Abstract
Purpose.
To evaluate the photopic negative response (PhNR) as an index of retinal ganglion cell (RGC) function in idiopathic intracranial hypertension (IIH).
Methods.
Amplitude and implicit time of the PhNR, as elicited by full-field, brief-luminance flashes, was measured in IIH (n = 10) and visually normal control (n = 15) subjects. Visual function was assessed in IIH subjects using standard automated perimetry mean deviation (SAP-MD) scores. Optic nerve structure was evaluated using the Frisén papilledema grading scale (FPG). Macula ganglion cell complex volume (GCCV) was extracted from optical coherence tomography images to assess RGC loss.
Results.
Median PhNR amplitude was significantly lower in IIH subjects compared with control subjects (P = 0.015, Mann-Whitney Rank Sum [MW]), but implicit time was similar (P = 0.54, MW). In IIH subjects, PhNR amplitude and SAP-MD were correlated (Pearson's r = 0.78, P = 0.008). Ganglion cell complex volume was correlated with both SAP-MD (r = 0.72, P = 0.019) and PhNR amplitude (r = 0.77, P = 0.009). Multivariate linear regression models demonstrated that the correlation between GCCV and PhNR amplitude was improved by accounting for FPG in the model (r = 0.94, P < 0.0001), but the correlation between GCCV and SAP-MD was not (r = 0.74, P = 0.009).
Conclusions.
Photopic negative response amplitude, which can be decreased in IIH subjects, correlates well with a clinical measure of visual function (SAP-MD). In multivariate models, it correlated with both an imaging measure of chronic ganglion cell injury (GCCV) and a clinical measure of acute optic nerve head pathology (FPG). Further studies are needed to determine the clinical utility of PhNR as a marker for diagnosis and monitoring of IIH.
Keywords: photopic negative response, papilledema, retinal ganglion cells, idiopathic intracranial hypertension
The photopic negative response amplitude, an index of retinal ganglion cell function, is reduced in subjects with idiopathic intracranial hypertension and correlates with ganglion cell complex thickness and papilledema grade.
Vision loss associated with papilledema, the anterior optic nerve swelling that occurs when elevated intracranial pressure (ICP) squeezes the retro-bulbar optic nerve, is the major morbidity of idiopathic intracranial hypertension (IIH), which affects 1:100,000 individuals annually and has a 20-fold higher incidence in young, obese females.1,2 Papilledema is caused by axoplasmic stasis in the retinal ganglion cells (RGCs) that comprise the optic nerve due to mechanical compression of the RGCs by elevated ICP and/or RGC ischemia due to mechanical compression of blood vessels supplying them.3,4 Visual dysfunction in papilledema is attributed to RGC dysfunction caused by these same mechanisms and is clinically characterized using standard automated perimetry (SAP) to quantify peripheral vision, as central vision is typically not affected until late in the disease course. Standard automated perimetry is the basis for most clinical management decisions in IIH.5,6 However, abnormalities on SAP measure only some subpopulations of RGCs in the central 24 degrees of the visual field and are likely a late manifestation of ganglion cell injury.7 Furthermore, as a psychophysical test, SAP is inherently subjective and prone to patient error. This introduces uncertainty into interpreting its results with respect to RGC function.8,9
Electrophysiological measures of RGC function offer advantages over SAP because they are objective, noninvasive, quick, and have minimal patient demands. Literature regarding other optic neuropathies suggests that abnormal or absent electrophysiological responses that originate from RGCs may precede changes in SAP.7 There are multiple electrophysiological tests that are abnormal when RGC function is compromised, including the visual evoked potential (VEP), pattern electroretinogram (PERG), and photopic negative response (PhNR). The VEP, which measures the integrated function of the visual pathway from the retina to the occipital lobe, has been shown to have prolonged latency in patients who have IIH.10,11 However, its use has not been widely adopted, as a clinically relevant cutoff could not be defined. The PERG has been shown to have specificity for inner retinal dysfunction associated with RGC injury and has been demonstrated to be abnormal in patients with IIH, particularly at intermediate and high spatial frequencies.12 However, the PERG is limited to assessing function within the central visual field, which is not typically affected until late in the disease course in IIH. The PhNR is a slow negative component of the photopic full-field ERG that has promise for assessing RGC function in patients with IIH. The PhNR is generated by the spiking activity of inner retinal neurons, primarily RGCs,13,14 and is reduced in human patients with glaucoma,15 acquired optic atrophy,16,17 OPA1-associated dominant optic atrophy,18 anterior ischemic optic neuropathy, compressive optic neuropathy,19 and optic neuritis.20,21 The PhNR also has been shown to correlate well with chronic structural changes of the retinal nerve fiber layer (RNFL), a measure related to RGC structure, in chronic optic neuropathies.15,16,22 The PhNR has advantages over the PERG by being a relatively brief test and not requiring refractive correction, features that make it less prone to test-taker and examiner errors.23 Importantly, the PhNR, elicited by a full-field flash, captures RGC function throughout the entire visual field, not just the central portion stimulated in PERG. This is theoretically relevant in papilledema, in which early vision loss localizes to the peripheral visual field. The PhNR has not been recorded from patients with IIH, but it may be useful for providing important information regarding RGC function in this population.
The purpose of the present study was to evaluate the PhNR in patients with IIH. The PhNR amplitude and timing, recorded in patients with IIH, were compared with measurements made in control subjects and correlated with clinical measures of visual function and optic nerve structure. The results are intended to lay the scientific and technical foundation for the use of the PhNR as a clinical tool and potential clinical trial outcome measure for IIH.
Methods
Subjects from the Neuro-ophthalmology Service at the University of Illinois at Chicago with current or prior papilledema and a known or suspected diagnosis of IIH were recruited prospectively (n = 10, age 33.0 ± 9.2 years, 9 females). Diagnosis of IIH was confirmed for all subjects based on medical record review. This occurred after study completion in one subject who was studied before diagnostic lumbar puncture. This included brain imaging not showing pathology that could elevate ICP and lumbar puncture with opening pressure greater than or equal to 25 cm H2O with normal cerebrospinal fluid constituents.24 No subjects had neurological or ophthalmic disease other than IIH or refractive error. Data also were obtained from 15 visually normal control subjects (age 39.3 ± 14.5 years, 4 females) without history of ophthalmic or neurological disease. The median age of the control and patient groups did not differ significantly (P = 0.37, Mann-Whitney Rank Sum). The research followed the tenets of the Declaration of Helsinki and was approved by an institutional review board of the University of Illinois at Chicago. All subjects provided informed consent.
For IIH subjects, papilledema grade at time of enrollment was evaluated by a fellowship-trained neuro-ophthalmologist according to the Frisén scale: low Frisén grade (0, 1, 2), high Frisén grade25 (3, 4, 5) or optic atrophy. Stage of IIH at time of enrollment was categorized as “untreated” if the subject had had a diagnostic spinal tap showing elevated ICP following the day of electrophysiological testing, “treated” if the subject was status post surgery for ICP management, such as venous sinus stent placement with subsequent spinal tap with normal opening pressure or ventriculoperitoneal shunt, or medical management with resolution of symptoms (headache, pulsatile tinnitus) of elevated ICP or “active” if the subject was on medical therapy with persistent signs or symptoms of elevated ICP (headache, pulsatile tinnitus, papilledema). Visual acuity for each eye was measured with best refraction using projected Snellen charts at 20 feet in a standard ophthalmology examination lane and was 20/20 or better for all eyes. Standard automated perimetry was performed with appropriate refractive correction using the 24-2 Swedish Interactive Testing Algorithm (SITA) (Humphrey Field Analyzer; Carl Zeiss Meditech, Jena, Thuringia, Germany). Mean deviation (SAP-MD) in decibels was recorded. The pattern of visual field loss was evaluated using the visual field classification schema of the IIH treatment trial (IIHTT).26 Ganglion cell complex volume (GCCV) was measured from 20° × 15° high-resolution optical coherence tomography (OCT) scans of the macula (Spectralis; Heidelberg Engineering, Inc., Heidelberg, Germany). Using Eye Explorer (Heidelberg Engineering, Inc.) the internal limiting membrane was automatically segmented and manually corrected.27 The boundary between the inner plexiform layer and inner nuclear layer was segmented by manual selection of key points, to which a series of splines were automatically fit. Ganglion cell complex volume was automatically calculated as the volume between these boundaries in a 3-mm diameter cylindrical volume centered on the fovea.
Electrophysiological testing was performed on the worse-seeing eye, assessed by SAP-MD, in all subjects, with the fellow eye patched. Two subjects were studied on a second occasion following unequivocal treatment of IIH. The procedure and stimuli that were used to obtain the PhNR measurements are described in detail elsewhere.28 In brief, the subjects' pupils were dilated using 1% tropicamide and 2.5% phenylephrine hydrochloride drops. A fiber Dawson-Trick-Litzkow (DTL) recording electrode was placed along the lower eyelid and the signal from this electrode was referenced to the earlobe, with a forehead ground electrode. The light-emitting diode–generated stimulus consisted of a full-field long-wavelength (640 nm, red) pulse (4 ms; 3.0 cd⋅s/m2) presented on a steady 465-nm (blue) adapting field (12.5 cd/m2). This combination of a long-wavelength pulse on a short-wavelength background has been shown to elicit large PhNRs in healthy subjects.13,28,29 Pulses were presented with an interpulse interval of approximately 2 seconds in a ColorDome desktop ganzfeld (Diagnosys, LLC, Lowell, MA, USA) until five or more ERG responses with minimal eye movement artifacts were recorded. These responses were averaged for analysis. Responses were obtained using an Espion E3 electrophysiology system (Diagnosys, LLC), with amplifier bandpass settings of 0.3 to 300 Hz; the sampling frequency was 2 kHz. The amplitude and implicit time of the PhNR were defined according to previous specifications.28 That is, the PhNR amplitude was calculated as the difference between the baseline amplitude and the mean amplitude of 11 consecutive ERG data points (5.5 ms) centered at the trough of the PhNR.
The median PhNR amplitudes of the IIH subjects and controls were compared using a Mann-Whitney Rank Sum (MW) test due to non-normalcy of the IIH PhNR amplitude distribution. Subjects with IIH with abnormal PhNR were defined as those outside the range of the laboratory normative data. Receiver operating characteristic analysis was used to identify the optimal cutoff to differentiate IIH subjects and controls. Functional measures (SAP-MD and PhNR) were compared using Pearson correlation analysis following log transformation of the PhNR amplitude values to match the log scale of the MD values. Structural assessments of the optic nerve (Frisén papilledema grading scale [FPG], atrophy) and RGCs (macula GCCV) were compared using MW and Spearman correlation analysis.
Associations between functional and structural measures were studied with multiple linear regression using forward techniques. A separate univariate model was constructed for each functional measure (log PhNR, SAP-MD) with GCCV as the independent variable. Multivariate models were constructed by adding FPG as a dichotomous independent variable (high grade = FPG 3, 4, 5; not high grade = atrophy or FPG 0, 1, 2) to each univariate model. The multivariate and univariate models for each functional measure were compared with regard to fit, as represented by the correlation coefficient, r, and statistical significance of coefficient estimates for independent variables. All analyses were performed using statistical software (SPSS Version 21; IBM SPSS Statistics, IBM Corporation, Chicago, IL, USA). Statistical significance was accepted at P < 0.05.
Results
The characteristics of the IIH subjects on the day of electrophysiological testing are summarized in the Table and the subjects' disease characteristics are summarized in Supplementary Table S1. All subjects had visual field loss per the IIHTT classification schema.26 Visual field loss patterns included three widespread, five arcuate (two with concurrent blind spot enlargement), one nasal step with blind spot enlargement, and one isolated blind spot enlargement. Visual function assessed by SAP-MD was normal (SAP-MD > −2) in one subject, mildly impaired (−2 ≥ SAP-MD > −5) in four subjects, and moderately to severely impaired (SAP-MD ≤ −5) in five subjects. The FPG was low grade in six subjects and high grade in two subjects. Two subjects had optic atrophy. Macula GCCV ranged from 0.44 to 0.87 mm3. The subjects with optic atrophy had the lowest GCCV. The difference in GCCV between IIH subjects with and without atrophy was significant (P = 0.04, MW). The GCCV did not correlate with papilledema grade (Spearman coefficient = 0.31, P = 0.46).
Table.
Functional and Structural Measures in Subjects With IIH at Time of Electrophysiological Testing
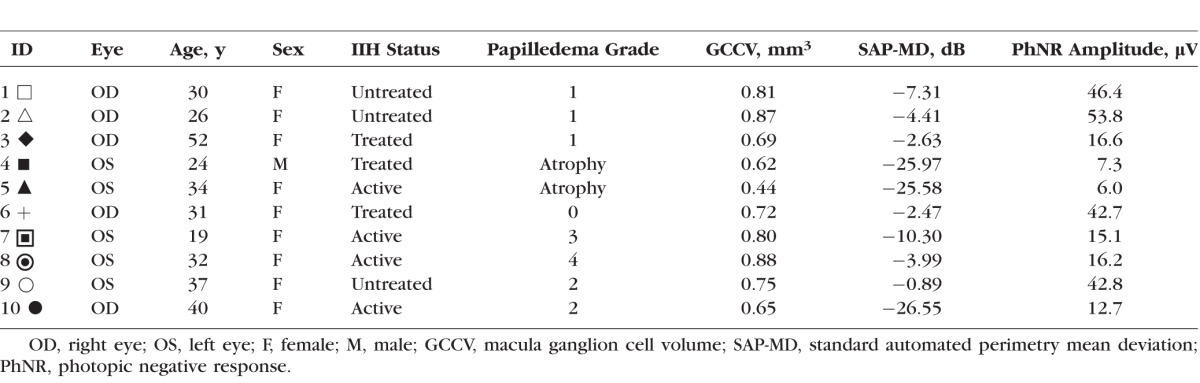
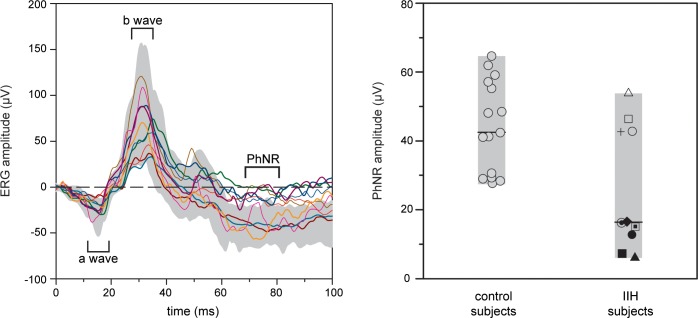
Figure 1 shows the ERG waveforms for the 10 IIH subjects (solid lines) and the range of normal (gray region). The a-wave (first negative deflection) and b-wave (first positive deflection) were within the range of normal in all 10 IIH subjects. However, the PhNR (second negative deflection) was abnormal in 6 of the 10 IIH subjects. The PhNR amplitude was quantified and is displayed in Figure 1. This figure shows the PhNR amplitude for the controls (left) and IIH subjects (right). The ranges of control and IIH subject data are indicated by the gray bars and the horizontal black lines mark the median PhNR amplitude values for the two groups. The median PhNR amplitude of the IIH subjects was significantly less than that of the controls (median 16.4 vs. 45.3 μV, P = 0.015, MW). Receiver operating characteristic analysis indicated 22 μV to be the optimal cutoff, giving 60% sensitivity and 100% specificity for IIH detection with an area under the curve of 0.79 (95% confidence interval 0.60–0.98, P = 0.015). The PhNR implicit time was not significantly different between controls and IIH subjects (median 75 vs. 72 ms, P = 0.54, MW). There was no association between PhNR amplitude or latency and subject age or sex for control subjects.
Figure 1.
Photopic negative response in IIH and control subjects. Each line on the left panel represents the photopic single-flash ERG response for a single IIH subject and the shaded area represents the range of responses for the control subjects. The right panel compares PhNR amplitudes in IIH and control subjects. Each marker represents a single subject. Horizontal lines represent the median for each group. The shaded areas are the ranges for each group.
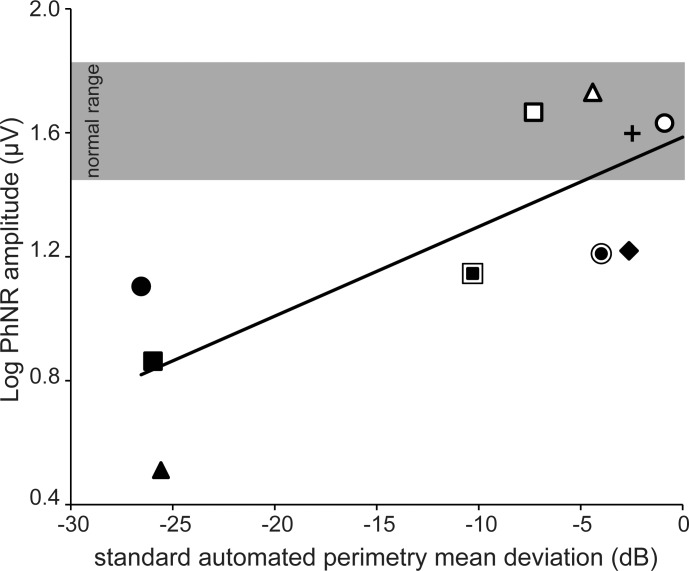
Figure 2 shows log PhNR amplitude as a function of SAP-MD amplitude. Each symbol represents a different IIH subject (symbols correspond to those given in the Table), the gray region represents the range of normal PhNR amplitude, and the solid line is a linear regression line fit to the data. There was a significant linear correlation between log PhNR amplitude and SAP-MD (Pearson correlation coefficient r = 0.78, P = 0.01). The four IIH subjects with PhNR amplitude within the range of normal had SAP-MD values that ranged from normal to moderately impaired (SAP-MD: −0.89 to −7.31 dB). For the six IIH subjects who had PhNR amplitude below the range of normal, SAP-MD was mildly impaired in two, moderately impaired in one, and severely impaired in three (SAP-MD: −2.63 to −25.55 dB). Thus, for subjects with abnormal PhNR amplitude, SAP-MD deficits ranged from mild to severe.
Figure 2.
Relationship between SAP-MD and log PhNR amplitude in IIH subjects. Each marker represents an IIH subject. Single markers are subjects with low-grade papilledema (Frisén Grades 0, 1, or 2) or optic atrophy. Markers surrounded by a ring indicate subjects with high-grade papilledema (Frisén Grades 3 or 4). The shaded area represents the range of normal PhNR amplitude based on control subject data (not shown). The solid line is the linear regression fit to the data.
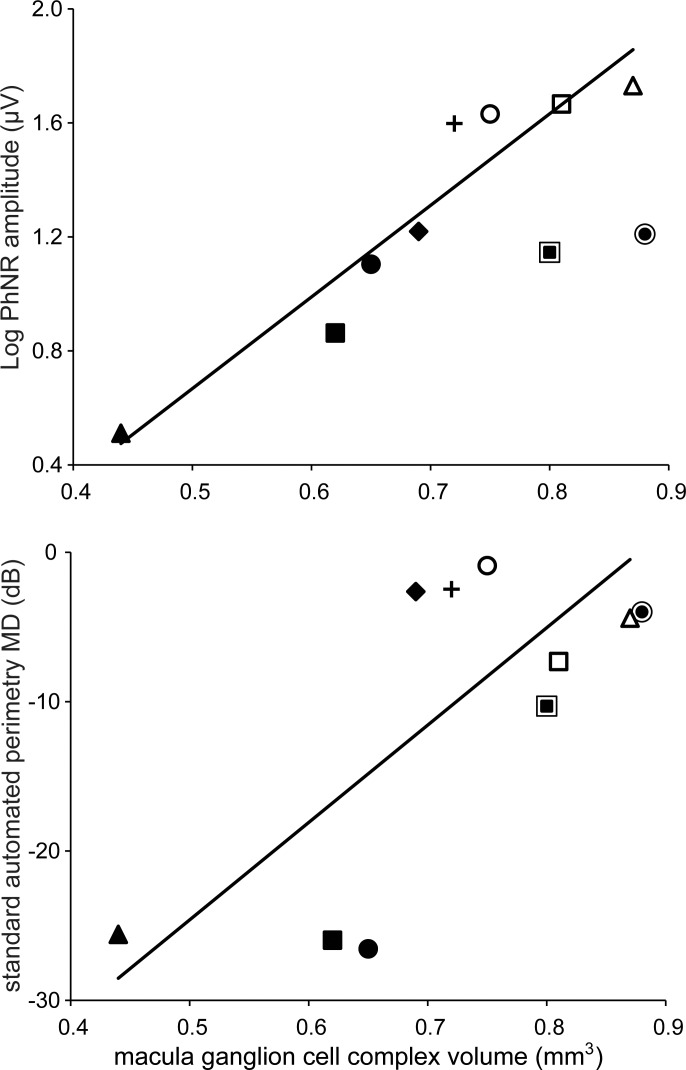
In Figure 3, log PhNR amplitude (top) and SAP-MD (bottom) are plotted as a function of GCCV. The solid lines represent linear regression fits to the data. Each data point represents a different subject (given in the Table) and subjects with high-grade papilledema are indicated with an outlined marker. There was a significant linear correlation between log PhNR amplitude and GCCV (r = 0.77, P < 0.01; top panel). However, the data points from the two patients with high-grade papilledema fell below the regression line, indicating a larger reduction in PhNR amplitude than would be expected from their GCCV. To account for papilledema grade (FPG), a multivariate regression model was developed that included FPG as a dichotomous independent variable. The addition of FPG improved the correlation between log PhNR amplitude and GCCV (r = 0.94, P < 0.0001).
Figure 3.
Relationship between GCCV and log PhNR amplitude (top) and SAP (bottom). Each marker represents an IIH subject. Single markers are subjects with low-grade papilledema (Frisén Grades 0, 1, or 2) or optic atrophy. Markers surrounded by a ring indicate subjects with high-grade papilledema (Frisén Grades 3 or 4). The solid lines are the univariate linear regression fit to the data.
There was also a significant linear correlation between SAP-MD and GCCV (r = 0.72, P < 0.01; bottom panel). The data points from the two patients with high-grade papilledema fell only slightly below the regression line, indicating GCCV provided a reasonable prediction of SAP-MD for these two patients. This suggests that consideration of FPG would not improve the predictive power of the model. To assess this suggestion quantitatively, a multivariate model was developed that included FPG as a dichotomous independent variable. After including FPG in the model, the correlation between SAP-MD and GCCV was essentially unchanged (r = 0.74, P < 0.009). Thus, accounting for FPG improved the model fit for the relationship between log PhNR and GCCV, but did not improve the model fit for the relationship between SAP-MD and GCCV.
For two subjects, a repeat measure of the PhNR was performed following a documented change in disease status. Figure 4 shows scanning laser ophthalmoscopy images of the optic nerve (top), Humphrey Visual Field total deviation plots (middle), and PhNR waveforms (bottom) for subject 8. This subject presented with Frisén grade 4 papilledema, mild vision loss (SAP-MD −3.99 dB), and abnormal PhNR amplitude (−14 μV). Following 2 months of aggressive medical intervention with acetazolamide and weight loss, headaches and pulsatile tinnitus resolved, and papilledema improved to Frisén grade 2. The SAP-MD was essentially unchanged (−3.34 dB), but the PhNR improved to normal (−39.15 μV). Subject 5 presented with atrophic papilledema (average peripapillary RNFL thickness was 60 μm using OCT [Spectralis; Heidelberg Engineering, Inc.]). One week after diagnosis she had stable severe vision loss (SAP-MD −25.6 dB) and abnormal PhNR amplitude (−6.0 μV). One month following definitive treatment of ICP with ventriculoperitoneal shunt, there was neither improvement in PhNR amplitude (undetectable) nor SAP-MD (−25.4 dB) (Supplementary Fig. S1).
Figure 4.
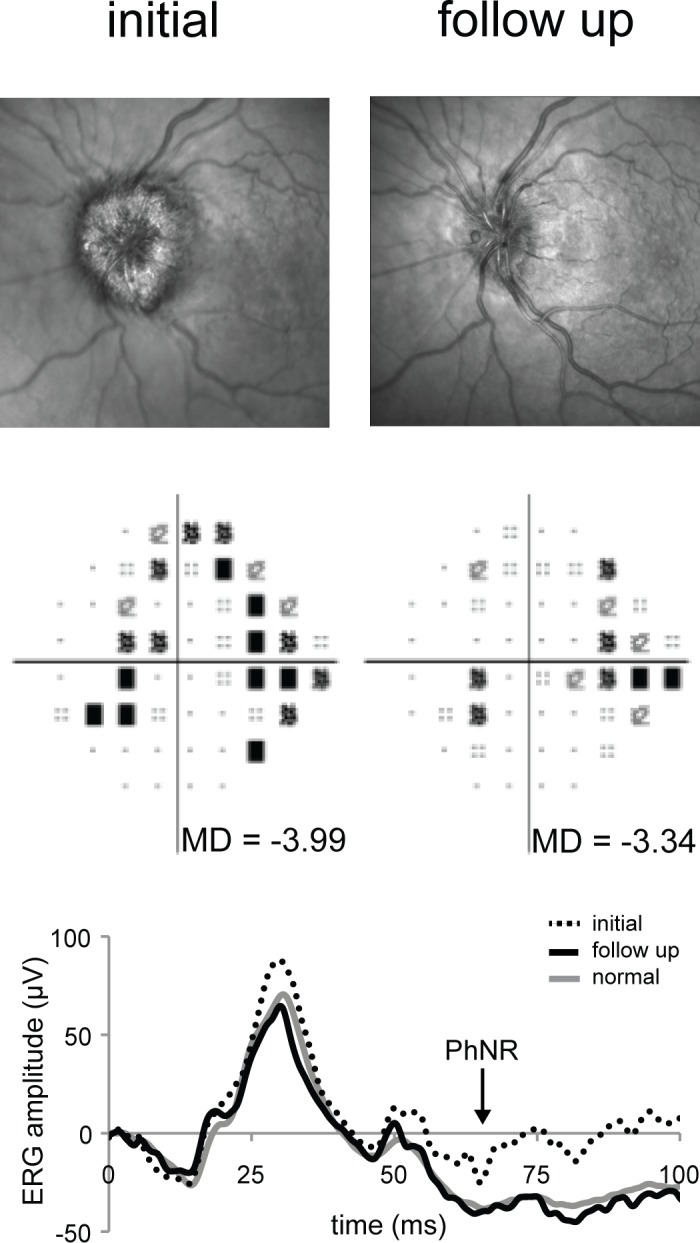
Comparison of initial and follow-up observations for subject 8. The top row shows initial (left) and follow-up (right) optic disc appearance as documented using scanning laser ophthalmoscopy (Spectralis). The middle row shows initial (left) and follow-up (right), SAP total deviation plots and MD values (Humphrey SITA 24-2). Thee bottom graph shows the photopic single-flash ERG responses. The arrow shows the time at which the PhNR amplitudes were calculated.
Discussion
We investigated an electrophysiological index of RGC activity, the PhNR, in 10 subjects with IIH and report correlations with visual function (SAP-MD) and optic nerve structure. The PhNR amplitude was reduced in 60% of IIH subjects in our sample, and the extent to which the PhNR was reduced correlated with visual field abnormalities assessed by SAP-MD. The PhNR amplitude was abnormal in all IIH subjects with optic atrophy or high-grade papilledema, including some with only mild visual field loss. Regression models showed GCCV, a measure of ganglion cell atrophy, to be moderately associated with both SAP-MD and PhNR amplitude. Multivariate regression models showed high-grade papilledema to be associated with PhNR amplitude but not SAP-MD. The results indicate that the PhNR is not a marker of elevated ICP per se, because normal PhNR amplitude was observed in one untreated subject with elevated ICP and impaired PhNR amplitudes were observed in treated subjects with normalized ICP. Rather, the PhNR appears to be a useful index of optic neuropathy in a group of individuals with IIH.
To our knowledge, this is the first investigation of the PhNR in subjects with IIH or papilledema. It builds on literature demonstrating PhNR abnormalities and correlations with clinical measures of visual function in overt optic neuropathies such as optic atrophy,16 optic neuritis,21 and glaucoma.15 Our observation that an anatomic measure of RGC loss (GCCV) is associated with PhNR amplitude parallels the report by Wang and colleagues20 that the PhNR amplitude correlated with chronic abnormalities in the peripapillary RNFL, as measured with OCT more than 6 months following optic neuritis. We report a novel observation of a multivariate association between PhNR amplitude, a measure of chronic RGC loss (GCCV), and a measure of acute RGC pathology (high FPG). This likely reflects the clinical pace of IIH and other acute optic neuropathies, in which vision loss and optic nerve abnormalities manifest over days to weeks, but measurable evidence of RGC loss does not manifest for weeks to months. The observation by Wang and colleagues20 that PhNR amplitude did not correlate with RNFL thickness performed less than 6 months following optic neuritis, but did correlate with RNFL thickness performed more than 6 months following optic neuritis, supports the notion of RGC loss measures such as RNFL thickness and GCCV as a marker of chronic, but not acute optic neuropathies. We propose that GCCV and high FPG provide complementary assessments of RGC impairment in IIH throughout the course of the disease. The association of both with PhNR amplitude supports the role of the PhNR as an integrated measure of both acute and chronic injury. Further investigation will determine if this finding is specific to IIH or common to all acute optic neuropathies.
Linear regression models indicated that PhNR amplitude varied as a function of both GCCV and high papilledema grade, whereas SAP-MD was associated only with GCCV. This suggests that the PhNR amplitude can be influenced by RGC dysfunction that is not associated with visual dysfunction as assessed by visual field perimetry and is similar to what has been demonstrated in patients with multiple sclerosis without history of optic neuritis,20 and pre-perimetric glaucoma.23 One possible explanation for this is that PhNR is a full-field test, whereas SAP measures only the central 48 degrees of the visual field. Consequently, if the effects of disease are localized beyond the central 48 degrees of the visual field, the PhNR amplitude may be reduced, but SAP-MD may be normal. There are also differences in the spatial extent of our structural measures in that FPG is an assessment of the entire optic nerve, whereas macular GCCV measures the region supplying the central 20 degrees of the visual field. Although peripapillary RNFL measurements may provide a broader assessment of RGC loss, this measurement is precluded in our subject population, in which the peripapillary region is often distorted due to papilledema.
Our observation in subject 8 that PhNR amplitude recovered in association with improving papilledema, but without significant change in SAP-MD, suggests that such pre-perimetric RGC dysfunction may be reversible. Improvement of the PhNR amplitude to normal during recovery from an acute optic neuropathy has not been previously reported. However, it is important to note that the improvement was observed in a single case and additional follow-up in a larger sample is needed to determine how commonly improvements in PhNR amplitude occur. In contrast to the results of subject 8, Nakamura and colleagues21 demonstrated decreased PhNR amplitudes during acute optic neuritis that did not recover despite improvement in visual function.
The PhNR may provide information to supplement standard clinical markers of optic neuropathy in IIH, such as visual field perimetry and ophthalmoscopy to advance clinical management of IIH. Due to its specificity for RGC activity, the PhNR may have application to monitoring RGC function in papilledema in situations when visual field perimetry is concurrently affected by both RGC and non-RGC lesions of the visual pathway. Furthermore, the PhNR is less impacted by purposeful or inadvertent patient error because it is an objective test that has minimal patient demands. For these reasons, it may have application for monitoring RGC activity in IIH in clinical situations in which visual field perimetry is unreliable, such as functional vision loss, young children, or cognitively impaired adults.8 The PhNR offers theoretical advantages over other electrophysiological tests that measure RGC activity. The brief, full-field stimulus captures function throughout the visual field and is less prone to patient errors and poor cooperation than PERG. Its specificity for RGC function reduces artifacts from other visual pathway lesions, compared to VEP. To our knowledge, a comparative study of these electrophysiological methods in IIH has not been performed, although the PhNR and PERG have been compared in glaucoma.23 Comparison of these methods, including identification of possible complementary roles in monitoring of IIH, is an important area of future research.
In summary, we have demonstrated that patients with IIH can have reduced PhNR amplitudes, which correlate significantly with visual function as assessed by SAP-MD and optic nerve structure. Future studies are needed to evaluate the suitability of the PhNR as a clinical monitoring tool in IIH and to explore the relationship between PhNR and the reversibility of RGC injury.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health Grants K12 EY021475, K23 EY024345, R00 EY019510, and P30 EY01792; an Illinois Society for the Prevention of Blindness Research Grant; and an unrestricted departmental grant from Research to Prevent Blindness.
Disclosure: H.E. Moss, None; J.C. Park, None; J.J. McAnany, None
References
- 1. Radhakrishnan K,, Thacker AK,, Bohlaga NH,, et al. Epidemiology of idiopathic intracranial hypertension: a prospective and case-control study. J Neurol Sci. 1993; 116: 18–28. [DOI] [PubMed] [Google Scholar]
- 2. Radhakrishnan K,, Ahlskog JE,, Cross SA, et al. Idiopathic intracranial hypertension (pseudotumor cerebri) descriptive epidemiology in Rochester, Minn, 1976 to 1990. Arch Neurol. 1993; 50: 78–80. [DOI] [PubMed] [Google Scholar]
- 3. Trobe JD. Papilledema: the vexing issues. J Neuroophthalmol. 2011; 31: 175. [DOI] [PubMed] [Google Scholar]
- 4. Tso MO,, Hayreh SS. Optic disc edema in raised intracranial pressure: IV. Axoplasmic transport in experimental papilledema. Arch Ophthalmol. 1977; 95: 1458. [DOI] [PubMed] [Google Scholar]
- 5. Rowe FJ,, Sarkies NJ. Assessment of visual function in idiopathic intracranial hypertension: a prospective study. Eye. 1998; 12: 111–118. [DOI] [PubMed] [Google Scholar]
- 6. Corbett JJ,, Savino PJ,, Thompson HS, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982; 39: 461–474. [DOI] [PubMed] [Google Scholar]
- 7. Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res. 2012; 31: 702–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Digre KB,, Nakamoto BK,, Warner JEA, et al. A comparison of idiopathic intracranial hypertension with and without papilledema. Headache. 2009; 49: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rao HL,, Yadav RK,, Begum VU,, et al. Role of visual field reliability indices in ruling out glaucoma. JAMA Ophthalmol. 2015; 133: 40–44. [DOI] [PubMed] [Google Scholar]
- 10. Sorensen PS,, Trojaborg W,, Gjerris F, et al. Visual evoked potentials in pseudotumor cerebri. Arch Neurol. 1985; 42: 150. [DOI] [PubMed] [Google Scholar]
- 11. Kesler A,, Vakhapova V,, Korczyn AD,, et al. Visual evoked potentials in idiopathic intracranial hypertension. Clin Neurol Neurosurg. 2009; 111: 433–436. [DOI] [PubMed] [Google Scholar]
- 12. Falsini B,, Tamburrelli C,, Porciatti V, et al. Pattern electroretinograms and visual evoked potentials in idiopathic intracranial hypertension. Ophthalmologica. 1992; 205: 194–203. [DOI] [PubMed] [Google Scholar]
- 13. Rangaswamy NV,, Shirato S,, Kaneko M,, et al. Effects of spectral characteristics of ganzfeld stimuli on the photopic negative response (PhNR) of the ERG. Invest Ophthalmol Vis Sci. 2007; 48: 4818–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viswanathan S,, Frishman LJ,, Robson JG, et al. The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci. 1999; 40: 1124–1136. [PubMed] [Google Scholar]
- 15. Viswanathan S,, Frishman LJ,, Robson JG,, et al. The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2001; 42: 514–522. [PubMed] [Google Scholar]
- 16. Gotoh Y,, Machida S,, Tazawa Y. Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol. 2004; 122: 341. [DOI] [PubMed] [Google Scholar]
- 17. Tamada K,, Machida S,, Yokoyama D, et al. Photopic negative response of full-field and focal macular electroretinograms in patients with optic nerve atrophy. Jpn J Ophthalmol. 2009; 53: 608–614. [DOI] [PubMed] [Google Scholar]
- 18. Miyata K,, Nakamura M,, Kondo M,, et al. Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Invest Ophthalmol Vis Sci. 2007; 48: 820–824. [DOI] [PubMed] [Google Scholar]
- 19. Rangaswamy NV,, Frishman LJ,, Dorotheo EU, et al. Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci. 2004; 45: 3827–3837. [DOI] [PubMed] [Google Scholar]
- 20. Wang J,, Cheng H,, Hu YS,, et al. The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci. 2012; 53: 1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakamura H,, Miyamoto K,, Yokota S, et al. Focal macular photopic negative response in patients with optic neuritis. Eye (Lond). 2011; 25: 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Machida S. Clinical applications of the photopic negative response to optic nerve and retinal diseases. J Ophthalmol. 2012; 2012: 397178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Preiser D,, Lagreze WA,, Bach M, et al. Photopic negative response versus pattern electroretinogram in early glaucoma. Invest Ophthalmol Vis Sci. 2013; 54: 1182–1191. [DOI] [PubMed] [Google Scholar]
- 24. Friedman DI,, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002; 59: 1492–1495. [DOI] [PubMed] [Google Scholar]
- 25. Frisen L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry. 1982; 45: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keltner JL,, Johnson CA,, Cello KE, et al. Baseline visual field findings in the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT). Invest Ophthalmol Vis Sci. 2014; 55: 3200–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krebs I,, Smretschnig E,, Moussa S,, et al. Quality and reproducibility of retinal thickness measurements in two spectral-domain optical coherence tomography machines. Invest Ophthalmol Vis Sci. 2011; 52: 6925–6933. [DOI] [PubMed] [Google Scholar]
- 28. Gowrisankaran S,, Genead MA,, Anastasakis A, et al. Characteristics of late negative ERG responses elicited by sawtooth flicker. Doc Ophthalmol. 2013; 126: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sustar M,, Cvenkel B,, Brecelj J. The effect of broadband and monochromatic stimuli on the photopic negative response of the electroretinogram in normal subjects and in open-angle glaucoma patients. Doc Ophthalmol. 2009; 118: 167–177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.