Abstract
Introduction
The constitutive androstane receptor (CAR) induces drug-metabolizing enzymes for xenobiotic metabolism.
Areas covered
This review covers recent advances in elucidating the biological functions of CAR and its modulation by a growing number of agonists and inhibitors.
Expert opinion
Extrapolation of animal CAR function to that of humans should be carefully scrutinized, particularly when rodents are used in evaluating the metabolic profile and carcinogenic properties of clinical drugs and environmental chemicals. Continuous efforts are needed to discover novel CAR inhibitors, with extensive understanding of their inhibitory mechanism, species selectivity, and discriminating power against other xenobiotic sensors.
Keywords: constitutive androstane receptor, drug-drug interactions, xenobiotic metabolism, small-molecule
1. Introduction
Constitutive androstane receptor (CAR), first identified as MB67 [1], now belongs to subfamily NR1I of the nuclear receptors (NRs) superfamily. CAR is unique in its ability to be constitutively active in the absence of direct binding ligands, and yet it retains its ability to be further activated or inhibited by various direct or indirect chemical modulators. The most frequently expressed isoform of human CAR protein (denoted hCAR1 or SV3) consists of 9 exons comprising 348 amino acid residues with a molecular weight of ~40 kDa. Similar to other NRs, the function of CAR is determined by its structural domains: the DNA-binding domain (DBD), dimerization domain, and the ligand-binding domain (LBD).
The CAR-DBD allows its binding to specific regulatory sequences of DNA (composed of direct repeats [DR] or inverted repeat motifs in the promoter regions of CAR target genes. For example, the gene promoter of cytochrome P450 2B6 (CYP2B6) has multiple xenobiotic-response elements concentrated mainly at two loci. The main binding sites for CAR are the phenobarbital-responsive enhancer module (PBREM), which is approximately 1.8 kb upstream and harbors two DR4 motifs, and the 400-bp distal xenobiotic responsive enhancer module (XREM), which contains one DR4 motif (and a few half sites) [2]. CAR shares these response elements with pregnane X receptor (PXR) [2, 3]; together, CAR and PXR regulate genes with important metabolic functions. CAR has also been shown in gel shift assays to bind estrogen responsive motifs [4].
CAR binds DNA as a heterodimeric complex with retinoid X receptors (RXRs) [1, 5], which in turn is the obligate heterodimeric partner of many of the class 1 nuclear receptors [6]. Among the RXR isoforms, CAR has been verified to interact with RXR alpha [1, 7] but whether or not it interacts with RXR beta or gamma is hitherto unknown. The dimerization interface between CAR and RXR is unique and unusually large as revealed by co-crystallization of the LBDs along with a coactivator peptide [8]. The CAR/RXR heterodimer has been shown to be permissive or non-permissive depending on the cellular context and CAR isoform [9, 10].
CAR is characterized by its broad specificity for both endogenous and exogenous ligands with varying chemical structures, which allows it to be a xenobiotic sensor. The CAR-LBD is partly responsible for this phenomenon and sanctions binding to a diverse variety of chemical structures. The LBD consists of ~250 amino acid residues, most of which are hydrophobic. In the case of CAR, the activation function 2 (AF2) domain is constantly fixed in an active conformation, and ligand binding further supports the conformational change causing the dissociation of the complex with corepressors and the formation of a new complex with coactivators and histone-acetyl transferases [11].
2. CAR agonists and activators
Since its identification in 1994, CAR has been known to be constitutively active in the absence of any activating ligand [1]. Researchers speculated about the existence of a natural activating ligand for CAR, and it has since been shown that CAR can be regulated by many endobiotics, including many steroids (androstanes, estrogens, and progestins), bile acids, and cholesterol metabolites. Additionally, many xenobiotics, such as clinical drugs, pesticides, food-derived flavonoids, and alcohol-derived polyphenols, are CAR modulators (reviewed in [12]). EC50 values for ligand binding to CAR vary from the nanomolar to micromolar range, suggesting that CAR-mediated ligand recognition can accommodate even low-affinity binding with a high capacity to induce and transduce cell signaling. CAR activation can occur through direct binding of ligands, as in the case of 6-(4-Chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl)oxime (CITCO) [13], or indirectly, as in the cases of phenobarbital (PB) [14] and others (Table 1). Ligand-dependent activation of CAR by direct binding seems to heavily rely on the nuclear translocation step, whereby once inside the nucleus, ligand-bound CAR adopts a conformation similar to that of the constitutively active CAR and maintains transcriptional activity. Table 1 summarizes a list of chemicals that activate CAR in various species either directly by binding or indirectly by inducing CAR dephosphorylation (such as in the case of PB). Note that published data were not available for some chemicals listed in Table 1 in their ability to activate PXR along with CAR.
Table 1.
Activators of CAR in various species
| Class | Type | Active chemical | Reference | Species* | Effect on PXR | Notes** |
|---|---|---|---|---|---|---|
| Drug | Analgesic | Acetaminophen | [132] | H, M | Strong PXR activator | |
| Analgesic (opiate) | Buprenorphine | [133] | H | Activates PXR | ||
| Anticonvulsant | Phenobarbital | [134] | H, M | Indirect CAR activator | ||
| Anticonvulsant | Phenytoin | [135] | H, M | Indirect CAR activator | ||
| Anticonvulsant | Carbamazepine | [136] | H | Mild PXR activator | ||
| Anticonvulsant | Valproic Acid | [137] | H, R | Activates PXR | ||
| Anti-malarial | Artemisinin and derivatives | [17] | H, M | Activates PXR | ||
| Anti-psychotic | Chlorpromazine | [138] | M | No PXR activity | ||
| Anti-retroviral | Efavirenz | [136, 139] | H | Activates PXR | ||
| Anti-retroviral | Nevirapine | [136, 140] | H | |||
| Anti-retroviral | Rilpivirine, Etravirine | [55, 140] | H | Activates PXR | ||
| Anxiolytic | Diazepam | [18] | H | Activates PXR | ||
| Chemotherapeutic | Cyclophosphamid, Ifosfamide | [141] | H | Activates PXR | ||
| HMG-CoA reductase inhibitors | Cerivastatin, Simvastatin, Fluvastatin | [142] | H, M, R | Activates PXR | ||
| Sulfonamide | Substituted sulfonamides, Thiazolidin-4-one | [33] | H | |||
| Steroid hormone | Estrogen | Estradiol, Estrone, and analogues | [67, 143] | M | Activates PXR | |
| Estrogen | 17α-ethinyl- 3,17β-estradiol (EE2) | [67, 143, 144] | M only | Activates PXR | hCAR inhibitor | |
| Hormone precursor | Dehydroepiandrost erone (DHEA) | [65, 145] | H, M | Activates PXR | ||
| Progestin | 5β-pregnane-3,20-dione | [146, 147] | H | Activates PXR | Superphysiologic concentrations required for hCAR activation | |
| Synthetic estrogen | Diethylstilbestrol | [66, 143] | H, M | Activates PXR | ||
| Natural compound | Flavonoid | Gingko biloba extract | [148] | H, M | Some components activate PXR | Some components activate AhR |
| Flavonoid | Galangin, chrysin, and baicalein | [149–151] | H, M | Activates PXR | Indirect activator of CAR | |
| Garlic extract | Diallyl sulfide | [152] | M, R | No PXR activity | ||
| Polyphenols | Ellagic acid, Resveratrol | [153] | H, M | No PXR activity | Activates hCAR better than mCAR | |
| Terpenoid | Squalestatin-1 | [154] | R | |||
| Terpenoid | Bilobalide | [148, 155] | R | No PXR activity | ||
| Environmental chemical | Anti-bacterial | Triclocarban | [100] | M | Indirect CAR activator, also activates ERα | |
| Antibacterial | Triclosan | [101, 156] | M | Mild PXR activator | Indirect activator | |
| Insecticide | Dichlorodiphenyltr ichloroethane (DDT) | [157–159] | H, M | Strong PXR activator | ||
| Insecticide | Dieldrin | [138] | M | Activates PXR | ||
| Insecticide | Methoxychlor | [160–162] | H, M | Activates PXR | ||
| Insecticide | Metofluthrin | [104] | R | |||
| Insecticide | Permethrin | [159, 163] | H | Activates PXR | ||
| Insecticide | Pyrethrins | [164] | R | Activates PXR | ||
| Insecticide | Sulfoxaflor | [165] | M, R | Activates PXR | ||
| Plasticizer | Diethylhexyl phthalate (DEHP) | [166] | H only | Activates PXR | May selectively activate CAR2 | |
| Triazole fungicide | Cyproconazole | [103, 167] | M | |||
| Triazole fungicide | Fluconazole | [167] | M | |||
| Triazole fungicide | Propiconazole | [105] | M | Activates PXR | ||
| Other | CITCO a | [13] | H only | Mildly activates PXR at high concentrations | ||
| FL81 | [159] | H | Activates PXR | |||
| TCPOBOP b | [10, 156] | M, R only | No PXR activity | |||
| Tri-p-methylphenyl phosphate (TMPP) | [168] | H | Activates hCAR, inhibits mCAR | |||
| UM104, UM145 | [77] | H | From a pharmacophore-based virtual screening |
H, human; M, mouse; R, rat
hCAR, human constitutive androstane receptor (CAR); mCAR, mouse CAR; AhR, aryl hydrocarbon receptor; ERα, estrogen receptor α
TCPOBOP, 1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene
Human and rodent CAR share several common characteristics, such as nuclear translocation after phenobarbital treatment and PBREM binding. But remarkable species selectivity is exhibited by CAR in its ligand binding and activation profiles. Moreover, many isoforms of human CAR (hCAR) have been identified: some are constitutively active, and others are strictly ligand inducible [15]. Because these CAR isoforms do not exist in rodents, data generated from rodent models may not reflect human CAR functions accurately, confounding the direct extrapolation of mouse/rat data to humans. For example, 1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) activates mouse but not human CAR, and pharmacological concentrations of androstanol repress mouse but not human CAR [10, 16]. Conversely, CITCO binds and activates human CAR but not mouse CAR (mCAR). Interestingly, in cell-based assays in which CAR is ectopically expressed, the activation of human CAR by CITCO is less than 2-fold because of high basal activation; yet, up to 10-fold activation can be observed with TCPOBOP on exogenous mouse CAR. In primary hepatocytes, however, both chemicals activate robust CAR-mediated gene expression in their respective species. Furthermore, chemicals such as CITCO, artemisinin, and diazepam that exhibit agonistic effects on hCAR by direct binding are also associated with some activation of human PXR (hPXR), albeit with higher EC50 values [13, 17, 18]. PXR and CAR regulate overlapping sets of genes, confounding the understanding of the mechanism of action of these receptors separately, especially in the liver, where both receptors are expressed and functional.
In addition to being activated by direct binding, CAR transactivation can be mediated by indirect activators. CAR activation seems to be heavily dependent on CAR phosphorylation status. Epidermal growth factor (EGF) and growth hormone have long been identified as suppressors of CYP2B1 mRNA induction by PB [19]. PB-mediated increase in CYP2B1 mRNA in rodent hepatocytes was previously observed and documented [20]. The discovery of CAR and subsequent identification of CAR binding to a PBREM upstream of the CYP2B gene led to a better understanding of the mechanism of action underlying this phenomenon [14]. The pathway of indirect CAR activation by PB involves the EGF receptor (EGFR) signaling pathway [21]. PB binds to EGFR to inhibit the binding of EGF, thus antagonizing EGF activation of EGFR which eventually leads to dephosphorylation of CAR at Thr38 [21, 22]. Dephosphorylation activates CAR nuclear translocation and subsequent transcriptional activity [22]. Recently, the dietary flavonoids chrysin, baicalein, and galangin were also shown to be indirect activators of hCAR [23] working through the EGFR-Elk1 signaling pathway.
In addition to small molecules, biologics may modulate xenobiotic receptor activity. Adnectins™ are a new family of therapeutic protein fragments designed to bind with high affinity to specific therapeutically relevant targets [24]. Adnectins™ are also being developed extensively for various protein targets indicated in cancers, lipoprotein maintenance, and other conditions. For example, the Adnectin™ CT-322 binds and inactivates vascular endothelial growth factor receptor 2 (VEGFR2) [25] and is being evaluated in Phase II clinical trials for efficacy in lung and metastatic colorectal cancer. In the first report of a xenobiotic receptor in complex with a biologic, Adnectin-1 was found to bind to PXR by competing with and effectively displacing steroid receptor coactivator-1 (SRC-1) peptide [26]. In this case, Adnectin-1 was shown to bind PXR, but whether it binds to CAR is unknown. However, the finding raises the possibility of xenobiotic receptor–mediated metabolic profile changes occurring with biologic treatment. Because of the structural similarity and functional overlaps between PXR and CAR, it is likely that both are capable of mediating biologic-induced alterations of drug metabolism.
3. CAR inhibitors
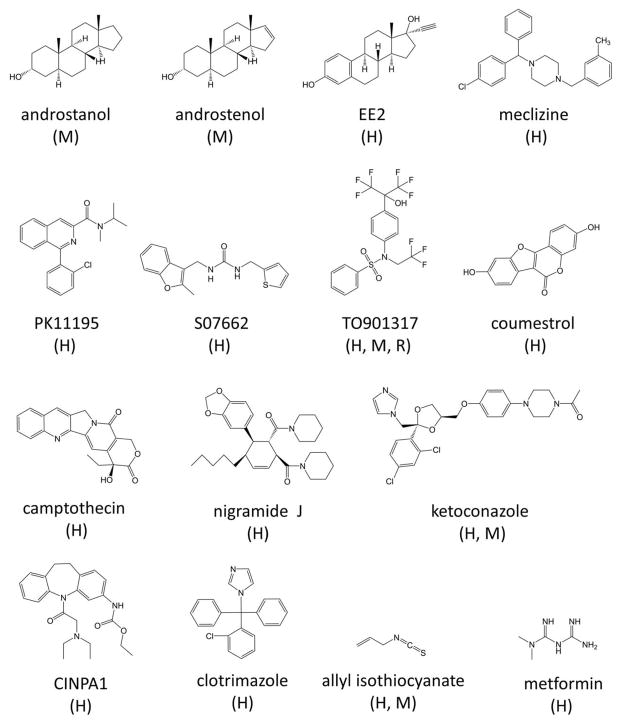
Because CAR regulates the expression of drug-metabolizing enzymes, there is great impetus to develop therapeutics that can prevent drug-drug interactions induced by elevated activity of CAR. Several compounds have been reported to decrease CAR activity (Figure 1), and some of these can competitively displace an agonist from the ligand-binding site (thus functioning as antagonists). Constitutive basal activity in the absence of an agonist can be abrogated by an inverse agonist. The term “inhibitor” is thus applied to any compound that decreases CAR activity, regardless of the mode of action (antagonism vs. inverse agonism).
Figure 1.
CAR inhibitors as reported for human (H), mouse (M) and rat (R) CAR.
Androstanol and the pheromone androstenol were among the first compounds to be validated as inhibitors of CAR basal activity [16]. This repression was not due to interference with protein-protein interactions of CAR and RXRα nor to disruption of DNA binding. Instead, it is believed that the androstanes cause dissociation of the co-activator from CAR. Although androstanol and androstenol share similar chemical structures, CAR was not inhibited by molecules with slight modifications, including 5α-androstan-3β-ol, 5α-androst-16-en-3β-ol, androstenone, 5α-androst-16-ene-3-one, dehydroepiandrosterone (DHEA), 5α-cholestan-3α-ol and pregnenolone.
The synthetic steroidal estrogen 17α-ethynyl-3,17 β-estradiol, also known as EE2, was described as a partial deactivator of hCAR, with 10 μM reducing transcriptional activity by 50–60% in reporter assays of cultured cells [27]. It was shown to promote the interaction between hCAR and the nuclear receptor co-repressor (NCoR). Conversely, it behaved as an agonist of mCAR, highlighting that slight sequence differences among orthologs can have contrasting regulatory effects.
Meclizine is an antiemetic and antihistamine drug of the piperazine class that targets H1 receptors. It was initially reported to be an hCAR inverse agonist based on reporter assays, with observed disruption of co-activator recruitment at high compound concentrations in a mammalian two-hybrid system [28]. By using humanized CAR mice, meclizine was shown to decrease acetaminophen-induced liver toxicity. Interestingly, this compound was reported to be an mCAR agonist instead. However, other studies did not validate meclizine as being an hCAR inverse agonist [29, 30].
The antifungal drug clotrimazole was reported to be a deactivator of hCAR but not of mCAR, displaying an EC50 of 700 nM in transcriptional reporter assays [31]. This compound was deemed to interact directly and with high affinity (Ki of 100 nM) with the protein in radioligand binding assays. By using a Foster/fluorescence resonance energy transfer (FRET) assay, clotrimazole was shown to disrupt the interaction of hCAR and SRC-1 with an IC50 of 100 nM. Nonetheless, these findings are challenged by contradicting reports that clotrimazole has no effect [32] or actually increases hCAR activity [27, 30, 33].
The peripheral benzodiazepine receptor ligand designated as PK11195 attenuates hCAR activity in different cell lines, with only a marginal response against mCAR [34]. In a mammalian one-hybrid system, the EC50 was approximately 0.8 μM [30]. Mammalian two-hybrid and GST pull-down assays demonstrated that PK11195 could repress the recruitment of the co-activators SRC-1 and glucocorticoid receptor-interacting protein 1 (GRIP1) without altering the interactions of CAR with the co-repressors NCoR and SMRT [34]. Contradictorily, another group reported that PK11195 increases CAR-NCoR interaction in a dose-dependent manner as a result of direct binding to the receptor as purified CAR is stabilized against proteolysis degradation to the same extent as several agonists [30]. Interestingly, in the presence of PK11195, hCAR localizes in the nucleus in the liver of mCAR-null mice [34].
By using virtual screening, the hCAR inverse agonist S07662 was identified as having an IC50 of 0.7 μM [30]. This compound could enhance NCoR recruitment, and it was suggested that it can directly bind to the LBD region, as it prevented protease digestion of the protein. Molecular dynamics simulations reaffirm the notion that this compound stabilizes the protein, modulating interactions of the receptor with cofactors. It is envisioned that S07662 increases the helical content of helix 12 and reorients it in such a way that prevents the adoption of the active form.
More recently, the potent LXR and hPXR agonist TO901317 was described as being an inverse agonist of the human, mouse, and rat orthologs of CAR, with an estimated IC50 of 2.2 μM [35]. The potent agonist CITCO reverses the inhibitory effect of TO901317 implying a competitive inhibition modality. TO901317 also is an antagonist of CAR splice variant 2 (CAR3), which does not possess constitutive activity and requires ligand-dependent activation. TO901317 prevented the interactions of hCAR with SRC-1 and increased interactions with the co-repressor NCoR.
Coumestrol is a phytoestrogen prevalent in legumes and soy beans [36]. The results of competition ligand binding assays using radiolabeled agonists show that coumestrol competes for binding to the CAR LBD with a Ki value of 54 μM. In cell-based reporter assays, the phytochemical displayed a profile consistent with a CAR inverse agonist, with an IC50 of 30 μM. Mechanistic studies are lacking for CAR, but the results of assays involving PXR mutants suggest that this compound binds to the outer surface of the PXR LBD. Because of structural similarities between PXR and CAR, it is also conceivable that coumestrol interacts with CAR LBD in a region other than the binding pocket.
The alkaloid nigramide J from Piper nigrum roots was discovered to be an hCAR inverse agonist via a mammalian one-hybrid screening of a library of natural compounds [37]. Cell-based assays displayed maximal activity suppression at a concentration of 5 μM. The compound had a lesser effect on rat CAR (rCAR), and no inhibition was observed in mCAR. Nigramide J is thought to occupy the ligand binding pocket because the inhibition can be reversed by the CAR agonist CITCO. This reversal would result in disengagement of CAR from the coactivator SRC-1, as the compound was shown to perturb the binding of SRC-1 but not that of the corepressor NCoR1. Interestingly, the alkaloids nigramide C and nigramide H, which are structurally similar to nigramide J, failed to attenuate CAR activity.
Several other compounds have been reported to reduce CAR transactivation, but detailed characterization is lacking, particularly concerning the inhibitory mechanism. In these reports, the main focus was the inhibition of PXR, and that of CAR was studied only for comparison purposes. These reported compounds include the quinoline alkaloid camptothecin [38], the antihyperglycemic agent metformin [39], the natural compound allyl isothiocyanate [40], and the antifungal ketoconazole [41]. We note that most known inhibitors of hCAR are also PXR activators, confounding the use of these molecules in instances of both receptors being present and functional.
4. CAR structure in complex with ligands
CAR displays several structural features shared among NRs [42], including a DBD linked to an LBD through a flexible hinge. The DBD, located at the N-terminus, binds to distinct target genes at the hormone response elements facilitated by zinc-finger motifs. CAR LBD is relatively large and flexible, which allows for the binding of ligands with a wide spectrum of chemical structures and molecular weights. Additionally, the LBD plays major roles in the interaction of CAR with co-regulatory proteins and RXR.
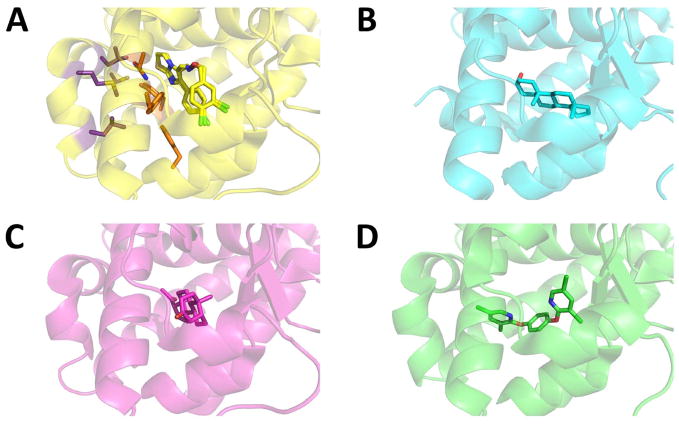
Only a few CAR crystal structures have been elucidated, but they provide invaluable information about the nature of CAR constitutive activity, ligand-mediated activation, and repression. All of these structures show the ligand to be positioned in the binding pocket within the LBD (Figure 2). The agonists 5β-pregnanedione and CITCO did not form direct contacts with the AF-2 helix, obstructed by a barrier comprising the residues Phe161, Asn165, Phe234, and Tyr326 [43]. In contrast, the high potency of the mCAR agonist TCPOBOP was attributed to its ability to penetrate this barrier [44].
Figure 2.
Crystal structures of the CAR LBD (cartoon representation) in complex with a ligand (stick representation) A) hCAR with CITCO (pdb 1XVP); B) mCAR with androstenol (pdb 1XNX); C) hCAR with 5β-pregnanedione (pdb 1XV9); D) mCAR with TCPOBOP (pdb 1XLS). Oxygen, nitrogen, and chlorine atoms are depicted in red, blue, and dark green, respectively. The residues Phe161, Asn165, Phe234, and Tyr326 (Figure 2A, carbon atoms in orange) form the barrier that obstructs ligand access to the AF-2 helix and helix-x residues Leu336, Met340, Leu343 and Cys347 (carbon atoms in purple). All structures are depicted in the same orientation for comparison.
The three-dimensional structure of mCAR LBD in complex with androstenol provides insight into the structural features leading to decreased basal activity [45]. In the active conformation, helices α10 and α11 appear to be a single continuous helix, which become two distinct helices separated by a “kink” upon androstenol binding [45]. The formation of the “kink” is thought to lead to an eventual destabilization of the active conformation by disrupting AF-2’s interaction with Lys205, which is an important characteristic of the active state of CAR.
5. Biological significance of CAR
5.1 CAR expression and function
CAR is predominantly expressed in the liver and intestine, but a low level of basal expression is also detected in the brain, heart, adrenal, testis, prostate, and kidney tissues [1, 46–49]. Many alternatively spliced variants of CAR have been identified [15, 47, 50, 51], with varying tissue expression profiles and different ligand specificities [5, 48, 52–55]. Although there is some preliminary data about the expression levels of the variants of CAR in liver [47, 56], further investigation of organ-specific induction of CAR target genes is needed to understand the tissue-specific variation in drug metabolism and drug–drug interactions. In one study of the splice-variant profile of CAR in the liver, researchers observed inter-ethnic and inter-individual variations among liver samples of Koreans and Caucasians [57]. In addition, single-nucleotide polymorphisms in the CAR gene have been indicated to confer differential functional effects (e.g., changes in bone mineral density or pharmacokinetics of the anti-HIV drug Efavirenz) [53, 58, 59]. Thus, any variation in CAR that is due to genetic polymorphisms or alternative splicing has the potential to influence the expression of CAR target genes and may contribute to the overall inter-individual variability in drug metabolism and the extent of drug–drug interactions.
CAR mRNA expression itself can be modulated by other nuclear receptors, such as hepatocyte nuclear factor 4 alpha (HNF4α), glucocorticoid receptor (GR), and possibly others [60–62]. It has been well documented that glucocorticoids (such as dexamethasone) increase the amount of CAR mRNA in the liver [62]. CAR is activated by a variety of xenobiotics and endobiotics (reviewed in [12]). CAR transactivation results in the expression of genes involved in the metabolism and efflux of these molecules. CAR target genes are often phase I, II, and III enzymes/transporters regulating the metabolism and secretion of endogenous and exogenous signaling molecules. These targets include many members of the cytochrome P450 family (CYPs), sulfotransferases (SULTs), UDP-glucoronyltransferases (UGTs), glutathione S-transferase (GST), membrane transporters, and many more genes that play crucial roles in absorption, distribution, metabolism, and excretion. CYP2B6 is considered to be a primary target gene of CAR activation. The response elements in this gene that bind activated CAR are also able to bind PXR, thus allowing this gene to be regulated by both receptors. Although an important phase I enzyme, CYP2B6 is considered to be minor compared to CYP3A4 in its ability to metabolize a broad spectrum of administered drugs and other xenobiotics. Although CYP3A4 is thought to be mainly regulated by PXR, activated CAR can enhance CYP3A4 levels [63, 64]. Therefore, there is a possibility of localized drug metabolism and removal in instances of tissues with increased expression and/or activation of CAR, even in the absence of PXR signaling. Extensive studies have revealed that CAR, much like PXR, plays a key role in xenobiotic and endobiotic metabolism, especially in liver and intestinal tissues, and in maintaining cellular and systemic homeostasis. Discussed below are a few such instances in which modulators of CAR could be vital players in sustaining normal physiology.
Hormone homeostasis
Androstanes were the first group of chemicals to be identified as CAR inverse agonists [16]. Micromolar concentrations of androstanes, which is several-fold higher than the physiological levels in circulation, are required to repress CAR-mediated transactivation. A major precursor of androgens and estrogens, dehydroepiandrosterone (DHEA) is a direct activator of CAR in mice, and its binding to CAR results in CAR nuclear accumulation and in increased transactivation of a CAR target gene [65]. Estrogens bind CAR with low binding affinity and promote CAR movement to the nucleus, similar to other CAR activators [66, 67]. But it is generally accepted that endogenous levels of estrogen in normal females might be too low to affect CAR. High concentrations of estrogens, which occur during pregnancy, are sufficient to activate CAR to induce CYP2B6 expression [68]. Estradiol and progesterone concentrations within this elevated physiological range during pregnancy may play a role in glucose tolerance and insulin resistance working through CAR [69]. Furthermore, CAR represses estrogen receptor (ER)-mediated transcriptional activation in cell-based transfection assays, and this action is proposed to occur by competition of CAR with ER for the existing coregulator pool, thus limiting availability for ER transactivation [70].
Androgens, estradiol, and their metabolites undergo sulfation by SULTs and glucuronidation by UGTs as part of their metabolic breakdown and excretion, and many of these Phase II enzymes are target genes of CAR [71, 72]. Thyroid hormone (TH) levels can also be regulated by xenobiotics working through CAR [73, 74], with CAR influencing TH activity by directly regulating synthesis of the hormone [74]. In this way, steroid hormone metabolism and clearance is tightly linked to the expression and activation status of CAR, amongst other receptors. These observations were made mostly in rodent models, and careful recapitulation in human tissue is needed. Nevertheless, cross-talk of CAR with other NRs could be a reason for the clinically observed alterations of physiological function and endocrine disruptions caused by xenobiotics.
Energy and heme homeostasis
Some clinical observations, especially those about PB treatments, were verified in the latter part of the 20th century to involve CAR signaling. Researchers have known for a long time that chronic PB treatment reduces plasma glucose levels in diabetic patients and decreases elevated bilirubin levels in patients with jaundice. Activation of CAR by PB causes a repression of hepatic gluconeogenic enzymes, such as phosphoenoylpyruvate carboxykinase 1 (PEPCK1) and glucose-6-phosphatase (G6Pase), and oxidative enzymes, such as carnitine palmitoyltransferase 1 (CPT1) and enoyl-CoA isomerase (ECI), suggesting a critical role for CAR in regulating hepatic energy metabolism in response to drug exposures [75, 76]. Important species differences in controlling hepatic energy metabolism could exist between hCAR and mCAR. For example, hCAR activation selectively inhibits gluconeogenesis without suppressing fatty acid synthesis, but mCAR activation results in downregulation of genes associated with gluconeogenesis (G6Pase and PEPCK1), fatty acid synthesis (Fas and Acc-α), and lipogenesis (Srebp-1c and Scd-1) [77]. CAR ligands can also induce Insig-1, an anti-lipogenic protein that controls triglyceride and cholesterol biosynthesis, which could explain the clinically reported alterations in lipid levels after certain drug therapies [78]. CAR activator TCPOBOP improved insulin sensitivity and glucose uptake and metabolism, inhibited lipogenesis and gluconeogenesis, and reduced adipose deposition induced by high fat diet in mice [79–82]. This suggests that CAR might have a role to play in preventing obesity and alleviating type 2 diabetes.
Bilirubin clearance is an important step in heme catabolism and reabsorption, misregulation of which could result in chronic accumulation associated with jaundice. CAR can regulate bilirubin breakdown and subsequent heme reabsorption by inducing UGTs and membrane transporters that promote bilirubin excretion [71, 83–85]. Liver-generated bile acids are required for micelle formation that promotes digestion and absorption of dietary fat. Bile secretion is also an important pathway for the elimination of large, hydrophobic endobiotic and xenobiotic metabolites, including many high–molecular weight conjugates. CAR activation has been consistently shown to reduce serum bile acid and bilirubin concentrations, with an elevation of the expression of the hepatic genes involved in their metabolism and excretion (CYPs, MRPs, UGT1A, GST, and SULTs [86–88]. Preliminary data from mice also indicate that CAR may be involved in the regulation of enzymes that produce bile acids [89]. These studies and others have thus broadened our understanding of CAR from being just a xenosensor to being a master regulator involved in several important physiological processes.
5.2 CAR regulation of cell growth and tumor resistance
In addition to being involved in maintenance of normal physiological processes, CAR regulation is also implicated in cell growth and tumor maintenance in a strictly species-specific manner. Phenobarbital is an anticonvulsant that has been successfully administered as anti-seizure medication globally since the early 1900s. Chronic phenobarbital exposure in rats has been historically linked to tumor formation in the liver [90, 91], and the discovery of CAR shed new light on possible mechanisms. In various strains of mice and rats, liver hyperplasia and tumors could be maintained with PB [92–96] or treatment with other CYP2B inducers [97–106] after exposure to liver tumor initiators such as diethylnitrosamine (DEN) or N-2-fluorenylacetamide (FAA). Subsequently, CAR knockout mice were noted to lack liver tumor formation after treatment with the tumor initiator DEN followed by PB, whereas tumors developed in normal mice [107]. Extrapolating from these compelling rodent studies, researchers pondered the possibility of CAR activators and CYP2B inducers (which includes a long list of clinical drugs and environmental agents) being tumor initiators or tumor promoters in humans. But results of multiple epidemiological studies in human patient cohorts treated with phenobarbital have demonstrated a lack of correlation between PB and an increased incidence of liver tumors in humans [108–110]. So, although tumorigenic events such as altered epigenetic changes, induction of hepatic P450 enzymes, liver hypertrophy, and decreased apoptosis were visible in PB-treated rodents (mice and rats), these changes are not observed in human hepatocytes or in patients treated with PB.
To address this species-specific variability in CAR activation and function, researchers have used chimeric mice with livers composed of human hepatocytes or humanized hCAR mice (with or without hPXR). In the chimeric mouse liver with human hepatocytes, a slight increase in liver weight was observed after PB treatment, which could be attributed to the small increase in cell volume (hypertrophy), but no hyperplasia was detected [111]. Furthermore, replicative DNA synthesis was not altered by PB treatment in the chimeric model, supporting the observation that rodent CYP2B inducers are not necessarily mitogenic agents in human hepatocytes. A change in methylation pattern of key genes in tumor development processes (angiogenesis, apoptosis, migration/invasion/metastasis) has been observed when comparing PB-treated normal mice (wild-type CAR) to CAR-knockout mice [112]. Studies have also been conducted in transgenic humanized knockout models (mice in which mCAR was replaced with hCAR), with interesting results. Although some researchers have reported that PB can produce proliferative transcriptional responses [113] and promote some tumor production [114] in hCAR/hPXR double-humanized mice, others have demonstrated liver hypertrophy without hyperplasia [115] and conclude that humanized mouse models are relatively resistant to tumorigenesis [92, 110, 114, 116, 117]. However, the hCAR/hPXR transgenic mouse, in which human-origin CAR and PXR are used with mouse-origin CAR target genes, may not be an appropriate model for extrapolating risk from rodent findings to humans. For example, PB and other CYP2B inducers can be non-genotoxic carcinogens and tumor promoters in mice and rats but appear to have a low likelihood of producing liver tumors in humans.
The reduced interspecies homology of CAR-LBDs contributes to the species-specific variability in CAR activation by small molecules (reviewed in [118]). It might be prudent to keep in mind that key species differences in the signaling of CAR target genes are likely to contribute to differences in the potency of hepatocellular proliferation and expression of drug-metabolizing enzymes. Although it is agreed upon that the presence of CAR activators can cause CAR-mediated activation of xenobiotic metabolizing enzymes and liver hypertrophy in human hepatocytes, these activators do not induce liver hyperplasia or hepatocellular carcinomas in humans [110, 116, 117]. Thus, particular considerations need to be made when evaluating human cancer risk and carcinogenic potential of chemicals in animal models [119].
CAR function in cell growth and tumorigenesis has also been evaluated in human primary hepatocytes and various human cancers. A divided opinion exists among researchers about the utility of CAR activators and CAR deactivators in their function as antineoplastic agents. Some reports point to CAR activation as a step in increasing chemotherapeutic efficacy. The chemotherapeutic cyclophosphamide (CPA) is widely used to treat many types of cancers. As a prodrug, CPA is converted in the body to its active form mainly by CYP2B6. Because CYP2B6 is a major target of CAR, in hepatocyte co-cultures with a leukemia cell line, CAR activation (with agonist CITCO) can enhance CPA-based antitumor activity and apoptosis [120] by increasing the availability of the active form. Recently, the common chemotherapeutic paclitaxel was used to determine that CAR agonists modestly increase paclitaxel-induced tumor depletion in 5 human lung cancer cell lines [121]. Interestingly, paclitaxel is a PXR agonist and a known inducer of CYP3A4 [122] and membrane transporters [123], which in turn could affect the breakdown and elimination of chemotherapeutics. Human CAR agonist CITCO inhibited the growth and expansion of brain tumor stem cells in isolated human gliospheres and in xenograft mouse studies by inducing cell-cycle arrest and apoptosis [124].
Consistent with the anticipated role of CAR in promoting drug breakdown and elimination, other reports point to CAR inhibition as an approach to increase chemotherapeutic efficacy. In four ovarian cell lines expressing CAR, treatment with CAR activator CITCO and anticancer agents caused an enhanced upregulation of MDR1 and UGT1A1 and induced significantly lower levels of apoptosis than did any single anticancer drug [125]. These observations were reversed when CAR was downregulated by RNA interference, indicating the possibility of increasing the efficacy of anticancer agents by downregulating CAR. Downregulation of CAR function can also be achieved by small molecules, underscoring the need for new CAR inhibitors that do not induce metabolic enzymes or membrane transporters through other mechanisms (for instance, by activating PXR). The divided opinion among researchers on the utility of CAR activators and CAR deactivators as antineoplastic agents, each supported by reasonable experimental data obtained from different experimental models by using therapeutic agents with different modes of action, indicates the need for further investigation of CAR regulation. There are many CAR isoforms. CAR isoform expression may vary between cancer types thus bringing up the possibility of isoform-specific regulation of cancer cells, which in turn enables them to respond to CAR activators or inhibitors differently. The feasibility of modulating the function of CAR by using small molecules with a specific mechanism of action will greatly facilitate such investigations.
6. Conclusion
CAR is known as a master xenobiotic sensor. Although we do not yet fully understand the significance of CAR other than its ADME and energy homeostatic functions, evidence suggests that CAR can regulate other important functions in the organism. In one study, neonatal activation of CAR in mice resulted in an epigenetic memory change, leading to permanent upregulation of certain liver drug–metabolic enzymes in the adult [126]. In other studies, CAR activation in the rodent liver increased cell proliferation and inhibited apoptosis, resulting in an increased incidence of hepatocellular carcinomas and liver injury from chronic exposure to CAR activators. In addition, CAR levels were markedly enhanced during the differentiation of hepatic-like cells derived from human embryonic stem cells, suggesting that CAR has a key functional role in directing human hepatogenesis [127].
Small-molecule modulators of CAR, including activators and inhibitors, exist. The reported CAR inhibitors encompass a wide range of chemical scaffolds, attesting to the promiscuous nature of CAR. This feature would provide ample opportunities for further modifications to improve potency and selectivity and reduce toxicity. The fact that several of these compounds are of natural sources, including some that are part of a routine diet, underscores the possibility of obtaining inhibitors with reduced side effects.
Although only a few structures exist of CAR LBD in complex with ligands, they cover some of the most important aspects of ligand-induced modulation of CAR activity, including agonism, superagonism, and inverse agonism. The structures also provide the basis for understanding the constitutive activation of CAR.
7. Expert opinion
The mouse CAR activator TCPOBOP has been shown to significantly suppress adipose deposition and weight gain induced by high fat diet in mice, and CAR activation was associated with improved serum lipid profile and insulin sensitivity [79–81]. In human studies, the anti-epileptic drug phenytoin, which is also a known indirect activator of CAR, has been shown to increase levels of high-density cholesterol [128, 129] suggesting a protective role of CAR activation in cardiovascular ailments [130]. Although CAR activators can cause CAR-mediated induction of xenobiotic metabolizing enzymes and liver hypertrophy in human hepatocytes, stronger evidence is required to determine the involvement of CAR activation in inducing hepatocellular carcinomas in humans [110, 116, 117]. Extrapolation of rodent CAR function to humans has to be done cautiously considering the species differences exhibited by CAR. Rodent models of xenobiotic receptors are used for evaluating the metabolic profile and carcinogenic properties of many clinical drugs and environmental chemicals, and special considerations need to be in place when evaluating human cancer risk and carcinogenic potential of chemicals in animal models. In addition, since activators of CAR promote carcinogenesis in rodent models in a CAR-dependent manner, when using such rodent tumor models that depend on activator of CAR to promote tumorigenesis to evaluate the anti-cancer properties of compounds, it is critical to first evaluate the effect of these compounds on CAR function in order to fully and appropriately interpret data obtained from such studies; a compound that modulates CAR function will behave differently from a compound that doesn’t, in such rodent tumor models. Some recent studies have hinted at the possibility of CAR inhibition as a therapeutic target for averting chemoresistance, possibly by reducing membrane transporters that are involved in the active efflux of chemotherapeutics; or CAR activation as a therapeutic target that induces CYP2B6, which increases the conversion of a prodrug CPA to its active form thus enhancing CPA-based antitumor activity and apoptosis [120, 125]. This possibility emphasizes the need for better understanding of CAR as a therapeutic target and for developing new small-molecule modulators (either activators or inhibitors, depending on the specific therapeutic need as discussed above) which are specific to CAR and able to modulate specific functions of CAR. One such small molecule capable of inhibiting CAR without activating PXR, named CINPA1, was recently identified and shown to reduce the co-activator recruitment and DNA binding properties of CAR [131] (Figure 1), supporting the feasibility of developing specific small molecule CAR modulators to meet a specific therapeutic need as required.
The ever-growing number of CAR inhibitors should be extensively assessed, particularly because target genes and various ligands of CAR are shared with PXR, another master xenobiotic sensor. Comparative analyses of CAR and PXR inhibitors that are performed by using purified proteins, careful selection of cell-based assay parameters, and structural/computational studies would be considerably beneficial.
CAR and PXR were discovered and initially defined as master xenobiotic receptors; they were since revealed to affect many other physiologic and pathologic processes. They not only are regulated, but also regulate other cellular signaling pathways. Therefore, to comprehensively understand the regulation and function of CAR, a systematic approach to investigate CAR by placing CAR in the context of all relevant signaling pathways is needed. Because of the increasing complexity of CAR regulation and function, novel small molecules with specific properties are needed to modulate the specific function of CAR under specific physiologic or pathologic context. Therefore, instead of passively evaluating whether existing compounds affect the function of CAR, it is critical to actively develop small molecules that specifically modulate CAR, guided by the newly discovered function of CAR. In the coming years, the comprehensive function of CAR will be revealed, assisted by large-scale genomic and proteomic studies, which will trigger the development of specific CAR modulators to further chemically validate the comprehensive function of CAR, some of which might become useful therapeutic leads. Continued study of CAR biology and that of other xenobiotic receptors, by taking a multidisciplinary and systematic approach, will be highly beneficial in better understanding their roles in drug metabolism and development of human diseases.
Article highlights.
CAR plays critical roles in the detoxification of xenobiotics and in the homeostasis of hormones, energy, and heme.
The unique features of CAR include constitutive activation, ligand promiscuity, species differences, and the ability to regulate a large number of genes involved in xenobiotic detoxification, either overlapping with or distinctive from those regulated by pregnane X receptor (PXR).
CAR is involved in the events leading to liver carcinogenesis in rodents, and possible implications of CAR in human diseases are considered.
Structural analysis provides insight into the constitutive activity of CAR and inverse agonism.
Acknowledgments
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC), St. Jude Children’s Research Hospital, National Institutes of Health National Institute of General Medical Sciences [Grants RO1-GM086415 & RO1-GM110034], and National Cancer Institute [Grant P30-CA21765]. The authors thank Cherise Guess PhD, ELS, for editing the manuscript.
Footnotes
Declaration of Interest
The authors declare no conflict of interest.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Baes M, Gulick T, Choi HS, et al. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol. 1994;14:1544–52. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Faucette S, Sueyoshi T, et al. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem. 2003;278:14146–52. doi: 10.1074/jbc.M212482200. [DOI] [PubMed] [Google Scholar]
- 3.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Min G, Kim H, Bae Y, et al. Inhibitory cross-talk between estrogen receptor (ER) and constitutively activated androstane receptor (CAR). CAR inhibits ER-mediated signaling pathway by squelching p160 coactivators. J Biol Chem. 2002;277:34626–33. doi: 10.1074/jbc.M205239200. [DOI] [PubMed] [Google Scholar]
- 5.Choi HS, Chung M, Tzameli I, et al. Differential transactivation by two isoforms of the orphan nuclear hormone receptor CAR. J Biol Chem. 1997;272:23565–71. doi: 10.1074/jbc.272.38.23565. [DOI] [PubMed] [Google Scholar]
- 6.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–50. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 7.Auerbach SS, Stoner MA, Su S, et al. Retinoid X receptor-alpha-dependent transactivation by a naturally occurring structural variant of human constitutive androstane receptor (NR1I3) Mol Pharmacol. 2005;68:1239–53. doi: 10.1124/mol.105.013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suino K, Peng L, Reynolds R, et al. The nuclear xenobiotic receptor CAR: structural determinants of constitutive activation and heterodimerization. Mol Cell. 2004;16:893–905. doi: 10.1016/j.molcel.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Wang K, Wan YJ. Retinoids activate RXR/CAR-mediated pathway and induce CYP3A. Biochem Pharmacol. 2010;79:270–6. doi: 10.1016/j.bcp.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzameli I, Pissios P, Schuetz EG, et al. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol. 2000;20:2951–8. doi: 10.1128/mcb.20.9.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Xu RX, Lambert MH, Wisely BB, et al. A structural basis for constitutive activity in the human CAR/RXRalpha heterodimer. Mol Cell. 2004;16:919–28. doi: 10.1016/j.molcel.2004.11.042. Important structural studies of CAR. [DOI] [PubMed] [Google Scholar]
- 12••.Molnar F, Kublbeck J, Jyrkkarinne J, et al. An update on the constitutive androstane receptor (CAR) Drug Metabol Drug Interact. 2013;28:79–93. doi: 10.1515/dmdi-2013-0009. A comprehensive review of CAR biology. [DOI] [PubMed] [Google Scholar]
- 13.Maglich JM, Parks DJ, Moore LB, et al. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J Biol Chem. 2003;278:17277–83. doi: 10.1074/jbc.M300138200. [DOI] [PubMed] [Google Scholar]
- 14.Honkakoski P, Zelko I, Sueyoshi T, et al. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18:5652–8. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auerbach SS, Ramsden R, Stoner MA, et al. Alternatively spliced isoforms of the human constitutive androstane receptor. Nucleic Acids Res. 2003;31:3194–207. doi: 10.1093/nar/gkg419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forman BM, Tzameli I, Choi HS, et al. Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature. 1998;395:612–5. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- 17.Burk O, Arnold KA, Nussler AK, et al. Antimalarial artemisinin drugs induce cytochrome P450 and MDR1 expression by activation of xenosensors pregnane X receptor and constitutive androstane receptor. Mol Pharmacol. 2005;67:1954–65. doi: 10.1124/mol.104.009019. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Ferguson SS, Negishi M, et al. Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol Pharmacol. 2003;64:316–24. doi: 10.1124/mol.64.2.316. [DOI] [PubMed] [Google Scholar]
- 19.Kietzmann T, Hirsch-Ernst KI, Kahl GF, et al. Mimicry in primary rat hepatocyte cultures of the in vivo perivenous induction by phenobarbital of cytochrome P-450 2B1 mRNA: role of epidermal growth factor and perivenous oxygen tension. Mol Pharmacol. 1999;56:46–53. doi: 10.1124/mol.56.1.46. [DOI] [PubMed] [Google Scholar]
- 20.Waxman DJ, Azaroff L. Phenobarbital induction of cytochrome P-450 gene expression. Biochem J. 1992;281 (Pt 3):577–92. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Mutoh S, Sobhany M, Moore R, et al. Phenobarbital indirectly activates the constitutive active androstane receptor (CAR) by inhibition of epidermal growth factor receptor signaling. Sci Signal. 2013;6:ra31. doi: 10.1126/scisignal.2003705. Mechanism of phenobarbital inhibition of CAR activity elucidated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutoh S, Osabe M, Inoue K, et al. Dephosphorylation of threonine 38 is required for nuclear translocation and activation of human xenobiotic receptor CAR (NR1I3) J Biol Chem. 2009;284:34785–92. doi: 10.1074/jbc.M109.048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez AC, Smutny T, Hyrsova L, et al. Chrysin, baicalein and galangin are indirect activators of the human constitutive androstane receptor (CAR) Toxicol Lett. 2015 doi: 10.1016/j.toxlet.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Lipovsek D. Adnectins: engineered target-binding protein therapeutics. Protein Eng Des Sel. 2011;24:3–9. doi: 10.1093/protein/gzq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dineen SP, Sullivan LA, Beck AW, et al. The Adnectin CT-322 is a novel VEGF receptor 2 inhibitor that decreases tumor burden in an orthotopic mouse model of pancreatic cancer. BMC Cancer. 2008;8:352. doi: 10.1186/1471-2407-8-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan JA, Camac DM, Low S, et al. Developing Adnectins That Target SRC Co-Activator Binding to PXR: A Structural Approach toward Understanding Promiscuity of PXR. J Mol Biol. 2015;427:924–42. doi: 10.1016/j.jmb.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Makinen JM, Frank C, Jyrkkarinne J, et al. Modulation of mouse and human phenobarbital-responsive enhancer module by nuclear receptors. Molecular pharmacology. 2002;62:366–78. doi: 10.1124/mol.62.2.366. [DOI] [PubMed] [Google Scholar]
- 28.Huang WD, Zhang J, Wei P, et al. Meclizine is an agonist ligand for mouse constitutive androstane receptor (CAR) and an inverse agonist for human CAR. Mol Endocrinol. 2004;18:2402–08. doi: 10.1210/me.2004-0046. [DOI] [PubMed] [Google Scholar]
- 29.Lau AJ, Yang GX, Rajaraman G, et al. Differential Effect of Meclizine on the Activity of Human Pregnane X Receptor and Constitutive Androstane Receptor. J Pharmacol Exp Ther. 2011;336:816–26. doi: 10.1124/jpet.110.175927. [DOI] [PubMed] [Google Scholar]
- 30.Kublbeck J, Jyrkkarinne J, Molnar F, et al. New in Vitro Tools to Study Human Constitutive Androstane Receptor (CAR) Biology: Discovery and Comparison of Human CAR Inverse Agonists. Mol Pharmaceut. 2011;8:2424–33. doi: 10.1021/mp2003658. [DOI] [PubMed] [Google Scholar]
- 31.Moore LB, Parks DJ, Jones SA, et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. Journal of Biological Chemistry. 2000;275:15122–27. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 32.Toell A, Kroncke KD, Kleinert H, et al. Orphan nuclear receptor binding site in the human inducible nitric oxide synthase promoter mediates responsiveness to steroid and xenobiotic ligands. J Cell Biochem. 2002;85:72–82. [PubMed] [Google Scholar]
- 33.Kublbeck J, Jyrkkarinne J, Poso A, et al. Discovery of substituted sulfonamides and thiazolidin-4-one derivatives as agonists of human constitutive androstane receptor. Biochem Pharmacol. 2008;76:1288–97. doi: 10.1016/j.bcp.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 34•.Li LH, Chen T, Stanton JD, et al. The peripheral benzodiazepine receptor ligand 1-(2-chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Molecular pharmacology. 2008;74:443–53. doi: 10.1124/mol.108.046656. Identified PK11195 as a potent CAR inverse agonist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanno Y, Tanuma N, Takahashi A, et al. T0901317, a potent LXR agonist, is an inverse agonist of CAR. J Toxicol Sci. 2013;38:309–15. doi: 10.2131/jts.38.309. [DOI] [PubMed] [Google Scholar]
- 36.Wang HW, Li H, Moore LB, et al. The phytoestrogen Coumestrol is a naturally occurring antagonist of the human pregnane x receptor. Mol Endocrinol. 2008;22:838–57. doi: 10.1210/me.2007-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanno Y, Tanuma N, Yatsu T, et al. Nigramide J is a novel potent inverse agonist of the human constitutive androstane receptor. Pharmacology Research & Perspectives. 2014;2:2–2. doi: 10.1002/prp2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen YK, Tang Y, Robbins GT, et al. Camptothecin Attenuates Cytochrome P450 3A4 Induction by Blocking the Activation of Human Pregnane X Receptor. J Pharmacol Exp Ther. 2010;334:999–1008. doi: 10.1124/jpet.110.168294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krausova L, Stejskaova L, Wang HW, et al. Metformin suppresses pregnane X receptor (PXR)-regulated transactivation of CYP3A4 gene. Biochemical pharmacology. 2011;82:1771–80. doi: 10.1016/j.bcp.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim YP, Cheng CH, Chen WC, et al. Allyl isothiocyanate (AITC) inhibits pregnane X receptor (PXR) and constitutive androstane receptor (CAR) activation and protects against acetaminophen- and amiodarone-induced cytotoxicity. Arch Toxicol. 2015;89:57–72. doi: 10.1007/s00204-014-1230-x. [DOI] [PubMed] [Google Scholar]
- 41.Huang H, Wang H, Sinz M, et al. Inhibition of drug metabolism by blocking the activation of nuclear receptors by ketoconazole. Oncogene. 2007;26:258–68. doi: 10.1038/sj.onc.1209788. [DOI] [PubMed] [Google Scholar]
- 42.Ingraham HA, Redinbo MR. Orphan nuclear receptors adopted by crystallography. Curr Opin Struc Biol. 2005;15:708–15. doi: 10.1016/j.sbi.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 43••.Xu RX, Lambert MH, Wisely BB, et al. A structural basis for constitutive activity in the human CAR/RXR alpha heterodimer. Mol Cell. 2004;16:919–28. doi: 10.1016/j.molcel.2004.11.042. Important structural studies of CAR. [DOI] [PubMed] [Google Scholar]
- 44••.Suino K, Peng L, Reynolds R, et al. The nuclear xenobiotic receptor CAR: Structural determinants of constitutive activation and heterodimerization. Mol Cell. 2004;16:893–905. doi: 10.1016/j.molcel.2004.11.036. Important structural studies of CAR. [DOI] [PubMed] [Google Scholar]
- 45••.Shan L, Vincent J, Brunzelle JS, et al. Structure of the murine constitutive androstane receptor complexed to androstenol: a molecular basis for inverse agonism. Mol Cell. 2004;16:907–17. doi: 10.1016/j.molcel.2004.11.037. Important structural studies of CAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shmueli O, Horn-Saban S, Chalifa-Caspi V, et al. GeneNote: whole genome expression profiles in normal human tissues. Comptes Rendus Biologies. 2003;326:1067–72. doi: 10.1016/j.crvi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Lamba JK, Lamba V, Yasuda K, et al. Expression of constitutive androstane receptor splice variants in human tissues and their functional consequences. J Pharmacol Exp Ther. 2004;311:811–21. doi: 10.1124/jpet.104.069310. [DOI] [PubMed] [Google Scholar]
- 48.Arnold KA, Eichelbaum M, Burk O. Alternative splicing affects the function and tissue-specific expression of the human constitutive androstane receptor. Nuclear receptor. 2004;2:1. doi: 10.1186/1478-1336-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.GeneCards: NR1I3 gene. Available at: http://www.genecards.org/cgi-bin/carddisp.pl?gene=NR1I3 [Last updated on 7 May 2014, cited on 7 April 2015]
- 50.Auerbach SS, Dekeyser JG, Stoner MA, et al. CAR2 displays unique ligand binding and RXRalpha heterodimerization characteristics. Drug Metab Dispos. 2007;35:428–39. doi: 10.1124/dmd.106.012641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanno Y, Aoki S, Nakahama T, et al. Role of the Defective Splicing of mRNA in the Lack of Pulmonary Expression of Constitutively Active Receptor in Rat. Journal of Health Science. 2003;49:541–46. [Google Scholar]
- 52.Omiecinski CJ, Coslo DM, Chen T, et al. Multi-species analyses of direct activators of the constitutive androstane receptor. Toxicol Sci. 2011;123:550–62. doi: 10.1093/toxsci/kfr191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikeda S, Kurose K, Jinno H, et al. Functional analysis of four naturally occurring variants of human constitutive androstane receptor. Mol Genet Metab. 2005;86:314–9. doi: 10.1016/j.ymgme.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Lau AJ, Chang TK. Fetal bovine serum and human constitutive androstane receptor: evidence for activation of the SV23 splice variant by artemisinin, artemether, and arteether in a serum-free cell culture system. Toxicol Appl Pharmacol. 2014;277:221–30. doi: 10.1016/j.taap.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 55.Sharma D, Lau AJ, Sherman MA, et al. Differential activation of human constitutive androstane receptor and its SV23 and SV24 splice variants by rilpivirine and etravirine. Br J Pharmacol. 2014 doi: 10.1111/bph.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jinno H, Tanaka-Kagawa T, Hanioka N, et al. Identification of novel alternative splice variants of human constitutive androstane receptor and characterization of their expression in the liver. Mol Pharmacol. 2004;65:496–502. doi: 10.1124/mol.65.3.496. [DOI] [PubMed] [Google Scholar]
- 57.Choi EJ, Jang YJ, Cha EY, et al. Identification and characterization of novel alternative splice variants of human constitutive androstane receptor in liver samples of Koreans and Caucasians. Drug Metab Dispos. 2013;41:888–96. doi: 10.1124/dmd.112.049791. [DOI] [PubMed] [Google Scholar]
- 58.Cortes CP, Siccardi M, Chaikan A, et al. Correlates of efavirenz exposure in Chilean patients affected with human immunodeficiency virus reveals a novel association with a polymorphism in the constitutive androstane receptor. Ther Drug Monit. 2013;35:78–83. doi: 10.1097/FTD.0b013e318274197e. [DOI] [PubMed] [Google Scholar]
- 59.Urano T, Usui T, Shiraki M, et al. Association of a single nucleotide polymorphism in the constitutive androstane receptor gene with bone mineral density. Geriatrics & gerontology international. 2009;9:235–41. doi: 10.1111/j.1447-0594.2009.00527.x. [DOI] [PubMed] [Google Scholar]
- 60.Ding X, Lichti K, Kim I, et al. Regulation of constitutive androstane receptor and its target genes by fasting, cAMP, hepatocyte nuclear factor alpha, and the coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha. J Biol Chem. 2006;281:26540–51. doi: 10.1074/jbc.M600931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tirona RG, Lee W, Leake BF, et al. The orphan nuclear receptor HNF4alpha determines PXR- and CAR-mediated xenobiotic induction of CYP3A4. Nat Med. 2003;9:220–4. doi: 10.1038/nm815. [DOI] [PubMed] [Google Scholar]
- 62.Pascussi JM, Gerbal-Chaloin S, Fabre JM, et al. Dexamethasone enhances constitutive androstane receptor expression in human hepatocytes: consequences on cytochrome P450 gene regulation. Mol Pharmacol. 2000;58:1441–50. doi: 10.1124/mol.58.6.1441. [DOI] [PubMed] [Google Scholar]
- 63.Goodwin B, Hodgson E, D’Costa DJ, et al. Transcriptional regulation of the human CYP3A4 gene by the constitutive androstane receptor. Mol Pharmacol. 2002;62:359–65. doi: 10.1124/mol.62.2.359. [DOI] [PubMed] [Google Scholar]
- 64.Li L, Chen T, Stanton JD, et al. The peripheral benzodiazepine receptor ligand 1-(2-chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Mol Pharmacol. 2008;74:443–53. doi: 10.1124/mol.108.046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kohalmy K, Tamasi V, Kobori L, et al. Dehydroepiandrosterone induces human CYP2B6 through the constitutive androstane receptor. Drug Metab Dispos. 2007;35:1495–501. doi: 10.1124/dmd.107.016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawamoto T, Kakizaki S, Yoshinari K, et al. Estrogen activation of the nuclear orphan receptor CAR (constitutive active receptor) in induction of the mouse Cyp2b10 gene. Mol Endocrinol. 2000;14:1897–905. doi: 10.1210/mend.14.11.0547. [DOI] [PubMed] [Google Scholar]
- 67.Makinen J, Reinisalo M, Niemi K, et al. Dual action of oestrogens on the mouse constitutive androstane receptor. Biochem J. 2003;376:465–72. doi: 10.1042/BJ20030553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koh KH, Jurkovic S, Yang K, et al. Estradiol induces cytochrome P450 2B6 expression at high concentrations: implication in estrogen-mediated gene regulation in pregnancy. Biochem Pharmacol. 2012;84:93–103. doi: 10.1016/j.bcp.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masuyama H, Hiramatsu Y. Potential role of estradiol and progesterone in insulin resistance through constitutive androstane receptor. J Mol Endocrinol. 2011;47:229–39. doi: 10.1530/JME-11-0046. [DOI] [PubMed] [Google Scholar]
- 70.Min G, Kemper JK, Kemper B. Glucocorticoid receptor-interacting protein 1 mediates ligand-independent nuclear translocation and activation of constitutive androstane receptor in vivo. J Biol Chem. 2002;277:26356–63. doi: 10.1074/jbc.M200051200. [DOI] [PubMed] [Google Scholar]
- 71.Sugatani J, Sueyoshi T, Negishi M, et al. Regulation of the Human UGT1A1 Gene by Nuclear Receptors Constitutive Active/Androstane Receptor, Pregnane X Receptor, and Glucocorticoid Receptor. In: Helmut S, Lester P, editors. Methods Enzymol. Academic Press; 2005. pp. 92–104. [DOI] [PubMed] [Google Scholar]
- 72.Fang HL, Strom SC, Cai H, et al. Regulation of human hepatic hydroxysteroid sulfotransferase gene expression by the peroxisome proliferator-activated receptor alpha transcription factor. Mol Pharmacol. 2005;67:1257–67. doi: 10.1124/mol.104.005389. [DOI] [PubMed] [Google Scholar]
- 73.Maglich JM, Watson J, McMillen PJ, et al. The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. J Biol Chem. 2004;279:19832–8. doi: 10.1074/jbc.M313601200. [DOI] [PubMed] [Google Scholar]
- 74.Qatanani M, Zhang J, Moore DD. Role of the constitutive androstane receptor in xenobiotic-induced thyroid hormone metabolism. Endocrinology. 2005;146:995–1002. doi: 10.1210/en.2004-1350. [DOI] [PubMed] [Google Scholar]
- 75.Kiyosawa N, Tanaka K, Hirao J, et al. Molecular mechanism investigation of phenobarbital-induced serum cholesterol elevation in rat livers by microarray analysis. Arch Toxicol. 2004;78:435–42. doi: 10.1007/s00204-004-0565-0. [DOI] [PubMed] [Google Scholar]
- 76.Ueda A, Hamadeh HK, Webb HK, et al. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- 77.Lynch C, Pan Y, Li L, et al. Activation of the constitutive androstane receptor inhibits gluconeogenesis without affecting lipogenesis or fatty acid synthesis in human hepatocytes. Toxicol Appl Pharmacol. 2014;279:33–42. doi: 10.1016/j.taap.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roth A, Looser R, Kaufmann M, et al. Regulatory cross-talk between drug metabolism and lipid homeostasis: constitutive androstane receptor and pregnane X receptor increase Insig-1 expression. Mol Pharmacol. 2008;73:1282–9. doi: 10.1124/mol.107.041012. [DOI] [PubMed] [Google Scholar]
- 79•.Gao J, He J, Zhai Y, et al. The constitutive androstane receptor is an anti-obesity nuclear receptor that improves insulin sensitivity. J Biol Chem. 2009;284:25984–92. doi: 10.1074/jbc.M109.016808. CAR activation suppresses the progression of obesity in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tamashiro KL, Terrillion CE, Hyun J, et al. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 2009;58:1116–25. doi: 10.2337/db08-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong B, Qatanani M, Moore DD. Constitutive androstane receptor mediates the induction of drug metabolism in mouse models of type 1 diabetes. Hepatology. 2009;50:622–9. doi: 10.1002/hep.23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82•.Dong B, Saha PK, Huang W, et al. Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc Natl Acad Sci U S A. 2009;106:18831–6. doi: 10.1073/pnas.0909731106. CAR activation improves symptoms of type 2 diabetes in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang W, Zhang J, Chua SS, et al. Induction of bilirubin clearance by the constitutive androstane receptor (CAR) Proc Natl Acad Sci U S A. 2003;100:4156–61. doi: 10.1073/pnas.0630614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kast HR, Goodwin B, Tarr PT, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–15. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 85.Sugatani J, Kojima H, Ueda A, et al. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology. 2001;33:1232–8. doi: 10.1053/jhep.2001.24172. [DOI] [PubMed] [Google Scholar]
- 86.Guo GL, Lambert G, Negishi M, et al. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem. 2003;278:45062–71. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- 87.Saini SP, Sonoda J, Xu L, et al. A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol Pharmacol. 2004;65:292–300. doi: 10.1124/mol.65.2.292. [DOI] [PubMed] [Google Scholar]
- 88.Zhang J, Huang W, Qatanani M, et al. The constitutive androstane receptor and pregnane X receptor function coordinately to prevent bile acid-induced hepatotoxicity. J Biol Chem. 2004;279:49517–22. doi: 10.1074/jbc.M409041200. [DOI] [PubMed] [Google Scholar]
- 89.Stedman CA, Liddle C, Coulter SA, et al. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci U S A. 2005;102:2063–8. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peraino C, Fry RJ, Staffeldt E. Reduction and enhancement by phenobarbital of hepatocarcinogenesis induced in the rat by 2-acetylaminofluorene. Cancer Res. 1971;31:1506–12. [PubMed] [Google Scholar]
- 91.Nishizumi M. Enhancement of diethylnitrosamine hepatocarcinogenesis in rats by exposure to polychlorinated biphenyls or phenobarbital. Cancer Lett. 1976;2:11–5. doi: 10.1016/s0304-3835(76)80004-3. [DOI] [PubMed] [Google Scholar]
- 92.Huang W, Zhang J, Washington M, et al. Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol. 2005;19:1646–53. doi: 10.1210/me.2004-0520. [DOI] [PubMed] [Google Scholar]
- 93.Ward JM. Increased susceptibility of livers of aged F344/NCr rats to the effects of phenobarbital on the incidence, morphology, and histochemistry of hepatocellular foci and neoplasms. J Natl Cancer Inst. 1983;71:815–23. [PubMed] [Google Scholar]
- 94.Ward JM, Rice JM, Creasia D, et al. Dissimilar patterns of promotion by di(2-ethylhexyl)phthalate and phenobarbital of hepatocellular neoplasia initiated by diethylnitrosamine in B6C3F1 mice. Carcinogenesis. 1983;4:1021–9. doi: 10.1093/carcin/4.8.1021. [DOI] [PubMed] [Google Scholar]
- 95.Diwan BA, Rice JM, Ohshima M, et al. Interstrain differences in susceptibility to liver carcinogenesis initiated by N-nitrosodiethylamine and its promotion by phenobarbital in C57BL/6NCr, C3H/HeNCrMTV- and DBA/2NCr mice. Carcinogenesis. 1986;7:215–20. doi: 10.1093/carcin/7.2.215. [DOI] [PubMed] [Google Scholar]
- 96.Kodama S, Negishi M. Phenobarbital confers its diverse effects by activating the orphan nuclear receptor car. Drug Metab Rev. 2006;38:75–87. doi: 10.1080/03602530600569851. [DOI] [PubMed] [Google Scholar]
- 97.Tinwell H, Rouquié D, Schorsch F, et al. Liver tumor formation in female rat induced by fluopyram is mediated by CAR/PXR nuclear receptor activation. Regul Toxicol Pharmacol. 2015 doi: 10.1016/j.yrtph.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 98.Ledda-Columbano GM, Pibiri M, Concas D, et al. Sex difference in the proliferative response of mouse hepatocytes to treatment with the CAR ligand, TCPOBOP. Carcinogenesis. 2003;24:1059–65. doi: 10.1093/carcin/bgg063. [DOI] [PubMed] [Google Scholar]
- 99.Takizawa D, Kakizaki S, Horiguchi N, et al. Constitutive active/androstane receptor promotes hepatocarcinogenesis in a mouse model of non-alcoholic steatohepatitis. Carcinogenesis. 2011;32:576–83. doi: 10.1093/carcin/bgq277. [DOI] [PubMed] [Google Scholar]
- 100.Yueh M-F, Li T, Evans RM, et al. Triclocarban Mediates Induction of Xenobiotic Metabolism through Activation of the Constitutive Androstane Receptor and the Estrogen Receptor Alpha. PLoS One. 2012;7:e37705. doi: 10.1371/journal.pone.0037705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yueh M-F, Taniguchi K, Chen S, et al. The commonly used antimicrobial additive triclosan is a liver tumor promoter. Proceedings of the National Academy of Sciences. 2014;111:17200–05. doi: 10.1073/pnas.1419119111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamada T, Uwagawa S, Okuno Y, et al. Case study: an evaluation of the human relevance of the synthetic pyrethroid metofluthrin-induced liver tumors in rats based on mode of action. Toxicol Sci. 2009;108:59–68. doi: 10.1093/toxsci/kfp007. [DOI] [PubMed] [Google Scholar]
- 103.Peffer RC, Moggs JG, Pastoor T, et al. Mouse liver effects of cyproconazole, a triazole fungicide: role of the constitutive androstane receptor. Toxicol Sci. 2007;99:315–25. doi: 10.1093/toxsci/kfm154. [DOI] [PubMed] [Google Scholar]
- 104.Deguchi Y, Yamada T, Hirose Y, et al. Mode of action analysis for the synthetic pyrethroid metofluthrin-induced rat liver tumors: evidence for hepatic CYP2B induction and hepatocyte proliferation. Toxicol Sci. 2009;108:69–80. doi: 10.1093/toxsci/kfp006. [DOI] [PubMed] [Google Scholar]
- 105.Currie RA, Peffer RC, Goetz AK, et al. Phenobarbital and propiconazole toxicogenomic profiles in mice show major similarities consistent with the key role that constitutive androstane receptor (CAR) activation plays in their mode of action. Toxicology. 2014;321:80–8. doi: 10.1016/j.tox.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morita R, Yafune A, Shiraki A, et al. Liver tumor promoting effect of orphenadrine in rats and its possible mechanism of action including CAR activation and oxidative stress. J Toxicol Sci. 2013;38:403–13. doi: 10.2131/jts.38.403. [DOI] [PubMed] [Google Scholar]
- 107.Yamamoto Y, Moore R, Goldsworthy TL, et al. The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 2004;64:7197–200. doi: 10.1158/0008-5472.CAN-04-1459. [DOI] [PubMed] [Google Scholar]
- 108.Lamminpaa A, Pukkala E, Teppo L, et al. Cancer incidence among patients using antiepileptic drugs: a long-term follow-up of 28,000 patients. Eur J Clin Pharmacol. 2002;58:137–41. doi: 10.1007/s00228-002-0429-6. [DOI] [PubMed] [Google Scholar]
- 109.Olsen JH, Schulgen G, Boice JD, Jr, et al. Antiepileptic treatment and risk for hepatobiliary cancer and malignant lymphoma. Cancer Res. 1995;55:294–7. [PubMed] [Google Scholar]
- 110.Lake BG. Species differences in the hepatic effects of inducers of CYP2B and CYP4A subfamily forms: relationship to rodent liver tumour formation. Xenobiotica. 2009;39:582–96. doi: 10.1080/00498250903098184. [DOI] [PubMed] [Google Scholar]
- 111.Yamada T, Okuda Y, Kushida M, et al. Human hepatocytes support the hypertrophic but not the hyperplastic response to the murine nongenotoxic hepatocarcinogen sodium phenobarbital in an in vivo study using a chimeric mouse with humanized liver. Toxicol Sci. 2014;142:137–57. doi: 10.1093/toxsci/kfu173. [DOI] [PubMed] [Google Scholar]
- 112.Phillips JM, Goodman JI. Multiple Genes Exhibit Phenobarbital-Induced Constitutive Active/Androstane Receptor–Mediated DNA Methylation Changes during Liver Tumorigenesis and in Liver Tumors. Toxicol Sci. 2009;108:273–89. doi: 10.1093/toxsci/kfp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luisier R, Lempiainen H, Scherbichler N, et al. Phenobarbital induces cell cycle transcriptional responses in mouse liver humanized for constitutive androstane and pregnane x receptors. Toxicol Sci. 2014;139:501–11. doi: 10.1093/toxsci/kfu038. [DOI] [PubMed] [Google Scholar]
- 114.Braeuning A, Gavrilov A, Brown S, et al. Phenobarbital-mediated tumor promotion in transgenic mice with humanized CAR and PXR. Toxicol Sci. 2014;140:259–70. doi: 10.1093/toxsci/kfu099. [DOI] [PubMed] [Google Scholar]
- 115.Ross J, Plummer SM, Rode A, et al. Human Constitutive Androstane Receptor (CAR) and Pregnane X Receptor (PXR) Support the Hypertrophic but not the Hyperplastic Response to the Murine Nongenotoxic Hepatocarcinogens Phenobarbital and Chlordane In Vivo. Toxicol Sci. 2010;116:452–66. doi: 10.1093/toxsci/kfq118. [DOI] [PubMed] [Google Scholar]
- 116.Elcombe CR, Peffer RC, Wolf DC, et al. Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: A case study with phenobarbital as a model constitutive androstane receptor (CAR) activator. Crit Rev Toxicol. 2014;44:64–82. doi: 10.3109/10408444.2013.835786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hirose Y, Nagahori H, Yamada T, et al. Comparison of the effects of the synthetic pyrethroid Metofluthrin and phenobarbital on CYP2B form induction and replicative DNA synthesis in cultured rat and human hepatocytes. Toxicology. 2009;258:64–9. doi: 10.1016/j.tox.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 118.Li H, Wang H. Activation of xenobiotic receptors: driving into the nucleus. Expert Opin Drug Metab Toxicol. 2010;6:409–26. doi: 10.1517/17425251003598886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Holsapple MP, Pitot HC, Cohen SH, et al. Mode of Action in Relevance of Rodent Liver Tumors to Human Cancer Risk. Toxicol Sci. 2006;89:51–56. doi: 10.1093/toxsci/kfj001. [DOI] [PubMed] [Google Scholar]
- 120.Wang D, Li L, Yang H, et al. The constitutive androstane receptor is a novel therapeutic target facilitating cyclophosphamide-based treatment of hematopoietic malignancies. Blood. 2013;121:329–38. doi: 10.1182/blood-2012-06-436691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fukumasu H, Rochetti AL, Pires PR, et al. Constitutive androstane receptor ligands modulate the anti-tumor efficacy of paclitaxel in non-small cell lung cancer cells. PLoS One. 2014;9:e99484. doi: 10.1371/journal.pone.0099484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nallani SC, Goodwin B, Maglich JM, et al. Induction of cytochrome P450 3A by paclitaxel in mice: pivotal role of the nuclear xenobiotic receptor, pregnane X receptor. Drug Metab Dispos. 2003;31:681–4. doi: 10.1124/dmd.31.5.681. [DOI] [PubMed] [Google Scholar]
- 123.Harmsen S, Meijerman I, Febus CL, et al. PXR-mediated induction of P-glycoprotein by anticancer drugs in a human colon adenocarcinoma-derived cell line. Cancer Chemother Pharmacol. 2010;66:765–71. doi: 10.1007/s00280-009-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chakraborty S, Kanakasabai S, Bright JJ. Constitutive androstane receptor agonist CITCO inhibits growth and expansion of brain tumour stem cells. Br J Cancer. 2011;104:448–59. doi: 10.1038/sj.bjc.6606064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125•.Wang Y, Masuyama H, Nobumoto E, et al. The inhibition of constitutive androstane receptor-mediated pathway enhances the effects of anticancer agents in ovarian cancer cells. Biochem Pharmacol. 2014;90:356–66. doi: 10.1016/j.bcp.2014.06.003. Downregulation of CAR activity results in increased chemosensitivity in ovarian cancer. [DOI] [PubMed] [Google Scholar]
- 126.Chen WD, Fu X, Dong B, et al. Neonatal activation of the nuclear receptor CAR results in epigenetic memory and permanent change of drug metabolism in mouse liver. Hepatology. 2012;56:1499–509. doi: 10.1002/hep.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen F, Zamule SM, Coslo DM, et al. The human constitutive androstane receptor promotes the differentiation and maturation of hepatic-like cells. Dev Biol. 2013;384:155–65. doi: 10.1016/j.ydbio.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goerdt C, Keith M, Rubins HB. Effects of Phenytoin on Plasma High-Density Lipoprotein Cholesterol Levels in Men with Low Levels of High-Density Lipoprotein Cholesterol. The Journal of Clinical Pharmacology. 1995;35:767–75. doi: 10.1002/j.1552-4604.1995.tb04118.x. [DOI] [PubMed] [Google Scholar]
- 129.Pelkonen R, Fogelholm R, Nikkila EA. Increase in serum cholesterol during phenytoin treatment. Br Med J. 1975;4:85. doi: 10.1136/bmj.4.5988.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Masson D, Qatanani M, Sberna AL, et al. Activation of the constitutive androstane receptor decreases HDL in wild-type and human apoA-I transgenic mice. J Lipid Res. 2008;49:1682–91. doi: 10.1194/jlr.M700374-JLR200. [DOI] [PubMed] [Google Scholar]
- 131•.Cherian MT, Lin W, Wu J, et al. CINPA1 is an inhibitor of constitutive androstane receptor that does not activate pregnane X receptor. Mol Pharmacol. 2015;87:878–89. doi: 10.1124/mol.115.097782. A newly identified small molecule inhibitor of CAR, which is mostly specific for CAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang J, Huang W, Chua SS, et al. Modulation of Acetaminophen-Induced Hepatotoxicity by the Xenobiotic Receptor CAR. Science. 2002;298:422–24. doi: 10.1126/science.1073502. [DOI] [PubMed] [Google Scholar]
- 133.Li L, Hassan HE, Tolson AH, et al. Differential Activation of Pregnane X Receptor and Constitutive Androstane Receptor by Buprenorphine in Primary Human Hepatocytes and HepG2 Cells. J Pharmacol Exp Ther. 2010;335:562–71. doi: 10.1124/jpet.110.173187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sueyoshi T, Kawamoto T, Zelko I, et al. The Repressed Nuclear Receptor CAR Responds to Phenobarbital in Activating the Human CYP2B6 Gene. J Biol Chem. 1999;274:6043–46. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- 135.Wang H, Faucette S, Moore R, et al. Human constitutive androstane receptor mediates induction of CYP2B6 gene expression by phenytoin. J Biol Chem. 2004;279:29295–301. doi: 10.1074/jbc.M400580200. [DOI] [PubMed] [Google Scholar]
- 136.Faucette SR, Zhang TC, Moore R, et al. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther. 2007;320:72–80. doi: 10.1124/jpet.106.112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cerveny L, Svecova L, Anzenbacherova E, et al. Valproic acid induces CYP3A4 and MDR1 gene expression by activation of constitutive androstane receptor and pregnane X receptor pathways. Drug Metab Dispos. 2007;35:1032–41. doi: 10.1124/dmd.106.014456. [DOI] [PubMed] [Google Scholar]
- 138.Wei P, Zhang J, Dowhan DH, et al. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacogenomics J. 2002;2:117–26. doi: 10.1038/sj.tpj.6500087. [DOI] [PubMed] [Google Scholar]
- 139.Meyer zu Schwabedissen HE, Oswald S, Bresser C, et al. Compartment-specific gene regulation of the CAR inducer efavirenz in vivo. Clin Pharmacol Ther. 2012;92:103–11. doi: 10.1038/clpt.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sharma D, Lau AJ, Sherman MA, et al. Agonism of human pregnane X receptor by rilpivirine and etravirine: comparison with first generation non-nucleoside reverse transcriptase inhibitors. Biochem Pharmacol. 2013;85:1700–11. doi: 10.1016/j.bcp.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 141.Wang D, Li L, Fuhrman J, et al. The role of constitutive androstane receptor in oxazaphosphorine-mediated induction of drug-metabolizing enzymes in human hepatocytes. Pharm Res. 2011;28:2034–44. doi: 10.1007/s11095-011-0429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kobayashi K, Yamanaka Y, Iwazaki N, et al. Identification of HMG-CoA reductase inhibitors as activators for human, mouse and rat constitutive androstane receptor. Drug Metab Dispos. 2005;33:924–9. doi: 10.1124/dmd.104.002741. [DOI] [PubMed] [Google Scholar]
- 143.Mnif W, Pascussi JM, Pillon A, et al. Estrogens and antiestrogens activate hPXR. Toxicol Lett. 2007;170:19–29. doi: 10.1016/j.toxlet.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 144.Repo S, Jyrkkarinne J, Pulkkinen JT, et al. Ligand specificity of constitutive androstane receptor as probed by induced-fit docking and mutagenesis. J Med Chem. 2008;51:7119–31. doi: 10.1021/jm800337r. [DOI] [PubMed] [Google Scholar]
- 145.Ripp SL, Fitzpatrick JL, Peters JM, et al. Induction of CYP3A expression by dehydroepiandrosterone: involvement of the pregnane X receptor. Drug Metab Dispos. 2002;30:570–5. doi: 10.1124/dmd.30.5.570. [DOI] [PubMed] [Google Scholar]
- 146.Moore LB, Parks DJ, Jones SA, et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–7. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 147.Lemaire G, de Sousa G, Rahmani R. A PXR reporter gene assay in a stable cell culture system: CYP3A4 and CYP2B6 induction by pesticides. Biochem Pharmacol. 2004;68:2347–58. doi: 10.1016/j.bcp.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 148.Li L, Stanton J, Tolson A, et al. Bioactive Terpenoids and Flavonoids from Ginkgo Biloba Extract Induce the Expression of Hepatic Drug-Metabolizing Enzymes Through Pregnane X Receptor, Constitutive Androstane Receptor, and Aryl hydrocarbon Receptor-Mediated Pathways. Pharm Res. 2009;26:872–82. doi: 10.1007/s11095-008-9788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yao R, Yasuoka A, Kamei A, et al. Dietary flavonoids activate the constitutive androstane receptor (CAR) J Agric Food Chem. 2010;58:2168–73. doi: 10.1021/jf903711q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sugatani J, Yamakawa K, Tonda E, et al. The induction of human UDP-glucuronosyltransferase 1A1 mediated through a distal enhancer module by flavonoids and xenobiotics. Biochem Pharmacol. 2004;67:989–1000. doi: 10.1016/j.bcp.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 151.Hiura Y, Satsu H, Hamada M, et al. Analysis of flavonoids regulating the expression of UGT1A1 via xenobiotic receptors in intestinal epithelial cells. Biofactors. 2014;40:336–45. doi: 10.1002/biof.1153. [DOI] [PubMed] [Google Scholar]
- 152.Fisher CD, Augustine LM, Maher JM, et al. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab Dispos. 2007;35:995–1000. doi: 10.1124/dmd.106.014340. [DOI] [PubMed] [Google Scholar]
- 153.Yao R, Yasuoka A, Kamei A, et al. Polyphenols in alcoholic beverages activating constitutive androstane receptor CAR. Biosci Biotechnol Biochem. 2011;75:1635–7. doi: 10.1271/bbb.110444. [DOI] [PubMed] [Google Scholar]
- 154.Kocarek TA, Mercer-Haines NA. Squalestatin 1-Inducible Expression of Rat CYP2B: Evidence That an Endogenous Isoprenoid Is an Activator of the Constitutive Androstane Receptor. Mol Pharmacol. 2002;62:1177–86. doi: 10.1124/mol.62.5.1177. [DOI] [PubMed] [Google Scholar]
- 155.Lau AJ, Yang G, Rajaraman G, et al. Species-Dependent and Receptor-Selective Action of Bilobalide on the Function of Constitutive Androstane Receptor and Pregnane X Receptor. Drug Metabolism and Disposition. 2012;40:178–86. doi: 10.1124/dmd.111.042879. [DOI] [PubMed] [Google Scholar]
- 156.Paul KB, Thompson JT, Simmons SO, et al. Evidence for triclosan-induced activation of human and rodent xenobiotic nuclear receptors. Toxicol In Vitro. 2013;27:2049–60. doi: 10.1016/j.tiv.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 157.Kiyosawa N, Kwekel JC, Burgoon LD, et al. Species-specific regulation of PXR/CAR/ER-target genes in the mouse and rat liver elicited by o, p’-DDT. BMC Genomics. 2008;9:487. doi: 10.1186/1471-2164-9-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kiyosawa N, Kwekel JC, Burgoon LD, et al. o, p’-DDT elicits PXR/CAR-, not ER-, mediated responses in the immature ovariectomized rat liver. Toxicol Sci. 2008;101:350–63. doi: 10.1093/toxsci/kfm275. [DOI] [PubMed] [Google Scholar]
- 159.Kublbeck J, Laitinen T, Jyrkkarinne J, et al. Use of comprehensive screening methods to detect selective human CAR activators. Biochem Pharmacol. 2011;82:1994–2007. doi: 10.1016/j.bcp.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 160.Blizard D, Sueyoshi T, Negishi M, et al. Mechanism of induction of cytochrome p450 enzymes by the proestrogenic endocrine disruptor pesticide-methoxychlor: interactions of methoxychlor metabolites with the constitutive androstane receptor system. Drug Metab Dispos. 2001;29:781–5. [PubMed] [Google Scholar]
- 161.Savary CC, Josse R, Bruyere A, et al. Interactions of endosulfan and methoxychlor involving CYP3A4 and CYP2B6 in human HepaRG cells. Drug Metab Dispos. 2014;42:1235–40. doi: 10.1124/dmd.114.057786. [DOI] [PubMed] [Google Scholar]
- 162.Mikamo E, Harada S, Nishikawa J, et al. Endocrine disruptors induce cytochrome P450 by affecting transcriptional regulation via pregnane X receptor. Toxicol Appl Pharmacol. 2003;193:66–72. doi: 10.1016/j.taap.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 163.Lemaire G, Mnif W, Pascussi JM, et al. Identification of new human pregnane X receptor ligands among pesticides using a stable reporter cell system. Toxicol Sci. 2006;91:501–9. doi: 10.1093/toxsci/kfj173. [DOI] [PubMed] [Google Scholar]
- 164.Price RJ, Walters DG, Finch JM, et al. A mode of action for induction of liver tumors by Pyrethrins in the rat. Toxicol Appl Pharmacol. 2007;218:186–95. doi: 10.1016/j.taap.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 165.LeBaron MJ, Geter DR, Rasoulpour RJ, et al. An integrated approach for prospectively investigating a mode-of-action for rodent liver effects. Toxicol Appl Pharmacol. 2013;270:164–73. doi: 10.1016/j.taap.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 166.DeKeyser JG, Stagliano MC, Auerbach SS, et al. Di(2-ethylhexyl) phthalate is a highly potent agonist for the human constitutive androstane receptor splice variant CAR2. Mol Pharmacol. 2009;75:1005–13. doi: 10.1124/mol.108.053702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tamura K, Inoue K, Takahashi M, et al. Involvement of constitutive androstane receptor in liver hypertrophy and liver tumor development induced by triazole fungicides. Food Chem Toxicol. 2015 doi: 10.1016/j.fct.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 168.Honkakoski P, Palvimo JJ, Penttila L, et al. Effects of triaryl phosphates on mouse and human nuclear receptors. Biochem Pharmacol. 2004;67:97–106. doi: 10.1016/j.bcp.2003.08.037. [DOI] [PubMed] [Google Scholar]