Abstract
Mother-to-child transmission (MTCT) of HIV-1 provides a model for studying the role of passively acquired antibodies in preventing HIV infection. We determined the titers of neutralizing antibodies (NAbs) against six primary isolates of clades B and CRF01_AE in sera from 45 transmitting and 45 nontransmitting mothers matched for the main independent factors associated with MTCT in Thailand. A lower risk of MTCT, particularly for intrapartum transmission, was associated only with higher NAb titers against the CRF01_AE strain, MBA. The envelope glycoprotein of this strain showed an unusually long V2 domain of 63 amino acids, encoding six potential N-linked glycosylation sites. We provided experimental data indicating that the extended V2 domain contributed to the higher level of resistance to neutralization by mothers' sera in this strain. Taken together the data suggest that some primary isolates with specific properties may be useful indicators for identifying protective antibodies.
Keywords: HIV, mother-to-child transmission, neutralizing antibodies
INTRODUCTION
Mother-to-child transmission (MTCT) is the leading source of human immunodeficiency virus (HIV) infection in children. In the absence of preventive measures, transmission may occur during pregnancy (in utero), labor or delivery (intrapartum) or after birth, through breastfeeding. Previous studies have shown that, despite a heterogeneous viral population in the mother, only homogeneous viral variants were generally transmitted to the infant (Ahmad et al., 1995; Samleerat et al., 2008; Scarlatti et al., 1993; Verhofstede et al., 2003; Wolinsky et al., 1992). Maternal neutralizing antibodies (NAbs) can cross the placental barrier, reaching high levels in the fetus at the end of pregnancy and protecting the infant against infection with numerous pathogens (Englund, Glezen, and Piedra, 1998; Safrit et al., 2004). Maternal antibodies are therefore among the selective host factors that could play a role in limiting transmission of neutralization-sensitive variants. However, conflicting results have been obtained concerning the role of maternal NAbs in reducing MTCT of HIV (Barin et al., 2006; Bongertz et al., 2001; Hengel et al., 1998; Lathey et al., 1999; Scarlatti et al., 1993). The differences in the results obtained may be due to differences in the methods used or populations studied. Particularly, analyses of the role of passively transferred antibodies should clearly separate cases of intrapartum transmission from in utero transmission: only in cases of intrapartum transmission can we be sure that exposure to the virus occurs in the presence of optimal levels of IgGs. Supporting a role of maternal NAbs in limiting MTCT, several recent molecular studies have shown that viruses transmitted are escape variants resistant to autologous maternal plasma (Dickover et al., 2006; Wu et al., 2006).
We previously hypothesized that broadly cross-neutralizing heterologous NAbs would protect babies against intrapartum HIV transmission. We measured NAb titers against primary isolates of various clades in sera from pregnant Thai women, for whom the time of transmission was known (Barin et al., 2006). We identified an association between higher titers of NAbs against a CRF01_AE primary isolate, MBA — belonging to the predominant clade in Thailand — and lower rates of intrapartum transmission. However, only one isolate per clade was used in this previous study. Here, we extended our previous study using several CRF01_AE strains in a different Thai population, selected on the basis of highly stringent criteria, and we confirmed the association previously observed. We also identified a specific feature of the envelope glycoprotein in this strain, accounting for this association, at least in part.
RESULTS AND DISCUSSION
Neutralizing activity of sera from mothers against six primary isolates
Neutralizing activity was tested against six heterologous primary isolates of different phenotypes in sera from 45 transmitting and 45 nontransmitting Thai mothers. Subjects were matched for baseline viral load and duration of zidovudine prophylaxis, the two main independent factors associated with MTCT in Thailand (Table 1A). Three of these primary isolates, MBA, LEA and C1712, belong to the predominant clade in Thailand, CRF01_AE. The other three, BIG, CHA and FRO, belong to the less prevalent clade B. None of the 90 sera displayed no neutralizing activity, whereas 15 sera (17%), including both transmitters and non-transmitters, showed neutralizing activity against all six strains. Although cross-clade NAbs were detected in several sera, subtype-specific neutralizing antibodies predominated (Table 1B, Figure 1A). Among the 80 CRF01_AE-infected mothers, 76 sera (95%) neutralized at least one strain of subtype CRF01_AE, a higher proportion than observed for strains of subtype B (50 of 80, 63 %). The opposite was previously observed when we explored serum samples from long-term non-progressors infected by subtype B variants (Braibant et al., 2006). However, the sensitivity to neutralization differed between CRF01_AE strains. The MBA strain was more resistant to neutralization than the other two CRF01_AE strains, LEA and C1712. Only 57 of 90 sera (63%) were able to neutralize MBA, whereas 75 (83%) and 81 (90%) sera neutralized C1712 and LEA, respectively (McNemar's test, P < 0.001 and P = 0.001, respectively). In contrast, the three subtype B isolates were each neutralized by a similar proportion of sera: BIG was neutralized by 39 (43%), CHA by 35 (39%) and FRO by 38 (42%) (McNemar's test not significant).
Table 1.
Baseline characteristics of transmitting and nontransmitting mothers (A) and comparison of detectable neutralizing antibodies against the six primary isolates in transmitting and nontransmitting mothers (B).
| A
| |||
|---|---|---|---|
| Characteristics | Nontransmitters (n = 45) | Transmitters (n = 45) | p value |
| Median (range) | Median (range) | Kruskal-Wallis test | |
| Age (years) | 26 (22–30) | 25 (23–30) | 0.61 |
| CD4 cell count (per mm3) | 352 (240–434) | 320 (220–450) | 0.96 |
| Viral load (log10 copies/mL) | 4.38 (3.89–4.75) | 4.41 (3.87–4.71) | 0.97 |
| Duration of zidovudine prophylaxis (weeks) | 6 (4–11) | 6 (4–11) | 0.92 |
| Hemoglobin level (g/dL) | 10.6 (10.1–11.5) | 10.4 (9.8–11.2) | 0.23 |
| Hematocrit (%) | 32 (30–35) | 32 (30–34) | 0.33 |
| Gestational age at sample acquisition (weeks) | 26 (22–26) | 26 (21–26) | 0.60 |
| Sample acquisition to delivery (weeks) | 14 (13–17) | 14 (12–19) | 0.98 |
| B
| ||||
|---|---|---|---|---|
| Presence of detectable NAbs against: | Nontransmitters (n = 45) | Transmitters (n = 45) | p value | |
| n (%) | n (%) | Fisher’s exact test | CLR | |
| BIG (B/R5) | 22 (49%) | 17 (38%) | 0.40 | 0.30 |
| CHA (B/R5X4) | 18 (40%) | 17 (38%) | 1.00 | 0.84 |
| FRO (B/X4) | 20 (44%) | 18 (40%) | 0.83 | 0.67 |
| LEA (CRF01_AE/R5) | 41 (91%) | 40 (89%) | 1.00 | 0.74 |
| MBA (CRF01_AE/R5X4) | 34 (76%) | 23 (51%) | 0.03 | 0.02 |
| C1712 (CRF01_AE/X4) | 37 (82%) | 38 (84%) | 1.00 | 0.78 |
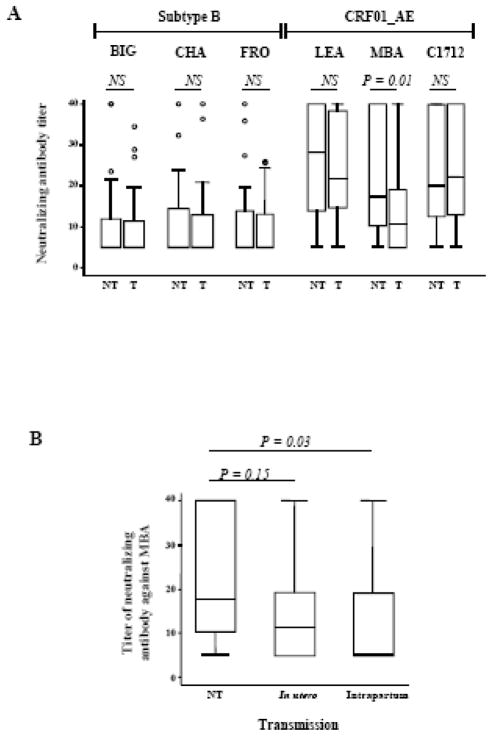
Figure 1. Comparison of neutralizing antibody titers in transmitting (T) and nontransmitting (NT) mothers.
(A) Comparison of NAb titers against the six strains in transmitting and nontransmitting mothers. Box plots show the distribution of maternal antibody titers; for each distribution, the horizontal lines represent the lower adjacent 25th, median, 75th, and upper adjacent percentiles. (B) Comparison of NAb titers against the MBA strain in nontransmitting mothers and transmitting mothers according to time of transmission (in utero or intrapartum). Nab titers were compared using the Wilcoxon matched-pairs signed-ranks test. NS: not significant.
The percentage of mothers with detectable NAbs against the three subtype B isolates and two of the CRF01_AE isolates (LEA and C1712) was similar between transmitting and nontransmitting mothers (Table 1B). In contrast, NAbs against MBA — the most resistant CRF01_AE strain to neutralization — were detected in 34 of 45 nontransmitting mothers (76%), a proportion significantly higher than that in transmitting mothers (23 of 45, 51%) (Fisher’s exact test: P=0.03; conditional logistic regression, CLR: P=0.02, odds ratio 3.8; 95% confidence interval: 1.2 to 11.3) (Table 1B). This difference remained significant if the analysis was restricted to the 80 CRF01_AE-infected mothers (CLR: P=0.01, odds ratio 6.5; 95% confidence interval: 1.5 to 28.8). Similarly, the distribution of titers against BIG, CHA, FRO, LEA and C1712 did not differ significantly between transmitting and nontransmitting mothers, whereas higher titers against MBA were observed in nontransmitting mothers (Wilcoxon signed rank, P = 0.01) (Figure 1A). High titers of NAbs against MBA remained associated with a lower rate of HIV-1 transmission when the analysis was restricted to CRF01_AE-infected mothers (Wilcoxon signed rank, P=0.009).
Neutralizing antibodies against MBA were undetectable in 15 of the 29 mothers who transmitted the virus intrapartum (52%) but in only seven of 29 (24%) matched nontransmitting mothers (Fisher’s exact test, P = 0.06; CRL, P = 0.05), and were undetectable in six (43%) of the 14 mothers who transmitted the virus in utero but only two of 14 (14%) matched nontransmitting mothers (Fisher’s exact test, P = 0.21; CRL, P = 1). Levels of neutralizing antibodies against MBA were significantly lower in mothers who transmitted the virus intrapartum than in matched nontransmitting mothers (Wilcoxon signed rank, P = 0.03). In contrast, they were not statistically different between mothers who transmitted the virus in utero and nontransmitting mothers (Wilcoxon signed rank, P = 0.15) (Figure 1B). These findings confirm the results of a previous study of a random sample of 28 transmitting and 62 nontransmitting mothers (Barin et al., 2006). Of the 45 transmitting and the 45 nontransmitting women who participed in the present study, only 14 (31%) and 3 (7%) were also included in the previous study. This indicates that the population was substantially different from the previous study, and therefore strengthens the finding. Such antibodies may thus be associated with a lower risk of MTCT. As intrapartum exposure to HIV resembles a natural challenge in the presence of preexisting antibodies, it might be possible to identify correlates of protection useful for ongoing or future vaccine studies.
Molecular characteristics of the MBA envelope glycoprotein
The molecular characteristics of the envelope glycoprotein of MBA may at least partially explain the association between a reduced risk of MTCT and the presence of NAbs against this isolate. We thus studied the env gene in the three CRF01_AE strains. Alignment of the Env amino-acid sequences from the three strains revealed a major difference between MBA and the other two strains (Figure 2). MBA Env had an unusually long V2 domain of 63 amino acids (23 and 25 amino acids longer than that of LEA and C1712, respectively), encoding six potential N-glycosylation sites (PNGS) versus only two PNGS in LEA and C1712. Searches of homology between MBA Env and sequences found in the HIV sequence database, using the HIV Blast program, revealed that this extended MBA V2 domain is unique. A detailed analysis of the V2 domains of the 68 CRF01_AE strains present in the web-alignment of the full spectrum of HIV sequences (http://www.hiv.lanl.gov/content/sequence/NEWALIGN/align.html) from the HIV sequence database, revealed that the longest of these V2 domains was composed of 53 amino acids (accession number U51188) and the V2 domain with the greatest number of potential glycosylation sites encoded five PNGS (accession number AY444805). In addition to this long MBA V2 domain, three PNGS were found in the C2 constant domain, the V4 variable loop and the membrane proximal external region (MPER) of the MBA envelope glycoproteins (Figure 2). We also identified a deletion of seven amino acids in the gp41 cytoplasmic tail in MBA, but not in LEA or C1712. The three PNGS observed in MBA are not specific to this strain. Indeed, two PNGS are present in the C2 and MPER domains in the envelope protein of the strain HxB2, which is highly sensitive to neutralization. Additionally, the envelope proteins of several other CRF01_AE strains possess the two PNGS in the V4 and MPER domains (data not shown).
Figure 2. Sequence characteristics, and their location, in the MBA envelope glycoprotein.
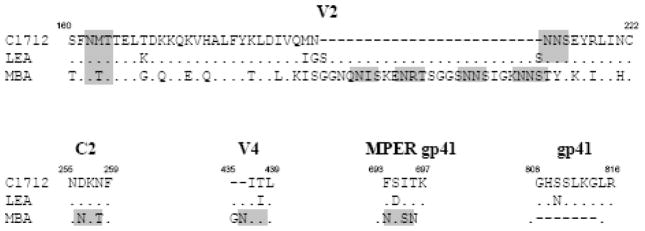
The Env amino-acid sequences of C1712, LEA and MBA were aligned using the BioEdit package version 5.0.9. Only regions showing characteristics specific to MBA are indicated. Amino-acid numbering is based on MBA amino-acid Env sequence. Identical amino acids and insertions are indicated by dots or dashes, respectively. PNGS (NXT or NXS) are highlighted in grey.
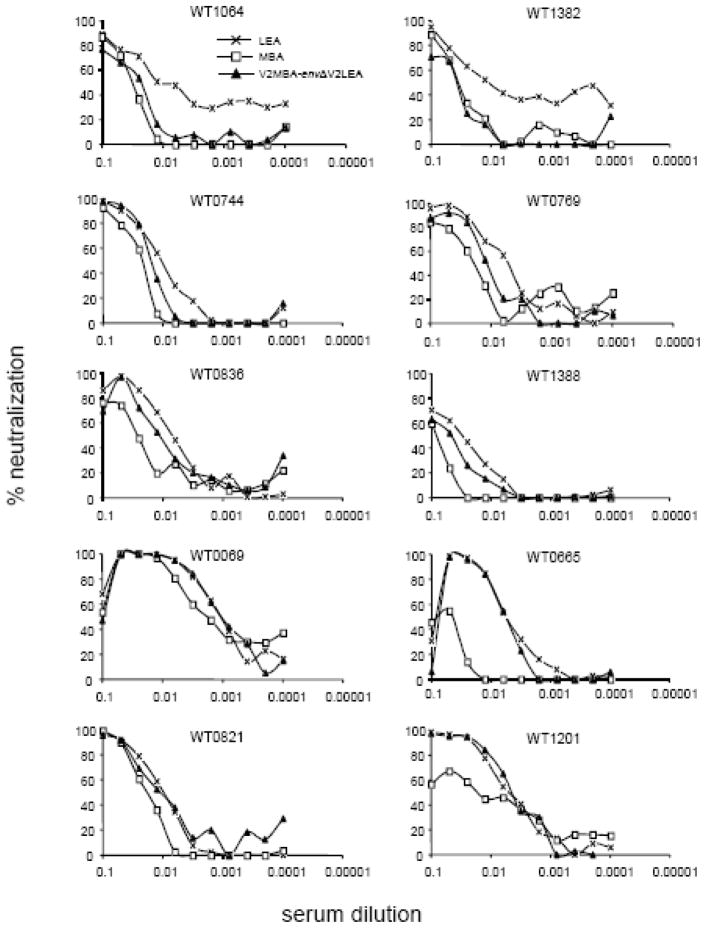
Several reports having shown that the V1V2 domain strongly influences sensitivity to neutralization by masking neutralizing targets (Pinter et al., 2004; Rong et al., 2007; Sagar et al., 2006; Shibata et al., 2007; Wolk and Schreiber, 2006), we therefore hypothesized that this unusual V2 domain contributes to the greater resistance to neutralization observed for MBA than that observed for LEA or C1712. To test this hypothesis, we produced pseudotyped viruses expressing the MBA and LEA wild-type Env proteins or two chimeric Env proteins, constructed by swapping the V2 domains (V2LEA–EnvΔV2MBA and V2MBA–EnvΔV2LEA). The chimeric V2LEA-EnvΔV2MBA pseudotyped virus, expressing the V2 domain of LEA in an MBA Env backbone, was not infectious. We therefore compared the neutralization profiles of the three other viruses, expressing MBA, LEA and V2MBA–EnvΔV2LEA, using 10 serum samples from mothers whose sera were more efficient at neutralizing LEA than MBA (Figure 3). The MBA V2 domain conferred a higher level of resistance to neutralization to LEA in the presence of six of the 10 sera (Figure 3; Table 2). Indeed, the chimeric virus displayed neutralization profiles for sera WT1064 and WT1382 similar to those of MBA, but with a level of sensitivity to neutralization approximately three times lower than that displayed by LEA. The neutralization profiles of the chimeric virus in the presence of sera WT0744, WT0769, WT0836 and WT1388 were intermediate between those of LEA and MBA, with LEA displaying a level of sensitivity to neutralization that was between 1.5 and 2.2 times higher. In contrast, the presence of the MBA V2 region did not affect the neutralization profile of the chimeric virus in the presence of sera WT0069, WT0665, WT0821 and WT1201. These results indicate that the V2 region of MBA is involved in determining neutralization resistance in this strain, but that other determinants may be involved.
Figure 3. Effect of the MBA V2 domain on LEA neutralization.
Neutralization activity in sera from 10 mothers was determined against viruses pseudotyped with the parental Env proteins from LEA (crosses) and MBA (open squares), and with the chimeric envelope protein V2MBAEnvΔV2LEA (closed triangles). Percentage of neutralization is plotted against serum dilution. The sera used are indicated at the top of each panel.
Table 2.
Neutralizing antibody titers in 10 serum samples against LEA, MBA and V2MBA-EnvΔV2LEA pseudotyped viruses.
| Serum | LEA | MBA | V2MBA–EnvΔV2LEA |
|---|---|---|---|
| WT1064 | 100 | 32.5 | 44.2 |
| WT1382 | 95 | 30.5 | 28.1 |
| WT0744 | 98.7 | 46.8 | 66.8 |
| WT0769 | 193.2 | 54.6 | 87 |
| WT0836 | 146.1 | 38.1 | 89.8 |
| WT1388 | 34 | 12.7 | 21.6 |
| WT0069 | 981 | 572.9 | 1014.7 |
| WT0665 | 182.9 | 22.3 | 183.2 |
| WT0821 | 108.7 | 57.3 | 93.5 |
| WT1201 | 217.1 | 65.1 | 240.3 |
Taken together, our data suggest that the antibodies involved in reducing the risk of MTCT would be potent antibodies targeting either epitopes exposed on the large V2 domain itself or, alternatively, distant epitopes exposed by a particular conformation imposed by this large heavily glycosylated structure. Interestingly, Pinter et al showed that the V1V2 domain is a global regulator of sensitivity of primary isolates to neutralization by antibodies commonly induced upon infection, but not by rare antibodies able to neutralize a broad range of primary isolates, such as the human monoclonal antibodies 2F5, 2G12 or b12 (Pinter et al., 2004). Further characterization of maternal antibodies with potent neutralizing activity against MBA and associated with a lower risk of MTCT, may therefore allow the identification of key epitopes involved in neutralization and protection.
In conclusion, this study confirms that higher titers of maternal antibodies against a CRF01_AE primary isolate, MBA, are associated with a lower intrapartum risk of HIV-1 transmission in Thailand, and that the V2 domain of gp120 seems to have a major role in the neutralization process. Our results suggest that certain primary isolates may be indicative of a protective antibody response. Further studies aiming to select strains indicative of neutralization/protection may facilitate the identification of correlates of protection crucial for the development of protective vaccines. The MTCT model could be beneficial for such studies.
MATERIALS AND METHODS
Study population and samples collection
Samples were obtained from HIV-1-infected pregnant women enrolled in a clinical trial assessing various zidovudine (ZDV) treatment regimens for the prevention of MTCT in Thailand (Perinatal HIV Prevention Trial, PHPT-1), in which infants were not breastfed (Lallemant et al., 2000). The HIV-1 infection status of the infants was determined by HIV-1 proviral DNA PCR as previously described (Barin et al., 2006; Lallemant et al., 2000). We selected 45 transmitting mothers (cases) and 45 nontransmitting mothers (controls) from the original study. Subjects were matched for baseline maternal viral load and duration of maternal zidovudine prophylaxis, the two main independent factors associated with MTCT (Table 1A). Of the 45 transmitting mothers, 14 transmitted HIV-1 to their infants in utero and 29 transmitted the virus intrapartum. The timing of transmission was not specified for two of the mothers. Blood samples were collected at baseline, before the start of ZDV prophylaxis, four to 11 weeks before delivery. Maternal viruses were subtyped, by both V3 serotyping (Barin et al., 2006) and phylogenetic analysis of genomic sequences (Brand et al., 2004). Samples with discrepant results in V3 serotyping and genetic analysis were defined as indeterminate. HIV-1 CRF01_AE was identified in 80 (89%) and subtype B, in four (4%) of the 90 mothers. Six samples were indeterminate.
Heterologous primary isolates neutralization assay
The heterologous primary isolates selected for this study belonged to the two prevalent clades in Thailand (CRF01_AE and B) and displayed various phenotypes (X4, R5, dual-tropic). There were three clade B strains, FRO (X4), BIG (R5) and CHA (R5X4), and three CRF01_AE strains, C1712 (X4), LEA (R5) and MBA (R5X4). The titers of NAbs against the six primary isolates in each maternal serum sample were determined using the P4P cell assay, as previously described (Barin et al., 2004; Barin et al., 2006; Charneau et al., 1994). The assay was performed in duplicate and results were expressed as mean values. The neutralization titer was defined as the reciprocal of the serum dilution resulting in a 90% decrease in the number of infected cells two days after infection with 100 TCID50 (50% tissue culture infectious dose).
Amplification, cloning and sequencing of env genes
Genomic RNA from C1712, LEA and MBA viruses was extracted from each virus stock using the QIAamp® viral RNA Mini Kit (Qiagen). Full-length (gp160) env gene was amplified by nested RT-PCR using subtype CRF01_AE env-specific primers. The outer primer pair was sensAEext (5’-GGTTARTTVARAGAATAAGAGAAAGAG-3’) and asAEext (5’-TRCTTTTTGACCAYTTGCYYCCCAT-3’), and the inner primers pair was sensAEint (5’-AGAAGACAGTGGAAATGAGAGTGA-3’) and asAEint (5’-ATDTTATRSCAAAGHCCTTTCDAAGCC-3’). Reverse transcription (RT) was carried out for 30 minutes at 50 °C. This was followed by the first round of PCR, using the SuperScript™ One-Step RT-PCR for Long Templates kit (Invitrogen) with the following conditions: 2 minutes at 94°C, then 35 cycles of 15 seconds at 94°C, 30 seconds at 50°C and 3 minutes at 68°C, and a final extension step of 10 minutes at 72°C. MBA env gene amplification differed from that of the two other viruses by the number of cycles (40 cycles) and annealing temperature (45°C). A 5μl aliquot of the products from the first round of PCR was then used as template for the second round of amplification under the same cycling conditions, with Platinum® PCR SuperMix High Fidelity (Invitrogen). PCR products were cloned into pCR2.1 (TOPO TA cloning® Kit; Invitrogen), before being excised by EcoRI restriction and transferred into the EcoRI site of the pCI expression vector (Invitrogen). PCR products and pCR2.1-env clones were sequenced, using a set of env-specific internal primers, according to the Dye Terminator cycle sequencing protocol (Applied Biosystems). Potential N-linked glycosylation sites (PNGS) were identified by N-GlycoSite (Zhang et al., 2004). MBA, C1712 and LEA env sequences have been submitted to GenBank and assigned accession numbers DQ518410, DQ518411 and DQ218412, respectively.
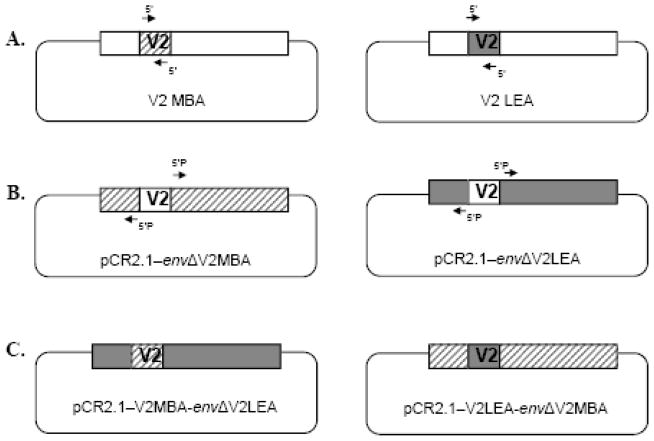
Construction of chimeric env genes
Two chimeric env genes, V2LEA–envΔV2MBA and V2MBA–envΔV2LEA, were constructed in the pCR2.1 vector. V2 domains of LEA and MBA env genes were inverted using a domain exchange strategy, as previously described (Rong et al., 2007). The donor V2 domains of LEA and MBA were amplified by PCR using specific primers annealing at both ends of the V2 domains (Figure 4A). The primer pairs used for the amplification of the V2 domains were: LEAV2S (5’-TCTTTTAATATGACCACAGAACTAAAAGATAAG-3’) and LEAV2AS (5’-ACAATTTATTAACCTATACTCACTATTATTACTACTTC-3’) for LEA, and MBAV2S (5’-ACTTTTAATACGACCACAGAACTAGGAGAT-3’) and MBV2AS (5’-ACAATGTATTAATATATACTTACTATAGGTACTATTGTTTTTCCC-3’) for MBA. The recipient env backbones (deleted of the V2 domain) within the pCR2.1 vector were amplified by PCR using specific primers that anneal to regions adjacent to the V2 primers (Figure 4B). A 5’ phosphate group (Phos) was added to these primers to allow ligation to the V2 amplicon. The primer pairs used for the amplification of the env backbones were: LEAEnvS (5’-Phos-AATACTTCAGTCATTAAGCAGGCTTGTC-3’) and LEAEnvAS (5’-Phos-ACAGTTTCTTACTTCATCTGTTATATTTCCTATTT-3’) for LEA, and MBAEnvS (5’-Phos-AATTCTTCAGTCATTAAGCAAGCATGTCC-3’) and MBAEnvAS (5’-Phos-GCAGTTTCTTACTGATTTTTCCATTTTTATTGTCTTAC-3’) for MBA.
Figure 4. Construction of the V2 chimeric Envs.
(A) Env sequences encoding donor V2 domains (MBA and LEA) were amplified by PCR using specific primers that anneal at both ends of the V2 domain. (B) Env sequences encoding recipient env backbones within the pCR2.1 vector (pCR2.1–envΔV2MBA and pCR2.1–envΔV2LEA) were amplified by PCR using specific primers that anneal to regions adjacent to the V2 primers. A 5’ phosphate group was added to these primers for ligation to the V2 amplicon. (C) Sequences encoding the V2 domains and recipient backbones were blunt-end ligated to generate chimeric env genes in pCR2.1 (pCR2.1–V2MBA-envΔV2LEA and pCR2.1–V2LEA-envΔV2MBA).
The PCR amplification conditions for the V2 domains were 30 seconds at 98°C, followed by 35 cycles of 10 seconds at 98°C, 30 seconds at 57°C and 30 seconds at 72°C, and a final extension step of 72°C for 10 minutes. The same amplification conditions were used for env backbones, with an extension time at 72°C increased to four minutes. PHUSION High Fidelity DNA Polymerase (BioLabs) was used to generate blunt-ended PCR amplicons. PCR amplicons were digested with DpnI to remove contaminating template DNA prior to ligation. Each donor V2 DNA fragment was ligated to its recipient env backbone to produce two pCR2.1 constructs containing chimeric env genes (Figure 4C). Chimeric env genes were then extracted from pCR2.1 by EcoRI digestion and transferred into the EcoRI site of the pCI expression vector (Invitrogen).
Env-pseudotyped virus neutralization assay
Env-pseudotyped viruses were generated by co-transfecting 3 x 106 293T cells with 12 μg of each pCI-env plasmid and 8 μg of pNL4.3.LUC.R−E− (Connor et al., 1995), using calcium phosphate (Invitrogen). After titration, pseudotyped virus stocks were diluted to obtain 600 TCID50/mL in growth medium. A volume of 25 μl corresponding to 15 TCID50 was then incubated for 1h at 37°C with 75 μl of two-fold serial dilutions of heat-inactivated serum. The virus/serum mixture was then used to infect 10,000 TZM-bl cells (Platt et al., 1998; Wei et al., 2002) in the presence of 30 μg/mL DEAE-dextran. Infection levels were determined after 48h, using the Bright-Glo luciferase assay (Promega) and a Centro LB 960 luminometer (Berthold Tecnhologies) to measure luciferase activity in cell lysates. The assay was performed in duplicate and results were expressed as mean values. Neutralizing antibody titers were defined as the reciprocal of the serum dilution required to reduce relative luminescence units (RLU) by 50%.
Statistical analysis
Categorical variables were compared using Fisher’s exact test and a conditional logistic regression (CLR) model to take into account the matching of the two groups on maternal baseline viral load and ZDV prophylaxis treatment duration (Barin et al., 2006). Continuous variables were compared using the Wilcoxon matched-pairs signed-ranks test. We used McNemar's chi-squared test for matched case-control data to compare the proportions of patient sera able to neutralize each strain.
Acknowledgments
We thank Dr E. Menu for providing us with the primary isolates LEA and C1712. pNL4.3.LUC.R−E− was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pNL4.3.LUC.R−E−from Dr. Nathaniel Landau. The TZM-bl cells were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl from Dr. John C. Kappes, Dr. Xiaoyun WU and Tranzyme Inc.
This work was supported by funds from the Agence Nationale de Recherches sur le Sida (ANRS, Paris, France), Sidaction (Paris, France), the Institut de Recherche pour le Développement (IRD, Paris, France), and National Institutes of Health (5 R01 HD 33326). T Samleerat was supported by a doctoral fellowship from the French Ministry of Foreign Affairs. Suzie Thenin was supported by a doctoral fellowship from the ANRS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad N, Baroudy BM, Baker RC, Chappey C. Genetic analysis of human immunodeficiency virus type 1 envelope V3 region isolates from mothers and infants after perinatal transmission. J Virol. 1995;69(2):1001–12. doi: 10.1128/jvi.69.2.1001-1012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barin F, Brunet S, Brand D, Moog C, Peyre R, Damond F, Charneau P, Barre-Sinoussi F. Interclade neutralization and enhancement of human immunodeficiency virus type 1 identified by an assay using HeLa cells expressing both CD4 receptor and CXCR4/CCR5 coreceptors. J Infect Dis. 2004;189(2):322–7. doi: 10.1086/380099. [DOI] [PubMed] [Google Scholar]
- Barin F, Jourdain G, Brunet S, Ngo-Giang-Huong N, Weerawatgoompa S, Karnchanamayul W, Ariyadej S, Hansudewechakul R, Achalapong J, Yuthavisuthi P, Ngampiyaskul C, Bhakeecheep S, Hemwutthiphan C, Lallemant M. Revisiting the role of neutralizing antibodies in mother-to-child transmission of HIV-1. J Infect Dis. 2006;193(11):1504–11. doi: 10.1086/503778. [DOI] [PubMed] [Google Scholar]
- Bongertz V, Costa CI, Veloso VG, Grinsztejn B, Joao Filho EC, Calvet G, Pilotto JH, Guimaraes ML, Morgado MG. Vertical HIV-1 transmission: importance of neutralizing antibody titer and specificity. Scand J Immunol. 2001;53(3):302–9. doi: 10.1046/j.1365-3083.2001.00866.x. [DOI] [PubMed] [Google Scholar]
- Braibant M, Brunet S, Costagliola D, Rouzioux C, Agut H, Katinger H, Autran B, Barin F. Antibodies to conserved epitopes of the HIV-1 envelope in sera from long-term non-progressors : prevalence and association with neutralizing activity. AIDS. 2006;20 (15):1923–1930. doi: 10.1097/01.aids.0000247113.43714.5e. [DOI] [PubMed] [Google Scholar]
- Brand D, Beby-Defaux A, Mace M, Brunet S, Moreau A, Godet C, Jais X, Cazein F, Semaille C, Barin F. First identification of HIV-1 groups M and O dual infections in Europe. Aids. 2004;18(18):2425–8. [PubMed] [Google Scholar]
- Charneau P, Mirambeau G, Roux P, Paulous S, Buc H, Clavel F. HIV-1 reverse transcription. A termination step at the center of the genome. J Mol Biol. 1994;241(5):651–62. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206(2):935–44. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- Dickover R, Garratty E, Yusim K, Miller C, Korber B, Bryson Y. Role of maternal autologous neutralizing antibody in selective perinatal transmission of human immunodeficiency virus type 1 escape variants. J Virol. 2006;80(13):6525–33. doi: 10.1128/JVI.02658-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund J, Glezen WP, Piedra PA. Maternal immunization against viral disease. Vaccine. 1998;16(14–15):1456–63. doi: 10.1016/s0264-410x(98)00108-x. [DOI] [PubMed] [Google Scholar]
- Hengel RL, Kennedy MS, Steketee RW, Thea DM, Abrams EJ, Lambert G, McDougal JS. Neutralizing antibody and perinatal transmission of human immunodeficiency virus type 1. New York City Perinatal HIV Transmission Collaborative Study Group. AIDS Res Hum Retroviruses. 1998;14(6):475–81. doi: 10.1089/aid.1998.14.475. [DOI] [PubMed] [Google Scholar]
- Lallemant M, Jourdain G, Le Coeur S, Kim S, Koetsawang S, Comeau AM, Phoolcharoen W, Essex M, McIntosh K, Vithayasai V. A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. Perinatal HIV Prevention Trial (Thailand) Investigators. N Engl J Med. 2000;343(14):982–91. doi: 10.1056/NEJM200010053431401. [DOI] [PubMed] [Google Scholar]
- Lathey JL, Tsou J, Brinker K, Hsia K, Meyer WA, 3rd, Spector SA. Lack of autologous neutralizing antibody to human immunodeficiency virus type 1 (HIV-1) and macrophage tropism are associated with mother-to-infant transmission. J Infect Dis. 1999;180(2):344–50. doi: 10.1086/314886. [DOI] [PubMed] [Google Scholar]
- Pinter A, Honnen WJ, He Y, Gorny MK, Zolla-Pazner S, Kayman SC. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J Virol. 2004;78(10):5205–15. doi: 10.1128/JVI.78.10.5205-5215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72(4):2855–64. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, Bibollet-Ruche F, Mulenga J, Allen S, Blackwell JL, Derdeyn CA. Role of V1V2 and other human immunodeficiency virus type 1 envelope domains in resistance to autologous neutralization during clade C infection. J Virol. 2007;81 (3):1350–9. doi: 10.1128/JVI.01839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safrit JT, Ruprecht R, Ferrantelli F, Xu W, Kitabwalla M, Van Rompay K, Marthas M, Haigwood N, Mascola JR, Luzuriaga K, Jones SA, Mathieson BJ, Newell ML. Immunoprophylaxis to prevent mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr. 2004;35(2):169–77. doi: 10.1097/00126334-200402010-00012. [DOI] [PubMed] [Google Scholar]
- Sagar M, Wu X, Lee S, Overbaugh J. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J Virol. 2006;80 (19):9586–98. doi: 10.1128/JVI.00141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samleerat T, Braibant M, Jourdain G, Moreau A, Ngo-Giang-Huong N, Leechanachai P, Hemvuttiphan J, Hinjiranandana T, Changchit T, Warachit B, Suraseranivong V, Lallemant M, Barin F. Characteristics of HIV type 1 (HIV-1) glycoprotein 120 env sequences in mother-infant pairs infected with HIV-1 subtype CRF01_AE. J Infect Dis. 2008;198(6):868–76. doi: 10.1086/591251. [DOI] [PubMed] [Google Scholar]
- Scarlatti G, Albert J, Rossi P, Hodara V, Biraghi P, Muggiasca L, Fenyo EM. Mother-to-child transmission of human immunodeficiency virus type 1: correlation with neutralizing antibodies against primary isolates. J Infect Dis. 1993;168(1):207–10. doi: 10.1093/infdis/168.1.207. [DOI] [PubMed] [Google Scholar]
- Shibata J, Yoshimura K, Honda A, Koito A, Murakami T, Matsushita S. Impact of V2 mutations on escape from a potent neutralizing anti-V3 monoclonal antibody during in vitro selection of a primary human immunodeficiency virus type 1 isolate. J Virol. 2007;81(8):3757–68. doi: 10.1128/JVI.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhofstede C, Demecheleer E, De Cabooter N, Gaillard P, Mwanyumba F, Claeys P, Chohan V, Mandaliya K, Temmerman M, Plum J. Diversity of the human immunodeficiency virus type 1 (HIV-1) env sequence after vertical transmission in mother-child pairs infected with HIV-1 subtype A. J Virol. 2003;77(5):3050–7. doi: 10.1128/JVI.77.5.3050-3057.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46(6):1896–905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky SM, Wike CM, Korber BT, Hutto C, Parks WP, Rosenblum LL, Kunstman KJ, Furtado MR, Munoz JL. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992;255(5048):1134–7. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- Wolk T, Schreiber M. N-Glycans in the gp120 V1/V2 domain of the HIV-1 strain NL4-3 are indispensable for viral infectivity and resistance against antibody neutralization. Med Microbiol Immunol. 2006;195(3):165–72. doi: 10.1007/s00430-006-0016-z. [DOI] [PubMed] [Google Scholar]
- Wu X, Parast AB, Richardson BA, Nduati R, John-Stewart G, Mbori-Ngacha D, Rainwater SM, Overbaugh J. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J Virol. 2006;80(2):835–44. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Gaschen B, Blay W, Foley B, Haigwood N, Kuiken C, Korber B. Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin. Glycobiology. 2004;14(12):1229–46. doi: 10.1093/glycob/cwh106. [DOI] [PubMed] [Google Scholar]