Significance
The association of hypertension and inflammation is clear, but mechanisms for this human finding remain elusive. The experiments in this article demonstrate that a highly local intramyocardial signaling pathway through ST2, the receptor for the proinflammatory cytokine interleukin 33 (IL-33), regulates the heart’s response to pressure overload. By generating and using new conditional deletion mice, we identified endothelial cells as the major source of systemic circulating IL-33. Our study reveals that endothelial cell secretion of IL-33 is crucial for translating myocardial pressure overload into a selective systemic inflammatory state.
Keywords: interleukin-33, cardiac hypertrophy, inflammation, endothelial cells
Abstract
Hypertension increases the pressure load on the heart and is associated with a poorly understood chronic systemic inflammatory state. Interleukin 33 (IL-33) binds to membrane-bound ST2 (ST2L) and has antihypertrophic and antifibrotic effects in the myocardium. In contrast, soluble ST2 appears to act as a decoy receptor for IL-33, blocking myocardial and vascular benefits, and is a prognostic biomarker in patients with cardiovascular diseases. Here we report that a highly local intramyocardial IL-33/ST2 conversation regulates the heart’s response to pressure overload. Either endothelial-specific deletion of IL33 or cardiomyocyte-specific deletion of ST2 exacerbated cardiac hypertrophy with pressure overload. Furthermore, pressure overload induced systemic circulating IL-33 as well as systemic circulating IL-13 and TGF-beta1; this was abolished by endothelial-specific deletion of IL33 but not by cardiomyocyte-specific deletion of IL33. Our study reveals that endothelial cell secretion of IL-33 is crucial for translating myocardial pressure overload into a selective systemic inflammatory response.
Hypertension is the most common cardiovascular risk factor and contributes to widespread morbidity and mortality worldwide (1), but the pathological and molecular mechanisms by which elevated blood pressure promotes vascular disease remain uncertain. Inflammation has been hypothesized to play a role in hypertension as well as the progression of vascular disease (2, 3). Although the association between hypertension and inflammation has now been clearly demonstrated, molecular mechanisms that link hypertension to systemic inflammation are unclear.
The soluble receptor ST2 is a prognostic biomarker in patients with cardiovascular disease (4, 5), and serum ST2 levels also predict changes in blood pressure in the community (6). ST2, also known as IL1RL1 (IL-1 receptor like 1), is a member of the IL-1 receptor family, which plays a major role in immune and inflammatory responses (7). At least two forms of ST2 are known, including the transmembrane receptor (ST2L) and the soluble form (sST2) that circulates in blood (8). Membrane-bound ST2L interacts with IL-33, an IL-1 family ligand (9), and IL-33 can have antihypertrophic and antifibrotic effects in the myocardium (10). In contrast, sST2 appears to act as a decoy receptor for IL-33, blocking myocardial and vascular benefits (10–12). IL-33 is also expressed in endothelial cells (ECs) (13–16), in which it induces angiogenesis (17), expression of adhesion molecules, and inflammatory activation (18). Here we report the surprising finding that endothelial IL-33 from pressure overload induces a selective systemic response, potentially linking hypertension with circulating factors that can affect the vasculature and other organs.
Results
ST2 Deficiency Exacerbates Pressure Overload-Induced Cardiac Hypertrophy.
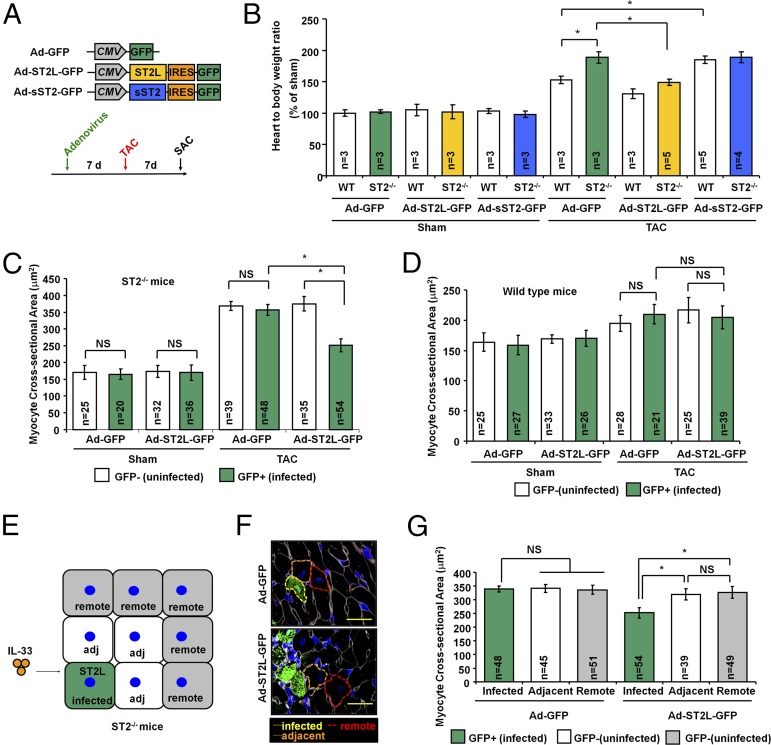
Communication between cardiomyocytes, fibroblasts, and ECs is important for normal cardiac function as well as pathophysiology (19, 20). We explored intercellular communication in the IL-33/ST2 pathway in cardiac hypertrophy induced by pressure overload. First, we performed adenoviral gene transfer to test whether delivery of exogenous ST2L or sST2 regulates cardiac hypertrophy. Adenoviral vectors (Ad-GFP, Ad-ST2L-GFP, or Ad-sST2-GFP) were injected into the left ventricular free wall in mice. Seven days after gene transfer, mice were subjected to Sham or transverse aortic constriction (TAC) surgery, which mimics the pressure overload of hypertension on the heart (Fig. 1A). We found that gene transfer of ST2L into ST2−/− mice reduced cardiac hypertrophy compared with Ad-GFP controls. In addition, gene transfer of sST2 to WT but not ST2−/− mice increased TAC-induced hypertrophy (Fig. 1B). These results indicate that ST2L signaling mediates antihypertrophic activity, whereas sST2 functions as a prohypertrophic factor.
Fig. 1.
ST2L controls pressure overload-induced hypertrophy in individual cardiomyocytes. (A) Adenovirus gene constructs and experiment design. SAC, sacrifice; TAC, transverse aortic constriction. (B) Adenoviral vectors (Ad-GFP, Ad-ST2L-GFP, or Ad-sST2-GFP) were injected into the left ventricular free wall in mice. Seven days after adenovirus gene transfer, the mice were subjected to Sham or TAC surgery. (C) Adenoviral transfer of ST2L to ST2−/− mice leads to reduced CSA in infected cardiomyocytes. (D) Adenoviral transfer of ST2L to WT mice had no effect on the CSAs between infected and uninfected cardiomyocytes. (E) Schematic representation of ST2L-transferred cardiomyocyte responses to local IL-33. Cardiomyocytes were grouped as infected or uninfected on the basis of GFP fluorescence. Adj, adjacent; infected, adenovirus infected cardiomyocyte; remote, remote cardiomyocyte. (F) Representative image showed the immunofluorescence staining of GFP and selection of the cells for CSA analysis. (Scale bar, 50 μm.) (G) ST2L-transferred cardiomyocytes develop lower cell size after TAC-induced hypertrophy. Only myocytes that had both a visible nucleus in the center and an intact cellular membrane were included in these studies. CSA was measured in ≥10 distinct microscope fields for each slide. Three hearts from each group were analyzed. Data are shown as mean ± SD. Statistical analysis was performed with one-way ANOVA. *P < 0.05. NS, no significance.
ST2L Controls Pressure Overload-Induced Hypertrophy in Individual Cardiomyocytes.
To determine whether the antihypertrophic effect of IL-33/ST2L signaling is highly spatially constrained, we took advantage of the nonuniform adenoviral gene transfer to cardiac myocytes in vivo, normally considered a pitfall of this gene delivery approach, to generate mosaic ST2L and sST2 expression. Adenovirus injection resulted in 40–60% mosaic expression at the injection site, and gene transfer efficiency decreased to 10–15% at distal sites (Fig. S1). Cardiomyocytes were grouped as infected or noninfected on the basis of GFP fluorescence. In ST2−/− mice, analysis of the myocyte cross-sectional area (MCA) blinded to the treatment group showed that Ad-GFP–infected cells (n = 48; 357 ± 16 μm2) had no change in cellular hypertrophy compared with noninfected adjacent cells (n = 39; 369 ± 13 μm2; P < 0.05 vs. noninfected adjacent cells) (Fig. 1C). In contrast, Ad-ST2L-GFP–infected cells (n = 54; 252 ± 19 μm2) had less cellular hypertrophy compared with noninfected adjacent cells (n = 35; 375 ± 21 μm2) or Ad-GFP–infected cells (n = 48; 357 ± 16 μm2; P < 0.05 vs. noninfected adjacent cells and P < 0.05 vs. remote cells). Interestingly, Ad-ST2L-GFP–infected WT cells (n = 39; 205 ± 19 μm2) did not have further reduction in cellular hypertrophy compared with noninfected adjacent (n = 25; 217 ± 21 μm2) cells or Ad-GFP–infected control cells (n = 21; 210 ± 16 μm2; P < 0.05 vs. noninfected adjacent cells and P < 0.05 vs. Ad-GFP–infected cells) (Fig. 1D), which suggests that endogenous cardiomyocyte ST2L signaling mediates an antihypertrophic response.
We further examined if IL-33/ST2L signaling affects nearby cells. Cardiomyocytes were grouped as infected (GFP+) cells, noninfected adjacent cells, and remote (cells adjacent to noninfected adjacent cells) in the same field (Fig. 1 E and F). In ST2−/− mice, Ad-ST2L-GFP–infected cells (n = 54; 252 ± 19 μm2) had less cellular hypertrophy compared with noninfected adjacent (n = 39; 319 ± 21 μm2) cells or remote cells (n = 49; 326 ± 23 μm2; P < 0.05 vs. noninfected adjacent cells and P < 0.05 vs. remote cells) (Fig. 1G). This result shows that IL-33/ST2L signaling functioned only in cardiomyocytes with gene transfer of ST2L, but this did not affect nearby cells, indicating that IL-33–mediated activity on cardiomyocytes is directly through that cardiomyocyte’s ST2L receptors.
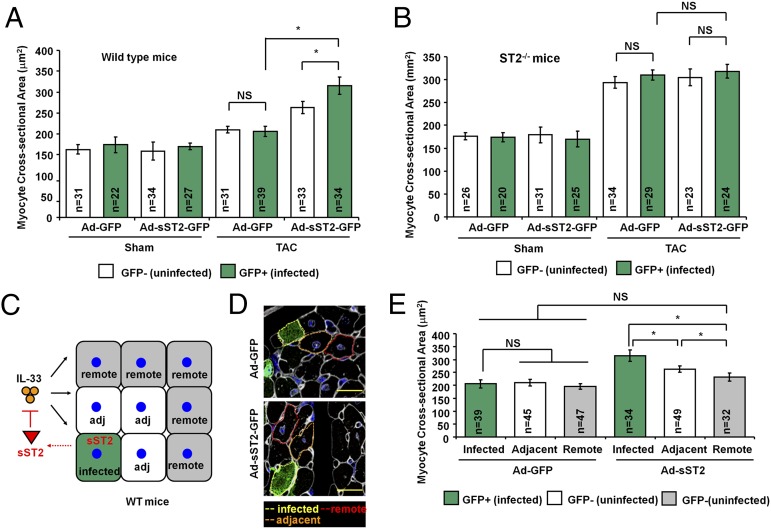
sST2 Exacerbates Pressure Overload-Induced Hypertrophy Locally in Myocardium.
We then evaluated if sST2 functions as an autocrine and paracrine factor and regulates local cellular hypertrophy. In WT mice, Ad-GFP–infected cells (n = 39; 206 ± 12 μm2) had no increase in cellular hypertrophy compared with noninfected adjacent cells (n = 31; 210 ± 8 μm2; P < 0.05 vs. noninfected adjacent cells) (Fig. 2A). Ad-sST2-GFP–infected cells (n = 34; 315 ± 21 μm2) had more hypertrophy compared with noninfected adjacent (n = 33; 263 ± 15 μm2) cells or Ad-GFP–infected cells (n = 39; 206 ± 12 μm2; P < 0.05 vs. noninfected adjacent cells and P < 0.05 vs. Ad-GFP–infected cells). Furthermore, Ad-sST2-GFP–infected ST2−/− cells (n = 24; 318 ± 15 μm2) did not result in enhanced hypertrophy compared with noninfected adjacent (n = 23; 305 ± 18 μm2) cells or Ad-GFP–infected control cells (n = 29; 310 ± 11 μm2; P < 0.05 vs. noninfected adjacent cells and P < 0.05 vs. Ad-GFP–infected cells) (Fig. 2B). sST2-exacerbated hypertrophy was observed only in WT mice but not in ST2−/− mice (which lack both sST2 and ST2L), demonstrating that sST2 is prohypertrophic but requires endogenous myocardial ST2L signaling for the sST2 effect, consistent with function of sST2 as a decoy receptor for IL-33.
Fig. 2.
sST2 exacerbates pressure overload-induced hypertrophy locally in myocardium. (A) Adenoviral transfer of sST2 to WT mice leads to greater CSA in infected cardiomyocytes after TAC. (B) Gene transfer of the sST2 gene to ST2−/− mice had no differences in CSAs between infected and uninfected cardiomyocytes. (C) Schematic representation of sST2-expressing cardiomyocytes affecting nearby cells by altering local IL-33 signaling via sST2. (D) Representative image showed the immunofluorescence staining of GFP and selection of the cells for CSA analysis. (Scale bar, 50 μm.) (E) sST2-expressing cardiomyocytes develop more hypertrophy and affect adjacent but not remote cardiomyocytes after TAC-induced hypertrophy. CSA was measured in ≥10 distinct microscope fields for each slide. Three hearts from each group were analyzed.
We then tested whether sST2 alters hypertrophy of nearby cells (Fig. 2 C and D). In WT mice, Ad-sST2-GFP–infected cells (n = 34; 315 ± 21 μm2) had more cellular hypertrophy compared with noninfected adjacent (n = 49; 263 ± 12 μm2) cells or remote cells (n = 32; 232 ± 15 μm2; P < 0.05 vs. noninfected adjacent cells and P < 0.05 vs. remote cells) (Fig. 2E). This result indicates that although IL-33/ST2L signaling directly controls hypertrophy of individual myocytes, sST2 can function as an autocrine and paracrine factor to regulate nearby cells. The paracrine effect of sST2, however, was restricted to directly adjacent cells (Fig. 2E).
Cardiomyocyte-Specific ST2 Deficiency Exacerbates Pressure Overload-Induced Hypertrophy.
To more specifically address whether endogenous myocardial-derived ST2 signaling regulates pressure overload-induced cardiac hypertrophy, we generated mice for conditional deletion of the ST2 gene. A cardiomyocyte-specific ST2 deletion mouse was generated by cross-breeding ST2fl/fl mice with myh6-mER-Cre-mER/ZEG double transgenic mice (21) to obtain ST2fl/fl/mER-Cre-mER/ZEG mice, in which cardiomyocyte-specific inducible Cre was driven by the myh6 promoter in cardiomyocytes and in which the ZEG reporter could be monitored as a marker of Cre recombination. Before 4-OH tamoxifen induction, cardiomyocytes expressed the LacZ and ST2 genes. After 4-OH tamoxifen-induced Cre recombination, LacZ and ST2 genes were deleted as evidenced by cellular expression of GFP (Fig. S2A). Cre-recombined cardiomyocytes expressing GFP were increased from 10% to 95% after 4-OH tamoxifen injection (Fig. S2B). ST2 expression in the untargeted (GFP−) and targeted (GFP+) cardiomyocytes was analyzed. Up to 90% of GFP-positive cells lacked ST2, indicating highly efficient inducible Cre recombination on both alleles and thus minimizing the possibility that the GFP-positive cells analyzed had an untargeted ST2 allele (Fig. S2C).
Given that mosaic Cre recombination could be induced in ST2fl/fl/mER-Cre-mER/ZEG mice, we next questioned whether the untargeted cardiomyocytes (GFP−ST2+) were less hypertrophied after TAC compared with targeted cardiomyocytes (GFP+ST2−). We performed TAC on ST2fl/fl/mER-Cre-mER/ZEG mice after 4-OH tamoxifen injection and determined the antihypertrophic effect of cardiomyocyte ST2. We found that mice with cardiomyocyte-specific ST2 deficiency developed more cardiac hypertrophy compared with control mice injected with vehicle (Fig. S2D). To further analyze local cellular hypertrophy, we compared cell size of untargeted cardiomyocytes (GFP−LacZ+ST2+), targeted cells adjacent to untargeted cardiomyocytes (GFP+LacZ−ST2−), and targeted remote cells far from the untargeted cells (GFP+LacZ−ST2−) (Fig. S2E). Untargeted cells (n = 24; 250 ± 15 μm2) had less cellular hypertrophy compared with targeted adjacent cells (n = 36; 323 ± 11 μm2) or targeted remote cells (n = 33; 349 ± 18 μm2; P < 0.05 vs. targeted adjacent cells and P < 0.05 vs. targeted remote cells) (Fig. S2F). Taken together, these data show that within the microenvironment, IL-33 mediates its antihypertrophic function through ST2-expressing cardiomyocytes (specifically ST2L), which is consistent with the above observation that Ad-ST2L gene transfer rescued local cellular hypertrophy in ST2−/− mice. Hence, ST2L-expressing cardiomyocytes are the direct target for the IL-33–mediated antihypertrophic response after pressure overload.
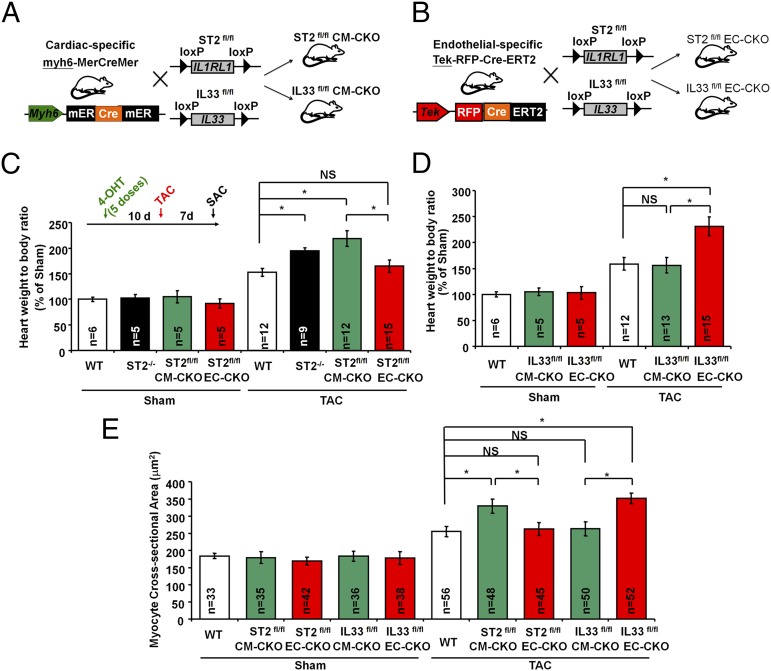
Endothelial-Specific IL33 and Cardiomyocyte-Specific ST2 Deficiency Exacerbate Pressure Overload-Induced Hypertrophy.
IL-33 is expressed in quiescent ECs, and IL-33 protein can be secreted in living cells after mechanical stress (9, 13–17). We therefore hypothesized that endothelial-derived IL-33 could represent a mechanism by which pressure overload of the myocardium can promote systemic inflammation, and thus we developed mice with conditional deletion of IL33. We then generated mice with cardiomyocyte-specific deletion (CM-CKO) or endothelial-specific deletion (EC-CKO) of the ST2 or IL33 genes by cross-breeding mice with floxed alleles (ST2fl/fl or IL33fl/fl) and mice with tissue-specific inducible Cre recombinase (myh6-mER-Cre-mER or Tek-RFP-CreERT2) (Fig. 3 A and B and Fig. S3). Mice were injected with 4-OH tamoxifen to induce Cre recombination, and then mice were subjected to Sham or TAC surgery. ST2fl/fl-CM-CKO mice but not ST2fl/fl-EC-CKO mice developed more cardiac hypertrophy after TAC compared with Sham controls (Fig. 3C). Interestingly, IL33fl/fl-EC-CKO but not IL33fl/fl-CM-CKO mice developed more hypertrophy after TAC (Fig. 3D). Mice with myh6-mER-Cre-mER or Tek-RFP-CreERT2 transgenes did not have differences in cardiac hypertrophy after TAC, indicating the observed effect was specifically caused by IL33 and ST2 deletion (Fig. S3F). MCAs in heart sections from the ST2fl/fl-CM-CKO and IL33fl/fl-EC-CKO mice were greater than those from ST2fl/fl-EC-CKO, IL33fl/fl-CM-CKO, and control mice (Fig. 3E). Echocardiography analysis showed that ST2fl/fl-CM-CKO and IL33fl/fl-EC-CKO mice had increased cardiac hypertrophy, impaired systolic function, increased left ventricular dilation, and reduced fractional shortening following TAC (Table S1).
Fig. 3.
Endothelial-specific IL33 and cardiomyocyte-specific ST2 deficiency exacerbate pressure overload-induced hypertrophy. Mice with (A) cardiomyocyte-specific deletion (CM-CKO) or (B) endothelial-specific deletion (EC-CKO) of the ST2 or IL33 gene were generated by cross-breeding the mice with floxed alleles (ST2fl/fl or IL33fl/fl) and the mice with tissue-specific Cre recombinase (myh6-mER-Cre-mER or Tek-RFP-CreERT2). (C) Mice were injected with 4-OH tamoxifen (five doses) to induce Cre recombination. After 10 d the mice were subjected to Sham or TAC surgery. ST2fl/fl-CM-CKO mice develop greater cardiac hypertrophy after TAC. (D) IL33fl/fl-EC-CKO but not IL33fl/fl-CM-CKO mice developed more hypertrophy after TAC. (E) TAC induces greater myocardial cellular hypertrophy in ST2fl/fl-CM-CKO and IL33fl/fl-EC-CKO mice. CSA was measured in ≥10 distinct microscope fields for each slide. Four hearts from each group were analyzed. Data are shown as mean ± SD. Statistical analysis was performed with one-way ANOVA. *P < 0.05. NS, no significance.
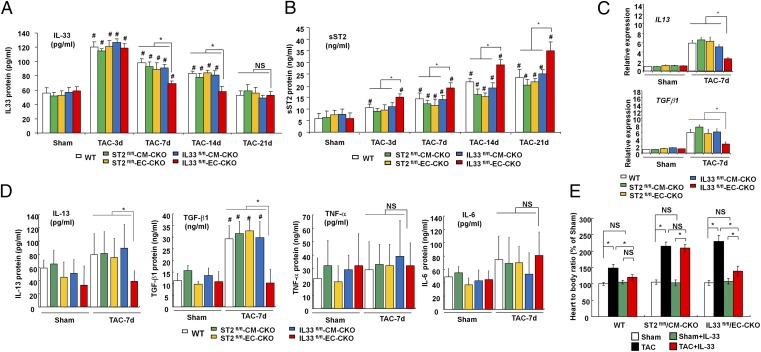
Myocardial Pressure Overload Leads to Systemic Endothelial-Derived IL-33.
To explore the possibility that the heart functions as a potential pressure sensor to induce a systemic inflammation state through IL-33, we next determined whether serum IL-33 and sST2 were elevated after TAC. We analyzed serum levels of IL-33 protein and found that systemic IL-33 peaked at 3 d after TAC and gradually reduced to normal at day 21 (Fig. 4A). Interestingly, deletion of the IL33 gene in ECs abolished the systemic elevation of IL-33 between day 7 and day 14 after TAC, whereas no significant difference was detected in serum IL-33 levels between groups on day 3 (Fig. 4A); this suggests that ECs are the major source of systemic IL-33 after pressure overload after day 3. In contrast to IL-33 levels, serum sST2 levels were elevated and gradually increased after TAC (Fig. 4B). Circulating sST2 levels were higher in IL33fl/fl-EC-CKO mice, but not in IL33fl/fl-CM-CKO mice, after TAC (from day 3 to day 21) compared with other groups, indicating that IL33 from ECs normally suppresses systemic sST2 levels.
Fig. 4.
EC IL-33 induces a selective systemic inflammatory response after myocardial pressure overload. (A) Serum IL-33 and (B) sST2 protein levels from the mice were analyzed by using ELISA (n = 6 in each group). #P < 0.05 compared with same genotype Sham controls. (C) mRNA expression of the IL13 and TGFβ1 gene in left ventricular tissues. (D) Serum levels of IL-13, TGF-β1, TNF-α, and IL-6 from the mice with TAC and Sham controls. (E) Treatment with IL-33 reduced hypertrophy only in WT and IL33fl/fl-EC-CKO but not ST2fl/fl-CM-CKO mice (n = 6 in each group). IL-33 caused no significant change under nonstress conditions in vivo. Data are shown as mean ± SD. Statistical analysis was performed with one-way ANOVA. *P < 0.05 and #P < 0.05 compared with same genotype Sham control group. NS, no significance.
EC IL-33 Mediates an Antihypertrophic Response and Selective Systemic Inflammation.
Expressions of hypertrophic genes (β-MHC, ANP, and BNP), fibrotic genes (Collagen 1a2 and Periostin), and angiogenic genes (eNOS and VEGF) were higher in left ventricular tissues from ST2fl/fl-CM-CKO and IL33fl/fl-EC-CKO mice than those from ST2fl/fl-EC-CKO, IL33fl/fl-CM-CKO, and control mice (Fig. S4). Conditional IL33 or ST2 deletion did not influence TAC-induced cardiac gene expression of some proinflammatory cytokines (TNF-α, IL-1β, and IL-6) (Fig. S5). In contrast, myocardial gene expression levels of IL13 and TGF-β1 were induced by TAC but lower in ventricular tissues of IL33fl/fl-EC-CKO mice (Fig. 4C). Moreover, TAC induced circulating IL-13 and TGF-β1 protein levels, and this was abolished in IL33fl/fl-EC-CKO mice (Fig. 4D). Conditional IL33 or ST2 deletion did not influence serum TNF-α and IL-6 levels in Sham or TAC groups (Fig. 4D). These data reveal that endothelial-derived IL-33 not only mediates local intramyocardial responses but also induces a selective systemic inflammatory response after pressure overload in vivo.
Administration of IL-33 Protects Against Pressure Overload-Induced Cardiac Hypertrophy.
We determined whether administration of exogenous IL-33 rescues exacerbated cardiac hypertrophy in IL33fl/fl-EC-CKO and ST2fl/fl-CM-CKO mice. Gross heart/body weight measurements showed that administration of IL-33 reduced cardiac hypertrophy in WT and IL33fl/fl-EC-CKO mice but not in ST2fl/fl-CM-CKO mice (Fig. 4E), showing that exogenous IL-33 can prevent pathological hypertrophy through ST2L signaling.
Myocardial Pressure Overload Induces IL-33 in Cardiac ECs.
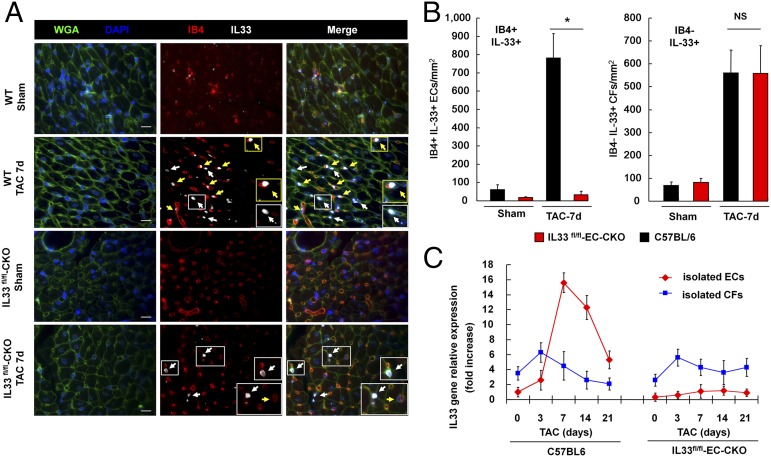
We next analyzed the IL33 mRNA levels in vital organs from mice with Sham or TAC and found that IL33 transcripts were elevated after TAC only in the heart, which suggested an intramyocardial source of IL-33 after pressure overload (Fig. S6A). To determine whether ECs are the major sources of IL-33 in cardiac tissues after myocardial pressure overload, we analyzed the expression and localization of IL-33 within the myocardium using immunofluorescence staining (Fig. 5A). Cells with positive staining with Isolectin B4 (IB4) were considered as ECs. In Sham controls, IL-33 protein was minimally detected in cardiac tissues from WT C57BL/6 and IL33fl/fl-EC-CKO mice. An increase in nuclear IL-33 immunoreactivity was detected in both IB4+ and IB4– interstitial cells in cardiac tissues from WT mice with TAC. IL-33 was detected in IB4– interstitial cells but not in IB4+ ECs in tissues from IL33fl/fl-EC-CKO mice, which confirmed the specificity of endothelial IL33 deletion.
Fig. 5.
Myocardial pressure overload induces IL-33 in cardiac ECs. (A) Expression of IL-33 in IB4+ cells (yellow arrowheads) and IB4– cells (white arrowheads) was analyzed using immunofluorescence staining. Endothelial IL-33 was not detected in cardiac tissues from IL33fl/fl-EC-CKO mice. Wheat germ agglutinin (WGA) staining was used for cellular membrane (green); DAPI staining was used for the nucleus (blue); IB4 staining for was used for ECs (red); IL-33 staining was detected in the nucleus (gray). (Scale bar, 50 μm.) (B) Quantification of IB4+IL-33+ and IB4–IL-33+ cells in cardiac tissues from mice with Sham or TAC (n = 3 mice from each group). Data are shown as mean ± SD. Statistical analysis was performed with one-way ANOVA. *P < 0.05. NS, no significance. (C) IL33 transcript levels in isolated cardiac ECs and CFs from mice with TAC surgery. Relative expression was normalized to the IL33 levels in ECs isolated from mice prior to TAC (day 0).
We further quantified the number of IB4+IL-33+ cells (ECs) and IB4–IL-33+ interstitial cells (cardiac fibroblasts, CFs) and found that IB4+IL-33+ cells comprised 62% of IL-33+ interstitial cells. Endothelial IL33 deletion diminished IL-33 protein expression in IB4+ ECs without affecting the expression of IL-33 in IB4– interstitial cells (Fig. 5B). Finally, we isolated cardiac ECs and CFs and compared IL33 gene expression levels between these two cell types (Fig. 5C). We found that, on day 0 prior to TAC and day 3 after TAC, isolated CFs expressed higher basal IL33 transcripts compared with that in isolated cardiac ECs. IL33 gene expression was dramatically induced in cardiac ECs compared with that in CFs isolated from mice with TAC for 7 d. Endothelial-specific deletion of IL33 abolished IL33 expression in isolated cardiac ECs without affecting CFs in IL33fl/fl-EC-CKO mice. These results indicated that myocardial pressure overload induces IL-33 expression and suggest that cardiac ECs are major sources of circulating IL-33 after pressure overload.
Discussion
In this study, we report that a highly local intramyocardial IL-33/ST2 conversation regulates the heart’s response to pressure overload. These experiments show that ST2L signaling directly regulates cardiomyocyte hypertrophy, whereas sST2 can only travel across a one-cell distance to reduce cellular hypertrophy within the myocardium. By generating and using new conditional deletion mice, we identified ECs as the major source of systemic circulating IL-33 after myocardial pressure overload. Either endothelial-specific deletion of IL33 or cardiomyocyte-specific deletion of ST2 exacerbated cardiac hypertrophy with pressure overload. Furthermore, pressure overload induced systemic circulating IL-33 as well as systemic circulating IL-13 and TGF-β1; this was abolished by endothelial-specific deletion of IL33 but not by cardiomyocyte-specific deletion of IL33. Our study reveals that EC secretion of IL-33 is crucial for translating myocardial pressure overload into a selective systemic inflammatory state.
In this study, we report that sST2 functions as a locally constrained factor within the myocardium, which suggests local activity, and thus circulating sST2 may not reflect the sST2 levels in local tissues. On the other hand, recent studies have revealed that ECs are sources of sST2 (16, 22, 23). In this case, endothelial-derived sST2 could be readily released into the blood stream and lead to high circulating sST2 levels.
One clinical study revealed that the source of circulating ST2 is extramyocardial in patients with cardiac hypertrophy (22). In another study, Mildner et al. also demonstrated that sST2 protein could be detected in multiple human tissues including lung, kidney, and the heart (23). In this study, we compared the mRNA levels of sST2 in vital organs from mice with Sham or TAC surgery and observed that the sST2 mRNA levels were elevated in the lung, heart, and kidney (Fig. S6B). Our result is consistent with the clinical observation showing extramyocardial sources of sST2 in human cardiac hypertrophy (22). It is of note that we still detected elevated sST2 mRNA levels in the mouse heart after TAC. Thus, although our study implicates ECs in the pathophysiology of an IL-33-ST2 systemic response, it is likely that extracardiac cells participate in this pathway.
In contrast to the broad specificity of sST2 induction, the induction of IL-33 is more restricted in injured tissue in the mouse TAC model (Fig. S6). Our results demonstrated that IL-33+ ECs comprise ∼62% of IL-33+ interstitial cells in cardiac tissues from mice after TAC (Fig. 5 A and B). We could not exclude a contribution of CFs to elevated systemic IL-33 after pressure overload, as CFs expressed higher basal levels of IL33 transcripts than that in cardiac ECs (Fig. 5C). However, our results showed that cardiac ECs dramatically induce IL33 in response to pressure overload after 7, 14, and 21 d of TAC compared with CFs (Fig. 5C). In IL33fl/fl-EC-CKO mice, endothelial IL33 deletion was sufficient to abolish the elevated circulating IL-33 levels at day 7 of TAC (Fig. 4A). These data together suggest that the elevated IL-33 at 3 d after TAC could be attributed to CFs, whereas ECs contribute to a later response.
Recently, Moore-Morris et al. showed that Tie2-Cre also labeled 19% of CFs, which are derived through endothelial-to-mesenchymal transition (EndMT) during cardiac development (24). The epicardial and endothelial origins of resident CFs appear to mediate pressure overload-induced fibrosis (24). In this study, we used the Tie2-CreERT2 system to induce endothelial-specific IL33 deletion in adult endothelium by injection of tamoxifen. After tamoxifen-induced Cre recombination, the IL33 floxed alleles are deleted in Tie2+ ECs. If EndMT occurs in the cardiac tissue after TAC, we could observe a decreased number of IB4–IL-33+ interstitial cells due to a dilution of the fibroblast pool by IL33−/−EC-derived CFs through EndMT. However, we did not find a difference in the quantity of IB4–IL-33+ interstitial cells in cardiac tissues between WT and IL33fl/fl-EC-CKO mice (Fig. 5B). This is consistent with the observation in Moore-Morris et al. that EndMT may contribute minimally to derive CFs in the model of cardiac pressure overload. We thus speculate that the IL-33+ CFs are derived from resident CFs but not through the process of EndMT.
In this study, we show direct evidence that ECs are the major source of systemic IL-33 after the heart is pressure overloaded. These data reveal the heart as a critical pressure sensor in response to biomechanical overload and that ECs provide a mechanistic link between pressure overload and subsequent inflammatory responses. Our study provides direct evidence that ECs can translate biomechanical overload into a selective systemic inflammatory state via IL-33.
Materials and Methods
Experimental details are provided in SI Materials and Methods.
Animals.
All experiments were conducted in accordance with the Guide for the Use and Care of Laboratory Animals and approved by the Harvard Medical School Standing Committee on Animals and by the Institutional Animal Care and Use Committee of Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan. ST2−/− mice were maintained as described previously (10). MerCreMer-ZEG mice were generated as previously described (21). The C57BL/6-Tg (Tek-RFP-Cre/ERT2)27Narl (RMRC13162) mouse was generated by genetic engineered murine model services (GEMMS), National Laboratory Animal Center, Taiwan. MerCreMer mice or Tek-RFP-CreERT2 mice were crossed with ST2fl/fl or IL33fl/fl conditional knockout mice (Table S2 and Dataset S1). TAC was produced as described previously (10). All sections were imaged with fluorescent microscopy to determine the cross-sectional area (CSA) of each cardiomyocyte. Only myocytes that had both a visible nucleus in the center and an intact cellular membrane were included in our studies. Details are provided in SI Materials and Methods.
ELISA.
Serum levels of IL-33 (R&D Systems, DY3626) or sST2 (R&D Systems, DY1004) were analyzed by using ELISA kit according to the manufacturer’s instructions.
Statistical Analysis.
All data are presented as mean ± SD. Statistical analysis was performed with the one-way ANOVA between groups.
Supplementary Material
Acknowledgments
The C57BL/6-Tg (Tek-RFP-Cre/ERT2)27Narl mouse (RMRC13162) was provided by Genetic Engineered Murine Model Services and Rodent Model Resource Center, National Laboratory Animal Center, Taiwan. W.-Y.C. was supported by Grants 101-2917-I-564-057 and 103-2320-B-182A-017 from the Taiwan Ministry of Science and Technology and Grant 13POST16940030 from the American Heart Association. R.T.L. was supported by Grant R01 HL092930 from the National Institutes of Health.
Footnotes
Conflict of interest statement: Brigham and Women’s Hospital holds patents on ST2, listing R.T.L. as inventor.
This article is a PNAS Direct Submission.
See Commentary on page 7113.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424236112/-/DCSupplemental.
References
- 1.Kearney PM, et al. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Harrison DG, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57(2):132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ky B, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4(2):180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimpo M, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109(18):2186–2190. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 6.Ho JE, et al. Soluble ST2 predicts elevated SBP in the community. J Hypertens. 2013;31(7):1431–1436. doi: 10.1097/HJH.0b013e3283611bdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cayrol C, Girard JP. IL-33: An alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Iwahana H, et al. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem. 1999;264(2):397–406. doi: 10.1046/j.1432-1327.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 9.Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. 2012;287(9):6941–6948. doi: 10.1074/jbc.M111.298703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanada S, et al. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117(6):1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seki K, et al. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. 2009;2(6):684–691. doi: 10.1161/CIRCHEARTFAILURE.109.873240. [DOI] [PubMed] [Google Scholar]
- 12.Miller AM, et al. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205(2):339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carriere V, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA. 2007;104(1):282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Küchler AM, et al. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol. 2008;173(4):1229–1242. doi: 10.2353/ajpath.2008.080014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: A novel ‘alarmin’? PLoS ONE. 2008;3(10):e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demyanets S, et al. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J Mol Cell Cardiol. 2013;60:16–26. doi: 10.1016/j.yjmcc.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi YS, et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009;114(14):3117–3126. doi: 10.1182/blood-2009-02-203372. [DOI] [PubMed] [Google Scholar]
- 18.Demyanets S, et al. Interleukin-33 induces expression of adhesion molecules and inflammatory activation in human endothelial cells and in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2011;31(9):2080–2089. doi: 10.1161/ATVBAHA.111.231431. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh PC, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol. 2006;68:51–66. doi: 10.1146/annurev.physiol.68.040104.124629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res. 2010;106(1):47–57. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh PC, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13(8):970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartunek J, et al. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J Am Coll Cardiol. 2008;52(25):2166–2174. doi: 10.1016/j.jacc.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mildner M, et al. Primary sources and immunological prerequisites for sST2 secretion in humans. Cardiovasc Res. 2010;87(4):769–777. doi: 10.1093/cvr/cvq104. [DOI] [PubMed] [Google Scholar]
- 24.Moore-Morris T, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124(7):2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.