Abstract
Peripheral coadministration of amylin and leptin produces enhanced suppression of food intake and body weight, but the central nuclei mediating these effects remain unclear. Because each of these peptides controls feeding via actions at the ventral tegmental area (VTA), we tested the hypothesis that the VTA is a site of action for the cooperative effects of leptin and amylin on energy balance control. First, we show that intra-VTA injection of amylin and leptin at doses of each peptide that are effective in reducing food intake and body weight when administered separately produces an enhanced suppression of feeding when administered in combination. We also demonstrate that subthreshold doses of both amylin and leptin cause significant hypophagia and body weight loss when coadministered into the VTA. Additionally, we provide evidence that VTA amylin receptor blockade significantly attenuates the ability of intra-VTA leptin to reduce feeding and body weight gain. Together, these data provide the first evidence that the VTA mediates the interaction of amylin and leptin to cooperatively promote negative energy balance.
Keywords: obesity, mesolimbic, reward, islet amyloid polypeptide
obesity represents a prevalent, costly health problem in the US and worldwide (11, 43, 44), yet effective noninvasive treatment options for this disease are extremely limited. Many pharmacotherapeutic strategies to date have attempted to treat obesity by targeting a single feeding-related neuroendocrine system, but the vast majority of these monotherapy approaches have failed to produce meaningful and sustained reductions in body weight (41). Therefore, attention has been turned to the notion that combination pharmacotherapies targeting more than one neuroendocrine feeding-relevant system may be more effective for the treatment of obesity (8, 49, 51).
The pancreatic hormone amylin has been identified as a leading candidate for the development of combination pharmaceutical strategies for the treatment of obesity (47, 51). Amylin acts centrally to promote negative energy balance (24, 50), and several reports have demonstrated that amylin can enhance the anorectic effects of other feeding-related signals in an additive if not synergistic manner (6, 10, 22, 35). Of particular interest is that amylin interacts cooperatively with the adipose-derived hormone leptin to produce enhanced suppression of food intake and body weight (13, 15). This amylin-leptin interaction has been observed in rodents (13, 42, 48, 56, 57, 59) and humans (46, 48). Indeed, several studies demonstrate that peripheral administration of amylin and leptin (or agonists for their receptors) produces greater weight loss than either compound alone (48, 56, 59). Of critical importance for the treatment of obesity is the fact that although humans and rodent models exhibit reduced sensitivity to the energy balance effects of leptin in the obese state (1, 39), amylin appears to restore leptin sensitivity (13, 48, 56), thereby improving weight loss when amylin and leptin are coadministered.
Although the body weight and food intake reductions produced by peripheral coadministration of amylin and leptin are clear, the central nervous system (CNS) nuclei mediating the interactive effects of these peptides to promote negative energy balance remain largely unknown. Much of the literature describing the hypophagic effects of amylin focuses on its ability to reduce food intake through actions at the area postrema of the hindbrain (26, 27, 34); however, this nucleus does not appear to mediate leptin's anorectic effects (18, 59), making it unlikely that this site supports an interaction between leptin and amylin. Thus, it remains critical to investigate the role of other central amylin-responsive sites as a potential point of interaction for leptin and amylin signaling. Of note is that the ventromedial nucleus of the hypothalamus has been shown to contribute in part to the interactive effects of amylin and leptin in the control of energy balance (20, 59), but the possible role of other amylin- and leptin-responsive CNS nuclei remains untested.
One potential site mediating the cooperative interaction between leptin and amylin is the ventral tegmental area (VTA) of the mesolimbic reward system. Recent studies have established the VTA as a physiologically and pharmacologically relevant site of action for the intake-suppressive effects of amylin (32, 33). Furthermore, leptin has been shown to act independently in the VTA to induce hypophagia in a physiologically relevant manner (14, 17). Together, these data highlight a possible role for the VTA in the interactive effects of amylin and leptin to suppress food intake and body weight. Here, we tested the ability of different dose combinations of intra-VTA amylin and leptin to reduce feeding and body weight gain. We also evaluated whether endogenous VTA amylin receptor signaling is required to mediate the food intake- and body weight-suppressive effects of VTA leptin signaling.
METHODS
Subjects.
Adult male Sprague-Dawley rats (346.5 ± 32.4 g at the start of experimental testing; Charles River Laboratories) were housed individually in hanging wire mesh cages in a temperature- and humidity-controlled environment (12:12-h light-dark cycle). Animals had ad libitum access to rodent chow (Laboratory Rodent Diet 5001; LabDiet) and water. All experimental procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee. All experiments were within subjects, with each rat receiving all treatments in a counterbalanced Latin squares design.
Drugs.
Rat amylin (American Peptide) and the amylin receptor antagonist AC187 (Bachem) were dissolved in artificial cerebrospinal fluid (aCSF; Harvard Apparatus). Leptin (NHPP) was dissolved in 0.01 M sodium bicarbonate. The selected doses of drugs were based on the literature (17, 32, 33).
Surgery.
Rats were anesthetized via intramuscular injection of a mixture containing ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) and placed into a stereotaxic apparatus. Each rat was surgically implanted with a bilateral guide cannula aimed at the VTA (coordinates: ± 0.5 mm lateral to midline; 6.8 mm posterior to bregma, with internal cannula aimed 8.6 mm ventral to skull). Guide cannulae (26-GA; Plastics One) were affixed to the skull with jeweler's screws and dental cement. Analgesic was provided for all surgeries (2 mg/kg meloxicam). VTA cannula placements were histologically verified postmortem by injection of 100 nl of pontamine sky blue ink. A representative image of unilateral VTA injection placement is shown in Fig. 1C.
Fig. 1.
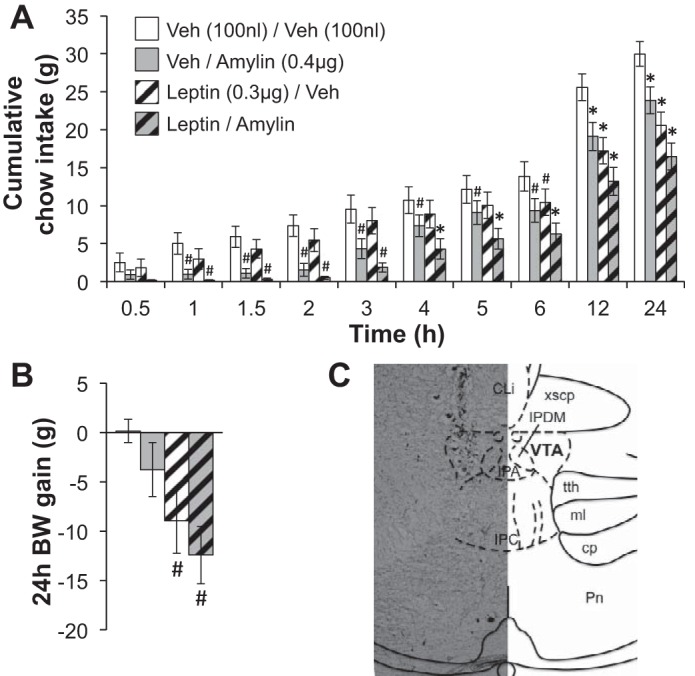
Cumulative food intake and 24-h body weight (BW) gain after intra-ventral tegmental area (VTA) administration of suprathreshold doses of amylin (0.4 μg) or its vehicle [Veh; 100 nl of artificial cerebrospinal fluid (aCSF)] plus leptin (0.3 μg) or its Veh (100 nl of 0.01 M sodium bicarbonate). A: VTA delivery of amylin alone or leptin alone reduces chow intake, but the suppression of feeding produced by the combination of amylin and leptin is greater than the effect of either peptide alone. B: the combination of amylin and leptin also significantly reduces body weight gain. Within a time bin: #statistical significance (P < 0.05) compared with Veh/Veh; *significant difference (P < 0.05) from all other conditions. All data shown as means ± SE. C: a representative image of VTA injection placement (30-μm coronal section) is shown.
Intra-VTA coadministration of amylin and leptin.
Rats with chronically implanted VTA cannula were given a unilateral VTA injection of leptin or its vehicle (100 nl of 0.01 M sodium bicarbonate), followed by a second unilateral VTA injection of amylin or its vehicle (100 nl of aCSF) just before the onset of the dark phase. In separate cohorts of animals, different doses of leptin and amylin were used to explore the effects of different dose combinations (experiment 1: 0.3 μg of leptin and 0.4 μg of amylin; experiment 2: 0.1 μg of leptin and 0.04 μg of amylin) on food intake and body weight. Both injections were given into the same hemisphere of the VTA, and injection days were separated by a minimum of 48 h. For these studies, rats were housed in a custom-made automated feedometer system in which a small access hole in the hanging wire mesh cage leads to a food cup resting on an electronic scale. Food cup weights were automatically recorded by computer software (LabView) every 10 s for the 24-h test period, allowing for detailed food intake and meal pattern analyses. The criteria for defining a meal were 0.25 g or more of food intake and a minimum of 10 min between feeding bouts (31–33). Food intake, meal size, and meal number were calculated over the course of the 24-h test period. Spillage was accounted for in food intake measurements by assigning a percentage of total crumbs to each meal/time bin corresponding to the percentage of total intake for that meal/time bin, as reported previously (31–33). Twenty-four-hour body weight gain was assessed by subtracting each rat's body weight at the beginning of the 24-h test period from its body weight at the end of the test (to the nearest 0.1 g).
Intra-VTA coadministration of AC187 and leptin.
Approximately 1 h before lights-off, rats with indwelling VTA cannulae were each given a unilateral intra-VTA injection of the amylin receptor antagonist AC187 (0.1 μg) or vehicle (100 nl aCSF). One hour later, just before lights off, each rat received a second unilateral VTA injection into the same hemisphere of the VTA containing leptin (0.6 μg) or its vehicle (100 nl 0.01 M sodium bicarbonate). Food was unavailable between injections. After the second injection, preweighed food was returned to the rats, and 24-h food intake (accounting for spillage) and change in body weight were measured. A minimum of 48 h separated subsequent treatments.
Statistical analyses.
All statistical analyses were run using Statistica (StatSoft). The α-level was set at P < 0.05 for all experiments. For each study, repeated-measures ANOVAs were conducted for each variable of interest (food intake, meal size/number, body weight). Each drug type (e.g., amylin, leptin, AC187) served as a within-subjects factor, with time as an additional within-subjects factor when multiple time points were assessed within a single experiment. Statistically significant main effects and interactions were probed using Student-Newman-Keuls post hoc analyses.
RESULTS
Intra-VTA doses of amylin and leptin that are independently effective to reduce food intake produce an enhanced suppression of food intake when combined.
We evaluated whether amylin and leptin signaling interact in the VTA to control food intake and body weight. We first selected doses of each peptide that, when administered independently into the VTA, are suprathreshold for energy balance effects (0.4 μg of amylin and 0.3 μg of leptin; Figs. 1 and 2). We tested the ability of these intra-VTA doses of amylin and leptin, alone or in combination, to reduce feeding and body weight gain in chow-fed rats (n = 11). As expected, amylin alone and leptin alone each reduced food intake throughout the 24-h test period (statistically significant main effects of amylin and of leptin, ANOVAs F1,13 ≥ 25.37, P < 0.001; main effect of time, F9,117 = 190.86, P < 0.0001; interactions between leptin/time and amylin/time, ANOVAs F9,117 ≥ 2.58, P < 0.01; planned comparisons of vehicle/vehicle vs. leptin/vehicle, P < 0.05 from 6–24 h; vehicle/vehicle vs. vehicle/amylin, P < 0.05 from 1–24 h; Fig. 1A). However, the combination of these doses of amylin and leptin produced a reduction in chow intake that was greater than the effect of either hormone alone (planned comparisons between leptin/amylin and all other conditions, P < 0.05 from 4–24 h), suggesting that the VTA mediates the interactive effect of these peptides on feeding. We also measured 24-h body weight change in this study and found that a combination of leptin and amylin significantly suppressed body weight gain over the 24-h test (main effect of leptin, F1,13 = 13.58, P < 0.01; planned comparisons between vehicle/vehicle and leptin/amylin, P < 0.05; Fig. 1B).
Fig. 2.

Meal patterns after intra-VTA administration of suprathreshold doses of amylin (0.4 μg) or its vehicle (100 nl of aCSF) plus leptin (0.3 μg) or its vehicle (100 nl of 0.01 M sodium bicarbonate). The hypophagia produced by VTA amylin combined with VTA leptin shown in Fig. 1 is driven primarily by reductions in meal size (A) rather than meal number (B). Within a time bin: #statistical significance (P < 0.05) compared with Veh/Veh; *significant difference (P < 0.05) from all other conditions. All data shown as means ± SE.
To probe the behavioral mechanisms underlying the suppression of food intake, we conducted meal pattern analyses. We found that meal size (Fig. 2A) was durably and robustly reduced by the amylin plus leptin combination (statistically significant main effects of amylin and of leptin, ANOVAs F1,13 ≥ 10.98, P < 0.01; main effect of time, F9,117 = 11.99, P < 0.0001; interaction between amylin/time, F9,117 = 4.19, P < 0.001; planned comparisons between vehicle/vehicle and leptin/amylin, P < 0.05 at 0.5–3 and 12 h), whereas effects on meal number were observed at only one early time point and were relatively minor (statistically significant main effect of amylin, F1,13 = 21.78, P < 0.001; main effect of time, F9,117 = 73.55, P < 0.0001; interaction between amylin/time, F9,117 = 1.96, P < 0.05; planned comparisons between vehicle/vehicle and leptin/amylin, P < 0.05 at 2 h; Fig. 2B). This suggests that the primary mechanism by which VTA amylin plus leptin reduces feeding is likely through an enhancement of within-meal satiation signaling, with fewer effects on intermeal satiety processes.
Combined intra-VTA injection of subthreshold doses of amylin and leptin significantly reduces chow intake and body weight gain.
The first study supported the hypothesis that the VTA is a site of action for the cooperative ability of amylin and leptin to promote hypophagia and suppress body weight gain. To further evaluate this effect, we conducted a similar experiment in a separate group of rats (n = 19) in which we delivered subthreshold doses of amylin (0.04 μg) and leptin (0.1 μg), alone or in combination, directly to the VTA. Although at these doses, neither amylin alone nor leptin alone reduced feeding or body weight gain over the 24-h postinjection, intra-VTA injection of the combination of the two peptides significantly suppressed both food intake [main effects of leptin (F1,18 = 19.01, P < 0.001) and amylin (F1,18 = 12.00, P < 0.01); interaction between amylin and leptin (F1,18 = 6.90, P < 0.02); planned comparisons of vehicle/vehicle vs. leptin/amylin, P < 0.05, vehicle/vehicle vs. leptin/vehicle or vehicle/amylin, P > 0.05; Fig. 3A] and body weight gain [main effect of leptin (F1,18 = 15.23, P < 0.01); planned comparisons of vehicle/vehicle vs. leptin/amylin, P < 0.05, vehicle/vehicle vs. leptin/vehicle or vehicle/amylin, P > 0.05; Fig. 3B]. We again analyzed meal patterns and found results similar to the first amylin-leptin experiment in that the combination of intra-VTA leptin and amylin significantly reduced meal size [main effect of leptin (F1,18 = 5.17, P < 0.04); planned comparisons of vehicle/vehicle vs. leptin/amylin, P < 0.05, vehicle/vehicle vs. leptin/vehicle or vehicle/amylin, P > 0.05; Fig. 3C] but had no effect on meal number (no main effect of leptin or amylin and no significant interaction; all ANOVAs F1,18 ≤ 1.60, P > 0.05; Fig. 3D). This further supports the notion that the VTA mediates the interactive effects of amylin and leptin on energy balance.
Fig. 3.
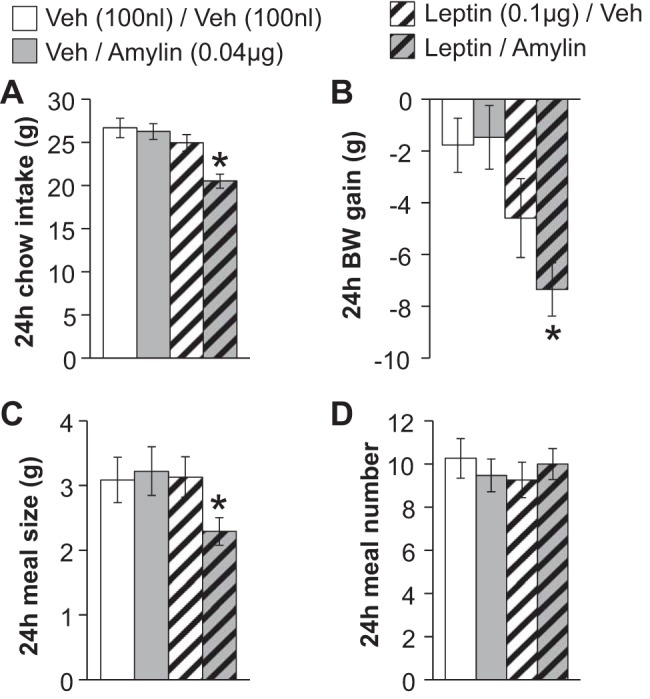
Food intake, BW gain, and meal patterns after intra-VTA administration of subthreshold doses of amylin (0.04 μg) or its vehicle (100 nl of aCSF) plus leptin (0.1 μg) or its vehicle (100 nl of 0.01 M sodium bicarbonate). A and C: although these doses of amylin and leptin do not impact 24-h food intake when delivered separately, the combination of the 2 peptides significantly reduces feeding (A) by suppressing meal size (C). D: no effect on meal number was observed. B: intra-VTA delivery of this dose combination of leptin and amylin also significantly reduces 24-h BW change. *P < 0.05 compared with Veh/Veh. All data are shown as means ± SE.
Pharmacological blockade of VTA amylin receptors attenuates the intake- and body weight-suppressive effects of intra-VTA leptin.
To begin to address the potential physiological relevance of the VTA for the cooperative energy balance effects of amylin and leptin, we tested whether endogenous amylin receptor activity is required for the ability of intra-VTA leptin to reduce feeding and body weight gain. Rats (n = 13) were given a direct VTA pretreatment injection of the amylin receptor antagonist AC187 (0.1 μg) or its vehicle (100 nl of aCSF), which was followed 1 h later by a second intra-VTA injection containing leptin (0.6 μg) or its vehicle (100 nl of 0.01 M sodium bicarbonate). This dose of intra-VTA-delivered AC187 was chosen for its ability to significantly attenuate suppression of food intake by systemic amylin agonist delivery, yet it is a subthreshold dose for independent effects when administered directly to the VTA (33). Chow intake and body weight gain were measured for 24 h posttreatment. The selected dose of intra-VTA leptin decreased both 24-h chow intake and body weight. However, VTA amylin receptor blockade with AC187 significantly attenuated both the hypophagia [main effect of leptin (F1,12 = 45.15, P < 0.0001); interaction between AC187 and leptin (F1,12 = 7.57, P < 0.02); post hoc comparisons, vehicle/vehicle vs. vehicle/leptin: P < 0.05, vehicle/leptin vs. AC187/leptin: P < 0.05; Fig. 4A] and suppression of body weight [main effect of leptin (F1,12 = 18.45, P < 0.01); main effect of AC187 (F1,12 = 13.07, P < 0.01); post hoc comparisons, vehicle/vehicle vs. vehicle/leptin: P < 0.05; vehicle/vehicle vs. AC187/vehicle: P > 0.05; vehicle/leptin vs. AC187/leptin: P < 0.05; Fig. 4B] produced by VTA leptin administration. This suggests that endogenous amylin is activating VTA amylin receptors to facilitate/enhance the anorectic and body weight-suppressive effects of VTA leptin signaling.
Fig. 4.
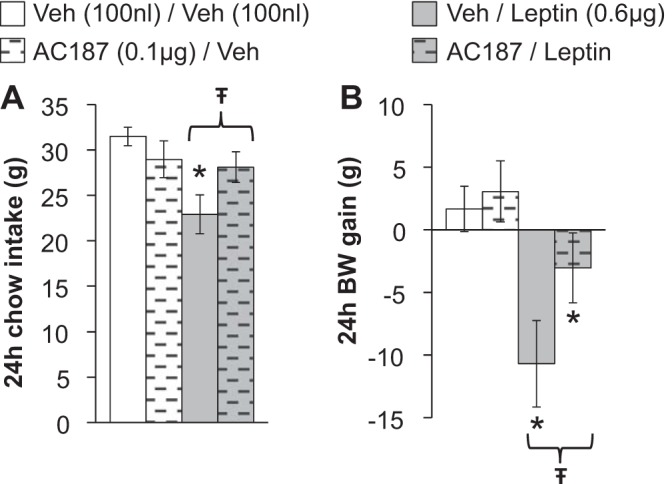
Chow intake (A) and body weight gain (B) after VTA administration of the amylin receptor antagonist AC187 (0.1 μg) or its vehicle (100 nl of aCSF) plus leptin (0.6 μg) or its vehicle (100 nl of 0.01 M sodium bicarbonate). The intake- and BW-suppressive effects of intra-VTA leptin are attenuated by VTA amylin receptor blockade. *P < 0.05 compared with Veh/Veh; *statistically significant difference between Veh/leptin and AC187/leptin (P < 0.05). All data shown as means ± SE.
DISCUSSION
Several studies to date have established that peripheral administration of a combination of amylin and leptin produces effects on feeding and body weight that are additive or potentially synergistic in nature (48, 56, 57, 59). Both peptides act within the CNS to promote negative energy balance, with the actions of amylin on energy balance thought to be mediated exclusively by central nuclei (24). Therefore, it is critical to identify sites within the brain that support the interaction between these signals. The current findings demonstrate that the VTA is a novel central nucleus mediating the interaction of amylin and leptin to promote negative energy balance. Our data show that when amylin and leptin are coadministered directly into the VTA, the hypophagic and body weight-suppressive effects of the combination of the two peptides are greater than that of either peptide alone. This was observed with doses of amylin and leptin that, when administered separately, were either suprathreshold or subthreshold for effects on food intake and body weight. Additionally, meal pattern analyses demonstrated that the reductions in feeding produced by the combination of intra-VTA leptin and amylin were due to suppression of meal size, consistent with the roles of both amylin and leptin acting as satiation signals (16, 18, 23, 25). In thinking about the reduction in food intake produced by VTA delivery of amylin and leptin, it is important to rule out alternative explanations for the observed hypophagia. This effect is unlikely to be due to impaired locomotion, as activation of VTA leptin receptors (17) or VTA amylin receptors (33) does not produce lasting reductions in locomotor activity. Furthermore, VTA amylin receptor activation does not cause pica (33) and does not produce conditioned taste avoidance (32), suggesting against the possibility that nausea/malaise causes the reductions in food intake observed with VTA amylin receptor activation. Together, these previous findings argue for a specific effect of VTA amylin plus leptin on feeding and body weight.
Leptin and amylin each can cross the blood-brain barrier (2–4), and within the VTA, both amylin receptor and leptin receptor signaling are physiologically relevant for energy balance control (17, 32, 33). Therefore, to begin to evaluate the potential physiological relevance of the VTA for the interaction between these peptides, we tested whether VTA amylin receptor blockade would attenuate the anorectic and body weight-suppressive effects of intra-VTA leptin. Indeed, our findings indicate that the reductions in chow intake and body weight gain produced by VTA delivery of leptin are blunted by intra-VTA pretreatment with the amylin receptor antagonist AC187. This hints at the possibility that activation of VTA amylin receptors by endogenous amylin may be required for the full expression of the food intake- and body weight-suppressive effects of VTA leptin receptor activation.
The present data describe a pharmacological and perhaps physiological role for the VTA in mediating the combined effects of amylin and leptin on food intake and body weight gain. The mechanism(s) underlying these effects is not yet known. Although our knowledge of the downstream effects of each peptide alone in the VTA is still somewhat limited, a few possible points of convergence are clear from the literature. One potential mechanism by which these two peptide signals could be integrated involves effects on dopaminergic signaling. Both amylin receptor and leptin receptor signaling within the VTA impact the activity of the mesolimbic dopamine system (14, 17, 32). Cooperative actions of VTA leptin and VTA amylin on dopaminergic signaling to key afferent targets such as the nucleus accumbens (14, 32) or central nucleus of the amygdala (21) could mediate the combined effects of these hormones on energy balance. A critical empirical question that remains to be tested is whether amylin receptors and leptin receptors are coexpressed on dopamine neurons within the VTA. Within the VTA, amylin receptors are highly expressed on dopamine neurons (32), and VTA dopamine neurons also express the leptin receptor at the mRNA level (17). However, the lack of a widely validated antibody against the leptin receptor limits the ability to test for colocalization of these receptors at the protein level. Given that leptin can regulate the activity of VTA dopamine neurons via a presynaptic mechanism (55), it is also possible that leptin acts presynaptically within the VTA to regulate dopamine signaling, whereas amylin acts via a direct postsynaptic mechanism.
A related possibility is that amylin receptor and leptin receptor activation may impinge on common intracellular signaling pathways within VTA neurons. The intracellular signaling response to leptin within the VTA includes phosphorylation of STAT3 (17) and ERK (58). Although the intracellular signaling cascade engaged by VTA amylin receptor activation has not yet been established, amylin receptor activation in other areas of the brain stimulates phosphorylated (p)-ERK (45) and also increases p-STAT (59). Because intracellular signaling pathways are known to be important points of convergence for energy status signals, increased activation of one or more of these signals through the actions of both amylin and leptin could contribute to the enhanced energy balance effects produced by the combination of the peptides. In fact, several in vitro reports demonstrate the ability of amylin and leptin to produce cooperative effects on common intracellular signaling pathways (36–38). Given that leptin can induce p-STAT3 in VTA dopamine neurons (17), an interesting possibility is that coactivation of the same dopamine neuron by amylin and leptin may result in a potentiated response through the activation of common intracellular molecules within a single dopaminergic cell. Such analyses are certainly warranted for future studies.
Obese animals have reduced sensitivity to the effects of leptin, which is indicated by a reduction in leptin-induced p-STAT3 in several key feeding-relevant nuclei (28, 39). This has limited the utility of leptin-targeting monotherapies for the treatment of obesity (5). However, amylin remains effective at reducing food intake and body weight in the obese state (7). In fact, the amylin analog pramlintide is FDA approved for the treatment of diabetes but has the additional effect of reducing food intake and body weight in obese individuals (12, 52, 53). This finding, along with the ability of amylin receptor signaling to enhance the body weight-lowering effects of other neuroendocrine signals (22), has led to the notion that the amylin system is a strong candidate for future antiobesity drug development (30, 47, 51). Interestingly, the available data on the interactive effects of peripherally administered amylin and leptin suggest that amylin may improve leptin sensitivity (48, 56), highlighting the potential clinical utility of a combination of amylin- and leptin-targeting pharmacotherapies for more effective obesity treatment (9, 54). Given that a contributing factor to the development of obesity is the overconsumption of highly palatable, energy-dense foods (19), our finding that the VTA mediates the cooperative effects of leptin and amylin on energy balance control is particularly intriguing, as this nucleus plays an important role in the control of palatable food intake and food reward (29, 40, 60). Although the current studies examined the effects of the VTA amylin-leptin combination on chow intake, an exciting extension of our findings will be to evaluate whether VTA delivery of amylin and leptin can also attenuate the intake of palatable foods, such as those high in fat and/or carbohydrate, in both lean and diet-induced obese animals.
Neuroendocrine signals are often studied in isolation, but in reality these hormones and neuropeptides interact in complex ways to regulate behavior and physiology. Understanding the mechanisms by which feeding-related peptides interact to control energy balance is critically important not only for furthering our understanding of the in vivo actions of the signals but also potentially to develop more effective pharmacotherapies for obesity. Our data indicate that the VTA is a central site of action mediating the cooperative effects of amylin and leptin for the control of energy balance. This finding, along with recent reports identifying the importance of the hypothalamus for the effects of the combination of amylin and leptin (20, 59), highlights the importance of further investigation of the distributed neural substrates and mechanisms underlying this interaction.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant NIH-K01-DK-103804 (E. G. Mietlicki-Baase), a Penn Medicine Neuroscience Center pilot award (M. R. Hayes), and NIDDK Grant NIH-R01-DK-096139 (M. R. Hayes).
DISCLOSURES
The authors have no conflicts of interest to disclose, financial or otherwise.
AUTHOR CONTRIBUTIONS
E.G.M.-B. and M.R.H. conception and design of research; E.G.M.-B., D.R.O., and B.A.J. performed experiments; E.G.M.-B. and B.A.J. analyzed data; E.G.M.-B., B.A.J., and M.R.H. interpreted results of experiments; E.G.M.-B. prepared figures; E.G.M.-B. drafted manuscript; E.G.M.-B., D.R.O., B.A.J., and M.R.H. edited and revised manuscript; E.G.M.-B., D.R.O., B.A.J., and M.R.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Amber Alhadeff, Ralph DiLeone, and Martin Myers for their valuable intellectual advice as well as David Reiner and Lauren McGrath for technical assistance.
REFERENCES
- 1.Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides 20: 1341–1345, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides 19: 883–889, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides 17: 305–311, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Banks WA, Kastin AJ, Maness LM, Huang W, Jaspan JB. Permeability of the blood-brain barrier to amylin. Life Sci 57: 1993–2001, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Bell-Anderson KS, Bryson JM. Leptin as a potential treatment for obesity: progress to date. Treat Endocrinol 3: 11–18, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bello NT, Kemm MH, Ofeldt EM, Moran TH. Dose combinations of exendin-4 and salmon calcitonin produce additive and synergistic reductions in food intake in nonhuman primates. Am J Physiol Regul Integr Comp Physiol 299: R945–R952, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle CN, Rossier MM, Lutz TA. Influence of high-fat feeding, diet-induced obesity, and hyperamylinemia on the sensitivity to acute amylin. Physiol Behav 104: 20–28, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Bray GA. Medical treatment of obesity: the past, the present and the future. Best Pract Res Clin Gastroenterol 28: 665–684, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Chan JL, Roth JD, Weyer C. It takes two to tango: combined amylin/leptin agonism as a potential approach to obesity drug development. J Investig Med 57: 777–783, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Clapper JR, Athanacio J, Wittmer C, Griffin PS, D'Souza L, Parkes DG, Roth JD. Effects of amylin and bupropion/naltrexone on food intake and body weight are interactive in rodent models. Eur J Pharmacol 698: 292–298, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Dee A, Kearns K, O'Neill C, Sharp L, Staines A, O'Dwyer V, Fitzgerald S, Perry IJ. The direct and indirect costs of both overweight and obesity: a systematic review. BMC Res Notes 7: 242, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunican KC, Adams NM, Desilets AR. The role of pramlintide for weight loss. Ann Pharmacother 44: 538–545, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Eiden S, Daniel C, Steinbrueck A, Schmidt I, Simon E. Salmon calcitonin - a potent inhibitor of food intake in states of impaired leptin signalling in laboratory rodents. J Physiol 541: 1041–1048, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51: 811–822, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Grabler V, Lutz TA. Chronic infusion of the amylin antagonist AC 187 increases feeding in Zucker fa/fa rats but not in lean controls. Physiol Behav 81: 481–488, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol 31: 61–78, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51: 801–810, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Kanoski SE, Zhao S, Guarnieri DJ, DiLeone RJ, Yan J, De Jonghe BC, Bence KK, Hayes MR, Grill HJ. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. Am J Physiol Endocrinol Metab 303: E496–E503, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karl JP, Roberts SB. Energy density, energy intake, and body weight regulation in adults. Adv Nutr 5: 835–850, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Foll C, Johnson MD, Dunn-Meynell A, Boyle CN, Lutz TA, Levin BE. Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes 64: 1621–1631, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, Munzberg H, Myers MG Jr. Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci 30: 5713–5723, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutz TA. The interaction of amylin with other hormones in the control of eating. Diabetes Obes Metab 15: 99–111, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Lutz TA. Roles of amylin in satiation, adiposity and brain development. Forum Nutr 63: 64–74, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Lutz TA, Del Prete E, Scharrer E. Subdiaphragmatic vagotomy does not influence the anorectic effect of amylin. Peptides 16: 457–462, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Lutz TA, Geary N, Szabady MM, Del Prete E, Scharrer E. Amylin decreases meal size in rats. Physiol Behav 58: 1197–1202, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int J Obes Relat Metab Disord 25: 1005–1011, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Lutz TA, Senn M, Althaus J, Del Prete E, Ehrensperger F, Scharrer E. Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene-related peptide (CGRP) in rats. Peptides 19: 309–317, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Matheny M, Shapiro A, Tumer N, Scarpace PJ. Region-specific diet-induced and leptin-induced cellular leptin resistance includes the ventral tegmental area in rats. Neuropharmacology 60: 480–487, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meye FJ, Adan RA. Feelings about food: the ventral tegmental area in food reward and emotional eating. Trends Pharmacol Sci 35: 31–40, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Mietlicki-Baase EG, Hayes MR. Amylin activates distributed CNS nuclei to control energy balance. Physiol Behav 136: 39–46, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, Hayes MR. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab 305: E1367–E1374, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mietlicki-Baase EG, Reiner DJ, Cone JJ, Olivos DR, McGrath LE, Zimmer DJ, Roitman MF, Hayes MR. Amylin modulates the mesolimbic dopamine system to control energy balance. Neuropsychopharmacology 40: 372–385, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mietlicki-Baase EG, Rupprecht LE, Olivos DR, Zimmer DJ, Alter MD, Pierce RC, Schmidt HD, Hayes MR. Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology 38: 1685–1697, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mollet A, Gilg S, Riediger T, Lutz TA. Infusion of the amylin antagonist AC 187 into the area postrema increases food intake in rats. Physiol Behav 81: 149–155, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Mollet A, Meier S, Grabler V, Gilg S, Scharrer E, Lutz TA. Endogenous amylin contributes to the anorectic effects of cholecystokinin and bombesin. Peptides 24: 91–98, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Moon HS, Chamberland JP, Diakopoulos KN, Fiorenza CG, Ziemke F, Schneider B, Mantzoros CS. Leptin and amylin act in an additive manner to activate overlapping signaling pathways in peripheral tissues: in vitro and ex vivo studies in humans. Diabetes Care 34: 132–138, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon HS, Chamberland JP, Mantzoros CS. Amylin and leptin activate overlapping signalling pathways in an additive manner in mouse GT1-7 hypothalamic, C2C12 muscle and AML12 liver cell lines. Diabetologia 55: 215–225, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moon HS, Dincer F, Mantzoros CS. Amylin-induced downregulation of hippocampal neurogenesis is attenuated by leptin in a STAT3/AMPK/ERK-dependent manner in mice. Diabetologia 56: 627–634, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol 31: 104–112, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen N, Champion JK, Ponce J, Quebbemann B, Patterson E, Pham B, Raum W, Buchwald JN, Segato G, Favretti F. A review of unmet needs in obesity management. Obes Surg 22: 956–966, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Osto M, Wielinga PY, Alder B, Walser N, Lutz TA. Modulation of the satiating effect of amylin by central ghrelin, leptin and insulin. Physiol Behav 91: 566–572, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Pérez Rodrigo C. Current mapping of obesity. Nutr Hosp 28, Suppl 5: 21–31, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Popkin BM, Slining MM. New dynamics in global obesity facing low- and middle-income countries. Obes Rev 14, Suppl 2: 11–20, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potes CS, Boyle CN, Wookey PJ, Riediger T, Lutz TA. Involvement of the extracellular signal-regulated kinase 1/2 signaling pathway in amylin's eating inhibitory effect. Am J Physiol Regul Integr Comp Physiol 302: R340–R351, 2012. [DOI] [PubMed] [Google Scholar]
- 46.Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 17: 1736–1743, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roth JD. Amylin and the regulation of appetite and adiposity: recent advances in receptor signaling, neurobiology and pharmacology. Curr Opin Endocrinol Diabetes Obes 20: 8–13, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA 105: 7257–7262, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roth JD, Trevaskis JL, Turek VF, Parkes DG. “Weighing in” on synergy: preclinical research on neurohormonal anti-obesity combinations. Brain Res 1350: 86–94, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Rushing PA, Hagan MM, Seeley RJ, Lutz TA, Woods SC. Amylin: a novel action in the brain to reduce body weight. Endocrinology 141: 850–853, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Sadry SA, Drucker DJ. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nat Rev Endocrinol 9: 425–433, 2013. [DOI] [PubMed] [Google Scholar]
- 52.Singh-Franco D, Perez A, Harrington C. The effect of pramlintide acetate on glycemic control and weight in patients with type 2 diabetes mellitus and in obese patients without diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 13: 169–180, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Smith SR, Blundell JE, Burns C, Ellero C, Schroeder BE, Kesty NC, Chen KS, Halseth AE, Lush CW, Weyer C. Pramlintide treatment reduces 24-h caloric intake and meal sizes and improves control of eating in obese subjects: a 6-wk translational research study. Am J Physiol Endocrinol Metab 293: E620–E627, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Tam CS, Lecoultre V, Ravussin E. Novel strategy for the use of leptin for obesity therapy. Expert Opin Biol Ther 11: 1677–1685, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson JL, Borgland SL. Presynaptic leptin action suppresses excitatory synaptic transmission onto ventral tegmental area dopamine neurons. Biol Psychiatry 73: 860–868, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Trevaskis JL, Coffey T, Cole R, Lei C, Wittmer C, Walsh B, Weyer C, Koda J, Baron AD, Parkes DG, Roth JD. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology 149: 5679–5687, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Trevaskis JL, Lei C, Koda JE, Weyer C, Parkes DG, Roth JD. Interaction of leptin and amylin in the long-term maintenance of weight loss in diet-induced obese rats. Obesity (Silver Spring) 18: 21–26, 2010. [DOI] [PubMed] [Google Scholar]
- 58.Trinko R, Gan G, Gao XB, Sears RM, Guarnieri DJ, DiLeone RJ. Erk1/2 mediates leptin receptor signaling in the ventral tegmental area. PLoS One 6: e27180, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turek VF, Trevaskis JL, Levin BE, Dunn-Meynell AA, Irani B, Gu G, Wittmer C, Griffin PS, Vu C, Parkes DG, Roth JD. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology 151: 143–152, 2010. [DOI] [PubMed] [Google Scholar]
- 60.Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med 2: 577–593, 2010. [DOI] [PubMed] [Google Scholar]


