Abstract
Rationale
A chromosomal haplotype producing cardiac overexpression of dipeptidyl peptidase-like protein-6 (DPP6) causes familial idiopathic ventricular fibrillation. The molecular basis of transient outward current (Ito) in Purkinje fibers (PFs) is poorly understood. We hypothesized that DPP6 contributes to PF Ito and that its overexpression might specifically alter PF Ito properties and repolarization.
Objective
To assess the potential role of DPP6 in PF Ito.
Methods and Results
Clinical data in 5 idiopathic ventricular fibrillation patients suggested arrhythmia origin in the PF-conducting system. PF and ventricular muscle Ito had similar density, but PF Ito differed from ventricular muscle in having tetraethylammonium sensitivity and slower recovery. DPP6 overexpression significantly increased, whereas DPP6 knockdown reduced, Ito density and tetraethylammonium sensitivity in canine PF but not in ventricular muscle cells. The K+-channel interacting β-subunit K+-channel interacting protein type-2, essential for normal expression of Ito in ventricular muscle, was weakly expressed in human PFs, whereas DPP6 and frequenin (neuronal calcium sensor-1) were enriched. Heterologous expression of Kv4.3 in Chinese hamster ovary cells produced small Ito; Ito amplitude was greatly enhanced by coexpression with K+-channel interacting protein type-2 or DPP6. Coexpression of DPP6 with Kv4.3 and K+-channel interacting protein type-2 failed to alter Ito compared with Kv4.3/K+-channel interacting protein type-2 alone, but DPP6 expression with Kv4.3 and neuronal calcium sensor-1 (to mimic PF Ito composition) greatly enhanced Ito compared with Kv4.3/neuronal calcium sensor-1 and recapitulated characteristic PF kinetic/pharmacological properties. A mathematical model of cardiac PF action potentials showed that Ito enhancement can greatly accelerate PF repolarization.
Conclusions
These results point to a previously unknown central role of DPP6 in PF Ito, with DPP6 gain of function selectively enhancing PF current, and suggest that a DPP6-mediated PF early-repolarization syndrome might be a novel molecular paradigm for some forms of idiopathic ventricular fibrillation.
Keywords: cardiac arrhythmia mechanisms, ECG, genetic arrhythmia syndromes, molecular electrophysiology, potassium channels, sudden death, ventricular tachycardia arrhythmia
Sudden cardiac death accounts for 300 000 deaths annually in North America.1,2 Almost 10% of patients with sudden cardiac death lack identifiable heart disease, manifesting so-called idiopathic ventricular fibrillation (IVF).3 The mechanisms underlying most IVF are unknown. Recently, a genome-wide haplotype-sharing analysis of Dutch families with IVF identified a founder haplotype on chromosome 7 (7q36), harboring the proximal and upstream sequences of the DPP6 gene as the genetic basis.4 IVF patients with this haplotype had markedly (~20-fold) increased cardiac tissue levels of DPP6 mRNA.4
Dipeptidyl aminopeptidase-like protein-6 (DPP6), a member of the dipeptidyl aminopeptidase family lacking enzymatic activity, is a potential β-subunit for neuronal A-type currents5,6 and cardiac transient outward potassium current (Ito)7 encoded by Kv4.x-subunits. DPP6 modulates trafficking, kinetics, and pharmacology of Kv4.x-encoded channels.6–8 Ito underlies the early-repolarization phase of cardiac action potentials (APs)9 and shows marked transmural variability.10 Alterations in myocardial Ito expression play important roles in cardiac arrhythmias11 and have been implicated in Brugada syndrome, although precise mechanisms remain controversial.12
Cardiac Purkinje fibers (PFs) form a specialized conducting system, with unique properties and important roles in cardiac physiology and arrhythmia generation.13 There are major differences in Ito between ventricular muscle (VM) and PFs in many species, including humans.13,14 PF Ito typically shows slower inactivation and much greater tetraethylammonium (TEA) sensitivity than VM.13,14 The molecular mechanisms underlying these differences are unclear. The β-subunit K+-channel interacting protein type-2 (KChIP2), which plays a crucial role in VM Ito, is weakly expressed in PF.15 In addition to KChIP2 and DPP6, other potential Kv4.2/4.3-interacting subunits include neuronal calcium sensor-1 (NCS-1) and KCNE1–5,16–19 many of which are expressed in the heart.20 DPP6 is known to confer TEA sensitivity on Kv4.x-subunit– based channels on heterologous expression.8 We hypothesized that DPP6 may contribute to PF Ito and that its overexpression might alter PF Ito properties in a way that contributes to IVF occurrence. The present study was designed (1) to assess the expression of various β-subunits in human VM and PF tissue, (2) to evaluate the result of DPP6 overexpression and knockdown by adenoviral gene transfer on VM and PF Ito, and (3) to study the properties of Ito resulting from heterologous coexpression of different combinations of putative β-subunits relevant to composition in human VM and PF. Our results point to an important contribution of DPP6 to PF Ito and implicate enhanced PF Ito as a novel candidate mechanism for IVF.
Methods
For further details, see the online Data Supplement.
Clinical Assessment of IVF Patients
Five IVF patients with confirmed 7q36 DPP6–associated haplotype were studied. Baseline clinical data were obtained for all. One patient underwent invasive electrophysiological study and long-term follow-up.
Human Cardiac Tissue Samples
Hearts from 15 nondiseased donors (8 for mRNA and 7 for protein extraction) were stored in cardioplegic solution at 4°C. PF false tendons, left ventricular (LV) epicardium, midmyocardium, and endocardium were dissected and snap-frozen in liquid N2.
mRNA Quantifcation
RNA was extracted with TRIzol, chlorofiorm extraction, and isopropanol precipitation. Genomic DNA was eliminated with DNase I, followed by phenol-chlorofiorm acid. First-strand cDNA was synthesized by reverse transcription with 1 µg RNA, random primers, and moloney murine leukemia virus reverse transcriptase. Real-time polymerase chain reaction was conducted (primers are given in Online Table I) with SYBR green. 18S rRNA was the internal standard. mRNA was quantified with comparative threshold cycle quantification (ΔΔCt).
Immunoblotting
Membrane protein fractions were run on 10% SDS-PAGE gels and transferred to polyvinylidene fluoride membranes. Blots were probed with primary antibodies against Kv4.3, KChIP2, NCS-1, DPP6, and GAPDH. Results were analyzed with Quantity-One, and data were normalized to GAPDH.
Native-Cell Isolation and Culture
Experiments were performed with canine cardiac tissues, which have relative VM and PF cell (PC) Ito properties similar to those of humans.13,14 Adult male mongrel dogs (19–30 kg, n=33) were anesthetized with pentobarbital (30 mg/kg IV). After excision of PF false tendons, the anterior LV (or in selected experiments, right ventricular [RV] free wall) was arterially perfused with Tyrode solution containing collagenase (120 U/mL, Worthington, type II). VM cardiomyocytes were isolated as previously described.21 PCs were obtained by digestion with elastase and collagenase (for details, see Online Methods). Isolated cells were either put in storage solution for study on the same day or placed in cell culture on laminin-coated glass coverslips. In some cases, attached PCs and VMs were subjected to adenovirus infection for 2 to 4 hours and incubated for 48 hours. After culture, PCs and VMs were washed and stored at 4°C for electrophysiological study.
Adenovirus Constructs
A bicistronic construct encoding triple FLAG-tagged DPP6 and green fluorescent protein (GFP) under control of the cytomegalovirus promoter was generated by inserting the cDNA into pShuttle-IRES-hrGFP-1 vector. The adenoviral vector containing DPP6 cDNA is designated Adv-GFP-DPP6, and the control vector containing only GFP is designated Adv-GFP-control.
DPP6 knockdown was obtained with a microRNA-embedded short-hairpin RNA (shRNA-mir57) sequence, targeted to the canine DPP6 mRNA (GeneBank identification, XM_532774). The shRNA-mir sequence was delivered into the cultured cells by a human adenovirus-based vector (Adv-GFP-DPP6-knockdown). A scrambled shRNA-mir carrying adenovirus was used as a negative control (Adv-GFP-Scr). KChIP2 knockdown virus and a scrambled control construct were prepared similarly. Adenovirus vector construction followed previous studies.22,23 For details, see online Data Supplement Methods. GFP expression was used to select cells with effective gene transfer.
Chinese Hamster Ovary Cell Culture and Transfection
Chinese hamster ovary (CHO) cells were cultured in F12-medium supplemented with bovine serum and penicillin/streptomycin. Transfection was performed with Lipofectamine 2000 and plasmid DNA encoding Kv4.3 or combinations of Kv4.3 with KChIP2b, DPP6, or NCS-1. Bicistronic vectors carrying DsRed, cyan fluorescence protein, and GFP were used to monitor gene transfer. Fluorescent cells were used for patch-clamp experiments within 1 to 2 days of transfection.
Immunoprecipitation Studies
Total proteins from CHO cells after 2-day transfections extracted with lysis buffer were fast-frozen and stored at −80°C. Immunoprecipitation was performed with a monoclonal anti-Kv4.3 antibody. Dynabeads M-280/sheep antimouse IgG were washed with PBS and preincubated with 1% BSA for 1 hour at room temperature to minimize nonspecific binding. Anti-Kv4.3 antibodies were incubated overnight at 4°C with 100 µL Dynabeads per sample. The anti-mouse IgG-coated beads were then washed 5 times with PBS and incubated overnight with 100 µg protein extracts from CHO cells, and supernatants were collected. The bead–antibody target protein complexes were washed and subjected to magnetic precipitation/resuspension. Bound Kv4.3 protein complexes were eluted and denatured.
Eluted proteins and supernatants were separated on 10% SDS-PAGE gel and transferred to polyvinylidene fluoride membranes. Blots were incubated overnight at 4°C with primary antibodies against Kv4.3, KChIP2, NCS-1, or DPP6. Protein bands were detected by chemiluminescence, and results were analyzed with Quantity-one software.
Confocal Microscopy
Two days after transfection, CHO cells were washed with PBS, then fixed with 2% paraformaldehyde, and washed 3 times with PBS. After blocking and permeabilization, cells were incubated overnight at 4°C with primary antibody against Kv4.3 in PBS containing 1% normal donkey serum and 0.05% Triton, followed by 3 washes and incubation with secondary antibody and wheatgerm agglutinin. Confocal microscopy was performed with a Zeiss LSM-710 system. Images were deconvolved using measured point-spread functions. Kv4.3 fluorescence densities were determined as the sum of the pixels within each region normalized to region area. Measurements were repeated in 5 Z stacks for each cell.
Electrophysiology
Whole-cell patch-clamp technique was applied for Ito recording at 36±0.5°C (native cells) or 22±0.5°C (CHO cells). Ito was always defined as the difference between peak and end-pulse current. Cell capacitances were not significantly different among groups (Online Table II). Recording solutions were as previously described14–16 (see online Data Supplement).
Data Analysis
Clampfit 9.0 (Axon) and GraphPad Prism 5.0 were used for basic data analysis. Real-time polymerase chain reaction results were analyzed with MXPro software. Statistical comparisons were performed with paired or unpaired Student t tests for 2-group-only analysis and by ANOVA followed by Bonferroni-corrected t tests for multiple-group comparisons. A 2-tailed value of P<0.05 indicated statistical significance; group data are mean±SEM.
PF AP Model
A previously described model of the electrophysiology of the PF cell was used,24 modified to reproduce behavior of canine cell recordings at physiological temperature. Current density was set to reproduce a peak current of 10 pA/pF at 30 mV.25
Results
IVF Patients
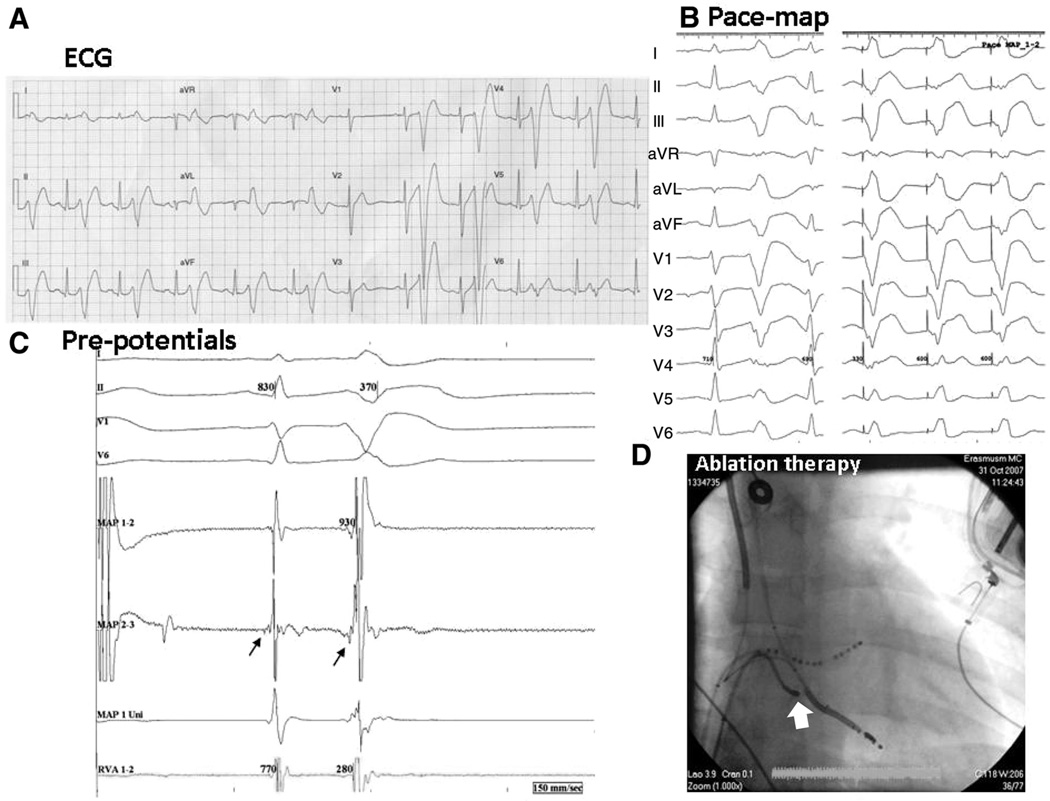
Baseline ECGs were normal in DPP6 risk-haplotype carriers (Online Table III). Ventricular arrhythmia manifested as short coupled ventricular extrasystoles (VESs) that sometimes initiated rapid polymorphic ventricular tachyarrhythmias (Online Figure I). VESs consistently displayed left bundle-branch block morphology and superior/leftward ECG axis, suggesting a lower RV origin. The short VES coupling intervals despite normal QTc, along with the relatively narrow QRS complexes, suggest an origin in the conduction system, as observed by Haïssaguerre et al26 in 25% of their IVF patients. In 1 patient undergoing ablation for repeated arrhythmia storm after implantation of a cardioverter-defibrillator (Figure 1), RV pace mapping produced a morphology similar to that of VESs (Figure 1A and 1B). Radiofrequency ablation was applied at a site with early diastolic PF potentials (Figure 1C) in the anterior lower RV (Figure 1D). During the 43-month follow-up, neither ventricular fibrillation nor typicalmorphology VESs occurred.
Figure 1. Radiofrequency ablation of idiopathic ventricular fibrillation in a DPP6 risk-haplotype carrier (patient E).
A, Early coupled ventricular extrasystoles (VESs) with a typical morphology in bigeminal pattern. B, left, Spontaneous VES; right, the pace map in the right ventricular (RV) apex. C, At the ablation site on the mapping ablation catheter, proximal recording (map 2–3) shows an early signal preceding the sinus beat, suggesting a Purkinje potential, which becomes clearer at the onset of a VES. D, Catheter position (arrow) at a successful ablation site (anteroposterior view). LV indicates left ventricle; and VF, ventricular fibrillation.
Differences Between PC and VM Ito
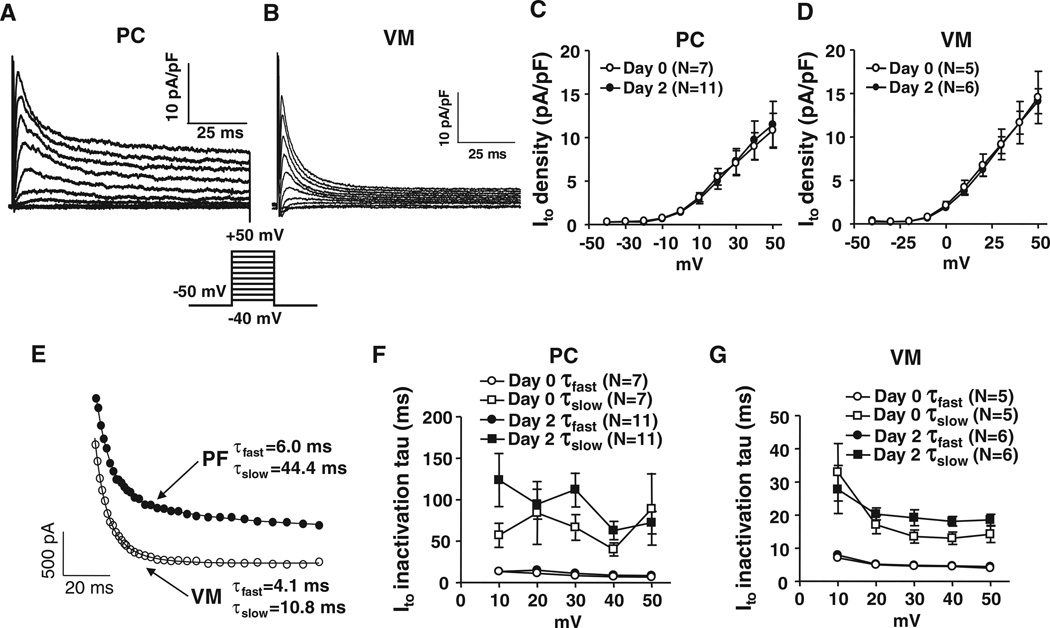
Figure 2A and 2B shows examples of Ito in freshly isolated canine PCs and VMs. The currents have similar overall morphologies, with PCs showing somewhat slower inactivation and larger end-pulse sustained currents compared with VM. Both PC Ito and VM Ito are completely blocked by 10 mmol/L 4-aminopyridine (Online Figure II). Overall current densities are of the same order for PCs and VMs (Figure 2C and 2D). Both PF Ito and VM Ito show biexponential inactivation (Figure 2E); however, the slow-phase inactivation time constants were slower for PF (Figure 2F and 2G). For example, the time constants at 30 mV averaged 8.3±1.0 milliseconds (fast phase) for PF versus 4.6±0.4 milliseconds for VM (P=0.005), and the slow-phase time constants averaged 66.5±15.4 milliseconds for PF versus 13.5±2.0 milliseconds for VM (P=0.044). The relative proportion of inactivation attributable to fast-phase inactivation was smaller in PF (57±6%) compared with VM (72±2%; P<0.05).
Figure 2. Comparison of Ito between Purkinje fiber cells (PCs) and ventricular muscles (VMs).
Representative recordings of Ito obtained with 100-millisecond depolarizations from a holding potential of −50 mV at 0.1 Hz in freshly isolated PCs (A) or VMs (B). C and D, Mean±SEM Ito density-voltage relations at day 0 (freshly isolated) and day 2 culture for PCs and VMs. E, Representative best-fit biexponentials to data from a PC and a VM during 100-millisecond depolarizations to 30 mV. F and G, Mean±SEM Ito inactivation time constants.
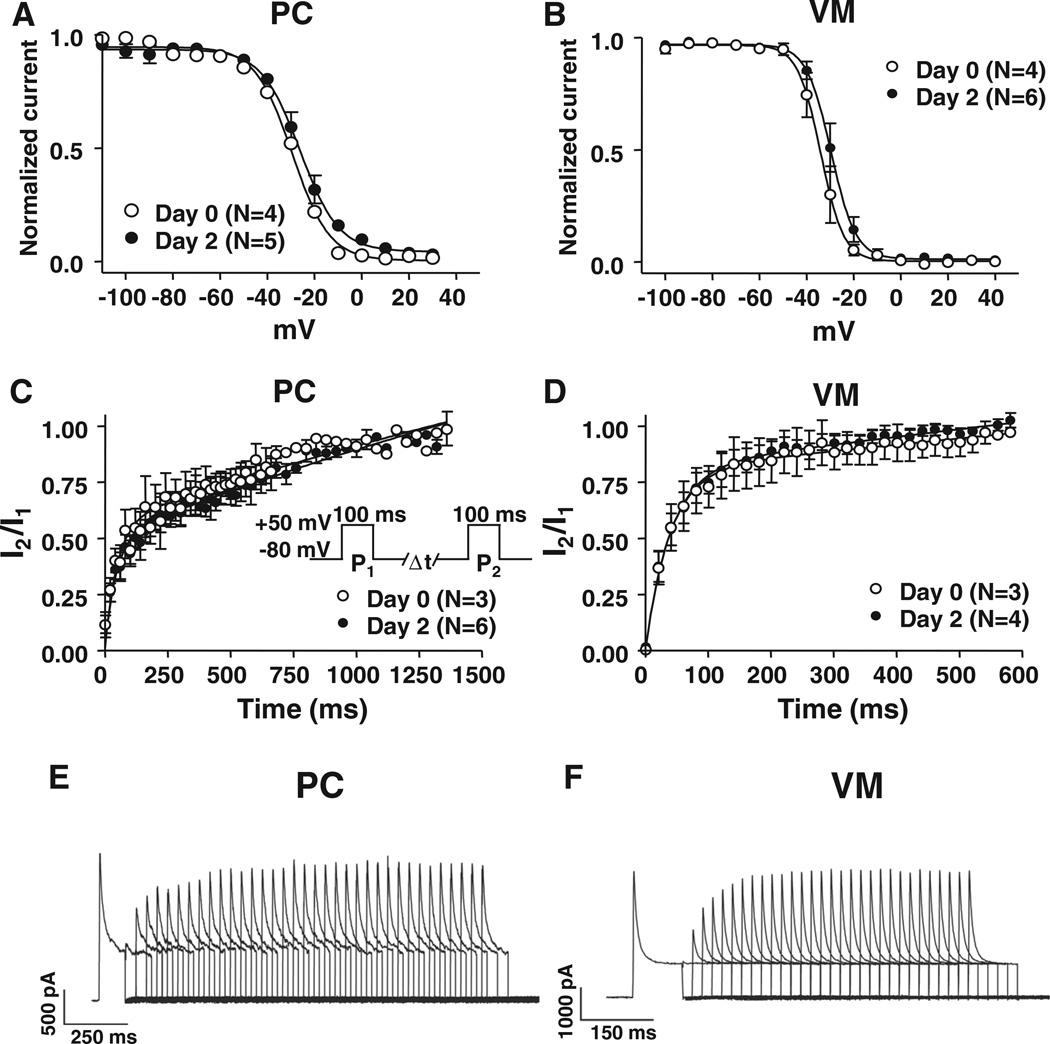
Figure 3 shows inactivation voltage dependence and recovery kinetics for PCs versus VM. Inactivation voltage dependence (Figure 3A and 3B) tended to be less negative in PCs: 50% inactivation voltages were −28.5±0.6 mV (PF) versus −34.0±2.8 mV (VM; P=0.184). Fast-phase recovery time constants were similar: 30±5 milliseconds for PF versus 21±4 milliseconds for VM (P=NS), but slow-phase recovery time constants were much slower for PF (691±139 milliseconds) compared with VM (166±48 milliseconds; P=0.04). The slow phase also comprised a larger portion of total inactivation in PF, 61±6%, compared with VM (39±7%; P=0.04). Ito properties were stable over 2 days in culture (Figures 2 and 3).
Figure 3. Inactivation voltage dependence and recovery kinetics of Purkinje fiber cell (PC) versus ventricular myocyte (VM) Ito.
Mean±SEM voltage dependence of PC (A) and VM (B) Ito inactivation voltage dependence obtained with a 200-millisecond test pulse to 50 mV preceded by 1-second conditioning pulses between −100 and 40 mV. Holding potential is −80 mV. Curves are best-ft Boltzmann relations. C and D, Time-dependent PC and VM Ito recovery from inactivation. Values are mean±SEM I2/I1 as a function of P1–P2 interval, obtained with the protocol shown in (C) at 0.1 Hz. Best-fit biexponential functions are shown. E and F, Representative recordings of Ito recovery from inactivation in PC and VM. Please note the different time scales.
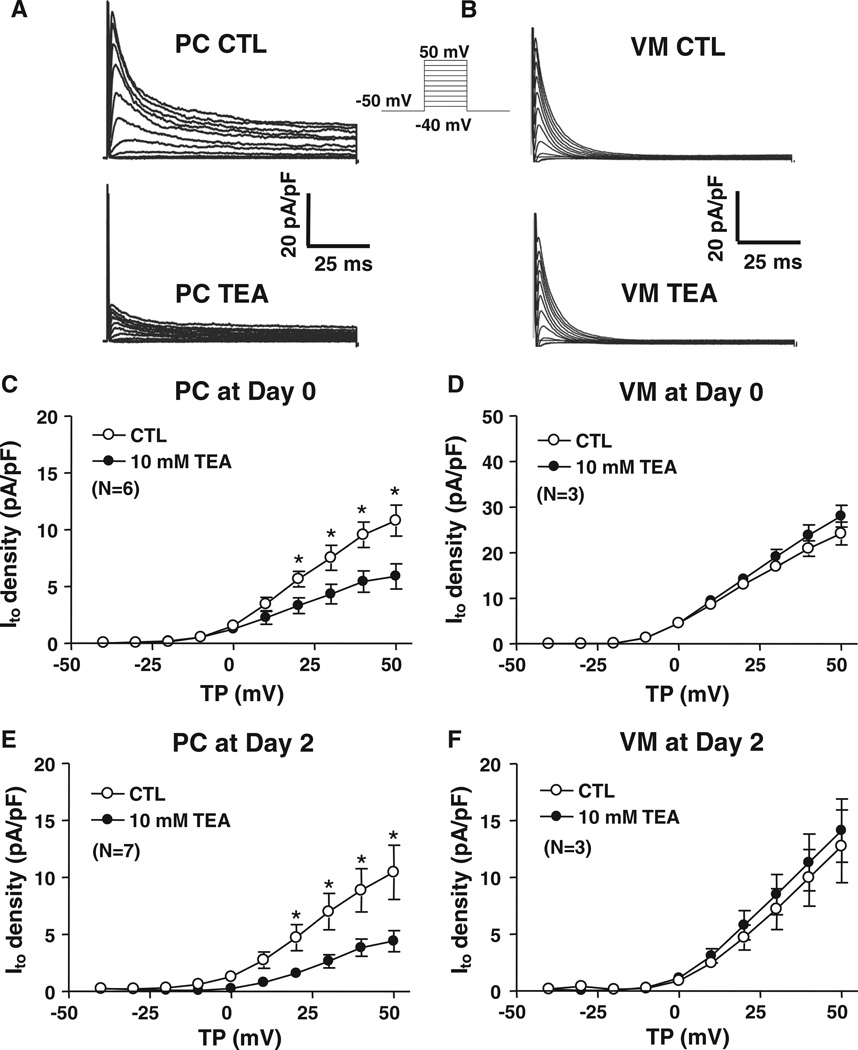
A signature property of PC Ito is TEA sensitivity.13,14 PC and VM responses to TEA (10 mmol/L) are illustrated in Figure 4A and 4B. TEA 10 mmol/L reduced PC Ito by ≈50% at 50 mV (Figure 4C). The dose-response relation for TEA inhibition of fresh PC Ito (Online Figure IIIA) showed a 50% inhibitory concentration (IC50) of 2.0±1.6 mmol/L. Neither inactivation time constants (Online Figure IIIB) nor the percentage of fast-versus slow-phase inactivation was changed by 100 mmol/L TEA. Recovery was accelerated by 10 mmol/L TEA (Online Figure IIIC) because of a decrease in the slow-phase proportion from 77±4% to 41±3% (P<0.01) without any change in the time constants per se. In contrast, VM Ito was unaffected by 10 mmol/L TEA (Figure 4D) and non-significantly decreased with 100 mmol/L TEA (Online Figure IIID). The relative responses of PCs versus VMs were unchanged after a 2-day culture (Figure 4E and 4F).
Figure 4. Effects of tetraethylammonium (TEA) on Purkinje fiber cell (PC) and ventricular myocyte (VM) Ito.
Representative recordings of Ito before (CTL, top) and after (bottom) 10-mmol/L tetraethylammonium (TEA) perfusion in freshly isolated PCs A, or VMs B. Currents were obtained with 100-millisecond depolarizations from a holding potential of −50 mV at 0.1 Hz. Mean±SEM Ito density-voltage relations before and after 10-mmol/L TEA at day 0 (C and D) and day 2 (E and F) from PCs (left) and VMs (right). *P<0.05, CTL vs 10 mmol/L TEA. CTL indicates control; TP, test potential.
All VM studies described above were performed in LV cells. We also compared PC Ito properties with those of RV cardiomyocytes. RV Ito was insensitive to 10 mmol/L TEA (Online Figure IVA), quite different from PCs (Online Figure IVB). Similar to LV VM, RV Ito inactived faster than PC (Online Figure VA and VB). The voltage dependence of Ito inactivation was less negative for PC Ito compared with RV (Online Figure VC). Recovery kinetics were also faster in RV cardiomyocytes compared with PCs (Online Figure VD). Overall, differences between PCs and RV cardiomyocytes paralleled those for LV cardiomyocytes.
Effects of DPP6 Expression Changes on PC and VM Ito
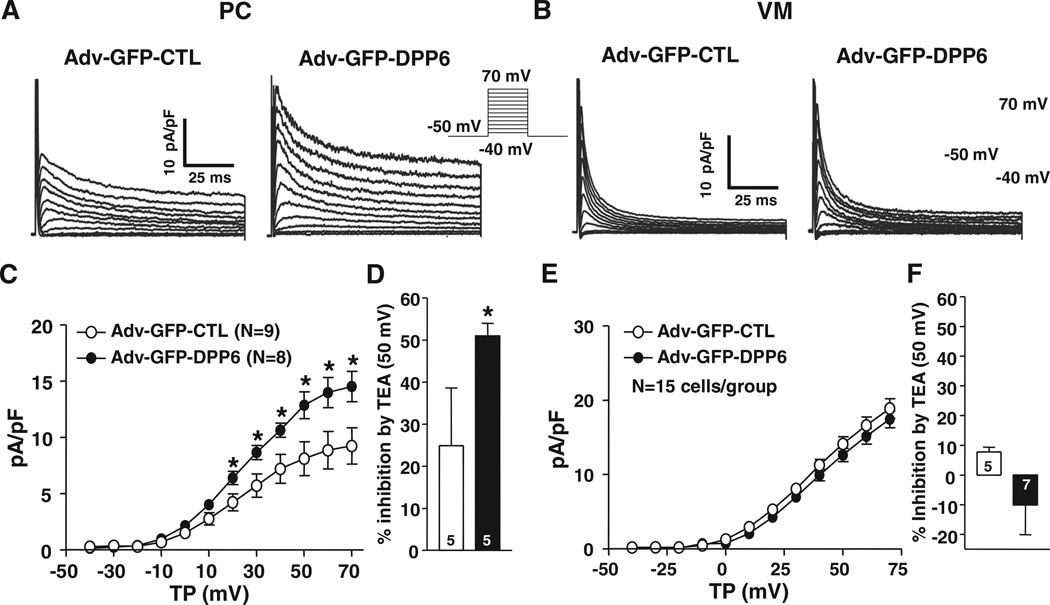
To assess the effect of DPP6 overexpression, as occurs in 7q36-IVF, on PC and VM Ito, we used adenovirus-based DPP6 gene transfer (Figure 5A and 5B). DPP6 overexpression significantly increased both Ito density (by ≈52%; Figure 5C) and TEA sensitivity (Figure 5D) in PCs but did not alter current density (Figure 5E) or TEA sensitivity (Figure 5F) in VMs. DPP6 overexpression did not alter Ito inactivation kinetics or voltage dependence (Online Figure VI). The time course of recovery from inactivation was similarly unaffected by DPP6 overexpression (Online Figure VII). Effective overexpression was confirmed at the mRNA level (Online Figure VIIIA).
Figure 5. Effects of dipeptidyl peptidase-like protein-6 (DPP6) overexpression on Purkinje fiber cells (PC) and ventricular muscle (VM) Ito.
A and B, Ito recordings obtained with 100-millisecond depolarizations from −50 mV at 0.1 Hz in VMs and PCs infected with Adv-GFP-CTL (CTL) or Adv-GFP-DPP6 (DPP6). C and E, Mean±SEM Ito density-voltage relations in CTL or DPP6 from VMs (C) and PCs (E). D and F, Percentage inhibition by 10 mmol/L tetraethylammonium (TEA) of VM (D) or PC (F) Ito at 50 mV. *P<0.05, CTL vs DPP6. GFP indicates green fluorescent protein; and T P, test potential.
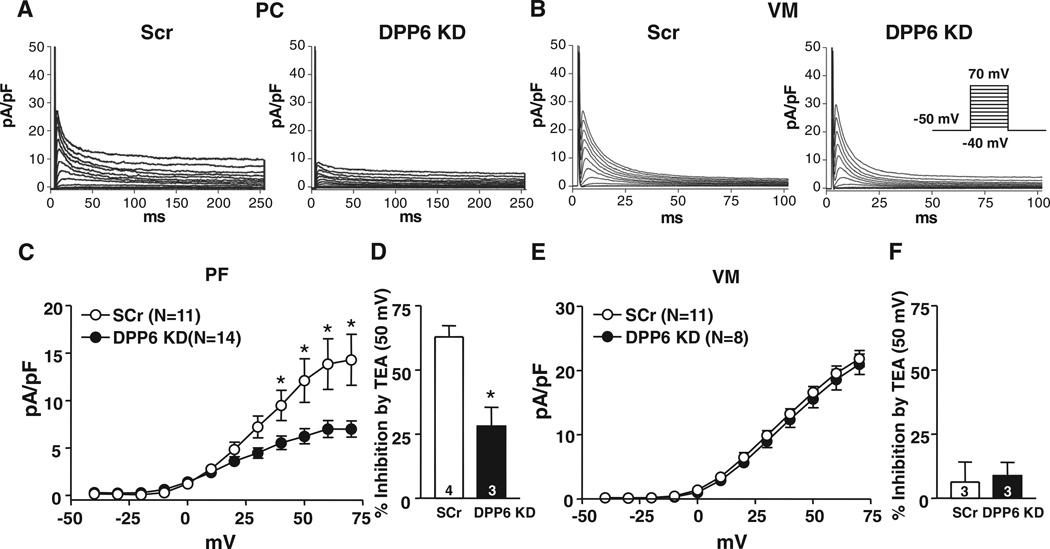
To further assess the potential role of DPP6 in PC and VM Ito, we studied the effects of DPP6 knockdown on native Ito. Effective DPP6 knockdown was confirmed by measuring DPP6 mRNA (online Figure VIIIB ). Examples of Ito in PCs and VMs infected with the scrambled control and DPP6-knockdown virus are shown in Figure 6A and 6B. DPP6 knockdown significantly decreased PC Ito density (Figure 6C) and TEA sensitivity (Figure 6D) but did not affect VM Ito (Figure 6E and 6F). Inactivation kinetics and voltage dependence, recovery kinetics, and relative amplitudes were not affected by DPP6 knockdown for both PC and VM Ito (Online Figure IX).
Figure 6. Effects of dipeptidyl peptidase-like protein-6 (DPP6) knockdown (KD) on Purkinje fiber cell (PC) and ventricular myocyte (VM) Ito.
Examples of Ito recordings from PC A, and VM B, infected with Adv-GFP-Scr (Scr) or Adv-GFP-DPP6 KD (DPP6 KD). Currents were obtained with 250- (PC) or 100-millisecond (VM) depolarizations at 0.1 Hz. C and E, Mean±SEM Ito density-voltage relations in Scr or DPP6 KD from PCs and VMs. D and F, Percentage inhibition by 10 mmol/L tetraethylammonium (TEA) of PC (D) or VM (F) Ito at 50 mV. *P<0.05, Scr vs DPP6 KD. GFP indicates green fluorescent protein.
Purkinje Fiber Versus Myocardial Expression of Ito-Related Subunits
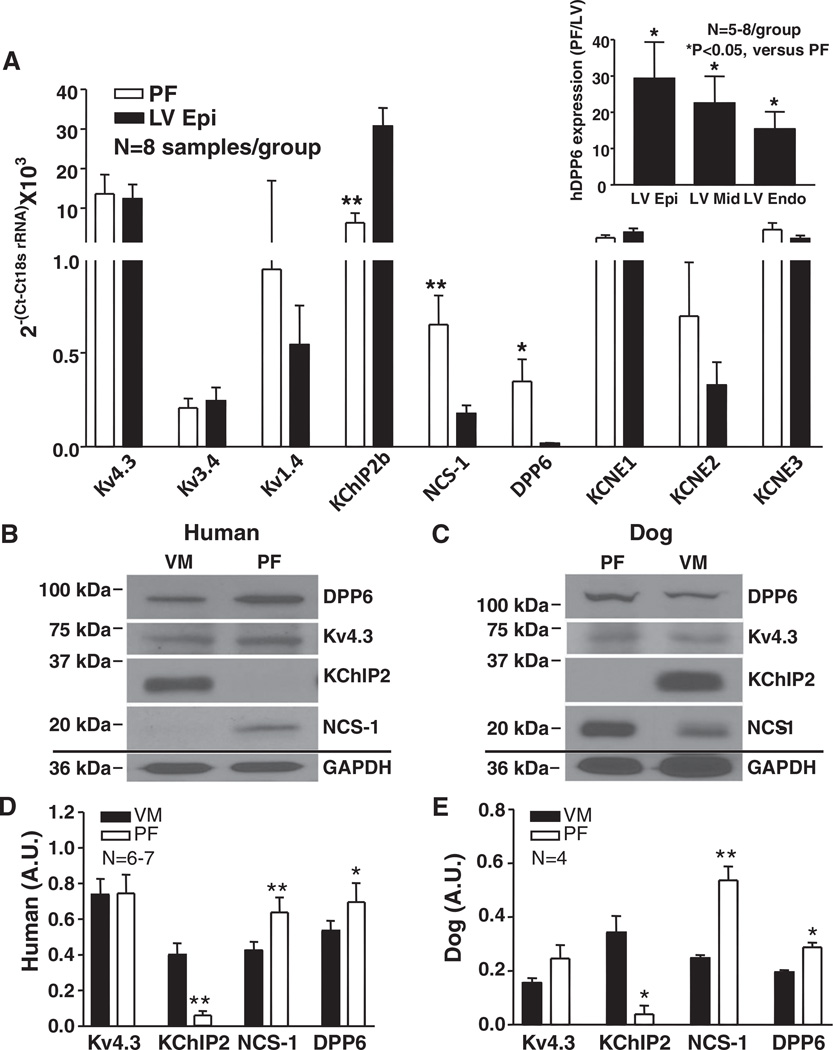
The studies shown in Figures 5 and 6 support the contention that DPP6 is an important contributor to PC (but not VM) Ito. To evaluate potential underlying mechanisms, we first assessed the differential expression of potential Ito subunits in PFs and VMs. Normal human hearts presented marked DPP6 mRNA expression gradients between PF and LV (Figure 7A). DPP6 mRNA expression was 29±10-, 23±7-, and 15±5-fold higher in PF compared wtih LV epicardium, midmyocardium, and endocardium, respectively (Figure 7A, inset). Kv4.3, Kv3.4, and Kv1.4 were similarly expressed in PF and LV, with Kv4.3 expression being by far the strongest. KChIP2b expression in LV epicardium was ≈5-fold greater than in PF, whereas NCS-1 was more strongly expressed (≈4-fold) in PF. KCNE1, KCNE2, and KCNE3 mRNA levels were not differential. We also measured the expression of Kv4.3, KChIP2, NCS-1, and DPP6 proteins in PF and VM membrane preparations from normal human hearts (Figure 7B and 7D) and from canine hearts (Figure 7C and 7E). The protein expression differences for Kv4.3, KChIP2, NCS-1, and DPP6 in PF and VM qualitatively paralleled relative transcript expression (Figure 7A), with KChIP2 more strongly expressed in VM and NCS-1 and DPP6 more strongly expressed in PF. Kv4.3 protein levels were not significantly different between PF and VM. Membrane extracts from CHO cells overexpressing the various subunits studied were probed with the antibodies used, showing good selectivity (Online Figure X).
Figure 7. mRNA and protein expression of Ito subunits.
A, Ito subunit mRNA expression in human heart. Mean±SEM normalized results for Kv4.3, Kv3.4, Kv1.4, K+-channel interacting protein type-2 (KChIP2)b, neuronal calcium sensor-1 (NCS-1), dipeptidyl peptidaselike protein-6 (DPP6), KCNE1, KCNE2, and KCNE3. *P<0.05, **P<0.01, Purkinje fibers (PF) vs left ventricle (LV) epicardium (Epi). Inset, DPP6 mRNA expression in PF as ratio of LV Epi (n=8), midmyocardium (LV Mid) (n=5), and endocardium (Endo) (n=6). *P<0.05, PF vs LV layers. B and C, Representative Western blot results in ventricular muscle (VM) and PF membrane fractions from human (B) and dog (C) hearts. All blots shown were from 1 VM and 1 PF sample on the same membrane for each, which was stripped before each antibody was applied. D and E, Mean±SEM protein levels normalized to GAPDH. *P<0.05, **P<0.01, PF vs VM.
Effects of β-Subunit Background on Heterologously Expressed Kv4.3
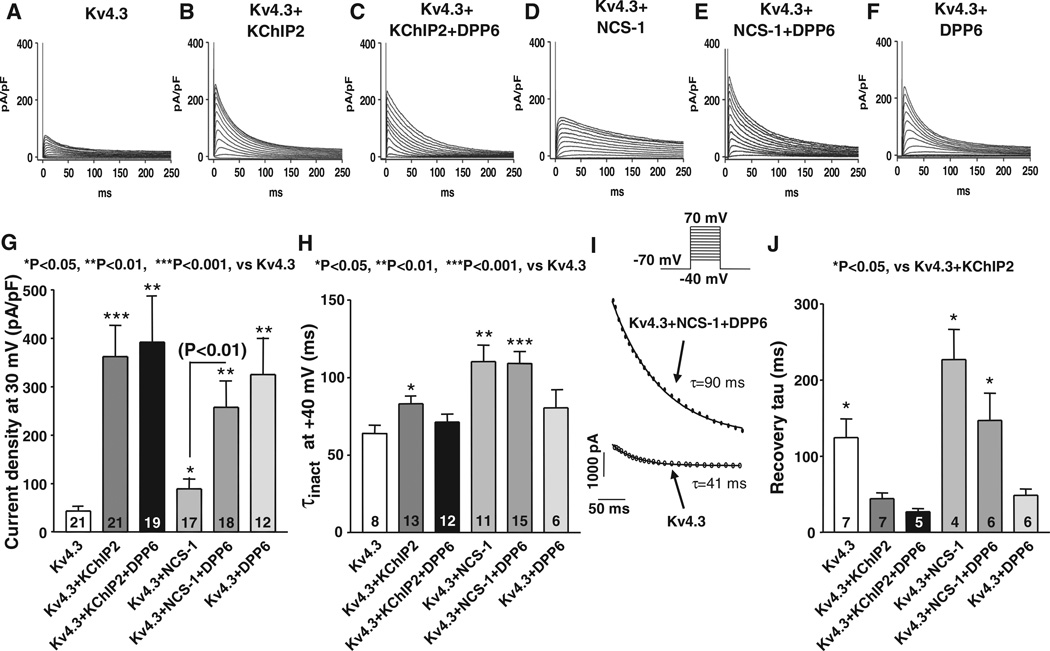
To relate the different properties of VM compared with PF Ito to the various subunits they express, we studied the effects of DPP6 on Kv4.3+KChIP2 (mimicking VM Ito subunit makeup) or Kv4.3+NCS-1 (mimicking PC Ito subunit makeup) in CHO cells. Figure 8A through 8F shows representative Ito recordings from CHO cells transfected with Kv4.3 alone, Kv4.3+KChIP2, Kv4.3+KChIP2+DPP6, Kv4.3+NCS-1, Kv4.3+NCS-1+DPP6, or Kv4.3+DPP6. Corresponding mean current density data are shown in Figure 8G. KChIP2 enhanced Ito current density (Figure 8B versus 8A), but the addition of DPP6 produced no further change (Figure 8C and 8G). NCS-1 cotransfection with Kv4.3 increased Ito slightly compared with Kv4.3 alone (Figure 8D), and the addition of DPP6 substantially enhanced Ito (Figure 8E). DPP6 alone enhanced Ito to about the same extent as DPP6 in the presence of NCS-1 (Figure 8F and 8G). Changes in mean inactivation time constants are shown in Figure 8H, with illustrative fits in Figure 8I. Currents encoded by Kv4.3+KChIP2 or Kv4.3+NCS-1 inactivated more slowly than Kv4.3-only current. Coexpression of DPP6 with Kv4.3+KChIP2 or with Kv4.3 alone did not significantly alter the rate of inactivation. However, τinact for currents encoded by Kv4.3+NCS-1+DPP6 was similar to τinact of Kv4.3+NCS-1 currents and slower than for Kv4.3+KChIP2 (P<0.05) or Kv4.3 alone. The voltage dependence of Kv4.3 inactivation was left-shifted by coexpression with DPP6 only but was not significantly altered by cotransfection with the other subunit combinations studied (Online Figure XIA and Online Table IV).
Figure 8. Comparison of Ito properties obtained from different subunit-combinations in heterologous expression system.
Examples of currents recorded in Chinese hamster ovary cells transiently transfected with Kv4.3 (A), Kv4.3+K+-channel interacting protein type-2 (KChIP2; B), Kv4.3+KChIP2+DPP6 (C), Kv4.3+neuronal calcium sensor-1 (NCS-1; D), Kv4.3+NCS-1+DPP6 (E), or Kv4.3+DPP6 (F). Currents were recorded during 250-millisecond depolarizations at 0.1 Hz. G, Mean±SEM current densities at 30 mV. *P<0.05, **P<0.01, ***P<0.001 vs Kv4.3 only. H, Mean±SEM inactivation time constants at 40 mV. *P<0.05, **P<0.01, ***P<0.001 vs Kv4.3 only. I, Representative fts from 2 experiments. J, Mean±SEM recovery time constants obtained as illustrated in Online Figure VIIIB. *P<0.05, vs Kv4.3+KChIP2.
Changes in subunit composition significantly affected Ito recovery (Figure 8J and Online Figure XIB). Cells transfected with Kv4.3 alone had slower recovery time constants than cells cotransfected with Kv4.3+KChIP2. Adding DPP6 to Kv4.3+KChIP2 did not significantly alter recovery kinetics compared with Kv4.3+KChIP2. Cotransfection of Kv4.3 with NCS-1, with or without DPP6, slowed recovery ≈3-fold relative to K4.3+KChIP2. Cotransfection of Kv4.3 with DPP6 alone accelerated Kv4.3 recovery to an extent similar to that of KChIP2.
Subunit composition significantly affected TEA sensitivity. Currents recorded before and after 5 mmol/L TEA in cells transfected with Kv4.3 alone and Kv4.3+DPP6 are shown in Online Figure XIIA and XIIB, respectively. Online Figure XIIC shows mean percentage inhibition by 5 mmol/L TEA of cells transfected with Kv4.3 and various combinations of β-subunits. Cells transfected with Kv4.3 alone, Kv4.3+KChIP2, and Kv4.3+KChIP2+DPP6 showed no significant effect of TEA. NCS-1 conferred slight TEA sensitivity. Cotransfection of DPP6 with Kv4.3 alone or with Kv4.3+NCS-1 significantly enhanced TEA sensitivity relative to Kv4.3+NCS-1. TEA sensitivity of cells cotransfected with Kv4.3+NCS-1+DPP6 was not significantly different from Kv4.3+DPP6. Thus, DPP6 enhances Kv4.3 currents and TEA sensitivity only in the absence of KChIP2.
Although the expression data do indicate stronger DPP6 expression in PF compared with VM, the protein data indicate relatively small quantitative rather than the qualitative differences seen with mRNA. We therefore considered the possibility that the prominent role of DPP6 in PF might be the result of interference with DPP6-Kv4.3 interaction by KChIP2, the predominant Ito β-subunit in VM that is weakly expressed in PCs, based on the lack of change in current density when DPP6 is added to Kv4.3+KChIP2 (Figure 8G) and the inability of DPP6 to induce TEA sensitivity when cotransfected with Kv4.3+KChIP2 (Online Figure XIIC). To study the physical interaction of various combinations of β-subunits with Kv4.3, we immunoprecipitated proteins from CHO cells expressing Kv4.3, Kv4.3+KChIP2, Kv4.3+KChIP2+DPP6, Kv4.3+DPP6, Kv4.3+NCS-1, or Kv4.3+NCS-1+DPP6 with a monoclonal anti-Kv4.3 antibody (Online Figure XIIIA). Kv4.3 was effectively and completely precipitated from each group because the 75-kDa Kv4.3 band was not observed in the supernatants. The 115-kDa DPP6 band was detected in the immunoprecipitates obtained from Kv4.3+KChIP2+DPP6, Kv4.3+DPP6, and Kv4.3+NCS-1+DPP6. Not all expressed DPP6 protein was bound to Kv4.3 because it was also detected in the supernatant. KChIP2 and NCS-1 similarly coimmunoprecipitated with Kv4.3, with significant amounts remaining in the supernatant. The amount of DPP6 that coimmunoprecipitated with Kv4.3 was significantly less when KChIP2 was expressed together with Kv4.3 and DPP6 compared with Kv4.3+DPP6 without KChIP2 (Online Figure XIIIB).
Potential Basis for Kv4.3 Current Enhancement by KChIP2 and DPP6
Either KChIP2 or DPP6 substantially enhances K4.3 current. To gain insights into possible mechanisms, we assessed CHO cell Kv4.3 expression in plasma membranes by immunohistochemistry (Online Figure XIV) and in crude membrane preparations with Western blot (Online Figure XV). Both approaches suggest that KChIP2 or DPP6 increases Kv4.3 membrane expression.
Effects of KChIP2 Knockdown on VM Ito
If KChIP2 prevents Kv4.3-DPP6 interaction and VM has significant DPP6 expression, DPP6 might be able to maintain Kv4.3 function when KChIP2 levels are decreased. To test this possibility, we knocked KChIP2 down with adenoviral gene transfer in VMs. Examples of Ito from VMs infected with scrambled control and KChIP2-knockdown viruses are shown in Online Figure XVIA. Effective KChIP2 knockdown was confirmed by an ≈50% decrease in KChIP2 Western blot signal (Online Figure XVIB). KChIP2 knockdown did not significantly affect Ito density (Online Figure XVIC).
Effects of Increased Ito on PC APs in a Mathematical Model
Our studies of the properties of PFs versus VM, of the effect of DPP6 overexpression and knockdown on native PCs and VMs, of putative Ito subunit composition, and of the results of cotransfection of Kv4.3 with different subunit combinations in CHO cells all point to an important role of DPP6 in PF Ito composition and suggest that increased DPP6 expression enhances PF Ito. We then sought to understand the potential functional consequences of DPP6 overexpression. We were unable to record physiological APs from cultured PCs. We therefore turned to a previously described computational model of the PC AP.24 Online Figure XVIIA shows the baseline model-derived Ito. The effects of Ito overexpression on PF repolarization are shown in Online Figure XVIIB. Increasing degrees of upregulation cause progressive deepening of the phase 1 notch and shortening of AP duration, leading to loss of the AP plateau and early repolarization directly from phase 1.
Discussion
The recent discovery of a founder risk haplotype on chromosome 7q36, which includes the proximal and upstream regions of the DPP6 gene, in Dutch familial IVF subjects brought the potential for new insights into our understanding of the mechanisms underlying IVF.4 In risk-haplotype carriers, IVF is highly linked to cardiac overexpression of the DPP6 gene,4 pointing to increased DPP6 expression as a potential molecular basis. However, the link between DPP6 upregulation and arrhythmogenesis has been unclear. In the present study, we examined clinical data from 5 DPP6 risk-haplotype carriers who had suffered IVF and found that DPP6-related IVF arrhythmias likely originated from the PF system. In nondiseased human hearts, we observed that DPP6 is more richly expressed in PFs compared with its expression in VMs. Besides DPP6, we found PF-VM differences in the expression of KChIP2 and NCS-1, with KChIP2 being more abundant in VMs and sparse in PFs, whereas NCS-1 is more strongly expressed in PFs than VMs. The biophysical and pharmacological properties of PF Ito are known to differ from those of VM Ito,13,14 but the underlying molecular mechanism has been unknown. In addition, the basis for substantial PF Ito density has been mysterious, in light of weak PF expression of KChIP2,15 known to be essential for VM Ito expression.27 The data presented here suggest that DPP6 performs a function in PFs comparable to that of KChIP2 in VMs, permitting normal current expression of Ito. In addition, NCS-1 and DPP6 recapitulated the specific functional PF Ito phenotype when coexpressed with the α-subunit Kv4.3, and DPP6 knockdown suppressed native PC Ito. Overexpression of DPP6 to mimic the particular cardiac gene expression phenotype observed in IVF patients enhances PF (but not VM) Ito; in vivo, this would translate into accelerated PF repolarization, which might cause a form of PF early-repolarization syndrome.
Possible Role in Arrhythmogenesis
Cardiac PFs participate in the initiation and maintenance of ventricular arrhythmias in the presence of pathology like congestive heart failure or myocardial infarction or in inheritable arrhythmic syndromes like the long-QT syndrome.28–31 They have also been implicated in catecholaminergic polymorphic ventricular tachycardia.32 PFs are particularly susceptible to early afterdepolarizations or delayed afterdepolarizations.33,34 Ion channel remodeling in PFs contributes to arrhythmogenic electrophysiological abnormalities in diseased hearts.14,24,35–37 Our discovery that upregulation of DPP6 expression specifically enhances Ito in cardiac PF but not VM is the first mechanistic clue to the pathogenesis of DPP6-related IVF. The absence of alterations in ventricular Ito with DPP6 overexpression potentially explains the normal ECG in risk-haplotype carriers because the Purkinje system is a small fraction of the myocardial mass.
Imbalances between Ito and inward currents have been suggested to underlie the development of ventricular arrhythmias. KCND3 (encoding Kv4.3) or KCNE3 gain-of-function mutations seen in Brugada syndrome patients enhance ventricular Ito and are presumed to cause steep transmural repolarization gradients that induce spontaneous generation of ectopic beats.38,39 In the risk haplotype for IVF, increased Purkinje Ito expression with DPP6 enhancement might similarly deepen phase 1 and appreciably accelerate repolarization (Online Figure XVII). Accelerated PF repolarization could cause strong local repolarization gradients with adjacent ventricular muscle (unaffected by DPP6 overexpression), thereby generating local ectopic activity that produces early coupled VESs without other evidence of electrocardiographic early-repolarization syndromes. This interesting possibility remains to be tested directly.
Ito Subunit Composition and Properties
The TEA sensitivity of PF Ito was a classic observation that contributed to the recognition that Ito is carried predominantly by K+.40 In contrast, VM Ito is TEA insensitive.13,14 Similarly, Ito recovery is markedly slower in PFs compared with VM,13,14 contributing to well-established differences in AP rate responsiveness.41 Nevertheless, the molecular basis for Purkinje Ito has not been established despite studies of PF Ito-related subunit composition.15,20,42,43 In addition to permitting normal Ito densities in the virtual absence of KChIP2, the sub-unit profiles we noted here may account for the unique TEA sensitivity and kinetic properties of PF Ito: DPP6 bequeathing TEA sensitivity and NCS-1 slow recovery. In previous studies, we found higher levels of Kv3.4 in PFs than in VM (findings we could not confirm here), identifying Kv3.4 as a potential contributor to Purkinje Ito.15 However, the TEA sensitivity of Kv3 channels is an order of magnitude greater than that of Purkinje Ito, and high concentrations of blood-depressing substance, a potent and specific Kv3.4 channel blocker, fail to inhibit Purkinje Ito.13 The results here present for the first time a plausible basis for the previously enigmatic molecular composition of Purkinje Ito.
KChIP2 and NCS-1 are members of the recoverin/NCS subfamily of calcium-binding proteins.44,45 Both proteins can interact with Kv4 channels and are recognized as regulatory subunits for Kv4 subunit channels in neuron and myocardium.5,10,16,17,44,45 The specific role of NCS-1 in the heart has not been determined. Nakamura et al16 initially suggested that NCS-1 regulates K4 currents. Guo et al17 showed that NCS-1 is expressed in mammalian myocardium, slows Kv4 current inactivation, and enhances current density. In mouse, NCS-1 is developmentally regulated and more abundant in immature hearts.45 Relatively little is known about the regional distribution of NCS-1 expression. Greener et al42 found greater frequenin (NCS-1) mRNA levels in the bundle of His (composed of PCs) than in VM. Extremely low-level KChIP2 expression in PFs is a consistent finding.15,20,42,43,46 Our finding of greater PF DPP6 abundance compared with VM is consistent with other recent observations.42,43 The interaction of DPP6 with Kv4.x subunits is also known to facilitate subunit trafficking and to alter current kinetics.5,6,8,47 The effect of DPP6 on Kv4 subunit channel TEA sensitivity is related to modified TEA binding to the external side of the pore.8,48 Our coexpression studies suggest that both NCS-1 and DPP6 contribute to the properties of PF Ito, with DPP6 required to enhance Kv4.3 current density and TEA sensitivity and NCS-1 necessary for the typical kinetic properties (slower inactivation and recovery). These observations provide new insights into the functional importance of differential PF-VM Ito subunit expression profiles.
Potential Limitations
In this study, we developed a novel in vitro system of cultured PCs and applied it to study the consequences of DPP6 overexpression, as seen in IVF, for PF Ito. We were unable to record physiologically relevant APs from cultured PCs, which prevented us from directly assessing the impact of DPP6 overexpression on PC APs. Instead, we used a mathematical PC-AP model to analyze the effect of PF Ito gain of function. Further work is needed to explore the electrophysiological phenotype associated with DPP6 overexpression. Because of the brief murine AP, studies in transgenic models more closely related to humans such as the rabbit49 may be needed. Interspecies differences in Ito also exist between the human and canine heart50 and need to be considered in the assessment of the application of our findings.
We compared Ito kinetics in native PCs and VMs with identical 100-millisecond depolarizing pulse protocols (Figure 3). Although these studies allowed us to demonstrate slower Ito inactivation in PCs compared with VM, because of the slow nature of PC Ito recovery, the slow-phase time constants could not be accurately quantified with such short pulses. We subsequently used a 1-second depolarizing pulse protocol and obtained results suggesting that the slow-phase time constant is at least on the order of 400 milliseconds (Online Figure XVIII).
Our DPP6 overexpression and knockdown data indicate that DPP6 plays a significant role in PF but not in VM Ito, but our studies do not establish a clear molecular basis for this highly PF-selective contribution. DPP6 and NCS-1 are more strongly expressed in human PF than VM at both the protein and mRNA levels (Figure 2); however, the PF-VM discrepancy is much greater in mRNA than protein expression. The mRNA data are more strictly quantitative than the protein data but are further removed from the functional molecule. The protein data are based on membrane preparations that include a variety of cell membranes and not solely the sarcolemma; in addition, the protein data do not reflect compartmentalization in potentially critical macromolecular complexes. Western blot analyses are limited by imperfect specificity and the detection of multiple molecular weight bands, particularly for polyclonal antibodies. KChIP2 seems to prevent Kv4.3 interaction with DPP6, as reflected by the lack of Kv4.3 current increase with DPP6 in the presence of KChIP2 compared with KChIP2-Kv4.3 alone (Figure 8G), the inability of DPP6 to confer TEA sensitivity in the presence of KChIP2 (Online Figure XIIC), and the reduction in DPP6 physical interaction with Kv4.3 in the presence of KChIP2 (Online Figure XIII). Thus, the low-level expression of KChIP2 in PF may contribute at least as much to the manifest role of DPP6 as the PF-VM differences in DPP6 expression per se. In contrast, coexpression with NCS-1 does not interfere with any DPP6 effects and is required to reproduce slow PC recovery kinetics. It must be noted that although our study clarifies the basis of unusual PF Ito properties by suggesting that the characteristic slowly recovering and TEA-sensitive components are likely caused by the involvement of NCS-1 and DPP6, by indicating that PF Ito current amplitude is maintained by DPP6 in the relative absence of KChIP2, and by pointing to PF early repo-larization as a mediator of arrhythmogenic consequences of DPP6-overexpressing arrhythmia syndromes, many questions about the molecular composition of PF Ito remain unanswered and need to be addressed in future work. It is likely that PF Ito consists of >1 channel type because TEA blocked a maximum of ≈75% of the current and selectively suppressed the slowly recovering kinetic component.
Studies in native cells are essential to assess the composition of native channels but are limited by the variability introduced by varying cell quality and ionic current densities. For this reason, we were as careful as possible to study all groups and interventions within each series of experiments in contemporaneous experiments and whenever possible within each set of cells. We studied native-cell properties at a physiological temperature (36°C) to work under as physiological conditions as possible. However, currents in heterologously expressing cells were too large at 36°C to be effectively voltage clamped. Therefore, heterologous cell work was done at room temperature, greatly slowing current kinetics. Comparisons with native-cell data are therefore based on qualitative findings rather than quantitative comparisons between corresponding kinetic components.
Translational Relevance
Our study is the first to address the pathophysiological mechanism of DPP6-related IVF. Familial IVF linked to the chromosome-7 locus including DPP6 presents as a malignant inheritable arrhythmia syndrome.4,51 The genetic findings enable risk stratifcation,4,51 and elucidation of the underlying electrophysiological mechanism is important to develop improved treatment. For example, our results provide a potential rationale for the efficacy of quinidine, an Ito blocker, in IVF patients, as reported previously.52 The concepts we elucidated may also aid in understanding potential mechanisms of IVF and in facilitating exploration of mechanisms associated with other novel genes53 and sudden arrhythmic death paradigms.
Supplementary Material
Novelty and Significance.
What Is Known?
The specialized ventricular conducting system consists of cardiac Purkinje fiber (PF) cells , which have an unusual form of transient outward K+ current (Ito with particularly slow recovery kinetics.
In cardiac cells, excessively rapid or excessively slow repolarization can lead to ventricular tachyarrhythmias, potentially lethal cardiac rhythm disturbances.
A familial idiopathic ventricular fibrillation sudden cardiac death (SCD) syndrome has as its basis a variant gene haplotype that leads to cardiac overexpression of dipeptidyl peptidase-like protein-6 (DPP6), which can act as a subunit component of Ito
What New Information Does This Article Contribute?
SCD patients with the DPP6-overexpressing genotype have ventricular tachyarrhythmias arising in the specialized PF-conducting system.
DPP6 plays an important role in constituting PF Ito, explaining many of its unusual properties and differences from Ito elsewhere in the heart.
DPP6 overexpression enhances PF Ito, which can accelerate PF repolarization, and this could explain the clinical idiopathic ventricular fibrillation/SCD syndrome origin in the PF system and the lack of abnormalities in other cardiac regions
PF Ito has a number of unusual properties like slow recovery kinetics and high tetraethylammonium sensitivity that suggest a potentially molecular basis distinct from atrial or ventricular muscle. This study assessed the role of DPP6 in PF Ito on the basis of evidence that patients with a genetic form of SCD show cardiac DPP6 overexpression, as well as arrhythmias that originate in the PF system. We show that DPP6 is preferentially enriched in PFs and that its overexpression and knockdown enhance and suppress, respectively, Ito in PF but not ventricular myocytes. In addition, DPP6 coexpression with the predominant Ito pore-forming subunit Kv4.3 alters Ito pharmacology, reproducing tetraethylammonium sensitivity. Furthermore, the accessory β-subunit K+-channel interacting protein type-2, essential for robust Ito formation by Kv4.3 subunits in the ventricule, is weakly expressed in PFs, where DPP6 plays a corresponding role in localizing functional Kv4.3 channels to the membrane. A mathematical PF model shows that DPP6 overex-pression–induced Ito enhancement can accelerate PF repolarization, potentially leading to ventricular arrhythmogenesis. Our study elucidates the previously cryptic basis for PF Ito and introduces a potential new paradigm for idiopathic ventricular fibrillation/SCD. These new insights have the potential to lead to improved understanding and treatment of life-threatening arrhythmias in humans.
Acknowledgments
We thank Louis Villeneuve for providing the fluorescent images; Dr Ange Maguy for technical consultation on coimmunoprecipitation; Nathalie L’Heureux, Chantal St-Cyr, and Audrey Bernard for technical assistance; and France Thériault for secretarial help with the article. We thank Dr Gordon Tomaselli for kindly providing human Kv4.3 plasmid and Drs Michael Morales, Harold Strauss, and Randall Rasmusson for the human KChIP2 plasmid.
Sources of Funding
This work was supported by the Canadian Institutes for Health Research (MOP68929), the Quebec Heart Foundation, Fondation Leducq (07CVD03), and the Netherlands Heart Foundation (2009B066).
Nonstandard Abbreviations and Acronyms
- AP
action potential
- CHO
Chinese hamster ovary
- DPP6
dipeptidyl aminopeptidase-like protein-6
- GFP
green fluorescent protein
- IVF
idiopathic ventricular fibrillation
- KChIP2
K+-channel interacting protein type-2
- LV
left ventricle
- NCS-1
neuronal calcium sensor-1
- PC
Purkinje fiber cell
- PF
Purkinje fiber
- RV
right ventricle
- TEA
tetraethylammonium
- VES
ventricular extrasystole
- VM
ventricular muscle
Footnotes
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.112.300227/-/DC1.
Disclosures
None.
References
- 1.Fishman GI, Chugh SS, Dimarco JP, et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 3.Survivors of out-of-hospital cardiac arrest with apparently normal heart: need for definition and standardized clinical evaluation: Consensus Statement of the Joint Committees of the Unexplained Cardiac Arrest Registry of Europe and of the Idiopathic Ventricular Fibrillation Registry in the United States. Circulation. 1996;95:265–272. doi: 10.1161/01.cir.95.1.265. [DOI] [PubMed] [Google Scholar]
- 4.Alders M, Koopmann TT, Christiaans I, Postema PG, Beekman L, Tanck MW, Zeppenfeld K, Loh P, Koch KT, Demolombe S, Mannens MM, Bezzina CR, Wilde AA. Haplotype-sharing analysis implicates chromosome 7q36 harboring DPP6 in familial idiopathic ventricular fibrillation. Am J Hum Genet. 2009;84:468–476. doi: 10.1016/j.ajhg.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maffie J, Rudy B. Weighing the evidence for a ternary protein complex mediating A-type K+ currents in neurons. J Physiol. 2008;586:5609–5623. doi: 10.1113/jphysiol.2008.161620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de Miera E, Ma Y, Mo W, Goldberg EM, Misumi Y, Ikehara Y, Neubert TA, Rudy B. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003;37:449–461. doi: 10.1016/s0896-6273(02)01185-6. [DOI] [PubMed] [Google Scholar]
- 7.Radicke S, Cotella D, Graf EM, Ravens U, Wettwer E. Expression and function of dipeptidyl-aminopeptidase-like protein 6 as a putative beta-subunit of human cardiac transient outward current encoded by Kv4.3. J Physiol. 2005;565:751–756. doi: 10.1113/jphysiol.2005.087312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colinas O, Pérez-Carretero FD, López-López JR, Pérez-García MT. A role for DPPX modulating external TEA sensitivity of Kv4 channels. J Gen Physiol. 2008;131:455–471. doi: 10.1085/jgp.200709912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antzelevitch C. Molecular basis for the transmural distribution of the transient outward current. J Physiol. 2001;533:1. doi: 10.1111/j.1469-7793.2001.0001b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niwa N, Nerbonne JM. Molecular determinants of cardiac transient outward potassium current (I(to)) expression and regulation. J Mol Cell Cardiol. 2010;48:12–25. doi: 10.1016/j.yjmcc.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 12.Antzelevitch C, Nof E. Brugada syndrome: recent advances and controversies. Curr Cardiol Rep. 2008;10:376–383. doi: 10.1007/s11886-008-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han W, Wang Z, Nattel S. A comparison of transient outward currents in canine cardiac Purkinje cells and ventricular myocytes. Am J Physiol Heart Circ Physiol. 2000;279:H466–H474. doi: 10.1152/ajpheart.2000.279.2.H466. [DOI] [PubMed] [Google Scholar]
- 14.Han W, Zhang L, Schram G, Nattel S. Properties of potassium currents in Purkinje cells of failing human hearts. Am J Physiol Heart Circ Physiol. 2002;283:H2495–H2503. doi: 10.1152/ajpheart.00389.2002. [DOI] [PubMed] [Google Scholar]
- 15.Han W, Bao W, Wang Z, Nattel S. Comparison of ion-channel subunit expression in canine cardiac Purkinje fibers and ventricular muscle. Circ Res. 2002;91:790–797. doi: 10.1161/01.res.0000039534.18114.d9. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura TY, Pountney DJ, Ozaita A, Nandi S, Ueda S, Rudy B, Coetzee WA. A role for frequenin, a Ca2+-binding protein, as a regulator of Kv4 K+-currents. Proc Natl Acad Sci U S A. 2001;98:12808–12813. doi: 10.1073/pnas.221168498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo W, Malin SA, Johns DC, Jeromin A, Nerbonne JM. Modulation of Kv4-encoded K(+) currents in the mammalian myocardium by neuronal calcium sensor-1. J Biol Chem. 2002;277:26436–26443. doi: 10.1074/jbc.M201431200. [DOI] [PubMed] [Google Scholar]
- 18.Radicke S, Cotella D, Graf EM, Banse U, Jost N, Varró A, Tseng GN, Ravens U, Wettwer E. Functional modulation of the transient outward current Ito by KCNE beta-subunits and regional distribution in human non-failing and failing hearts. Cardiovasc Res. 2006;71:695–703. doi: 10.1016/j.cardiores.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Pourrier M, Schram G, Nattel S. Properties, expression and potential roles of cardiac K+ channel accessory subunits: MinK, MiRPs, KChIP, and KChAP. J Membr Biol. 2003;194:141–152. doi: 10.1007/s00232-003-2034-8. [DOI] [PubMed] [Google Scholar]
- 20.Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol. 2007;582:675–693. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao L, Zhang L, Han W, Wang Z, Nattel S. Sex-based transmural differences in cardiac repolarization and ionic-current properties in canine left ventricles. Am J Physiol Heart Circ Physiol. 2006;291:H570–H580. doi: 10.1152/ajpheart.01288.2005. [DOI] [PubMed] [Google Scholar]
- 22.Paddison PJ, Cleary M, Silva JM, Chang K, Sheth N, Sachidanandam R, Hannon GJ. Cloning of short hairpin RNAs for gene knockdown in mammalian cells. Nat Methods. 2004;1:163–167. doi: 10.1038/nmeth1104-163. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, Vogelstein B, He TC. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2:1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 24.Sampson KJ, Iyer V, Marks AR, Kass RS. A computational model of Purkinje fibre single cell electrophysiology: implications for the long QT syndrome. J Physiol. 2010;588:2643–2655. doi: 10.1113/jphysiol.2010.187328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeck C, Pinto J, Boyden P. Transient outward currents in subendocardial Purkinje myocytes surviving in the infarcted heart. Circulation. 1995;92:465–473. doi: 10.1161/01.cir.92.3.465. [DOI] [PubMed] [Google Scholar]
- 26.Haïssaguerre M, Shoda M, Jaïs P, et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106:962–967. doi: 10.1161/01.cir.0000027564.55739.b1. [DOI] [PubMed] [Google Scholar]
- 27.Rosati B, Pan Z, Lypen S, Wang HS, Cohen I, Dixon JE, McKinnon D. Regulation of KChIP2 potassium channel beta subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. J Physiol. 2001;533:119–125. doi: 10.1111/j.1469-7793.2001.0119b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bode K, Hindricks G, Piorkowski C, Sommer P, Janousek J, Dagres N, Arya A. Ablation of polymorphic ventricular tachycardias in patients with structural heart disease. Pacing Clin Electrophysiol. 2008;31:1585–1591. doi: 10.1111/j.1540-8159.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 29.Bogun F, Good E, Reich S, Elmouchi D, Igic P, Tschopp D, Dey S, Wimmer A, Jongnarangsin K, Oral H, Chugh A, Pelosi F, Morady F. Role of Purkinje fibers in post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;48:2500–2507. doi: 10.1016/j.jacc.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 30.Chiang CE, Roden DM. The long QT syndromes: genetic basis and clinical implications. J Am Coll Cardiol. 2000;36:1–12. doi: 10.1016/s0735-1097(00)00716-6. [DOI] [PubMed] [Google Scholar]
- 31.Ben Caref E, Boutjdir M, Himel HD, El-Sherif N. Role of subendocardial Purkinje network in triggering torsade de pointes arrhythmia in experimental long QT syndrome. Europace. 2008;10:1218–1223. doi: 10.1093/europace/eun248. [DOI] [PubMed] [Google Scholar]
- 32.Kang G, Giovannone SF, Liu N, Liu FY, Zhang J, Priori SG, Fishman GI. Purkinje cells from RyR2 mutant mice are highly arrhythmogenic but responsive to targeted therapy. Circ Res. 2010;107:512–519. doi: 10.1161/CIRCRESAHA.110.221481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nattel S, Quantz MA. Pharmacological response of quinidine induced early afterdepolarisations in canine cardiac Purkinje fibres: insights into underlying ionic mechanisms. Cardiovasc Res. 1988;22:808–817. doi: 10.1093/cvr/22.11.808. [DOI] [PubMed] [Google Scholar]
- 34.Maruyama M, Joung B, Tang L, Shinohara T, On YK, Han S, Choi EK, Kim DH, Shen MJ, Weiss JN, Lin SF, Chen PS. Diastolic intracellular calcium-membrane voltage coupling gain and postshock arrhythmias: role of purkinje fibers and triggered activity. Circ Res. 2010;106:399–408. doi: 10.1161/CIRCRESAHA.109.211292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corrias A, Giles W, Rodriguez B. Ionic mechanisms of electrophysiological properties and repolarization abnormalities in rabbit Purkinje fibers. Am J Physiol Heart Circ Physiol. 2011;300:H1806–H1813. doi: 10.1152/ajpheart.01170.2010. [DOI] [PubMed] [Google Scholar]
- 36.Han W, Chartier D, Li D, Nattel S. Ionic remodeling of cardiac Purkinje cells by congestive heart failure. Circulation. 2001;104:2095–2100. doi: 10.1161/hc4201.097134. [DOI] [PubMed] [Google Scholar]
- 37.Maguy A, Le Bouter S, Comtois P, Chartier D, Villeneuve L, Wakili R, Nishida K, Nattel S. Ion channel subunit expression changes in cardiac Purkinje fibers: a potential role in conduction abnormalities associated with congestive heart failure. Circ Res. 2009;104:1113–1122. doi: 10.1161/CIRCRESAHA.108.191809. [DOI] [PubMed] [Google Scholar]
- 38.Delpón E, Cordeiro JM, Núñez L, Thomsen PE, Guerchicoff A, Pollevick GD, Wu Y, Kanters JK, Larsen CT, Hofman-Bang J, Burashnikov E, Christiansen M, Antzelevitch C. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol. 2008;1:209–218. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giudicessi JR, Ye D, Tester DJ, Crotti L, Mugione A, Nesterenko VV, Albertson RM, Antzelevitch C, Schwartz PJ, Ackerman MJ. Transient outward current (I(to)) gain-of-function mutations in the KCND3-encoded Kv4.3 potassium channel and Brugada syndrome. Heart Rhythm. 2011;8:1024–1032. doi: 10.1016/j.hrthm.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenyon JL, Gibbons WR. Influence of chloride, potassium, and tetra-ethylammonium on the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979;73:117–138. doi: 10.1085/jgp.73.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller JP, Wallace AG, Feezor MD. A quantitative comparison of the relation between the shape of the action potential and the pattern of stimulation in canine ventricular muscle and Purkinje fibers. J Mol Cell Cardiol. 1971;2:3–19. doi: 10.1016/0022-2828(71)90074-5. [DOI] [PubMed] [Google Scholar]
- 42.Greener ID, Monfredi O, Inada S, Chandler NJ, Tellez JO, Atkinson A, Taube MA, Billeter R, Anderson RH, Efimov IR, Molenaar P, Sigg DC, Sharma V, Boyett MR, Dobrzynski H. Molecular architecture of the human specialised atrioventricular conduction axis. J Mol Cell Cardiol. 2011;50:642–651. doi: 10.1016/j.yjmcc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 43.Atkinson A, Inada S, Li J, Tellez JO, Yanni J, Sleiman R, Allah EA, Anderson RH, Zhang H, Boyett MR, Dobrzynski H. Anatomical and molecular mapping of the left and right ventricular His-Purkinje conduction networks. J Mol Cell Cardiol. 2011;51:689–701. doi: 10.1016/j.yjmcc.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 44.An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura TY, Sturm E, Pountney DJ, Orenzoff B, Artman M, Coetzee WA. Developmental expression of NCS-1 (frequenin), a regulator of Kv4 K+ channels, in mouse heart. Pediatr Res. 2003;53:554–557. doi: 10.1203/01.PDR.0000057203.72435.C9. [DOI] [PubMed] [Google Scholar]
- 46.Nattel S, Frelin Y, Gaborit N, Louault C, Demolombe S. Ion-channel mRNA-expression profling: insights into cardiac remodeling and arrhythmic substrates. J Mol Cell Cardiol. 2010;48:96–105. doi: 10.1016/j.yjmcc.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Radicke S, Cotella D, Sblattero D, Ravens U, Santoro C, Wettwer E. The transmembrane beta-subunits KCNE1, KCNE2, and DPP6 modify pharmacological effects of the antiarrhythmic agent tedisamil on the transient outward current Ito. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:617–626. doi: 10.1007/s00210-008-0389-1. [DOI] [PubMed] [Google Scholar]
- 48.Wada K, Yokotani N, Hunter C, Doi K, Wenthold RJ, Shimasaki S. Differential expression of two distinct forms of mRNA encoding members of a dipeptidyl aminopeptidase family. Proc Natl Acad Sci U S A. 1992;89:197–201. doi: 10.1073/pnas.89.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brunner M, Peng X, Liu GX, et al. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest. 2008;118:2246–2259. doi: 10.1172/JCI33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akar FG, Wu RC, Deschenes I, Armoundas AA, Piacentino V3rd, Houser SR, Tomaselli GF. Phenotypic differences in transient outward K+ current of human and canine ventricular myocytes: insights into molecular composition of ventricular Ito. Am J Physiol Heart Circ Physiol. 2004;286:H602–H609. doi: 10.1152/ajpheart.00673.2003. [DOI] [PubMed] [Google Scholar]
- 51.Postema PG, Christiaans I, Hofman N, Alders M, Koopmann TT, Bezzina CR, Loh P, Zeppenfeld K, Volders PG, Wilde AA. Founder mutations in the Netherlands: familial idiopathic ventricular fibrillation and DPP6. Neth Heart J. 2011;19:290–296. doi: 10.1007/s12471-011-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belhassen B, Viskin S, Fish R, Glick A, Setbon I, Eldar M. Effects of electrophysiologic-guided therapy with Class IA antiarrhythmic drugs on the long-term outcome of patients with idiopathic ventricular fibrillation with or without the Brugada syndrome. J Cardiovasc Electrophysiol. 1999;10:1301–1312. doi: 10.1111/j.1540-8167.1999.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 53.Ohno S, Zankov DP, Ding WG, Itoh H, Makiyama T, Doi T, Shizuta S, Hattori T, Miyamoto A, Naiki N, Hancox JC, Matsuura H, Horie M. KCNE5 (KCNE1L) variants are novel modulators of Brugada syndrome and idiopathic ventricular fibrillation. Circ Arrhythm Electrophysiol. 2011;4:352–361. doi: 10.1161/CIRCEP.110.959619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.