor p53
- Wnt
Wnt oncogene analog
- WNT3A
Wingless-type MMTV integration site family, member 3A
- ZEB1
Zinc finger E-box binding homeobox 1
- ZNF652
Zinc finger protein 652
- 3′UTR
3′ Untranslated region
Footnotes
Conflict of interest statement
The authors have no conflict of interest with any company or financial organization.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsuno S, Egawa S, Fukuyama S, Motoi F, Sunamura M, Isaji S, Imaizumi T, Okada S, Kato H, Suda K, Nakao A, Hiraoka T, Hosotani R, Takeda K. Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas. 2004;28:219–230. doi: 10.1097/00006676-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Sultana A, Tudur SC, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses. Br J Cancer. 2008;99:6–13. doi: 10.1038/sj.bjc.6604436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 7.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hornstein E, Mansfield JH, Yekta S, Hu JK, Harfe BD, McManus MT, Baskerville S, Bartel DP, Tabin CJ. The microRNA miR-196 acts upstream of Hoxb8 and Shh in limb development. Nature. 2005;438:671–674. doi: 10.1038/nature04138. [DOI] [PubMed] [Google Scholar]
- 9.Rachagani S, Kumar S, Batra SK. MicroRNA in pancreatic cancer: pathological, diagnostic and therapeutic implications. Cancer Lett. 2010;292:8–16. doi: 10.1016/j.canlet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szafranska AE, Davison TS, John J, Cannon T, Sipos B, Maghnouj A, Labourier E, Hahn SA. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;26:4442–4452. doi: 10.1038/sj.onc.1210228. [DOI] [PubMed] [Google Scholar]
- 12.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Momi N, Kaur S, Rachagani S, Ganti AK, Batra SK. Smoking and microRNA dysregulation: a cancerous combination. Trends Mol Med. 2014;20:36–47. doi: 10.1016/j.molmed.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, Taccioli C, Zanesi N, Garzon R, Aqeilan RI, Alder H, Volinia S, Rassenti L, Liu X, Liu CG, Kipps TJ, Negrini M, Croce CM. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci U S A. 2008;105:5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng M, Tang H, Zhou Y, Zhou M, Xiong W, Zheng Y, Ye Q, Zeng X, Liao Q, Guo X, Li X, Ma J, Li G. miR-216b suppresses tumor growth and invasion by targeting KRAS in nasopharyngeal carcinoma. J Cell Sci. 2011;124:2997–3005. doi: 10.1242/jcs.085050. [DOI] [PubMed] [Google Scholar]
- 17.Diaz R, Silva J, Garcia JM, Lorenzo Y, Garcia V, Pena C, Rodriguez R, Munoz C, Garcia F, Bonilla F, Dominguez G. Deregulated expression of miR-106a predicts survival in human colon cancer patients. Genes Chromosomes Cancer. 2008;47:794–802. doi: 10.1002/gcc.20580. [DOI] [PubMed] [Google Scholar]
- 18.Drakaki A, Iliopoulos D. MicroRNA-gene signaling pathways in pancreatic cancer. Biomed J. 2013;36:200–208. doi: 10.4103/2319-4170.119690. [DOI] [PubMed] [Google Scholar]
- 19.Hirata H, Ueno K, Shahryari V, Tanaka Y, Tabatabai ZL, Hinoda Y, Dahiya R. Oncogenic miRNA-182-5p targets Smad4 and RECK in human bladder cancer. PLoS One. 2012;7:e51056. doi: 10.1371/journal.pone.0051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 21.Macha MA, Seshacharyulu P, Krishn SR, Pai P, Rachagani S, Jain M, Batra SK. MicroRNAs (miRNA) as Biomarker(s) for Prognosis and Diagnosis of Gastrointestinal (GI) Cancers. Curr Pharm Des. 2014 doi: 10.2174/1381612820666140128213117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radhakrishnan P, Mohr AM, Grandgenett PM, Steele MM, Batra SK, Hollingsworth MA. MicroRNA-200c modulates the expression of MUC4 and MUC16 by directly targeting their coding sequences in human pancreatic cancer. PLoS One. 2013;8:e73356. doi: 10.1371/journal.pone.0073356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 24.Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, Bentwich Z. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 25.Carthew RW. Gene regulation by microRNAs. Curr Opin Genet Dev. 2006;16:203–208. doi: 10.1016/j.gde.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 28.Babak T, Zhang W, Morris Q, Blencowe BJ, Hughes TR. Probing microRNAs with microarrays: tissue specificity and functional inference. RNA. 2004;10:1813–1819. doi: 10.1261/rna.7119904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sood P, Krek A, Zavolan M, Macino G, Rajewsky N. Cell-type-specific signatures of microRNAs on target mRNA expression. Proc Natl Acad Sci U S A. 2006;103:2746–2751. doi: 10.1073/pnas.0511045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, Calin GA, Liu CG, Croce CM, Harris CC. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 31.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, DePinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 32.Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 33.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C, Croce CM. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 34.Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ, Schmittgen TD. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang Y, Yao Q, Chen Z, Xiang J, William FE, Gibbs RA, Chen C. Genetic and molecular alterations in pancreatic cancer: implications for personalized medicine. Med Sci Monit. 19:916–26. doi: 10.12659/MSM.889636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan S, Ansarullah, Kumar D, Jaggi M, Chauhan SC. Targeting microRNAs in pancreatic cancer: microplayers in the big game. Cancer Res. 2013;73:6541–6547. doi: 10.1158/0008-5472.CAN-13-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J, Wang F. Recent progress in microRNA study: benefits from technique advance. Sci China Life Sci. 2012;55:649–650. doi: 10.1007/s11427-012-4342-7. [DOI] [PubMed] [Google Scholar]
- 38.Jiang J, Lee EJ, Gusev Y, Schmittgen TD. Real-time expression profiling of microRNA precursors in human cancer cell lines. Nucleic Acids Res. 2005;33:5394–5403. doi: 10.1093/nar/gki863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A, Croce CM. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677–4684. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava SK, Bhardwaj A, Singh S, Arora S, Wang B, Grizzle WE, Singh AP. MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis. 2011;32:1832–1839. doi: 10.1093/carcin/bgr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohuchida K, Mizumoto K, Lin C, Yamaguchi H, Ohtsuka T, Sato N, Toma H, Nakamura M, Nagai E, Hashizume M, Tanaka M. MicroRNA-10a is overexpressed in human pancreatic cancer and involved in its invasiveness partially via suppression of the HOXA1 gene. Ann Surg Oncol. 2012;19:2394–2402. doi: 10.1245/s10434-012-2252-3. [DOI] [PubMed] [Google Scholar]
- 42.Munding JB, Adai AT, Maghnouj A, Urbanik A, Zollner H, Liffers ST, Chromik AM, Uhl W, Szafranska-Schwarzbach AE, Tannapfel A, Hahn SA. Global microRNA expression profiling of microdissected tissues identifies miR-135b as a novel biomarker for pancreatic ductal adenocarcinoma. Int J Cancer. 2012;131:E86–E95. doi: 10.1002/ijc.26466. [DOI] [PubMed] [Google Scholar]
- 43.Papaconstantinou IG, Manta A, Gazouli M, Lyberopoulou A, Lykoudis PM, Polymeneas G, Voros D. Expression of microRNAs in patients with pancreatic cancer and its prognostic significance. Pancreas. 2013;42:67–71. doi: 10.1097/MPA.0b013e3182592ba7. [DOI] [PubMed] [Google Scholar]
- 44.Schultz NA, Andersen KK, Roslind A, Willenbrock H, Wojdemann M, Johansen JS. Prognostic microRNAs in cancer tissue from patients operated for pancreatic cancer--five microRNAs in a prognostic index. World J Surg. 2012;36:2699–2707. doi: 10.1007/s00268-012-1705-y. [DOI] [PubMed] [Google Scholar]
- 45.Ikenaga N, Ohuchida K, Mizumoto K, Yu J, Kayashima T, Sakai H, Fujita H, Nakata K, Tanaka M. MicroRNA-203 expression as a new prognostic marker of pancreatic adenocarcinoma. Ann Surg Oncol. 2010;17:3120–3128. doi: 10.1245/s10434-010-1188-8. [DOI] [PubMed] [Google Scholar]
- 46.Yu J, Li A, Hong SM, Hruban RH, Goggins M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin Cancer Res. 2012;18:981–992. doi: 10.1158/1078-0432.CCR-11-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Li M, Wang H, Fisher WE, Lin PH, Yao Q, Chen C. Profiling of 95 microRNAs in pancreatic cancer cell lines and surgical specimens by real-time PCR analysis. World J Surg. 2009;33:698–709. doi: 10.1007/s00268-008-9833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park JK, Henry JC, Jiang J, Esau C, Gusev Y, Lerner MR, Postier RG, Brackett DJ, Schmittgen TD. miR-132 and miR-212 are increased in pancreatic cancer and target the retinoblastoma tumor suppressor. Biochem Biophys Res Commun. 2011;406:518–523. doi: 10.1016/j.bbrc.2011.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang S, Hao J, Xie F, Hu X, Liu C, Tong J, Zhou J, Wu J, Shao C. Downregulation of miR-132 by promoter methylation contributes to pancreatic cancer development. Carcinogenesis. 2011;32:1183–1189. doi: 10.1093/carcin/bgr105. [DOI] [PubMed] [Google Scholar]
- 50.Morimura R, Komatsu S, Ichikawa D, Takeshita H, Tsujiura M, Nagata H, Konishi H, Shiozaki A, Ikoma H, Okamoto K, Ochiai T, Taniguchi H, Otsuji E. Novel diagnostic value of circulating miR-18a in plasma of patients with pancreatic cancer. Br J Cancer. 2011;105:1733–1740. doi: 10.1038/bjc.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ali S, Almhanna K, Chen W, Philip PA, Sarkar FH. Differentially expressed miRNAs in the plasma may provide a molecular signature for aggressive pancreatic cancer. Am J Transl Res. 2010;3:28–47. [PMC free article] [PubMed] [Google Scholar]
- 52.Ali S, Saleh H, Sethi S, Sarkar FH, Philip PA. MicroRNA profiling of diagnostic needle aspirates from patients with pancreatic cancer. Br J Cancer. 2012;107:1354–1360. doi: 10.1038/bjc.2012.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144:1220–1229. doi: 10.1053/j.gastro.2013.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spaargaren M, Bischoff JR, McCormick F. Signal transduction by Ras-like GTPases: a potential target for anticancer drugs. Gene Expr. 1995;4:345–356. [PMC free article] [PubMed] [Google Scholar]
- 55.Yu S, Lu Z, Liu C, Meng Y, Ma Y, Zhao W, Liu J, Yu J, Chen J. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 2010;70:6015–6025. doi: 10.1158/0008-5472.CAN-09-4531. [DOI] [PubMed] [Google Scholar]
- 56.Jiao LR, Frampton AE, Jacob J, Pellegrino L, Krell J, Giamas G, Tsim N, Vlavianos P, Cohen P, Ahmad R, Keller A, Habib NA, Stebbing J, Castellano L. MicroRNAs targeting oncogenes are down-regulated in pancreatic malignant transformation from benign tumors. PLoS One. 2012;7:e32068. doi: 10.1371/journal.pone.0032068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kent OA, Chivukula RR, Mullendore M, Wentzel EA, Feldmann G, Lee KH, Liu S, Leach SD, Maitra A, Mendell JT. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev. 2010;24:2754–2759. doi: 10.1101/gad.1950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao WG, Yu SN, Lu ZH, Ma YH, Gu YM, Chen J. The miR-217 microRNA functions as a potential tumor suppressor in pancreatic ductal adenocarcinoma by targeting KRAS. Carcinogenesis. 2010;31:1726–1733. doi: 10.1093/carcin/bgq160. [DOI] [PubMed] [Google Scholar]
- 59.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 60.Torrisani J, Bournet B, du Rieu MC, Bouisson M, Souque A, Escourrou J, Buscail L, Cordelier P. let-7 MicroRNA transfer in pancreatic cancer-derived cells inhibits in vitro cell proliferation but fails to alter tumor progression. Hum Gene Ther. 2009;20:831–844. doi: 10.1089/hum.2008.134. [DOI] [PubMed] [Google Scholar]
- 61.Kopp F, Wagner E, Roidl A. The proto-oncogene KRAS is targeted by miR-200c. Oncotarget. 2014;5:185–95. doi: 10.18632/oncotarget.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 63.Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15:2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 65.Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B, Jamieson NB, Oien KA, Lowy AM, Brunton VG, Frame MC, Evans TR, Sansom OJ. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107:246–251. doi: 10.1073/pnas.0908428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Redston MS, Caldas C, Seymour AB, Hruban RH, da CL, Yeo CJ, Kern SE. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res. 1994;54:3025–3033. [PubMed] [Google Scholar]
- 67.Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- 68.Sigal A, Rotter V. Oncogenic mutations of the p53 tumor suppressor: the demons of the guardian of the genome. Cancer Res. 2000;60:6788–6793. [PubMed] [Google Scholar]
- 69.Thukral SK, Lu Y, Blain GC, Harvey TS, Jacobsen VL. Discrimination of DNA binding sites by mutant p53 proteins. Mol Cell Biol. 1995;15:5196–5202. doi: 10.1128/mcb.15.9.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sampath J, Sun D, Kidd VJ, Grenet J, Gandhi A, Shapiro LH, Wang Q, Zambetti GP, Schuetz JD. Mutant p53 cooperates with ETS and selectively up-regulates human MDR1 not MRP1. J Biol Chem. 2001;276:39359–39367. doi: 10.1074/jbc.M103429200. [DOI] [PubMed] [Google Scholar]
- 71.Stambolsky P, Tabach Y, Fontemaggi G, Weisz L, Maor-Aloni R, Siegfried Z, Shiff I, Kogan I, Shay M, Kalo E, Blandino G, Simon I, Oren M, Rotter V. Modulation of the vitamin D3 response by cancer-associated mutant p53. Cancer Cell. 2010;17:273–285. doi: 10.1016/j.ccr.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strano S, Dell’Orso S, Di AS, Fontemaggi G, Sacchi A, Blandino G. Mutant p53: an oncogenic transcription factor. Oncogene. 2007;26:2212–2219. doi: 10.1038/sj.onc.1210296. [DOI] [PubMed] [Google Scholar]
- 73.Weisz L, Oren M, Rotter V. Transcription regulation by mutant p53. Oncogene. 2007;26:2202–2211. doi: 10.1038/sj.onc.1210294. [DOI] [PubMed] [Google Scholar]
- 74.Dittmer D, Pati S, Zambetti G, Chu S, Teresky AK, Moore M, Finlay C, Levine AJ. Gain of function mutations in p53. Nat Genet. 1993;4:42–46. doi: 10.1038/ng0593-42. [DOI] [PubMed] [Google Scholar]
- 75.Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, El-Naggar AK, Lozano G. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 76.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 77.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 78.Xi Y, Shalgi R, Fodstad O, Pilpel Y, Ju J. Differentially regulated micro-RNAs and actively translated messenger RNA transcripts by tumor suppressor p53 in colon cancer. Clin Cancer Res. 2006;12:2014–2024. doi: 10.1158/1078-0432.CCR-05-1853. [DOI] [PubMed] [Google Scholar]
- 79.Hunten S, Siemens H, Kaller M, Hermeking H. The p53/microRNA network in cancer: experimental and bioinformatics approaches. Adv Exp Med Biol. 2013;774:77–101. doi: 10.1007/978-94-007-5590-1_5. [DOI] [PubMed] [Google Scholar]
- 80.Lin CP, Choi YJ, Hicks GG, He L. The emerging functions of the p53-miRNA network in stem cell biology. Cell Cycle. 2012;11:2063–2072. doi: 10.4161/cc.20207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 82.Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, MacDougald OA, Cho KR, Fearon ER. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 83.Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007;67:8433–8438. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- 84.He L, He X, Lim LP, de SE, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12:414–418. doi: 10.1016/j.ccr.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 86.Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, Bentwich Z, Oren M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26:731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 87.Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A, Meister G, Hermeking H. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6:1586–1593. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- 88.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26:5017–5022. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 89.Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Korner H, Knyazev P, Diebold J, Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 90.Vogt M, Munding J, Gruner M, Liffers ST, Verdoodt B, Hauk J, Steinstraesser L, Tannapfel A, Hermeking H. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458:313–322. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- 91.Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dong P, Karaayvaz M, Jia N, Kaneuchi M, Hamada J, Watari H, Sudo S, Ju J, Sakuragi N. Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis. Oncogene. 2013;32:3286–3295. doi: 10.1038/onc.2012.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neilsen PM, Noll JE, Mattiske S, Bracken CP, Gregory PA, Schulz RB, Lim SP, Kumar R, Suetani RJ, Goodall GJ, Callen DF. Mutant p53 drives invasion in breast tumors through up-regulation of miR-155. Oncogene. 2013;32:2992–3000. doi: 10.1038/onc.2012.305. [DOI] [PubMed] [Google Scholar]
- 94.Tucci P, Agostini M, Grespi F, Markert EK, Terrinoni A, Vousden KH, Muller PA, Dotsch V, Kehrloesser S, Sayan BS, Giaccone G, Lowe SW, Takahashi N, Vandenabeele P, Knight RA, Levine AJ, Melino G. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci U S A. 2012;109:15312–15317. doi: 10.1073/pnas.1110977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang XJ, Ye H, Zeng CW, He B, Zhang H, Chen YQ. Dysregulation of miR-15a and miR-214 in human pancreatic cancer. J Hematol Oncol. 2010;3:46. doi: 10.1186/1756-8722-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, Elble R, Watabe K, Mo YY. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci U S A. 2009;106:3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen PS, Su JL, Cha ST, Tarn WY, Wang MY, Hsu HC, Lin MT, Chu CY, Hua KT, Chen CN, Kuo TC, Chang KJ, Hsiao M, Chang YW, Chen JS, Yang PC, Kuo ML. miR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humans. J Clin Invest. 2011;121:3442–3455. doi: 10.1172/JCI45390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 99.Kim T, Veronese A, Pichiorri F, Lee TJ, Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, Alder H, Liu CG, Dejean A, Croce CM. p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med. 2011;208:875–883. doi: 10.1084/jem.20110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soubani O, Ali AS, Logna F, Ali S, Philip PA, Sarkar FH. Re-expression of miR-200 by novel approaches regulates the expression of PTEN and MT1-MMP in pancreatic cancer. Carcinogenesis. 2012;33:1563–1571. doi: 10.1093/carcin/bgs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J, Brabletz T. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011;30:770–782. doi: 10.1038/emboj.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Braun CJ, Zhang X, Savelyeva I, Wolff S, Moll UM, Schepeler T, Orntoft TF, Andersen CL, Dobbelstein M. p53-Responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res. 2008;68:10094–10104. doi: 10.1158/0008-5472.CAN-08-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao C, Zhang J, Zhang S, Yu D, Chen Y, Liu Q, Shi M, Ni C, Zhu M. Diagnostic and biological significance of microRNA-192 in pancreatic ductal adenocarcinoma. Oncol Rep. 2013;30:276–284. doi: 10.3892/or.2013.2420. [DOI] [PubMed] [Google Scholar]
- 104.Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 105.Wang YA, Kamarova Y, Shen KC, Jiang Z, Hahn MJ, Wang Y, Brooks SC. DNA methyltransferase-3a interacts with p53 and represses p53-mediated gene expression. Cancer Biol Ther. 2005;4:1138–1143. doi: 10.4161/cbt.4.10.2073. [DOI] [PubMed] [Google Scholar]
- 106.Guo R, Wang Y, Shi WY, Liu B, Hou SQ, Liu L. MicroRNA miR-491-5p targeting both TP53 and Bcl-XL induces cell apoptosis in SW1990 pancreatic cancer cells through mitochondria mediated pathway. Molecules. 2012;17:14733–14747. doi: 10.3390/molecules171214733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chim CS, Wong KY, Qi Y, Loong F, Lam WL, Wong LG, Jin DY, Costello JF, Liang R. Epigenetic inactivation of the miR-34a in hematological malignancies. Carcinogenesis. 2010;31:745–750. doi: 10.1093/carcin/bgq033. [DOI] [PubMed] [Google Scholar]
- 108.Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer. 2009;9:123–128. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Okada N, Lin CP, Ribeiro MC, Biton A, Lai G, He X, Bu P, Vogel H, Jablons DM, Keller AC, Wilkinson JE, He B, Speed TP, He L. A positive feedback between p53 and miR-34 miRNAs mediates tumor suppression. Genes Dev. 2014;28:438–450. doi: 10.1101/gad.233585.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Penna E, Orso F, Cimino D, Tenaglia E, Lembo A, Quaglino E, Poliseno L, Haimovic A, Osella-Abate S, De PC, Pinatel E, Stadler MB, Provero P, Bernengo MG, Osman I, Taverna D. microRNA-214 contributes to melanoma tumour progression through suppression of TFAP2C. EMBO J. 2011;30:1990–2007. doi: 10.1038/emboj.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang H, Kong W, He L, Zhao JJ, O’Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 113.Xu CX, Xu M, Tan L, Yang H, Permuth-Wey J, Kruk PA, Wenham RM, Nicosia SV, Lancaster JM, Sellers TA, Cheng JQ. MicroRNA miR-214 regulates ovarian cancer cell stemness by targeting p53/Nanog. J Biol Chem. 2012;287:34970–34978. doi: 10.1074/jbc.M112.374611. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B, Korzh V, Lodish HF, Lim B. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009;23:862–876. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reif K, Gout I, Waterfield MD, Cantrell DA. Divergent regulation of phosphatidylinositol 3-kinase P85 alpha and P85 beta isoforms upon T cell activation. J Biol Chem. 1993;268:10780–10788. [PubMed] [Google Scholar]
- 116.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009;16:23–29. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- 117.Hu W, Chan CS, Wu R, Zhang C, Sun Y, Song JS, Tang LH, Levine AJ, Feng Z. Negative regulation of tumor suppressor p53 by microRNA miR-504. Mol Cell. 2010;38:689–699. doi: 10.1016/j.molcel.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fornari F, Gramantieri L, Giovannini C, Veronese A, Ferracin M, Sabbioni S, Calin GA, Grazi GL, Croce CM, Tavolari S, Chieco P, Negrini M, Bolondi L. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69:5761–5767. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- 119.Herrera-Merchan A, Cerrato C, Luengo G, Dominguez O, Piris MA, Serrano M, Gonzalez S. miR-33-mediated downregulation of p53 controls hematopoietic stem cell self-renewal. Cell Cycle. 2010;9:3277–3285. doi: 10.4161/cc.9.16.12598. [DOI] [PubMed] [Google Scholar]
- 120.Naveena B, Mohammed Altaf, Qian Li, Steele Vernon E, Rao Chinthalapally V. Cancer miRNA profiling and target identification in PanINs and pancreatic cancer in LSL-KrasG12D/+ mice: implication for human pancreatic cancer chemoprevention and treatment. 2014 [Google Scholar]
- 121.Pichiorri F, Suh SS, Rocci A, De LL, Taccioli C, Santhanam R, Zhou W, Benson DM, Jr, Hofmainster C, Alder H, Garofalo M, Di LG, Volinia S, Lin HJ, Perrotti D, Kuehl M, Aqeilan RI, Palumbo A, Croce CM. Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell. 2010;18:367–381. doi: 10.1016/j.ccr.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122.Xiao J, Lin H, Luo X, Luo X, Wang Z. miR-605 joins p53 network to form a p53:miR-605:Mdm2 positive feedback loop in response to stress. EMBO J. 2011;30:524–532. doi: 10.1038/emboj.2010.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mohr AM, Bailey JM, Lewallen ME, Liu X, Radhakrishnan P, Yu F, Tapprich W, Hollingsworth MA. MUC1 regulates expression of multiple microRNAs involved in pancreatic tumor progression, including the miR-200c/141 cluster. PLoS One. 2013;8:e73306. doi: 10.1371/journal.pone.0073306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol. 1996;16:859–867. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 126.Okamoto A, Demetrick DJ, Spillare EA, Hagiwara K, Hussain SP, Bennett WP, Forrester K, Gerwin B, Serrano M, Beach DH. Mutations and altered expression of p16INK4 in human cancer. Proc Natl Acad Sci U S A. 1994;91:11045–11049. doi: 10.1073/pnas.91.23.11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang L, Jamaluddin MS, Weakley SM, Yao Q, Chen C. Roles and mechanisms of microRNAs in pancreatic cancer. World J Surg. 2011;35:1725–1731. doi: 10.1007/s00268-010-0952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lal A, Kim HH, Abdelmohsen K, Kuwano Y, Pullmann R, Jr, Srikantan S, Subrahmanyam R, Martindale JL, Yang X, Ahmed F, Navarro F, Dykxhoorn D, Lieberman J, Gorospe M. p16(INK4a) translation suppressed by miR-24. PLoS One. 2008;3:e1864. doi: 10.1371/journal.pone.0001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakata K, Ohuchida K, Mizumoto K, Kayashima T, Ikenaga N, Sakai H, Lin C, Fujita H, Otsuka T, Aishima S, Nagai E, Oda Y, Tanaka M. MicroRNA-10b is overexpressed in pancreatic cancer, promotes its invasiveness, and correlates with a poor prognosis. Surgery. 2011;150:916–922. doi: 10.1016/j.surg.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 130.Gabriely G, Yi M, Narayan RS, Niers JM, Wurdinger T, Imitola J, Ligon KL, Kesari S, Esau C, Stephens RM, Tannous BA, Krichevsky AM. Human glioma growth is controlled by microRNA-10b. Cancer Res. 2011;71:3563–3572. doi: 10.1158/0008-5472.CAN-10-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Venkataraman S, Alimova I, Fan R, Harris P, Foreman N, Vibhakar R. MicroRNA 128a increases intracellular ROS level by targeting Bmi-1 and inhibits medulloblastoma cancer cell growth by promoting senescence. PLoS One. 2010;5:e10748. doi: 10.1371/journal.pone.0010748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Poliseno L, Pitto L, Simili M, Mariani L, Riccardi L, Ciucci A, Rizzo M, Evangelista M, Mercatanti A, Pandolfi PP, Rainaldi G. The proto-oncogene LRF is under post-transcriptional control of MiR-20a: implications for senescence. PLoS One. 2008;3:e2542. doi: 10.1371/journal.pone.0002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Braconi C, Huang N, Patel T. MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor suppressor gene expression by interleukin-6 in human malignant cholangiocytes. Hepatology. 2010;51:881–890. doi: 10.1002/hep.23381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chien WW, Domenech C, Catallo R, Kaddar T, Magaud JP, Salles G, Ffrench M. Cyclin-dependent kinase 1 expression is inhibited by p16(INK4a) at the post-transcriptional level through the microRNA pathway. Oncogene. 2011;30:1880–1891. doi: 10.1038/onc.2010.570. [DOI] [PubMed] [Google Scholar]
- 135.Al-Khalaf HH, Mohideen P, Nallar SC, Kalvakolanu DV, Aboussekhra A. The cyclin-dependent kinase inhibitor p16INK4a physically interacts with transcription factor Sp1 and cyclin-dependent kinase 4 to transactivate microRNA-141 and microRNA-146b-5p spontaneously and in response to ultraviolet light-induced DNA damage. J Biol Chem. 2013;288:35511–35525. doi: 10.1074/jbc.M113.512640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Whitman M. Smads and early developmental signaling by the TGFbeta superfamily. Genes Dev. 1998;12:2445–2462. doi: 10.1101/gad.12.16.2445. [DOI] [PubMed] [Google Scholar]
- 137.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 138.Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J. 2003;17:1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- 139.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6:635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 140.Donovan J, Slingerland J. Transforming growth factor-beta and breast cancer: Cell cycle arrest by transforming growth factor-beta and its disruption in cancer. Breast Cancer Res. 2000;2:116–124. doi: 10.1186/bcr43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nicolas FJ, Hill CS. Attenuation of the TGF-beta-Smad signaling pathway in pancreatic tumor cells confers resistance to TGF-beta-induced growth arrest. Oncogene. 2003;22:3698–3711. doi: 10.1038/sj.onc.1206420. [DOI] [PubMed] [Google Scholar]
- 142.Blahna MT, Hata A. Smad-mediated regulation of microRNA biosynthesis. FEBS Lett. 2012;586:1906–1912. doi: 10.1016/j.febslet.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chung AC, Huang XR, Meng X, Lan HY. miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc Nephrol. 2010;21:1317–1325. doi: 10.1681/ASN.2010020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kong W, Yang H, He L, Zhao JJ, Coppola D, Dalton WS, Cheng JQ. MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol. 2008;28:6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Naito Y, Sakamoto N, Oue N, Yashiro M, Sentani K, Yanagihara K, Hirakawa K, Yasui W. MicroRNA-143 regulates collagen type III expression in stromal fibroblasts of scirrhous type gastric cancer. Cancer Sci. 2014;105:228–235. doi: 10.1111/cas.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang Y, Fan KJ, Sun Q, Chen AZ, Shen WL, Zhao ZH, Zheng XF, Yang X. Functional screening for miRNAs targeting Smad4 identified miR-199a as a negative regulator of TGF-beta signalling pathway. Nucleic Acids Res. 2012;40:9286–9297. doi: 10.1093/nar/gks667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu L, Nie J, Chen L, Dong G, Du X, Wu X, Tang Y, Han W. The oncogenic role of microRNA-130a/301a/454 in human colorectal cancer via targeting Smad4 expression. PLoS One. 2013;8:e55532. doi: 10.1371/journal.pone.0055532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen Z, Chen LY, Dai HY, Wang P, Gao S, Wang K. miR-301a promotes pancreatic cancer cell proliferation by directly inhibiting Bim expression. J Cell Biochem. 2012;113:3229–3235. doi: 10.1002/jcb.24200. [DOI] [PubMed] [Google Scholar]
- 150.Lu Z, Li Y, Takwi A, Li B, Zhang J, Conklin DJ, Young KH, Martin R, Li Y. miR-301a as an NF-kappaB activator in pancreatic cancer cells. EMBO J. 2011;30:57–67. doi: 10.1038/emboj.2010.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hruban RH, Offerhaus GJ, Kern SE, Goggins M, Wilentz RE, Yeo CJ. Tumor-suppressor genes in pancreatic cancer. J Hepatobiliary Pancreat Surg. 1998;5:383–391. doi: 10.1007/s005340050062. [DOI] [PubMed] [Google Scholar]
- 152.Singh P, Srinivasan R, Wig JD. Major molecular markers in pancreatic ductal adenocarcinoma and their roles in screening, diagnosis, prognosis, and treatment. Pancreas. 2011;40:644–652. doi: 10.1097/MPA.0b013e31821ff741. [DOI] [PubMed] [Google Scholar]
- 153.Singh P, Srinivasan R, Wig JD, Radotra BD. A study of Smad4, Smad6 and Smad7 in Surgically Resected Samples of Pancreatic Ductal Adenocarcinoma and Their Correlation with Clinicopathological Parameters and Patient Survival. BMC Res Notes. 2011;4:560–564. doi: 10.1186/1756-0500-4-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gaspar NJ, Li L, Kapoun AM, Medicherla S, Reddy M, Li G, O’Young G, Quon D, Henson M, Damm DL, Muiru GT, Murphy A, Higgins LS, Chakravarty S, Wong DH. Inhibition of transforming growth factor beta signaling reduces pancreatic adenocarcinoma growth and invasiveness. Mol Pharmacol. 2007;72:152–161. doi: 10.1124/mol.106.029025. [DOI] [PubMed] [Google Scholar]
- 155.Smith AL, Robin TP, Ford HL. Molecular pathways: targeting the TGF-beta pathway for cancer therapy. Clin Cancer Res. 2012;18:4514–4521. doi: 10.1158/1078-0432.CCR-11-3224. [DOI] [PubMed] [Google Scholar]
- 156.Yao G, Yin M, Lian J, Tian H, Liu L, Li X, Sun F. MicroRNA-224 is involved in transforming growth factor-beta-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Mol Endocrinol. 2010;24:540–551. doi: 10.1210/me.2009-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Du J, Wang J, Tan G, Cai Z, Zhang L, Tang B, Wang Z. Aberrant elevated microRNA-146a in dendritic cells (DC) induced by human pancreatic cancer cell line BxPC-3-conditioned medium inhibits DC maturation and activation. Med Oncol. 2012;29:2814–2823. doi: 10.1007/s12032-012-0175-2. [DOI] [PubMed] [Google Scholar]
- 158.Hao J, Zhang S, Zhou Y, Hu X, Shao C. MicroRNA 483-3p suppresses the expression of DPC4/Smad4 in pancreatic cancer. FEBS Lett. 2011;585:207–213. doi: 10.1016/j.febslet.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 159.Hao J, Zhang S, Zhou Y, Liu C, Hu X, Shao C. MicroRNA 421 suppresses DPC4/Smad4 in pancreatic cancer. Biochem Biophys Res Commun. 2011;406:552–557. doi: 10.1016/j.bbrc.2011.02.086. [DOI] [PubMed] [Google Scholar]
- 160.Li L, Li Z, Kong X, Xie D, Jia Z, Jiang W, Cui J, Du Y, Wei D, Huang S, Xie K. Down-regulation of microRNA-494 via loss of SMAD4 increases FOXM1 and beta-catenin signaling in pancreatic ductal adenocarcinoma cells. Gastroenterology. 2014;147:485–497. doi: 10.1053/j.gastro.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 161.Geraldo MV, Yamashita AS, Kimura ET. MicroRNA miR-146b-5p regulates signal transduction of TGF-beta by repressing SMAD4 in thyroid cancer. Oncogene. 2012;31:1910–1922. doi: 10.1038/onc.2011.381. [DOI] [PubMed] [Google Scholar]
- 162.Hui CC, Angers S. Gli proteins in development and disease. Annu Rev Cell Dev Biol. 2011;27:513–537. doi: 10.1146/annurev-cellbio-092910-154048. [DOI] [PubMed] [Google Scholar]
- 163.Dosch JS, Pasca di MM, Simeone DM. Pancreatic cancer and hedgehog pathway signaling: new insights. Pancreatology. 2010;10:151–157. doi: 10.1159/000225923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernandez-del CC, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, Hollingsworth MA. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sheehy NT, Cordes KR, White MP, Ivey KN, Srivastava D. The neural crest-enriched microRNA miR-452 regulates epithelial-mesenchymal signaling in the first pharyngeal arch. Development. 2010;137:4307–4316. doi: 10.1242/dev.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Uziel T, Karginov FV, Xie S, Parker JS, Wang YD, Gajjar A, He L, Ellison D, Gilbertson RJ, Hannon G, Roussel MF. The miR-17~92 cluster collaborates with the Sonic Hedgehog pathway in medulloblastoma. Proc Natl Acad Sci U S A. 2009;106:2812–2817. doi: 10.1073/pnas.0809579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tsuda N, Ishiyama S, Li Y, Ioannides CG, Abbruzzese JL, Chang DZ. Synthetic microRNA designed to target glioma-associated antigen 1 transcription factor inhibits division and induces late apoptosis in pancreatic tumor cells. Clin Cancer Res. 2006;12:6557–6564. doi: 10.1158/1078-0432.CCR-06-0588. [DOI] [PubMed] [Google Scholar]
- 169.Ahmad A, Maitah MY, Ginnebaugh KR, Li Y, Bao B, Gadgeel SM, Sarkar FH. Inhibition of Hedgehog signaling sensitizes NSCLC cells to standard therapies through modulation of EMT-regulating miRNAs. J Hematol Oncol. 2013;6:77–6. doi: 10.1186/1756-8722-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Luo G, Long J, Cui X, Xiao Z, Liu Z, Shi S, Liu L, Liu C, Xu J, Li M, Yu X. Highly lymphatic metastatic pancreatic cancer cells possess stem cell-like properties. Int J Oncol. 2013;42:979–984. doi: 10.3892/ijo.2013.1780. [DOI] [PubMed] [Google Scholar]
- 171.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 172.Lee KH, Lotterman C, Karikari C, Omura N, Feldmann G, Habbe N, Goggins MG, Mendell JT, Maitra A. Epigenetic silencing of MicroRNA miR-107 regulates cyclin-dependent kinase 6 expression in pancreatic cancer. Pancreatology. 2009;9:293–301. doi: 10.1159/000186051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pramanik D, Campbell NR, Karikari C, Chivukula R, Kent OA, Mendell JT, Maitra A. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol Cancer Ther. 2011;10:1470–1480. doi: 10.1158/1535-7163.MCT-11-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun F, Fu H, Liu Q, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett. 2008;582:1564–1568. doi: 10.1016/j.febslet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 175.Sanchez-Diaz PC, Hsiao TH, Chang JC, Yue D, Tan MC, Chen HI, Tomlinson GE, Huang Y, Chen Y, Hung JY. De-regulated microRNAs in pediatric cancer stem cells target pathways involved in cell proliferation, cell cycle and development. PLoS One. 2013;8:e61622. doi: 10.1371/journal.pone.0061622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, Banerjee S, Azmi AS, Miele L, Sarkar FH. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307:26–36. doi: 10.1016/j.canlet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 177.Bao B, Ali S, Banerjee S, Wang Z, Logna F, Azmi AS, Kong D, Ahmad A, Li Y, Padhye S, Sarkar FH. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012;72:335–345. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bao B, Wang Z, Ali S, Ahmad A, Azmi AS, Sarkar SH, Banerjee S, Kong D, Li Y, Thakur S, Sarkar FH. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res (Phila) 2012;5:355–364. doi: 10.1158/1940-6207.CAPR-11-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Basu A, Alder H, Khiyami A, Leahy P, Croce CM, Haldar S. MicroRNA-375 and MicroRNA-221: Potential Noncoding RNAs Associated with Antiproliferative Activity of Benzyl Isothiocyanate in Pancreatic Cancer. Genes Cancer. 2011;2:108–119. doi: 10.1177/1947601911409212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bhutia YD, Hung SW, Patel B, Lovin D, Govindarajan R. CNT1 expression influences proliferation and chemosensitivity in drug-resistant pancreatic cancer cells. Cancer Res. 2011;71:1825–1835. doi: 10.1158/0008-5472.CAN-10-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Greither T, Grochola LF, Udelnow A, Lautenschlager C, Wurl P, Taubert H. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int J Cancer. 2010;126:73–80. doi: 10.1002/ijc.24687. [DOI] [PubMed] [Google Scholar]
- 182.Heyn H, Schreek S, Buurman R, Focken T, Schlegelberger B, Beger C. MicroRNA miR-548d is a superior regulator in pancreatic cancer. Pancreas. 2012;41:218–221. doi: 10.1097/MPA.0b013e318224b701. [DOI] [PubMed] [Google Scholar]
- 183.Ivanovska I, Ball AS, Diaz RL, Magnus JF, Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson AL, Linsley PS, Cleary MA. MicroRNAs in the miR-106b family regulate p21/CDKN1A and promote cell cycle progression. Mol Cell Biol. 2008;28:2167–2174. doi: 10.1128/MCB.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li Y, VandenBoom TG, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liffers ST, Munding JB, Vogt M, Kuhlmann JD, Verdoodt B, Nambiar S, Maghnouj A, Mirmohammadsadegh A, Hahn SA, Tannapfel A. MicroRNA-148a is down-regulated in human pancreatic ductal adenocarcinomas and regulates cell survival by targeting CDC25B. Lab Invest. 2011;91:1472–1479. doi: 10.1038/labinvest.2011.99. [DOI] [PubMed] [Google Scholar]
- 186.Ma Y, Yu S, Zhao W, Lu Z, Chen J. miR-27a regulates the growth, colony formation and migration of pancreatic cancer cells by targeting Sprouty2. Cancer Lett. 2010;298:150–158. doi: 10.1016/j.canlet.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 187.Nalls D, Tang SN, Rodova M, Srivastava RK, Shankar S. Targeting epigenetic regulation of miR-34a for treatment of pancreatic cancer by inhibition of pancreatic cancer stem cells. PLoS One. 2011;6:e24099. doi: 10.1371/journal.pone.0024099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Park JK, Lee EJ, Esau C, Schmittgen TD. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas. 2009;38:e190–e199. doi: 10.1097/MPA.0b013e3181ba82e1. [DOI] [PubMed] [Google Scholar]
- 189.Su A, He S, Tian B, Hu W, Zhang Z. MicroRNA-221 mediates the effects of PDGF-BB on migration, proliferation, and the epithelial-mesenchymal transition in pancreatic cancer cells. PLoS One. 2013;8:e71309. doi: 10.1371/journal.pone.0071309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sun M, Estrov Z, Ji Y, Coombes KR, Harris DH, Kurzrock R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol Cancer Ther. 2008;7:464–473. doi: 10.1158/1535-7163.MCT-07-2272. [DOI] [PubMed] [Google Scholar]
- 191.Yu J, Ohuchida K, Mizumoto K, Sato N, Kayashima T, Fujita H, Nakata K, Tanaka M. MicroRNA, hsa-miR-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol Cancer. 2010;9:169. doi: 10.1186/1476-4598-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Petrocca F, Vecchione A, Croce CM. Emerging role of miR-106b-25/miR-17-92 clusters in the control of transforming growth factor beta signaling. Cancer Res. 2008;68:8191–8194. doi: 10.1158/0008-5472.CAN-08-1768. [DOI] [PubMed] [Google Scholar]
- 193.Smith AL, Iwanaga R, Drasin DJ, Micalizzi DS, Vartuli RL, Tan AC, Ford HL. The miR-106b-25 cluster targets Smad7, activates TGF-beta signaling, and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer. Oncogene. 2012;31:5162–5171. doi: 10.1038/onc.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26:1268–1286. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Weissmueller S, Manchado E, Saborowski M, Morris JP, Wagenblast E, Davis CA, Moon SH, Pfister NT, Tschaharganeh DF, Kitzing T, Aust D, Markert EK, Wu J, Grimmond SM, Pilarsky C, Prives C, Biankin AV, Lowe SW. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor beta signaling. Cell. 2014;157:382–394. doi: 10.1016/j.cell.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Morin RD, O’Connor MD, Griffith M, Kuchenbauer F, Delaney A, Prabhu AL, Zhao Y, McDonald H, Zeng T, Hirst M, Eaves CJ, Marra MA. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res. 2008;18:610–621. doi: 10.1101/gr.7179508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Castoldi M, Schmidt S, Benes V, Noerholm M, Kulozik AE, Hentze MW, Muckenthaler MU. A sensitive array for microRNA expression profiling (miChip) based on locked nucleic acids (LNA) RNA. 2006;12:913–920. doi: 10.1261/rna.2332406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Davison TS, Johnson CD, Andruss BF. Analyzing micro-RNA expression using microarrays. Methods Enzymol. 2006;411:14–34. doi: 10.1016/S0076-6879(06)11002-2. [DOI] [PubMed] [Google Scholar]
- 199.Liang RQ, Li W, Li Y, Tan CY, Li JX, Jin YX, Ruan KC. An oligonucleotide microarray for microRNA expression analysis based on labeling RNA with quantum dot and nanogold probe. Nucleic Acids Res. 2005;33:e17. doi: 10.1093/nar/gni019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Thomson JM, Parker J, Perou CM, Hammond SM. A custom microarray platform for analysis of microRNA gene expression. Nat Methods. 2004;1:47–53. doi: 10.1038/nmeth704. [DOI] [PubMed] [Google Scholar]
- 201.Valoczi A, Hornyik C, Varga N, Burgyan J, Kauppinen S, Havelda Z. Sensitive and specific detection of microRNAs by northern blot analysis using LNA-modified oligonucleotide probes. Nucleic Acids Res. 2004;32:e175. doi: 10.1093/nar/gnh171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Duncan DD, Eshoo M, Esau C, Freier SM, Lollo BA. Absolute quantitation of microRNAs with a PCR-based assay. Anal Biochem. 2006;359:268–270. doi: 10.1016/j.ab.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 203.Raymond CK, Roberts BS, Garrett-Engele P, Lim LP, Johnson JM. Simple, quantitative primer-extension PCR assay for direct monitoring of microRNAs and short-interfering RNAs. RNA. 2005;11:1737–1744. doi: 10.1261/rna.2148705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shi Z, Johnson JJ, Stack MS. Fluorescence In Situ Hybridization for MicroRNA Detection in Archived Oral Cancer Tissues. J Oncol. 2012:903581. doi: 10.1155/2012/903581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Neely LA, Patel S, Garver J, Gallo M, Hackett M, McLaughlin S, Nadel M, Harris J, Gullans S, Rooke J. A single-molecule method for the quantitation of microRNA gene expression. Nat Methods. 2006;3:41–46. doi: 10.1038/nmeth825. [DOI] [PubMed] [Google Scholar]
- 206.Deo M, Yu JY, Chung KH, Tippens M, Turner DL. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev Dyn. 2006;235:2538–2548. doi: 10.1002/dvdy.20847. [DOI] [PubMed] [Google Scholar]
- 207.Kloosterman WP, Wienholds E, de BE, Kauppinen S, Plasterk RH. In situ detection of miRNAs in animal embryos using LNA-modified oligonucleotide probes. Nat Methods. 2006;3:27–29. doi: 10.1038/nmeth843. [DOI] [PubMed] [Google Scholar]
- 208.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13:358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lu C, Meyers BC, Green PJ. Construction of small RNA cDNA libraries for deep sequencing. Methods. 2007;43:110–117. doi: 10.1016/j.ymeth.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 210.Kawahara Y, Zinshteyn B, Sethupathy P, Iizasa H, Hatzigeorgiou AG, Nishikura K. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science. 2007;315:1137–1140. doi: 10.1126/science.1138050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer ML, Jarvie TP, Jirage KB, Kim JB, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasz A, Vogt KA, Volkmer GA, Wang SH, Wang Y, Weiner MP, Yu P, Begley RF, Rothberg JM. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Berezikov E, Cuppen E, Plasterk RH. Approaches to microRNA discovery. Nat Genet. 2006;38(Suppl):S2–S7. doi: 10.1038/ng1794. [DOI] [PubMed] [Google Scholar]
- 213.Berezikov E, Thuemmler F, van Laake LW, Kondova I, Bontrop R, Cuppen E, Plasterk RH. Diversity of microRNAs in human and chimpanzee brain. Nat Genet. 2006;38:1375–1377. doi: 10.1038/ng1914. [DOI] [PubMed] [Google Scholar]
- 214.Varallyay E, Burgyan J, Havelda Z. MicroRNA detection by northern blotting using locked nucleic acid probes. Nat Protoc. 2008;3:190–196. doi: 10.1038/nprot.2007.528. [DOI] [PubMed] [Google Scholar]
- 215.Habbe N, Koorstra JB, Mendell JT, Offerhaus GJ, Ryu JK, Feldmann G, Mullendore ME, Goggins MG, Hong SM, Maitra A. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol Ther. 2009;8:340–346. doi: 10.4161/cbt.8.4.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ho AS, Huang X, Cao H, Christman-Skieller C, Bennewith K, Le QT, Koong AC. Circulating miR-210 as a Novel Hypoxia Marker in Pancreatic Cancer. Transl Oncol. 2010;3:109–113. doi: 10.1593/tlo.09256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ganepola GA, Rutledge JR, Suman P, Yiengpruksawan A, Chang DH. Novel blood-based microRNA biomarker panel for early diagnosis of pancreatic cancer. World J Gastrointest Oncol. 2014;6:22–33. doi: 10.4251/wjgo.v6.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Frampton AE, Giovannetti E, Jamieson NB, Krell J, Gall TM, Stebbing J, Jiao LR, Castellano L. A microRNA meta-signature for pancreatic ductal adenocarcinoma. Expert Rev Mol Diagn. 2014;14:267–71. doi: 10.1586/14737159.2014.893192. [DOI] [PubMed] [Google Scholar]
- 219.Schultz NA, Dehlendorff C, Jensen BV, Bjerregaard JK, Nielsen KR, Bojesen SE, Calatayud D, Nielsen SE, Yilmaz M, Hollander NH, Andersen KK, Johansen JS. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA. 2014;311:392–404. doi: 10.1001/jama.2013.284664. [DOI] [PubMed] [Google Scholar]
- 220.Li A, Yu J, Kim H, Wolfgang CL, Canto MI, Hruban RH, Goggins M. MicroRNA array analysis finds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls. Clin Cancer Res. 2013;19:3600–3610. doi: 10.1158/1078-0432.CCR-12-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang WS, Liu LX, Li GP, Chen Y, Li CY, Jin DY, Wang XL. Combined serum CA19-9 and miR-27a-3p in peripheral blood mononuclear cells to diagnose pancreatic cancer. Cancer Prev Res (Phila) 2013;6:331–338. doi: 10.1158/1940-6207.CAPR-12-0307. [DOI] [PubMed] [Google Scholar]
- 222.Liu J, Gao J, Du Y, Li Z, Ren Y, Gu J, Wang X, Gong Y, Wang W, Kong X. Combination of plasma microRNAs with serum CA19-9 for early detection of pancreatic cancer. Int J Cancer. 2012;131:683–691. doi: 10.1002/ijc.26422. [DOI] [PubMed] [Google Scholar]
- 223.Liu R, Chen X, Du Y, Yao W, Shen L, Wang C, Hu Z, Zhuang R, Ning G, Zhang C, Yuan Y, Li Z, Zen K, Ba Y, Zhang CY. Serum microRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clin Chem. 2012;58:610–618. doi: 10.1373/clinchem.2011.172767. [DOI] [PubMed] [Google Scholar]
- 224.Wan C, Shen Y, Yang T, Wang T, Chen L, Wen F. Diagnostic value of microRNA for pancreatic cancer: a meta-analysis. Arch Med Sci. 2012;8:749–755. doi: 10.5114/aoms.2012.31609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kawaguchi T, Komatsu S, Ichikawa D, Morimura R, Tsujiura M, Konishi H, Takeshita H, Nagata H, Arita T, Hirajima S, Shiozaki A, Ikoma H, Okamoto K, Ochiai T, Taniguchi H, Otsuji E. Clinical impact of circulating miR-221 in plasma of patients with pancreatic cancer. Br J Cancer. 2013;108:361–369. doi: 10.1038/bjc.2012.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bauer AS, Keller A, Costello E, Greenhalf W, Bier M, Borries A, Beier M, Neoptolemos J, Buchler M, Werner J, Giese N, Hoheisel JD. Diagnosis of pancreatic ductal adenocarcinoma and chronic pancreatitis by measurement of microRNA abundance in blood and tissue. PLoS One. 2012;7:e34151. doi: 10.1371/journal.pone.0034151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Szafranska AE, Doleshal M, Edmunds HS, Gordon S, Luttges J, Munding JB, Barth RJ, Jr, Gutmann EJ, Suriawinata AA, Marc PJ, Tannapfel A, Korc M, Hahn SA, Labourier E, Tsongalis GJ. Analysis of microRNAs in pancreatic fine-needle aspirates can classify benign and malignant tissues. Clin Chem. 2008;54:1716–1724. doi: 10.1373/clinchem.2008.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yabushita S, Fukamachi K, Tanaka H, Sumida K, Deguchi Y, Sukata T, Kawamura S, Uwagawa S, Suzui M, Tsuda H. Circulating microRNAs in serum of human K-ras oncogene transgenic rats with pancreatic ductal adenocarcinomas. Pancreas. 2012;41:1013–1018. doi: 10.1097/MPA.0b013e31824ac3a5. [DOI] [PubMed] [Google Scholar]
- 229.Wang J, Raimondo M, Guha S, Chen J, Diao L, Dong X, Wallace MB, Killary AM, Frazier ML, Woodward TA, Wang J, Sen S. Circulating microRNAs in Pancreatic Juice as Candidate Biomarkers of Pancreatic Cancer. J Cancer. 2014;5:696–705. doi: 10.7150/jca.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Slater EP, Strauch K, Rospleszcz S, Ramaswamy A, Esposito I, Kloppel G, Matthai E, Heeger K, Fendrich V, Langer P, Bartsch DK. MicroRNA-196a and -196b as Potential Biomarkers for the Early Detection of Familial Pancreatic Cancer. Transl Oncol. 2014;7:464–471. doi: 10.1016/j.tranon.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ding Z, Wu H, Zhang J, Huang G, Ji D. MicroRNAs as novel biomarkers for pancreatic cancer diagnosis: a meta-analysis based on 18 articles. Tumour Biol. 2014 doi: 10.1007/s13277-014-2133-4. [DOI] [PubMed] [Google Scholar]
- 232.Bose D, Jayaraj GG, Kumar S, Maiti S. A molecular-beacon-based screen for small molecule inhibitors of miRNA maturation. ACS Chem Biol. 2013;8:930–938. doi: 10.1021/cb300650y. [DOI] [PubMed] [Google Scholar]
- 233.Pai P, Rachagani S, Are C, Batra SK. Prospects of miRNA-based therapy for pancreatic cancer. Curr Drug Targets. 2013;14:1101–1109. doi: 10.2174/13894501113149990181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Davis S, Lollo B, Freier S, Esau C. Improved targeting of miRNA with antisense oligonucleotides. Nucleic Acids Res. 2006;34:2294–2304. doi: 10.1093/nar/gkl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Davis S, Propp S, Freier SM, Jones LE, Serra MJ, Kinberger G, Bhat B, Swayze EE, Bennett CF, Esau C. Potent inhibition of microRNA in vivo without degradation. Nucleic Acids Res. 2009;37:70–77. doi: 10.1093/nar/gkn904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Esau CC, Monia BP. Therapeutic potential for microRNAs. Adv Drug Deliv Rev. 2007;59:101–114. doi: 10.1016/j.addr.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 237.Esau CC. Inhibition of microRNA with antisense oligonucleotides. Methods. 2008;44:55–60. doi: 10.1016/j.ymeth.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 238.Petersen M, Wengel J. LNA: a versatile tool for therapeutics and genomics. Trends Biotechnol. 2003;21:74–81. doi: 10.1016/S0167-7799(02)00038-0. [DOI] [PubMed] [Google Scholar]
- 239.Stenvang J, Kauppinen S. MicroRNAs as targets for antisense-based therapeutics. Expert Opin Biol Ther. 2008;8:59–81. doi: 10.1517/14712598.8.1.59. [DOI] [PubMed] [Google Scholar]
- 240.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 241.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 242.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 243.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Orum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gumireddy K, Young DD, Xiong X, Hogenesch JB, Huang Q, Deiters A. Small-molecule inhibitors of microrna miR-21 function. Angew Chem Int Ed Engl. 2008;47:7482–7484. doi: 10.1002/anie.200801555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Young DD, Connelly CM, Grohmann C, Deiters A. Small molecule modifiers of microRNA miR-122 function for the treatment of hepatitis C virus infection and hepatocellular carcinoma. J Am Chem Soc. 2010;132:7976–7981. doi: 10.1021/ja910275u. [DOI] [PubMed] [Google Scholar]
- 246.Staton AA, Giraldez AJ. Use of target protector morpholinos to analyze the physiological roles of specific miRNA-mRNA pairs in vivo. Nat Protoc. 2011;6:2035–2049. doi: 10.1038/nprot.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Park JK, Kogure T, Nuovo GJ, Jiang J, He L, Kim JH, Phelps MA, Papenfuss TL, Croce CM, Patel T, Schmittgen TD. miR-221 silencing blocks hepatocellular carcinoma and promotes survival. Cancer Res. 2011;71:7608–7616. doi: 10.1158/0008-5472.CAN-11-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW, Weinberg RA. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28:341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ebert MS, Sharp PA. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043–2050. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kluiver J, Gibcus JH, Hettinga C, Adema A, Richter MK, Halsema N, Slezak-Prochazka I, Ding Y, Kroesen BJ, van den Berg A. Rapid generation of microRNA sponges for microRNA inhibition. PLoS One. 2012;7:e29275. doi: 10.1371/journal.pone.0029275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bader AG, Brown D, Stoudemire J, Lammers P. Developing therapeutic microRNAs for cancer. Gene Ther. 2011;18:1121–1126. doi: 10.1038/gt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Montgomery RL, van RE. Therapeutic advances in MicroRNA targeting. J Cardiovasc Pharmacol. 2011;57:1–7. doi: 10.1097/FJC.0b013e3181f603d0. [DOI] [PubMed] [Google Scholar]
- 253.Trang P, Wiggins JF, Daige CL, Cho C, Omotola M, Brown D, Weidhaas JB, Bader AG, Slack FJ. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther. 2011;19:1116–1122. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Li L, Xie X, Luo J, Liu M, Xi S, Guo J, Kong Y, Wu M, Gao J, Xie Z, Tang J, Wang X, Wei W, Yang M, Hung MC, Xie X. Targeted expression of miR-34a using the T-VISA system suppresses breast cancer cell growth and invasion. Mol Ther. 2012;20:2326–2334. doi: 10.1038/mt.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Nair VS, Maeda LS, Ioannidis JP. Clinical outcome prediction by microRNAs in human cancer: a systematic review. J Natl Cancer Inst. 2012;104:528–540. doi: 10.1093/jnci/djs027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- 258.Sicard F, Gayral M, Lulka H, Buscail L, Cordelier P. Targeting miR-21 for the therapy of pancreatic cancer. Mol Ther. 2013;21:986–994. doi: 10.1038/mt.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Passadouro M, Pedroso de Lima MC, Faneca H. MicroRNA modulation combined with sunitinib as a novel therapeutic strategy for pancreatic cancer. Int J Nanomedicine. 2014;9:3203–3217. doi: 10.2147/IJN.S64456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Arora S, Swaminathan SK, Kirtane A, Srivastava SK, Bhardwaj A, Singh S, Panyam J, Singh AP. Synthesis, characterization, and evaluation of poly (D, L-lactide-co-glycolide)-based nanoformulation of miRNA-150: potential implications for pancreatic cancer therapy. Int J Nanomedicine. 2014;9:2933–2942. doi: 10.2147/IJN.S61949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Garchow BG, Bartulos EO, Leung YT, Tsao PY, Eisenberg RA, Caricchio R, Obad S, Petri A, Kauppinen S, Kiriakidou M. Silencing of microRNA-21 in vivo ameliorates autoimmune splenomegaly in lupus mice. EMBO Mol Med. 2011;3:605–615. doi: 10.1002/emmm.201100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D, Fearon ER, Lawrence TS, Xu L. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 264.Trang P, Medina PP, Wiggins JF, Ruffino L, Kelnar K, Omotola M, Homer R, Brown D, Bader AG, Weidhaas JB, Slack FJ. Regression of murine lung tumors by the let-7 microRNA. Oncogene. 2010;29:1580–1587. doi: 10.1038/onc.2009.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Esquela-Kerscher A, Trang P, Wiggins JF, Patrawala L, Cheng A, Ford L, Weidhaas JB, Brown D, Bader AG, Slack FJ. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7:759–764. doi: 10.4161/cc.7.6.5834. [DOI] [PubMed] [Google Scholar]
- 266.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, Bader AG. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70:5923–5930. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Takeshita F, Patrawala L, Osaki M, Takahashi RU, Yamamoto Y, Kosaka N, Kawamata M, Kelnar K, Bader AG, Brown D, Ochiya T. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol Ther. 2010;18:181–187. doi: 10.1038/mt.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta. 2010;1803:1231–1243. doi: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 270.van RE, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hullinger TG, Montgomery RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, Dalby CM, Robinson K, Stack C, Latimer PA, Hare JM, Olson EN, van RE. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res. 2012;110:71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, Sadek HA. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci U S A. 2013;110:187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Patrick DM, Zhang CC, Tao Y, Yao H, Qi X, Schwartz RJ, Jun-Shen HL, Olson EN. Defective erythroid differentiation in miR-451 mutant mice mediated by 14-3-3zeta. Genes Dev. 2010;24:1614–1619. doi: 10.1101/gad.1942810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 275.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 276.Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, Krichevsky AM. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28:5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Swarbrick A, Woods SL, Shaw A, Balakrishnan A, Phua Y, Nguyen A, Chanthery Y, Lim L, Ashton LJ, Judson RL, Huskey N, Blelloch R, Haber M, Norris MD, Lengyel P, Hackett CS, Preiss T, Chetcuti A, Sullivan CS, Marcusson EG, Weiss W, L’Etoile N, Goga A. miR-380-5p represses p53 to control cellular survival and is associated with poor outcome in MYCN-amplified neuroblastoma. Nat Med. 2010;16:1134–1140. doi: 10.1038/nm.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Huynh C, Segura MF, Gaziel-Sovran A, Menendez S, Darvishian F, Chiriboga L, Levin B, Meruelo D, Osman I, Zavadil J, Marcusson EG, Hernando E. Efficient in vivo microRNA targeting of liver metastasis. Oncogene. 2011;30:1481–1488. doi: 10.1038/onc.2010.523. [DOI] [PubMed] [Google Scholar]
- 279.Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3:120. doi: 10.3389/fgene.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Hou B, Jian Z, Chen S, Ou Y, Li S, Ou J. Expression of miR-216a in pancreatic cancer and its clinical significance. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:1628–1631. [PubMed] [Google Scholar]
- 281.Moulton JD, Jiang S. Gene knockdowns in adult animals: PPMOs and vivo-morpholinos. Molecules. 2009;14:1304–1323. doi: 10.3390/molecules14031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Okamoto A, Demetrick DJ, Spillare EA, Hagiwara K, Hussain SP, Bennett WP, Forrester K, Gerwin B, Serrano M, Beach DH. Mutations and altered expression of p16INK4 in human cancer. Proc Natl Acad Sci U S A. 1994;91:11045–11049. doi: 10.1073/pnas.91.23.11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lal A, Kim HH, Abdelmohsen K, Kuwano Y, Pullmann R, Jr, Srikantan S, Subrahmanyam R, Martindale JL, Yang X, Ahmed F, Navarro F, Dykxhoorn D, Lieberman J, Gorospe M. p16(INK4a) translation suppressed by miR-24. PLoS One. 2008;3:e1864. doi: 10.1371/journal.pone.0001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gabriely G, Yi M, Narayan RS, Niers JM, Wurdinger T, Imitola J, Ligon KL, Kesari S, Esau C, Stephens RM, Tannous BA, Krichevsky AM. Human glioma growth is controlled by microRNA-10b. Cancer Res. 2011;71:3563–3572. doi: 10.1158/0008-5472.CAN-10-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Venkataraman S, Alimova I, Fan R, Harris P, Foreman N, Vibhakar R. MicroRNA 128a increases intracellular ROS level by targeting Bmi-1 and inhibits medulloblastoma cancer cell growth by promoting senescence. PLoS One. 2010;5:e10748. doi: 10.1371/journal.pone.0010748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Poliseno L, Pitto L, Simili M, Mariani L, Riccardi L, Ciucci A, Rizzo M, Evangelista M, Mercatanti A, Pandolfi PP, Rainaldi G. The proto-oncogene LRF is under post-transcriptional control of MiR-20a: implications for senescence. PLoS One. 2008;3:e2542. doi: 10.1371/journal.pone.0002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Braconi C, Huang N, Patel T. MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor suppressor gene expression by interleukin-6 in human malignant cholangiocytes. Hepatology. 2010;51:881–890. doi: 10.1002/hep.23381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Chien WW, Domenech C, Catallo R, Kaddar T, Magaud JP, Salles G, Ffrench M. Cyclin-dependent kinase 1 expression is inhibited by p16(INK4a) at the post-transcriptional level through the microRNA pathway. Oncogene. 2011;30:1880–1891. doi: 10.1038/onc.2010.570. [DOI] [PubMed] [Google Scholar]
- 289.Al-Khalaf HH, Mohideen P, Nallar SC, Kalvakolanu DV, Aboussekhra A. The cyclin-dependent kinase inhibitor p16INK4a physically interacts with transcription factor Sp1 and cyclin-dependent kinase 4 to transactivate microRNA-141 and microRNA-146b-5p spontaneously and in response to ultraviolet light-induced DNA damage. J Biol Chem. 2013;288:35511–35525. doi: 10.1074/jbc.M113.512640. [DOI] [PMC free article] [PubMed] [Google Scholar]