Abstract
When living organisms become sick as a result of a bacterial infection, a suite of brain-mediated responses occur, including fever, anorexia and sleepiness. Systemic administration of lipopolysaccharide (LPS), a common constituent of bacterial cell walls, increases body temperature and non-rapid eye movement (NREM) sleep in animals and induces the production of pro-inflammatory prostaglandins (PGs). Prostaglandin E2 (PGE2) is the principal mediator of fever, and both PGE2 and PGD2 regulate sleep–wake behavior. The extent to which PGE2 and PGD2 are involved in the effect of LPS on NREM sleep remains to be clarified. Therefore, we examined LPS-induced changes in body temperature and NREM sleep in mice with nervous system-specific knockouts (KO) for the PGE2 receptors, EP3 or EP4; in mice with total body KO of microsomal PGE synthase-1, or the PGD2 receptor DP; and in mice treated with the cyclooxygenase (COX) inhibitor meloxicam. We observed that LPS-induced NREM sleep was slightly attenuated in mice lacking EP4 receptors in the nervous system, but was not affected in any of the other KO mice or in mice pretreated with the COX inhibitor. These results suggest that the effect of LPS on NREM sleep is partially dependent on PGs and is likely mediated mainly by other pro-inflammatory substances. In addition, our data show that the main effect of LPS on body temperature is hypothermia in the absence of nervous system EP3 receptors or in the presence of a COX inhibitor.
Keywords: EEG, Delta power, Nestin-Cre, LoxP, Thromboxane A, Prostacyclin, Cytokine, Nonsteroidal anti-inflammatory drugs, Sleepiness, Fever
1. Introduction
During infection, it is critical for mammals to detect bacteria rapidly and mount a vigorous immune response. This response is mediated by a cascade of pro-inflammatory mediators, including a wide range of cytokines and prostaglandins (PGs). These mediators trigger an array of physiological responses, termed the acute phase reaction, among which is included “sickness behavior”: fever, malaise, increased pain sensitivity, changes in sleep–wake cycles, and feeding which are mediated by the central nervous system (CNS) (see Saper et al., 2012). Through these responses, PGs protect the integrity of the organism and improve survival.
PGs are formed from unsaturated fatty acids, primarily arachidonic acid, which are converted to cyclic endoperoxides (i.e. PGH2 derived from arachidonic acid) by cyclooxygenases (COXs). PGH2 constitutes an important branching point from which the stable PGs, including PGE2, PGD2 and PGF2α, as well as the more unstable thromboxane A2 (TXA2) and prostacyclin (PGI2) are formed by specific synthases. PGE2 is considered to be the most important link between the peripheral immune system and the brain. Produced by microsomal PGE synthase-1 (mPGES-1) at the boundaries between the bloodstream and brain tissue, PGE2 is thought to penetrate the blood-brain barrier and enter the CNS. PGE2 is, therefore, an ideal candidate to translate a peripheral immune signal into an acute phase response by modulating neural activity (Saper et al., 2012). PGE2 acts on neuronal receptors of the EP family in thermoregulation circuitry to trigger fever. Focal deletion of EP3 receptors in the median preoptic nucleus reduces the fever normally produced by systemic lipopolysaccharide (LPS) (Lazarus et al., 2007). Perfusion of an EP4 receptor agonist (ONO-AE1329) into the tuberomammillary nucleus (TMN), a wake-promoting area in the hypothalamus, induces wakefulness (Huang et al., 2003), but the role of EP3 receptors in sleep–wake regulation remains unknown. PGD2 is the major prostanoid produced in the CNS and functions as a neuromodulator of sleep–wake regulation and neuroinflammation (Eguchi et al., 1999; Mohri et al., 2006; Urade and Hayaishi, 2011). The somnogenic activity of PGD2 was discovered when PGD2 was microinjected in nanomolar amounts into rats (Ueno et al., 1982) and non-human primates (Onoe et al., 1988). It is now well accepted that PGD2 produced by lipocalin-type PGD synthase (Urade et al., 1985) acts through DP receptors (DPRs) (Kabashima and Narumiya, 2003) to promote sleep (Qu et al., 2006).
In rats and mice, low to moderate doses of intraperitoneal LPS reduce rapid eye movement (REM) sleep, a state characterized by dreaming, fast cortical activity and muscle paralysis, and increase non-REM (NREM) sleep, a state of slow cortical activity and low metabolism (Toth and Opp, 2001). It remains unknown whether increased NREM sleep during sickness is mediated by PGE2 or PGD2. By using nervous system (NS)-specific knockout (KO) mice for EP3 and EP4 receptors, total body KO mice for mPGES-1 and DPR and a COX inhibitor, we investigated the roles of PGE2 and PGD2 in LPS-mediated changes in body temperature and sleep.
2. Material and methods
2.1. Animals
All procedures followed National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center and Osaka Bioscience Institute. We used male EP3 flox mice (Lazarus et al., 2007), EP4 flox mice (Schneider et al., 2004), rat nestin (Nes)-Cre mice (Jackson Laboratory 003771), mPGES-1 KO mice (Trebino et al., 2003) and DPR KO mice (Matsuoka et al., 2000), weighing 20–30 g (10–20 weeks old). EP3 or EP4 flox/Nestin-Cre mice (129sv, C57BL/6 background) were maintained by mating homozygous EP3 or EP4 flox mice with Nestin-Cre mice. Littermate mice lacking the Nestin-Cre allele were used in control experiments. mPGES-1 KO mice and DPR KO mice were backcrossed to the C57BL/6 strain for more than 10 generations. Mice were housed at an ambient temperature of approximately 22°C on a 12:12 light/dark cycle (lights on at 07:00) with ad libitum access to food and water.
2.2. Surgeries, drug injections and recording of sleep
We anesthetized mice with ketamine/xylazine (100 and 10 mg/kg, i.p.) and implanted them with epidural screw electrodes for recording of the electroencephalogram (EEG), and fine, stainless steel wires in the neck extensor muscles to record the electromyogram (EMG) as described previously (Oishi et al., 2013). In addition, we placed a telemetric temperature transmitter (TA10TA-F20; Data Sciences International, St. Paul, MN) in the peritoneal cavity. After 7 days recovery, mice were habituated to the EEG/EMG recording cables for 3 days before the first recording day. Thirty minutes before dark onset, we injected mice with intraperitoneal saline or 15 µg LPS (Escherichia. coli 055:B5, Sigma–Aldrich, St. Louis, MO). For the COX inhibitor experiments, we injected saline or 10 mg/kg meloxicam (Metacam, Boehringer Ingelheim, St. Joseph, MO) 1 h before dark onset, followed by saline or 15 µg LPS at 30 min before dark onset. Each mouse received only one LPS injection to avoid responses being influenced by LPS tolerance. The EEG/EMG signals were amplified, filtered (EEG, 0.5–30 Hz; EMG, 5–50 Hz), digitized at a sampling rate of 128 Hz, and recorded with SleepSign software (Kissei Comtec). We used SleepSign for the preliminary scoring of wakefulness, REM sleep, and NREM sleep in 10-s epochs and then examined all epochs and made corrections when necessary.
2.3. Statistical analysis
All data are expressed as the mean ± SEM. Statistical analyses were carried out using GraphPad Prism software (GraphPad). We compared groups using paired or unpaired two-tailed Student’s t tests or using mixed model ANOVA. In all cases, p < 0.05 was taken as the level of significance.
3. Results
To examine the effect of EP3 deficiency on sleep under baseline conditions and after LPS administration, we crossed conditional EP3 flox mice with mice expressing Cre recombinase under the Nes promoter, which is expressed selectively in neuronal and glial-cell precursors (Tronche et al., 1999), to generate NS-specific KO mice for the EP3 receptor (EP3 flox/Nes-Cre mice) as previously described (Lazarus et al., 2007). We recorded EEG and EMG in the EP3 flox/Nes-Cre mice and their EP3 flox littermates (control mice) and classified vigilance states offline into three stages: waking and REM and NREM sleep.
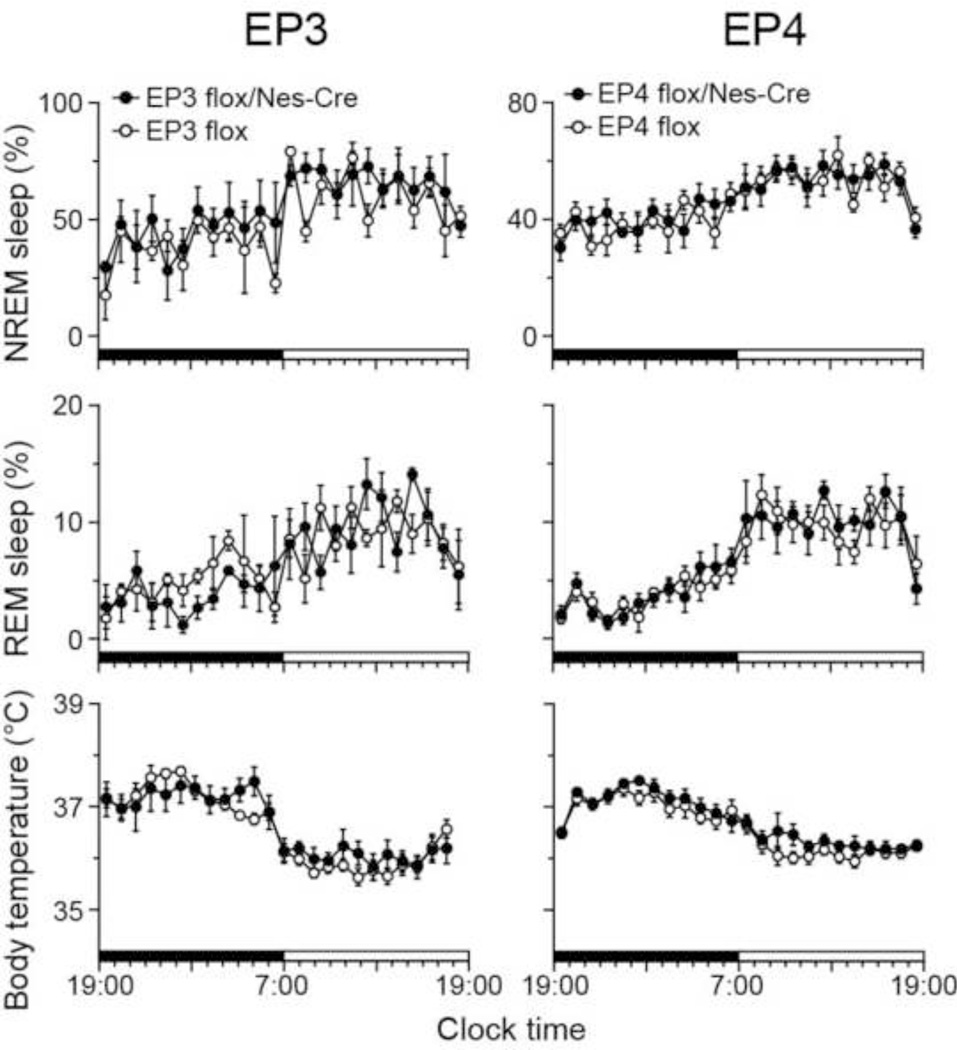
Under baseline condition, we found a clear circadian sleep–wake rhythm, with more sleep in the light period than the dark period, in both EP3 flox/Nes-Cre and EP3 flox mice, and no significant difference for NREM and REM sleep amounts between EP3 flox/Nes-Cre and EP3 flox mice, as assessed by mixed model ANOVA (genotype effect: independent; time effect: repeated; F(1,4) = 1.14, p = 0.35 for NREM sleep; F(1,4) = 0.064, p = 0.81 for REM sleep; Fig. 1). Body temperature of both EP3 flox/Nes-Cre and EP3 flox mice also showed a circadian rhythm with higher body temperature during the dark period, but no significant difference between EP3 flox/Nes-Cre and EP3 flox mice, as assessed by mixed model ANOVA (genotype effect: independent; time effect: repeated; F(1,7) = 0.132, p = 0.73).
Fig. 1.
Sleep profile and body temperature of mice deficient for EP3 (EP3 flox/Nes-Cre) and EP4 (EP4 flox/Nes-Cre) receptors in the NS and their littermates (EP3 flox and EP4 flox, respectively) under baseline conditions. Data are presented as the mean ± SEM (n = 3–5, left panel) or (n = 6–7, right panel). Black and white bars above the x-axes indicate the dark and light periods.
Because whole body EP4 KO mice die immediately after birth from failure of the ductus arteriosus to close (Nguyen et al., 1997; Segi et al., 1998), we also crossed EP4 flox mice with Nes-Cre mice to generate NS-specific EP4 KO mice (EP4 flox/Nes-Cre mice). Both EP4 flox/Nes-Cre mice and their littermate EP4 flox mice (control mice) showed clear circadian rhythms of sleep–wake states and body temperature with no significant differences between the two groups for NREM and REM sleep amounts and body temperature, as assessed by mixed model ANOVA (genotype effect: independent; time effect: repeated; NREM sleep: F(1,12) = 0.172, p = 0.69; REM sleep: F(1,12) = 0.150, p = 0.71; body temperature: F(1,11) = 0.974, p = 0.34; Fig. 1). These results suggest that EP3 or EP4 deletion in the central and peripheral nervous system does not affect the amounts of NREM and REM sleep or body temperature under baseline conditions.
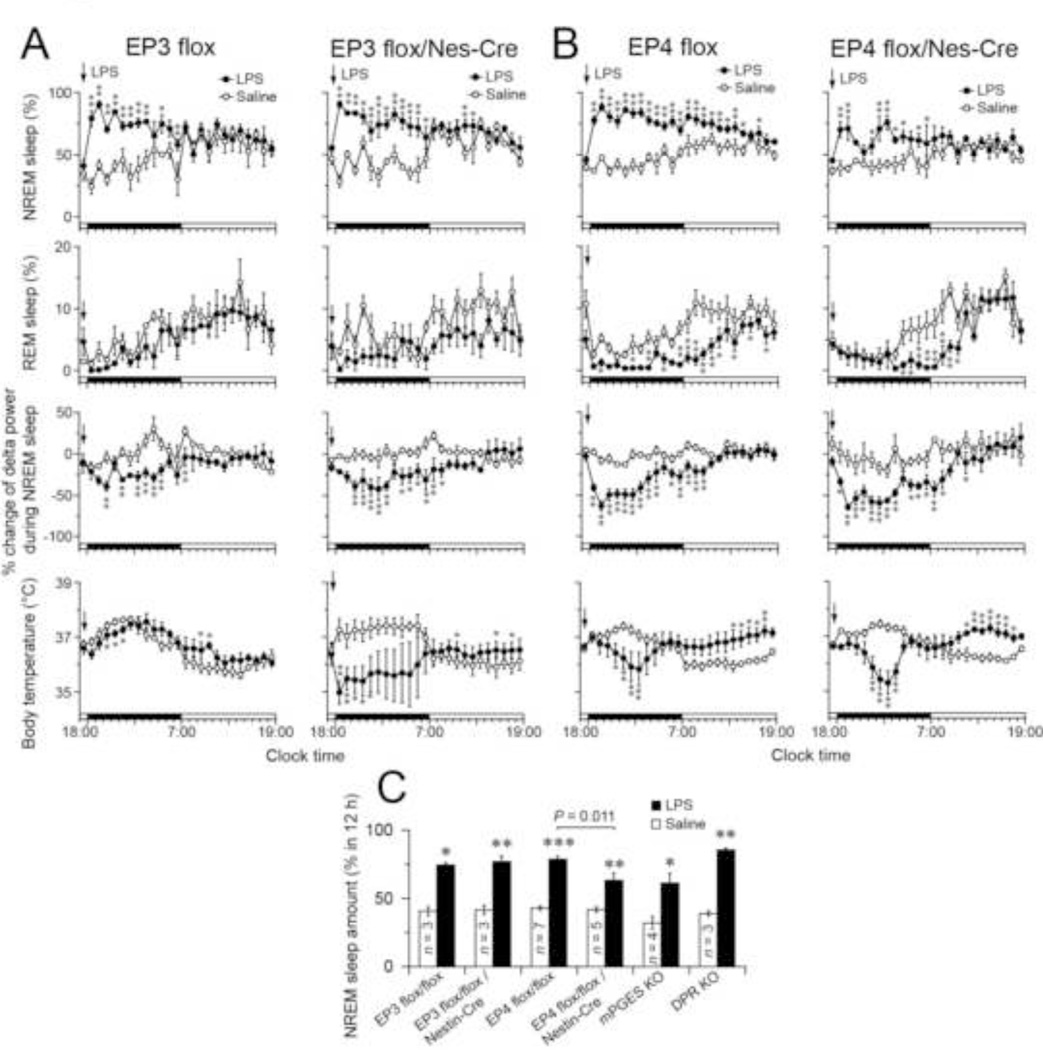
Next, we administered 15 µg/mouse LPS i.p. to EP3 flox/Nes-Cre or EP3 flox mice 30 min before dark onset to examine the effect of EP3 deficiency on sleep architecture during sickness behavior (Fig. 2A). The dose and route of LPS administration was chosen to be comparable to previous studies (Jakubcakova et al., 2011; Morrow and Opp, 2005; Toth and Opp, 2001; Zager et al., 2009). Intraperitoneal LPS injection significantly increased the amount of NREM sleep and reduced EEG delta power for 12 h after the injection in both EP3 flox control mice and EP3 flox/Nes-Cre mice, as compared to the vehicle control. Whereas EP3 flox mice showed little change in body temperature for 24 h after LPS injection, EP3 flox/Nes-Cre mice showed a very significant hypothermic response during the 12 h after LPS administration.
Fig. 2.
(A and B) Time courses of NREM and REM sleep, changes in NREM sleep delta power and body temperature in EP3 flox/Nes-Cre (A), EP4 flox/Nes-Cre (B) mice and their control littermates (EP3 flox and EP4 flox, respectively) after LPS administration (15 µg/mouse 30 min before dark onset) during the 12-h light and dark periods. Percent change of NREM sleep delta power was calculated based on the average of the NREM sleep delta power during 24 h under the baseline condition. Data are presented as the mean ± SEM (A, n = 3–5, and B, n = 5–7). *p < 0.05, **p < 0.01 compared to saline injection, as assessed by paired two-tailed Student’s t tests. Black and white bars above the x-axes indicate the dark and light periods. (C) Total amount of NREM sleep during the dark period after LPS or saline injection in NS-specific KO mice for EP3 and EP4 receptors and their control littermates (EP3 flox and EP4 flox, respectively) as well as total body KO mice for the microsomal PGE synthase-1 and PGD2 receptors. Data are presented as the mean ± SEM. *p < 0.05, **p < 0.01 compared to saline injection, as assessed by paired two-tailed Student’s t tests.
To examine the effect of EP4 deficiency on sleep or body temperature changes during systemic inflammation, we injected 15 µg/mouse LPS i.p. into EP4 flox/Nes-Cre mice and their EP4 flox littermates. LPS administration increased NREM sleep amount, reduced NREM sleep delta power and decreased REM sleep amount for more than 12 h in both EP4 flox and EP4 flox/Nes-Cre mice, as compared to the saline treatment (Fig. 2B). While LPS significantly increased the total amount of NREM sleep in both the EP4 flox and EP4 flox/Nes-Cre mice during the 12-h dark period (by 1.8- and 1.5-fold, respectively, when compared with the saline injection), the NREM sleep amount was 20% lower in the EP4 flox/Nes-Cre mice than the EP4 flox mice during the 12-h dark period (unpaired t test: t(10) = 3.11, p = 0.011). These findings suggest that EP4 receptors are partially involved in promoting NREM sleep after LPS administration. The total REM sleep amount during the dark period was significantly reduced by 77% and 59% in LPS-treated EP4 flox and EP4 flox/Nes-Cre mice, respectively, as compared to the saline-treated animals, with no significant difference between the two groups. LPS injection significantly decreased body temperature for 12 hours after LPS injection but increased body temperature during the 12-h light period of the next day in both EP4 flox control and EP4 flox/Nes-Cre mice, as compared to the vehicle control.
Our observations indicate that the EP3 receptor in the brain does not play a role in the increase of NREM sleep after LPS administration, and that the EP4 receptor only partially mediates this response. However, PGE2 acting on other EP receptors or PGD2 acting on DP receptors could be responsible for the remaining component of LPS-induced NREM sleep. We therefore recorded EEG and EMG after the administration of 15 µg LPS in mPGES-1 and DPR KO mice, which would eliminate all EP and DP receptor-mediated effects. We found that LPS significantly increased NREM sleep by 1.9- or 2.2-fold in the mPGES-1 and DPR KO mice, respectively, as compared to saline injections (Fig. 2C). Our results show that increased NREM sleep after LPS administration for mPGES-1 KO mice is very similar to EP4 flox/Nes-Cre mice, and the DPR KO mice were similar to the EP3 and EP4 flox mice, suggesting that PGE2 appears to play a small role in the increased NREM sleep after systemic LPS and this is mediated by EP4 receptors, but PGD2 action on DP receptors does not contribute to the LPS effect on sleep.
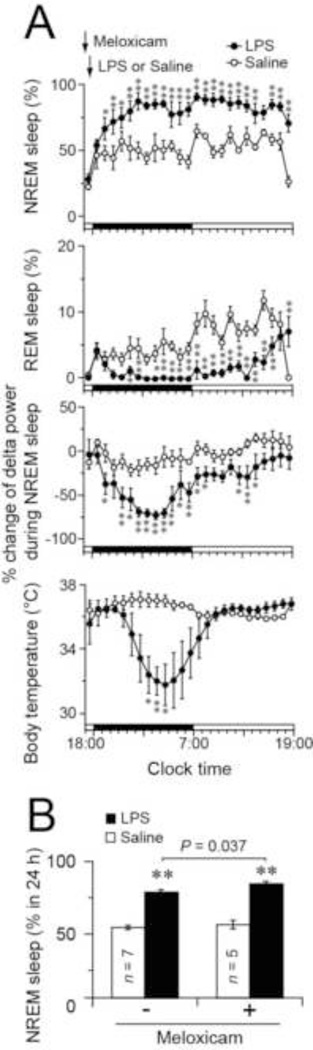
It is however possible that prostanoids other than PGE2 and PGD2 are involved in prolonged NREM sleep by systemic LPS. Therefore, we used COX inhibitor meloxicam in another group of the control (EP4 flox) mice to reduce the production of prostanoids after LPS (Fig. 3). We found that pretreatment with 10 mg/kg meloxicam 1 h before dark onset followed by 15 µg LPS administration 30 min later increased the amount of NREM sleep, reduced NREM sleep delta power, and decreased REM sleep for more than 20 h, and induced hypothermia during the dark period after the LPS injection compared to treatment of mice with only meloxicam (Fig. 3A), who did not show any difference in the sleep behavior from EP4 flox mice treated with saline (F(1,8) = 0.238, p = 0.64 assessed by mixed model ANOVA; treatment effect: independent; time effect: repeated). On the other hand, comparing the mice that received meloxicam before LPS with mice that received LPS alone, there was an increase in the total amount of NREM sleep during 24 h after LPS (unpaired t test: t(10) = 2.40, p = 0.037; Fig. 3B), suggesting that some COX product may suppress the NREM increase. These unexpected results indicate that in addition to the limited role played by PGE2 and PGD2, other prostanoids may decrease NREM sleep after LPS administration.
Fig. 3.
(A) Time courses of NREM and REM sleep, changes in NREM sleep delta power and body temperature in EP4 flox mice pretreated with meloxicam (10 mg/kg 1 h before dark onset) after LPS administration (15 µg/mouse 30 min before dark onset) during the 12-h light and dark periods. Percent change of NREM sleep delta power was calculated based on the average of the NREM sleep delta power during 24 h under the baseline condition. (B) Total amount of NREM sleep during the 24 h after LPS or saline injection in EP4 flox mice pretreated with or without meloxicam. Data are presented as the mean ± SEM (n = 5–7). *p < 0.05, **p < 0.01 compared to saline injection, as assessed by paired two-tailed Student’s t tests. Black and white bars above the x-axes indicate the dark and light periods.
4. Discussion
In this study, we used i.p. LPS as a model of bacterial invasion in mice lacking either EP3 or EP4 receptors in the NS, or lacking mPGES-1 and DPR in the entire body, as well as mice pretreated with COX inhibitor meloxicam to examine whether PGE2 or PGD2 is involved in the effect of LPS on prolonged NREM sleep, a hallmark response of sickness behavior. Our results showed that LPS-induced PGs, mainly by the effect of PGE2 on the EP4 receptor, contribute only a small amount (perhaps 10–20%) to the increase in NREM sleep after i.p. administration of a moderate dose (15 µg) of LPS.
The endotoxin LPS, a bacterial cell wall component, has been widely used as a model immune stimulant. We chose to use i.p. LPS so that our results would be comparable to earlier studies of the effects of LPS administration on sleep. Intraperitoneal injection has been shown to activate responses rapidly, in part via the vagus nerve (Simons et al., 1998) and in fact, a recent study (Zielinski et al., 2013) showed that low doses of LPS (<0.1 µg/mouse) induce sleep via the vagus nerve. Therefore, responses using higher doses of LPS may mask PG-dependent effects on NREM sleep by the vagus nerve.
LPS binds to the CD14 receptor on leukocytes and macrophages. By interaction with the Toll-like receptor 4, this induces secretion of cytokines, including interleukin (IL)-1β, tumor necrosis factor α (TNFα), and IL-6, as well as PGs (Fitzgerald and O'Neill, 2000; Rivest, 2003). In an effort to use a less complex immune stimulus, many studies have used systemically injected cytokines such as IL-1β and TNFα to stimulate the immune system. However, circulating levels of IL-1β are not elevated in the bloodstream during systemic infection (Hopkins and Rothwell, 1995), and individual cytokines are rarely if ever elevated in isolation of other components of the pro-inflammatory response. Most cytokines are likely produced locally to act in a paracrine manner (Konsman et al., 2002), where they inevitably induce the secretion of the remaining pro-inflammatory cytokines and PGs. Investigations in humans and in animal models have elucidated the role of cytokines in the physiological and inflammatory regulation of sleep (Krueger et al., 2001; Krueger and Majde, 2003; Mullington et al., 2001). Whereas the sleep patterns of humans in response to elevated levels of cytokines are more complicated and dose-dependent, leading to increased or decreased NREM sleep, there is typically a pronounced enhancement of NREM sleep and a decrease in REM sleep in rodents treated with proinflammatory cytokines. IL-6 and IL-10 mediate some of the effects of LPS on NREM sleep (Morrow and Opp, 2005; Toth and Opp, 2001). It is therefore possible that LPS-induced sleep is regulated primarily by a wide range of cytokines rather than PGs, despite the facts that (1) LPS results in increased expression of COX-2 (Breder and Saper, 1996) and subsequent production of PGs (Ueno et al., 1982b) and (2) PGE2 and PGD2 have been shown to regulate sleep–wake behavior (Huang et al., 2007; Urade and Lazarus, 2013).
Our results indicate that the effects of LPS on NREM sleep are slightly but significantly attenuated in mice lacking EP4 receptors in the central and peripheral nervous system, suggesting a minor contribution of PGE2 to LPS-induced NREM sleep via EP4 receptors. PGE2 has contrasting effects on sleep–wake regulation in different areas of the brain. For example, Hayaishi and colleagues have attributed a wake-promoting effect of PGE2 in the posterior hypothalamus to EP4 receptors in the TMN (Huang et al., 2003; Matsumura et al., 1989a; Matsumura et al., 1989b). They showed by in situ hybridization that EP4 receptor mRNA is expressed in histaminergic TMN neurons (Huang et al., 2003) and that injection of the EP4 receptor agonist (ONO-AE1329) into the TMN mimicked the excitatory effect of PGE2 on histaminergic TMN neurons and induced wakefulness. By contrast, when PGE2 was infused into the subarachnoid space underlying the ventral surface area of the rostral basal forebrain, the sleeping time of rats was significantly increased (Ram et al., 1997). The infusion site at the ventral surface zone of the rostral basal forebrain is adjacent to the preoptic area including the sleep-promoting ventrolateral preoptic neurons, an area known for its high expression of EP4 receptors (Oka et al., 2000; Zhang and Rivest, 1999). Therefore, it is reasonable to argue that effects of EP4 receptors on sleep and wakefulness are site-dependent and that LPS may partially promote NREM sleep through PGE2 via EP4 receptors localized to the ventrolateral preoptic region.
In contrast to NREM sleep under baseline conditions or saline treatment, LPS-induced NREM sleep in mice is characterized by low delta power. By contrast, such an effect has never been observed during sleep induced by PGD2 from its physiological (Onoe et al., 1988) to pathological range (Kaushik et al., 2014) in the brain. The EEG delta power after i.p. LPS administration was not different in the animals that lacked EP4 receptors in the NS or control (EP4 flox) mice given meloxicam, so this aspect of sleep is apparently independent of the PGs.
Although it has been known since the 19th century that aspirin could prevent many components of sickness behavior, and this compound is widely used for this purpose, the mechanism of action was not understood until Sir John Vane in 1971 made the fundamental discovery that anti-inflammatory compounds such as aspirin act by blocking the formation of PGs (Vane, 1971). Our observation that only a small part of the effect of systemic LPS on increasing NREM sleep is dependent on PGs suggests that nonsteroidal anti-inflammatory drugs, usually abbreviated to NSAIDs, are unlikely to be useful to remedy sleepiness or low sleep quality during sickness. Moreover, it appears that the EP3 receptor is necessary not only to produce fever responses, but also to prevent profound hypothermic responses, particularly at higher doses of LPS. In the absence of CNS EP3 receptors or in the presence of a COX inhibitor, the main effect of LPS is hypothermia, which appears to be due to other proinflammatory cytokines such as TNFα (Kozak et al., 1997).
In conclusion, by using NS-specific KO mice for EP3 and EP4 receptors, total body KO mice for mPGES-1 and DPR and a COX inhibitor, we found that in contrast to the fever response, the increase of NREM sleep and decrease of NREM sleep delta power after i.p. administration of a moderate dose of LPS is largely independent of prostaglandins, with only the EP4 receptor contributing a small amount to the increase in NREM sleep. Our results also clearly show that there is little relationship between the changes in body temperature and NREM sleep in response to LPS and thus, we conclude that fever does not cause sleep.
Highlights.
We studied whether increased NREM sleep during sickness is mediated by PGE2 or PGD2
LPS-induced increase of NREM sleep is largely independent of prostaglandins
Only the EP4 receptor contributes slightly to the NREM sleep increase after LPS
Thermoregulatory effect of LPS is hypothermia after inhibition of prostaglandins
Acknowledgments
We thank Matthew and Richard Breyer (Vanderbilt University) for providing EP4 flox mice, Shuh Narumiya (Kyoto University) for providing DPR KO mice and Takatoshi Mochizuki (Beth Israel Deaconess Medical Center) for technical advice. This work was supported by a Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellowship for Research Abroad (to Y.O.), the G. Harold and Leila Y. Mathers Foundation (to C.B.S.), Nestlé Nutrition Council of Japan (to M.L.), VA merit, National Institutes of Health grants DK37097 and NS072337 (to C.B.S.), JSPS grant 24300129 (to M.L.) and 25890005 (to Y.O.) and World Premier International Research Center Initiative (WPI) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to M.L., Y.O. and Y.U.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Breder CD, Saper CB. Expression of inducible cyclooxygenase mRNA in the mouse brain after systemic administration of bacterial lipopolysaccharide. Brain Research. 1996;713:64–69. doi: 10.1016/0006-8993(95)01474-8. [DOI] [PubMed] [Google Scholar]
- Eguchi N, Minami T, Shirafuji N, Kanaoka Y, Tanaka T, Nagata A, Yoshida N, Urade Y, Ito S, Hayaishi O. Lack of tactile pain (allodynia) in lipocalin-type prostaglandin D synthase-deficient mice. Proc Natl Acad Sci U S A. 1999;96:726–730. doi: 10.1073/pnas.96.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, O'Neill LA. The role of the interleukin-1/Toll-like receptor superfamily in inflammation and host defence. Microbes and Infection/Institut Pasteur. 2000;2:933–943. doi: 10.1016/s1286-4579(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system. I: Expression and recognition. Trends in Neurosciences. 1995;18:83–88. [PubMed] [Google Scholar]
- Huang ZL, Sato Y, Mochizuki T, Okada T, Qu WM, Yamatodani A, Urade Y, Hayaishi O. Prostaglandin E2 activates the histaminergic system via the EP4 receptor to induce wakefulness in rats. The Journal of Neuroscience. 2003;23:5975–5983. doi: 10.1523/JNEUROSCI.23-14-05975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZL, Urade Y, Hayaishi O. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Current Opinion in Pharmacology. 2007;7:33–38. doi: 10.1016/j.coph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Jakubcakova V, Flachskamm C, Deussing JM, Kimura M. Deficiency of corticotropin-releasing hormone type-2 receptor alters sleep responses to bacterial lipopolysaccharide in mice. Brain, Behavior, and Immunity. 2011;25:1626–1636. doi: 10.1016/j.bbi.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Kabashima K, Narumiya S. The DP receptor, allergic inflammation and asthma. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2003;69:187–194. doi: 10.1016/s0952-3278(03)00080-2. [DOI] [PubMed] [Google Scholar]
- Kaushik MK, Aritake K, Kamauchi S, Hayaishi O, Huang ZL, Lazarus M, Urade Y. Prostaglandin D2 is crucial for seizure suppression and postictal sleep. Experimental Neurology. 2014;253:82–90. doi: 10.1016/j.expneurol.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends in Neurosciences. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Kozak W, Soszynski D, Rudolph K, Leon LR, Conn CA, Kluger MJ. Soluble tumor necrosis factor alpha receptor prevents decrease of body temperature in mice treated with indomethacin and lipopolysaccharide. Annals of the New York Academy of Sciences. 1997;813:264–271. doi: 10.1111/j.1749-6632.1997.tb51704.x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Majde JA. Humoral links between sleep and the immune system: research issues. Annals of the New York Academy of Sciences. 2003;992:9–20. doi: 10.1111/j.1749-6632.2003.tb03133.x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Annals of the New York Academy of Sciences. 2001;933:211–221. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Matsumura H, Honda K, Choi WS, Inoue S, Sakai T, Hayaishi O. Evidence that brain prostaglandin E2 is involved in physiological sleep-wake regulation in rats. Proc Natl Acad Sci U S A. 1989a;86:5666–5669. doi: 10.1073/pnas.86.14.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Honda K, Goh Y, Ueno R, Sakai T, Inoue S, Hayaishi O. Awaking effect of prostaglandin E2 in freely moving rats. Brain Research. 1989b;481:242–249. doi: 10.1016/0006-8993(89)90800-7. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, Sugimoto Y, Kobayashi T, Ushikubi F, Aze Y, Eguchi N, Urade Y, Yoshida N, Kimura K, Mizoguchi A, Honda Y, Nagai H, Narumiya S. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- Mohri I, Taniike M, Taniguchi H, Kanekiyo T, Aritake K, Inui T, Fukumoto N, Eguchi N, Kushi A, Sasai H, Kanaoka Y, Ozono K, Narumiya S, Suzuki K, Urade Y. Prostaglandin D2-mediated microglia/astrocyte interaction enhances astrogliosis and demyelination in twitcher. The Journal of Neuroscience. 2006;26:4383–4393. doi: 10.1523/JNEUROSCI.4531-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Opp MR. Diurnal variation of lipopolysaccharide-induced alterations in sleep and body temperature of interleukin-6-deficient mice. Brain, Behavior, and Immunity. 2005;19:40–51. doi: 10.1016/j.bbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Hinze-Selch D, Pollmacher T. Mediators of inflammation and their interaction with sleep: relevance for chronic fatigue syndrome and related conditions. Annals of the New York Academy of Sciences. 2001;933:201–210. doi: 10.1111/j.1749-6632.2001.tb05825.x. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Camenisch T, Snouwaert JN, Hicks E, Coffman TM, Anderson PA, Malouf NN, Koller BH. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Williams RH, Agostinelli L, Arrigoni E, Fuller PM, Mochizuki T, Saper CB, Scammell TE. Role of the medial prefrontal cortex in cataplexy. The Journal of Neuroscience. 2013;33:9743–9751. doi: 10.1523/JNEUROSCI.0499-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Oka K, Scammell TE, Lee C, Kelly JF, Nantel F, Elmquist JK, Saper CB. Relationship of EP1–4 prostaglandin receptors with rat hypothalamic cell groups involved in lipopolysaccharide fever responses. The Journal of Comparative Neurology. 2000;428:20–32. doi: 10.1002/1096-9861(20001204)428:1<20::aid-cne3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Onoe H, Ueno R, Fujita I, Nishino H, Oomura Y, Hayaishi O. Prostaglandin D2, a cerebral sleep-inducing substance in monkeys. Proc Natl Acad Sci U S A. 1988;85:4082–4086. doi: 10.1073/pnas.85.11.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Aritake K, Eguchi N, Nambu F, Narumiya S, Urade Y, Hayaishi O. Lipocalin-type prostaglandin D synthase produces prostaglandin D2 involved in regulation of physiological sleep. Proc Natl Acad Sci U S A. 2006;103:17949–17954. doi: 10.1073/pnas.0608581103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram A, Pandey HP, Matsumura H, Kasahara-Orita K, Nakajima T, Takahata R, Satoh S, Terao A, Hayaishi O. CSF levels of prostaglandins, especially the level of prostaglandin D2, are correlated with increasing propensity towards sleep in rats. Brain Research. 1997;751:81–89. doi: 10.1016/s0006-8993(96)01401-1. [DOI] [PubMed] [Google Scholar]
- Rivest S. Molecular insights on the cerebral innate immune system. Brain, Behavior, and Immunity. 2003;17:13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Saper CB, Romanovsky AA, Scammell TE. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci. 2012;15:1088–1095. doi: 10.1038/nn.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Guan YF, Zhang YH, Magnuson MA, Pettepher C, Loftin CD, Langenbach R, Breyer RM, Breyer MD. Generation of a conditional allele of the mouse prostaglandin EP4 receptor. Genesis. 2004;40:7–14. doi: 10.1002/gene.20048. [DOI] [PubMed] [Google Scholar]
- Segi E, Sugimoto Y, Yamasaki A, Aze Y, Oida H, Nishimura T, Murata T, Matsuoka T, Ushikubi F, Hirose M, Tanaka T, Yoshida N, Narumiya S, Ichikawa A. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochemical and Biophysical Research Communications. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- Simons CT, Kulchitsky VA, Sugimoto N, Homer LD, Szekely M, Romanovsky AA. Signaling the brain in systemic inflammation: which vagal branch is involved in fever genesis? The American Journal of Physiology. 1998;275:R63–R68. doi: 10.1152/ajpregu.1998.275.1.R63. [DOI] [PubMed] [Google Scholar]
- Toth LA, Opp MR. Cytokine- and microbially induced sleep responses of interleukin-10 deficient mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2001;280:R1806–R1814. doi: 10.1152/ajpregu.2001.280.6.R1806. [DOI] [PubMed] [Google Scholar]
- Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, Pandher K, Lapointe JM, Saha S, Roach ML, Carter D, Thomas NA, Durtschi BA, McNeish JD, Hambor JE, Jakobsson PJ, Carty TJ, Perez JR, Audoly LP. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9044–9049. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nature Genetics. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Ueno R, Ishikawa Y, Nakayama T, Hayaishi O. Prostaglandin D2 induces sleep when microinjected into the preoptic area of conscious rats. Biochemical and Biophysical Research Communications. 1982;109:576–582. doi: 10.1016/0006-291x(82)91760-0. [DOI] [PubMed] [Google Scholar]
- Ueno R, Narumiya S, Ogorochi T, Nakayama T, Ishikawa Y, Hayaishi O. Role of prostaglandin D2 in the hypothermia of rats caused by bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1982b;79:6093–6097. doi: 10.1073/pnas.79.19.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urade Y, Fujimoto N, Hayaishi O. Purification and characterization of rat brain prostaglandin D synthetase. The Journal of Biological Chemistry. 1985;260:12410–12415. [PubMed] [Google Scholar]
- Urade Y, Hayaishi O. Prostaglandin D2 and sleep/wake regulation. Sleep Medicine Reviews. 2011;15:411–418. doi: 10.1016/j.smrv.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Urade Y, Lazarus M. Prostaglandin D2 in the regulation of sleep. In: Shaw PJ, Tafti M, Thorpy MJ, editors. The Genetic Basis of Sleep & Sleep Disorders. Cambridge: Cambridge University Press; 2013. pp. 73–83. [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirinlike drugs. Nature: New biology. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Zager A, Andersen ML, Lima MM, Reksidler AB, Machado RB, Tufik S. Modulation of sickness behavior by sleep: the role of neurochemical and neuroinflammatory pathways in mice. European Neuropsychopharmacology. 2009;19:589–602. doi: 10.1016/j.euroneuro.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Rivest S. Distribution, regulation and colocalization of the genes encoding the EP2- and EP4-PGE2 receptors in the rat brain and neuronal responses to systemic inflammation. The European Journal of Neuroscience. 1999;11:2651–2668. doi: 10.1046/j.1460-9568.1999.00682.x. [DOI] [PubMed] [Google Scholar]
- Zielinski MR, Dunbrasky DL, Taishi P, Souza G, Krueger JM. Vagotomy attenuates brain cytokines and sleep induced by peripherally administered tumor necrosis factor-alpha and lipopolysaccharide in mice. Sleep. 2013;36:1227–1238. doi: 10.5665/sleep.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]