Abstract
The medial prefrontal cortex (mPFC) has been implicated in multiple disorders characterized by clear sex differences, including schizophrenia, attention deficit hyperactivity disorder, post-traumatic stress disorder, depression, and drug addiction. These sex differences likely represent underlying differences in connectivity and/or the balance of neuronal excitability within the mPFC. Recently, we demonstrated that signaling via the metabotropic γ-aminobutyric acid receptor (GABABR) and G proteingated inwardly-rectifying K+ (GIRK/Kir3) channels modulates the excitability of the key output neurons of the mPFC, the layer 5/6 pyramidal neurons. Here, we report a sex difference in the GABABR-GIRK signaling pathway in these neurons. Specifically, GABABR-dependent GIRK currents recorded in the prelimbic region of the mPFC were larger in adolescent male mice than in female counterparts. Interestingly, this sex difference was not observed in layer 5/6 pyramidal neurons of the adjacent infralimbic cortex, nor was it seen in young adult mice. The sex difference in GABABR-GIRK signaling is not attributable to different expression levels of signaling pathway components, but rather to a phosphorylation-dependent trafficking mechanism. Thus, sex differences related to some diseases associated with altered mPFC function may be explained in part by sex differences in GIRK-dependent signaling in mPFC pyramidal neurons.
Keywords: GIRK, Kir3, GABA, medial prefrontal cortex, slice electrophysiology, sex differences
1. Introduction
The prefrontal cortex (PFC) is involved in high-order cognitive functions such as regulation of emotional responses and decision-making (Wood and Grafman 2003). In rodents, the medial PFC (mPFC) is highly interconnected with limbic, cortical, and subcortical brain regions (Heidbreder and Groenewegen 2003; Uylings et al. 2003). The mPFC consists of an aggregate of sub-divisions including the infralimbic (ILC), prelimbic (PrLC), and cingulate cortices (CG) (Seamans et al. 2008). While inter-species differences in mPFC function remains a debated topic, anatomical and electrophysiological findings in rodents and non-human primates suggest that these sub-regions display important similarities with analogous regions in the human brain, particularly with respect to their function and connectivity (Uylings et al. 2003; Seamans et al. 2008).
Interest in mPFC function and physiology has grown substantially in recent years, as dysfunction of this region is implicated in a number of clinical disorders such as schizophrenia, attention deficit hyperactivity disorder (ADHD), post-traumatic stress disorder (PTSD), depression, and drug addiction (Sesack and Carr 2002; Godsil et al. 2013; Ishii-Takahashi et al. 2014; Szczepanski and Knight 2014). Moreover, converging evidence from clinical and preclinical studies has highlighted the importance of intrinsic sex differences in mPFC physiology and function, and how these distinctions may have translational significance for individual resilience or susceptibility to pathological disorders such as ADHD, PTSD, depression, and addiction (Godsil et al. 2013; Ishii-Takahashi et al. 2014; Szczepanski and Knight 2014).
Several cell types exist in the mPFC, including glutamatergic pyramidal neurons and both GABAergic and cholinergic interneurons (Kawaguchi 1993; Kawaguchi and Kondo 2002). The glutamatergic pyramidal neurons found in layer 5/6 are the primary output neurons of the mPFC and represent a major source of excitatory input to many cortical and subcortical structures (Hearing et al. 2012). The excitability of these neurons is dependent on their intrinsic physiological properties in conjunction with synaptic input (excitatory and inhibitory) (Lee et al. 2014).
Inhibitory (GABAergic) input to layer 5/6 mPFC pyramidal neurons activates ionotropic (GABAAR) and metabotropic (GABABR) receptors. GABABRs exert inhibitory effects at both the pre- and post-synaptic level through the Gi/o G protein-dependent modulation of multiple ion channels, including Ca2+ and K+ channels (Hearing et al. 2012). Recently, we demonstrated that the GABABR-dependent inhibition of layer 5/6 mPFC pyramidal neurons from male mice is attributable largely (~60%) to activation of G protein-gated inwardly-rectifying K+ (GIRK/Kir3) channels (Hearing et al. 2013). Neuronal GIRK channels are formed by homo- and hetero-assembly among GIRK1, GIRK2 and GIRK3 subunits (Lujan et al. 2014). They play a critical role in modulating neuronal excitability throughout the brain (Luscher and Slesinger 2010; Lujan et al. 2014), have been linked to sex-differences in cellular function (Kelly et al. 2003), and are implicated in a number of neurological disorders (Luscher and Slesinger 2010).
Given the impact of GIRK channels on the excitability of layer 5/6 mPFC pyramidal neurons, the contribution of the mPFC to neuropsychiatric disorders with documented sex biases, and previously-noted sex differences in inhibitory G protein-dependent signaling in the mPFC (Sun et al. 2010), we sought to determine whether sex differences in GABABR-GIRK signaling exist in layer 5/6 mPFC pyramidal neurons. Using a combination of electrophysiological, biochemical, and ultrastructural approaches, we show that GABABR-GIRK signaling is more prominent in layer 5/6 PrLC pyramidal neurons from adolescent (P30–40) male mice as compared to female mice, a difference attributable to a phosphorylation-dependent difference in the trafficking of GIRK channels to the cell surface.
2. Materials and methods
2.1. Animals
Animal usage was approved by the Institutional Animal Care and Use Committee and was in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). All efforts were made to minimize animal suffering and to reduce the number of animals used in this study. Generation of constitutive Girk1−/−, Girk2−/−, and Girk3−/− mice was described previously (Signorini et al. 1997; Bettahi et al. 2002; Torrecilla et al. 2002). Girk−/− mice were backcrossed for >20 rounds against the C57BL/6J strain prior to initiating these studies. Mice were housed on a 12 h light/dark cycle, with food and water available ad libitum. In order to ascertain estrous cycle stages, vaginal lumen samples were collected and analyzed as described (Caligioni 2009). Briefly, lumen samples were collected by gently flushing 10 µL of saline using a fine-tip plastic pipette and placed into 48-well plates for visualization under light microscope. Differentiation of the estrous phases was based on the presence of stage specific epithelial cells (nucleated epithelial cells, cornified cells and leucocytes) in ³90% of the cell population.
2.1. Slice electrophysiology
Coronal slices (300 µm) containing the mPFC were prepared from male and female mice (30–40 d, unless otherwise noted) in an ice-cold solution containing (in mM): 229 mM sucrose, 1.9 KCl, 1.2 Na2HPO4, 33 NaHCO3, 6 MgCl2, 0.5 CaCl2, 10 glucose, 0.4 ascorbic acid, bubbled with 95% O2/5% CO2. Slices were transferred to pre-warmed (32–35°C) ACSF (in mM): 125 NaCl, 2.5 KCl, 1.25 Na2HPO4, 25 NaHCO3, 4 MgCl2, 1 CaCl2, 10 glucose, 0.4 ascorbic acid (pH 7.3–7.4), and gradually acclimated to room temperature over the course of ≥1 h. Slices were transferred to a recording chamber and superfused with oxygenated ACSF (in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.3 MgCl2, 2.0 CaCl2, 10 glucose, 0.4 ascorbic acid (pH 7.3–7.4) at a flow rate of 2–2.5 mL/min. Bath and chamber temperatures were maintained at 29–30°C.
Borosilicate (2.7–3.5 MΩ) electrodes were filled with (in mM): 140 K-gluconate, 2 MgCl2, 1.1 EGTA, 5 HEPES, 2 Na2-ATP, 0.3 Na-GTP, and 5 phosphocreatine, pH 7.2. The predicted EK for these conditions is −105 mV. In some experiments, either okadaic acid (100 nM) or vehicle (DMSO, 14 µM) was added to the pipette solution. Currents, resistances, and potentials were measured using a Multiclamp 700A amplifier and pCLAMP v.9 software (Molecular Devices; Foster City, CA) or an EPC10 HEKA amplifier and Patchmaster 2×73.2 software (HEKA Elektronik; Bellmore, NY) and stored on hard disk. All measured and command potentials factored in a junction potential (-15 mV) predicted using JPCalc software (Molecular Devices).
Layer 5/6 pyramidal neurons were identified by a pyramidal-shape soma, long and superficially-extending apical dendrite, a resting membrane potential ≤ −60 mV, lack of spontaneous activity, and a capacitance of ≥100 pF, as described (Hearing et al. 2013). Agonist-induced changes in holding current were measured at a holding potential of −60 mV. Input and series resistance values were monitored throughout the recording using 0.2 Hz voltage steps (-5 mV, 800 ms). Only experiments with stable (<20% variation) and low series resistances (<30 MΩ) were analyzed. For the current/spike experiments, only those cells with data points for all the current injection steps were included in the final analysis.
2.2. qRT-PCR
Punches (2-mm diameter, 2-mm thick) containing the mPFC were taken from male and female mice (30–40 d). Punches were frozen on crushed dry ice and stored at −80°C. Quantitative analysis of mRNA levels was performed as described (Arora et al. 2011) using the 2[-ΔCt] method. The primers used were: Girk1 (forward) 5’-GAGGGACGGAAAACTCACTCT-3’; Girk1 (reverse) 5’-TCAGGTGTCTGCCGAGATT-3’; Girk2 (forward) 5’- CGTGGAGTGAATTATTGAATCT-3’; Girk2(reverse) 5’ -GTCATTTCTTCTTTGTGCTTTT-3’; Girk3 (forward) 5’-CAGAGGGAACCTAGGGTACTG-3’; Girk3 (reverse) 5’-TTCCTAGGCTTTCAGGGTC-3’; GABAB1R(forward) 5’-GCTCCCGGAGCATCTGTAGT-3’; GABAB1R(reverse) 5’-CTGAGTGTGGCGTTCGATTCA-3’; GABAB2R(forward) 5’-ATGGAAGGCTACATCGGA-3’; GABAB2R(reverse) 5’-GCTTGCTGTTGTATTCTCTTTC-3’. GAPDH was used as control using GAPDH QuantiTect oligonucleotides (Qiagen; Valencia, CA). The following amplification program was used: 95°C/5min followed by 45 cycles of 95°C/10s, 60°C/30s, 72°C/10s. A melting curve at the end of the program confirmed the specificity of the reaction.
2.3. Quantitative immunoblotting
Immunoblotting procedures and quantitative analysis of protein levels from mPFC punches (2 mm diameter, 2 mm thick) were performed as described (Hearing et al. 2013), using the following primary antibodies diluted in 5% milk/TBS/0.1% Tween-20 or 1% milk/TBS/0.1% Tween-20: GIRK1 (1:100, Alomone Labs; Jerusalem, Israel), GIRK2 (1:200, Alomone Labs), GIRK3 (Frontier Institute Co., Ltd.; Ishikari, Hokkaido; Japan), GABABR1 (1:500)(Kulik et al. 2003), GABABR2 (1:10, NeuroMab; UC Davis/NIH, CA), GABABR2 (pSer-783) (1:200, PhosphoSolutions; Aurora, CO), or β-actin (1:10,000; Abcam; Cambridge, MA). Donkey anti-mouse #926–32212 (1:1000–5000, LI-COR Biosciences; Lincoln, NE) or anti-rabbit #926–68072 (1:5000, LI-COR) secondary antibodies were used with the Odyssey infrared imaging system (LI-COR) and an integrated density of each band was measured using Image J software (NIH; Bethesda, MD).
2.4. Surface biotinylation
mPFC punches (2-mm diameter, 2-mm thick) were dissected into smaller pieces using a razor blade. Fragments were transferred into 1 mL of EZlink NHS-SS-Biotin (1 mg/mL, Pierce; Rockford, IL) in PBS and incubated for 1 h at 4°C with gentle agitation. Slices were washed in 100 mM glycine/PBS followed by 2 washes in PBS for 20 min at 4°C. The washed slices were centrifuged (10,000 × g for 1 min) and sonicated in 100 µL of lysis buffer (25 mM HEPES, 150 mM NaCl, 1% Triton X-100, 0.1% SDS) containing Halt phosphatase inhibitor cocktail (Pierce). Homogenates were centrifuged at 1000 × g at 4°C for 7 min, the supernatant was collected and protein concentrations were measured using a BCA assay kit (Pierce). Lysates (100 µg) were incubated overnight at 4°C with 50 µL streptavidin agarose beads (Pierce), saving the remaining homogenate as a total protein fraction. The following day, samples were centrifuged at 10,000 × g for 5 min and the supernatant was collected (non-biotinylated fraction). Beads were washed 3 times in ice-cold lysis buffer followed by ice-cold 50 mM Tris-HCl, pH 7.4. All protein bound to beads was extracted by heating samples to 90°C for 7 min in 4x SDS-sample buffer containing 100 mM DTT followed by centrifugation for 1 min at 10,000 × g and supernatant containing biotinylated proteins was collected for immunoblotting.
2.5. Immunoelectron microscopy
The subcellular distribution of GIRK2 and GABABR1 was assessed using pre-embedding immunoelectron microscopy, as described (Arora et al. 2011).
2.5. Data analysis
Data are presented throughout as mean ± SEM, unless otherwise stated. Statistical analyses were performed using Prism 5 software (GraphPad Software, Inc.; La Jolla, CA) or SigmaPlot (Systat Software; San Jose, CA). Electrophysiological data were analyzed with Student’s t-test or ANOVA (one-way, two-way, or two-way repeated measures), as appropriate. Holm-Sidak post hoc test was used for pair-wise comparisons, if warranted. Electron-microscopy data were analyzed with Mann-Whitney rank sum test. Differences were considered significant if P<0.05.
3. Results
3.1. Sex differences in GABABR-dependent signaling in layer 5/6 PrLC pyramidal neurons
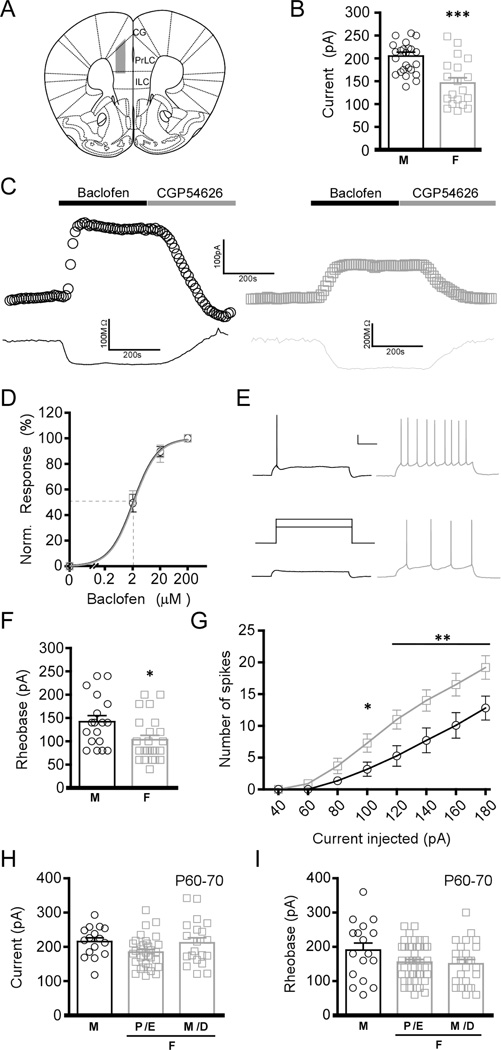
We began by evaluating GABABR-dependent signaling in layer 5/6 PrLC pyramidal neurons from adolescent (P30–40) male and female C57BL/6J mice (Fig. 1A). Bath application of a saturating concentration (200 µM) of the GABABR agonist baclofen triggered outward currents that correlated with a decrease in input resistance, and were reversed by the GABABR antagonist CGP54626 (Fig. 1B,C). Baclofen-induced somatodendritic currents were significantly (~30%) larger in layer 5/6 PrLC pyramidal neurons from male as compared to female mice (Fig. 1B). This was not attributable to a sex difference in baclofen potency, as EC50 values for baclofen-induced current activation in male and female neurons were indistinguishable (Fig. 1D). Importantly, the larger baclofen-induced currents in layer 5/6 PrLC pyramidal neurons from adolescent males correlated with a higher rheobase, the amount of current required to evoke an action potential (Fig. 1E,F). A corresponding sex difference was also observed in the number of action potentials evoked by increasing amounts of current (Fig. 1G). In contrast, no difference in resting membrane potential was observed for layer 5/6 PrLC pyramidal neurons from adolescent male (-81 ± 1 mV) and female (-80 ± 1 mV) mice (t32=0.45, P=0.66; n=14–20/sex). Similarly, no difference in action potential threshold was observed between male (-52 ± 1 mV) and female (-50 ± 1 mV) mice (t9=1.8, P=0.11; n=5–6/sex).
Figure 1. Sex differences in GABABR-dependent signaling in layer 5/6 PrLC pyramidal neurons.
A) Schematic depiction of the region targeted for electrophysiological characterization. Abbreviations: PrLC, prelimbic cortex; IL, infralimbic cortex; CG, cingulate cortex. B) Summary of baclofen-induced currents in PrLC pyramidal neurons from adolescent male (left, black) and female (right, gray) mice (t42=4.16, P<0.001; n=19–25/sex). C) Representative baclofen-induced currents (upper traces) and concomitant decreases in input resistance (lower traces) in PrLC pyramidal neurons from adolescent male (left, black) and female (right, grey) mice. D) Baclofen dose-response (0.2–200 µM) and EC50 determination for adolescent male (black) and female (gray) PrLC pyramidal neurons (t17=1.07, P=0.3; n=9–10/sex). E) Representative rheobase traces from adolescent male (left, black) and female (right, gray) layer 5/6 PrLC pyramidal neurons. The schematic (middle) shows the current injection protocol, with steps corresponding to 100 and 140 pA. Scale bar: 20 mV, 250 ms. F) Summary of rheobase data from adolescent male (M) and female (F) mice (t40=2.48, P<0.05; n=18–24/sex). G) Current-spike plots for adolescent male (black) and female (gray) layer 5/6 PrLC pyramidal neurons (F7,167=4.7, P<0.001; n=10–11/sex). Symbols: *,** P<0.05 and 0.01, respectively. H) Summary of baclofen-induced currents in layer 5/6 PrLC pyramidal neurons from young adult (P60–70) mice. No differences were observed in neurons from young adult male (M) and female (F) mice in the different phases of the estrous cycle (F2,68=2.7, P=0.07; n=16–33/group). Abbreviations: P/E, proestrous/estrous; M/D, metestrous/diestrous. I) Rheobase in layer 5/6 PrLC pyramidal neurons from young adult mice. No differences were observed between male and female mice in the different phases of the estrous cycle (F2,84=2.4, P=0.1; n=17–43/group).
Gonadal steroid hormones and aging have a significant impact on brain neurochemistry and physiology (Anyanwu 2007; Gillies and McArthur 2010). Therefore, we next examined whether the sex difference in GABABR-dependent signaling was observed in layer 5/6 PrLC pyramidal neurons from young adult (P60–70) mice. No difference in baclofen-induced current was observed in young adult males when compared to females in either the proestrous/estrous or metestrous/diestrous stages, when estrogen levels are at their highest and lowest, respectively (Fig. 1H). Moreover, no significant difference was found in the rheobase of layer 5/6 PrLC pyramidal neurons from young adult male and female mice (Fig. 1I).
3.2. Anatomic specificity of the sex difference in GABABR-dependent signaling
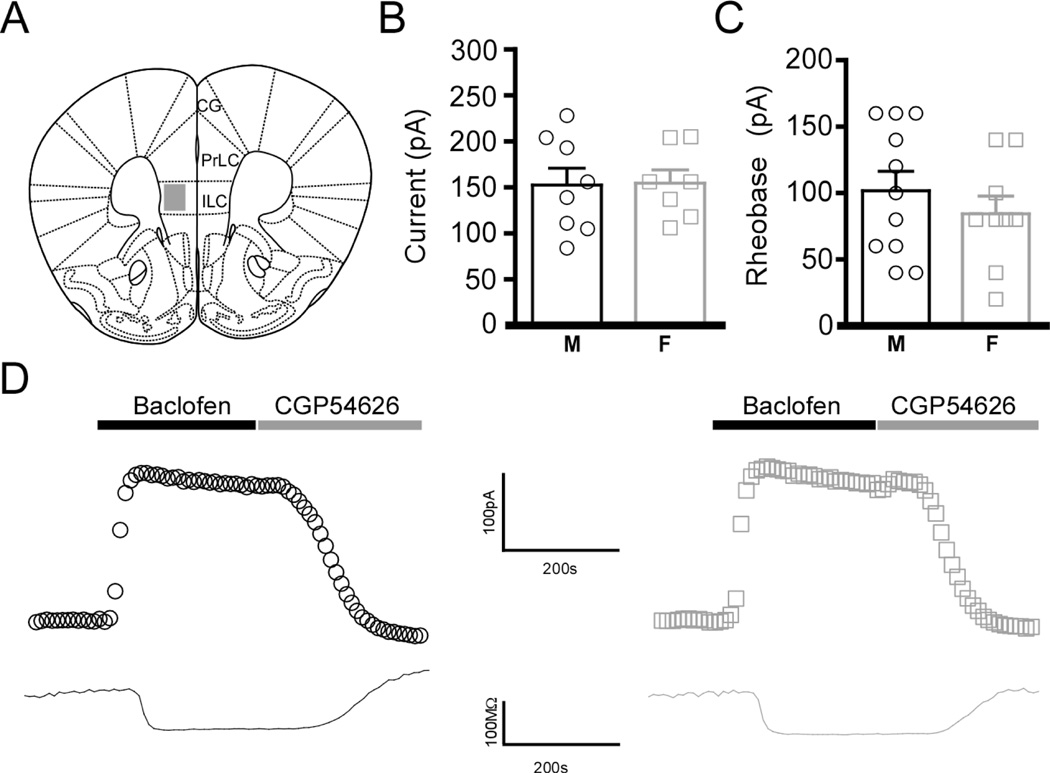
We next evaluated baclofen-evoked currents in layer 5/6 pyramidal neurons in the infralimbic cortex (ILC), a ventral sub-region of the mPFC that displays similarity with the PrLC in cytoarchitecture, but differs in terms of connectivity and functionality (Vertes 2004; Seamans et al. 2008) (Fig. 2A). In contrast to the PrLC, no sex differences in baclofen-induced currents (Fig. 2B,D) or rheobase (Fig. 2C) were observed in layer 5/6 ILC pyramidal neurons in adolescent mice, indicating that such differences in GABABR-dependent signaling are specific to the dorsal aspect of the mPFC.
Figure 2. Anatomic specificity of the sex differences in layer 5/6 pyramidal neurons.
A) Schematic depiction of the region targeted for electrophysiological characterization. Abbreviations: PrLC, prelimbic cortex; ILC, infralimbic cortex; CG, cingulate cortex. B) Summary of baclofen-induced currents in layer 5/6 ILC pyramidal neurons from adolescent male (M) and female (F) mice (t13=0.09, P=0.93; n=7–8/sex). C) Summary of rheobase data in layer 5/6 ILC pyramidal neurons from adolescent male (M) and female (F) mice (t18=0.86, P=0.4; n=9–11/sex). D) Representative baclofen-induced currents (upper traces) and concomitant decreases in input resistance (lower traces) in layer 5/6 ILC pyramidal neurons from adolescent male (left, black) and female (right, gray) mice.
3.3. GIRK channel contribution to sex differences in layer 5/6 PrLC pyramidal neurons
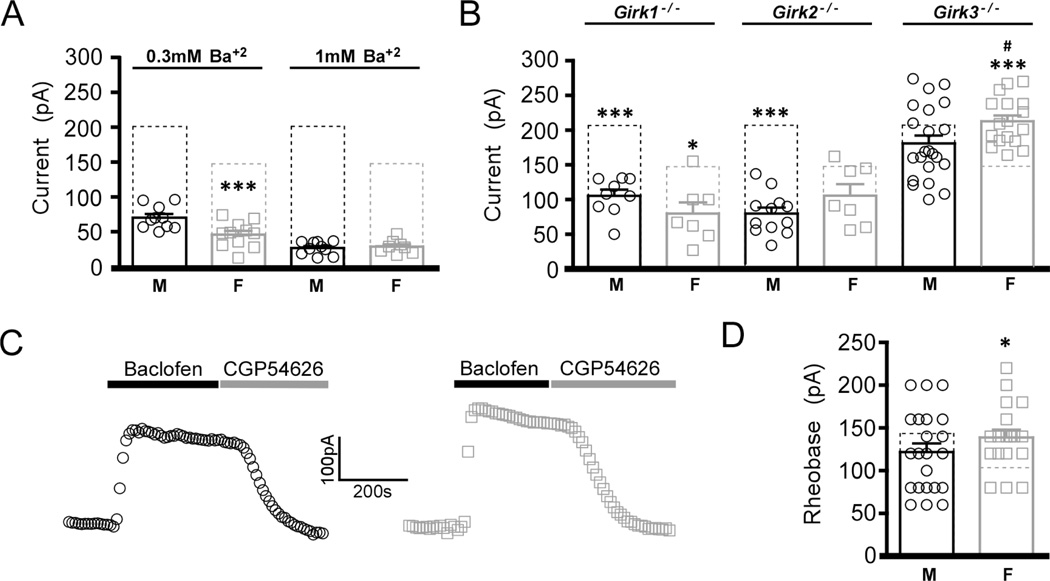
GIRK channels mediate most (60%) of the baclofen-induced somatodendritic current in layer 5/6 PrLC neurons of male mice (Hearing et al. 2013). To determine whether the sex difference in GABABR-dependent signaling is linked to the GIRK-dependent component of this composite conductance, we evaluated baclofen-induced currents in layer 5/6 PrLC pyramidal neurons from adolescent male and female mice in the presence of Ba2+, a non-selective GIRK channel blocker. While baclofen-induced currents were comparably reduced by 0.3 mM Ba2+ (65%) in male and female pyramidal neurons, the currents remained significantly higher in slices from male mice (Fig. 3A). No sex difference was observed in the baclofen-induced current, however, when measured in the presence of 1 mM Ba2+, suggesting that the sex difference is linked primarily to the GIRK component of the GABABR-dependent current.
Figure 3. Contribution of GIRK channels to sex differences in layer 5/6 PrLC pyramidal neurons.
A) Summary of Ba2+-resistant baclofen-induced currents in layer 5/6 PrLC pyramidal neurons from adolescent male (M) and female (F) wild-type mice. Dotted rectangles show the average baclofen-induced currents measured in the absence of Ba2+ (from Fig. 1B). A significant interaction between treatment and sex was found (F1,39=8.4, P<0.001; n=8–11/group). Symbols: ***P<0.001 vs. male (within treatment). B) Summary of residual baclofen-induced currents in layer 5/6 PrLC pyramidal neurons from adolescent male (M) and female (F) Girk−/− mice, and comparison to currents measured in neurons from wild-type mice (dotted rectangles, from Fig. 1B). A significant interaction between genotype and sex was observed (F3,118=8.2, P<0.001; n=7–25/group). Symbols: *,*** P<0.05 & 0.001, respectively, vs. wild-type; #P<0.05 vs. male Girk3−/− mice. C) Representative baclofen-induced currents in layer 5/6 PrLC pyramidal neurons from adolescent male (left, black) and female (right, grey) Girk3−/− mice. D) Summary of rheobase data in layer 5/6 PrLC pyramidal neurons from adolescent male (M) and female (F) Girk3−/− mice. An interaction was found between sex and genotype (F1,81=7.1, P<0.01; n=18–24/group). Symbols: *P<0.05 vs. wild-type female.
To test whether specific GIRK channel subunits contribute to the sex difference in GABABR-GIRK signaling in layer 5/6 PrLC pyramidal neurons, we next compared baclofen-induced currents in neurons from adolescent male and female Girk−/− mice. Genetic ablation of either Girk1 or Girk2 correlated with attenuated baclofen-induced currents in layer 5/6 PrLC pyramidal neurons from both adolescent male and female mice; no sex difference was observed for the residual baclofen-induced current in these neurons (Fig. 3B). Interestingly, while ablation of Girk3 had no effect on baclofen-induced currents in male neurons, loss of Girk3 in females produced a significant increase in baclofen-evoked currents, as compared to wild-type females (Fig. 3B,C). This increase also correlated with an increase in rheobase (Fig. 3D). Collectively, these data suggest that the difference in baclofen-induced current and rheobase in adolescent male and female layer 5/6 PrLC pyramidal neurons may be linked to GIRK3.
3.4. Membrane trafficking of GABABR1 and GIRK2 in male and female mice
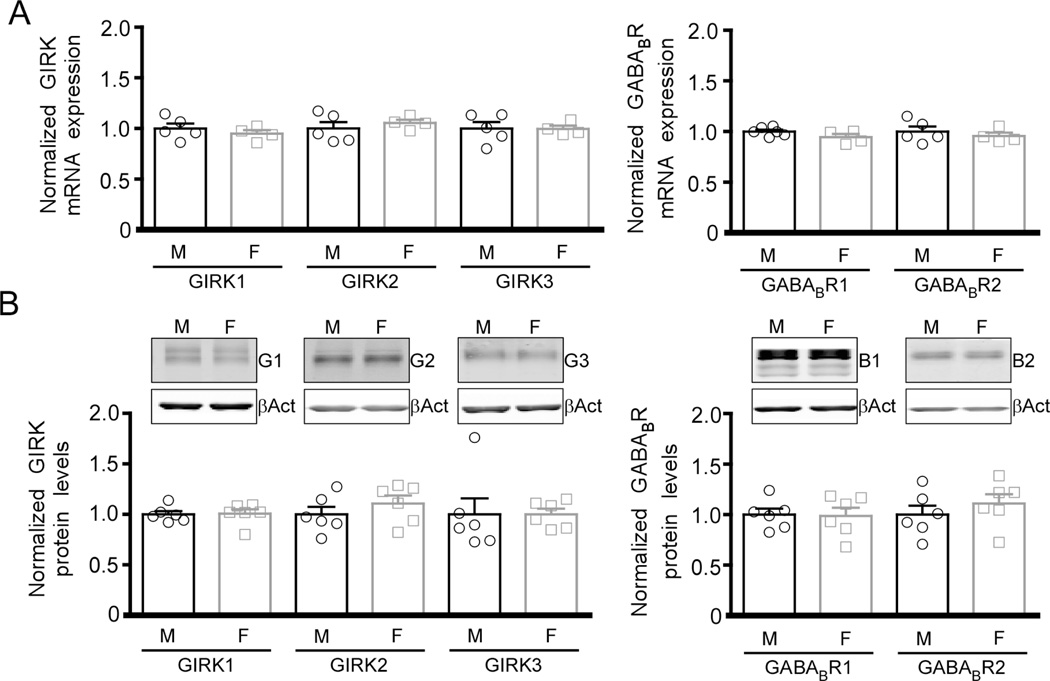
We next tested whether adolescent sex differences in baclofen-evoked currents in layer 5/6 PrLC pyramidal neurons is linked to altered expression of GIRK channel subunits. qRT-PCR analysis of micropunches containing the mPFC showed no significant difference in GIRK1, GIRK2, or GIRK3 mRNA levels, or in the expression of the two formative GABABR subunits, GABABR1 and GABABR2 (Fig. 4A). Similarly, total protein levels for GIRK1, GIRK2, GIRK3, GABABR1, and GABABR2 did not differ between adolescent male and female mice (Fig. 4B).
Figure 4. Expression of GABABR-GIRK signaling components in the male and female mPFC.
A) mRNA levels of GABABR-GIRK signaling pathway constituents (GIRK1, GIRK2, GIRK3, GABABR1 and GABABR2) in micropunches containing the mPFC from adolescent male (M) and female (F) wild-type mice. Data are normalized to the expression of GAPDH and to mRNA levels in samples from male mice (n=4–6/sex). B) Top: representative immunoblots showing total protein levels of the key GABABR-GIRK signaling pathway constituents (G1: GIRK1, G2: GIRK2, G3: GIRK3, B1: GABABR1, B2: GABABR2, βAct: β-actin) in micropunches containing the mPFC from adolescent male (M) and female (F) wild-type mice. Bottom: quantification of GIRK and GABABR protein levels, with normalization to the levels of β-actin and to protein levels in samples from male mice (n=6/sex).
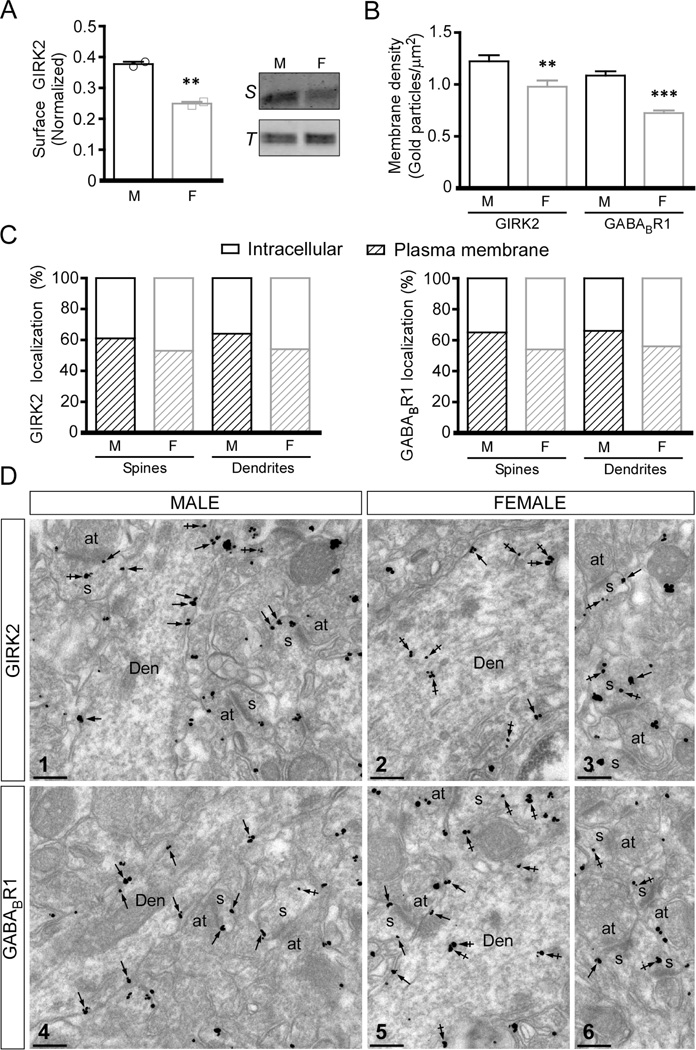
Changes in the subcellular distribution of GABABR1 and/or GIRK2 have been linked to adaptations in GABABR-GIRK signaling in layer 5/6 PrLC pyramidal neurons and other neuron populations (Arora et al. 2011; Padgett et al. 2012; Hearing et al. 2013). To determine whether differential trafficking of the GABABR-GIRK signaling components underlies the adolescent sex difference in baclofen-induced currents in layer 5/6 PrLC pyramidal neurons, we evaluated surface membrane expression of GIRK2 using a biotinylation assay and micropunches of the mPFC. We observed a modest but significant sex difference in the level of GIRK2 on the cell surface, with samples from female mice showing lower surface GIRK2 levels (Fig. 5A). Using a quantitative immunoelectron microscopy approach, we observed a greater immunoparticle density at the plasma membrane of dendrites of layer 5/6 pyramidal neurons from adolescent male as compared to female mice, for both GIRK2 and GABABR1 (Fig. 5B,D). Immunoparticles were found both in dendrites and spines, with a higher percentage of GIRK2 and GABABR1 labeling on the cell surface relative to intracellular sites in adolescent male as compared to female mice (Fig. 5C,D). These data suggest that the differential surface trafficking of GABABR and/or GIRK channels likely explains the adolescent sex differences in baclofen-induced current of layer 5/6 PrLC pyramidal neurons.
Figure 5. Subcellular localization of GIRK2 and GABABR1 in layer 5/6 pyramidal neurons of male and female mice.
A) Left: Quantification of surface GIRK2 protein levels in mPFC micropunches from adolescent male (M) and female (F) mice, with normalization to total GIRK2 protein (t2=13, P<0.01; n=2/group). Right: Representative immunoblots showing surface (S) and total GIRK2 (T) protein levels in mPFC micropunches from adolescent male and female wild-type mice. B) Plasma membrane-associated immunogold particle density for GIRK2 (Mann-Whitney rank sum test; T=5499, n=80; P<0.01) and GABABR1 (Mann-Whitney rank sum test; T=4380, n=80; P<0.001) in dendrites from adolescent male (M) and female (F) mice (n=4/sex) **,*** P < 0.01 & 0.001, respectively, vs. male. C) Distribution of GIRK2 and GABABR1 immunoparticles at the plasma membrane and intracellular sites in layer 5/6 PrLC pyramidal neuron spines and dendrites from adolescent male (M) and female (F) mice (n=4/sex), expressed as a percentage of total particles (n=4/sex). D) Representative images of GIRK2 (Image 1) and GABABR1 (Image 4) immunoreactivity in mPFC Layer 5/6 pyramidal neuron dendrites and spines from a male mouse. Immunoparticles for GIRK2 or GABABR1 were mainly detected along the extrasynaptic plasma membrane (arrows) of dendritic shafts (Den) and spines (s), and at low levels at intracellular sites (crossed arrows) in these compartments. (Images 2,3,5,6) Representative images of GIRK2 (Images 2,3) and GABABR1 (Images 5,6) immunoreactivity in mPFC Layer 5/6 pyramidal neuron dendrites and spines from a female mouse. Immunoparticles for GIRK2 or GABABR1 were detected along the extrasynaptic plasma membrane (arrows) of dendritic shafts (Den) and spines (s), and more frequently observed at intracellular sites (crossed arrows) in these compartments. Abbreviation: at, axon terminal. Scale bars: 0.2 µm.
3.5. A phosphorylation-dependent mechanism underlies the sex difference
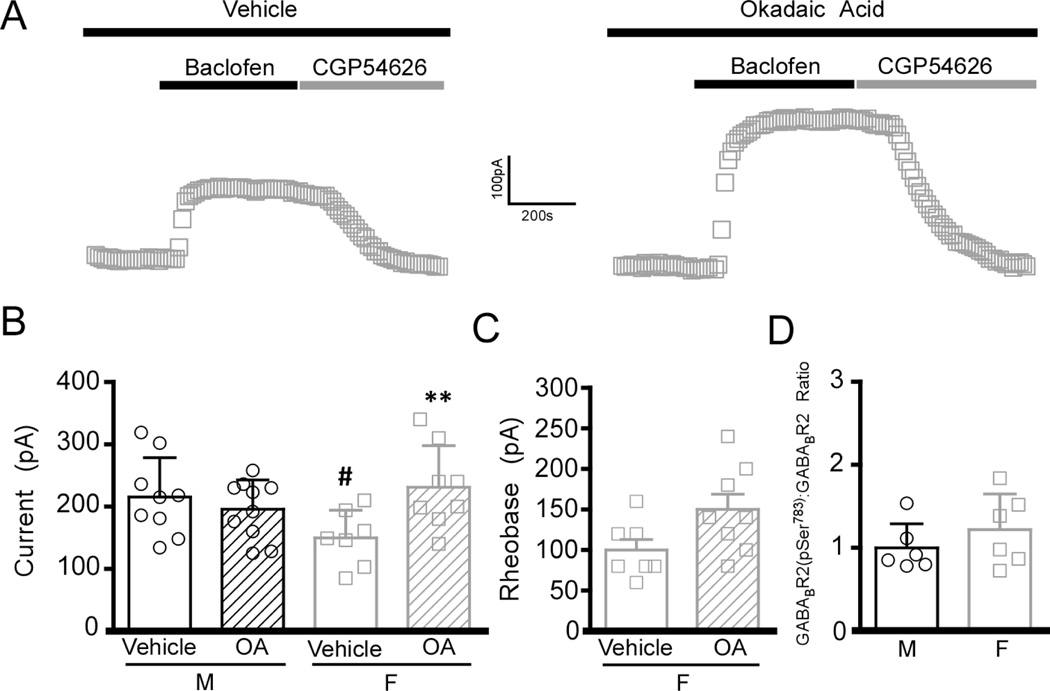
Surface trafficking of GIRK channels and GABABR is regulated by phosphorylation (Chung et al. 2009; Terunuma et al. 2010a; Padgett et al. 2012; Hearing et al. 2013). Acute intracellular application of okadaic acid (OA), a potent PP1/PP2A phosphatase inhibitor, has been shown to normalize drug-induced adaptations in GABABR-GIRK signaling (Padgett et al. 2012; Hearing et al. 2013). In the present study, inclusion of OA (but not vehicle control) in the pipette solution enhanced baclofen-induced currents in layer 5/6 PrLC pyramidal neurons from adolescent female but not male mice (Fig. 6A,B). Moreover, OA treatment tended to increase the rheobase in female neurons (t13=2.1, P=0.06; n=7–8/group) (Fig. 6C). Previous studies have shown that the phosphorylation of Ser783 in GABABR2 can influence the strength of GABABR-GIRK signaling in multiple neuron populations, including layer 5/6 PrLC neurons (Padgett et al. 2012; Hearing et al. 2013). Using phospho-specific antibodies and quantitative immunoblotting of micropunches of the PFC, however, we found that the levels of GABABR2-(pSer783) were not different between adolescent males and females (Fig. 6D). Thus, while our data suggest that a phosphorylation-dependent mechanism underlies the sex difference in GIRK-dependent signaling seen in layer 5/6 PrLC pyramidal neurons from adolescent mice, the critical phosphorylation site(s) is/are unknown.
Figure 6. Phosphorylation and the sex difference in GIRK-dependent signaling in layer 5/6 PrLC pyramidal neurons.
A) Representative traces showing effect of vehicle (DMSO, 14 µM) or OA (added via the patch pipette) on baclofen-induced currents in layer 5/6 PrLC pyramidal neurons from adolescent female mice. B) Summary of baclofen-induced currents in layer 5/6 PrLC pyramidal neurons from adolescent male (M) and female (F) mice (n=7–10/sex). A significant interaction was found between sex and treatment (F1,33=6.7, P<0.05). Symbols: **P<0.01 vs. female vehicle (DMSO); #P<0.05 vs. male vehicle (DMSO). C) Summary of rheobase data for vehicle- (DMSO) and OA-treated layer 5/6 PrLC pyramidal cells from adolescent female (F) mice (t13=2.1, P=0.06; n=7–8/treatment). D) Quantification of GABABR2(pSer783) protein levels, with normalization to the level of total GABABR2 in micropunches containing the mPFC from adolescent male (M) and female (F) wild-type mice (n=6/sex).
4. Discussion
Over the past decade, it has become increasingly apparent that some neurological and neuropsychiatric disorders show significant sex differences with respect to susceptibility, pathophysiology, and response to treatments (Becker et al. 2012; McCarthy et al. 2012; Bangasser and Valentino 2014). In addition to hormonal influences, evidence suggests that intrinsic sex differences in neural connectivity, neurotransmission, and cell physiology within a number of brain regions, including the PFC, contribute to these differences (Baran et al. 2010; Westberry and Wilson 2012; Godsil et al. 2013). The mPFC plays an integral role in high-order cognitive functions important for behavioral inhibition, attention gating, and processing of emotional- and reward-related information (Wood and Grafman 2003). Here, we describe a sex difference in the strength of inhibitory GABABR-dependent signaling in layer 5/6 pyramidal neurons in the PrLC of adolescent mice.
Elevated GABABR-GIRK signaling seen in layer 5/6 PrLC pyramidal neurons from female Girk3−/− mice correlated with increased rheobase, suggesting a strong association between the strength of GABABR-GIRK signaling in, and excitability, of these neurons. Indeed, our findings are consistent with the possibility that GABABR-GIRK signaling exerts a tonic inhibitory influence on the excitability of layer 5/6 PrLC pyramidal neurons. In support of this contention, a tonically-active A1 adenosine receptor-GIRK channel signaling pathway was shown to contribute to the intrinsic membrane properties of CA1 pyramidal neurons in the dorsal hippocampus (Kim and Johnston 2015). No evidence for tonically-active GABABR-GIRK signaling, however, was observed in CA1 pyramidal neurons. While receptor-independent (basal) activity of GIRK channels is generally considered to be low, it is also possible that the reduced surface expression of GIRK channels in female layer 5/6 PrLC pyramidal neurons, and the correspondingly lower level of basal GIRK channel activity, could account for the lower rheobase in female neurons. We did not, however, observe any difference in resting membrane potential of layer 5/6 PrLC pyramidal neurons from adolescent male and female mice, suggesting that basal GIRK channel activity contributes minimally to the excitability of these neurons, or that compensatory conductances are masking what would otherwise be a GIRK-dependent sex difference. Finally, the difference in rheobase in layer 5/6 PrLC pyramidal neurons from adolescent male and female could reflect sex differences in another conductance(s) that influences the excitability of these neurons.
Sex differences in GIRK-dependent signaling have been reported previously within more subcortical regions such as the dorsal raphe, where smaller currents in females as compared to males was attributed, at least in part, to sex-related variations in receptor-G protein signaling mechanisms (Loucif et al. 2006). In the present study, however, similar EC50 profiles were observed for baclofen-induced currents in adolescent male and female neurons, suggesting that GABABR-GIRK coupling efficiency is not different across sexes. Rather, biochemical and ultrastructural data support the contention that the sex difference in layer 5/6 PrLC pyramidal neurons is largely due to differences in the trafficking of GABABR and/or GIRK channels.
Surface trafficking of GABABR and GIRK channels is regulated by phosphorylation-dependent mechanisms. For example, phosphorylation of Ser9 on GIRK2 promotes internalization of GIRK2-containing channels, while dephosphorylation of Ser783 on GABABR2 is associated with reduced surface expression of the GABAB receptor (Chung et al. 2009; Terunuma et al. 2010b; Padgett et al. 2012). In the present study, intracellular blockade of PP1/PP2A phosphatase activity with OA negated the sex difference in GABABR-GIRK currents, suggesting that a sex difference in phosphatase activity in layer 5/6 PrLC pyramidal neurons may underlie the difference. Consistent with this hypothesis, previous studies have demonstrated sex-specific differences in basal kinase and phosphatase signaling within other limbic brain regions (Nazarian et al. 2009; Zhou et al. 2009). While no significant differences were observed in Ser783-GABABR protein phosphorylation, we cannot rule out a role for this residue in the sex-dependent differences reported herein, as the lack of observed difference could reflect our inability to selectively isolate pyramidal neurons within the PrLC from other cell types and mPFC sub-regions (i.e., ILC).
Constitutive ablation of Girk1 and Girk2 reduced GABABR-GIRK currents in layer 5/6 PrLC pyramidal neurons from both male and female mice. And while the loss of Girk3 did not correlate with altered currents in neurons from male mice, it did correlate with larger baclofen-induced currents in female mice. GIRK3 contains a unique lysosomal targeting motif that affects its membrane expression (Ma et al. 2002). Expression of GIRK3 can increase the level of trafficking of GIRK channels to lysosomes by virtue of its interaction with sorting nexin 27 (SNX27) (Lunn et al. 2007). Thus, the sex difference in GIRK-dependent signaling in layer 5/6 PrLC pyramidal neurons may be linked to altered expression/function of SNX27 or related trafficking proteins. Though no sex differences in SNX27 expression or function have been reported, SNX2 expression has been shown to be sexually-dimorphic; it is up-regulated in males and co-localizes in neurons expressing the androgen receptor (Wu et al. 2010).
There are a number of intriguing possibilities when considering the selective nature of the sexually-dimorphic physiology of PrLC layer 5/6 pyramidal neurons. First, these differences were only observed in adolescent mice. As the PFC is slower to develop in comparison with other brain regions, it remains in a highly-plastic state during adolescence (Tsujimoto 2008; Kolb et al. 2012; Galvan 2014). This protracted development is believed to render it more susceptible to environmental influences that are thought to promote the emergence of emotionally-reactive and risk-related behavior, as well as heightened reward seeking that is often observed during adolescence (Romer 2010; Somerville et al. 2011). Therefore, it is tempting to speculate that reductions in GIRK-dependent inhibition may permit greater plasticity in females compared to males during this developmental period, which may in turn explain differences in susceptibility (or resilience) to certain disorders.
The mPFC is a part of the mesocorticolimbic reward circuitry. The PrLC, in particular, plays a critical role in the acquisition, maintenance, and reinstatement of psychostimulant place preference and self-administration (Freeman et al. 2010; Van den Oever et al. 2010; Ary et al. 2013). Studies have shown a sexually-dimorphic pattern of behavioral responding to drugs of abuse in females. For example, females display more rapid acquisition of psychostimulant self-administration, develop place preference at lower doses and with fewer conditioning sessions, and demonstrate increased sensitivity to the motor activating effects of drugs (Hu et al. 2004; Anker and Carroll 2011; Becker et al. 2012; Bobzean et al. 2014). We demonstrated recently that repeated cocaine exposure reduces GIRK-dependent signaling in layer 5/6 pyramidal neurons of the PrLC cortex, and that this reduction facilitates behavioral sensitization to cocaine (Hearing et al. 2013). Thus, it will be interesting to determine whether the increased excitability of layer 5/6 pyramidal neurons in adolescent female mice alters the processing of reward-related information and facilitates increased responding to drugs of abuse and associated stimuli.
While overlap in function and anatomical connectivity does exist between the PrLC and the ILC, overwhelming evidence suggests that these two divisions play dissociable roles in behaviors related to learning, stress, anxiety, and addiction. For example, alterations in PrLC function are important for modulating anxiety responses during stressful situations and gating of fear-related memories, the latter of which is associated with development of PTSD (Sotres-Bayon et al. 2012; Fenton et al. 2014). In females, heightened activation of the PrLC is believed to underlie increased anxiety-like behavior and enhanced expression of learned fear (Fenton et al. 2014; Saitoh et al. 2014). Moreover, reductions in excitatory signaling in the PrLC promote anxiolytic effects (Ohashi et al. 2014), while reductions in intra-PrLC GABAergic signaling increase pyramidal cell activity and promote fear responding (Sotres-Bayon et al. 2012). Therefore, it is possible that reduced inhibitory signaling in PrLC output neurons has a role in pre-disposing females to certain aspects of emotional disorders. Given the recent development of subtype-selective modulators for GIRK channels (Kaufmann et al. 2013; Wydeven et al. 2014), GIRK channels within the mPFC may prove to be effective targets for treating neuropsychiatric disorders in the future.
5. Conclusions
The present study reports sex differences in GABABR-GIRK signaling in, and the excitability of, layer 5/6 PrLC pyramidal neurons in adolescent mice. The sex differences could contribute to sex differences in susceptibility to nervous system disorders associated with altered mPFC function. A better understanding of these differences, and the underlying mechanisms, could lead to a more effective, sex specific, approach for the treatment of these disorders.
HIGHLIGHTS.
-
-
There is a sex difference in GABABR-dependent signaling in the mouse mPFC
-
-
The difference involves prelimbic layer 5/6 pyramidal neurons from adolescent mice
-
-
The GIRK channel branch of the GABABR signaling pathway is implicated
-
-
Surface trafficking of the GABABR and/or GIRK channel explains this phenomenon
-
-
Phosphorylation and the GIRK3 subunit underlie this sex difference
ACKNOWLEDGEMENTS
The authors would like to thank Daniele Young, Jennifer Kutzke, and Alex Shnaydruk for maintaining the mouse colony. This work was supported by NIH grants to MH (DA007097), NCV (DA007234), and KW (MH061933 and DA034696), and by grants from the Spanish Ministry of Education and Science to RL (BFU-2012-38348).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Anyanwu EC. Neurochemical changes in the aging process: implications in medication in the elderly. Scientific World Journal. 2007;7:1603–1610. doi: 10.1100/tsw.2007.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora D, Hearing M, Haluk DM, Mirkovic K, Fajardo-Serrano A, Wessendorf MW, Watanabe M, Lujan R, Wickman K. Acute cocaine exposure weakens GABA(B) receptor-dependent G-protein-gated inwardly rectifying K+ signaling in dopamine neurons of the ventral tegmental area. J Neurosci. 2011;31(34):12251–12257. doi: 10.1523/JNEUROSCI.0494-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ary AW, Lominac KD, Wroten MG, Williams AR, Campbell RR, Ben-Shahar O, von Jonquieres G, Klugmann M, Szumlinski KK. Imbalances in prefrontal cortex CC-Homer1 versus CC-Homer2 expression promote cocaine preference. J Neurosci. 2013;33(19):8101–8113. doi: 10.1523/JNEUROSCI.1727-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35(3):303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Conrad CD. Prefrontal cortex lesions and sex differences in fear extinction and perseveration. Learn Mem. 2010;17(5):267–278. doi: 10.1101/lm.1778010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3(1):14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettahi I, Marker CL, Roman MI, Wickman K. Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh. J Biol Chem. 2002;277(50):48282–48288. doi: 10.1074/jbc.M209599200. [DOI] [PubMed] [Google Scholar]
- Bobzean SA, DeNobrega AK, Perrotti LI. Sex differences in the neurobiology of drug addiction. Exp Neurol. 2014;259:64–74. doi: 10.1016/j.expneurol.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix. 2009;4 doi: 10.1002/0471142301.nsa04is48. Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Qian X, Ehlers M, Jan YN, Jan LY. Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc Natl Acad Sci U S A. 2009;106(2):629–634. doi: 10.1073/pnas.0811615106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton GE, Pollard AK, Halliday DM, Mason R, Bredy TW, Stevenson CW. Persistent prelimbic cortex activity contributes to enhanced learned fear expression in females. Learn Mem. 2014;21(2):55–60. doi: 10.1101/lm.033514.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, Lull ME, Patel KM, Brucklacher RM, Morgan D, Roberts DC, Vrana KE. Gene expression changes in the medial prefrontal cortex and nucleus accumbens following abstinence from cocaine self-administration. BMC Neurosci. 2010;11:29. doi: 10.1186/1471-2202-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Insights about adolescent behavior, plasticity, and policy from neuroscience research. Neuron. 2014;83(2):262–265. doi: 10.1016/j.neuron.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62(2):155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol. 2013;23(10):1165–1181. doi: 10.1016/j.euroneuro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Hearing M, Kotecki L, Marron Fernandez de Velasco E, Fajardo-Serrano A, Chung HJ, Lujan R, Wickman K. Repeated cocaine weakens GABA(B)-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron. 2013;80(1):159–170. doi: 10.1016/j.neuron.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Zink AN, Wickman K. Cocaine-induced adaptations in metabotropic inhibitory signaling in the mesocorticolimbic system. Rev Neurosci. 2012;23(4):325–351. doi: 10.1515/revneuro-2012-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27(6):555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29(1):81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Ishii-Takahashi A, Takizawa R, Nishimura Y, Kawakubo Y, Kuwabara H, Matsubayashi J, Hamada K, Okuhata S, Yahata N, Igarashi T, Kawasaki S, Yamasue H, Kato N, Kasai K, Kano Y. Prefrontal activation during inhibitory control measured by near-infrared spectroscopy for differentiating between autism spectrum disorders and attention deficit hyperactivity disorder in adults. Neuroimage Clin. 2014;4:53–63. doi: 10.1016/j.nicl.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K, Romaine I, Days E, Pascual C, Malik A, Yang L, Zou B, Du Y, Sliwoski G, Morrison RD, Denton J, Niswender CM, Daniels JS, Sulikowski GA, Xie XS, Lindsley CW, Weaver CD. ML297 (VU0456810), the first potent and selective activator of the GIRK potassium channel, displays antiepileptic properties in mice. ACS Chem Neurosci. 2013;4(9):1278–1286. doi: 10.1021/cn400062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol. 1993;69(2):416–431. doi: 10.1152/jn.1993.69.2.416. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31(3–5):277–287. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Rønnekleiv OK. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann N Y Acad Sci. 2003;1007:6–16. doi: 10.1196/annals.1286.001. [DOI] [PubMed] [Google Scholar]
- Kim CS, Johnston D. A1 adenosine receptor-mediated GIRK channels contributes to the resting conductance of CA1 neurons in the dorsal hippocampus. J Neurophysiol. 2015 doi: 10.1152/jn.00951.2014. jn.00951.02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R. Experience and the developing prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17186–17193. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik A, Vida I, Lujan R, Haas CA, Lopez-Bendito G, Shigemoto R, Frotscher M. Subcellular localization of metabotropic GABAB receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus. J Neurosci. 2003;23(35):11026–11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AT, Gee SM, Vogt D, Patel T, Rubenstein JL, Sohal VS. Pyramidal neurons in prefrontal cortex receive subtype-specific forms of excitation and inhibition. Neuron. 2014;81(1):61–68. doi: 10.1016/j.neuron.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucif AJ, Bonnavion P, Macri B, Golmard JL, Boni C, Melfort M, Leonard G, Lesch KP, Adrien J, Jacquin TD. Gender-dependent regulation of G-protein-gated inwardly rectifying potassium current in dorsal raphe neurons in knock-out mice devoid of the 5-hydroxytryptamine transporter. J Neurobiol. 2006;66(13):1475–1488. doi: 10.1002/neu.20321. [DOI] [PubMed] [Google Scholar]
- Lujan R, Marron Fernandez de Velasco E, Aguado C, Wickman K. New insights into the therapeutic potential of Girk channels. Trends Neurosci. 2014;37(1):20–29. doi: 10.1016/j.tins.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn ML, Nassirpour R, Arrabit C, Tan J, McLeod I, Arias CM, Sawchenko PE, Yates JR, 3rd, Slesinger PA. A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat Neurosci. 2007;10(10):1249–1259. doi: 10.1038/nn1953. [DOI] [PubMed] [Google Scholar]
- Luscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11(5):301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY. Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron. 2002;33(5):715–729. doi: 10.1016/s0896-6273(02)00614-1. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32(7):2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian A, Sun WL, Zhou L, Kemen LM, Jenab S, Quinones-Jenab V. Sex differences in basal and cocaine-induced alterations in PKA and CREB proteins in the nucleus accumbens. Psychopharmacology (Berl) 2009;203(3):641–650. doi: 10.1007/s00213-008-1411-5. [DOI] [PubMed] [Google Scholar]
- Ohashi M, Saitoh A, Yamada M, Oka JI, Yamada M. Riluzole in the prelimbic medial prefrontal cortex attenuates veratrine-induced anxiety-like behaviors in mice. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3676-1. [DOI] [PubMed] [Google Scholar]
- Padgett CL, Lalive AL, Tan KR, Terunuma M, Munoz MB, Pangalos MN, Martinez-Hernandez J, Watanabe M, Moss SJ, Lujan R, Luscher C, Slesinger PA. Methamphetamine-evoked depression of GABA(B) receptor signaling in GABA neurons of the VTA. Neuron. 2012;73(5):978–989. doi: 10.1016/j.neuron.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D. Adolescent risk taking, impulsivity, and brain development: implications for prevention. Dev Psychobiol. 2010;52(3):263–276. doi: 10.1002/dev.20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh A, Ohashi M, Suzuki S, Tsukagoshi M, Sugiyama A, Yamada M, Oka J, Inagaki M, Yamada M. Activation of the prelimbic medial prefrontal cortex induces anxiety-like behaviors via N-Methyl-D-aspartate receptor-mediated glutamatergic neurotransmission in mice. J Neurosci Res. 2014;92(8):1044–1053. doi: 10.1002/jnr.23391. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res. 2008;14(2–3):249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav. 2002;77(4–5):513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- Signorini S, Liao YJ, Duncan SA, Jan LY, Stoffel M. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci U S A. 1997;94(3):923–927. doi: 10.1073/pnas.94.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76(4):804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WL, Festa ED, Jenab S, Quinones-Jenab V. Sex differences in dopamine D2-like receptor-mediated G-protein activation in the medial prefrontal cortex after cocaine. Ethn Dis. 2010;20(Suppl 1):88–91. [PubMed] [Google Scholar]
- Szczepanski SM, Knight RT. Insights into human behavior from lesions to the prefrontal cortex. Neuron. 2014;83(5):1002–1018. doi: 10.1016/j.neuron.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Pangalos MN, Moss SJ. Advances in Pharmacology. Vol. 58. Elsevier; 2010a. Functional Modulation of GABAB Receptors by Protein Kinases and Receptor Trafficking; pp. 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Vargas KJ, Wilkins ME, Ramirez OA, Jaureguiberry-Bravo M, Pangalos MN, Smart TG, Moss SJ, Couve A. Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc Natl Acad Sci U S A. 2010b;107(31):13918–13923. doi: 10.1073/pnas.1000853107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci. 2002;22(11):4328–4334. doi: 10.1523/JNEUROSCI.22-11-04328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto S. The prefrontal cortex: functional neural development during early childhood. Neuroscientist. 2008;14(4):345–358. doi: 10.1177/1073858408316002. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146(1–2):3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010;35(2):276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Wilson ME. Regulation of estrogen receptor alpha gene expression in the mouse prefrontal cortex during early postnatal development. neurogenetics. 2012;13(2):159–167. doi: 10.1007/s10048-012-0323-z. [DOI] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci. 2003;4(2):139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Wu D, Tang YP, Wade J. Co-localization of sorting nexin 2 and androgen receptor in the song system of juvenile zebra finches. Brain Res. 2010;1343:104–111. doi: 10.1016/j.brainres.2010.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydeven N, Marron Fernandez de Velasco E, Du Y, Benneyworth MA, Hearing MC, Fischer RA, Thomas MJ, Weaver CD, Wickman K. Mechanisms underlying the activation of G-protein-gated inwardly rectifying K+ (GIRK) channels by the novel anxiolytic drug, ML297. Proc Natl Acad Sci U S A. 2014;111(29):10755–10760. doi: 10.1073/pnas.1405190111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Nazarian A, Sun WL, Jenab S, Quinones-Jenab V. Basal and cocaine-induced sex differences in the DARPP-32-mediated signaling pathway. Psychopharmacology (Berl) 2009;203(1):175–183. doi: 10.1007/s00213-008-1388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]