Abstract
Galectin-1 (Gal-1)-binding to Gal-1 ligands on immune and endothelial cells can influence melanoma development through dampening anti-tumor immune responses and promoting angiogenesis. However, whether Gal-1 ligands are functionally expressed on melanoma cells to help control intrinsic malignant features remains poorly understood. Here, we analyzed expression, identity and function of Gal-1 ligands in melanoma progression. Immunofluorescent analysis of benign and malignant human melanocytic neoplasms revealed that Gal-1 ligands were abundant in severely-dysplastic nevi as well as in primary and metastatic melanomas. Biochemical assessments indicated that melanoma cell adhesion molecule (MCAM) was a major Gal-1 ligand on melanoma cells that was largely dependent on its N-glycans. Other melanoma cell Gal-1 ligand activity conferred by O-glycans was negatively regulated by α2,6 sialyltransferase ST6GalNAc2. In Gal-1-deficient mice, MCAM-silenced (MCAMKD) or ST6GalNAc2-overexpressing (ST6O/E) melanoma cells exhibited slower growth rates, underscoring a key role for melanoma cell Gal-1 ligands and host Gal-1 in melanoma growth. Further analysis of MCAMKD or ST6O/E melanoma cells in cell migration assays indicated that Gal-1 ligand-dependent melanoma cell migration was severely inhibited. These findings provide a refined perspective on Gal-1 – melanoma cell Gal-1 ligand interactions as contributors to melanoma malignancy.
Keywords: sialyltransferase, cell surface carbohydrates, tumor cell adhesion, galectin, melanoma
Introduction
Galectin-1 (Gal-1) is one of 15 evolutionarily-conserved S-type lectins that bind lactosamine sugars on discrete cell membrane proteins and ECM components (Camby et al., 2006; Cho and Cummings, 1995). Gal-1 is expressed by effector T and B cells, inflammatory macrophages, decidual natural killer (NK) cells, FoxP3+ regulatory T cells and endothelial cells (EC), where it plays a key role in suppressing innate and adaptive immune responses (Baum et al., 1995; Blois et al., 2007; Garin et al., 2007; Koopman et al., 2003; Kopcow et al., 2008; Ouyang et al., 2011; Rabinovich et al., 1998; Thijssen et al., 2008; Zuniga et al., 2001). Gal-1 is also elevated in certain tumor cells, where it promotes tumor growth and cancer progression by immune tolerizing effects on dendritic cells (DC) and effector T cells and by angiogenesis via direct interactions with ECs (Cedeno-Laurent et al., 2012b; Cedeno-Laurent et al., 2012c; Demydenko and Berest, 2009; Ilarregui et al., 2009; Laderach et al., 2013; Lefranc et al., 2011; Mathieu et al., 2012; Rubinstein et al., 2004; Thijssen et al., 2010; Thijssen et al., 2006). One recent report identified Gal-1 on mesenchymal stem cells (MSC) as a positive regulator of tumor growth (Szebeni et al., 2012). Gal-1 elicits its effects via binding to glycoprotein (or glycolipid) counter-receptor ligands that confers a Gal-1 ligand activity and subsequent initiation of functional activities, including adhesion/migration, immune suppression and angiogenesis. Our descriptions herein define a Gal-1 ligand as a preferred membrane protein bearing poly-N-acetyllactosamine(s) on asparagine (N)- and/or serine/threonine (O)-glycans in an optimal orientation for Gal-1-binding. Understanding how Gal-1 ligands regulate tumor growth could provide important insights on the development of anti-cancer therapeutics and lay the foundation for generation of reliable diagnostic markers for tumor growth and metastasis.
Malignant melanoma is a well-documented tumor model leveraging Gal-1 – Gal-1 ligand interactions. Complete ablation of melanoma- and host-derived Gal-1 expression severely limits melanoma growth (Banh et al., 2011; Cedeno-Laurent et al., 2012b; Rubinstein et al., 2004; Thijssen et al., 2010; Thijssen et al., 2006; Toscano et al., 2007). Gal-1 facilitates melanoma immune evasion by reducing the number of IFN-γ-producing T helper (Th) cells and cytolytic T cells (CTLs), including melanoma-specific CTLs (Cedeno-Laurent et al., 2012b; Ilarregui et al., 2009; Rubinstein et al., 2004; Toscano et al., 2007). Depending on local concentrations, Gal-1 can engage DC/T cell Gal-1 ligands CD7, CD43 and/or CD45 and either initiate a pro-apoptotic activity or a regulatory signaling circuit (Cedeno-Laurent et al., 2010; Cedeno-Laurent et al., 2012a; Cedeno-Laurent et al., 2012b; Fulcher et al., 2009; Hernandez et al., 2006; Nguyen et al., 2001; Pace et al., 2000; Perillo et al., 1995; Suzuki et al., 2005a). Alternatively, melanoma- and host-derived Gal-1 bind ECs and support a number of pro-angiogenic activities, including EC survival, migration, and capillary formation in vitro and in vivo (Croci et al., 2014; Laderach et al., 2013; Mathieu et al., 2012; Szebeni et al., 2012; Thijssen et al., 2010; Thijssen et al., 2008; Thijssen et al., 2006). Gal-1-binding to CD146, otherwise known as melanoma cell adhesion molecule (MCAM), on ECs, in fact, can encourage survival (Jouve et al., 2013). While Gal-1 – Gal-1 ligand interactions clearly promote melanoma growth through immunosuppressive and pro-angiogenic mechanisms, Gal-1’s direct impact on melanoma cells is not fully understood. One study shows that Gal-1 on melanoma cells can mediate homotypic cell-cell interactions, in part, via binding to Gal-1 ligand, 90K/MAC-2BP (Tinari et al., 2001).
Here, we performed a comprehensive assessment into expression, identity and regulation of Gal-1 ligands on melanoma cells and related malignant behavior. Dual immunofluorescence (IF) analysis of Gal-1 ligand expression using an innovative Gal-1 probe showed that malignant melanomas, including melanoma in situ, radial and vertical growth phase melanomas and melanoma metastases, contained an abundance of Gal-1 ligand, which was largely absent on epidermal melanocytes in normal human skin, in benign nevi and in uninvolved skin adjacent to the malignant lesion. Of note, dermal melanocytic nests in an atypical nevus with spindle cell proliferation, inflammation and features of regression also were also positive for Gal-1 ligands. Biochemical analysis showed that MCAM, which, itself, has been implicated in melanomagenesis (Jean et al., 1998; Mills et al., 2002; Xie et al., 1997), was one of the major melanoma cell Gal-1 ligands and was largely dependent on its N-glycans for Gal-1-binding. We found that O-glycans, to a lesser extent, also contributed to total melanoma cell Gal-1 ligand activity. Gene expression analysis revealed that α2,6 sialyltransferase ST6GalNAc2, whose α2,6 sialylation activity on a Core 1 O-glycan prevents synthesis of extended Core 2 O-glycans that bind Gal-1 (Earl et al., 2010; Nguyen et al., 2001), was significantly downregulated in malignant melanoma cells compared with human epidermal melanocytes (HEM). When MCAM-silenced (MCAMKD) or ST6GalNAc2-overexpressing (ST6O/E) melanoma cells were grown in mice deficient in Gal-1, tumor growth was significantly reduced. Likewise, Gal-1 ligand-dependent migration of MCAMKD or ST6O/E melanoma cells on ECM was inhibited, suggesting that Gal-1 ligand activity may be promoted by high MCAM and low ST6GalNAc expression. Together, these data demonstrate a key role for melanoma cell Gal-1 ligands, including MCAM, and of Gal-1 ligand regulator, ST6GalNAc2, as functional correlates with malignant behavior.
Results
Expression of Gal-1 ligands is elevated in malignant melanomas
To investigate the relationship between Gal-1 ligand expression and malignant melanoma, we used dual immunofluorescence (IF) to determine Gal-1 ligand expression on benign and malignant melanocytes in human biological specimens. We stained for S100 (in red), a marker of melanocyte-lineage cells and Gal-1 ligands with mouse Gal-1 – human immunoglobulin chimera (Gal-1hFc) (in green) or dmGal-1hFc (a non-binding double mutant control) as described (Cedeno-Laurent et al., 2012b). Because S100 is also found in Langerhans cells, this staining strategy was intentionally implemented to encompass a predominant epidermal immune cell subset that could potentially bear Gal-1 ligand.
We found that S100+ cells encompassing both Langerhans cells and melanocytes in the epidermis of normal skin and a benign nevus were negative for Gal-1 ligand expression (Figure 1a and b). However, melanocyte-lineage cells in a melanoma in situ were positive for both S100 and Gal-1 ligand (merged in yellow) (Figure 1c). Western blotting lysates from normal human epidermal melanocytes (HEM) and human melanoma G361 lysates (Figure 1d) and FACS staining primary human metastatic melanoma cells and human G361 melanoma cells (Figure 1e) with Gal-1hFc revealed conspicuous elevation in Gal-1 ligand(s) on melanomas. Of note, detection of surface Gal-1 ligands was not significantly masked by well-described melanoma cell galectins, Gal-1, -3 and -9 (Braeuer et al., 2012), as we did not stain appreciable levels of Gal-1, -3 and -9 on the melanoma cell surface (Supplemental Figure 1). To verify Gal-1 ligand staining with statistical significance, IF analysis was performed on tissue microarrays (containing 56 primary and 20 metastatic melanomas and 24 benign pigmented nevi) using Gal-1hFc (in green). In this case, dual IF staining was not employed due to the potential variations in S100 expression by metastatic melanoma cells (Aisner et al., 2005). Data demonstrated significantly higher mean intensities of Gal-1 ligand staining on primary and metastatic melanomas compared levels on benign nevi (p<0.001) (Figure 1f).
Figure 1. Gal-1 ligands are differentially expressed on normal human melanocytes and human melanoma cells.
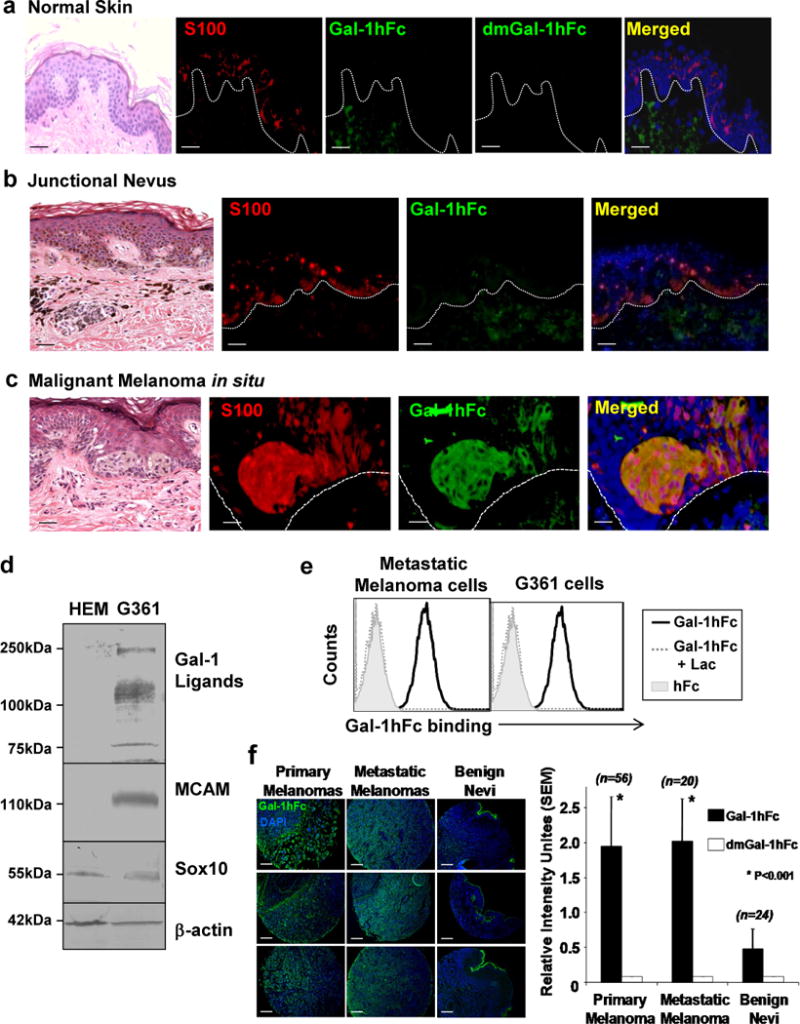
Dual IF analysis of Gal-1 ligands with Gal-1hFc (in green) or dmGal1-hFc control and S100 with anti-S100A-B (in red) was performed on FFPE-sections of normal human skin (a), a benign junctional nevus (b) and melanoma in situ (c). In (d), Western blot analysis of Gal-1 ligands (with Gal-1hFc), MCAM polypeptide, SOX10 and ß-actin in HEM and G361 melanoma cell lysates was performed. Primary melanoma cells and G361 melanoma cells were FACS analyzed with Gal-1hFc or controls (e). In (f), IF analysis of Gal-1 ligands was performed on TMAs containing primary (n=56) and metastatic melanomas (n=20) and benign nevi (n=24). (*p<0.001; Statistically significance compared with benign nevi). Scale bars = 100μm.
Additional dual IF staining of a premalignant nevus with atypia and inflammation showed that dermal S100+ melanocytic nests were strongly positive for Gal-1 ligand, suggesting that melanocyte localization to dermis may correspond with Gal-1 ligand up-regulation. Yet, dual IF staining of malignant melanomas, including radial and vertical growth phase subsets, showed that malignant melanocytic nests located in the epidermis and dermis were strongly positive for Gal-1 ligand(s) (Figure 2b–d). Of note, epidermal melanocytes outside of the tumor margin did not stain for Gal-1 ligand (Figure 2b and c), highlighting the capacity of this method to distinguish Gal-1 ligand+ malignant melanocytes from benign epidermal counterparts.
Figure 2. A pre-malignant melanocytic tumor and malignant melanomas are strongly positive for Gal-1 ligands.
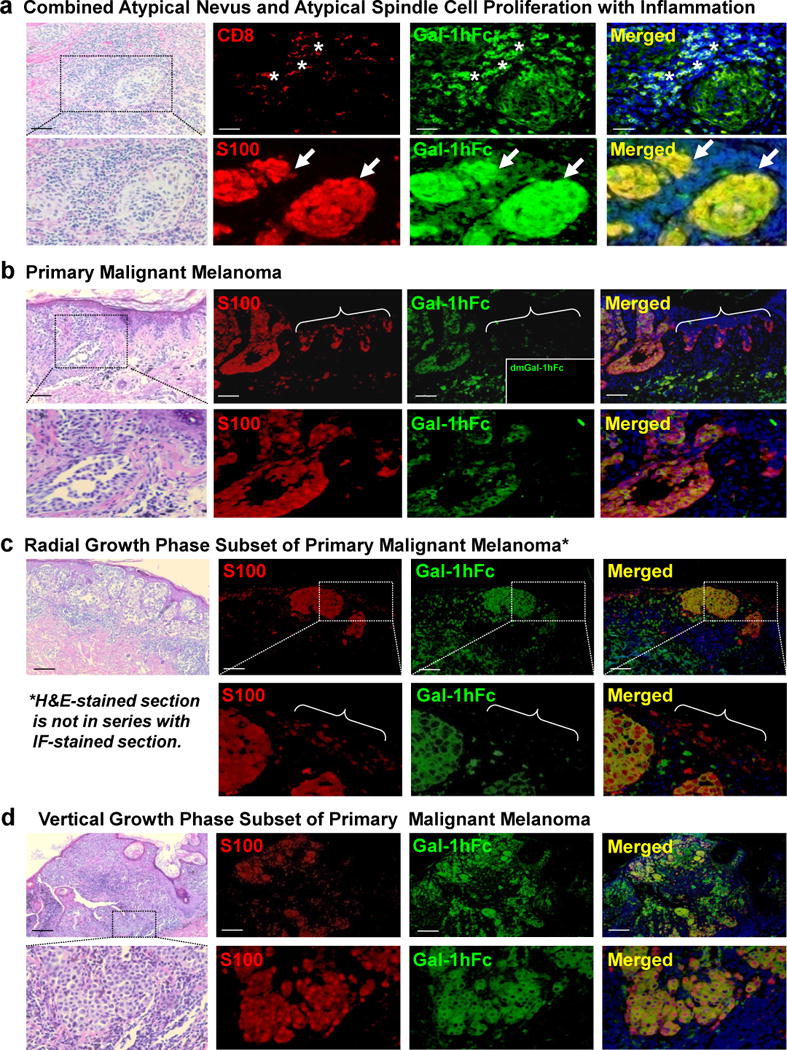
Dual IF analysis of Gal-1 ligands (in green) and CD8 or S100 (in red) was performed on FFPE-sections of (a) a combined atypical nevus and an atypical spindle cell proliferation with inflammation (Asterisks= Gal-1 ligand+ CD8+ cells) (Arrows= Gal-1 ligand+ S100+ dermal nests). Dual IF staining of primary cutaneous melanomas (b), including radial (c) and vertical (d) growth phase subsets, was also performed. Brackets in (b) (Upper Panel) and (c) (Lower Panel) indicated margin tissue where non-malignant epidermal S100+ cells were Gal-1 ligand−. Scale bars = 100μm and photomicrographs enlarged in the lower panels as indicated.
MCAM is a Gal-1 ligand on human melanoma cells
To identify potential Gal-1 ligands on melanoma cells, we used protein G-affinity chromatography and Western blotting to interrogate Gal-1-binding proteins in human melanoma cells using Gal-1hFc as a probe (Cedeno-Laurent et al., 2010; Cedeno-Laurent et al., 2012a). We detected major Gal-1-stained bands in the range of 110–150kDa as well as at 250kDa in melanoma short-term cultures and SK-MEL, SK-MEL2 and G361 melanoma cell lines (Figure 1d and 3a). Blotting MCAM was performed in parallel to control for detection of a common melanoma-specific marker (Figure 3a). Negative control blots probed with secondary Ab alone, with Gal-1hFc and 50mM lactose, or with dmGal-1hFc showed no staining, confirming carbohydrate dependence and Gal-1 ligand authenticity.
Figure 3. Affinity-purification of candidate Gal-1 ligands from human melanoma cells implicates MCAM as a major Gal-1 ligand.
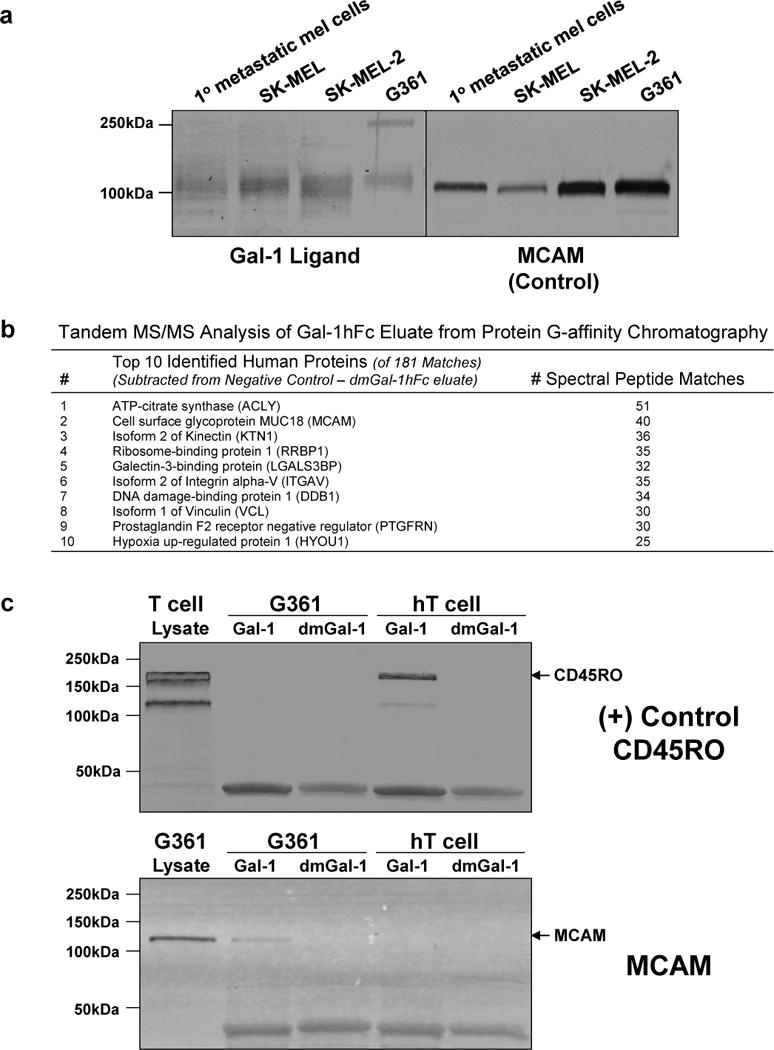
In (a), primary metastatic melanoma cells or melanoma cell line lysates were blotted with Gal-1hFc or control anti-MCAM. As shown in (b), the top 10 proteins and corresponding number of peptide matches identified by tandem MS/MS of elutes from protein G affinity chromatography with Gal-1hFc or negative control dmGal-1hFc and G361 cell lysate are listed. In (c), control activated human T cell or melanoma G361 cell lysate and eluates from protein G affinity chromatography with Gal-1hFc or negative control dmGal-1hFc were blotted with anti-CD45RO or anti-MCAM. Arrows indicate the presence of T cell CD45RO at 190kDa and melanoma cell MCAM at 120kD.
Protein G-affinity chromatography of G361 melanoma cell lysate with Gal-1hFc or dmGal-hFc control and tandem mass spectrometry (MS/MS) analysis of corresponding eluates revealed several potential Gal-1 ligand candidates. The candidate with highest number of peptide matches corresponding to a membrane protein was melanoma cell adhesion molecule (MCAM) (Figure 3b). Examination of the entire list of protein matches indicated the presence of other known human Gal-1 ligand(s), including, in descending order, galectin-3-binding protein (90K/MAC-2BP), lysosomal-associated membrane protein-1 and -2 (LAMP-1/2) and carcinoembryonic antigen (Tinari et al., 2001; Woynarowska et al., 1996) (Supplemental Figure 2). Validation of MCAM as a Gal-1 ligand was ascertained by blotting Gal-1hFc eluate from G361 cell lysates with anti-MCAM antibody. Eluates from non-binding dmGal-1hFc control and from Gal-1hFc-affinity chromatography of Gal-1 ligand+ human activated T cell lysate were examined in parallel to control for Gal-1 specificity. In Figure 3c, a known human T cell Gal-1 ligand, CD45RO, was purified in Gal-1hFc-eluate, while MCAM was isolated from G361 cell lysate. On the other hand, dmGal-1hFc did not purify CD45RO or MCAM, showing dependence on functional Gal-1 (Figure 3c). We additionally immunoprecipitated 90k/MAC-2BP from human A375 melanoma cells and blotted with Gal-1hFc to demonstrate that Gal-1hFc could recognize a human Gal-1 ligand previously identified on A375 cells using human Gal-1 (Supplemental Figure 3) (Iacobelli et al., 1986; Tinari et al., 2001).
To solidify MCAM as a Gal-1 ligand, Western blotting anti-MCAM immunoprecipitates of G361 (Figure 4a) or primary melanoma (Figure 4b) cell lysates with Gal-1hFc was also performed and demonstrated that MCAM indeed binds Gal-1. Anti-MCAM immunoprecipitates from A375 cells were blotted with Gal-1hFc, demonstrating that Gal-1-binding glycans were similarly displayed on MCAM (Figure 4c). Anti-MCAM immunoprecipitates blotted with anti-MCAM confirmed presence of MCAM protein at 120kDa (Figure 4a and c). Control immunoprecipitates with either anti-MCAM or anti-CD45RO in the absence of lysate revealed non-specific stained-Ig bands at 100kDa and 150kDa (Figure 4c). As expected, anti-CD45RO immunoprecipitate from human T cells was blotted with Gal-1hFc, confirming Gal-1hFc’s capacity to authenticate a hallmark human Gal-1 ligand (Figure 4c) (Cedeno-Laurent et al., 2012a). In all, affinity chromatography, Western blotting and immunoprecipitation approaches helped identify MCAM as a putative Gal-1 ligand.
Figure 4. N-glycosylated MCAM binds Gal-1 and is a major contributor of total melanoma cell ligand activity.
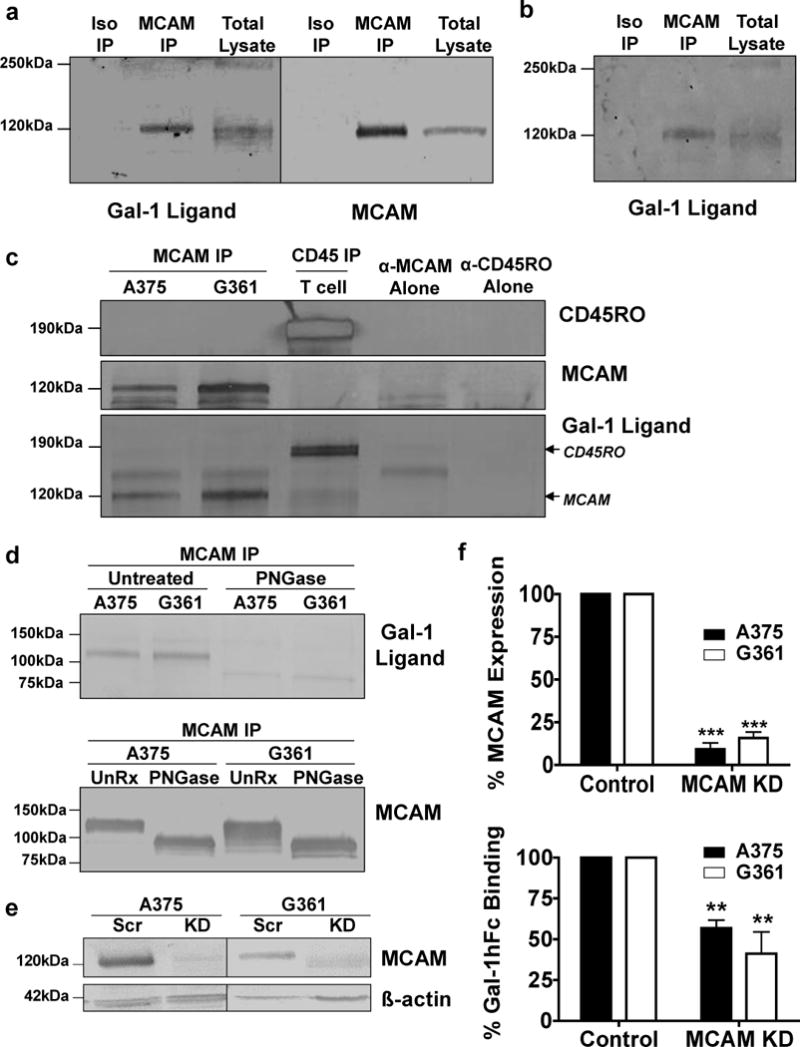
Anti-MCAM immunoprecipitates from G361 (a) or primary melanoma cell lysates (b) were blotted with Gal-1hFc or anti-MCAM. In (c), anti-CD45RO immunoprecipitate from activated T cell lysate or anti-MCAM immunoprecipitate from A375 and G316 cell lysates were blotted with anti-CD45RO, anti-MCAM or Gal-1hFc. Anti-MCAM immunoprecipitate from A375 and G361 cell lysates were treated with PNGase and blotted with Gal-1hFc or anti-MCAM (d). Scr or MCAMKD A375 and G361 cell lysates were blotted with anti-MCAM or anti-ß-actin (e). In (f), Scr or MCAMKD A375 and G361 cells were analyzed for MCAM and Gal-1 ligand by flow cytometry (**p<0.01 and ***p<0.001; statistically significance compared with Scr control). All experiments were performed three-times.
Since MCAM has 8 potential N-glycosylation sites (Lehmann et al., 1989), we examined whether MCAM’s Gal-1-binding determinants resided on N-linked glycans. We treated anti-MCAM immunoprecipitates with PNGase, separated products by SDS-PAGE and blotted with Gal-1hFc or controls to detect Gal-1-binding MCAM. PNGase-treated anti-MCAM immunoprecipitates were also blotted with anti-MCAM to control for MCAM detection. PNGase treatment lowered MCAM’s size to ~85kDa, which is indicative of de-N-glycosylation, and eliminated its Gal-1-binding activity (Figure 4d). To determine the relative contribution of MCAM’s Gal-1 ligand activity, we analyzed Gal-1 ligand expression in A375 and G361 melanoma cells knocked down (KD) for MCAM expression. Using Western blot and FACS analysis, we showed a significant reduction in MCAM expression by 90% (p<0.001) (Figure 4e) and, compared with Scr controls, MCAMKD cell variants exhibited a 40% reduction in Gal-1 ligand activity (Figure 4f). Furthermore, dependency of MCAM for binding human Gal-1 was validated by FACS analysis and confirmed the capacity of our Gal-1hFc formulation to similarly detect human Gal-1 ligands (Supplemental Figure 4) (Tsai et al., 2008). Control treatments containing 50mM lactose or probing with dmGal-1hFc did not detect any measurable Gal-1 ligand. These data suggested that MCAM through its N-glycosylations was a major Gal-1 ligand on melanoma cells.
Alpha2,6 sialyltransferase ST6GalNAc2 is a negative regulator of Gal-1 ligand activity in melanoma cells
Since MCAM-silencing on melanoma cells did not completely lower Gal-1 ligand activity, we subsequently ascertained whether other glycoconjugates could contribute to total cellular ligand activity. Bromelain protease treatment prior to assaying for Gal-1 ligand activity on A375 and G361 melanoma cells indicated that nearly all of the cellular activity was contributed by glycoproteins with a negligible contribution by glycolipids (Supplemental Figure 5a). So, we then treated melanoma cells with an effective complex N-glycan inhibitor, kifunensine, and found that Gal-1 ligand activity was significantly reduced by 80% and 50% in A375 and G361 cells, respectively (p<0.001) (Figure 5a). This suggested that any residual activity above protease treatment level was likely due to O-glycans. In a control experiment, binding of PHA-L, which binds tetra-antennae of complex N-glycans, was completely eliminated, validating N-glycan removal (Supplemental Figure 5b). We next examined whether the membrane protein LAMP-1 identified by Gal-1 affinity chromatography and bearer of putative O-glycan sites, could also serve as a Gal-1 ligand. We observed that LAMP-1 immunoprecipitates from melanoma cell avidly bound Gal-1 (Supplemental Figure 6). These data suggested that LAMP-1, which has been shown to display O-glycans and bind Gal-1 (Ohannesian et al., 1994; Skrincosky et al., 1993), could contribute to melanoma cell Gal-1 ligand activity.
Figure 5. ST6GalNAc2 is downregulated in melanoma cells and is a putative regulator of O-glycan-dependent Gal-1 ligand activity.
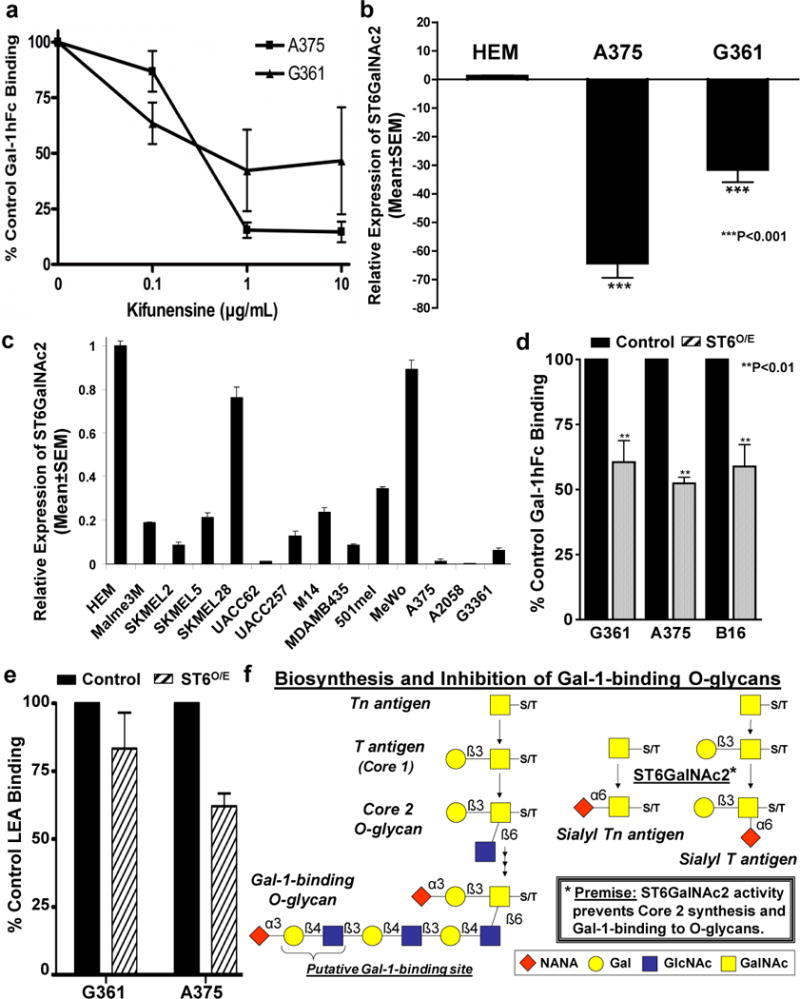
Kifunensine-treated A375 and G361 cells were assayed for Gal-1 ligand activity by flow cytometry with Gal-1hFc or controls (a). Real-time RT-qPCR analysis was performed on HEM, G361 and A375 cells (b) and 11 other melanoma cell lines (c). Relative ST6GalNAc2 expression level was normalized to expression in HEM over 3-experiments and expressed as Mean±SEM. Gal-1hFc- (d) and LEA-binding (e) of control or ST6O/E melanoma cells were analyzed by flow cytometry. In (f), an illustration of ST6GalNAc2’s role on the biosynthesis and putative inhibition of Gal-1-binding to O-glycans. **p<0.01 and ***p<0.001 – statistical significance compared with untreated controls or HEM.
Recent data suggest that high expression of α2,6 sialyltransferase ST6GalNAc2, which transfers sialic acid in a α2,6 linkage to N-acetylgalactosamine on Core 1 O-glycans (Marcos et al., 2004), prevents Gal-3-binding to unmodified Core 1 O-glycan (Murugaesu et al., 2014). Since Gal-1 binds extended Core 2 O-glycans, we investigated whether ST6GalNAc2 could neutralize O-glycan-dependent Gal-1 ligand activity. We hypothesized that ST6GalNAc2 was differentially expressed between Gal-1 ligand− HEM cells and Gal-1 ligand+ melanoma cells. Real-time RT-qPCR analysis revealed that, compared with expression in HEM, ST6GalNAc2 was downregulated in A375 and G361 cells by 65- and 30-fold, respectively (Figure 5b). In fact, ST6GalNAc2 was uniformly downregulated in 13 melanoma cell lines (Figure 5c). To examine the negative role of ST6GalNAc2 in Gal-1 ligand activity, we generated G361 and A375 cells, along with B16 melanoma cells stably overexpressing ST6GalNAc2 and assayed for Gal-1 ligand activity. ST6GalNAc2-overexpressing (ST6O/E) cell variants exhibited ~30% lower Gal-1 ligand activity than vector control cells (p<0.01) (Figure 5d). Assaying for binding of Lycopersicon esculentum lectin (LEA), which binds poly-N-acetyllactosamines known for binding Gal-1 (Earl et al., 2010; Nguyen et al., 2001; Ohannesian et al., 1994; Skrincosky et al., 1993), further showed that ST6O/E cell variants expressed reduced levels of poly-N-acetyllactosamines (Figure 5e). These data suggested that ST6GalNAc2 could potentially serve as a negative regulator of Gal-1-binding to O-glycans (Figure 5f).
Melanoma cell Gal-1 ligands contribute to tumor formation in mice
To investigate whether Gal-1 collaborates with MCAM or other Gal-1 ligands regulated by ST6GalNAc2 to trigger melanoma growth, we assayed the growth of MCAM-silenced (MCAMKD) (Supplemental Figure 7) or ST6O/E B16 melanoma cells in mice deficient in Gal-1. To rule out intrinsic alterations in proliferation due to silencing/overexpression methods, we compared MCAMKD or ST6O/E B16 cell proliferation with vector controls in a CFSE-dilution assay and found no differences in proliferation rates (Supplemental Figure 8). Assessments on longitudinal growth of control, MCAMKD and ST6O/E B16 cells in wild type (wt) mice showed that MCAMKD tumors grew at similar rates as control cells, whereas ST6O/E tumors exhibited slower growth (p<0.01) (Figure 6a). Prior data, in fact, show that melanoma cells expressing variable levels of MCAM grow at similar rates in mice (Wu et al., 2008), which may be associated with MCAM’s pleiotropic role in cancer development (Wang and Yan, 2013). However, as expected, MCAMKD or ST6O/E tumors when inoculated in Gal-1−/− mice grew at significantly slower velocities (p<0.001) (Figure 6a). These data suggested that collaboration of host-derived Gal-1 and melanoma cell Gal-1 ligands, governed by either MCAM or ST6GalNAc2 expression, was necessary for optimal melanoma growth.
Figure 6. In vivo growth of melanoma cells and migration of melanoma cells on Matrigel is regulated, in part, by host Gal-1 and on melanoma cell Gal-1 ligands.
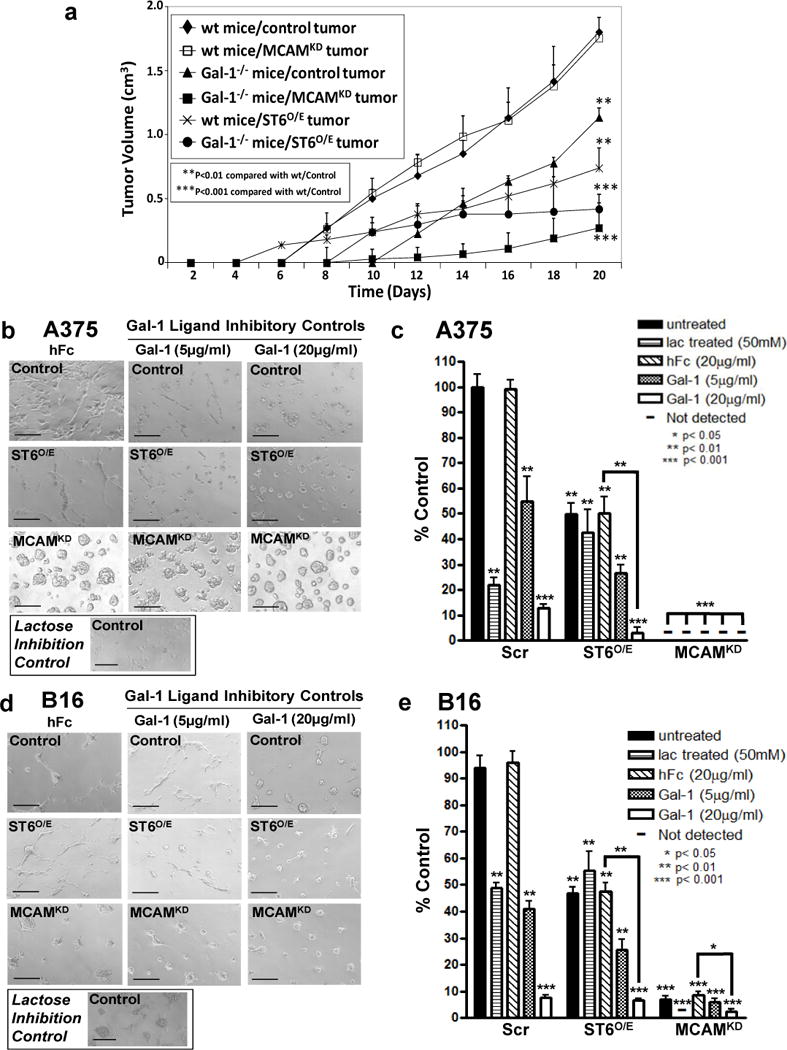
Wt or Gal-1−/− mice were inoculated s.c. with control, ST6O/E or MCAMKD B16 cells and monitored for tumor growth. Mean tumor volumes (SEM) (n=8/group) were calculated and plotted against time (a). In (b–e), control, ST6O/E or MCAMKD A375 and B16 melanoma cells pre-blocked with Gal-1hFc, hFc or lactose were assayed for formation of tube-like structures on Matrigel. Tube-like structures were illustrated in representative phase photomicrographs (Scale bars=100μm). The number of tube-like structures was expressed as % Control hFc-treated cells (*p<0.05, **p<0.01 and ***p<0.001; statistically significance compared with hFc-control cells). Data were collected from at least 3-experiments.
To further investigate MCAM and ST6GalNAc2 in malignant potential of melanoma cells, we examined the ability of MCAMKD and ST6O/E melanoma cells to migrate in a well-described Matrigel assay (Frank et al., 2011). Since the ECM used is rich in Gal-1 (Croci et al., 2012) (Supplemental Figure 9), we were able to assay MCAMKD or ST6O/E melanoma cell migration in a Gal-1 ligand-dependent manner. Though Gal-3 and Gal-9 ligands could also potentially bind Gal-1, requirement for melanoma cell Gal-1 ligands was established by pre-incubating and growing cells in the presence of Gal-1hFc (or hFc control) to bind ligand and interfere with native Gal-1-binding in the Matrigel. Moreover, Gal-1-dependence and galectins in general were substantiated by adding lactose in the assay buffer and in the ECM preparation. While migration of control A375 and B16 cells treated with hFc was observed, pre-incubation with Gal-1hFc significantly reduced migratory activity (Figure 6b–e). To our surprise, MCAMKD A375 cell migratory activity was severely blunted even in the absence of Gal-1hFc, implicating additional non-Gal-1-binding functions (Figure 6b and c) (p<0.001). MCAMKD B16 cells also exhibited blunted migratory activity when compared with control cells (p<0.001), but migration was further reduced in the presence of Gal-1hFc (p<0.01) (Figure 6d and e). ST6O/E A375 and ST6O/E B16 cell migration was also attenuated compared with control cells, and was further inhibited by Gal-1hFc pretreatment (p<0.01). These results suggested that melanoma cell Gal-1 ligands and Gal-1 in ECM were important, in part, for effective migration, which mirrored data on Gal-1’s role in ovarian tumor cell adhesion to ECM (Skrincosky et al., 1993).
Discussion
Studies from a number of laboratories show that Gal-1, whether distributed from tumor cells or the host, is critical for melanoma growth. Gal-1 can suppress effector T cell function and anti-tumor immunity (Banh et al., 2011; Ilarregui et al., 2009) as well as trigger pro-angiogenic activity in melanomas (Thijssen et al., 2010; Thijssen et al., 2008; Thijssen et al., 2006). There are corresponding binding activities between Gal-1 and its counter-receptor glycoprotein ligand(s) to convey these pro-tumorigenic properties. As demonstrated on immune cells and ECs, Gal-1 ligands commonly display poly-N-acetyllactosamines on their N- and/or O-glycans. CD7, CD43, CD45, CD146 and VEGFR1, for example, are well-described T cell, DC or EC Gal-1 ligands that, upon Gal-1-binding, transmit signals that help induce immunoregulatory, pro-apoptotic, pro-survival or pro-angiogenic activities (Cedeno-Laurent et al., 2010; Cedeno-Laurent et al., 2012a; Cedeno-Laurent et al., 2012c; Croci et al., 2014; Fulcher et al., 2009; Hernandez et al., 2006; Jouve et al., 2013; Suzuki et al., 2005a, b). Interestingly, analysis of melanoma cell Gal-1 ligands and their glycosyltransferase regulator(s) and relationship to melanoma malignancy has not been formally addressed.
Here, we studied the expression, identity and regulation of Gal-1 ligands on melanoma cells. Our data implicate melanoma Gal-1 ligands, notably N-glycosylated MCAM, and Gal-1-binding O-glycans negatively regulated by α2,6 sialyltransferase ST6GalNAc2 as pro-tumorigenic factors on melanoma cells. Prior data, in fact, show that MCAM expression directly correlates with melanoma metastasis (Kim et al., 2012; Luca et al., 1993; Mills et al., 2002; Xie et al., 1997) and ST6GalNAc2 also acts as a negative regulator of breast cancer metastasis by forming non-Gal-3-binding sialylated Core 1 O-glycans (Murugaesu et al., 2014). Our findings further highlight MCAM as a Gal-1 ligand and ST6GalNAc2 as a regulator of Gal-1 ligand activity in the glyco-pathogenesis of melanoma growth.
Using Gal-1hFc chimera, we probed Gal-1 ligands on melanoma cells by IF, flow cytometry and Western blotting. While the glycan-binding repertoire of Gal-1hFc is not identical to human Gal-1 (Cedeno-Laurent et al., 2010), Gal-1hFc upholds hallmark N-acetyllactosamine-binding activity and binds the same glycoproteins as human Gal-1 as shown here and elsewhere (Barthel et al., 2011; Cedeno-Laurent et al., 2010; Cedeno-Laurent et al., 2012a; Cedeno-Laurent et al., 2012b). Though a potential limitation of this study, Gal-1hFc can interrogate human Gal-1 ligands without the need for structure stabilizers. While efforts using native Gal-1 do exist (Andre et al., 1999; Kaltner et al., 1997; Plzak et al., 2000), native human Gal-1 is problematic for use in bioassays due to rapid oxidative deactivation and the need for reducing chemicals, which complicates interpretation of ligand-binding data. Previous studies using alkylation-induced stabilization or cysteine-less Gal-1 mutants illustrate other methods used to circumvent drawbacks of probing with native Gal-1 (Inagaki et al., 2000; Nishi et al., 2008; Powell and Whitney, 1984; Stowell et al., 2009).
Our initial assessments focused on whether Gal-1 ligands were differentially-expressed on melanocytes in normal human skin and in human benign and malignant melanocytic specimens. While routinely detecting Gal-1 ligands on melanoma cells, including metastases, RGP and VGF subsets and melanoma in situ, we did not observe a similar high level of Gal-1 ligand staining on S100+ cells in the epidermis of normal skin or adjacent uninvolved skin in melanoma lesions. As normal melanocytes are part of the S100+ cell population in skin, these data indicated that normal melanocytes expressed low levels of Gal-1 ligand. The lack of Gal-1 ligand detection in HEM cell lysates supported this notion. We did, however, detect dermal nests of Gal-1 ligand+ S100+ cells in a premalignant atypical nevus lesion, suggesting that Gal-1 ligand expression may correspond with transition to malignancy. Further IF studies on other premalignant lesions, including melanoma mimics, are needed to strengthen the speculation that Gal-1 ligands are biomarkers of malignancy.
By performing Gal-1-affinity chromatography and MS of protein isolates, we were able to identify MCAM was a major ligand on melanoma cells. LAMP-1 and -2 and CEA were also identified though were relatively less abundant. Analysis of Gal-1 ligand activity on MCAMKD melanoma cells revealed that MCAM, indeed, contributed to a significant portion (35%) of total cellular ligand activity. Furthermore, Gal-1’s weak binding to de-N-glycosylated MCAM demonstrated that MCAM’s Gal-1 ligand activity was largely dependent on its N-glycans.
Since de-N-glycosylation and protease treatment data suggested that a residual Gal-1-binding activity was expressed on melanoma cells, we explored the potential contribution of O-glycans. Given that α2,6 sialyltransferase ST6GalNAc2 can prevent Core 2 O-glycan formation (Marcos et al., 2004) and related Gal-1-binding Core 2 structures (Earl et al., 2010; Nguyen et al., 2001), we first examined whether ST6GalNAc2 was differentially expressed in Gal-1 ligandlo HEM and Gal-1 ligandhi melanoma cells. We observed consistent downregulation of ST6GalNAc2 in Gal-1 ligandhi melanoma cells compared with HEM, implicating its potential role in blocking Gal-1 ligand activity conferred by O-glycans (As Illustrated in Figure 5f). This notion was solidified by assaying for Gal-1 ligand expression and LEA-binding in ST6O/E A375, G361 and/or B16 cells, whose Gal-1 ligand activity and LEA binding was lowered, implicating other non-MCAM O-glycan-bearing proteins, such as LAMP-1, as constituents of cellular ligand activity (Carlsson et al., 1993; Ohannesian et al., 1994).
In vivo data using MCAMKD and ST6O/E melanoma cells suggested that MCAM functioned as a pro-tumorigenic factor and ST6GalNAc2 served as a negative tumorigenic regulator in collaboration with host Gal-1. While Gal-1 produced by melanoma cells plays a role in immunoregulation and angiogenesis (Cedeno-Laurent et al., 2012b; Cedeno-Laurent et al., 2012c; Rubinstein et al., 2004; Thijssen et al., 2010; Thijssen et al., 2006), in vivo results shown here indicated that host Gal-1 was critical for MCAM- and ST6GalNAc2-dependent tumor growth. Growth of MCAMKD or ST6O/E melanoma cells in wt mice suggested that melanoma-derived Gal-1 was incapable of fully compensating for the lack of host Gal-1. In fact, our MCAMKD tumorigenicity data in wt mice paralleled prior work (Wu et al., 2008) and strengthened our contention that, when binding partner Gal-1 is deficient in mice can dependency on MCAM’s Gal-1 ligand activity for robust melanoma growth be appreciated.
In migration assays, Gal-1 ligand neutralization and lactose treatments supported the concept that melanoma Gal-1 ligands helped confer migratory activity. Hence, evaluations on the relative migratory activity of MCAMKD and ST6O/E melanoma cells indicated that MCAM expression and ST6GalNAc2 downregulation were critical for optimal Gal-1 ligand-mediated migratory activity. Because MCAM-deficiency abrogated migration below Gal-1 ligand neutralization of control cells, we speculate that additional non-Gal-1 effects could have been impacted by MCAM-deficiency. Indeed, MCAM has been shown to impact cell morphogenesis (Zeng et al., 2012) or the function of VEGFR (Jiang et al., 2012), which is required for optimal migration in this assay system (Frank et al., 2011). Of note, Gal-1hFc-binding of melanoma cell Gal-1 ligands in solution did not, itself, promote migration, suggesting that Gal-1 immobilized within ECM may be more efficient at forming lattices and triggering a migratory activity on melanoma cells. Further studies are underway to dissect Gal-1-dependent signaling in melanoma cells through MCAM and other Gal-1 ligands.
In summary, observations herein advance the hypothesis that Gal-1 – Gal-1 ligand axis is critical for melanoma development, while providing firm insights on the intrinsic role of Gal-1 ligands on melanoma cells. Our data now implicate Gal-1’s influence on the malignant behavior of melanoma cells through engagement of its Gal-1 ligands. Results now raise the possibility that malignant progression is controlled by expression of Gal-1 ligands, such as MCAM among other membrane glycoproteins, and partially by negative regulator, ST6GalNAc2. These findings have invigorated further inquiry on the glyco-molecular transition of normal and premalignant melanocytes to malignant melanocytes and whether Gal-1 ligand expression can help discriminate malignant melanoma from tumor mimics. This report expands our perspective on the glyco-pathogenesis of malignant melanoma and strengthens the use of Gal-1 antagonists, such as neutralizing Abs, as therapeutically efficacious reagents to treat malignant melanoma.
Materials and Methods
Cells
Please see Supplemental Material section for extensive list of human and mouse cells, methods of cell acquisition, validation of authenticity and institutional approvals.
Immunofluorescence
Archival FFPE normal human skin, benign melanocytic tumors and malignant melanoma specimens were obtained in accordance with IRB approval, stained with H&E and analyzed by IF. Studies consisted of tissues from human normal skin (n=3), benign nevi (n=3), a combined atypical nevus and atypical spindle cell proliferation with inflammation, a malignant melanoma in situ, and primary malignant melanomas (n=5). In addition, tissue microarray (TMA) sections containing 56 primary melanomas, 20 metastatic melanomas and 24 benign pigmented lesions were obtained commercially (BioMax, Inc.; Rockville, MD). Following deparaffinization and antigen retrieval using EDTA (pH 8), sections were treated with hydrogen peroxide for 5 min, protein block for 30 min and then dual stained with rabbit polyclonal anti-S100 (clone Z0311) (1:400) (Dako; Carpinteria, CA) or rabbit IgG anti-human CD8 (1:2000) (Abcam; San Francisco, CA) and/or Gal-1hFc or dmGal-1hFc (each at 50μg/ml) for 1h at room temperature as described (Cedeno-Laurent et al., 2010; Cedeno-Laurent et al., 2012b). Slides were incubated for 30 min with a cocktail of Cy-3 anti-rabbit IgG (1:500) (Invitrogen) and with APC-goat Fab anti-hFc (1:500) (Jackson ImmunoResearch, Inc.; West Grove, PA) and counterstained with DAPI. Slides were treated with ProLong Gold Anti-Fade (Invitrogen) prior to fluorescence microscopy. Staining was analyzed with a BX51/BX52 microscope and images were acquired using a Nikon eclipse Ti microscope and a Nikon FDX-35 digital camera and analyzed using CytoVision 3.6 software (Applied Imaging; San Jose, CA).
Fluorescence analysis of TMA-stained slides was performed using Spot Advanced software. Representative core fields at 10X magnification (encompassing >85% of each core) were analyzed using semi-quantitative raw intensity analysis with NIH Image J software. Please see Supplemental Material section for detailed description of immunofluorescence procedures, type and number of analyzed tissues and method of quantification.
Lectin-affinity Chromatographic, Mass Spectrometry (MS) and Western Blot Analysis
Protein G-affinity chromatography of Gal-1 ligands was performed on human G361 melanoma or control human activated T cell lysates using Gal-1hFc or non-binding dmGal-1hFc probes (Cedeno-Laurent et al., 2010) incubated at a ratio of 100μg lysate:2μg for 18hr at 4°C. Eluates were washed extensively in lysis buffer containing 2% NP-40 and in PBS. For Gal-1 ligand identification, eluates from Gal-1hFc- or dmGal-1hFc – protein G chromatography of G361 lysates were analyzed by tandem MS/MS by the Beth-Israel Deaconess Medical Center Mass Spectrometry Core Facility (Boston, MA).
For Western blot analyses, whole cell lysates, Matrigel, eluates from dmGal-1hFc/Gal-1hFc – protein G chromatography and immunoprecipitates using anti-MCAM (clone P1H12) (Lifespan Biosciences, Inc.; Seattle, WA), anti-MCAM (EPR3208) (EMD Millipore; Billerica, MA), anti-CD45RO (UCH-L1) (Santa Cruz Biotechnology, Inc.; Dallas, TX), anti-LAMP-1 (clone H4A3) (BioLegend, Inc.), anti-90k/MAC-2BP (clone SP-2) (a generous gift from Dr. Stefano Iacobelli, MediaPharma S.r.l.) (Iacobelli et al., 1986) or isotype control Ab were prepared, separated on reducing 4–20% SDS-PAGE gradient gels (Bio-Rad, Inc.; Hercules, CA) and transferred to immunoblot PVDF membrane (Bio-Rad) as described (Cedeno-Laurent et al., 2012a). Where indicated, anti-MCAM immunoprecipitates were treated with Peptide-N-Glycosidase F (PNGase) as per manufacturer’s protocol (New England Biolabs, Inc.; Ipswich, MA). MCAM, CD45RO, Gal-1 ligands, SOX10, Gal-1, and LAMP-1, MAC-2BP and ß-actin were Western blotted with anti-MCAM (P1H12 or EPR3208) (1μg/ml), anti-CD45RO (UCH-L1) (1μg/ml), Gal-1hFc (10μg/ml), non-binding mutant dmGal-1hFc (10μg/ml), goat polyclonal anti-SOX10 (Santa Cruz Biotechnology, Inc.) (1μg/ml), goat polyclonal anti-mouse/human Gal-1 (R&D Systems) (2μg/ml), anti-LAMP-1 (H4A3), anti-90k/MAC-2BP (clone SP-2) or anti-ß-actin (BD Biosciences, Inc.; San Jose, CA) (1μg/ml), respectively; then incubated with relevant alkaline phosphatase (AP)-conjugated secondary Ab (Jackson ImmunoResearch, Inc.; West Grove, PA) and developed with Western Blue® AP-substrate (Promega; Madison, WI) as described (Cedeno-Laurent et al., 2010). Alternatively, blots were incubated with IRDye®-800CW anti-hIgG, IRDye-800CW anti-rabbit IgG or IRDye®-680RD anti-mouse IgG and analyzed on a LI-COR Odyssey Imaging System (LI-COR Biosciences; Lincoln, NE).
Real-time quantitative RT-PCR Analysis of Galectins
Please see Supplemental Material section for detailed procedures on real-time qRT-PCR.
Silencing of MCAM
Please see Supplemental Material section for detailed procedures on stable MCAM silencing in cells.
Overexpression of ST6GalNAc2
Please see Supplemental Material section for detailed procedures on stable ST6GalNAc2 overexpression in cells.
Flow Cytometry
Please see Supplemental Material section for detailed procedures on FACS analysis and glyco-metabolic inhibitor treatments.
Melanoma Cell Migration Assay
Melanoma cells were plated and cultured on Matrigel, which is an ECM preparation from a mouse sarcoma – a rich source of Gal-1 (Croci et al., 2012) as described (Frank et al., 2011). Presence of Gal-1 in Matrigel preparations was validated by Western blot analysis (Supplemental Figure 9). Negative controls consisted of adding 50mM lactose to Matrigel and assay medium or pre-treating melanoma cells with saturating levels of Gal-1hFc (Cedeno-Laurent et al., 2010).
Prior to assays, melanoma cells were cultured for 24h in RPMI 1640/10%FBS/1% Pen/Strep with 50mM lactose to elute pre-bound melanoma-derived Gal-1, inhibit melanoma cell Gal-1 ligand engagement and silence Gal-1 ligand-dependent cellular events. Cells were then harvested with 1mM EDTA, washed 3X in PBS, suspended in RPMI 1640/10% FBS/100ng/ml rhVEGF or rmVEGF (R&D Systems, Inc.; Minneapolis, MN) and seeded at 2×104/well in 24-well plates coated with growth factor-depleted Matrigel (BD Biosciences). Where indicated, Gal-1hFc, hFc control or 50mM lactose control was added to occupy and competitively inhibit melanoma cell Gal-1 ligand binding to native Gal-1 in Matrigel. Tube-like cellular formation, which corresponds with melanoma virulence (Frank et al., 2011), was examined by phase-contrast microscopy after 72h. Tube-like formations defined as ≥2 cells forming elongated structures were counted at 10X magnification from 4 different fields for each condition. Experiments were done at least 3-times.
In vitro Melanoma Cell Proliferation Assay
Please see Supplemental Material section for detailed procedures on melanoma cell proliferation assay.
Melanoma Growth in Mice
Scr or vector control, MCAMKD or ST6O/E B16 melanoma cells (1×105) were inoculated subcutaneously (s.c.) in left flank of wt or Gal-1−/− C57BL/6 mice (The Jackson Laboratory; Bar Harbor, ME). Tumor growth (n=8/exp) was measured every other day using calipers. All animal experiments were authorized by according to IACUC and mice were euthanized as per IACUC guidelines. Experiments were repeated 3-times.
Statistical Analysis
Statistical significant comparisons were ascertained by two-tailed Student’s t-test, paired t-test, one-way ANOVA with Dunnett’s post test, or contingency table on GraphPad Prism (GraphPad Software, La Jolla, CA).
Supplementary Material
Acknowledgments
We would like to thank Dr. John Asara and Ming Yuan from the BIDMC Mass Spectrometry Core facility, which is partially funded by NIH shared instrumentation grant 1S10OD010612 (J. Asara), for acquiring and analyzing MS identification data.
Footnotes
Grant support: NIH/NCI grant (R01CA173610, C. Dimitroff) and (RO1CA158467, G. Murphy); NIH Kirschstein-NRSA Post-doctoral Fellowship award (F32CA144219, S. Barthel); NIH Pre-doctoral Fellowship Award (F31CA171520, J. Geddes-Sweeney); Dermatology Foundation Research Grant (S. Barthel); American Cancer Society New England Division Postdoctoral Fellowship (PF-10-227-01-CSM, S. Barthel); BWH Department of Dermatology Developmental SPORE Grant (P50CA093683, S. Barthel and T. Schatton); Research Career Development Award, Dermatology Foundation (T. Schatton); and Innovative Research Grant, Melanoma International Foundation (T. Schatton).
Conflict of Interest
While all other authors declare no conflicts of interest, Dr. Abrar A. Qureshi consults for Abbvie, Inc., Amgen, Inc., Novartis Corp, the Centers for Disease Control and Prevention, Janssen, Inc. and Merck & Co., Inc., and serves as an investigator for Amgen, Inc.
References
- Aisner DL, Maker A, Rosenberg SA, et al. Loss of S100 antigenicity in metastatic melanoma. Human pathology. 2005;36:1016–9. doi: 10.1016/j.humpath.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre S, Kojima S, Yamazaki N, et al. Galectins-1 and -3 and their ligands in tumor biology. Non-uniform properties in cell-surface presentation and modulation of adhesion to matrix glycoproteins for various tumor cell lines, in biodistribution of free and liposome-bound galectins and in their expression by breast and colorectal carcinomas with/without metastatic propensity. Journal of cancer research and clinical oncology. 1999;125:461–74. doi: 10.1007/s004320050303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banh A, Zhang J, Cao H, et al. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Cancer research. 2011;71:4423–31. doi: 10.1158/0008-5472.CAN-10-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel SR, Antonopoulos A, Cedeno-Laurent F, et al. Peracetylated 4-fluoro-glucosamine reduces the content and repertoire of N- and O-glycans without direct incorporation. The Journal of biological chemistry. 2011;286:21717–31. doi: 10.1074/jbc.M110.194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum LG, Seilhamer JJ, Pang M, et al. Synthesis of an endogeneous lectin, galectin-1, by human endothelial cells is up-regulated by endothelial cell activation. Glycoconjugate journal. 1995;12:63–8. doi: 10.1007/BF00731870. [DOI] [PubMed] [Google Scholar]
- Blois SM, Ilarregui JM, Tometten M, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nature medicine. 2007;13:1450–7. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- Braeuer RR, Shoshan E, Kamiya T, et al. The sweet and bitter sides of galectins in melanoma progression. Pigment cell & melanoma research. 2012;25:592–601. doi: 10.1111/j.1755-148X.2012.01026.x. [DOI] [PubMed] [Google Scholar]
- Camby I, Le Mercier M, Lefranc F, et al. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–57R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- Carlsson SR, Lycksell PO, Fukuda M. Assignment of O-glycan attachment sites to the hinge-like regions of human lysosomal membrane glycoproteins lamp-1 and lamp-2. Archives of biochemistry and biophysics. 1993;304:65–73. doi: 10.1006/abbi.1993.1322. [DOI] [PubMed] [Google Scholar]
- Cedeno-Laurent F, Barthel SR, Opperman MJ, et al. Development of a nascent galectin-1 chimeric molecule for studying the role of leukocyte galectin-1 ligands and immune disease modulation. Journal of immunology. 2010;185:4659–72. doi: 10.4049/jimmunol.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedeno-Laurent F, Opperman M, Barthel SR, et al. Galectin-1 triggers an immunoregulatory signature in Th cells functionally defined by IL-10 expression. Journal of immunology. 2012a;188:3127–37. doi: 10.4049/jimmunol.1103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedeno-Laurent F, Opperman MJ, Barthel SR, et al. Metabolic inhibition of galectin-1-binding carbohydrates accentuates antitumor immunity. The Journal of investigative dermatology. 2012b;132:410–20. doi: 10.1038/jid.2011.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedeno-Laurent F, Watanabe R, Teague JE, et al. Galectin-1 inhibits the viability, proliferation, and Th1 cytokine production of nonmalignant T cells in patients with leukemic cutaneous T-cell lymphoma. Blood. 2012c;119:3534–8. doi: 10.1182/blood-2011-12-396457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. I. Physical and chemical characterization. The Journal of biological chemistry. 1995;270:5198–206. doi: 10.1074/jbc.270.10.5198. [DOI] [PubMed] [Google Scholar]
- Croci DO, Cerliani JP, Dalotto-Moreno T, et al. Glycosylation-Dependent Lectin-Receptor Interactions Preserve Angiogenesis in Anti-VEGF Refractory Tumors. Cell. 2014;156:744–58. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- Croci DO, Salatino M, Rubinstein N, et al. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. The Journal of experimental medicine. 2012;209:1985–2000. doi: 10.1084/jem.20111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demydenko D, Berest I. Expression of galectin-1 in malignant tumors. Exp Oncol. 2009;31:74–9. [PubMed] [Google Scholar]
- Earl LA, Bi S, Baum LG. N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. The Journal of biological chemistry. 2010;285:2232–44. doi: 10.1074/jbc.M109.066191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank NY, Schatton T, Kim S, et al. VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth. Cancer research. 2011;71:1474–85. doi: 10.1158/0008-5472.CAN-10-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher JA, Chang MH, Wang S, et al. Galectin-1 co-clusters CD43/CD45 on dendritic cells and induces cell activation and migration through Syk and protein kinase C signaling. The Journal of biological chemistry. 2009;284:26860–70. doi: 10.1074/jbc.M109.037507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin MI, Chu CC, Golshayan D, et al. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–65. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- Hernandez JD, Nguyen JT, He J, et al. Galectin-1 binds different CD43 glycoforms to cluster CD43 and regulate T cell death. Journal of immunology. 2006;177:5328–36. doi: 10.4049/jimmunol.177.8.5328. [DOI] [PubMed] [Google Scholar]
- Iacobelli S, Arno E, D’Orazio A, et al. Detection of antigens recognized by a novel monoclonal antibody in tissue and serum from patients with breast cancer. Cancer research. 1986;46:3005–10. [PubMed] [Google Scholar]
- Ilarregui JM, Croci DO, Bianco GA, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nature immunology. 2009;10:981–91. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Sohma Y, Horie H, et al. Oxidized galectin-1 promotes axonal regeneration in peripheral nerves but does not possess lectin properties. European journal of biochemistry/FEBS. 2000;267:2955–64. doi: 10.1046/j.1432-1033.2000.01311.x. [DOI] [PubMed] [Google Scholar]
- Jean D, Gershenwald JE, Huang S, et al. Loss of AP-2 results in up-regulation of MCAM/MUC18 and an increase in tumor growth and metastasis of human melanoma cells. The Journal of biological chemistry. 1998;273:16501–8. doi: 10.1074/jbc.273.26.16501. [DOI] [PubMed] [Google Scholar]
- Jiang T, Zhuang J, Duan H, et al. CD146 is a coreceptor for VEGFR-2 in tumor angiogenesis. Blood. 2012;120:2330–9. doi: 10.1182/blood-2012-01-406108. [DOI] [PubMed] [Google Scholar]
- Jouve N, Despoix N, Espeli M, et al. The involvement of CD146 and its novel ligand Galectin-1 in apoptotic regulation of endothelial cells. The Journal of biological chemistry. 2013;288:2571–9. doi: 10.1074/jbc.M112.418848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltner H, Lips KS, Reuter G, et al. Quantitation and histochemical localization of galectin-1 and galectin-1-reactive glycoconjugates in fetal development of bovine organs. Histology and histopathology. 1997;12:945–60. [PubMed] [Google Scholar]
- Kim HJ, Jeon HK, Cho YJ, et al. High galectin-1 expression correlates with poor prognosis and is involved in epithelial ovarian cancer proliferation and invasion. European journal of cancer. 2012;48:1914–21. doi: 10.1016/j.ejca.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. The Journal of experimental medicine. 2003;198:1201–12. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopcow HD, Rosetti F, Leung Y, et al. T cell apoptosis at the maternal-fetal interface in early human pregnancy, involvement of galectin-1. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18472–7. doi: 10.1073/pnas.0809233105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laderach DJ, Gentilini LD, Giribaldi L, et al. A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease. Cancer research. 2013;73:86–96. doi: 10.1158/0008-5472.CAN-12-1260. [DOI] [PubMed] [Google Scholar]
- Lefranc F, Mathieu V, Kiss R. Galectin-1 as an oncotarget in gliomas and melanomas. Oncotarget. 2011;2:892–3. doi: 10.18632/oncotarget.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Riethmuller G, Johnson JP. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:9891–5. doi: 10.1073/pnas.86.24.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca M, Hunt B, Bucana CD, et al. Direct correlation between MUC18 expression and metastatic potential of human melanoma cells. Melanoma research. 1993;3:35–41. doi: 10.1097/00008390-199304000-00006. [DOI] [PubMed] [Google Scholar]
- Marcos NT, Pinho S, Grandela C, et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer research. 2004;64:7050–7. doi: 10.1158/0008-5472.CAN-04-1921. [DOI] [PubMed] [Google Scholar]
- Mathieu V, de Lassalle EM, Toelen J, et al. Galectin-1 in melanoma biology and related neo-angiogenesis processes. The Journal of investigative dermatology. 2012;132:2245–54. doi: 10.1038/jid.2012.142. [DOI] [PubMed] [Google Scholar]
- Mills L, Tellez C, Huang S, et al. Fully human antibodies to MCAM/MUC18 inhibit tumor growth and metastasis of human melanoma. Cancer research. 2002;62:5106–14. [PubMed] [Google Scholar]
- Murugaesu N, Iravani M, van Weverwijk A, et al. An in vivo functional screen identifies ST6GalNAc2 sialyltransferase as a breast cancer metastasis suppressor. Cancer discovery. 2014;4:304–17. doi: 10.1158/2159-8290.CD-13-0287. [DOI] [PubMed] [Google Scholar]
- Nguyen JT, Evans DP, Galvan M, et al. CD45 modulates galectin-1-induced T cell death: regulation by expression of core 2 O-glycans. Journal of immunology. 2001;167:5697–707. doi: 10.4049/jimmunol.167.10.5697. [DOI] [PubMed] [Google Scholar]
- Nishi N, Abe A, Iwaki J, et al. Functional and structural bases of a cysteine-less mutant as a long-lasting substitute for galectin-1. Glycobiology. 2008;18:1065–73. doi: 10.1093/glycob/cwn089. [DOI] [PubMed] [Google Scholar]
- Ohannesian DW, Lotan D, Lotan R. Concomitant increases in galectin-1 and its glycoconjugate ligands (carcinoembryonic antigen, lamp-1, and lamp-2) in cultured human colon carcinoma cells by sodium butyrate. Cancer research. 1994;54:5992–6000. [PubMed] [Google Scholar]
- Ouyang J, Juszczynski P, Rodig SJ, et al. Viral induction and targeted inhibition of galectin-1 in EBV+ posttransplant lymphoproliferative disorders. Blood. 2011;117:4315–22. doi: 10.1182/blood-2010-11-320481. [DOI] [PubMed] [Google Scholar]
- Pace KE, Hahn HP, Pang M, et al. CD7 delivers a pro-apoptotic signal during galectin-1-induced T cell death. Journal of immunology. 2000;165:2331–4. doi: 10.4049/jimmunol.165.5.2331. [DOI] [PubMed] [Google Scholar]
- Perillo NL, Pace KE, Seilhamer JJ, et al. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–9. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- Plzak J, Smetana K, Jr, Betka J, et al. Endogenous lectins (galectins-1 and -3) as probes to detect differentiation-dependent alterations in human squamous cell carcinomas of the oropharynx and larynx. International journal of molecular medicine. 2000;5:369–72. doi: 10.3892/ijmm.5.4.369. [DOI] [PubMed] [Google Scholar]
- Powell JT, Whitney PL. Endogenous ligands of rat lung beta-galactoside-binding protein (galaptin) isolated by affinity chromatography on carboxyamidomethylated-galaptin-Sepharose. The Biochemical journal. 1984;223:769–74. doi: 10.1042/bj2230769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich GA, Iglesias MM, Modesti NM, et al. Activated rat macrophages produce a galectin-1-like protein that induces apoptosis of T cells: biochemical and functional characterization. Journal of immunology. 1998;160:4831–40. [PubMed] [Google Scholar]
- Rubinstein N, Alvarez M, Zwirner NW, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer cell. 2004;5:241–51. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- Skrincosky DM, Allen HJ, Bernacki RJ. Galaptin-mediated adhesion of human ovarian carcinoma A121 cells and detection of cellular galaptin-binding glycoproteins. Cancer research. 1993;53:2667–75. [PubMed] [Google Scholar]
- Stowell SR, Cho M, Feasley CL, et al. Ligand reduces galectin-1 sensitivity to oxidative inactivation by enhancing dimer formation. The Journal of biological chemistry. 2009;284:4989–99. doi: 10.1074/jbc.M808925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki O, Nozawa Y, Abe M. Altered N-glycosylation in CD45 and regulatory roles of altered N-glycosylation in galectin-1-induced growth inhibition in human diffuse large B cell lymphoma. Oncology reports. 2005a;13:109–14. [PubMed] [Google Scholar]
- Suzuki O, Nozawa Y, Abe M. Regulatory roles of altered N- and O-glycosylation of CD45 in galectin-1-induced cell death in human diffuse large B cell lymphoma. International journal of oncology. 2005b;26:1063–8. [PubMed] [Google Scholar]
- Szebeni GJ, Kriston-Pal E, Blazso P, et al. Identification of galectin-1 as a critical factor in function of mouse mesenchymal stromal cell-mediated tumor promotion. PloS one. 2012;7:e41372. doi: 10.1371/journal.pone.0041372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen VL, Barkan B, Shoji H, et al. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer research. 2010;70:6216–24. doi: 10.1158/0008-5472.CAN-09-4150. [DOI] [PubMed] [Google Scholar]
- Thijssen VL, Hulsmans S, Griffioen AW. The galectin profile of the endothelium: altered expression and localization in activated and tumor endothelial cells. The American journal of pathology. 2008;172:545–53. doi: 10.2353/ajpath.2008.070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen VL, Postel R, Brandwijk RJ, et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15975–80. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinari N, Kuwabara I, Huflejt ME, et al. Glycoprotein 90K/MAC-2BP interacts with galectin-1 and mediates galectin-1-induced cell aggregation. International journal of cancer Journal international du cancer. 2001;91:167–72. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1022>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- Toscano MA, Bianco GA, Ilarregui JM, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nature immunology. 2007;8:825–34. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- Tsai CM, Chiu YK, Hsu TL, et al. Galectin-1 promotes immunoglobulin production during plasma cell differentiation. Journal of immunology. 2008;181:4570–9. doi: 10.4049/jimmunol.181.7.4570. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yan X. CD146, a multi-functional molecule beyond adhesion. Cancer letters. 2013;330:150–62. doi: 10.1016/j.canlet.2012.11.049. [DOI] [PubMed] [Google Scholar]
- Woynarowska B, Dimitroff CJ, Sharma M, et al. Inhibition of human HT-29 colon carcinoma cell adhesion by a 4-fluoro-glucosamine analogue. Glycoconjugate journal. 1996;13:663–74. doi: 10.1007/BF00731455. [DOI] [PubMed] [Google Scholar]
- Wu GJ, Fu P, Wang SW, et al. Enforced expression of MCAM/MUC18 increases in vitro motility and invasiveness and in vivo metastasis of two mouse melanoma K1735 sublines in a syngeneic mouse model. Molecular cancer research: MCR. 2008;6:1666–77. doi: 10.1158/1541-7786.MCR-07-2200. [DOI] [PubMed] [Google Scholar]
- Xie S, Luca M, Huang S, et al. Expression of MCAM/MUC18 by human melanoma cells leads to increased tumor growth and metastasis. Cancer research. 1997;57:2295–303. [PubMed] [Google Scholar]
- Zeng Q, Li W, Lu D, et al. CD146, an epithelial-mesenchymal transition inducer, is associated with triple-negative breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1127–32. doi: 10.1073/pnas.1111053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga E, Rabinovich GA, Iglesias MM, et al. Regulated expression of galectin-1 during B-cell activation and implications for T-cell apoptosis. Journal of leukocyte biology. 2001;70:73–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


