Abstract
Microparticles or microvesicles (MV) are sub-cellular membrane blebs shed from all cells in response to various stimuli. MVs carry a battery of signaling molecules, many of them related to redox-regulated processes. The role of MVs, either as a cause or result of cellular redox signaling has been increasingly recognized over the past decade. This is in part due to advances in flow cytometry and its detection of MVs. Notably, recent studies have shown circulating MVs from platelets and endothelial cells drive reactive species-dependent angiogenesis; circulating MVs in cancer alter the microenvironment and enhance invasion through horizontal transfer of mutated proteins and nucleic acids, and harbor redox-regulated matrix metalloproteinases and pro-coagulative surface molecules; and circulating MVs from RBCs and other cells modulate cell-cell interactions through scavenging or production of nitric oxide and other free radicals. While our recognition of MVs in redox-related processes is growing, especially in the vascular biology field, much remains unknown regarding the various biologic and pathologic functions of MVs. Like reactive oxygen and nitrogen species, MVs were originally believed to have a solely a pathological role in biology. And like our understanding of reactive species, it is now clear that MVs also play an important role in normal growth, development, and homeostasis. We are just beginning to understand how MVs are involved in various biological processes—developmental, homeostatic and pathological—and the role of MVs in redox signaling is an rich and exciting area of investigation.
Keywords: Microparticles, microvesicles, redox signaling, pathology, phospholipids
Introduction
Microvesicles or microparticles are small plasma membrane fragments that are present in biological fluids. They are shed from many, if not all, cell-types and have been shown to be important in a range of biological and pathobiological processes. The formation and shedding of membrane vesicles provides a means of complex cell-cell communication [1–3] but can also be an avenue to dispose of unwanted/unneeded macromolecular components [4–6]. Furthermore, these structures can provide surfaces or substrates for biological processes [7–10]. Finally microvesicle-shedding is a component of controlled cell death pathways [11,12]. Often the term ‘microparticle’ and ‘microvesicle’ are used interchangeably. In this review we have used the term microvesicle to avoid confusion with non-membrane-derived micro- or nano-particles used for diagnostics or drug delivery.
The literature provides many examples of connections between microvesicles and redox processes. Some of these connections are circumstantial and some are more purposeful. It is the intention of this review to collate and discuss this information, highlighting the possible importance of these sub-cellular structures to oxidative pathology and redox signaling.
What are microvesicles?
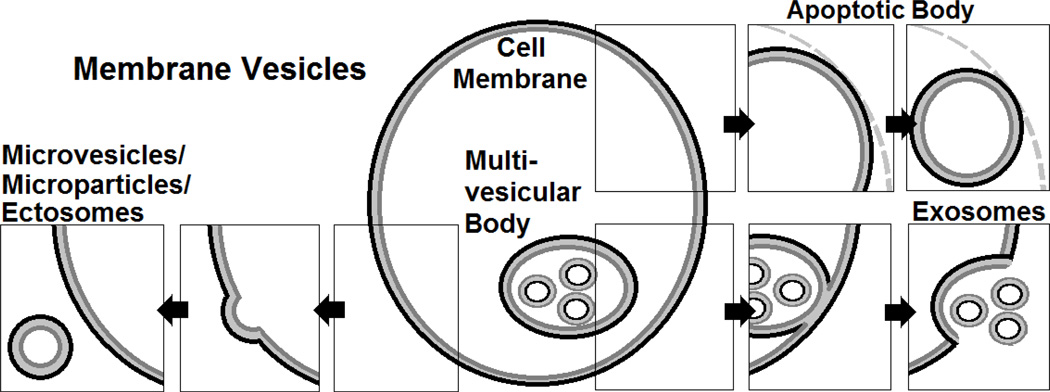
Cells can shed a number of vesicular structures, and debate exists over the classification schemes of these different subpopulations (Figure 1). Classification based on both size and vesicle membrane constituents, namely phosphatidylserine (PS), have been used. Microvesicles (MVs) are concertedly shed from cell membranes and are typically between 0.1 and 2 µm in diameter. One µm is typically the maximum size for platelet-derived MVs to assuredly discriminate them from their small parent cells [13–16]. MVs are larger than exosomes or nanovesicles that arise from multi-vesicular bodies with diameters between 40 to 100 nm [17–19], but smaller than apoptotic bodies, which are roughly 3 to 6 µm in diameter (Figure 1) [1]. Exosomes are more constitutively generated, stored, and shed, whereas MVs seem to be generated in response to specific stimuli [17]; apoptotic bodies are the result of cell fragmentation during apoptotic cell death. There is considerable overlap in size, since moribund cells shed “apoptotic microparticles” as well [19], obfuscating attempts to neatly and orderly classify biological phenomena by size alone. In terms of membrane constituents, MVs have been defined by their ability to bind to annexin V, a PS-binding protein; however, this definition is incomplete, as certain MV populations fail to bind annexin V [20] or even the more sensitive PS probe, lactadherin while binding to the novel phosphatidylethanolamine (PE) probe, duramycin [21] or other surface markers [20]. As virtually all cells shed MVs in various degrees in response to a myriad of stimuli, there is much heterogeneity in their functions. This review focuses on circulating MVs and their emerging role in redox processes.
Figure 1. Membrane Vesicles.
Schematic depicting the difference between exosomes (smallest), microparticles/microvesicles, and apoptotic bodies (largest).
Approaches to defining and studying MVs
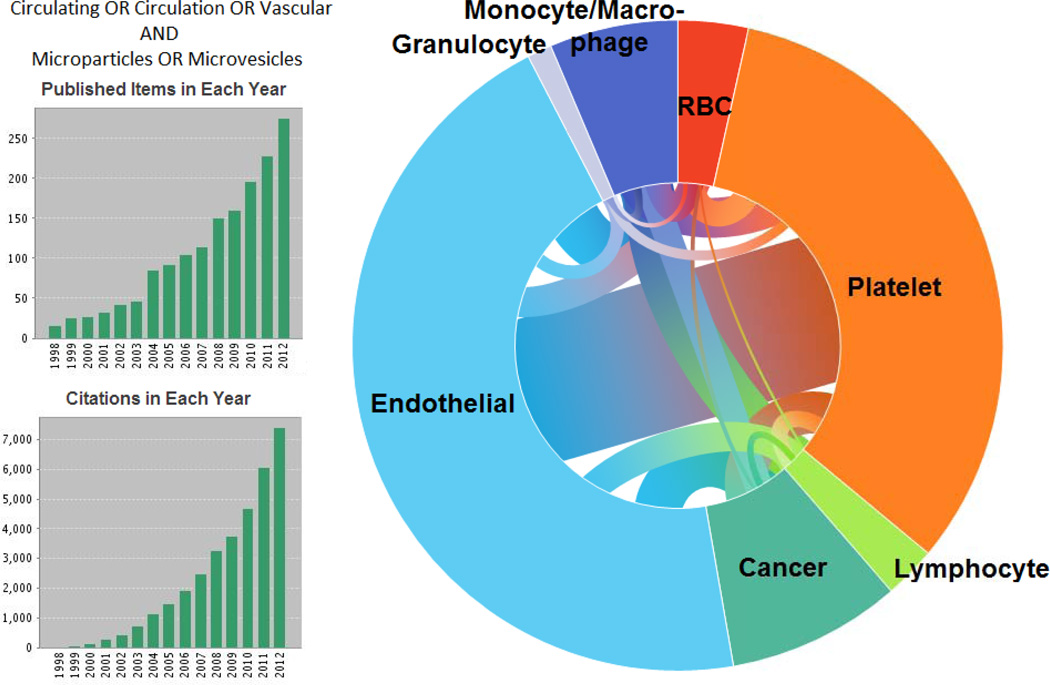
A literature search in Thomson Reuters’ Web of Science up to December 2012 for the topics “vascular,” “circulating,” or “circulation” and “microparticles” or “microvesicles” resulted in over 1000 articles. Articles were then classified by the type of MV, including cancer or tumor, platelet, endothelium or endothelial, erythrocyte or RBC, lymphocyte, granulocyte, and lastly monocyte or macrophage. Figure 2 shows the results, the number of publications and citations per year, as well as the parent cell source(s) of the MV.
Figure 2. Web of Science literature search.
Results of a search for circulating, vascular, or circulation and MVs. Left, top) publications per year and left, bottom) citations per year. Right) Modified pie chart showing studies with MV types mentioned. Connecting lines are weighted to the number of publications listing multiple MV types.
MVs were originally considered cellular debris, since their small size and transient nature did not lend itself to easy isolation, detection or characterization. Isolation of MVs is not trivial; centrifugation, freezing, buffers and vigorous pipetting can activate or damage cells and “artifactually” generate MVs [22,23]. Thus, when obtaining specimens of MVs from their parent cells, care must be taken not to induce the formation of more MVs.
The detection of MVs has been expanding thanks to new technology. Because of their small size, electron microscopy remains the most sensitive way to visualize individual MVs. Enzyme-linked immunoassays, immunoblot, and functional assays have also been used [1,24]; however, these sensitive methods require knowledge of the system under investigation a priori and only provide data on populations of MVs being studied.
The most commonly used method for studying MVs is flow cytometry. While typical applications of flow cytometry rely on the forward scatter intensity to characterize the size of cells, the wavelength of the interrogating laser is on the same order of magnitude of MVs’ diameters, making forward scatter intensity largely dependent upon the MV refractive index rather than their size [25]. Recent data suggests that fluorescence-based flow cytometry of MVs only detects the “tip of the iceberg,” and that, depending on the cytometer and its settings, a “swarm” of small vesicles may register as a single MV cytometer event [26]. Flow cytometry has other limitations; it is difficult to distinguish background “noise” from MVs, so fluorescent labeling is essential. Unfortunately, fluorescently-labeled antibodies may form immune complexes resembling MVs [27,28]. Also, calcium-phosphate precipitates can mimic MVs’ flow cytometry signature and even bind non-specifically to fluorescently-labeled antibodies [29]. Fluorescently-labeled annexin V is the most widely used generic MP marker, although annexin V binding of MVs requires millimolar calcium and is relatively insensitive compared to other phospholipid-binding probes, such as lactadherin [30,31] and duramycin [21]. Additionally, surface marker exposure on MVs is dependent on the stimulus used to generate the MVs [32]. Thus, detection of MVs is not trivial and requires consideration of the method used to isolate MVs, working buffers, molecular labels, and cytometer capabilities. Despite these limitations, flow cytometry provides quantitative as well as qualitative examination of MVs and, according to Lacroix et al, is a “highly competitive analytical method to measure microparticles [microvesicles].” [33]
Microvesicles and Redox Biology
Microvesicles in health
In a study of 16 healthy individuals, proteomic analysis revealed that plasma microparticles contained numerous proteins central to redox processes, including glutathione peroxidase, glutathione S-transferase, peroxiredoxins 1, 2 and 3, protein disulfide isomerase, and manganese superoxide dismutase [34]. Many of these had not been identified before in the plasma proteome [34]. A different study found endothelial nitric oxide synthase (NOS3) located in circulating MVs from 12 healthy individuals. MV-associated NOS3 converted [3H]-l-arginine to [3H]-l-citrulline, was inhibited by the NOS inhibitor, L-NAME, and increased nitrite accumulation. Both the MV-NOS3 levels and activity decreased in patients with endothelial dysfunction [35]. These examples illustrate that MVs contain many of the enzymes associated with redox control mechanisms, even in healthy individuals.
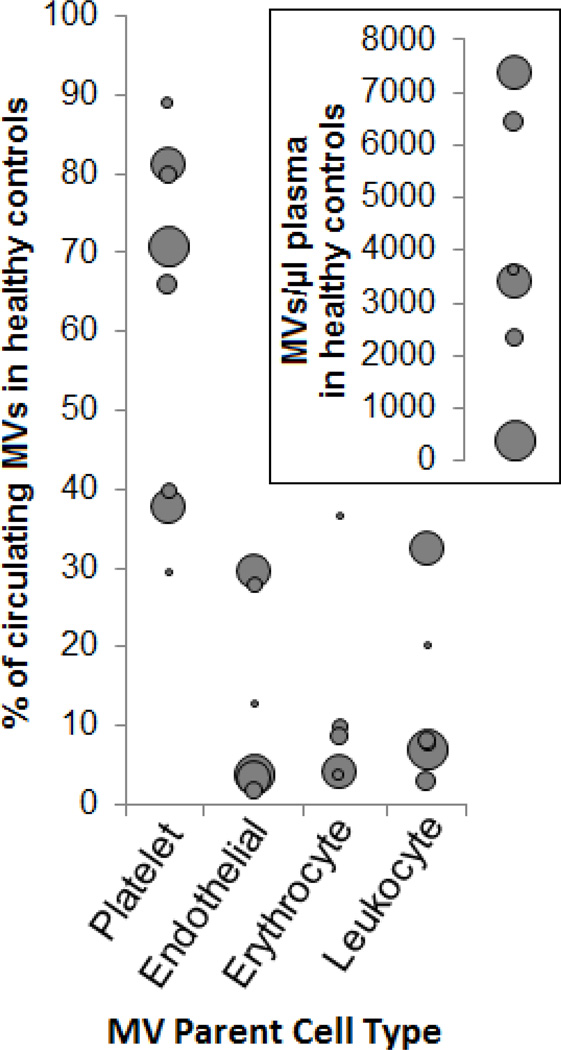
The methods to isolate MVs vary widely, as do the immunolabeling antibodies and protocols. However, there are at least 1000’s of MVs per microliter in platelet-free plasma from “healthy” individuals [35–42] (Figure 3), most of which are platelet-derived. However, the physiologic and demographic state of “healthy” subjects can vary widely—circulating levels of MVs correlate to age and blood pressure [42], vary by gender, the menstrual cycle [43], and are influenced by meals [44,45] and smoking [46]. Additionally, MVs generated from HUVECs in vitro differed by the race of the donor [47].
Figure 3. MVs in healthy individuals.
Results from searching for “circulating microparticles” and “healthy controls”. The percent of circulating MVs by type in controls is shown. Points are sized relative to the number of control plasmas examined. Insert) MVs per microliter. The patient demographics, methods for drawing, clarification to remove cells, centrifugation to remove platelets, labeling, and detection all differed among the studies, highlighting the variability of the results across the studies. Taken from refs. [36–41]
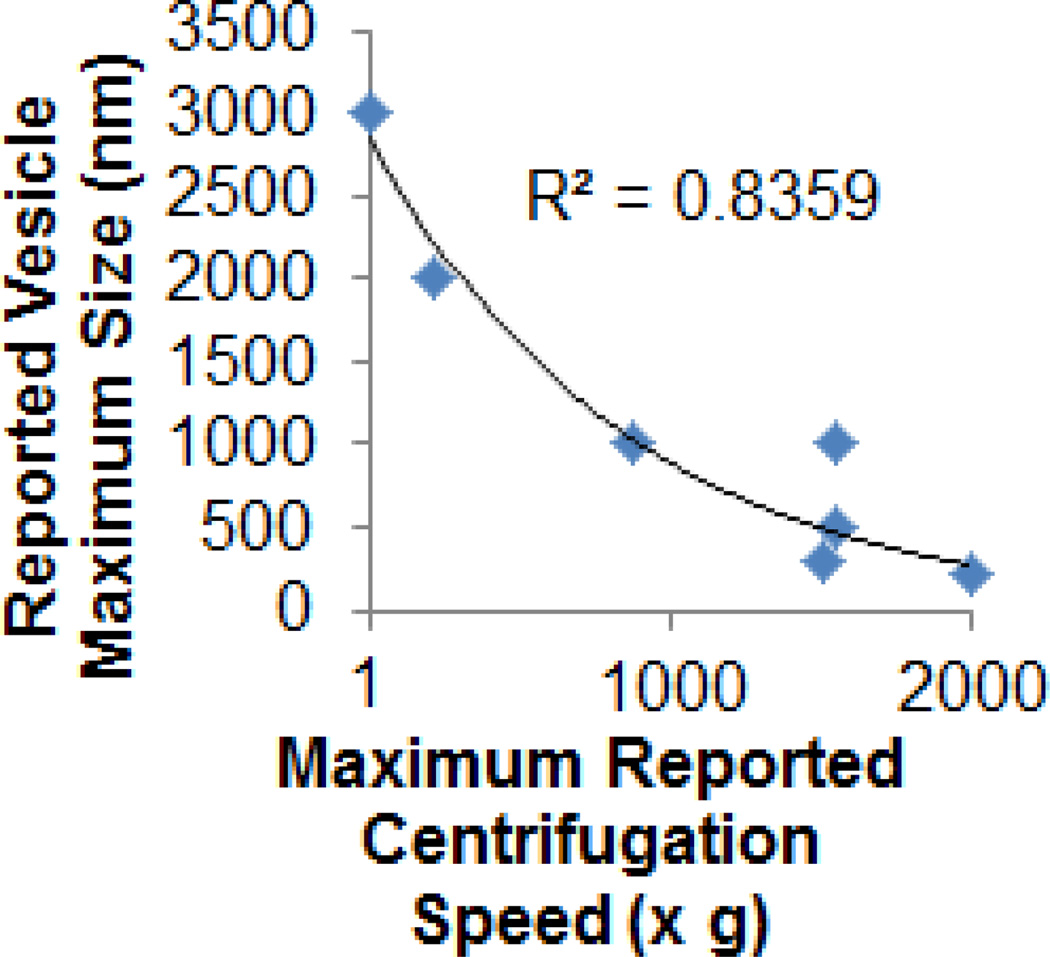
Of note, even gentle centrifugation used to obtain platelet-rich plasma (500g for 5 minutes) can sediment large RBC-derived vesicles (Larson et al, manuscript in preparation), likely making the above numbers an underestimate of the true concentration of circulating MVs (Figure 4).
Figure 4. Centrifugation and resulting MV sizing.
Variability seen in characterization of vesicles may in part be explained by the method used to isolate them. The reported maximum centrifugation speed used to remove parent cells was compared against the maximum size or diameter of the resulting RBC-derived MVs. The trend fit an exponential decay curve (R2=0.83). Taken from > [5,6,154,195–197] (and Larson et al., manuscript in preparation for “1g” sedimentation).
Pathologies associated with circulating MVs and their relevance to redox processes
Since their initial description as “platelet dust” decades ago [48], MVs have been increasingly recognized as more than just cellular debris. As methods for detecting MVs have become more practical and widespread, their presence in numerous diseases has also been reported. And while we now know of their presence, we have only just begun to elucidate their role in these pathological states. Table 1 describes a few examples of common diseases in which the role of MVs has been investigated.
Table 1.
Select diseases with elevated MVs, their cellular origin, and redox relevance.
| Select diseases with elevated MVs |
Cell type that shed MVs (in order of relative abundance) |
Notable redox relevance |
|---|---|---|
| Cardio/vascular diseases | ||
| Acute myocardial infarction | Platelet, Endothelium | Vitamin C significantly decreased circulating MVs in diabetic patients following an acute MI[50] |
| Vascular diseases | ||
| Atherosclerotic plaques | Macrophage > RBC > Lymphocyte > Vasc. Smooth Muscle > Endothelium, Granulocyte | MVs were enriched in taurine, a putative antioxidant[51] |
| Diabetes mellitus | Platelet > Endothelium > Leukocyte | MVs exacerbate inflammatory cell activation[38] |
| Metabolic syndrome | Platelet > RBC > Endothelium | MVs correlated with increased oxidative stress markers[36] |
| Preeclampsia | Platelet > Endothelium, Leukocyte | Platelet MVs enhanced NO release; Leukocyte MVs drove COX-2-mediated vasoconstriction[53] |
| Erythrocyte/vascular diseases | ||
| Sickle cell disease (stable baseline) | RBC > Platelet > Monocyte > Endothelium | RBC MVs contained hemoglobin, which scavenged NO nearly as rapidly as soluble hemoglobin[59,60] |
| Sickle cell crisis | Elevated levels of all the above[59] | |
| Neoplastic/vascular diseases | ||
| Cancer | Platelets > Tumor, Tumor Vasculature | Platelet and Tumor MVs carry angiogenic growth factors that stimulate redox-regulated processes (VEGF, PI3K, Akt, ERK signaling; increased MMP activity)[1,54] |
| Infectious diseases | ||
| HIV | Endothelial[64], Platelets[65,194], Immune cells[66,67] | T cell MPs mediate immune suppression by bearing HIV-1 Nef, inducing lymphocyte apoptosis[67] |
| Platelet MVs activate ROS-mediated pathways[65] | ||
| Severe Malaria | Platelet > RBC > Endothelium, Leukocyte | MVs from infected RBCs activate macrophages, initiate inflammation[70] |
| Neoplastic/vascular diseases | ||
| Cancer | Platelets > Tumor, Tumor Vasculature | Platelet and Tumor MVs carry angiogenic growth factors that stimulate redox-regulated processes (VEGF, PI3K, Akt, ERK signaling; increased MMP activity)[1,54] |
| Inflammatory diseases | ||
| Arthritis | Lymphocyte>Monocyte>Platelet | MVs were positive for RANK/RANKL, which are important in causing ROS production driving monocytes into osteoclasts[27] |
Ischemic heart diseases are the leading cause of death world-wide. During acute myocardial infarction (MI), there are elevated levels of circulating platelet- and endothelium-derived MVs [49,50]. Vitamin C (1g/day for 5 days), a water soluble antioxidant, significantly decreased the levels of circulating MVs in diabetic patients and patients with other cardiovascular risk factors, which further highlights the link between oxidative stress during MI and MV formation [50].
Stroke and other cerebrovascular diseases are the 2nd leading cause of death worldwide. Atherosclerosis is one such disease in which the role of free radicals has been extensively investigated, and which the role of MVs is receiving more attention. A study of MVs within atherosclerotic plaques found thrombogenic MVs from macrophages, RBCs, lymphocytes, vascular smooth muscle cells, endothelial cells, and granulocytes [8]. The MVs were enriched in taurine, a putative antioxidant, which scavenges myeloperoxidase-generated oxidants [51].
Endothelial dysfunction is a contributor to both of the above-listed pathologies. MVs from a variety of cellular sources enhance oxidative stress and endothelial dysfunction in numerous ways. Patients with metabolic syndrome had elevated platelet, RBC, and endothelial MVs correlating with increased oxidative stress markers, including plasma glutathione peroxidase and urinary 8-iso-prostaglandin F2α, but not the inflammatory markers CRP, TNFα, IL-6, sICAM, or sVCAM [52]. Also, MVs from women with preeclampsia upregulated inducible NOS (iNOS) and cyclo-oxygenase 2 (COX-2) expression, activated NFkB, and enhanced reactive species-mediated cell injury when added to experimental vessels ex vivo. Platelet MVs enhanced NO release, while leukocyte MVs drove COX-2-mediated vasoconstriction [53].
Cancer is another leading cause of death, especially in developed countries. While cancer is often thought of as a situation of uncontrolled growth, it is the uncontrolled spread of neoplastic cells that typically mediates morbidity and mortality. Cancer is associated with a hypercoaguable state, and MVs bearing Tissue Factor (TF) and/or phosphatidylserine (PS) contribute to this prothrombotic state [1,9,54]. Tumor cells and the tumor vasculature both shed pro-coagulant MVs and activate platelets, causing further generation of MVs [2]. Platelet MVs in cancer enhance matrix metalloproteinase activity [10,55] and stimulate redox-regulated signaling pathways involved in angiogenesis, including VEGF and PI3/AKT [55–57].
Sickle cell disease (SCD) was the first disease in which a point mutation was linked to a cell phenotype and subsequent pathology [58], yet, there remain unanswered questions of how the mutant molecule causes the sever and varied vascular pathologies observed in this disease. MVs from numerous cell types likely play an important role in the vascular dysfunction seen in SCD. Patients with SCD have elevated RBC, platelet, monocyte, and endothelial MVs that increase during crisis [59]. RBC-derived MVs were shown to house hemoglobin, which scavenged NO with comparable kinetics to soluble hemoglobin [60]. Sickle cell monocyte and endothelial MVs carry tissue factor, a key initiator of the coagulation cascade that is [59] expressed in response to numerous oxidative stressors [61]. Another unique link in sickle cell to redox biology is the use of hydroxyurea as a therapeutic. Hydroxyurea is a nitric oxide (NO) donor [62], and one clinical observation indicated that children with sickle cell anemia on hydroxyurea therapy had decreased RBC and platelet-derived MVs than their untreated counterparts. The authors speculate that NO derived from hydroxyurea may be an important mediator of this phenomenon, since NO inhibits platelet aggregation and activation, and thus, MV formation [63].
Infectious diseases are a major cause of premature death world-wide. HIV/AIDS is the leading individual cause of infectious disease-related mortality, and HIV, too, has been shown to change circulating MV levels. Patients with HIV-1 had increased circulating endothelial-derived [64] and platelet-derived MVs, which decreased with antiretroviral therapy [65]. HIV may utilize vesiculation pathways to enhance immune suppression and viral spread [66,67]. HIV-1-induced MVs carried Nef [67], which can biphasically modulate superoxide release of human phagocytic cells [68].
Malaria is an infectious disease with significant global health burden. Malaria, especially P. falciparum malaria, is characterized by elevated levels of RBC-derived MVs [69], platelet MVs [7], endothelial MVs, and leukocyte MVs [70]. Increased erythrocyte oxidative stress may be critical for RBC MP formation in malaria [69].
Lastly, MVs likely play a role in chronic inflammatory diseases. In arthritis, monocytes utilize ROS-mediated signaling pathways. MVs isolated from the synovial fluid of arthritic patients contained RANKL [71]; RANK/RANKL downstream effectors include ROS [72].
Table 1 and the above discussion provide a few examples of common diseases with elevated MVs, and how these MVs are interlinked with redox signaling pathways. Next, we will discuss general redox-related MV formation and functions from specific cells types.
General MV formation
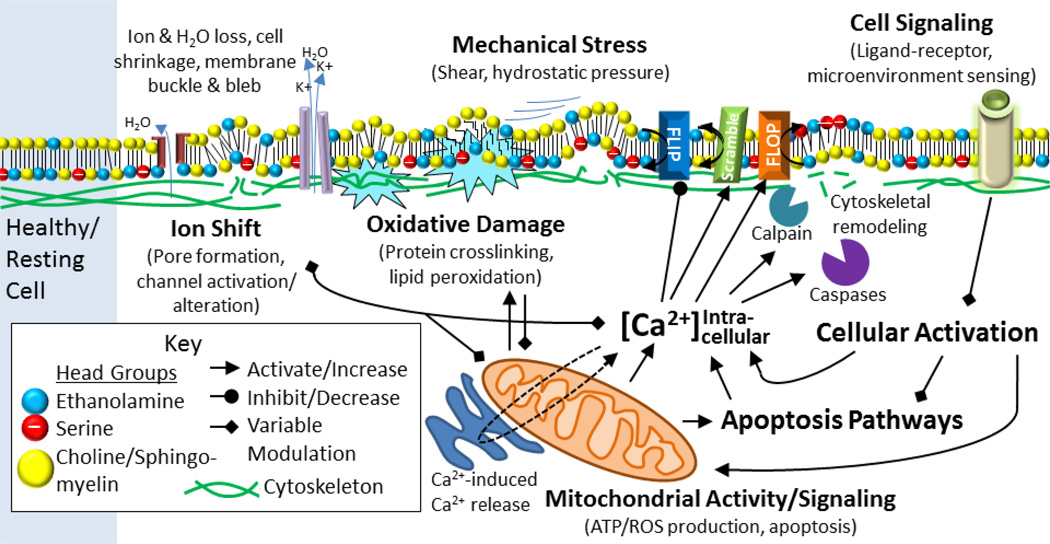
The generation of MVs is dependent on both the cell and the stimulus, with lipid bilayer rearrangement (or derangement) being a critical component of MV formation [13,73]. Bilayer derangement is exquisitely linked to redox biology. This is suggested by the current “gold standard” defining a MV, which is surface exposure of phosphatidylserine (PS), a phospholipid primarily on the cytosolic face of the (quiescent) membrane bilayer [13]. Maintenance of PS on the inner leaflet requires an aminophospholipid translocase that transports outer-leaflet aminophospholipids to the inner-cellular face, also known as a “flippase”. These phospholipid-flipping proteins requires ATP and reduced sulfhydryl groups to “flip” phospholipids [74], and are classically identified by being sensitive to vanadate [75]. PS exposure occurs in response to agonist-induced increases in intracellular Ca2+ [76]. Increasing intracellular Ca2+ modulates mitochondrial permeability[77], inhibits “flippases” [78], activates “floppases” (outward-flopping transporters), and enables caspase- and calpain-dependent cytoskeletal cleavage and reorganization [77]. Mitochondria are centrally involved in MV release (see ref. [77] for a detailed review on mechanistic aspects of mitochondria-driven MV formation). Calcium’s role in MP formation is highlighted in Figure 5. Another phospholipid species found largely on the inner membrane is PE; PE is enriched on the surface of MVs [21] and its location is important in membrane curvature dynamics [79] and vesiculation [73]. PE internalization is maintained by the P4 type ATPase (a “flippase” that transports outer-leaflet phospholipids to the inner-cellular face), TAT-5; loss of TAT-5 in C. elegans results in “large-scale shedding” of PE-positive MVs. These MVs did not show appreciable PS externalization, and their formation required viral budding proteins Rab-11 and ESCRT complex [73]. While PE and PS externalization may be important in the formation of MVs from different cell types, there may also be redox-related mechanisms at play. For example, giant artificial lipid bilayer vesicles comprised of unsaturated fatty acids with phosphatidylcholine headgroups vesiculated in response to redox stimuli. Interestingly, photo-oxidation of the inner leaflet of these giant vesicles induced budding and the release of smaller vesicles [80]. Thus, MV formation through lipid oxidation and/or mitochondrial, caspase, and calcium signaling is often inherently linked to redox processes.
Figure 5. Mechanisms of MV formation.
Shown are general mechanisms underlying cellular MV formation, including electrolyte/hydration shift, membrane (bilayer and structural support) oxidative damage, physical stress, pharmacological signaling, and mitochondrial and apoptotic signaling pathways.
Platelet microvesicles
Platelet MV formation
Platelet MVs are generally formed after an increase in cytoplasmic calcium. This can be achieved with various agonists (such as thrombin or collagen), pore formation (via complement proteins C5b-9 or calcium ionophore) and subsequent calcium influx, or shear stress [81]. With increased calcium follows activation of calpain [82], a cysteine protease susceptible to oxidative stress [83]. Calpain then fragments the cytoskeleton, initiating vesicluation [81]. Additionally, the calcium influx activates scramblase, which randomly redistributes bilayer phospholipids between cytoplasmic and extracellular leaflets in an energy-independent manner [84]. Alteration of the lipid bilayer is a critical component of platelet vesiculation [85], since in a rare bleeding disorder known as “Scott Syndrome,” platelets are unable to externalize PS and shed MVs [86]. Platelet MVs provide essential phospholipid binding surfaces for coagulant proteins involved in hemostasis [84] and inflammation [87].
Platelet MVs and redox processes
Megakaryocyte and platelet-derived MVs are the most abundant circulating MVs in healthy humans, where they likely participate in vascular homeostasis and angiogenesis. These MVs are elevated in chronic diseases characterized by a pro-thrombotic state, including cancer [56], sickle cell disease [59], and rheumatoid arthritis [88] (see Table 1 for more examples). During conditions of acute platelet activation, such as heparin-induced thrombocytopenia, immune thrombotic thrombocytopenia, and arterial thrombosis, levels of circulating platelet MVs also increase dramatically [88]. The composition of platelet MVs varies depending on the stimulus [89] and differs by microparticle sub-type [90], but the following demonstrates their importance as vehicles, drivers and passengers in redox processes (Figure 6).
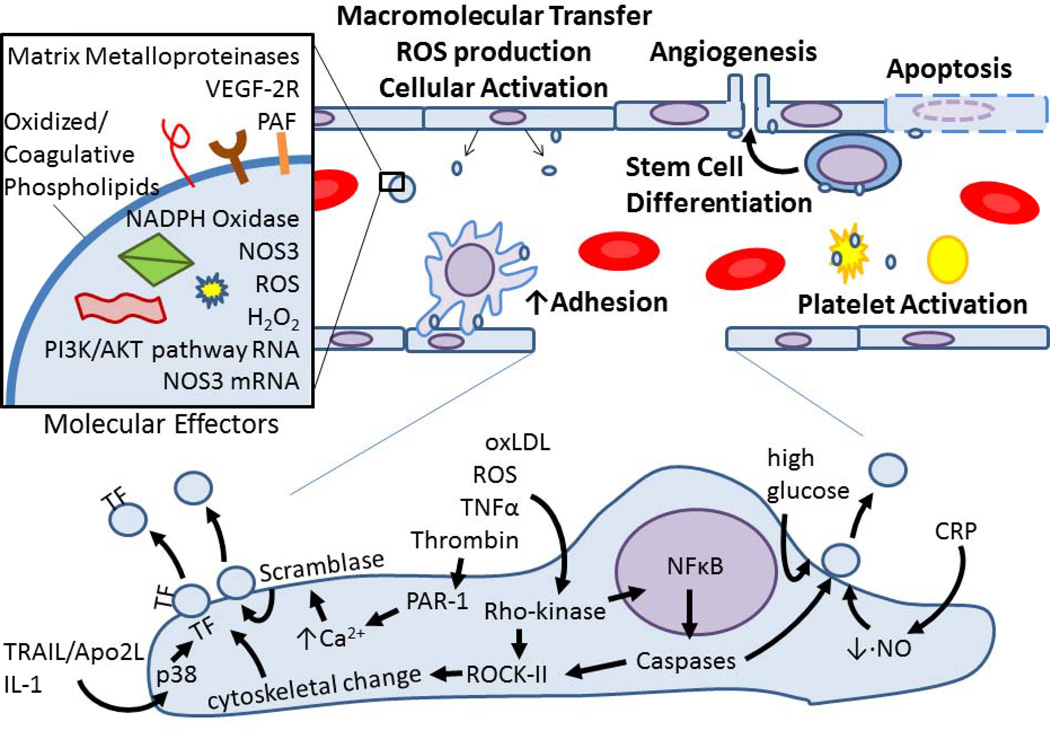
Figure 6. Platelet MVs participate in redox processes.
Platelet activation results in MV formation. Platelet MVs act upon endothelial cells, WBCs, cancer cells, and other platelets via the various known redox effectors listed in the inset. These MV-cell interactions cause enhanced adhesion and cellular activation, which result in ROS production, intracellular signaling, apoptosis, and angiogenesis to name a few redox-regulated processes.
Platelet-derived vesicles from patients with sepsis contained increased levels of NADPH oxidase subunits on Western blot and could generate superoxide; when these MVs were incubated with endothelial cells, apoptosis was enhanced [91]. Platelets exposed to an NO donor and LPS generated MVs similar to those isolated from septic patients; these contained NO synthase II, but not isoforms I or III, as well as protein disulfide-isomerase, and NADPH oxidase subunits. When these MVs were incubated on endothelial cells, apoptosis was again enhanced. In the presence of NO synthase inhibitors, an SOD mimetic, or 1mM urate, endothelial cell apoptosis was inhibited. Interestingly, these redox characteristics and pro-apoptotic effects were not seen in platelets exposed to thrombin or TNFα [92], giving one example of how diverse MV content may be dependent on the specific parent cell stimulus.
The membrane of platelet MVs has been shown to house bioactive lipids, including arachidonic acid [93], which is metabolized by stereospecific lipid peroxidation to generate various signaling molecules [94]; oxidized phospholipids, which can stimulate the plasma membrane receptor for platelet-activating factor (PAF) to activate cells or interact with mitochondria to initiate apoptosis [95]; as well as release PAF [96], which is a potent phagocyte, endothelial cell and platelet activator in several diseases [97]. Furthermore, the clearance of PAF is oxidatively regulated—PAF acetylhydrolase, responsible for inactivating PAF and modified phospholipids, is itself inactivated by peroxynitrite [98].
CD36 (platelet glycoprotein IV) is an abundant transmembrane scavenger receptor that binds oxidized phospholipids among other ligands. Oxidized LDL induced generation of platelet MVs via a CD36- and PS-dependent manner through MKK4/JNK2, which is a pathway known to be involved in the oxidative stress response. Platelets treated with these MVs generated 8-iso-prostaglandin F2α, a marker of lipid peroxidation. Thus, oxidized LDL activation of platelets can to amplify coagulation and oxidative stress [99].
Platelet MVs also carry CD154 on their surface [100]; this tumor necrosis factor super-family member modulates inflammatory and immune cell responses. CD154 bound to its ligand, CD40 on human hepatocytes, stimulates ROS as well as apoptosis. This response is amplified following ischemia-reperfusion injury, resulting in NADPH oxidase-dependent cell death. Inhibiting JNK and p38 signaling pathways attenuates CD154-mediated apoptosis [101]. These MV-triggered signaling pathways are mediated, in part, by reactive species [101,102].
Platelet MVs are also important in angiogenesis and cancer progression. Platelet MVs promote angiogenesis through VEGF [103], which signaling pathway is mediated largely by ROS [104]. Platelet MVs can transfer macromolecules to cells [55,93] to induce expression of COX-2 and angiogenic factors, such as matrix metalloproteinases (MMPs) and VEGF [55,56]. MMPs are redox-regulated [105], demonstrating another link between MVs and redox processes.
Endothelial cell microvesicles
Endothelial MV formation
Endothelial MVs are produced in response to a myriad of stimuli, including ROS, oxidized LDL, and other molecules with strong ties to redox biology [3] (Figure 7). MVs formed after TNFα or thrombin stimulation involves caspases, Rho-kinase, and NFκβ [3], proteins known to be modulated by redox factors. Stimulating HUVECs with C-reactive protein (CRP) decreased NO production while concurrently increasing MP release. This effect was reversed with pretreatment of tetrahydrobiopterin [106]. The mechanism by which the endothelial cell sheds MVs in response to thrombin has been partially characterized in vitro [107,108]. In an early phase, thrombin activates PAR-1, which increases intracellular Ca2++ and subsequent scramblase activation, cell contraction, and cytoskeletal reorganization additionally mediated by Rho-kinase and caspase 2 activation of ROCK-II. In the later stages following thrombin stimulation, TF-expressing MVs are shed dependent on the cytokines TRAIL/Apo2L [107], IL-1 [3,11], and the p38 pathway [109]. Additionally, endothelial MVs are produced in response to mechanical forces in a caspase-dependent, yet apoptosis-independent fashion [110], further linking MVs with redox processes.
Figure 7. Endothelial MVs mediate oxidative stress and vascular injury.
Various redox-regulated effectors, proteins and pathways result in endothelial cell vesicluation, with the MV cargo varying depending on the stimulus. Endothelial MVs then influence vasculature cells by enhancing adhesion, triggering hemostasis, orchestrating cell activation or differentiation, and enhancing or inducing apoptosis by various beneficial or damaging mediators as shown in the inset. TF, Tissue Factor; CRP, C-Reactive Protein.
Endothelial MVs and redox processes
Endothelial-derived MVs are elevated in vascular diseases by oxidative stress, including hypertension [111], atherosclerosis [8,12], and diabetes [112]. Circulating platelet MVs are the most abundant MVs in healthy individuals, and platelet MVs are further elevated in thrombotic conditions. Interestingly, levels of endothelial MVs in individuals with primarily vascular disorders approach the number of platelet MVs per unit volume [113,114]. These endothelial MVs are a rich source of biologically active macromolecules within the vasculature. The parental cell type, injury or health status, and the stimulus on the parent endothelial cell influences the macromolecules packaged into the endothelial-derived MP [12,32,115]. For example, proteomic analysis of HUVEC-derived MVs treated with TNFα, plasminogen activator inhibitor type 1 (PAI-1), or vehicle revealed that, while there was considerable overlap of the proteome, nearly half of the identified proteins were unique to the agonist-specific population. These MVs contained numerous redox-related proteins, including 40–50 unique proteins with oxidoreductase activity and some (<10) with protein disulfide isomerase activity. The authors noted how glutathione peroxidase was differentially expressed in the different populations of MVs, highlighting the variability seen from various MV-producing stimuli [115].
Endothelial MVs generated in vitro from HUVECS that were serum starved for 4 hours decreased acetylcholine-dependent vasorelaxation and NO production, and increased superoxide production when incubated with healthy HUVEC cultures [15]. Additionally, endothelial MVs decreased cell proliferation and increased apoptosis in a dose- and time-dependent manner. These effects were reversible with co-incubation of a cell permeable SOD mimetic, and blunted with an NOS3 inhibitor [112], suggesting that antioxidants can modulate MV function. Another group recently showed that endothelial MVs increase endothelial cell senescence [116]. Primary mouse aortic endothelial MVs were generated by stimulating parent cells with 100uM H2O2. These MVs were then incubated on early-passage endothelial cells, which increased the production of superoxide and hydrogen peroxide. The MVs also caused premature senescence reversed by 1) a superoxide scavenger (tiron), 2) apocynin to inhibit NADPH oxidase, or 3) rotenone to block mitochondrial respiration, but not allopurinol to inhibit xanthine oxidase activity [116]. When similar aortic endothelial cells were treated with angiotensin II, the cells produced more superoxide and MVs; these effects were inhibited with an angiotensin II receptor blocker. The generation of MVs was mediated by NADPH oxidase, ROS and Rho kinase, and was inhibited by molecules to disrupt lipid rafts/caveolae [117].
Endothelial MVs from high glucose-injured human coronary artery endothelial cells generated ROS. The MVs also activated p38 and caused ROS formation when incubated on endothelial cells. This activation was also attenuated with the addition of antioxidant enzymes [118].
Enzymes directly related to redox processes have been identified within endothelial-derived MVs, including NADPH oxidase subunits p22(phox) [15] and gp91(phox). Endothelial MVs generated in response to ischemia had increased NADPH oxidase p47(phox) and p67(phox). The isolated MVs generated ROS, which production was inhibited by apocynin (an antioxidant [119]), DPI (an purported inhibitor of NADPH oxidase [120]), or in gp91-KO mice-derived MVs [121]. High glucose-injured human coronary artery endothelial cells produced MVs with more NADPH oxidase activity and intra-microparticle H2O2 than spontaneously-produced MVs [118]. Lastly, endothelial MVs from ischemic mouse limb vessels contained NOS3 [121].
In addition to cytosolic proteins, MVs from endothelial cells also carry nucleic acid messages [122]. MVs from endothelial progenitor cells contained mRNA specific for NOS3 and other PI3K/AKT signaling pathway transcripts. These MVs were incorporated into human microvascular endothelial cells (HMECs), and ultimately stimulated AKT activation and NOS3 expression. These progenitor endothelial MVs promoted cell growth and survival in vitro, and were proangiogenic in vivo [122].
MVs generated from tert-butyl hydroperoxide or apoptotic, but not Ca2+ ionophore-treated, endothelial cells promoted monocyte adhesion to endothelial cells. An oxidized phospholipid was responsible for this biological activity [12]. MVs from activated or apoptotic endothelial cells directly interact with or fuse with bone marrow stem cells in, presumably, a PS-dependent manner. These MVs caused the bone marrow stem cells to develop an endothelial phenotype; this process was inhibited by apocynin or by incubation with MVs from gp91 KO mice [121]. Lastly, PAF-containing MVs were generated by treating endothelial cells with H2O2 [123].
MMPs, which are redox-regulated [105], were localized to the extracellular face of endothelial MVs [124]. Endothelial MVs from ischemic mouse limb vessels also exposed VEGF-2R [121]. Additionally, endothelial MVs bear other surface molecules indirectly involving redox-regulated processes, including adhesion molecules, cadherins, coagulation and cell survival molecules [3].
WBC microvesicles
Leukocyte MV formation
While leukocytes represent a diverse population of cells, one commonality is that their shedding of MVs has been consistently linked with NO-mediated signaling. Monocyte/macrophage MV generation was studied in RAW 264.7 murine macrophage-like cells. Monocyte MVs were generated in response to various TLR ligands, most robustly through TLR3 and TLR4. MV generation paralleled nitrite (and presumably NO) production. Addition of NO donors also caused MV formation in a dose-dependent manner, and inhibition of caspases increased MVs while decreasing nitrite formation. Inhibition of NOS2 with the NOS2-specific inhibitor, 1400W, diminished MV production to basal levels [125]. The reverse was true in neutrophils. Neutrophils treated with the NOS inhibitor L-NAME enhanced MV formation and subsequent neutrophil migration. This enhancement of MV production was diminished by calpain and SOD inhibition [126].
Neutrophil MVs and redox processes
The cell type and microenvironment of MV targets may also modify the resultant outcome. Neutrophils shed MVs that activate HUVECs and induce production of cytokines, such as IL-6 [127]. These cytokines can then orchestrate inflammation and subsequent “oxidative stress.” Neutrophil-derived MVs contain myeloperoxidase and MMP-9 [128], which may be a means by which neutrophils localize antimicrobial activity or mediate tissue damage [129]. In other settings, neutrophil MVs may also induce inactivating or anti-inflammatory functions. For example, neutrophil MVs reduced dendritic cell phagocytosis and inhibited up-regulation of markers of activation [130] as well as pro-inflammatory cytokine production [131].
Lymphocyte MVs and redox processes
The relation of MVs to redox biology has been investigated using a proteomic approach. Thymocytes isolated from healthy BALB/c mice shed MVs over a 24 hour period. These MVs were harvested, and their proteome was examined. The proteome contained numerous proteins important for various redox processes, including CuZn-SOD, Peroxiredoxins 1, 2 and 6, and Cytochrome C oxidase subunits 2 and 5A. This proteomic approach has also been used on MVs generated from malignant human T cells. Proteomic analysis of malignant human lymphoid CEM (acute lymphoblastic leukemia) T cell-derived MVs also contained various redox-related proteins, including Peroxiredoxins 1,2 5, and 6; Thioredoxin; and numerous mitochondrial proteins [132]. MVs generated after stimulating CEM T cells with hemagglutinin or actinomycin D contained the NADPH oxidase subunit p22(phox), but not p47(phox) and gp91(phox) subunits [133]. CEM T cell MVs also expressed NADPH oxidase isoforms NOX1 and NOX4 on their surface [134].
In addition to platelet- and endothelial-derived MVs, lymphocyte MVs are also involved in angiogenesis. Sonic hedgehog-bearing MVs from stimulated CEM T cells induced NO release and decreased ROS production from endothelial cells in vitro and ultimately improved endothelial function after ischemia/reperfusion in vivo [135]. These MVs also inhibited cell migration, but induced the formation of capillary-like structures of endothelial cells in vitro [136] and enhanced angiogenesis and blood flow after ischemic injury in vivo [137].
While the lymphoid MVs display pro-angiogenic properties, the reverse is also true. Stimulated CEM T cells generated MVs that decreased NO- and prostacyclin-mediated vasodilation after 12–24 hours of incubation on isolated vessels ex vivo. Additionally, when similarly incubated on HUVECs, the lymphocyte MVs decreased NOS3 expression [138]. A different group showed actinomycin D-generated lymphocyte MVs decreased NO production through PI3K and MAPK pathway modulation, and increased ROS production dependent on xanthine oxidase and NF-kB [134]. These MVs suppressed angiogenesis in both ex vivo and in vivo assays. When incubated on HUVECs, the MVs decreased survival, proliferation, and migration of the endothelial cells. The anti-proliferative effect was partially blunted with antioxidants, and the anti-migratory effect was reversed by NOX inhibitors. The MVs increased HUVEC expression and activity of NOX and production of ROS, as well as expression of the scavenger receptor, CD36, while decreasing the VEGF2R [133]. Thus, the redox effects of these MVs are largely dependent on the generating stimulus, injury or environment of the effector cells.
Jurkat cells (from a different human T cell leukemia) generated MVs after being treated with a variety of stimuli (LPS, TNFα, Con A, staurosporin, actionomycin D, and Fas ligand). These MVs activated fibroblasts as demonstrated by increased expression and activity of MMPs of fibroblasts, as well as up-regulation of IL-6, IL-8, MCP-1, and MCP-2. The cytokine production results were duplicated in primary T cells from peripheral blood. The increase in MMPs was mediated by NFκB but not TNFα or IL-1 [139].
Monocyte/Macrophage MVs and redox processes
The properties of monocytic MVs also differed based on the generating stimulus [140]. For example, THP-1 cells stimulated with LPS or P-selectin (via a P-selectin Ig chimera) contained SOD and cytochrome C, but not those generated spontaneously or with control Ig only [140].
U937 monocytes and peripheral monocyte-derived MVs activated fibroblasts, increasing the fibroblasts’ production of cytokines IL-6, IL-8, MCP-1, and MCP-2, which downstream effectors are modulated, in part, by reactive species, as well as production and activity of numerous redox-sensitive MMPs [139].
Peripheral blood monocytes treated with Fas-L for 6 hours shed numerous MVs [141]. These MVs were then incubated on HUVECs, which resulted in ROS formation inhibited by antioxidant enzymes (catalase and SOD), small molecule antioxidants (N-acetylcystein and vitamin C) or a host of inhibitors of free radical-producing enzymes. Such enzymes included cyclooxygenase COX-2 (inhibited by diclofenac), NADPH oxidase (inhibited by DPI), mitochondrial complexes I and III (inhibited by rotenone and antimycine), xanthine oxidase (inhibited by allopurinol), and NOS3 (inhibited by L-NAME and BH4). No single free radical-producing enzyme inhibitor was able to prevent ROS formation to basal levels. The monocyte MVs also induces p38 MAPK in a superoxide-dependent manner, highlighting the link between MVs and inflammation [141].
Neoplastic microvesicles
Cancer MV formation
Cancerous cell vesiculation results in a wide variety of MVs with diverse makeup and function that are regulated in a cell-specific manner according to the microenvironment and specific stimuli. Furthermore, cancer MV secretion is regulated by oncogenic pathways [54]. Additionally, the differences in genetic alterations and the microenvironment of the cancer cell alters both the quantity and characteristics of MVs arising from similar tissue types [54,142–144]. p53, which involves redox regulation [145], is one such example. In human non-small cell lung cancer cells, non-lethal irradiation with gamma-rays resulted in p53-dependent production of MVs that was not accompanied by increased apoptosis. Secretion of MVs from similarly-irradiated mouse embryo fibroblasts was also dependent on p53 [144]. However, in colorectal cancer cells, p53 deletion or mutant K-ras expression increased MV production [143].
Another example of redox signaling and cancer MVs is illustrated by EGFR, which works via a well-characterized ROS-dependent signaling cascade [146]. Glioma cells naturally shed MVs, shed MVs more abundantly upon transfection with EGFRvIII [142]; EGFRvIII downstream signaling proteins, such as AKT, are sensitive to reactive species [147]. Also, prostate cancer cells shed MVs upon activation of EGFR and AKT. These MVs enhanced proliferation and migration of MV-recipient tumor cells [148].
Cancer MVs and redox processes
Cancer cells have been shown to horizontally transfer oncogenic cargo via MVs [1], including nucleic acids for various redox-related proteins, such as EGFRvIII [142], GAPDH [149], mitochondrial DNA [150]. Cancer MVs also carried mutated oncoproteins, whose pathways are redox mediated, including EGFR, HER2, Akt, K-ras [1].
Cancer cells shed MVs displaying active Fas antigen and Fas/L [151]. They also carry TF, the expression of which can be modulated by ROS [61]. Cancer-derived MVs also bear redox-sensitive MMPs, angiogenic growth factors, and cytokine receptors [1].
Cancer cells also tend to activate cells in the local microenvironment, inducing further MP shedding from surrounding healthy tissues. As already described above, cancer is associated with increased platelet and endothelial MVs. Additionally, cancer-surrounding stromal fibroblasts shed MVs in response to tumor MP-activation. These fibroblast MVs facilitated migration and invasion of the cancer cells by bearing the chemokine CX3CL1 [152].
Vascular Smooth Muscle MVs and redox processes
Cultured human vascular smooth muscle cells incubated with LDL produced MVs bearing TF [153]. As previously discussed, TF expression is linked to NADPH Oxidase and ROS-dependent processes [61] in addition to its critical role in coagulation cascade activation.
RBC microvesicles
Normal erythrocyte MVs
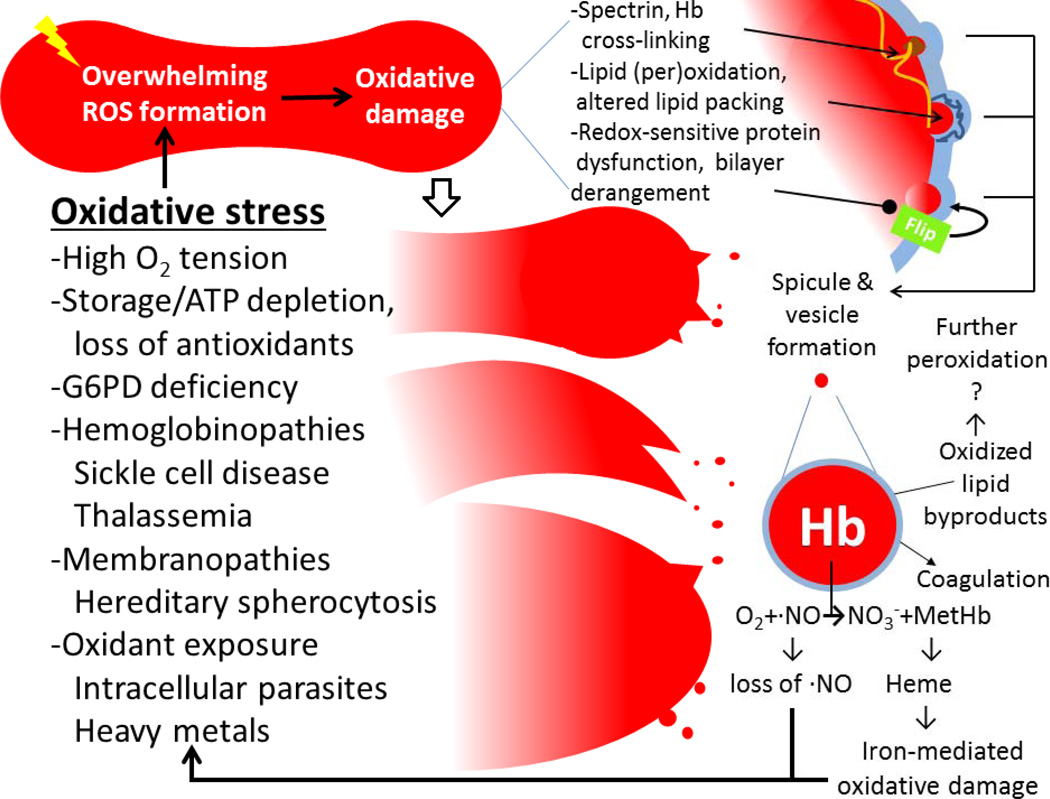
RBC MPs have unique ties to redox biology, as circulating RBCs lack mitochondria and nuclei typically needed to orchestrate MV release. RBC MVs are generated constitutively throughout the life of the RBC [5,6,154–156] and are normally cleared by Kupffer cells via scavenger receptors recognizing PS [157]. Hemoglobin continually binds and releases molecular oxygen; occasional “imperfect” hemoglobin-O2 electron transfers result in the formation of superoxide [158]. This auto-oxidation of hemoglobin happens in 0.5–3% of total hemoglobin every day [155]. Superoxide, methemoglobin and subsequent hemichromes, or other related reactive species may then oxidize and/or cross-link hemoglobin, cytoskeletal proteins [159], membrane proteins [155] and membrane lipids [160,161] (Figure 8). Studies have demonstrated that oxidation of the cytosolic face of band-3, a transmembrane chloride/bicarbonate ion exchanger which also binds to the underlying cytoskeleton, leads to tyrosine phosphorylation on band-3. This phosphorylation dissociates band-3 from the underlying cytoskeleton, leading to vesiculation [162].
Figure 8. RBC MV formation and function relating to oxidative stress.
RBCs experiencing overwhelming oxidative stress/damage shed MVs with hemoglobin (Hb). These RBC MVs bear Hb capable of scavenging nitric oxide (NO) and pro-coagulative phospholipids, leading to a pro-thrombotic state.
Much of what we know about RBC vesiculation is from studies of donor RBC units. Oxidation of the cytoskeletal protein, spectrin, directly correlates with vesiculation of RBCs in donor blood units [159]. Oxidative damage to the membrane bilayer resulted in accumulation of fatty acid peroxidation byproducts and derangement of the membrane bilayer [160,161,163] which preceded vesiculation and correlated with increased coagulation activity [163]. Oxidative damage, primarily due to thiol depletion by mercury, inhibited flippase and caused the release of pro-coagulant MVs [164]. Oxidation and aggregation of band-3 resulted in enhanced binding of auto-antibodies [165] and complement to the RBC [156]. This aggregation may then enhance either 1) phagocytosis of senescent RBCs [165] or 2) membrane budding and vesicluation in order to preserve the otherwise healthy RBC [5,6]. Indeed, up to 30% of RBC volume is lost as it ages, both in vivo [166,167] and in vitro [168,169]. During storage prior to transfusion, these RBC MVs accumulate in the blood bag [170,171].
While the RBC normally balances regulators of oxidation and reduction important in normal circulation in vivo, stored blood MVs contain more proteins involved in “oxidative cellular processes,” which increases as the duration of storage lengthens [172]. Additional changes in RBC MVs over the time of storage have been described, including size, morphology, and “oxidative index” [5]. This latter study also showed that RBC MVs carried Fas-related signaling molecules, including Fas/CD95, FADD, and caspases 3 and 8; these macromolecules are influenced by the redox environment.
In addition to carrying apoptotic signaling molecules, RBC MPs accrued in stored blood house hemoglobin capable of scavenging NO at a rate comparable to soluble cell-free hemoglobin [60]. As NO normally vasodilates, inhibits platelet aggregation and leukocyte adhesion [173], consumption of NO lends to vasoconstriction, platelet aggregation and increased cell-cell adhesion. Coupled with pro-coagulant aminophospholipid exposure and other bioeffector molecules such as Ig, complement, adhesion molecules on the surface of RBC MPs, the stage is set to initiate or propagate thromboses. These processes are likely underpinning non-immune mediated transfusion complications [174] and vasculature problems, such as hemostatic activation and endothelial dysfunction in other scenarios of hemolysis, including various hemoglobinopathies and membranopathies as discussed next.
MVs from diseased RBCs
As stored RBCs lack replenishment of antioxidants, similarly, G6PD deficient RBCs are susceptible to oxidative stress. G6PD RBCs lack sufficient NADPH as a glutathione reductase substrate during oxidative insults (as from fava bean ingestion or exposure to moth ball fumes), and this results in accelerated “senescence” and hemolysis of RBCs [156] and (not surprisingly) MV formation [175]. Specifically in G6PD RBCs, oxidant exposure leads to hemichrome (denatured hemoglobin) formation, which induces phosphorylation of band-3 by Syk kinase. Phosphorylation of band-3 destabilizes the membrane [176] and causes hemolysis and vesiculation, which can be reduced with a specific kinase inhibitor [177].
RBCs in those with sickle cell disease (SCD) are also prone to oxidative stress and vesiculation. In vitro, oxidants (e.g. phenylhydrazine, H2O2) can induce RBCs to sickle [178]. In vivo and in vitro, SCD RBCs sickle in response to hypoxia, and subsequent reoxygenation causes the loss of 2–3% of sickled cells’ lipids in the form of MVs [179]. These MVs can promote activation of the coagulation cascade, providing one explanation for the hypercoaguable state seen in SCD [180].
Likewise, thalassemia, caused by an imbalanced ratio of hemoglobin alpha and beta chains, results in RBCs more sensitive to oxidative damage. This is likely due to increased release of iron from unstable hemoglobin multimers, rendering the iron active to redox cycling [181]. Those with thalassemia have elevated circulating RBC MVs, which have also been suggested as contributors to the hypercoaguable state of thalassemic individuals [182]. Individuals with thalassemia intermedia have a direct correlation between the hemichrome content and the vesiculation tendency of their erythrocytes [183]. Hemichromes bind to the cytoplasmic face of band-3, which results in disulfide-linked band-3 dimers that are subsequently phosphorylated by p72Syk kinase. Inhibiting this kinase decreases MV formation. Proteomic analysis demonstrate these MVs carried various redox-related proteins, including hemichromes, oxidiezed band-3, catalase, heat shock protein70, and peroxiredoxin 2 [183].
While the underlying pathologies originate from totally different causes, there are similarities between genetic RBC disorders and malarial-infected RBCs in the oxidative stress-vesiculation process. Falciparum (malaria)-infected RBCs experience increased oxidative stress [184], and shed >10 times the MVs compared to uninfected RBCs [69]. RBC-MV production is also increased by non-parasitized RBCs in malaria-infected individuals, suggesting systemic signaling induces shedding from “healthy” RBCs. This is inhibited by N-acetylcysteine, which led the authors to propose heme-mediated oxidative stress as a pathway for MV generation in malaria [69]. Early in the malaria infection, RBCs experience increased phosphorylation of band-3 [185], with subsequent increased vesiculation [186]. In fact, enhancing the redox-mediated signaling underlying vesiculation and hemolysis early in the course of malaria-RBC infection is a suggested mechanism of action of a new antimalarial drug [186]. Thus, RBC MV formation in a variety of processes and pathologies is linked to redox biology.
Lastly, RBC MVs from a mouse model of sickle cell disease induced ROS formation by endothelial cells likely via NADPH oxidase or PKC as the effect was inhibited by DPI and apocynin or PKC inhibitors [187]. Interestingly, the ROS formation was also inhibited by pre-treating the MVs with annexin V to “cover” MP anionic phospholipids [187]. The same RBC MVs were injected into a mouse model of sickle cell disease and caused “vaso-occlusion” of the kidneys [187]. Additionally, ROS were implicated in the pathology seen in ischemia/reperfusion injury after transient vascular occlusion. RBC MVs are also found in mouse models of hereditary spherocytosis, along with a high oxidative potential in the plasma, and endothelial oxidative damage from NO scavenging [188].
Conclusion
Oxidative stress and MV formation go hand-in-hand across multiple cell types. There is a distinct link in RBCs between oxidative stress, whether from malarial parasites or unstable and pro-oxidant hemoglobin, to MV formation. There is a similar correlation between oxidative stress and MV formation in other cells, with redox and mitochondrial signaling orchestrating the generation of MVs.
An increased understanding of the roles circulating MVs play in cardiovascular diseases, thromboses, cancer, inflammation and other pathological processes will undoubtedly lead to enhanced diagnostics and treatments.
For example, D-dimer testing as a measure of thrombosis in cancer patients lacks specificity to be used to screen cancer patients at risk for venous thromboembolism (VTE) and prophylaxis thereof, but MV measurements were a more promising biomarker in retrospective [189] and prospective studies [190]. Other serum antigen testing provides highly sensitive data on some cancers [191]; the lack of perfect specificity, however, can lead to false positives and clinical harm [192]. The concentration of circulating MVs, whether from cancer cells, stromal cells, or activated platelets, could provide additional useful clinical data bridging the gap between tissue- and molecule- level diagnostic or prognostic indicators.
Additionally, washing donor RBCs to remove hemolysate, including RBC MVs, improved biomarkers of inflammation and resulted in less transfusions needed compared to unwashed RBC (and RBC MV) recipients undergoing cardiac surgery [193].
We are just beginning to understand how MVs are involved in various biological processes, both homeostatic and pathologic. The role of MVs in redox signaling is an exciting new area of research. Like reactive species, MVs were originally thought to have a primarily detrimental role in biology. And like reactive species, there appears to be an important role for MVs play in normal growth, development, and homeostasis.
Cells shed microparticles/microvesicles (MVs) largely by redox-regulated pathways
MVs, in turn, affect cells through bearing, inducing, or altering reactive species
The specific role of MVs in various disease processes are under intense study
MVs are increasingly being recognized important mediators of growth and homeostasis
Cell-specific MVs may serve as diagnostic/prognostic biomarkers in various diseases
Acknowledgments
This work was supported by the Midwest Athletes against Childhood Cancer (MACC) fund and R01 NS070711 (to CAH), Midwest Basic and Translational Program U54 HL090503 (to NH and CAH), and ML is a member of the MCW-MSTP, which is partially supported by a T32 grant from NIGMS, GM080202.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee T, D’Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer—the emerging science of cellular “debris.”. Seminars in Immunopathology. 2011;33:455–467. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- 2.Hugel B, Martínez MC, Kunzelmann C, Freyssinet J-M. Membrane Microparticles: Two Sides of the Coin. Physiology. 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 3.Dignat-George F, Boulanger CM. The Many Faces of Endothelial Microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 4.Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin. Immunopathol. 2005;27:375–387. doi: 10.1007/s00281-005-0004-1. [DOI] [PubMed] [Google Scholar]
- 5.Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signaling components. Transfusion. 2008;48:1943–1953. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 6.Willekens FLA, Werre JM, Groenen-Döpp YAM, Roerdinkholder-Stoelwinder B, de Pauw B, Bosman GJCGM. Erythrocyte vesiculation: a self-protective mechanism? Br. J. Haematol. 2008;141:549–556. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 7.Faille D, Combes V, Mitchell AJ, Fontaine A, Juhan-Vague I, Alessi M-C, Chimini G, Fusaï T, Grau GE. Platelet microparticles: a new player in malaria parasite cytoadherence to human brain endothelium. FASEB J. 2009;23:3449–3458. doi: 10.1096/fj.09-135822. [DOI] [PubMed] [Google Scholar]
- 8.Leroyer AS, Isobe H, Lesèche G, Castier Y, Wassef M, Mallat Z, Binder BR, Tedgui A, Boulanger CM. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J. Am. Coll. Cardiol. 2007;49:772–777. doi: 10.1016/j.jacc.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 9.Furie B, Zwicker J, LaRocca T, Kos C, Bauer B, Furie B. Tissue factor-bearing microparticles and cancer-associated thrombosis. Haemotologica Reports 2005. 2005;1:5–8. [Google Scholar]
- 10.Dashevsky O, Varon D, Brill A. Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production. International Journal of Cancer. 2009;124:1773–1777. doi: 10.1002/ijc.24016. [DOI] [PubMed] [Google Scholar]
- 11.Berda-Haddad Y, Robert S, Salers P, Zekraoui L, Farnarier C, Dinarello CA, Dignat-George F, Kaplanski G. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1α. PNAS. 2011;108:20684–20689. doi: 10.1073/pnas.1116848108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber J, Vales A, Mitulovic G, Blumer M, Schmid R, Witztum JL, Binder BR, Leitinger N. Oxidized Membrane Vesicles and Blebs From Apoptotic Cells Contain Biologically Active Oxidized Phospholipids That Induce Monocyte-Endothelial Interactions. Arterioscler Thromb Vasc Biol. 2002;22:101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 13.Morel O, Jesel L, Freyssinet J-M, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler. Thromb. Vasc. Biol. 2011;31:15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 14.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Brodsky SV, Zhang F, Nasjletti A, Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol. 2004;286:H1910–H1915. doi: 10.1152/ajpheart.01172.2003. [DOI] [PubMed] [Google Scholar]
- 16.Lacroix R, Robert S, Poncelet P, Kasthuri RS, Key NS, Dignat-george F. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. Journal of Thrombosis and Haemostasis. 2010;8:2571–2574. doi: 10.1111/j.1538-7836.2010.04047.x. [DOI] [PubMed] [Google Scholar]
- 17.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends in Cell Biology. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Johnstone RM. Exosomes biological significance: A concise review. Blood Cells, Molecules, and Diseases. 2006;36:315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 19.György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, Nagy G, Falus A, Buzás EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connor DE, Exner T, Ma DDF, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb. Haemost. 2010;103:1044–1052. doi: 10.1160/TH09-09-0644. [DOI] [PubMed] [Google Scholar]
- 21.Larson MC, Woodliff JE, Hillery CA, Kearl TJ, Zhao M. Phosphatidylethanolamine is externalized at the surface of microparticles. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2012;1821:1501–1507. doi: 10.1016/j.bbalip.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shet AS. Characterizing blood microparticles: technical aspects and challenges. Vasc Health Risk Manag. 2008;4:769–774. doi: 10.2147/vhrm.s955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erdbruegger. Detection of circulating microparticles by flow cytometry: influence of centrifugation, filtration of buffer, and freezing. Vascular Health and Risk Management. 2010:1125. doi: 10.2147/VHRM.S13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enjeti AK, Lincz LF, Seldon M. Detection and measurement of microparticles: an evolving research tool for vascular biology. Semin. Thromb. Hemost. 2007;33:771–779. doi: 10.1055/s-2007-1000369. [DOI] [PubMed] [Google Scholar]
- 25.Chandler WL, Yeung W, Tait JF. A new microparticle size calibration standard for use in measuring smaller microparticles using a new flow cytometer. Journal of Thrombosis and Haemostasis. 2011;9:1216–1224. doi: 10.1111/j.1538-7836.2011.04283.x. [DOI] [PubMed] [Google Scholar]
- 26.Van Der POL E, Van GEMERT MJC, Sturk A, Nieuwland R, Van LEEUWEN TG. Single vs. swarm detection of microparticles and exosomes by flow cytometry. Journal of Thrombosis and Haemostasis. 2012;10:919–930. doi: 10.1111/j.1538-7836.2012.04683.x. [DOI] [PubMed] [Google Scholar]
- 27.Gyorgy B, Modos K, Pallinger E, Paloczi K, Pasztoi M, Misjak P, Deli MA, Sipos a, Szalai A, Voszka I, Polgar A, Toth K, Csete M, Nagy G, Gay S, Falus A, Kittel a, Buzas EI. Flow cytometric diagnostic assessment of cell-derived microparticles is severely confounded by immune complexes in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2011;70:A11–A12. [Google Scholar]
- 28.György B, Módos K, Pállinger É, Pálóczi K, Pásztói M, Misják P, Deli MA, Sipos Á, Szalai A, Voszka I, Polgár A, Tóth K, Csete M, Nagy G, Gay S, Falus A, Kittel Á, Buzás EI. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117:e39–e48. doi: 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- 29.Larson MC, Luthi MR, Hogg N, Hillery CA. Calcium phosphate microprecipitates mimic microparticles when examined by flow cytometry. Cytometry. 2013;83A:242–250. doi: 10.1002/cyto.a.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi J, Heegaard CW, Rasmussen JT, Gilbert GE. Lactadherin binds selectively to membranes containing phosphatidyl-L-serine and increased curvature. Biochim. Biophys. Acta. 2004;1667:82–90. doi: 10.1016/j.bbamem.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Hou J, Fu Y, Zhou J, Li W, Xie R, Cao F, Gilbert GE, Shi J. Lactadherin functions as a probe for phosphatidylserine exposure and as an anticoagulant in the study of stored platelets. Vox Sang. 2011;100:187–195. doi: 10.1111/j.1423-0410.2010.01375.x. [DOI] [PubMed] [Google Scholar]
- 32.Jimenez JJ, Jy W, Mauro LM, Soderland C, Horstman LL, Ahn YS. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb. Res. 2003;109:175–180. doi: 10.1016/s0049-3848(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 33.Lacroix R, Robert S, Poncelet P, Dignat-George F. Overcoming limitations of microparticle measurement by flow cytometry. Semin. Thromb. Hemost. 2010;36:807–818. doi: 10.1055/s-0030-1267034. [DOI] [PubMed] [Google Scholar]
- 34.Jin M, Drwal G, Bourgeois T, Saltz J, Wu HM. Distinct proteome features of plasma microparticles. PROTEOMICS. 2005;5:1940–1952. doi: 10.1002/pmic.200401057. [DOI] [PubMed] [Google Scholar]
- 35.Horn P, Cortese-Krott MM, Amabile N, Hundsdörfer C, Kröncke K-D, Kelm M, Heiss C. Circulating Microparticles Carry a Functional Endothelial Nitric Oxide Synthase That Is Decreased in Patients With Endothelial Dysfunction. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.112.003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, Mostefai HA, Draunet-Busson C, Leftheriotis G, Heymes C, Martinez MC, Andriantsitohaina R. Endothelial Dysfunction Caused by Circulating Microparticles from Patients with Metabolic Syndrome. The American Journal of Pathology. 2008;173:1210–1219. doi: 10.2353/ajpath.2008.080228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieuwland R, Berckmans RJ, McGregor S, Böing AN, Romijn FPHTM, Westendorp RGJ, Hack CE, Sturk A. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930–935. [PubMed] [Google Scholar]
- 38.Diamant M, Nieuwland R, Pablo RF, Sturk A, Smit JWA, Radder JK. Elevated Numbers of Tissue-Factor Exposing Microparticles Correlate With Components of the Metabolic Syndrome in Uncomplicated Type 2 Diabetes Mellitus. Circulation. 2002;106:2442–2447. doi: 10.1161/01.cir.0000036596.59665.c6. [DOI] [PubMed] [Google Scholar]
- 39.Beers EJ, van Schaap MCL, Berckmans RJ, Nieuwland R, Sturk A, Doormaal FF, van Meijers JCM, Biemond BJ. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94:1513–1519. doi: 10.3324/haematol.2009.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sellam J, Proulle V, Jüngel A, Ittah M, Miceli Richard C, Gottenberg J-E, Toti F, Benessiano J, Gay S, Freyssinet J-M, Mariette X. Increased levels of circulating microparticles in primary Sjögren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res. Ther. 2009;11:R156. doi: 10.1186/ar2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y, Brunet P, Dignat-George F. Elevation of circulating endothelial microparticles in patients with chronic renal failure. Journal of Thrombosis and Haemostasis. 2006;4:566–573. doi: 10.1111/j.1538-7836.2005.01780.x. [DOI] [PubMed] [Google Scholar]
- 42.Feng B, Chen Y, Luo Y, Chen M, Li X, Ni Y. Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis. 2010;208:264–269. doi: 10.1016/j.atherosclerosis.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 43.Toth B, Nikolajek K, Rank A, Nieuwland R, Lohse P, Pihusch V, Friese K, Thaler CJ. Gender-specific and menstrual cycle dependent differences in circulating microparticles†. Platelets. 2007;18:515–521. doi: 10.1080/09537100701525843. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland WHF, de Jong SA, Hessian PA, Williams MJA. Ingestion of native and thermally oxidized polyunsaturated fats acutely increases circulating numbers of endothelial microparticles. Metabolism. 2010;59:446–453. doi: 10.1016/j.metabol.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 45.Tushuizen ME, Nieuwland R, Rustemeijer C, Hensgens BE, Sturk A, Heine RJ, Diamant M. Elevated Endothelial Microparticles Following Consecutive Meals Are Associated With Vascular Endothelial Dysfunction in Type 2 Diabetes. Dia Care. 2007;30:728–730. doi: 10.2337/dc06-1473. [DOI] [PubMed] [Google Scholar]
- 46.Gordon C, Gudi K, Krause A, Sackrowitz R, Harvey B-G, Strulovici-Barel Y, Mezey JG, Crystal RG. Circulating Endothelial Microparticles as a Measure of Early Lung Destruction in Cigarette Smokers. Am. J. Respir. Crit. Care Med. 2011;184:224–232. doi: 10.1164/rccm.201012-2061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown MD, Feairheller DL, Thakkar S, Veerabhadrappa P, Park J-Y. Racial differences in tumor necrosis factor-α-induced endothelial microparticles and interleukin-6 production. Vasc Health Risk Manag. 2011;7:541–550. doi: 10.2147/VHRM.S22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 49.Bernal-Mizrachi L, Jy W, Jimenez JJ, Pastor J, Mauro LM, Horstman LL, de Marchena E, Ahn YS. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am. Heart J. 2003;145:962–970. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 50.Morel O, Jesel L, Hugel B, Douchet M-P, Zupan M, Chauvin M, Freyssinet J-M, Toti F. Protective effects of vitamin C on endothelium damage and platelet activation during myocardial infarction in patients with sustained generation of circulating microparticles. J. Thromb. Haemost. 2003;1:171–177. doi: 10.1046/j.1538-7836.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 51.Mayr M, Grainger D, Mayr U, Leroyer AS, Leseche G, Sidibe A, Herbin O, Yin X, Gomes A, Madhu B, Griffiths JR, Xu Q, Tedgui A, Boulanger CM. Proteomics, Metabolomics, and Immunomics on Microparticles Derived From Human Atherosclerotic Plaques CLINICAL PERSPECTIVE. Circ Cardiovasc Genet. 2009;2:379–388. doi: 10.1161/CIRCGENETICS.108.842849. [DOI] [PubMed] [Google Scholar]
- 52.Helal O, Defoort C, Robert S, Marin C, Lesavre N, Lopez-Miranda J, Risérus U, Basu S, Lovegrove J, McMonagle J, Roche HM, Dignat-George F, Lairon D. Increased levels of microparticles originating from endothelial cells, platelets and erythrocytes in subjects with metabolic syndrome: relationship with oxidative stress. Nutr Metab Cardiovasc Dis. 2011;21:665–671. doi: 10.1016/j.numecd.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Meziani F, Tesse A, David E, Martinez MC, Wangesteen R, Schneider F, Andriantsitohaina R. Shed membrane particles from preeclamptic women generate vascular wall inflammation and blunt vascular contractility. Am. J. Pathol. 2006;169:1473–1483. doi: 10.2353/ajpath.2006.051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rak J. Microparticles in cancer. Semin. Thromb. Hemost. 2010;36:888–906. doi: 10.1055/s-0030-1267043. [DOI] [PubMed] [Google Scholar]
- 55.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int. J. Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 56.Varon D, Shai E. Role of platelet-derived microparticles in angiogenesis and tumor progression. Discov Med. 2009;8:237–241. [PubMed] [Google Scholar]
- 57.Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp. Hematol. 2002;30:450–459. doi: 10.1016/s0301-472x(02)00791-9. [DOI] [PubMed] [Google Scholar]
- 58.Pauling L, Itano HA, Singer SJ, Wells Ibert C. Sickle Cell Anemia, A Molecular Disease. Science. 1949;110:543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 59.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 60.Donadee C, Raat NJH, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric Oxide Scavenging by Red Blood Cell Microparticles and Cell-Free Hemoglobin as a Mechanism for the Red Cell Storage Lesion. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herkert O, Djordjevic T, BelAiba RS, Görlach A. Insights into the redox control of blood coagulation: role of vascular NADPH oxidase-derived reactive oxygen species in the thrombogenic cycle. Antioxid. Redox Signal. 2004;6:765–776. doi: 10.1089/1523086041361695. [DOI] [PubMed] [Google Scholar]
- 62.King SB. Nitric oxide production from hydroxyurea. Free Radical Biology and Medicine. 2004;37:737–744. doi: 10.1016/j.freeradbiomed.2004.02.073. [DOI] [PubMed] [Google Scholar]
- 63.Nébor D, Romana M, Santiago R, Vachiery N, Picot J, Broquere C, Chaar V, Doumdo L, Odièvre M-H, Benkerrou M, Elion J. Fetal hemoglobin and hydroxycarbamide modulate both plasma concentration and cellular origin of circulating microparticles in sickle cell anemia children. Haematologica. 2013 doi: 10.3324/haematol.2012.073619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Da Silva EFR, Fonseca FAH, França CN, Ferreira PRA, Izar MCO, Salomão R, Camargo LM, Tenore SB, Lewi DS. Imbalance between endothelial progenitors cells and microparticles in HIV-infected patients naive for antiretroviral therapy. AIDS. 2011;25:1595–1601. doi: 10.1097/QAD.0b013e32834980f4. [DOI] [PubMed] [Google Scholar]
- 65.Holme PA, Müller F, Solum NO, Brosstad F, Frøland SS, Aukrust P. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEBJ. 1998;12:79–90. doi: 10.1096/fasebj.12.1.79. [DOI] [PubMed] [Google Scholar]
- 66.Krishnamoorthy L, Bess JW, Preston AB, Nagashima K, Mahal LK. HIV-1 and microvesicles from T cells share a common glycome, arguing for a common origin. Nat Chem Biol. 2009;5:244–250. doi: 10.1038/nchembio.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raymond AD, Campbell-Sims TC, Khan M, Lang M, Huang MB, Bond VC, Powell MD. HIV Type 1 Nef is released from infected cells in CD45(+) microvesicles and is present in the plasma of HIV-infected individuals. AIDS Res. Hum. Retroviruses. 2011;27:167–178. doi: 10.1089/aid.2009.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olivetta E, Pietraforte D, Schiavoni I, Minetti M, Federico M, Sanchez M. HIV-1 Nef regulates the release of superoxide anions from human macrophages. Biochem J. 2005;390:591–602. doi: 10.1042/BJ20042139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nantakomol D, Dondorp AM, Krudsood S, Udomsangpetch R, Pattanapanyasat K, Combes V, Grau GE, White NJ, Viriyavejakul P, Day NPJ, Chotivanich K. Circulating red cell-derived microparticles in human malaria. J. Infect. Dis. 2011;203:700–706. doi: 10.1093/infdis/jiq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pankoui Mfonkeu JB, Gouado I, Fotso Kuaté H, Zambou O, Amvam Zollo PH, Grau GER, Combes V. Elevated cell-specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS ONE. 2010;5:e13415. doi: 10.1371/journal.pone.0013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.György B, Szabó TG, Turiák L, Wright M, Herczeg P, Lédeczi Z, Kittel A, Polgár A, Tóth K, Dérfalvi B, Zelenák G, Böröcz I, Carr B, Nagy G, Vékey K, Gay S, Falus A, Buzás EI. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS ONE. 2012;7:e49726. doi: 10.1371/journal.pone.0049726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim MS, Yang Y-M, Son A, Tian YS, Lee S-I, Kang SW, Muallem S, Shin DM. RANKL-mediated Reactive Oxygen Species Pathway That Induces Long Lasting Ca2+ Oscillations Essential for Osteoclastogenesis. J. Biol. Chem. 2010;285:6913–6921. doi: 10.1074/jbc.M109.051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wehman AM, Poggioli C, Schweinsberg P, Grant BD, Nance J. The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Curr. Biol. 2011;21:1951–1959. doi: 10.1016/j.cub.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Comfurius P, Senden JMG, Tilly RHJ, Schroit AJ, Bevers EM, Zwaal RFA. Loss of membrane phospholipid asymmetry in platelets and red cells may be associated with calcium-induced shedding of plasma membrane and inhibition of aminophospholipid translocase. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1990;1026:153–160. doi: 10.1016/0005-2736(90)90058-v. [DOI] [PubMed] [Google Scholar]
- 75.Ding J, Wu Z, Crider BP, Ma Y, Li X, Slaughter C, Gong L, Xie XS. Identification and functional expression of four isoforms of ATPase II, the putative aminophospholipid translocase. Effect of isoform variation on the ATPase activity and phospholipid specificity. J. Biol. Chem. 2000;275:23378–23386. doi: 10.1074/jbc.M910319199. [DOI] [PubMed] [Google Scholar]
- 76.Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM. Appearance of Phosphatidylserine on Apoptotic Cells Requires Calcium-mediated Nonspecific Flip-Flop and Is Enhanced by Loss of the Aminophospholipid Translocase. J. Biol. Chem. 1997;272:26159–26165. doi: 10.1074/jbc.272.42.26159. [DOI] [PubMed] [Google Scholar]
- 77.Morel O, Toti F, Jesel L, Freyssinet J-M. Mechanisms of Microparticle Generation: On the Trail of the Mitochondrion. Seminars in Thrombosis and Hemostasis. 2010;36:833–844. doi: 10.1055/s-0030-1267037. [DOI] [PubMed] [Google Scholar]
- 78.Morrot G, Zachowski A, Devaux PF. Partial purification and characterization of the human erythrocyte Mg2+ -ATPase A candidate aminophospholipid translocase. FEBS Letters. 1990;266:29–32. doi: 10.1016/0014-5793(90)81498-d. [DOI] [PubMed] [Google Scholar]
- 79.Morris RJ. Ionic control of the metastable inner leaflet of the plasma membrane: Fusions natural and artefactual. FEBS Lett. 2010;584:1665–1669. doi: 10.1016/j.febslet.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 80.Heuvingh J, Bonneau S. Asymmetric Oxidation of Giant Vesicles Triggers Curvature-Associated Shape Transition and Permeabilization. Biophys J. 2009;97:2904–2912. doi: 10.1016/j.bpj.2009.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nomura S. Function and Clinical Significance of Platelet-Derived Microparticles. International Journal of Hematology. 2001;74:397–404. doi: 10.1007/BF02982082. [DOI] [PubMed] [Google Scholar]
- 82.Pasquet J-M, Dachary-Prigent J, Nurden AT. Calcium Influx is a Determining Factor of Calpain Activation and Microparticle Formation in Platelets. European Journal of Biochemistry. 1996;239:647–654. doi: 10.1111/j.1432-1033.1996.0647u.x. [DOI] [PubMed] [Google Scholar]
- 83.Guttmann RP, Johnson GVW. Oxidative Stress Inhibits Calpain Activity in Situ. J. Biol. Chem. 1998;273:13331–13338. doi: 10.1074/jbc.273.21.13331. [DOI] [PubMed] [Google Scholar]
- 84.Lhermusier T, Chap H, Payrastre B. Platelet membrane phospholipid asymmetry: from the characterization of a scramblase activity to the identification of an essential protein mutated in Scott syndrome. J. Thromb. Haemost. 2011;9:1883–1891. doi: 10.1111/j.1538-7836.2011.04478.x. [DOI] [PubMed] [Google Scholar]
- 85.Devaux PF, Herrmann A, Ohlwein N, Kozlov MM. How lipid flippases can modulate membrane structure. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2008;1778:1591–1600. doi: 10.1016/j.bbamem.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 86.Sims PJ, Wiedmer T, Esmon CT, Weiss HJ, Shattil SJ. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane. Studies in Scott syndrome: an isolated defect in platelet procoagulant activity. J. Biol. Chem. 1989;264:17049–17057. [PubMed] [Google Scholar]
- 87.Assinger A, Koller F, Schmid W, Zellner M, Babeluk R, Koller E, Volf I. Specific binding of hypochlorite-oxidized HDL to platelet CD36 triggers proinflammatory and procoagulant effects. Atherosclerosis. 2010;212:153–160. doi: 10.1016/j.atherosclerosis.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 88.Italiano JE, Mairuhu ATA, Flaumenhaft R. Clinical Relevance of Microparticles from Platelets and Megakaryocytes. CurrOpin Hematol. 2010;17:578–584. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perez-Pujol S, Marker PH, Key NS. Platelet microparticles are heterogeneous and highly dependent on the activation mechanism: studies using a new digital flow cytometer. Cytometry A. 2007;71:38–45. doi: 10.1002/cyto.a.20354. [DOI] [PubMed] [Google Scholar]
- 90.Dean WL, Lee MJ, Cummins TD, Schultz DJ, Powell DW. Proteomic and functional characterisation of platelet microparticle size classes. Thromb Haemost. 2009;102:711–718. doi: 10.1160/TH09-04-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janiszewski M, Do Carmo AO, Pedro MA, Silva E, Knobel E, Laurindo FRM. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: A novel vascular redox pathway. Crit. Care Med. 2004;32:818–825. doi: 10.1097/01.ccm.0000114829.17746.19. [DOI] [PubMed] [Google Scholar]
- 92.Gambim MH, do Carmo A, de O, Marti L, Veríssimo-Filho S, Lopes LR, Janiszewski M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care. 2007;11:R107. doi: 10.1186/cc6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barry OP, Pratico D, Lawson JA, FitzGerald GA. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J. Clin. Invest. 1997;99:2118–2127. doi: 10.1172/JCI119385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kalyankrishna S, Parmentier J-H, Malik KU. Arachidonic acid-derived oxidation products initiate apoptosis in vascular smooth muscle cells. Prostaglandins & Other Lipid Mediators. 2002;70:13–29. doi: 10.1016/s0090-6980(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 95.McIntyre TM. Bioactive oxidatively truncated phospholipids in inflammation and apoptosis: Formation, targets, and inactivation. Biochim. Biophys. Acta. 2012;1818:2456–2464. doi: 10.1016/j.bbamem.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iwamoto S, Kawasaki T, Kambayashi J, Ariyoshi H, Monden M. Platelet Microparticles: A Carrier of Platelet-Activating Factor? Biochemical and Biophysical Research Communications. 1996;218:940–944. doi: 10.1006/bbrc.1996.0166. [DOI] [PubMed] [Google Scholar]
- 97.Yost CC, Weyrich AS, Zimmerman GA. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie. 2010;92:692–697. doi: 10.1016/j.biochi.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.MacRitchie AN, Gardner AA, Prescott SM, Stafforini DM. Molecular basis for susceptibility of plasma platelet-activating factor acetylhydrolase to oxidative inactivation. FASEB J. 2007;21:1164–1176. doi: 10.1096/fj.06-6743com. [DOI] [PubMed] [Google Scholar]
- 99.Wang H, Wang Z-H, Kong J, Yang M-Y, Jiang G-H, Wang X-P, Zhong M, Zhang Y, Deng J-T, Zhang W. Oxidized Low-Density Lipoprotein-Dependent Platelet-Derived Microvesicles Trigger Procoagulant Effects and Amplify Oxidative Stress. Mol Med. 2011;18:159–166. doi: 10.2119/molmed.2011.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sprague DL, Elzey BD, Crist SA, Waldschmidt TJ, Jensen RJ, Ratliff TL. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood. 2008;111:5028–5036. doi: 10.1182/blood-2007-06-097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhogal RH, Weston CJ, Curbishley SM, Adams DH, Afford SC. Activation of CD40 with Platelet Derived CD154 Promotes Reactive Oxygen Species Dependent Death of Human Hepatocytes during Hypoxia and Reoxygenation. PLoS ONE. 2012;7:e30867. doi: 10.1371/journal.pone.0030867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shen H-M, Liu Z. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 103.Kim HK, Song KS, Chung J-H, Lee KR, Lee S-N. Platelet microparticles induce angiogenesis in vitro. Br. J. Haematol. 2004;124:376–384. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- 104.Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signal. 2007;9:731–739. doi: 10.1089/ars.2007.1556. [DOI] [PubMed] [Google Scholar]
- 105.Buhimschi IA, Kramer WB, Buhimschi CS, Thompson LP, Weiner CP. Reduction-oxidation (redox) state regulation of matrix metalloproteinase activity in human fetal membranes. Am. J. Obstet. Gynecol. 2000;182:458–464. doi: 10.1016/s0002-9378(00)70239-0. [DOI] [PubMed] [Google Scholar]
- 106.Wang J-M, Wang Y, Huang J-Y, Yang Z, Chen L, Wang L-C, Tang A-L, Lou Z-F, Tao J. C-Reactive protein-induced endothelial microparticle generation in HUVECs is related to BH4-dependent NO formation. J. Vasc. Res. 2007;44:241–248. doi: 10.1159/000100558. [DOI] [PubMed] [Google Scholar]
- 107.Simoncini S, Njock M-S, Robert S, Camoin-Jau L, Sampol J, Harlé J-R, Nguyen C, Dignat-George F, Anfosso F. TRAIL/Apo2L Mediates the Release of Procoagulant Endothelial Microparticles Induced by Thrombin In Vitro A Potential Mechanism Linking Inflammation and Coagulation. Circulation Research. 2009;104:943–951. doi: 10.1161/CIRCRESAHA.108.183285. [DOI] [PubMed] [Google Scholar]
- 108.Sapet C, Simoncini S, Loriod B, Puthier D, Sampol J, Nguyen C, Dignat-George F, Anfosso F. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood. 2006;108:1868–1876. doi: 10.1182/blood-2006-04-014175. [DOI] [PubMed] [Google Scholar]
- 109.Curtis AM, Wilkinson PF, Gui M, Gales TL, Hu E, Edelberg JM. p38 mitogen-activated protein kinase targets the production of proinflammatory endothelial microparticles. J. Thromb. Haemost. 2009;7:701–709. doi: 10.1111/j.1538-7836.2009.03304.x. [DOI] [PubMed] [Google Scholar]
- 110.Vion A, Birukova A, Boulanger C, Birukov K. Mechanical forces stimulate endothelial microparticle generation via caspase-dependent apoptosis-independent mechanism. Pulmonary Circulation. 2013;3:95–99. doi: 10.4103/2045-8932.109921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ferroni P, Basili S, Paoletti V, Davì G. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr Metab Cardiovasc Dis. 2006;16:222–233. doi: 10.1016/j.numecd.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 112.Mezentsev A, Merks RMH, O’Riordan E, Chen J, Mendelev N, Goligorsky MS, Brodsky SV. Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H1106–H1114. doi: 10.1152/ajpheart.00265.2005. [DOI] [PubMed] [Google Scholar]
- 113.Brogan PA, Shah V, Brachet C, Harnden A, Mant D, Klein N, Dillon MJ. Endothelial and platelet microparticles in vasculitis of the young. Arthritis & Rheumatism. 2004;50:927–936. doi: 10.1002/art.20199. [DOI] [PubMed] [Google Scholar]
- 114.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of Severe Hypertension on Endothelial and Platelet Microparticles. Hypertension. 2003;41:211–217. doi: 10.1161/01.hyp.0000049760.15764.2d. [DOI] [PubMed] [Google Scholar]
- 115.Peterson DB, Sander T, Kaul S, Wakim BT, Halligan B, Twigger S, Pritchard KA, Jr., Oldham KT, Ou J-S. Comparative proteomic analysis of PAI-1 and TNF-alpha-derived endothelial microparticles. Proteomics. 2008;8:2430–2446. doi: 10.1002/pmic.200701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burger D, Kwart DG, Montezano AC, Read NC, Kennedy CRJ, Thompson CS, Touyz RM. Microparticles Induce Cell Cycle Arrest Through Redox-Sensitive Processes in Endothelial Cells: Implications in Vascular Senescence. J Am Heart Assoc. 2012;1 doi: 10.1161/JAHA.112.001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burger D, Montezano AC, Nishigaki N, He Y, Carter A, Touyz RM. Endothelial Microparticle Formation by Angiotensin II Is Mediated via Ang II Receptor Type I/NADPH Oxidase/ Rho Kinase Pathways Targeted to Lipid Rafts. Arterioscler Thromb Vasc Biol. 2011;31:1898–1907. doi: 10.1161/ATVBAHA.110.222703. [DOI] [PubMed] [Google Scholar]
- 118.Jansen F, Yang X, Franklin BS, Hoelscher M, Schmitz T, Bedorf J, Nickenig G, Werner N. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc Res. 2013;98:94–106. doi: 10.1093/cvr/cvt013. [DOI] [PubMed] [Google Scholar]
- 119.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HHHW, Busse R, Schröder K, Brandes RP. Apocynin Is Not an Inhibitor of Vascular NADPH Oxidases but an Antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 120.Kono H, Rusyn I, Uesugi T, Yamashina S, Connor HD, Dikalova A, Mason RP, Thurman RG. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1005–G1012. doi: 10.1152/ajpgi.2001.280.5.G1005. [DOI] [PubMed] [Google Scholar]
- 121.Leroyer AS, Ebrahimian TG, Cochain C, Récalde A, Blanc-Brude O, Mees B, Vilar J, Tedgui A, Levy BI, Chimini G, Boulanger CM, Silvestre J-S. Microparticles From Ischemic Muscle Promotes Postnatal Vasculogenesis. Circulation. 2009;119:2808–2817. doi: 10.1161/CIRCULATIONAHA.108.816710. [DOI] [PubMed] [Google Scholar]
- 122.Deregibus MC, Cantaluppi V, Calogero R, Iacono ML, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell-derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 123.Lewis MS, Whatley RE, Cain P, McIntyre TM, Prescott SM, Zimmerman GA. Hydrogen peroxide stimulates the synthesis of platelet-activating factor by endothelium and induces endothelial cell-dependent neutrophil adhesion. J Clin Invest. 1988;82:2045–2055. doi: 10.1172/JCI113825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 2002;160:673–680. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gauley J, Pisetsky DS. The release of microparticles by RAW 264.7 macrophage cells stimulated with TLR ligands. J Leukoc Biol. 2010;87:1115–1123. doi: 10.1189/jlb.0709465. [DOI] [PubMed] [Google Scholar]
- 126.Nolan S, Dixon R, Norman K, Hellewell P, Ridger V. Nitric Oxide Regulates Neutrophil Migration through Microparticle Formation. Am J Pathol. 2008;172:265–273. doi: 10.2353/ajpath.2008.070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mesri M, Altieri DC. Endothelial Cell Activation by Leukocyte Microparticles. J Immunol. 1998;161:4382–4387. [PubMed] [Google Scholar]
- 128.Gasser O, Hess C, Miot S, Deon C, Sanchez J-C, Schifferli JA. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 2003;285:243–257. doi: 10.1016/s0014-4827(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 129.Angelillo-Scherrer A. Leukocyte-Derived Microparticles in Vascular Homeostasis. Circulation Research. 2012;110:356–369. doi: 10.1161/CIRCRESAHA.110.233403. [DOI] [PubMed] [Google Scholar]
- 130.Eken C, Gasser O, Zenhaeusern G, Oehri I, Hess C, Schifferli JA. Polymorphonuclear Neutrophil-Derived Ectosomes Interfere with the Maturation of Monocyte-Derived Dendritic Cells. J Immunol. 2008;180:817–824. doi: 10.4049/jimmunol.180.2.817. [DOI] [PubMed] [Google Scholar]
- 131.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate antiinflammatory microparticles by ectocytosis. Blood. 2004;104:2543–2548. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 132.Miguet L, Pacaud K, Felden C, Hugel B, Martinez MC, Freyssinet J-M, Herbrecht R, Potier N, van Dorsselaer A, Mauvieux L. Proteomic analysis of malignant lymphocyte membrane microparticles using double ionization coverage optimization. PROTEOMICS. 2006;6:153–171. doi: 10.1002/pmic.200500133. [DOI] [PubMed] [Google Scholar]
- 133.Yang C, Mwaikambo BR, Zhu T, Gagnon C, Lafleur J, Seshadri S, Lachapelle P, Lavoie J-C, Chemtob S, Hardy P. Lymphocytic microparticles inhibit angiogenesis by stimulating oxidative stress and negatively regulating VEGF-induced pathways. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R467–R476. doi: 10.1152/ajpregu.00432.2007. [DOI] [PubMed] [Google Scholar]
- 134.Mostefai HA, Agouni A, Carusio N, Mastronardi ML, Heymes C, Henrion D, Andriantsitohaina R, Martinez MC. Phosphatidylinositol 3-kinase and xanthine oxidase regulate nitric oxide and reactive oxygen species productions by apoptotic lymphocyte microparticles in endothelial cells. J. Immunol. 2008;180:5028–5035. doi: 10.4049/jimmunol.180.7.5028. [DOI] [PubMed] [Google Scholar]
- 135.Agouni A, Mostefai HA, Porro C, Carusio N, Favre J, Richard V, Henrion D, Martínez MC, Andriantsitohaina R. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J. 2007;21:2735–2741. doi: 10.1096/fj.07-8079com. [DOI] [PubMed] [Google Scholar]
- 136.Soleti R, Benameur T, Porro C, Panaro MA, Andriantsitohaina R, Martínez MC. Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis. 2009;30:580–588. doi: 10.1093/carcin/bgp030. [DOI] [PubMed] [Google Scholar]
- 137.Benameur T, Soleti R, Porro C, Andriantsitohaina R, Martínez MC. Microparticles Carrying Sonic Hedgehog Favor Neovascularization through the Activation of Nitric Oxide Pathway in Mice. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martin S, Tesse A, Hugel B, Martínez MC, Morel O, Freyssinet J-M, Andriantsitohaina R. Shed Membrane Particles From T Lymphocytes Impair Endothelial Function and Regulate Endothelial Protein Expression. Circulation. 2004;109:1653–1659. doi: 10.1161/01.CIR.0000124065.31211.6E. [DOI] [PubMed] [Google Scholar]
- 139.Distler JHW, Jüngel A, Huber LC, Seemayer CA, Reich CF, Gay RE, Michel BA, Fontana A, Gay S, Pisetsky DS, Distler O. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. PNAS. 2005;102:2892–2897. doi: 10.1073/pnas.0409781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bernimoulin M, Waters EK, Foy M, Steele BM, Sullivan M, Falet H, Walsh MT, Barteneva N, Geng J-G, Hartwig JH, Maguire PB, Wagner DD. Differential stimulation of monocytic cells results in distinct populations of microparticles. Journal of Thrombosis and Haemostasis. 2009;7:1019–1028. doi: 10.1111/j.1538-7836.2009.03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Essayagh S, Xuereb J-M, Terrisse A-D, Tellier-Cirioni L, Pipy B, Sié P. Microparticles from apoptotic monocytes induce transient platelet recruitment and tissue factor expression by cultured human vascular endothelial cells via a redox-sensitive mechanism. Thromb. Haemost. 2007;98:831–837. [PubMed] [Google Scholar]
- 142.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 143.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, Coomber BL, Mackman N, Rak JW. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–1741. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 144.Yu X, Harris SL, Levine AJ. The Regulation of Exosome Secretion: a Novel Function of the p53 Protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 145.Maillet A, Pervaiz S. Redox regulation of p53, redox effectors regulated by p53: a subtle balance. Antioxid. Redox Signal. 2012;16:1285–1294. doi: 10.1089/ars.2011.4434. [DOI] [PubMed] [Google Scholar]
- 146.Truong TH, Carroll KS. Redox Regulation of Epidermal Growth Factor Receptor Signaling through Cysteine Oxidation. Biochemistry. 2012;51:9954–9965. doi: 10.1021/bi301441e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Trachootham D, Lu W, Ogasawara MA, Valle NR-D, Huang P. Redox Regulation of Cell Survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Di Vizio D, Kim J, Hager MH, Morello M, Yang W, Lafargue CJ, True LD, Rubin MA, Adam RM, Beroukhim R, Demichelis F, Freeman MR. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 2009;69:5601–5609. doi: 10.1158/0008-5472.CAN-08-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Skog J, Wurdinger T, van Rijn S, Meijer D, Gainche L, Sena-Esteves M, Curry WT, Carter RS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 151.Albanese J, Meterissian S, Kontogiannea M, Dubreuil C, Hand A, Sorba S, Dainiak N. Biologically Active Fas Antigen and Its Cognate Ligand Are Expressed on Plasma Membrane-Derived Extracellular Vesicles. Blood. 1998;91:3862–3874. [PubMed] [Google Scholar]
- 152.Castellana D, Zobairi F, Martinez MC, Panaro MA, Mitolo V, Freyssinet J-M, Kunzelmann C. Membrane Microvesicles as Actors in the Establishment of a Favorable Prostatic Tumoral Niche: A Role for Activated Fibroblasts and CX3CL1-CX3CR1 Axis. Cancer Res. 2009;69:785–793. doi: 10.1158/0008-5472.CAN-08-1946. [DOI] [PubMed] [Google Scholar]
- 153.Llorente-Cortés V, Otero-Viñas M, Camino-López S, Llampayas O, Badimon L. Aggregated Low-Density Lipoprotein Uptake Induces Membrane Tissue Factor Procoagulant Activity and Microparticle Release in Human Vascular Smooth Muscle Cells. Circulation. 2004;110:452–459. doi: 10.1161/01.CIR.0000136032.40666.3D. [DOI] [PubMed] [Google Scholar]
- 154.Wagner GM, Chiu DT, Yee MC, Lubin BH. Red cell vesiculation-a common membrane physiologic event. J. Lab. Clin. Med. 1986;108:315–324. [PubMed] [Google Scholar]
- 155.Yamaguchi T, Yamada S, Kimoto E. Effects of cross-linking of membrane proteins on vesiculation induced by dimyristoylphosphatidylcholine in human erythrocytes. J. Biochem. 1994;115:659–663. doi: 10.1093/oxfordjournals.jbchem.a124392. [DOI] [PubMed] [Google Scholar]
- 156.Arese P, Turrini F, Schwarzer E. Band 3/complement-mediated recognition and removal of normally senescent and pathological human erythrocytes. Cell. Physiol. Biochem. 2005;16:133–146. doi: 10.1159/000089839. [DOI] [PubMed] [Google Scholar]
- 157.Willekens FLA, Werre JM, Kruijt JK, Roerdinkholder-Stoelwinder B, Groenen-Döpp YAM, van den Bos AG, Bosman GJCGM, van Berkel TJC. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood. 2005;105:2141–2145. doi: 10.1182/blood-2004-04-1578. [DOI] [PubMed] [Google Scholar]
- 158.Balagopalakrishna C, Manoharan PT, Abugo OO, Rifkind JM. Production of Superoxide from Hemoglobin-Bound Oxygen Under Hypoxic Conditions. Biochemistry. 1996;35:6393–6398. doi: 10.1021/bi952875+. [DOI] [PubMed] [Google Scholar]
- 159.Wagner GM, Chiu DT, Qju JH, Heath RH, Lubin BH. Spectrin oxidation correlates with membrane vesiculation in stored RBCs. Blood. 1987;69:1777–1781. [PubMed] [Google Scholar]
- 160.Szostek R, Kucuk O, Lis LJ, Tracy D, Mata R, Dey T, Kauffman JW, Yachnin S, Westerman MP. Effect of inserted oxysterols on phospholipid packing in normal and sickle red blood cell membranes. Biochemical and Biophysical Research Communications. 1991;180:730–734. doi: 10.1016/s0006-291x(05)81126-x. [DOI] [PubMed] [Google Scholar]
- 161.Jain SK. The accumulation of malonyldialdehyde, a product of fatty acid peroxidation, can disturb aminophospholipid organization in the membrane bilayer of human erythrocytes. J. Biol. Chem. 1984;259:3391–3394. [PubMed] [Google Scholar]
- 162.Ferru E, Giger K, Pantaleo A, Campanella E, Grey J, Ritchie K, Vono R, Turrini F, Low PS. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood. 2011;117:5998–6006. doi: 10.1182/blood-2010-11-317024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jain SK. In vivo externalization of phosphatidylserine and phosphatidylethanolamine in the membrane bilayer and hypercoagulability by the lipid peroxidation of erythrocytes in rats. J Clin Invest. 1985;76:281–286. doi: 10.1172/JCI111958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lim K-M, Kim S, Noh J-Y, Kim K, Jang W-H, Bae O-N, Chung S-M, Chung J-H. Low-level mercury can enhance procoagulant activity of erythrocytes: a new contributing factor for mercury-related thrombotic disease. Environ. Health Perspect. 2010;118:928–935. doi: 10.1289/ehp.0901473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Low PS, Waugh SM, Zinke K, Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985;227:531–533. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- 166.Mohandas N, Groner W. Cell membrane and volume changes during red cell development and aging. Ann. N. Y. Acad. Sci. 1989;554:217–224. doi: 10.1111/j.1749-6632.1989.tb22423.x. [DOI] [PubMed] [Google Scholar]
- 167.Dumaswala UJ, Greenwalt TJ. Human erythrocytes shed exocytic vesicles in vivow. Transfusion. 1984;24:490–492. doi: 10.1046/j.1537-2995.1984.24685066807.x. [DOI] [PubMed] [Google Scholar]
- 168.Greenwalt TJ. The how and why of exocytic vesicles. Transfusion. 2006;46:143–152. doi: 10.1111/j.1537-2995.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 169.Greenwalt TJ, Sostok CZ, Dumaswala UJ. Studies in Red Blood Cell Preservation 2. Comparison of Vesicle Formation, Morphology, and Membrane Lipids during Storage in AS-1 and CPDA-1. Vox Sanguinis. 1990;58:90–93. doi: 10.1111/j.1423-0410.1990.tb02068.x. [DOI] [PubMed] [Google Scholar]
- 170.Vlaar APJ, Hofstra JJ, Levi M, Kulik W, Nieuwland R, Tool ATJ, Schultz MJ, de Korte D, Juffermans NP. Supernatant of aged erythrocytes causes lung inflammation and coagulopathy in a “two-hit” in vivo syngeneic transfusion model. Anesthesiology. 2010;113:92–103. doi: 10.1097/ALN.0b013e3181de6f25. [DOI] [PubMed] [Google Scholar]
- 171.Tissot J-D, Rubin O, Canellini G. Analysis and clinical relevance of microparticles from red blood cells. Curr. Opin. Hematol. 2010;17:571–577. doi: 10.1097/moh.0b013e32833ec217. [DOI] [PubMed] [Google Scholar]
- 172.Bosman GJCGM, Lasonder E, Luten M, Roerdinkholder-Stoelwinder B, Novotný VMJ, Bos H, De Grip WJ. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–835. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 173.Bian K, Murad F. Nitric oxide signaling in vascular biology. Journal of the American Society of Hypertension. 2007;1:17–29. doi: 10.1016/j.jash.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 174.Jy W, Ricci M, Shariatmadar S, Gomez-Marin O, Horstman LH, Ahn YS. Microparticles in stored red blood cells as potential mediators of transfusion complications. Transfusion. 2011;51:886–893. doi: 10.1111/j.1537-2995.2011.03099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nantakomol D, Palasuwan A, Chaowanathikhom M, Soogarun S, Imwong M. Red cell and platelet-derived microparticles are increased in G6PD-deficient subjects. Eur. J. Haematol. 2012;89:423–429. doi: 10.1111/ejh.12010. [DOI] [PubMed] [Google Scholar]
- 176.Pantaleo A, Ferru E, Giribaldi G, Mannu F, Carta F, Matte A, de Franceschi L, Turrini F. Oxidized and poorly glycosylated band 3 is selectively phosphorylated by Syk kinase to form large membrane clusters in normal and G6PD-deficient red blood cells. Biochemical Journal. 2009;418:359. doi: 10.1042/BJ20081557. [DOI] [PubMed] [Google Scholar]
- 177.Pantaleo A, Ferru E, Carta F, Mannu F, Simula LF, Khadjavi A, Pippia P, Turrini F. Irreversible AE1 Tyrosine Phosphorylation Leads to Membrane Vesiculation in G6PD Deficient Red Cells. PLoS ONE. 2011;6:e15847. doi: 10.1371/journal.pone.0015847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Melhorn DK, Gross S, Lake GA, Leu JA. The Hydrogen Peroxide Fragility Test and Serum Tocopherol Level in Anemias of Various Etiologies. Blood. 1971;37:438–446. [PubMed] [Google Scholar]
- 179.Allan D, Limbrick AR, Thomas P, Westerman MP. Release of spectrin-free spicules on reoxygenation of sickled erythrocytes. Nature. 1982;295:612–613. doi: 10.1038/295612a0. [DOI] [PubMed] [Google Scholar]
- 180.Westerman MP, Cole ER, Wu K. The effect of spicules obtained from sickle red cells on clotting activity. British Journal of Haematology. 1984;56:557–562. doi: 10.1111/j.1365-2141.1984.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 181.Comporti M, Signorini C, Buonocore G, Ciccoli L. Iron release, oxidative stress and erythrocyte ageing. Free Radical Biology and Medicine. 2002;32:568–576. doi: 10.1016/s0891-5849(02)00759-1. [DOI] [PubMed] [Google Scholar]
- 182.Westerman M, Pizzey A, Hirschman J, Cerino M, Weil-Weiner Y, Ramotar P, Eze A, Lawrie A, Purdy G, Mackie I, Porter J. Microvesicles in haemoglobinopathies offer insights into mechanisms of hypercoagulability, haemolysis and the effects of therapy. British Journal of Haematology. 2008;142:126–135. doi: 10.1111/j.1365-2141.2008.07155.x. [DOI] [PubMed] [Google Scholar]
- 183.Ferru E, Pantaleo A, Carta F, Mannu F, Khadjavi A, Gallo V, Ronzoni L, Graziadei G, Cappellini MD, Turrini F. Thalassemic erythrocytes release microparticles loaded with hemichromes by redox activation of p72Syk kinase. Haematologica. 2013 doi: 10.3324/haematol.2013.084533. haematol.2013.084533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Percário S, Moreira DR, Gomes BAQ, Ferreira MES, Gonçalves ACM, Laurindo PSOC, Vilhena TC, Dolabela MF, Green MD. Oxidative stress in malaria. Int J Mol Sci. 2012;13:16346–16372. doi: 10.3390/ijms131216346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pantaleo A, Ferru E, Carta F, Mannu F, Giribaldi G, Vono R, Lepedda AJ, Pippia P, Turrini F. Analysis of changes in tyrosine and serine phosphorylation of red cell membrane proteins induced by P. falciparum growth. PROTEOMICS. 2010;10:3469–3479. doi: 10.1002/pmic.201000269. [DOI] [PubMed] [Google Scholar]
- 186.Pantaleo A, Ferru E, Vono R, Giribaldi G, Lobina O, Nepveu F, Ibrahim H, Nallet J-P, Carta F, Mannu F, Pippia P, Campanella E, Low PS, Turrini F. New antimalarial indolone-N-oxides, generating radical species, destabilize the host cell membrane at early stages of Plasmodium falciparum growth: role of band 3 tyrosine phosphorylation. Free Radical Biology and Medicine. 2012;52:527–536. doi: 10.1016/j.freeradbiomed.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Camus SM, Gausserès B, Bonnin P, Loufrani L, Grimaud L, Charue D, Moraes JAD, Renard J-M, Tedgui A, Boulanger CM, Tharaux P-L, Blanc-Brude OP. Erythrocyte microparticles can induce kidney vaso-occlusions in a murine model of sickle cell disease. Blood. 2012;120:5050–5058. doi: 10.1182/blood-2012-02-413138. [DOI] [PubMed] [Google Scholar]
- 188.Frei AC, Guo Y, Jones DW, Pritchard KA, Jr., Fagan KA, Hogg N, Wandersee NJ. Vascular dysfunction in a murine model of severe hemolysis. Blood. 2008;112:398–405. doi: 10.1182/blood-2007-12-126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B. Tumor-Derived Tissue Factor-Bearing Microparticles are Associated with Venous Thromboembolic Events in Malignancy. Clin Cancer Res. 2009;15:6830–6840. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zwicker JI, Liebman HA, Bauer KA, Caughey T, Campigotto F, Rosovsky R, Mantha S, Kessler CM, Eneman J, Raghavan V, Lenz H-J, Bullock A, Buchbinder E, Neuberg D, Furie B. Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor-bearing microparticles: a randomized-controlled phase II trial (the Microtec study) British Journal of Haematology. 2013;160:530–537. doi: 10.1111/bjh.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Duffy MJ. Serum Tumor Markers in Breast Cancer: Are They of Clinical Value? Clinical Chemistry. 2006;52:345–351. doi: 10.1373/clinchem.2005.059832. [DOI] [PubMed] [Google Scholar]
- 192.Heleno B, Thomsen MF, Rodrigues DS, Jorgensen KJ, Brodersen J. Quantification of harms in cancer screening trials: literature review. BMJ. 2013;347:f5334–f5334. doi: 10.1136/bmj.f5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cholette JM, Henrichs KF, Alfieris GM, Powers KS, Phipps R, Spinelli SL, Swartz M, Gensini F, Daugherty LE, Nazarian E, Rubenstein JS, Sweeney D, Eaton M, Lerner NB, Blumberg N. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: results of a prospective, randomized, controlled clinical trial. Pediatr Crit Care Med. 2012;13:290–299. doi: 10.1097/PCC.0b013e31822f173c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Corrales-Medina VF, Simkins J, Chirinos JA, Serpa JA, Horstman LL, Jy W, Ahn Y-S. Increased levels of platelet microparticles in HIV-infected patients with good response to highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2010;54:217–218. doi: 10.1097/QAI.0b013e3181c8f4c9. [DOI] [PubMed] [Google Scholar]
- 195.Zucker-Franklin D, Karpatkin S. Red-Cell and Platelet Fragmentation in Idiopathic Autoimmune Thrombocytopenic Purpura. New England Journal of Medicine. 1977;297:517–523. doi: 10.1056/NEJM197709082971001. [DOI] [PubMed] [Google Scholar]
- 196.Mrvar-Brečko A, Šuštar V, Janša V, Štukelj R, Janša R, Mujagić E, Kruljc P, Iglič A, Hägerstrand H, Kralj-Iglič V. Isolated microvesicles from peripheral blood and body fluids as observed by scanning electron microscope. Blood Cells, Molecules, and Diseases. 2010;44:307–312. doi: 10.1016/j.bcmd.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 197.Rubin O, Crettaz D, Canellini G, Tissot J-D, Lion N. Microparticles in stored red blood cells: an approach using flow cytometry and proteomic tools. Vox Sanguinis. 2008;95:288–297. doi: 10.1111/j.1423-0410.2008.01101.x. [DOI] [PubMed] [Google Scholar]