Abstract
Patients presenting for treatment of chronic pain often believe that pain reduction must be achieved before returning to normal functioning. However, treatment programs for chronic pain typically take a rehabilitative approach, emphasizing decreasing pain-related disability first with the expectation that pain reduction will follow. This information is routinely provided to patients, yet no studies have systematically examined the actual trajectories of pain and disability in a clinical care setting. In this study of youth with chronic pain (N = 94, 8 to 18 years), it was hypothesized that 1) functional disability and pain would decrease over the course of psychological treatment for chronic pain and 2) functional disability would decrease more quickly than pain intensity. Participants received cognitive behavioral therapy (CBT) for pain management (M = 5.6 sessions) plus standard medical care. The Functional Disability Inventory and a Numeric Rating Scale of average pain intensity were completed by the child at every CBT session. Hierarchical linear modeling was conducted to examine the longitudinal trajectories of disability and pain. Standardized estimates of the slopes of change were obtained to test differences in rates of change between pain and disability. Results showed an overall significant decline in functional disability over time. Although pain scores reduced slightly from pretreatment to posttreatment, the longitudinal decline over treatment was not statistically significant. As expected, the rate of change of disability was significantly more rapid than pain. Evidence for variability in treatment response was noted, suggesting the need for additional research into individual trajectories of change in pediatric pain treatment.
Keywords: Functional disability, Pain intensity, Pediatric chronic pain, Symptom trajectories
1. Introduction
Clinical experience and anecdotal reports suggest that some patients and families presenting for treatment of chronic pain initially believe that pain reduction should be achieved before increased functioning can be expected. In practice, outpatient treatments for chronic pain emphasize a rehabilitative approach including decreasing pain-related disability, and it has been a common dictum among clinicians that “improvement is first measured by increased functioning” [38, p. 262]. In research including clinical trials, pain intensity typically has been identified as the primary treatment outcome; however, there is an increasing focus on demonstrating functional improvement as well as pain reduction [17,35] as recommended by leading pediatric pain experts [23]. However, there is a challenge to providers in the clinic setting to assure patients that increased function in the absence of pain reduction is a positive sign, and that functional progress should not wait on pain resolution. The evidence to support this position is sparse.
Research has shown differential patterns of change in pain and functioning in response to behavioral intervention over time. In the adult pain literature, there is some support that functioning may improve before or in the absence of improvements in pain. For example, a case series of graded exercise exposure in 8 adults with complex regional pain syndrome demonstrated that fear of induced pain and pain-related functional disability improved before a decrease in pain intensity [8]. In another case series, 4 adults with chronic low back pain referred for outpatient rehabilitation showed reduced pain-related fear of movement and functional disability in the presence of only partial pain control [36]. Across the pain literature, different aspects of pain intensity and interference have been investigated, including daily variability in symptoms [31] as well as changes after intensive pediatric treatment programs [9,19,32]. However, none of these studies have addressed rates of changes between both pain and disability. Leading pediatric pain researchers and practitioners recognize a gap in the literature regarding whether it can be stipulated that painrelated functional disability truly improves before pain decreases, and on what grounds we can counsel patients to that extent [24]. This study serves as a preliminary step to address that gap using a dataset of 94 pediatric patients with chronic pain. It takes advantage of a systematic method of data collection via electronic medical record (EMR), which has become increasingly popular as medical institutions take advantage of available technology to provide data of high quality and consistency to aid in documenting and improving patient outcomes.
The primary aim of this study was to investigate longitudinal trajectories of change in pain-related functional disability and average pain intensity for patients completing a typical course of cognitive behavioral therapy (CBT) with standard medical care for chronic pain. It was hypothesized that both functional disability and average pain intensity would improve over time. Moreover, it was predicted that pain-related functional disability would improve at a faster rate than average pain intensity over the course of treatment.
2. Materials and methods
2.1. Participants
Participants were children and adolescents with chronic pain referred to a pediatric psychology clinic at a large Midwestern children’s hospital for behavioral pain management. Participants were included in the research study if they met the following criteria: 1) primary diagnosis of chronic pain (>3 months), 2) age between 8 and 18 years, 3) patient completion of a course of CBT for pain management (described in detail later) in 2012, and 4) outcome data collected for at least 3 sessions. Patients were typically referred from other subspecialty clinics (e.g., neurology, pain management, gastroenterology, rheumatology). Clinical data were obtained from the institution’s EMR using the aforementioned criteria. Institutional review board approval was obtained for electronic chart review and creation of a de-identified database.
2.2. Measures
2.2.1. Demographic information
For each participant, the following demographic information was collected from the EMR: sex, age, race/ethnicity, zip code (proxy variable for geographic distance from the hospital), pain diagnosis, and insurance type (private versus public as a proxy for economic status).
2.2.2. Functional disability
The Functional Disability Inventory (FDI) [37] is a 15-item self-report instrument assessing a child’s perception of her difficulty completing common, daily activities due to pain. It has been classified as a well-established measure, has good evidence of psychometric validity and reliability [5,27], and has limited clinician burden in terms of length, administration, scoring, and interpretation [27]. The FDI has been used in multiple pediatric pain populations including headache, abdominal pain, and generalized pain [5,14,18,21,22,29]. Items are scored on a 5-point Likert scale, ranging from 0 to 4 and categorized by descriptors “no trouble” to “impossible.” A total score is created by summing the items (range 0 to 60). Higher scores indicate greater functional disability. Clinical cut-off scores have been developed to represent no/minimal (≤12), moderate (13 to 29), and severe (≥30) levels of disability [14].
2.2.3. Average pain intensity
Average pain intensity was collected on a 0 to 10 numeric rating scales (NRS) based on the Brief Pain Inventory [6,34], a measure developed in part to provide information on the intensity of pain. Patients were asked to rate their average pain for the last week.
2.3. Procedure
The FDI and NRS were administered as clinical outcome measures as part of standard clinical care for every patient referred to a pediatric psychology clinic for CBT for pain management. Patient data were completed electronically (via tablets or kiosk) at each session and saved directly into the EMR. This process was repeated at every visit by the patient.
CBT is an empirically validated psychological intervention for treating pediatric chronic pain [10] and typically includes the following components: psychoeducation about pain perception, behavioral strategies for parents, relaxation training with or without the use of biofeedback technology, behavioral activation and activity pacing, self-monitoring of symptoms, and cognitive reframing techniques. Although treatment was not manualized, a clinician toolkit used across providers allowed for an organized approach to the instruction and delivery of treatment components. Active CBT was deemed complete based on mutual agreement by patient and provider that formal instruction of materials and coping techniques was finished (i.e., all standard components from the toolkit were taught) and/or the patient had made sufficient treatment gains. This was called end of active treatment and was designated as such by the treating clinician in the EMR. For some patients, end of active treatment was also the termination of psychological intervention. For other patients, maintenance/follow up sessions occurred to monitor symptoms and treatment concluded at a later end point. This was called termination of treatment, indicating that no further meetings with a treatment provider transpired. Thus, the number of sessions until termination may not be equivalent to the number of sessions in active treatment. For example, a patient might participate in 6 sessions of active CBT learning pain coping skills (session 6 is end of active treatment) and then return monthly 2 more times for symptom monitoring and problem solving before terminating psychological services (session 8 is termination of treatment). As such, session lengths varied and were tailored to meet the individualized needs of the patient taking into account age, distance from the clinic, and degree of functional impairment.
Patients also participated in standard medical care as prescribed. Although the EMR did not easily allow for consistent, quantifiable data about each patient’s medical plan, general summaries are provided for reference. For patients with headaches, common treatment plans include acute therapy (abortive medication plan), preventative medication, and behavioral/lifestyle modifications to reduce headache triggers [26]. Patients with chronic abdominal pain are frequently managed with a combination of dietary therapy (dietary restrictions, fiber supplementation, probiotics) and pharmacotherapy to address various symptoms such as visceral hyperalgesia, smooth muscle spasms, and gastric motility [3]. For joint-based and/or musculoskeletal-based pain complaints, treatment recommendations often include medication for symptom management of pain, muscle tension, and/or inflammation as well as aerobic exercise, strength training, and physical therapy [16].
2.4. Statistical analysis plan
Initially, 100 patients met inclusion criteria. Six patients were deemed outliers based on session length and psychiatric comorbidity (e.g., eating disorder, obsessive compulsive disorder). The final dataset contained 94 patients for analysis. All sessions through treatment termination were used in longitudinal data analysis to provide the most robust dataset for investigating changes in outcome variables over time.
For each selected patient, the following data were extracted from the EMR and imported into an Excel database: demographic data, FDI total score for every session, and NRS average pain intensity rating for every session. Data were de-identified, coded, cleaned (e.g., assessed for duplicate entries), and imported into SPSS version 20 [7] for preliminary analysis. Variables were examined for missing data to ensure completeness. When missing data could be obtained from other sources in the EMR (e.g., pain diagnosis at first visit based on the physician report), these data were used to supplement fields that were omitted. Descriptive analyses were conducted regarding patient and pain characteristics.
All further data analyses were conducted in Mplus version 7.11 [25]. For the FDI and NRS average pain intensity, 3.3% of data were missing for each measure, respectively. Missing data on these measures were handled in Mplus through maximum likelihood parameter estimation under a missing at random assumption (e.g., Enders [11]). It should be noted that the total number of sessions until termination of treatment for each patient varied (range 3 to 10 sessions), which constitutes unbalanced longitudinal data best analyzed by multilevel longitudinal modeling techniques rather than with structural equation longitudinal growth models [28]. Thus hierarchical linear modeling (HLM) was conducted separately for pain-related functional disability and average pain intensity to test the hypothesis that these variables changed over time and to investigate the nature of their trajectories.
Model building for both functional disability and pain intensity proceeded along 4 general steps. The first 3 steps involved properly modeling change over time (i.e., specifying the level 1 model); the final step involved the addition of control covariates (i.e., specifying the level 2 model). In the first step, an unconditional linear model was estimated to quantify average linear change (fixed effect: linear slope) over time. The second step tested for significant variation across participants in linear change (random effect: linear slope) across participants. The third step tested for significant nonlinear change over time (fixed effect: quadratic change), then tested for significant variation across participants in nonlinear change over time (random effect: quadratic change). Covariates were added to the model in the final step as control variables: sex, age, and insurance type. In a separate analysis, the same model building approach was taken to model the form of change in average pain intensity over time. Added fixed and random effect components were retained only if the model log likelihood statistic showed improvement (i.e., decreased) across successive models [28].
The value of the slope coefficients shows the expected rate of linear change per treatment session, with larger values reflecting greater rates of change. Comparison of slopes was necessary to test the hypothesis that functional disability improved at a more rapid rate than average pain intensity. However, slope comparisons between the 2 variables could not be done directly given their different unstandardized scales. Standardized estimates of the slopes for the FDI and NRS, as well as their respective standard errors (SE), were obtained to compute 95% confidence intervals (CI) around both slope estimates. Significant differences in rates of change between functional disability and pain intensity were tested by examining the CIs of both slopes. Nonoverlapping 95% CIs indicate statistically significant slope differences at P < .05.
3. Results
3.1. Participant characteristics
Participants were 94 children and adolescents with a mean age of 14.1 (SD = 2.8). Adolescents were predominantly Caucasian (N = 81, 86.2%) and female (N = 70, 74.5%). These patient characteristics are consistent across other studies in pediatric chronic pain [10,22,23]. A minority of families had public/state insurance, indicative of lower financial status (N = 11, 11.7%). Our sample seems consistent with those reported by studies from other pediatric pain centers with respect to parents typically completing higher levels of education, being employed, and employment characterized as skilled or semiskilled [4,13,17]. Families primarily lived locally in a large metropolitan area (N = 70, 74.5%), defined as residing in the county of the medical center or 6 surrounding counties.
3.2. Pain, functional disability, and treatment characteristics
Most common pain problems included headache (N = 45, 47.9%), abdominal pain (N = 19, 20.2%), joint(s) pain (N = 11, 11.7%), and other pain problems (N = 19, 20.2%). At initiation of treatment, patients reported moderate levels of pain (average pain intensity M = 4. 6, SD = 2.2, sample range 0 to 9), with a small subset of patients reporting very high (7 to 10 of 10) levels of average pain (N = 16, 17.0%). Patient-reported functional disability was in the moderate range (M = 15.9, SD = 10.5, sample range 0 to 48) [14], with 14 patients (14.9%) endorsing severe levels of functional disability (FDI 30 to 60). Over 90% of patients (91.5%) ended active CBT treatment in 3 to 7 sessions (M = 5.6, SD = 1.3, range = 3 to 9) (Table 1).
Table 1.
Number of treatment sessions in active treatment and until treatment termination.
| Variable | Percent | N |
|---|---|---|
| Number of sessions: end of active cognitive behavioral therapy | ||
| 3 | 5.3 | 5 |
| 4 | 14.9 | 14 |
| 5 | 21.3 | 20 |
| 6 | 34.0 | 32 |
| 7 | 16.0 | 15 |
| 8 | 6.4 | 6 |
| 9 | 2.1 | 2 |
| Number of sessions: treatment termination | ||
| 3 to 4 | 16.0 | 15 |
| 5 to 6 | 48.9 | 46 |
| 7 to 8 | 26.6 | 27 |
| 9 to 10 | 8.6 | 8 |
3.3. Hierarchical linear modeling
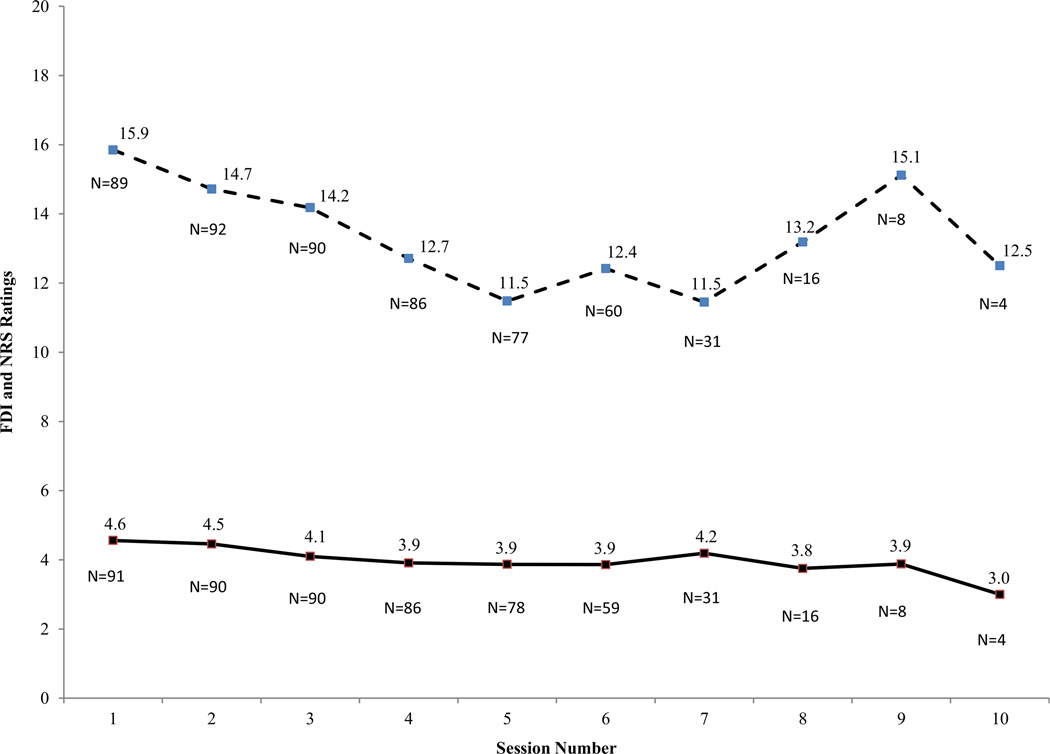
As shown in Table 2, in each step of model building, there was evidence of improved fit of the model to the sample data as shown by successively decreasing log likelihood values. Results showed that change in FDI over time is best described by a negative fixed effect linear slope (−1.11) and a positive quadratic fixed effect (0.01). This finding reflects an overall decline in functional disability over time by approximately 1 point per session. Specifically, change in functional disability is best described by a slightly (but significantly) nonlinear decrease over time (Fig. 1). A significant linear slope random effect (20.87, P < .01) and a significant quadratic random effect (0.28, P < .01) both indicated notable variation in longitudinal FDI changes across participants, meaning that although some patients’ functional disability was improving, others worsened or changed minimally. As shown in Table 3, change in average pain intensity over time across participants was best described with an unconditional (i.e., an intercept only) model. Despite an overall reduction in average pain scores over time (M = 4.6 at pretreatment to M = 3.4 at termination of treatment), no specific variation in linear slope, nonlinear trend, or nonlinear variation was present in average pain scores. (Models containing additional fixed and random effects did not improve the model log likelihood value.) This result indicates that pain intensity may be relatively slow to change in a brief course of CBT.
Table 2.
Model summary: functional disability inventory.
| Parameters | Fixed linear | Random linear | Fixed quadratic | Random quadratic | Covariates |
|---|---|---|---|---|---|
| Regression coefficients (fixed effects) | |||||
| Intercept | 16.74* | 16.98* | 16.35* | 16.92* | 18.62* |
| Time | −1.01* | −1.11* | −0.68† | −1.13 | −1.11 |
| Time2 | 0.06‡ | 0.01 | 0.01 | ||
| Age | 0.37 | ||||
| Insurance type | −0.76 | ||||
| Sex | −3.43 | ||||
| Variance components (random effects) | |||||
| Residual | 23.50* | 15.83* | 15.64* | 11.31* | 11.31* |
| Intercept | 90.76* | 120.92* | 120.52* | 123.43* | 122.21* |
| Linear slope | 1.77* | 1.85* | 20.83* | 20.87* | |
| Intercept/linear slope covariance | −7.20† | −7.22† | −21.07‡ | −22.83† | |
| Quadratic trend | 0.28* | 0.28* | |||
| Intercept/quadratic trend covariance | 1.47 | 1.72 | |||
| Linear slope/quadratic trend covariance | −2.33* | −2.33* | |||
| Model summary | |||||
| Log likelihood | −1804.77 | −1765.17 | −1764.35 | −1737.14 | −1734.92 |
| Number of estimated parameters | 4 | 6 | 7 | 10 | 13 |
P < .01.
P < .05.
P < .10.
Fig. 1.
FDI and NRS scores across sessions for patients (N = 94) completing CBT. Dashed line, FDI; Solid line, NRS of average pain intensity. FDI = functional disability inventory; NRS = Numeric rating scale.
Table 3.
Model summary: average pain intensity.
| Parameters | Fixed linear | Covariates |
|---|---|---|
| Regression coefficients (fixed effects) | ||
| Intercept | 3.78* | 2.92 |
| Time | −0.06 | −0.06 |
| Age | 0.21 | |
| Insurance type | 0.89 | |
| Sex | −0.54 | |
| Variance components (random effects) | ||
| Residual | 59.80† | 59.69† |
| Intercept | 2.97 | 2.61 |
| Linear slope | ||
| Intercept/linear slope covariance | ||
| Model summary | ||
| Log likelihood | −1938.08 | −1936.31 |
| Number of estimated parameters | 4 | 7 |
P < .01.
P < .05.
3.4. Slope comparisons between FDI and NRS average pain intensity
To test the hypothesis that functional disability improved at a more rapid rate than average pain intensity, standardized estimates of FDI and NRS slopes were obtained to compute 95% CIs (i.e., 95% CI = βLinearSlope ± 1.96 [SE]). Results showed that FDI and NRS CIs did not overlap (FDI: β = −0.49, SE = 0.11, 95% CI: −0.70 to −0.27; NRS: β = −0.02, SE = 0.10, 95% CI: −0.20 to 0.17). Thus, findings suggest that functional disability and pain intensity changed at different rates over time, and that functional disability was in fact changing at a more rapid pace (Fig. 1).
4. Discussion
To our knowledge, this is the first study to investigate the temporal changes of functional disability and average pain intensity systematically in a pediatric clinical sample. It provides empirical evidence to partially support what providers have anecdotally believed—that functional disability decreases independent of changes in pain intensity, although it remains unclear how and when pain reduction may occur. These results begin to explain a vital gap in the field of pediatric chronic pain research and clinical work. Strengths of this study include systematic data collection from the EMR, use of evidence-based outcome measures in clinical care, and multiple data points for each patient across treatment. This rich data set allowed for the use of statistical techniques that provided a more in-depth portrayal of the changes in functional disability and pain over time. Because data were collected in a real-world setting with broad eligibility criteria, patients who had variable presenting pain problems, pain severity, and pain-related disability were included, which increases the generalizability of the findings.
We were particularly interested in whether rates of change for functional disability and pain intensity differed over time, which in fact was shown by nonoverlapping CIs around the standardized slope estimates for the FDI and NRS. This result begins to support the long-held belief among providers that patients can have improvement in functional limitations independent of marked reductions in pain intensity. Notably, this sample was selected from patients with chronic pain who were participating in psychological interventions as part of multidisciplinary care. The goal of providers is to improve both functioning and pain, and psychological interventions specifically focus on techniques to enhance pain coping and decrease functional disability, with less explicit intervention for pain reduction solely (as might be associated with medical interventions). Thus, future studies should investigate whether trajectories might be different for patients pursuing only medication management and/or physical therapy.
Given our longitudinal data set, HLM was conducted to investigate patients’ symptom trajectories, which is a robust way to model change over time and augments results from other research studies that may conduct evaluations at only 3 or 4 time points (e.g., [17,30,35]). HLM showed overall statistically significant reductions in functional disability over time, in which scores from baseline to session 10 reduced a severity grade from moderate to no/minimal disability [14]. Despite this reduction in severity grade, the clinical significance of such a decline remains unclear. Notably, there were few patients contributing data at sessions 8 to 10 given that the typical patient completed active CBT within an average of 6 sessions and terminated altogether within 7 sessions. Thus, the significant positive quadratic fixed effect observed for functional disability may represent a subset of patients experiencing a different pattern of symptom improvement at later treatment sessions, which is hidden by broad-based analyses, or a subgroup of nonresponders requiring a more intensive level of treatment. Additional evidence for pain subgroups is suggested by the significant variability in linear slope for functional disability, meaning the model-estimated trajectories for patients in the sample varied considerably from one another in terms of positive or negative change.
HLM results for average pain intensity showed nonsignificant changes over the course of psychological treatment, which contrasts the reported efficacy of these interventions in reducing pain intensity [10]. However, our result is not necessarily incompatible with the existing evidence. During brief treatment, functioning improved without an increase in average pain intensity, suggesting some degree of successful pain control. Average pain intensity may be slow to improve, with meaningful changes in this outcome variable better reflected in the long-term follow-up inherent to the design of clinical trials (e.g., 6- or 12-month posttreatment evaluations) and not clinical practice.
Given the variability that is present within this heterogeneous sample, there may be unobserved but qualitatively different subgroups (pain subtypes) or other potential factors such as age, severity of baseline functional disability or pain ratings, and length of treatment (with longer courses of treatment indicative of slower treatment responders or patients who are more disabled) that need to be further examined. Interestingly, many of the reviews that cite reductions in pain intensity upon completion of psychological intervention are driven by pediatric headache studies [10]. A recent randomized clinical trial of CBT for functional abdominal pain also showed reductions in pain intensity and functional disability from baseline to posttreatment and at 6- and 12-month follow- up [35]. However, a clinical trial investigating the efficacy of CBT for adolescents with juvenile-onset fibromyalgia reported improvements in functional disability and depression but minimal reductions in pain intensity [17], suggesting that youth with generalized pain conditions may not see the same benefits in pain reduction. As a whole, baseline levels of functional disability in our sample were mild to moderate (approximately 80% of patients) despite moderate levels of pain intensity. How our sample compares with the pediatric pain literature remains somewhat unclear because other studies including clinical trials report varying levels of baseline disability in their samples. For example, samples of youth with abdominal pain and chronic daily headaches reported baseline FDI scores in the mild range [15,35], whereas other trials of abdominal pain [30] and fibromyalgia [17] showed moderate levels of functional disability at baseline. It is conceivable that the length of time until disability and/or pain improvements are noted may be dependent on baseline symptom severity, regardless of type of presenting pain problem.
Several additional patient and contextual factors may be implicated in the differential rates of change between functional disability and pain intensity. It is well established that impaired functioning is a consequence of multiple variables beyond pain levels; depression, catastrophizing, and family history of pain all can affect functional levels [22]. In the adult pain literature, a few case studies have shown a temporal relationship between functional disability and pain intensity, with noted reductions in functional disability before pain intensity in the context of pain-related anxiety [8]. It is plausible that kinesiophobia, or fear of movement, is a powerful influence on functional disability, above and beyond pain levels [1]. Thus, addressing and reducing kinesiophobia, fear of pain, and/or pain-related avoidance is the suspected process by which functional disability decreases without direct correlation to intensity of pain symptoms. Additionally, there is strong evidence that CBT improves mood [2], which is modestly correlated with pain and disability [12,15,18]. Although depressive symptoms are generally mild in pediatric pain populations [20], studies have shown mood improvements after treatment [17,35], although there is no direct evidence suggesting that reduction in depressive symptoms drives functional improvements [17].
Results should be interpreted in the context of this being a retrospective study from a convenience sample stemming from clinical work, which does not allow for comparison to a no-treatment group as is done in clinical trials. Because of the patient heterogeneity and the unavoidable limitations of EMR-based research, quantifying multidisciplinary care was difficult. All patients had some degree of medical treatment; however, the quantity, intensity, and length of treatment by medical and allied health services remain difficult to measure. For example, some patients may pursue additional care (i.e., physical therapy) at an outside institution, which would not be readily captured in our internal EMR. As such, designing a prospective study to investigate temporal changes would better identify trajectories for specific pain diagnoses and quantify patients’ medical plans. Additionally, the current sample size for any single pain diagnosis was too small to conduct the complex model-building processes undertaken in this project. Nevertheless, trends of improvement in functioning seem to match those recently reported in the literature for other chronic pain conditions [17,35]. Finally, there was a lack of available information to assess treatment expectations or objective measures of functional disability (e.g., school attendance data). Without formal evaluation of patient or provider expectancies at the initiation of services, it is unknown and worth future study whether any source of bias about final outcomes or purpose of treatment (reduction in pain, disability, or both) existed. It is possible that patients reported on symptom improvements in a manner based on their perception of their providers’ expectations [33] (i.e., being the “good patient”).
This was the first systematic evaluation of the relative rates of improvement of functional disability and pain in a mixed sample of pediatric patients with chronic pain. Functional disability showed significant and faster improvements over time compared with pain intensity, which did not change significantly in the course of treatment. The results have clinical utility to providers, whose patients often ask them to speculate about the course and timeline of recovery. Providers may begin to have more confidence in telling their patients that indeed improved functioning seems to be a promising outcome in and of itself during treatment of chronic pain. However, further research is needed to better understand differential trajectories of improvement and their predictors to help clinicians form specific prognoses and provide targeted education and expectations for patients and families.
Acknowledgements
The authors acknowledge our team of collaborators who assisted in making this study a success: Kimberly Barnett, BS, research coordinator, as well as staff from the Pain Management Center including Alexandra Szabova, MD, and John Rose, MD; advanced practice nurses Debbie Wolf, RN, MSN, Holly Stahlman, RN, MSN, CNP, and Tracy Rogers, RN, MSN, FNP; and nurses Mary Pat Burke, RN, BSN, and Michelle Tate, RN, BSN. This project was partially supported by National Institutes of Health K24 Midcareer Awards in Patient-Oriented Research to Susmita Kashikar-Zuck, PhD (K24 #AR056687).
Footnotes
Conflict of interest statement
The authors have no conflicts of interest related to this study.
References
- 1.Al-Obaidi SM, Nelson RM, Al-Awadhi S, Al-Shuwaie N. The role of anticipation and fear of pain in the persistence of avoidance behavior in patients with chronic low back pain. Spine (Phila Pa 1976) 2000;25:1126–1131. doi: 10.1097/00007632-200005010-00014. [DOI] [PubMed] [Google Scholar]
- 2.Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Chiou E, Nurko S. Management of functional abdominal pain and irritable bowel syndrome in children and adolescents. Exp Rev Gastroenterol Hepatol. 2010;4:293–304. doi: 10.1586/egh.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claar RL, Baber KF, Simons LE, Logan DE, Walker LS. Pain coping profiles in adolescents with chronic pain. PAIN®. 2008;140:368–375. doi: 10.1016/j.pain.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. PAIN®. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 7.IBM Corp. IBM SPSS Statistics for Windows, version 20.0. Armonk, NY: IBM Corp; Released 2011. [Google Scholar]
- 8.de Jong JR, Vlaeyen JW, Onghena P, Cuypers C, den Hollander M, Ruijgrok J. Reduction of pain-related fear in complex regional pain syndrome type I: the application of graded exposure in vivo. PAIN®. 2005;116:264–275. doi: 10.1016/j.pain.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Eccleston C, Malleson PN, Clinch J, Connell H, Sourbut C. Chronic pain in adolescents: evaluation of a programme of interdisciplinary cognitive behaviour therapy. Arch Dis Child. 2003;88:881–885. doi: 10.1136/adc.88.10.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eccleston C, Palermo TM, de C Williams AC, Lewandowski A, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2012;12:CD003968. doi: 10.1002/14651858.CD003968.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enders CK. Applied missing data analysis. New York: Guilford; 2010. [Google Scholar]
- 12.Gauntlett-Gilbert J, Eccleston C. Disability in adolescents with chronic pain: patterns and predictors across different domains of functioning. PAIN®. 2007;131:132–141. doi: 10.1016/j.pain.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Guite JW, Logan DE, McCue R, Sherry DD, Rose JB. Parental beliefs and worries regarding adolescent chronic pain. Clin J Pain. 2009;25:223–232. doi: 10.1097/AJP.0b013e31818a7467. [DOI] [PubMed] [Google Scholar]
- 14.Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, Palermo TM, Wilson AC. Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. PAIN®. 2011;152:1600–1607. doi: 10.1016/j.pain.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and functional disability in chronic pediatric pain. Clin J Pain. 2001;17:341–349. doi: 10.1097/00002508-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Kashikar-Zuck S, Ting TV. Juvenile fibromyalgia: current status of research and future developments. Nat Rev Rheumatol. 2014;10:89–96. doi: 10.1038/nrrheum.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, Passo MH, Schikler KN, Hashkes PJ, Spalding S, Lynch-Jordan AM, Banez G, Richards MM, Lovell DJ. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: a multisite, single-blind, randomized, controlled clinical trial. Arthritis Rheum. 2012;64:297–305. doi: 10.1002/art.30644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewandowski AS, Palermo TM, Peterson CC. Age-dependent relationships among pain, depressive symptoms, and functional disability in youth with recurrent headaches. Headache. 2006;46:656–662. doi: 10.1111/j.1526-4610.2006.00363.x. [DOI] [PubMed] [Google Scholar]
- 19.Logan DE, Carpino EA, Chiang G, Condon M, Firn E, Gaughan VJ, Hogan M, Leslie DS, Olson K, Sager S, Sethna N, Simons LE, Zurakowski D, Berde CB. A day-hospital approach to treatment of pediatric complex regional pain syndrome: initial functional outcomes. Clin J Pain. 2012;28:766–774. doi: 10.1097/AJP.0b013e3182457619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan DE, Claar RL, Guite JW, Kashikar-Zuck S, Lynch-Jordan A, Palermo TM, Wilson AC, Zhou C. Factor structure of the children’s depression inventory in a multisite sample of children and adolescents with chronic pain. J Pain. 2013;14:689–698. doi: 10.1016/j.jpain.2013.01.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logan DE, Scharff L. Relationships between family and parent characteristics and functional abilities in children with recurrent pain syndromes: an investigation of moderating effects on the pathway from pain to disability. J Pediatr Psychol. 2005;30:698–707. doi: 10.1093/jpepsy/jsj060. [DOI] [PubMed] [Google Scholar]
- 22.Lynch AM, Kashikar-Zuck S, Goldschneider KR, Jones BA. Psychosocial risks for disability in children with chronic back pain. J Pain. 2006;7:244–251. doi: 10.1016/j.jpain.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 23.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9:771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Morley S. Trial design of psychological treatments in chronic pain: what can we tell patients? In: McQuay HJ, Kalso E, Moore RA, editors. Systematic reviews in pain research: methodology refined. Seattle, WA: IASP; 2008. pp. 217–232. [Google Scholar]
- 25.Muthen MK, Muthen BO. Mplus user’s guide. Los Angeles, CA: Muthen & Muthen; 1998–2012. [Google Scholar]
- 26.O’Brien HL, Kabbouche MA, Hershey AD. Treating pediatric migraine: an expert opinion. Exp Opin Pharmacother. 2012;13:959–966. doi: 10.1517/14656566.2012.677434. [DOI] [PubMed] [Google Scholar]
- 27.Palermo TM, Long AC, Lewandowski AS, Drotar D, Quittner AL, Walker LS. Evidence-based assessment of health-related quality of life and functional impairment in pediatric psychology. J Pediatr Psychol. 2008;33:983–996. doi: 10.1093/jpepsy/jsn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 29.Reid GJ, Lang BA, McGrath PJ. Primary juvenile fibromyalgia: psychological adjustment, family functioning, coping, and functional disability. Arthritis Rheum. 1997;40:752–760. doi: 10.1002/art.1780400423. [DOI] [PubMed] [Google Scholar]
- 30.Robins PM, Smith SM, Glutting JJ, Bishop CT. A randomized controlled trial of a cognitive-behavioral family intervention for pediatric recurrent abdominal pain. J Pediatr Psychol. 2005;30:397–408. doi: 10.1093/jpepsy/jsi063. [DOI] [PubMed] [Google Scholar]
- 31.Schneider S, Junghaenel DU, Keefe FJ, Schwartz JE, Stone AA, Broderick JE. Individual differences in the day-to-day variability of pain, fatigue, and wellbeing in patients with rheumatic disease: associations with psychological variables. PAIN®. 2012;153:813–822. doi: 10.1016/j.pain.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simons LE, Sieberg CB, Pielech M, Conroy C, Logan DE. What does it take? Comparing intensive rehabilitation to outpatient treatment for children with significant pain-related disability. J Pediatr Psychol. 2013;38:213–223. doi: 10.1093/jpepsy/jss109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, Cleeland C, Dionne R, Farrar JT, Galer BS, Hewitt DJ, Jadad AR, Katz NP, Kramer LD, Manning DC, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robinson JP, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Witter J. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. PAIN®. 2003;106:337–345. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Turk DC, Dworkin RH, Revicki D, Harding G, Burke LB, Cella D, Cleeland CS, Cowan P, Farrar JT, Hertz S, Max MB, Rappaport BA. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. PAIN®. 2008;137:276–285. doi: 10.1016/j.pain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 35.van der Veek SMC, Derkx BHF, Benninga MA, Boer F, de Haan E. Cognitive behavior therapy for pediatric functional abdominal pain: a randomized controlled trial. Pediatr. 2013;132(5):e1163–e1172. doi: 10.1542/peds.2013-0242. [DOI] [PubMed] [Google Scholar]
- 36.Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. Graded exposure in vivo in the treatment of pain-related fear: a replicated single-case experimental design in four patients with chronic low back pain. Behav Res Ther. 2001;39:151–166. doi: 10.1016/s0005-7967(99)00174-6. [DOI] [PubMed] [Google Scholar]
- 37.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 38.Zeltzer LK, Schlank CB. Conquering your child’s chronic pain: a pediatrician’s guide for reclaiming a normal childhood. New York, NY: HarperCollins; 2005. [Google Scholar]