Abstract
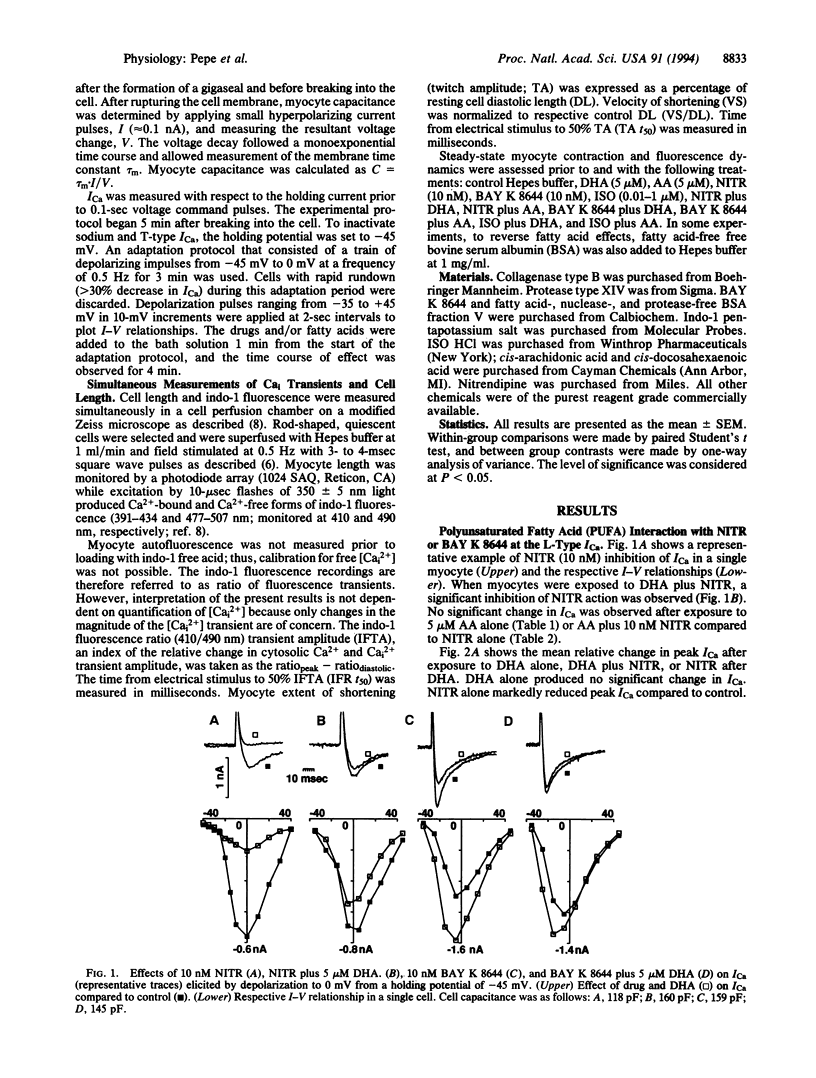
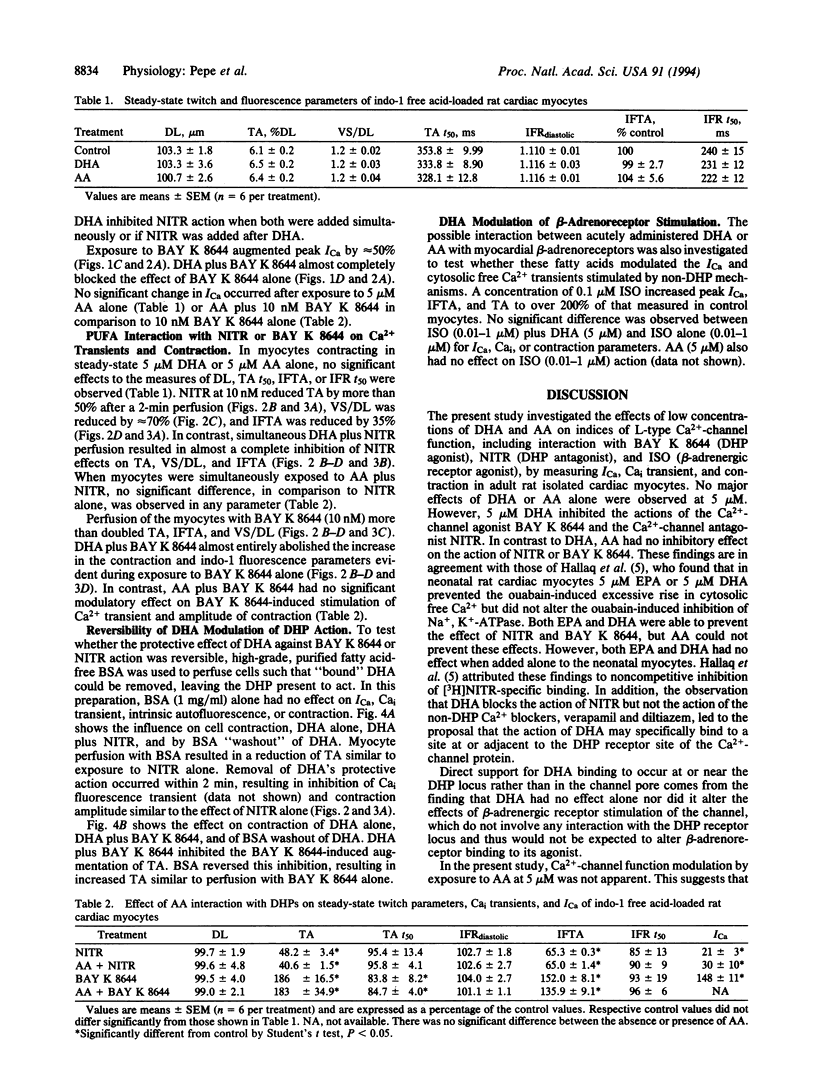
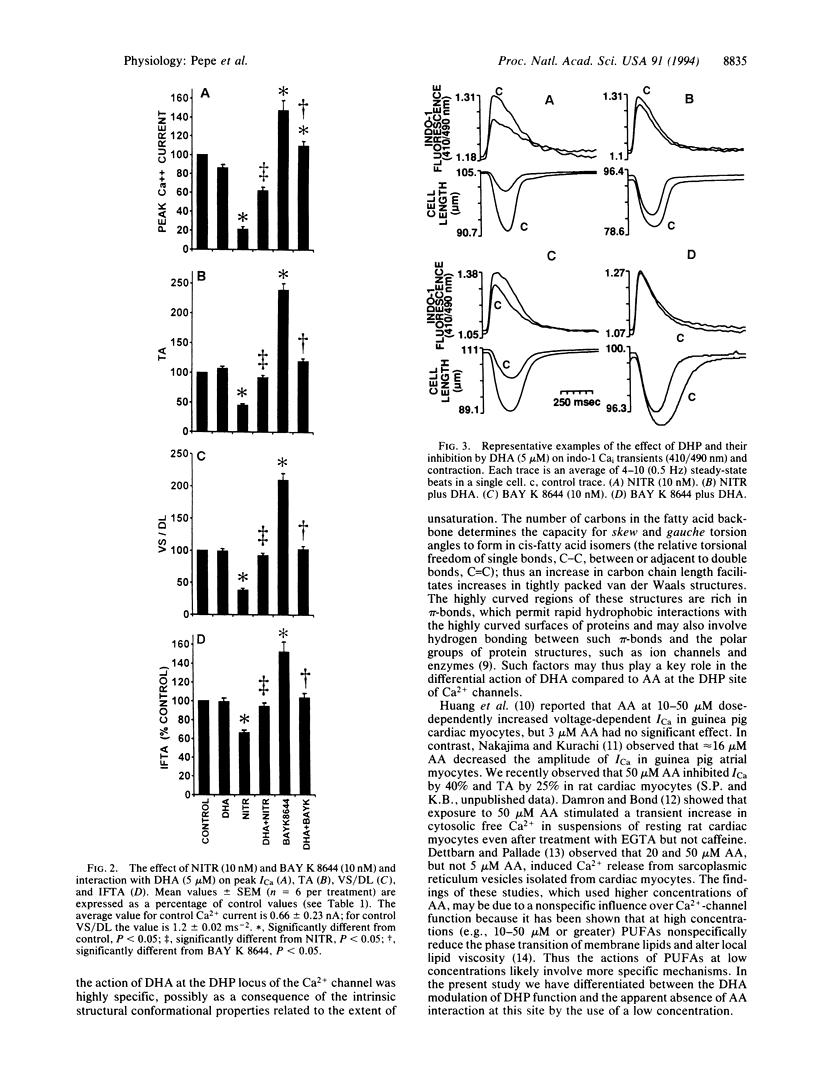
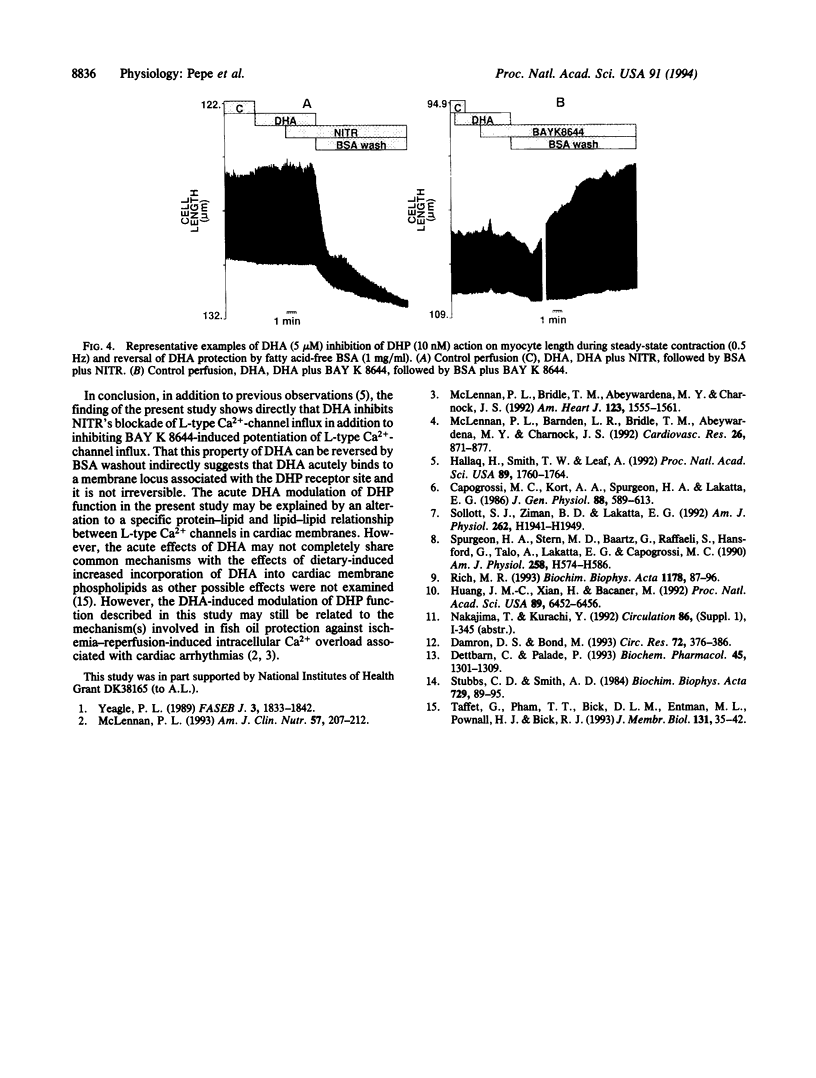
The effect of docosahexaenoic acid (DHA; C22:6) on dihydropyridine (DHP) interaction with L-type Ca2+ channel current (ICa), cytosolic Ca2+ (Cai), and cell contraction in isolated adult rat cardiac myocytes was studied. The DHP L-type Ca(2+)-channel blocker nitrendipine (10 nM) reduced peak ICa (measured by whole-cell voltage clamp from -45 to 0 mV) and reduced the amplitude of the Ca2+ transient (measured as the transient in indo-1 fluorescence, 410/490 nm) and the twitch amplitude (measured via photodiode array) during steady-state electrical stimulation (0.5 Hz). The DHP L-type Ca2+ channel agonist BAY K 8644 (10 nM) significantly increased ICa, the amplitude of the Cai transient, and contraction. When cells were exposed to DHA (5 microM) simultaneously with either BAY K 8644 or nitrendipine, the drug effects were abolished. Arachidonic acid (C20:4) at 5 microM did not block the inhibitory effects of nitrendipine nor did it prevent the potentiating effects of BAY K 8644. DHA modulation of DHP action could be reversed by cell perfusion with fatty acid-free bovine serum albumin at 1 mg/ml. Neither DHA nor arachidonic acid alone (5 microM) had any apparent effect on the parameters measured. DHA (5 microM) had no influence over beta-adrenergic receptor stimulation (isoproterenol, 0.01-1 microM)-induced increases in ICa, Cai, or contraction. The findings that DHA inhibits the effect of DHP agonists and antagonists on Ca(2+)-channel current but has no effect alone or on beta-adrenergic-induced increases in ICa suggests that DHA specifically binds to Ca2+ channels at or near DHP binding sites and interferes with ICa modulation.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capogrossi M. C., Kort A. A., Spurgeon H. A., Lakatta E. G. Single adult rabbit and rat cardiac myocytes retain the Ca2+- and species-dependent systolic and diastolic contractile properties of intact muscle. J Gen Physiol. 1986 Nov;88(5):589–613. doi: 10.1085/jgp.88.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damron D. S., Bond M. Modulation of Ca2+ cycling in cardiac myocytes by arachidonic acid. Circ Res. 1993 Feb;72(2):376–386. doi: 10.1161/01.res.72.2.376. [DOI] [PubMed] [Google Scholar]
- Dettbarn C., Palade P. Arachidonic acid-induced Ca2+ release from isolated sarcoplasmic reticulum. Biochem Pharmacol. 1993 Mar 24;45(6):1301–1309. doi: 10.1016/0006-2952(93)90283-3. [DOI] [PubMed] [Google Scholar]
- Hallaq H., Smith T. W., Leaf A. Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1760–1764. doi: 10.1073/pnas.89.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. M., Xian H., Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6452–6456. doi: 10.1073/pnas.89.14.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan P. L., Barnden L. R., Bridle T. M., Abeywardena M. Y., Charnock J. S. Dietary fat modulation of left ventricular ejection fraction in the marmoset due to enhanced filling. Cardiovasc Res. 1992 Sep;26(9):871–877. doi: 10.1093/cvr/26.9.871. [DOI] [PubMed] [Google Scholar]
- McLennan P. L., Bridle T. M., Abeywardena M. Y., Charnock J. S. Dietary lipid modulation of ventricular fibrillation threshold in the marmoset monkey. Am Heart J. 1992 Jun;123(6):1555–1561. doi: 10.1016/0002-8703(92)90809-a. [DOI] [PubMed] [Google Scholar]
- McLennan P. L. Relative effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on cardiac arrhythmias in rats. Am J Clin Nutr. 1993 Feb;57(2):207–212. doi: 10.1093/ajcn/57.2.207. [DOI] [PubMed] [Google Scholar]
- Rich M. R. Conformational analysis of arachidonic and related fatty acids using molecular dynamics simulations. Biochim Biophys Acta. 1993 Jul 28;1178(1):87–96. doi: 10.1016/0167-4889(93)90113-4. [DOI] [PubMed] [Google Scholar]
- Sollott S. J., Ziman B. D., Lakatta E. G. Novel technique to load indo-1 free acid into single adult cardiac myocytes to assess cytosolic Ca2+. Am J Physiol. 1992 Jun;262(6 Pt 2):H1941–H1949. doi: 10.1152/ajpheart.1992.262.6.H1941. [DOI] [PubMed] [Google Scholar]
- Spurgeon H. A., Stern M. D., Baartz G., Raffaeli S., Hansford R. G., Talo A., Lakatta E. G., Capogrossi M. C. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am J Physiol. 1990 Feb;258(2 Pt 2):H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- Stubbs C. D., Smith A. D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984 Jan 27;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Taffet G. E., Pham T. T., Bick D. L., Entman M. L., Pownall H. J., Bick R. J. The calcium uptake of the rat heart sarcoplasmic reticulum is altered by dietary lipid. J Membr Biol. 1993 Jan;131(1):35–42. doi: 10.1007/BF02258532. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Lipid regulation of cell membrane structure and function. FASEB J. 1989 May;3(7):1833–1842. [PubMed] [Google Scholar]



