Abstract
Substantial genetic, biochemical, and in vivo data indicate that progressive accumulation of amyloid-β (Aβ) plays a central role in the pathogenesis of Alzheimer’s disease (AD). Historically centered in the importance of parenchymal plaques, the role of cerebral amyloid angiopathy (CAA)—a frequently neglected amyloid deposit present in >80% of AD cases—for the mechanism of disease pathogenesis is now starting to emerge. CAA consistently associates with microvascular modifications, ischemic lesions, micro- and macro-hemorrhages, and dementia, progressively affecting cerebral blood flow, altering blood-brain barrier permeability, interfering with brain clearance mechanisms and triggering a cascade of deleterious pro-inflammatory and metabolic events that compromise the integrity of the neurovascular unit. New evidence highlights the contribution of pre-fibrillar Aβ in the induction of cerebral endothelial cell dysfunction. The recently discovered interaction of oligomeric Aβ species with TRAIL DR4 and DR5 cell surface death receptors mediates the engagement of mitochondrial pathways and sequential activation of multiple caspases, eliciting a cascade of cell death mechanisms while unveiling an opportunity for exploring mechanistic-based therapeutic interventions to preserve the integrity of the neurovascular unit.
Keywords: Amyloid-β genetic variants, amyloidosis, cerebral amyloid angiopathy, cerebral microvascular cells, death receptors, familial Alzheimer’s disease, inflammation, mitochondrial dysfunction
Amyloid diseases are considered part of an emerging group of chronic and progressive entities collectively known as “Disorders of Protein Folding” [1–5], in which structural transitions of specific proteinaceous components from soluble monomeric species normally present in body fluids into polymeric aggregates that generate poorly soluble tissue deposits significantly contribute to the mechanism of disease pathogenesis. Many factors are known to destabilize the structure of soluble proteins and contribute to the mechanism of amyloidogenesis, among them high protein concentration, the presence of certain metal ions, the existence of amino acid substitutions, N- and C-terminal truncations, post-translational modifications, or a favorable mildly acidic pH [6–8]. The poor solubility of the deposits in conjunction with their high resistance to proteolytic degradation impairs effective tissue removal leading to cell damage, organ dysfunction, and eventually death. At the present time, 30 different proteins (Table 1) and more than 100 genetic variants are known to be associated with systemic and localized forms of the disease in humans; however, the pathogenic mechanism(s) ultimately responsible for triggering the formation of fibrils remain largely unidentified. Puzzling as well is the fact that only a fraction of those amyloid subunits, seven out of thirty (Aβ, ACys, ATTR, APrP, AGel, ABri and ADan), are associated with the formation of fibrillar deposits in the central nervous system (CNS), and of those seven, only one (Aβ, amyloid-β) seems to be specifically restricted to the CNS.
Table 1.
Amyloid proteins associated with disease in humans
| Biological function of the precursor protein | Precursor protein | Chrom | Amyloid subunit | Syst (S)/restrict (R) | CAA |
|---|---|---|---|---|---|
| Apolipoproteins & lipopeptides | apoSAA | 11 | AA | S | No |
| apoA-I | 11 | AApoA-I | S | No | |
| apoA-II | 1 | AApoA-II | S | No | |
| apoA-IV | 11 | AApoA-IV | S | No | |
| Lung Surfactant Protein C | 8 | ASPC | R | No | |
| Cell adhesion proteins | Keratoepithelin | 5 | AKep | R | No |
| Lactadherin | 15 | AMed | R | No | |
| Galectin-7 | 19 | AGal7 | R | No | |
| Corneodesmosin | 6 | ACor | R | No | |
| Cell Receptor | Oncostatin M Receptor | 5 | AOMR | R | No |
| Chemotaxis | Leukocyte Chemotactic Factor-2 | 5 | ALect2 | R | No |
| Coagulation factors | Fibrinogen α-chain | 4 | AFib | S | No |
| Cytoskeletal protein | Keratin | 12,17 | AKer | R | No |
| Enzymatic inhibitors | Cystatin C | 20 | ACys | S | Yes |
| Enzymes | Lysozyme | 12 | ALys | S | No |
| Hormones | Calcitonin | 11 | ACal | R | No |
| Prolactin | 6 | APro | R | No | |
| Atrial Natriuretic Factor | 1 | AANF | R | No | |
| Amylin | 12 | AIAPP | R | No | |
| Insulin | 11 | AIns | R | No | |
| Immune System associated proteins | Light chain λ | 22 | ALλ | S, R | No |
| Light chain κ | 2 | ALκ | S, R | No | |
| Heavy chain γ | 14 | AHγ | S, R | No | |
| Heavy chain μ | 14 | AHμ | S, R | No | |
| Heavy chain α | 14 | AHα | S, R | No | |
| β2-microglobulin | 15 | Aβ2M | S | No | |
| Infectious agents | Prion protein | 20 | APrPSC | R | Yes |
| Regulatory proteins | Gelsolin | 9 | AGel | S | Yes |
| Semenogelin I | 20 | ASem | R | No | |
| Transport proteins | Transtyretin | 18 | ATTR | S | Yesa |
| Lactoferrin | 3 | ALac | R | No | |
| Unknown biological function | Amyloid β precursor protein | 21 | Aβ | R | Yes |
| Bri2 protein | 13 | ABri | S | Yes | |
| Bri2 protein | 13 | ADan | S | Yes |
Amyloid nomenclature follows the recommendations of the Nomenclature Committee of the International Society of Amyloidosis [79].
Specific variants.
The amyloid precursor proteins listed in Table 1 do not share sequence homology; they are codified by different chromosomes and exhibit a wide variety of biological functions. However, in spite of these differences, all the unrelated amyloid subunits derived from these precursors assemble into morphologically indistinguishable bundles of long twisted 6–8 nm wide Congo red positive filaments, irrespective of their biochemical nature or the topographic distribution of the deposits. Although not definitively settled, cumulative evidence indicate that these fibrillar lesions are rather inert deposits, causing only the expected physical disruption of tissue architecture but little or no additional biological effects on the cells that surround the lesions. Thus, studies of toxic effects on different cell types have focused lately on intermediate conformations (oligomers and protofibrils) rather than in the final fibrillar assemblies. The bulk of these studies have emerged from research on Aβ, the peptide associated with the most common form of amyloidosis in humans, Alzheimer’s disease (AD). In AD, intermediate oligomeric and protofibrillar forms of Aβ seem to display the most potent toxic activity in neuronal cells, inducing synaptic disruption and neurotoxicity [9, 10]; a similar dependence on the aggregation state of the amyloidogenic peptides also exist for cells composing the vessel wall [11–14]. For studying the latter, investigators have focused their attention on disorders primarily associated with cerebrovascular amyloid deposits, broadly known as cerebral amyloid angiopathy (CAA), the most frequent condition associated with focal ischemia, cerebral hemorrhage, and neurovascular dysfunction. It compromises medium and small size arteries and arterioles as well as capillary endothelium leading to endothelial degeneration, decreased cerebral blood flow and ischemic metabolic changes [15–18]. Progressive build-up of amyloid in and around vessels induces alterations in blood-brain barrier permeability and release of inflammatory mediators that chronically limits blood supply resulting in focal deprivation of oxygen and nutrients. These changes trigger a secondary cascade of metabolic events involving generation of free radicals and oxidative stress damage, ion channel-disruption, release of proteases, disturbances of intracellular Ca+2 homeostasis, and induction of cell death mechanisms, events that further compromise the integrity of the neurovascular unit [19, 20].
CAA-ASSOCIATED Aβ GENETIC VARIANTS
A diverse group of unrelated proteins, mostly derived from hereditary conditions, are known to produce cerebrovascular amyloid lesions in humans [2, 21]; however, the most frequent form of CAA is related to Aβ deposition in sporadic AD. Vascular deposits in AD—albeit biochemically highly heterogeneous—are largely constituted by the 40-residues-long peptide Aβ40, contrasting with the classic parenchymal plaques which are predominantly associated with deposition of Aβ42 [2]. Today, substantial genetic, biochemical, and in vivo data suggest that progressive accumulation of Aβ plays a central role in the pathogenesis of AD [22]. While historically centered on Aβ plaques, the lack of correlation between plaque load and neuronal loss triggered a shift in attention toward the role of non-fibrillar oligomeric Aβ assemblies, particularly in the process of synaptic dysfunction and cell toxicity. The contribution of vascular amyloid deposits, a frequently neglected feature present in >80% of AD cases and associated with microvascular modifications, ischemic lesions, micro-and macro-hemorrhages, and dementia, adds further complexity to the molecular pathogenesis of the disease [23]. A plethora of Aβ genetic variants (Table 2), although infrequent, provide unique paradigms to further examine the role of amyloid in the mechanism of disease pathogenesis and to dissect the link between vascular and parenchymal amyloid deposition and their differential contribution to neurodegeneration. Particular biophysical characteristics of these Aβ genetic variants, e.g., accelerated oligomerization and fibrillization propensity in comparison to their wild-type counterpart, make them unique valuable models to better understand their effects on vascular cells and the molecular mechanisms associated with their detrimental effect on the cell functionality [13, 14, 24, 25]. As indicated in Table 2, several of these Aβ mutants are largely associated with the formation of CAA deposits, particularly those involving amino acids 21–23 and 34.
Table 2.
Mutations in the AβPP gene located within the Aβ sequence
| Codon | Nucleotide substitution | Amino Acid substitution | Aβ mutation | Kindred | Clinical phenotype | Ref. |
|---|---|---|---|---|---|---|
| 673 | C > T | A > V | A2V | Italian | Aggressive, early onset dementia | [80] |
| 677 | A > G | H > R | H6R | British | AD-like dementia, early onset | [81] |
| 678 | G > A | D > N | D7N | Tottori | Progressive AD-like dementia | [82] |
| 682 | G > A | E > K | E11K | Leuven | AD-like dementia, early onset | [83] |
| 692 | C > G | A > G | A21G | Flemish | CAA, dementia, cerebral hemorrhage | [84] |
| 693 | G > C | E > Q | E22Q | Dutch | CAA, fatal cerebral hemorrhages | [26] |
| 693 | A > G | E > G | E22G | Artic | AD-like dementia, early onset | [85] |
| 693 | G > A | E > K | E22K | Italian | CAA, cerebral hemorrhage | [86] |
| 693 | delAGA | ΔE | ΔE22 | Japanese | AD-like dementia | [87] |
| 694 | G > A | D > N | D23N | Iowa | CAA, early onset AD-like dementia | [88] |
| 705 | D > G | L > V | L34V | Piedmont | CAA, recurrent cerebral hemorrhages | [89] |
| 713 | G > A | A > T | A42T | Italian/Spanish | Dementia, stroke, early onset | [90] |
The AβE22Q substitution, the first mutation reported in the AβPP gene [26], is one of the most widely studied. This genetic variant was reported in members of a Dutch kindred affected with a condition known as Hereditary Cerebral Hemorrhage with Amyloidosis (HCHWA-D) that presents with recurrent episodes of cerebral hemorrhage that are fatal on about two-thirds of the cases with the rest developing vascular dementia [21]. Neuropathologically, extensive deposition of amyloid affecting leptomeningeal, cortical arteries, and arterioles co-exist with parenchymal diffuse (Congo red negative) deposits and rare or even absent neuritic plaques and neurofibrillary tangle pathology [27, 28]. The presence of the AβE22Q mutation with a loss of a negatively charged residue drastically alters the Aβ aggregation pattern and results in the accelerated formation of oligomeric/fibrillar assemblies. In turn, this structural change hampers its transport and clearance across capillaries and leptomeningeal vessels in vivo, resulting in an enhanced accumulation of AβE22Q in the brain [29]. Together these features parallel the early onset and aggressiveness of the disease in vivo [13, 30].
AMYLOID-MEDIATED MICROVASCULAR CELL DEATH MECHANISMS
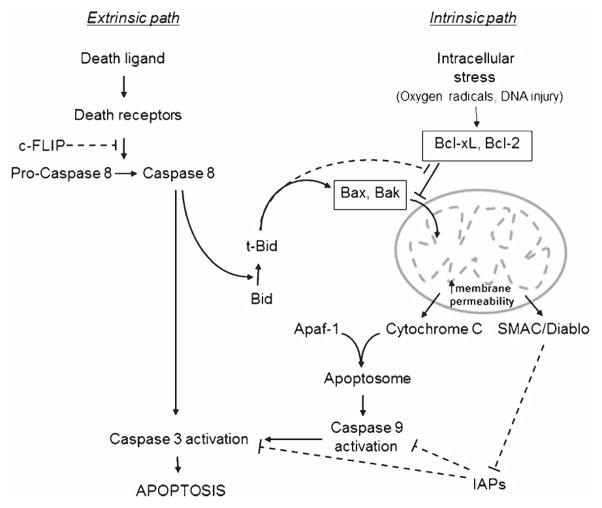
Amyloid accumulation around cerebral vessels is known to induce degeneration of the entire neurovascular unit [31]. Not only do insoluble amyloid species accumulating at the vascular walls cause alterations of the smooth muscle and endothelial cell layers but amyloid deposition and concomitant microhemorrhages also do take place in small capillary vessels lacking the smooth muscle layer, emphasizing the relevance of CAA-dependent mechanisms in brain endothelial cells. Increasing evidence suggests that apoptotic biochemical cascades play pivotal roles in the neuronal dysfunction and death observed in AD [32, 33]. Recent findings demonstrate the induction of analogous Aβ-mediated cell death mechanisms in vascular cells as those described in neurons in which mitochondrial dysfunction [32, 34] and engagement of apoptotic pathways involving cell death receptors have been postulated [35]. Two main pathways, extrinsic and intrinsic, lead to apoptosis in mammalian cells (Fig. 1). The latter, modulated by the Bcl-2 family of proteins and typically initiated by oxidative stress and calcium dysregulation, involves mitochondrial outer membrane permeabilization allowing the release of proteins, including cytochrome c, to the cytoplasm with subsequent formation of the apoptosome [36]. These events in turn facilitate downstream cell death cascades leading to sequential activation of caspase-9 followed by caspase-3, DNA fragmentation, and formation of apoptotic bodies. The extrinsic path, normally activated through specific cell receptors, involves multiple partners and complex mechanisms and is typically centered in receptor-mediated activation of the initiator caspase-8 prior to the downstream activation of the effector caspases common to both intrinsic and extrinsic pathways [32]. Both mechanisms are not completely independent and activation of caspase-8, step regulated by cFLIP, also results in the engagement of the mitochondrial path through its proteolytic effect on Bid. This mechanistic cascade leads to Bax translocation, oligomerization, and insertion onto the mitochondrial membrane with subsequent leakage of cytochrome c and downstream formation of the apoptosome [36]. In fact, the participation of both pathways was recently demonstrated in Aβ-challenged smooth muscle and endothelial cells [12, 13, 37]. As illustrated in Fig. 2, treatment of endothelial cells with the aggressive AβE22Q genetic variant which exhibits accelerated formation of pathogenic oligomeric assemblies, resulted in severe induction of apoptosis. Analysis of the conformational state of the amyloid peptides triggering cell death mechanisms indicated that early stages of apoptosis preceded fibril formation correlating with the presence of intermediate-size oligomeric assemblies. In this sense, although the apoptotic effect was fastest and most prominent with AβE22Q, which showed accelerated and enhanced oligomerization, it was also relevant for less aggressive variants as well as for wild-type Aβ40 associated with sporadic forms of AD, albeit with a delayed kinetics in parallel with the age of onset and the aggressiveness of the different clinical phenotypes in vivo. The limited biochemical analysis of CAA lesions available supports the existence of oligomeric assemblies in vivo, not only in Aβ-related disorders [38] but also in non-Aβ cerebral amyloidosis [39]. In spite of all data highlighting the importance of oligomeric assemblies for the induction of cell toxicity, it is important to note that similar structures may not always necessarily evoke the same cellular responses. In some cases the peptide behavior appears to be dictated by the intrinsic properties of the genetic variant which correlate with the inherent clinical phenotype associated with the mutation. Albeit limited research is available, this may explain the lack of endothelial cell toxicity exhibited by pre-fibrillar aggregates of the AβE22 G Artic mutant linked to an AD-like phenotype with very mild CAA in absence of micro-/macro-bleeds [11], cautioning that the toxic effect in some cases may go beyond the mere peptide multimerization.
Fig. 1.
Schematic diagram of extrinsic and intrinsic cell death pathways. The diagram illustrates both intrinsic and receptor-mediated cell death mechanisms, highlighting secondary engagement of mitochondrial paths through the caspase-8 mediated cleavage of Bid. Inhibitors are depicted by broken lines. c-FLIP indicates cellular FLICE-like inhibitory protein, an endogenous inhibitor of caspase-8 activation; IAPs indicates inhibitor of apoptosis proteins.
Fig. 2.
Receptor-mediated mitochondrial pathways elicited by Aβ on cerebral microvascular endothelial cells. A) Phase contrast microscopy illustrates apoptotic changes in E22Q-challenged (Q22) microvascular endothelial cells compared to untreated control cells (Ctrl). B) Anexin V immunofluorescence signal highlights phosphatidylserine translocation to the outer layer of the cell membrane, indicative of apoptotic processes. C) Caspase-3 activation is illustrated by the appearance of cleaved caspase-3 bands in E22Q-treated cells as assessed by western blot analysis. D) Immunofluorescence deconvolution microscopy evaluation of cytochrome c subcellular localization depicts the mitochondrial punctuated association of the protein in control cells and its release from the organelle to the cytoplasm in E22Q-treated cells (Q22). E) Nucleosome formation/DNA fragmentation evaluated by cell death ELISA in presence/absence of the pancaspase inhibitor ZVAD, and specific inhibitors of caspase-8 (ZIETD) and caspase-9 (ZLEHD) demonstrates the caspase-dependency of Aβ-induced cell death mechanisms. F) Time-course assessment illustrates the activation of caspase-8 and caspase-9 after only few hours of peptide challenge and suggests a slower kinetics or lower intensity for caspase-9, highlighting the early engagement of receptor-mediated pathways. G) Confocal microscopy evaluation of DR4 upregulation and colocalization with AβE22Q (Q22) on the cell membrane in endothelial cells challenged with the variant peptide depicts the engagement of the TRAIL DR4 receptor. Insets illustrate the respective fluorescence signals in control untreated cells. Green fluorescence: DR4 staining; red fluorescence: Aβ signal; yellow fluoresence indicates merge of both signals. H) Silencing DR4 (siDR4) and DR5 (siDR5) results in inhibition of caspase-8 activation. siNC illustrates endothelial cells transfected with non-silencing siRNA controls. I) Silencing DR4 (siDR4), DR5 (siDR5), or both receptors (siDR4/5) results in inhibition of apoptosis induced by incubation with Q22 and evaluated by cell death ELISA. Silencing of the receptors had no effect on untreated control cells. Transfection with non-silencing siRNA controls (siNC) had no effect per se on the cell viability or on the apoptotic effect of the Aβ peptide.
The initiation of apoptosis in endothelial cells challenged with the CAA-associated AβE22Q is evidenced by the abnormal cell morphology with evidence of cell shrinking, and appearance of apoptotic bodies in phase-contrast microscopy, as well by phosphatidylserine translocation to the outer layer of the cell membrane, indicated by annexin V staining, clear signs of the initiation of apoptosis (Fig. 2A, B). The mitochondrial dysfunction induced by oligomeric Aβ resulted in cytochrome c release to the cytoplasm (Fig. 2D), an early stage in the apoptotic cascade, and involved downstream activation of terminal caspase 3, depicted in Fig. 2C by the appearance of the 17–20 kDa cleaved caspase-3 bands. Time-course evaluation demonstrated early activation of both caspase-8 and caspase-9 confirming the engagement of mitochondrial pathways and suggesting the upstream involvement of cell death receptors. The activation of both caspases, with caspase-8 either preceding or exhibiting a faster kinetics than caspase-9, also took place with oligomeric assemblies of the less aggregation prone wild-type Aβ40 (Fig. 2F), indicating that, once a certain degree of oligomerization is achieved, these structures elicit common cellular pathways. More detailed studies unveiled a key role for the TRAIL (TNF-related-apoptosis-inducing-ligand) death receptors DR4 and DR5 in the mitochondrial dysfunction elicited by Aβ, with upregulation of both receptors and their colocalization with Aβ on the plasma membrane (Fig. 2G). Direct binding assays using receptor chimeras confirmed the specific interaction of oligomeric Aβ with DR4 and DR5 [40] pointing out to a novel mechanism by which brain cells—through the engagement of the receptors upon binding to Aβ oligomers—become susceptible to DR4- and DR5-mediated apoptosis, in absence of the canonical ligand of the receptors, TRAIL, which is not constitutively expressed in the brain, and it is only released under conditions of neuroinflammation [40, 41]. Prevention of caspase 8 activation and apoptosis protection achieved through RNA silencing of both DR4 and DR5 receptors (Fig. 2H and I, respectively) validated their crucial role in the initiation of downstream apoptotic pathways.
DR4 and DR5 are members of the death receptor family which include Fas (also known as death receptor 2, CD95, and APO-1) and TNFR1 (tumor necrosis factor receptor 1) as well as other members of the TNF death receptor group, DR3, DR6 and p75/NGFR (nerve growth factor receptor). Notably, albeit only recently reported in cerebral microvascular cells, death receptors of the TNFR family have previously been implicated in Aβ-mediated responses in neuronal cells. Both the p75 neurotrophin receptor and TNF-α receptors mediated Aβ42-induced neuritic dystrophy and neuronal death [42, 43]. Supporting a mechanistic involvement of apoptotic extrinsic pathways in AD pathogenesis, both Aβ and AβPP were shown to activate neuronal death receptor signaling through direct binding to p75, DR6, or a complex of both receptors [44–46]. In AD cases, many of the reported apoptosis-associated genes transcriptionally upregulated are active participants in the mitochondrial cascade, with elevated levels of Bax and Bak coexisting with downregulated expression of the anti-apoptotic Bcl-2 family members in brains of affected individuals [32, 47]. Active forms of effector caspases were found co-localizing with neurofibrillary tangles, senile plaques, and dystrophic neurites [32, 33] while mRNA evaluation corroborated the upregulation in AD temporal cortex of caspase-3 and -7, as well as of the death receptor-related caspase-8 [48], in agreement with findings in cerebrovascular cells [13, 37, 40]. The involvement of death receptors on the oligomeric Aβ-induced apoptosis in brain vascular cells pinpoints to DR4 and DR5 as major players in CAA-related dysfunction and suggests that analogous cellular pathways may be elicited in different cell populations by common structural amyloid assemblies.
CEREBROVASCULAR AMYLOID AND INFLAMMATION-MEDIATED PATHWAYS
Compelling evidence continues to accumulate for a significant role of local inflammatory processes in the progression of AD [49]. In this sense, complement activation and its proinflammatory consequences have been demonstrated to contribute extensively to disease pathogenesis [50], and inflammation-related cytokines are considered today a driving force in the neuropathological cascade associated with AD [49, 51]. Complement activation products co-localize with cerebral parenchymal and vascular deposits in AD and non-Aβ amyloidosis thereby indicating that the chronic inflammatory response, most likely initiated by the deposits, is probably a general phenomenon [49, 52–55]. These deposit-associated components originate from direct activation of the system by Aβ as well as by non-Aβ amyloid peptides and, once generated, seem to participate in several key steps of amyloidogenesis including aggregation, microglial activation and phagocytosis [52, 53, 56–60]. In addition, to those related to activation of the complement system, the presence of other markers of inflammation in AD brains has been amply documented by numerous studies [61, 62]. Elevated cytokines and chemokines as well as accumulation of activated cytokine-expressing microglia are found in or near the pathologic lesions not only in AD [49, 63] but also in other non-Aβ neurodegenerative disorders [51, 64]. Notably, in AD the increased numbers of clustered activated cells appear to correlate with the progression of dementia at early stages of the disease in which tau pathology is moderate [65, 66].
Most studies related to inflammation-driven mechanisms in neurodegeneration focus on the role of microglial cells, while more limited information is available for the contribution of vessel wall cells, in spite of the potent inflammatory potential of both endothelial cells and smooth muscle cells. Both cells are intricately involved in inflammation, capable of secreting a wide array of mediators ranging from pro-inflammatory and regulatory interleukins (e.g., IL-1, 6, 8, 10), colony-, granulocyte-, and macrophage-stimulating factors, and chemokines (monocyte chemotactic protein-1, RANTES), to important mediators in cytokine regulation such as TNF-α, and IFG-γ [67–69]. Because of their strategic location, endothelial cells in particular, are able to interact with other cells, in both the bloodstream and the vessel wall, while the vast surface area of the endothelium provides ample sites for cell-cell and cell-matrix interactions. On exposure to various environmental stimuli, endothelial cells undergo profound changes in gene expression and function participating in numerous inflammatory reactions. As a result, endothelial cells are both the target of cytokines secreted by other cells as well as the direct generators of a number of cytokines as a consequence of stress and other stimuli including hypoxia, oxidized lipoproteins, systemic/vascular infections, and vascular cell injury [67]. Among the factors affecting cerebral endothelial cells, exposure to Aβ has been shown to evoke an array of pro-inflammatory responses with elevated secretion of various cytokines including the pro-inflammatory mediators IL-1 and IL-6 [70].
A robust elevation in inflammatory mediators has been observed in the cerebral microcirculation in AD [71]. Compared to microvessels from age-matched controls, AD brain microvessels release significantly higher levels of a number of inflammatory factors including nitric oxide, thrombin, TNF-α, transforming growth factor-β, IL-1β, IL-6, IL-8, and matrix metalloproteinases [71–74]. The latter comprises a multifactorial family of proteins capable of affecting disease pathogenesis through various mechanisms including disruption of permeability barriers and regulation of the activity of factors indispensable for neuronal survival such as NGF [75]. In contrast to the detailed Aβ-mediated cell death pathways illustrated above, the specific mechanisms elicited by the diverse inflammatory mediators involved in CAA-related paths, remain to be clearly defined and are likely to differ depending on the specific inflammatory elements/pathways affected. One of the most studied downstream mechanisms is the IL-1 mediated path, a self-amplifying cycle capable of increasing neuropathological changes, neuronal stress, and neuroinflammation, in part through its effect on the synthesis of the precursors that originate the Aβ lesions and the neurofibrillary tangles [76]. This IL-1 mediated mechanism has been shown to elicit Aβ-induced neuronal apoptosis through activation of NFκB pathways [76, 77]. In spite of the absence of mechanistic information on cells composing the vessel wall, it is clear that the synergistic effect of glial activation, vascular amyloid deposition, and the pro-inflammatory nature of endothelial cells all combine together to create a complex self-activating vicious circle capable of disrupting basement membranes, increasing vessel leakage, and decreasing vessel contractibility, all elements that contribute to the amyloidogenesis process and lead to further dysfunction of the neurovascular unit.
CONCLUDING REMARKS
The mechanisms leading to amyloid deposition are highly complex and interlink an array of molecular pathways ultimately resulting in cell toxicity and death. Histopathologic, genetic, biochemical, and physicochemical studies, together with information obtained from transgenic animal models, strongly support the notion that abnormal aggregation/fibrillization and subsequent Aβ tissue accumulation are key players in AD pathogenesis. The presence of mutations affecting the mean hydrophobicity of proteins, the propensity to generate β-structures or those reducing the net charge of the molecule favor peptide aggregation from unfolded states which exist in dynamic equilibrium with folded structures [78]. The AβE22Q vasculotropic variant, exhibiting the loss of a negatively charged residue of critical relevance for the maintenance of intramolecular interactions, rapidly assembles in solution to form high molecular mass oligomeric/protofibrillar species with subsequent formation of the characteristic amyloid fibrils, thereby providing a unique paradigm to examine the role of amyloid in general, and CAA in particular, in the mechanisms of disease pathogenesis. New evidence highlights the contribution of these prefibrillar Aβ species to the initiation of cerebral microvascular cell dysfunction through the interaction with TRAIL DR4 and DR5 death receptors, and subsequent engagement of mitochondrial pathways. Through the induction of microvascular cell dysfunction and exacerbation of inflammation-related processes, Aβ undoubtedly plays a key role in altering the functionality of the neurovascular unit, a dynamic entity that modulates cerebral blood flow, influences the permeability properties of the blood-brain barrier, is indispensable for the maintenance of brain homeostasis, and ultimately responsible for normal neuronal function. By delineating the Aβ-elicited molecular mechanisms engaging downstream mitochondrial pathways, the new findings unveil novel targets for future pharmacological intervention in CAA.
Acknowledgments
This work was supported by National Institute of Health grants AG030539 and NS051715, the American Heart Association, and the Alzheimer’s Association.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=2162).
References
- 1.Dobson CM. Protein folding and its links with human disease. Biochem Soc Symp. 2001;68:1–26. [PubMed] [Google Scholar]
- 2.Rostagno A, Holton JL, Lashley T, Revesz T, Ghiso J. Cerebral amyloidosis: Amyloid subunits, mutants and phenotypes. Cell Mol Life Sci. 2010;67:581–600. doi: 10.1007/s00018-009-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–1995. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- 4.Temussi PA, Masino L, Pastore A. From Alzheimer to Huntington: Why is a structural understanding so difficult? EMBO J. 2003;22:355–361. doi: 10.1093/emboj/cdg044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovestone S, McLoughlin DM. Protein aggregates and dementia: Is there a common toxicity? J Neurol Neurosurg Psychiatry. 2002;72:152–161. doi: 10.1136/jnnp.72.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghiso J, Frangione B. Amyloidosis and Alzheimer’s disease. Adv Drug Delivery Rev. 2002;54:1539–1551. doi: 10.1016/s0169-409x(02)00149-7. [DOI] [PubMed] [Google Scholar]
- 7.Rostagno A, Lal R, Ghiso J. Protein misfolding, aggregation, and fibril formation: Common features of cerebral and non-cerebral amyloid diseases. In: Dawbarn D, Allen S, editors. The Neurobiology of Alzheimer’s disease. Oxford University Press; Oxford, United Kingdom: 2007. pp. 133–160. [Google Scholar]
- 8.Rostagno A, Vidal R, Kaplan B, Chuba J, Kumar A, Elliott JI, Frangione B, Gallo G, Ghiso J. pH-dependent fibrillogenesis of a VkIII Bence Jones protein. Br J Haematology. 1999;107:835–843. doi: 10.1046/j.1365-2141.1999.01778.x. [DOI] [PubMed] [Google Scholar]
- 9.Caughey B, Lansbury PTJ. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 10.Walsh DM, Selkoe DJ. A beta oligomers – a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 11.Solito R, Corti F, Fossati S, Mezhericher E, Donnini S, Ghiso J, Giachetti A, Rostagno A, Ziche M. Dutch and Arctic mutant peptides of beta amyloid(1-40) differentially affect the FGF-2 pathway in brain endothelium. Exp Cell Res. 2009;315:385–395. doi: 10.1016/j.yexcr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viana RJ, Nunes AF, Castro RE, Ramalho RM, Meyerson J, Fossati S, Ghiso J, Rostagno A, Rodrigues CM. Tau-roursodeoxycholic acid prevents E22Q Alzheimer’s Abeta toxicity in human cerebral endothelial cells. Cell Mol Life Sci. 2009;66:1094–1104. doi: 10.1007/s00018-009-8746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fossati S, Cam J, Meyerson J, Mezhericher E, Romero IA, Couraud P-O, Weksler B, Ghiso J, Rostagno A. Differential activation of mitochondrial apoptotic pathways by vasculotropic amyloid-β variants in cells composing the cerebral vessel walls. FASEB J. 2010;24:229–241. doi: 10.1096/fj.09-139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fossati S, Todd K, Sotolongo K, Ghiso J, Rostagno A. Differential contribution of isoaspartate post-translational modifications to the fibrillization and toxic properties of amyloid β and the Asn23 Iowa mutation. Biochem J. 2013;456:347–360. doi: 10.1042/BJ20130652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Revesz T, Holton JL, Lashley T, Plant G, Frangione B, Rostagno A, Ghiso J. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 2009;118:115–130. doi: 10.1007/s00401-009-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frangione B, Revesz T, Vidal R, Holton J, Lashley T, Houlden H, Wood N, Rostagno A, Plant G, Ghiso J. Familial cerebral amyloid angiopathy related to stroke and dementia. Amyloid. 2001;8(Suppl 1):36–42. [PubMed] [Google Scholar]
- 17.Auriel E, Greenberg SM. The pathophysiology and clinical presentation of cerebral amyloid angiopathy. Curr Atheroscler Rep. 2012;14:343–350. doi: 10.1007/s11883-012-0254-z. [DOI] [PubMed] [Google Scholar]
- 18.Attems J, Jellinger K, Thal DR, Van Nostrand W. Review: Sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2011;37:75–93. doi: 10.1111/j.1365-2990.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 19.Sagare AP, Bell RD, Zlokovic BV. Neurovascular defects and faulty amyloid-β vascular clearance in Alzheimer’s disease. J Alzheimers Dis. 2013;33:S87–S100. doi: 10.3233/JAD-2012-129037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang-Nunes SX, Maat-Schieman M, van Duinen S, Roos R, Frosch MP, Greenberg SM. The cerebral β-amyloid angiopathies: Hereditary and sporadic. Brain Pathol. 2006;16:30–39. doi: 10.1111/j.1750-3639.2006.tb00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selkoe DJ. Alzheimer’s disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004457. pii: a004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada M, Naiki H. Cerebral amyloid angiopathy. Prog Mol Biol Transl Sci. 2012;107:41–78. doi: 10.1016/B978-0-12-385883-2.00006-0. [DOI] [PubMed] [Google Scholar]
- 24.van Nostrand WE, Melchor JP, Cho HS, Greenberg SM, Rebeck GW. Pathogenic effects of D23N Iowa mutant amyloid β-protein. J Biol Chem. 2001;276:32860–32866. doi: 10.1074/jbc.M104135200. [DOI] [PubMed] [Google Scholar]
- 25.Davis JB, van Nostrand WE. Enhanced pathologic properties of Dutch-type mutant amyloid beta-protein. Proc Natl Acad Sci U S A. 1996;93:2996–3000. doi: 10.1073/pnas.93.7.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy E, Carman MD, Fernandez Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Frangione B. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 27.Maat-Schieman M, Yamaguchi H, van Duinen S, Natte R, Roos RA. Age-related plaque morphology and C-terminal heterogeneity of amyloid β in Dutch-type hereditary cerebral hemorrhage with amyloidosis. Acta Neuropathol. 2000;99:409–419. doi: 10.1007/s004010051143. [DOI] [PubMed] [Google Scholar]
- 28.Maat-Schieman M, Roos R, van Duinen S. Hereditary cerebral hemorrhage with amyloidosis-Dutch type. Neuropathology. 2005;25:288–297. doi: 10.1111/j.1440-1789.2005.00631.x. [DOI] [PubMed] [Google Scholar]
- 29.Monro OR, Mackic JB, Yamada S, Segal MB, Ghiso J, Maurer C, Calero M, Frangione B, Zlokovic B. Substitution at codon 22 reduces clearance of Alzheimer’s amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol Aging. 2002;23:405–412. doi: 10.1016/s0197-4580(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 30.Melchor JP, van Nostrand WE. Charge alterations of E22 enhance the pathogenic properties of the amyloid beta-protein. J Biol Chem. 2000;275:9782–9791. doi: 10.1046/j.1471-4159.2000.0742209.x. [DOI] [PubMed] [Google Scholar]
- 31.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 33.Cribbs DH, Poon WW, Rissman RA, Blurton-Jones M. Caspase-mediated degeneration in Alzheimer’s disease. Am J Pathol. 2004;165:353–355. doi: 10.1016/S0002-9440(10)63302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takuma K, Yan S-D, Stern D, Yamada K. Mitochondrial dysfunction, endoplasmic reticulum stress, and apoptosis in Alzheimer’s disease. J Pharmacol Sci. 2005;97:312–316. doi: 10.1254/jphs.cpj04006x. [DOI] [PubMed] [Google Scholar]
- 35.Folin M, Baiguera S, Fioravanzo L, Conconi MT, Grandi C, Nussdorfer GG, Parnigotto PP. Caspase-8 activation and oxidative stress are involved in the cytotoxic effect of beta-amyloid on rat brain microvascular endothelial cells. Int J Mol Med. 2006;17:431–435. [PubMed] [Google Scholar]
- 36.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death in disease: Mechanisms and emerging therapeutic concepts. N Engl J Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fossati S, Ghiso J, Rostagno A. Insights into caspase-mediated apoptotic pathways induced by amyloid-β in cerebral microvascular endothelial cells. Neurodegener Dis. 2012;10:324–328. doi: 10.1159/000332821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomidokoro Y, Rostagno A, Neubert TA, Lu Y, Rebeck GW, Frangione B, Greenberg SM, Ghiso J. Iowa variant of familial Alzheimer’s disease: Accumulation of posttranslationally modified AbD23N in parenchymal and cerebrovascular amyloid deposits. Am J Pathol. 2010;176:1841–1854. doi: 10.2353/ajpath.2010.090636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomidokoro Y, Lashley T, Rostagno A, Neubert TA, Bojsen-Moller M, Braendgaard H, Plant G, Holton J, Frangione B, Revesz T, Ghiso J. Familial Danish dementia: Coexistence of ADan and Aβ amyloid subunits in the absence of compact plaques. J Biol Chem. 2005;280:36883–36894. doi: 10.1074/jbc.M504038200. [DOI] [PubMed] [Google Scholar]
- 40.Fossati S, Ghiso J, Rostagno A. TRAIL death receptors DR4 and DR5 mediate cerebral microvascular endothelial cell apoptosis induced by oligomeric Alzheimer’s Aβ. Cell Death Dis. 2012;3:e321. doi: 10.1038/cddis.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorr J, Bechmann I, Waiczies S, Aktas O, Walczak H, Krammer PH, Nitsch R, Zipp F. Lack of tumor necrosis factor-related apoptosis-inducing ligand but presence of its receptors in the human brain. J Neurosci. 2002;22:RC209. doi: 10.1523/JNEUROSCI.22-04-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knowles JK, Rajadas J, Nguyen TV, Yang T, LeMieux MC, Vander Griend L, Ishikawa C, Massa SM, Wyss-Coray T, Longo FM. The p75 neurotrophin receptor promotes amyloid-beta(1-42)-induced neuritic dystrophy in vitro and in vivo. J Neurosci. 2009;29:10627–10637. doi: 10.1523/JNEUROSCI.0620-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sotthibundhu A, Sykes AM, Fox B, Underwood CK, Thangnipon W, Coulson EJ. Beta-amyloid(1-42) induces neuronal death through the p75 neurotrophin receptor. J Neurosci. 2008;28:3941–3946. doi: 10.1523/JNEUROSCI.0350-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Yaar M, Zhai S, Fine RE, Eisenhauer PB, Arble BL, Stewart KB, Gilchrest BA. Amyloid beta binds trimers as well as monomers of the 75-kDa neurotrophin receptor and activates receptor signaling. J Biol Chem. 2002;277:7720–7725. doi: 10.1074/jbc.M110929200. [DOI] [PubMed] [Google Scholar]
- 46.Hu Y, Lee X, Shao Z, Apicco D, Huang G, Gong BJ, Pepinsky RB, Mi S. A DR6/p75NTR complex is responsible for β-amyloid-induced cortical neuron death. Cell Death Dis. 2013;4:e579. doi: 10.1038/cddis.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sajan FD, Martiniuk F, Marcus DL, Frey WH, Hite R, Bordayo EZ, Freedman ML. Apoptotic gene expression in Alzheimer’s disease hippocampal tissue. Am J Alzheimer Dis Other Dement. 2007;22:319–328. doi: 10.1177/1533317507302447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsui T, Ramasamy K, Ingelsson M, Fukumoto H, Conrad C, Frosch MP, Irizarry M, Yuan J, Hyman BT. Coordinated expression of caspase8, 3 and 7 mRNA in temporal cortex of Alzheimer disease: Relationship to formic acid extractable Aβ levels. J Neuropathol Exp Neurol. 2006;65:508–515. doi: 10.1097/01.jnen.0000229238.05748.12. [DOI] [PubMed] [Google Scholar]
- 49.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling MR, Fiebich BL, Finch C, Frautschy S, Griffin WST, Hampel H, Hull M, Landreth G, Lue L-F, Mrak RE, Mackenzie IR, McGeer EG, O’Banion MK, Pachter J, Pasinetti GM, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyama I, Van Muiswinkel FL, Veerhuis R, Walker DG, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tenner AJ. Complement in Alzheimer’s disease: Opportunities for modulating protective and pathogenic events. Neurobiol Aging. 2001;22:849–861. doi: 10.1016/s0197-4580(01)00301-3. [DOI] [PubMed] [Google Scholar]
- 51.Griffin WST, Sheng JG, Royston MC, Gentleman SM, McKenzie J, Graham DI, Roberts GW, Mrak RE. Glial-neuronal interactions in Alzheimer’s disease: The potential role of a “cytokine cycle” in disease progression. Brain Pathol. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emmerling MR, Watson MD, Raby CA, Spiegel K. The role of complement in Alzheimer’s disease pathology. Biochim Biophys Acta. 2000;1502:158–171. doi: 10.1016/s0925-4439(00)00042-9. [DOI] [PubMed] [Google Scholar]
- 53.Rostagno A, Revesz T, Lashley T, Tomidokoro Y, Magnotti L, Braendgaard H, Bojsen-Moller M, Holton J, Frangione B, Ghiso J. Complement activation in chromosome 13 dementias: Similarities with Alzheimer’s disease. J Biol Chem. 2003;277:49782–49790. doi: 10.1074/jbc.M206448200. [DOI] [PubMed] [Google Scholar]
- 54.Iseki E, Marui W, Akiyama H, Ueda K, Kosaka K. Degeneration process of Lewy bodies in the brains of patients with dementia with Lewy bodies using a-synuclein-immunohistochemistry. Neurosci Lett. 2000;286:69–73. doi: 10.1016/s0304-3940(00)01090-9. [DOI] [PubMed] [Google Scholar]
- 55.Ishii T, Haga S, Yagishita S, Tateishi J. The presence of complement in amyloid plaques of Creutzfeldt-Jakob disease and Gerstmann-Straussler-Scheinker disease. Appl Pathol. 1984;2:370–379. [PubMed] [Google Scholar]
- 56.Bradt B, Kolb WP, Cooper NR. Complement-dependent proinflammatory properties of the Alzheimer’s disease β-peptide. J Exp Med. 1998;188:431–438. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang H, Burdick D, Glabe CG, Cotman CW, Tenner AJ. β amyloid activates complement by binding to a specific region of the collagen-like domain of the C1qA chain. J Immunol. 1994;152:5050–5059. [PubMed] [Google Scholar]
- 58.Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, Styren SD, Civin WH, Brachova L, Bradt B, Ward P, Lieberburg I. Complement activation by β-amyloid in Alzheimer’s disease. Proc Natl Acad Sci U S A. 1992;89:10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Webster S, O’Barr S, Rogers J. Enhanced aggregation and β structure of amyloid β peptide after coincubation with C1q. J Neurosci Res. 1994;39:448–456. doi: 10.1002/jnr.490390412. [DOI] [PubMed] [Google Scholar]
- 60.Webster S, Yang AJ, Margol L, Garzon-Rodriguez W, Glabe CG, Tenner AJ. Complement component C1q modulates the phagocytosis of Aβ by microglia. Exp Neurol. 2000;161:127–138. doi: 10.1006/exnr.1999.7260. [DOI] [PubMed] [Google Scholar]
- 61.Cooper N, Bradt B, O’Barr S, Yu J. Focal inflammation in the brain: Role in Alzheimer’s disease. Immunol Res. 2000;21:159–165. doi: 10.1385/IR:21:2-3:159. [DOI] [PubMed] [Google Scholar]
- 62.Lee YJ, Han SB, Nam SY, Oh KW, Hong JT. Inflammation and Alzheimer’s disease. Arch Pharmacol Res. 2010;33:1539–1555. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- 63.Wyss-Coray T. Inflammation in Alzheimer disease: Driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 64.Ghiso J, Revesz T, Holton J, Rostagno A, Lashley T, Houlden H, Gibb G, Anderton B, Bek T, Bojsen-Moller M, Wood N, Vidal R, Braendgaard H, Plant G, Frangione B. Chromosome 13 dementia syndromes as models of neurodegeneration. Amyloid. 2001;8:277–284. doi: 10.3109/13506120108993826. [DOI] [PubMed] [Google Scholar]
- 65.Arends YM, Duyckaerts C, Rozemuller J, Eikelenboom P, Hauw JJ. Microglia, amyloid and dementia in Alzheimer’s disease. A correlative study. Neurobiol Aging. 2000;21:39–47. doi: 10.1016/s0197-4580(00)00094-4. [DOI] [PubMed] [Google Scholar]
- 66.Griffin WST, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer’s disease: Significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 67.Krishnaswamy G, Kelley J, Yerra L, Smith JK, Chi DS. Human endothelium as a source of multifunctional cytokines: Molecular regulation and possible role in human disease. J Interferon Cytokine Res. 1999;19:91–104. doi: 10.1089/107999099314234. [DOI] [PubMed] [Google Scholar]
- 68.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 69.von der Thussen JH, Kuiper J, van Berkel TJC, Biessen EA. Interleukins in atherosclerosis: Molecular pathways and therapeutic potential. Pharmacol Rev. 2003;55:133–166. doi: 10.1124/pr.55.1.5. [DOI] [PubMed] [Google Scholar]
- 70.Vukic V, Callaghan D, Walker D, Lue LF, Liu QY, Couraud PO, Romero IA, Weksler B, Stanimirovic DB, Zhang W. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer’s brain is mediated by the JNK-AP1 signaling pathway. Neurobiol Dis. 2009;34:95–106. doi: 10.1016/j.nbd.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: Implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation. 2011;8:26. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dorheim MA, Tracey WR, Pollock JS, Grammas P. Nitric oxide is elevated in Alzheimer’s brain microvessels. Biochem Biophys Res Commun. 1994;205:659–665. doi: 10.1006/bbrc.1994.2716. [DOI] [PubMed] [Google Scholar]
- 73.Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol Aging. 2001;22:837–842. doi: 10.1016/s0197-4580(01)00276-7. [DOI] [PubMed] [Google Scholar]
- 74.Grammas P, Ovase R. Cerebrovascular TGF-β contributes to inflammation in the Alzheimer’s brain. Am J Pathol. 2002;160:1583–1587. doi: 10.1016/s0002-9440(10)61105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Broussard GJ, Mytar J, Li R-C, Klapstein GJ. The role of inflammatory processes in Alzheimer’s disease. Inflammopharmacology. 2012;20:109–126. doi: 10.1007/s10787-012-0130-z. [DOI] [PubMed] [Google Scholar]
- 76.Wilcock DM, Griffin WST. Down’s syndrome, neuroinflammation, and Alzheimer neuropathogenesis. J Neuroinflammation. 2013;10:84. doi: 10.1186/1742-2094-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuner P, Schubenel R, Hertel C. β-Amyloid binds to p75NTR and activates NFkB in human neuroblastoma cells. J Neurosci Res. 1998;54:798–804. doi: 10.1002/(SICI)1097-4547(19981215)54:6<798::AID-JNR7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 78.Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature. 2003;424:805–808. doi: 10.1038/nature01891. [DOI] [PubMed] [Google Scholar]
- 79.Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, Westermark P. Amyloid fibril protein nomenclature: 2012 recommendations from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2012;19:167–170. doi: 10.3109/13506129.2012.734345. [DOI] [PubMed] [Google Scholar]
- 80.Di Fede G, Catania M, Morbin M, Rossi G, Suardi S, Mazzoleni G, Merlin M, Giovagnoli AR, Prioni S, Erbetta A, Falcone C, Gobbi M, Colombo L, Bastone A, Beeg M, Manzoni C, Francescucci B, Spagnoli A, Cantù L, Del Favero E, Levy E, Salmona M, Tagliavini F. A recessive mutation in the APP gene with dominant-negative effect on amyloidogenesis. Science. 2009;323:1473–1477. doi: 10.1126/science.1168979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Janssen JC, Beck JA, Campbell TA, Dickinson A, Fox NC, Harvey RJ, Houlden H, Rossor MN, Collinge J. Early onset familial Alzheimer’s disease. Mutation frequency in 31 families. Neurology. 2003;60:235–239. doi: 10.1212/01.wnl.0000042088.22694.e3. [DOI] [PubMed] [Google Scholar]
- 82.Wakutani Y, Watanabe K, Adachi Y, Wada-Isoe K, Urakami K, Ninomiya H, Saido TC, Hashimoto T, Iwatsubo T, Nakashima K. Novel amyloid precursor protein gene missense mutation (D678N) in probable familial Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1039–1042. doi: 10.1136/jnnp.2003.010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brouwers N, Sleegers K, Van Broeckhoven C. Molecular genetics of Alzheimer’s disease: An update. Ann Med. 2008;40:562–583. doi: 10.1080/07853890802186905. [DOI] [PubMed] [Google Scholar]
- 84.Hendriks L, van Duijn CM, Cras P, Cruts M, Van Hul W, van Harskamp F, Warren A, McInnis MG, Antonarakis SE, Martin JJ, Hofman A, Van Broeckhoven C. Presenile dementia and cerebral haemorrhage linked to a mutation at codon 692 of the beta-amyloid precursor protein gene. Nat Genet. 1992;1:218–221. doi: 10.1038/ng0692-218. [DOI] [PubMed] [Google Scholar]
- 85.Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, Naslund J, Lannfelt L. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat Neurosci. 2001;4:887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- 86.Miravalle L, Tokuda T, Chiarle R, Giaccone G, Bugiani O, Tagliavini F, Frangione B, Ghiso J. Substitution at codon 22 of Alzheimer’s Aβ peptide induce diverse conformational changes and apoptotic effects in human cerebral endothelial cells. J Biol Chem. 2000;275:27110–27116. doi: 10.1074/jbc.M003154200. [DOI] [PubMed] [Google Scholar]
- 87.Tomiyama T, Nagata T, Shimada H, Teraoka R, Fukushima A, Kanemitsu H, Takuma H, Kuwano R, Imagawa M, Ataka S, Wada Y, Yoshioka E, Nishizaki T, Watanabe Y, Mori H. A new amyloid beta variant favoring oligomerization in Alzheimer’s-type dementia. Ann Neurol. 2008;63:377–387. doi: 10.1002/ana.21321. [DOI] [PubMed] [Google Scholar]
- 88.Grabowski TJ, Cho HS, Vonsattel JPG, Rebeck GW, Greenberg SM. A novel APP mutation in an Iowa family with dementia and severe cerebral amyloid angiopathy. Ann Neurol. 2001;49:697–705. doi: 10.1002/ana.1009. [DOI] [PubMed] [Google Scholar]
- 89.Obici L, Demarchi A, de Rosa G, Bellotti V, Marciano S, Donadei S, Arbustini E, Palladini G, Diegoli M, Genovese E, Ferrari G, Coverlizza S, Merlini G. A novel AbetaPP mutation exclusively associated with cerebral amyloid angiopathy. Ann Neurol. 2005;58:639–644. doi: 10.1002/ana.20571. [DOI] [PubMed] [Google Scholar]
- 90.Rossi G, Giaccone G, Maletta R, Morbin M, Capobianco R, Mangieri M, Giovagnoli AR, Bizzi A, Tomaino C, Perri M, Di Natale M, Tagliavini F, Bugiani O, Bruni AC. A family with Alzheimer disease and strokes associated with A713T mutation of the APP gene. Neurology. 2004;63:910–912. doi: 10.1212/01.wnl.0000137048.80666.86. [DOI] [PubMed] [Google Scholar]