Significance
The role of signal transducer and activator of transcription 3 (STAT3) pathway in hepatic stellate cells (HSCs) remains unclear. Using sorafenib and its derivative SC-1, we demonstrated the significant role of STAT3 pathway in liver fibrogenesis. We further found that STAT3 pathway of HSCs overexpressed in chronic hepatitis B patients with advanced fibrosis, therefore STAT3 may serve as a promising fibrotic biomarker and target. Furthermore, SHP-1 phosphatase-direct STAT3 inhibition may represent a previously unidentified strategy for antifibrotic drug discovery.
Keywords: hepatic stellate cell, STAT3, SHP-1, hepatitis B, liver fibrosis
Abstract
Signal transducer and activator of transcription 3 (STAT3) had been involved in liver fibrogenesis. We aimed to explore the antifibrotic activities of sorafenib and its derivative SC-1 (devoid of Raf kinase inhibition activity) both in vivo and in vitro with special focus on the STAT3 pathway in hepatic stellate cells (HSCs). The clinical role of STAT3 in chronic hepatitis B (CHB) was also investigated. Experimental fibrosis mouse models were established by thioacetamide injection and bile duct ligation in Balb/C mice and treated with sorafenib and SC-1. Rat and human HSCs were used for mechanistic investigations. Forty CHB patients were enrolled to quantify the hepatic phospho-STAT3 (p-STAT3) levels and correlated with liver fibrosis. Both sorafenib and SC-1 ameliorated liver fibrosis in vivo and promoted HSC apoptosis in vitro. p-STAT3 and downstream signals were down-regulated after sorafenib and SC-1 treatment in HSC. STAT3 overexpression in HSC enhanced cell proliferation and undermined the apoptotic effects of sorafenib and SC-1, whereas STAT3-specific inhibition promoted HSC apoptosis. Sorafenib and SC-1 activated Src-homology protein tyrosine phosphatase-1 (SHP-1) and STAT3 inhibition followed. Of particular interest, in CHB patients with advanced liver fibrosis, p-STAT3 in HSC was significantly overexpressed and positively correlated with the severity of liver fibrosis and plasma IL-6 levels. In conclusion, sorafenib and SC-1 ameliorate liver fibrosis through STAT3 inhibition in HSC and STAT3 may potentially serve as a promising fibrotic biomarker and target in liver fibrosis. SHP-1 phosphatase-directed STAT3 inhibition may represent a previously unidentified strategy for antifibrotic drug discovery.
More than 400 million people worldwide are infected with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. They are the major causes of chronic hepatitis, which often leads to fibrosis progression, cirrhosis, and hepatocellular carcinoma (HCC) and, thus, poses a great threat to public health (1). Several potent antiviral agents with a minimal drug resistance profile are shown to persistently suppress HBV replication in patients with chronic hepatitis B (CHB), leading to a reduction or even a reversal of hepatic fibrosis in the treated patients (2). This fibrosis reduction is also true for chronic HCV infection after effective therapy (3). Nevertheless, active therapy directed against liver fibrogenesis is not yet available and is thus urgently needed in clinical practice (4).
Liver fibrogenesis represents a wound-healing response to a variety of chronic stimuli, including viral hepatitis. Fibrosis is characterized by an excessive deposition of several extracellular matrix proteins, which disrupts the normal architecture of the liver, resulting in fibrosis progression and subsequent cirrhosis. Hepatic stellate cells (HSCs) play a major role in liver fibrogenesis. A comprehensive understanding of the molecular mechanisms involved in HSC activation, proliferation, and fibrosis-related gene expression will provide invaluable insight to ameliorate fibrosis progression of liver diseases with various etiology (5, 6).
Sorafenib, a multikinase inhibitor, is the only Food and Drug Administration-approved molecular targeted agent against advanced HCC. Interestingly, sorafenib has been reported to attenuate liver fibrosis, and reduce HSC proliferation by enhancing cell apoptosis (7). However, the underlying mechanisms of the antifibrogenic effects remain unclear. The signal transducer and activator of transcription 3 (STAT3) is a transcription factor associated with liver injury, inflammation, and regeneration (8, 9). Several studies have shown that interleukin-6 (IL-6) activates STAT3 in HSCs and promotes their survival, proliferation, and activation, thus contributing to liver fibrogenesis (8, 10). Recently, we found that sorafenib could down-regulate phospho-STAT3 (p-STAT3) in HCC cells (11). In addition, we also found that sorafenib and its derivative SC-1 inhibit HCC via a Raf kinase-independent mechanism: Src-homology protein tyrosine phosphatase-1 (SHP-1)-dependent STAT3 inactivation (12).
Taking these lines of evidence together, we hypothesized that STAT3 activation in HSCs is involved in liver fibrogenesis and is associated with the antifibrotic mechanism of sorafenib. We further investigated the antifibrotic activity of SC-1 and the role of STAT3 as a potential target of antifibrotic therapy in CHB patients with liver fibrosis.
Results
Sorafenib and SC-1 Ameliorated Experimental Liver Fibrosis.
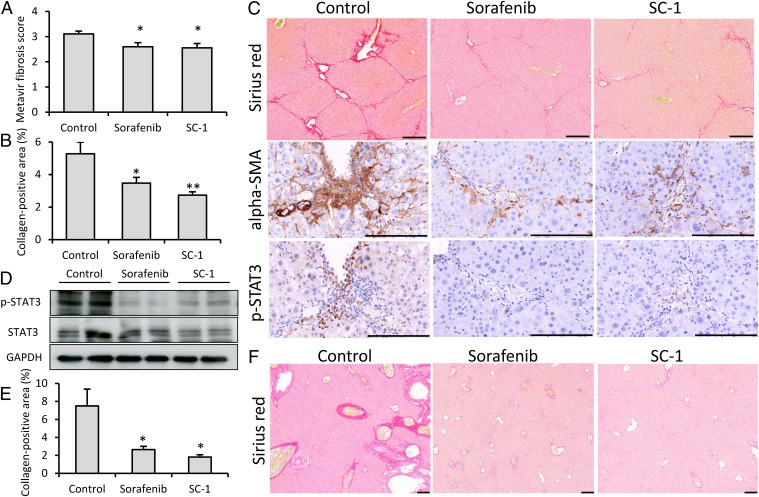
To investigate the antifibrotic effects of sorafenib and SC-1, we administered these agents in hepatotoxic and cholestatic models. In the thioacetamide model, moderate fibrosis (Metavir score 2–3) was achieved after 2 wk of thioacetamide administration. After 8 wk of thioacetamide induction, hepatic fibrosis significantly reduced after sorafenib and SC-1 treatment in terms of Metavir fibrosis score (P = 0.022 and 0.017, respectively, compared with the control group) (Fig. 1A), and collagen-positive area (P = 0.033 and 0.004, respectively, compared with the control group) (Fig. 1B). The histological images from a representative mouse of each group are shown in Fig. 1C. α-Smooth muscle actin (α-SMA) and p-STAT3 were both down-regulated after sorafenib and SC-1 treatment. After sorafenib and SC-1 treatment, significant fibrosis regression was found by Sirius Red staining. Most p-STAT3–positive nuclei were located in portal areas and in areas of significant inflammation and fibrosis. In addition, after staining with α-SMA [an activated HSC (myofibroblast) marker], the p-STAT3–positive cells were largely HSCs. Hepatic p-STAT3 was down-regulated in Western blot after sorafenib and SC-1 treatment. (Fig. 1D). In the bile duct ligation (BDL) model, hepatic fibrosis was also significantly reduced after sorafenib and SC-1 treatment according to collagen-positive area (P = 0.020 and 0.031, respectively, compared with the control group) (Fig. 1E). The histological images from a representative mouse of each group are shown in Fig. 1F.
Fig. 1.
Sorafenib and SC-1 treatment ameliorate liver fibrosis in hepatotoxic and cholestatic mouse models. In the thioacetamide-induced liver fibrosis mouse model (n = 9–10 in each group), significant fibrosis regression was found after sorafenib and SC-1 treatment by Metavir fibrosis scoring (A) and collagen-positive area quantification (B). (C) Representative liver tissues after Sirius Red, α-SMA, and p-STAT3 immunohistochemical staining. Significant fibrosis regression after sorafenib and SC-1 treatment was observed as indicated by Sirius Red staining. α-SMA and p-STAT3 overexpression was reduced after sorafenib and SC-1 treatment. (D) Hepatic p-STAT3 was down-regulated after sorafenib and SC-1 treatment. In the bile duct ligation mouse model (n = 4–6 in each group), significant fibrosis regression was observed after sorafenib and SC-1 treatment by collagen-positive area quantification (E) and Sirius Red staining (F). Columns, mean; bars, SE. *P < 0.05, **P < 0.01. (Scale bars: 200 μm.)
HSC Viability Decreased After Sorafenib and SC-1 Treatment.
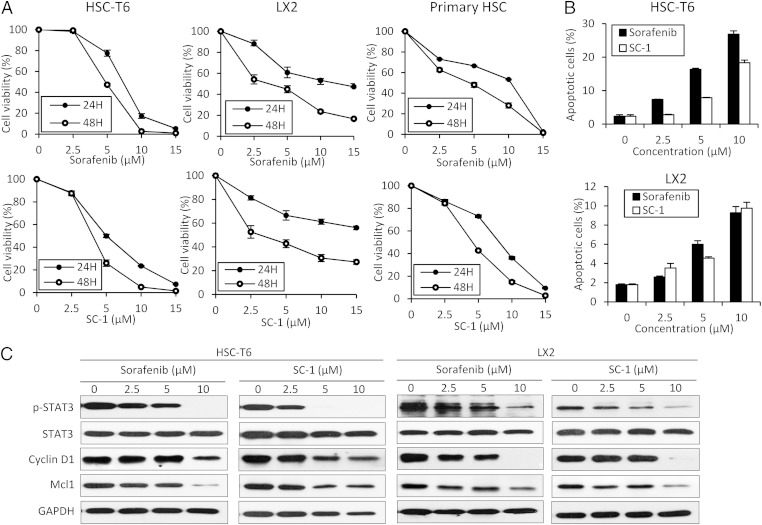
Both sorafenib and SC-1 had dose- and time-dependent effects against cell viability on HSC-T6, LX2, and mouse primary HSCs (Fig. 2A). In addition, apoptotic HSC cells were significantly increased after sorafenib and SC-1 treatment in a dose-dependent manner by flow cytometry (Fig. 2B) and by increasing cleaved poly (ADP ribose) polymerase (PARP) fragments (Fig. S1).
Fig. 2.
Sorafenib and SC-1 treatment induce apoptosis of HSCs via the STAT3 pathway. (A) Dose-escalation and time-dependent effects of sorafenib and SC-1 for 24 or 48 h on cell viability in HSC-T6, LX2, and mouse primary HSCs. Circles, mean; bars, SE (n = 3). (B) Dose-escalation effects of sorafenib and SC-1 for 24 h on apoptosis in HSC-T6 and LX2 cells. Columns, mean; bars, SE (n = 3). (C) Dose-escalation effects of sorafenib or SC-1 for 24 h on STAT3-related proteins in HSC-T6 and LX2 cells.
Sorafenib or SC-1 Down-Regulated p-STAT3 in HSCs.
We then investigated the STAT3-related signaling pathway in both sorafenib- and SC-1–treated HSC-T6 and LX2 cells. Cell death/apoptosis-related molecules including myeloid cell leukemia sequence 1 (Mcl-1) and cyclin D1 were examined. Both sorafenib and SC-1 down-regulated p-STAT3 and suppressed Mcl-1 and cyclin D1 in both HSC-T6 and LX2 cell lines in a dose-dependent manner (Fig. 2C), but total STAT3 protein was not affected. In addition, sorafenib and SC-1 down-regulated the p-Smad2 and p-Smad3 in the TGF-β pathway (Fig. S2) and down-regulated the p-PDGFR and p-Akt in the PDGF pathway (Fig. S3 A and B). The p-ERK was unaltered after treatment with SC-1 because it lacked the Raf kinase activity.
Overexpression of STAT3 Undermined the Apoptotic Effect of Sorafenib and SC-1.
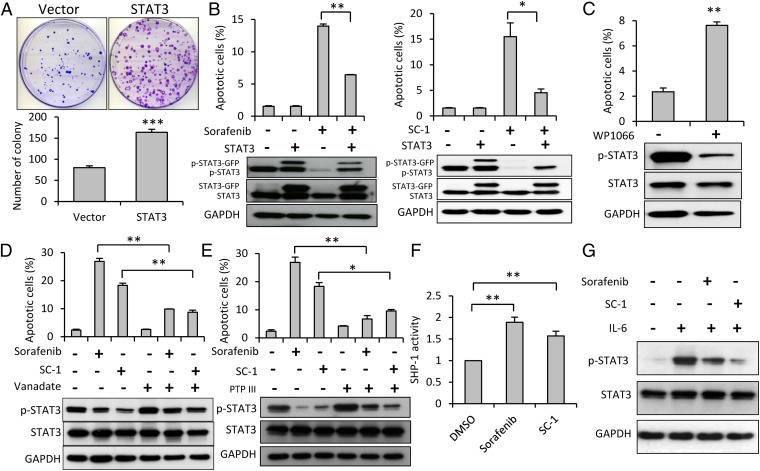
To validate the apoptotic effect of sorafenib and SC-1 in HSC cells, stable clones of HSC-T6 cells that overexpressed STAT3 were established. First, the colony formation assay demonstrated the enhanced cell proliferation after overexpression of STAT3 (Fig. 3A). As shown in Fig. 3B, both sorafenib- and SC-1–induced apoptosis were abolished in STAT3-overexpressing HSC cells, as evidenced by the flow cytometry data, suggesting STAT3 may be a major mediator of sorafenib- and SC-1–induced apoptosis. To investigate whether STAT3 inhibition is directly associated with apoptosis of HSC cells, we administered WP1066, a STAT3-specific inhibitor and found a significant increase in apoptotic cells after WP1066 treatment (Fig. 3C).
Fig. 3.
Protective effect of STAT3 on apoptosis induced by sorafenib and SC-1 in HSC-T6 cells. (A) The colony formation assay demonstrated the significantly enhanced cell proliferation after overexpression of STAT3 compared with vector control. (B) Cells (with wild-type or ectopic expression of STAT3) were exposed to sorafenib (Left) or SC-1 (Right). Apoptotic cells were analyzed by flow cytometry. Both sorafenib- and SC-1–induced apoptosis were abolished in STAT3-overexpressing HSCs. (C) Treatment with WP1066, a STAT3 pathway-specific inhibitor, increased apoptosis. (D) Treatment with vanadate, a nonspecific phosphatase inhibitor, up-regulated p-STAT3 and reduced apoptosis. (E) SHP-1 specific inhibitor (PTP inhibitor III) up-regulated p-STAT3 and reduced apoptosis. (F) SHP-1 activity increased after sorafenib and SC-1 treatment. (G) IL-6 stimulation up-regulates p-STAT3 and suppressed by sorafenib and SC-1 treatment. Columns, mean; bars, SE (n = 3) *P < 0.05, **P < 0.01, ***P < 0.001.
SHP-1 Phosphatase Has a Role in STAT3 Pathway-Associated Apoptosis in HSCs.
SHP-1 phosphatase is involved in down-regulation of p-STAT3 (12). To investigate whether this protein phosphatase is also involved in p-STAT3 pathway-associated apoptosis in HSC cells, we used sodium vanadate, a nonspecific phosphatase inhibitor. Sodium vanadate up-regulated p-STAT3 and abolished sorafenib- or SC-1–induced apoptosis (Fig. 3D). Furthermore, we found that a specific SHP-1 phosphatase inhibitor (PTP inhibitor III) could reverse sorafenib- or SC-1–induced HSC apoptosis and down-regulation of p-STAT3 (Fig. 3E). Both sorafenib and SC-1 up-regulated SHP-1 activity up to 1.9-fold compared with the control cells (P < 0.01) (Fig. 3F). Finally, IL-6 is the main activating signal of STAT3, and we demonstrated IL-6 stimulation activated the STAT3 pathway in HSC, which can be suppressed by sorafenib and SC-1 (Fig. 3G).
P-STAT3 Overexpression in CHB Patients with Advanced Liver Fibrosis.
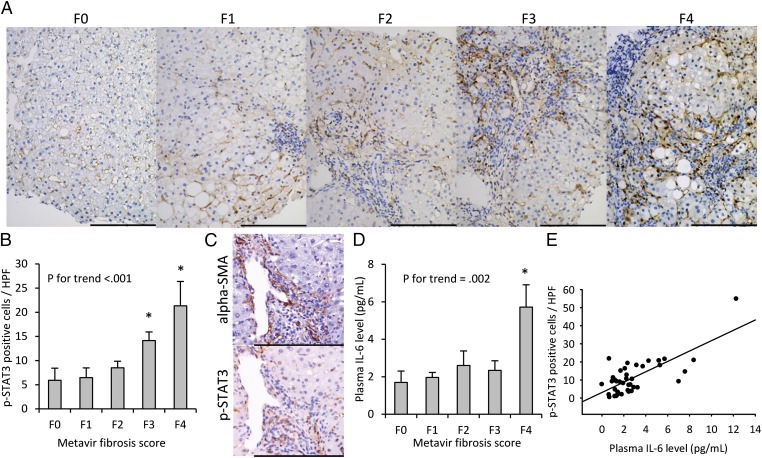
We further explored whether STAT3 could be a potential antifibrotic target in 40 patients with CHB (Table S1). The mean histology activity index score (HAI) was 7.5, and it was positively correlated with the Metavir scores (P for trend < 0.001). There was no correlation between viral factors (HBsAg and HBV DNA levels) and the Metavir score. The images of p-STAT3 nuclear staining from a representative patient of each Metavir score (F0–F4) are shown in Fig. 4A. The p-STAT3 nuclear staining according to the Metavir score is shown in Fig. 4B. Compared with patients without fibrosis (F0), those with advanced fibrosis had p-STAT3 overexpression (P for trend < 0.001). In addition, p-STAT3–positive cells were largely HSCs, rather than hepatocytes (Fig. 4C and Fig. S4). We further measured the IL-6 level in the patients’ plasma and found plasma IL-6 concentration increased significantly with higher Metavir scores (P for trend = 0.002) (Fig. 4D). A good correlation of 0.73 (P < 0.001) between hepatic p-STAT3 nuclear staining and plasma IL-6 level was found (Fig. 4E).
Fig. 4.
Overexpression of p-STAT3 in HSCs in CHB patients with advanced fibrosis. Fibrosis was graded after Metavir fibrosis score. (A) p-STAT3 nuclear immunohistochemical staining increased significantly with advancing fibrosis. (B) Nuclear p-STAT3 expression increased with severity of fibrosis. *P < 0.05, compared with patients at F0. P for trend <0.001. (C) Selected areas with α-SMA [an activated HSC (myofibroblast) marker] and p-STAT3 immunohistochemical staining. The p-STAT3–positive cells were mostly HSCs. (D) Plasma IL-6 levels increased with severity of fibrosis according to Metavir scores. P for trend = 0.002. Columns, mean; bars, SE. *P < 0.05, compared with patients at F0. (E) Plasma IL-6 levels positively correlated with hepatic p-STAT3. (Pearson’s r = 0.73, P < 0.001). (Scale bars: 200 μm.)
Discussion
In this study, we demonstrated that sorafenib and its derivative SC-1 could successfully ameliorate liver fibrosis. In addition to the TGF-β and PDGF pathways, we further identified that the STAT3 pathway is critical in the apoptosis and survival of HSCs. In addition, we also showed p-STAT3 overexpression in the HSCs of CHB patients with advanced liver fibrosis. Our results suggested STAT3 might be a promising fibrotic biomarker and target, and STAT3 inhibition might ameliorate liver fibrosis both in vitro and in vivo. Furthermore, SHP-1 phosphatase–directed therapy (e.g., SC-1) might be a previously unidentified strategy for the discovery of antifibrotic drugs.
STAT3 activation has been detected in several human liver diseases, and STAT3 signaling is involved in liver injury, steatosis, inflammation, regeneration, fibrosis, and hepatocarcinogenesis (8, 9). The role of STAT3 in liver inflammation and fibrosis is cell type-specific and model-dependent. STAT3 activation in hepatocytes induces acute phase responses, promoting liver regeneration, hepatocyte survival, and ameliorating fatty liver (8, 13). In hepatocyte-specific STAT3 knockout mice, CCl4 injection induced greater liver damage than wild-type mice (14). In another alcoholic liver injury model, STAT3 in hepatocytes otherwise promotes inflammation, whereas STAT3 in macrophages/Kupffer cells suppresses inflammation (15). In Kupffer cells, the transient activation of STAT3 by IL-6 is proinflammatory, promoting HSC survival and proliferation (10). The activation of STAT3 in HSCs by IL-6 or leptin stimulates HSC survival, proliferation, and activation (8, 16, 17). Nevertheless, the exact mechanisms of IL-6/STAT3 in HSC during liver fibrogenesis remain to be determined.
Sorafenib inhibits tyrosine kinases, vascular endothelial growth factor receptor 2, PDGFR-β, and Raf kinases (18). The microenvironment in a fibrotic liver is rather complex, including hepatocytes, Kupffer cells, liver sinusoidal endothelial cells (LSEC), and HSC. Sorafenib may have various effects on different cell types. Mejias et al. first reported sorafenib ameliorated portal hypertension, intrahepatic fibrosis, inflammation, and angiogenesis in a cirrhotic rat model (19). Subsequent studies demonstrated sorafenib might reduce HSC proliferation, and inhibit the synthesis of fibrogenesis-related proteins and extracellular matrix (7, 20, 21). Sorafenib inhibits the KLF6/angiopoietin-1/fibronectin to disrupt the LSEC–HSC interactions, affecting the matrix reconstruction and vascular remodeling (22). Although sorafenib suppresses HSC by p-STAT3 inhibition in our study, a recent study suggested that sorafenib suppressed TGF-β1 induced epithelial-mesenchymal transition and apoptosis of hepatocytes, and also inhibited TGF-β1–induced STAT3 phosphorylation (23). Recently, Deng et al. demonstrated that STAT3 in hepatocytes was critical for sorafenib-mediated protection against liver fibrosis, by Kupffer cell-derived IL-6–dependent p-STAT3 up-regulation (24). In our study, we consistently confirmed the antifibrotic effect of sorafenib and further verified that sorafenib inhibited the STAT3 pathway and downstream cyclin D1 and Mcl-1 expression, which are involved in the cell-cycle regulation and apoptosis (25). Of particular note, p-STAT3 inhibition was associated with HSC apoptosis, which was clearly reversed by p-STAT3 overexpression. Deng et al. showed the nuclear translocation of STAT3 in hepatocytes after sorafenib treatment; however, after thioacetamide induction, we found nuclear p-STAT3 overexpressed largely in HSC and reduced by sorafenib. One possible explanation of these findings is a time-dependent hepatic p-STAT3 inhibition and followed by activation during fibrogenesis, indicating a dynamic regulation by IL-6 from Kupffer cells (24). These results highlight the impact of the different cell types on the STAT3-dependent effects of sorafenib and STAT3 may be a key player in liver fibrosis. However, the beneficial results of sorafenib from these cellular or animal studies in oversimplified conditions should be carefully examined in patients.
We further introduced SC-1, a sorafenib analog devoid of Raf kinase inhibition activity due to the trimming of the functional amide group or pyridine ring that are critical to the hydrogen bond interactions between sorafenib and the ATP binding pocket of B-Raf (26). Our data showed that SC-1 exerted potent antifibrotic activity through STAT3 inhibition. This kinase-independent p-STAT3 inhibition possibly occurs through the up-regulation of SHP-1 with protein phosphatase and cell growth suppression activities (27). SHP-1 belongs to a family of nonreceptor protein tyrosine phosphatases with two Src homology region 2 (SH2) domains, a catalytic protein tyrosine phosphatases (PTP) domain, and a C-terminal tail. The N-SH2 domain protrudes into the PTP domain to block the entrance of phosphopeptide activators. Sorafenib interacts with the inhibitory N-SH2 domain and relieves the autoinhibition, leading to increased SHP-1 activity. The deletion or a point mutation (D61A) of N-SH2 domain abolishes the effect of sorafenib on SHP-1 and p-STAT3 (28). The SHP-1 down-regulates p-STAT3 by a direct interaction and dephosphorylation of p-STAT3 (28). SHP-1 also dephosphorylates JAK2 kinase (29), the upstream of JAK2/STAT3 pathway, and thus represses the p-STAT3 expression.
TGF-β and PDGFR are two major pathways in fibrogenesis, promoting HSC activation and extracellular matrix synthesis (5). In our study, sorafenib and SC-1 also down-regulated TGF-β/Smad2/Smad3 signaling. The Raf and Akt are two canonical downstream pathways of PDGFR-β. Although SC-1 devoid of Raf kinase activity, both sorafenib and SC-1 still down-regulate PDGFR-β/Akt and further contribute to their antifibrotic effects.
Sorafenib-induced liver dysfunction is more common in patients with Child B/C cirrhosis than those with Child A cirrhosis (30), which limits its clinical usefulness as an antifibrotic agent. In addition, sorafenib-induced multikinase inhibition causes various cutaneous adverse reactions through its off-target effects (31). In the mouse model, we also found elevation of alanine aminotransferase levels in the sorafenib group, compared with the SC-1 and control groups (209 vs. 143 vs. 120 U/L, n = 2 in each group). The hepatotoxicity of tyrosine kinase inhibitors should be monitored after marketing (32). From the perspective of drug discovery, kinase-independent STAT3 inhibitors like SC-1 may be more specific and safer agents for antifibrotic therapy.
To explore the clinical implications of STAT3 in liver fibrogenesis, we enrolled CHB patients with liver fibrosis. We clearly demonstrated p-STAT3 overexpression in HSC of CHB patients with advanced liver fibrosis. This p-STAT3 overexpression correlated well with fibrotic score and plasma IL-6 levels. In line with another clinical observation (33), plasma IL-6 levels correlated well with the severity of liver fibrosis. Therefore, the STAT3 signaling pathway in HSCs might be a potential previously unidentified target for antifibrotic therapy, functioning in a similar manner to AG490, a specific JAK2 inhibitor that inhibits JAK2/STAT3 activation and prevents early activation of HSCs (34). In addition, plasma IL-6 level, an indicator of liver fibrogenesis and p-STAT3 expression, may potentially serve as a noninvasive biomarker to monitor the antifibrotic efficacy of novel STAT3 inhibitors; nevertheless, further studies are required for verification.
In conclusion, in vitro and in vivo studies indicate that sorafenib and its derivative SC-1 can ameliorate liver fibrosis through STAT3 inhibition in HSC. p-STAT3 is overexpressed in CHB patients with advanced liver fibrosis. Therefore, STAT3 may be a promising fibrotic biomarker and target, and SHP-1 phosphatase-directed antifibrotic therapy may represent a novel strategy for antifibrotic drug discovery.
Materials and Methods
See SI Materials and Methods for more information.
Reagents.
Sorafenib (Nexavar) was kindly provided by Bayer HealthCare AG. SC-1 was synthesized by the replacement of N-methylpicolinamide by a phenylcyano group in sorafenib, which abolished Raf kinase inhibition activity without compromising SHP-1–activating activity (26).
Liver Fibrosis Mouse Model.
Male Balb/C mice (8 wk old) were obtained from the National Laboratory Animal Center Taiwan. Two murine liver fibrosis models were used. In the thioacetamide model, they were administered with triweekly i.p. injection of thioacetamide (200 mg/kg) for 8 wk. Vehicle, sorafenib (10 mg/kg), or SC-1 (10 mg/kg) was administered via oral gavage for 5 d a week from the third week until sacrifice. In the BDL model, the common bile duct was double-ligated and followed by resection. Vehicle, sorafenib, (10 mg/kg) or SC-1 (10 mg/kg) was administered via oral gavage daily from day 8 until killing at day 14. All experimental procedures performed on these mice were in accordance with protocols approved by the Institutional Laboratory Animal Care and Use Committee of National Taiwan University.
Cell Culture.
HSC-T6 and LX2 cells are immortalized rat and human HSC cell lines, respectively, and were kindly provided by Scott Friedman (Mount Sinai Hospital, New York). Mouse primary HSC was isolated according to the procedures described with modification (35). For in vitro studies, sorafenib and SC-1 at various concentrations were dissolved in 0.1% DMSO and then added to the cells in 5% (vol/vol) FBS in Waymouth's or DMEM medium for the scheduled incubation duration.
Patient Enrollment.
A total of 193 CHB patients who received liver biopsies and plasma collection at National Taiwan University Hospital between 2006 and 2009 were enrolled, and their plasma samples were stored at −80 °C until use. According to Metavir fibrosis scores, we randomly selected eight patients with F0, F1, F2, F3, and F4, respectively. Finally, 40 patients were included in this study and their clinical information was retrieved. Their biopsied liver tissues were stained with p-STAT3 and α-SMA. Cells with positive p-STAT3 nuclear staining were counted over 10 high-power fields (200× magnification) for each patient. The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of National Taiwan University Hospital. Written informed consent was obtained from all patients at enrollment.
Statistical Analysis.
Continuous variables are presented as mean (SE) and categorical data are presented as number (percentage) as appropriate. Differences between subgroups were evaluated by Student’s t test. Pearson’s correlation was used to compare the p-STAT3 vs. IL-6 levels. The statistical analysis was performed by STATA (version 10.0; Stata Corp). All tests were two-sided and a P value <0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Prof. Scott Friedman of the Mount Sinai Hospital, New York for kindly providing us with HSC-T6 and LX2 cells, and the 4th Core Laboratory of the Department of Medical Research, National Taiwan University Hospital and the Taiwan Mouse Clinic (MOST 103-2325-B-001-015) for technical assistance. This work was supported by National Health Research Institutes Grant NHRI-EX103-10319PC; Ministry of Science and Technology, Taiwan, Grant NSC 102-2314-B-002-160-MY3; and National Taiwan University Hospital Grants 102-N2256 and 103-N2540.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507499112/-/DCSupplemental.
References
- 1.Chen DS. Fighting against viral hepatitis: Lessons from Taiwan. Hepatology. 2011;54(2):381–392. doi: 10.1002/hep.24500. [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Liver 2012. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 57(1):167–185, and correction (2013) 58(1):201. [DOI] [PubMed]
- 3.Lee YA, Friedman SL. Reversal, maintenance or progression: What happens to the liver after a virologic cure of hepatitis C? Antiviral Res. 2014;107:23–30. doi: 10.1016/j.antiviral.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: Nearing the starting line. Sci Transl Med. 2013;5(167):167sr161. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- 5.Su TH, Kao JH, Liu CJ. Molecular mechanism and treatment of viral hepatitis-related liver fibrosis. Int J Mol Sci. 2014;15(6):10578–10604. doi: 10.3390/ijms150610578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134(6):1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, et al. New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J Hepatol. 2010;53(1):132–144. doi: 10.1016/j.jhep.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Lafdil F, Kong X, Gao B. Signal transducer and activator of transcription 3 in liver diseases: A novel therapeutic target. Int J Biol Sci. 2011;7(5):536–550. doi: 10.7150/ijbs.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao B, Wang H, Lafdil F, Feng D. STAT proteins - key regulators of anti-viral responses, inflammation, and tumorigenesis in the liver. J Hepatol. 2012;57(2):430–441. doi: 10.1016/j.jhep.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieto N. Oxidative-stress and IL-6 mediate the fibrogenic effects of [corrected] Kupffer cells on stellate cells. Hepatology. 2006;44(6):1487–1501. doi: 10.1002/hep.21427. [DOI] [PubMed] [Google Scholar]
- 11.Chen KF, et al. Sorafenib overcomes TRAIL resistance of hepatocellular carcinoma cells through the inhibition of STAT3. Clin Cancer Res. 2010;16(21):5189–5199. doi: 10.1158/1078-0432.CCR-09-3389. [DOI] [PubMed] [Google Scholar]
- 12.Tai WT, et al. Signal transducer and activator of transcription 3 is a major kinase-independent target of sorafenib in hepatocellular carcinoma. J Hepatol. 2011;55(5):1041–1048. doi: 10.1016/j.jhep.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 13.Mair M, et al. Signal transducer and activator of transcription 3 protects from liver injury and fibrosis in a mouse model of sclerosing cholangitis. Gastroenterology. 2010;138(7):2499–2508. doi: 10.1053/j.gastro.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 14.Horiguchi N, et al. Dissociation between liver inflammation and hepatocellular damage induced by carbon tetrachloride in myeloid cell-specific signal transducer and activator of transcription 3 gene knockout mice. Hepatology. 2010;51(5):1724–1734. doi: 10.1002/hep.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horiguchi N, et al. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134(4):1148–1158. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: Evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35(4):762–771. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, et al. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009;137(2):713–723. doi: 10.1053/j.gastro.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilhelm SM, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 19.Mejias M, et al. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49(4):1245–1256. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 20.Hennenberg M, et al. Hepatic and HSC-specific sorafenib effects in rats with established secondary biliary cirrhosis. Lab Invest. 2011;91(2):241–251. doi: 10.1038/labinvest.2010.148. [DOI] [PubMed] [Google Scholar]
- 21.Hong F, Chou H, Fiel MI, Friedman SL. Antifibrotic activity of sorafenib in experimental hepatic fibrosis: Refinement of inhibitory targets, dosing, and window of efficacy in vivo. Dig Dis Sci. 2013;58(1):257–264. doi: 10.1007/s10620-012-2325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thabut D, et al. Complementary vascular and matrix regulatory pathways underlie the beneficial mechanism of action of sorafenib in liver fibrosis. Hepatology. 2011;54(2):573–585. doi: 10.1002/hep.24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen YL, et al. Sorafenib inhibits transforming growth factor β1-mediated epithelial-mesenchymal transition and apoptosis in mouse hepatocytes. Hepatology. 2011;53(5):1708–1718. doi: 10.1002/hep.24254. [DOI] [PubMed] [Google Scholar]
- 24.Deng YR, et al. STAT3-mediated attenuation of CCl4-induced mouse liver fibrosis by the protein kinase inhibitor sorafenib. J Autoimmun. 2013;46:25–34. doi: 10.1016/j.jaut.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem. 2002;277(32):28411–28417. doi: 10.1074/jbc.M202807200. [DOI] [PubMed] [Google Scholar]
- 26.Chen KF, et al. Sorafenib derivatives induce apoptosis through inhibition of STAT3 independent of Raf. Eur J Med Chem. 2011;46(7):2845–2851. doi: 10.1016/j.ejmech.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Chen KF, et al. Blockade of STAT3 activation by sorafenib derivatives through enhancing SHP-1 phosphatase activity. Eur J Med Chem. 2012;55:220–227. doi: 10.1016/j.ejmech.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 28.Tai WT, et al. Discovery of novel Src homology region 2 domain-containing phosphatase 1 agonists from sorafenib for the treatment of hepatocellular carcinoma. Hepatology. 2014;59(1):190–201. doi: 10.1002/hep.26640. [DOI] [PubMed] [Google Scholar]
- 29.Jiao H, et al. Direct association with and dephosphorylation of Jak2 kinase by the SH2-domain-containing protein tyrosine phosphatase SHP-1. Mol Cell Biol. 1996;16(12):6985–6992. doi: 10.1128/mcb.16.12.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wörns MA, et al. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma in consideration of concomitant stage of liver cirrhosis. J Clin Gastroenterol. 2009;43(5):489–495. doi: 10.1097/MCG.0b013e31818ddfc6. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors - a review on pharmacology, metabolism and side effects. Curr Drug Metab. 2009;10(5):470–481. doi: 10.2174/138920009788897975. [DOI] [PubMed] [Google Scholar]
- 32.Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: Clinical and regulatory perspectives. Drug Saf. 2013;36(7):491–503. doi: 10.1007/s40264-013-0048-4. [DOI] [PubMed] [Google Scholar]
- 33.Sripa B, et al. Advanced periductal fibrosis from infection with the carcinogenic human liver fluke Opisthorchis viverrini correlates with elevated levels of interleukin-6. Hepatology. 2009;50(4):1273–1281. doi: 10.1002/hep.23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakner AM, Moore CC, Gulledge AA, Schrum LW. Daily genetic profiling indicates JAK/STAT signaling promotes early hepatic stellate cell transdifferentiation. World J Gastroenterol. 2010;16(40):5047–5056. doi: 10.3748/wjg.v16.i40.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macdonald JM, et al. Liver cell culture and lineage biology. In: Atala A, Lanza RP, editors. Methods of Tissue Engineering. Academic; New York: 2002. pp. 155–160. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.