Abstract
We are living exciting times in the field of beta cell replacement therapies for the treatment of diabetes. While steady progress has been recorded thus far in clinical islet transplantation, novel approaches are needed to make cell-based therapies more reproducible and leading to long-lasting success. The multiple facets of diabetes impose the need for a transdisciplinary approach to attain this goal, by targeting immunity, promoting engraftment and sustained functional potency. We discuss herein the emerging technologies applied to beta cell replacement therapies.
Keywords: Diabetes, Islets of Langerhans, Islet Transplantation, Biomaterials, Implantable Devices, Tissue Engineering, Cellular Therapies, Beta Cells, Insulin, Stem Cells
1. Introduction
1.1. Diabetes Mellitus
Diabetes is a metabolic disorder characterized by elevated glucose levels in the blood (hyperglycemia). Type 1 diabetes mellitus (T1DM) is caused by an autoimmune-mediated destruction of the insulin-producing β-cells in the pancreatic islets [1]. Type 2 diabetes mellitus (T2DM) is associated with dyslipidemia, obesity, initial hyperinsulinemia and insulin resistance in target tissues (fat, liver and muscle), resulting in progressive islet cell dysfunction and ultimately in insulinopenia and need for exogenous insulin therapy [2]. Glycemic metabolism can be controlled, at least to a certain extent, by daily administration of exogenous insulin, frequent monitoring of blood sugar levels combined with diet and exercise. Achieving tight glycemic control is desirable in patients with diabetes [3]. Unfortunately, even with a careful insulin treatment based on the use of improved insulin formulations, infusion systems and continuous glucose monitoring systems, daily glycemic excursions are difficult to keep tightly in the normal range. Thus, chronic and degenerative complications, such as retinopathy, nephropathy, neuropathy, and atherosclerosis, still occur in a considerable fraction of patients with diabetes, contributing to the poor quality of life, reduced life expectancy and to the elevated medical costs associated with diabetes.
1.2. Restoration of Physiologic Metabolic Control
Restoration of physiologic glucose metabolic control is highly desirable in patients with diabetes. Replacement of islet β-cells can be performed either by whole pancreas or isolated pancreatic islet transplantation. The experience of the last three decades supports the positive impact on metabolic control of the biologic replacement of β-cells via allogeneic islet and whole pancreas transplantation. Notably, islet transplantation requires less risky implantation approaches than invasive surgery. Moreover, the possibility of engineering the islet transplant to promote its engraftment and long-term function makes of islet transplantation an appealing therapeutic approach to restore β-cell function.
1.2.1. Islet Transplantation
The islet transplantation procedure is currently performed with a minimally invasive approach consisting of a percutaneous cannulation of the portal vein, through which islets are infused into the recipient’s liver [4–7]. This technique has been utilized since the 1970’s mainly to prevent or ameliorate metabolic control in patients with chronic pancreatitis requiring pancreatectomy (autologous islet transplantation) [8,9], and to restore metabolic control in patients with unstable T1DM associated with frequent severe hypoglycemic episodes [7,10]. Recently, autologous islet transplantation has also been proposed for patients with resectable neoplastic lesions of the pancreas [11–14]. Clinical islet allogeneic transplantation trials performed in patients with brittle T1DM demonstrated restoration of metabolic control with complete independence from (when adequate islets are implanted) or dramatic reduction of exogenous insulin requirements (i.e., transplantation of suboptimal islet numbers or development of graft dysfunction), as well as prevention of severe hypoglycemic episodes paralleled by improved quality of life [10,15,16]. Interestingly, preliminary data suggest improvement of diabetes complications following islet transplantation [10,17–19].
1.2.2. Current Challenges of Islet Transplantation
Even though islet transplantation has become a promising clinical therapeutic option in recent years, several challenges currently limit its application to the most severe cases of unstable diabetes characterized by hypoglycemia unawareness and frequent debilitating, severe hypoglycemia, at times life-threatening. Amongst the key hurdles recognized to the widespread application of islet transplantation is the variable long-term success of intra-hepatic implantation, which may result from a combination of variables likely secondary to lack of vasculature in the early peri-transplant period and to nonspecific inflammation triggered by islet isolation and transplantation procedures, collectively resulting in reduced islet engraftment (β-cell death and functional impairment), as well as in triggering of adaptive immunity affecting graft survival.
Cadaveric human donor pancreata represent an unsustainable source of transplantable islets since variables related to donor (i.e., age, sex, cause of death and duration of intensive care) and organ characteristics (i.e., warm and cold ischemia, preservation technique utilized, presence of intra-parenchymal fat infiltration, etc.), islet isolation (i.e., enzyme and purification methods) and culture conditions may yield quite disparate results in terms of islet integrity, numbers, potency and immunogenicity, all ultimately determining graft outcome after transplantation [20].
Development of reliable tests and algorithms able to predict the success of islet isolation and transplantation based on donor variables [20–22] or final cell product assessment [23–31] may be of assistance in achieving higher success rates after islet transplantation more reproducibly. However, even by refining and expanding donor selection criteria (i.e., use of organ donation after cardiac arrest and marginal donors) and improving the efficiency of organ recovery, the number of pancreata suitable for transplant may fall short of the needs of the large patient population potentially benefiting of a biologic replacement of β-cell function.
Another challenge is the immunosuppression utilized in islet transplant recipients, which relies on agents that may impair tissue remodeling and neovascularization (e.g., mTOR inhibitors) as well as affect β-cell function over time (e.g., calcineurin inhibitors, amongst other). Moreover, the immunosuppression required to prevent rejection may not adequately target autoreactive immune responses, in turn allowing progressive loss of graft function to autoimmunity recurrence in patients with T1DM. Development of novel approaches to promote and enhance islet engraftment and long-term function, as well as to modulate immunity are needed to make islet transplantation a more reproducible therapeutic option in the near future.
1.3. Alternative Sources for Transplantable Islet Cells
Undeniably, there is an urgent need for unlimited source of transplantable ‘islets’, which may come from xenogeneic donors (e.g., porcine islet cells), conversion of adult or embryonic stem cells into endocrine pancreatic cells. Islet transplantation of islets obtained from xenogeneic donors is appealing. Porcine islets may represent a readily available source, and pilot human clinical trials have been attempted, with demonstration of transient function of implanted islets without adventitious effects related to zoonotic diseases (i.e., porcine endogenous retrovirus infections, PERV) [32–35]. Moreover, remarkable progress has been documented with the development of specific pathogen-free herds, as well as of genetically modified strains that aim at overcoming the immunological barriers [36,37], which may enable achieving long-term restoration of metabolic control in combination of safe immunotherapies and bioengineering of the transplant microenvironment (i.e., immunoisolation).
Embryonic stem cells (ESCs) are a promising alternative cell source for treating diabetes. They are pluripotent stem cells capable of unlimited replicative capacity and the potential to differentiate into different cell phenotypes. Differentiation of insulin-producing cells from mouse and human ESCs has been demonstrated. A milestone in the field was the work by D’Amour et al. demonstrating the differentiation of human ESCs into endocrine cells able to produce insulin, glucagon, somatostatin, pancreatic polypeptide and ghrelin using a five step protocol mimicking the pancreatic development in vitro through a series of endoderm intermediates [38]. However, the release of C-peptide by these cells in response to glucose in vitro was marginal. Interestingly, these immature cells can subsequently differentiate in vivo into endocrine cells capable to support metabolic function in chemically-induced diabetic mice [39]. These studies have stimulated the field and led to a phase 1/2 clinical trial in patients with T1DM currently underway (Table 1).
Table 1.
Islet Clinical Trials (clinicaltrials.gov, accessed Jan 31, 2015)
| Sponsor | Title of Trial | Phase | Registry No.* |
|---|---|---|---|
| Viacyte, San Diego, CA, USA | ESC in Macrodevice | I/II | NCT02239354 |
| University of Miami/Diabetes Research Institute, Miami, FL, USA | Allogeneic Islet Transplant into the Omentum | I/II | NCT 02213003 |
| Vrije Universiteit Brussel, Brussels, Belgium | Long Term Function of Beta Cell Allografts in Non-Uremic T1D Patients | I/II | NCT00798785 |
| Ospedale San Raffaele, Milan, Italy | Bone Marrow as an Alternative Site for Islet Transplantation | I | NCT01345227 |
| Ospedale San Raffaele, Milan, Italy | Bone Marrow vs Liver as Site for Islet Transplantation (IsletBOM 2) | I/II | NCT01722682 |
| The Nordic Network For Clinical Islet Transplantation, Sweden and Norway | Intraportal or Intramuscular Site for Islets in Simultaneous Islet and Kidney Transplantation | II | NCT01967186 |
| Uppsala University Hospital, Sweden | Autologous Mesenchymal Stem Cell Transplantation | II | NCT02057211 |
| Uppsala University Hospital, Sweden | Beta/Air Device for Encapsulation of Transplanted Human islets | I/II | NCT02064309 |
| Cliniques Universitaires Saint-Luc-Université Catholique de Louvain, Leuven, Belgium | Encapsulated Human Islets in a "Monolayer Cellular Device" | I | NCT00790257 |
| Novocell, Irvine, CA, USA | Allogeneic Cultured Islet Cells (Human); Encapsulated | I/II | NCT00260234 |
| Living Cell Technologies, Auckland, New Zealand | Alginate-Encapsulated Porcine Islets for Xenotransplantation | I/II |
NCT00940173 NCT01736228 NCT01739829 |
| Vrije Universiteit Brussel, Brussels, Belgium | Transplantation of Encapsulated Beta Cells | II | NCT01379729 |
| Fuzhou General Hospital, Xiamen University, China | Cotransplantation of Islet and Mesenchymal Stem Cell | I/II | NCT00646724 |
| University of Alberta, Edmonton, BC, Canada | Sernova Cell Pouch | I/II | NCT01652911 |
| University of Perugia, Italy | Transplantation of Immunoprotected Pancreatic Islets for the Therapy of Type 1 Diabetes Mellitus (T1DM) | I | ISRCTN43557935 |
| Technische Universität Dresden, Germany | Beta O2 Immunoisolation Device | “individual treatment attempt” | PMID24167261 |
NCT: clincialtrials.gov; ISRCTN: isrctn.com; PMID: ncbi.nlm.nih.gov/PubMed
Conversion of pancreatic exocrine cells into insulin-producing cells in adult mouse pancreas has been achieved by specific combination of transcription factors (namely, Ngn3, Pdx1, and Mafa) [40]. Moreover, pancreatic acinar cells can be converted into somatostatin and glucagon cells by Ngn3 and Ngn3+Mafa respectively [41]. It has also been demonstrated that pancreatic ductal structure may contain precursor cells that can yield to insulin-producing cells [42–45]. Collectively, these studies point to the potential of developing protocols for the large scale production of pancreatic endocrine cells for transplantation in vitro from tissue that is currently considered waste product of islet isolation processing. Additionally, it may lead to optimization of approaches to promote endocrine cell differentiation and/or expansion in vivo targeting impure (e.g., containing acinar and ductal structures) islet preparations transplanted in engineered sites that can be targeted selectively with the appropriate treatment for controlled (time and amounts) delivery.
1.4. Cell-Based Immunomodulation
Immunity represents one of the critical hurdles for islet grafts, particularly since their fate depends on the combination of allogeneic and autoreactive immune responses in patients with T1DM [46,47]. Restoration of self-tolerance and/or donor-specific hyporesponsiveness/tolerance is the desirable goal of diabetes and transplant immunobiology. Different approaches have been proposed that may be of assistance in achieving this ambitious objective, likely by combining immunotherapy and cell-based treatments. A growing body of literature supports the potential beneficial impact of the use of hematopoietic stem cells (HSC) and of regulatory immune cell subsets (i.e., T regulatory cells, Tregs; Mesenchymal Stromal Cells, MSC; Dendritic Cells, DC; amongst others) to achieve such goal in patients with T1D [48–52] and in organ transplant recipients [53–60]. In the context of clinical islet transplantation, the use of donor-specific HSC was shown to associate with the achievement of graft function and transient hematopoietic chimerism, though the discontinuation of immunosuppression by protocol (in the absence of tests predictive of immunomodulation) invariably resulted in loss of graft function [4,54,61]. Combination of islets with MSC treatment was shown to improve islet engraftment in rodents [62–65] and large animal models [66], and is currently under evaluation in the clinical settings (Table 1).
The initial reports on the use of cell-based immunotherapies are quite encouraging, though current challenges include the lack of reliable immune markers predictive of patient response, as well as the fact that different trials rely on quite different protocols for cell isolation and concomitant immunotherapy utilized, rendering cumbersome the comparison of outcomes. A coordinated effort amongst Centers is desirable, along with the use of standardized approaches and readouts is paramount towards the development of successful cell-based therapies for the treatment of diabetes and their widespread clinical application.
1.5. Bioengineering Approaches for Islet Grafts
The critical importance of creating a microenvironment suitable for islet cell has prompted many tissue engineering approaches for the development of a bioartificial pancreas, which can ensure an optimal site of implantation to favor both engraftment and long-term function of islet cells. Ultimate goal of engineering the transplant microenvironment is to enhance islet cell viability, to promote prompt neo-angiogenesis, and to modulate immunity.
1.5.1. Extra-Hepatic Islet Transplantation
The choice of the site of implant for islet grafts may ultimately influence clinical outcomes. For the last three decades, clinical islet transplantation has relied on their embolization in the hepatic sinusoids of the portal system (intra-hepatic islet transplantation) [67]. It has been recognized that the liver microenvironment may not be ideal for islet cells. The portal system is exposed to high concentrations of immunosuppressive drug levels that may result in impaired islet cell potency [68]. Moreover, glycemic levels are higher than in the liver than in the pancreas, which may contribute to increase endocrine cell basal activity leading to exhaustion. Contact of islets with blood induces an instant blood-mediated inflammatory reaction (IBMIR) that results from the activation of coagulation and complement cascades, neutrophils and leukocyte recruitment [69,70] that contributes to amplifying the activation of transplant microenvironment via endothelial cell and macrophage activation associated with islet embolization in the hepatic sinusoids [71] and hypoxia negatively affecting islet cell viability and engraftment. Notably, prompt neovascularization of islet grafts is essential to achieve functional competence after transplantation. The isolation process results in damage of intrainsular vascular structures [72] that may contribute to immunogenicity and delayed neovascularization of transplanted islets, which may require weeks to be fully re-established [73,74]. It is conceivable also that the microvascular complications of diabetes may altered endothelial repair capabilities in the transplant microenvironment [75]. Progressive loss of functional islet mass has been reported also in large animal models of intrahepatic autologous islet transplantation in the absence of chronic rejection and autoimmunity recurrence or toxicity of immunosuppressive drugs [76], further supporting the need for the development of alternative, extra-hepatic sites for islet grafts.
An ideal site for islet transplantation may include: sufficient capacity to host large volumes of tissue containing impure (non-endocrine) fractions, portal drainage, ease of access using minimally invasive techniques, potential of microenvironment manipulation (tissue engineering), and accessible for noninvasive imaging and biopsy [77]. In recent years, different studies have explored extrahepatic implantation sites for islet cells in experimental models [77,78]. Implantation sites such as the omentum may meet the requirements of ideal portal venous drainage and space pliable for tissue engineering approaches [79–84]. Also, the intestinal subserosa [85–87], excluded vascular structures [88], amongst other sites, were shown to represent an interesting implantation site for islet grafts (Figure 1).
Figure 1. Implantation sites for islet cells.
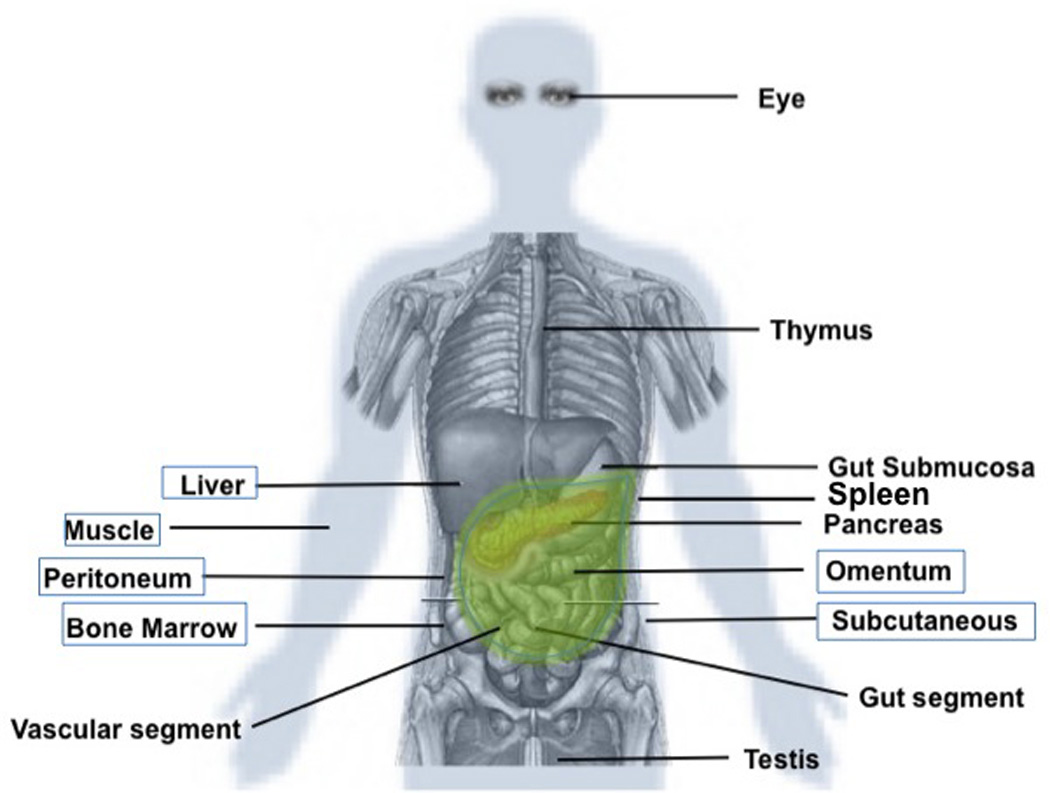
Several implantation sites have been proposed for islet cells, besides the intrahepatic portal system. An ideal site may be spacious, easily accessible for minimally invasive implantation and explant, have portal venous drainage, modifiable to enhance engraftment and modulate immunity, and allow for graft monitoring via noninvasive or minimally invasive approaches. The sites in the boxes have been tested in humans.
Evaluation of the feasibility and efficacy of extrahepatic sites in the clinical setting is also underway. Allogeneic and xenogeneic islets incorporated in immunoisolating microcapsules have been implanted in the peritoneal cavity of patients with diabetes, but the achievement of sustained function has been difficult to attain so far in the clinical settings, which may require better characterization of the suitability of the site for islet cells [89,90]. The subcutaneous space is easily accessible with minimally invasive surgery, and initial attempts with implantable devices have been reported [34,91,92]. Engraftment and functional competence of autologous islets implanted in the intramuscular site (forearm) has also been reported in human subjects and may represent a viable approach [93–96]. More recently, the feasibility of intra-bone marrow islet transplantation has been demonstrated in a pilot series of human islet autografts in patients with contraindication for intrahepatic route, in which successful engraftment without adverse events were reported [77,97]. Collectively, it is likely that future clinical trials will increasingly rely on extrahepatic implantation sites to enhance the long-term success of β-cell replacement therapies (Table 1).
1.5.2. Scaffolds
Given the high metabolic demand of islets, it would be desirable to design a device that allows for both intra-device vascularization and to achieve spatial distribution of the cells within the device to avoid clumping and generation of hypoxic conditions. The use of a biomaterial to spatially distribute the cells would allow for a more desirable three-dimensional arrangement of the cells and result in a more efficient delivery of nutrients. Moreover, devices may be engineered to incorporate drugs, generate oxygen and several biomaterials have been used, both for in-vitro and in-vivo studies, to generate macroporous scaffolds, including collagen, chitosan, hydroxyapatite, poly(α- hydroxy esters) like poly(glycolic acid) (PGA), poly(lactic acid) (PLA), and poly(lacticco- glycolic acid) (PLGA).
In general, natural materials have the benefit of high cellular recognition and tissue compatibility, but are costly, variable, biodegradable, and require high levels of purification [98]. Synthetic materials, on the other hand, can be fabricated with uniformity between batches, precise control over rates of degradation, and modified chemical properties.
Progressive nonimmunologic loss of functioning islets may be related to the lack of a functional islet microenvironment after isolation from the whole pancreas. Developing technologies that provide a better microenvironment for islet grafts may represent a viable strategy to extend islet longevity as well as reduce the islet mass required to achieve insulin independence [99]. Problems associated with the hepatic transplantation of islets may preclude the broad application of islet transplantation. Thus, new approaches to the extrahepatic transplantation of islets using a synthetic biodegradable polymer scaffold have been developed.
Several basic requirements for cell transplantation on macro- and micro-porous scaffolds have been identified, including biocompatibility, a high surface area/volume ratio with sufficient mechanical integrity, and a suitable environment for new tissue formation that can integrate with the surrounding tissue [100]. Porous polymer-based scaffolds are and not intended to serve as an immune barrier, like macro- and micro-encapsulation. Rather, they were specifically designed to provide a solid support for islets that would allow cellular infiltration and formation of a vascular network within the transplant graft. Scaffolds with a high surface area/volume ratio not only have sufficient surface area to support cell adhesion, but can also support nutrient transport by diffusion from surrounding tissue. Moreover, they can be fabricated from material that has sufficient mechanical properties to resist collapse while maintaining an interconnected pore structure that allows for cell infiltration from the surrounding tissue. This is important not only for integration of the engineered tissue with the host, but also for development of a vascular network throughout the tissue to supply the needed metabolites once the transplanted cells are engrafted.
Reversal of diabetes in diabetic rodents was shown after seeding islets onto a highly porous Poly(D,L14 lactide-co-glycolide) (PLG) scaffold implanted in the intraperitoneal fat [101]. Similar results were obtained using porous scaffolds fabricated in biocompatible poly(dimethylsiloxane) (PDMS) that supported adequate neovascularization and functional potency of syngeneic islets in rodents [84,102]. These findings indicate that a synthetic polymer scaffold can serve as a platform for islet transplantation. Notably, the scaffold may also be useful to deliver bioactive molecules such as oxygen, trophic factors (i.e., proangiogenic molecules) or immunomodulatory agents to modify the microenvironment surrounding the transplanted islets and, thus, enhance islet survival and function [102–104].
Synthetic biodegradable polymers have been extensively utilized in tissue engineering [100], as their degradation kinetics and mechanical properties can be tailored to meet the needs of a specific application [105]. Successful engraftment of islets loaded on a synthetic (VICRYL (Polyglactin 910) and PDS (poly-P-Dioxanon) biodegradable scaffold implanted an omental pouch of diabetic monkeys supported the feasibility in preclinical settings [81].
In recent years, there has been a growing interest in developing biocompatible scaffolds containing extracellular matrix (ECM) components to improve islet culture and transplantation [106]. Cultured on collagen, fibrin or surrogate matrices, islets show a decrease in apoptosis, better survival in culture and improved and prolonged function in vitro [107]. A key factor in choosing this compound is that fibrin is already available as a clinical grade tissue sealant. Alternatively, sufficient quantities of fibrinogen-rich plasma can potentially be obtained from islet recipients prior to transplantation [82]. In addition, fibrin scaffolds have been shown to be beneficial for islets in culture: they increase human islet cell mass in vitro [108], stimulate endothelial cell proliferation [109], and have been used successfully in experimental islet transplantation [110]. An additional and appealing source for extracellular matrices applicable to islet grafts are decellularized pancreata (human or from other species) that may be used as 3-D structures for implantation or as lyophilized matrices for co-implantation with islet cells [111– 113].
1.5.3. Macrodevices
Macrodevices may be designed to support engraftment and physical containment of the cellular grafts. Prevascularization of the islet implantation site may be achieved by using spacers (e.g., Polytetrafluoroethylene, PTFE) containing or not pro-angiogenic factors to prime the environment and that are replaced before islet transplantation. While this approach does not confer immune isolation, it may help mitigating the local inflammation and allow adequate engraftment of the cellular implants [34,91,114–123].
Amongst devices that provide a physical barrier to the graft is the TheraCyte™ macroencapsulation system, a planar pouch featuring a bilaminar PTFE semipermeable membrane. The outer layer promotes tissue integration, whereas an inner, cell impermeable, membrane has a 0.4µm pore size. Its subcutaneous placement allows cells to be transplanted in a minimally-invasive manner and retrieved if necessary. The device is biologically inert and when transplanted into human patients for a year, there were no adverse effects reported [124]. This type of device has been utilized to protect cells from immune reaction, but also to prevent dissemination of the cell implant in the recipient body. Feasibility of this approach was demonstrated for the implantation of genetically engineered cells specially designed for the in vivo delivery of therapeutic proteins, such as endostatin, which circumvents the problem of limited half-life and variation in circulating levels [125]. This device limits direct contact of host immune cells with the implanted cells, which could reduce or delay rejection while allowing the exchange of subcellular materials. The use of TheraCyte device has yielded some degree of successes reported with stem cell derived endocrine cells [126], as well as with allogeneic and xenogeneic pancreatic islet transplantation in experimental animals [127], including in the presence of autoimmunity in NOD mice [128]. Immunoisolation of pancreatic islets using the TheraCyte device has shown to protect against allograft rejection in non-immunized recipients [129]. A similar device, aimed at both confining and immunoprotecting human embryonic stem cell-derived endocrine cells is under evaluation for clinical testing (Table 1).
1.5.4. Islet Encapsulation
Islet encapsulation offers a means to create a physical barrier around islet clusters to shield them from the immune system, while allowing free exchange of insulin and nutrients throughout the cells [comprehensive review [130]]. Amongst the challenges in this field is the selection of materials that protect islets from the immune system. The encapsulation material must perform two vital functions: permit diffusion of insulin and waste products, and isolate the encapsulated islets from the immune system (i.e., macrophage and lymphocyte infiltration)[131]. Different approaches have been proposed that rely on macrodevices and microdevices containing multiple islets or thin-layer (conformal) coating of individual islets [132,133]. One of the materials widely used for islets encapsulation is alginate, an anionic polysaccharide produced by seaweed, whose biocompatibility and gelling properties make it useful for macro- and micro-encapsulation [126,134]. To maximize biocompatibility and elimination of potential pyrogenic contaminants (e.g., endotoxin), rigorous purification of the naturally occurring compound is required [135]. In addition to alginate, polysulphone (PSU) has also been proposed as a possible encapsulation material because it does not limit the secretion and diffusion of insulin in macroencapsulated islets [136,137]. Other hydrogels, such as poly-ethylene glycol (PEG) [138] or methacrylate copolymers, have been proposed for islets encapsulation [131,139].
Many materials in different configurations have been tested in experimental models of islet transplantation in recent years, but only few have been evaluated in humans. Thus, little is known on their effective biocompatibility and suitability for long-term implantation as part of islet transplantation approaches in the clinical settings. New approaches are focused on the conjugation or incorporation of biomolecules, such as extracellular matrices that may reduce anoikis and stabilize cell membranes, to the biomaterials for encapsulation [140,141].
Intravascular macrocapsules consist of a synthetic hollow fiber semipermeable membrane that passes through a compartment seeded with pancreatic islets [142,143], where blood flows through the lumen of the hollow fibers allowing islets to promptly sense changes in glucose homeostasis and timely release of insulin, while they are protected from immunity by the membrane. This kind of device provides transport of nutrients and oxygen to the islets, but with a trade-off increased risk of damaging a blood vessel during surgery.
Extravascular macrocapsules are referred to macroencapsulated cells that are implanted outside the vasculature (e.g., in the peritoneal cavity as well as subcutaneously). The easy implant and explant relying on minimally invasive techniques reduce the risk of the procedure. The major drawback is the limitation of oxygen diffusion and nutrients transport related to the thickness of the device and tissue reaction around the foreign material. Encouraging results have been reported on the use of immunoisolation macrodevices in preclinical models of diabetes, supporting the ability to immune protect xenogeneic islets and improve metabolic control for extended periods of times [144–146]. Another approach consisting of the incorporation of an oxygen delivery system to the immunoisolation macrodevice to support the graft and help overcoming potential diffusion limitation from surrounding tissue, showed sustained human islet function in a pilot clinical case [92,147].
In microcapsules, islets are immobilized inside microspheres of a biocompatible material, usually alginate, coated with a semipermeable membrane, and implanted in the recipient – generally, as free intraperitoneal grafts. This kind of device has been the most studied thus far because of the simplicity of manufaturing and flexibility for modifying key components, which allowed researchers to play with key parameters like wall thickness or pore size to overcome significant nutrient and oxygen diffusion limitations seen in all extravascular implantation sites. Drawbacks of microencapsulation are mainly related to their size (500 – >1000um) that limits oxygen diffusion to the core of the capsule and in turn favors the survival of islets close to its surface. Also, the large volume of the capsules requires a large implantation site such as the peritoneal cavity, where generally the capsules are injected and tend to pellet in the pelvis by gravity expoosing the islets to unfavorable hypoxic conditions. Also, removal of the implants from the peritoneal cavity is cumbersome.
The concept of microencapsulated islets was first introduced in 1980 by Lim and Sun [148], who encapsulated pancreatic islets inside calcium-gelated alginate microcapsules: alginate microcapsules were cross-linked with polycationic poly-L-lysine (PLL) and an outer layer of alginate. However, these capsules were found to cause fibrosis when engrafted inside an animal. Islet encapsulation based on Ba2+ ions as the gelating cation combined with protamine sulfate (PS) residues as alginate cross linker have been tested. Ba2+ ions have a higher affinity than Ca2+ ions, so the mixing barium and alginate produces a stronger barium-alginate hydrogel and it supported a higher cell viability as compared to conventional alginate caspules [149]. Interestingly, unlike calcium–gelled capsules, barium capsules are intrinsically radiopaque, which could be easily imaged in vivo with micro-computed tomography. Improved survival of xenogeneic islets was demonstrated in the stringent NOD mouse model by combining bariumgelled alginate microcapsules with co-stimulation blockade [150,151].
Drawbacks of macrocapsules include loss of islet cells to hypoxia due to poor diffusion of vital molecules such as oxygen and nutrients into the central core of the constructs, and the unfavorable transplants volumes for the clinically preferred intraportal route of transplantation [152]. Incorporation of emulsions with oxygen carrying moieties, such as perfluorinated chemicals has been proposed to help overcoming oxygen diffusion within macro- and microcapsules, [153,154].
As an alternative approach that may overcome the limitations of macro- and microencapsulation, conformal coating of pancreatic islets may represent a viable strategy to allow for a more physiologic release of insulin into the bloodstream, while improving oxygen and nutrient diffusion supporting their survival and function after implantation. The use of polyhetylene glycol (PEG) coating obtained by photopolymerization for islet immunoisolation showed great promise in rodents [155] and nonhuman primate models of diabetes [130]. Multilayered nanofilms can be applied directly to the surface of the islet clusters to confer immunoislation after transplantation [135,156]. Improved islet allograft survival has been reported for conformally-coated islets in experimental animal models in recent years [157].
Novel techniques are needed in order to improve the efficiency of conformal coating of pancreatic islets, which are challenging due to the variability of size of the clusters comprised in the final preparation. The use of fluid dynamics approach may be of assistance to address such limitations, and encouraging initial results have been recently reported using PEG hydrogels [138]. Translation of these approaches to the clinical setting is appealing, and hopefully will allow attaining sustained graft function with lower or no requirement for lifelong immunosuppression.
1.6. Conclusions
We are living exciting times in the field of beta cell replacement therapies for the treatment of diabetes. While steady progress has been recorded thus far in clinical islet transplantation, novel approaches are needed to make cell-based therapies more reproducible and leading to long-lasting success. The multiple facets of diabetes impose the need for a transdisciplinary approach to attain this goal, by targeting immunity, promoting engraftment and sustained functional potency.
For stem cell based therapies to become a definitive solution to the shortage of transplantable endocrine cells, it may be desirable to fully recapitulate the complex structure and function leading to the tight regulation of human islets, which are in fact micro-organs. This goal should parallel the long-term safety of stem cell-based therapies (namely, no tumor formation).
Exploring novel sites for islet implantation that are accessible and pliable to modification of the microenvironment may allow for targeted interventions to enhance engraftment, reduce immune cell activation and islet cell loss at the time of transplant. The choice of the ideal site for islet implantation should take into account safety concerns and sustainability of implanted cell function. With the emerging evidence that non-endocrine pancreatic tissue represents a potential source for precursor cells able to maintain functional beta cell mass, it may be desirable to transplant impure islet cell preparations. This would require the availability of implantation sites that can accommodate relatively large volumes of tissues while avoiding competition for nutrients and oxygen.
The use of scaffolds or macrodevices is appealing for the potential to modify the local transplant microenvironment. The choice for biodegradable or non-biodegradable materials should be weighted, since the immune reaction for degradation and persistence of foreign material, respectively, may affect the fate of the graft long-term.
Physical modifications of the islet surface using biologics, gene therapy or polymers may be of assistance in also preventing immune mediated destruction, and allow lowering the immunotherapy needs to sustain graft function, and even enable the success of protocols aimed at immune tolerance. The use of transient coating of islet surface with polymers and/or ECM products may contribute to stabilize the graft (i.e., addressing ainoikis) and possibly protect it during the early post-transplant period, resulting in reduced attrition and microenvironment activation ultimately leading to improved outcome. Thus far, immunoisolation approaches have not met the desirable goal of a long-term function of transplanted islets. This may be the result of attrition over time due to inadequate adaptation of the graft to the implant conditions (i.e., lack of vascularization, inadequate nutrients and oxygen, waste products accumulation inside the constructs, amongst others).
Encouraging experimental data supports the potential value of strategies that combine different cellular types (i.e., mesenchymal stromal cells, endothelial cell precursors, or others) to promote engraftment and modulate immunity, which may synergize with the bioengineering approaches discussed herein. In fact combination products (e.g., comprising biomaterials, cell products and drugs) along with rationale systemic immunotherapy may lead to a permanent restoration of beta cell function in people with insulin-requiring diabetes. Only through a sequential, integrated approach, and a collective effort of the research community this ambitious goal can be achieved.
Figure 2. Schematic representation of immunoisolation approaches.
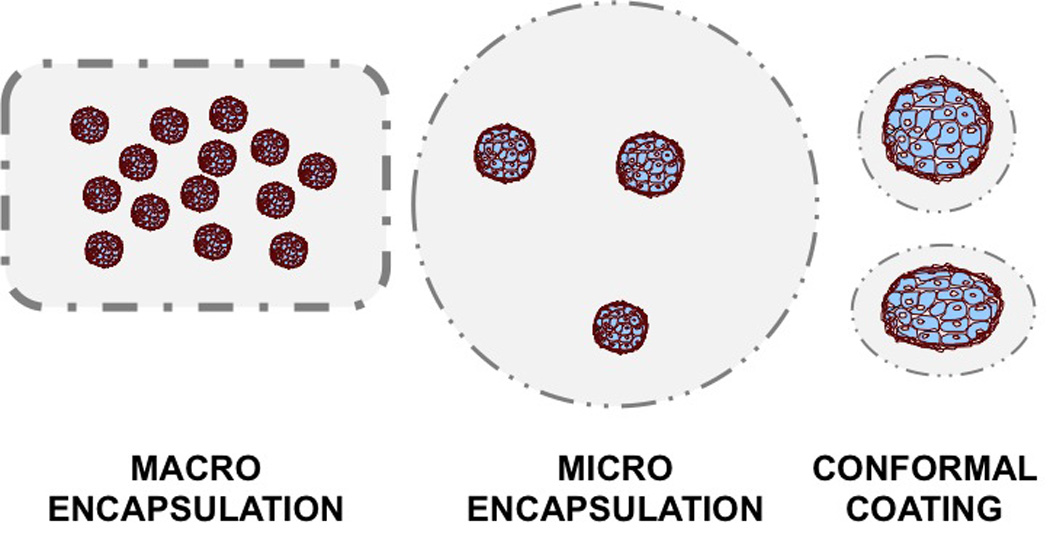
Macrodevices may house large amount of islet cell products and may be implanted subcutaneously or intraabdominally (i.e., intraomental pouch). Macro- and Micro-encapsulation devices are 400–1000um in diameter and may accommodate different numbers of islet clusters within each capsule. The diameter and overall volume of the graft, limits the possibility of implantation to the intraperitoneal cavity. Conformal (thin) coating consists of a thin polymer layering (e.g., hydrogel) on the islet clusters’ surface to confer immunoprotection.
Figure 3. Differences between conformal coating and microencapsulation.
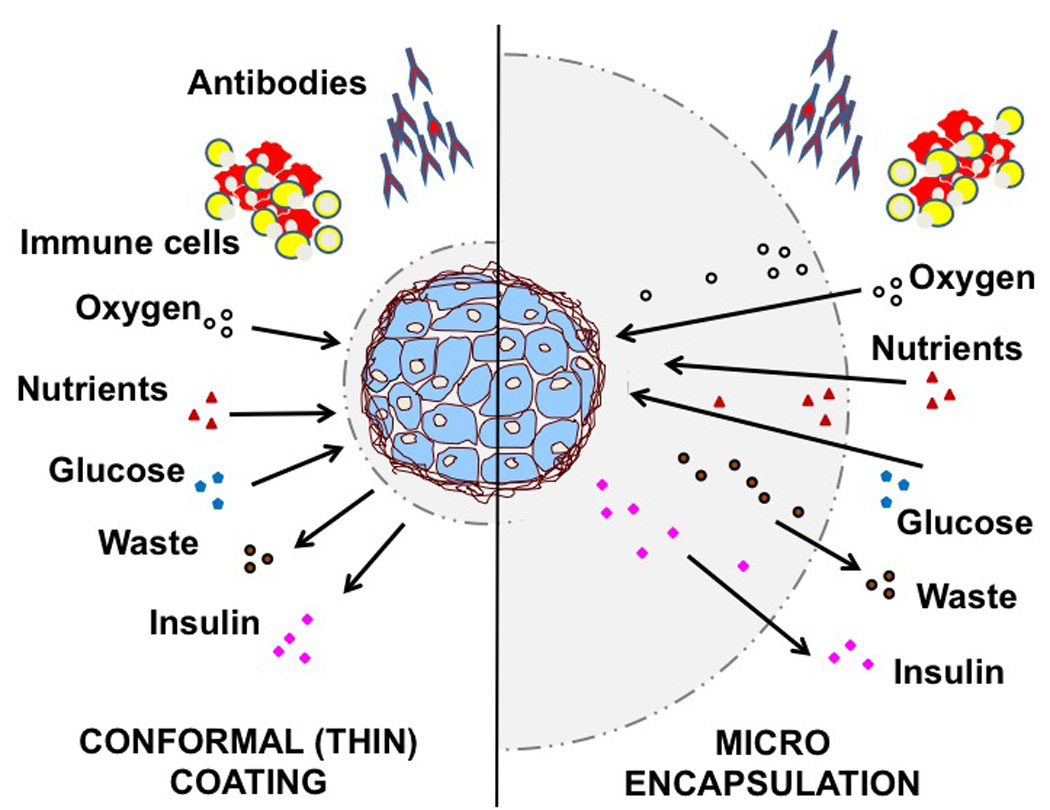
Effective immunoisolation should rely on biocompatible materials to protect the islets from immune cells and humoral factors (i.e., antibodies), while allowing adequate exchange of oxygen and nutrients from the surrounding microenvironment, elimination of cellular waste, and timely inflow of glucose and outflow of insulin. In conformal coating immunoisolation (Left panel), a thin layer of polymer is applied on each islet cluster. The thickness of the coating is considered to represent an advantage when compared to micro- and macro- encapsulation where the distance between islet surface and the outer environment may hamper or delay inflow diffusion of oxygen and nutrients, as well as the outflow of cellular waste that may result in death or impairment of islet cell function (Right panel). Novel formulations of polymers able to enhance intracapsular oxygen generation or diffusion, and incorporation of oxidative scavenging molecules may be of assistance in overcoming the current limitations of larger constructs.
Acknowledgements
We acknowledge support by grants from the American Diabetes Association (7-13-IN-32), the Juvenile Diabetes Research Foundation International (17-2012-361, 17-2010-5, 4-2008-811, 4-2004-361), The Leona M. and Harry B. Helmsley Charitable Trust, the National Institutes of Health (5U19AI050864-10; U01DK089538; 5U42RR016603-08S1; 1U01DK70460-02; 5R01DK25802-24; 5R01DK56953-05), the University of Miami Interdisciplinary Research Development Initiative, and the Diabetes Research Institute Foundation, and Converge Biotech. C.F. received a Travel Fellowship from the Federation Of Clinical Immunology Societies (FOCIS). The funding agencies had no role in the design and conduct of the study, collection, management, analysis and interpretation of the data, content, presentation, decision to publish, or preparation of the manuscript.
Abbreviations
- DC
Dendritic Cells
- ECM
Extracellular matrix
- ESC
Embryonic Stem Cells
- HSC
Hematopoietic Stem Cells
- MSC
Mesenchymal Stromal Cells
- PDMS
Poly(dimethylsiloxane)
- PEG
Poly-ethylene glycol
- PERV
Porcine Endogenous Retrovirus
- PGA
Poly(glycolic acid)
- PLA
Poly(lactic acid)
- PLGA
Poly(lacticco- glycolic acid)
- PLL
Polycationic poly-L-Lysine
- PS
Protamine sulfate
- PSU
Polysulphone
- PTFE
Polytetrafluoroethylene
- T1DM
Type 1 Diabetes Mellitus
- T2DM
Type 2 Diabetes Mellitus
- Tregs
T regulatory cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest. N.F. has no conflict of interest to disclose. C.F. and A.P. own intellectual property that may be related to some of the topics discussed in this article. A.P. is member of the scientific advisory board and stock option holders in Converge Biotech, licensee of some of the intellectual property that may be related to the topic discussed in this article.
References
- 1.Pugliese A. The multiple origins of type 1 diabetes. Diabetic medicine. a journal of the British Diabetic Association. 2012 doi: 10.1111/dme.12081. [DOI] [PubMed] [Google Scholar]
- 2.Lencioni C, Lupi R, Del Prato S. Beta-cell failure in type 2 diabetes mellitus. Current diabetes reports. 2008;8:179–184. doi: 10.1007/s11892-008-0031-0. [DOI] [PubMed] [Google Scholar]
- 3.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The diabetes control and complications trial research group. The New England journal of medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Pileggi A, Ricordi C, Kenyon NS, Froud T, Baidal DA, Kahn A, Selvaggi G, Alejandro R. Twenty years of clinical islet transplantation at the diabetes research institute--university of miami. Clinical transplants. 2004:177–204. [PubMed] [Google Scholar]
- 5.Baidal DA, Froud T, Ferreira JV, Khan A, Alejandro R, Ricordi C. The bag method for islet cell infusion. Cell transplantation. 2003;12:809–813. doi: 10.3727/000000003108747280. [DOI] [PubMed] [Google Scholar]
- 6.Fiorina P, Secchi A. Pancreatic islet cell transplant for treatment of diabetes. Endocrinology and metabolism clinics of North America. 2007;36:999–1013. ix. doi: 10.1016/j.ecl.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Mineo D, Pileggi A, Alejandro R, Ricordi C. Point: Steady progress and current challenges in clinical islet transplantation. Diabetes care. 2009;32:1563–1569. doi: 10.2337/dc09-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Najarian JS, Sutherland DE, Matas AJ, Steffes MW, Simmons RL, Goetz FC. Human islet transplantation: A preliminary report. Transplantation proceedings. 1977;9:233–236. [PubMed] [Google Scholar]
- 9.Bellin MD, Balamurugan AN, Pruett TL, Sutherland DE. No islets left behind: Islet autotransplantation for surgery-induced diabetes. Current diabetes reports. 2012;12:580–586. doi: 10.1007/s11892-012-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pileggi A. Islet transplantation. In: De Groot LJ, editor. Endotext org. South Dartmouth, MA: USA: MDText. com; 2011. [Google Scholar]
- 11.Oberholzer J, Mathe Z, Bucher P, Triponez F, Bosco D, Fournier B, Majno P, Philippe J, Morel P. Islet autotransplantation after left pancreatectomy for non-enucleable insulinoma. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2003;3:1302–1307. doi: 10.1046/j.1600-6143.2003.00218.x. [DOI] [PubMed] [Google Scholar]
- 12.Balzano G, Maffi P, Nano R, Zerbi A, Venturini M, Melzi R, Mercalli A, Magistretti P, Scavini M, Castoldi R, Carvello M, Braga M, Del Maschio A, Secchi A, Staudacher C, Piemonti L. Extending indications for islet autotransplantation in pancreatic surgery. Annals of surgery. 2013;258:210–218. doi: 10.1097/SLA.0b013e31829c790d. [DOI] [PubMed] [Google Scholar]
- 13.Balzano G, Carvello M, Piemonti L, Nano R, Ariotti R, Mercalli A, Melzi R, Maffi P, Braga M, Staudacher C. Combined laparoscopic spleen-preserving distal pancreatectomy and islet autotransplantation for benign pancreatic neoplasm. World journal of gastroenterology : WJG. 2014;20:4030–4036. doi: 10.3748/wjg.v20.i14.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balzano G, Piemonti L. Autologous islet transplantation in patients requiring pancreatectomy for neoplasm. Current diabetes reports. 2014;14:512. doi: 10.1007/s11892-014-0512-2. [DOI] [PubMed] [Google Scholar]
- 15.Poggioli R, Faradji RN, Ponte G, Betancourt A, Messinger S, Baidal DA, Froud T, Ricordi C, Alejandro R. Quality of life after islet transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6:371–378. doi: 10.1111/j.1600-6143.2005.01174.x. [DOI] [PubMed] [Google Scholar]
- 16.Cure P, Pileggi A, Froud T, Messinger S, Faradji RN, Baidal DA, Cardani R, Curry A, Poggioli R, Pugliese A, Betancourt A, Esquenazi V, Ciancio G, Selvaggi G, Burke GW, 3rd, Ricordi C, Alejandro R. Improved metabolic control and quality of life in seven patients with type 1 diabetes following islet after kidney transplantation. Transplantation. 2008;85:801–812. doi: 10.1097/TP.0b013e318166a27b. [DOI] [PubMed] [Google Scholar]
- 17.Del Carro U, Fiorina P, Amadio S, De Toni Franceschini L, Petrelli A, Menini S, Martinelli Boneschi F, Ferrari S, Pugliese G, Maffi P, Comi G, Secchi A. Evaluation of polyneuropathy markers in type 1 diabetic kidney transplant patients and effects of islet transplantation: Neurophysiological and skin biopsy longitudinal analysis. Diabetes care. 2007;30:3063–3069. doi: 10.2337/dc07-0206. [DOI] [PubMed] [Google Scholar]
- 18.Venturini M, Fiorina P, Maffi P, Losio C, Vergani A, Secchi A, Del Maschio A. Early increase of retinal arterial and venous blood flow velocities at color doppler imaging in brittle type 1 diabetes after islet transplant alone. Transplantation. 2006;81:1274–1277. doi: 10.1097/01.tp.0000208631.63235.6a. [DOI] [PubMed] [Google Scholar]
- 19.Bassi R, Fiorina P. Impact of islet transplantation on diabetes complications and quality of life. Current diabetes reports. 2011;11:355–363. doi: 10.1007/s11892-011-0211-1. [DOI] [PubMed] [Google Scholar]
- 20.Ponte GM, Pileggi A, Messinger S, Alejandro A, Ichii H, Baidal DA, Khan A, Ricordi C, Goss JA, Alejandro R. Toward maximizing the success rates of human islet isolation: Influence of donor and isolation factors. Cell transplantation. 2007;16:595–607. doi: 10.3727/000000007783465082. [DOI] [PubMed] [Google Scholar]
- 21.O'Gorman D, Kin T, Murdoch T, Richer B, McGhee-Wilson D, Ryan EA, Shapiro JA, Lakey JR. The standardization of pancreatic donors for islet isolations. Transplantation. 2005;80:801–806. doi: 10.1097/01.tp.0000172216.47547.d5. [DOI] [PubMed] [Google Scholar]
- 22.Hubert T, Strecker G, Gmyr V, Arnalsteen L, Garrigue D, Ezzouaoui R, Caiazzo R, Dezfoulian G, Averland B, Vandewalle B, Vantyghem MC, Kerr-Conte J, Pattou F. Acute insulin response to arginine in deceased donors predicts the outcome of human islet isolation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8:872–876. doi: 10.1111/j.1600-6143.2007.02131.x. [DOI] [PubMed] [Google Scholar]
- 23.Ichii H, Inverardi L, Pileggi A, Molano RD, Cabrera O, Caicedo A, Messinger S, Kuroda Y, Berggren PO, Ricordi C. A novel method for the assessment of cellular composition and betacell viability in human islet preparations. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5:1635–1645. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 24.Fraker C, Timmins MR, Guarino RD, Haaland PD, Ichii H, Molano D, Pileggi A, Poggioli R, Presnell SC, Inverardi L, Zehtab M, Ricordi C. The use of the bd oxygen biosensor system to assess isolated human islets of langerhans: Oxygen consumption as a potential measure of islet potency. Cell transplantation. 2006;15:745–758. doi: 10.3727/000000006783981440. [DOI] [PubMed] [Google Scholar]
- 25.Papas KK, Colton CK, Nelson RA, Rozak PR, Avgoustiniatos ES, Scott WE, 3rd, Wildey GM, Pisania A, Weir GC, Hering BJ. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7:707–713. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweet IR, Gilbert M, Scott S, Todorov I, Jensen R, Nair I, Al-Abdullah I, Rawson J, Kandeel F, Ferreri K. Glucose-stimulated increment in oxygen consumption rate as a standardized test of human islet quality. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8:183–192. doi: 10.1111/j.1600-6143.2007.02041.x. [DOI] [PubMed] [Google Scholar]
- 27.Cabrera O, Jacques-Silva MC, Berman DM, Fachado A, Echeverri F, Poo R, Khan A, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. Automated, high-throughput assays for evaluation of human pancreatic islet function. Cell transplantation. 2008;16:1039–1048. [PMC free article] [PubMed] [Google Scholar]
- 28.Ichii H, Miki A, Yamamoto T, Molano RD, Barker S, Mita A, Rodriguez-Diaz R, Klein D, Pastori R, Alejandro R, Inverardi L, Pileggi A, Ricordi C. Characterization of pancreatic ductal cells in human islet preparations. Laboratory investigation; a journal of technical methods and pathology. 2008;88:1167–1177. doi: 10.1038/labinvest.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goto M, Abe H, Ito-Sasaki T, Goto M, Inagaki A, Ogawa N, Fujimori K, Kurokawa Y, Matsue T, Satomi S. A novel predictive method for assessing the quality of isolated pancreatic islets using scanning electrochemical microscopy. Transplantation proceedings. 2009;41:311–313. doi: 10.1016/j.transproceed.2008.10.075. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, Park SG, Lee HN, Lee YY, Park HS, Kim HI, Yu JE, Kim SH, Park CG, Ha J, Kim SJ, Park KS. Atp measurement predicts porcine islet transplantation outcome in nude mice. Transplantation. 2009;87:166–169. doi: 10.1097/TP.0b013e318191e925. [DOI] [PubMed] [Google Scholar]
- 31.Hanson MS, Park EE, Sears ML, Greenwood KK, Danobeitia JS, Hullett DA, Fernandez LA. A simplified approach to human islet quality assessment. Transplantation. 2010;89:1178–1188. doi: 10.1097/TP.0b013e3181d54bce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groth CG, Korsgren O, Tibell A, Tollemar J, Moller E, Bolinder J, Ostman J, Reinholt FP, Hellerstrom C, Andersson A. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344:1402–1404. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 33.Elliott RB, Escobar L, Garkavenko O, Croxson MC, Schroeder BA, McGregor M, Ferguson G, Beckman N, Ferguson S. No evidence of infection with porcine endogenous retrovirus in recipients of encapsulated porcine islet xenografts. Cell transplantation. 2000;9:895–901. doi: 10.1177/096368970000900616. [DOI] [PubMed] [Google Scholar]
- 34.Valdes-Gonzalez RA, Dorantes LM, Garibay GN, Bracho-Blanchet E, Davila-Perez R, Teran L, Ormsby CE, Ayala-Sumuano JT, Copeman L, White DJ. Unexpected immunoresponse to gal and apa antigens in diabetic type 1 patients receiving neonatal pig islets after 6 years. Journal of clinical immunology. 2007;27:266–274. doi: 10.1007/s10875-007-9079-x. [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Mo Z, Ye B, Hu P, Liu S, Yi S. A clinical trial of xenotransplantation of neonatal pig islets for diabetic patients. Zhong nan da xue xue bao Yi xue ban = Journal of Central South University Medical sciences. 2011;36:1134–1140. doi: 10.3969/j.issn.1672-7347.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Nagaraju S, Bottino R, Wijkstrom M, Hara H, Trucco M, Cooper DK. Islet xenotransplantation from genetically engineered pigs. Current opinion in organ transplantation. 2013;18:695–702. doi: 10.1097/MOT.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 37.Wijkstrom M, Bottino R, Iwase H, Hara H, Ekser B, van der Windt D, Long C, Toledo FG, Phelps CJ, Trucco M, Cooper DK, Ayares D. Glucose metabolism in pigs expressing human genes under an insulin promoter. Xenotransplantation. 2014 doi: 10.1111/xen.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nature biotechnology. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 39.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucoseresponsive insulin-secreting cells in vivo. Nature biotechnology. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li W, Nakanishi M, Zumsteg A, Shear M, Wright C, Melton DA, Zhou Q. In vivo reprogramming of pancreatic acinar cells to three islet endocrine subtypes. eLife. 2014;3:e01846. doi: 10.7554/eLife.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gmyr V, Kerr-Conte J, Vandewalle B, Proye C, Lefebvre J, Pattou F. Human pancreatic ductal cells: Large-scale isolation and expansion. Cell transplantation. 2001;10:109–121. [PubMed] [Google Scholar]
- 44.Lysy PA, Weir GC, Bonner-Weir S. Making beta cells from adult cells within the pancreas. Current diabetes reports. 2013;13:695–703. doi: 10.1007/s11892-013-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Lanzoni G, Carpino G, Cui CB, Dominguez-Bendala J, Wauthier E, Cardinale V, Oikawa T, Pileggi A, Gerber D, Furth ME, Alvaro D, Gaudio E, Inverardi L, Reid LM. Biliary tree stem cells, precursors to pancreatic committed progenitors: Evidence for possible life-long pancreatic organogenesis. Stem cells. 2013;31:1966–1979. doi: 10.1002/stem.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burke GW, 3rd, Vendrame F, Pileggi A, Ciancio G, Reijonen H, Pugliese A. Recurrence of autoimmunity following pancreas transplantation. Current diabetes reports. 2011;11:413–419. doi: 10.1007/s11892-011-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vendrame F, Pileggi A, Laughlin E, Allende G, Martin-Pagola A, Molano RD, Diamantopoulos S, Standifer N, Geubtner K, Falk BA, Ichii H, Takahashi H, Snowhite I, Chen Z, Mendez A, Chen L, Sageshima J, Ruiz P, Ciancio G, Ricordi C, Reijonen H, Nepom GT, Burke GW, 3rd, Pugliese A. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive cd4 t-cells. Diabetes. 2010;59:947–957. doi: 10.2337/db09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Francese R, Fiorina P. Immunological and regenerative properties of cord blood stem cells. Clinical immunology. 2010;136:309–322. doi: 10.1016/j.clim.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Fiorina P, Voltarelli J, Zavazava N. Immunological applications of stem cells in type 1 diabetes. Endocrine reviews. 2011;32:725–754. doi: 10.1210/er.2011-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Couri CE, de Oliveira MC, Simoes BP. Risks, benefits, and therapeutic potential of hematopoietic stem cell transplantation for autoimmune diabetes. Current diabetes reports. 2012;12:604–611. doi: 10.1007/s11892-012-0309-0. [DOI] [PubMed] [Google Scholar]
- 51.D'Addio F, Valderrama Vasquez A, Ben Nasr M, Franek E, Zhu D, Li L, Ning G, Snarski E, Fiorina P. Autologous nonmyeloablative hematopoietic stem cell transplantation in new-onset type 1 diabetes: A multicenter analysis. Diabetes. 2014;63:3041–3046. doi: 10.2337/db14-0295. [DOI] [PubMed] [Google Scholar]
- 52.Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64:587–592. doi: 10.2337/db14-0656. [DOI] [PubMed] [Google Scholar]
- 53.Rybka WB, Fontes PA, Rao AS, Winkelstein A, Ricordi C, Ball ED, Starzl TE. Hematopoietic progenitor cell content of vertebral body marrow used for combined solid organ and bone marrow transplantation. Transplantation. 1995;59:871–874. doi: 10.1097/00007890-199503270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fotino C, Ricordi C, Lauriola V, Alejandro R, Pileggi A. Bone marrow-derived stem cell transplantation for the treatment of insulin-dependent diabetes. The review of diabetic studies : RDS. 2010;7:144–157. doi: 10.1900/RDS.2010.7.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, Pileggi A, Ricordi C. Induction therapy with autologous mesenchymal stem cells in livingrelated kidney transplants: A randomized controlled trial. Jama. 2012;307:1169–1177. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- 56.Pileggi A. Mesenchymal stem cells for the treatment of diabetes. Diabetes. 2012;61:1355–1356. doi: 10.2337/db12-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciancio G, Sageshima J, Akpinar E, Gaynor JJ, Chen L, Zarak A, Hanson L, Tueros L, Guerra G, Mattiazzi A, Kupin W, Roth D, Ricordi C, Burke GW., 3rd A randomized pilot study of donor stem cell infusion in living-related kidney transplant recipients receiving alemtuzumab. Transplantation. 2013;96:800–806. doi: 10.1097/TP.0b013e3182a0f68c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krzystyniak A, Golab K, Witkowski P, Trzonkowski P. Islet cell transplant and the incorporation of tregs. Current opinion in organ transplantation. 2014;19:610–615. doi: 10.1097/MOT.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yolcu ES, Leventhal JR, Ildstad ST. Facilitating cells in tolerance induction for kidney transplantation. Current opinion in organ transplantation. 2015;20:57–63. doi: 10.1097/MOT.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 60.Cabello-Kindelan C, Mackay S, Bayer AL. Adoptive t regulatory cell therapy for tolerance induction. Curr Transplant Rep. 2015 doi: 10.1007/s40472-015-0058-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mineo D, Ricordi C, Xu X, Pileggi A, Garcia-Morales R, Khan A, Baidal DA, Han D, Monroy K, Miller J, Pugliese A, Froud T, Inverardi L, Kenyon NS, Alejandro R. Combined islet and hematopoietic stem cell allotransplantation: A clinical pilot trial to induce chimerism and graft tolerance. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8:1262–1274. doi: 10.1111/j.1600-6143.2008.02230.x. [DOI] [PubMed] [Google Scholar]
- 62.Duprez IR, Johansson U, Nilsson B, Korsgren O, Magnusson PU. Preparatory studies of composite mesenchymal stem cell islets for application in intraportal islet transplantation. Upsala journal of medical sciences. 2011;116:8–17. doi: 10.3109/03009734.2010.524320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rackham CL, Chagastelles PC, Nardi NB, Hauge-Evans AC, Jones PM, King AJ. Co-transplantation of mesenchymal stem cells maintains islet organisation and morphology in mice. Diabetologia. 2011;54:1127–1135. doi: 10.1007/s00125-011-2053-4. [DOI] [PubMed] [Google Scholar]
- 64.Chen J, Ye YF, Liao LM, Cai JQ, Huang LH, Yang SL, Ma YJ, Fu YF, Xu XM, Tan JM. Mesenchymal stem cells promote islet survival in vitro and function in vivo. CellR4. 2013;1:e382. [Google Scholar]
- 65.Hamilton DC, Shih HH, Schubert RA, Michie SA, Staats PN, Kaplan DL, Fontaine MJ. A silk-based encapsulation platform for pancreatic islet transplantation improves islet function in vivo. Journal of tissue engineering and regenerative medicine. 2015 doi: 10.1002/term.1990. [DOI] [PubMed] [Google Scholar]
- 66.Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, Karl JA, Wiseman RW, O'Connor DH, Bartholomew AM, Kenyon NS. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes. 2010;59:2558–2568. doi: 10.2337/db10-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piemonti L, Pileggi A. 25 years of the ricordi automated method for islet isolation. CellR4. 2013;1:8–22. [PMC free article] [PubMed] [Google Scholar]
- 68.Shapiro AM, Gallant HL, Hao EG, Lakey JR, McCready T, Rajotte RV, Yatscoff RW, Kneteman NM. The portal immunosuppressive storm: Relevance to islet transplantation? Therapeutic drug monitoring. 2005;27:35–37. doi: 10.1097/00007691-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 69.Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: Implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Upsala journal of medical sciences. 2000;105:125–133. doi: 10.1517/03009734000000059. [DOI] [PubMed] [Google Scholar]
- 70.Johansson H, Lukinius A, Moberg L, Lundgren T, Berne C, Foss A, Felldin M, Kallen R, Salmela K, Tibell A, Tufveson G, Ekdahl KN, Elgue G, Korsgren O, Nilsson B. Tissue factor produced by the endocrine cells of the islets of langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54:1755–1762. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 71.Bottino R, Fernandez LA, Ricordi C, Lehmann R, Tsan MF, Oliver R, Inverardi L. Transplantation of allogeneic islets of langerhans in the rat liver: Effects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998;47:316–323. doi: 10.2337/diabetes.47.3.316. [DOI] [PubMed] [Google Scholar]
- 72.Nyqvist D, Kohler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes. 2005;54:2287–2293. doi: 10.2337/diabetes.54.8.2287. [DOI] [PubMed] [Google Scholar]
- 73.Speier S, Nyqvist D, Cabrera O, Yu J, Molano RD, Pileggi A, Moede T, Kohler M, Wilbertz J, Leibiger B, Ricordi C, Leibiger IB, Caicedo A, Berggren PO. Noninvasive in vivo imaging of pancreatic islet cell biology. Nature medicine. 2008;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nyqvist D, Speier S, Rodriguez-Diaz R, Molano RD, Lipovsek S, Rupnik M, Dicker A, Ilegems E, Zahr-Akrawi E, Molina J, Lopez-Cabeza M, Villate S, Abdulreda MH, Ricordi C, Caicedo A, Pileggi A, Berggren PO. Donor islet endothelial cells in pancreatic islet revascularization. Diabetes. 2011;60:2571–2577. doi: 10.2337/db10-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bassi R, Trevisani A, Tezza S, Ben Nasr M, Gatti F, Vergani A, Farina A, Fiorina P. Regenerative therapies for diabetic microangiopathy. Experimental diabetes research. 2012;2012:916560. doi: 10.1155/2012/916560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alejandro R, Cutfield RG, Shienvold FL, Polonsky KS, Noel J, Olson L, Dillberger J, Miller J, Mintz DH. Natural history of intrahepatic canine islet cell autografts. The Journal of clinical investigation. 1986;78:1339–1348. doi: 10.1172/JCI112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pileggi A, Ricordi C. A new home for pancreatic islet transplants: The bone marrow. Diabetes. 2013;62:3333–3335. doi: 10.2337/db13-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Current diabetes reports. 2011;11:364–374. doi: 10.1007/s11892-011-0216-9. [DOI] [PubMed] [Google Scholar]
- 79.Yasunami Y, Lacy PE, Finke EH. A new site for islet transplantation--a peritonealomental pouch. Transplantation. 1983;36:181–182. doi: 10.1097/00007890-198308000-00014. [DOI] [PubMed] [Google Scholar]
- 80.Guan J, Zucker PF, Atkison P, Behme MT, Dupre J, Stiller CR. Liver-omental pouch and intrahepatic islet transplants produce portal insulin delivery and prevent hyperinsulinemia in rats. Transplantation proceedings. 1995;27:3236. [PubMed] [Google Scholar]
- 81.Berman DM, O'Neil JJ, Coffey LC, Chaffanjon PC, Kenyon NM, Ruiz P, Jr, Pileggi A, Ricordi C, Kenyon NS. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:91–104. doi: 10.1111/j.1600-6143.2008.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hefty TR, Kuhr CS, Chong KT, Guinee DG, Wang W, Reems JA, Greenbaum CJ. Omental roll-up: A technique for islet engraftment in a large animal model. The Journal of surgical research. 2010;161:134–138. doi: 10.1016/j.jss.2008.11.842. [DOI] [PubMed] [Google Scholar]
- 83.Bartholomeus K, Jacobs-Tulleneers-Thevissen D, Shouyue S, Suenens K, In't Veld PA, Pipeleers-Marichal M, Pipeleers DG, Hellemans K. Omentum is better site than kidney capsule for growth, differentiation, and vascularization of immature porcine beta-cell implants in immunodeficient rats. Transplantation. 2013;96:1026–1033. doi: 10.1097/TP.0b013e3182a6ee41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pedraza E, Brady AC, Fraker CA, Molano RD, Sukert S, Berman DM, Kenyon NS, Pileggi A, Ricordi C, Stabler CL. Macroporous three-dimensional pdms scaffolds for extrahepatic islet transplantation. Cell transplantation. 2013;22:1123–1135. doi: 10.3727/096368912X657440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van der Windt DJ, Echeverri GJ, Ijzermans JN, Cooper DK. The choice of anatomical site for islet transplantation. Cell transplantation. 2008;17:1005–1014. [PubMed] [Google Scholar]
- 86.Echeverri GJ, McGrath K, Bottino R, Hara H, Dons EM, van der Windt DJ, Ekser B, Casu A, Houser S, Ezzelarab M, Wagner R, Trucco M, Lakkis FG, Cooper DK. Endoscopic gastric submucosal transplantation of islets (endo-sti): Technique and initial results in diabetic pigs. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:2485–2496. doi: 10.1111/j.1600-6143.2009.02815.x. [DOI] [PubMed] [Google Scholar]
- 87.Kakabadze Z, Gupta S, Pileggi A, Molano RD, Ricordi C, Shatirishvili G, Loladze G, Mardaleishvili K, Kakabadze M, Berishvili E. Correction of diabetes mellitus by transplanting minimal mass of syngeneic islets into vascularized small intestinal segment. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:2550–2557. doi: 10.1111/ajt.12412. [DOI] [PubMed] [Google Scholar]
- 88.Kakabadze Z, Shanava K, Ricordi C, Shapiro AM, Gupta S, Berishvili E. An isolated venous sac as a novel site for cell therapy in diabetes mellitus. Transplantation. 2012;94:319–324. doi: 10.1097/TP.0b013e31825e4a83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, Racanicchi L, Mancuso F, Brunetti P. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: First two cases. Diabetes care. 2006;29:137–138. doi: 10.2337/diacare.29.1.137. [DOI] [PubMed] [Google Scholar]
- 90.Basta G, Montanucci P, Luca G, Boselli C, Noya G, Barbaro B, Qi M, Kinzer KP, Oberholzer J, Calafiore R. Long-term metabolic and immunological follow-up of nonimmunosuppressed patients with type 1 diabetes treated with microencapsulated islet allografts: Four cases. Diabetes care. 2011;34:2406–2409. doi: 10.2337/dc11-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Valdes-Gonzalez RA, Dorantes LM, Garibay GN, Bracho-Blanchet E, Mendez AJ, Davila-Perez R, Elliott RB, Teran L, White DJ. Xenotransplantation of porcine neonatal islets of langerhans and sertoli cells: A 4-year study. European journal of endocrinology / European Federation of Endocrine Societies. 2005;153:419–427. doi: 10.1530/eje.1.01982. [DOI] [PubMed] [Google Scholar]
- 92.Ludwig B, Reichel A, Steffen A, Zimerman B, Schally AV, Block NL, Colton CK, Ludwig S, Kersting S, Bonifacio E, Solimena M, Gendler Z, Rotem A, Barkai U, Bornstein SR. Transplantation of human islets without immunosuppression. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19054–19058. doi: 10.1073/pnas.1317561110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weber CJ, Hardy MA, Pi-Sunyer F, Zimmerman E, Reemtsma K. Tissue culture preservation and intramuscular transplantation of pancreatic islets. Surgery. 1978;84:166–174. [PubMed] [Google Scholar]
- 94.Stegall MD, Lafferty KJ, Kam I, Gill RG. Evidence of recurrent autoimmunity in human allogeneic islet transplantation. Transplantation. 1996;61:1272–1274. doi: 10.1097/00007890-199604270-00027. [DOI] [PubMed] [Google Scholar]
- 95.Rafael E, Tibell A, Ryden M, Lundgren T, Savendahl L, Borgstrom B, Arnelo U, Isaksson B, Nilsson B, Korsgren O, Permert J. Intramuscular autotransplantation of pancreatic islets in a 7-year-old child: A 2-year follow-up. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8:458–462. doi: 10.1111/j.1600-6143.2007.02060.x. [DOI] [PubMed] [Google Scholar]
- 96.Dardenne S, Sterkers A, Leroy C, Da Mata L, Zerbib P, Pruvot FR, Pattou F, Truant S. Laparoscopic spleen-preserving distal pancreatectomy followed by intramuscular autologous islet transplantation for traumatic pancreatic transection in a young adult. JOP : Journal of the pancreas. 2012;13:285–288. [PubMed] [Google Scholar]
- 97.Maffi P, Balzano G, Ponzoni M, Nano R, Sordi V, Melzi R, Mercalli A, Scavini M, Esposito A, Peccatori J, Cantarelli E, Messina C, Bernardi M, Del Maschio A, Staudacher C, Doglioni C, Ciceri F, Secchi A, Piemonti L. Autologous pancreatic islet transplantation in human bone marrow. Diabetes. 2013;62:3523–3531. doi: 10.2337/db13-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vacik J, Dvorankova B, Michalek J, Pradny M, Krumbholcova E, Fenclova T, Smetana K., Jr Cultivation of human keratinocytes without feeder cells on polymer carriers containing ethoxyethyl methacrylate: In vitro study. Journal of materials science Materials in medicine. 2008;19:883–888. doi: 10.1007/s10856-007-3225-0. [DOI] [PubMed] [Google Scholar]
- 99.Kin T, O'Neil JJ, Pawlick R, Korbutt GS, Shapiro AM, Lakey JR. The use of an approved biodegradable polymer scaffold as a solid support system for improvement of islet engraftment. Artificial organs. 2008;32:990–993. doi: 10.1111/j.1525-1594.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- 100.Putnam AJ, Mooney DJ. Tissue engineering using synthetic extracellular matrices. Nature medicine. 1996;2:824–826. doi: 10.1038/nm0796-824. [DOI] [PubMed] [Google Scholar]
- 101.Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, Powers AC, Kaufman DB, Shea LD, Lowe WL., Jr Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82:452–459. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brady AC, Martino MM, Pedraza E, Sukert S, Pileggi A, Ricordi C, Hubbell JA, Stabler CL. Proangiogenic hydrogels within macroporous scaffolds enhance islet engraftment in an extrahepatic site. Tissue engineering Part A. 2013;19:2544–2552. doi: 10.1089/ten.tea.2012.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song Y, Margolles-Clark E, Fraker CA, Weaver JD, Ricordi C, Pileggi A, Stabler CL, Buchwald P. Feasibility of localized immunosuppression: 3. Preliminary evaluation of organosilicone constructs designed for sustained drug release in a cell transplant environment using dexamethasone. Die Pharmazie. 2012;67:394–399. [PubMed] [Google Scholar]
- 104.Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4245–4250. doi: 10.1073/pnas.1113560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim WS, Vacanti JP, Cima L, Mooney D, Upton J, Puelacher WC, Vacanti CA. Cartilage engineered in predetermined shapes employing cell transplantation on synthetic biodegradable polymers. Plastic and reconstructive surgery. 1994;94:233–237. discussion 238–240. [PubMed] [Google Scholar]
- 106.Shimizu H, Ohashi K, Utoh R, Ise K, Gotoh M, Yamato M, Okano T. Bioengineering of a functional sheet of islet cells for the treatment of diabetes mellitus. Biomaterials. 2009;30:5943–5949. doi: 10.1016/j.biomaterials.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 107.Andrades P, Asiedu C, Rodriguez C, Goodwin KJ, McCarn J, Thomas JM. Subcutaneous pancreatic islet transplantation using fibrin glue as a carrier. Transplantation proceedings. 2007;39:191–192. doi: 10.1016/j.transproceed.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 108.Beattie GM, Montgomery AM, Lopez AD, Hao E, Perez B, Just ML, Lakey JR, Hart ME, Hayek A. A novel approach to increase human islet cell mass while preserving beta-cell function. Diabetes. 2002;51:3435–3439. doi: 10.2337/diabetes.51.12.3435. [DOI] [PubMed] [Google Scholar]
- 109.Johansson U, Rasmusson I, Niclou SP, Forslund N, Gustavsson L, Nilsson B, Korsgren O, Magnusson PU. Formation of composite endothelial cell-mesenchymal stem cell islets: A novel approach to promote islet revascularization. Diabetes. 2008;57:2393–2401. doi: 10.2337/db07-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lim JY, Min BH, Kim BG, Han HJ, Kim SJ, Kim CW, Han SS, Shin JS. A fibrin gel carrier system for islet transplantation into kidney subcapsule. Acta diabetologica. 2009;46:243–248. doi: 10.1007/s00592-008-0073-4. [DOI] [PubMed] [Google Scholar]
- 111.De Carlo E, Baiguera S, Conconi MT, Vigolo S, Grandi C, Lora S, Martini C, Maffei P, Tamagno G, Vettor R, Sicolo N, Parnigotto PP. Pancreatic acellular matrix supports islet survival and function in a synthetic tubular device: In vitro and in vivo studies. International journal of molecular medicine. 2010;25:195–202. [PubMed] [Google Scholar]
- 112.Mirmalek-Sani SH, Orlando G, McQuilling JP, Pareta R, Mack DL, Salvatori M, Farney AC, Stratta RJ, Atala A, Opara EC, Soker S. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials. 2013;34:5488–5495. doi: 10.1016/j.biomaterials.2013.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goh SK, Bertera S, Olsen P, Candiello JE, Halfter W, Uechi G, Balasubramani M, Johnson SA, Sicari BM, Kollar E, Badylak SF, Banerjee I. Perfusion-decellularized pancreas as a natural 3d scaffold for pancreatic tissue and whole organ engineering. Biomaterials. 2013;34:6760–6772. doi: 10.1016/j.biomaterials.2013.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Vos P, Hillebrands JL, De Haan BJ, Strubbe JH, Van Schilfgaarde R. Efficacy of a prevascularized expanded polytetrafluoroethylene solid support system as a transplantation site for pancreatic islets. Transplantation. 1997;63:824–830. doi: 10.1097/00007890-199703270-00006. [DOI] [PubMed] [Google Scholar]
- 115.Valdes R, Martin S, Cravioto A, Tenopala J. Biological encapsulation as a new model for preservation of islets of langerhans. Transplantation proceedings. 1998;30:481. doi: 10.1016/s0041-1345(97)01365-1. [DOI] [PubMed] [Google Scholar]
- 116.Halberstadt CR, Williams D, Emerich D, Goddard M, Vasconcellos AV, Curry W, Bhatia A, Gores PF. Subcutaneous transplantation of islets into streptozocin-induced diabetic rats. Cell transplantation. 2005;14:595–605. doi: 10.3727/000000005783982792. [DOI] [PubMed] [Google Scholar]
- 117.Kawakami Y, Iwata H, Gu Y, Miyamoto M, Murakami Y, Yamasaki T, Cui W, Ikada Y, Imamura M, Inoue K. Modified subcutaneous tissue with neovascularization is useful as the site for pancreatic islet transplantation. Cell transplantation. 2000;9:729–732. doi: 10.1177/096368970000900523. [DOI] [PubMed] [Google Scholar]
- 118.Marzorati S, Bocca N, Molano RD, Hogan AR, Doni M, Cobianchi L, Inverardi L, Ricordi C, Pileggi A. Effects of systemic immunosuppression on islet engraftment and function into a subcutaneous biocompatible device. Transplantation proceedings. 2009;41:352–353. doi: 10.1016/j.transproceed.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 119.Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation. 2006;81:1318–1324. doi: 10.1097/01.tp.0000203858.41105.88. [DOI] [PubMed] [Google Scholar]
- 120.Bocca N, Buchwald P, Molano RD, Marzorati S, Stabler C, Pileggi A, Ricordi C. Biohybrid devices and local immunomodulation as opposed to systemic immunosuppression. In: Hallé JP, De Vos P, Rosenberg L, editors. The bioartificial pancreas and other biohybrid therapies: Research Signpost. 2009. pp. 309–327. [Google Scholar]
- 121.Kriz J, Vilk G, Mazzuca DM, Toleikis PM, Foster PJ, White DJ. A novel technique for the transplantation of pancreatic islets within a vascularized device into the greater omentum to achieve insulin independence. American journal of surgery. 2012;203:793–797. doi: 10.1016/j.amjsurg.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 122.Fotino C, Molano RD, Ricordi C, Pileggi A. Transdisciplinary approach to restore pancreatic islet function. Immunologic research. 2013;57:210–221. doi: 10.1007/s12026-013-8437-4. [DOI] [PubMed] [Google Scholar]
- 123.Pileggi A, Klein D, Fotino C, Bravo-Egana V, Rosero S, Doni M, Podetta M, Ricordi C, Molano RD, Pastori RL. Micrornas in islet immunobiology and transplantation. Immunologic research. 2013;57:185–196. doi: 10.1007/s12026-013-8436-5. [DOI] [PubMed] [Google Scholar]
- 124.Lee SH, Hao E, Savinov AY, Geron I, Strongin AY, Itkin-Ansari P. Human beta-cell precursors mature into functional insulin-producing cells in an immunoisolation device: Implications for diabetes cell therapies. Transplantation. 2009;87:983–991. doi: 10.1097/TP.0b013e31819c86ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Malavasi NV, Rodrigues DB, Chammas R, Chura-Chambi RM, Barbuto JA, Balduino K, Nonogaki S, Morganti L. Continuous and high-level in vivo delivery of endostatin from recombinant cells encapsulated in theracyte immunoisolation devices. Cell transplantation. 2010;19:269–277. doi: 10.3727/096368909X480927. [DOI] [PubMed] [Google Scholar]
- 126.Motte E, Szepessy E, Suenens K, Stange G, Bomans M, Jacobs-Tulleneers-Thevissen D, Ling Z, Kroon E, Pipeleers D. Beta Cell Therapy Consortium E-F. Composition and function of macroencapsulated human embryonic stem cell-derived implants: Comparison with clinical human islet cell grafts. American journal of physiology Endocrinology and metabolism. 2014;307:E838–E846. doi: 10.1152/ajpendo.00219.2014. [DOI] [PubMed] [Google Scholar]
- 127.Rafael E, Wernerson A, Arner P, Tibell A. In vivo studies on insulin permeability of an immunoisolation device intended for islet transplantation using the microdialysis technique. European surgical research Europaische chirurgische Forschung Recherches chirurgicales europeennes. 1999;31:249–258. doi: 10.1159/000008700. [DOI] [PubMed] [Google Scholar]
- 128.Yang Z, Chen M, Fialkow LB, Ellett JD, Wu R, Nadler JL. Survival of pancreatic islet xenografts in nod mice with the theracyte device. Transplantation proceedings. 2002;34:3349–3350. doi: 10.1016/s0041-1345(02)03685-0. [DOI] [PubMed] [Google Scholar]
- 129.Kumagai-Braesch M, Jacobson S, Mori H, Jia X, Takahashi T, Wernerson A, Flodstrom-Tullberg M, Tibell A. The theracyte device protects against islet allograft rejection in immunized hosts. Cell transplantation. 2013;22:1137–1146. doi: 10.3727/096368912X657486. [DOI] [PubMed] [Google Scholar]
- 130.Scharp DW, Marchetti P. Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution. Advanced drug delivery reviews. 2014;67–68:35–73. doi: 10.1016/j.addr.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 131.Beck J, Angus R, Madsen B, Britt D, Vernon B, Nguyen KT. Islet encapsulation: Strategies to enhance islet cell functions. Tissue engineering. 2007;13:589–599. doi: 10.1089/ten.2006.0183. [DOI] [PubMed] [Google Scholar]
- 132.Basta G, Calafiore R. Immunoisolation of pancreatic islet grafts with no recipient's immunosuppression: Actual and future perspectives. Current diabetes reports. 2011;11:384–391. doi: 10.1007/s11892-011-0219-6. [DOI] [PubMed] [Google Scholar]
- 133.Veriter S, Gianello P, Dufrane D. Bioengineered sites for islet cell transplantation. Current diabetes reports. 2013;13:745–755. doi: 10.1007/s11892-013-0412-x. [DOI] [PubMed] [Google Scholar]
- 134.Paredes Juarez GA, Spasojevic M, Faas MM, de Vos P. Immunological and technical considerations in application of alginate-based microencapsulation systems. Frontiers in bioengineering and biotechnology. 2014;2:26. doi: 10.3389/fbioe.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.O'Sullivan ES, Vegas A, Anderson DG, Weir GC. Islets transplanted in immunoisolation devices: A review of the progress and the challenges that remain. Endocrine reviews. 2011;32:827–844. doi: 10.1210/er.2010-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cornolti R, Figliuzzi M, Remuzzi A. Effect of micro- and macroencapsulation on oxygen consumption by pancreatic islets. Cell transplantation. 2009;18:195–201. doi: 10.3727/096368909788341252. [DOI] [PubMed] [Google Scholar]
- 137.Petersen P, Lembert N, Zschocke P, Stenglein S, Planck H, Ammon HP, Becker HD. Hydroxymethylated polysulphone for islet macroencapsulation allows rapid diffusion of insulin but retains perv. Transplantation proceedings. 2002;34:194–195. doi: 10.1016/s0041-1345(01)02724-5. [DOI] [PubMed] [Google Scholar]
- 138.Tomei AA, Manzoli V, Fraker CA, Giraldo J, Velluto D, Najjar M, Pileggi A, Molano RD, Ricordi C, Stabler CL, Hubbell JA. Device design and materials optimization of conformal coating for islets of langerhans. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:10514–10519. doi: 10.1073/pnas.1402216111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hisano N, Morikawa N, Iwata H, Ikada Y. Entrapment of islets into reversible disulfide hydrogels. Journal of biomedical materials research. 1998;40:115–123. doi: 10.1002/(sici)1097-4636(199804)40:1<115::aid-jbm13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 140.Thomas FT, Contreras JL, Bilbao G, Ricordi C, Curiel D, Thomas JM. Anoikis, extracellular matrix, and apoptosis factors in isolated cell transplantation. Surgery. 1999;126:299–304. [PubMed] [Google Scholar]
- 141.Beenken-Rothkopf LN, Karfeld-Sulzer LS, Davis NE, Forster R, Barron AE, Fontaine MJ. The incorporation of extracellular matrix proteins in protein polymer hydrogels to improve encapsulated beta-cell function. Annals of clinical and laboratory science. 2013;43:111–121. [PubMed] [Google Scholar]
- 142.Monaco AP. Transplantation of pancreatic islets with immunoexclusion membranes. Transplantation proceedings. 1993;25:2234–2236. [PubMed] [Google Scholar]
- 143.Prochorov AV, Tretjak SI, Goranov VA, Glinnik AA, Goltsev MV. Treatment of insulin dependent diabetes mellitus with intravascular transplantation of pancreatic islet cells without immunosuppressive therapy. Advances in medical sciences. 2008;53:240–244. doi: 10.2478/v10039-008-0045-5. [DOI] [PubMed] [Google Scholar]
- 144.Storrs R, Dorian R, King SR, Lakey J, Rilo H. Preclinical development of the islet sheet. Annals of the New York Academy of Sciences. 2001;944:252–266. doi: 10.1111/j.1749-6632.2001.tb03837.x. [DOI] [PubMed] [Google Scholar]
- 145.Dufrane D, Goebbels RM, Gianello P. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation. 2010;90:1054–1062. doi: 10.1097/TP.0b013e3181f6e267. [DOI] [PubMed] [Google Scholar]
- 146.Krishnan R, Arora RP, Alexander M, White SM, Lamb MW, Foster CE, 3rd, Choi B, Lakey JR. Noninvasive evaluation of the vascular response to transplantation of alginate encapsulated islets using the dorsal skin-fold model. Biomaterials. 2014;35:891–898. doi: 10.1016/j.biomaterials.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ludwig B, Rotem A, Schmid J, Weir GC, Colton CK, Brendel MD, Neufeld T, Block NL, Yavriyants K, Steffen A, Ludwig S, Chavakis T, Reichel A, Azarov D, Zimermann B, Maimon S, Balyura M, Rozenshtein T, Shabtay N, Vardi P, Bloch K, de Vos P, Schally AV, Bornstein SR, Barkai U. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5022–5027. doi: 10.1073/pnas.1201868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 149.Arifin DR, Manek S, Call E, Arepally A, Bulte JW. Microcapsules with intrinsic barium radiopacity for immunoprotection and x-ray/ct imaging of pancreatic islet cells. Biomaterials. 2012;33:4681–4689. doi: 10.1016/j.biomaterials.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cui H, Tucker-Burden C, Cauffiel SM, Barry AK, Iwakoshi NN, Weber CJ, Safley SA. Long-term metabolic control of autoimmune diabetes in spontaneously diabetic nonobese diabetic mice by nonvascularized microencapsulated adult porcine islets. Transplantation. 2009;88:160–169. doi: 10.1097/TP.0b013e3181abbfc1. [DOI] [PubMed] [Google Scholar]
- 151.Safley SA, Cui H, Cauffiel SM, Xu BY, Wright JR, Jr, Weber CJ. Encapsulated piscine (tilapia) islets for diabetes therapy: Studies in diabetic nod and nod-scid mice. Xenotransplantation. 2014;21:127–139. doi: 10.1111/xen.12086. [DOI] [PubMed] [Google Scholar]
- 152.de Vos P, Spasojevic M, Faas MM. Treatment of diabetes with encapsulated islets. Advances in experimental medicine and biology. 2010;670:38–53. doi: 10.1007/978-1-4419-5786-3_5. [DOI] [PubMed] [Google Scholar]
- 153.Fraker CA, Mendez AJ, Inverardi L, Ricordi C, Stabler CL. Optimization of perfluoro nano-scale emulsions: The importance of particle size for enhanced oxygen transfer in biomedical applications. Colloids and surfaces B, Biointerfaces. 2012;98:26–35. doi: 10.1016/j.colsurfb.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 154.Gattas-Asfura KM, Fraker CA, Stabler CL. Perfluorinated alginate for cellular encapsulation. Journal of biomedical materials research Part A. 2012;100:1963–1971. doi: 10.1002/jbm.a.34052. [DOI] [PubMed] [Google Scholar]
- 155.Hill RS, Cruise GM, Hager SR, Lamberti FV, Yu X, Garufis CL, Yu Y, Mundwiler KE, Cole JF, Hubbell JA, Hegre OD, Scharp DW. Immunoisolation of adult porcine islets for the treatment of diabetes mellitus. The use of photopolymerizable polyethylene glycol in the conformal coating of mass-isolated porcine islets. Annals of the New York Academy of Sciences. 1997;831:332–343. doi: 10.1111/j.1749-6632.1997.tb52208.x. [DOI] [PubMed] [Google Scholar]
- 156.Wilson JT, Cui W, Chaikof EL. Layer-by-layer assembly of a conformal nanothin peg coating for intraportal islet transplantation. Nano letters. 2008;8:1940–1948. doi: 10.1021/nl080694q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhi ZL, Kerby A, King AJ, Jones PM, Pickup JC. Nano-scale encapsulation enhances allograft survival and function of islets transplanted in a mouse model of diabetes. Diabetologia. 2012;55:1081–1090. doi: 10.1007/s00125-011-2431-y. [DOI] [PubMed] [Google Scholar]


