Abstract
Epilepsy and other neurological deficits are common, disabling manifestations of the genetic disorder, Tuberous Sclerosis Complex (TSC). Brain inflammation has been implicated in contributing to epileptogenesis in acquired epilepsy due to brain injury, but the potential role of inflammatory mechanisms in genetic epilepsies is relatively unexplored. In this study, we investigated activation of inflammatory mediators and tested the effects of anti-inflammatory treatment on epilepsy in the Tsc1-GFAP conditional knock-out mouse model of TSC (Tsc1GFAPCKO mice). Real-time quantitative RT-PCR, immunohistochemistry, and western blotting demonstrated increased expression of specific cytokines and chemokines, particularly IL-1β and CXCL10, in the neocortex and hippocampus of Tsc1GFAPCKO mice, which was reversed by treatment with a mammalian target of rapamycin complex 1 (mTORC1) inhibitor. Double-labeling immunohistochemical studies indicated that the increased IL-1β was localized primarily to astrocytes. Importantly, the increase in inflammatory markers was also observed in astrocyte culture in vitro and at 2 weeks of age in Tsc1GFAPCKO mice before the onset of epilepsy in vivo, indicating that the inflammatory changes were not secondary to seizures. Epicathechin-3-gallate, an inhibitor of IL-1β and CXCL10, at least partially reversed the elevated cytokine and chemokine levels, reduced seizure frequency, and prolonged survival of Tsc1GFAPCKO mice. These findings suggest that mTOR-mediated inflammatory mechanisms may be involved in epileptogenesis in the genetic epilepsy, TSC.
Keywords: epilepsy, seizure, tuberous sclerosis, inflammation, mice, interleukin, cytokine, chemokine
INTRODUCTION
Tuberous sclerosis complex (TSC) is a genetic disorder, characterized by tumor or hamartoma formation in multiple organs (Crino et al., 2006; Orlova and Crino, 2010). Neurological involvement often accounts for the most disabling symptoms of TSC, including drug-resistant epilepsy, intellectual disability, and autism (Chu-Shore et al., 2010; Holmes et al., 2007). TSC is caused by mutations in the TSC1 or TSC2 genes, which leads to hyperactivation of the mammalian target of rapamycin complex 1 (mTORC1) pathway and stimulates cell growth and proliferation, promoting tumor growth. The use of mTOR inhibitors represents a rational, proven approach for treating tumors in TSC (Franz et al., 2013; Krueger et al., 2010).
Although TSC patients can develop brain tumors, the chronic neurological symptoms of epilepsy, intellectual disability, and autism are generally not directly caused by tumor growth per se. Cortical tubers, which represent static, developmental malformations or hamartomas of the brain, may contribute to some of the chronic neurological manifestations of TSC, especially epilepsy. However, there is also accumulating evidence that non-tuber, structurally normal-appearing regions of the brain possess cellular and molecular abnormalities that promote neurological dysfunction (Wong, 2008).
Independent of tumor growth, the mTORC1 pathway has also been implicated in promoting epilepsy and intellectual disability in TSC patients, and mTOR inhibitors are being tested in clinical trials as potential treatments for these neurological symptoms (Krueger et al., 2013). Even if mTOR inhibitors are effective against neurological manifestations of TSC, the critical mechanisms downstream from mTORC1 causing epilepsy and neurocognitive dysfunction in TSC are poorly understood. As mTORC1 inhibitors have significant side effects, such as immunosuppression, identification of these downstream mechanisms may lead to more targeted therapies, with more specific efficacy and fewer side effects.
Brain inflammation has been strongly implicated in the pathophysiology of epilepsy and other neurological disorders (Vezzani et al., 2013a, 2013b; Xu et al., 2013). While activation of inflammatory mechanisms in response to acquired brain injury is perhaps not surprising, a more novel idea is that brain inflammation could also be important in the pathophysiology of developmental or genetic neurological disorders. In fact, inflammatory markers, such as cytokines and chemokines, have been found in brain specimens from patients with genetic malformations of cortical development, including TSC (Boer et al., 2008, 2010; Maldonado et al., 2003; Prabowo et al., 2013), but the pathophysiological significance of inflammation in TSC is poorly understood. Thus, the purpose of this study is to identify specific inflammatory mechanisms, downstream from mTOR, activated in the brain of a mouse model of TSC and determine the effect of modulating these mechanisms.
MATERIALS AND METHODS
Animals and drug treatment
Care and use of animals were conducted according to an animal protocol approved by the Washington University Animal Studies Committee. Tsc1flox/flox-GFAP-Cre knock-out (Tsc1GFAPCKO) mice with conditional inactivation of the Tsc1 gene predominantly in glia were generated as described previously (Uhlmann et al., 2002). Tsc1flox/+-GFAP-Cre and Tsc1flox/flox littermates have previously been found to have no abnormal phenotype and were used as control animals in these experiments.
In some experiments, three-week-old Tsc1GFAPCKO mice were treated with rapamycin (3 mg/kg/day) or vehicle for one week, and brain tissues were then harvested for western blot, real-time quantitative RT-PCR, or immunohistochemistry analysis. Rapamycin (LC Labs, Woburn, MA) was initially dissolved in 100% ethanol, stored at −20°C, and diluted in a vehicle solution containing 5% Tween 80, 5% PEG 400 (Sigma, St. Louis, MO), and 4% ethanol immediately before injection or adding to the culture medium.
In other experiments, three-week-old Tsc1GFAPCKO mice were treated with vehicle (saline) or Epicatechin-3-gallate (ECG, Sigma, St Louis, MO). ECG dissolved in saline was administrated by peritoneal injection at the dose of 12.5 mg/kg/d for one week for western blot and immunohistochemistry analysis, for four weeks for histology, and for up to 12 weeks for video-EEG monitoring. Vehicle-treated non-KO littermates served as additional controls. Other vehicle or ECG-treated Tsc1GFAPCKO mice and control mice were monitored for body and brain weight measurements or for survival analysis.
Real-time Quantitative RT-PCR
Real-time quantitative RT-PCR was used to screen a panel of inflammatory markers (Table S1). Total RNA was prepared from brain of two or four week old Tsc1GFAPCKO or control mice, or from cultured astrocytes of Tsc1GFAPCKO or control mice. Following DNase I treatment (Invitrogen, Grand Island, NY), cDNA was synthesized using iScript Reverse Transcription Kit (BIO-RAD, Hercules, CA). The following conditions were used for reverse transcription: 25°C for 10 min, 48°C for 30 min, and 95°C for 5 min.
Each 25 μl PCR contained 2 μl cDNA, 12.5 μl of 2X SYBR Green PCR Master Mix (Applied Biosystems, Foster city, CA), and 12.5 pmol of each primer. Real-time quantitative PCR was performed in 96-well optical reaction plates on the AB1 7000 Real-Time PCR System (Applied Biosystems) under the following conditions: 50°C for 2 min, 95°C for 10 min, and then 40 cycles of 95°C for 15 s and 60°C for 1 min. Emitted fluorescence for each reaction was measured at the annealing/extension phase. All oligonucleotide primers used for quantitative PCR were designed using Primer Express v2.0 (Applied Biosystems). Calculated copies were normalized against copies of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Forward and reverse primer sets are listed in Table S1.
Western blotting
Western blot analysis was used to measure protein levels of CXCL10 in the brains of Tsc1GFAPCKO mice, using standard methods as described previously (Zeng et al., 2008). Briefly, brains were dissected and homogenized separately. Equal amounts of total protein extract were separated by gel electrophoresis and transferred to nitrocellulose membranes. β-actin was used as a loading control. After incubating with primary antibodies to CXCL10 (1:1,000; Abcam, Cambridge, MA), or β-actin (1:2,000; Cell Signaling Technology), the membranes were reacted with a peroxidase-conjugated secondary antibody (1:1,000, Cell Signaling Technology). Signals were detected by enzyme chemiluminescence (GE healthcare life science, Little Chalfont Buckinghamshire, UK) and quantitatively analyzed with ImageJ software (NIH, Bethesda, MD).
Immunohistochemistry/Histology
Histological analysis was performed to assess glial proliferation and neuronal organization by standard methods, as previously described (Zeng et al., 2008). In brief, brains were perfusion-fixed with 4% paraformaldehyde and cut into 45 μm sections with a cryotome. Some sections were stained with 0.5% cresyl violet. Other sections were labeled with GFAP antibody (anti–GFAP, mouse; 1:500; Cell Signaling Technology, Beverly, MA), and then Cy3-conjugated goat anti–mouse IgG (1:500; Jackson Immuno. West Grove, PA).
In other experiments, immunohistochemistry was performed for IL-1β, by labeling with primary antibody (anti-IL-1β, rabbit, 1:500; Abcam), followed by labeling with secondary antibody Alexa-488 conjugated goat anti-rabbit IgG (1:500; Life Technologies, Grand Island, NY). Some sections were double stained with GFAP antibody (anti–GFAP, mouse; 1:500; Cell Signaling Technology), NeuN antibody (anti-NeuN, mouse, 1:500; Millipore, Billerica, MA), or Iba1 antibody (anti-Iba1, mouse, 1:500; Millipore), followed by labeling with secondary antibodies: Alexa-488 conjugated goat anti-rabbit IgG (1:500; Life Technologies, Grand Island, NY) or Cy3 conjugated goat anti-mouse IgG (1:500; Jackson Immuno, West Grove, PA). In addition, some sections were counterstained with TO-PRO-3 Iodide (1:1,000; Life Technologies) for the nonspecific nuclear staining of all cells.
For blinded analysis, images were acquired with a Zeiss LSM PASCAL confocal microscope (Zeiss Thornwood, NY), or with a Nanozoomer HT system (Hamamatsu, Bridgewater, NJ). In images from coronal sections at approximately 2 mm posterior to bregma and approximately 1 mm from midline, regions of interest were marked in neocortex by a 200μm-wide box spanning from the neocortical surface to the bottom of layer VI and in hippocampus by areas up to 0.04 mm2 within the striatum radiatum of CA1 and dentate gyrus. GFAP-immunoreactive cells were quantified in the regions of interest from two sections per mouse from a total of eight mice per group. Similarly, IL-1β positive cells and TO-PRO-3 Iodide stained cells numbers were counted in the sections of each groups, and IL-1β positive cell numbers were normalized to TO-PRO-3 Iodide stained cell number, and were presented as IL-1β positive cells/100 TO-PRO-3 positive cells.
ELISA of serum IL-1β and CXCL10 expression
IL-1β and CXCL10 proteins were assayed in duplicate from serum of four week old Tsc1GFAPCKO mice and control mice with the mouse IL-1β and CXCL10 enzyme-linked immunosorbent assay (ELISA) kits (R&D System, Minneapolis, MN, USA) according to the manufacturer’s instructions. Additional control mice were treated with lipopolysaccharide (LPS, 250 μg/kg, i.p.), as a positive control. Blood was collected 6 h following i.p. LPS administration and serum was separated from clotted blood overnight at 4 °C
Astrocyte culture and measurement of inflammatory markers in vitro
Astrocytes were obtained from mixed cell cultures of the forebrains as described previously (Zhang et al., 2002), with slight modification to remove microglial cells completely. Briefly, the forebrains of newborn Tsc1GFAPCKO mice and non-KO littermates were dissected and the dissociated brain cells were seeded in a poly-D-lysine-coated 75 cm2-culture flask (Becton Dickinson Labware, Franklin Lakes, NJ). Cells were cultured for 8–10 days until confluent, then they were vigorously hand-shaken for 0.5–1 min to remove microglial cells present on the astrocyte monolayer, followed by medium exchange and incubation overnight in a CO2 incubator. The purification procedure was repeated three times during the subsequent 3 days. Finally, the flask was washed with DMEM several times and the medium replaced with Neurobasal medium for 6 hours before treatment.
Rapamycin at a concentration of 2 ng/ml or vehicle were added to the medium of prepared astrocytes and incubated for 16 hours. Samples were collected after trypsinization with 0.25% trypsin-EDTA (Invitrogen, Grand Island, NY), and then Western blotting analysis was performed to measure the ratio of CXCL10 and β-actin as described above, or real-time RT-PCR was used to measure the mRNA level of CCL2, IL-1β and CXCL10.
Video-electroencephalography monitoring
Vehicle- and ECG-treated Tsc1GFAPCKO mice underwent continuous video-EEG monitoring starting at 3 weeks of age, using established methods for implanting epidural electrodes and performing continuous video-EEG recordings, as described previously (Erbayat-Altay et al., 2007; Zeng et al., 2008). Briefly, mice were anesthetized with isoflurane and placed in a stereotaxic frame. Epidural screw electrodes were surgically implanted and secured using dental cement for long term EEG recordings. Four electrodes were placed on the skull: one right and one left central electrodes (1 mm lateral to midline, 2 mm posterior to bregma), one frontal electrode (0.5 mm anterior and 0.5 mm to the right or left of bregma) and one occipital electrode (0.5 mm posterior and 0.5 mm to the right or left lambda). The typical recording montage involved two EEG channels with the right and left central “active” electrodes being compared to either the frontal or occipital “reference” electrode. Video and EEG data were acquired simultaneously with an AD Instruments PowerLab video-EEG system. Continuous 24/7 video-EEG data were obtained every week from each mouse, for the life of the animal or until 12 weeks of age, and were analyzed for seizures. Electrographic seizures were identified by their characteristic pattern of discrete periods of rhythmic spike discharges that evolved in frequency and amplitude lasting at least 10 seconds, typically ending with repetitive burst discharges and voltage suppression. On video analysis, the behavioral correlate to these seizures typically involved head bobbing, rearing with forelimb clonus, and occasional generalized convulsive activity. Seizure frequency (number of seizures per week period, based on analysis of the entire EEG record) was calculated from each week epoch.
Statistics
All statistical analysis was performed using GraphPad Prism (GraphPad Software). Quantitative differences between groups were analyzed by Student’s t test or one-way ANOVA with Turkey’s multiple comparisons post hoc tests when comparing one factor over more than two groups or by repeated measures two-way ANOVA when comparing multiple treatment variables (e.g. effect of treatment and genotype). Comparable non-parametric tests were used when data did not fit a normal distribution. Chi-Square test was used for survival analysis. Quantitative data are expressed as mean ± SEM. Statistical significance was defined as p<0.05.
RESULTS
Proinflammatory cytokines and chemokines are up-regulated in Tsc1GFAPCKO mice and inhibited by rapamycin
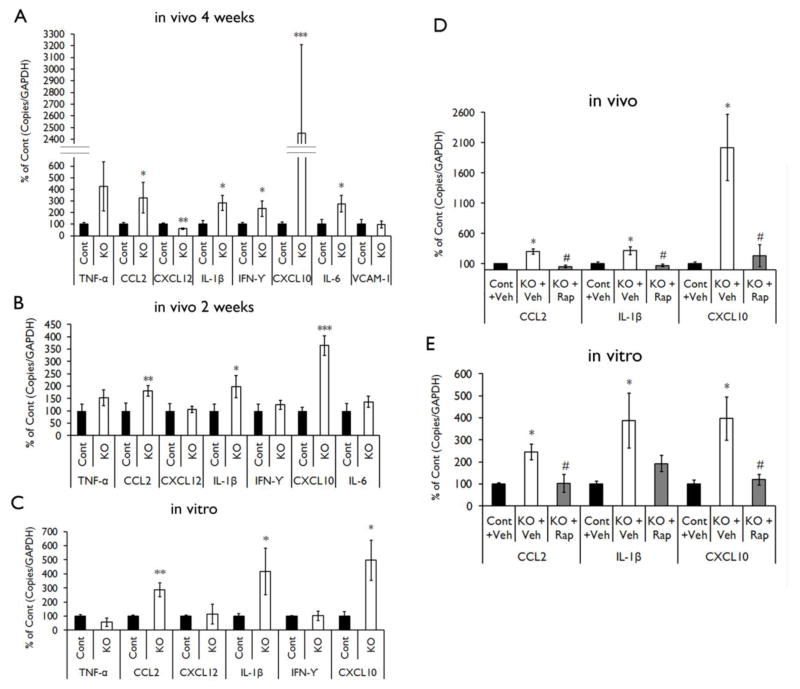
We first used real-time quantitative RT-PCR to screen for inflammatory markers activated in Tsc1GFAPCKO mice. The mRNA levels of CCL2, IL-1β, IFN-γ, CXCL10 and IL-6 were increased in the brains of four-week-old Tsc1GFAPCKO mice compared to control mice, while the mRNA level of CXCL12 was decreased (Fig. 1A). As seizures in Tsc1GFAPCKO mice start around 3–4 weeks of age (Erbayat-Altay et al., 2007; Zeng et al., 2011) and could secondarily affect inflammation, we then performed real-time quantitative RT-PCR in two-week old Tsc1GFAPCKO mice. Only the mRNA levels of CCL2, IL-1β, and CXCL10 were increased in two-week-old Tsc1GFAPCKO mice compared to control mice; no significant difference was found in IFN-γ, IL-6 and CXCL12 in two-week-old Tsc1GFAPCKO mice (Fig. 1B). Similar to the results of two-week-old mice, the mRNA levels of CCL2, IL-1β, and CXCL10 were increased in the cultured astrocytes of Tsc1GFAPCKO mice compared to the astrocytes of control mice (Fig. 1C).
Figure 1. Proinflammatory cytokines and chemokines are up-regulated in Tsc1GFAPCKO mice and inhibited by rapamycin.
mRNA expression of cytokines and chemokines was evaluated by real-time quantitative RT-PCR in the brains or cultured astrocytes from Tsc1GFAPCKO and control mice. (A) The mRNA levels of CCL2, IL-1β, IFN-γ, CXCL10 and IL-6 were increased in four-week old Tsc1GFAPCKO mice compared with control mice, but CXCL12 was decreased (n = 8–10 mice/group). (B) The mRNA levels of CCL2, IL-1β and CXCL10 were increased in two-week old Tsc1GFAPCKO mice compared with control mice (n = 8–11 mice/group). (C) The mRNA levels of CCL2, IL-1β and CXCL10 were increased in cultured astrocytes from Tsc1GFAPCKO mice compared with astrocytes from control mice (n = 5–9 mice/group). * p < 0.05, ** p < 0.01, *** p < 0.001, versus control mice by Student’s t test. (D) Seven days of rapamycin treatment (3 mg/kg/d i.p.) significantly inhibited the mRNA levels of CCL2, IL-1β and CXCL10 in the brains of Tsc1GFAPCKO mice compared with vehicle-treated Tsc1GFAPCKO mice (n = 4–12 mice/group). (E) Rapamycin treatment (2 ng/ml for 16 hours) significantly inhibited the mRNA levels of CCL2 and CXCL10, but not IL-1β, in cultured astrocytes from Tsc1GFAPCKO mice (n = 5–12 mice/group). * p<0.05 versus control mice, # p < 0.05 versus vehicle-treated Tsc1GFAPCKO mice by one-way ANOVA. Cont = control mice, KO = Tsc1GFAPCKO, Veh = vehicle, Rap = Rapamycin.
To assess whether mTOR activation may be involved in the upregulation of inflammatory markers in Tsc1GFAPCKO mice, we tested the effect of rapamycin on mRNA levels of those markers that were consistently elevated in the previous experiments. Rapamycin treatment at the dose of 3 mg/kg/d for seven days significantly inhibited the mRNA levels of CCL2, IL-1β and CXCL10 in the brains of Tsc1GFAPCKO mice in vivo compared with vehicle treated Tsc1GFAPCKO mice (Fig. 1D). For in vitro experiments, treatment of astrocytes with rapamycin at the dose of 2 ng/ml, down-regulated the mRNA expression of CCL2 and CXCL10, although the effect on IL-1β did not reach significance (Fig. 1E).
IL-1β protein is upregulated in Tsc1GFAPCKO mice and inhibited by anti-inflammatory treatments
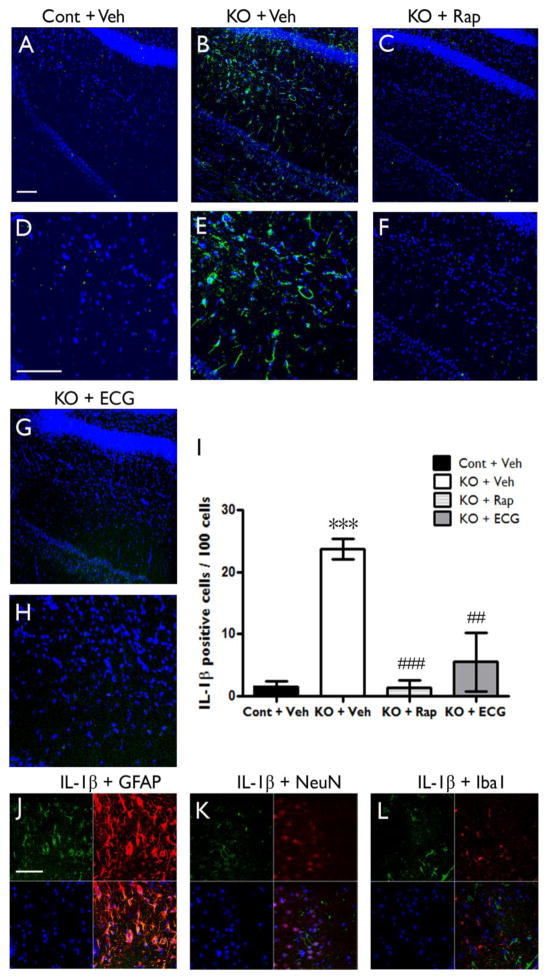
We next tested whether inflammatory markers increased on the mRNA level translated to upregulated protein in Tsc1GFAPCKO mice. Immunohistochemistry of IL-1β was performed in brain sections of four-week old mice treated with vehicle, rapamycin, or ECG for one week starting at three weeks of age. IL-1β protein expression was strongly detected in hippocampal brain sections of Tsc1GFAPCKO mice (Fig. 2B, E), but rarely in control mice (Fig. 2A, D). Treatment with rapamycin (3 mg/kg/d i.p.) significantly inhibited the IL-1β expression in Tsc1GFAPCKO mice (Fig. 2C, F, I). Epicatechins have been reported to inhibit IL-1β and CXCL10 expression (Crouvezier et al., 2001; Hosokawa et al., 2010). ECG treatment (12.5 mg/kg/d i.p) also inhibited expression of IL-1β expression in Tsc1GFAPCKO mice (Fig. 2G, H, I).
Figure 2. IL-1β protein is upregulated in Tsc1GFAPCKO mice and inhibited by anti-inflammatory treatments.
Protein expression of IL-1β was assessed by immunohistochemistry in Tsc1GFAPCKO and control mice, as well as in rapamycin or ECG treated Tsc1GFAPCKO mice. (A–H) Confocal images of immunohistochemical staining of IL-1β (green) and TO-PRO-3 Iodide (blue). TO-PRO-3 Iodide (blue) was used as an optimal fluorescence dye for nuclear counterstaining. IL-1β protein expression was detected in brain sections of Tsc1GFAPCKO mice (B,E KO + Veh), but not in control mice (A,D Cont + Veh). Rapamycin (C,F KO + Rap) and ECG (G,H KO + ECG) treatment inhibited the IL-1β expression. Scale bars = 100 μm. (I) Quantitative analysis confirmed an increase in IL-1β-positive cells (per 100 TO-PRO-3 positive cells) in vehicle-treated Tsc1GFAPCKO group (KO + Veh) compared with vehicle-treated control group (Cont + Veh). Rapamycin (3 mg/kg/d i.p. for one week) or ECG treatment (12.5 mg/kg/d i.p. for one week) significantly decreased IL-1β-positive cells. ***p<0.05 versus vehicle-treated control mice (n=4–5 mice/group); ## p<0.01, ### p<0.001 versus vehicle-treated Tsc1GFAPCKO mice by two-way ANOVA. (J, K, L) Double label immunofluorescence confocal microscopy for expression of IL-1β protein (green) with GFAP (F, red for astrocytes), NeuN (G, red for neurons) and Iba1 (H, red for microglia) within brain sections of four-week-old Tsc1GFAPCKO mice. All sections were also labeled with TO-PRO-3 Iodide (blue) as an optimal fluorescence dye for nuclear counterstaining. IL-1β was found co-localized with GFAP, but not with NeuN and Iba1. Scale bar = 50 μm. Cont = control, KO = Tsc1GFAPCKO, TO PRO3 = TO-PRO-3 Iodide, ECG = Epicatechin-3-gallate, Rap = Rapamycin.
To define the cellular localization of IL-1β, we double labeled sections with three different combinations of double immunofluorescence staining: IL-1β with GFAP for astrocytes (Fig. 2J), with NeuN for neurons (Fig. 2K) and with Iba1 for microglia (Fig. 2L). IL-1β colocalized with GFAP positive cells, but not with NeuN or Iba1 positive cells in the brains of four-week-old Tsc1GFAPCKO mice.
CXCL10 protein is upregulated in Tsc1GFAPCKO mice and reversed by anti-inflammatory treatment in vivo and in vitro
Western blot analysis was used to measure protein levels of CXCL10. CXCL10 was increased in the brains of four-week-old Tsc1GFAPCKO mice compared to control mice (Fig. 3A), as well as in cultured astrocytes from Tsc1GFAPCKO mice (Fig. 3B). Rapamycin treatment at the dose of 3 mg/kg/d significantly reversed the up-regulated protein levels of CXCL10 in the brain of the four-week-old Tsc1GFAPCKO mice in vivo, compared with vehicle treated Tsc1GFAPCKO mice (Fig. 3A). Similarly, rapamycin treatment at the dose of 2 ng/ml for 16 hours, down-regulated the protein level of CXCL10 in cultured astrocytes of Tsc1GFAPCKO mice compared to vehicle treated astrocytes of Tsc1GFAPCKO mice (Fig. 3B). Furthermore, ECG treatment at the dose of 12.5 mg/kg/d started at the age of three weeks for seven days, significantly down-regulated the protein levels of CXCL10 in the brains of Tsc1GFAPCKO mice compared to vehicle-treated Tsc1GFAPCKO mice (Fig. 3C).
Figure 3. CXCL10 protein is upregulated in Tsc1GFAPCKO mice and reversed by anti-inflammatory treatment in vivo and in vitro.
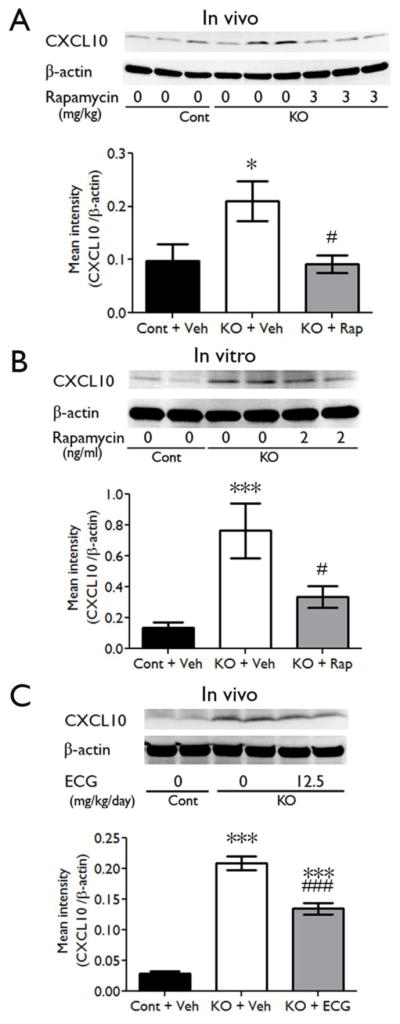
Protein expression of CXCL10 was assessed by western blotting in the brains and cultured astrocytes of Tsc1GFAPCKO mice, and the effects of rapamycin and ECG were tested. (A) Vehicle-treated Tsc1GFAPCKO mice have significantly increased CXCL10 levels, compared with control mice. Rapamycin treatment (3 mg/kg/d i.p. for seven days) significantly inhibited the upregulated-CXCL10 in Tsc1GFAPCKO mice (7–11 mice/group). (B) Vehicle-treated cultured astrocytes from Tsc1GFAPCKO mice showed increased CXCL10 expression compared with astrocytes from control mice. Rapamycin treatment (2 ng/ml added to the culture medium for 16 hours) blocked the up-regulation of CXCL10 in Tsc1GFAPCKO astrocyte (n=8–12 mice/group). (C) Vehicle-treated Tsc1GFAPCKO mice have significantly increased CXCL10 protein expression compared with control mice. ECG treatment (12.5 mg/kg/d i.p. for seven days) inhibited the increased CXCL10 in Tsc1GFAPCKO mice, but this was still significantly higher than control mice (n=8 mice/group). * p<0.05, *** p<0.001, versus vehicle-treated control mice or astrocytes by one-way ANOVA; # p<0.05, ### p<0.001, versus vehicle-treated Tsc1GFAPCKO mice or astrocytes by one-way ANOVA (n = 9–12 mice/group). Cont = control, KO = Tsc1GFAPCKO, Veh = vehicle, Rap = Rapamycin, ECG = Epicatechin-3-gallate.
Lack of evidence of peripheral inflammation in Tsc1GFAPCKO mice
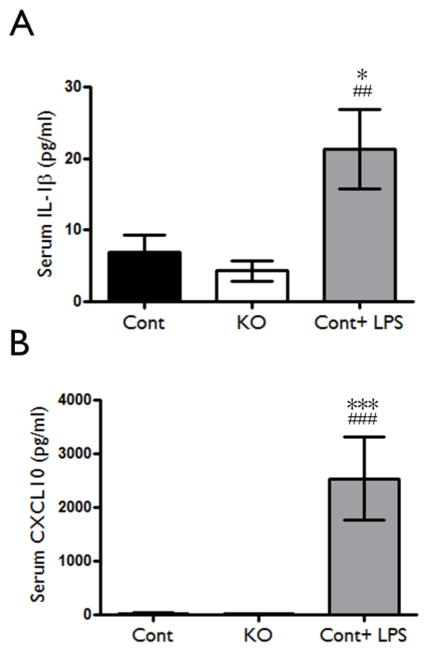
In other models of epilepsy, systemic inflammation and breakdown of the blood-brain barrier may promote brain inflammation. In Tsc1GFAPCKO mice, the targeted genetic manipulation of Tsc1 gene inactivation in GFAP-expressing cells in the brain and the demonstration of inflammatory markers in cultured cells in vitro make this possibility unlikely, especially as an early event before seizure onset. However, to investigate the potential contribution of systemic inflammatory factors in Tsc1GFAPCKO mice, serum expression of inflammatory markers was assessed. Although lipopolysaccharide injection in control mice caused an expected increase in serum IL-1β and CXCL10 levels assayed by ELISA, 4 week old Tsc1GFAPCKO mice had no increase in these inflammatory markers in the serum (Fig. 4A, B). Thus, the early inflammatory changes observed in the brains of Tsc1GFAPCKO mice are most likely due to modulation of innate brain immunity, not infiltration of peripheral immune mediators.
Figure 4. Lack of evidence for systemic inflammation in Tsc1GFAPCKO mice.
Serum inflammatory markers were assessed in Tsc1GFAPCKO mice to evaluate the potential involvement of the peripheral immune system in contributing to brain inflammation. There was no significant difference in serum IL-1β (A) and CXCL10 (B) protein levels as assayed by ELISA in 4 week-old Tsc1GFAPCKO mice compared with control mice, while LPS induced a significant increase in these markers in control mice. * p<0.05, *** p<0.001, versus vehicle-treated control mice by one-way ANOVA; ## p<0.01, ### p<0.001, versus vehicle-treated Tsc1GFAPCKO mice by one-way ANOVA (n = 5–9 mice/group). Cont = control, KO = Tsc1GFAPCKO.
ECG treatment slightly decreases brain weight and glial proliferation, but does not affect body weight or neuronal organization of Tsc1GFAPCKO mice
The effect of rapamycin on pathological abnormalities and epilepsy has been previously reported (Zeng et al., 2008). We similarly tested the effects of anti-inflammatory treatment with ECG on the neurological phenotype of Tsc1GFAPCKO mice. Consistent with previous studies (Uhlmann et al., 2002; Zeng et al., 2008), vehicle-treated Tsc1GFAPCKO mice developed dramatic, diffuse megalencephaly (brain weight = 519.0±10.6 mg at 7 weeks of age) compared with non-KO control mice (389.2±3.2 mg; p<0.001). ECG treatment at the dose of 12.5 mg/kg/d for four weeks slightly improved the megalencephaly in Tsc1GFAPCKO mice (485.0±8.7; p<0.05 compared with vehicle-treated Tsc1GFAPCKO mice). At 7 weeks of age, there was a trend towards a decrease in body weight in vehicle-treated Tsc1GFAPCKO mice (15.7±0.5 g) compared with non-KO control mice (18.3±0.9 g). ECG treatment had no significant effect on body weight of Tsc1GFAPCKO mice (14.9±0.8 g).
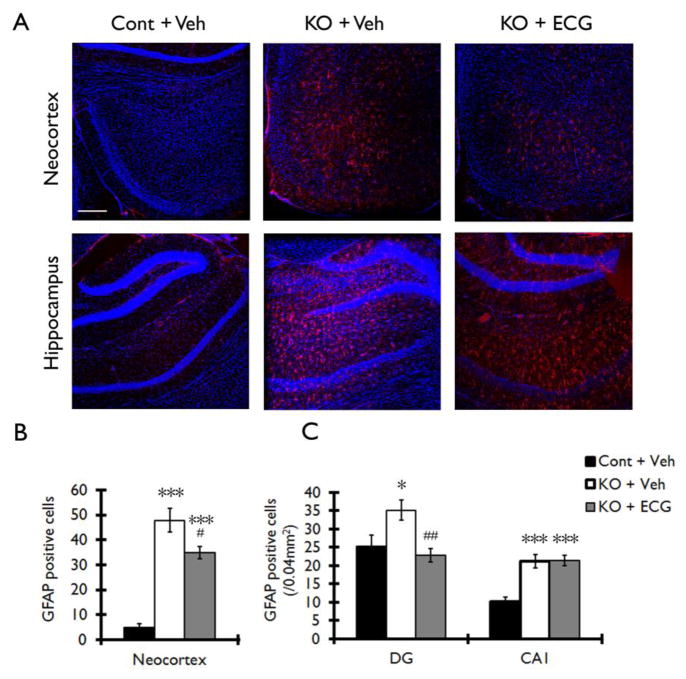
Tsc1GFAPCKO mice exhibit a progressive excessive glial proliferation (Uhlmann et al. 2002; Zeng et al., 2008). Consistent with previous studies, vehicle-treated Tsc1GFAPCKO mice showed a large increase in GFAP-positive cells in neocortex and hippocampus compared with non-KO control mice (Fig. 5A). ECG treatment at the dose of 12.5 mg/kg/d for four weeks caused a significant decrease in the number of GFAP positive cells in neocortex and dentate gyrus, but not in CA1 of hippocampus, of Tsc1GFAPCKO mice (Fig. 5B, C).
Figure 5. ECG treatment decreases the number of GFAP positive cells in neocortex and hippocampus of Tsc1GFAPCKO mice.
The effect of ECG on the number of GFAP positive cells was assessed in Tsc1GFAPCKO mice by immunohistochemistry. (A) Vehicle-treated Tsc1GFAPCKO mice (KO + Veh) displayed a diffuse increase in GFAP-positive cells (red) in neocortex (upper middle panel) and hippocampus (lower middle panel) compared with the vehicle-treated control mice (Cont + Veh). ECG treatment partially prevented this increase in GFAP-positive cells in Tsc1GFAPCKO mice (KO + ECG). TO-PRO-3 Iodide (blue) was used as an optimal fluorescence dye for nuclear counterstaining. (B, C) Quantitative analysis confirmed an increase in GFAP-positive cells in vehicle-treated Tsc1GFAPCKO group (KO + Veh) compared with vehicle-treated control group (Cont + Veh) in neocortex, dentate gyrus (DG) and CA1 of hippocampus. ECG treatment (12.5 mg/kg/d i.p. for four weeks) decreased GFAP-positive cells in neocortex and DG, but not in CA1 (KO + ECG; n=8 mice/group). *p<0.05, *** p<0.001 versus vehicle-treated control mice by two-way ANOVA; #p<0.05, ## p<0.01 versus vehicle-treated Tsc1GFAPCKO mice by two-way ANOVA. Scale bar = 200 μm. Cont = control mice, KO = Tsc1GFAPCKO mice, Veh = vehicle, ECG = Epicatechin-3-gallate, DG = dentate gyrus, CA1 = CA1 pyramidal cell layer of hippocampus.
Tsc1GFAPCKO mice exhibit disorganization and dispersion of the pyramidal cell layer of hippocampus (Uhlmann et al., 2002; Zeng et al., 2008). Consistent with previous studies, Cresyl violet staining demonstrated that vehicle-treated Tsc1GFAPCKO mice had widely dispersed pyramidal cell layers (Fig. 6B) in all regions of hippocampus (CA1–CA4) compared with control mice (Fig. 6A). ECG treatment had no apparent effect on this neuronal disorganization in Tsc1GFAPCKO mice (Fig. 6C). Quantitative analysis showed a significant increase in the width of the CA1 pyramidal layer in vehicle-treated Tsc1GFAPCKO mice (114.8 ±1.6 μm) compared with control mice (67.0 ± 2.4 μm; p<0.001 by one-way ANOVA, n=8 mice/group), but no effect of ECG in Tsc1GFAPCKO mice (109.8 ± 0.9 μm).
Figure 6. ECG treatment does not prevent neuronal disorganization in Tsc1GFAPCKO mice.
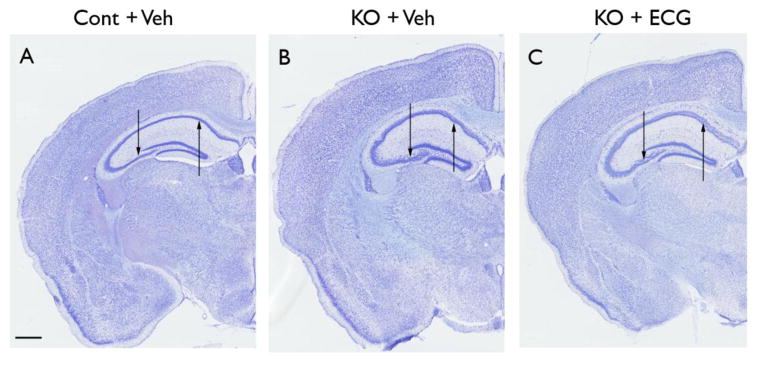
The effect of ECG on neuronal organization was assessed in Tsc1GFAPCKO mice by cresyl violet staining. Compared with control mice (A), vehicle-treated Tsc1GFAPCKO mice (B) exhibited widely dispersed pyramidal cell layers (arrows) in all regions of hippocampus (CA1–CA4). ECG treated Tsc1GFAPCKO mice (C) had a similar pattern as vehicle-treated Tsc1GFAPCKO group (B), with no apparent effect on this neuronal disorganization. Scale bar = 500 μm. Cont = control mice, KO = Tsc1GFAPCKO mice, Veh = vehicle, ECG = Epicatechin-3-gallate.
ECG treatment moderately decreases the development of seizures and improves survival in Tsc1GFAPCKO mice
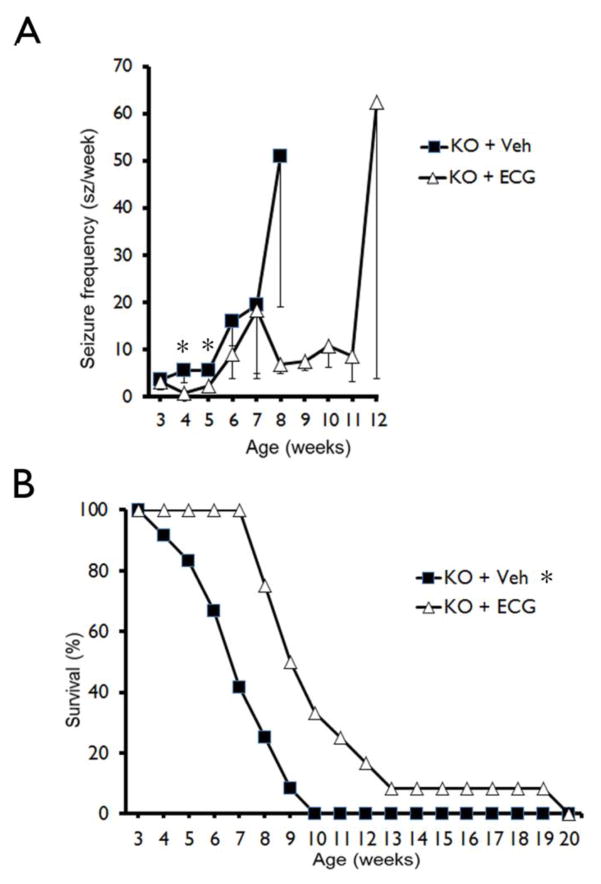
Tsc1GFAPCKO mice develop progressive epilepsy starting around three to four weeks of life and then die prematurely by around seven to ten weeks of age (Erbayat-Altay et al, 2007; Zeng et al., 2011). In the present study, video-EEG monitoring again showed that seizures developed in vehicle-treated Tsc1GFAPCKO mice around 3–5 weeks of life (3.6±2.1 seizures/week at 3 weeks) and became progressively more frequent with age (19.6±14.7 at 7 weeks). ECG treatment (12.5 mg/kg/d i.p.) caused a small, but significant decrease in seizure frequency in Tsc1GFAPCKO mice (Fig. 7A). However, all mice had at least one seizure in both vehicle-treated and ECG-treated Tsc1GFAPCKO mice.
Figure 7. ECG treatment slightly decreases the development of seizures and improves survival in Tsc1GFAPCKO mice.
(A) Seizures start to develop in vehicle-treated Tsc1GFAPCKO mice (A, KO + Veh) around 3 weeks and become progressively more frequent with age. ECG treatment (12.5 mg/kg/d i.p. starting at 3 weeks of age (KO + ECG) slightly decreased seizure frequency in Tsc1GFAPCKO mice at 4 and 5 weeks of age compared with vehicle-treated Tsc1GFAPCKO mice. (*p<0.05 by one-way ANOVA, n = 12 mice/group). However, all mice were found to have at least one seizure in both vehicle-treated and ECG-treated Tsc1GFAPCKO mice. (B) Survival analysis showed that vehicle-treated Tsc1GFAPCKO mice die prematurely with 50% mortality between 6–7 weeks of age and 100% mortality by 10 weeks. ECG treatment (12.5 mg/kg/d i.p. starting at 3 weeks of age) significantly improved the survival of Tsc1GFAPCKO mice compared to vehicle treated Tsc1GFAPCKO mice, but all ECG-treated mice still died by 20 weeks of age. *p<0.05 by Chi-Square test, comparing the two groups (n = 12 mice/group). KO = Tsc1GFAPCKO, Veh = vehicle, ECG = Epicatechin-3-gallate.
Survival analysis confirmed previous studies demonstrating that vehicle-treated Tsc1GFAPCKO mice die prematurely, with 50% mortality between six to seven weeks of age and 100% mortality by ten weeks of age. ECG treatment caused a significant increase in survival of Tsc1GFAPCKO mice compared to vehicle-treated Tsc1GFAPCKO mice; all animals survived to seven weeks of age, and 50% of mice still survived at nine weeks of age. However, all ECG-treated mice eventually died by twenty weeks of age (Fig. 7B).
DISCUSSION
TSC is one of the most common single-gene disorders causing drug-resistant epilepsy, intellectual disability, and autism. Proinflammatory mechanisms have been implicated in contributing to the pathophysiology of epilepsy, especially acquired epilepsy due to brain injury. However, the role of brain inflammation in developmental or genetic epilepsies is relatively unexplored. In this study, we provide evidence that inflammatory signaling mechanisms, particularly the cytokine IL-1β and chemokine CXCL10, are abnormally activated in a mouse model of TSC. These inflammatory mediators were reversed by the mTORC1 inhibitor, rapamycin, indicating that these mechanisms are downstream from mTORC1, and occurred in astrocyte culture in vitro and before epilepsy onset in vivo, indicating that these changes were not secondary to seizures. Furthermore, inhibition of IL-1β and CXCL10 by ECG at least partially reduced seizure frequency and prolonged survival of Tsc1GFAPCKO mice, suggesting a potential role of anti-inflammatory treatments for epilepsy and other neurological manifestations in TSC.
Mechanisms of epileptogenesis in TSC are still poorly understood. In many cases, epilepsy may be caused by the focal malformations of cortical development, the tubers, which are the pathological hallmarks of TSC. However, beyond tubers, a variety of cellular and molecular abnormalities have been implicated in epileptogenesis in mouse models of TSC and pathological specimens from TSC patients (Wong, 2008). Independent of tumor growth, the mTORC1 pathway may regulate specific cellular and molecular mechanisms of epileptogenesis, such as neuronal death, synaptic reorganization, and expression of ion channels and neurotransmitter receptors (Wong, 2010, 2013). mTORC1 inhibitors can prevent the development of epilepsy and inhibit ongoing seizures in mouse models of TSC (Goto et al., 2011; Meikle et al., 2008; Zeng et al., 2008, 2011), as well as in some models of acquired epilepsy due to brain injury (Berdichevsky et al., 2013; Guo et al., 2013; Huang et al., 2010; van Vliet et al., 2012; Zeng et al., 2009). Preliminary clinical trials suggest that mTOR inhibitors may be effective in reducing seizures in TSC patients with refractory epilepsy (Krueger et al., 2013). Even if mTOR plays a critical role in epilepsy in TSC, the specific mechanisms downstream from mTOR causing epileptogenesis are poorly understood. The results from the present study suggest that inflammatory processes may represent downstream mTOR-mediated mechanisms that contribute to epileptogenesis in our mouse model of TSC. There is substantial evidence for interactions between the mTOR pathway and inflammatory mechanisms in the periphery (Weichhart and Saemann, 2009), but much less is known about this relationship in innate brain immunity.
The role of brain inflammation in the pathophysiology of various types of epilepsy has received increasing attention, especially in response to epileptogenic brain injuries (Vezzani et al., 2013a, 2013b; Xu et al., 2013). Different inflammatory mediators and pathways, such as cytokines and chemokines, are activated in a variety of animal models of epilepsy, including chemoconvulsant and electrical kindling models of epilepsy, as well as in human tissue obtained from epilepsy patients, such as with mesial temporal sclerosis (De Simoni et al., 2000; Fabene et al., 2010; Li et al., 2011; Ravizza et al., 2008a). Furthermore, anti-inflammatory treatments targeting these pathways have begun to be explored. For example, selective pharmacological inhibition of the cytokine IL-1β production in astrocytes inhibits seizures in rats (Maroso et al., 2011; Ravizza et al., 2008b). Although similar inflammatory markers have also been found in tubers from TSC patients (Boer et al., 2008, 2010; Maldonado et al., 2003; Prabowo et al., 2013), the pathophysiological significance of inflammation in TSC, such as for epilepsy, is unknown. The findings from our mouse study support a potential pathogenic role of specific cytokines and chemokines in TSC. These cytokines and chemokines, such as IL-1β and CXCL10, may directly regulate neuronal excitability and other processes involved in epileptogenesis (Fabene et al., 2010; Li et al., 2011). As ECG, an inhibitor of IL-1β and CXCL10, could reduce seizures and prolong survival in Tsc1GFAPCKO mice, this suggests that these inflammatory processes, at least partially, contribute to epileptogenesis in these mice. Furthermore, the reversal of these inflammatory mediators by rapamycin indicates that cytokine and chemokine signaling is downstream from mTORC1 and may partially account for the antiepileptogenic effects of mTORC1 inhibition previously reported in Tsc1GFAPCKO mice (Zeng et al., 2008). The possibility that ECG could have other off-target effects is a limitation of this study and should be further evaluated by additional pharmacological and genetic approaches manipulating cytokine and chemokine signaling. However, ECG does not appear to directly inhibit mTOR activity (Zhang and Wong, unpublished data), indicating that its effects in Tsc1GFAPCKO mice are mediated by mechanisms independent of, or more likely, downstream from mTOR.
Many inflammatory reactions in the brain, such as in the cytokine system, appear to be most closely associated with glial cells, including reactive astrocytes and activated microglia. Our data indicate that at least IL-1β activation occurs predominantly in astrocytes from Tsc1GFAPCKO mice. Previous studies have demonstrated a number of cellular and molecular abnormalities in astrocytes that contribute to epileptogenesis in these mice (Jansen et al., 2005; Uhlmann et al., 2002; Wong et al., 2003; Xu et al., 2009). Thus, the current findings suggest that innate immunity or inflammatory mechanisms specifically in glia may mediate and coordinate epileptogenic mechanisms in TSC and support the recent trend emphasizing the novel role of non-neuronal cells in epilepsy. Future studies using additional cell-targeted manipulations can help define the contribution of different brain cell types in mediating inflammatory responses and epileptogenesis in TSC.
In addition to innate immunity within the brain, systemic inflammation and associated breakdown of the blood-brain barrier has also been strongly implicated in some forms of epilepsy (Kim et al., 2012; Marchi et al., 2012; Riazi et al. 2010). For example, status epilepticus and other acquired brain injuries that lead to epilepsy may elicit systemic inflammatory mechanisms, including cytokines, which lead to breakdown of the blood-brain barrier (Marchi et al., 2009). This breakdown of the blood brain barrier can lead to infiltration of pathogenic systemic inflammatory mediators, immune cells or other toxic substances into the brain that may promote epileptogenesis (Fabene et al., 2008; Ivens et al., 2007). In fact, astrocytic proteins may be involved in triggering peripheral immune responses in the context of brain injury and blood brain barrier breakdown (Bargerstock et al. 2014; Marchi et al. 2013; Zhang et al. 2014). In contrast to epilepsy related to acquired brain injuries, much less is known about a potential role of systemic inflammation in genetic epilepsies such as TSC. Despite the presence of brain inflammation in Tsc1GFAPCKO mice, we did not find any concurrent evidence of peripheral inflammation, suggesting that systemic inflammation is not directly involved in epileptogenesis in these mice. The importance of innate brain immunity in Tsc1GFAPCKO mice is further supported by the finding of inflammatory changes in astrocyte culture and their reversal by rapamycin in vitro. Future studies can further address whether there is any evidence of blood brain barrier breakdown in these mice.
Limitations of this study include uncertainties about the causative role of inflammatory mechanisms in epileptogenesis. Based on the timing of cytokine and chemokine elevation occurring before onset of epilepsy, as well as similar findings in vitro, the inflammation changes appear to be primary events that may contribute to epileptogenesis, not secondary to seizures. However, most of this data are simply correlative. Again, the effect of ECG in the Tsc1GFAPCKO mice suggests that inflammatory mechanisms do contribute to the neurological phenotype of these mice, but need to be replicated with other anti-inflammatory treatments. In terms of the translational application of these findings, Tsc1GFAPCKO mice have some limitations in not fully recapitulating human TSC, in particular focal tuber-like lesions. As there are now a number of TSC mouse models available, examining inflammation in other brain-targeted TSC models is indicated.
Finally, this pre-clinical study provides initial proof-of-concept supporting potential translational applications of anti-inflammatory treatments for neurological manifestations of TSC. mTOR inhibitors are already being tested in clinical trials as treatments for epilepsy and cognitive deficits in TSC patients (Krueger et al., 2013). However, mTOR inhibitors potentially have significant side effects, such as immunosuppression and inhibition of mechanisms of normal growth, development, and learning. Thus, targeting mechanisms downstream from mTOR may maintain efficacy but reduce side effects. Anti-inflammatory drugs may represent rational candidates for developing novel antiepileptogenic or antiseizure treatments for TSC.
In summary, this study identifies inflammatory mechanisms involving specific cytokines and chemokines which are abnormally activated in a mouse model of TSC. Inhibition of these mechanisms was associated with a decrease in seizures and improved survival in these mice, providing proof-of-concept that anti-inflammatory treatments represent potential therapy for this genetic epilepsy.
Supplementary Material
Highlights.
Cytokines and chemokines are activated in a mouse model of tuberous sclerosis.
An antinflammatory agent, epicatechin, decreases seizures and prolongs survival in this model.
This study implicates inflammatory mechanisms in contributing to a genetic epilepsy.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 NS056872 to MW and S10 RR027552 to Washington University) and the Department of Defense (W81XWH-12-1-0190 to MW), and by the Alafi Neuroimaging Lab at Washington University.
ABBREVIATIONS
- ANOVA
analysis of variance
- CKO
conditional knock-out
- ECG
epicatechin-3-gallatel
- EEG
electroencephalography
- GFAP
glial fibrillary acidic protein
- IL-1 β
interleukin-1β
- KO
knock-out
- mTOR
mammalian target of rapamycin
- mTORC1
mammmalian target of rapamycin complex 1
- RT-PCR
reverse transcriptase polymerase chain reaction
- SEGA
subependymal giant cell astrocytoma
- TSC
tuberous sclerosis complex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bargerstock E, Puvenna V, Iffland P, Falcone T, Hossain M, Vetter S, Man S, Dickstein L, Marchi N, Ghosh C, Carvalho-Tavares J, Janigro D. Is peripheral immunity regulated by blood-brain barrier permeability changes? PLoS One. 2014;9:e101477. doi: 10.1371/journal.pone.0101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevsky Y, Dryer AM, Saponijian Y, Mahoney MM, Pimentel CA, Lucini CA, Usenovic M, Staley KJ. PI3K-Akt signaling activates mTOR-mediated epileptogenesis in organotypic hippocampal culture model of post-traumatic epilepsy. J Neurosci. 2013;33:9056–9067. doi: 10.1523/JNEUROSCI.3870-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer K, Jansen F, Nellist M, Redeker S, van den Ouweland AM, Spliet WG, van Nieuwenhuizen O, Troost D, Crino PB, Aronica E. Inflammatory processes in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Epilepsy Res. 2008;78:7–21. doi: 10.1016/j.eplepsyres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Boer K, Crino PB, Gorter JA, Nellist M, Jansen FE, Spliet WG, van Rijen PC, Wittink FR, Breit TM, Troost D, Wadman WJ, Aronica E. Gene expression analysis of tuberous sclerosis complex cortical tubers reveals increased expression of adhesion and inflammatory factors. Brain Pathol. 2010;20:704–719. doi: 10.1111/j.1750-3639.2009.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele E. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–1241. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- Crouvezier S, Powell B, Keir D, Yaqoob P. The effects of phenolic components of tea on the production of pro- and anti-inflammatory cytokines by human leukocytes in vitro. Cytokine. 2001;13:280–286. doi: 10.1006/cyto.2000.0837. [DOI] [PubMed] [Google Scholar]
- De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, De Luigi A, Garattini S, Vezzani A. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci. 2000;12:2623–2633. doi: 10.1046/j.1460-9568.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- Erbayat-Altay E, Zeng LH, Xu L, Gutmann D, Wong M. The natural history and treatment of epilepsy in a murine model of tuberous sclerosis. Epilepsia. 2007;48:1470–1476. doi: 10.1111/j.1528-1167.2007.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabene PF, Bramanti P, Constantin G. The emerging role for chemokines in epilepsy. J Neuroimmunol. 2010;224:22–27. doi: 10.1016/j.jneuroim.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Fabene PF, Navarro Mora G, Martinello M, Rossi B, Merigo F, Ottoboni L, Bach S, Angiari S, Benati D, Chakir A, Zanetti L, Schio F, Osculati A, Marzola P, Nicolato E, Homeister JW, Xia L, Lowe JB, McEver RP, Osculati F, Sbarbati A, Butcher EC, Constantin G. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14:1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, Witt O, Kohrman MH, Flamini JR, Wu JY, Curatolo P, de Vries PJ, Whittemore VH, Thiele EA, Ford JP, Shah G, Cauwel H, Lebwohl D, Sahmoud T, Jozwiak S. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomized, placebo-controlled phase 3 trial. Lancet. 2013;381:125–132. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- Goto J, Talos DM, Klein P, Qin W, Chekaluk YI, Anderl S, Malinowska IA, Di Nardo A, Bronson RT, Chan JA, Vinters HV, Kernie SG, Jensen FE, Sahin M. Regulable neural progenitor-specific TSC1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex. Proc Natl Acad Sci USA. 2011;108:1070–1079. doi: 10.1073/pnas.1106454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo DG, Zeng LH, Brody DL, Wong M. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS One. 2013;8:e64078. doi: 10.1371/journal.pone.0064078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL, Stafstrom CE the Tuberous Sclerosis Study Group. Tuberous Sclerosis Complex and epilepsy: recent developments and future challenges. Epilepsia. 2007;48:617–630. doi: 10.1111/j.1528-1167.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- Hosokawa Y, Hosokawa I, Ozaki K, Nakanishi T, Nakae H, Matsuo T. Catechins inhibit CXCL10 production from oncostatin M-stimulated human gingival fibroblasts. J Nutr Biochem. 2010;21:659–664. doi: 10.1016/j.jnutbio.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, Seiffert E, Heinemann U, Friedman A. TFG-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130:535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- Jansen LA, Uhlmann EJ, Gutmann DH, Wong M. Epileptogenesis and reduced inward rectifier potassium current in Tuberous Sclerosis Complex-1 deficient astrocytes. Epilepsia. 2005;46:1871–1880. doi: 10.1111/j.1528-1167.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Kim SY, Buckwalter M, Soreq H, Vezzani A, Kaufer D. Blood-brain barrier dysfunction-induced inflammatory signaling in brain pathology and epileptogenesis. Epilepsia. 2012;53(Suppl 6):37–44. doi: 10.1111/j.1528-1167.2012.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T, Franz DN. Everolimus for subependymal giant-cell astrocytomas in Tuberous Sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- Krueger DA, Wilfong AA, Holland-Bouley K, Anderson AE, Agricola K, Tudor C, Mays M, Lopez CM, Kim MO, Franz DN. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol. 2013;74:679–687. doi: 10.1002/ana.23960. [DOI] [PubMed] [Google Scholar]
- Li G, Bauer S, Nowak M, Norwood B, Tackenberg B, Rosenow F, Knake S, Oertel WH, Hamer HM. Cytokines and epilepsy. Seizure. 2011;20:249–256. doi: 10.1016/j.seizure.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Maldonado M, Baybis M, Newman D, Kolson DL, Chen W, McKhann G, Gutmann DH, Crino PB. Expression of ICAM-1, TNF-α, NFκB, and MAP kinase in tubers of the tuberous sclerosis complex. Neurobiol Dis. 2003;14:279–290. doi: 10.1016/s0969-9961(03)00127-x. [DOI] [PubMed] [Google Scholar]
- Marchi N, Bazarian JJ, Puvenna V, Janigro M, Ghosh C, Zhong J, Zhu T, Blackman E, Stewart D, Ellis J, Butler R, Janigro D. Consequences of repeated blood-brain barrier disruption in football players. PLoS One. 2013;8:e56805. doi: 10.1371/journal.pone.0056805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Fan Q, Ghosh C, Fazio V, Berolini F, Betto G, Batra A, Carlton E, Najm I, Granata T, Janigro D. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis. 2009;33:171–181. doi: 10.1016/j.nbd.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Granatam T, Ghosh C, Janigro D. Blood-brain barrier dysfunction and epilepsy: pathophysiologic roles and therapeutic approaches. Epilepsia. 2012;53:1877–1886. doi: 10.1111/j.1528-1167.2012.03637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Bianchi ME, Vezzani A. Interleukin-1β biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics. 2011;8:304–315. doi: 10.1007/s13311-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of Tuberous Sclerosis to mammalian target of Rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova KA, Crino PB. The tuberous sclerosis complex. Ann NY Acad Sci. 2010;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabowo AS, Anink JJ, Lammens M, Mellist M, van den Ouweland AM, Adle-Biassette H, Sarnat HB, Flores-Sarnat L, Crino PB, Aronica E. Fetal brain lesions in tuberous sclerosis complex: TORC1 activation and inflammation. Brain Pathol. 2013;23:45–59. doi: 10.1111/j.1750-3639.2012.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza T, Boer K, Redeker S, Spliet WGM, van Rijen PC, Troost D, Vezzani A, Aronica E. The IL-1β system in epilepsy-associated malformations of cortical development. Neurobiol Dis. 2006;24:128–143. doi: 10.1016/j.nbd.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008a;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Noe F, Zardoni D, Vaghi V, Sifringer M, Vezzani A. Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1β production. Neurobiol Dis. 2008b;31:327–333. doi: 10.1016/j.nbd.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Riazi K, Galic MA, Pitmann QJ. Contributions of peripheral inflammation to seizure susceptibility: cytokines and brain excitability. Epilepsy Res. 2010;89:34–43. doi: 10.1016/j.eplepsyres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada KA, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Forte G, Holtman L, den Burger JC, Sinjewel A, de Vries HE, Aronica E, Gorter JA. Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood-brain barrier leakage but not microglia activation. Epilepsia. 2012;53:1254–1263. doi: 10.1111/j.1528-1167.2012.03513.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Aronica E, Mazarati A, Pittman QJ. Epilepsy and brain inflammation. Exp Neurol. 2013a;244:11–21. doi: 10.1016/j.expneurol.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2013b;69:16–24. doi: 10.1016/j.neuropharm.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Miller SD, Koh S. Immune mechanisms in epileptogenesis. Front Cell Neurosci. 2013;7:195. doi: 10.3389/fncel.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zeng LH, Wong M. Impaired astrocyte gap junction coupling and potassium buffering in a mouse model of Tuberous Sclerosis Complex. Neurobiol Dis. 2009;34:291–299. doi: 10.1016/j.nbd.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weicchart T, Saemann MD. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30:218–226. doi: 10.1016/j.it.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Wong M. Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development with abnormal glioneuronal proliferation. Epilepsia. 2008;49:8–21. doi: 10.1111/j.1528-1167.2007.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. Mammalian target of rapamycin (mTOR) inhibition as potential antiepileptogenic therapy: from tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:27–36. doi: 10.1111/j.1528-1167.2009.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. A critical review of mTOR inhibitors and epilepsy: from basic science to clinical trials. Expert Rev Neurotherap. 2013;13:657–669. doi: 10.1586/ern.13.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Ess KE, Uhlmann EJ, Jansen LA, Li W, Crino PB, Mennerick S, Yamada KA, Gutmann DH. Impaired astrocyte glutamate transport in a mouse epilepsy model of tuberous sclerosis complex. Ann Neurol. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–72. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Rensing NR, Zhang B, Gutmann DH, Gambello MJ, Wong M. TSC2 gene inactivation causes a more severe epilepsy phenotype than TSC1 inactivation in a mouse model of Tuberous Sclerosis Complex. Hum Mol Genet. 2011;20:445–454. doi: 10.1093/hmg/ddq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Yang L, Konishi Y, Maeda N, Sakanaka M, Tanaka J. Suppressive effects of phosphodiesterase type IV inhibitors on rat cultured microglial cells: comparison with other types of cAMP-elevating agents. Neuropharm. 2002;42:262–269. doi: 10.1016/s0028-3908(01)00174-5. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zoltewicz JS, Mondello S, Newsom KJ, Yang Z, Yang B, Kobeissay F, Guingab J, Glushakova O, Robicsek S, Heaton S, Buki A, Hannay J, Gold MS, Rubenstein R, Lu XC, Dave JR, Schmid K, Tortella F, Robertson CS, Wang KK. Human traumatic brain injury induces autoantibody response against glial fibrillary acidic protein and its breakdown products. PLoS One. 2014;9:e92698. doi: 10.1371/journal.pone.0092698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.