Abstract
Ethnopharmacological relevance
Renealmia alpinia is native to the American continent and can be found from Mexico to Brazil, and in the Caribbean islands. It is known as “matandrea” in Colombia, and it has been commonly used in traditional medicine to treat painful diseases and ailments. Based on its traditional uses, it is of interest to evaluate the pharmacologic effects of this plant and its secondary metabolites.
Materials and methods
Methanol and aqueous extracts of wild and micropropagated R. alpinia (leaves) were obtained and chemically compared by High Performance Thin Layer Chromatography (HPTLC). The antinociceptive activity of these extracts was examined using an in vivo assay (Siegmund test). Additionally, the dichloromethane extract of R. alpinia was fractionated and pure compounds were isolated by chromatographic methods. The structure elucidation of isolated compounds was performed by NMR experiments and spectroscopic techniques and comparison with the literature data. Purified compounds were evaluated for their in vitro binding affinity for opioids and cannabinoids receptors.
Results
The dichloromethane extract of the plant’s aerial part afforded sinostrobin (1), naringenin 7,4′-dimethyl ether (2), 2′,6′-dihydroxy-4′-methoxychalcone (3), 4-methoxy-6-(2-phenylethenyl)-2H-pyran-2-one (4), naringenin 7-methyl ether (5) and 3,5-heptanediol, 1,7-diphenyl (6), which were isolated using chromatographic methods. Their chemical structures were established by physical and spectroscopic techniques. The antinociceptive effects observed in mice by extracts of wild and micropropagated plants were similar. The compounds isolated from R. alpinia do not show affinity to opioid or cannabinoid receptors.
Conclusion
Aqueous and methanol extracts of R. alpinia provide antinociceptive and analgesic effects in an in vivo model. These results contribute additional insight as to why this plant is traditionally used for pain management. Also, this is the first comprehensive report of a phytochemical study of R. alpinia.
Keywords: Renealmia alpinia, Antinociceptive, In vitro propagation, HPTLC, Cannabinoid receptor, Opioid receptor
1. Introduction
Renealmia alpinia (Zingiberaceae) is a plant found in many tropical rainforests of Central and South America, among other geographical rainforest. It is distributed from Mexico through Central America and the North of South America including: Peru, Brazil, Guyana, Suriname, Venezuela and Colombia. The plant is used as a febrifuge, analgesic, antiemetic, antiulcer, anticonvulsant and to treat snakebites and other injuries (Otero et al., 2000; Macía, 2003; Ruysschaert et al., 2009; Milliken and Ibert, 1996; Gomez-Betancur and Benjumea, 2014). The ethanolic extract of R. alpinia has been explored for its analgesic activity (Patiño et al., 2013). Later, it was found that the main component of the dichloromethane extract is the flavanone pinostrobin and it possessed inhibitory properties against Bothrops atrox-asper poison (Gomez-Betancur et al., 2014). in fact, three of the pure compounds were tested (all three major compounds), with Siegmund test, to assess activity in animal model on the peripheral nervous system, however, only statistically significant activity was obtained with the isolated major component, we mean with Pinostrobin (Gomez-Betancur et al., 2014). In order to improve the productivity and uniformity of its extracts, the plant was grown in large scale under greenhouse conditions, which allows for the cultivation of more plant material than could be obtained from wild sources (Patiño et al., 2013).
Herein, we describe the identification of some known compounds from the dichloromethane extract of leaves of R. alpinia, as well as, the comparison of the aqueous and methanol extracts obtained from vegetal material of wild and micropropagated plants for their antinociceptive effects in murine pain models. HPTLC was used as a comparative model to profile the dichloromethane and methanol extracts. This is the first attempt to investigate the mechanism involved with the analgesia effects of R. alpinia.
2. Materials and methods
2.1. General experimental procedures
1H and 13C NMR spectra were obtained on Bruker models AMX 400, 500 and 600 MHz NMR spectrometers with standard pulse sequences, operating at 400, 500 and 600 MHz in 1H and 100,125 and 150 MHz in 13C. The chemical shift values were reported in parts per million units (ppm) from tetramethylsilane using known solvent chemical shifts. Coupling constants were recorded in hertz (Hz). Standard pulse sequences were used for COSY, HMQC, HMBC, TOCSY, NOESY, and DEPT. High resolution mass spectra (HRMS) were measured on a Micromass QT of a Micromass spectrometer with a lock spray source.
Column chromatography was carried out on silica gel (70–230 mesh, Merck) and Sephadex LH-20 (Mitsubishi Kagaku, Tokyo, Japan). TLC (silica gel 60 F254) was used to monitor the fractions from column chromatography. Preparative TLC was carried out on silica gel 60 PF254+366 plates (20 × 20 cm2, 1 mm thick). Visualization of the TLC plates was achieved with a UV lamp (λ=254 and 365 nm) and after spraying with anisaldehyde/acid reagent and heating at 100 °C for 1 min.
2.2. Plant material
For this study, two types of plant material were used: one collected from wild flora, and the other obtained through micropropagation. The wild plant material was collected from a farm in the village of Dantas, municipality of San Rafael (Eastern Antioquia- Colombia) and its identification was performed at the Herbarium of the University of Antioquia by Francisco Roldán, Voucher 107316, registration number 6456 RF.
The vegetative material was obtained from three hundred sixty (360) seedlings collected in the village of Titumate, Unguía, Chocó, Colombia and were germinated in a biological station, at the University of Antioquia and identified at the Herbarium of the University of Antioquia. These seedlings were washed once with iodine soap and running water, were disinfected by exposure to NaClO (2% v/v, 25 min), then aseptically transferred to culture flasks with a nutrient medium previously sterilized by moist heat (C/15 psi/15 121 min). The medium is composed of basic salts for Murashige Skoog (Murashige and Skoog 1962; Abdelnour and Escalant 1994): pH=5.8, sucrose (30 g/l) and agar (7g/l). Once established the micropropagated seedlings were subjected to subculture every four weeks and kept under controlled temperature (25 ± 1 °C) and photoperiods corresponding to a long day (16 h light, 50 mol m−2 s−1). Both, the wild cultivar and micropropagated plants were dried at 38 °C for four hours and then pulverized in a mill (Electro Motor Pact at rpm 50 Hz), after which they were deposited and stored in airtight containers in a cool, dry place until ready to use.
2.3. Extracts preparation
Extracts were prepared as follows: 1) wild plants; dried plant material (1500 g) was extracted by maceration with 80% methanol (1000 ml) at room temperature (three times). The extract was concentrated and lyophilized (50 g, yield: 3.3% w/w) and 2) micropropagation plants; dried plant material (150 g) was extracted by maceration with 80% methanol (200 ml) at room temperature (three times). The extract was concentrated and lyophilized (4 g, yield: 2.7% w/w) and stored at 4 °C until use.
Aqueous extract; 50 g dry plant material of wild plant mixed with 400 ml of water at 70 °C for 30 min, was filtered and immediately administered to the mice. For the aqueous extract of micropropagated plants, it was prepared the same way for the wild material. Finally, the dichloromethane extract was prepared by taking wild dried leaves (1500 g) and exposing them to dichloromethane (3L×3) at room temperature. The dichloromethane was removed using a rotary evaporator, and the extract (74.0 g) was stored at 4 °C until used.
2.4. Analysis by High Performance Thin Layer Chromatography (HPTLC)
Methanol and dichloromethane extracts (1.0 mg) of wild and micropropagated plants were redissolved in 1 ml of methanol. HPTLC Silica gel plates 60 F-254 pre-coated with aluminum (Merck, Germany 10 × 10 cm2, and thickness 250 μm) were used. The samples were applied with 100 mL syringe (Hamilton, Bona-duz Schweiz, Camag, and Switzerland) using a Linomat V (Camag, Switzerland) system. 20 μL of sample were applied in bands of 10 mm on the slopes of the plates. The plates were developed vertically in a glass chamber (20 × 10 cm2, Camag, Muttenz, and Switzerland), using the mobile phase hexane-acetone 7:3 at 25 ± 2 °C. After development, the plates were dried and the components were visualized by irradiation of energy at different UV wavelengths, and sprayed with anisaldehyde to visualize the complete profile. The chromatograms were integrated using win CATS evaluation software (Version 1.4.4.6337; Camag, Muttenz, Switzerland). The analysis was carried out in duplicate.
2.5. Isolation of secondary metabolites
Vacuum Liquid Chromatography (VLC) was performed with dichloromethane extract (20 g) of the wild material using different solvent mixtures, eluting sequentially with hexane-dichloromethane (3:1, 1:1, 1:3), dichloromethane–acetone (3:1, 1:1), and acetone-methanol (3:1,1:1,1:3) gradients (1 Leach), successively, to obtain fractions 1 to 8: 1 (0.125 g), 2 (0.471 g), 3 (1.9 g), 4 (3.0 g), 5 (1.0 g), 6 (7.0 g), 7 (1.6 g), and 8 (1.6 g). Fraction 4 (3.0 g) was applied to a silica gel column, eluting with hexane-dichloromethane (6:4) to obtain subfractions 4-1 to 4-4. Subfraction 4-3 (1.8 g) was recrystallized using ethyl acetate-methanol affording colorless crystals of compound 1 (1300 mg). Fraction 5 (1.0 g) was subject to a silica gel column, eluting with hexane-dichloromethane (6:4) to obtain subfractions 5-1 to 5-9. Fraction 5-2 (0.075 g) was applied to a Sephadex LH-20 column eluted with a 1:1 mixture CHCl3-MeOH, and the compound 2, (8.0 mg) was separated as colorless crystals. Fraction 6 (6 g) was recrystallized using methanol to yield orange crystals of compound 3 (500 mg). The mother liquor of the fraction 6 was fractionated by Vacuum Liquid Chromatography (VLC) using different solvent mixtures, eluting sequentially with hexane-dichloromethane (3:1,1:1, 1:3), dichloromethane-ethyl acetate (3:1, 1:1, 1:3), and ethyl acetatemethanol (3:1, 1:1, 1:3) gradients (0.5 L each), successively, to obtain subfractions 6-1 to 6-10. Subfraction 6.6 (5.0 g) was applied to a silica gel column, eluting with hexane-acetone (8:2) to obtain fractions from 6.6-1 to 6.6-20. Fraction 6.6-10 (0.720 g) was applied to a silica gel column, eluting with hexane-ethyl acetate (6:4) to obtain fractions from 6.6.10-1 to 6.6.10-5. Fraction 6.6.10.4 (0.5 g) was applied to a Sephadex LH-20 column, eluting with chloroform-methanol (1:1) to obtain compound 4 (21 mg). The fraction 6.6-13 afforded colorless crystals of compound 5 (700 mg). Fraction 7 (1.5 g) was applied to a silica gel column, eluting with hexane-acetone (7:3) to obtain subfractions 7-1 to 7-4. After evaporation of the solvent subfraction 7-3 afforded 12 mg of compound 6.
2.6. Cell culture
HEK-293 cells stably transfected with opioid receptor subtypes μ, δ, and κ were used to perform the opioid receptor binding assays. These cells were maintained at 37 °C and 5% CO2 in a Dulbecco’s modified Eagle medium (DMEM) nutrient mixture supplemented with 2 mM L-glutamine, 10% fetal bovine serum, penicillin–streptomycin, and either G418 or hygromycin B antibiotic solutions. Membranes for the radioligand binding assays were prepared by scraping the cells in a 50 mM Tris-HCl buffer, followed by homogenization, sonication, and centrifugation for 40 min at 13,650 rpm at 4 °C. These were kept at − 80 °C until used for bioassays. Protein concentration was determined via Bio-Rad Protein Assay (Bradford, 1976).
2.7. Radioligand binding for cannabinoids and opioid receptor subtypes
Compounds 1–6 isolated, from R. alpinia, were submitted for biological evaluation. They were run in competition binding assays against both cannabinoid receptor subtypes and all three opioid receptor subtypes (León et al., 2013). Cannabinoid binding took place under the following conditions: 10 μM of each compound was incubated with 0.6 nM [3H] CP 55.940 and 10 μg of CB1 or CB2 membranes for 90 min in a silanized 96-well plate. The reaction was terminated via rapid vacuum filtration through GF/B filters presoaked with 0.3% bovine serum albumin (BSA) using a Perkin-Elmer 96-well Unifilter followed by 10 washes with 50 mM Tris-HCl. Plates were read using a Perkin-Elmer Topcount. Opioid binding assays were performed under the following conditions: 10 μM of each compound was incubated with [3H]-DAMGO (μ), [3H]-U-69,593 (κ), or [3H]-enkephalin (δ) for 60 min in a 96-well plate. Percent binding was calculated as the average of the triplicate tested at 10 μM. Each sample concentration point of the compounds tested in dose response was in triplicate, and each compound showing activity was tested at least three times. The reaction was terminated via rapid vacuum filtration through GF/B filters presoaked with 0.3% bovine serum albumin (BSA) using a Perkin-Elmer 96-well Unifilter followed by 10 washes with 50 mM Tris-HCl. Plates were read using a Perkin-Elmer Topcount. Total binding was defined as binding in the presence of 1.0% DMSO. Nonspecific binding was the binding observed in the presence of 10 μM DAMGO (μ), nor-binaltorphimine (κ), or DPDPE (δ). Specific binding was defined as the difference between total and nonspecific binding. Percent binding was calculated using the following formula: (binding of compound – nonspecific binding) × 100/specific binding.
2.8. Experimental animals
Swiss mice, male and female, weighing between 20–25 g were used after an adaptation period of 3 days, forming 18 groups of 4 mice each. The animals were obtained from the vivarium of the Research Office of the University of Antioquia and maintained according to the conditions contained in the Guidelines of the Canadian Council for the care of experimental animals and the Guide for the Care and Use Laboratory Animals of the National Institutes of Health in the United States (Consejo Canadiense, 1998). Mice were placed in transparent cages propylene, the temperature in the experimental room was 22 ± 3 °C with a relative humidity of 60–70% and artificial lighting and alternating cycles of 12 h light and 12 h of darkness. Animals were fasted 6 h before the experiment and provided “ad libitum” water.
2.9. Test of Siegmund
This test assesses the peripherally analgesic activity according to the procedure described by Siegmund (Siegmund et al., 1957). Mice were treated orally, with different doses of the aqueous extracts (infusions 50,100, 200 and 300), and the methanol extract at doses of 50,100, 200 and 300 mg/kg. Control group was treated with PBS (phosphate buffer solution). After 30 min, the animals were given phenylquinone (4 mg/Kg) i.p; after a latency period of 5 min, the writhing of the hind legs and torsion of the back–abdominal musculature in each mouse was counted for 10 min. A treaty with the vehicle solution as a negative control and a positive control group treated with ibuprofen as reference drug at a dose of 75 mg/Kg group was used.
2.10. Statistical analysis
Results were analyzed using one-way ANOVA followed by a test of Dunnett’s (Dunnett and Gent, 1977) to compare the control group (vehicle) with the treated groups was performed. All results are expressed as mean±SEM (standard error of the mean). Significant differences were considered when the p values were at least p<0.001.
3. Results and discussion
3.1. Structural elucidation
Chromatographic separation of the dichloromethane extract of the aerial part of wild R. alpinia aerial parts fforded six compounds (Fig. 1). Structure elucidation of all compounds was performed by 1D and 2D NMR, EIMS, and by comparison with data from the literature. The compounds were identified as pinostrobin (1) (González et al., 1989), naringenin 7,4′-dimethyl ether (2) (Iwu and Chiori, 1984), 2′,6′-dihydroxy-4′-methoxychalcone (3) (Hu et al., 2006), 4-methoxy-6-(2-phenylethenyl)-2H-pyran-2-one (4) (Orapin et al., 2005), naringenin 7-methyl ether (5) (Agrawal, 1989) and 3,5-heptanediol, 1,7-diphenyl (6) (Kuroyanagi et al., 1983). The absolute stereochemistry for the flavanone 1, was found to be 2S by comparing the specific rotation value of our isolate (− 48.7°; c 0.41, CHCl3) with the value reported by Kinghorn (− 57.5°; c 0.80, CHCl3) (Su et al., 2003). The stereochemistry for the flavanone 2 was assumed to be S for biogenetic considerations. Compound 5 was isolated as a racemic mixture. A literature search showed that most of the flavanones isolated from natural sources have the 2S absolute configuration, which is in agreement with our results (Su et al., 2003; Yoshikawa et al., 1998). The relative stereochemistry of the double bond in the compounds 2 and 4 was determined as E based on the coupling constant values and comparison with published values (Hu et al., 2006; Orapin et al., 2005). Therefore, the phytochemical composition of R. alpinia showed the presence of flavonoids, diarylheptanoids, and kavalactones. Also, several saponins were detected by NMR and TLC’s analysis, but were not isolated. In a previous study, it was demonstrated that 1 is responsible, in part, for the analgesic and anti-inflammatory activity of R. alpinia (Gomez-Betancur et al., 2014). Compounds 2–6 are reported for first time from this species.
Fig. 1.
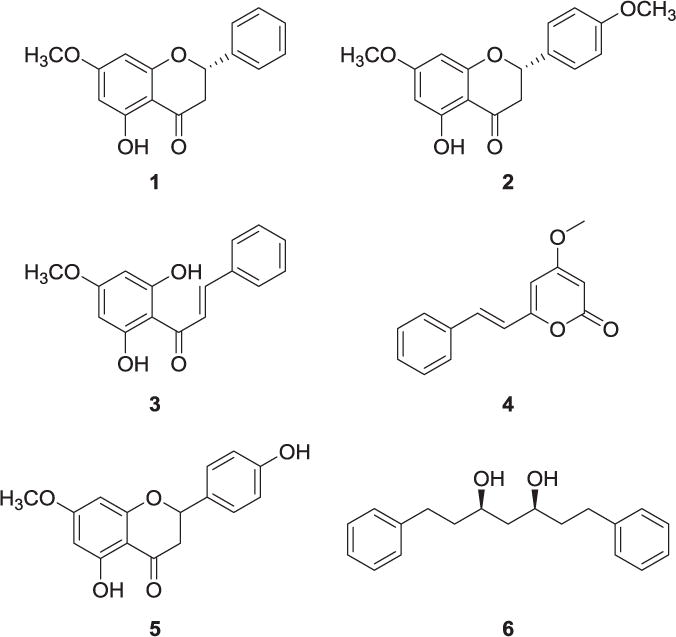
Chemical structure of metabolites isolated from Renealmia alpinia.
3.2. Analysis by High Performance Thin Layer Chromatography (HPTLC)
In order to compare the typical profile of the dichloromethane and methanol extracts of R. alpinia, we carried out an HPTLC study. HPTLC methodology is reliable, fast and cost efficient protocol that can be used to do qualitative and quantitative analysis of secondary metabolites extracted from natural sources (Lebot et al., 2014; Sagi et al., 2014). In Fig. 2 (upper part), the densitograms obtained at 200 nm, 254 nm and 365 nm showed differences between the analyzed extracts. It is clear a greater chemical wealth exists in methanol extracts obtained from wild than micropropagated plants, showing less concentration of metabolites with an unclear profile. These qualitative differences between the amounts of compounds in wild plants and micropropagated cultures can be explained by the differences between in vivo and in vitro growth conditions. Since abiotic factors such as humidity, temperature, pH and soil nutrients create environmental stress on the plant, this is likely to result in production of additional secondary metabolites (Dudareva and Pichersky, 2000). In addition to the abiotic factors, the plants exposed to abiotic stimulus with which they have co-evolved, also promote the development of different biosynthetic pathways through which a large variety of secondary metabolites are biosynthesized (Dudareva and Pichersky, 2000). These results in a variety of compounds that were not observed in the extracts obtained under micropropagation conditions. However, when the comparison is between dichloromethane and methanol extracts from wild plants, a greater enrichment in dichloromethane extract is observed (Fig. 2, right part), showing the extraction efficiency of this organic solvent. Comparison of the pure compounds 1–3, 5 to the extract is showed in Fig. 3.
Fig. 2.
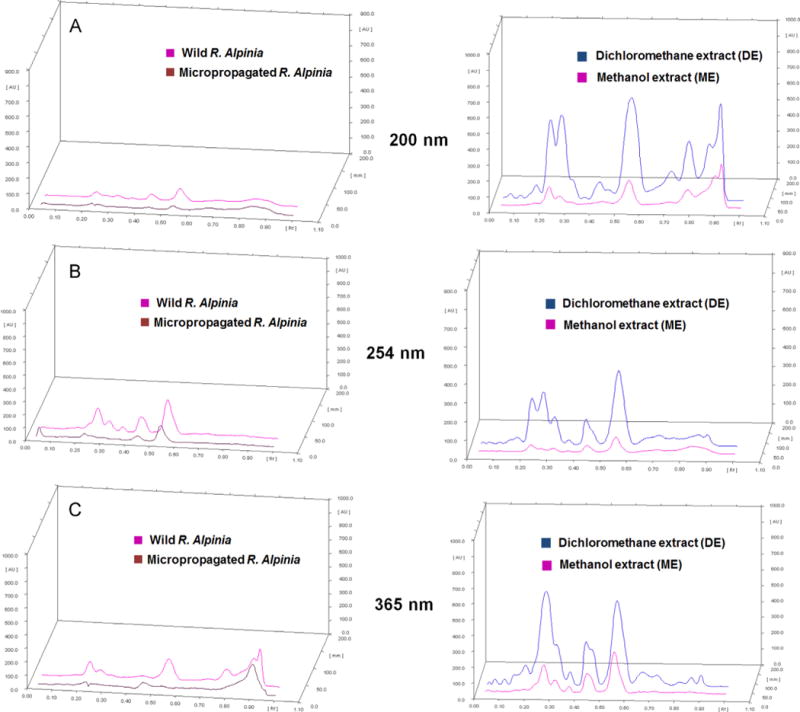
Chromatographic analysis by HPTLC. Left part: comparison of the methanol extracts of R. alpinia collected from the wild and micropropagation. Right part: comparison of dichloromethane (DE) and methanol (ME) extracts of R. alpinia (wild). Mobile phase: n-hexane: acetone 7:3 vertical linear development chamber saturated with vapor of the mobile phase, anisaldehyde/H2SO4 treatment. A: 200 nm; B: 254 nm; C: 365 nm.
Fig. 3.
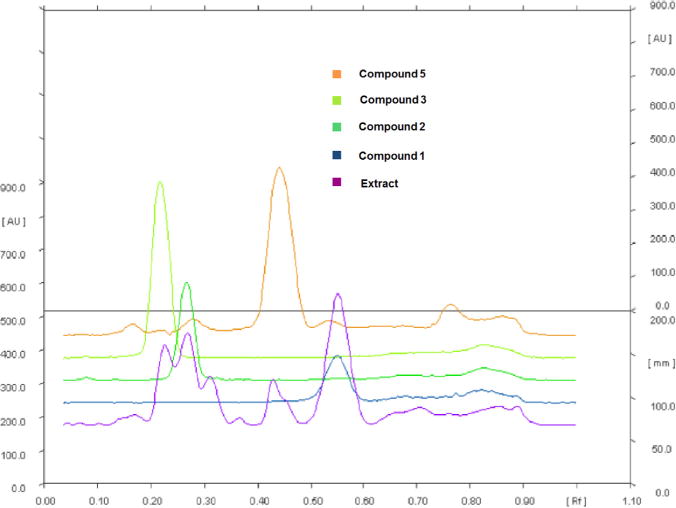
Chromatographic analysis by HPTLC. Compounds 1, 2, 3 and 5 of R. alpinia and the dichloromethane extract collected from the wild. Mobile phase: n-hexane: acetone 7:3 vertical linear development chamber saturated with vapor of the mobile phase, anisaldehyde/H2SO4 treatment. at 254 nm.
3.3. Antinociceptive activity of aqueous and methanol extracts from R. alpinia, obtained from wild flora and micropropagation
A decrease in pain manifestation from phenylquinone-induced writhing was observed in all animals treated with both aqueous and methanol extracts of R alpinia from the wild and micropropagated plant material (Table 1). However, methanol extracts prepared from wild material demonstrated higher percentages of pain inhibition. The methanol extract of wild R. alpinia, at doses of 50,100,200 and 300 mg/Kg, showed dose-dependent inhibition percentages of 57.4%, 63.8%, 66.2% and 74.0%, respectively when compared with the control group. The methanol extract of R. alpinia prepared from micropropagation, doses of 50,100, 200 and 300 mg/Kg, showed dependent inhibition of 31.8%, 60.9%, 62.1% and 63.3%, respectively (Table 1). Percentage inhibition of writhing was calculated using the following formula:
Table 1.
Effect of oral administration of methanol extract at doses of 50, 100, 200 and 300 mg/Kg of R. alpinia collected from the wild flora (RAs) and micropropagated cultured (RAmp), on the number of phenylquinone-induced writhing produced in experimental animals.
| Group | Extract | N | Doses (mg/kg) | No. of writhing (SEM±SD) | Percent of inhibition (%) |
|---|---|---|---|---|---|
| Control − | – | 4 | – | 42.25±6.4 | – |
| Ibuprofen (+) | – | 4 | 75 | 9.50±0.6* | 77.5 |
| RAs | MeOH | 4 | 50 | 18.0±2.6** | 57.4 |
| RAs | MeOH | 4 | 100 | 15.3±2.6** | 63.8 |
| RAs | MeOH | 4 | 200 | 14.3±3.1** | 66.2 |
| RAs | MeOH | 4 | 300 | 11.0±0.7** | 74.0 |
| RAmp | MeOH | 4 | 50 | 28.80±3.1** | 31.8 |
| RAmp | MeOH | 4 | 100 | 16.52±3.3** | 60.9 |
| RAmp | MeOH | 4 | 200 | 16.0±1.4** | 62.1 |
| RAmp | MeOH | 4 | 300 | 15.5±2.8** | 63.3 |
SEM±SD: Standard deviation more or less error; Significant differences were considered when p is at least.
p<0.001.
p<0.0001.
Meanwhile, infusions prepared at 50, 100, 200 and 300 mg/Kg obtained from wild plant material, the results showed pain dose-dependent protection of 46.7%, 62.6%, 66.2% and 77.5% respectively. In contrast, infusions prepared at 50, 100, 200 and 300 mg/Kg from micropropagated plant material, the results showed pain dose-dependent protection of 42.0%, 50.8%, 65.0% and 63.3%, respectively (Table 2).
Table 2.
Effect of oral administration of aqueous extracts at 50, 100, 200 and 300 mg/Kg of RA collected from the wild flora (RAs) and micropropagated cultured (RAmp), the number of phenylquinone-induced writhing produced in experimental animals.
| Group | Extract | N | Doses (mg/kg) | No. of writhing (SEM±SD) | Percent of inhibition (%) |
|---|---|---|---|---|---|
| Control | – | 4 | – | 42.25±6.4 | – |
| Ibuprofen | – | 4 | 75 | 9.50±0.3** | 77.5 |
| RAs | Infusion | 4 | 50 | 22.5±4.9** | 46.7 |
| RAs | Infusion | 4 | 100 | 15.8±1.7** | 62.6 |
| RAs | Infusion | 4 | 200 | 14.3±2.3** | 66.2 |
| RAs | Infusion | 4 | 300 | 9.50±0.6** | 77.5 |
| RAiv | Infusion | 4 | 50 | 24.5±3.1** | 42.0 |
| RAiv | Infusion | 4 | 100 | 20.8±1.9** | 50.8 |
| RAiv | Infusion | 4 | 200 | 14.8±2.0** | 65.0 |
| RAiv | Infusion | 4 | 300 | 11.3±1.4** | 73.3 |
SEM±SD: Standard deviation more or less error; Significant differences were considered when p is at least.
p<0.001.
p<0.0001.
3.4. Radioligand binding of secondary metabolites isolated from R. alpinia
The test results of binding affinity of the secondary metabolites isolated from R. alpinia with cannabinoids (CB1 and CB2 subtypes) and opioid receptors (subtypes δ, κ, and μ) are shown in Table 3. This data indicates that the pain relieving property of the metabolites is most likely not due to the endocannabinoid or opioid systems. Given that these compounds are found in both dichloromethane and methanolic extracts, the analgesic effect of this plant could be associated with the anti-inflammatory action and mediating its effects through a peripheral mechanism.
Table 3.
Cannabinoids (subtypes CB1 and CB2) and opioids receptors (subtypes δ, κ, and μ) binding affinity assay of the secondary metabolites isolated from R. alpinia. Concentration Tested (10 μM).
| Compound | Cannabinoid receptors (%)
|
Opioid receptors (%)
|
|||
|---|---|---|---|---|---|
| CB1 | CB2 | δ Delta | κ Kappa | μ Mu | |
| 1 | – | 6.6 | – | – | – |
| 2 | – | 2.5 | – | 14.9 | – |
| 3 | 9.3 | 3.4 | – | 13.3 | 4.4 |
| 4 | 33.3 | 16.7 | 2.8 | 23.7 | 37.4 |
| 5 | – | – | – | 33.5 | 32.0 |
| 6 | – | 7. 8 | – | – | 12.7 |
| AM251 | 97.6 | 83.3 | – | – | – |
| AM630 | 77.6 | 95.9 | – | – | – |
| Naloxone | – | – | 99.3 | 98.9 | 99.8 |
AM-251 is an inverse agonist at the CB1 cannabinoid receptor; AM-630 (6-Iodopravadoline) is a compound that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2.
R. alpinia is a plant traditionally used to treat pain, such as headache, stomach ache, etc. The present study confirmed its traditional use and identified therapeutic possibilities as alternatives to pain management. Analgesic studies in animals showed that R. alpinia has a protective effect on pain induced by anti-analgesic substances. These effects are most likely related to the presence of flavonoids, substances which occasionally possess analgesic effects (Mekonnen et al., 2010; Leal et al., 2000; Borgi et al., 2008). In fact, we found earlier that pinostrobin (1), a well-known flavanone, could be responsible for some of the analgesic activity attributed to this plant (Gómez-Betancur et al., 2014). This phytochemical study found that dichloromethane and methanolic extracts from R. alpinia, contains the flavonoids 1, 2, 3 and 5. Flavonoids are known to target prostaglandins and be involved in the late phase of acute inflammation and pain sensation (Rathee et al., 2009). Therefore, the presence of flavonoids might contribute to the analgesic activity of R. alpinia.
These results suggest a significant role of its analgesic action with a mechanism similar to ibuprofen, which inhibits prostaglandin synthesis by inhibiting cyclooxygenase 1 and 2 (COX1 and COX2), leading to decreased formation of precursors of prostaglandins and thromboxanes, resulting in less pain. These results are also supported in the previous study in which a model of acute inflammation was used to test the activity of R. alpinia and pinostrobin (Gómez-Betancur et al., 2014). This model is sensitive to cyclooxygenase enzyme (COX) and has been used to evaluate the effect of non-steroidal anti-inflammatory drug (NSAID), that inhibit the COX enzyme, which is involved in the synthesis of prostaglandins (Ricciotti and Fitzgerald, 2011). Nevertheless, further studies must be performed to determine its exact mechanism.
4. Conclusion
The present study demonstrated that methanol and aqueous extracts of R. alpinia provide antinociceptive effects with significant results in an in vivo model and thus contributes to knowledge about the use of this plant in traditional medicine to treat pain. Also, this is the first comprehensive report of a phytochemical study of R. alpinia of Colombia. Future study should be related to the continued isolation of secondary metabolites of R. alpinia, on the other hand, should continue to explore the analgesic activity to elucidate the mechanism of action of the compound or compounds responsible for this activity on the ground which, apparently, is not related to opioid or cannabinoid receptors.
Supplementary Material
Acknowledgments
This work was supported, in part, by the Comité para el Desarrollo de la Investigación (CODI) of the University of Antioquia (Project CIQF-139) and by Grant number P20GM104932 from the National Institute of General Medical Sciences (NIGMS) a component of the National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIGMS or NIH. Part of this investigation was conducted in a facility constructed with support from research facilities improvement program C06RR14503 from the NIH National Center for Research Resources. The authors also acknowledge the financial support from the Sustainability Strategy 2014–2015 of the Ophidism/Scorpionism Program of University of Antioquia.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.jep.2015.02.012.
Footnotes
Chemical compounds studied in this article: Pinostrobin (1) (PubChem CID: 4101463) naringenin 7,4′-dimethyl ether (2) (PubChem CID: 14057196) 2′,6′-dihydroxy-4′-methoxychalcone (3) (PubChem CID: 5316793) 4-methoxy-6-(2-phenylethenyl)-2H-pyran-2-one (4) (PubChem CID: 164901) naringenin 7-methyl ether (5) (PubChem CID: 14057196)
Conflict of interest
The authors have declared that there is no conflict of interest.
References
- Abdelnour A, Escalant JV. CONCEPTOS BÁSICOS DEL CULTIVO DE TEJIDOS VEGETALES. CATIE (Centro agronómico tropical de investigación y enseñanza); Costa Rica: 1994. [Google Scholar]
- Agrawal PK. Carbon-13 NMR of Flavonoids. Elsevier Publishers; Amsterdam: 1989. [Google Scholar]
- Borgi W, Recio MC, Ríos JL, Chouchane N. Anti-inflammatory and analgesic activities of flavonoid and saponin fractions from Zizyphus lotus (L.) Lam. South African Journal of Botany. 2008;74:320–324. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Olfert ED, Cross BM, McWilliam AA, editors. Consejo Canadiense de Protección de los Animales. Manual sobre el cuidado y uso de los animales de experimentación. Vol. 1. Ottawa, Canada: 1998. Spanish Version. [Google Scholar]
- Dudareva N, Pichersky E. Biochemical and molecular genetic aspects of floral scents. Plant Physiology. 2000;122:627–633. doi: 10.1104/pp.122.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett CW, Gent M. Significance testing to establish equivalence between treatments, with special reference to data in the form of 2 × 2 tables. Biometrics. 1977;33:593–602. [PubMed] [Google Scholar]
- Gómez-Betancur I, Benjumea D. Traditional use of the Renealmia genus and Renealmia alpinia (Rottb.) Maas (Zingiberaceae) (“matandrea”). A review in the treatment of snakebite. Asian Pacific Journal of Tropical Biomedicine. 2014;11:841–849. doi: 10.1016/S1995-7645(14)60292-3. [DOI] [PubMed] [Google Scholar]
- Gómez-Betancur I, Benjumea D, Patiño A, Jiménez N, Osorio E. Inhibition of the toxic effects of Bothrops asper venom by pinostrobin, a flavanone isolated from Renealmia alpinia (Rottb.) MAAS. Journal of Ethnopharmacology. 2014;155:1609–1615. doi: 10.1016/j.jep.2014.08.002. [DOI] [PubMed] [Google Scholar]
- González A, Aguiar ZE, Luis JG, Ravelo AG, Vázquez JT, Domínguez XA. Flavonoids from Salvia texana. Phytochemistry. 1989;28:2871–2872. [Google Scholar]
- Hu YM, Ye WC, Li Q, Tian HY, Wang H, Du HYC. C-Glycosylflavones from Stellaria media. Chinese Journal of Natural Medicines. 2006;4:420–425. [Google Scholar]
- Iwu MM, Chiori CO. Antimicrobial activity of Eupaturium odoratum extracts. Fitoterapia. 1984;55:354–356. [Google Scholar]
- Kuroyanagi M, Noro T, Fukushima S, Aiyama R, Ikuta A, Itokawa H, Morita M. Studies on the constituents of the seeds of Alpinia katsumadai Hayata. Chemical & Pharmaceutical Bulletin. 1983;31:1544–1550. [Google Scholar]
- Leal LK, Ferreira AA, Bezerra GA, Matos FJ, Viana GS. Antinociceptive, anti-inflammatory and bronchodilator activities of Brazilian medicinal plants containing coumarin: a comparative study. Journal of Ethnopharmacology. 2000;70:151–159. doi: 10.1016/s0378-8741(99)00165-8. [DOI] [PubMed] [Google Scholar]
- Lebot V, Do TKT, Legendre L. Detection of flavokavins (A, B, C) in cultivars of kava (Piper methysticum) using high performance thin layer chromatography (HPTLC) Food Chemistry. 2014;151:554–560. doi: 10.1016/j.foodchem.2013.11.120. [DOI] [PubMed] [Google Scholar]
- León F, Gao J, Dale OR, Wu Y, Habib E, Husni AS, Hill RA, Cutler SJ. Secondary metabolites from Eupenicillium parvum and their in vitro binding affinity for human opioid and cannabinoid receptors. Planta Medica. 2013;79:1756–1761. doi: 10.1055/s-0033-1351099. [DOI] [PubMed] [Google Scholar]
- Macía MJ. Renealmia alpinia (Rottb.) Maas (Zingiberaceae): planta comestible de la Sierra Norte de Puebla (México) Anales Jardin Botanico Madrid. 2003;60:183–187. [Google Scholar]
- Mekonnen T, Urga K, Engidawork E. Evaluation of the diuretic and analgesic activities of the rhizomes of Rumex abyssinicus Jacq in mice. Journal of Ethnopharmacology. 2010;127:433–439. doi: 10.1016/j.jep.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Milliken W, lbert B. The use of medicinal plants by the Yanomami Indians of Brazil. Economic Botany. 1996;50:10–25. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Otero R, Fonnegra R, Jiménez SL, Núñez V, Evans N, Alzate SP, García ME, Saldarriaga M, Valle GD, Osorio RG, Díaz A, Valderrama R, Duque A, Vélez HN. Snakebites and Ethnobotany in the northwest region of Colombia. Part I: traditional use of plants. Journal of Ethnopharmacology. 2000;71:493–504. doi: 10.1016/s0378-8741(00)00243-9. [DOI] [PubMed] [Google Scholar]
- Patiño AC, Benjumea DC, Pereañez JA. Inhibition of venom serine proteinase and metalloproteinase activities by Renealmia alpinia (Zingiberaceae) extracts: comparison of wild and in vitro propagated plants. Journal of Ethnopharmacology. 2013;149:590–596. doi: 10.1016/j.jep.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Rathee P, Chaudhary H, Rathee S, Rhatee D, Kumar V, Kohli K. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflammation & Allergy: Drug Targets. 2009;8:229–235. doi: 10.2174/187152809788681029. [DOI] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arteriosclerosis, Thrombosis and Vascular Biology. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruysschaert S, Van Andel T, Van de Putte K, Van Damme P. Bathe the baby to make it strong and healthy: plant use and child care among Saramaccan Maroons in Suriname. Journal of Ethnopharmacology. 2009;121:148–170. doi: 10.1016/j.jep.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Sagi S, Avula B, Wang Y-H, Zhao J, Khan IA. Quantitative determination of seven chemical constituents and chemo-type differentiation of chamomiles using high-performance thin-layer chromatography. Journal of Separation Science. 2014;37:2797–2804. doi: 10.1002/jssc.201400646. [DOI] [PubMed] [Google Scholar]
- Siegmund E, Cadmus R, Lu G. A method for evaluating both non-narcotic and narcotic analgesics. Experimental Biology and Medicine. 1957;95:729–731. doi: 10.3181/00379727-95-23345. [DOI] [PubMed] [Google Scholar]
- Su B-N, Park EJ, Vigo JS, Graham JG, Cabieses F, Fong HHS, Pezzuto JM, Kinghorn AD. Activity-guided isolation of the chemical constituents of Muntingia calabura using a quinone reductase induction assay. Phytochemistry. 2003;63:335–341. doi: 10.1016/s0031-9422(03)00112-2. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Shimada H, Nishida N, Li Y, Toguchida I, Yamahara J, Matsuda H. Antidiabetic principles of natural medicines II. Aldose reductase and α-glucosidases inhibitors from Brazilian natural medicines, the leaves of Myrcia multiflora DC. Myrtaceae): structures of myrciacitrins I and II and myrciaphenones A and B. Chemical & Pharmaceutical Bulletin. 1998;46:113–119. doi: 10.1248/cpb.46.113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


