Abstract
Inflammation is associated with peripheral neuropathy, however the interplay among cytokines, chemokines, and neurons is still unclear. We hypothesized that this neuroinflammatory interaction can be defined by computational modeling based on the dynamics of protein expression in the sciatic nerve of rats subjected to chronic constriction injury. Using Dynamic Bayesian Network inference, we identified interleukin (IL)-18 as a central node associated with neuropathic pain in this animal model. Immunofluorescence supported a role for inflammasome activation and induction of IL-18 at the site of injury. Combined in vivo and in silico approaches may thus highlight novel targets in peripheral neuropathy.
Keywords: Neuroinflammation, Dynamic bayesian network, Chronic constriction injury, Neuropathic pain, IL-18
1. Introduction
Pain arises with multiple etiologies and when pain persists beyond the natural course of tissue healing, it is considered to be chronic pain (IASP, 2003). Chronic pain is now considered of public health importance due to its wide impact on society. Chronic pain affects around 100 million American adults and leads to economic loss of approximately $600 billion annually (IOM, 2011). Neuropathic pain is one of the causes of chronic pain, and affects more than 6 million Americans (IOM, 2011). Neuropathic pain occurs in response to injury of the somatosensory nervous system (Jensen et al., 2011), and may cause pain in the joints, muscles, internal organs, or skin. Furthermore, neuropathic pain may occur in the central or peripheral nervous system based upon the site of lesion.
Following nerve injury, Wallerian degeneration or anterograde degeneration of the neurons occurs leading to disintegration of the myelin and the axons distal to the injury causing inflammation and neuropathic pain (Gaudet et al., 2011). This axonal degeneration causes disorientation of the axon–Schwann cell organization, which initiates activation of the Schwann cells (Dubovy et al., 2014), as well as resident immune cells including mast cells, and macrophages. Activated Schwann cells as well as the immune cells secrete chemokines and cytokines that accelerate the recruitment of other immune cells from the bloodstream. These recruited cells accumulate at the site of injury and include neutrophils, monocytes, lymphocytes, and natural killer cells (Cui et al., 2000; Monk et al., 2007; Dubovy, 2011), likely in an attempt to promote tissue healing (Ji et al., 2014). However, this inflammatory response often becomes self-sustaining and thus a deleterious part of the process of post-injury pain (Costigan et al., 2009). Indeed, due to demyelination, axonopathy, and the local inflammatory responses at the lesion, neuronal firing for the affected peripheral nerves is increased at the site of injury (peripheral sensitization). This leads to hypersensitivity or reduced threshold to a given stimuli also known as allodynia (Kajander et al., 1992; Tal and Eliav, 1996; Chen and Devor, 1998). In the past few years, various studies have been performed to explore the molecular dialogue during inflammation following peripheral nerve injury (neuroinflammation) (Austin and Moalem-Taylor, 2010; Clark et al., 2013; Ji et al., 2014; Kiguchi et al., 2012; White et al., 2005; Zhang et al., 2013) including chronic constriction injury (CCI) rat model as developed in the 1980s (Bennett and Xie, 1988); however, details about the interaction among various inflammatory mediators and neurons during neuroinflammation still need to be elucidated.
Numerous immune cells and inflammatory mediators are involved at different anatomical levels in the pain axis, and hence various strategies targeting inflammation have been employed to dampen neuropathic pain (Austin and Moalem-Taylor, 2010). One strategy is to diminish the infiltration or activation of immune cells such as mast cells, Schwann cells, neutrophils, macrophages, and microglia in an effort to reduce the over-exuberant and self-sustaining production of inflammatory mediators (Austin and Moalem-Taylor, 2010). Even though this strategy has shown some success in limiting neuropathic pain associated hypersensitivity in animal models, doing so poses a major side effect of causing a delay in the nerve repair and regeneration (Fu and Gordon, 1997; Barrette et al., 2008; Niemi et al., 2013). An alternative strategy is to specifically target the pain stimulating mediators from the immune cells without disrupting the beneficial functions of other inflammatory mediators (Dubovy, 2011; Ji et al., 2014). In recent years, multiple studies have documented dynamic changes in various inflammatory mediators’ post-CCI, including cytokines (TNF-α, IL-2, IL-6, IL-1β, IL-4) and chemokines (MCP-1/CCL2 and KC/CXCL1). The expression of these mediators is elevated in the sciatic nerve after CCI in rats, and induced peripheral hypersensitivity post injury (Austin and Moalem-Taylor, 2010; Cui et al., 2000; Kleinschnitz et al., 2004; Moalem et al., 2004; Okamoto et al., 2001; Ramesh et al., 2013; Rittner et al., 2005). Cytokines such as IL-1β and TNF-α contribute to inflammation and pain during early stages of nerve injury, but later they exhibit a beneficial role and are expressed during nerve repair/regeneration (Nadeau et al., 2011). Therefore, studying the complex interplay among various inflammatory mediators at different time points during the disease progression is more relevant than studying the discrete mediators as singular products functioning at one time point in the disease (Vodovotz, 2006).
One method for studying the changes of complex pathophysiological responses in a time series experiment is to employ data driven computational modeling. Such an approach does not rely on an a priori understanding of the biological system, but rather only the input and the data measured over time (Vodovotz and Billiar, 2013; Aerts et al., 2014). Among several different approaches, network-centric models such as Dynamic Bayesian Network (DBN) inference can be used to identify dependent interactions. Furthermore, network-based models may reveal how related variables interact with one another (Aerts et al., 2014; Mi et al., 2011). For example, DBN inference has recently been used to study the interactions among various inflammatory mediators in multiple clinical and pre-clinical settings (Azhar et al., 2013; Emr et al., 2014; Zaaqoq et al., 2014).
In the present study, we utilized a combination of in vivo and DBN modeling approaches to predict an inflammatory network that is active during chronic neuropathic pain as evident in chronic constriction injury (CCI). The expression of 14 different cytokines/chemokines in the CCI sciatic nerve was assayed using multiplex ELISA, over a period of 18 days post-surgery. These protein expression levels were used to calculate the interplay of inflammatory mediators based on DBN inference. Immunohistological examination of the CCI sciatic nerve for several inflammasome markers supports the DBN prediction. To the best of our knowledge, this is the first study to define an inflammatory target using computational modeling in the setting of chronic neuropathic pain.
2. Materials and methods
2.1. Animals
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health, and Institutional Animal Care and Use Committee (IACUC) at Duquesne University approved the protocol (# 1109-10). Male Sprague–Dawley rats weighing 250–350 g were used in this study (Hilltop Lab Animals, Inc., Scottdale, PA). Rats were maintained on a 12:12 hour light dark cycle and were given ad libitum access to food and water.
2.2. Surgery and anesthesia
Surgery was performed as described by Bennett and Xie Bennett and Xie (1988) (Vasudeva et al., 2014), which induces neuropathic pain in rats. Rats were divided into three groups, CCI, sham controls and naïve non-surgical control rats. Briefly, under isoflurane anesthesia, sciatic nerve was exposed via incision through skin followed by muscle separation and then four ligatures approximately 1 mm apart were tied around the common sciatic nerve of the CCI rat, using 4-0 chromic gut suture. The ligatures were neither tight nor loose. The muscle layer was closed using 4-0 chromic gut suture followed by skin closure using stainless steel wound clips. Sham rats underwent identical surgery except that the ligatures were not applied to the sciatic nerve. Naïve control rats did not undergo any surgical procedures and are referred to as non-surgical controls.
2.3. Behavioral analysis
Naïve control, sham surgical control and CCI were tested for mechanical allodynia a day before surgery and day 11 post-surgery as described previously (Vasudeva et al., 2014). Briefly, the calibrated Semmes-Weinstein monofilaments were applied to the plantar surface of the right hindpaw in the region of sciatic nerve innervation. The filaments were applied in the ascending order of gram force ranging from 0.41 to 15.13 gram force. Each filament was applied 10 times and the withdrawal responses were recorded. The force at which the rat withdrew a paw on 5 of 10 pushes (50% withdrawal threshold) was predicted by using the regression equation. Normalized 50% withdrawal threshold (WT) for the ipsilateral hindpaw was calculated by using the formula: Normalized 50% WT = Post-Surgery 50% WT − Baseline 50% WT. The normalized withdrawal latencies were averaged for CCI, sham and naïve non-surgical controls, and their means were compared using Kruskal–Wallis 1-way ANOVA by ranks test (Graphpad PRISM version 6.0c). Differences were considered significant at p ≤ 0.05.
2.4. Tissue dissection
The ipsilateral sciatic nerves were collected at the region of injury from CCI, sham and naïve control rats. The tissue collection was performed at four time points spanned over the period of 18 days post-surgery i.e. 2–3 h post-surgery and day 8, 11 and 18 post surgery for the CCI and sham rats, along with the naïve non-surgical control rats. Four rats were used for every given time point. Immediately post-dissection, the tissue was preserved in RNA later solution (AM7024, Ambion) and stored at 4 °C for 24 h and thereafter stored at −20 °C freezer until further processing. For histology, the tissue was preserved in PBS buffered (pH 7.4) 4% paraformaldehyde solution.
2.5. Histology and confocal microscopy
The fixed nerves from control, sham and CCI rats were sliced into 20 μm thick longitudinal cryosections. Double immunofluorescence studies were performed to examine the infiltration of macrophages (CD68) along with the presence of caspase-1, IL-18, IL-1β and IL-1α using mouse anti-CD68, 1:100 (MCA341R, AbD Serotec), rabbit anti-caspase-1, 1:50 (caspase-1 p10 (M20) sc514, Santa Cruz Biotechnology), rabbit anti-IL-18, 1:100 (R1186, Acris Antibodies), rabbit anti-IL-1β, 1:100 (ab9787, Abcam) and goat anti-IL-1α, 1:100 (ab9875, Abcam) primary antibodies. The secondary antibodies used were Alexa fluor 488 donkey anti mouse, 1:200 (A-21202, Invitrogen), Alexa fluor 546 donkey anti goat, 1:200 (A-11056, Invitrogen) and Alexa fluor 647 donkey anti rabbit, 1:200 (A-31573, Invitrogen). All the antibody dilutions were prepared using 1:20 normal donkey serum in 1× PBS, pH 7.4. Antigen retrieval was performed using sodium citrate buffer (10 mM sodium citrate, 0.1% Tween 20, pH 6.0 and pH 8.5). Confocal microscopy was performed on a Leica SP2 spectral Laser Scanning Confocal microscope.
2.6. Processing of rat sciatic nerve
Sciatic nerve sections were obtained from rats (Hilltop Lab Animals, Scottdale, Pennsylvania) and immediately preserved in RNAlater® solution (Ambion). Nerve tissue was then homogenized in a 2× lysis buffer (Cell Signaling, Danvers, MA) with 1 mM PMSF using a handheld homogenizer. Samples were then centrifuged at 12,000 g for 10 min at 4 °C. After centrifugation, collected supernatant was measured for total protein concentration using a Pierce™ BCA Protein Assay Kit (Pierce, Rockford, IL). Next, 100 μg of total protein in a volume of 100 μl per sample were subjected to analysis of rat cytokines and chemokines as described below.
2.7. Analysis of inflammatory mediators
Prepared nerve sample protein extracts were added to a Millipore (rat cytokine/chemokine 14-plex, cat. #RECYTMAG-25K (Billerica, MA)) assay kit as per manufacturer’s specifications, for use on a Luminex™100 instrument (Luminex Corp., Austin, TX). Briefly, 14 cytokines and chemokines, granulocyte-macrophage colony stimulating factor [GM-CSF], interferon [IFN]-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-18, GRO/KC, monocyte chemotactic protein-1 [MCP-1], and TNF-α, were assessed using the MiraiBio CT/QT multiplexing software platform (MiraiBio, Alameda, CA) as per manufacturer’s specifications. Reported values in pg/ml were then normalized to pg analyte/mg total protein. The cytokine concentrations obtained (pg/mg) were averaged for each group of rat at a given time point and their means were compared among different groups at various time points using two-way ANOVA. Differences were considered significant at p ≤ 0.05.
2.8. Dynamic Bayesian Network inference
It was assumed that the cytokine levels obtained at four different time points are the result of ongoing neuroinflammation post CCI. The time course of cytokines/chemokines at four different time points over a period of 18 days after CCI was first averaged across experimental repeats and then used as input for a Dynamic Bayesian Network (DBN) inference algorithm, implemented in Matlab® essentially as described previously (Azhar et al., 2013; Emr et al., 2014; Grzegorczyk and Husmeier, 2011; Zaaqoq et al., 2014). Briefly, the algorithm assumes the data to be drawn from a multivariate normal distribution, with interactions between nodes modeled as piece-wise linear Gaussian distributions. Network structures were sampled using a Gibbs sampling scheme and scored according to their fit with experimental data. The Gibbs sampling procedure was run for 25 steps, and marginal posterior probabilities for each edge were then averaged over these networks, and reported as an indicator of the confidence in the inferred interactions. Only edges with marginal probabilities greater than 0.5 were included in the reported network structure (Aderhold et al., 2014).
3. Results
3.1. Mechanical allodynia and inflammatory mediators during CCI
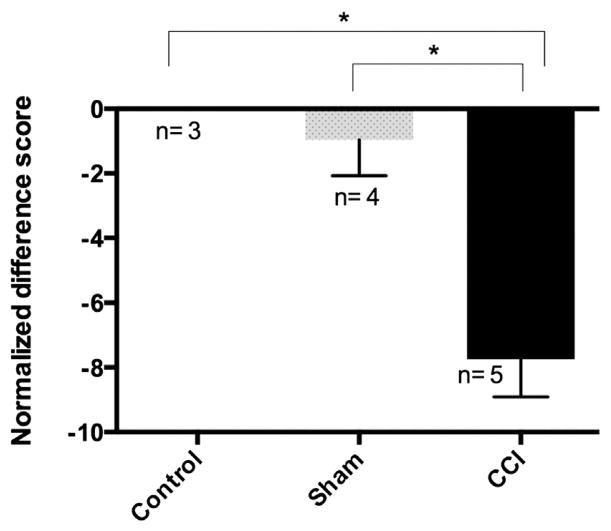
Mechanical allodynia is one of the classic signs of neuropathic pain development (Bennett and Xie, 1988). In the current study, an experimental paradigm of Chronic Constriction Injury (CCI) was used to induce neuropathic pain in rats (Bennett and Xie, 1988; Vasudeva et al., 2014). Similar to prior reports (Bennett and Xie, 1988; Vasudeva et al., 2014), CCI resulted in a significant increase (p ≤ 0.05) in tactile pain sensitivity (mechanical allodynia) during the second week after CCI surgery as compared to sham and control rats (Fig. 1, tactile sensitivity measured on day 11 post-surgery). The increased sensitivity to the tactile stimulus was observed only on the affected ipsilateral side (right hindlimb) of the rat and no such signs were observed on the unaffected contralateral side (left hindlimb, data not shown). Animals subjected to CCI also exhibited guarding behaviors for their right hindlimbs. These findings confirmed the development of hypersensitivity, which is an indication of neuropathic pain in the rat’s post-CCI.
Fig. 1.
Mechanical allodynia develops on ipsilateral hindpaw post CCI surgery: The mechanical allodynia is represented as normalized difference scores between the baseline values before the surgery and day 11 post-surgery for three treatment groups; naïve control, sham control and CCI rats. More negative scores represent higher mechanical sensitivity towards Semmes-Weinstein filaments. CCI rats developed mechanical allodynia and were significantly different from both sham and naïve control groups. Bars represent the mean ± SEM and * represents significant difference at p ≤ 0.05 (Kruskal–Wallis 1-way ANOVA by ranks). Sample size for each treatment group is indicated on the graph.
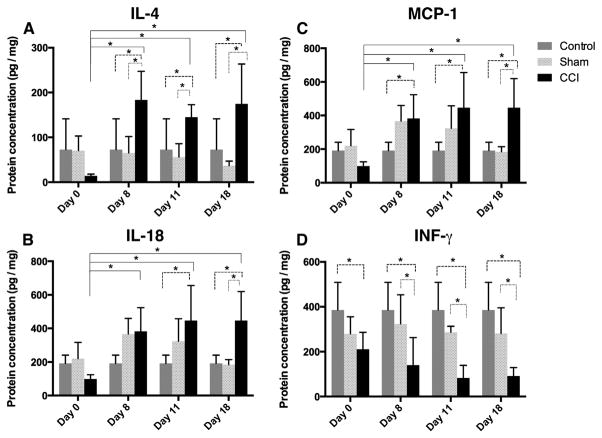
As mentioned earlier, inflammation at the site of nerve lesion contributes to the development of peripheral sensitization (Kiguchi et al., 2012; Vasudeva et al., 2014). To investigate the dynamics of neuroinflammation in this animal model, fourteen different inflammatory mediators were assayed simultaneously at multiple time points using Luminex™ assessment of protein extracts from the dissected sciatic nerve (Extended Data Table 1). Inflammatory mediators whose expression was determined to be significantly different between CCI and sham rats included IL-1β, IL-2, IL-4, IL-5, IL-6, IL-18, IFN-γ, TNF-α, MCP-1 and IL-12p70. Four mediators exhibited significant difference across the entire time course of the experiment (Fig. 2). For example, in CCI rats, the expression of IL-4 was significantly elevated by day 8 and high expression persisted through day 11 and day 18 as compared to both sham-surgery rats and the naïve control (Fig. 2A). In sham-surgery rats, IL-18 expression was significantly higher than naïve control from day 8 to day 11 post-surgery, followed by a return to baseline levels on day 18. In contrast, the CCI rats exhibited IL-18 expression that was significantly higher than normal on day 8 and that persisted at a significantly high level through day 18 (Fig. 2B). Similar to IL-18, MCP-1 expression was elevated at day 8 for both the sham-surgery and CCI rats. In contrast, at day 11 and day 18 MCP-1 expression trajectories of the sham and CCI animals diverged: MCP-1 expression in the sham animals returned to baseline levels, while MCP-1 expression in CCI animals persisted at a significantly high level (Fig. 2C). IFN-γ levels were significantly lower in CCI rats as compared to sham and naïve rats starting on day 8, and remained low through day 18 (Fig. 2D).
Fig. 2.
Cytokine and chemokine protein levels at ipsilateral sciatic nerve suggest sustained inflammasome activation in CCI rats. Protein concentration measured on ipsilateral sciatic nerves by multiplex ELISA for three treatment groups; naïve control, sham-surgery control and CCI rats at four time points; day 0 (2–3 h post surgery), days 8, 11 and 18 post surgery. A. IL-4 protein is elevated in a statistically significant manner in the CCI condition as compared to sham and naïve rats from day 8 through day 18. B. For CCI, IL-18 concentration increased from day 8 through day 18 and was significantly different from day 0 values at all time points. Significant difference between CCI & sham rats was observed on day 18. Significant difference for CCI and naïve control rats was observed on day 8, 11 and 18. For sham rats, the IL-18 levels increased from day 8 to day 11, however unlike CCI rats, the levels returned to baseline levels by day 18 post surgery. C. MCP-1 protein is elevated in a statistically significant manner similar to what was observed for IL-18 in the CCI condition as compared to sham and naïve rats. D. IFN-γ protein drops in the CCI condition in a statistically significant manner over the time course of the experiment as compared to sham and naïve rats. Bars represent the mean ± SEM and * represents significant difference at p ≤ 0.05 (two-way ANOVA). Four rats were used in each treatment group at every given time point.
3.2. Dynamic Bayesian Network (DBN) inference
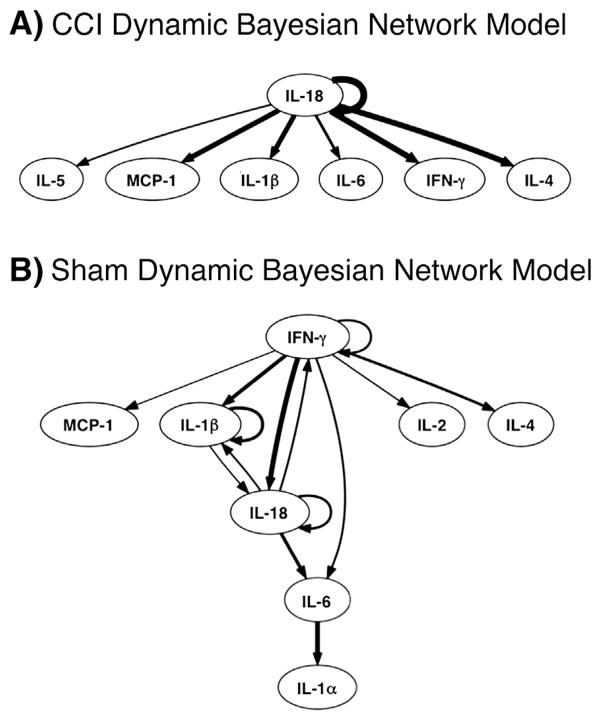
Based on the tissue-level protein expression data (Extended Data Table 1), DBN inference (Azhar et al., 2013; Emr et al., 2014; Zaaqoq et al., 2014) was utilized to calculate the most likely central nodes and regulatory interactions (Fig. 3). This analysis suggested that elevated pain sensitivity in CCI rats occurs in parallel to changes in the tissue expression of key inflammatory mediators. In CCI animals (Fig. 3A), this analysis suggested a network in which IL-18 serves as a central node exhibiting auto-regulatory feedback. Furthermore, IL-18 was inferred to affect the expression of multiple inflammatory mediators including IL-5, MCP-1, IL-1β, IL-6, IFN-γ, and IL-4. In sham-surgery animals (Fig. 3B), DBN inference suggested a network centered on IFN-γ, IL-1β, and IL-18, with each exhibiting auto-regulation.
Fig. 3.
Dynamic Bayesian Network (DBN) inference suggests a central role for the inflammasome in CCI: Data on inflammatory mediators from CCI (A) and sham (B) rats (see Fig. 2 & Extended Data Table 1) were used to carry out DBN inference. Inflammatory mediators are represented as network nodes. The thickness of connections among nodes or to themselves (edges) is thicker based on how often they are represented in individual experiments. Nodes with auto-regulatory feedback and connections to other nodes are considered central.
3.3. Immunohistochemical analysis
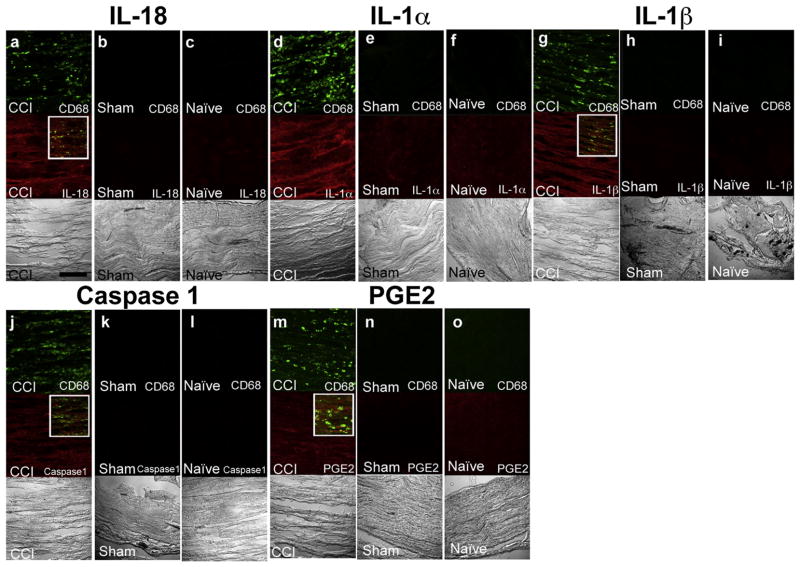
Fig. 3 raised the hypothesis that inflammasome activation – known to drive the post-translational processing of pro-IL-18 and pro-IL-1β to IL-18 and IL-1, respectively (Franchi et al., 2009; Schroder and Tschopp, 2010) – occurred in the setting of CCI. To test this hypothesis, multi-target immunofluorescence was performed on the ipsilateral sciatic nerve tissue sections from CCI, sham, and naïve control rats. The analysis of CCI tissue sections demonstrated the elevated expression of prostaglandin E2 (PGE2), IL-18, IL-1β, IL-1α, and the inflammasome product caspase-1 in CCI, whereas these proteins were not detected in sham or naïve control nerve sections (Fig. 4).
Fig. 4.
Double immunofluorescence for macrophages and cytokines on ipsilateral sciatic nerve cryosections suggests inflammasome activation in CCI rats: IL-18 a shows infiltration of CD68 positive macrophages and presence of IL-18 in CCI sciatic nerve. Sham and naïve control sciatic nerves did not exhibit presence of macrophages and IL-18 (b, c). IL-1α ipsilateral CCI sciatic nerves exhibit presence of macrophages and IL-1α (D), which are both absent from sham and naïve control tissue (e, f). IL-1β macrophages and IL-1β are evident in the affected sciatic nerve (g), but are absent from sham and naïve control tissue (h, i). Caspase-1 macrophages and caspase-1 are evident in the CCI sciatic nerve (j), but are not evident in the sham and naïve control tissue (k, l). PGE2 macrophages and PGE2 are evident in the affected CCI sciatic nerve (m). No macrophages or PGE2 expression are evident in the sham and naïve control tissue (n, o). For each experiment group (a, b, c), (d, e, f), (g, h, i), (j, k, l) and (m, n, o), the confocal images were acquired in the same sitting with the same image acquisition parameters. Bar = 150 μm.
4. Discussion
Within hours following the peripheral nerve injury, several physiological responses can be observed including involvement of innate and adaptive immune responses (Austin and Moalem-Taylor, 2010). Within days of the peripheral nerve injury, the physiological changes become manifested as hyperexcitability of nociceptive neurons in response to innocuous tactile stimuli. This phenomenon becomes evident as mechanical allodynia, as described by Bennett and Xie (1988). Similar to our previous report (Vasudeva et al., 2014), we have confirmed the development of mechanical allodynia in this study. Inflammation begins at the site of injury, and later involves the dorsal root ganglia (DRG), spinal cord and brain (Austin and Moalem-Taylor, 2010). Conventional immune cells such as mast cells, neutrophils, macrophages and T lymphocytes, along with other resident cells such as Schwann cells, microglia and astrocytes, exhibit activated profiles of gene expression producing a variety of inflammatory mediators that contribute to the initiation and maintenance of neuropathic pain (Austin and Moalem-Taylor, 2010; Dubovy et al., 2014; Ren and Dubner, 2010; Scholz and Woolf, 2007). Based upon previous studies, the cytokines that are consistently elevated in expression following peripheral nerve injury are TNF-α, IL-1β, and IL-6 (Sacerdote et al., 2013).
Neuroinflammation is a complex physiological response. While extensive research on inflammatory mediators has led to some useful clinical therapies, satisfactory treatment of neuropathic pain remains elusive (Baron et al., 2010). Under experimental conditions of neuroinflammation, neutralizing antibodies have been used to attack several inflammatory mediators including TNF-α, IL-1β, IL-6, IL-15, IL-18, and MCP-1 resulting in limited success in reducing neuropathic pain (Austin and Moalem-Taylor, 2010). Furthermore, stimulation of nominally anti-inflammatory cytokines (IL-4, IL-10, TGF-β1) was reported to reduce hypersensitivity in various animal models of neuropathic pain (Austin and Moalem-Taylor, 2010).
In the present study, we hypothesized that a better understanding of the dynamic interconnections among tissue inflammatory mediators would facilitate the search for inflammation-modulating therapies in the setting of neuropathic pain. In clinical settings, neuropathic pain is mostly diagnosed in its chronic stages. Therefore, we determined the tissue levels of inflammatory mediators at both acute (2–3 h post-CCI) and subacute as well as chronic stages (day 8 to day 18 post-CCI). Based on DBN inference carried out with these data, we hypothesize key interactions among inflammatory mediators that are operant in CCI-induced neuroinflammation, as well as the transition from acute to chronic pain. Specifically, we infer a central role for IFN-γ, IL-1β, and IL-18 following general tissue injury, since these mediators appeared as central nodes (those which are feeding back on themselves as well as affecting the expression of other mediators) in the sham surgical acute inflammatory network. In contrast, IL-18 was inferred as the central node in the CCI animals, appearing to control its own expression along with affecting other mediators involved in neuroinflammation. This IL-18-centered network correlates with the maintenance of neuropathic pain (mechanical allodynia) throughout the period of study.
The inflammasome is a caspase-activating complex that serves critical roles in innate immunity (Franchi et al., 2009; Lechtenberg et al., 2014; Li et al., 2013; Schroder and Tschopp, 2010; Wang et al., 2014). It is known that inflammasome induction in macrophages leads to secretion of IL-18 as well as IL-1β. Both IL-18 and IL-1β are cleaved proteolytically from their respective precursors via activation of caspase-1 enzyme. Both IL-18 and IL-1β can induce cyclooxygenase and PGE2 (Fiebich et al., 2000; Franchi et al., 2009; Futani et al., 2002; Han et al., 2002; Kashiwamura et al., 2002; Olee et al., 1999). Both IL-18 and IL-1β are elevated in the spinal cord (central nervous system) following CCI (Li et al., 2013; Zhang et al., 2013). Similarly, we found IL-18 expression to be elevated consistently at the site of injury (peripheral nervous system) from day 8 to day 18 post-CCI. However, no significant difference was found for IL-1β baseline and post-CCI protein levels at the site of injury. Immunofluorescence analysis carried out on ipsilateral sciatic nerve sections further confirmed the expression of IL-18, caspase-1, and PGE2 after the CCI surgery.
Among the many inflammatory pathways induced by inflammasome activation, the cyclooxygenase pathway and attendant production of PGE2 play a key role in the setting of inflammatory pain following peripheral nerve injuries (Ma and Eisenach, 2003; Ren and Dubner, 2010). Separate studies have also demonstrated that IL-18 can induce both cyclooxygenase and PGE2 from macrophages and chondrocytes (Futani et al., 2002; Olee et al., 1999). Our immunohistological studies confirm the expression of IL-18 in the sciatic nerve, supporting our Luminex™data and supporting the hypothesis that inflammasome activation occurs in damaged nerves in the setting of CCI.
DBN inference along with our other data suggests that as a consequence of CCI, besides driving its own expression, IL-18 also drives the expression of IL-4 and MCP-1 along with the down regulation of IFN-γ. In the context of nerve injury, IFN-γ has been shown to exhibit elevated expression in the central nervous system, and contribute to central sensitization (Ramesh et al., 2013). However in the present study, we observed a gradual decline in IFN-γ at the site of chronic constriction injury on the sciatic nerve over the entire duration of the study. IFN-γ is induced in macrophages either by the synergistic action of both IL-18 and IL-12 or in the presence of active IL-12p70 homodimer (Munder et al., 1998; Yoshimoto et al., 1998; Tominaga et al., 2000; Schindler et al., 2001). We did not observe an up regulation in the IL-12p70 levels for the CCI rats. With regard to IL-4, this cytokine can play an anti-inflammatory role in many settings of inflammation, as well as exerting anti-nociceptive properties in the setting of neuropathic pain (Hao et al., 2006). Moreover, IL-4 is a known inhibitor of IFN-γ production by the macrophages (Schindler et al., 2001). Here, we observed a consistent up regulation of IL-4 in the CCI rats from day 8 to day 18 post-CCI. Importantly, IL-18 is also known to perform other immune functions that are not directly related to the IFN-γ production. Furthermore, IFN-γ production is not dependent entirely on IL-18 (Dinarello et al., 1998). Therefore, even though IL-18 (which was initially named as ‘IFN-γ inducing factor’) is up regulated, this does not necessarily cause an up regulation of IFN-γ in all circumstances.
Dynamic Bayesian Network inference also suggested that IL-18 may drive the expression of the chemokine MCP-1/CCL2 during peripheral neuropathy. Consistent with previous studies (Abbadie et al., 2009), we found elevated MCP-1 expression as a consequence of CCI, and it is likely that MCP-1 expression also contributes to the development of mechanical allodynia. These findings further support the activation of inflammasome pathways post-CCI injury, leading to activation of caspase-1 and the production of IL-18 and IL-1β. Similar observations have been made for neuropathic pain models and a model of complex regional pain syndrome (Li et al., 2009, 2013).
5. Conclusion
This study highlights the potential for computational modeling as a tool to infer key interactions among inflammatory mediators at the tissue level in the context of chronic neuropathic pain. The results of this combined in vivo/in silico study suggest a central role for inflammasome activation and subsequent production of IL-18 in the induction and maintenance of neuropathic pain during peripheral nerve injury. Furthermore, our analyses suggest that the response to general tissue injury in this context involves interactions among IFN-γ, IL-1β and IL-18. Recent studies have demonstrated that spinal blockade of IL-18 led to improvement in mechanical allodynia and thermal hyperalgesia during CCI in rats (Zhang et al., 2013). Therefore, these results raise the possibility that IL-18 or inflammasome inhibition may lead to significant neuropathic pain relief; however, these studies must be extended and the exact underlying mechanisms still need to be revealed. Finally, these studies demonstrate the power of combined in vivo/in silico studies in the setting of chronic neuropathic pain.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jneuroim.2015.04.012.
6. Uncited reference
http://biosupport.licor.com/docs/InVivoDiet-Considerations.pdf, n.a
Extended Data
Extended Data Table 1.
Cytokine concentrations for 14 different inflammatory mediators in three treatment groups; naïve control, sham control and CCI rats at four time points; day 0, day 8, day 11 and day 18 post surgery.
| Results in pg/mg protein | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Sample ID | Sample | Time Point of tissue collection (hrs.) | IL-5 | MCP-1 | TNF-α | IL-1β | IL-6 | GM-CSF | GRO/KC | IFN-γ | IL-1α | IL-10 | IL-12p70 | IL-18 | IL-2 | IL-4 |
| Baseline | Baseline-1 | 0 | 26.02 | 86.8 | 6.06 | 272.13 | 164.04 | 10.58 | 8.16 | 277.18 | 31.72 | 38.32 | 32.29 | 207.14 | 114.97 | 174.14 |
| Baseline-2 | 0 | 20.86 | 129.14 | 1.11 | 164.04 | 214.59 | 0 | 4.76 | 422.55 | 18.06 | 5.08 | 31.04 | 176.91 | 86.98 | 50.51 | |
| Baseline-3 | 0 | 23 | 208.26 | 14.95 | 264.41 | 333.79 | 11.85 | 30.12 | 543.56 | 28.39 | 30.89 | 33.52 | 249.61 | 121.2 | 44.38 | |
| Baseline-4 | 0 | 19.73 | 86.8 | 1.11 | 189.08 | 214.59 | 9.3 | 9.82 | 298.79 | 37.44 | 3.46 | 31.04 | 132.45 | 126.58 | 22.82 | |
|
| ||||||||||||||||
| Sham Day 0 |
Sham (0)1 | 2 hr. | 21.95 | 2533.15 | 10.15 | 546.64 | 6841.53 | 14.35 | 901.84 | 243.7 | 19.83 | 20.07 | 28.45 | 203.36 | 107.94 | 49.64 |
| Sham (0)2 | 2 hr. | 19.73 | 1454.84 | 0 | 240.43 | 7285.84 | 3.9 | 3208.15 | 228.9 | 34.19 | 50.31 | 31.04 | 93.37 | 144.85 | 117.41 | |
| Sham (0)3 | 2 hr. | 26.02 | 1490.07 | 6.83 | 530.11 | 5466.47 | 14.35 | 1658.14 | 392.1 | 21.57 | 61.76 | 32.29 | 262.91 | 117.19 | 47.04 | |
| Sham (0)4 | 2 hr. | 20.86 | 2736.26 | 9.84 | 588.89 | 5485.88 | 8.65 | 1482.66 | 253.41 | 22.44 | 42.41 | 29.76 | 320.89 | 101.86 | 68.44 | |
|
| ||||||||||||||||
| Sham Day 8 |
Sham (8)1 | 192 hr. | 24.03 | 86.8 | 7.56 | 384.93 | 124.44 | 13.11 | 3.22 | 201.76 | 35.82 | 17.99 | 32.29 | 381.52 | 123.43 | 55.16 |
| Sham (8)2 | 192 hr. | 19.73 | 0 | 3.27 | 332.05 | 137.84 | 0 | 4.35 | 225.56 | 42.65 | 20.84 | 32.29 | 478.26 | 86.56 | 117.08 | |
| Sham (8)3 | 192 hr. | 21.95 | 116.43 | 1.98 | 251.36 | 251.26 | 10.58 | 8.11 | 463.04 | 39.05 | 40.62 | 31.66 | 252.65 | 124.78 | 31.2 | |
| Sham (8)4 | 192 hr. | 27.11 | 0.00 | 11.04 | 317.08 | 181.68 | 0.00 | 4.88 | 404.68 | 26.37 | 33.83 | 45.32 | 355.55 | 178.25 | 58.17 | |
|
| ||||||||||||||||
| Sham Day 11 |
Sham (11)1 | 264 hr. | 27.88 | 0.00 | 8.47 | 448.57 | 170.92 | 17.03 | 3.55 | 312.23 | 194.99 | 58.91 | 38.49 | 519.00 | 114.64 | 41.75 |
| Sham (11)2 | 264 hr. | 20.86 | 129.14 | 0 | 220.11 | 226.92 | 9.3 | 11.5 | 301.84 | 36.63 | 50.31 | 32.29 | 292.24 | 118.97 | 27.11 | |
| Sham (11)3 | 264 hr. | 18.57 | 44.22 | 5.22 | 195.5 | 124.44 | 0 | 4.19 | 251.8 | 30.89 | 32.95 | 29.76 | 267.1 | 173.13 | 62.11 | |
| Sham (11)4 | 264 hr. | 20.86 | 68.32 | 0 | 106.73 | 164.04 | 0 | 3.8 | 280.3 | 37.44 | 4.54 | 28.45 | 217.74 | 106.2 | 94.99 | |
|
| ||||||||||||||||
| Sham Day 18 |
Sham (18)1 | 432 hr. | 19.73 | 44.22 | 11.59 | 261.73 | 151.03 | 0 | 4.85 | 322.89 | 11.19 | 11.2 | 32.29 | 161.82 | 69.36 | 32.19 |
| Sham (18)2 | 432 hr. | 16.09 | 102.49 | 1.11 | 207.21 | 82.64 | 0 | 4.17 | 191.28 | 18.94 | 0 | 27.11 | 221.53 | 79.51 | 41.67 | |
| Sham (18)3 | 432 hr. | 14.76 | 0 | 0 | 112.51 | 67.99 | 0 | 3.45 | 187.74 | 11.67 | 0 | 27.11 | 159.18 | 56.41 | 26.06 | |
| Sham (18)4 | 432 hr. | 23 | 140.91 | 8.41 | 125.8 | 226.92 | 11.85 | 6.73 | 423.92 | 24.16 | 27.81 | 32.29 | 195.04 | 108.81 | 48.78 | |
|
| ||||||||||||||||
| CCI Day 0 |
CCI (0)1 | 2 hr. | 17.35 | 1159.21 | 0 | 405.17 | 9125.13 | 11.85 | 2300.08 | 201.76 | 71.41 | 34.74 | 25.74 | 64.85 | 36.82 | 17.04 |
| CCI (0)2 | 2 hr. | 18.57 | 841.11 | 0 | 189.95 | 3613.15 | 0 | 558.11 | 319.91 | 39.05 | 0 | 30.4 | 124.93 | 50.33 | 11.93 | |
| CCI (0)3 | 2 hr. | 20.86 | 1633.73 | 0 | 501.66 | 15927.95 | 11.22 | 13484.15 | 167.87 | 29.23 | 65.57 | 31.04 | 105.38 | 34.72 | 10.54 | |
| CCI (0)4 | 2 hr. | 18.57 | 1480.39 | 0 | 247.52 | 9016.66 | 6.66 | 2698.09 | 152.92 | 54.38 | 30.64 | 29.76 | 100.88 | 32.63 | 18.24 | |
|
| ||||||||||||||||
| CCI Day 8 |
CCI (8)1 | 192 hr. | 51.95 | 311.08 | 0 | 167.53 | 176.89 | 0 | 13.19 | 274.05 | 59.36 | 9.61 | 32.29 | 556 | 34.02 | 213.69 |
| CCI (8)2 | 192 hr. | 99.25 | 140.91 | 0 | 103.26 | 124.44 | 0 | 4.63 | 208.65 | 13.53 | 0 | 29.76 | 378.83 | 41.11 | 137.94 | |
| CCI (8)3 | 192 hr. | 111.15 | 116.43 | 0 | 93.44 | 82.64 | 0 | 7.65 | 70.16 | 31.72 | 0 | 29.76 | 209.04 | 36.12 | 258.99 | |
| CCI (8)4 | 192 hr. | 86.89 | 140.91 | 0 | 152.43 | 52.81 | 0 | 6.16 | 8.06 | 30.89 | 0 | 27.11 | 384.22 | 28.54 | 123.94 | |
|
| ||||||||||||||||
| CCI Day 11 |
CCI (11)1 | 264 hr. | 67.79 | 68.32 | 0 | 73.18 | 52.81 | 0 | 3.45 | 115.2 | 13.53 | 0 | 27.11 | 194.28 | 27.21 | 111.44 |
| CCI (11)2 | 264 hr. | 110.13 | 208.26 | 0 | 261.73 | 96.89 | 0 | 8.03 | 98.05 | 81.74 | 39.6 | 32.29 | 441 | 36.82 | 178.79 | |
| CCI (11)3 | 264 hr. | 51.31 | 216.51 | 0 | 246.93 | 52.81 | 0 | 9.53 | 0 | 63.91 | 0 | 25.74 | 449.91 | 29.89 | 139.19 | |
| CCI (11)4 | 264 hr. | 59.6 | 190.95 | 0 | 214.83 | 96.89 | 0 | 10.4 | 119.35 | 47.78 | 0 | 29.76 | 703.95 | 29.89 | 150.91 | |
|
| ||||||||||||||||
| CCI Day 18 |
CCI (18)1 | 432 hr. | 18.57 | 190.95 | 0 | 123.78 | 52.81 | 0 | 6.24 | 43.57 | 27.55 | 0 | 28.45 | 461.55 | 27.21 | 155.15 |
| CCI (18)2 | 432 hr. | 29.73 | 129.14 | 0 | 78.39 | 67.99 | 0 | 2.55 | 115.2 | 9.76 | 16.69 | 32.29 | 667.72 | 31.26 | 104.34 | |
| CCI (18)3 | 432 hr. | 107.06 | 102.49 | 0 | 80.71 | 82.64 | 0 | 3.51 | 127.51 | 15.36 | 0 | 28.45 | 249.61 | 31.26 | 304.08 | |
| CCI (18)4 | 432 hr. | 82.61 | 349.58 | 0 | 309.18 | 96.89 | 0 | 15.03 | 79.81 | 83.93 | 2.64 | 32.29 | 408.12 | 30.23 | 136.06 | |
Acknowledgments
This work was supported by grants to J.A.P. and J.M.J. from the Pittsburgh Tissue Engineering Initiative and NIH grant P50-GM-53789 to Y.V. K.V. was supported with pre-doctoral teaching and research fellowships from Duquesne University. Additional support was provided by the Duquesne University Chronic Pain Research Consortium, and the Provost’s Interdisciplinary Research Consortia Grant, Duquesne University.
Contributor Information
Kiran Vasudeva, Email: vasudevak@duq.edu.
Yoram Vodovotz, Email: vodovotzy@upmc.edu.
Nabil Azhar, Email: naa42@pitt.edu.
Derek Barclay, Email: barcdx2@upmc.edu.
Jelena M. Janjic, Email: janjicj@duq.edu.
John A. Pollock, Email: pollock@duq.edu.
References
- Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res Rev. 2009;60:125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderhold A, Husmeier D, Grzegorczyk M. Statistical inference of regulatory networks for circadian regulation. Stat Appl Genet Mol Biol. 2014;13:227–273. doi: 10.1515/sagmb-2013-0051. [DOI] [PubMed] [Google Scholar]
- Aerts JM, Haddad WM, An G, Vodovotz Y. From data patterns to mechanistic models in acute critical illness. J Crit Care. 2014;29:604–610. doi: 10.1016/j.jcrc.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229:26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Azhar N, Ziraldo C, Barclay D, Rudnick DA, Squires RH, Vodovotz Y, et al. Analysis of serum inflammatory mediators identifies unique dynamic networks associated with death and spontaneous survival in pediatric acute liver failure. PLoS One. 2013;8:e78202. doi: 10.1371/journal.pone.0078202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- Barrette B, Hebert MA, Filali M, Lafortune K, Vallieres N, Gowing G, et al. Requirement of myeloid cells for axon regeneration. J Neurosci. 2008;28:9363–9376. doi: 10.1523/JNEUROSCI.1447-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Chen Y, Devor M. Ectopic mechanosensitivity in injured sensory axons arises from the site of spontaneous electrogenesis. Eur J Pain. 1998;2:165–178. doi: 10.1016/s1090-3801(98)90009-x. [DOI] [PubMed] [Google Scholar]
- Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res. 2013;6:803–814. doi: 10.2147/JPR.S53660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui JG, Holmin S, Mathiesen T, Meyerson BA, Linderoth B. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain. 2000;88:239–248. doi: 10.1016/S0304-3959(00)00331-6. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Novick D, Puren AJ, Fantuzzi G, Shapiro L, Muhl H, et al. Overview of interleukin-18: more than an interferon-gamma inducing factor. J Leukoc Biol. 1998;63:658–664. [PubMed] [Google Scholar]
- Dubovy P. Wallerian degeneration and peripheral nerve conditions for both axonal regeneration and neuropathic pain induction. Ann Anat. 2011;193:267–275. doi: 10.1016/j.aanat.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Dubovy P, Klusakova I, Hradilova Svizenska I. Inflammatory profiling of Schwann cells in contact with growing axons distal to nerve injury. BioMed Res Int. 2014;2014:691041. doi: 10.1155/2014/691041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr B, Sadowsky D, Azhar N, Gatto LA, An G, Nieman GF, et al. Removal of inflammatory ascites is associated with dynamic modification of local and systemic inflammation along with prevention of acute lung injury: in vivo and in silico studies. Shock. 2014;41:317–323. doi: 10.1097/SHK.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebich BL, Mueksch B, Boehringer M, Hull M. Interleukin-1beta induces cyclooxygenase-2 and prostaglandin E(2) synthesis in human neuroblastoma cells: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB. J Neurochem. 2000;75:2020–2028. doi: 10.1046/j.1471-4159.2000.0752020.x. [DOI] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Futani H, Okayama A, Matsui K, Kashiwamura S, Sasaki T, Hada T, et al. Relation between interleukin-18 and PGE2 in synovial fluid of osteoarthritis: a potential therapeutic target of cartilage degradation. J Immunother. 2002;25 (Suppl 1):S61–S64. doi: 10.1097/00002371-200203001-00009. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzegorczyk M, Husmeier D. Improvements in the reconstruction of time-varying gene regulatory networks: dynamic programming and regularization by information sharing among genes. Bioinformatics. 2011;27:693–699. doi: 10.1093/bioinformatics/btq711. [DOI] [PubMed] [Google Scholar]
- Han R, Tsui S, Smith TJ. Up-regulation of prostaglandin E2 synthesis by interleukin-1beta in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent prostaglandin E2 synthase expression. J Biol Chem. 2002;277:16355–16364. doi: 10.1074/jbc.M111246200. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Glorioso JC, Fink DJ. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain. 2006;2:6. doi: 10.1186/1744-8069-2-6. http://biosupport.licor.com/docs/InVivoDiet-Considerations.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IASP. International association for the study of pain. Pain XI. 2003:1–4. [Google Scholar]
- IOM. Institute of Medicine, Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- Jensen TS, Baron R, Haanpaa M, Kalso E, Loeser JD, Rice AS, et al. A new definition of neuropathic pain. Pain. 2011;152:2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett. 1992;138:225–228. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- Kashiwamura S, Ueda H, Okamura H. Roles of interleukin-18 in tissue destruction and compensatory reactions. J Immunother. 2002;25 (Suppl 1):S4–S11. doi: 10.1097/00002371-200203001-00002. [DOI] [PubMed] [Google Scholar]
- Kiguchi N, Kobayashi Y, Kishioka S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol. 2012;12:55–61. doi: 10.1016/j.coph.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Brinkhoff J, Zelenka M, Sommer C, Stoll G. The extent of cytokine induction in peripheral nerve lesions depends on the mode of injury and NMDA receptor signaling. J Neuroimmunol. 2004;149:77–83. doi: 10.1016/j.jneuroim.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Lechtenberg BC, Mace PD, Riedl SJ. Structural mechanisms in NLR inflammasome signaling. Curr Opin Struct Biol. 2014;29C:17–25. doi: 10.1016/j.sbi.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Guo TZ, Liang D, Shi X, Wei T, Kingery WS, et al. The NALP1 inflammasome controls cytokine production and nociception in a rat fracture model of complex regional pain syndrome. Pain. 2009;147:277–286. doi: 10.1016/j.pain.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Tian Y, Wang ZF, Liu SB, Mi WL, Ma HJ, et al. Involvement of the spinal NALP1 inflammasome in neuropathic pain and aspirin-triggered-15-epi-lipoxin A4 induced analgesia. Neuroscience. 2013;254:230–240. doi: 10.1016/j.neuroscience.2013.09.028. [DOI] [PubMed] [Google Scholar]
- Ma W, Eisenach JC. Cyclooxygenase 2 in infiltrating inflammatory cells in injured nerve is universally up-regulated following various types of peripheral nerve injury. Neuroscience. 2003;121:691–704. doi: 10.1016/s0306-4522(03)00495-0. [DOI] [PubMed] [Google Scholar]
- Mi Q, Constantine G, Ziraldo C, Solovyev A, Torres A, Namas R, et al. A dynamic view of trauma/hemorrhage-induced inflammation in mice: principal drivers and networks. PLoS One. 2011;6:e19424. doi: 10.1371/journal.pone.0019424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moalem G, Xu K, Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience. 2004;129:767–777. doi: 10.1016/j.neuroscience.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Monk KR, Wu J, Williams JP, Finney BA, Fitzgerald ME, Filippi MD, et al. Mast cells can contribute to axon-glial dissociation and fibrosis in peripheral nerve. Neuron Glia Biol. 2007;3:233–244. doi: 10.1017/S1740925X08000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, et al. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1beta and TNF: implications for neuropathic pain. J Neurosci. 2011;31:12533–12542. doi: 10.1523/JNEUROSCI.2840-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi JP, DeFrancesco-Lisowitz A, Roldan-Hernandez L, Lindborg JA, Mandell D, Zigmond RE. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J Neurosci. 2013;33:16236–16248. doi: 10.1523/JNEUROSCI.3319-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Martin DP, Schmelzer JD, Mitsui Y, Low PA. Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp Neurol. 2001;169:386–391. doi: 10.1006/exnr.2001.7677. [DOI] [PubMed] [Google Scholar]
- Olee T, Hashimoto S, Quach J, Lotz M. IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol. 1999;162:1096–1100. [PubMed] [Google Scholar]
- Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediat Inflamm. 2013;2013:480739. doi: 10.1155/2013/480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittner HL, Machelska H, Stein C. Leukocytes in the regulation of pain and analgesia. J Leukoc Biol. 2005;78:1215–1222. doi: 10.1189/jlb.0405223. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Franchi S, Moretti S, Castelli M, Procacci P, Magnaghi V, et al. Cytokine modulation is necessary for efficacious treatment of experimental neuropathic pain. J Neuroimmune Pharmacol. 2013;8:202–211. doi: 10.1007/s11481-012-9428-2. [DOI] [PubMed] [Google Scholar]
- Schindler H, Lutz MB, Rollinghoff M, Bogdan C. The production of IFN-gamma by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. J Immunol. 2001;166:3075–3082. doi: 10.4049/jimmunol.166.5.3075. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Tal M, Eliav E. Abnormal discharge originates at the site of nerve injury in experimental constriction neuropathy (CCI) in the rat. Pain. 1996;64:511–518. doi: 10.1016/0304-3959(95)00175-1. [DOI] [PubMed] [Google Scholar]
- Tominaga K, Yoshimoto T, Torigoe K, Kurimoto M, Matsui K, Hada T, et al. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int Immunol. 2000;12:151–160. doi: 10.1093/intimm/12.2.151. [DOI] [PubMed] [Google Scholar]
- Vasudeva K, Andersen K, Zeyzus-Johns B, Hitchens TK, Patel SK, Balducci A, et al. Imaging neuroinflammation in vivo in a neuropathic pain rat model with near-infrared fluorescence and (19)f magnetic resonance. PLoS One. 2014;9:e90589. doi: 10.1371/journal.pone.0090589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodovotz Y. Deciphering the complexity of acute inflammation using mathematical models. Immunol Res. 2006;36:237–245. doi: 10.1385/IR:36:1:237. [DOI] [PubMed] [Google Scholar]
- Vodovotz Y, Billiar TR. In silico modeling: methods and applications to trauma and sepsis. Crit Care Med. 2013;41:2008–2014. doi: 10.1097/CCM.0b013e31829a6eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lei T, Zhang K, Zhao W, Fang L, Lai B, et al. Xenobiotic receptor PXR regulates innate immunity via activation of NLRP3 inflammasome in vascular endothelial cells. J Biol Chem. 2014 doi: 10.1074/jbc.M114.578781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov. 2005;4:834–844. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- Zaaqoq AM, Namas R, Almahmoud K, Azhar N, Mi Q, Zamora R, et al. Inducible protein-10, a potential driver of neurally controlled interleukin-10 and morbidity in human blunt trauma. Crit Care Med. 2014 doi: 10.1097/CCM.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YK, Huang ZJ, Liu S, Liu YP, Song AA, Song XJ. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Investig. 2013;123:2268–2286. doi: 10.1172/JCI65364. [DOI] [PMC free article] [PubMed] [Google Scholar]